The Roles of Micronutrition and Nutraceuticals in Enhancing Wound Healing and Tissue Regeneration: A Systematic Review
Abstract
1. Introduction
1.1. Objectives
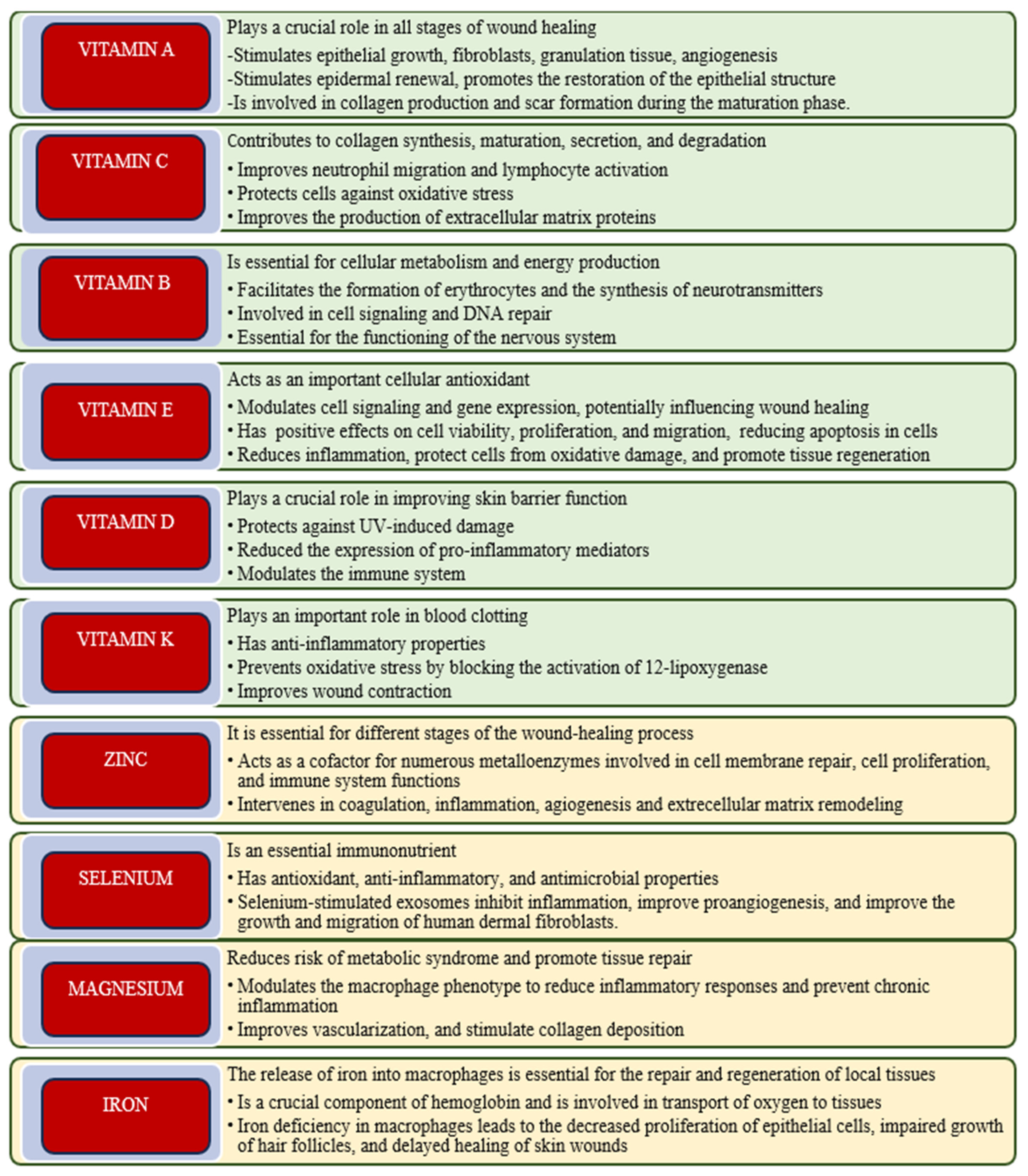
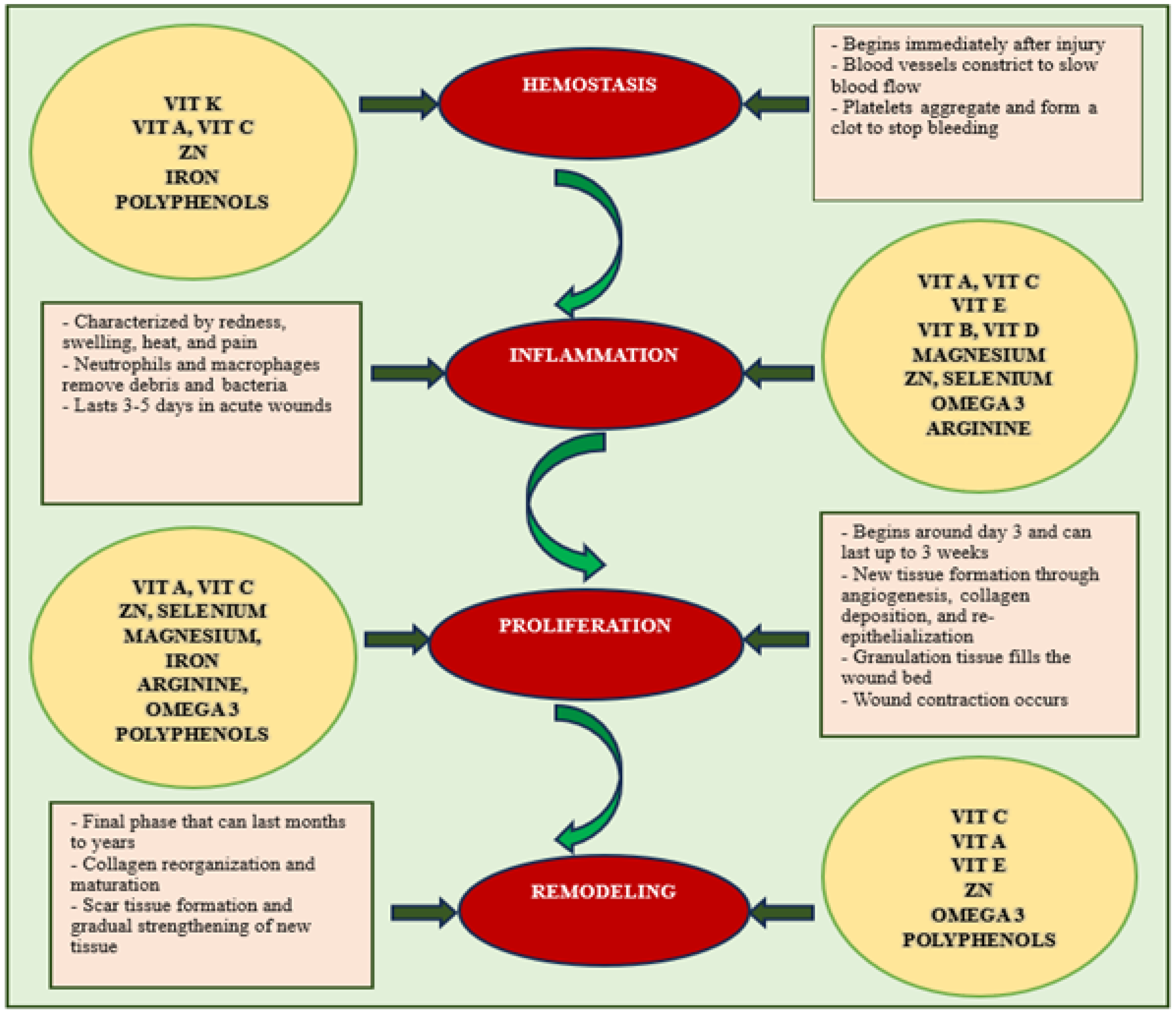
1.2. Vitamins and Their Role in Wound Healing
1.2.1. Vitamin A
1.2.2. Vitamin C
1.2.3. B-Complex Vitamins
1.2.4. Vitamin D
1.2.5. Vitamin E
1.2.6. Vitamin K
1.3. Essential Minerals Involved in Wound Healing
1.3.1. Zinc
1.3.2. Selenium
1.3.3. Magnesium
1.3.4. Iron
1.4. Probiotics Influence Skin Health and Wound Healing Processes
1.5. Effects of Omega-3 Fatty Acids on Wound Healing
1.5.1. Omega-3 Fatty Acids
1.5.2. Arginine and Glutamine
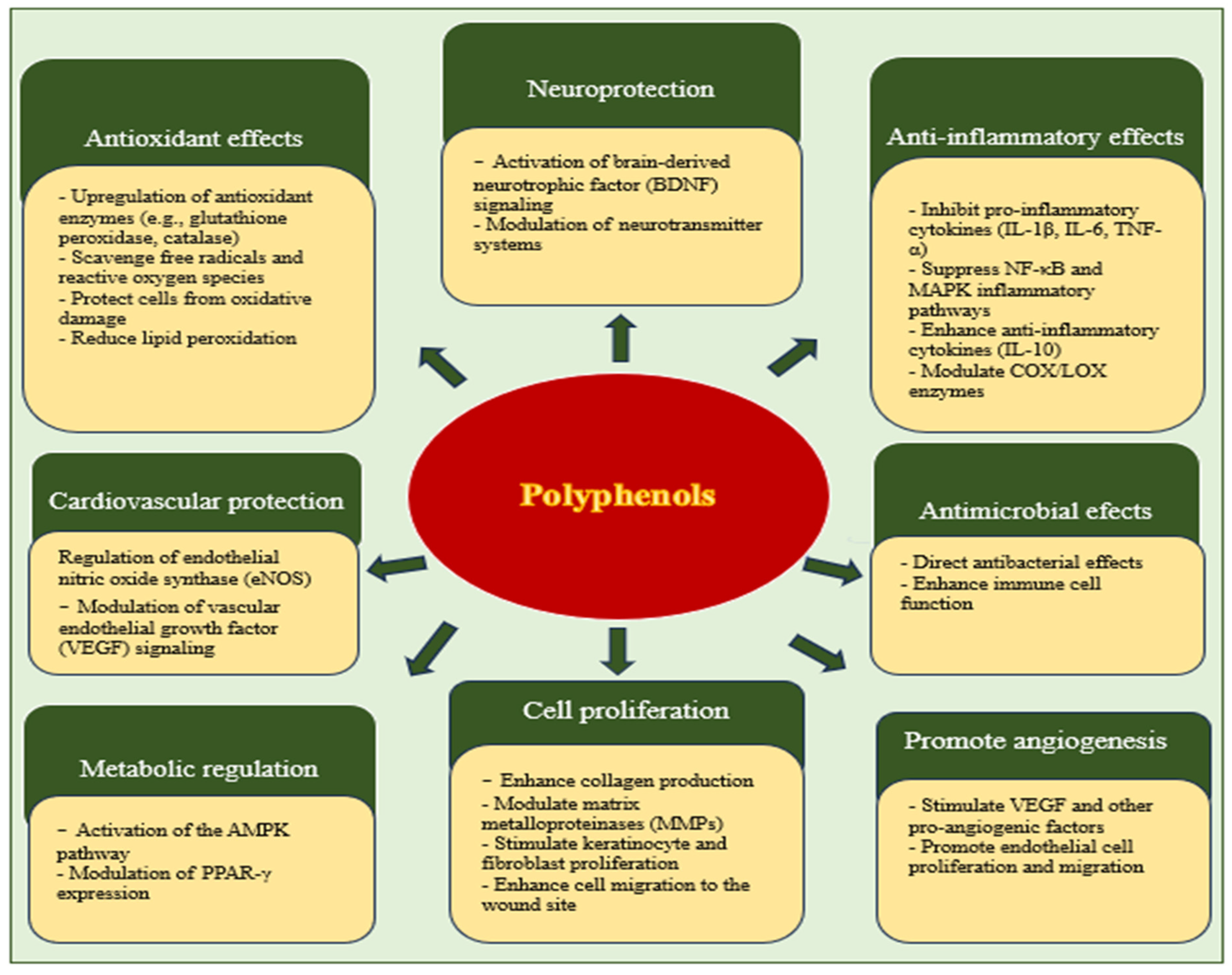
1.6. The Effectiveness of Polyphenols in Wound Healing
1.6.1. Resveratrol
1.6.2. Curcumin
1.7. Flavonoids
1.7.1. Proanthocyanidins
1.7.2. Quercetin
