Airborne Cyanobacterial Toxins and Their Links to Neurodegenerative Diseases
Abstract
1. Introduction
2. Cyanobacterial Neurotoxins
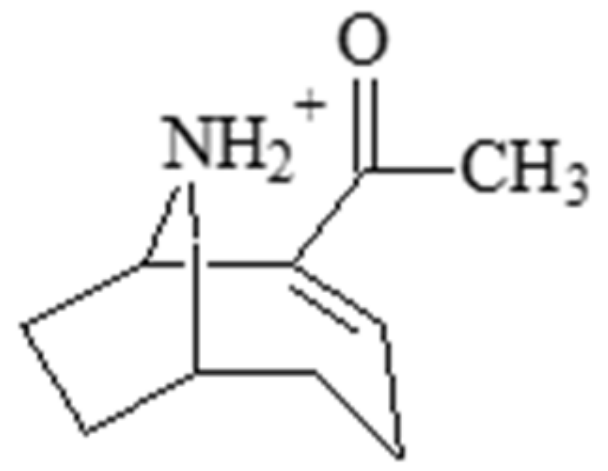
2.1. Anatoxin-a
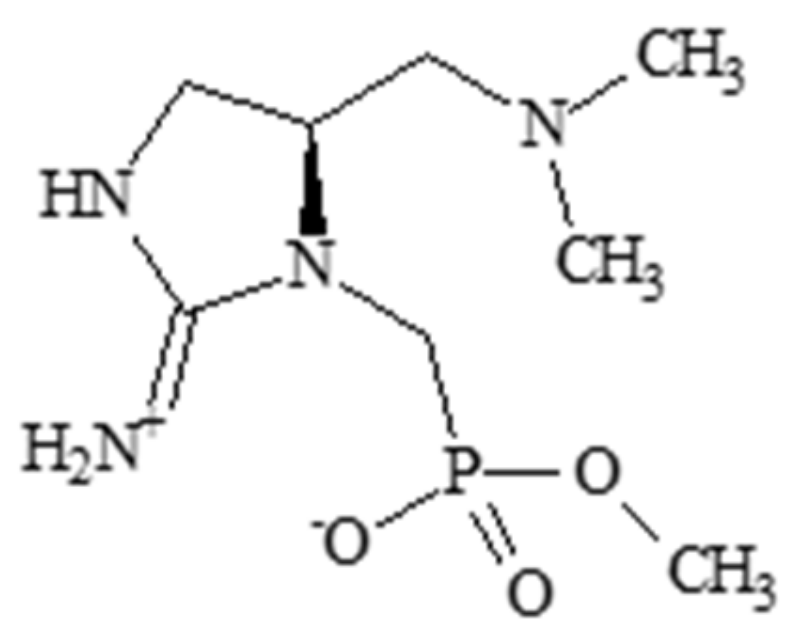
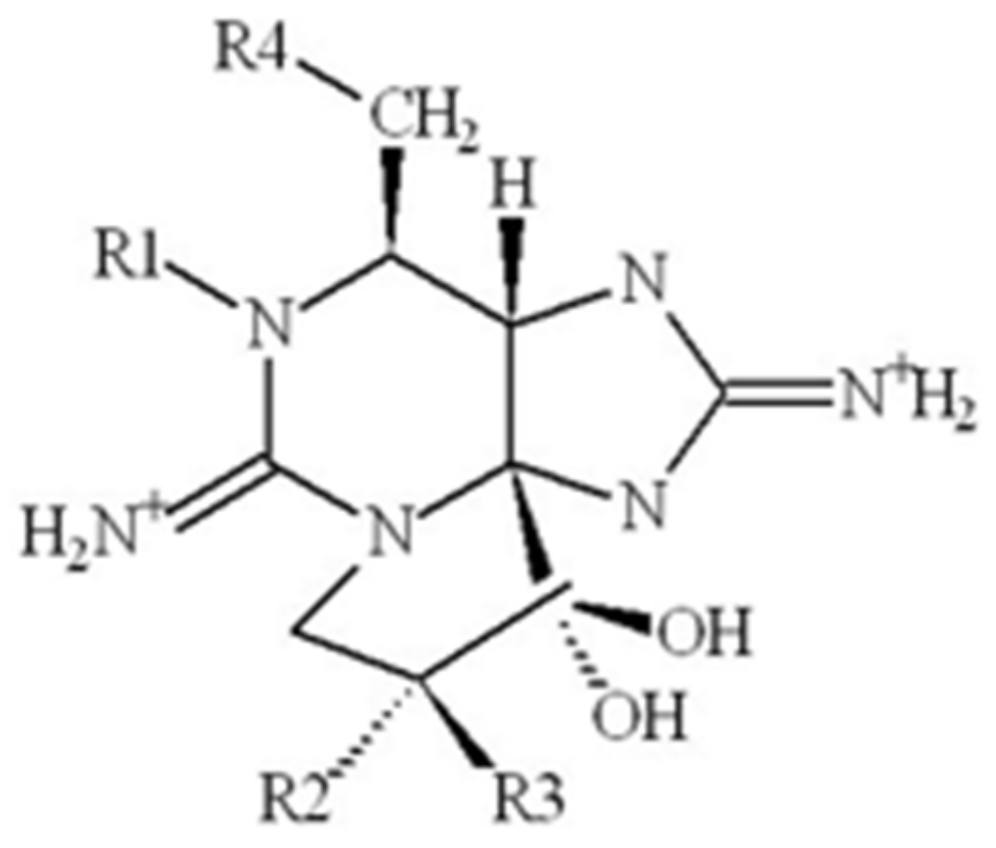
2.2. Guanitoxin
2.3. Saxitoxin
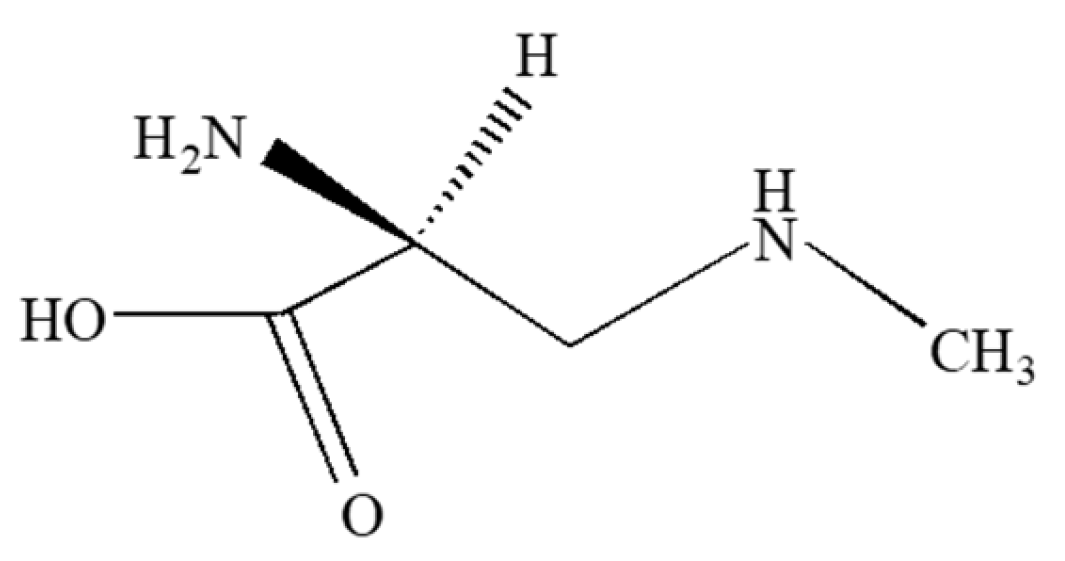
2.4. Neurotoxic Amino Acids
2.5. Microcystin
2.6. Cylindrospermopsin
3. Cyanotoxin Exposure Routes
3.1. Exposure to Cyanotoxins from Aquatic Environments
3.2. Exposure to Cyanotoxins from Terrestrial Environments
3.3. Exposure to Cyanotoxins from Food and Dietary Supplements
3.4. Exposure to Cyanotoxins in the Atmosphere
4. Cyanobacteria and Cyanotoxins in the Atmosphere
5. Health Implications of Atmospheric Cyanobacteria and Cyanotoxins
5.1. Possible Consequences of Exposure to Atmospheric Cyanobacteria and Associated Neurotoxins
5.2. Atmospheric Cyanobacteria and the Spread of Toxins
5.3. Occupational Exposure
5.4. Cyanobacteria and Cyanotoxins in Indoor Environments
5.5. Dose-Response for Inhaled Cyanotoxins
5.6. Biomarkers for Inhalation of Cyanotoxins
6. Mitigation of Cyanobacteria and Cyanotoxins in the Atmosphere
7. Research Gaps
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schopf, J.W.; Packer, B.M. Early Archean (3.3-billion to 3.5-billion-year-old) microfossils from Warrawoona Group, Australia. Science 1987, 237, 70. [Google Scholar] [CrossRef] [PubMed]
- Papke, R.T.; Ramsing, N.B.; Bateson, M.M.; Ward, D.M. Geographical isolation in hot spring cyanobacteria. Environ. Microbiol. 2003, 5, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Kulasooriya, S.A. Cyanobacteria: Pioneers of planet earth. Ceylon J. Sci. (Biol. Sci.) 2012, 40, 71–88. [Google Scholar] [CrossRef]
- Álvarez, C.; Jiménez-Ríos, L.; Iniesta-Pallarés, M.; Jurado-Flores, A.; Molina-Heredia, F.P.; Ng, C.K.; Mariscal, V. Symbiosis between cyanobacteria and plants: From molecular studies to agronomic applications. J. Exp. Bot. 2023, 74, 6145–6157. [Google Scholar] [CrossRef]
- Gaysina, L.A.; Saraf, A.; Singh, P. Cyanobacteria in diverse habitats. In Cyanobacteria; Academic Press: Cambridge, MA, USA, 2019; pp. 1–28. [Google Scholar]
- Polyak, Y.; Zaytseva, T.; Medvedeva, N. Response of toxic cyanobacterium Microcystis aeruginosa to environmental pollution. Water Air Soil Pollut. 2013, 224, 1494. [Google Scholar] [CrossRef]
- Ramakrishnan, B.; Megharaj, M.; Venkateswarlu, K.; Naidu, R.; Sethunathan, N. The impacts of environmental pollutants on microalgae and cyanobacteria. Crit. Rev. Environ. Sci. Technol. 2010, 40, 699–821. [Google Scholar] [CrossRef]
- Fujimoto, N.; Sudo, R.; Sugiura, N.; Inamori, Y. Nutrient-limited growth of Microcystis aeruginosa and Phormidium tenue and competition under various N: P supply ratios and temperatures. Limnol. Oceanogr. 1997, 42, 250–256. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Grizzetti, B.; Bouraoui, F.; Aloe, A. Changes of nitrogen and phosphorus loads to European seas. Glob. Change Biol. 2012, 18, 769–782. [Google Scholar] [CrossRef]
- Grizzetti, B.; Billen, G.; Davidson, E.A.; Winiwarter, W.; de Vries, W.; Fowler, D.; Howard, C.M.; Bleeker, A.; Sutton, M.A.; Lassaletta, L.; et al. Global nitrogen and phosphorus pollution. In Just Enough Nitrogen: Perspectives on How to Get There for Regions with Too Much and Too Little Nitrogen; Springer: Berlin/Heidelberg, Germany, 2020; pp. 421–431. [Google Scholar]
- Michalak, A.M.; Anderson, E.J.; Beletsky, D.; Boland, S.; Bosch, N.S.; Bridgeman, T.B.; Chaffin, J.D.; Cho, K.; Confesor, R.; Daloğlu, I. Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 6448–6452. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.B.; Miller, C.; Arhonditsis, G.; Boyer, G.L.; Carmichael, W.; Charlton, M.N.; Confesor, R.; Depew, D.; Höök, T.O.; Ludsin, S.A. The re-eutrophication of Lake Erie: Harmful algal blooms and hypoxia. Harmful Algae 2016, 56, 44–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Boegman, L.; Scavia, D.; Culver, D.A. Spatial distributions of external and internal phosphorus loads in Lake Erie and their impacts on phytoplankton and water quality. J. Great Lakes Res. 2016, 42, 1212–1227. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Li, Z.; Yu, J.; Yang, M.; Zhang, J.; Burch, M.D.; Han, W. Cyanobacterial population and harmful metabolites dynamics during a bloom in Yanghe Reservoir, North China. Harmful Algae 2010, 9, 481–488. [Google Scholar] [CrossRef]
- Lomeo, D.; Tebbs, E.J.; Babayani, N.D.; Chadwick, M.A.; Gondwe, M.J.; Jungblut, A.D.; McCulloch, G.P.; Morgan, E.R.; Schillereff, D.N.; Simis, S.G.H.; et al. Remote sensing and spatial analysis reveal unprecedented cyanobacteria bloom dynamics associated with elephant mass mortality. Sci. Total Environ. 2024, 957, 177525. [Google Scholar] [CrossRef] [PubMed]
- Backer, L.C.; Landsberg, J.H.; Miller, M.; Keel, K.; Taylor, T.K. Canine cyanotoxin poisonings in the United States (1920s–2012): Review of suspected and confirmed cases from three data sources. Toxins 2013, 5, 1597–1628. [Google Scholar] [CrossRef]
- Falconer, I.R.; Humpage, A.R. Health risk assessment of cyanobacterial (blue-green algal) toxins in drinking water. Int. J. Environ. Res. Public Health 2005, 2, 43–50. [Google Scholar] [CrossRef]
- Negri, A.P.; Jones, G.J.; Hindmarsh, M. Sheep mortality associated with paralytic shellfish poisons from the cyanobacterium Anabaena circinalis. Toxicon 1995, 33, 1321–1329. [Google Scholar] [CrossRef]
- Thomas, A.; Saker, M.; Norton, J.; Olsen, R. Cyanobacterium Cylindrospermopsis raciborskii as a probable cause of death in cattle in northern Queensland. Aust. Vet. J. 1998, 76, 592–594. [Google Scholar] [CrossRef]
- Wood, S.A.; Heath, M.W.; Holland, P.T.; Munday, R.; McGregor, G.B.; Ryan, K.G. Identification of a benthic microcystin-producing filamentous cyanobacterium (Oscillatoriales) associated with a dog poisoning in New Zealand. Toxicon 2010, 55, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Chatziefthimiou, A.D.; Metcalf, J.S.; Glover, W.B.; Powell, J.T.; Banack, S.A.; Cox, P.A.; Ladjimi, M.; Sultan, A.A.; Chemaitelly, H.; Richer, R.A. Cyanotoxin accumulation and growth patterns of biocrust communities under variable environmental conditions. Toxicon X 2024, 23, 100199. [Google Scholar] [CrossRef] [PubMed]
- Koksharova, O.A.; Butenko, I.O.; Pobeguts, O.V.; Safronova, N.A.; Govorun, V.M. The first proteomic study of Nostoc sp. PCC 7120 exposed to cyanotoxin BMAA under nitrogen starvation. Toxins 2020, 12, 310. [Google Scholar] [CrossRef] [PubMed]
- Koksharova, O.A.; Butenko, I.O.; Pobeguts, O.V.; Safronova, N.A.; Govorun, V.M. Proteomic insights into starvation of nitrogen-replete cells of Nostoc sp. PCC 7120 under β-N-methylamino-L-alanine (BMAA) treatment. Toxins 2020, 12, 372. [Google Scholar] [CrossRef]
- Downing, S.; Banack, S.A.; Metcalf, J.S.; Cox, P.A.; Downing, T.G. Nitrogen starvation of cyanobacteria results in the production of β-N-methylamino-L-alanine. Toxicon 2011, 58, 187–194. [Google Scholar] [CrossRef]
- Song, L.; Jia, Y.; Qin, B.; Li, R.; Carmichael, W.W.; Gan, N.; Xu, H.; Shan, K.; Sukenik, A. Harmful cyanobacterial blooms: Biological traits, mechanisms, risks, and control strategies. Annu. Rev. Environ. Resour. 2023, 48, 123–147. [Google Scholar] [CrossRef]
- Scott, L.L.; Downing, S.; Phelan, R.R.; Downing, T.G. Environmental modulation of microcystin and β-N-methylamino-L-alanine as a function of nitrogen availability. Toxicon 2014, 87, 1–5. [Google Scholar] [CrossRef]
- Cox, P.A.; Banack, S.A.; Murch, S.J. Biomagnification of cyanobacterial neurotoxins and neurodegenerative disease among the Chamorro people of Guam. Proc. Natl. Acad. Sci. USA 2003, 100, 13380–13383. [Google Scholar] [CrossRef]
- Murch, S.J.; Cox, P.A.; Banack, S.A. A mechanism for slow release of biomagnified cyanobacterial neurotoxins and neurodegenerative disease in Guam. Proc. Natl. Acad. Sci. USA 2004, 101, 12228–12231. [Google Scholar] [CrossRef]
- Jonasson, S.; Eriksson, J.; Berntzon, L.; Spácil, Z.; Ilag, L.L.; Ronnevi, L.-O.; Rasmussen, U.; Bergman, B. Transfer of a cyanobacterial neurotoxin within a temperate aquatic ecosystem suggests pathways for human exposure. Proc. Natl. Acad. Sci. USA 2010, 107, 9252–9257. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Rydberg, S. Transfer of the neurotoxin β-N-methylamino-L-alanine (BMAA) in the agro-aqua cycle. Mar. Drugs 2020, 18, 244. [Google Scholar] [CrossRef] [PubMed]
- Roney, B.R.; Renhui, L.; Banack, S.A.; Murch, S.; Honegger, R.; Cox, P.A. Consumption of fa cai Nostoc soup: A potential for BMAA exposure from Nostoc cyanobacteria in China? Amyotroph. Lateral Scler. 2009, 10 (Suppl. S2), 44–49. [Google Scholar] [CrossRef] [PubMed]
