Antioxidant Activity and Phytochemical Profiling of Steam-Distilled Oil of Flaxseed (Linum usitatissimum): Therapeutic Targeting Against Glaucoma, Oxidative Stress, Cholinergic Imbalance, and Diabetes
Abstract
1. Introduction
2. Results
2.1. Polyphenol Profile of Volatile Constituents of L. usitatissimum
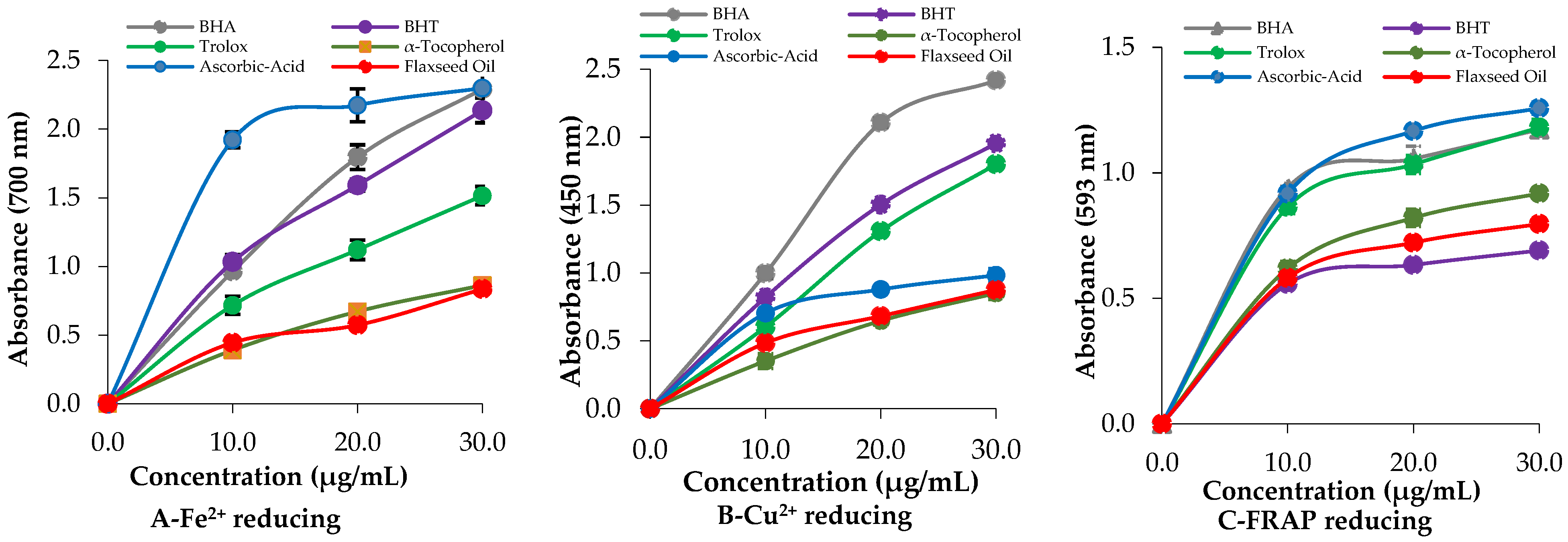
2.2. Determination of Reducing Power in Steam-Distilled Oil of L. usitatissimum
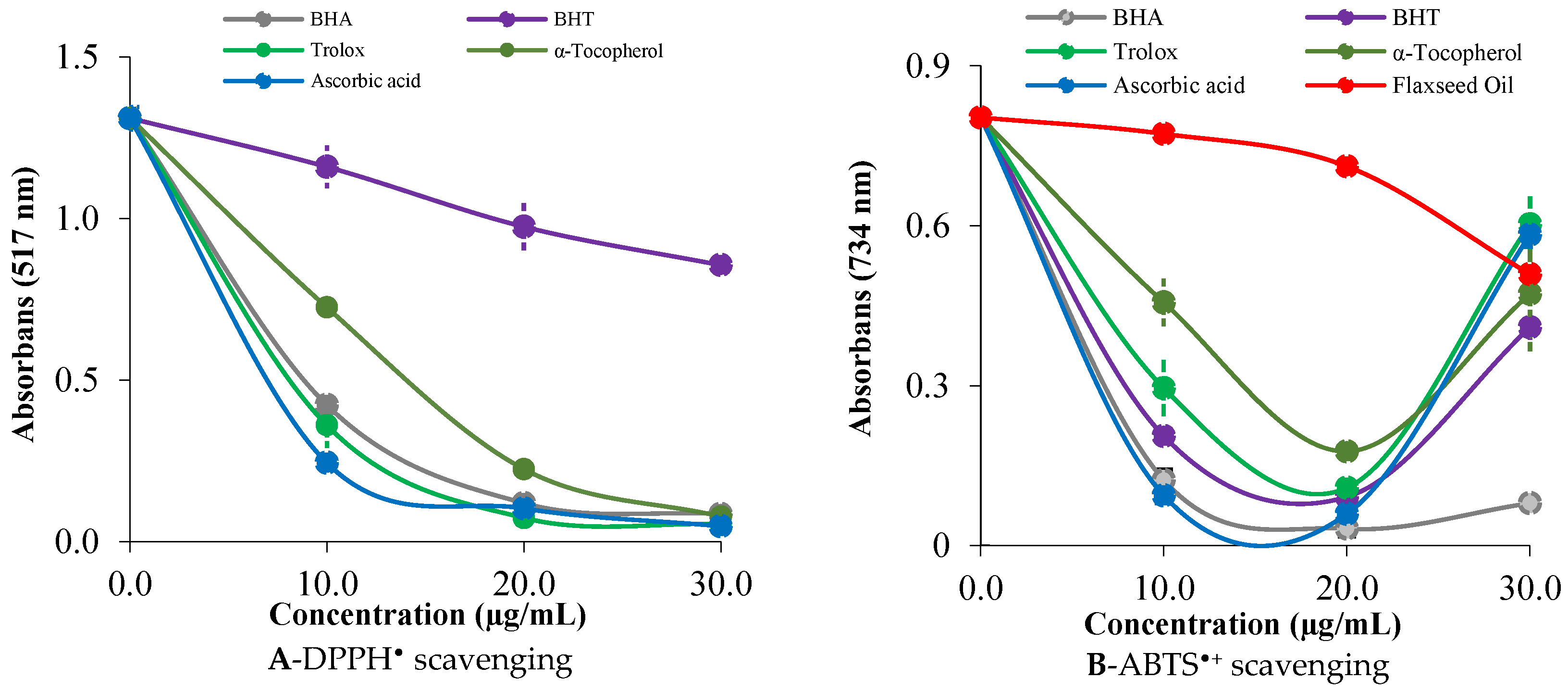
2.3. Radical Scavenging Activity of the Steam-Distilled Oil of L. usitatissimum
2.4. Evaluation of Enzyme Inhibition Effects of Steam-Distilled Oil of L. usitatissimum
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Extraction of Steam-Distilled Oil of L. usitatissimum
4.3. Profiling of Polyphenols in Steam-Distilled Oil of L. usitatissimum by LC-HRMS
4.4. Analysis of Steam-Distilled Oil of L. usitatissimum by GC/MS and GC-FID
4.5. Reducing Capacity of Steam-Distilled Oil of L. usitatissimum
4.6. Free Radical Scavenging Capacity of Steam-Distilled Oil of L. usitatissimum
4.7. Acetylcholinesterase Inhibitory Effects of Steam-Distilled Oil of L. usitatissimum
4.8. α-Amylase Inhibition Potential of Steam-Distilled Oil of L. usitatissimum
4.9. Carbonic Anhydrase II (hCA II) Inhibition Effects of Steam-Distilled Oil of L. usitatissimum
4.10. IC50 Value Determination
4.11. Data Analysis and Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tel, A.Z.; Aslan, K.; Yılmaz, M.A.; Gulcin, İ. A multidimensional study for design of phytochemical profiling, antioxidant potential, and enzyme inhibition effects of ışgın (Rheum telianum) as an edible plant. Food Chem. X 2025, 25, 102125. [Google Scholar] [CrossRef]
- Li, X.; Kong, W.; Shi, W.; Shen, Q. A combination of chemometrics methods and GC–MS for the classification of edible vegetable oils. Chemom. Intell. Lab. Syst. 2016, 155, 145–150. [Google Scholar] [CrossRef]
- Tian, M.; Bai, Y.; Tian, H.; Zhao, X. The chemical composition and health-promoting benefits of vegetable oils—A review. Molecules 2023, 28, 6393. [Google Scholar] [CrossRef] [PubMed]
- Gogus, U.; Smith, C. N-3 omega fatty acids: A review of current knowledge. Int. J. Food Sci. Technol. 2010, 45, 417–436. [Google Scholar] [CrossRef]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Gill, S.; Sihag, M. Flax and flaxseed oil: An ancient medicine & modern functional food. J. Food Sci. Technol. 2014, 51, 1633–1653. [Google Scholar] [CrossRef]
- Deshpande, R.; Raina, P.; Shinde, K.; Mansara, P.; Karandikar, M.; Kaul-Ghanekar, R. Flax seed oil reduced tumor growth, modulated immune responses and decreased HPV E6 and E7 oncoprotein expression in a murine model of ectopic cervical cancer. Prostaglandins Other Lipid Mediat. 2019, 143, 106332. [Google Scholar] [CrossRef]
- Bayr, H. Reactive oxygen species. Crit. Care Med. 2005, 33, S498. [Google Scholar] [CrossRef]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Dickinson, B.C.; Chang, C.J. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol. 2011, 7, 504–511. [Google Scholar] [CrossRef]
- Sindhi, V.; Gupta, V.; Sharma, K.; Bhatnagar, S.; Kumari, R.; Dhaka, N. Potential applications of antioxidants—A review. J. Pharm. Res. 2013, 7, 828–835. [Google Scholar] [CrossRef]
- Çakmakçı, S.; Topdaş, E.F.; Kalın, P.; Han, H.; Şekerci, P.; Polat Kose, L.; Gulcin, İ. Antioxidant capacity and functionality of oleaster (Elaeagnus angustifolia L.) flour and crust in a new kind of fruity ice cream. Int. J. Food Sci. Technol. 2015, 50, 472–481. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidant and antiradical activities of L-carnitine. Life Sci. 2006, 78, 803–811. [Google Scholar] [CrossRef]
- Cherian, C.; Vennila, J.; Sharan, L. Marine bromophenols as an effective inhibitor of virulent proteins (peptidyl arginine deiminase, gingipain R and hemagglutinin A) in Porphyromas gingivalis. Arch. Oral Biol. 2019, 100, 119–128. [Google Scholar] [CrossRef]
