Discovery of Novel Benzamide-Based Sigma-1 Receptor Agonists with Enhanced Selectivity and Safety
Abstract
1. Introduction
2. Results and Discussion
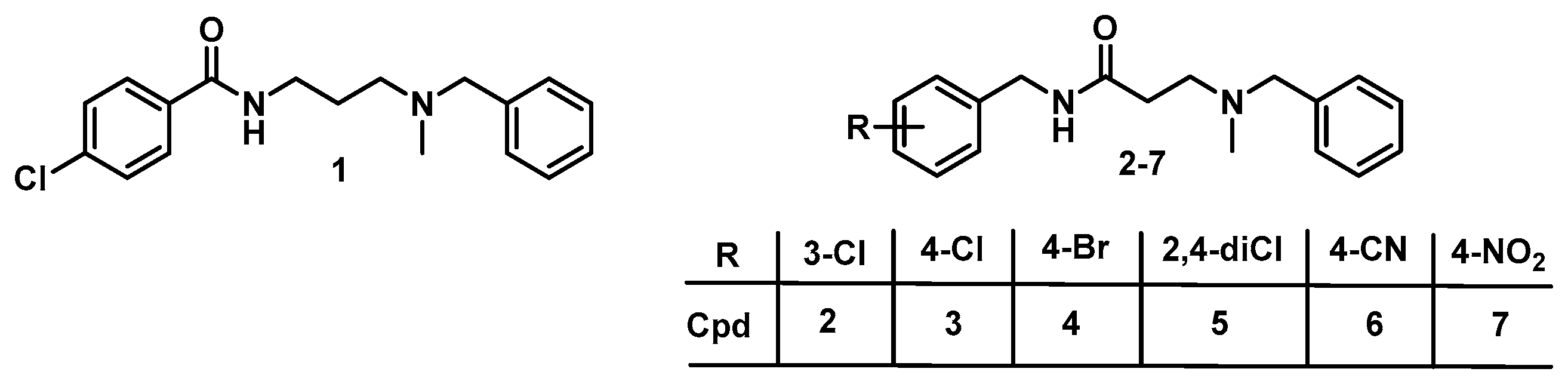
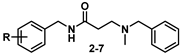
2.1. Chemistry
2.2. Biological Evaluation and SAR
2.3. Docking Study of Compounds
2.4. Off-Target Analysis over a Panel of 58 Recombinant Human Receptors
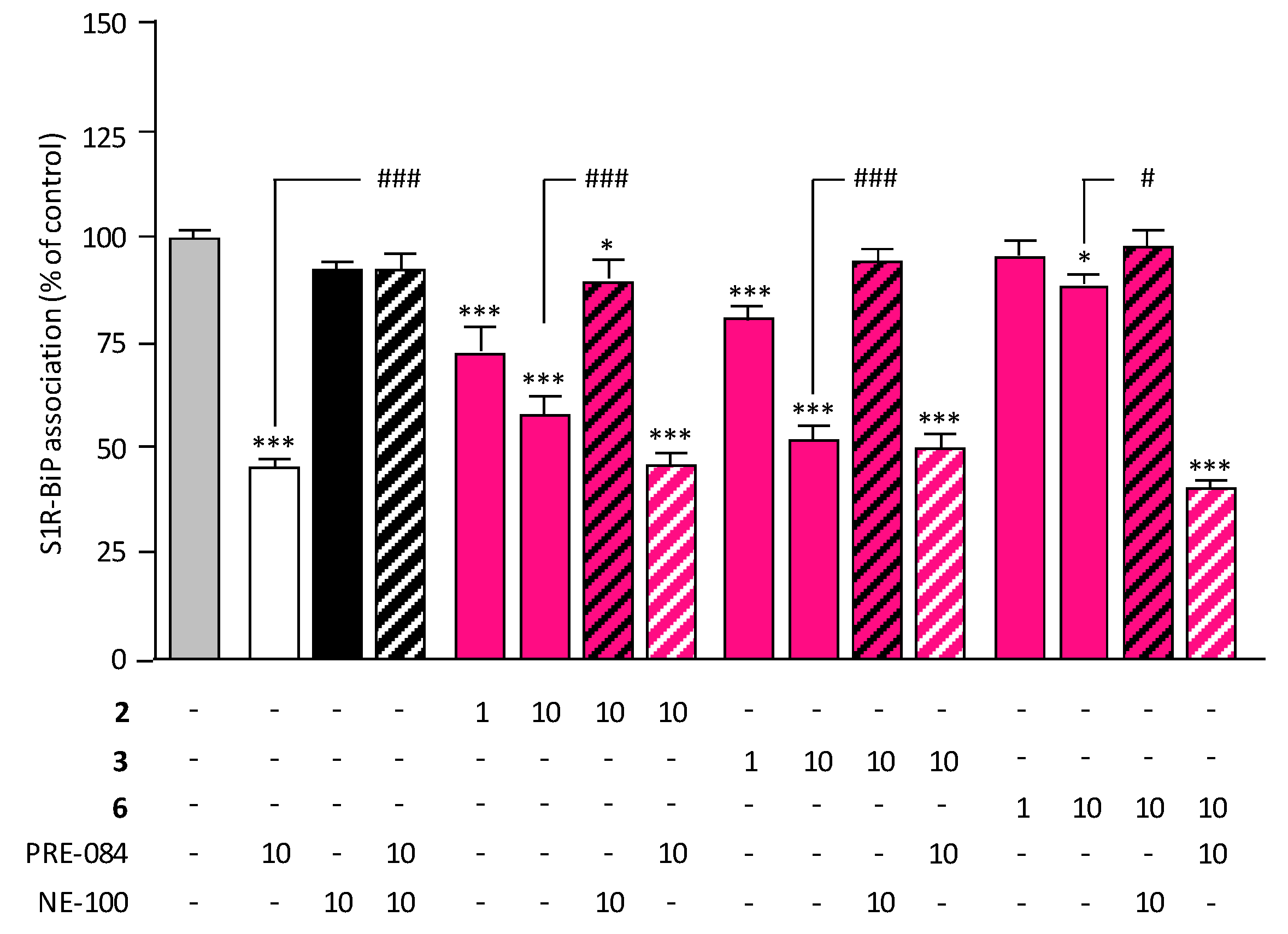
2.5. Target Engagement and Agonist Profile of Selected Compounds
2.6. ADME Studies of Selected Compounds
3. Materials and Methods
3.1. Chemistry
3.2. Biological Activity
3.2.1. Assay for Binding to Sigma Receptors
3.2.2. Assay for Cytotoxicity
3.2.3. Selectivity Profile (Agonist and Antagonist Activities) over a Panel of 58 Recombinant Human Receptors
3.2.4. In Vitro Evaluation of S1R Functionality
3.2.5. Physicochemical Properties
3.3. Molecular Docking
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zang, Y.; Rao, X.; Wang, J.; Liu, H.; Wang, Q.; Wang, X.; Hua, F.; Guan, X.; Lin, Y. Mitochondria-Associated Membranes: A Key Point of Neurodegenerative Diseases. CNS Neurosci. Ther. 2025, 31, e70378. [Google Scholar] [CrossRef]
- Jia, Z.; Li, H.; Xu, K.; Li, R.; Yang, S.; Chen, L.; Zhang, Q.; Li, S.; Sun, X. MAM-mediated mitophagy and endoplasmic reticulum stress: The hidden regulators of ischemic stroke. Front. Cell. Neurosci. 2024, 18, 1470144. [Google Scholar] [CrossRef]
- Watanabe, S.; Yamanaka, K. Mitochondria and Endoplasmic Reticulum Contact Site as a Regulator of Proteastatic Stress Responses in Neurodegenerative Diseases. BioEssays 2025, 47, e70016. [Google Scholar] [CrossRef] [PubMed]
- Delprat, B.; Crouzier, L.; Su, T.P.; Maurice, T. At the Crossing of ER Stress and MAMs: A Key Role of Sigma-1 Receptor ? Adv. Exp. Med. Biol. 2020, 1131, 699–718. [Google Scholar]
- Marlar, D.S.; Thitilertdecha, P.; Ruckvongacheep, K.S.; Brimson, S.; Tencomnao, T.; Brimson, J.M. Targeting Sigma Receptors for the Treatment of Neurodegenerative and Neurodevelopmental disorders. CNS Drugs 2023, 37, 399–440. [Google Scholar] [CrossRef] [PubMed]
- Ngo, A.; Fattakhov, N.; Toborek, M. Sigma-1 receptor signaling: A Potential therapeutic approach for ischemic stroke. J. Cereb. Blood Flow Metab. 2024, 44, 1430–1440. [Google Scholar] [CrossRef]
