The Elicitation of an Antigen-Specific Antibody Immune Response Using a Nanoparticulate Adjuvant Derived from Saponaria officinalis
Abstract
1. Introduction
2. Results
2.1. Characterization of Saponin-Containing Complexes
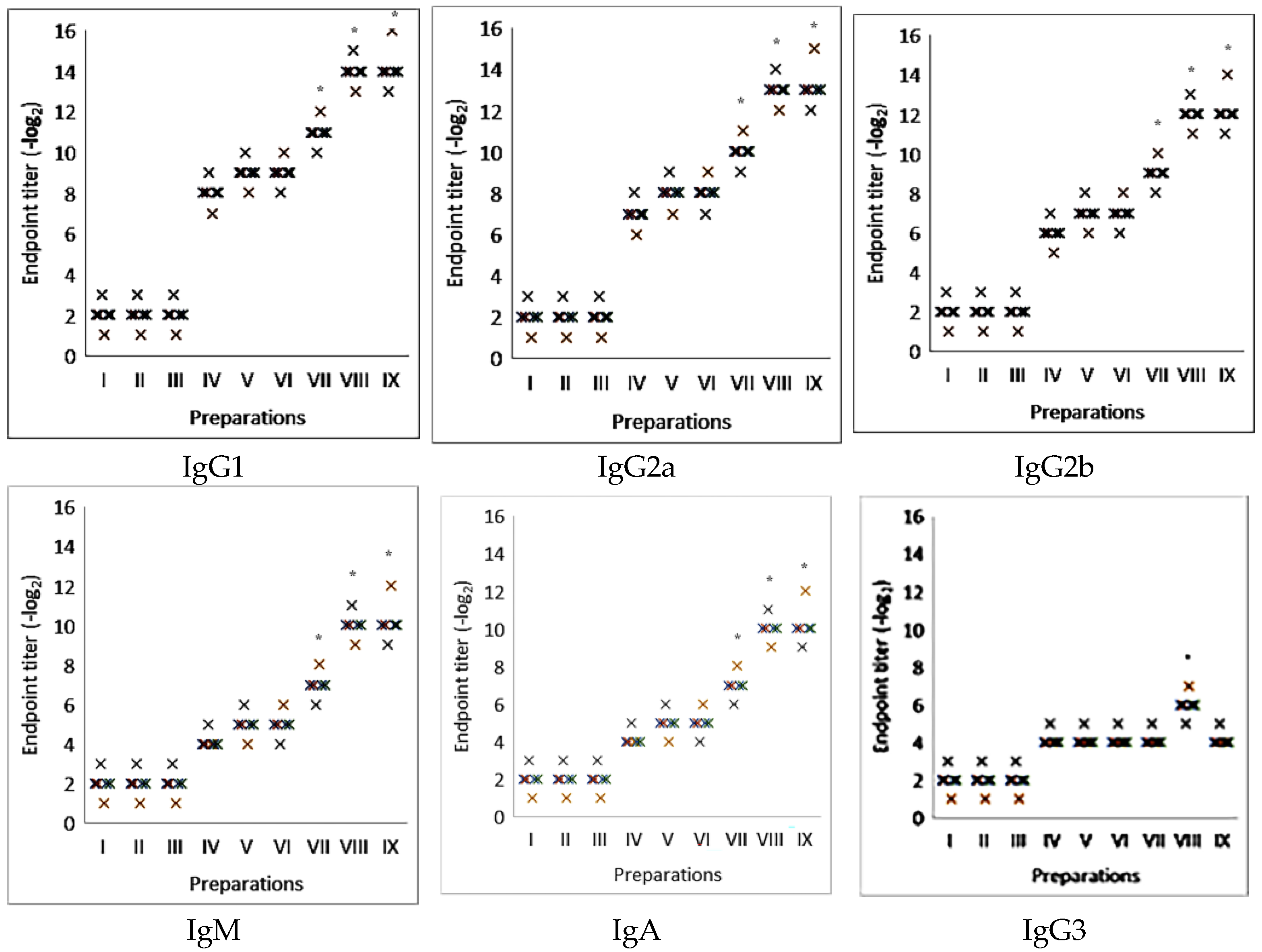
2.2. Induction of an Antigen-Specific Antibody Immune Response Using Influenza Virus Glycoprotein Antigens Combined with Adjuvants
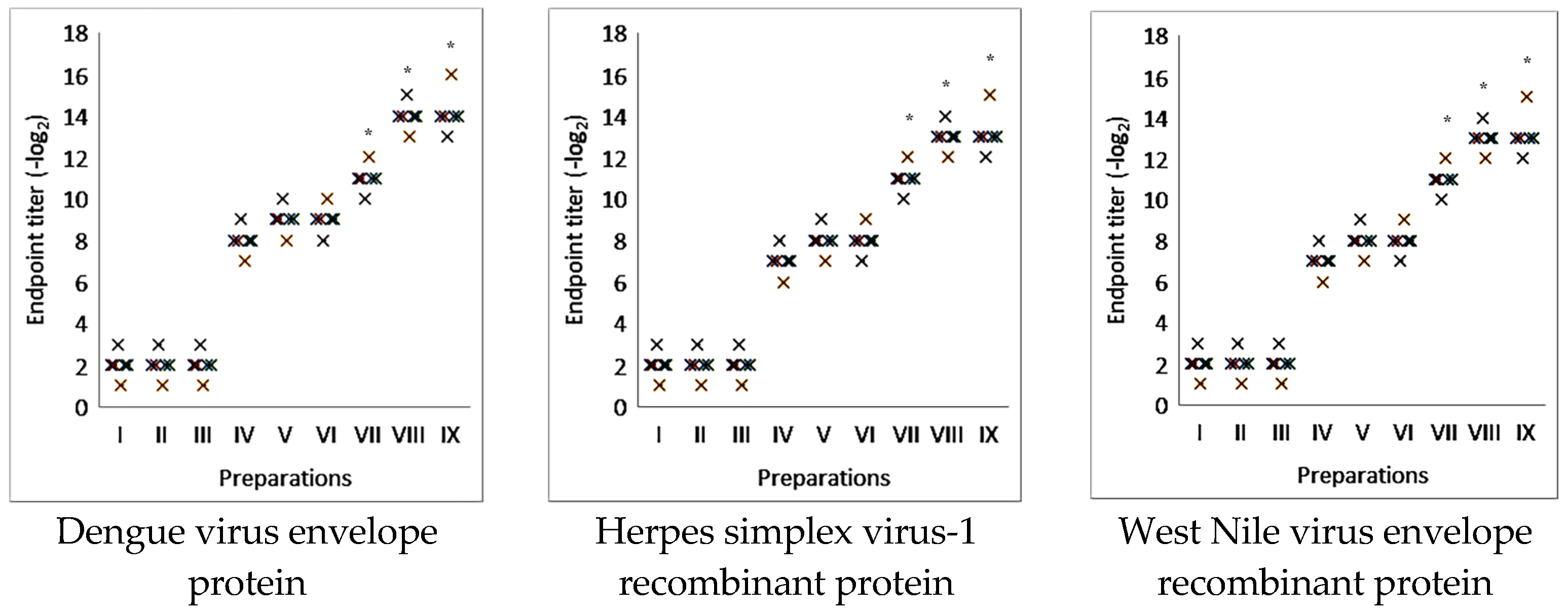
2.3. The Effectiveness of Antigen-Specific Antibody Immune Response Induction Using Nanoparticulate Adjuvant from Saponaria officinalis Saponin in the Model of Commercial Viral Antigens
2.4. Induction of Virus Neutralization Immune Response with Influenza Virus Glycoprotein Antigens or Purified Whole Influenza Virions Mixed with Adjuvants
3. Discussion
4. Materials and Methods
4.1. Main Reagents
4.2. Viruses and Antigens
4.3. Preparation and Characterization of ISCOMATRIX
4.4. Cytotoxicity
4.5. Animals and Immunization
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Micro-Neutralization Assay
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hadian, S.A.; Rezayatmand, R. Economic impact of acute respiratory disease pandemics: A scoping review. J. Res. Med. Sci. 2022, 27, 88. [Google Scholar] [CrossRef]
- Hanage, W.P.; Schaffner, W. Burden of Acute Respiratory Infections Caused by Influenza Virus, Respiratory Syncytial Virus, and SARS-CoV-2 with Consideration of Older Adults: A Narrative Review. Infect. Dis. Ther. 2025, 14 (Suppl. 1), 5–37. [Google Scholar] [CrossRef]
- Filipić, B.; Pantelić, I.; Nikolić, I.; Majhen, D.; Stojić-Vukanić, Z.; Savić, S.; Krajišnik, D. Nanoparticle-Based Adjuvants and Delivery Systems for Modern Vaccines. Vaccines 2023, 11, 1172. [Google Scholar] [CrossRef]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Ho, M.; Hu, Y.; Shi, Y. Vaccine adjuvants: Current status, research and development, licensing, and future opportunities. J. Mater. Chem. B. 2024, 12, 4118–4137. [Google Scholar] [CrossRef] [PubMed]
- Korchowiec, B.; Korchowiec, J.; Kwiecińska, K.; Gevrenova, R.; Bouguet-Bonnet, S.; Deng, C.; Henry, M.; Rogalska, E. The Molecular Bases of the Interaction between a Saponin from the Roots of Gypsophila paniculata L. and Model. Lipid Membranes. Int. J. Mol. Sci. 2022, 23, 3397. [Google Scholar] [CrossRef]
