Applications of Hydrophilic Interaction Chromatography in Pharmaceutical Impurity Profiling: A Comprehensive Review of Two Decades
Abstract
1. Introduction
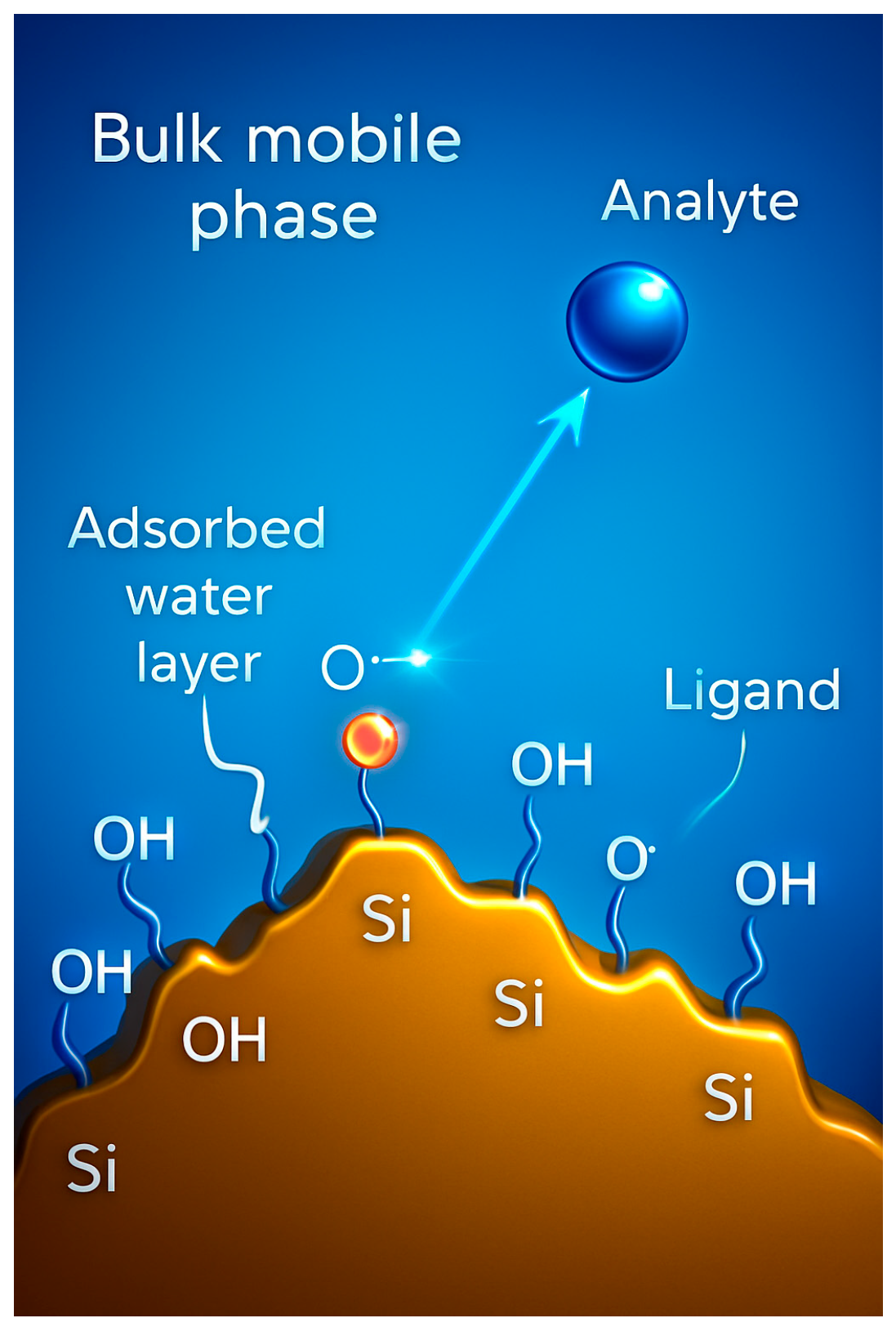
2. HILIC
2.1. General Considerations
2.2. Structure-Guided Stationary Phase Selection
- (i)
- Neutral polar groups (–OH, –NH2, –CONH2): Compounds containing neutral polar groups interact strongly with the water-enriched layer on HILIC stationary phases. For example, nucleosides and “sugar-like” drugs (i.e., macrolide drugs) often exhibit robust retention on bare silica or zwitterionic phases due to hydrogen bonding and partitioning into the aqueous layer.
- (ii)
- Acidic groups (–COOH, –SO3H): Carboxylic acids and sulfonic acids exhibit retention through both hydrophilic partitioning and electrostatic interactions. Their behavior is highly pH-dependent: under partially ionized conditions, acidic analytes benefit from zwitterionic or amino-type phases that provide complementary ionic interactions.
- (iii)
- Basic groups (–NH2, heteroaromatic nitrogens): Basic analytes (e.g., many antibiotics, nucleobases) are prone to strong electrostatic interactions with residual silanols, leading to poor peak shape if not properly controlled. Zwitterionic or amide-type HILIC phases can minimize this issue by balancing ionic and hydrogen-bonding interactions, improving reproducibility.
- (iv)
- Zwitterionic or amphoteric compounds: Molecules with both acidic and basic groups (e.g., amino acids, peptides) benefit from stationary phases that contain zwitterionic ligands. These phases stabilize both charge states and offer consistent retention while minimizing peak tailing.
3. Applications
3.1. HPLC-UV/FLD
| Analyte | Analytical Column | Mobile Phase | Flow Rate (mL/min) | UV/FLD Wavelength(s) (nm) | LODs/LOQs | Recovery (%) | Reference |
|---|---|---|---|---|---|---|---|
| Prulifloxacin, ulifloxacin and four process impurities | Diol-bonded silica (250 × 4.6 mm, 5 μm) | NH4Ac buffer (5 mM, pH = 5.8) and ACN | 1 | 218–283 (UV) | 0.15/0.25 μg/mL (3/5 μg/mL for ulifloxacin) | NA | [33] |
| Amino acids and amino acid-like molecules | Diol-bonded core–shell silica (100 × 4.6 mm, 2.5 μm) | Potassium phosphate buffer (12.5 mM, pH = 2.8) and ACN | 1.4 | 200 (UV) | 0.02–8.0/0.05–20.0 μg/mL | 97.3–110.6 | [38] |
| Polar API and three impurities | Diol-bonded silica (250 × 4.6 mm, 5 μm) C18 (100 × 4.6 mm, 3.5 μm) | 0.09% phosphoric acid and ACN Ammonium chloride (10 mM) and ACN | 1.5 1.0 | 215 (UV) | NA/0.05% (for impurities) | 99.2–100.6 | [39] |
| Bilastine and two impurities | Diol-bonded silica (100 × 4.6 mm, 5 μm) | NH4Ac buffer (50 Mm, pH = 5.3) | 1.0 | 275 (UV) | 0.16/0.5 μg/mL (for impurity 1) 0.08/0.25 μg/mL (for impurity 2) | 98.7–101.8 | [40] |
| Acetylsalicylic acid, amlodipine, one impurity and atenolol | Diol-bonded silica (100 × 4.6 mm, 2.6 μm, 100 Å) | NH4Ac buffer (75 mM, pH = 5.3) and ACN | 1.0 | 254 (UV) | NA/NA | NA | [41] |
| Succinylcholine and impurities | HILIC–silica (250 × 4.6 mm, 5 μm) | 30% phosphate buffer (pH = 4.0; 0.05 M) in ACN | 1.0 | 214 (UV) | 2.4–11.5/7.3–24.9 μg/mL | 95.7–98.9 | [34] |
| Sodium cromoglicate | HILIC–silica (250 × 4.6 mm, 5 μm) | NH4Ac buffer (30 mM, pH = 3.0) and ACN | 2.0 | 326 (UV) | 25/100 ng/mL | 100.4 | [42] |
| Dalfampridine and five process impurities | Bare silica (250 × 4.6 mm, 5 μm) | Ammonium formate (10 mM, pH = 5.0) and MeOH:ACN (10:90 v/v) | 1.0 | 280 (UV) | 7.19–7.64/21.57–22.93 μg/mL | 88.1–107.7 | [35] |
| Linagliptin and its impurity 3-aminopyridine | EXTRASIL Silica (150 × 4.0 mm, 3 μm) | NH4Ac buffer (10 mM, pH = 6.0) and ACN | 1.0 | 298 (UV) | 7.5/25 μg/mL (with respect to linagliptin) | 97.3–101.3 | [43] |
| Metformin hydrochloride and its related impurities, cyanoguanidine and melamine | HILIC–silica (250 × 4.6 mm, 5 μm) | Sodium phosphate buffer (25 mM, pH = 3.0) and ACN | 2.0 | 218 (UV) | 5.0–100/25–350 ng/mL | 98.1–101.0 | [44] |
| 2-aminoisobutyric acid | HILIC–silica (250 × 4.6 mm, 5 μm) | Potassium acetate buffer (25 mM, pH = 5.5) and ACN | 0.8 | 345 (excitation)/ 450 (emission) (FLD) | 4.5/15 ng/mL | 99.9 | [45] |
| Moxonidine and four impurities | RX–silica (250 × 4.6 mm, 5 μm) | Ammonium formate buffer (40 mM, pH = 2.8) and ACN | 1.0 | 255 (UV) | 0.012/0.04 μg/mL (for impurities A,B) 0.024/0.08 μg/mL (for impurities C,D) | 93.7–114.1 | [46] |
| Salicylic acid, acetyl salicylic acid and Ascorbic acid | RX–silica column (250 × 4.6 mm, 5 μm) | NH4Ac buffer (22 mM, pH = 4.4) and ACN | N/A | 285 (UV) | 0.03 μg/mL (for salicylic acid)/0.09–2.5 μg/mL | 93.9–105.8 | [41] |
| Iodixanol and three impurities | Core–shell silica (150 × 4.6 mm, 2.6 μm) | Formic acid solution (1 mM, pH = 3.2) and ACN | 0.8 | 243 (UV) | 0.024–0.19/0.061–0.48 μg/mL | 97.5–103 | [47] |
| Tofacitinib citrate and related substances | Zwitterionic HILIC (250 × 4.6 mm, 5 μm) | Potassium phosphate buffer (10 mM, pH = 7.0) and ACN | 0.5 | 210 (UV) | 0.03/0.05 and 0.06% w/w | 86.0–100.0 | [48] |
| Sulfaquinoxaline sodium and related compound A | Zwitterionic HILIC (250 × 4.6 mm, 5 μm) | NH4Ac buffer (200 mM, pH = 5.7) and ACN | 0.5 | 263 (UV) | 0.04/0.13 μg/mL (for sulfaquinoxaline sodium) | 98.9–100.9 | [49] |
| Iohexol, one isomer and three impurities | Zwitterionic HILIC (100 × 4.6 mm, 5 μm) | NH4Ac buffer (72 mM, 6.5) and ACN | 1.0 | 254 (UV) | 0.08/0.25 μg/mL | 94.2–110.2 | [50] |
| N-hydroxysuccinimide and N-hydroxysulfosuccinimide | Ζwitterionic silica-based HILIC (150 × 3 mm, 3 μm) | NH4Ac buffer (10 mM, pH = 7.5) and ACN | 0.4 | 220 and 260 (UV) | 1/3 mg/L (for N-hydroxysuccinimide) and 0.5/1.5 mg/L (for N-hydroxysulfosuccinimide) | NA | [51] |