- Ye, N.; Qin, W.; Tian, S.; Xu, Q.; Wold, E.A.; Zhou, J.; Zhen, X.C. Small Molecules Selectively Targeting Sigma-1 Receptor for the Treatment of Neurological Diseases. J. Med. Chem. 2020, 63, 15187–15217. [Google Scholar] [CrossRef]
- Cummings, J.; Zhou, Y.; Lee, G.; Zhong, K.; Fonseca, J.; Cheng, F. Alzheimer’s disease drug development pipeline: 2024. Alzheimers Dement. 2024, 10, e12465. [Google Scholar] [CrossRef]
- Macfarlane, S.; Grimmer, T.; Teo, K.; O’Brien, T.J.; Woodward, M.; Grunfeld, J.; Mander, A.; Brodtmann, A.; Brew, B.J.; Morris, P.; et al. Blarcamesine for the treatment of Early Alzheimer’d Disease: Results from the ANAVEX2-73-AD-004 Phase IIB/III trial. J. Prev. Alzheimers Dis. 2025, 12, 100016. [Google Scholar] [CrossRef]
- Cenci, M.A.; Skovgard, K.; Odin, P. Non-dopaminergic approaches to the treatment of motor complications in Parkinson’s disease. Neuropharmacology 2022, 210, 109027. [Google Scholar] [CrossRef] [PubMed]
- Van de Roovaart, H.J.; Nguyen, N.; Veenstra, T.D. Huntington’s Disease Drug Development: A Phase 3 Pipeline Analysis. Pharmaceuticals 2023, 16, 1513. [Google Scholar] [CrossRef]
- Cazenave-Gassiot, A.; Charton, J.; Girault-Mizzi, S.; Gilleron, P.; Debreu-Fontaine, M.A.; Sergheraert, C.; Melnyk, P. Synthesis and pharmacological evaluation of Tic-Hydantoin derivatives as selective σ1 ligands. Part 2. Bioorganic Med. Chem. Lett. 2005, 15, 4828–4832. [Google Scholar] [CrossRef]
- Venna, V.R.; Deplancke, D.; Melnyk, P.; Bordet, R. Neuroprotective and antidepressant-like effects of LC 03/55, a novel sigma-1 receptor ligand. Fundam. Clin. Pharmacol. 2008, 22, 1. [Google Scholar]
- Oxombre, B.; Lee-Chang, C.; Duhamel, A.; Toussaint, M.; Giroux, M.; Donnier-Maréchal, M.; Carato, P.; Lefranc, D.; Zephir, H.; Prin, L.; et al. High affinity sigma-1 receptor agonist reduces clinical and pathological signs of experimental auto-immune encephalomyelitis. Br. J. Pharmacol. 2015, 172, 1769–1782. [Google Scholar] [CrossRef] [PubMed]
- Donnier-Maréchal, M.; Carato, P.; Larchanché, P.E.; Ravez, S.; Boulahjar, R.; Barczyk, A.; Oxombre, B.; Vermersch, P.; Melnyk, P. Synthesis and pharmacological evaluation of benzamide derivatives as potent and selective sigma-1 ligands. Eur. J. Med. Chem. 2017, 138, 964–978. [Google Scholar] [CrossRef] [PubMed]