- Eygeris, Y.; Jozic, A.; Henderson, M.I.; Nelson, D.; Sahay, G. Exploring the potential of saponins as adjuvants in lipid-nanoparticle-based mRNA vaccines. Mol. Ther. Methods Clin. Dev. 2025, 33, 101495. [Google Scholar] [CrossRef] [PubMed]
- Magedans, Y.V.; Yendo, A.C.; Costa, F.; Gosmann, G.; Fett-Neto, A.G. Foamy matters: An update on Quillaja saponins and their use as immunoadjuvants. Future Med. Chem. 2019, 11, 1485–1499. [Google Scholar] [CrossRef]
- Sun, H.X.; Xie, Y.; Ye, Y.P. ISCOMs and ISCOMATRIX. Vaccine 2009, 27, 4388–4401. [Google Scholar] [CrossRef]
- Osterhaus, A.D.; Rimmelzwaan, G.F. Induction of virus-specific immunity by iscoms. Dev. Biol. Stand. 1998, 92, 49–58. [Google Scholar]
- Petkov, V.; Tsibranska, S.; Manoylov, I.; Kechidzhieva, L.; Ilieva, K.; Bradyanova, S.; Ralchev, N.; Mihaylova, N.; Denkov, N.; Tchorbanov, A.; et al. ISCOM-type matrix from beta-escin and glycyrrhizin saponins. Heliyon 2025, 11, e41935. [Google Scholar] [CrossRef]
- Stertman, L.; Palm, A.E.; Zarnegar, B.; Carow, B.; Lunderius Andersson, C.; Magnusson, S.E.; Carnrot, C.; Shinde, V.; Smith, G.; Glenn, G.; et al. The Matrix-M™ adjuvant: A critical component of vaccines for the 21st century. Hum. Vaccin. Immunother. 2023, 19, 2189885. [Google Scholar] [CrossRef]
- Fries, L.; Cho, I.; Krähling, V.; Fehling, S.K.; Strecker, T.; Becker, S.; Hooper, J.W.; Kwilas, S.A.; Agrawal, S.; Wen, J.; et al. Randomized, blinded, dose-ranging trial of an ebola virus glycoprotein nanoparticle vaccine with Matrix-M adjuvant in healthy adults. J. Infect. Dis. 2020, 222, 572–582. [Google Scholar] [CrossRef]
- Datoo, M.S.; Natama, M.H.; Somé, A.; Traoré, O.; Rouamba, T.; Bellamy, D.; Yameogo, P.; Valia, D.; Tegneri, M.; Ouedraogo, F.; et al. Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M.; with seasonal administration to children in Burkina Faso: A randomised controlled trial. Lancet 2021, 397, 1809–1818. [Google Scholar] [CrossRef]
- Shinde, V.; Cai, R.; Plested, J.; Cho, I.; Fiske, J.; Pham, X.; Zhu, M.; Cloney-Clark, S.; Wang, N.; Zhou, H.; et al. Induction of cross-reactive hemagglutination inhibiting antibody and polyfunctional CD4+ T-cell responses by a recombinant matrix-M-adjuvanted hemagglutinin nanoparticle influenza vaccine. Clin. Infect. Dis. 2021, 73, e4278–e4287. [Google Scholar] [CrossRef] [PubMed]