- Demirtaş, İ.; Atalar, M.N.; Bingöl, Z.; Köktürk, M.; Ozhan, G.; Abdelsalam, E.H.; Arslan, Ş.; Gulcin, İ. Evaluation of in vivo and in vitro toxicity of chestnut (Castanea mollissima blume) plant: Developmental toxicity in zebrafish embryos cytotoxicity, antioxidant activity and phytochemical composition by LC-ESI-MS/MS. Food Sci. Nutr. 2025, 13, e70387. [Google Scholar] [CrossRef]
- Cetinkaya, Y.; Gocer, H.; Menzek, A.; Gulcin, İ. Synthesis and antioxidant properties of (3,4-dihydroxyphenyl)(2,3,4-trihydroxyphenyl)methanone and its derivatives. Arch. Pharm. 2012, 345, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chetelat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- D’Cruz, M.M.; Banerjee, D. The person is not the disease-Revisiting Alzheimer’s dementia after 120 years. J. Geriat. Ment. Health 2021, 8, 136. [Google Scholar] [CrossRef]
- Qiu, C.; Kivipelto, M.; von Strauss, E. Epidemiology of Alzheimer’s disease: Occurrence, determinants, and strategies toward intervention. Dialogues Clin. Neurosci. 2009, 11, 111–128. [Google Scholar] [CrossRef]
- Dumurgier, J.; Tzourio, C. Epidemiology of neurological diseases in older adults. Rev. Neurol. 2020, 176, 642–648. [Google Scholar] [CrossRef]
- Twarowski, B.; Herbet, M. Inflammatory processes in Alzheimer’s disease—Pathomechanism, diagnosis and treatment: A review. Int. J. Mol. Sci. 2023, 24, 6518. [Google Scholar] [CrossRef]
- Guay, C.; Regazzi, R. Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat. Rev. Endocrinol. 2013, 9, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Leon, L.E.; Rani, S.; Fernandez, M.; Larico, M.; Calligaris, S.D. Subclinical detection of diabetic cardiomyopathy with MicroRNAs: Challenges and perspectives. J. Diab. Res. 2016, 2016, 6143129. [Google Scholar] [CrossRef] [PubMed]
- Kaštelan, M.S.; Tomić, A.; Antunica, J.G.; Rabatić, S.; Ljubić, S. Inflammation and pharmacological treatment in diabetic retinopathy. Mediat. Inflamm. 2013, 2013, 213130. [Google Scholar] [CrossRef] [PubMed]
- Aghaei Zarch, S.M.; Dehghan Tezerjani, M.; Talebi, M.; Vahidi Mehrjardi, M.Y. Molecular biomarkers in diabetes mellitus (DM). Med. J. Islam. Repub. Iran. 2020, 34, 28. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. 7IDF diabetes atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Khaw, P.T. Primary open-angle glaucoma. Lancet 2004, 363, 1711–1720. [Google Scholar] [CrossRef]
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef]
- Hennis, A.; Wu, S.Y.; Nemesure, B.; Honkanen, R.; Leske, M.C. Barbados eye studies group, awareness of incident open-angle glaucoma in a population study: The Barbados eye studies. Ophthalmology 2007, 114, 1816–1821. [Google Scholar] [CrossRef]
- Day, A.C.; Baio, G.; Gazzard, G.; Bunce, C.; Azuara-Blanco, A.; Munoz, B.; Friedman, D.S.; Foster, P.J. The prevalence of primary angle closure glaucoma in European derived populations: A systematic review. Br. J. Ophthalmol. 2012, 96, 1162–1167. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef]
- Jayaram, H.; Kolko, M.; Friedman, D.S.; Gazzard, G. Glaucoma: Now and beyond. Lancet 2023, 402, 1788–1801. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ.; Gören, A.C.; Taslimi, P.; Alwasel, S.H.; Kilic, O.; Bursal, E. Anticholinergic, antidiabetic and antioxidant activities of Anatolian pennyroyal (Mentha pulegium)—Analysis of its polyphenol contents by LC-MS/MS. Biocat. Agric. Biotechnol. 2020, 23, 101441. [Google Scholar] [CrossRef]
- Coban, T.A.; Beydemir, S.; Gulcin, I.; Ekinci, D. The effect of ethanol on erythrocyte carbonic anhydrase isoenzymes activity: An in vitro and in vivo study. J. Enzym. Inhib. Med. Chem. 2008, 23, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Gören, A.C.; Kiraz Kinoğlu, B.; Gulcin, İ. Quantification of secondary metabolites of Satureja pilosa (Lamiaceae) by LC-HRMS and evaluation of antioxidant and cholinergic activities. Rec. Nat. Prod. 2024, 6, 674–686. [Google Scholar] [CrossRef]
- Sun, X.L.; Wan, Y.L.; Liu, W.Y.; Wei, C.Q. Effects of different extraction methods on volatile profiles of flaxseed oils. J. Food Sci. 2023, 88, 4988–5001. [Google Scholar] [CrossRef]