| Sildenafil citrate and its impurity imidazole | Zwitterionic sulfobetaine-bonded silica (150 × 4.6 mm, 5 μm, 200 Å) | NH4Ac buffer (10 mM, pH = 5.5) and ACN | 0.85 | 210 (UV) | 0.025/0.125 μg/mL (for imidazole) | NA | [52] |
| N,N’-ethylenebis-l-cysteine diethyl ester (Bicisate) and impurities | Mixed-mode HILIC (150 × 4.6 mm, 5 μm, 100 Å) | TFA solution (7.5 mM) and ACN | 1.0 | 215 (UV) | NA/NA | 85.0–109.0 | [53] |
| l-ascorbic acid 2-phosphate magnesium | Mixed-mode HILIC (150 × 3.2 mm, 5 μm) | Potassium phosphate buffer (15 mM, pH = 2.5) and ACN | 0.4 | 240 (UV) | 0.017/0.052% | 97.1–101.2 | [54] |
| Capreomycin sulfate and four impurities | Mixed-mode HILIC (250 × 4.6 mm, 5 μm) | Ammonium trifluoroacetate buffer (20 mM, pH 5.0) and ACN | 1.0 | 260 (UV) | NA/NA | NA | [55] |
| 2-chloromalonaldehyde | Amide HILIC (150 × 2.1 mm, 1.7 μm) | NH4Ac buffer (200 mM) and ACN | 0.5 | 273 (UV) | 0.004/0.01 mg/mL | 79.1–106.6 | [56] |
| Amlodipine besylate and three impurities | Aminopropyl-bonded silica (250 × 4.6 mm, 5 μm) | NH4Ac buffer (50 mM, pH = 4.0) and ACN | 1.0 | 230 (UV) | 0.010/0.025 μg/mL | 96.4–101.2 | [57] |
| Amitriptyline and six impurities | Aminopropyl-bonded silica Amide-bonded silica Bare silica Diol-bonded silica (250 × 4.6 mm, 5 μm) | NH4Ac buffer (various pH values) and ACN | 1.0 | 254 (UV) | NA/NA | NA | [58] |
| Amlodipine besilate and two impurities bisoprolol fumarate and four impurities | Aminopropyl-bonded silica (250 × 4.6 mm, 5 μm) Bare silica (100 × 4.6 mm, 5 μm) Diol-bonded silica (100 × 4.6 mm, 5 μm, 200 Å) | NH4Ac buffer (20 mM, pH = 4.0) and ACN | 1.0 | 230 (UV) | NA/NA | NA | [59] |
| Amitriptyline and four impurities | Aminopropyl-bonded silica (100 × 4.6 mm, 5 μm) | NH4Ac buffer (60 mM, pH = 4.5) and ACN | 1.0 | 254 (UV) | 0.025–0.50/0.05–1 μg/mL | 96.4–102.1 | [60] |
| Eleven nicotinamide metabolites | Pentyl-bromide (3PBr) (150 × 3 mm, 3 μm) | Ammonium formate buffer (20 mM, pH = 6.4) and MeOH | 0.4 | 260 (UV) | NA/NA | NA | [61] |
| Four cyclic synthetic pharmaceutical peptides | Silica-based (150 × 2.1 mm, 1.7 μm) Acidic (tetrazole), basic (pyridyl), zwitterionic (phosphorylcholine) (250 × 4.6 mm, 3 µm) | NH4Ac buffer (20 mM, pH = 4.7) and ACN | 1.0 | 220 and 254 (UV) | NA/NA | NA | [62] |
| Olopatadine hydrochloride, one isomer and benzalkonium chloride | Cyano-bonded silica (100 × 4.6 mm, 5 μm) | NH4Ac buffer (5 mM, pH = 4.5) and ACN | 1.0 | 257 (UV) | 0.1/0.375 μg/mL (for isomer) 6/20 μg/mL (for benzalkonium chloride) | 83.2–101.5 | [63] |
| Acarbose and seven impurities | Pentafluorophenyl (PFP)-bonded core–shell silica (100 × 4.6 mm, 2.6 μm) | Ammonium formate buffer | 1.0 | 210 (UV) | NA/NA | NA | [64] |
| Oxaliplatin enantiomers | Cellulose-based silica (100 × 4.6 mm, 3 μm) | Water and ACN | 1.0 | 210 (UV) | 0.07/0.21 μg/mL | NA | [65] |
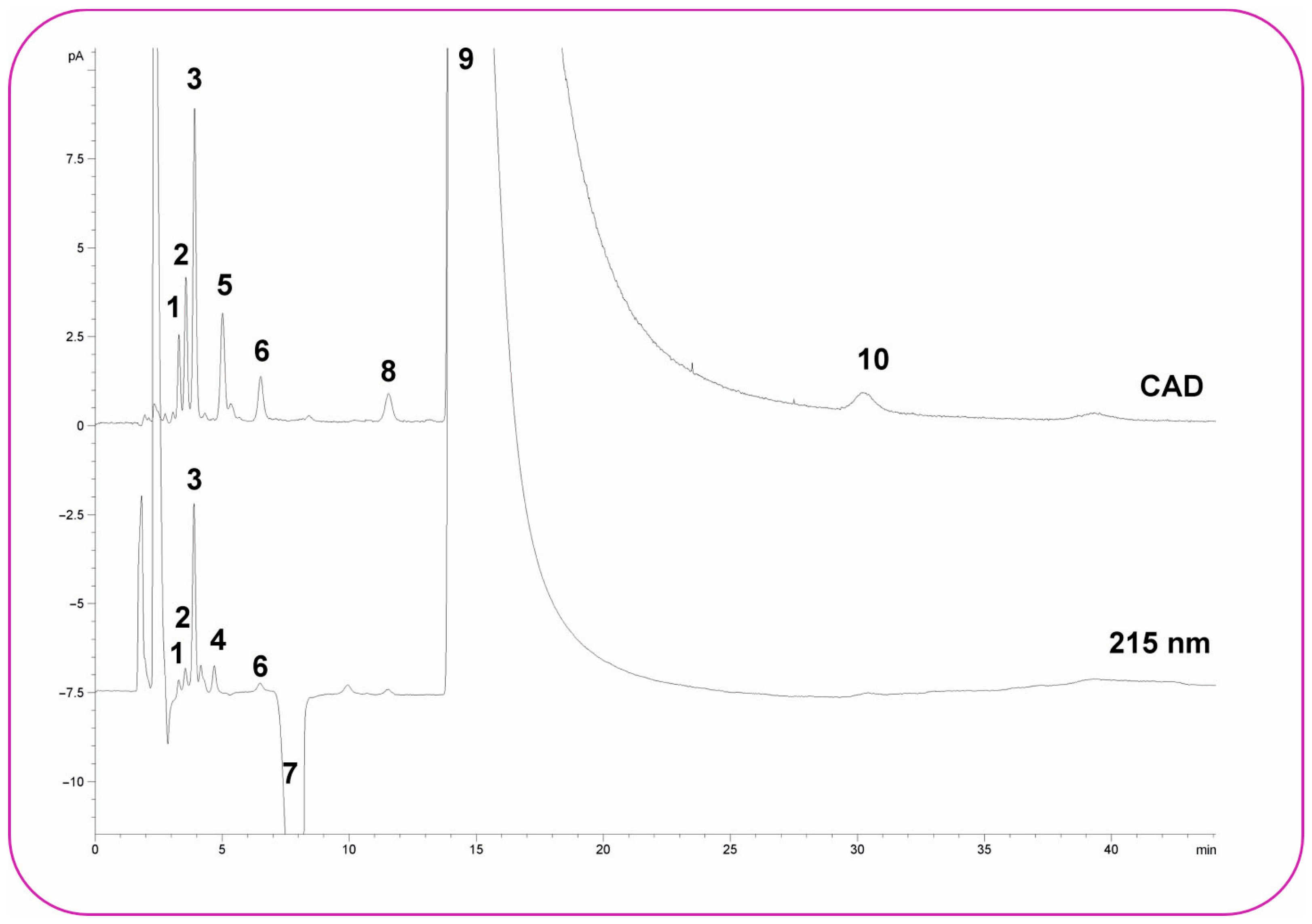
3.2. HPLC-CAD
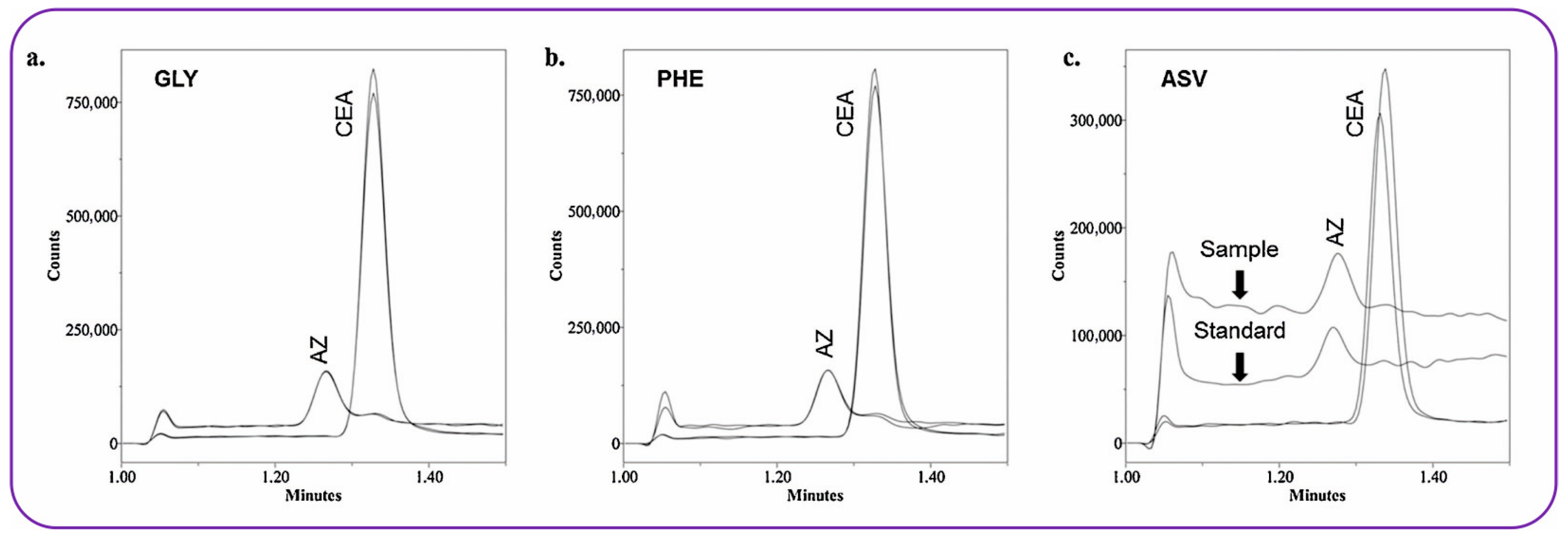
3.3. HPLC-MS
| Analyte | Analytical Column | Mobile Phase | Flow Rate (mL/min) | MS Parameters | LODs/LOQs | Recovery (%) | Reference |
|---|---|---|---|---|---|---|---|
| Dimethyl sulfate | Dihydroxypropane-bonded silica (150 × 2 mm, 3 μm) | Ammonium formate buffer (50 mM, pH = 3.2) and ACN | 0.8 | ESI (+ve), capillary voltage: 4 kV, SIM (m/z): 94.2 | NA/0.04–0.13 μg/mL | 85.4–114.5 | [77] |
| Dimethyl sulfate | Mixed-mode silica (100 × 2.1 mm, 3 μm) | NH4Ac buffer (10 mM, pH = 3.2) and ACN | 0.3 | ESI (+ve), cone voltage: 40 V, MRM (m/z): 246.2/97.1, 230.2 | 0.5/1.15 ng/mL | 94.9–106.4 | [78] |
| Dimethyl sulfate, diethyl sulfate | Bare silica (50 × 2.1 mm, 3 μm) | Ammonium formate buffer (20 mM) and ACN | 0.2 | NA | NA | 80.3–102.3 90.3–100 | [81] |
| Dimethyl sulfate | Bare silica (100 × 2.1 mm, 1.6 μm) | Ammonium formate buffer (50 mM) and ACN | 0.35 | ESI (+ve), capillary voltage: 0.8 kV, SIM (m/z): 116 | 0.19/0.65 ng/mL | 96.46–105.98 | [79] |
| Alkyl sulfonate dialkyl sulfates | Bare silica (50 × 2.1 mm, 3 μm) | Ammonium formate buffer (50 mM) and ACN | 0.3 | ESI (+ve), capillary voltage: 3 kV, SIM (m/z): 88, 102, 106 | NA/NA | 74–137 | [80] |
| Dimethylamine | Bare silica (150 × 3.0 mm, 2.7 μm) | Ammonium formate buffer (10 mM, pH = 4.8) and ACN | 0.8 | ESI (+ve), capillary voltage: 0.5 kV, SIM (m/z): 46 | 0.75/2.5 ng/mL | 99–104.2 | [84] |
| 1,1-dimethyl-3-hydroxy-pyrrolidinium bromide | Bare silica (100 × 4.6 mm, 5 μm) | Ammonium formate buffer (10 mM) and ACN | 1.2 | ESI (+ve), collision energy: 27 V, MRM (m/z): 116.1/88.1 | 17/51 ng/mL | 89.7–103.2 | [85] |
| Degradation products of citicoline | Bare silica (50 × 4.6 mm, 3 μm) | Ammonium formate buffer (20 mM, pH = 3) and ACN | NA | ESI (+ve), capillary voltage: 4 kV, SCAN (m/z): 50–800 | 0.03–0.45/0.11–1.35 μg/mL | 99.0–99.9 | [86] |
| 2-chloro-N-(2-chloroethyl)ethanamine | Mixed-mode (150 × 4.6 mm, 5 μm) | Ammonium formate buffer (20 mM) and ACN | 0.8 | ESI (+ve), capillary voltage: 0.5 V, SIM (m/z): 142 | 0.3/1 ng/mL | NA | [82] |
| Aziridine, 2-chloroethylamine | Bridged-ethyl hybrid silica (50 × 2.1 mm, 1.7 μm) | Ammonium formate buffer (100 mM) and ACN | 0.5 | ESI (+ve), capillary voltage: 3 kV, SIM (m/z): 44.1, 79.9 | 0.5 ng/mL | 84–105 | [83] |
| 2,3-dichloroaniline, bis(2-chloroethyl) amine, 2-chloroethylamine | Polyhydroxyl-based silica (100 × 4.6 mm, 5 μm) | Ammonium formate buffer (100 mM, pH = 6) and ACN | 0.8 | ESI (+ve), capillary voltage: 3 kV, MRM (m/z): 163.1/126.3 (for 2,3-dichloroaniline), 143.1/63.1 (for bis(2-chloroethyl) amine, 80.5/63.1 (for 2-chloroethylamine) | 2–30 ng/mL | 96.4–98.2 | [87] |