- Chatziefthimiou, A.D.; Deitch, E.J.; Glover, W.B.; Powell, J.T.; Banack, S.A.; Richer, R.A.; Cox, P.A.; Metcalf, J.S. Analysis of neurotoxic amino acids from marine waters, microbial mats, and seafood destined for human consumption in the Arabian Gulf. Neurotox. Res. 2018, 33, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, J.; Duy, S.V.; Troncy, C.; Dinh, Q.T.; Simon, D.F.; Munoz, G.; Sauvé, S. Screening of multi-class cyanotoxins in algal dietary supplements marketed in North America. Algal Res. 2023, 73, 103162. [Google Scholar] [CrossRef]
- Roy-Lachapelle, A.; Solliec, M.; Bouchard, M.F.; Sauvé, S. Detection of cyanotoxins in algae dietary supplements. Toxins 2017, 9, 76. [Google Scholar] [CrossRef]
- Wynne, T.T.; Stumpf, R.P. Spatial and temporal patterns in the seasonal distribution of toxic cyanobacteria in Western Lake Erie from 2002–2014. Toxins 2015, 7, 1649–1663. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andersson, M.; Karlsson, O.; Bergström, U.; Brittebo, E.B.; Brandt, I. Maternal transfer of the cyanobacterial neurotoxin β-N-methylamino-L-alanine (BMAA) via milk to suckling offspring. PLoS ONE 2013, 8, e78133. [Google Scholar] [CrossRef]
- Andersson, M.; Karlsson, O.; Banack, S.A.; Brandt, I. Transfer of developmental neurotoxin β-N-methylamino-L-alanine (BMAA) via milk to nursed offspring: Studies by mass spectrometry and image analysis. Toxicol. Lett. 2016, 258, 108–114. [Google Scholar] [CrossRef]
- Andersson, M.; Ersson, L.; Brandt, I.; Bergström, U. Potential transfer of neurotoxic amino acid β-N-methylamino-alanine (BMAA) from mother to infant during breast-feeding: Predictions from human cell lines. Toxicol. Appl. Pharmacol. 2017, 320, 40–50. [Google Scholar] [CrossRef]
- Garamszegi, S.P.; Banack, S.A.; Duque, L.L.; Metcalf, J.S.; Stommel, E.W.; Cox, P.A.; Davis, D.A. Detection of β-N-methylamino-L-alanine in postmortem olfactory bulbs of Alzheimer’s disease patients using UHPLC-MS/MS: An autopsy case-series study. Toxicol. Rep. 2023, 10, 87–96. [Google Scholar] [CrossRef]
- Facciponte, D.N.; Bough, M.W.; Seidler, D.; Carroll, J.L.; Ashare, A.; Andrew, A.S.; Tsongalis, G.J.; Vaickus, L.J.; Henegan, P.L.; Butt, T.H. Identifying aerosolized cyanobacteria in the human respiratory tract: A proposed mechanism for cyanotoxin-associated diseases. Sci. Total Environ. 2018, 645, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Barnard, M.A.; Chaffin, J.D.; Plaas, H.E.; Boyer, G.L.; Wei, B.; Wilhelm, S.W.; Rossignol, K.L.; Braddy, J.S.; Bullerjahn, G.S.; Bridgeman, T.B.; et al. Roles of nutrient limitation on Western Lake Erie cyanoHAB toxin production. Toxins 2021, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Soliakov, L.; Gallagher, T.; Wonnacott, S. Anatoxin-a-evoked [3H] dopamine release from rat striatal synaptosomes. Neuropharmacology 1995, 34, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Lakshmana Rao, P.; Bhattacharya, R.; Gupta, N.; Parida, M.; Bhaskar, A.; Dubey, R. Involvement of caspase and reactive oxygen species in cyanobacterial toxin anatoxin-a-induced cytotoxicity and apoptosis in rat thymocytes and Vero cells. Arch. Toxicol. 2002, 76, 227–235. [Google Scholar] [CrossRef]
- Zhong, Y.; Shen, L.; Ye, X.; Zhou, D.; He, Y.; Li, Y.; Ding, Y.; Zhu, W.; Ding, J.; Zhang, H. Neurotoxic anatoxin-a can also exert immunotoxicity by the induction of apoptosis on Carassius auratus lymphocytes in vitro when exposed to environmentally relevant concentrations. Front. Physiol. 2020, 11, 316. [Google Scholar] [CrossRef]
- Fiore, M.F.; de Lima, S.T.; Carmichael, W.W.; McKinnie, S.M.K.; Chekan, J.R.; Moore, B.S. Guanitoxin, re-naming a cyanobacterial organophosphate toxin. Harmful Algae 2020, 92, 101737. [Google Scholar] [CrossRef]
- Passos, L.S.; Gomes, L.C.; Pereira, T.M.; Sadauskas-Henrique, H.; Dal Pont, G.; Ostrensky, A.; Pinto, E. Response of Oreochromis niloticus (Teleostei: Cichlidae) exposed to a guanitoxin-producing cyanobacterial strain using multiple biomarkers. Sci. Total Environ. 2022, 835, 155471. [Google Scholar] [CrossRef]
- Cusick, K.D.; Sayler, G.S. An overview on the marine neurotoxin, saxitoxin: Genetics, molecular targets, methods of detection and ecological functions. Mar. Drugs 2013, 11, 991–1018. [Google Scholar] [CrossRef]
- Llewellyn, L.E. Saxitoxin, a toxic marine natural product that targets a multitude of receptors. Nat. Prod. Rep. 2006, 23, 200–222. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Tischbein, M.; Cox, P.A.; Stommel, E.W. Cyanotoxins and the nervous system. Toxins 2021, 13, 660. [Google Scholar] [CrossRef]
- Ramos, P.B.; Diehl, F.; dos Santos, J.M.; Monserrat, J.M.; Yunes, J.S. Oxidative stress in rats induced by consumption of saxitoxin contaminated drink water. Harmful Algae 2014, 37, 68–74. [Google Scholar] [CrossRef]
- Wu, H.; Prithiviraj, B.; Tan, Z. Physiological effects of oxidative stress caused by saxitoxin in the nematode Caenorhabditis elegans. Mar. Drugs 2023, 21, 544. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.A.; de Morais, E.C.P.; Costa, M.D.M.; Ribas, J.L.C.; Guiloski, I.C.; Ramsdorf, W.A.; Zanata, S.M.; Cestari, M.M.; Ribeiro, C.A.O.; Magalhães, V.F. Saxitoxins induce cytotoxicity, genotoxicity and oxidative stress in teleost neurons in vitro. Toxicon 2014, 86, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.A.; Davis, D.A.; Mash, D.C.; Metcalf, J.S.; Banack, S.A. Dietary exposure to an environmental toxin triggers neurofibrillary tangles and amyloid deposits in the brain. Proc. R. Soc. B Biol. Sci. 2016, 283, 20152397. [Google Scholar] [CrossRef]
- Dunlop, R.A.; Cox, P.A.; Banack, S.A.; Rodgers, K.J. The non-protein amino acid BMAA is misincorporated into human proteins in place of L-Serine causing protein misfolding and aggregation. PLoS ONE 2013, 8, e75376. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Lobner, D.; Banack, S.A.; Cox, G.A.; Nunn, P.B.; Wyatt, P.B.; Cox, P.A. Analysis of BMAA enantiomers in cycads, cyanobacteria, and mammals: In vivo formation and toxicity of d-BMAA. Amino Acids 2017, 49, 1427–1439. [Google Scholar] [CrossRef]
- Schneider, T.; Simpson, C.; Desai, P.; Tucker, M.; Lobner, D. Neurotoxicity of isomers of the environmental toxin L-BMAA. Toxicon 2020, 184, 175–179. [Google Scholar] [CrossRef]
- Esterhuizen-Londt, M.; Wiegand, C.; Downing, T.G. β-N-methylamino-L-alanine (BMAA) uptake by the animal model, Daphnia magna and subsequent oxidative stress. Toxicon 2015, 100, 20–26. [Google Scholar] [CrossRef]
- Esterhuizen-Londt, M.; Pflugmacher, S.; Downing, T.G. The effect of β-N-methylamino-L-alanine (BMAA) on oxidative stress response enzymes of the macrophyte Ceratophyllum demersum. Toxicon 2011, 57, 803–810. [Google Scholar] [CrossRef]
- Laugeray, A.; Oummadi, A.; Jourdain, C.; Feat, J.; Meyer-Dilhet, G.; Menuet, A.; Plé, K.; Gay, M.; Routier, S.; Mortaud, S.; et al. Perinatal exposure to the cyanotoxin β-N-methylamino-L-alanine (BMAA) results in long-lasting behavioral changes in offspring: Potential involvement of DNA damage and oxidative stress. Neurotox. Res. 2018, 33, 87–112. [Google Scholar] [CrossRef]
- Tian, K.W.; Jiang, H.; Wang, B.B.; Zhang, F.; Han, S. Intravenous injection of L-BMAA induces a rat model with comprehensive characteristics of amyotrophic lateral sclerosis/Parkinson–dementia complex. Toxicol. Res. 2016, 5, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.F.; Candeias, E.; Esteves, A.R.; Magalhães, J.D.; Ferreira, I.L.; Nunes-Costa, D.; Rego, A.C.; Empadinhas, N.; Cardoso, S.M. Microbial BMAA elicits mitochondrial dysfunction, innate immunity activation, and Alzheimer’s disease features in cortical neurons. J. Neuroinflamm. 2020, 17, 332. [Google Scholar] [CrossRef] [PubMed]
- Botha, N.; Gehringer, M.M.; Downing, T.G.; van de Venter, M.; Shephard, E.G. The role of microcystin-LR in the induction of apoptosis and oxidative stress in CaCo2 cells. Toxicon 2004, 43, 85–92. [Google Scholar] [CrossRef]
- Pflugmacher, S. Promotion of oxidative stress in the aquatic macrophyte Ceratophyllum demersum during biotransformation of the cyanobacterial toxin microcystin-LR. Aquat. Toxicol. 2004, 70, 169–178. [Google Scholar] [CrossRef]
- Jayaraj, R.; Anand, T.; Rao, P.L. Activity and gene expression profile of certain antioxidant enzymes to microcystin-LR induced oxidative stress in mice. Toxicology 2006, 220, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Gu, X.; Song, R.; Zhang, Q.; Geng, J.; Wang, X.; Yang, L. Time-dependent oxidative stress and histopathological changes in Cyprinus carpio L. exposed to microcystin-LR. Ecotoxicology 2011, 20, 1000–1009. [Google Scholar] [CrossRef]
- Pinho, G.L.L.; Da Rosa, C.M.; Maciel, F.E.; Bianchini, A.; Yunes, J.S.; Proença, L.A.D.O.; Monserrat, J.M. Antioxidant responses and oxidative stress after microcystin exposure in the hepatopancreas of an estuarine crab species. Ecotoxicol. Environ. Saf. 2005, 61, 353–360. [Google Scholar] [CrossRef]