- Yuan, B.; Wei, X.; Yang, Y.; Zhou, Q. Lipidomics reveals the formation of key aroma compounds in flaxseed oil during seed roasting. Food Chem. 2025, 490, 145089. [Google Scholar] [CrossRef]
- Yang, J.; Huang, Q.; Qi, H.; Feng-hong, H.; Deng, Q. Comparative research on volatile compounds in cold & hot pressed flaxseed oils. Chin. J. Oil Crop. Sci. 2013, 35, 321–325. [Google Scholar]
- Han, Y.; Wang, X.; Li, Y.; Wang, S.; Chen, Y.; Wang, J. Analysis and identification of volatile profiles in Qinghai flaxseed oil by SPME-GC-MS. Sci. Technol. Food Ind. 2021, 42, 255–260. [Google Scholar]
- Ma, X.L.; Wang, X.C.; Zhang, J.N.; Liu, J.N.; Ma, M.H.; Ma, F.L.; She, Y.B. A study of flavor variations during the flaxseed roasting procedure by developed real-time SPME GC-MS coupled with chemometrics. Food Chem. 2023, 410, 135453. [Google Scholar] [CrossRef]
- Yuan, B.; Jia, Y.; Yang, Y.; Chen, Y.; Zhou, Q. Analysis of the aroma characteristics of different varieties of fragrant linseed oil. J. Food Saf. Qual. 2023, 14, 90–100. [Google Scholar]
- Tietel, Z.; Hammann, S.; Meckelmann, S.W.; Ziv, C.; Pauling, J.K.; Wolk, M.; Domingues, M.R. An overview of food lipids toward food lipidomics. Comp. Rev. Food Sci. Food Saf. 2023, 22, 4302–4354. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.C.; Zhang, K.; Xu, C.W.; Lai, C.R.; Liu, Y.; Cao, Y.; Zhao, L.C. Contribution of lipid to the formation of characteristic volatile flavor of peanut oil. Food Chem. 2024, 442, 138496. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, X.N.; Li, T.; Wang, X.; Zhong, C.; Wang, X.P.; Li, P.W. Identification of glycerophospholipids using self-built recognition software based on positive and negative ion high-resolution mass spectrometric fragmentation experiments. Talanta 2022, 238, 123006. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Hossain, A. Role of lipids in food flavor generation. Molecules 2022, 27, 5014. [Google Scholar] [CrossRef]
- Liao, M.; Ren, H.; Jin, R.; Kang, J.; Shang, J.; Ning, X.; Yao, S.; Liu, N. Screen lipidmolecular markers of flaxseed oils by UHPLC-QTOF-MS technology. Trans. Chin. Soc. Agric. Eng. 2021, 37, 338–346. [Google Scholar] [CrossRef]
- Shi, C.P.; Guo, H.; Wu, T.T.; Tao, N.P.; Wang, X.C.; Zhong, J. Effect of three types of thermal processing methods on the lipidomics profile of tilapia fillets by UPLC-Q-extractive orbitrap mass spectrometry. Food Chem. 2019, 298, 125029. [Google Scholar] [CrossRef]
- Çelik, Ş.; Dervişoğlu, G.; İzol, E.; Seczyk, L.; Özdemir, F.A.; Yilmaz, M.E.; Yilmaz, M.A.; Gulcin, İ.; Al-Anazi, K.M.; Farah, M.A.; et al. Comprehensive phytochemical analysis of Salvia hispanica L. callus extracts using LC-MS/MS. Biomed. Chromatogr. 2024, 38, e5975. [Google Scholar] [CrossRef]
- Prasad, K.; Dhar, A. Flaxseed and diabetes. Curr. Pharm. Des. 2016, 22, 141–144. [Google Scholar] [CrossRef]
- Ahmed, S.H.; Meyer, H.E.; Kjøllesdal, M.K.; Marjerrison, N.; Mdala, I.; Htet, A.S.; Bjertness, E.; Madar, A.A. The prevalence of selected risk factors for non-communicable diseases in Hargeisa, Somaliland: A cross-sectional study. BMC Public Health 2019, 19, 878. [Google Scholar] [CrossRef]
- Jackson, S.E.; Llewellyn, C.H.; Smith, L. The obesity epidemic—Nature via nurture: A narrative review of high-income countries. SAGE Open Med. 2020, 8, 2050312120918265. [Google Scholar] [CrossRef]
- Zulet, M.A.; Puchau, B.; Navarro, C.; Martí, A.; Martínez, J.A. Inflammatory biomarkers: The link between obesity and associated pathologies. Nutr. Hosp. 2007, 22, 511–527. [Google Scholar]
- Hutchins, A.M.; Brown, B.D.; Cunnane, S.C.; Domitrovich, S.G.; Adams, E.R.; Bobowiec, C.E. Daily flaxseed consumption improves glycemic control in obese men and women with pre-diabetes: A randomized study. Nutr. Res. 2013, 33, 367–375. [Google Scholar] [CrossRef]