| Impurities in streptomycin and dihydrostreptomycin | Fused-core silica (100 × 2.1 mm, 2.7 μm) | Ammonium formate buffer (200 mM) and ACN | 0.4 | ESI (+ve), voltage: 4.5 kV, SCAN (m/z): 100–1000 | NA/NA | NA | [88] |
| Amino acids, non-amino acids | Ζwitterionic silica-based HILIC (150 × 3 mm, 3 μm) | Ammonium formate buffer (100 mM) and ACN | 0.5 | ESI, capillary voltage: 0.3 kV (for (+)ve mode), 0.8 kV (for (−)ve mode), SIM (m/z): 139.0 (urocanic acid), 127.0 (4-imidazoleacetic acid), 133.0 (asparagine), 112.1 (histamine). 142.1 (histidinol), 156.1 (histidine), 175.1 (arginine) 147.1 (lysine), 132.0 (aspartic acid), 157.1 (β-Imidazolelactic acid) | 1.1–18.2/2.5–42.6 ng/mL | 76.1–114.3 | [89] |
| Various impurities in parenteral solutions | Polysulfoethyl A-based HILIC (150 × 4.6 mm, 5 μm) Quinine-based HILIC (150 × 4.0 mm, 5 μm) C18 (150 × 3.0 mm, 3 μm) | Carbonate buffer (NaHCO3, Na2CO3, 100 mM each, pH 9.5)/ACN FA (0.1%) in water and ACN | 1 mL/min 0.5 mL/min 0.3 mL/min | ESI, capillary voltage: 0.3 kV (for (+)ve mode), 0.8 kV (for (-)ve mode), MRM mode | NA/5–500 ng/mL | NA | [90] |
| Trimethylammonium bromide, 1,1,1-trimethylhydrazinium bromide, 3-hydroxy-1,1-dimethyl-4,5-dihydro-1H-pirazolium-1-betaine hydrate, 3-(2,2,2-trimethylhydrazinium)methylpropionate bromide, 3-(2,2,2-trimethylhydrazinium)ethylpropionate bromide and 3-(2,2,2-trimethylhydrazinium)prop-2-yl propionate bromide | Cyano-based HILIC (100 × 2.1 mm, 5 μm) Amino-based HILIC (150 × 3.2 mm, 3 μm) Silica-based HILIC (150 × 2.1 mm, 3 μm) Zwitterionic HILIC (100 × 2.1 mm, 5 μm) | Various buffers containing ammonium formate, FA, and ACN | 0.2 mL/min | ESI, capillary voltage: 3 kV, MRM mode | 0.00006–0.003/0.0002–0.01% | 96.9–116.4 | [91] |
| Methanesulfonic acid | Triazole-based HILIC (150 × 4.6 mm, 5 μm) Amino-based HILIC (150 × 4.6 mm, 3 μm) | Ammonium formate buffer (100 mM, pH = 3.5) and ACN | 1 mL/min | ESI (-ve), voltage: 4 kV, SIM (m/z): 95 (methanesulfonic acid) | NA/NA | 97.2–98.2 | [92] |
| Semi-synthetic glycoproteins | Amide-based HILIC (150 × 2.0 mm, 3 μm) | TFA in water and ACN (0.05%) | 0.3 mL/min | ESI (+ve), voltage: 4 kV, SIM (m/z): 95 (methanesulfonic acid) | NA/NA | NA | [93] |
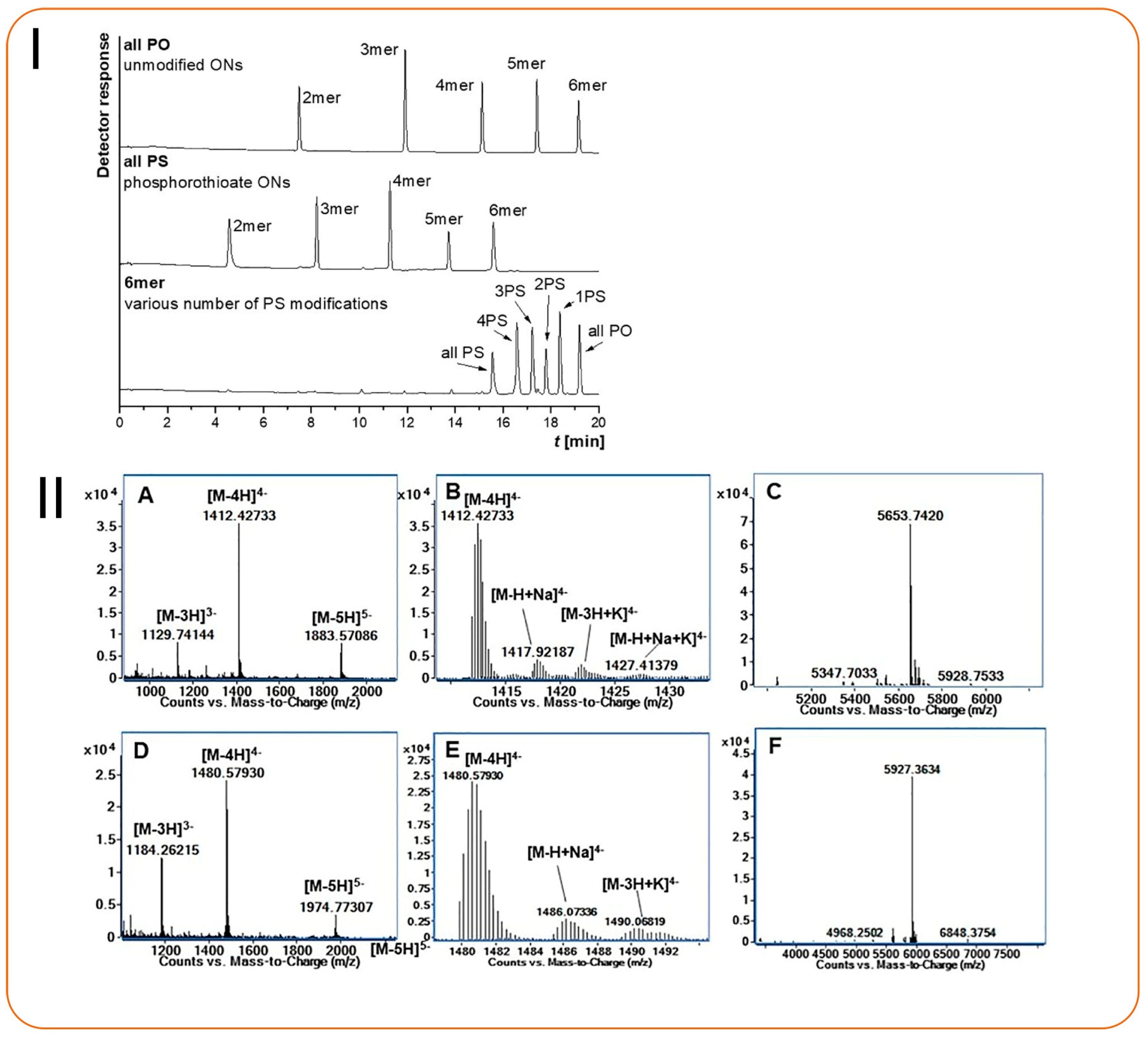
| Oligonucleotide impurities and nusinersen metabolites | Amide-based HILIC (150 × 2.1 mm, 1.7 μm) | NH4Ac buffers at various pH values with ACN | 0.4 mL/min | ESI (+ve), capillary voltage: 4 kV, SCAN (m/z): 50–1250 | NA/NA | NA | [94] |
| Characterization of DNA and RNA oligonucleotides | Amide-based HILIC (150 × 2.1 mm, 1.7 μm) | Various NH4Ac and ammonium formate buffers and ACN | 0.25 mL/min | ESI (-ve), capillary voltage: 3 kV, SCAN (m/z): 400–2000, MRM (m/z): 94.23 (PSO2−) | 2 pmol/NA | NA | [95] |
| Enantiomeric impurities of cabotegravir | Cellulose-tris (4-chloro-3-methyl phenyl carbamate)-based HILIC (150 × 4.6 mm, 3 μm) | 0.1% FA/ACN | 1 mL/min | ESI, interface voltage: 4.5 kV (for (+)ve), −4.5 kV (for (-)ve mode), SCAN (m/z): 100–2000 | 0.01–0.02/0.03–0.06 μg/mL | 99.2–114.9 | [96] |
| Sequencing of oligomers | Polyvinyl alcohol-based HILIC (150 × 2.1 mm, 1.7 μm) | NH4Ac buffer (1 mM) and ACN | 0.2 mL/min | ESI (-ve), capillary voltage: 3.5 kV, SCAN (m/z): 100–3200 | NA/NA | NA | [97] |
| Characterization of impurities in therapeutic monoclonal antibodies | Glycoprotein amide-based HILIC (150 × 2.1 mm, 1.7 μm) | 0.1% TFA in water and ACN | 0.2 mL/min | ESI, capillary voltage: 4 kV, SCAN (m/z): 800–4000 | NA/NA | NA | [98] |
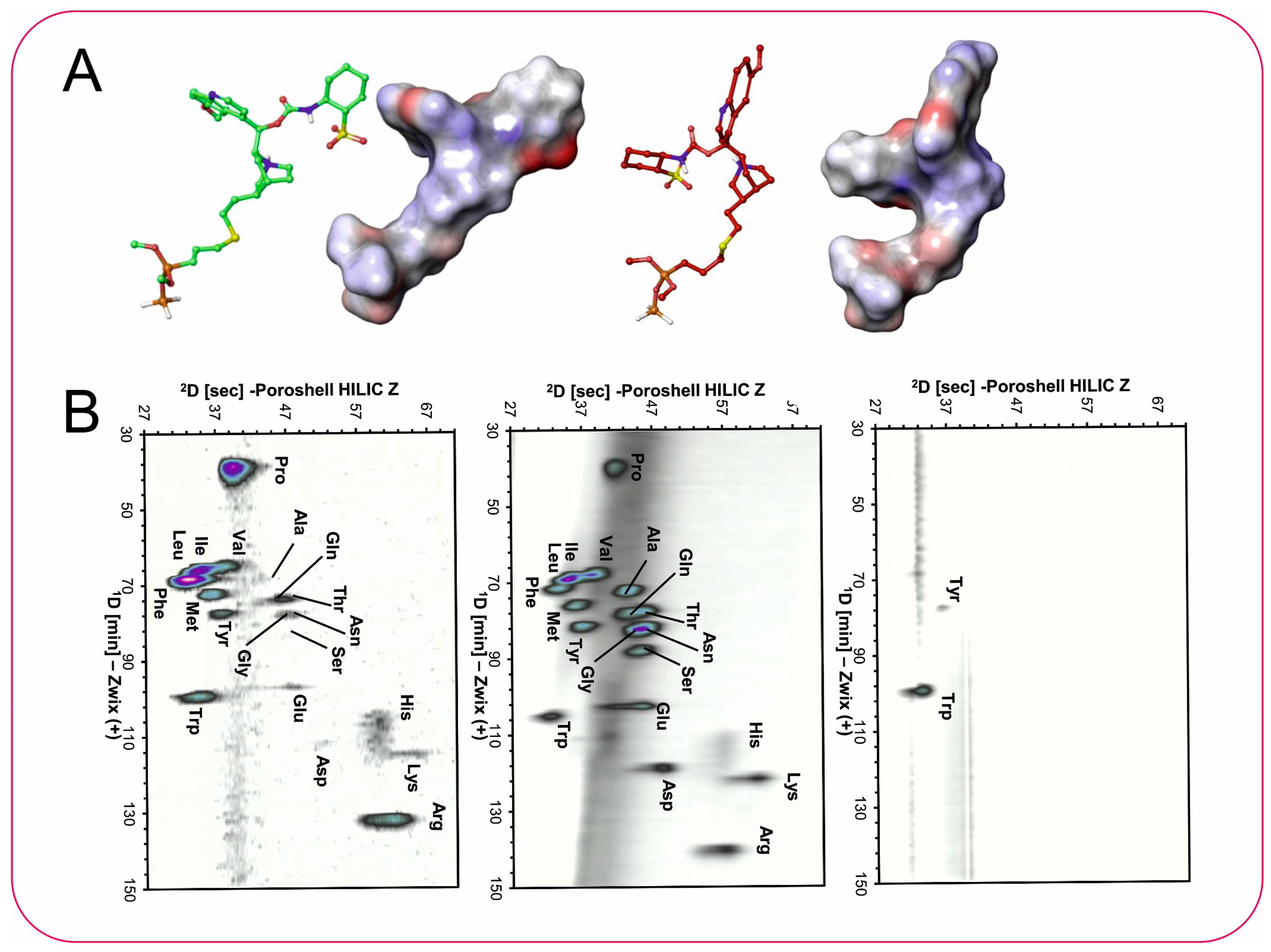
3.4. Two-Dimensional LC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2-AIBA | 2-aminoisobutyric acid |
| 2-CIMA | 2-chloromalonaldehyde |
| ACN | Acetonitrile |
| AEX | Anion-exchange chromatography |
| API | Active pharmaceutical ingredients |
| AQbD | Analytical quality by design |
| CAD | Charged aerosol detector |
| DAD | Diode array detector |
| DoE | Design of experiments |
| ESI | Electrospray |
| ELSD | Evaporative light-scattering detector |
| EP | European Pharmacopoeia |
| FA | Formic acid |
| FLD | Fluorescence detector |
| GPC | Gel permeation chromatography |
| HILIC | Hydrophilic interaction chromatography |
| HPLC | High-performance liquid chromatography |
| ICH | International Council for Harmonization |
| IPRP | Ion-pair reversed phase |
| LOD | Limit of detection |
| LOQ | Limit of quantitation |
| MS | Mass spectrometry |
| NA | Not applicable |
| NH4Ac | Ammonium acetate |
| NHS | N-hydroxysuccinimide |
| QTOF | Quadrupole time-of-flight |
| RP | Reversed phase |
| RP-HPLC | Reversed-phase high-performance liquid chromatography |
| SIM | Selected ion monitoring |
| SPE | Solid phase extraction |
| sulfo-NHS | N-hydroxysulfosuccinimide |
| TFA | Trifluoroacetic acid |
| TLC | Thin-layer chromatography |
| USP | United States Pharmacopeia |
References
- ICH Topic Q 3 A (R2) Impurities in New Drug Substances ICH Step 5 Note for Guidance on Impurities Testing: Impurities in New Drug Substances; European Medicines Agency: London, UK, 2006.
- ICH Topic Q 1 A (R2) Stability Testing of New Drug Substances and Products Step 5 Note for Guidance on Stability Testing: Stability Testing of New Drug Substances and Products; European Medicines Agency: London, UK, 2003.
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Ich Harmonised Tripartite Guideline Impurities in New Drug Products Q3B(R2); European Medicines Agency: London, UK, 2006.