- Mondal, A.; Saha, P.; Bose, D.; Chatterjee, S.; Seth, R.K.; Xiao, S.; Porter, D.E.; Brooks, B.W.; Scott, G.I.; Nagarkatti, M. Environmental microcystin exposure in underlying NAFLD-induced exacerbation of neuroinflammation, blood-brain barrier dysfunction, and neurodegeneration are NLRP3 and S100B dependent. Toxicology 2021, 461, 152901. [Google Scholar] [CrossRef]
- Yan, M.; Jin, H.; Pan, C.; Han, X. Chronic microcystin-LR-induced α-synuclein promotes neuroinflammation through activation of the NLRP3 inflammasome in microglia. Mol. Neurobiol. 2023, 60, 884–900. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Zhu, J.; Ding, J.; Chen, Y.; Han, X. Blood-brain barrier disruption and inflammation reaction in mice after chronic exposure to microcystin-LR. Sci. Total Environ. 2019, 689, 662–678. [Google Scholar] [CrossRef]
- Mondal, A.; Bose, D.; Saha, P.; Seth, R.; Scott, G.; Xiao, S.; Porter, D.; Brooks, B.; Chatterjee, S. Microcystin-LR exacerbates neuroinflammation and neurodegeneration in nonalcoholic fatty liver disease. FASEB J. 2021, 35. [Google Scholar] [CrossRef]
- Li, X.B.; Zhang, X.; Ju, J.; Li, Y.; Yin, L.; Pu, Y. Alterations in neurobehaviors and inflammation in hippocampus of rats induced by oral administration of microcystin-LR. Environ. Sci. Pollut. Res. 2014, 21, 12419–12425. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Y.; Zhang, C.; Xiang, Z.; Ding, J.; Han, X. Learning and memory deficits and alzheimer’s disease-like changes in mice after chronic exposure to microcystin-LR. J. Hazard. Mater. 2019, 373, 504–518. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, M.; Endo, S.; Critz, S.D.; Shenolikar, S.; Byrne, J.H. Microcystin-LR, a potent protein phosphatase inhibitor, prolongs the serotonin-and cAMP-induced currents in sensory neurons of Aplysia californica. Brain Res. 1990, 533, 137–140. [Google Scholar] [CrossRef]
- Wang, J.; Lin, F.; Cai, F.; Yan, W.; Zhou, Q.; Xie, L. Microcystin-LR inhibited hippocampal long-term potential via regulation of the glycogen synthase kinase-3β pathway. Chemosphere 2013, 93, 223–229. [Google Scholar] [CrossRef]
- Pichardo, S.; Devesa, V.; Puerto, M.; Vélez, D.; Cameán, A.M. Intestinal transport of cylindrospermopsin using the Caco-2 cell line. Toxicol. Vitro 2017, 38, 142–149. [Google Scholar] [CrossRef]
- Guzmán-Guillén, R.; Prieto, A.I.; Vasconcelos, V.M.; Cameán, A.M. Cyanobacterium producing cylindrospermopsin cause oxidative stress at environmentally relevant concentrations in sub-chronically exposed tilapia (Oreochromis niloticus). Chemosphere 2013, 90, 1184–1194. [Google Scholar] [CrossRef]
- Žegura, B.; Gajski, G.; Štraser, A.; Garaj-Vrhovac, V. Cylindrospermopsin induced DNA damage and alteration in the expression of genes involved in the response to DNA damage, apoptosis and oxidative stress. Toxicon 2011, 58, 471–479. [Google Scholar] [CrossRef]
- López-Alonso, H.; Rubiolo, J.A.; Vega, F.; Vieytes, M.R.; Botana, L.M. Protein synthesis inhibition and oxidative stress induced by cylindrospermopsin elicit apoptosis in primary rat hepatocytes. Chem. Res. Toxicol. 2013, 26, 203–212. [Google Scholar] [CrossRef]
- Hinojosa, M.G.; Cascajosa-Lira, A.; Prieto, A.I.; Gutiérrez-Praena, D.; Vasconcelos, V.; Jos, A.; Cameán, A.M. Cytotoxic effects and oxidative stress produced by a cyanobacterial cylindrospermopsin producer extract versus a cylindrospermopsin non-producing extract on the neuroblastoma SH-SY5Y cell line. Toxins 2023, 15, 320. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, J.; Jian, J.; Liu, H.; Zhou, X.; Zhang, Y.; Zou, G.; Zhou, L.; Wang, J. Exploring the neurotoxic effects of cylindrospermopsin in early development of zebrafish: An integrated impact of oxidative stress, inflammatory response, and apoptosis. Ecotoxicol. Environ. Saf. 2025, 293, 118021. [Google Scholar] [CrossRef] [PubMed]
- Puerto, M.; Jos, A.; Pichardo, S.; Moyano, R.; Blanco, A.; Cameán, A.M. Acute exposure to pure cylindrospermopsin results in oxidative stress and pathological alterations in tilapia (Oreochromis niloticus). Environ. Toxicol. 2014, 29, 371–385. [Google Scholar] [CrossRef]
- Sieroslawska, A.; Rymuszka, A. Cylindrospermopsin induces oxidative stress and genotoxic effects in the fish CLC cell line. J. Appl. Toxicol. 2015, 35, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa, M.G.; Prieto, A.I.; Muñoz-Castro, C.; Sánchez-Mico, M.V.; Vitorica, J.; Cameán, A.M.; Jos, Á. Cytotoxicity and effects on the synapsis induced by pure cylindrospermopsin in an E17 embryonic murine primary neuronal culture in a concentration- and time-dependent manner. Toxins 2022, 14, 175. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.d.C.d.; Grötzner, S.R.; Costa, D.D.M.; Garcia, J.R.E.; Muelbert, J.; de Magalhães, V.F.; Neto, F.F.; Ribeiro, C.A.d.O. Comparative bioaccumulation and effects of purified and cellular extract of cylindrospermopsin to freshwater fish Hoplias malabaricus. J. Toxicol. Environ. Health Part A 2018, 81, 620–632. [Google Scholar] [CrossRef]
- Guzmán-Guillén, R.; Manzano, I.L.; Moreno, I.M.; Ortega, A.I.P.; Moyano, R.; Blanco, A.; Cameán, A.M. Cylindrospermopsin induces neurotoxicity in tilapia fish (Oreochromis niloticus) exposed to Aphanizomenon ovalisporum. Aquat. Toxicol. 2015, 161, 17–24. [Google Scholar] [CrossRef]
- Rabelo, J.C.S.; Hanusch, A.L.; de Jesus, L.W.O.; Mesquita, L.A.; Franco, F.C.; Silva, R.A.; Sabóia-Morais, S.M.T. DNA damage induced by cylindrospermopsin on different tissues of the biomonitor fish Poecilia reticulata. Environ. Toxicol. 2021, 36, 1125–1134. [Google Scholar] [CrossRef]
- Takser, L.; Benachour, N.; Husk, B.; Cabana, H.; Gris, D. Cyanotoxins at low doses induce apoptosis and inflammatory effects in murine brain cells: Potential implications for neurodegenerative diseases. Toxicol. Rep. 2016, 3, 180–189. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Biggs, D.F.; Gorham, P.R. Toxicology and pharmacological action of Anabaena flos-aquae toxin. Science 1975, 187, 542–544. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Gorham, P.R. Anatoxins from clones of Anabaena flos-aquae isolated from lakes of western Canada. Int. Ver. Für Theor. Angew. Limnol. Mitteilungen 1978, 21, 285–295. [Google Scholar]
- Devlin, J.P.; Edwards, O.E.; Gorham, P.R.; Hunter, N.R.; Pike, R.K.; Stavric, B. Anatoxin-a, a toxic alkaloid from Anabaena flos-aquae NRC-44h. Can. J. Chem. 1977, 55, 1367–1371. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G. Harmful cyanobacterial blooms: Causes, consequences, and controls. Microb Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Wonnacott, S.; Gallagher, T. The chemistry and pharmacology of anatoxin-a and related homotropanes with respect to nicotinic acetylcholine receptors. Mar. Drugs 2006, 4, 228–254. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Biggs, D.F.; Peterson, M.A. Pharmacology of anatoxin-a, produced by the freshwater cyanophyte Anabaena flos-aquae NRC-44-1. Toxicon 1979, 17, 229–236. [Google Scholar] [CrossRef]
- Heath, M.; Wood, S.A.; Young, R.G.; Ryan, K.G. The role of nitrogen and phosphorus in regulating Phormidium sp. (cyanobacteria) growth and anatoxin production. FEMS Microbiol. Ecol. 2016, 92, fiw021. [Google Scholar] [CrossRef]
- Kramer, B.J.; Jankowiak, J.G.; Nanjappa, D.; Harke, M.J.; Gobler, C.J. Nitrogen and phosphorus significantly alter growth, nitrogen fixation, anatoxin-a content, and the transcriptome of the bloom-forming cyanobacterium, Dolichospermum. Front. Microbiol. 2022, 13, 955032. [Google Scholar] [CrossRef]
- Gagnon, A.; Pick, F.R. Effect of nitrogen on cellular production and release of the neurotoxin anatoxin-a in a nitrogen-fixing cyanobacterium. Front. Microbiol. 2012, 3, 211. [Google Scholar] [CrossRef]
- Edwards, C.; Beattie, K.A.; Scrimgeour, C.M.; Codd, G.A. Identification of anatoxin-a in benthic cyanobacteria (blue-green algae) and in associated dog poisonings at Loch Insh, Scotland. Toxicon 1992, 30, 1165–1175. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Morrison, L.F.; Krienitz, L.; Ballot, A.; Krause, E.; Kotut, K.; Pütz, S.; Wiegand, C.; Pflugmacher, S.; Codd, G.A. Analysis of the cyanotoxins anatoxin-a and microcystins in Lesser Flamingo feathers. Toxicol. Environ. Chem. 2006, 88, 159–167. [Google Scholar] [CrossRef]