- Oxombre, B.; Madouri, F.; Journé, A.S.; Ravez, S.; Woitrain, E.; Odou, P.; Duhal, N.; Ninni, S.; Montaigne, D.; Delhem, N.; et al. Safe and efficient sigma1 ligand: A potential drug candidate for Multiple Sclerosis. Int. J. Mol. Sci. 2022, 23, 11893. [Google Scholar] [CrossRef]
- Ganapathy, M.E.; Prasad, P.D.; Huang, W.; Seth, P.; Leibach, F.H.; Ganapathy, V. Molecular and ligand-binding characterization of the sigma-receptor in the Jurkat human T lymphocyte cell line. J. Pharmacol. Exp. Ther. 1999, 289, 251–260. [Google Scholar] [CrossRef]
- Schmidt, H.R.; Zheng, S.; Gurpinar, E.; Koehl, A.; Manglik, A.; Kruse, A.C. Crystal structure of the human σ1 receptor. Nature 2016, 532, 527–530. [Google Scholar] [CrossRef]
- Le Poul, E.; Hisada, S.; Mizuguchi, Y.; Dupriez, V.J.; Burgeon, E.; Detheux, M. Adaptation of Aequorin Functional Assay to High Throughput Screening. J. Biomol. Screen. 2002, 7, 57–65. [Google Scholar] [CrossRef][Green Version]
- Degorce, F.; Card, A.; Soh, S.; Trinquet, E.; Knapik, G.P.; Xie, B. HTRF: A Technology Tailored for Drug Discovery—A Review of Theoretical Aspects and Recent Applications. Curr. Chem. Genom. 2009, 3, 22–32. [Google Scholar] [CrossRef]
- Ferrer, M.; Kolodin, G.D.; Zuck, P.; Peltier, R.; Berry, K.; Mandala, S.M.; Rosen, H.; Ota, H.; Ozaki, S.; Inglese, J.; et al. A fully automated [35S]GTPgammaS scintillation proximity assay for the high-throughput screening of Gi-linked G protein-coupled receptors. Assay Drug Dev. Technol. 2003, 1, 261–273. [Google Scholar] [CrossRef]
- Hayashi, T.; Su, T.-P. Sigma-1 Receptor Chaperones at the ER-Mitochondrion Interface Regulate Ca2+ Signaling and Cell Survival. Cell 2007, 131, 596–610. [Google Scholar] [CrossRef]
- Maurice, T.; Hiramatsu, M.; Itoh, J.; Kameyama, T.; Hasegawa, T.; Nabeshima, T. Behavioral Evidence for a Modulating Role of Sigma Ligands in Memory Processes. I. Attenuation of Dizocilpine (MK-801)-Induced Amnesia. Brain Res. 1994, 647, 44–56. [Google Scholar] [CrossRef]
