Bioactive Power of Black Chokeberry Pomace as Affected by Advanced Extraction Techniques and Cryogrinding
Abstract
1. Introduction
2. Results and Discussion
2.1. Determination of PLE, MAE, and UAE Optimal Process Conditions
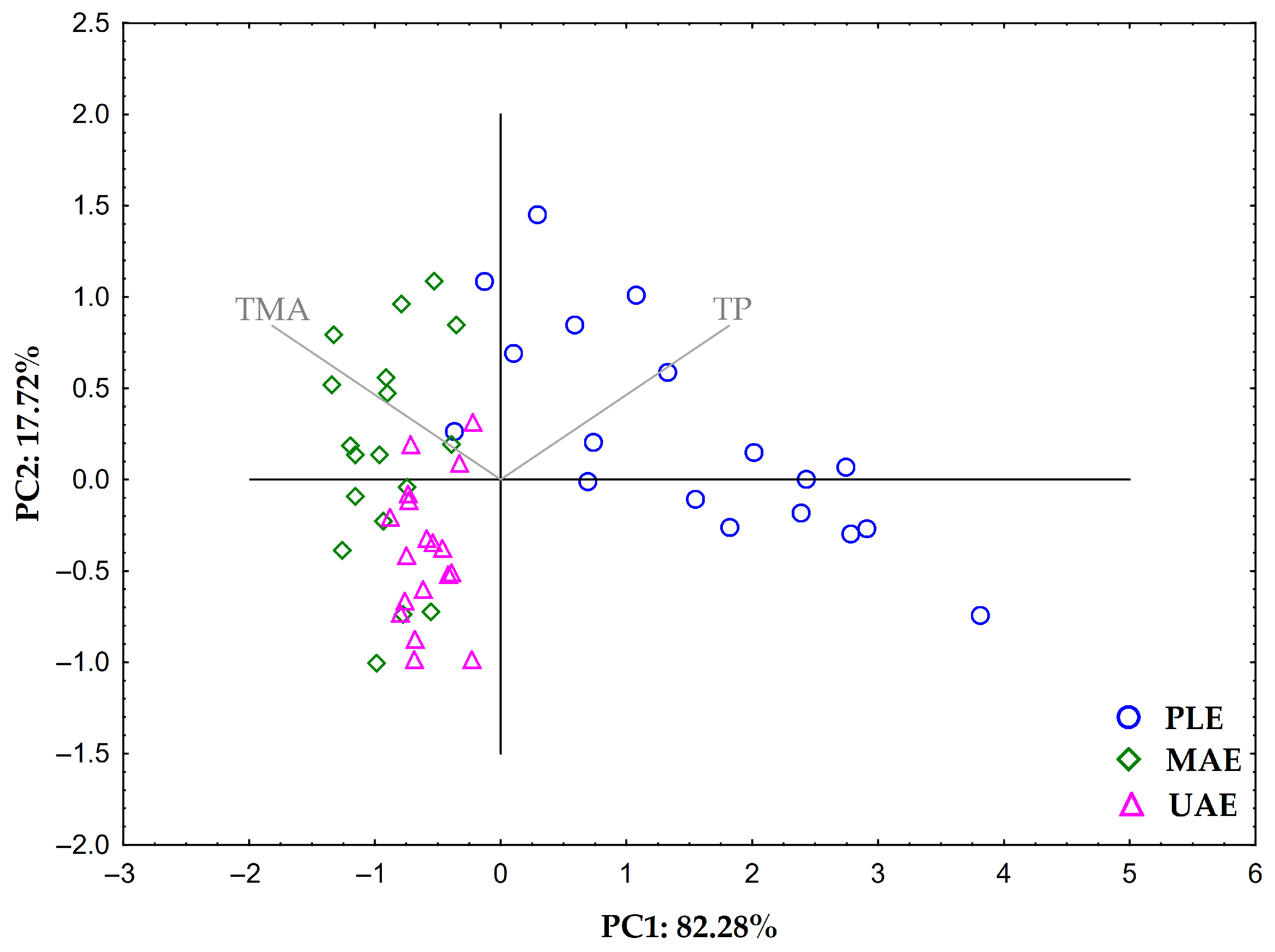
2.2. Efficiency Comparison of PLE, MAE, UAE, and Reflux
2.3. Effects of Cryogrinding
2.4. Phenolic Profile of BCP Extract Obtained Under Optimal MAE Conditions in Combination with Cryogrinding
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Extraction Procedure
3.3.1. PLE
3.3.2. MAE
3.3.3. UAE
3.3.4. Reflux
3.4. Cryogrinding
3.5. Particle Size Measurement
3.6. Determination of Total Polyphenols
3.7. Determination of Total Monomeric Anthocyanins
- A = (A520nm − A700nm)pH=1.0 − (A520nm − A700nm)pH=4.5;
- MW = molecular weight of cyanidin-3-glucoside (449.2 g/mol);
- DF = dilution factor;
- 103 = factor for conversion g to mg;
- ε = molar absorption extinction coefficient of cyanidin-3-glucoside (26,900 L/mol cm);
- l = cuvette thickness (1 cm).