1.7.3. Fisetin
1.8. Saponins
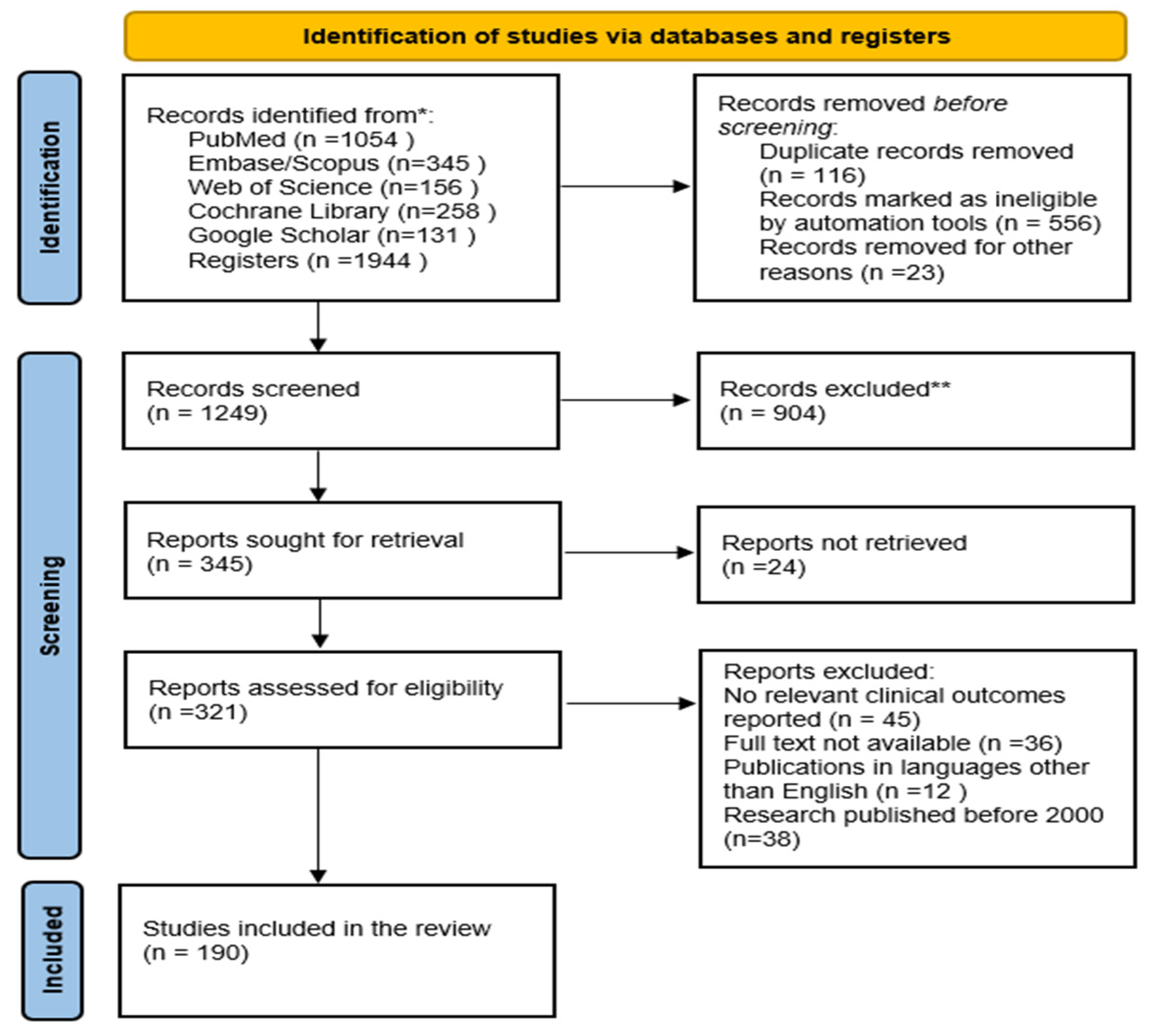
2. Methods and Materials
2.1. Eligibility Criteria
- Inclusion and Exclusion Criteria
- Inclusion Criteria:
- Peer-reviewed original research, clinical trials, and reviews examining the impacts of nutraceuticals on wound healing and tissue regeneration.
- Previous studies that focused on the effects of vitamins, minerals, and amino acids on wound healing and tissue regeneration.
- Articles written in English and available in full-text format.
- Human and animal model studies providing mechanistic or clinical insights.
- Exclusion Criteria:
- Publications in languages other than English.
- Editorials, opinion pieces, or conference abstracts without full data.
- Studies lacking specific outcome measures related to wound healing or tissue regeneration.
- Research published before 2000.
