Essential Oils from Wild Albanian Lamiaceae: GC-MS Profiling, Biological Activity, and Enhanced Delivery via Nanoencapsulation
Abstract
1. Introduction
2. Results and Discussion
2.1. Yield of Extracted EOs
2.2. Quantitative and Qualitative Analysis of the EOs
2.3. Antioxidant Activity
2.4. Antimicrobial Activity
2.5. EOs Nanoemulsions
3. Materials and Methods
3.1. Plant Material
3.2. EOs Extraction
3.3. Reagents and Microbial Strains
3.4. Gas Chromatography–Mass Spectrometry
3.5. Free Radical Scavenging Activity
3.6. Evaluation of Antimicrobial Activity by Microdilution Broth Method
3.7. Encapsulation of EOs
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teshome, E.; Forsido, S.F.; Rupasinghe, H.P.V.; Keyata, E.O. Potentials of Natural Preservatives to Enhance Food Safety and Shelf Life: A Review. Sci. World J. 2022, 2022, 9901018. [Google Scholar] [CrossRef]
- Dong, H.; Xu, Y.; Zhang, Q.; Li, H.; Chen, L. Activity and Safety Evaluation of Natural Preservatives. Food Res. Int. 2024, 190, 114548. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Sun, P.; Kang, C.; Zhang, L.; Guo, L.; Kou, Y. Chemical Composition and Biological Activities of Essential Oils from Six Lamiaceae Folk Medicinal Plants. Front. Plant Sci. 2022, 13, 919294. [Google Scholar] [CrossRef]
- Ietswaart, J.H. A Taxonomic Revision of the Genus Origanum (Labiatae); Leiden Botanical Series 4; Leiden University Press: The Hague, The Netherlands, 1980. [Google Scholar]
- Vangjeli, J.; Ruci, B.; Mullaj, A. Red Book of Albanian Flora; Institute of Biological Research, Academy of Sciences of Albania: Tirana, Albania, 1996. [Google Scholar]
- Kokkini, S. Taxonomy, Diversity and Distribution of Origanum Species. In Proceedings of the IPGRI International Workshop on Oregano, Bari, Italy, 8–12 May 1996; Padulosi, S., Ed.; CIHEAM Valenzano: Bari, Italy, 1997; pp. 122–132. [Google Scholar]
- Elezi, F.; Plaku, F.; Ibraliu, A.; Stefkov, G.; Karapandzova, M.; Kulevanova, S.; Aliu, S. Genetic Variation of Oregano (Origanum vulgare L.) for Etheric Oil in Albania. Agric. Sci. 2013, 4, 449–454. [Google Scholar] [CrossRef][Green Version]
- Koukoulitsa, C.; Karioti, A.; Bergonzi, M.C.; Pescitelli, G.; Di Bari, L.; Skaltsa, H. Polar Constituents from the Aerial Parts of Origanum vulgare L. ssp. hirtum Growing Wild in Greece. J. Agric. Food Chem. 2006, 54, 5388–5392. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Salgueiro, L.; Miguel, M.G.; Faleiro, M.L. Portuguese Thymbra and Thymus Species Volatiles: Chemical Composition and Biological Activities. Curr. Pharm. Des. 2008, 14, 3120–3140. [Google Scholar] [CrossRef] [PubMed]
- Pirintsos, S.A.; Bariotakis, M.; Kampa, M.; Sourvinos, G.; Lionis, C.; Castanas, E. The Therapeutic Potential of the Essential Oil of Thymbra capitata (L.) Cav., Origanum dictamnus L. and Salvia fruticosa Mill. and a Case of Plant-Based Pharmaceutical Development. Front. Pharmacol. 2020, 11, 522213. [Google Scholar] [CrossRef] [PubMed]
- Ibraliu, A.; Mi, X.; Elezi, F.; Sala, F. Analysis of Essential Oils of Three Wild Medicinal Plants in Albania. J. Med. Plants Res. 2011, 5, 58–62. [Google Scholar]
- Saoulajan, H.; Boujida, N.; El Mihyaoui, A.; El Baakili, A.; Alshahrani, M.M.; Lee, L.-H.; Bouyahya, A. Phytochemistry, Pharmacological Investigations, Industrial Applications, and Encapsulation of Thymbra capitata L.: A Review. Trends Food Sci. Technol. 2022, 129, 463–491. [Google Scholar] [CrossRef]
- Chorianopoulos, N.; Kalpoutzakis, E.; Aligiannis, N.; Mitaku, S.; Nychas, G.-J.E.; Haroutounian, S.A. Essential Oils of Satureja, Origanum, and Thymbra Species: Chemical Composition and Antibacterial Activities Against Foodborne Pathogens. J. Agric. Food Chem. 2004, 52, 8261–8267. [Google Scholar] [CrossRef] [PubMed]
- Ozogul, Y.; Karsli, G.T.; Yazgan, H.; Ozogul, F.; Regenstein, J.M. Enhanced Pathogen Control Through Thymol and Carvacrol Nanoemulsions: A Microfluidization Approach. Food Bioprocess Technol. 2025, 18, 5377–5387. [Google Scholar] [CrossRef]