3.8. Phenolic Characterization by UPLC-MS/MS
3.9. Determination of Antioxidant Capacity
3.9.1. Ferric Reducing Antioxidant Power (FRAP) Analysis
3.9.2. 2,2-Diphenyl-1-picrylhydrazyl Radical (DPPH) Scavenging Analysis
3.9.3. 2,2-Azinobis(3-ethylbenzothiazoline-6-sulfonic Acid) (ABTS) Analysis
3.9.4. Oxygen Radical Absorbance Capacity (ORAC) Analysis
3.10. Experimental Design and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCP | Black chokeberry pomace |
| PLE | Pressurized liquid extraction |
| MAE | Microwave-assisted extraction |
| UAE | Ultrasound-assisted extraction |
| TP | Total polyphenols |
| TMA | Total monomeric anthocyanins |
| SSR | Solid–solvent ratio |
| PCA | Principal component analysis |
| PC1, PC2 | Principal components |
References
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Kaloudi, T.; Tsimogiannis, D.; Oreopoulou, V. Aronia melanocarpa: Identification and exploitation of its phenolic components. Molecules 2022, 27, 4375. [Google Scholar] [CrossRef]
- Elez Garofulić, I.; Repajić, M.; Zorić, Z.; Jurendić, T.; Dragović-Uzelac, V. Evaluation of microwave-and ultrasound-assisted extraction techniques for revalorization of black chokeberry (Aronia melanocarpa) fruit pomace anthocyanins. Sustainability 2023, 15, 7047. [Google Scholar] [CrossRef]
- Oszmiański, J.; Lachowicz, S. Effect of the production of dried fruits and juice from chokeberry (Aronia melanocarpa L.) on the content and antioxidative activity of bioactive compounds. Molecules 2016, 21, 1098. [Google Scholar] [CrossRef]
- Sójka, M.; Kołodziejczyk, K.; Milala, J. Polyphenolic and basic chemical composition of black chokeberry industrial by-products. Ind. Crops Prod. 2013, 51, 77–86. [Google Scholar] [CrossRef]
- Tena, N.; Asuero, A.G. Up-to-date analysis of the extraction methods for anthocyanins: Principles of the techniques, optimization, technical progress, and industrial application. Antioxidants 2022, 11, 286. [Google Scholar] [CrossRef] [PubMed]
- Elmas, E.; Şen, F.B.; Kublay, İ.Z.; Baş, Y.; Tüfekci, F.; Derman, H.; Bekdeşer, B.; Aşçı, Y.S.; Capanoglu, E.; Bener, M. Green Extraction of Antioxidants from Hazelnut By-products Using Microwave-Assisted Extraction, Ultrasound-Assisted Extraction, and Pressurized Liquid Extraction. Food Bioprocess Technol. 2025, 18, 5388–5406. [Google Scholar] [CrossRef]
- Pereira, D.T.V.; Tarone, A.G.; Cazarin, C.B.B.; Barbero, G.F.; Martinez, J. Pressurized liquid extraction of bioactive compounds from grape marc. J. Food Eng. 2019, 240, 105–113. [Google Scholar] [CrossRef]
- Machado, A.P.D.F.; Pasquel-Reátegui, J.L.; Barbero, G.F.; Martínez, J. Pressurized liquid extraction of bioactive compounds from blackberry (Rubus fruticosus L.) residues: A comparison with conventional methods. Food Res. Int. 2015, 77, 675–683. [Google Scholar] [CrossRef]
- Taha, M.; Dimitrov, K.; Samaillie, J.; Caux, B.; Sahpaz, S.; Blanchemain, N.; West, C.; Rivière, C. Optimizing the Extraction of Bioactive Compounds (Polyphenols, Lipids, and Alpha-Tocopherol) from Almond Okara to Unlock Its Potential as Functional Food. Foods 2024, 13, 2828. [Google Scholar] [CrossRef]