- Shareghfarid, E.; Nadjarzadeh, A.; Heidarzadeh-Esfahani, N.; Azamian, Y.; Hajiahmadi, S. The effect of flaxseed oil supplementation on body composition and inflammation indices in overweight adults with pre-diabetes. Nutr. Metab. Insights 2022, 15, 11786388221090083. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ.; Ozden, E.M.; Mutlu, M.; Mirzaee, Z.; Bingol, Z.; Köksal, E.; Alwasel, S.; Goren, A.C. Exploring of biological activity and diverse metabolites in hemp (Cannabis sativa) seed oil by GC/MS, GC-FID, and LC-HR/MS chromatographies. Futur. J. Pharm. Sci. 2024, 10, 130. [Google Scholar] [CrossRef]
- Haliga, R.; Mocanu, V.; Păduraru, I.; Stoica, B.; Oboroceanu, T.; Luca, V. Effects of dietary flaxseed supplementation on renal oxidative stress in experimental diabetes. Rev. Med. Chir. Soc. Med. Nat. Iasi 2009, 113, 1200–1204. [Google Scholar] [PubMed]
- Badawy, E.A.; Rasheed, W.I.; Elias, T.R.; Hussein, J.; Harvi, M.; Morsy, S.; Mahmoud Yel, L. Flaxseed oil reduces oxidative stress and enhances brain monoamines release in streptozotocin-induced diabetic rats. Hum. Exp. Toxicol. 2015, 34, 1133–1138. [Google Scholar] [CrossRef]
- Ulven, T.; Christiansen, E. Dietary fatty acids and their potential for controlling metabolic diseases through activation of FFA4/GPR120. Annu. Rev. Nutr. 2015, 35, 239–263. [Google Scholar] [CrossRef]
- Demir, Y.; Durmaz, L.; Taslimi, P.; Gulcin, İ. Anti-diabetic properties of dietary phenolic compounds: Inhibition effects on α-amylase, aldose reductase and α-glycosidase. Biotechnol. Appl. Biochem. 2019, 66, 781–786. [Google Scholar] [CrossRef]
- Zhu, L.; Sha, L.; Li, K.; Wang, Z.; Wang, T.; Li, Y.; Liu, P.; Dong, X.; Dong, Y.; Zhang, X.; et al. Dietary flaxseed oil rich in omega-3 suppresses severity of type 2 diabetes mellitus via anti-inflammation and modulating gut microbiota in rats. Lipids Health Dis. 2020, 19, 20. [Google Scholar] [CrossRef]
- Aslan, K.; Erden Koparır, E.; Kelle, K.; Karageçili, H.; Yılmaz, M.A.; Çakır, O.; Alwasel, S.; Gulcin, İ. Phytochemical profile and bioactive properties of sage (Salvia fruticosa) and thyme (Thymus vulgaris) extracts. Int. J. Food Proper. 2025, 28, 2481148. [Google Scholar] [CrossRef]
- İzol, E.; Yılmaz, M.A.; Gülçin, İ. Chemical characterization by chromatography techniques and comprehensive biological activities of Artvin bee products. ChemistrySelect 2025, 10, e202501545. [Google Scholar] [CrossRef]
- Hisar, O.; Beydemir, Ş.; Gulcin, İ.; Küfrevioğlu, Ö.İ.; Supuran, C.T. Effect of low molecular weight plasma inhibitors of rainbow trout (Oncorhyncytes mykiss) on human erythrocytes carbonic anhydrase-II isozyme activity in vitro and rat erythrocytes in vivo. J. Enzym. Inhib. Med. Chem. 2005, 20, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Duffy, A. Factors influencing the gut microbiota, inflammation, and type 2 diabetes. J. Nutr. 2017, 147, 1468S–1475S. [Google Scholar] [CrossRef]
- Festa, A.; D’Agostino, R.; Howard, G.; Mykkänen, L.; Tracy, R.P.; Haffner, S.M. Chronic subclinical inflammation as part of the insulin resistance syndrome: The insulin resistance atherosclerosis study (IRAS). Circulation 2000, 102, 42–47. [Google Scholar] [CrossRef]
- White, P.J.; Marette, A. Potential role of omega-3-derived resolution mediators in metabolic inflammation. Immunol. Cell Biol. 2014, 92, 324–330. [Google Scholar] [CrossRef]
- Morey, M.; O’Gaora, P.; Pandit, A.; Hélary, C. Hyperglycemia acts in synergy with hypoxia to maintain the pro-inflammatory phenotype of macrophages. PLoS ONE 2019, 14, e0220577. [Google Scholar] [CrossRef]
- Moura-Assis, A.; Afonso, M.S.; de Oliveira, V.; Morari, J.; Dos Santos, G.A.; Koike, M.; Lottenberg, A.M.; Catharino, R.; Velloso, L.A.; Sanchez Ramos da Silva, A.; et al. Flaxseed oil rich in omega-3 protects aorta against inflammation and endoplasmic reticulum stress partially mediated by GPR120 receptor in obese, diabetic and dyslipidemic mice models. J. Nutr. Biochem. 2018, 53, 9–19. [Google Scholar] [CrossRef]