- Shinde, V.; Cho, I.; Plested, J.S.; Agrawal, S.; Fiske, J.; Cai, R.; Zhou, H.; Pham, X.; Zhu, M.; Cloney-Clark, S.; et al. Comparison of the safety and immunogenicity of a novel Matrix-M-adjuvanted nanoparticle influenza vaccine with a quadrivalent seasonal influenza vaccine in older adults: A phase 3 randomised controlled trial. Lancet Infect. Dis. 2022, 22, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Huis in’t Veld, L.G.; Cornelissen, L.A.; van den Bogaard, L.; Ansems, M.; Ho, N.I.; Adema, G.J. Saponin-based adjuvant uptake and induction of antigen cross-presentation by CD11b+ dendritic cells and macrophages. NPJ Vaccines 2025, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- Ou, B.S.; Baillet, J.; Filsinger Interrante, M.V.; Adamska, J.Z.; Zhou, X.; Saouaf, O.M.; Yan, J.; Klich, J.H.; Jons, C.K.; Meany, E.L.; et al. Saponin nanoparticle adjuvants incorporating Toll-like receptor agonists drive distinct immune signatures and potent vaccine responses. Sci Adv. 2024, 10, eadn7187. [Google Scholar] [CrossRef]
- Berezin, V.E.; Bogoyavlenskiy, A.P.; Tolmacheva, V.P.; Makhmudova, N.R.; Khudyakova, S.S.; Levandovskaya, S.V.; Omirtaeva, E.S.; Zaitceva, I.A.; Tustikbaeva, G.B.; Ermakova, O.S.; et al. Immunostimulating complexes incorporating Eimeria tenella antigens and plant saponins as effective delivery system for coccidia vaccine immunization. J. Parasitol. 2008, 94, 381–385. [Google Scholar] [CrossRef]
- Turmagambetova, A.S.; Alexyuk, P.G.; Bogoyavlenskiy, A.P.; Zaitseva, I.A.; Omirtaeva, E.S.; Alexyuk, M.S.; Sokolova, N.S.; Berezin, V.E. Adjuvant activity of saponins from Kazakhstani plants on the immune responses to subunit influenza vaccine. Arch. Virol. 2017, 162, 3817–3826. [Google Scholar] [CrossRef] [PubMed]
- Alexyuk, P.G.; Bogoyavlenskiy, A.P.; Alexyuk, M.S.; Turmagambetova, A.S.; Zaitseva, I.A.; Omirtaeva, E.S.; Berezin, V. Adjuvant activity of multimolecular complexes based on Glycyrrhiza glabra saponins, lipids, and influenza virus glycoproteins. Arch. Virol. 2019, 164, 1793–1803. [Google Scholar] [CrossRef] [PubMed]