- Kraljić, K.; Škevin, D.; Čukelj Mustač, N.; Benković, M.; Drakula, S.; Balbino, S.; Mandura Jarić, A.; Mamilović, K.; Ramljak, I.; Ćurić, D. Influence of cryogenic grinding on the nutritional and antinutritional components of rapeseed cake. Appl. Sci. 2023, 13, 5841. [Google Scholar] [CrossRef]
- Cvitković, D.; Lisica, P.; Zorić, Z.; Pedisić, S.; Repajić, M.; Dragović-Uzelac, V.; Balbino, S. The influence of cryogrinding on essential oil, phenolic compounds and pigments extraction from myrtle (Myrtus communis L.) leaves. Processes 2022, 10, 2716. [Google Scholar] [CrossRef]
- Saxena, S.N.; Saxena, P.; Rathore, S.S.; Sharma, L.K.; Saxena, R.; Barnwal, P. Effect of cryogenic grinding on phenolic compounds and antioxidant properties of fenugreek (Trigonella foenum-graecum L.) seed extract. J. Spices Aromat. Crops 2016, 25, 73–78. [Google Scholar]
- Jung, J.-Y.; Rhee, J.-K. Roasting and cryogenic grinding enhance the antioxidant property of sword beans (Canavalia gladiata). J. Microbiol. Biotechnol. 2020, 30, 1706. [Google Scholar] [CrossRef]
- Woźniak, Ł.; Marszałek, K.; Skąpska, S.; Jędrzejczak, R. The application of supercritical carbon dioxide and ethanol for the extraction of phenolic compounds from chokeberry pomace. Appl. Sci. 2017, 7, 322. [Google Scholar] [CrossRef]
- Simić, V.M.; Rajković, K.M.; Stojičević, S.S.; Veličković, D.T.; Nikolić, N.Č.; Lazić, M.L.; Karabegović, I.T. Optimization of microwave-assisted extraction of total polyphenolic compounds from chokeberries by response surface methodology and artificial neural network. Sep. Purif. Technol. 2016, 160, 89–97. [Google Scholar] [CrossRef]
- D’Alessandro, L.G.; Dimitrov, K.; Vauchel, P.; Nikov, I. Kinetics of ultrasound assisted extraction of anthocyanins from Aronia melanocarpa (black chokeberry) wastes. Chem. Eng. Res. Des. 2014, 92, 1818–1826. [Google Scholar] [CrossRef]
- Roda-Serrat, M.C.; Andrade, T.A.; Rindom, J.; Lund, P.B.; Norddahl, B.; Errico, M. Optimization of the recovery of anthocyanins from chokeberry juice pomace by homogenization in acidified water. Waste Biomass Valorization 2021, 12, 1815–1827. [Google Scholar] [CrossRef] [PubMed]
- Dobroslavić, E.; Elez Garofulić, I.; Šeparović, J.; Zorić, Z.; Pedisić, S.; Dragović-Uzelac, V. Pressurized Liquid Extraction as a Novel Technique for the Isolation of Laurus nobilis L. Leaf Polyphenols. Molecules 2022, 27, 5099. [Google Scholar] [CrossRef]
- Ju, Z.Y.; Howard, L.R. Effects of solvent and temperature on pressurized liquid extraction of anthocyanins and total phenolics from dried red grape skin. J. Agric. Food Chem. 2003, 51, 5207–5213. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Yan, J.; Liu, R.; Marcone, M.F.; Aisa, H.A.; Tsao, R. Optimization of microwave-assisted extraction of phenolics from potato and its downstream waste using orthogonal array design. Food Chem. 2012, 133, 1292–1298. [Google Scholar] [CrossRef]