2.2. Information Sources and Search Strategies
2.3. Selection Process
2.4. Data Collection Process and Data Items
2.5. Study Risk of Bias Assessment
2.6. Effect Measures and Statistical Analysis
2.7. Study Selection
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghaly, P.; Iliopoulos, J.; Ahmad, M. The role of nutrition in wound healing: An overview. Br. J. Nurs. 2021, 30, 38–42. [Google Scholar] [CrossRef]
- Moore, Z.E.; Corcoran, M.A.; Patton, D. Nutritional interventions for treating foot ulcers in people with diabetes. Cochrane Database Syst. Rev. 2020, 7, CD011378. [Google Scholar] [CrossRef]
- Clark, R.K.; Stampas, A.; Kerr, K.W.; Nelson, J.L.; Sulo, S.; Leon-Novelo, L.; Nigan, E.; Pandya, D. Evaluating the impact of using a wound-specific oral nutritional supplement to support wound healing in a rehabilitation setting. Int. Wound J. 2023, 20, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Vidhya, C.S.; Komal, O.; Yashwanth, B.S.; Singh, B.; Gupta, S.; Pandey, S.K. The Role of Functional Foods and Nutraceuticals in Disease Prevention and Health Promotion. Eur. J. Nutr. Food Saf. 2024, 16, 61–83. [Google Scholar] [CrossRef]
- Yang, F.; Bai, X.; Dai, X.; Li, Y. The Biological Processes During Wound Healing. Regen. Med. 2021, 16, 373–390. [Google Scholar] [CrossRef]
- Stanescu, C.; Boev, M.; Avram, O.A.; Ciubara, A. Investigating the Connection Between Skin Cancers and Mental Disorders: A Thorough Analysis. BRAIN. Broad Res. Artif. Intell. Neurosci. 2024, 15, 118–131. [Google Scholar] [CrossRef]
- Cioce, A.; Cavani, A.; Cattani, C.; Scopelliti, F. Role of the Skin Immune System in Wound Healing. Cells 2014, 13, 624. [Google Scholar] [CrossRef]
- Polcz, M.E.; Barbul, A. The Role of Vitamin A in Wound Healing. Nutr. Clin. Pract. 2019, 34, 695–700. [Google Scholar] [CrossRef]
- Brossaud, J.; Pallet, V.; Corcuff, J.-B. Vitamin A, endocrine tissues and hormones: Interplay and interactions. Endocr. Connect. 2017, 6, R121–R130. [Google Scholar] [CrossRef]
- Zinder, R.; Cooley, R.; Lucian, G.; Vlad, M.D. Vitamin A and Wound Healing. Nutr. Clin. Pract. 2019, 34, 839–849. [Google Scholar] [CrossRef]
- Wicke, C.; Halliday, B.; Allen, D.; Roche, N.S.; Scheuenstuhl, H.; Spencer, M.M.; Roberts, A.; Kunt, T.K. Effects of steroids and retinoids on wound healing. Arch. Surg. 2000, 135, 1265–1270. [Google Scholar] [CrossRef]
- Wu, Z.-H.; Wang, Y.-X.; Song, J.-J.; Zhao, L.-Q.; Zhai, Y.-J.; Liu, Y.-F.; Guo, W.-J. LncRNA SNHG26 promotes gastric cancer progression and metastasis by inducing c-Myc protein translation and an energy metabolism positive feedback loop. Cell Death Dis. 2024, 15, 236. [Google Scholar] [CrossRef] [PubMed]
- Stephensen, C.B. Vitamin A, infection, and immune function. Annu. Rev. Nutr. 2001, 21, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.R.D. The neurotoxic effects of vitamin A and retinoids. An. Da Acad. Bras. De Ciências 2015, 87, 1361–1373. [Google Scholar] [CrossRef] [PubMed]
- Chaitrakoonthong, T.; Kamolratanakul, P.; Ampornaramveth, R. Rinsing with L-Ascorbic Acid Exhibits Concentration-Dependent Effects on Human Gingival Fibroblast In Vitro Wound Healing Behavior. Int. J. Dent. 2020, 1, 1–7. [Google Scholar] [CrossRef]
- Iqbal, K.; Khan, A.; Muzaffar Ali Khan Khattak, M. Biological Significance of Ascorbic Acid (Vitamin C) in Human Health—A Review. Pak. J. Nutr. 2004, 3, 5–13. [Google Scholar] [CrossRef]
- Bechara, N.; Flood, V.M.; Gunton, J.E. A Systematic Review on the Role of Vitamin C in Tissue Healing. Antioxidants 2022, 11, 1605. [Google Scholar] [CrossRef]
- Waibel, J.S.; Mi, Q.; Ozog, D.; Qu, L.; Zhou, L.; Rudnick, A.; Al-Niaimi, F.; Woodward, J.; Campo, V.; Mordon, S. Laser-assisted delivery of vitamin C, vitamin E, and ferulic acid formula serum decreases fractional laser postoperative recovery by increased beta fibroblast growth factor expression. Lasers Surg. Med. 2015, 48, 238–244. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Mohammed, B.M.; Fisher, B.J.; Kraskauskas, D.; Ward, S.; Wayne, J.S.; Brophy, D.F.; Fower, A.A.; Yager, D.R.; Natarajan, R. Vitamin C promotes wound healing through novel pleiotropic mechanisms. Int. Wound J. 2015, 13, 572–584. [Google Scholar] [CrossRef]
- Ellinger, S.; Stehle, P. Efficacy of vitamin supplementation in situations with wound healing disorders: Results from clinical intervention studies. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Khan, S.; Pathak, S.; Mazhar, M.; Singh, H. Vitamin B-Complex and its Relationship with the Health of Vegetarian People. Nat. Resour. Hum. Health 2023, 3, 342–354. [Google Scholar] [CrossRef]
- Rembe, J.-D.; Stuermer, E.K.; Fromm-Dornieden, C. Effects of Vitamin B Complex and Vitamin C on Human Skin Cells: Is the Perceived Effect Measurable? Adv. Ski. Wound Care 2018, 31, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Neiva, R.F.; Al-Shammari, K.; Wang, H.; Soehren, S.; Nociti, F.H. Effects of Vitamin-B Complex Supplementation on Periodontal Wound Healing. J. Periodontol. 2005, 76, 1084–1091. [Google Scholar] [CrossRef]
- Daher, G.S.; Choi, K.Y.; Wells, J.W.; Goyal, N. A Systematic Review of Oral Nutritional Supplement and Wound Healing. Ann. Otol. Rhinol. Laryngol. 2022, 131, 1358–1368. [Google Scholar] [CrossRef]
- Kavalukas, S.L.; Barbul, A. Nutrition and Wound Healing: An Update. Plast. Reconstr. Surg. 2011, 127, 38S–43S. [Google Scholar] [CrossRef]
- Saeg, F.; Orazi, R.; Janis, J.E.; Gerald, B.; Jeffrey, J. Evidence-Based Nutritional Interventions in Wound Care. Plast. Reconstr. Surg. 2021, 148, 226–238. [Google Scholar] [CrossRef]
- Coelho, S.C.; Estevinho, B.N.; Giron, A.; Rocha, F. Electrosprayed B-complex vitamins/zein microparticles for drug sustained release and antioxidant applications. J. Chem. Technol. Biotechnol. 2023, 99, 217–226. [Google Scholar] [CrossRef]
- Schwalfenberg, G.K. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol. Nutr. Food Res. 2010, 55, 96–108. [Google Scholar] [CrossRef]
- Bikle, D.D. Role of vitamin D and calcium signaling in epidermal wound healing. J. Endocrinol. Investig. 2023, 46, 205–212. [Google Scholar] [CrossRef]
- Ambagaspitiya, S.S.; Dassanayake, R.S.; Appuhamillage, G.A. Impact of vitamin D on ultraviolet-induced photoaging and skin diseases. Explor. Med. 2024, 5, 363–383. [Google Scholar] [CrossRef]
- Palermo, N.E.; Holick, M.F. Vitamin D, bone health, and other health benefits in pediatric patients. J. Pediatr. Rehabil. Med. 2014, 7, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, K.; Dilisio, M.; Agrawal, D. Vitamin D and the immunomodulation of rotator cuff injury. J. Inflamm. Res. 2016, 9, 123. [Google Scholar] [CrossRef]
- Winzenberg, T.; Jones, G. Vitamin D and Bone Health in Childhood and Adolescence. Calcif. Tissue Int. 2012, 92, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Muresan, G.C.; Hedesiu, M.; Lucaciu, O.; Boca, S.; Petrescu, N. Effect of Vitamin D on Bone Regeneration: A Review. Medicina 2022, 58, 1337. [Google Scholar] [CrossRef] [PubMed]
- Basit, S. Vitamin D in health and disease: A literature review. Br. J. Biomed. Sci. 2013, 70, 161–172. [Google Scholar] [CrossRef]
- Tanaka, K.; Ao, M.; Tamaru, J.; Kuwabara, A. Vitamin D insufficiency and disease risk in the elderly. J. Clin. Biochem. Nutr. 2024, 74, 9–16. [Google Scholar] [CrossRef]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Y.-G. Vitamin D Receptor Influences Intestinal Barriers in Health and Disease. Cells 2022, 11, 1129. [Google Scholar] [CrossRef]
- Ricca, C.; Aillon, A.; Bergandi, L.; Alotto, D.; Castagnoli, C.; Silvagno, F. Vitamin D Receptor Is Necessary for Mitochondrial Function and Cell Health. Int. J. Mol. Sci. 2018, 19, 1672. [Google Scholar] [CrossRef]
- Fawzy El-Sayed, K.M.; Cosgarea, R.; Sculean, A.; Doerfer, C. Can vitamins improve periodontal wound healing/regeneration? Periodontology 2000 2024, 94, 539–602. [Google Scholar] [CrossRef]