- Mahmood, N.A.; Carmichael, W.W. The pharmacology of anatoxin-a(s), a neurotoxin produced by the freshwater cyanobacterium Anabaena flos-aquae NRC 525-17. Toxicon 1986, 24, 425–434. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Richer, R.; Cox, P.A.; Codd, G.A. Cyanotoxins in desert environments may present a risk to human health. Sci. Total Environ. 2012, 421–422, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.A.; Fadul, J.C.; Fiore, M.F.; Pinto, E. A systematic review on guanitoxin: General characteristics and ecological risks. Chemosphere 2024, 352, 141277. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W.; Evans, W.R.; Yin, Q.Q.; Bell, P.; Moczydlowski, E. Evidence for paralytic shellfish poisons in the freshwater cyanobacterium Lyngbya wollei (Farlow ex Gomont) comb. nov. Appl. Environ. Microbiol. 1997, 63, 3104–3110. [Google Scholar] [CrossRef] [PubMed]
- Wiese, M.; D’Agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic alkaloids: Saxitoxin and its analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef]
- Lipkind, G.M.; Fozzard, H.A. A structural model of the tetrodotoxin and saxitoxin binding site of the Na+ channel. Biophys. J. 1994, 66, 1–13. [Google Scholar] [CrossRef]
- O’Neill, K.; Musgrave, I.F.; Humpage, A. Low dose extended exposure to saxitoxin and its potential neurodevelopmental effects: A review. Environ. Toxicol. Pharmacol. 2016, 48, 7–16. [Google Scholar] [CrossRef]
- Pinto, A.; Macário, I.P.; Marques, S.M.; Lourenço, J.; Domingues, I.; Botelho, M.J.; Asselman, J.; Pereira, P.; Pereira, J.L. A short-term exposure to saxitoxin triggers a multitude of deleterious effects in Daphnia magna at levels deemed safe for human health. Sci. Total Environ. 2024, 951, 175431. [Google Scholar] [CrossRef]
- Banack, S.A.; Metcalf, J.S.; Jiang, L.; Craighead, D.; Ilag, L.L.; Cox, P.A. Cyanobacteria produce N-(2-aminoethyl) glycine, a backbone for peptide nucleic acids which may have been the first genetic molecules for life on earth. PLoS ONE 2012, 7, e49043. [Google Scholar] [CrossRef]
- Cox, P.A.; Banack, S.A.; Murch, S.J.; Rasmussen, U.; Tien, G.; Bidigare, R.R.; Metcalf, J.S.; Morrison, L.F.; Codd, G.A.; Bergman, B. Diverse taxa of cyanobacteria produce β-N-methylamino-L-alanine, a neurotoxic amino acid. Proc. Natl. Acad. Sci. USA 2005, 102, 5074–5078. [Google Scholar] [CrossRef]
- Esterhuizen, M.; Downing, T.G. β-N-methylamino-L-alanine (BMAA) in novel South African cyanobacterial isolates. Ecotoxicol. Environ. Saf. 2008, 71, 309–313. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Banack, S.A.; Lindsay, J.; Morrison, L.F.; Cox, P.A.; Codd, G.A. Co-occurrence of β-N-methylamino-L-alanine, a neurotoxic amino acid with other cyanobacterial toxins in British waterbodies, 1990–2004. Environ. Microbiol. 2008, 10, 702–708. [Google Scholar] [CrossRef]
- Violi, J.P.; Mitrovic, S.M.; Colville, A.; Main, B.J.; Rodgers, K.J. Prevalence of β-methylamino-L-alanine (BMAA) and its isomers in freshwater cyanobacteria isolated from eastern Australia. Ecotoxicol. Environ. Saf. 2019, 172, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Banack, S.A.; Caller, T.; Henegan, P.; Haney, J.; Murby, A.; Metcalf, J.S.; Powell, J.; Cox, P.A.; Stommel, E. Detection of cyanotoxins, β-N-methylamino-L-alanine and microcystins, from a lake surrounded by cases of amyotrophic lateral sclerosis. Toxins 2015, 7, 322–336. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, O.; Berg, A.-L.; Lindström, A.-K.; Hanrieder, J.; Arnerup, G.; Roman, E.; Bergquist, J.; Lindquist, N.G.; Brittebo, E.B.; Andersson, M. Neonatal exposure to the cyanobacterial toxin BMAA induces changes in protein expression and neurodegeneration in adult hippocampus. Toxicol. Sci. Off. J. Soc. Toxicol. 2012, 130, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.H.; Choi, D.W. Beta-N-methylamino-L-alanine neurotoxicity: Requirement for bicarbonate as a cofactor. Science 1988, 241, 973–975. [Google Scholar] [CrossRef]
- Weiss, J.H.; Koh, J.Y.; Choi, D.W. Neurotoxicity of β-N-methylamino-L-alanine (BMAA) and β-N-oxalylamino-L-alanine (BOAA) on cultured cortical neurons. Brain Res. 1989, 497, 64–71. [Google Scholar] [CrossRef]
- Lobner, D.; Piana, P.M.T.; Salous, A.K.; Peoples, R.W. β-N-methylamino-L-alanine enhances neurotoxicity through multiple mechanisms. Neurobiol. Dis. 2007, 25, 360–366. [Google Scholar] [CrossRef]
- Rao, S.D.; Banack, S.A.; Cox, P.A.; Weiss, J.H. BMAA selectively injures motor neurons via AMPA/kainate receptor activation. Exp. Neurol. 2006, 201, 244–252. [Google Scholar] [CrossRef]
- Murch, S.J.; Cox, P.A.; Banack, S.A.; Steele, J.C.; Sacks, O.W. Occurrence of β-methylamino-L-alanine (BMAA) in ALS/PDC patients from Guam. Acta Neurol. Scand. 2004, 110, 267–269. [Google Scholar] [CrossRef]
- Burton, B.; Collins, K.; Brooks, J.; Marx, K.; Renner, A.; Wilcox, K.; Moore, E.; Osowski, K.; Riley, J.; Rowe, J.; et al. The biotoxin BMAA promotes dysfunction via distinct mechanisms in neuroblastoma and glioblastoma cells. PLoS ONE 2023, 18, e0278793. [Google Scholar] [CrossRef]
- Main, B.J.; Rodgers, K.J. Assessing the combined toxicity of BMAA and its isomers 2,4-DAB and AEG in vitro using human neuroblastoma cells. Neurotox. Res. 2018, 33, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, J.S.; Banack, S.A.; Richer, R.; Cox, P.A. Neurotoxic amino acids and their isomers in desert environments. J. Arid Environ. 2015, 112, 140–144. [Google Scholar] [CrossRef]
- Spasic, S.; Stanojevic, M.; Nesovic Ostojic, J.; Kovacevic, S.; Prostran, M.; Lopicic, S. Extensive depolarization and lack of recovery of leech Retzius neurons caused by 2,4 diaminobutyric acid. Aquat. Toxicol. 2018, 199, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Harke, M.J.; Steffen, M.M.; Gobler, C.J.; Otten, T.G.; Wilhelm, S.W.; Wood, S.A.; Paerl, H.W. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef]
- Kurmayer, R. The toxic cyanobacterium Nostoc sp. strain 152 produces highest amounts of microcystin and nostophycin under stress conditions 1. J. Phycol. 2011, 47, 200–207. [Google Scholar] [CrossRef]
- Bouaïcha, N.; Miles, C.O.; Beach, D.G.; Labidi, Z.; Djabri, A.; Benayache, N.Y.; Nguyen-Quang, T. Structural diversity, characterization and toxicology of microcystins. Toxins 2019, 11, 714. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, Z. Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production. Green Process. Synth. 2022, 11, 64–70. [Google Scholar] [CrossRef]
- Lone, Y.; Koiri, R.K.; Bhide, M. An overview of the toxic effect of potential human carcinogen microcystin-LR on testis. Toxicol. Rep. 2015, 2, 289–296. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, W.; Hu, X.; Su, H.; Wen, G.; Yang, K.; Cao, Y. Toxicity of the microcystin-producing cyanobacteria Microcystis aeruginosa to shrimp Litopenaeus vannamei. Ecotoxicology 2022, 31, 1403–1412. [Google Scholar] [CrossRef]
- Zhou, M.; Tu, W.; Xu, J. Mechanisms of microcystin-LR-induced cytoskeletal disruption in animal cells. Toxicon 2015, 101, 92–100. [Google Scholar] [CrossRef]
- Javadpour, P.; Dargahi, L.; Ahmadiani, A.; Ghasemi, R. To be or not to be: PP2A as a dual player in CNS functions, its role in neurodegeneration, and its interaction with brain insulin signaling. Cell. Mol. Life Sci. 2019, 76, 2277–2297. [Google Scholar] [CrossRef] [PubMed]
- Nematullah, M.; Hoda, M.N.; Khan, F. Protein phosphatase 2A: A double-faced phosphatase of cellular system and its role in neurodegenerative disorders. Mol. Neurobiol. 2018, 55, 1750–1761. [Google Scholar] [CrossRef] [PubMed]
- Verbinnen, I.; Vaneynde, P.; Reynhout, S.; Lenaerts, L.; Derua, R.; Houge, G.; Janssens, V. Protein phosphatase 2A (PP2A) mutations in brain function, development, and neurologic disease. Biochem. Soc. Trans. 2021, 49, 1567–1588. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, H.; Wei, L.; Ma, Y.; Jiang, H.; Yuen, C.N.T.; Zhang, J.; Wu, H.; Shu, Y. Microcystin-leucine arginine causes brain injury and functional disorder in Lithobates catesbeianus tadpoles by oxidative stress and inflammation. Aquat. Toxicol. 2023, 258, 106509. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, I.; Moore, R.E.; Runnegar, M.T.C. Cylindrospermopsin: A potent hepatotoxin from the blue-green alga Cylindrospermopsis raciborskii. J. Am. Chem. Soc. 1992, 114, 7941–7942. [Google Scholar] [CrossRef]
- Gutiérrez-Praena, D.; Pichardo, S.; Jos, Á.; Cameán, A.M. Toxicity and glutathione implication in the effects observed by exposure of the liver fish cell line PLHC-1 to pure cylindrospermopsin. Ecotoxicol. Environ. Saf. 2011, 74, 1567–1572. [Google Scholar] [CrossRef]
- Gutiérrez-Praena, D.; Pichardo, S.; Jos, Á.; Moreno, F.J.; Cameán, A.M. Biochemical and pathological toxic effects induced by the cyanotoxin cylindrospermopsin on the human cell line Caco-2. Water Res. 2012, 46, 1566–1575. [Google Scholar] [CrossRef]
- Hinojosa, M.G.; Gutiérrez-Praena, D.; Prieto, A.I.; Guzmán-Guillén, R.; Jos, A.; Cameán, A.M. Neurotoxicity induced by microcystins and cylindrospermopsin: A review. Sci. Total Environ. 2019, 668, 547–565. [Google Scholar] [CrossRef]