- Mamo, T.; Poland, G.A. Nanovaccinology: The next generation of vaccines meets 21st century materials science and engineering. Vaccine 2012, 30, 6609–6611. [Google Scholar] [CrossRef] [PubMed]
- Sadr, S.; Poorjafari Jafroodi, P.; Haratizadeh, M.J.; Ghasemi, Z.; Borji, H.; Hajjafari, A. Current status of nano-vaccinology in veterinary medicine science. Vet. Med. Sci. 2023, 9, 2294–2308. [Google Scholar] [CrossRef]
- Yasamineh, S.; Kalajahi, H.G.; Yasamineh, P.; Yazdani, Y.; Gholizadeh, O.; Tabatabaie, R.; Afkhami, H.; Davodabadi, F.; Farkhad, A.K.; Pahlevan, D.; et al. An overview on nanoparticle-based strategies to fight viral infections with a focus on COVID-19. J. Nanobiotechnol. 2022, 20, 440. [Google Scholar] [CrossRef]
- Zaheer, T.; Pal, K.; Zaheer, I. Topical review on nano-vaccinology: Biochemical promises and key challenges. Process Biochem. 2021, 100, 237–244. [Google Scholar] [CrossRef]
- Li, X.; Sloat, B.R.; Yanasarn, N.; Cui, Z. Relationship between the size of nanoparticles and their adjuvant activity: Data from a study with an improved experimental design. Eur. J. Pharm. Biopharm. 2011, 78, 107–116. [Google Scholar] [CrossRef]
- Bo, C.; Wei, X.; Wang, X.; Ji, W.; Yang, H.; Zhao, Y.; Wang, H. Physicochemical properties and adsorption state of aluminum adjuvants with different processes in vaccines. Heliyon 2023, 9, e18800. [Google Scholar] [CrossRef]
- Zhong, L.; Fu, S.; Peng, X.; Zhan, H.; and Sun, R. Colloidal stability of negatively charged cellulose nanocrystalline in aqueous systems. Carbohydr. Polym. 2012, 90, 644–649. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, X.; Wu, X.; Wang, S.; Lu, C. Cellulose nanocrystals mediated assembly of graphene in rubber composites for chemical sensing applications. Carbohydr. Polym. 2016, 140, 88–95. [Google Scholar] [CrossRef]
- Silveira, F.; Cibulski, S.P.; Varela, A.P.; Marqués, J.M.; Chabalgoity, A.; de Costa, F.; Yendo, A.C.; Gosmann, G.; Roehe, P.M.; Fernández, C.; et al. Quillaja brasiliensis saponins are less toxic than Quil A and have similar properties when used as an adjuvant for a viral antigen preparation. Vaccine 2011, 29, 9177–9182. [Google Scholar] [CrossRef] [PubMed]