- Hobson, R. Vitamin E and wound healing: An evidence-based review. Int. Wound J. 2014, 13, 331–335. [Google Scholar] [CrossRef]
- Dibaise, M.; Tarleton, S.M. Hair, Nails, and Skin: Differentiating Cutaneous Manifestations of Micronutrient Deficiency. Nutr. Clin. Pract. 2019, 34, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Caccavale, C.; Della Sala, F.; Zeppetelli, S.; Veneziano, R.; Borzacchiello, A. Natural Formulations Provide Antioxidant Complement to Hyaluronic Acid-Based Topical Applications Used in Wound Healing. Polymers 2020, 12, 1847. [Google Scholar] [CrossRef] [PubMed]
- Musalmah, M.; Nizrana, M.Y.; Fairuz, A.H.; Nooraini, A.H.; Azian, A.L.; Gapor, M.T.; Wan Ngah, W.Z. Comparative effects of palm vitamin E and α-tocopherol on healing and wound tissue antioxidant enzyme levels in diabetic rats. Lipids 2005, 40, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Hoff, J.; Karl, B.; Gerstmeier, J.; Beekmann, U.; Schmölz, L.; Börner, F.; Kralisch, D.; Bauer, M.; Werz, O.; Fischer, D.; et al. Controlled Release of the α-Tocopherol-Derived Metabolite α-13′-Carboxychromanol from Bacterial Nanocellulose Wound Cover Improves Wound Healing. Nanomaterials 2021, 11, 1939. [Google Scholar] [CrossRef]
- Parks, E.; Traber, M.G. Mechanisms of vitamin E regulation: Research over the past decade and focus on the future. Antioxid. Redox Signal. 2000, 2, 405–412. [Google Scholar] [CrossRef]
- Sylvester, P.W.; Sumit, J.; Shah, S.J. Mechanisms mediating the antiproliferative and apoptotic effects of vitamin E in mammary cancer cells. Front. Biosci. (Landmark Ed.) 2005, 10, 699–709. [Google Scholar] [CrossRef]
- Yang, C.S.; Luo, P.; Zeng, Z.; Wang, H.; Malafa, M.; Suh, N. Vitamin E and cancer prevention: Studies with different forms of tocopherols and tocotrienols. Mol. Carcinog. 2020, 59, 365–389. [Google Scholar] [CrossRef]
- Comitato, R.; Ambra, R.; Virgili, F. Tocotrienols: A Family of Molecules with Specific Biological Activities. Antioxidants 2017, 6, 93. [Google Scholar] [CrossRef]
- Yam, M.; Abdul Hafid, S.R.; Cheng, H.; Nesaretnam, K. Tocotrienols Suppress Proinflammatory Markers and Cyclooxygenase-2 Expression in RAW264.7 Macrophages. Lipids 2009, 44, 787–797. [Google Scholar] [CrossRef]
- Szewczyk, K.; Chojnacka, A.; Górnicka, M. Tocopherols and Tocotrienols—Bioactive Dietary Compounds; What Is Certain, What Is Doubt? Int. J. Mol. Sci. 2021, 22, 6222. [Google Scholar] [CrossRef]
- Müller, L.; Theile, K.; Böhm, V. In vitro antioxidant activity of tocopherols and tocotrienols and comparison of vitamin E concentration and lipophilic antioxidant capacity in human plasma. Mol. Nutr. Food Res. 2010, 54, 731–742. [Google Scholar] [CrossRef]
- Brenner, B.; Kuperman, A.; Watzka, M.; Oldenburg, J. Vitamin K–Dependent Coagulation Factors Deficiency. Semin. Thromb. Hemost. 2009, 35, 439–446. [Google Scholar] [CrossRef]
- Pazyar, N.; Houshmand, G.; Yaghoobi, R.; Hemmati, A.A.; Zeineli, Z.; Ghorbanzadeh, B. Wound healing effects of topical Vitamin K: A randomized controlled trial. Indian J. Pharmacol. 2019, 51, 88–92. [Google Scholar] [CrossRef]
- Vermeer, C.V. Vitamin K: The effect on health beyond coagulation—An overview. Food Nutr. Res. 2012, 56, 5329. [Google Scholar] [CrossRef]
- Nuszkiewicz, J.; Sutkowy, P.; Wróblewski, M.; Pawłowska, M.; Wesołowski, R.; Wróblewska, J.; Woźniak, A. Links between Vitamin K, Ferroptosis and SARS-CoV-2 Infection. Antioxidants 2023, 12, 733. [Google Scholar] [CrossRef]
- Li, J.; Lin, J.C.; Wang, H.; Peterson, J.W.; Furie, B.C.; Furie, B.; Booth, S.L.; Volpe, J.J.; Rosenberg, P.A. Novel Role of Vitamin K in Preventing Oxidative Injury to Developing Oligodendrocytes and Neurons. J. Neurosci. 2003, 23, 5816–5826. [Google Scholar] [CrossRef] [PubMed]
- Maurya, R.; Namdeo, M. Superoxide Dismutase: A Key Enzyme for the Survival of Intracellular Pathogens in Host; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Lim, Y.; Levy, M.; Bray, T.M. Dietary zinc alters early inflammatory responses during cutaneous wound healing in weanling CD-1 mice. J. Nutr. 2004, 134, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Krężel, A.; Maret, W. The Functions of Metamorphic Metallothioneins in Zinc and Copper Metabolism. Int. J. Mol. Sci. 2017, 18, 1237. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Elebeedy, D.; Fahmy Mohamed Elsayed, A.; Abd El Maksoud, A.I.; Matar, E.R. Investigation of Angiogenesis and Wound Healing Potential Mechanisms of Zinc Oxide Nanorods. Front. Pharmacol. 2021, 12, 661217. [Google Scholar] [CrossRef]
- Hamza, R.T.; Sallam, M.T.; Hamed, A.I. Effect of zinc supplementation on growth Hormone Insulin growth factor axis in short Egyptian children with zinc deficiency. Ital. J. Pediatr. 2012, 38, 21. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Le, G.; Shi, Y. Effect of zinc sulphate and zinc methionine on growth, plasma growth hormone concentration, growth hormone receptor and insulin-like growth factor-I gene expression in mice. Clin. Exp. Pharmacol. Physiol. 2005, 32, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Bhang, S.H.; Jang, W.S.; Han, J.; Yoon, J.-K.; La, W.-G.; Lee, E.; Kim, Y.S.; Shin, J.Y.; Lee, T.J.; Baik, H.K.; et al. Zinc Oxide Nanorod-Based Piezoelectric Dermal Patch for Wound Healing. Adv. Funct. Mater. 2016, 27, 1603497. [Google Scholar] [CrossRef]
- Piperi, C.; Papavassiliou, G.A. Molecular Mechanisms Regulating Matrix Metalloproteinases. Curr. Top. Med. Chem. 2012, 12, 1095–1112. [Google Scholar] [CrossRef]
- Rahmat, A.; Norman, J.N.; Smith, G. The effect of zinc deficiency on wound healing. Br. J. Surg. 2005, 61, 271–273. [Google Scholar] [CrossRef]
- Schoofs, H.; Schmit, J.; Rink, L. Zinc Toxicity: Understanding the Limits. Molecules 2024, 29, 3130. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Bilgic, E.; Alili, L.; Sies, H.; Brenneisen, P. Selenoprotein P protects endothelial cells from oxidative damage by stimulation of glutathione peroxidase expression and activity. Free Radic. Res. 2006, 40, 936–943. [Google Scholar] [CrossRef]
- Wang, N.; Tan, H.-Y.; Li, S.; Xu, Y.; Guo, W.; Feng, Y. Supplementation of Micronutrient Selenium in Metabolic Diseases: Its Role as an Antioxidant. Oxidative Med. Cell. Longev. 2017, 2017, 7478523. [Google Scholar] [CrossRef]
- Bera, S.; De Rosa, V.; Rachidi, W.; Diamond, A.M. Does a role for selenium in DNA damage repair explain apparent controversies in its use in chemoprevention? Mutagenesis 2012, 28, 127–134. [Google Scholar] [CrossRef]
- Heo, J.S. Selenium-Stimulated Exosomes Enhance Wound Healing by Modulating Inflammation and Angiogenesis. Int. J. Mol. Sci. 2022, 23, 11543. [Google Scholar] [CrossRef]
- Karas, R.A.; Alexeree, S.; Elsayed, H.; Attia, Y.A. Assessment of wound healing activity in diabetic mice treated with a novel therapeutic combination of selenium nanoparticles and platelets rich plasma. Sci. Rep. 2024, 14, 5346. [Google Scholar] [CrossRef]
- Nejati, O.; Tışlı, B.; Yaşayan, G.; Zaman, B.T.; Torkay, G.; Dönmez, M.; Kayin, I.; Bakirdere, S.; Bal-Oztürk, A. Microwave-assisted hydrothermal green synthesis of selenium nanoparticles incorporated with hyaluronic acid methacrylate/gelatin methacrylate hydrogels for wound healing applications. Polym. Eng. Sci. 2023, 64, 316–327. [Google Scholar] [CrossRef]