- Puerto, M.; Prieto, A.I.; Maisanaba, S.; Gutiérrez-Praena, D.; Mellado-García, P.; Jos, Á.; Cameán, A.M. Mutagenic and genotoxic potential of pure cylindrospermopsin by a battery of in vitro tests. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 121, 413–422. [Google Scholar] [CrossRef]
- Roos, W.P.; Kaina, B. DNA damage-induced cell death by apoptosis. Trends Mol. Med. 2006, 12, 440–450. [Google Scholar] [CrossRef]
- Brittain, S.M.; Wang, J.; Babcock-Jackson, L.; Carmichael, W.W.; Rinehart, K.L.; Culver, D.A. Isolation and characterization of microcystins, cyclic heptapeptide hepatotoxins from a Lake Erie Strain of Microcystis aeruginosa. J. Great Lakes Res. 2000, 26, 241–249. [Google Scholar] [CrossRef]
- Veerman, J.; Kumar, A.; Mishra, D.R. Exceptional landscape-wide cyanobacteria bloom in Okavango Delta, Botswana in 2020 coincided with a mass elephant die-off event. Harmful Algae 2022, 111, 102145. [Google Scholar] [CrossRef] [PubMed]
- Henrikson, T.; Ekberg, E.C.; Nilner, M. Symptoms and signs of temporomandibular disorders in girls with normal occlusion and Class II malocclusion. Acta Odontol. Scand. 1997, 55, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.A.; Mondo, K.; Stern, E.; Annor, A.K.; Murch, S.J.; Coyne, T.M.; Brand, L.E.; Niemeyer, M.E.; Sharp, S.; Bradley, W.G.; et al. Cyanobacterial neurotoxin BMAA and brain pathology in stranded dolphins. PLoS ONE 2019, 14, e0213346. [Google Scholar] [CrossRef]
- Jiang, L.; Kiselova, N.; Rosén, J.; Ilag, L.L. Quantification of neurotoxin BMAA (β-N-methylamino-L-alanine) in seafood from Swedish markets. Sci. Rep. 2014, 4, 6931. [Google Scholar] [CrossRef]
- Brand, L.E.; Pablo, J.; Compton, A.; Hammerschlag, N.; Mash, D.C. Cyanobacterial blooms and the occurrence of the neurotoxin, beta-N-methylamino-L-alanine (BMAA), in South Florida aquatic food webs. Harmful Algae 2010, 9, 620–635. [Google Scholar] [CrossRef]
- Sandhu, P.K.; Solonenka, J.T.; Murch, S.J. Neurotoxic non-protein amino acids in commercially harvested lobsters (Homarus americanus H. Milne-Edwards). Sci. Rep. 2024, 14, 8017. [Google Scholar] [CrossRef]
- Reside, A. Characterizing the Neurotoxic Potential of the algal toxin beta-methylamino-L-alanine (BMAA) in fish. Doctoral Dissertation, University of Guelph, Guelph, ON, Canada, 2022. [Google Scholar]
- Ai, Y.; Lee, S.; Lee, J. Drinking water treatment residuals from cyanobacteria bloom-affected areas: Investigation of potential impact on agricultural land application. Sci. Total Environ. 2020, 706, 135756. [Google Scholar] [CrossRef]
- Codd, G.; Bell, S.; Kaya, K.; Ward, C.; Beattie, K.; Metcalf, J. Cyanobacterial toxins, exposure routes and human health. Eur. J. Phycol. 1999, 34, 405–415. [Google Scholar] [CrossRef]
- Contardo-Jara, V.; Schwanemann, T.; Pflugmacher, S. Uptake of a cyanotoxin, β-N-methylamino-L-alanine, by wheat (Triticum aestivum). Ecotoxicol. Environ. Saf. 2014, 104, 127–131. [Google Scholar] [CrossRef]
- Contardo-Jara, V.; Schwanemann, T.; Esterhuizen-Londt, M.; Pflugmacher, S. Protein association of β-N-methylamino-L-alanine in Triticum aestivum via irrigation. Food Addit. Contam. Part A 2018, 35, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Levizou, E.; Papadimitriou, T.; Papavasileiou, E.; Papadimitriou, N.; Kormas, K.A. Root vegetables bioaccumulate microcystins-LR in a developmental stage-dependent manner under realistic exposure scenario: The case of carrot and radish. Agric. Water Manag. 2020, 240, 106274. [Google Scholar] [CrossRef]
- Esterhuizen-Londt, M.; Pflugmacher, S. Vegetables cultivated with exposure to pure and naturally occurring β-N-methylamino-L-alanine (BMAA) via irrigation. Environ. Res. 2019, 169, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yu, S.; Li, G.; Chen, X.; Huang, M.; Liao, X.; Li, H.; Hu, F.; Wu, J. Transfer of a cyanobacterial neurotoxin, β-methylamino-L-alanine from soil to crop and its bioaccumulation in Chinese cabbage. Chemosphere 2019, 219, 997–1001. [Google Scholar] [CrossRef]
- Mohamed, Z.A.; Elnour, R.O.; Alamri, S.; Hashem, M.; Alshehri, A.M.; Campos, A.; Vasconcelos, V.; Badawye, H. Occurrence of β-N-methylamino-L-alanine (BMAA) toxin in irrigation water and field vegetable plants and assessing its potential risk to human health. Water Air Soil Pollut. 2024, 235, 72. [Google Scholar] [CrossRef]
- Samardzic, K.; Steele, J.R.; Violi, J.P.; Colville, A.; Mitrovic, S.M.; Rodgers, K.J. Toxicity and bioaccumulation of two non-protein amino acids synthesised by cyanobacteria, β-N-Methylamino-L-alanine (BMAA) and 2,4-diaminobutyric acid (DAB), on a crop plant. Ecotoxicol. Environ. Saf. 2021, 208, 111515. [Google Scholar] [CrossRef]
- Weralupitiya, C.; Wanigatunge, R.P.; Gunawardana, D.; Vithanage, M.; Magana-Arachchi, D. Cyanotoxins uptake and accumulation in crops: Phytotoxicity and implications on human health. Toxicon 2022, 211, 21–35. [Google Scholar] [CrossRef]
- Reed, D.M.; Brody, J.A. Amyotrophic Lateral Sclerosis and Parkinsonism-Dementia on Guam, 1945–1972: I. Descriptive Epidemiology. Am. J. Epidemiol. 1975, 101, 287–301. [Google Scholar] [CrossRef]
- Spencer, P.S. Parkinsonism and motor neuron disorders: Lessons from Western Pacific ALS/PDC. J. Neurol. Sci. 2022, 433, 120021. [Google Scholar] [CrossRef]
- Belnap, J. Biological soil crusts and wind erosion. In Biological Soil Crusts: Structure, Function, and Management; Springer: Berlin/Heidelberg, Germany, 2003; pp. 339–347. [Google Scholar]
- Weber, B.; Belnap, J.; Büdel, B.; Antoninka, A.J.; Barger, N.N.; Chaudhary, V.B.; Darrouzet-Nardi, A.; Eldridge, D.J.; Faist, A.M.; Ferrenberg, S.; et al. What is a biocrust? A refined, contemporary definition for a broadening research community. Biol. Rev. 2022, 97, 1768–1785. [Google Scholar] [CrossRef]
- Chatziefthimiou, A.D.; Banack, S.A.; Cox, P.A. Biocrust-produced cyanotoxins are found vertically in the desert soil profile. Neurotox. Res. 2021, 39, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.A.; Richer, R.; Metcalf, J.S.; Banack, S.A.; Codd, G.A.; Bradley, W.G. Cyanobacteria and BMAA exposure from desert dust: A possible link to sporadic ALS among Gulf War veterans. Amyotroph. Lateral Scler. 2009, 10 (Suppl. S2), 109–117. [Google Scholar] [CrossRef] [PubMed]
- Dulić, T.; Meriluoto, J.; Malešević, T.P.; Gajić, V.; Važić, T.; Tokodi, N.; Obreht, I.; Kostić, B.; Kosijer, P.; Khormali, F.; et al. Cyanobacterial diversity and toxicity of biocrusts from the Caspian Lowland loess deposits, North Iran. Quat. Int. 2017, 429, 74–85. [Google Scholar] [CrossRef]
- Richer, R.; Banack, S.A.; Metcalf, J.S.; Cox, P.A. The persistence of cyanobacterial toxins in desert soils. J. Arid Environ. 2015, 112, 134–139. [Google Scholar] [CrossRef]
- Powell, J.T.; Chatziefthimiou, A.D.; Banack, S.A.; Cox, P.A.; Metcalf, J.S. Desert crust microorganisms, their environment, and human health. J. Arid Environ. 2015, 112, 127–133. [Google Scholar] [CrossRef]
- Chrapusta, E.; Węgrzyn, M.; Zabaglo, K.; Kaminski, A.; Adamski, M.; Wietrzyk, P.; Bialczyk, J. Microcystins and anatoxin-a in Arctic biocrust cyanobacterial communities. Toxicon 2015, 101, 35–40. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Banack, S.A.; Cox, P.A. Cyanotoxin analysis of air samples from the Great Salt Lake. Toxins 2023, 15, 659. [Google Scholar] [CrossRef]
- Jungblut, A.D.; Wilbraham, J.; Banack, S.A.; Metcalf, J.S.; Codd, G.A. Microcystins, BMAA and BMAA isomers in 100-year-old Antarctic cyanobacterial mats collected during Captain RF Scott’s Discovery Expedition. Eur. J. Phycol. 2018, 53, 115–121. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Dunlop, R.A.; Banack, S.A.; Souza, N.R.; Cox, P.A. Cyanotoxin analysis and amino acid profiles of cyanobacterial food items from Chad. Neurotox. Res. 2021, 39, 72–80. [Google Scholar] [CrossRef]
- Johnson, H.E.; King, S.R.; Banack, S.A.; Webster, C.; Callanaupa, W.J.; Cox, P.A. Cyanobacteria (Nostoc commune) used as a dietary item in the Peruvian highlands produce the neurotoxic amino acid BMAA. J. Ethnopharmacol. 2008, 118, 159–165. [Google Scholar] [CrossRef]
- Brown, R.M., Jr.; Larson, D.A.; Bold, H.C. Airborne algae: Their abundance and heterogeneity. Science 1964, 143, 583–585. [Google Scholar] [CrossRef] [PubMed]
- Trout-Haney, J.V.; Wood, Z.T.; Cottingham, K.L. Presence of the cyanotoxin microcystin in Arctic Lakes of Southwestern Greenland. Toxins 2016, 8, 256. [Google Scholar] [CrossRef]
- Plaas, H.E.; Paerl, H.W. Toxic cyanobacteria: A growing threat to water and air quality. Environ. Sci. Technol. 2020, 55, 44–64. [Google Scholar] [CrossRef] [PubMed]
- Sahu, N.; Tangutur, A.D. Airborne algae: Overview of the current status and its implications on the environment. Aerobiologia 2015, 31, 89–97. [Google Scholar] [CrossRef]
- Sharma, N.K.; Singh, S.; Rai, A.K. Diversity and seasonal variation of viable algal particles in the atmosphere of a subtropical city in India. Environ. Res. 2006, 102, 252–259. [Google Scholar] [CrossRef]