- García-Lupo, L.; Crouzier, L.; Bencomo-Martínez, A.; Meunier, J.; Morilleau, A.; Delprat, B.; Carrazana, M.S.; Menéndez Soto Del Valle, R.; Maurice, T.; Rodríguez-Tanty, C. Amylovis-201 is a dual-target ligand, acting as an anti-amyloidogenic compound and a potent agonist of the sigma-1 chaperone protein. Acta Pharm. Sin. B 2024, 14, 4345–4359. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Hidalgo, I.; Raub, T.; Borchardt, R. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Banker, M.J.; Clark, T.H.; Williams, J.A. Development and validation of a 96-well equilibrium dialysis apparatus for measuring plasma protein binding. J. Pharm. Sci. 2003, 92, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Dierks, E.A.; Stams, K.R.; Lim, H.K.; Cornelius, G.; Zhang, H.; Ball, S.E. A method for the simultaneous evaluation of the activities of seven major human drug-metabolizing cytochrome P450s using an in vitro cocktail of probe substrates and fast gradient liquid chromatography tandem mass spectrometry. Drug Metab. Dispos. 2001, 29, 23–29. [Google Scholar]
- Kido, Y.; Matsson, P.; Giacomini, K.M. Profiling of a Prescription Drug Library for Potential Renal Drug–Drug Interactions Mediated by the Organic Cation Transporter 2. J. Med. Chem. 2011, 54, 4548–4558. [Google Scholar] [CrossRef]
- Craddock, A.L.; Love, M.W.; Daniel, R.W.; Kirby, L.C.; Walters, H.C.; Wong, M.H.; Dawson, P.A. Expression and transport properties of the human ileal and renal sodium-dependent bile acid transporter. Am. J. Physiol. Liver Physiol. 1998, 274, G157–G169. [Google Scholar] [CrossRef]
- Paturi, D.K.; Kwatra, D.; Ananthula, H.K.; Pal, D.; Mitra, A.K. Identification and functional characterization of breast cancer resistance protein in human bronchial epithelial cells (Calu-3). Int. J. Pharm. 2010, 384, 32–38. [Google Scholar] [CrossRef]
- Hamilton, O.K.; Topp, E.; Makagiansar, I.; Siahaan, T.; Yazdanian, M.; Audus, K.L. Multidrug resistance-associated protein-1 functional activity in Calu-3 cells. J. Pharmacol. Exp. Ther. 2001, 298, 1199–1205. [Google Scholar] [CrossRef]
- Matsson, P.; Pedersen, J.M.; Norinder, U.; Bergström, C.A.S.; Artursson, P. Identification of novel specific and general inhibitors of the three major human ATP-binding cassette transporters P-gp, BCRP and MRP2 among registered drugs. Pharm. Res. 2009, 26, 1816–1831. [Google Scholar] [CrossRef]