- Zwolak, I.; Zaporowska, H. Selenium interactions and toxicity: A review. Cell Biol. Toxicol. 2012, 28, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, Y.; Teruya, T.; Miyamoto, C.; Hirose, M.; Endo, S.; Ikari, A. Unraveling the Mechanisms Involved in the Beneficial Effects of Magnesium Treatment on Skin Wound Healing. Int. J. Mol. Sci. 2024, 25, 4994. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Zou, X.-B.; Chai, Y.-F.; Yao, Y.-M. Macrophage polarization in inflammatory diseases. Int. J. Biol. Sci. 2014, 10, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.F.; Haider, A.J.; Al-Musawi, S. Antimicrobial and Wound Healing Effects of Metal Oxide Nanoparticles-Enriched Wound Dressing. Nano 2023, 18. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, W.; Wang, L.; Jiang, H.; Wang, S.; Jia, X.; Wang, Z.; Wang, H.; Zhang, B.; Ding, G. Relationship between Dietary Magnesium Intake and Metabolic Syndrome. Nutrients 2022, 14, 2013. [Google Scholar] [CrossRef]
- Dibaba, D.T.; Xun, P.; Fly, A.D.; Yokota, K.; He, K. Dietary magnesium intake and risk of metabolic syndrome: A meta-analysis. Diabet. Med. 2014, 31, 1301–1309. [Google Scholar] [CrossRef]
- Bleizgys, A. Zinc, Magnesium and Vitamin K Supplementation in Vitamin D Deficiency: Pathophysiological Background and Implications for Clinical Practice. Nutrients 2024, 16, 834. [Google Scholar] [CrossRef]
- Süntar, I.; Çetinkaya, S.; Panieri, E.; Saha, S.; Buttari, B.; Profumo, E.; Saso, L. Regulatory Role of Nrf2 Signaling Pathway in Wound Healing Process. Molecules 2021, 26, 2424. [Google Scholar] [CrossRef]
- Nielsen, F.H. Magnesium deficiency and increased inflammation: Current perspectives. J. Inflamm. Res. 2018, 11, 25–34. [Google Scholar] [CrossRef]
- Peña, O.A.; Martin, P. Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Schreml, S.; Szeimies, R.M.; Prantl, L.; Karrer, S.; Landthaler, M.; Babilas, P. Oxygen in acute and chronic wound healing. Br. J. Dermatol. 2010, 163, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Rehak, L.; Giurato, L.; Meloni, M.; Panunzi, A.; Manti, G.M.; Uccioli, L. The Immune-Centric Revolution in the Diabetic Foot: Monocytes and Lymphocytes Role in Wound Healing and Tissue Regeneration—A Narrative Review. J. Clin. Med. 2022, 11, 889. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Xu, X.; Guo, Y.; Sun, C.; Yang, Y.; Liu, H. Repair and regeneration: Ferroptosis in the process of remodeling and fibrosis in impaired organs. Cell Death Discov. 2024, 10, 424. [Google Scholar] [CrossRef]
- Zhou, Y.; Que, K.T.; Zhang, Z.; Yi, Z.J.; Zhao, P.X.; You, Y.; Gong, J.P.; Liu, Z.J. Iron overloaded polarizes macrophage to proinflammation phenotype through ROS/acetyl-p53 pathway. Cancer Med. 2018, 7, 4012–4022. [Google Scholar] [CrossRef]
- Saboor, M.; Zehra, A.; Hamali, H.; Mobarki, A. Revisiting Iron Metabolism, Iron Homeostasis and Iron Deficiency Anemia. Clin. Lab. 2021, 67. [Google Scholar] [CrossRef]
- Avnee, M.; Sood, S.; Chaudhary, D.R.; Jhorar, P.; Rana, R.S. Biofortification: An approach to eradicate micronutrient deficiency. Front. Nutr. 2023, 10, 1233070. [Google Scholar] [CrossRef]
- Bădăluță, V.A.; Curuțiu, C.; Dițu, L.M.; Holban, A.M.; Lazăr, V. Probiotics in Wound Healing. Int. J. Mol. Sci. 2024, 25, 5723. [Google Scholar] [CrossRef]
- Piazzesi, A.; Scanu, M.; Ciprandi, G.; Putignani, L. Modulations of the skin microbiome in skin disorders: A narrative review from a wound care perspective. Int. Wound J. 2024, 21, e70087. [Google Scholar] [CrossRef] [PubMed]
- Barra, N.G.; Anhê, F.F.; Cavallari, J.F.; Singh, A.M.; Chan, D.Y.; Schertzer, J.D. Micronutrients impact the gut microbiota and blood glucose. J. Endocrinol. 2021, 250, R1–R21. [Google Scholar] [CrossRef] [PubMed]
- Mohtashami, M.; Mohamadi, M.; Azimi-Nezhad, M.; Saeidi, J.; Nia, F.F.; Ghasemi, A. Lactobacillus bulgaricus and Lactobacillus plantarum improve diabetic wound healing through modulating inflammatory factors. Biotechnol. Appl. Biochem. 2020, 68, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Boev, M.; Stănescu, C.; Turturică, M.; Cotârleţ, M.; Batîr-Marin, D.; Maftei, N.; Chiţescu, C.; Grigore-Gurgu, L.; Barbu, V.; Enachi, E.; et al. Bioactive Potential of Carrot-Based Products Enriched with Lactobacillus plantarum. Molecules 2024, 29, 917. [Google Scholar] [CrossRef]
- Yin, Z.; Wang, Y.; Feng, X.; Liu, C.; Guan, X.; Liu, S.; Long, Z.; Miao, Z.; He, F.; Cheng, R.; et al. Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis BB-12 promote infected wound healing via regulation of the wound microenvironment. Microb. Biotechnol. 2024, 17, e70031. [Google Scholar] [CrossRef]
- Panagiotou, D.; Filidou, E.; Gaitanidou, M.; Tarapatzi, G.; Spathakis, M.; Kandilogiannakis, L.; Stavrou, G.; Arvanitidis, K.; Tsetis, J.K.; Gionga, P.; et al. Role of Lactiplantibacillus plantarum UBLP-40, Lactobacillus rhamnosus UBLR-58 and Bifidobacterium longum UBBL-64 in the Wound Healing Process of the Excisional Skin. Nutrients 2023, 15, 1822. [Google Scholar] [CrossRef]
- Zielińska, M.; Pawłowska, A.; Orzeł, A.; Sulej, L.; Muzyka-Placzyńska, K.; Baran, A.; Filipecka-Tyczka, D.; Pawłowska, P.; Nowińska, A.; Bogusławska, J.; et al. Wound Microbiota and Its Impact on Wound Healing. Int. J. Mol. Sci. 2023, 24, 17318. [Google Scholar] [CrossRef]
- Togo, C.; Zidorio, A.P.; Gonçalves, V.; Botelho, P.; de Carvalho, K.; Dutra, E. Does Probiotic Consumption Enhance Wound Healing? A Systematic Review. Nutrients 2021, 14, 111. [Google Scholar] [CrossRef]
- Barthe, M.; Gillot, L.; Perdigon, L.; Jacobs, A.; Schoonbroodt, G.; Mauhin, P.; Bouhajja, E.; Osman-Ponchet, H. Topical Probiotic Formulation Promotes Rapid Healing in Dog Keratinocyte Cells: A Promising Approach for Wound Management. Int. J. Mol. Sci. 2023, 24, 12360. [Google Scholar] [CrossRef]
- Johnson, T.R.; Gómez, B.I.; McIntyre, M.K.; Dubick, M.A.; Christy, R.J.; Nicholson, S.E.; Burmeister, D.M. The Cutaneous Microbiome and Wounds: New Molecular Targets to Promote Wound Healing. Int. J. Mol. Sci. 2018, 19, 2699. [Google Scholar] [CrossRef] [PubMed]
- Bekiaridou, A.; Karlafti, E.; Oikonomou, I.M.; Ioannidis, A.; Papavramidis, T.S. Probiotics and Their Effect on Surgical Wound Healing: A Systematic Review and New Insights into the Role of Nanotechnology. Nutrients 2021, 13, 4265. [Google Scholar] [CrossRef] [PubMed]
- Knackstedt, R.; Knackstedt, T.; Gatherwright, J. The role of topical probiotics on wound healing: A review of animal and human studies. Int. Wound J. 2020, 17, 1687–1694. [Google Scholar] [CrossRef] [PubMed]
- Atabati, H.; Esmaeili, S.; Saburi, E.; Akhlaghi, M.; Raoofi, A.; Rezaei, N.; Momtazi-Borojeni, A.A. Probiotics with ameliorating effects on the severity of skin inflammation in psoriasis: Evidence from experimental and clinical studies. J. Cell. Physiol. 2020, 235, 8925–8937. [Google Scholar] [CrossRef]
- Ersanli, C.; Tzora, A.; Voidarou, C.; Skoufos, S.; Zeugolis, D.I.; Skoufos, I. Biodiversity of Skin Microbiota as an Important Biomarker for Wound Healing. Biology 2023, 12, 1187. [Google Scholar] [CrossRef]
- Arribas-López, E.; Zand, N.; Ojo, O.; Snowden, M.J.; Kochhar, T. The Effect of Amino Acids on Wound Healing: A Systematic Review and Meta-Analysis on Arginine and Glutamine. Nutrients 2021, 13, 2498. [Google Scholar] [CrossRef]
- Kwon, Y. Immuno-Resolving Ability of Resolvins, Protectins, and Maresins Derived from Omega-3 Fatty Acids in Metabolic Syndrome. Mol. Nutr. Food Res. 2019, 64, 1900824. [Google Scholar] [CrossRef]
- Burger, B.; Kühl, C.M.C.; Candreva, T.; Cardoso, R.S.; Silva, J.R.; Castelucci, B.G.; Consonni, S.R.; Fisk, H.L.; Calder, P.C.; Vinolo, M.A.R.; et al. Oral administration of EPA-rich oil impairs collagen reorganization due to elevated production of IL-10 during skin wound healing in mice. Sci. Rep. 2019, 9, 9119. [Google Scholar] [CrossRef]
- Kjaer, M.; Frederiksen, A.K.S.; Nissen, N.I.; Willumsen, N.; van Hall, G.; Jorgensen, L.N.; Andersen, J.R.; Agren, J.R. Multinutrient Supplementation Increases Collagen Synthesis during Early Wound Repair in a Randomized Controlled Trial in Patients with Inguinal Hernia. J. Nutr. 2020, 150, 792–799. [Google Scholar] [CrossRef]