- Jokić, S.; Cvjetko, M.; Božić, Đ.; Fabek, S.; Toth, N.; Vorkapić-Furač, J.; Redovniković, I.R. Optimisation of microwave-assisted extraction of phenolic compounds from broccoli and its antioxidant activity. Int. J. Food Sci. Technol. 2012, 47, 2613–2619. [Google Scholar] [CrossRef]
- Zhao, C.-N.; Zhang, J.-J.; Li, Y.; Meng, X.; Li, H.-B. Microwave-assisted extraction of phenolic compounds from Melastoma sanguineum fruit: Optimization and identification. Molecules 2018, 23, 2498. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Chen, H.; Zhou, X.; Deng, Q.; Zhao, Y.; Zhao, C.; Gong, X. Optimization of the microwave-assisted extraction and antioxidant activities of anthocyanins from blackberry using a response surface methodology. RSC Adv. 2015, 5, 19686–19695. [Google Scholar] [CrossRef]
- Pap, N.; Beszédes, S.; Pongrácz, E.; Myllykoski, L.; Gábor, M.; Gyimes, E.; Hodúr, C.; Keiski, R.L. Microwave-assisted extraction of anthocyanins from black currant marc. Food Bioprocess Technol. 2013, 6, 2666–2674. [Google Scholar] [CrossRef]
- Borrás-Enríquez, A.J.; Reyes-Ventura, E.; Villanueva-Rodríguez, S.J.; Moreno-Vilet, L. Effect of ultrasound-assisted extraction parameters on total polyphenols and its antioxidant activity from mango residues (Mangifera indica L. var. Manililla). Separations 2021, 8, 94. [Google Scholar] [CrossRef]
- González, M.J.A.; Carrera, C.; Barbero, G.F.; Palma, M. A comparison study between ultrasound–assisted and enzyme–assisted extraction of anthocyanins from blackcurrant (Ribes nigrum L.). Food Chem. X 2022, 13, 100192. [Google Scholar] [CrossRef]
- He, B.; Zhang, L.-L.; Yue, X.-Y.; Liang, J.; Jiang, J.; Gao, X.-L.; Yue, P.-X. Optimization of ultrasound-assisted extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace. Food Chem. 2016, 204, 70–76. [Google Scholar] [CrossRef]
- Osorio-Tobón, J.F. Recent advances and comparisons of conventional and alternative extraction techniques of phenolic compounds. J. Food Sci. Technol. 2020, 57, 4299–4315. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef]
- Christodoulou, M.C.; Orellana Palacios, J.C.; Hesami, G.; Jafarzadeh, S.; Lorenzo, J.M.; Domínguez, R.; Moreno, A.; Hadidi, M. Spectrophotometric methods for measurement of antioxidant activity in food and pharmaceuticals. Antioxidants 2022, 11, 2213. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef]
- Chaovanalikit, A.; Wrolstad, R.E. Total anthocyanins and total phenolics of fresh and processed cherries and their antioxidant properties. J. Food Sci. 2004, 69, FCT67–FCT72. [Google Scholar] [CrossRef]
- Balbino, S.; Dorić, M.; Vidaković, S.; Kraljić, K.; Škevin, D.; Drakula, S.; Voučko, B.; Čukelj, N.; Obranović, M.; Ćurić, D. Application of cryogenic grinding pretreatment to enhance extractability of bioactive molecules from pumpkin seed cake. J. Food Process Eng. 2019, 42, e13300. [Google Scholar] [CrossRef]