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Impurities: Guideline for Residual Solvents Q3C(R9); European Medicines Agency: London, UK, 2004.
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Ich Harmonised Guideline Guideline for Elemental Impurities Q3D(R2); European Medicines Agency: London, UK, 2022.
- Khandale, N.; Rajge, R.R.; Singh, S.K.; Singh, G. Advances of Hyphenated Technique in Impurity Profiling of Active Pharmaceutical Ingredients and Pharmaceutical Products. Sep. Sci. Plus 2023, 6, 2300018. [Google Scholar] [CrossRef]
- Finotti Cordeiro, C.; Lopardi Franco, L.; Teixeira Carvalho, D.; Bonfilio, R. Impurities in Active Pharmaceutical Ingredients and Drug Products: A Critical Review. Crit. Rev. Anal. Chem. 2024, 1–21. [Google Scholar] [CrossRef] [PubMed]
- D’Atri, V.; Barrientos, R.C.; Losacco, G.L.; Rudaz, S.; Delobel, A.; Regalado, E.L.; Guillarme, D. Trends in Pharmaceutical Analysis: The Evolving Role of Liquid Chromatography. Anal. Chem. 2025, 97, 4706–4727. [Google Scholar] [CrossRef]
- Singh, D.K.; Sahu, A.; Kumar, S.; Singh, S. Critical Review on Establishment and Availability of Impurity and Degradation Product Reference Standards, Challenges Faced by the Users, Recent Developments, and Trends. TrAC Trends Anal. Chem. 2018, 101, 85–107. [Google Scholar] [CrossRef]
- Görög, S. Critical Review of Reports on Impurity and Degradation Product Profiling in the Last Decade. TrAC Trends Anal. Chem. 2018, 101, 2–16. [Google Scholar] [CrossRef]
- Zhang, L.; Dai, Q.; Qiao, X.; Yu, C.; Qin, X.; Yan, H. Mixed-Mode Chromatographic Stationary Phases: Recent Advancements and Its Applications for High-Performance Liquid Chromatography. TrAC Trends Anal. Chem. 2016, 82, 143–163. [Google Scholar] [CrossRef]
- Kartsova, L.A.; Bessonova, E.A.; Somova, V.D. Hydrophilic Interaction Chromatography. J. Anal. Chem. 2019, 74, 415–424. [Google Scholar] [CrossRef]
- Olsen, B.A.; Pack, B.W. Hydrophilic Interaction Chromatography: A Guide for Practitioners; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Sheng, Q.; Liu, M.; Lan, M.; Qing, G. Hydrophilic Interaction Liquid Chromatography Promotes the Development of Bio-Separation and Bio-Analytical Chemistry. TrAC Trends Anal. Chem. 2023, 165, 117148. [Google Scholar] [CrossRef]
- Erkmen, C.; Gebrehiwot, W.H.; Uslu, B. Hydrophilic Interaction Liquid Chromatography (HILIC): Latest Applications in the Pharmaceutical Researches. Curr. Pharm. Anal. 2020, 17, 316–345. [Google Scholar] [CrossRef]
- Wei, B.; Dai, L.; Zhang, K. Applications of Hydrophilic Interaction and Mixed-Mode Liquid Chromatography in Pharmaceutical Analysis. J. Chromatogr. A 2025, 1739, 465524. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, F.Q.; Ge, L.; Hu, Y.J.; Xia, Z.N. Recent Applications of Hydrophilic Interaction Liquid Chromatography in Pharmaceutical Analysis. J. Sep. Sci. 2017, 40, 49–80. [Google Scholar] [CrossRef]
- Goyon, A.; Yehl, P.; Zhang, K. Characterization of Therapeutic Oligonucleotides by Liquid Chromatography. J. Pharm. Biomed. Anal. 2020, 182, 113105. [Google Scholar] [CrossRef] [PubMed]
- Lardeux, H.; D’Atri, V.; Guillarme, D. Recent Advances and Current Challenges in Hydrophilic Interaction Chromatography for the Analysis of Therapeutic Oligonucleotides. TrAC Trends Anal. Chem. 2024, 176, 117758. [Google Scholar] [CrossRef]
- Guo, Y. Separation of Nucleobases, Nucleosides, Nucleotides and Oligonucleotides by Hydrophilic Interaction Liquid Chromatography (HILIC): A State-of-the-Art Review. J. Chromatogr. A 2024, 1738, 465467. [Google Scholar] [CrossRef] [PubMed]
- Alpert, A.J. Hydrophilic-Interaction Chromatography for the Separation of Peptides, Nucleic Acids and Other Polar Compounds. J. Chromatogr. A 1990, 499, 177–196. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Baran, D. Hydrophilic Partitioning or Surface Adsorption? A Quantitative Assessment of Retention Mechanisms for Hydrophilic Interaction Chromatography (HILIC). Molecules 2023, 28, 6459. [Google Scholar] [CrossRef]
- Buszewski, B.; Noga, S. Hydrophilic Interaction Liquid Chromatography (HILIC)—A Powerful Separation Technique. Anal. Bioanal. Chem. 2012, 402, 231–247. [Google Scholar] [CrossRef]
- Guo, Y.; Baran, D.; Ryan, L. Quantitative Assessment of Retention Mechanisms for Ionized Compounds in Hydrophilic Interaction Chromatography (HILIC). Anal. Chem. 2025, 97, 4057–4065. [Google Scholar] [CrossRef]
- Guo, Y. Recent Progress in the Fundamental Understanding of Hydrophilic Interaction Chromatography (HILIC). Analyst 2015, 140, 6452–6466. [Google Scholar] [CrossRef]
- Jandera, P. Stationary and Mobile Phases in Hydrophilic Interaction Chromatography: A Review. Anal. Chim. Acta 2011, 692, 1–25. [Google Scholar] [CrossRef]
- Wang, L.; Wei, W.; Xia, Z.; Jie, X.; Xia, Z.Z. Recent Advances in Materials for Stationary Phases of Mixed-Mode High-Performance Liquid Chromatography. TrAC Trends Anal. Chem. 2016, 80, 495–506. [Google Scholar] [CrossRef]
- Guo, Y. A Survey of Polar Stationary Phases for Hydrophilic Interaction Chromatography and Recent Progress in Understanding Retention and Selectivity. Biomed. Chromatogr. 2022, 36, e5332. [Google Scholar] [CrossRef]
- Qiao, L.; Shi, X.; Xu, G. Recent Advances in Development and Characterization of Stationary Phases for Hydrophilic Interaction Chromatography. TrAC Trends Anal. Chem. 2016, 81, 23–33. [Google Scholar] [CrossRef]