- Sarac, N.; Ugur, A. Antimicrobial Activities of the Essential Oils of Origanum onites L., Origanum vulgare L. Subspecies hirtum (Link) Ietswaart, Satureja thymbra L., and Thymbra cilicicus Boiss. & Bal. Growing Wild in Turkey. J. Med. Food 2008, 11, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Baratta, M.T.; Dorman, H.J.D.; Deans, S.G.; Figueiredo, A.C.; Barroso, J.G.; Ruberto, G. Antimicrobial and Antioxidant Properties of Some Commercial Essential Oils. Flavour Fragr. J. 1998, 13, 235–244. [Google Scholar] [CrossRef]
- Stoilova, I.; Bail, S.; Buchbauer, G.; Stoyanova, A.; Krastanov, A.; Staneva, D. Chemical Composition, Olfactory Evaluation and Antioxidant Effects of the Essential Oil of Satureja montana L. Nat. Prod. Commun. 2008, 3, 1035–1042. [Google Scholar] [CrossRef]
- Chroho, M.; Rouphael, Y.; Petropoulos, S.A.; Bouissane, L. Carvacrol and Thymol Content Affects the Antioxidant and Antibacterial Activity of Origanum compactum and Thymbra zygis Essential Oils. Antibiotics 2024, 13, 139. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- D’Amato, S.; Rossi, C.; Maggio, F.; Valbonetti, L.; Savini, V.; Paparella, A.; Serio, A. Antilisterial Effectiveness of Origanum vulgare var. hirtum and CoridoThymbra capitata Essential Oils and Hydrolates Alone and in Combination. Foods 2024, 13, 860. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Friedman, M.; Henika, P.R.; Mandrell, R.E. Bactericidal Activities of Plant Essential Oils and Some of Their Isolated Constituents Against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2002, 65, 1545–1560. [Google Scholar] [CrossRef]
- de Castro, R.D.; de Souza, T.M.; Bezerra, L.M.; Ferreira, G.L.; Costa, E.M.; Cavalcanti, A.L. Antifungal Activity and Mode of Action of Thymol and Its Synergism with Nystatin Against Candida Species Involved with Infections in the Oral Cavity: An In Vitro Study. BMC Complement. Altern. Med. 2015, 15, 417. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus Microemulsions: Terminology, Differences, and Similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Maurya, A.; Singh, V.K.; Das, S.; Prasad, J.; Kedia, A.; Upadhyay, N.; Dubey, N.K.; Dwivedy, A.K. Essential Oil Nanoemulsion as Eco-Friendly and Safe Preservative: Bioefficacy Against Microbial Food Deterioration and Toxin Secretion, Mode of Action, and Future Opportunities. Front. Microbiol. 2021, 12, 751062. [Google Scholar] [CrossRef]
- Singh, I.R.; Pulikkal, A.K. Preparation, Stability and Biological Activity of Essential Oil-Based Nanoemulsions: A Comprehensive Review. OpenNano 2022, 8, 100066. [Google Scholar] [CrossRef]
- Enayatifard, R.; Akbari, J.; Babaei, A.; Rostamkalaei, S.S.; Hashemi, S.M.H.; Habibi, E. AnTC-Microbial Potential of Nano-Emulsion Form of Essential Oil Obtained from Aerial Parts of Origanum vulgare L. as Food Additive. Adv. Pharm. Bull. 2021, 11, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Jayari, A.; Donsì, F.; Ferrari, G.; Maaroufi, A. Nanoencapsulation of Thyme Essential Oils: Formulation, Characterization, Storage Stability, and Biological Activity. Foods 2022, 11, 1858. [Google Scholar] [CrossRef]
- Rinaldi, F.; Maurizi, L.; Conte, A.L.; Marazzato, M.; Maccelli, A.; Crestoni, M.E.; Hanieh, P.N.; Forte, J.; Conte, M.P.; Zagaglia, C.; et al. Nanoemulsions of Satureja montana Essential Oil: Antimicrobial and Antibiofilm Activity Against Avian Escherichia coli Strains. Pharmaceutics 2021, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Hodaj-Çeliku, E.; Tsiftsoglou, O.; Shuka, L.; Abazi, S.; Hadjipavlou-Litina, D.; Lazari, D. Antioxidant Activity and Chemical Composition of Essential Oils of Some Aromatic and Medicinal Plants from Albania. Nat. Prod. Commun. 2017, 12, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Giannoulis, K.D.; Kamvoukou, C.-A.; Gougoulias, N.; Wogiatzi, E. Irrigation and Nitrogen Application Affect Greek Oregano (Origanum vulgare ssp. hirtum) Dry Biomass, Essential Oil Yield and Composition. Ind. Crops Prod. 2020, 150, 112392. [Google Scholar] [CrossRef]