- Magalhães, M.S.; Fechine, F.V.; Macedo, R.N.; Monteiro, D.L.; Oliveira, C.C.; Brito, G.A.; Moraes, M.E.; Moraes, M.O. Effect of a combination of medium chain triglycerides, linoleic acid, soy lecithin and vitamins A and E on wound healing in rats. Acta Cir. Bras. 2008, 23, 262–269. [Google Scholar] [CrossRef]
- Choudhary, V.; Choudhary, M.; Bollag, W.B. Exploring Skin Wound Healing Models and the Impact of Natural Lipids on the Healing Process. Int. J. Mol. Sci. 2024, 25, 3790. [Google Scholar] [CrossRef]
- Mori, T.A.; Beilin, L.J. Omega-3 fatty acids and inflammation. Curr. Atheroscler. Rep. 2004, 6, 461–467. [Google Scholar] [CrossRef]
- Carracedo, M.; Artiach, G.; Arnardottir, H.; Bäck, M. The resolution of inflammation through omega-3 fatty acids in atherosclerosis, intimal hyperplasia, and vascular calcification. Semin. Immunopathol. 2019, 41, 757–766. [Google Scholar] [CrossRef]
- Silva, J.R.; Burger, B.; Kühl, C.M.C.; Candreva, T.; Dos Anjos, M.B.P.; Rodrigues, H.G. Wound Healing and Omega-6 Fatty Acids: From Inflammation to Repair. Mediat. Inflamm. 2018, 5, 2503950. [Google Scholar] [CrossRef] [PubMed]
- Forchhammer, K. Glutamine signalling in bacteria. Front. Biosci. (Landmark Ed.) 2007, 12, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, A.T.; Wei, Y.; Alneamah, S.J.A.; Al-Shawi, S.G.; Kadir, S.Y.A.; Zainol, J.; Liu, X. What Is New in the Preventive and Therapeutic Role of Dairy Products as Nutraceuticals and Functional Foods? BioMed Res. Int. 2021, 2021, 8823222. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Gokhale, J. Immunity boosting nutraceuticals: Current trends and challenges. J. Food Biochem. 2021, 46, e13902. [Google Scholar] [CrossRef]
- Catorce, M.N.; Gevorkian, G. Evaluation of Anti-inflammatory Nutraceuticals in LPS-induced Mouse Neuroinflammation Model: An Update. Curr. Neuropharmacol. 2020, 18, 636–654. [Google Scholar] [CrossRef]
- Young, G.; Conquer, J. Omega-3 fatty acids and neuropsychiatric disorders. Reprod. Nutr. Dev. 2005, 45, 1–28. [Google Scholar] [CrossRef]
- Chow, O.; Barbul, A. Immunonutrition: Role in Wound Healing and Tissue Regeneration. Adv. Wound Care 2014, 3, 46–53. [Google Scholar] [CrossRef]
- Alexander, J.W.; Supp, D.M. Role of Arginine and Omega-3 Fatty Acids in Wound Healing and Infection. Adv. Wound Care 2014, 3, 682–690. [Google Scholar] [CrossRef]
- Witte, M.B.; Barbul, A. Arginine physiology and its implication for wound healing. Wound Repair Regen. 2003, 11, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.W.S.; Long, Y.C.; Sim, A.Y.L. Glutamine metabolism regulates autophagy-dependent mTORC1 reactivation during amino acid starvation. Nat. Commun. 2017, 8, 338. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Casanella, O.; Núñez, O.; Granados, M.; Saurina, J.; Sentellas, S. Analytical Methods for Exploring Nutraceuticals Based on Phenolic Acids and Polyphenols. Appl. Sci. 2021, 11, 8276. [Google Scholar] [CrossRef]
- Chu, A. Antagonism by Bioactive Polyphenols Against Inflammation: A Systematic View. Inflamm. Allergy-Drug Targets 2014, 13, 34–64. [Google Scholar] [CrossRef]
- Rodrigo, R.; Libuy, M.; Feliu, F.; Hasson, D. Polyphenols in disease: From diet to supplements. Curr. Pharm. Biotechnol. 2014, 15, 304–317. [Google Scholar] [CrossRef]
- Singh, A.; Gade, A.S.W.N.; Vats, T.; Lenardi, C.; Milani, P.; Aditi, A.S. Nanomaterials: New Generation Therapeutics in Wound Healing and Tissue Repair. Curr. Nanosci. 2010, 6, 577–586. [Google Scholar] [CrossRef]
- Hu, M.S.; Maan, Z.N.; Wu, J.C.; Rennert, R.C.; Hong, W.X.; Lai, T.S.; Cheung, A.T.M.; Walmsley, G.G.; Chung, M.T.; McArdle, A.; et al. Tissue Engineering and Regenerative Repair in Wound Healing. Ann. Biomed. Eng. 2014, 42, 1494–1507. [Google Scholar] [CrossRef]
- Utpal, B.K.; Sutradhar, B.; Zehravi, M.; Sweilam, S.H.; Panigrahy, U.P.; Urs, D.; Fatima, A.F.; Nallasivan, P.K.; Chhabra, G.S.; Sayed, M.; et al. Polyphenols in wound healing: Unlocking prospects with clinical applications. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 398, 2459–2485. [Google Scholar] [CrossRef]
- Jia, Y.; Shao, J.-H.; Zhang, K.-W.; Zou, M.-L.; Teng, Y.-Y.; Tian, F.; Chen, M.-N.; Chen, W.-W.; Yuan, Z.-D.; Wu, J.-J.; et al. Emerging Effects of Resveratrol on Wound Healing: A Comprehensive Review. Molecules 2022, 27, 6736. [Google Scholar] [CrossRef]
- De Sá Coutinho, D.; Pacheco, M.T.; Frozza, R.L.; Bernardi, A. Anti-Inflammatory Effects of Resveratrol: Mechanistic Insights. Int. J. Mol. Sci. 2018, 19, 1812. [Google Scholar] [CrossRef]
- Bi, M.; Qin, Y.; Zhang, J.; Wang, L. The protective role of resveratrol in diabetic wound healing. Phytother. Res. 2023, 37, 5193–5204. [Google Scholar] [CrossRef]
- Hecker, A.; Schellnegger, M.; Hofmann, E.; Luze, H.; Nischwitz, S.P.; Kamolz, L.; Kotzbeck, P. The impact of resveratrol on skin wound healing, scarring, and aging. Int. Wound J. 2021, 19, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-C.; Wu, Z.; Zhu, L.; Yang, R.; Xu, N.; Liang, L. Resveratrol-loaded peptide-hydrogels inhibit scar formation in wound healing through suppressing inflammation. Regen. Biomater. 2019, 7, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Pignet, A.-L.; Schellnegger, M.; Hecker, A.; Kohlhauser, M.; Kotzbeck, P.; Kamolz, L.-P. Resveratrol-Induced Signal Transduction in Wound Healing. Int. J. Mol. Sci. 2021, 22, 12614. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhou, J.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Health Benefits and Molecular Mechanisms of Resveratrol: A Narrative Review. Foods 2020, 9, 340. [Google Scholar] [CrossRef]
- Bishayee, A. Cancer Prevention and Treatment with Resveratrol: From Rodent Studies to Clinical Trials. Cancer Prev. Res. 2009, 2, 409–418. [Google Scholar] [CrossRef]
- Tejada, S.; Manayi, A.; Daglia, M.; Nabavi, S.; Sureda, A.; Hajheydari, Z.; Gortzi, O.; Pazoki-Toroui, H.; Nabavi, S.M. Wound Healing Effects of Curcumin: A Short Review. Curr. Pharm. Biotechnol. 2016, 17, 1002–1007. [Google Scholar] [CrossRef]
- Santos, M.D.; Campos, E.C.R.; Gonçalves Junior, R.; Koga, A.Y.; Kono, P.A.; Salina, M.V.J.; Filho, E.D.; Toledo Junior, A.O.; Lipinski, L.C. Effects of curcumin supplementation on abdominal surgical wound healing. Acta Cir. Bras. 2024, 39, e392124. [Google Scholar] [CrossRef]
- Haddad, J.J.; Harb, H.L. L-gamma-Glutamyl-L-cysteinyl-glycine (glutathione; GSH) and GSH-related enzymes in the regulation of pro- and anti-inflammatory cytokines: A signaling transcriptional scenario for redox(y) immunologic sensor(s)? Mol. Immunol. 2005, 42, 987–1014. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Favara, G.; Magnano San Lio, R.; Evola, G.; Agodi, A.; Basile, G. Nutrition and Wound Healing: An Overview Focusing on the Beneficial Effects of Curcumin. Int. J. Mol. Sci. 2019, 20, 1119. [Google Scholar] [CrossRef]
- Phan, T.-T.; See, P.; Lee, S.-T.; Chan, S.-Y. Protective effects of curcumin against oxidative damage on skin cells in vitro: Its implication for wound healing. J. Trauma Inj. Infect. Crit. Care 2001, 51, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Omidian, H.; Wilson, R.L.; Chowdhury, S.D. Enhancing Therapeutic Efficacy of Curcumin: Advances in Delivery Systems and Clinical Applications. Gels 2023, 9, 596. [Google Scholar] [CrossRef] [PubMed]
- Elkhateeb, O.; Badawy, M.E.I.; Tohamy, H.G.; Abou-Ahmed, H.; El-Kammar, M.; Elkhenany, H. Curcumin-infused nanostructured lipid carriers: A promising strategy for enhancing skin regeneration and combating microbial infection. BMC Vet. Res. 2023, 19, 206. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Dhanka, M.; Yadav, I.; Gautam, S.; Bashir, S.M.; Mishra, N.C.; Arora, T.; Hassan, S. Technological Interventions Enhancing Curcumin Bioavailability in Wound-Healing Therapeutics. Tissue Eng. Part B Rev. 2023, 30, 230–253. [Google Scholar] [CrossRef]