- Fochetti, A.A.; Ciogli, A.; Mazzoccanti, G.A.; Guarducci, M.A.; Fochetti, A.; Ciogli, A.; Mazzoccanti, G. A Compendium of the Principal Stationary Phases Used in Hydrophilic Interaction Chromatography: Where Have We Arrived? Separations 2023, 10, 22. [Google Scholar] [CrossRef]
- Hao, Z.; Xiao, B.; Weng, N. Impact of Column Temperature and Mobile Phase Components on Selectivity of Hydrophilic Interaction Chromatography (HILIC). J. Sep. Sci. 2008, 31, 1449–1464. [Google Scholar] [CrossRef]
- Taraji, M.; Haddad, P.R.; Amos, R.I.J.; Talebi, M.; Szucs, R.; Dolan, J.W.; Pohl, C.A. Chemometric-Assisted Method Development in Hydrophilic Interaction Liquid Chromatography: A Review. Anal. Chim. Acta 2018, 1000, 20–40. [Google Scholar] [CrossRef]
- Locatelli, M.; De Lutiis, F.; Carlucci, G. High Performance Liquid Chromatography Determination of Prulifloxacin and Five Related Impurities in Pharmaceutical Formulations. J. Pharm. Biomed. Anal. 2013, 78–79, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Szterk, A.; Zmys1owski, A.; Gorinstein, S. Application of Hydrophilic Interaction Liquid Chromatography for the Quantification of Succinylcholine in Active Pharmaceutical Ingredient and Medicinal Product. Identification of New Impurities of Succinylcholine Chloride. Heliyon 2018, 4, e01097. [Google Scholar] [CrossRef]
- Jain, M.; Srivastava, V.; Kumar, R.; Dangi, V.; Hiriyanna, S.G.; Kumar, A.; Kumar, P. Determination of Five Potential Genotoxic Impurities in Dalfampridine Using Liquid Chromatography. J. Pharm. Biomed. Anal. 2017, 133, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Donnely, R. Chemical Stability of 4-Aminopyridine Capsules. Can. J. Hosp. Pharm. 2004, 57, 283–287. [Google Scholar]
- Thomas, S.; Shandilya, S.; Bharti, A.; Agarwal, A. A Stability Indicating Simultaneous Dual Wavelength UV-HPLC Method for the Determination of Potential Impurities in Fampridine Active Pharmaceutical Ingredient. J. Pharm. Biomed. Anal. 2012, 58, 136–140. [Google Scholar] [CrossRef]
- Themelis, T.; Gotti, R.; Gatti, R. A Novel Hydrophilic Interaction Liquid Chromatography Method for the Determination of Underivatized Amino Acids in Alimentary Supplements. J. Pharm. Biomed. Anal. 2017, 145, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, W.; Rasmussen, H.T. Orthogonal Method Development Using Hydrophilic Interaction Chromatography and Reversed-Phase High-Performance Liquid Chromatography for the Determination of Pharmaceuticals and Impurities. J. Chromatogr. A 2005, 1083, 58–62. [Google Scholar] [CrossRef]
- Terzić, J.; Popović, I.; Stajić, A.; Tumpa, A.; Jančić-Stojanovic, B. Application of Analytical Quality by Design Concept for Bilastine and Its Degradation Impurities Determination by Hydrophilic Interaction Liquid Chromatographic Method. J. Pharm. Biomed. Anal. 2016, 125, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Hatambeygi, N.; Abedi, G.; Talebi, M. Method Development and Validation for Optimised Separation of Salicylic, Acetyl Salicylic and Ascorbic Acid in Pharmaceutical Formulations by Hydrophilic Interaction Chromatography and Response Surface Methodology. J. Chromatogr. A 2011, 1218, 5995–6003. [Google Scholar] [CrossRef]
- Ali, M.S.; Rafluddin, S.; Al-Jawi, D.A.; Al-Hetari, Y.; Ghori, M.U.H.; Roshanall Khatri, A.; Khatri, A.R. Stability-Indicating Assay of Sodium Cromoglicate in Ophthalmic Solution Using Mixed-Mode Hydrophilic Interaction Chromatography. J. Sep. Sci. 2008, 31, 1645–1650. [Google Scholar] [CrossRef]
- Al-Sabti, B.; Harbali, J. Quantitative Determination of Potential Genotoxic Impurity 3-Aminopyridine in Linagliptin Active Pharmaceutical Ingredient Using HILIC-UV. Biomed. Chromatogr. 2020, 34, e4930. [Google Scholar] [CrossRef]
- Ali, M.S.; Rafiuddin, S.; Ghori, M.; Khatri, A.R. Simultaneous Determination of Metformin Hydrochloride, Cyanoguanidine and Melamine in Tablets by Mixed-Mode HILIC. Chromatographia 2008, 67, 517–525. [Google Scholar] [CrossRef]
- Douša, M. Quantification of 2-Aminoisobutyric Acid Impurity in Enzalutamide Bulk Drug Substance Using Hydrophilic Interaction Chromatography with Fluorescence Detection. J. Pharm. Biomed. Anal. 2019, 164, 296–301. [Google Scholar] [CrossRef]
- Filipic, S.; Elek, M.; Popović, M.; Nikolic, K.; Agbaba, D. Development of Hydrophilic Interaction Liquid Chromatography Method for the Analysis of Moxonidine and Its Impurities. J. Anal. Methods Chem. 2016, 2016, 3715972. [Google Scholar] [CrossRef]
- Rondon, B.D.; Cassiano, N.M.; Cass, Q.B. An Isocratic Hydrophilic Interaction Liquid Chromatographic Method for Simultaneous Determination of Iodixanol and Its Related Impurities in Drug Substance. J. Pharm. Biomed. Anal. 2017, 140, 342–346. [Google Scholar] [CrossRef]
- Sivaprasadu, G.; Pamerla, M.; Rao, P.A.; Rao, A.V.; Salakolusu, S.; Padhy, H.; Ganta, R.K. Stability-Indicating HPLC Method Development and Validation for the Quantification of Tofacitinib Citrate and Its Related Substances Using Hydrophilic Liquid Interaction Chromatography. Sep. Sci. Plus 2024, 7, e202400048. [Google Scholar] [CrossRef]
- Ghanem, M.; Abu-Lafi, S.; Mohammad, D. Development and validation of a stability-indicating hydrophilic interaction liquid chromatographic method for the determination of sulfaquinoxaline sodium in water soluble powder formulation. Int. J. Pharm. Pharm. Sci. 2014, 6 (Suppl. 2), 652–657. [Google Scholar]
- Jovanović, M.; Rakić, T.; Tumpa, A.; Jančić Stojanović, B. Quality by Design Approach in the Development of Hydrophilic Interaction Liquid Chromatographic Method for the Analysis of Iohexol and Its Impurities. J. Pharm. Biomed. Anal. 2015, 110, 42–48. [Google Scholar] [CrossRef]