- Sharma, N.K.; Rai, A.K.; Singh, S.; Brown, R.M., Jr. Airborne algae: Their present status and relevance 1. J. Phycol. 2007, 43, 615–627. [Google Scholar] [CrossRef]
- Sharma, N.K.; Singh, S. Differential aerosolization of algal and cyanobacterial particles in the atmosphere. Indian J. Microbiol. 2010, 50, 468–473. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Chatziefthimiou, A.D.; Souza, N.R.; Cox, P.A. Desert Dust as a Vector for Cyanobacterial Toxins. The Arabian Seas: Biodiversity, Environmental Challenges and Conservation Measures; Springer: Berlin/Heidelberg, Germany, 2021; pp. 161–178. [Google Scholar]
- Bernstein, I.L.; Safferman, R.S. Viable algae in house dust. Nature 1970, 227, 851–852. [Google Scholar] [CrossRef]
- Chu, W.L.; Tneh, S.Y.; Ambu, S. A survey of airborne algae and cyanobacteria within the indoor environment of an office building in Kuala Lumpur, Malaysia. Grana 2013, 52, 207–220. [Google Scholar] [CrossRef]
- Holland, R.D.; Walne, P.L.; Richardson, C.B.; Hornsby, R.P. Viable Algae from Housedust-Possible Causal Agents in Human Allergenicity. J. Phycol. 1973, 9, 11–12. [Google Scholar]
- Tiberg, E.; Bergman, B.; Wictorin, B.; Willen, T. Occurrence of microalgae in indoor and outdoor environments in Sweden. Nord. Aerobiol. 1983, 24–29. [Google Scholar]
- Blanchard, D.C.; Syzdek, L.D. Electrostatic collection of jet and film drops 1. Limnol. Oceanogr. 1975, 20, 762–774. [Google Scholar] [CrossRef]
- Blanchard, D.C.; Syzdek, L.D. Water-to-air transfer and enrichment of bacteria in drops from bursting bubbles. Appl. Environ. Microbiol. 1982, 43, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, D.C. The size and height to which jet drops are ejected from bursting bubbles in seawater. J. Geophys. Res. Ocean. 1989, 94, 10999–11002. [Google Scholar] [CrossRef]
- Olson, N.E.; Cooke, M.E.; Shi, J.H.; Birbeck, J.A.; Westrick, J.A.; Ault, A.P. Harmful algal bloom toxins in aerosol generated from inland lake water. Environ. Sci. Technol. 2020, 54, 4769–4780. [Google Scholar] [CrossRef]
- Shi, J.H.; Olson, N.E.; Birbeck, J.A.; Pan, J.; Peraino, N.J.; Holen, A.L.; Ledsky, I.R.; Jacquemin, S.J.; Marr, L.C.; Schmale, D.G. Aerosolized Cyanobacterial Harmful Algal Bloom Toxins: Microcystin Congeners Quantified in the Atmosphere. Environ. Sci. Technol. 2023, 57, 21801–21814. [Google Scholar] [CrossRef]
- Oksanen, I.; Jokela, J.; Fewer, D.P.; Wahlsten, M.; Rikkinen, J.; Sivonen, K. Discovery of rare and highly toxic microcystins from lichen-associated cyanobacterium Nostoc sp. strain IO-102-I. Appl. Environ. Microbiol. 2004, 70, 5756–5763. [Google Scholar] [CrossRef]
- Kaasalainen, U.; Fewer, D.P.; Jokela, J.; Wahlsten, M.; Sivonen, K.; Rikkinen, J. Cyanobacteria produce a high variety of hepatotoxic peptides in lichen symbiosis. Proc. Natl. Acad. Sci. USA 2012, 109, 5886–5891. [Google Scholar] [CrossRef]
- Zhang, Y.; Husk, B.R.; Duy, S.V.; Dinh, Q.T.; Sanchez, J.S.; Sauvé, S.; Whalen, J.K. Quantitative screening for cyanotoxins in soil and groundwater of agricultural watersheds in Quebec, Canada. Chemosphere 2021, 274, 129781. [Google Scholar] [CrossRef]
- Whitman, W.B.; Coleman, D.C.; Wiebe, W.J. Prokaryotes: The unseen majority. Proc. Natl. Acad. Sci. USA 1998, 95, 6578–6583. [Google Scholar] [CrossRef]
- Chatziefthimiou, A.D.; Metcalf, J.S.; Glover, W.B.; Banack, S.A.; Dargham, S.R.; Richer, R.A. Cyanobacteria and cyanotoxins are present in drinking water impoundments and groundwater wells in desert environments. Toxicon 2016, 114, 75–84. [Google Scholar] [CrossRef]
- Ehrenberg, C.G. Bericht über die zur Bekanntmachung geeigneten Verhandlungen der Königlich-Preussischen. Akad. Wiss. Berl. 1844, 9, 182–207. [Google Scholar]
- Murby, A.L.; Haney, J.F. Field and laboratory methods to monitor lake aerosols for cyanobacteria and microcystins. Aerobiologia 2016, 32, 395–403. [Google Scholar] [CrossRef]
- Genitsaris, S.; Kormas, K.A.; Moustaka-Gouni, M. Airborne algae and cyanobacteria: Occurrence and related health effects. Front. Biosci (Elite Ed.) 2011, 3, 772–787. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, A.U.; Śliwińska-Wilczewska, S.; Woźniczka, D. Identification of cyanobacteria and microalgae in aerosols of various sizes in the air over the Southern Baltic Sea. Mar. Pollut. Bull. 2017, 125, 30–38. [Google Scholar] [CrossRef]
- Brookes, J.D.; Ganf, G.G. Variations in the buoyancy response of Microcystis aeruginosa to nitrogen, phosphorus and light. J. Plankton Res. 2001, 23, 1399–1411. [Google Scholar] [CrossRef]
- Ahern, K.S.; Ahern, C.R.; Savige, G.M.; Udy, J.W. Mapping the distribution, biomass and tissue nutrient levels of a marine benthic cyanobacteria bloom (Lyngbya majuscula). Mar. Freshw. Res. 2007, 58, 883–904. [Google Scholar] [CrossRef]
- Vignati, E.; de Leeuw, G.; Schulz, M.; Plate, E. Characterization of aerosols at a coastal site near Vindeby (Denmark). J. Geophys. Res. Ocean. 1999, 104, 3277–3287. [Google Scholar] [CrossRef]
- Dueker, M.E.; O’Mullan, G.D.; Martínez, J.M.; Juhl, A.R.; Weathers, K.C. Onshore wind speed modulates microbial aerosols along an urban waterfront. Atmosphere 2017, 8, 215. [Google Scholar] [CrossRef]
- Wood, S.A.; Dietrich, D.R. Quantitative assessment of aerosolized cyanobacterial toxins at two New Zealand lakes. J. Environ. Monit. 2011, 13, 1617–1624. [Google Scholar] [CrossRef]
- Backer, L.C.; Carmichael, W.; Kirkpatrick, B.; Williams, C.; Irvin, M.; Zhou, Y.; Johnson, T.B.; Nierenberg, K.; Hill, V.R.; Kieszak, S.M. Recreational exposure to low concentrations of microcystins during an algal bloom in a small lake. Mar. Drugs 2008, 6, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Backer, L.C.; McNeel, S.V.; Barber, T.; Kirkpatrick, B.; Williams, C.; Irvin, M.; Zhou, Y.; Johnson, T.B.; Nierenberg, K.; Aubel, M. Recreational exposure to microcystins during algal blooms in two California lakes. Toxicon 2010, 55, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.S.; Zhou, Y.; Irvin, C.M.; Kirkpatrick, B.; Backer, L.C. Characterization of aerosols containing microcystin. Mar. Drugs 2007, 5, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Gambaro, A.; Barbaro, E.; Zangrando, R.; Barbante, C. Simultaneous quantification of microcystins and nodularin in aerosol samples using high-performance liquid chromatography/negative electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2012, 26, 1497–1506. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, J.; Fan, H.; Xie, P.; He, J. A review of the neurotoxicity of microcystins. Environ. Sci. Pollut. Res. 2016, 23, 7211–7219. [Google Scholar] [CrossRef]
- Sutherland, J.W.; Turcotte, R.J.; Molden, E.; Moriarty, V.; Kelly, M.; Aubel, M.; Foss, A. The detection of airborne anatoxin-a (ATX) on glass fiber filters during a harmful algal bloom. Lake Reserv. Manag. 2021, 37, 113–119. [Google Scholar]
- Dabney, A.D. Detection of Microcystin Aerosol Particles. Master’s Thesis, University of South Carolina, Columbia, SC, USA, 2022. [Google Scholar]
- Yu, S.; Zhou, X.; Hu, P.; Chen, H.; Shen, F.; Yu, C.; Meng, H.; Zhang, Y.; Wu, Y. Inhalable particle-bound marine biotoxins in a coastal atmosphere: Concentration levels, influencing factors and health risks. J. Hazard. Mater. 2022, 434, 128925. [Google Scholar] [CrossRef]
- Caller, T.A.; Doolin, J.W.; Haney, J.F.; Murby, A.J.; West, K.G.; Farrar, H.E.; Ball, A.; Harris, B.T.; Stommel, E.W. A cluster of amyotrophic lateral sclerosis in New Hampshire: A possible role for toxic cyanobacteria blooms. Amyotroph. Lateral Scler. 2009, 10 (Suppl. S2), 101–108. [Google Scholar] [CrossRef]
- Wiśniewska, K.; Lewandowska, A.U.; Śliwińska-Wilczewska, S. The importance of cyanobacteria and microalgae present in aerosols to human health and the environment: Review study. Environ. Int. 2019, 131, 104964. [Google Scholar] [CrossRef]
- Kirso, U.; Paalme, L.; Voll, M.; Urbas, E.; Irha, N. Accumulation of carcinogenic hydrocarbons at the sediment-water interface. Mar. Chem. 1990, 30, 337–341. [Google Scholar] [CrossRef]
- Kowalewska, G.; Konat, J. Distribution of polynuclear aromatic hydrocarbons (PAHs) in sediments of the southern Baltic Sea. Oceanogr. Lit. Rev. 1997, 12, 1559. [Google Scholar]
- Baird, W.M.; Hooven, L.A.; Mahadevan, B. Carcinogenic polycyclic aromatic hydrocarbon-DNA adducts and mechanism of action. Env. Mol Mutagen. 2005, 45, 106–114. [Google Scholar] [CrossRef] [PubMed]
- ATSDR Agency for Toxic Substances and Disease Registry. Public Health Statement for Polycyclic Aromatic Hydrocarbons (PAHs). Polycyclic Aromatic Hydrocarbons (PAHs)|Public Health Statement|ATSDR. Available online: https://www.scirp.org/reference/referencespapers?referenceid=202673 (accessed on 28 August 2014).