- Merchant, A.T.; Curhan, G.C.; Rimm, E.B.; Willett, W.C.; Fawzi, W.W. Intake of n-6 and n-3 fatty acids and fish and risk of community-acquired pneumonia in US men. Am. J. Clin. Nutr. 2005, 82, 668–674. [Google Scholar] [CrossRef]
- Jamilian, M.; Tabassi, Z.; Reiner, Ž.; Panahandeh, I.; Naderi, F.; Aghadavod, E.; Amirani, E.; Taghizadeh, M.; Shafabakhsh, R.; Satari, M.; et al. The effects of n-3 fatty acids from flaxseed oil on genetic and metabolic profiles in patients with gestational diabetes mellitus: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2020, 123, 792–799. [Google Scholar] [CrossRef]
- Muhammad, A.; Chukwuma, C.I.; Erukainure, O.L.; Islam, M.S. Editorial: Therapeutic potential of natural products in oxidative and metabolic diseases. Front. Pharmacol. 2024, 15, 1375788. [Google Scholar] [CrossRef]
- Shukla, S.K.; Gupta, S.; Ojha, S.K.; Sharma, S.B. Cardiovascular friendly natural products: A promising approach in the management of CVD. Nat. Prod. Res. 2010, 24, 873–898. [Google Scholar] [CrossRef]
- Shrivastav, D.; Kumbhakar, S.K.; Srivastava, S.; Singh, D.D. Natural product-based treatment potential for type 2 diabetes mellitus and cardiovascular disease. World J. Diab. 2024, 15, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Deng, Q.; Tang, Y.; Xiao, L.; Liu, L.; Yao, P.; Tang, H.; Dong, X. Flaxseed oil attenuates hepatic steatosis and insulin resistance in mice by rescuing the adaption to ER stress. J. Agric. Food Chem. 2018, 66, 10729–10740. [Google Scholar] [CrossRef] [PubMed]
- Jangale, N.M.; Devarshi, P.P.; Bansode, S.B.; Kulkarni, M.J.; Harsulkar, A.M. Dietary flaxseed oil and fish oil ameliorates renal oxidative stress, protein glycation, and inflammation in streptozotocin–nicotinamide-induced diabetic rats. J. Physiol. Biochem. 2016, 72, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Ocaña-Sánchez, M.F.; Soto-Ojeda, G.A.; Cocotle-Ronzón, Y.; Soria-Fregozo, C.; Sánchez-Medina, A.; García-Rodríguez, R.V.; Rodríguez-Landa, J.F.; Corro-Méndez, E.J.; Hernández-Lozano, M. Flaxseed oil (Linum usitatissimum) prevents cognitive and motor damage in rats with hyperammonemia. Nutrients 2023, 15, 4550. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.K.; Awasthi, H.; Srivastava, D.; Fatima, Z.; Mohapatra, L. Flaxseed oil: Safeguarding neurological health through apoptosis and oxidative damage defense. Cells 2024, 13, 1184. [Google Scholar] [CrossRef]
- Al-Madhagy, S.; Ashmawy, N.S.; Mamdouh, A.; Eldahshan, O.A.; Farag, M.A. A comprehensive review of the health benefits of flaxseed oil in relation to its chemical composition and comparison with other omega-3-rich oils. Eur. J. Med. Res. 2023, 28, 240. [Google Scholar] [CrossRef]
- Mitrovic, N.; Dragić, M.; Zarić, M.; Nedeljković, N.; Grkovic, I. Flaxseed oil attenuates trimethyltin-induced neurodegeneration via down-regulation of inflammatory activity of astrocytes. Res. Sq. 2021, 13, 1184. [Google Scholar] [CrossRef]
- Nowak, W.; Jeziorek, M. The role of flaxseed in improving human health. Healthcare 2023, 11, 395. [Google Scholar] [CrossRef]
- Aktas, A.; Barut Celepci, D.; Gok, Y.; Taslimi, P.; Akincioglu, H.; Gulcin, İ. A novel Ag-N-heterocyclic carbene complex bearing the hydroxyethyl ligand: Synthesis, characterization, crystal and spectral structures and bioactivity properties. Crystals 2020, 10, 171. [Google Scholar] [CrossRef]
- Gulcin, İ.; Trofimov, B.; Kaya, R.; Taslimi, P.; Sobenina, L.; Schmidt, E.; Petrova, O.; Malysheva, S.; Gusarova, N.; Farzaliyev, V.; et al. Synthesis of nitrogen, phosphorus, selenium and sulfur-containing heterocyclic compounds–Determination of their carbonic anhydrase, acetylcholinesterase, butyrylcholinesterase and α-glycosidase inhibition properties. Bioorg. Chem. 2020, 103, 104171. [Google Scholar] [CrossRef] [PubMed]