- Klykov, O.; Weller, M.G. Quantification of N-Hydroxysuccinimide and N-Hydroxysulfosuccinimide by Hydrophilic Interaction Chromatography (HILIC). Anal. Methods 2015, 7, 6443–6448. [Google Scholar] [CrossRef]
- Ntontis, S.; Tsanaktsidou, E.; Tzanavaras, P.D.; Kachrimanis, K.; Markopoulou, C.K.; Zacharis, C.K. Analytical Quality by Design Approach for the Determination of Imidazole in Sildenafil API and Its Formulations Using Zwitterionic HILIC Stationary Phase. J. Pharm. Biomed. Anal. 2023, 224, 115186. [Google Scholar] [CrossRef]
- Wahl, O.; Cleynhens, J.; Verbruggen, A.M.; Holzgrabe, U. Impurity Profiling of N,N′-Ethylenebis-L-Cysteine Diethyl Ester (Bicisate). J. Pharm. Biomed. Anal. 2018, 150, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Urlaub, J.; Woźniczka, M.; Wynendaele, E.; Herman, K.; Schollmayer, C.; Diehl, B.; Van Calenbergh, S.; Holzgrabe, U.; De Spiegeleer, B. Zwitterionic-Hydrophilic Interaction Liquid Chromatography for L-Ascorbic Acid 2-Phosphate Magnesium, a Raw Material in Cell Therapy. J. Pharm. Biomed. Anal. 2019, 165, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Luan, B.; Liang, G.; Xing, L.; Huang, L.; Wang, C.; Xu, Y. Isolation and Identification of Four Major Impurities in Capreomycin Sulfate. J. Chromatogr. A 2018, 1571, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Denton, J.R.; Berwick, L.; Loughlin, T.P. Development of a Low Level Detection Method for 2-Chloromalonaldehyde in Active Pharmaceutical Ingredients by HILIC Separation. Anal. Methods 2016, 8, 4659–4663. [Google Scholar] [CrossRef]
- Kasagić-Vujanović, I.; Knežević, D. Design of Experiments in Optimization and Validation of Hydrophilic Interaction Liquid Chromatography Method for Determination of Amlodipine Besylate and Its Impurities. Acta Chromatogr. 2022, 34, 41–52. [Google Scholar] [CrossRef]
- Knežević Ratković, D.; Kukobat, R.; Kasagić-Vujanović, I. Retention Mechanisms of Amitriptyline and Its Impurities on Amide, Amino, Diol, and Silica Columns in Hydrophilic Interaction Liquid Chromatography. J. Sep. Sci. 2024, 47, 202300949. [Google Scholar] [CrossRef] [PubMed]
- Kasagić-Vujanović, I.; Jančić-Stojanović, B.; Ivanović, D. Investigation of the Retention Mechanisms of Amlodipine Besylate, Bisoprolol Fumarate, and Their Impurities on Three Different HILIC Columns. J. Liq. Chromatogr. Relat. Technol. 2018, 41, 523–531. [Google Scholar] [CrossRef]
- Kasagić-Vujanović, I.; Jančić-Stojanović, B. Quality by Design Oriented Development of Hydrophilic Interaction Liquid Chromatography Method for the Analysis of Amitriptyline and Its Impurities. J. Pharm. Biomed. Anal. 2019, 173, 86–95. [Google Scholar] [CrossRef]
- Ozaki, M.; Shimotsuma, M.; Hirose, T. Separation of Nicotinamide Metabolites Using a PBr Column Packed with Pentabromobenzyl Group Modified Silica Gel. Anal. Biochem. 2022, 655, 114837. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Kato, S.; Nagai, K.; Shimamoto, S.; Onishi, T.; Ohnishi, A. Impurity Profiling of Synthetic Cyclic Peptides Based on Orthogonality between Hydrophilic-Interaction and Reversed-Phase Liquid Chromatography. J. Chromatogr. A 2025, 1745, 465748. [Google Scholar] [CrossRef]
- Maksić, J.; Jovanović, M.; Rakić, T.; Popović, I.; Ivanović, D.; Jancic-Stojanovic, B. Chromatographic Analysis of Olopatadine in Hydrophilic Interaction Liquid Chromatography. J. Chromatogr. Sci. 2015, 53, 680–686. [Google Scholar] [CrossRef]
- Leistner, A.; Holzgrabe, U. Alternative Methods to Assess the Impurity Profile of a Monographed API Using Acarbose as an Example. J. Pharm. Biomed. Anal. 2022, 221, 115063. [Google Scholar] [CrossRef]
- Gallinella, B.; Bucciarelli, L.; Zanitti, L.; Ferretti, R.; Cirilli, R. Direct Separation of the Enantiomers of Oxaliplatin on a Cellulose-Based Chiral Stationary Phase in Hydrophilic Interaction Liquid Chromatography Mode. J. Chromatogr. A 2014, 1339, 210–213. [Google Scholar] [CrossRef]
- Rabel, F.; Olsen, B.A. Advances in Hydrophilic Interaction Chromatography (HILIC) for Biochemical Applications. In Hydrophilic Interaction Chromatography: A Guide for Practitioners; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 195–217. [Google Scholar] [CrossRef]
- Mallampati, S.; Huang, S.; Ashenafi, D.; Van Hemelrijck, E.; Hoogmartens, J.; Adams, E. Development and Validation of a Liquid Chromatographic Method for the Analysis of Capreomycin Sulfate and Its Related Substances. J. Chromatogr. A 2009, 1216, 2449–2455. [Google Scholar] [CrossRef]
- Lardeux, H.; Guillarme, D.; D’Atri, V. Comprehensive Evaluation of Zwitterionic Hydrophilic Liquid Chromatography Stationary Phases for Oligonucleotide Characterization. J. Chromatogr. A 2023, 1690, 463785. [Google Scholar] [CrossRef] [PubMed]
- Almeling, S.; Ilko, D.; Holzgrabe, U. Charged Aerosol Detection in Pharmaceutical Analysis. J. Pharm. Biomed. Anal. 2012, 69, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Guo, Z.; Liu, X.; Zhang, Q.; Liu, X.; Jin, Y.; Liang, L.; Li, H.; Wei, J.; Wu, N. A Sensitive Non-Derivatization Method for Apramycin and Impurities Analysis Using Hydrophilic Interaction Liquid Chromatography and Charged Aerosol Detection. Talanta 2016, 146, 423–429. [Google Scholar] [CrossRef]
- Xu, Q.; Tan, S.; Petrova, K. Development and Validation of a Hydrophilic Interaction Chromatography Method Coupled with a Charged Aerosol Detector for Quantitative Analysis of Nonchromophoric α–Hydroxyamines, Organic Impurities of Metoprolol. J. Pharm. Biomed. Anal. 2016, 118, 242–250. [Google Scholar] [CrossRef]