- EPA United States Environmental Protection Agency. Metals. Metals|US EPA. Available online: https://www.researchgate.net/figure/United-State-Environmental-Protection-Agency-USEPA-maximum-contamination-levels-for_tbl1_349899836 (accessed on 7 February 2025).
- Andrew, A.S.; O’Brien, K.M.; Jackson, B.P.; Sandler, D.P.; Kaye, W.E.; Wagner, L.; Stommel, E.W.; Horton, D.K.; Mehta, P.; Weinberg, C.R. Keratinous biomarker of mercury exposure associated with amyotrophic lateral sclerosis risk in a nationwide U.S. study. Amyotroph Lateral Scler Front. Degener. 2020, 21, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.I.; Bradley, W.G.; Chen, C.Y.; Pioro, E.P.; Stommel, E.W.; Andrew, A.S. Amyotrophic lateral sclerosis risk, family income, and fish consumption estimates of mercury and omega-3 PUFAs in the United States. Int. J. Environ. Res. Public Health 2021, 18, 4528. [Google Scholar] [CrossRef]
- Tao, Y.; Xue, B.; Yang, Z.; Yao, S.; Li, S. Effects of heavy metals on the sorption of polycyclic aromatic hydrocarbons by. J. Environ. Qual. 2014, 43, 1953–1962. [Google Scholar] [CrossRef]
- Stewart, I.; Carmichael, W.W.; Sadler, R.; McGregor, G.B.; Reardon, K.; Eaglesham, G.K.; A Wickramasinghe, W.; A Seawright, A.; Shaw, G.R. Occupational and environmental hazard assessments for the isolation, purification and toxicity testing of cyanobacterial toxins. Environ. Health 2009, 8, 52. [Google Scholar] [CrossRef]
- Stewart, I.; Webb, P.M.; Schluter, P.J.; Shaw, G.R. Recreational and occupational field exposure to freshwater cyanobacteria–a review of anecdotal and case reports, epidemiological studies and the challenges for epidemiologic assessment. Environ. Health 2006, 5, 6. [Google Scholar] [CrossRef]
- Turner, P.C.; Gammie, A.J.; Hollinrake, K.; Codd, G.A. Pneumonia associated with contact with cyanobacteria. Br. Med. J. 1990, 300, 1440. [Google Scholar] [CrossRef]
- Richer, R.; Anchassi, D.; El-Assaad, I.; El-Matbouly, M.; Ali, F.; Makki, I.; Metcalf, J.S. Variation in the coverage of biological soil crusts in the State of Qatar. J. Arid Environ. 2012, 78, 187–190. [Google Scholar] [CrossRef]
- Horner, R.; Kamins, K.; Feussner, J.; Grambow, S.; Hoff-Lindquist, J.; Harati, Y.; Mitsumoto, H.; Pascuzzi, R.; Spencer, P.; Tim, R.; et al. Occurrence of amyotrophic lateral sclerosis among Gulf War veterans. Neurology 2003, 61, 742–749. [Google Scholar] [CrossRef]
- Horner, R.D.; Grambow, S.C.; Coffman, C.J.; Lindquist, J.H.; Oddone, E.Z.; Allen, K.D.; Kasarskis, E.J. Amyotrophic lateral sclerosis among 1991 Gulf War veterans: Evidence for a time-limited outbreak. Neuroepidemiology 2008, 31, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Coffman, C.J.; Horner, R.D.; Grambow, S.C.; Lindquist, J. Estimating the occurrence of amyotrophic lateral sclerosis among Gulf War (1990–1991) veterans using capture-recapture methods. Neuroepidemiology 2005, 24, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.L.; Galeano, M.A.O.; Tassone, E.; Allen, K.D.; Horner, R.D. Spatial analysis of the etiology of amyotrophic lateral sclerosis among 1991 Gulf War veterans. Neurotoxicology 2008, 29, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Vernham, G.; Bailey, J.J.; Chase, J.M.; Hjort, J.; Field, R.; Schrodt, F. Understanding trait diversity: The role of geodiversity. Trends Ecol. Evol. 2023, 38, 736–748. [Google Scholar] [CrossRef]
- Fitzgeorge, R.B.; Clark, S.A.; Keevil, C.W. Routes of Intoxication. In Detection Methods for Cyanobacterial Toxins; The Royal Society of Chemistry: London, UK, 1994; pp. 69–74. [Google Scholar]
- Creasia, D.A. Acute inhalation toxicity of microcystin-LR with mice. Toxicon 1990, 28, 605. [Google Scholar]
- Fawell, J.K.; Mitchell, R.E.; Hill, R.E.; Everett, D.J. The toxicity of cyanobacterial toxins in the mouse: II anatoxin-a. Hum. Exp. Toxicol. 1999, 18, 168–173. [Google Scholar] [CrossRef]
- Benson, J.M.; Hutt, J.A.; Rein, K.; Boggs, S.E.; Barr, E.B.; Fleming, L.E. The toxicity of microcystin-LR in mice following 7 days of inhalation exposure. Toxicon 2005, 45, 691–698. [Google Scholar] [CrossRef]
- Scott, L.L.; Downing, S.; Downing, T.G. The evaluation of BMAA inhalation as a potential exposure route using a rat model. Neurotox. Res. 2018, 33, 6–14. [Google Scholar] [CrossRef]
- Pierozan, P.; Piras, E.; Brittebo, E.; Karlsson, O. The cyanobacterial neurotoxin β-N-methylamino-L-alanine (BMAA) targets the olfactory bulb region. Arch. Toxicol. 2020, 94, 2799–2808. [Google Scholar] [CrossRef]
- Ziesemer, S.; Meyer, S.; Edelmann, J.; Vennmann, J.; Gudra, C.; Arndt, D.; Effenberg, M.; Hayas, O.; Hayas, A.; Thomassen, J.S. Target mechanisms of the cyanotoxin cylindrospermopsin in immortalized human airway epithelial cells. Toxins 2022, 14, 785. [Google Scholar] [CrossRef]
- Kubickova, B.; Laboha, P.; Hildebrandt, J.P.; Hilscherová, K.; Babica, P. Effects of cylindrospermopsin on cultured immortalized human airway epithelial cells. Chemosphere 2019, 220, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Ojima, Y.; Nguyen, M.H.; Yajima, R.; Taya, M. Flocculation of Escherichia coli cells in association with enhanced production of outer membrane vesicles. Appl. Environ. Microbiol. 2015, 81, 5900–5906. [Google Scholar] [CrossRef] [PubMed]
- Lürling, M.; Kang, L.; Mucci, M.; van Oosterhout, F.; Noyma, N.P.; Miranda, M.; Huszar, V.L.; Waajen, G.; Marinho, M.M. Coagulation and precipitation of cyanobacterial blooms. Ecol. Eng. 2020, 158, 106032. [Google Scholar] [CrossRef]
- Sukenik, A.; Kaplan, A. Cyanobacterial harmful algal blooms in aquatic ecosystems: A comprehensive outlook on current and emerging mitigation and control approaches. Microorganisms 2021, 9, 1472. [Google Scholar] [CrossRef]
- Zang, X.; Zhang, H.; Liu, Q.; Li, L.; Li, L.; Zhang, X. Harvesting of Microcystis flos-aquae using chitosan coagulation: Influence of proton-active functional groups originating from extracellular and intracellular organic matter. Water Res. 2020, 185, 116272. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Khalaf, R.; Ulén, B.; Bergkvist, G. Potential phosphorus release from catch crop shoots and roots after freezing-thawing. Plant Soil 2013, 371, 543–557. [Google Scholar] [CrossRef]
- EPA United States Environmental Protection Agency. Lake Erie Binational Nutrient Management Strategy. Lake Erie Binational Nutrient Management Strategy|US EPA. Available online: https://www.epa.gov/greatlakes/lake-erie-binational-nutrient-management-strategy (accessed on 16 September 2024).
- Smith, D.R.; King, K.W.; Williams, M.R. What is causing the harmful algal blooms in Lake Erie? J. Soil Water Conserv. 2015, 70, 27A–29A. [Google Scholar] [CrossRef]
- White, C.M.; Weil, R.R. Forage radish cover crops increase soil test phosphorus surrounding radish taproot holes. Soil Sci. Soc. Am. J. 2011, 75, 121–130. [Google Scholar] [CrossRef]
- Carver, R.E.; Nelson, N.O.; Roozeboom, K.L.; Kluitenberg, G.J.; Tomlinson, P.J.; Kang, Q.; Abel, D.S. Cover crop and phosphorus fertilizer management impacts on surface water quality from a no-till corn-soybean rotation. J. Environ. Manag. 2022, 301, 113818. [Google Scholar] [CrossRef]