- Wei, S.-D.; Zhou, H.-C.; Lin, Y.-M. Antioxidant activities of fractions of polymeric procyanidins from stem bark of Acacia confusa. Int. J. Mol. Sci. 2011, 12, 1146–1160. [Google Scholar] [CrossRef]
- Schofield, P.; Mbugua, D.M.; Pell, A.N. Analysis of condensed tannins: A review. Anim. Feed Sci. Technol. 2001, 91, 21–40. [Google Scholar] [CrossRef]
- Rosa, N.N.; Barron, C.; Gaiani, C.; Dufour, C.; Micard, V. Ultra-fine grinding increases the antioxidant capacity of wheat bran. J. Cereal Sci. 2013, 57, 84–90. [Google Scholar] [CrossRef]
- Sapna, I.; Kamaljit, M.; Priya, R.; Jayadeep, P.A. Milling and thermal treatment induced changes on phenolic components and antioxidant activities of pigmented rice flours. J. Food Sci. Technol. 2019, 56, 273–280. [Google Scholar] [CrossRef]
- Lin, S.; Meng, X.; Tan, C.; Tong, Y.; Wan, M.; Wang, M.; Zhao, Y.; Deng, H.; Kong, Y.; Ma, Y. Composition and antioxidant activity of anthocyanins from Aronia melanocarpa extracted using an ultrasonic-microwave-assisted natural deep eutectic solvent extraction method. Ultrason. Sonochem. 2022, 89, 106102. [Google Scholar] [CrossRef] [PubMed]
- Slimestad, R.; Torskangerpoll, K.; Nateland, H.S.; Johannessen, T.; Giske, N.H. Flavonoids from black chokeberries, Aronia melanocarpa. J. Food Compos. Anal. 2005, 18, 61–68. [Google Scholar] [CrossRef]
- Vagiri, M.; Jensen, M. Influence of juice processing factors on quality of black chokeberry pomace as a future resource for colour extraction. Food Chem. 2017, 217, 409–417. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res. Mol. Mech. Mutagen. 2005, 579, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Elez Garofulić, I.; Repajić, M.; Cegledi, E.; Dobroslavić, E.; Dobrinčić, A.; Zorić, Z.; Pedisić, S.; Franković, T.; Breški, M.; Dragović-Uzelac, V. Green Approach to Enhance the Recovery of Polyphenols from Blackcurrant and Bilberry Leaves: Evaluation of Microwave-Assisted and Pressurized Liquid Extraction. Molecules 2024, 29, 1351. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Barnes, K.W.; Eisele, T.; Giusti, M.M.; Haché, J.; Hofsommer, H.; Koswig, S.; Krueger, D.A.; et al. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Elez Garofulić, I.; Zorić, Z.; Pedisić, S.; Brnčić, M.; Dragović-Uzelac, V. UPLC-MS2 Profiling of Blackthorn Flower Polyphenols Isolated by Ultrasound-Assisted Extraction. J. Food Sci. 2018, 83, 2782–2789. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Braca, A.; De Tommasi, N.; Di Bari, L.; Pizza, C.; Politi, M.; Morelli, I. Antioxidant principles from Bauhinia tarapotensis. J. Nat. Prod. 2001, 64, 892–895. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.A. Cinnamates and hydroxybenzoates in the diet: Antioxidant activity assessed using the ABTS•+ radical cation. Br. Food J. 1997, 99, 57–62. [Google Scholar] [CrossRef]
- Elez Garofulić, I.; Kruk, V.; Martić, A.; Martić, I.; Zorić, Z.; Pedisić, S.; Dragović, S.; Dragović-Uzelac, V. Evaluation of polyphenolic profile and antioxidant activity of Pistacia lentiscus L. leaves and fruit extract obtained by optimized microwave-assisted extraction. Foods 2020, 9, 1556. [Google Scholar] [CrossRef]