- Rapti, E.; Adamantidi, T.; Efthymiopoulos, P.; Kyzas, G.Z.; Tsoupras, A. Potential Applications of the Anti-Inflammatory, Antithrombotic and Antioxidant Health-Promoting Properties of Curcumin: A Critical Review. Nutraceuticals 2024, 4, 562–595. [Google Scholar] [CrossRef]
- Soares, R.D.F.; Campos, M.G.N.; Ribeiro, G.P.; Salles, B.C.C.; Cardoso, N.S.; Ribeiro, J.R.; Souza, R.M.; Leme, K.C.; Soares, C.B.; Oliveira, C.M.; et al. Development of a chitosan hydrogel containing flavonoids extracted from Passiflora edulis leaves and the evaluation of its antioxidant and wound healing properties for the treatment of skin lesions in diabetic mice. J. Biomed. Mater. Res. 2019, 108, 654–662. [Google Scholar] [CrossRef]
- Zulkefli, N.; Che Zahari, C.N.M.; Sayuti, N.H.; Kamarudin, A.A.; Saad, N.; Hamezah, H.S.; Bunawan, H.; Baharum, S.N.; Mediani, A.; Ahmed, Q.U.; et al. Flavonoids as Potential Wound-Healing Molecules: Emphasis on Pathways Perspective. Int. J. Mol. Sci. 2023, 24, 4607. [Google Scholar] [CrossRef]
- Pang, Y.; Zhang, Y.; Huang, L.; Xu, L.; Wang, K.; Wang, D.; Guan, L.; Zhang, Y.; Yu, F.; Chen, Z.; et al. Effects and Mechanisms of Total Flavonoids from Blumea balsamifera (L.) DC. on Skin Wound in Rats. Int. J. Mol. Sci. 2017, 18, 2766. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, L.; Ding, Q.; Zhang, S.; Sun, S.; Liu, W.; Liu, J.; Han, X.; Ding, C. Flavonoid-Loaded Biomaterials in Bone Defect Repair. Molecules 2023, 28, 6888. [Google Scholar] [CrossRef]
- Antunes-Ricardo, M.; Gutierrez-Uribe, J.; Serna-Saldivar, S. Anti-inflammatory glycosylated flavonoids as therapeutic agents for treatment of diabetes-impaired wounds. Curr. Top. Med. Chem. 2015, 15, 2456–2463. [Google Scholar] [CrossRef]
- Cos, P.; Bruyne, T.; Berghe, D.; Apers, S.; Hermans, N.; Vlietinck, A. Proanthocyanidins in health care: Current and new trends. Curr. Med. Chem. 2004, 11, 1345–1359. [Google Scholar] [CrossRef]
- Bladé, C.; Salvadó, M.; Arola, L. Hypolipidemic effects of proanthocyanidins and their underlying biochemical and molecular mechanisms. Mol. Nutr. Food Res. 2010, 54, 37–59. [Google Scholar] [CrossRef]
- Yang, L.; Xian, D.; Xiong, X.; Lai, R.; Song, J.; Zhong, J. Proanthocyanidins against Oxidative Stress: From Molecular Mechanisms to Clinical Applications. BioMed Res. Int. 2018, 2, 1–11. [Google Scholar] [CrossRef]
- Liang, L.; Meng, F.; Zhong, W.; Weng, L.; Qiu, H.; Wu, L.; Liu, Y. Advances in Extraction Protocols, Degradation Methods, and Bioactivities of Proanthocyanidins. Molecules 2024, 29, 2179. [Google Scholar] [CrossRef]
- De Pascual-Teresa, S.; Moreno, D.A.; García-Viguera, C. Flavanols and Anthocyanins in Cardiovascular Health: A Review of Current Evidence. Int. J. Mol. Sci. 2010, 11, 1679–1703. [Google Scholar] [CrossRef] [PubMed]
- McKay, T.B.; Emmitte, K.A.; German, C.; Karamichos, D. Quercetin and Related Analogs as Therapeutics to Promote Tissue Repair. Bioengineering 2023, 10, 1127. [Google Scholar] [CrossRef] [PubMed]
- Yadav, E.; Neupane, N.P.; Otuechere, C.A.; Yadav, J.P.; Bhat, M.A.; Al-Omar, M.A.; Yadav, P.; Verma, A. Cutaneous Wound-Healing Activity of Quercetin-Functionalized Bimetallic Nanoparticles. Chem. Biodivers. 2024, 22, e202401551. [Google Scholar] [CrossRef] [PubMed]
- Beken, B.; Serttas, R.; Yazicioglu, M.; Turkekul, K.; Erdogan, S. Quercetin Improves Inflammation, Oxidative Stress, and Impaired Wound Healing in Atopic Dermatitis Model of Human Keratinocytes. Pediatr. Allergy Immunol. Pulmonol. 2020, 33, 69–79. [Google Scholar] [CrossRef]
- Huang, H.-C.; Chen, Y.; Hu, J.; Guo, X.-T.; Zhou, S.-R.; Yang, Q.-Q.; Du, Y.; Jin, Y.; Liu, G.; Peng, Y. Quercetin and its derivatives for wound healing in rats/mice: Evidence from animal studies and insight into molecular mechanisms. Int. Wound J. 2023, 21, e14389. [Google Scholar] [CrossRef]
- Alharbi, H.O.A.; Alshebremi, M.; Babiker, A.Y.; Rahmani, A.H. The Role of Quercetin, a Flavonoid in the Management of Pathogenesis Through Regulation of Oxidative Stress, Inflammation, and Biological Activities. Biomolecules 2025, 15, 151. [Google Scholar] [CrossRef]
- Yin, G.; Wang, Z.; Wang, Z.; Wang, X. Topical application of quercetin improves wound healing in pressure ulcer lesions. Exp. Dermatol. 2018, 27, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Nayak, M.; Kumar, V.; Banerjee, D.; Pradhan, L.; Kamath, P.; Mukherjee, S. Quercetin nanocrystal-loaded alginate hydrogel patch for wound healing applications. J. Mater. Chem. B 2025, 13, 1690–1703. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Jia, C.; Li, J.; Zhou, Y.; Luo, Y.; Liu, J.; Zhao, Z.; Zhang, J.; Lin, S.; Chen, Y. Fisetin Attenuates Diabetic Nephropathy-Induced Podocyte Injury by Inhibiting NLRP3 Inflammasome. Front. Pharmacol. 2022, 13, 783706. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Hao, Z.; Wang, H.; Su, Y.; Yang, W.; Li, X.; Duan, L.; Guan, F.; Ma, S. Fisetin Promotes Functional Recovery after Spinal Cord Injury by Inhibiting Microglia/Macrophage M1 Polarization and JAK2/STAT3 Signaling Pathway. J. Agric. Food Chem. 2024, 72, 17964–17976. [Google Scholar] [CrossRef]
- Sun, X.; Ma, X.; Li, Q.; Yang, G.; Xu, X.; Sun, J.; Yu, M.; Cao, K.; Yang, L.; Yang, G.; et al. Anti-cancer effects of fisetin on mammary carcinoma cells via regulation of the PI3K/Akt/mTOR pathway: Invitro and in vivo studies. Int. J. Mol. Med. 2018, 42, 811–820. [Google Scholar] [CrossRef]
- Yang, H.; Hong, Y.; Gong, M.; Cai, S.; Yuan, Z.; Feng, S.; Chen, Q.; Liu, X.; Mei, Z. Fisetin exerts neuroprotective effects in vivo and in vitro by inhibiting ferroptosis and oxidative stress after traumatic brain injury. Front. Pharmacol. 2024, 15, 1480345. [Google Scholar] [CrossRef]
- Sun, A.; Xu, X.; Lin, J.; Xu, R.; Cui, X. Neuroprotection by saponins. Phytother. Res. 2014, 29, 187–200. [Google Scholar] [CrossRef]
- Men, S.; Huo, Q.; Shi, L.; Yan, Y.; Yang, C.; Yu, W.; Liu, B. Panax notoginseng saponins promotes cutaneous wound healing and suppresses scar formation in mice. J. Cosmet. Dermatol. 2019, 19, 529–534. [Google Scholar] [CrossRef]
- Shen, L.; Luo, H.; Fan, L.; Tian, X.; Tang, A.; Wu, X.; Dong, K.; Su, Z. Potential Immunoregulatory Mechanism of Plant Saponins: A Review. Molecules 2024, 29, 113. [Google Scholar] [CrossRef]
- Barone, M.; D’Amico, F.; Brigidi, P.; Turroni, S. Gut microbiome-micronutrient interaction: The key to controlling the bioavailability of minerals and vitamins? BioFactors 2022, 48, 307–314. [Google Scholar] [CrossRef]
- Chelmow, D.; Cabana, M.; Nicholson, W.K.; Davis, E.M.; Donahue, K.E.; Ogedegbe, G.; Ruiz, J.; Bibins-Domingo, K. Vitamin, Mineral, and Multivitamin Supplementation to Prevent Cardiovascular Disease and Cancer. JAMA 2022, 327, 2326. [Google Scholar] [CrossRef]
- Godswill, A.G.; Somtochukwu, I.V.; Ikechukwu, A.O.; Kate, E.C. Health Benefits of Micronutrients (Vitamins and Minerals) and their Associated Deficiency Diseases: A Systematic Review. Int. J. Food Sci. 2020, 3, 1–32. [Google Scholar] [CrossRef]
- Wieringa, F.T.; Van Der Meer, J.W.M.; Dijkhuizen, M.A.; Van Der Ven-Jongekrijg, J.; Muhilal, M.; West, C.E. Reduced production of immunoregulatory cytokines in vitamin A- and zinc-deficient Indonesian infants. Eur. J. Clin. Nutr. 2004, 58, 1498–1504. [Google Scholar] [CrossRef]
- Van Ballegooijen, A.J.; Pilz, S.; Grübler, M.R.; Verheyen, N.; Tomaschitz, A. The Synergistic Interplay between Vitamins D and K for Bone and Cardiovascular Health: A Narrative Review. Int. J. Endocrinol. 2017, 4, 1–12. [Google Scholar] [CrossRef]
- Razzaque, M.S.; Wimalawansa, S.J. Minerals and Human Health: From Deficiency to Toxicity. Nutrients 2025, 17, 454. [Google Scholar] [CrossRef] [PubMed]
- Sang, L.; Yang, S.; Zhu, Y.; Zhu, Z.; Yang, B.; Li, Z.; Mao, X.; Chen, S.; Li, C.; Du, J.; et al. The combined use of B vitamins and probiotics promotes B vitamin absorption and increases Akkermansia abundance. Food Funct. 2024, 15, 7017–7031. [Google Scholar] [CrossRef] [PubMed]