- Akbaba, Y.; Akıncıoğlu, A.; Göçer, H.; Göksu, S.; Gülçin, I.; Supuran, C.T. Carbonic anhydrase inhibitory properties of novel sulfonamide derivatives of aminoindanes and aminotetralins. J. Enzym. Inhib. Med. Chem. 2014, 29, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Erdemir, F.; Barut Celepci, D.; Aktaş, A.; Gök, Y.; Kaya, R.; Taslimi, P.; Demir, Y.; Gulcin, İ. Novel 2-aminopyridine liganded Pd(II) N-heterocyclic carbene complexes: Synthesis, characterization, crystal structure and bioactivity properties. Bioorg. Chem. 2019, 91, 103134. [Google Scholar] [CrossRef]
- Lolak, N.; Akocak, S.; Turkes, C.; Taslimi, P.; Işık, M.; Beydemir, Ş.; Gulcin, İ.; Durgun, M. Synthesis, characterization, inhibition effects, and molecular docking studies as acetylcholinesterase, α-glycosidase, and carbonic anhydrase inhibitors of novel benzenesulfonamides incorporating 1,3,5-triazine structural motifs. Bioorg. Chem. 2020, 100, 103897. [Google Scholar] [CrossRef]
- Özbey, F.; Taslimi, P.; Gulcin, İ.; Maraş, A.; Göksu, S.; Supuran, C.T. Synthesis of diaryl ethers with acetylcholinesterase, butyrylcholinesterase and carbonic anhydrase inhibitory actions. J. Enzym. Inhib. Med. Chem. 2016, 31, 79–85. [Google Scholar] [CrossRef]
- Akbaş, F.; Ozaydin, A.; Polat, E.; Onaran, I. Lucilia sericata larval secretions stimulating wound healing effects on rat dermal fibroblast cells. Rec. Nat. Prod. 2020, 14, 340–354. [Google Scholar] [CrossRef]
- Patiño-Bayona, W.R.; Plazas, E.; Bustos-Cortes, J.J.; Prieto-Rodríguez, J.A.; Patino-Ladino, O.J. Essential oils of three hypericum species from Colombia: Chemical composition, insecticidal and repellent activity against Sitophilus zeamais motsch. (coleoptera: Curculionidae). Rec. Nat. Prod. 2021, 15, 111–121. [Google Scholar] [CrossRef]
- Süzgeç, S.; Özek, T.; Özek, G.; Yur, S.; Göger, F.; Gürdal, M.B.; Gülsoy Toplan, G.; Meriçli, A.H.; Başer, K.H.C. The leaf and the gall volatiles of Salvia fruticosa Miller from Turkey: Chemical composition and biological activities. Rec. Nat. Prod. 2021, 15, 10–24. [Google Scholar] [CrossRef]
- Altun, M.; Goren, A.C. Essential oil composition of Satureja cuneifolia by simultaneous distillation-extraction and thermal desorption GC-MS techniques. J. Essent. Oil Bear. Plants 2007, 10, 139–144. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH radical scavenging assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Ozden, E.M.; Bingöl, Z.; Mutlu, M.; Karageçili, H.; Koksal, E.; Gören, A.C.; Alwasel, S.H.; Gülçin, İ. Antioxidant, antiglaucoma, anticholinergic, and antidiabetic effects of kiwifruit (Actinidia deliciosa) oil: Metabolite profile analysis using LC-HR/MS, GC/MS and GC-FID. Life 2023, 13, 1939. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I.; Büyükokuroglu, M.E.; Oktay, M.; Küfrevioglu, O.I. Antioxidant and analgesic activities of turpentine of Pinus nigra Arn. subsp. pallsiana (Lamb.) Holmboe. J. Ethnopharmacol. 2003, 86, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Bayrak, Ç.; Taslimi, P.; Gulcin, İ.; Menzek, A. The first synthesis of 4-phenylbutenone derivative bromophenols including natural products and their inhibition profiles for carbonic anhydrase, acetylcholinesterase and butyrylcholinesterase enzymes. Bioorg. Chem. 2017, 72, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Güney, M.; Coşkun, A.; Topal, F.; Daştan, A.; Gulcin, İ.; Supuran, C.T. Oxidation of cyanobenzocycloheptatrienes: Synthesis, photooxygenation reaction and carbonic anhydrase isoenzymes inhibition properties of some new benzotropone derivatives. J. Enzym. Inhib. Med. Chem. 2014, 22, 3537–3543. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidant activity of L-adrenaline: A structure-activity insight. Chem. Biol. Interact. 2009, 179, 71–80. [Google Scholar] [CrossRef]
- Xiao, Z.; Storms, R.; Tsang, A. A quantitative starch-iodine method for measuring alpha-amylase and glucoamylase activities. Anal. Biochem. 2006, 351, 146–148. [Google Scholar] [CrossRef]
- Taslimi, P.; Gulcin, İ. Antidiabetic potential: In vitro inhibition effects of some natural phenolic compounds on α-glycosidase and α-amylase enzymes. J. Biochem. Mol. Toxicol. 2017, 31, e21956. [Google Scholar] [CrossRef]