| Extraction Technique | Temperature (°C) | Amplitude (%) | Time (min) | SSR (g/mL) | TP (mg GAE/g dm) | TMA (mg C3GE/g dm) |
|---|---|---|---|---|---|---|
| PLE | 100 | 5 | 1:40 | 86.0 ± 0.2 | 22.5 ± 0.1 | |
| 100 | 5 | 1:60 | 101.6 ± 1.6 | 24.1 ± 0.1 | ||
| 100 | 5 | 1:80 | 113.1 ± 4.6 | 23.9 ± 0.3 | ||
| 100 | 10 | 1:40 | 99.2 ± 4.6 | 22.4 ± 0.1 | ||
| 100 | 10 | 1:60 | 108.6 ± 5.6 | 21.5 ± 0.5 | ||
| 100 | 10 | 1:80 | 118.1 ± 6.7 | 20.6 ± 0.1 | ||
| 125 | 5 | 1:40 | 101.3 ± 8.7 | 19.3 ± 0.2 | ||
| 125 | 5 | 1:60 | 97.6 ± 3.0 | 18.2 ± 0.3 | ||
| 125 | 5 | 1:80 | 115.6 ± 0.8 | 18.7 ± 0.1 | ||
| 125 | 10 | 1:40 | 110.4 ± 4.4 | 15.0 ± 0.1 | ||
| 125 | 10 | 1:60 | 119.2 ± 3.4 | 15.6 ± 0.4 | ||
| 125 | 10 | 1:80 | 108.7 ± 1.0 | 16.2 ± 0.1 | ||
| 150 | 5 | 1:40 | 119.8 ± 1.3 | 13.7 ± 0.3 | ||
| 150 | 5 | 1:60 | 123.1 ± 5.0 | 14.1 ± 0.1 | ||
| 150 | 5 | 1:80 | 128.7 ± 0.2 | 13.4 ± 0.1 | ||
| 150 | 10 | 1:40 | 126.2 ± 4.2 | 12.0 ± 0.1 | ||
| 150 | 10 | 1:60 | 123.9 ± 3.9 | 12.3 ± 0.2 | ||
| 150 | 10 | 1:80 | 132.5 ± 7.4 | 8.2 ± 0.1 | ||
| MAE | 40 | 5 | 1:40 | 58.5 ± 1.5 | 20.7 ± 0.4 | |
| 40 | 5 | 1:60 | 69.3 ± 5.9 | 23.7 ± 0.2 | ||
| 40 | 5 | 1:80 | 75.5 ± 1.0 | 25.9 ± 0.7 | ||
| 40 | 10 | 1:40 | 63.5 ± 0.4 | 23.1 ± 1.3 | ||
| 40 | 10 | 1:60 | 72.7 ± 2.1 | 24.3 ± 0.8 | ||
| 40 | 10 | 1:80 | 79.8 ± 1.8 | 26.6 ± 0.1 | ||
| 60 | 5 | 1:40 | 65.4 ± 1.7 | 20.8± 0.1 | ||
| 60 | 5 | 1:60 | 72.8 ± 1.8 | 24.5 ± 0.5 | ||
| 60 | 5 | 1:80 | 81.3 ± 4.5 | 24.5 ± 0.1 | ||
| 60 | 10 | 1:40 | 70.6 ± 0.1 | 22.7 ± 0.2 | ||
| 60 | 10 | 1:60 | 75.5 ± 2.7 | 23.8 ± 0.5 | ||
| 60 | 10 | 1:80 | 90.1 ± 4.3 | 25.6 ± 0.2 | ||
| 80 | 5 | 1:40 | 68.9 ± 0.8 | 20.3 ± 0.2 | ||
| 80 | 5 | 1:60 | 84.7 ± 2.0 | 22.3 ± 0.5 | ||
| 80 | 5 | 1:80 | 82.4 ± 6.7 | 24.8 ± 0.1 | ||
| 80 | 10 | 1:40 | 76.1 ± 1.2 | 22.7 ± 0.2 | ||
| 80 | 10 | 1:60 | 95.7± 6.52 | 25.2 ± 0.4 | ||
| 80 | 10 | 1:80 | 94.8 ± 8.3 | 24.0 ± 0.5 | ||
| UAE | 50 | 5 | 1:40 | 65.2 ± 2.2 | 20.9 ± 0.7 | |
| 50 | 5 | 1:60 | 79.9 ± 2.7 | 23.2 ± 0.9 | ||
| 50 | 5 | 1:80 | 75.2 ± 1.5 | 22.4 ± 0.4 | ||
| 50 | 10 | 1:40 | 69.7 ± 1.0 | 20.8 ± 0.1 | ||
| 50 | 10 | 1:60 | 74.4 ± 4.3 | 20.4 ± 0.1 | ||
| 50 | 10 | 1:80 | 73.9 ± 1.8 | 20.5 ± 0.5 | ||
| 75 | 5 | 1:40 | 70.6 ±2.8 | 21.7 ± 0.2 | ||
| 75 | 5 | 1:60 | 75.3 ± 6.6 | 21.0 ± 0.1 | ||
| 75 | 5 | 1:80 | 74.3 ± 1.2 | 21.5 ± 0.3 | ||
| 75 | 10 | 1:40 | 66.7 ± 1.0 | 21.0 ± 0.1 | ||
| 75 | 10 | 1:60 | 71.7 ± 1.6 | 22.6 ± 0.3 | ||
| 75 | 10 | 1:80 | 84.1 ± 2.2 | 21. 9 ± 0.1 | ||
| 100 | 5 | 1:40 | 64.8 ± 2.2 | 20.2 ± 0.1 | ||
| 100 | 5 | 1:60 | 75.7 ± 3.6 | 22.5 ± 0.4 | ||
| 100 | 5 | 1:80 | 74.6 ± 5.7 | 21.3 ± 0.1 | ||
| 100 | 10 | 1:40 | 63.1 ± 0.4 | 19.9 ± 1.3 | ||
| 100 | 10 | 1:60 | 69.8 ± 5.7 | 18.7 ± 0.2 | ||
| 100 | 10 | 1:80 | 88.9 ± 2.0 | 22.2 ± 0.1 |
| Source of Variation | TP (mg GAE/g dm) | TMA (mg C3GE/g dm) |
|---|---|---|
| PLE | ||