- Tucker, I.M.; Burley, A.; Petkova, R.E.; Hosking, S.L.; Webster, J.R.P.; Li, P.X.; Ma, K.; Doutch, J.; Penfold, J.; Thomas, R.K. Self-assembly in saponin mixtures: Escin / tea, tea / glycyrrhizic acid, and escin / glycyrrhizic acid mixtures. Colloids Surf. A 2021, 629, 127420. [Google Scholar] [CrossRef]
- Wang, D.; Sha, L.; Xu, C.; Huang, Y.; Tang, C.; Xu, T.; Li, X.; Di, D.; Liu, J.; Yang, L. Natural saponin and cholesterol assembled nanostructures as the promising delivery method for saponin. Colloids Surf. B Biointerfaces 2022, 214, 112448. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.Y.; Coyle, E.M.; Jani, D.; King, L.R.; Bhardwaj, R.; Fries, L.; Smith, G.; Glenn, G.; Golding, H.; Khurana, S. ISCOMATRIX™ adjuvant promotes epitope spreading and antibody affinity maturation of influenza A H7N9 virus like particle vaccine that correlate with virus neutralization in humans. Vaccine 2015, 33, 3953–3962. [Google Scholar] [CrossRef]
- Hägglund, S.; Hu, K.; Blodörn, K.; Makabi-Panzu, B.; Gaillard, A.L.; Ellencrona, K.; Chevret, D.; Hellman, L.; Bengtsson, K.L.; Riffault, S.; et al. Characterization of an experimental vaccine for bovine respiratory syncytial virus. Clin. Vaccine Immunol. 2014, 21, 997–1004. [Google Scholar] [CrossRef][Green Version]
- Fossum, C.; Hjertner, B.; Ahlberg, V.; Charerntantanakul, W.; McIntosh, K.; Fuxler, L.; Balagunaseelan, N.; Wallgren, P.; Bengtsson, K.L. Early inflammatory response to the saponin adjuvant Matrix-M in the pig. Vet. Immunol. Immunopathol. 2014, 158, 53–61. [Google Scholar] [CrossRef]
- Oda, K.; Matsuda, H.; Murakami, T.; Katayama, S.; Ohgitani, T.; Yoshikawa, M. Adjuvant and haemolytic activities of 47 saponins derived from medicinal and food plants. Biol. Chem. 2000, 381, 67–74. [Google Scholar] [CrossRef]
- Ojiako, C.M.; Okoye, E.I.; Oli, A.N.; Ike, C.J.; Esimone, C.O.; Attama, A.A. Preliminary studies on the formulation of immune stimulating complexes using saponin from Carica papaya leaves. Heliyon 2019, 5, e01962. [Google Scholar] [CrossRef]
- Wang, P.; Ding, X.; Kim, H.; Michalek, S.M.; Zhang, P. Structural Effect on Adjuvanticity of Saponins. J. Med. Chem. 2020, 63, 3290–3297. [Google Scholar] [CrossRef]