- Weiss, J.; Theile, D.; Ketabi-Kiyanvash, N.; Lindenmaier, H.; Haefeli, W.E. Inhibition of MRP1/ABCC1, MRP2/ABCC2, and MRP3/ABCC3 by nucleoside.; nucleotide.; and non-nucleoside reverse transcriptase inhibitors. Drug Metab. Dispos. 2007, 35, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.B.; Leake, B.; Cvetkovic, M.; Roden, M.M.; Nadeau, J.; Walubo, A.; Wilkinson, G.R. Modulation by drugs of human hepatic sodium-dependent bile acid transporter (sodium taurocholate cotransporting polypeptide) activity. J. Pharmacol. Exp. Ther. 1999, 291, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Cihlar, T.; Ho, E.S. Fluorescence-based assay for the interaction of small molecules with the human renal organic anion transporter 1. Anal. Biochem. 2000, 283, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Gui, C.; Obaidat, A.; Chaguturu, R.; Hagenbuch, B. Development of a Cell-Based High-Throughput Assay to Screen for Inhibitors of Organic Anion Transporting Polypeptides 1B1 and 1B3. Curr. Chem. Genom. 2010, 4, 1–8. [Google Scholar] [CrossRef]
- Polli, J.; Wring, S.A.; Humphreys, J.E.; Huang, L.; Morgan, J.B.; Webster, L.O.; Serabjit-Singh, C.S. Rational use of in vitro P-glycoprotein assays in drug discovery. J. Pharmacol. Exp. Ther. 2001, 299, 620–628. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Prusoff, W.H. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. [Google Scholar] [CrossRef]
- Fahmi, O.A.; Kish, M.; Boldt, S.; Obach, R.S. Cytochrome P450 3A4 mRNA Is a More Reliable Marker than CYP3A4 Activity for Detecting Pregnane X Receptor-Activated Induction of Drug-Metabolizing Enzymes. Drug Metab. Dispos. 2010, 38, 1605–1611. [Google Scholar] [CrossRef]
- Mathes, C. QPatch: The past, present and future of automated patch clamp. Expert Opin. Ther. Targets 2006, 10, 319–327. [Google Scholar] [CrossRef]
- McNutt, A.; Li, Y.; Meli, R.; Aggarwal, R.; Koes, D.R. GNINA 1.3: The next increment in molecular docking with deep learning (Primary application citation). J. Cheminformatics 2025, 17, 28. [Google Scholar] [CrossRef]
- Melnyk, P.; Vermersch, P.; Carato, P.; Vanteghem-Oxombre, B.; Zephir, H.; Donnier-Maréchal, M. Compounds, Pharmaceutical Composition and Their Use in Treating Neurodegenerative Diseases. Patent WO/2015/193255, 16 June 2014. [Google Scholar]


 | ||||||
|---|---|---|---|---|---|---|
| Cpd. | R | S1R (Ki nM) a | S2R (Ki nM) a | Ki (S2R)/ Ki (S1R) | SY5Y % Cytotox b | IC50 (SY5Y) c/ Ki (S1R) |
| 1 | 4-Cl | 3.2 | 190 | 60 | 28 | >31,250 |