| Temperature (°C) | p < 0.001 * | p < 0.001 * |
| 100 | 104.4 ± 3.3 a | 22.5 ± 0.4 c |
| 125 | 108.8 ± 2.5 a | 17.3 ± 0.5 b |
| 150 | 125.7 ± 1.5 b | 12.3 ± 0.6 a |
| Time (min) | p = 0.155 | p = 0.070 |
| 5 | 109.6 ± 3.2 a | 18.7 ± 1.0 a |
| 10 | 116.3 ± 2.5 a | 16.0 ± 1.1 a |
| SSR (g/mL) | p = 0.053 | p = 0.953 |
| 1:40 | 107.2 ± 4.2 a | 17.5 ± 1.3 a |
| 1:60 | 112.3 ± 3.2 a | 17.7 ± 1.3 a |
| 1:80 | 119.4 ± 2.7 a | 16.8 ± 1.5 a |
| MAE | ||
| Temperature (°C) | p = 0.003 * | p = 0.552 |
| 40 | 69.9 ± 2.2 a | 24.0 ± 0.6 a |
| 60 | 76.0 ± 2.5 ab | 23.6 ± 0.5 a |
| 80 | 83.8 ± 3.1 b | 23.2 ± 0.5 a |
| Time (min) | p = 0.050 * | p = 0.054 |
| 5 | 73.2 ± 2.1 a | 23.1 ± 0.5 a |
| 10 | 79.9 ± 2.7 b | 24.2 ± 0.3 a |
| SSR (g/mL) | p < 0.001 * | p < 0.001 * |
| 1:40 | 67.2 ± 1.7 a | 21.7 ± 0.4 a |
| 1:60 | 78.5 ± 2.9 b | 24.0 ± 0.3 b |
| 1:80 | 84.0 ± 2.2 b | 25.2 ± 0.3 c |
| UAE | ||
| Amplitude (%) | p = 0.944 | p = 0.228 |
| 50 | 73.1 ± 1.5 a | 21.4 ± 0.3 a |
| 75 | 73.8 ± 1.8 a | 21.6 ± 0.2 a |
| 100 | 72.8 ± 2.7 a | 20.8 ± 0.4 a |
| Time (min) | p = 0.749 | p = 0.050 * |
| 5 | 72.9 ± 1.3 a | 21.6 ± 0.2 b |
| 10 | 73.6 ± 1.9 a | 20.9 ± 0.3 a |
| SSR (g/mL) | p < 0.001 * | p = 0.161 |
| 1:40 | 66.7 ± 0.9 a | 20.7 ± 0.2 a |
| 1:60 | 74.5 ± 1.4 b | 21.4 ± 0.5 a |
| 1:80 | 78.5 ± 1.9 b | 21.6 ± 0.2 a |
| Extraction Technique | TP (mg GAE/g dm) | TMA (mg C3GE/g dm) | FRAP (µmol TE/g dm) | DPPH (µmol TE/g dm) | ABTS (µmol TE/g dm) | ORAC (µmol TE/g dm) |
|---|---|---|---|---|---|---|
| p = 0.012 * | p = 0.007 * | p = 0.002 * | p = 0.001 * | p < 0.001 * | p < 0.001 * | |
| PLE | 101.3 ± 8.7 ab | 19.3 ± 0.2 a | 1052.1 ± 48.9 b | 297.1 ± 2.5 a | 519.2 ± 9.4 a | 92.2 ± 2.2 a |
| MAE | 94.8 ± 8.3 a | 24.0 ± 0.5 b | 983.7 ± 60.5 b | 420.4 ± 8.0 b | 681.3 ± 24.8 b | 199.6 ± 1.6 c |
| UAE | 79.9 ± 2.7 a | 23.2 ± 0.9 b | 637.4 ± 28.1 a | 315.7 ± 3.2 a | 461.3 ± 15.0 a | 142.7 ± 1.9 b |
| REFLUX | 120.8 ± 0.8 b | 22.9 ± 0.8 b | 1033.4 ± 16.7 b | 472.9 ± 25.3 b | 790.2 ± 14.4 c | 222.6 ± 2.9 d |
| Cryogrinding Time (min) | d(0.1) (µm) | d(0.5) (µm) | d(0.9) (µm) | Span |
|---|---|---|---|---|
| p < 0.001 * | p < 0.001 * | p < 0.001 * | p < 0.001 * | |
| 0 | 37.0 ± 2.8 b | 238.1 ± 10.9 d | 712.9 ± 43.4 c | 2.8 ± 0.0 a |
| 1 | 5.0 ± 0.2 a | 75.3 ± 2.3 c | 455.6 ± 17.3 b | 6.0 ± 0.1 b |
| 3 | 3.7 ± 0.1 a | 53.1 ± 4.2 b | 419.7 ± 70.1 b | 7.8 ± 0.7 bc |
| 5 | 2.6 ± 0.1 a | 24.9 ± 1.1 a | 245.1 ± 29.2 a | 9.7 ± 0.7 c |
| 7 | 2.3 ± 0.1 a | 19.4 ± 0.7 a | 186.7 ± 19.8 a | 9.5 ± 0.7 c |
| 9 | 2.1 ± 0.1 a | 15.4 ± 0.8 a | 135.6 ± 13.5 a | 8.7 ± 0.5 c |
| Cryogrinding Time (min) | TP (mg GAE/g dm) | TMA (mg C3GE/g dm) | FRAP (µmol TE/g dm) | DPPH (µmol TE/g dm) | ABTS (µmol TE/g dm) | ORAC (µmol TE/g dm) |
|---|---|---|---|---|---|---|
| p = 0.001 * | p = 0.980 | p = 0.003 * | p = 0.046 * | p = 0.004 * | p = 0.006 * | |
| 1 | 115.1 ± 0.2 b | 22.0 ± 0.7 a | 816.5 ± 6.7 ab | 380.3 ± 3.3 a | 631.5 ± 11.7 a | 204.1 ± 4.1 b |
| 3 | 103.4 ± 0.7 a | 22.3 ± 0.9 a | 822.8 ± 2.2 ab | 395.3 ± 4.7 ab | 634.6 ± 7.3 a | 192.6 ± 3.4 b |