- Mutlu, M.; Bingöl, Z.; Ozden, E.M.; Koksal, E.; Erturk, A.; Gören, A.C.; Alwasel, S.H.; Gülçin, İ. Antioxidant, and enzyme inhibition effects of chia (Salvia hispánica) seed oil: A comprehensive phytochemical screening using LC-HR/MS. Elec. J. Biotechnol. 2025, 74, 41–53. [Google Scholar] [CrossRef]
- Duysak, L.; Ertürk, A.; Becit-Kızılkaya, M.; Gulcin, İ. Comprehensive UPLC-MS/MS-based metabolic profiling, antioxidant potential, enzyme ınhibition, and molecular docking studies of Ficus carica L. leaves: Potential for functional food applications. Food Biosci. 2025, 69, 106835. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Durmaz, L.; Gulcin, İ.; Taslimi, P.; Tüzün, B. Isofraxidin: Antioxidant, anti-carbonic anhydrase, anti-cholinesterase, anti-diabetic, and in silico properties. ChemistrySelect 2023, 8, e202300170. [Google Scholar] [CrossRef]
- Scozzafava, A.; Passaponti, M.; Supuran, C.T.; Gülçin, İ. Carbonic anhydrase inhibitors: Guaiacol and catechol derivatives effectively inhibit certain human carbonic anhydrase isoenzymes (hCA I, II, IX and XII). J. Enzym. Inhib. Med. Chem. 2015, 30, 586–591. [Google Scholar] [CrossRef]
- Taslimi, P.; Gulcin, İ.; Oztaskın, N.; Cetinkaya, Y.; Goksu, S.; Alwasel, S.H.; Supuran, C.T. The effects of some bromophenol derivatives on human carbonic anhydrase isoenzymes. J. Enzym. Inhib. Med. Chem. 2016, 31, 603–607. [Google Scholar] [CrossRef]
- Akıncıoglu, A.; Topal, M.; Gulcin, I.; Goksu, S. Novel sulphamides and sulphonamides incorporating the tetralin scaffold as carbonic anhydrase and acetylcholine esterase inhibitors. Arch. Pharm. 2014, 347, 68–76. [Google Scholar] [CrossRef]

| Compounds | Formula | RT | Area (%) | Identification |
|---|---|---|---|---|
| α-Pinene | C10H16 | 3.36 | 0.53 | RT, ST, MS |
| Cymene | C10H14 | 4.88 | t | RT, ST, MS |
| 1-Dodecanol | C12H26O | 7.92 | 0.25 | RT, MS |
| 1-Tetradecene | C14H28 | 12.13 | 0.54 | RT, MS |
| 2,4,-Di-t-buthylphenol | C14H22O | 14.63 | 1.24 | RT, MS |
| Hexaadecanol (cetal) | C16H34O | 16.15 | 0.83 | RT, MS |
| Nonadecene | C19H38 | 19.83 | 0.94 | RT, MS |
| Palmitic acid | C16H32O2 | 22.64 | 4.91 | RT, MS, ST |
| Linoleic acid | C18H32O2 | 25.36 | 13.21 | RT, MS, ST |
| Linolenic acid | C18H30O2 | 25.46 | 57.97 | RT, MS, ST |
| 9-Octadecenoic acid | C18H34O2 | 26.21 | t | RT, MS |
| Stearic acid | C18H36O2 | 26.23 | 0.44 | RT, MS, ST |
| Octacosanol | C28H58O | 29.04 | 0.27 | RT, MS |
| Campesterol | C28H48O | 39.19 | 2.49 | RT, MS |
| Stigmasterol | C29H48O | 39.59 | 0.4 | RT, MS |
| Sitosterol | C29H50O | 40.40 | 5.2 | RT, MS |
| Cycloartenol | C30H50O | 41.55 | 6.56 | RT, MS |
| TOTAL | 95.78 |
| Essential Oils | Fe3+ Reducing | Cu2+ Reducing | Fe3+-TPTZ Reducing | |||
|---|---|---|---|---|---|---|
| λ700 * | r2 | λ450 * | r2 | λ 593 * | r2 | |
| BHA | 2.292 ± 0.012 | 0.9993 | 2.418 ± 0.018 | 0.9887 | 1.172 ± 0.014 | 0.9605 |
| BHT | 2.136 ± 0.090 | 0.9957 | 1.953 ± 0.045 | 0.9998 | 0.690 ± 0.008 | 0.9645 |
| Trolox | 1.514 ± 0.066 | 0.9963 | 1.800 ± 0.096 | 0.9974 | 1.180 ± 0.032 | 0.9732 |
| α-Tocopherol | 0.862 ± 0.038 | 0.9996 | 0.851 ± 0.046 | 0.9994 | 0.918 ± 0.011 | 0.9904 |
| Ascorbic acid | 2.298 ± 0.086 | 0.9659 | 0.983 ± 0.048 | 0.9822 | 1.257 ± 0.024 | 0.9869 |
| SDOLU | 1.005 ± 0.043 | 0.9997 | 0.875 ± 0.028 | 0.9907 | 0.796 ± 0.010 | 0.9821 |
| Essential Oils | DPPH Scavenging | ABTS + Scavenging | ||
|---|---|---|---|---|
| IC50 (µg/mL) | r2 | IC50 (µg/mL) | r2 | |
| BHA | 6.86 | 0.9949 | 6.36 | 0.9746 |
| BHT | 49.50 | 0.9957 | 12.60 | 0.9995 |
| Trolox | 6.03 | 0.9925 | 16.50 | 0.9775 |
| α-Tocopherol | 7.70 | 0.9961 | 18.73 | 0.9347 |
| Ascorbic acid | 5.82 | 0.9668 | 11.75 | 0.9983 |
| SDOLU | 19.80 | 0.9998 | 57.75 | 0.9887 |
| Enzymes | SDOLU | Standards | ||
|---|---|---|---|---|
| IC50 | r2 | IC50 | r2 | |
| CA II * | 281.02 | 0.9148 | 9.96 | 0.9930 |
| AChE * | 13.23 | 0.9839 | 8.82 | 0.9836 |
| α-Amylase * | 531.44 | 0.9194 | 7.54 | 0.9074 |
| Phenolics | Molecular Formula | m/z | Ionization Mode | Linear Range | Linear Regression Equation | LOD/LOQ | R2 | Recovery | Phenolics | U% |