- Stevens, H.; Sanz Rodriguez, E.; Lai, M.; Vance, T.R.; Curran, M.; Ritchie, H.; Bowie, A.R.; Paull, B. Highly Sensitive Tandem Mass Spectrometry Detection for High Resolution HILIC Separation of Biomass Burning Markers. J. Chromatogr. A 2025, 1748, 465878. [Google Scholar] [CrossRef]
- Xu, Q.; Tan, S. Quantitative Analysis of 3-Isopropylamino-1,2-Propanediol as a Degradation Product of Metoprolol in Pharmaceutical Dosage Forms by HILIC-CAD. J. Pharm. Anal. 2019, 9, 431–436. [Google Scholar] [CrossRef]
- Pawellek, R.; Holzgrabe, U. Performance of Ion Pairing Chromatography and Hydrophilic Interaction Liquid Chromatography Coupled to Charged Aerosol Detection for the Analysis of Underivatized Amino Acids. J. Chromatogr. A 2021, 1659, 462613. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, Q.; Wei, X.; Zhu, B.; Wang, J.; Wang, F. Quantitative Analysis of the Impurities in Etimicin Using Hydrophilic Interaction Liquid Chromatography Coupled with Charged Aerosol Detector. J. Pharm. Biomed. Anal. 2024, 249, 116384. [Google Scholar] [CrossRef]
- Kohler, I.; Verhoeven, M.; Haselberg, R.; Gargano, A.F.G. Hydrophilic Interaction Chromatography—Mass Spectrometry for Metabolomics and Proteomics: State-of-the-Art and Current Trends. Microchem. J. 2022, 175, 106986. [Google Scholar] [CrossRef]
- Grinberg, N.; Albu, F.; Fandrick, K.; Iorgulescu, E.; Medvedovici, A. Assay at Low Ppm Level of Dimethyl Sulfate in Starting Materials for API Synthesis Using Derivatization in Ionic Liquid Media and LC–MS. J. Pharm. Biomed. Anal. 2013, 75, 1–6. [Google Scholar] [CrossRef] [PubMed]
- GONG, L.; HANG, B.; XIAN, R.; YANG, M.; ZHANG, X.; WEI, X. Determination of Dimethyl Sulfate Genotoxic Impurities in Tertiary Amine Drugs by Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry. Chin. J. Chromatogr. 2022, 40, 854–859. [Google Scholar] [CrossRef]
- Çeğil, T.; Yardımcı, A.; Sevinç, N.; Pamukçu, Z. Determination of Dimethyl Sulfate Genotoxic Impurity in Pantoprazole Sodium Sesquihydrate by Derivatization Method and UPLC/MS. Int. J. Mass. Spectrom. 2024, 506, 117330. [Google Scholar] [CrossRef]
- An, J.; Sun, M.; Bai, L.; Chen, T.; Liu, D.Q.; Kord, A. A Practical Derivatization LC/MS Approach for Determination of Trace Level Alkyl Sulfonates and Dialkyl Sulfates Genotoxic Impurities in Drug Substances. J. Pharm. Biomed. Anal. 2008, 48, 1006–1010. [Google Scholar] [CrossRef]
- Guo, Z.; Li, T.; Sun, Y.; Li, P.; Wang, Q. Determination of Two Genotoxic Impurities in Sulconazole Nitrate by Combining Derivatization with LC-MS Method. Chem. Ind. Eng. 2025, 42, 106–112. [Google Scholar] [CrossRef]
- Douša, M.; Klvaňa, R.; Doubský, J.; Srbek, J.; Richter, J.; Exner, M.; Gibala, P. HILIC–MS Determination of Genotoxic Impurity of 2-Chloro-N-(2-Chloroethyl)Ethanamine in the Vortioxetine Manufacturing Process. J. Chromatogr. Sci. 2016, 54, 119–124. [Google Scholar] [CrossRef]
- Shackman, J.G. Rapid, Direct, and Sensitive Determination of Aziridine and 2-Chloroethylamine by Hydrophilic Interaction Liquid Chromatography-Mass Spectrometry. MethodsX 2019, 6, 2176–2180. [Google Scholar] [CrossRef] [PubMed]
- Douša, M.; Jireš, J. HILIC-MS Determination of Dimethylamine in the Active Pharmaceutical Ingredients and in the Dosage Forms of Metformin. J. Pharm. Biomed. Anal. 2020, 191, 113573. [Google Scholar] [CrossRef] [PubMed]
- Chawla, R.K.; Rao, G.S.N.K.; Kulandaivelu, U.; Panda, S.P.; Alavala, R.R. A Selective and Sensitive Method Development and Validation of 1,1-Dimethyl-3-Hydroxy-Pyrrolidinium Bromide Impurity in Glycopyrrolate Oral Solution by Liquid Chromatography-Tandem Mass Spectroscopy. J. Chromatogr. Sci. 2021, 59, 566–575. [Google Scholar] [CrossRef]
- Derbouz, S.; Guermouche, M.H.; Guermouche, S. Stability-Indicating HILIC Method for the Determination of Citicoline and Characterization of Its Degradation Products by LC–MS/TOF, 1H and 13C NMR. Chromatographia 2017, 80, 265–274. [Google Scholar] [CrossRef]
- Mullangi, S.; Ravindhranath, K.; Panchakarla, R.K. An Efficient HILIC-MS/MS Method for the Trace Level Determination of Three Potential Genotoxic Impurities in Aripiprazole Active Drug Substance. J. Anal. Sci. Technol. 2021, 12, 21. [Google Scholar] [CrossRef]
- Kawano, S.I. Analysis of Impurities in Streptomycin and Dihydrostreptomycin by Hydrophilic Interaction Chromatography/Electrospray Ionization Quadrupole Ion Trap/Time-of-Flight Mass Spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 907–914. [Google Scholar] [CrossRef]
- Xu, Q.; Tadjimukhamedov, F.K. Development and Evaluation of a HILIC-MS Method for the Determination of Amino Acid and Non-Amino Acid Impurities in Histidine. J. Pharm. Biomed. Anal. 2022, 219, 114936. [Google Scholar] [CrossRef]
- Schiesel, S.; Lämmerhofer, M.; Leitner, A.; Lindner, W. Quantitative High-Performance Liquid Chromatography-Tandem Mass Spectrometry Impurity Profiling Methods for the Analysis of Parenteral Infusion Solutions for Amino Acid Supplementation Containing l-Alanyl-l-Glutamine. J. Chromatogr. A 2012, 1259, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Hmelnickis, J.; Pugovičs, O.; Kažoka, H.; Viksna, A.; Susinskis, I.; Kokums, K. Application of Hydrophilic Interaction Chromatography for Simultaneous Separation of Six Impurities of Mildronate Substance. J. Pharm. Biomed. Anal. 2008, 48, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Francis, R.; Zha, Y.; Ruan, J. Development of a Simple Method for Quantitation of Methanesulfonic Acid at Low Ppm Level Using Hydrophilic Interaction Chromatography Coupled with ESI-MS. J. Pharm. Biomed. Anal. 2014, 102, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Tengattini, S.; Domínguez-Vega, E.; Temporini, C.; Bavaro, T.; Rinaldi, F.; Piubelli, L.; Pollegioni, L.; Massolini, G.; Somsen, G.W. Hydrophilic Interaction Liquid Chromatography-Mass Spectrometry as a New Tool for the Characterization of Intact Semi-Synthetic Glycoproteins. Anal. Chim. Acta 2017, 981, 94–105. [Google Scholar] [CrossRef]