| 5 | 100.7 ± 2.5 a | 22.2 ± 0.9 a | 945.2 ± 13.5 c | 403.9 ± 4.4 ab | 651.3 ± 16.0 ab | 190.9 ± 1.1 ab |
| 7 | 99.1 ± 0.2 a | 22.4 ± 0.7 a | 786.6 ± 22.4 a | 401.3 ± 13.1 ab | 688.4 ± 13.1 bc | 189.6 ± 4.4 ab |
| 9 | 104.2 ± 2.5 a | 22.1 ± 0.4 a | 881.5 ± 35.9 bc | 411.7 ± 5.5 b | 718.1 ± 14.6 c | 177.1 ± 4.0 a |
| Polyphenol | Precursor Ion (m/z) | Product Ion (m/z) | mg/g dm |
|---|---|---|---|
| Anthocyanins | |||
| Cyanidin-3-O-galactoside | 449 | 287 | 20.67 ± 0.03 |
| Cyanidin-3-O-glucoside | 449 | 287 | 20.81 ± 0.01 |
| Cyanidin-3-O-arabinoside | 419 | 287 | 11.37 ± 0.06 |
| Cyanidin-3-O-xyloside | 419 | 287 | 11.31 ± 0.04 |
| Total | 64.16 | ||
| Phenolic acids | |||
| Isochlorogenic acid A | 515 | 353 | 0.70 ± 0.01 |
| Chlorogenic acid | 353 | 191 | 0.80 ± 0.04 |
| Neochlorogenic acid | 353 | 191 | 0.91 ± 0.02 |
| Total | 2.41 | ||
| Flavonols | |||
| Quercetin-3-O-dihexoside | 627 | 303 | 0.15 ± 0.01 |
| Isorhamnetin-3-rutinoside | 625 | 317 | 0.06 ± 0.01 |
| Quercetin-3-O-rutinoside | 611 | 303 | 0.91 ± 0.02 |
| Isorhamnetin-pentosylhexoside | 611 | 317 | 0.02 ± 0.04 |
| Quercetin-3-O-vicianoside | 597 | 434, 303 | 0.16 ± 0.01 |
| Kaempferol-3-rutinoside | 595 | 287 | 0.03 ± 0.05 |
| Isorhamnetin-3-O-glucoside | 479 | 317 | 0.02 ± 0.01 |
| Quercetin-3-O-glucuronide | 479 | 303 | 0.03 ± 0.02 |
| Isorhamnetin-3-O-galactoside | 479 | 317 | 0.02 ± 0.02 |
| Quercetin-3-O-galactoside | 465 | 303 | 1.54 ± 0.04 |
| Quercetin-3-glucoside | 465 | 303 | 1.61 ± 0.03 |
| Kaempferol-3-glucoside | 449 | 287 | 24.53 ± 0.03 |
| Total | 29.08 | ||
| Flavan-3-ols and Procyanidins | |||
| Epicatechin | 291 | 139, 123 | 0.22 ± 0.05 |
| Procyanidin B2 | 577 | 289 | 0.04 ± 0.04 |
| Total | 0.26 | ||
| Total UPLC-MS/MS polyphenols | 95.91 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Repajić, M.; Zorić, M.; Magnabosca, I.; Pedisić, S.; Dragović-Uzelac, V.; Elez Garofulić, I. Bioactive Power of Black Chokeberry Pomace as Affected by Advanced Extraction Techniques and Cryogrinding. Molecules 2025, 30, 3383. https://doi.org/10.3390/molecules30163383
Repajić M, Zorić M, Magnabosca I, Pedisić S, Dragović-Uzelac V, Elez Garofulić I. Bioactive Power of Black Chokeberry Pomace as Affected by Advanced Extraction Techniques and Cryogrinding. Molecules. 2025; 30(16):3383. https://doi.org/10.3390/molecules30163383
Chicago/Turabian StyleRepajić, Maja, Marija Zorić, Ivan Magnabosca, Sandra Pedisić, Verica Dragović-Uzelac, and Ivona Elez Garofulić. 2025. "Bioactive Power of Black Chokeberry Pomace as Affected by Advanced Extraction Techniques and Cryogrinding" Molecules 30, no. 16: 3383. https://doi.org/10.3390/molecules30163383
APA StyleRepajić, M., Zorić, M., Magnabosca, I., Pedisić, S., Dragović-Uzelac, V., & Elez Garofulić, I. (2025). Bioactive Power of Black Chokeberry Pomace as Affected by Advanced Extraction Techniques and Cryogrinding. Molecules, 30(16), 3383. https://doi.org/10.3390/molecules30163383