- Klimov, A.; Balish, A.; Veguilla, V.; Sun, H.; Schifferet, J.; Lu, X.; Katz, J.M.; Hancock, K. Influenza virus titration, antigenic characterization, and serological methods for antibody detection. Methods Mol. Biol. 2012, 865, 25–51. [Google Scholar] [CrossRef]
- Carlsson, N.; Borde, A.; Wölfel, S.; Åkerman, B.; Larsson, A. Quantification of protein concentration by the Bradford method in the presence of pharmaceutical polymers. Anal. Biochem. 2011, 411, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Golali, E.; Jaafari, M.R.; Khamesipour, A.; Abbasi, A.; Saberi, Z.; Badiee, A. Comparison of in vivo Adjuvanticity of Liposomal PO CpG ODN with Liposomal PS CpG ODN:Soluble Leishmania Antigens as a Model. Iran. J. Basic. Med. Sci. 2012, 15, 1032–1045. [Google Scholar]
- Jaafari, M.R.; Ghafarian, A.; Farrokh-Gisour, A.; Samiei, A.; Kheiri, M.T.; Mahboudi, F.; Barkhordari, F.; Khamesipour, A.; McMaster, W.R. Immune response and protection assay of recombinant major surface glycoprotein of leishmania (rgp63) reconstituted with liposomes in BALB/c mice. Vaccine 2006, 24, 5708–5717. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, S.; Paliwal, R.; Paliwal, S.R.; Vyas, S.P. Hyaluronic acid modified chitosan nanoparticles for effective management of glaucoma: Development, characterization, and evaluation. J. Drug Target. 2010, 18, 292–302. [Google Scholar] [CrossRef]
- Stepanenko, A.A.; Dmitrenko, V.V. Pitfalls of the MTT assay:direct and off-target effects of inhibitors can result in over/underestimation of cell viability. Gene 2015, 574, 193–203. [Google Scholar] [CrossRef]
- He, P.; Zou, Y.; Hu, Z. Advances in aluminium hydroxide-based adjuvant research and its mechanism. Hum. Vaccin. Immunother. 2015, 11, 477–488. [Google Scholar] [CrossRef]
- Berezin, V.E.; Zaides, V.M.; Isaeva, E.S.; Artamonov, A.F.; Zhdanov, V.M. Controlled organization of multimolecular complexes of enveloped virus glycoproteins: Study of immunogenicity. Vaccine 1988, 6, 450–456. [Google Scholar] [CrossRef]
- Verschoor, C.P.; Singh, P.; Russell, M.L.; Bowdish, D.M.; Brewer, A.; Cyr, L.; Ward, B.J.; Loeb, M. Correction: Microneutralization assay titres correlate with protection against seasonal influenza H1N1 and H3N2 in children. PLoS ONE 2016, 11, e0163830. [Google Scholar] [CrossRef]