- DiPietro, L.A. Angiogenesis and wound repair: When enough is enough. J. Leukoc. Biol. 2016, 100, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Shu, F.; Zhang, W.; Huang, H.; Liu, W.; Lu, J.; Lin, J.; Liu, T.; Xiao, S.; Zheng, Y.; Xia, Z. Junctional adhesion molecule A orchestrates endothelial cell-driven angiogenesis and wound healing in diabetes. Pharmacol. Res. 2025, 217, 107796. [Google Scholar] [CrossRef]
- Yoon, J.H.; Baek, S.J. Molecular Targets of Dietary Polyphenols with Anti-inflammatory Properties. Yonsei Med. J. 2005, 46, 585–596. [Google Scholar] [CrossRef]
- Mohammadi, N.; Farrell, M.; O’Sullivan, L.; Langan, A.; Franchin, M.; Azevedo, L.; Granato, D. Effectiveness of anthocyanin-containing foods and nutraceuticals in mitigating oxidative stress, inflammation, and cardiovascular health-related biomarkers: A systematic review of animal and human interventions. Food Funct. 2024, 15, 3274–3299. [Google Scholar] [CrossRef]
- Anghel, L.; Boev, M.; Stanescu, C.; Mitincu Caramfil, S.; Luca, L.; Muşat, C.L.; Ciubara, A. Depression in the diabetic patient. BRAIN Broad Res. Artif. Intell. Neurosci. 2023, 14, 658–672. [Google Scholar] [CrossRef]
- Batir-Marin, D.; Ștefan, C.S.; Boev, M.; Gurău, G.; Popa, G.V.; Matei, M.N.; Ursu, M.; Nechita, A.; Maftei, N.-M. A Multidisciplinary Approach of Type 1 Diabetes: The Intersection of Technology, Immunotherapy, and Personalized Medicine. J. Clin. Med. 2025, 14, 2144. [Google Scholar] [CrossRef] [PubMed]
- Leenders, M.; Van Loon, L.J. Leucine as a pharmaconutrient to prevent and treat sarcopenia and type 2 diabetes. Nutr. Rev. 2011, 69, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Boniakowski, A.E.; Kimball, A.S.; Jacobs, B.N.; Kunkel, S.L.; Gallagher, K.A. Macrophage-Mediated Inflammation in Normal and Diabetic Wound Healing. J. Immunol. 2017, 199, 17–24. [Google Scholar] [CrossRef]
- Stechmiller, J.K. Understanding the Role of Nutrition and Wound Healing. Nutr. Clin. Pract. 2010, 25, 61–68. [Google Scholar] [CrossRef]
- Palmieri, B.; Laurino, C.; Vadalà, M. Nutrition in wound healing: Investigation of the molecular mechanisms, a narrative review. J. Wound Care 2019, 28, 683–693. [Google Scholar] [CrossRef]
- Stanescu, C.; Anghel, L.; Tamas, C.; Ciubara, A. Education of Patients and Their Families to Manage Emotional Impact of Skin Scars. BRAIN Broad Res. Artif. Intell. Neurosci. 2025, 16, 324–341. [Google Scholar] [CrossRef]
- Seth, I.; Lim, B.; Cevik, J.; Gracias, D.; Chua, M.; Kenney, P.S.; Rozen, W.M.; Cuomo, R. Impact of nutrition on skin wound healing and aesthetic outcomes: A comprehensive narrative review. JPRAS Open 2024, 39, 291–302. [Google Scholar] [CrossRef]
| Nutraceuticals | Role in Wound Healing | Mechanisms of Action |
|---|---|---|
| Polyphenols and flavonoids | Supports vascular regeneration and prevents chronic inflammation | Inhibits the NF-κB pathway, pro-motes angiogenesis, and cell prolif-eration |
| Saponins | Anti-inflammatory, antioxidant, antibacterial, and pro-collagen syn-thesis properties | Exerts anti-inflammatory effects by regulating the NF-κB and MAPK pathways |
| Arginine and glutamine | Stimulates collagen formation and immune defense | Boosts nitric oxide synthesis, en-hances fibroblast activity, and im-proves collagen synthesis |
| Omega-3 Fatty Acids | Promotes cell migration and tissue regeneration | Suppress pro-inflammatory cyto-kines, activate high-conductance voltage-activated (BK) calcium (Ca2+) and potassium (K+) channels, which contribute to vasodilation |
| Probiotics | Supports systemic immune balance via gut-skin axis, improves skin barrier, and reduces infection risk | Reduces pro-inflammatory cytokine levels, stimulates local cell growth and repair, and protects against pathogens |
| Compounds | Physiological Doses | Toxic Doses | Toxicity Effects |
|---|---|---|---|
| Vitamin A | 700–900 µg/day | >3000 µg/day | Hepatotoxicity, headaches, osteoporosis |
| Vitamin C | 75–90 mg/day | >2000 mg/day | Gastrointestinal upset and kidney stones |
| Vitamin B1 | 1.1–1.2 mg/day | No UL | Generally safe |
| Vitamin B6 | 1.3–2.0 mg/day | >100 mg/day | Neuropathic symptoms, abdominal pain |
| Vitamin B12 | 2.4 -3 µg/day | No UL | Generally safe |
| Vitamin E | 15 mg/day | >1000 mg/day | Bleeding risk, amplified risks of cardiovas-cular events, and fatigue |
| Vitamin D | 15–20 µg/day (600–800 IU) | >100 µg/day (>4000 IU) | Hypercalcemia, nausea, vomiting, polyuria, polydipsia, dehydration, confusion, apathy |
| Vitamin K | 50–200 µg/day | Low toxicity | Generally safe |
| Zinc | 8–11 mg/day | >40 mg/day | Copper deficiency, anemia, leukopenia, nausea, and memory impairment |
| Selenium | 55 µg/day | >400 µg/day | Hair loss, nail brittleness, gastrointestinal disturbances |
| Magnesium | 310–420 mg/day | >350 mg/day | Diarrhea, hypotension |
| Iron | 8–18 mg/day | >45 mg/day | Gastrointestinal distress, hemosiderosis |
| Omega-3 (EPA/DHA) | 250–500 mg/day | >3000 mg/day | Bleeding risk, immune suppression |
| Arginine | 6–18 g/day | >30 g/day | Gastrointestinal upset, hypotension, and viral reactivation risk |
| Glutamine | 5–10 g/day-30 g/day | >0.75 g/kg/day | Elevated ammonia, Gastrointestinal dis-tress, liver strain |
| Resveratrol | 100–500 mg/day | >1000 mg/day | Unknown long-term toxicity |
| Curcumin | 500–2000 mg/day | >8000 mg/day | Gastrointestinal distress, liver enzyme ele-vation |
| Proanthocyanidis | 50–300 mg/day | Generally safe up to 1000 mg/day | Very high doses may cause gastrointestinal disturbances or interfere with nutrient ab-sorption |
| Quercetin | 500–1000 mg/day | >1000 mg/day | Headaches, kidney stress (high doses) |
| Fisetin | 100–200 mg/day | >1000 mg/day | Possible liver damage at high doses |
| Saponins | Varies by source; ~10–100 mg/day | >10 g/kg | Gastrointestinal distress, diarrhea, and possible liver/kidney effects |
| Category | Synergistic Component | Role in Wound Healing and Tissue Regeneration |
|---|---|---|
| Colagenogenesis | Vitamin C Vitamin A Zinc | Stimulates collagen synthesis and tis-sue remodeling |
| Regenerate Tissue | Arginine Glutamine Vitamin B complex | Promotes cell proliferation and tissue restoration |
| Local immunity | Probiotics Vitamin D Zinc Selenium | Improves immune response and pro-tects the epithelial barrier |
| Antioxidant, Anti-inflammatory | Omega 3 Curcumin Vitamin E | Reduces inflammation and oxidative stress |
| Angiogenesis | Vitamin D Arginine Polyphenols | Stimulates the formation of blood vessels in the affected tissues |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanescu, C.; Chiscop, I.; Mihalache, D.; Boev, M.; Tamas, C.; Stoleriu, G. The Roles of Micronutrition and Nutraceuticals in Enhancing Wound Healing and Tissue Regeneration: A Systematic Review. Molecules 2025, 30, 3568. https://doi.org/10.3390/molecules30173568
Stanescu C, Chiscop I, Mihalache D, Boev M, Tamas C, Stoleriu G. The Roles of Micronutrition and Nutraceuticals in Enhancing Wound Healing and Tissue Regeneration: A Systematic Review. Molecules. 2025; 30(17):3568. https://doi.org/10.3390/molecules30173568
Chicago/Turabian StyleStanescu, Cristina, Iulia Chiscop, Daniela Mihalache, Monica Boev, Camelia Tamas, and Gabriela Stoleriu. 2025. "The Roles of Micronutrition and Nutraceuticals in Enhancing Wound Healing and Tissue Regeneration: A Systematic Review" Molecules 30, no. 17: 3568. https://doi.org/10.3390/molecules30173568
APA StyleStanescu, C., Chiscop, I., Mihalache, D., Boev, M., Tamas, C., & Stoleriu, G. (2025). The Roles of Micronutrition and Nutraceuticals in Enhancing Wound Healing and Tissue Regeneration: A Systematic Review. Molecules, 30(17), 3568. https://doi.org/10.3390/molecules30173568