| 2 | 3-Cl | 0.6 | 200 | 317 | 12 | >166,666 |
| 3 | 4-Cl | 1.7 | 410 | 241 | 1 | >58,823 |
| 4 | 3-Br | >200 | >200 | nd | 6 | nd |
| 5 | 2,4-diCl | 2.3 | 120 | 52 | 37 | >43,478 |
| 6 | 4-CN | 5.6 | 1800 | 331 | 8 | >17,857 |
| 7 | 4-NO2 | 110 | 6500 | 59 | 9 | >909 |
| Cpd | 5-HT1A | 5-HT1B | 5-HT2A | 5-HT2B | 5-HT2C | 5-HT3 | 5-HT4e | 5-HT5a | 5-HT6 | 5-HT7 | A1 | A2A | A3 | β1 | β2 | α1a | α1b | |||||||||||||||||
| [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | |
| 1 [19] | 92 | 73 | ||||||||||||||||||||||||||||||||
| 2 | 72 | 68 | 64 | 70 | ||||||||||||||||||||||||||||||
| 3 | 76 | 72 | 76 | 88 | 65 | |||||||||||||||||||||||||||||
| 6 | 93 | 56 | 52 | |||||||||||||||||||||||||||||||
| 7 | 80 | 61 | ||||||||||||||||||||||||||||||||
| Cpd | α2a | α2b | α2c | AT1 | CB1 | CB2 | CCK1 | CCK2 | CGRP | CXCR1 | CysLT1 | D1 | D2 | D3 | D4.4 | Eta | ETb | |||||||||||||||||
| [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | |
| 1 [19] | ||||||||||||||||||||||||||||||||||
| 2 | 61 | 59 | 64 | |||||||||||||||||||||||||||||||
| 3 | 54 | 84 | ||||||||||||||||||||||||||||||||
| 6 | ||||||||||||||||||||||||||||||||||
| 7 | ||||||||||||||||||||||||||||||||||
| Cpd | GHS-R | H1 | H2 | H3 | MTL | M1 | M2 | M3 | M4 | M5 | ORL1 | NK1 | NK2 | NPY1 | NMDA | |||||||||||||||||||
| [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [b] | ||||||
| 1 [19] | ||||||||||||||||||||||||||||||||||
| 2 | ||||||||||||||||||||||||||||||||||
| 3 | ||||||||||||||||||||||||||||||||||
| 6 | ||||||||||||||||||||||||||||||||||
| 7 | ||||||||||||||||||||||||||||||||||
| Cpd | δ | κ | µ | SST4 | Uroll | V1a | V1b | VPAC1 | B1 | |||||||||||||||||||||||||
| [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | [a] | [b] | |||||||||||||||||
| 1 [19] | ||||||||||||||||||||||||||||||||||
| 2 | ||||||||||||||||||||||||||||||||||
| 3 | ||||||||||||||||||||||||||||||||||
| 6 | ||||||||||||||||||||||||||||||||||
| 7 | ||||||||||||||||||||||||||||||||||
| 1 [16] | 2 | 6 | |
|---|---|---|---|
| Solubility (µM) | |||
| PBSpH7.4 | 185 | 167 | 190 |
| SGF | 193 | 186 | 178 |
| SIF | 188 | 181 | 190 |
| Permeability (×10−6 cm/s) (10 µM) | |||
| A/B pH7.4/7.4 | 29.1 | 39 | 49.8 |
| B/A pH7.4/7.4 | 66.1 | 67.4 | 60.9 |
| e-ratio | 2.3 | 1.7 | 1.2 |
| Plasma protein binding (10 µM) % | 94 | 98 | 63 |