| Formulation | Size (nm) | PDI | Zeta Potential (mv) | Encapsulation Efficiency, % |
|---|---|---|---|---|
| ISCOMATRIX QuilA | 75 ± 9.5 | 0.21 ± 0.08 * | −13.4 ± 5.6 | 92.34 ± 3.5 |
| Complexes with saponins from Saponaria officinalis | 70.2 ± 11.1 | 0.30 ± 0.05 | −15.8 ± 5.2 | 94.2 ± 4.1 |
| Saponin | CC50 (μg/mL) Macrophages * | CC50 (μg/mL) MDCK * | CC50 (μg/mL) Caco2 * | CC50 (μg/mL) HT29 * |
|---|---|---|---|---|
| Quil-A | 21.69 ± 3.5 | 28.13 ± 8.3 | 15.3 ± 2.8 | 18.25 ± 2.9 |
| Saponin from Saponaria officinalis | 161.00 ± 39.8 | 187.50 ± 33.1 | 142.24 ± 31.11 | 151.12 ± 28.23 |
| Iscommatrix | 65.00 ± 5.7 | 90.12 ± 7.9 | 100.13 ± 9.34 | 110.26 ± 8.67 |
| Complexes with saponin from Saponaria officinalis | 480.23 ± 32.25 | 570.36 ± 36.33 | 342.45 ± 34.52 | 456.47 ± 37.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogoyavlenskiy, A.; Alexyuk, M.; Alexyuk, P.; Omirtayeva, E.; Zaitseva, I.; Moldakhanov, Y.; Anarkulova, E.; Berezin, V. The Elicitation of an Antigen-Specific Antibody Immune Response Using a Nanoparticulate Adjuvant Derived from Saponaria officinalis. Molecules 2025, 30, 3328. https://doi.org/10.3390/molecules30163328
Bogoyavlenskiy A, Alexyuk M, Alexyuk P, Omirtayeva E, Zaitseva I, Moldakhanov Y, Anarkulova E, Berezin V. The Elicitation of an Antigen-Specific Antibody Immune Response Using a Nanoparticulate Adjuvant Derived from Saponaria officinalis. Molecules. 2025; 30(16):3328. https://doi.org/10.3390/molecules30163328
Chicago/Turabian StyleBogoyavlenskiy, Andrey, Madina Alexyuk, Pavel Alexyuk, Elmira Omirtayeva, Irina Zaitseva, Yergali Moldakhanov, Elmira Anarkulova, and Vladimir Berezin. 2025. "The Elicitation of an Antigen-Specific Antibody Immune Response Using a Nanoparticulate Adjuvant Derived from Saponaria officinalis" Molecules 30, no. 16: 3328. https://doi.org/10.3390/molecules30163328
APA StyleBogoyavlenskiy, A., Alexyuk, M., Alexyuk, P., Omirtayeva, E., Zaitseva, I., Moldakhanov, Y., Anarkulova, E., & Berezin, V. (2025). The Elicitation of an Antigen-Specific Antibody Immune Response Using a Nanoparticulate Adjuvant Derived from Saponaria officinalis. Molecules, 30(16), 3328. https://doi.org/10.3390/molecules30163328