|---|---|---|---|---|---|---|---|---|---|---|
| Epigallocatechin | C15H14O7 | 307.0812 | Positive | 0.3–5 | y = 0.00317x + 0.000443 | 0.17/0.57 | 0.9947 | 102.22 | 1.94 | 3.09 |
| Chlorogenic acid | C16H18O9 | 353.0878 | Negative | 0.05–10 | y = 0.00817x + 0.000163 | 0.02/0.06 | 0.9994 | 96.68 | 0.42 | 3.58 |
| Fumaric acid | C4H4O4 | 115.0037 | Negative | 0.1–10 | y = 0.00061x − 0.0000329 | 0.05/0.17 | 0.9991 | 97.13 | - | 2.88 |
| Orientin | C21H20O11 | 447.0933 | Negative | 0.1–10 | y = 0.00757x + 0.000347 | 0.01/0.03 | 0.9993 | 96.22 | 0.26 | 3.67 |
| Caffeic acid | C9H8O4 | 179.0350 | Negative | 0.3–10 | y = 0.0304x + 0.00366 | 0.08/0.27 | 0.9993 | 94.51 | - | 3.74 |
| Luteolin 7-glycoside | C21H20O11 | 447.0933 | Negative | 0.1–7 | y = 0.0162x + 0.00226 | 0.01/0.03 | 0.9961 | 96.31 | 0.17 | 4.14 |
| Rutin | C27H30O16 | 609.1461 | Negative | 0.05–10 | y = 0.00329x − 0.00005576 | 0.01/0.03 | 0.999 | 96.97 | 0.19 | 3.07 |
| Hyperoside | C21H20O12 | 463.0882 | Negative | 0.05–10 | y = 0.0072x − 0.00003096 | 0.01/0.03 | 0.9995 | 96.62 | 0.28 | 3.46 |
| Apigenin 7-glycoside | C21H20O10 | 431.0984 | Negative | 0.3–7 | y = 0.0246x + 0.00306 | 0.01/0.03 | 0.9962 | 96.07 | 0.08 | 3.59 |
| Ellagic acid | C14H6O8 | 300.9990 | Negative | 0.05–10 | y = 0.0085x − 0.000612 | 0.03/1 | 0.9994 | 101.49 | - | 4.20 |
| Quercitrin | C21H20O11 | 447.0933 | Negative | 0.05–10 | y = 0.0179 + 0.0003331 | 0.01/0.03 | 0.999 | 97.00 | 0.12 | 3.78 |
| Quercetin | C15H10O7 | 301.0354 | Negative | 0.1–10 | y = 0.0509x + 0.00467 | 0.01/0.03 | 0.9978 | 96.41 | - | 2.95 |
| Herniarin | C10H8O3 | 177.0546 | Positive | 0.1–7 | y = 0.309x + 0.0266 | 0.01/0.03 | 0.9983 | 92.92 | - | 3.89 |
| Naringenin | C15H12O5 | 271.0612 | Negative | 0.1–10 | y = 0.0281x + 0.00182 | 0.01/0.03 | 0.9995 | 86.65 | 1.22 | 4.20 |
| Luteolin | C15H10O6 | 285.0405 | Negative | 0.1–10 | y = 0.117x + 0.00848 | 0.01/0.03 | 0.9981 | 96.98 | 0.11 | 3.42 |
| Apigenin | C15H10O5 | 269.0456 | Negative | 0.3–10 | y = 0.104x + 0.0199 | 0.01/0.03 | 0.9998 | 81.55 | 0.20 | 2.87 |
| Hispidulin | C16H12O6 | 301.0707 | Positive | 0.05–10 | y = 0.02614x + 0.0003114 | 0.01/0.03 | 0.9993 | 98.36 | 0.72 | 3.41 |
| Penduletin | C18H16O7 | 343.0823 | Negative | 0.3–10 | y = 0.0258x + 0.00253 | 0.01/0.03 | 0.9991 | 83.43 | 0.03 | 3.20 |
| CAPE | C17H16O4 | 283.0976 | Negative | 0.3–7 | y = 0.255x + 0.0477 | 0.01/0.03 | 0.9964 | 94.42 | - | 3.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulcin, İ.; Mutlu, M.; Bingol, Z.; Ozden, E.M.; Mirzaee, Z.; Goren, A.C.; Köksal, E. Antioxidant Activity and Phytochemical Profiling of Steam-Distilled Oil of Flaxseed (Linum usitatissimum): Therapeutic Targeting Against Glaucoma, Oxidative Stress, Cholinergic Imbalance, and Diabetes. Molecules 2025, 30, 3384. https://doi.org/10.3390/molecules30163384
Gulcin İ, Mutlu M, Bingol Z, Ozden EM, Mirzaee Z, Goren AC, Köksal E. Antioxidant Activity and Phytochemical Profiling of Steam-Distilled Oil of Flaxseed (Linum usitatissimum): Therapeutic Targeting Against Glaucoma, Oxidative Stress, Cholinergic Imbalance, and Diabetes. Molecules. 2025; 30(16):3384. https://doi.org/10.3390/molecules30163384
Chicago/Turabian StyleGulcin, İlhami, Muzaffer Mutlu, Zeynebe Bingol, Eda Mehtap Ozden, Ziba Mirzaee, Ahmet C. Goren, and Ekrem Köksal. 2025. "Antioxidant Activity and Phytochemical Profiling of Steam-Distilled Oil of Flaxseed (Linum usitatissimum): Therapeutic Targeting Against Glaucoma, Oxidative Stress, Cholinergic Imbalance, and Diabetes" Molecules 30, no. 16: 3384. https://doi.org/10.3390/molecules30163384
APA StyleGulcin, İ., Mutlu, M., Bingol, Z., Ozden, E. M., Mirzaee, Z., Goren, A. C., & Köksal, E. (2025). Antioxidant Activity and Phytochemical Profiling of Steam-Distilled Oil of Flaxseed (Linum usitatissimum): Therapeutic Targeting Against Glaucoma, Oxidative Stress, Cholinergic Imbalance, and Diabetes. Molecules, 30(16), 3384. https://doi.org/10.3390/molecules30163384