| Mouse plasma t1/2 (h) | 25.9 | 35 | >48 |
| Metabolic stability (10 µM) | |||
| Human liver microsome | |||
| Intrinsic clearance (µL/min/pmol) | <115.5 | 135.3 | <115.5 |
| Half-life t1/2 (min) | >60 | 51 | >60 |
| CYP inhibition (% at 10 µM) | |||
| 1A | 2.1 | 23.3 | 13.2 |
| 2B6 | −4.3 | 16.9 | 17.7 |
| 2C8 | −9.2 | −16.8 | −12.1 |
| 2C9 | −7.2 | 13.1 | 10 |
| 2C19 | 36.2 | 43.9 | 30 |
| 2D6 | 95.1 | 80.5 | 87.7 |
| 3A | 36.9 | 30.9 | 17.4 |
| Drug transport inhibition (% at 10 µM) | |||
| ABC family | |||
| P-gp | 10.6 | 4.7 | 4.7 |
| BCRP | 8.3 | −9 | −5.8 |
| MRP1 | 1.9 | −0.3 | −1.1 |
| MRP2 | −18.6 | 5.6 | 3.3 |
| MRP3 | −0.3 | 0.7 | 0.4 |
| SLC family | |||
| OAT1 | −9.6 | −23.1 | −25.8 |
| OAT3 | 34.3 | 33.3 | 27.8 |
| OATP1B1 | 12 | 3.9 | 1.3 |
| OCT1 | 41.2 | 19.9 | 2.4 |
| OCT2 | 49.4 | 54.1 | 59.3 |
| ABST | 5.3 | 21.8 | −8.9 |
| NTCP | −1.7 | 8.7 | −12.5 |
| hERG inhibition | |||
| IC50 (µM) | 1.0 | 6.8 | 0.8 |
| IC50 (hERG)/Ki (σ1) | 312 | 11,333 | 143 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carato, P.; Oxombre, B.; Ravez, S.; Boulahjar, R.; Donnier-Maréchal, M.; Barczyk, A.; Liberelle, M.; Vermersch, P.; Melnyk, P. Discovery of Novel Benzamide-Based Sigma-1 Receptor Agonists with Enhanced Selectivity and Safety. Molecules 2025, 30, 3584. https://doi.org/10.3390/molecules30173584
Carato P, Oxombre B, Ravez S, Boulahjar R, Donnier-Maréchal M, Barczyk A, Liberelle M, Vermersch P, Melnyk P. Discovery of Novel Benzamide-Based Sigma-1 Receptor Agonists with Enhanced Selectivity and Safety. Molecules. 2025; 30(17):3584. https://doi.org/10.3390/molecules30173584
Chicago/Turabian StyleCarato, Pascal, Bénédicte Oxombre, Séverine Ravez, Rajaa Boulahjar, Marion Donnier-Maréchal, Amélie Barczyk, Maxime Liberelle, Patrick Vermersch, and Patricia Melnyk. 2025. "Discovery of Novel Benzamide-Based Sigma-1 Receptor Agonists with Enhanced Selectivity and Safety" Molecules 30, no. 17: 3584. https://doi.org/10.3390/molecules30173584
APA StyleCarato, P., Oxombre, B., Ravez, S., Boulahjar, R., Donnier-Maréchal, M., Barczyk, A., Liberelle, M., Vermersch, P., & Melnyk, P. (2025). Discovery of Novel Benzamide-Based Sigma-1 Receptor Agonists with Enhanced Selectivity and Safety. Molecules, 30(17), 3584. https://doi.org/10.3390/molecules30173584