- Soltangheisi, A.; Teles, A.P.B.; Sartor, L.R.; Pavinato, P.S. Cover cropping may alter legacy phosphorus dynamics under long-term fertilizer addition. Front. Environ. Sci. 2020, 8, 13. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.; Hou, J.; Wang, P.; Miao, L.; Wang, X.; Guo, L. Optimization of cyanobacterial harvesting and extracellular organic matter removal utilizing magnetic nanoparticles and response surface methodology: A comparative study. Algal Res. 2020, 45, 101756. [Google Scholar] [CrossRef]
- Nie, C.; Geng, X.; Zhang, R.; Wang, L.; Li, L.; Chen, J. Abundant cyanobacteria in autumn adhering to the heating, ventilation, and air-conditioning (HVAC) in Shanghai. Microorganisms 2023, 11, 1835. [Google Scholar] [CrossRef] [PubMed]
- Gaston, C.J.; Royer, H.M.; Leibensperger, R.J., III; Maizel, D.; Lanpher, K.B.; Solo-Gabriele, H.; Brand, L.E.; Zhai, R.G.; Caban-Martinez, A.J.; Popendorf, K.J. Filtration efficiency of air conditioner filters and face masks to limit exposure to aerosolized algal toxins. Aerosol Air Qual. Res. 2021, 21, 210016. [Google Scholar] [CrossRef]
- Hardison, D.R.; Holland, W.C.; Currier, R.D.; Kirkpatrick, B.; Stumpf, R.; Fanara, T.; Burris, D.; Reich, A.; Kirkpatrick, G.J.; Litaker, R.W. HABscope: A tool for use by citizen scientists to facilitate early warning of respiratory irritation caused by toxic blooms of Karenia brevis. PLoS ONE 2019, 14, e0218489. [Google Scholar] [CrossRef]
- Campbell, L.; Henrichs, D.W.; Olson, R.J.; Sosik, H.M. Continuous automated imaging-in-flow cytometry for detection and early warning of Karenia brevis blooms in the Gulf of Mexico. Environ. Sci. Pollut. Res. 2013, 20, 6896–6902. [Google Scholar] [CrossRef]
- Ko, B.; Park, J.; Nam, J.Y. Spatiotemporal bag-of-features for early wildfire smoke detection. Image Vis. Comput. 2013, 31, 786–795. [Google Scholar] [CrossRef]
- Bridgeman, T.B.; Chaffin, J.D.; Filbrun, J.E. A novel method for tracking western Lake Erie Microcystis blooms, 2002–2011. J. Great Lakes Res. 2013, 39, 83–89. [Google Scholar] [CrossRef]
- Coffer, M.M.; Schaeffer, B.A.; Darling, J.A.; Urquhart, E.A.; Salls, W.B. Quantifying national and regional cyanobacterial occurrence in US lakes using satellite remote sensing. Ecol. Indic. 2020, 111, 105976. [Google Scholar] [CrossRef]
- Ganf, G.G. Phytoplankton biomass and distribution in a shallow eutrophic lake (Lake George, Uganda). Oecologia 1974, 16, 9–29. [Google Scholar] [CrossRef]
- Vareschi, E. The ecology of Lake Nakuru (Kenya) I. Abundance and feeding of the Lesser Flamingo. Oecologia 1978, 32, 11–35. [Google Scholar] [CrossRef]
- Vareschi, E. The ecology of Lake Nakuru (Kenya) III. Abiotic factors and primary production. Oecologia 1982, 55, 81–101. [Google Scholar] [CrossRef] [PubMed]
- Vareschi, E.; Jacobs, J. The ecology of Lake Nakuru (Kenya) V. Production and consumption of consumer organisms. Oecologia 1984, 61, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Vaeth, M.J.E.; Cheema, M.; Omer, S.; Gupta, I.; Sun, K.J.; Mitchell, A.; Elhabashy, M.; Foyez, M.; Cheema, A.; Javed, B. Self-administered versus clinician-performed BinaxNOW COVID rapid test: A comparison of accuracy. Microbiol. Spectr. 2024, 12, e02525-23. [Google Scholar] [CrossRef] [PubMed]
- Hodinka, R.L.; Kaiser, L. Point-Counterpoint: Is the era of viral culture over in the clinical microbiology laboratory? J. Clin. Microbiol. 2013, 51, 2–8. [Google Scholar] [CrossRef]
- Pacheco, A.B.F.; Guedes, I.A.; Azevedo, S.M. Is qPCR a reliable indicator of cyanotoxin risk in freshwater? Toxins 2016, 8, 172. [Google Scholar] [CrossRef]
- Rudko, S.P.; Reimink, R.L.; Peter, B.; White, J.; Hanington, P.C. Democratizing water monitoring: Implementation of a community-based qPCR monitoring program for recreational water hazards. PLoS ONE 2020, 15, e0229701. [Google Scholar] [CrossRef]
- Clements, E.; Thompson, K.A.; Hannoun, D.; Dickenson, E.R. Classification machine learning to detect de facto reuse and cyanobacteria at a drinking water intake. Sci. Total Environ. 2024, 948, 174690. [Google Scholar] [CrossRef]
- Fournier, C.; Quesada, A.; Cirés, S.; Saberioon, M. Discriminating bloom-forming cyanobacteria using lab-based hyperspectral imagery and machine learning: Validation with toxic species under environmental ranges. Sci. Total Environ. 2024, 932, 172741. [Google Scholar] [CrossRef]
- Mellios, N.; Moe, S.J.; Laspidou, C. Machine learning approaches for predicting health risk of cyanobacterial blooms in northern european lakes. Water 2020, 12, 1191. [Google Scholar] [CrossRef]
- Hill, A.B. The environment and disease: Association or causation? Proc. R. Soc. Med. 1965, 58, 295–300. [Google Scholar] [CrossRef]
- Newell, M.E.; Adhikari, S.; Halden, R.U. Systematic and state-of the science review of the role of environmental factors in Amyotrophic Lateral Sclerosis (ALS) or Lou Gehrig’s Disease. Sci. Total Environ. 2022, 817, 152504. [Google Scholar] [CrossRef] [PubMed]
- Sini, P.; Dang, T.B.C.; Fais, M.; Galioto, M.; Padedda, B.M.; Lugliè, A.; Iaccarino, C.; Crosio, C. Cyanobacteria, cyanotoxins, and neurodegenerative diseases: Dangerous liaisons. Int. J. Mol. Sci. 2021, 22, 8726. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, J.S.; Codd, G.A. Co-occurrence of cyanobacteria and cyanotoxins with other environmental health hazards: Impacts and implications. Toxins 2020, 12, 629. [Google Scholar] [CrossRef] [PubMed]
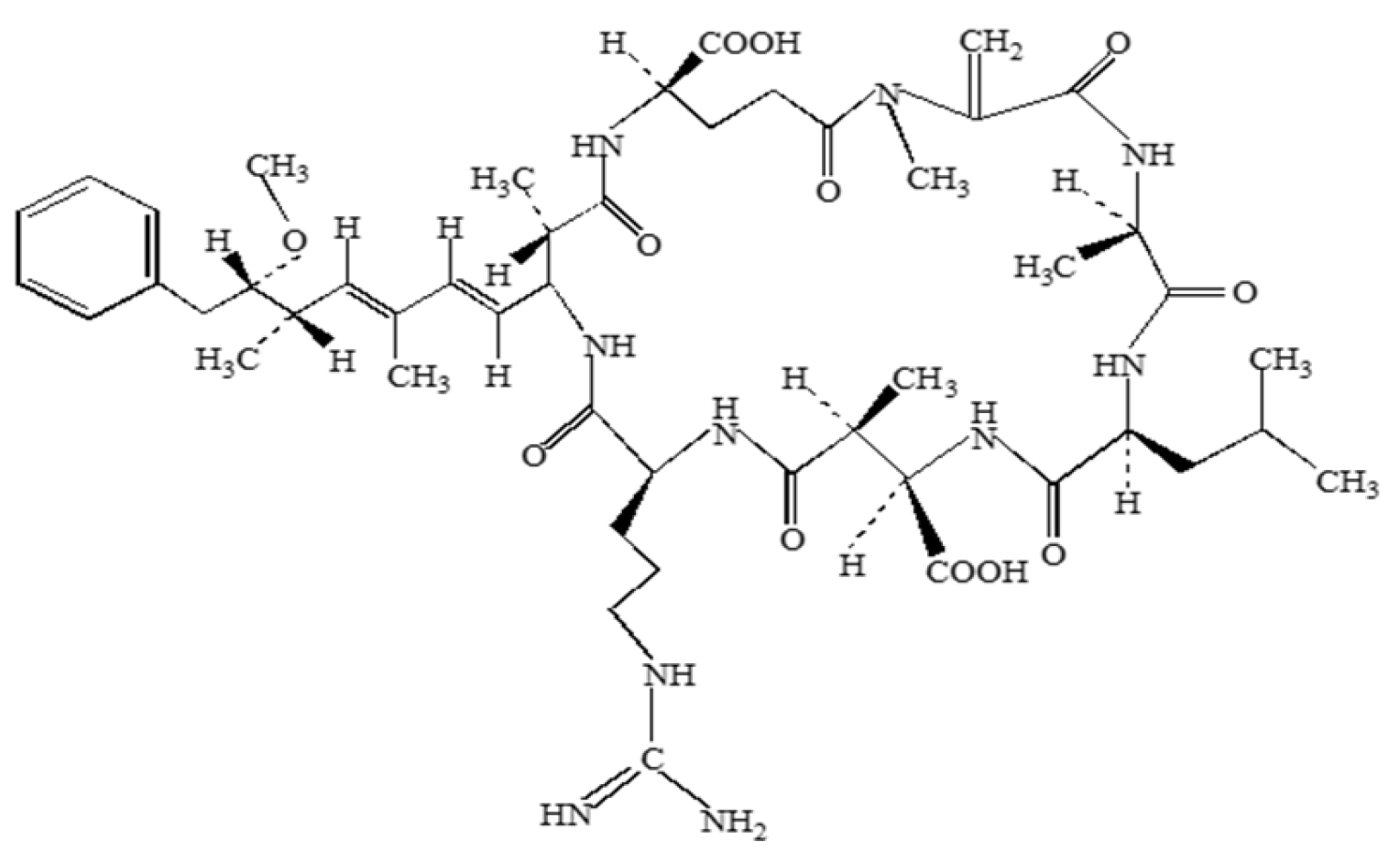
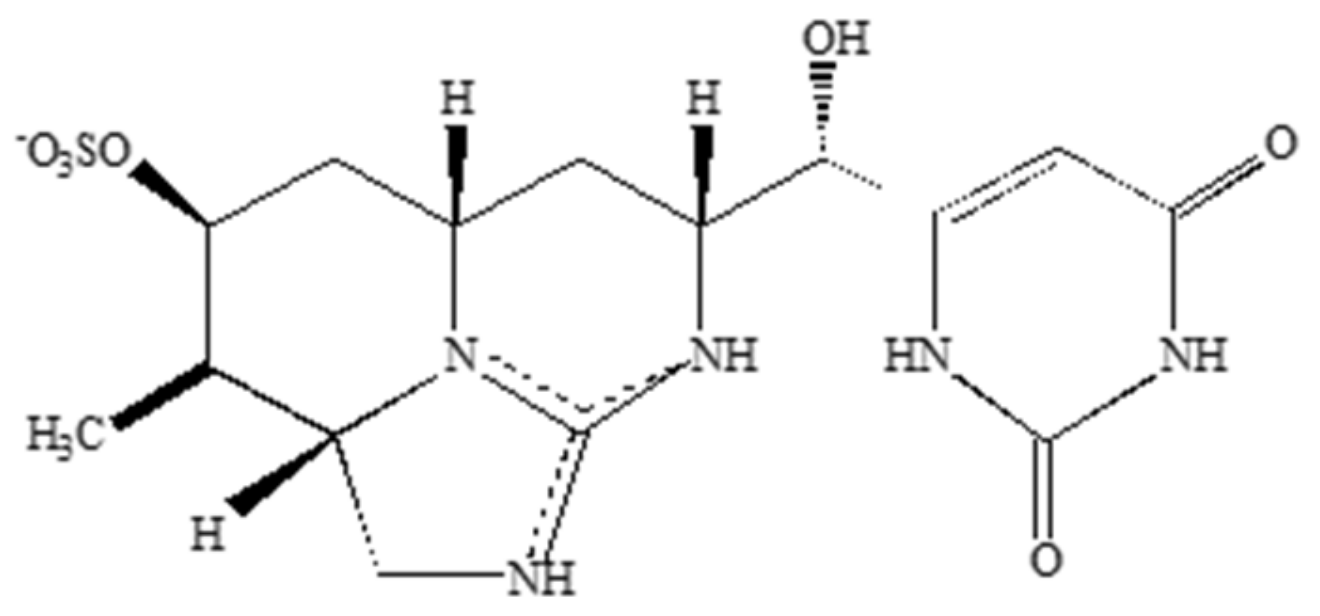
| Cyanotoxin | Oxidative Stress | Neuro-Inflammation | Protein Misfolding | Neuro-Stimulation | Neuro-Inhibition | References |
|---|---|---|---|---|---|---|
| Anatoxin-a | + | - | - | + | - | [44,45,46,47] |
| Guanitoxin | + | - | - | + | - | [48,49] |
| Saxitoxin | + | + | - | - | + | [50,51,52,53,54,55] |
| Amino Acids * | + | + | + | + | - | [56,57,58,59,60,61,62,63,64] |
| Microcystin | + | + | - | + | + | [65,66,67,68,69,70,71,72,73,74,75,76,77] |
| Cylindrospermopsin | + | + | - | - | + | [78,79,80,81,82,83,84,85,86,87,88,89,90] |
| Cyanotoxin | Route | LD50 | Test Organism | Reference |
|---|---|---|---|---|
| Anatoxin-a | Inhalation | 2000 µg/kg | Mouse | [233] |
| Microcystin-LR | Inhalation | 250 µg/kg | Mouse | [233] |
| Microcystin-LR | Aerosol | NA * | Mouse | [233] |
| Microcystin-LR | Inhalation | 43 µg/kg | Mouse | [234] |
| BMAA | Inhalation | NA | Rat | [236] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morris, Z.J.; Stommel, E.W.; Metcalf, J.S. Airborne Cyanobacterial Toxins and Their Links to Neurodegenerative Diseases. Molecules 2025, 30, 2320. https://doi.org/10.3390/molecules30112320
Morris ZJ, Stommel EW, Metcalf JS. Airborne Cyanobacterial Toxins and Their Links to Neurodegenerative Diseases. Molecules. 2025; 30(11):2320. https://doi.org/10.3390/molecules30112320
Chicago/Turabian StyleMorris, Zachary James, Elijah W. Stommel, and James Spencer Metcalf. 2025. "Airborne Cyanobacterial Toxins and Their Links to Neurodegenerative Diseases" Molecules 30, no. 11: 2320. https://doi.org/10.3390/molecules30112320
APA StyleMorris, Z. J., Stommel, E. W., & Metcalf, J. S. (2025). Airborne Cyanobacterial Toxins and Their Links to Neurodegenerative Diseases. Molecules, 30(11), 2320. https://doi.org/10.3390/molecules30112320