- Vosáhlová, Z.; Kalíková, K.; Gilar, M.; Szymarek, J.; Mazurkiewicz-Bełdzińska, M.; Studzińska, S. Hydrophilic Interaction Liquid Chromatography with Mass Spectrometry for the Separation and Identification of Antisense Oligonucleotides Impurities and Nusinersen Metabolites. J. Chromatogr. A 2024, 1713, 464535. [Google Scholar] [CrossRef]
- Huang, M.; Xu, X.; Qiu, H.; Li, N. Analytical Characterization of DNA and RNA Oligonucleotides by Hydrophilic Interaction Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2021, 1648, 462184. [Google Scholar] [CrossRef]
- Krishna Murthy Kasa, S.R.; Venkatanarayana, M.; Chennuru, L.N.; Chandra Sekhara Rao, B.; Vemparala, M.; Chaman, A.F.; Talluri, M.V.N.K. Chiral LC Method Development: Stereo-Selective Separation, Characterization, and Determination of Cabotegravir and Related RS, RR, and SS Isomeric Impurities on Coated Cellulose-Based Chiral Stationary Phase by HILIC-LC and LC-MS. J. Pharm. Biomed. Anal. 2023, 222, 115062. [Google Scholar] [CrossRef]
- Wang, M.; O’Day, B.; Michaels, B.; Jurayj, J.; Cai, B.Z.; Wei, T. Sequencing of Phosphorodiamidate Morpholino Oligomers by Hydrophilic Interaction Chromatography Coupled to Tandem Mass Spectrometry. J. Am. Soc. Mass. Spectrom. 2024, 35, 2490–2498. [Google Scholar] [CrossRef]
- Wang, S.; Liu, A.P.; Yan, Y.; Daly, T.J.; Li, N. Characterization of Product-Related Low Molecular Weight Impurities in Therapeutic Monoclonal Antibodies Using Hydrophilic Interaction Chromatography Coupled with Mass Spectrometry. J. Pharm. Biomed. Anal. 2018, 154, 468–475. [Google Scholar] [CrossRef]
- Mitch, W.A.; Sedlak, D.L. Formation of N-Nitrosodimethylamine (NDMA) from Dimethylamine during Chlorination. Environ. Sci. Technol. 2002, 36, 588–595. [Google Scholar] [CrossRef]
- Maekawa, K.; Sawa, R.; Matsui, M.; Konda, T.; Maeda, Y.K.A.; Takahashi, C.; Matsuo, A.; Tanimoto, T.; Nakagawa, Y.; Yoneda, S.; et al. Quality Evaluation of Gentamicin Sulfate Reference Standards in Japanese Pharmacopoeia Using Hydrophilic Interaction Chromatography Combined With Tandem Mass Spectrometry. J. AOAC Int. 2024, 107, 234–241. [Google Scholar] [CrossRef]
- Kuijper, E.C.; Bergsma, A.J.; Pijnappel, W.W.M.P.; Aartsma-Rus, A. Opportunities and Challenges for Antisense Oligonucleotide Therapies. J. Inherit. Metab. Dis. 2021, 44, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Goyon, A.; Fan, Y.; Zhang, K. Analysis of Oligonucleotides by Liquid Chromatography. In Liquid Chromatography: Applications; Elsevier: Amsterdam, The Netherlands, 2023; pp. 357–380. ISBN 9780323999694. [Google Scholar]
- van den Hurk, R.S.; Pursch, M.; Stoll, D.R.; Pirok, B.W.J. Recent Trends in Two-Dimensional Liquid Chromatography. TrAC Trends Anal. Chem. 2023, 166, 117166. [Google Scholar] [CrossRef]
- Stoll, D.R.; Carr, P.W. Two-Dimensional Liquid Chromatography: A State of the Art Tutorial. Anal. Chem. 2017, 89, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Goyon, A.; Zhang, K. Characterization of Antisense Oligonucleotide Impurities by Ion-Pairing Reversed-Phase and Anion Exchange Chromatography Coupled to Hydrophilic Interaction Liquid Chromatography/Mass Spectrometry Using a Versatile Two-Dimensional Liquid Chromatography Setup. Anal. Chem. 2020, 92, 5944–5951. [Google Scholar] [CrossRef]
- Bäurer, S.; Ferri, M.; Carotti, A.; Neubauer, S.; Sardella, R.; Lämmerhofer, M. Mixed-Mode Chromatography Characteristics of Chiralpak ZWIX(+) and ZWIX(−) and Elucidation of Their Chromatographic Orthogonality for LC × LC Application. Anal. Chim. Acta 2020, 1093, 168–179. [Google Scholar] [CrossRef]
- Tang, S.; Venkatramani, C.J. Resolving Solvent Incompatibility in Two-Dimensional Liquid Chromatography with In-Line Mixing Modulation. Anal. Chem. 2022, 94, 16142–16150. [Google Scholar] [CrossRef]
- Gu, X.; Yang, L.; Tao, Q.; Ai, J.; Yan, C.; Zheng, J.; Hong, L. Application of Heart-Cutting Two-Dimensional Liquid Chromatography-Mass Spectrometry to the Characterization of Highly Polar Impurities in Calcium Gluconate Injection. J. Chromatogr. A 2022, 1685, 463632. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntorkou, M.; Zacharis, C.K. Applications of Hydrophilic Interaction Chromatography in Pharmaceutical Impurity Profiling: A Comprehensive Review of Two Decades. Molecules 2025, 30, 3567. https://doi.org/10.3390/molecules30173567
Ntorkou M, Zacharis CK. Applications of Hydrophilic Interaction Chromatography in Pharmaceutical Impurity Profiling: A Comprehensive Review of Two Decades. Molecules. 2025; 30(17):3567. https://doi.org/10.3390/molecules30173567
Chicago/Turabian StyleNtorkou, Marianna, and Constantinos K. Zacharis. 2025. "Applications of Hydrophilic Interaction Chromatography in Pharmaceutical Impurity Profiling: A Comprehensive Review of Two Decades" Molecules 30, no. 17: 3567. https://doi.org/10.3390/molecules30173567
APA StyleNtorkou, M., & Zacharis, C. K. (2025). Applications of Hydrophilic Interaction Chromatography in Pharmaceutical Impurity Profiling: A Comprehensive Review of Two Decades. Molecules, 30(17), 3567. https://doi.org/10.3390/molecules30173567