- Baser, K.; Can, H. Aromatic biodiversity among the flowering plant taxa of Turkey. Pure Appl. Chem. 2002, 74, 527–545. [Google Scholar] [CrossRef]
- Adam, K.; Sivropoulou, A.; Kokkini, S.; Lanaras, T.; Arsenakis, M. Antifungal activities of Origanum vulgare subsp. hirtum, Mentha spicata, Lavandula angustifolia and Salvia fruticosa Essential Oils against Human Pathogenic Fungi. J. Agric. Food Chem. 1998, 46, 1738–1745. [Google Scholar] [CrossRef]
- Mugao, L.G. Factors Influencing Yield, Chemical Composition and Efficacy of Essential Oils. Int. J. Multidiscip. Res. Growth Eval. 2024, 5, 169–178. [Google Scholar] [CrossRef]
- Hedhili, L.; Romdhane, M.; Abderrabba, A.; Planche, H.; Cherif, I. Variability in essential oil composition of Tunisian Thymbra capitata (L.) Hoffmanns. et Link. Flavour Fragr. J. 2002, 17, 26–28. [Google Scholar] [CrossRef]
- Tagnaout, I.; Zerkani, H.; Hadi, N.; El Moumen, B.; El Makhoukhi, F.; Bouhrim, M.; Al-Salahi, R.; Nasr, F.A.; Mechchate, H.; Zair, T. Chemical Composition, Antioxidant and Antibacterial Activities of Thymbra broussonetii Boiss and Thymbra capitata (L.) Hoffmann and Link Essential Oils. Plants 2022, 11, 954. [Google Scholar] [CrossRef]
- Hajdari, A.; Elezaj, R.; Mulla, D.; Mustafa, B.; Quave, C.L.; Novak, J. Chemical Composition of the Essential Oil, Total Phenolics, Total Flavonoids and Antioxidant Activity of Methanolic Extracts of Satureja montana L. Rec. Nat. Prod. 2016, 10, 750–760. [Google Scholar]
- Said-Al Ahl, H.A.H.; Kačániova, M.; Mahmoud, A.A.; Hikal, W.M.; Čmiková, N.; Szczepanek, M.; Błaszczyk, K.; Al-Balawi, S.M.; Bianchi, A.; Smaoui, S.; et al. Phytochemical Characterization and Biological Activities of Essential Oil from Satureja montana L., a Medicinal Plant Grown under the Influence of Fertilization and Planting Dates. Biology 2024, 13, 328. [Google Scholar] [CrossRef]
- Zawiślak, G.; Nurzyńska-Wierdak, R. Variation in winter savory (Satureja montana L.) yield and essential oil production as affected by different plant density and number of harvests. Acta Sci. Pol. Hortorum Cultus 2017, 16, 159–168. [Google Scholar] [CrossRef]
- Serrano, C.; Matos, O.; Teixeira, B.; Ramos, C.; Neng, N.; Nogueira, J.; Nunes, M.L.; Marques, A. Antioxidant and Antimicrobial Activity of Satureja montana L. Extracts. J. Sci. Food Agric. 2011, 91, 1554–1560. [Google Scholar] [CrossRef]
- Kokkini, S.; Karousou, R.; Vokou, D. Pattern of Geographic Variations of Origanum vulgare Trichomes and Essential Oil Content in Greece. Biochem. Syst. Ecol. 1994, 22, 517–528. [Google Scholar] [CrossRef]
- Mączka, W.; Twardawska, M.; Grabarczyk, M.; Wińska, K. Carvacrol—A Natural Phenolic Compound with Antimicrobial Properties. Antibiotics 2023, 12, 824. [Google Scholar] [CrossRef]
- Maniki, E.; Kostoglou, D.; Paterakis, N.; Nikolaou, A.; Kourkoutas, Y.; Papachristoforou, A.; Giaouris, E. Chemical Composition, Antioxidant, and Antibiofilm Properties of Essential Oil from Thymbra capitata Plants Organically Cultured on the Greek Island of Lemnos. Molecules 2023, 28, 1154. [Google Scholar] [CrossRef] [PubMed]
- Salgueiro, L.; Pinto, E.; Gonçalves, M.J.; Pina-Vaz, C.; Cavaleiro, C.; Rodrigues, A.G.; Palmeira, A.; Tavares, C.; Costa-de-Oliveira, S.; Martinez-de-Oliveira, J. Chemical Composition and Antifungal Activity of the Essential Oil of Thymbra capitata. Planta Med. 2004, 70, 572–575. [Google Scholar] [CrossRef] [PubMed]
- ISO 14717:2008; Oil of Origanum, Spanish Type [Coridothymus capitatus (L.) Rchb.f.]. ISO: Geneva, Switzerland, 2008.
- Salihila, J.; Nuro, A. Chemical Characterisation of Essential Oil for Satureja montana Population from Burreli Area, Albania. Madridge J. Anal. Sci. Instrum. 2018, 4, 88–91. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and Antifungal Activities of Thymol: A Brief Review of the Literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Krause, S.T.; Liao, P.; Crocoll, C.; Boachon, B.; Förster, C.; Leidecker, F.; Wiese, N.; Zhao, D.; Wood, J.C.; Buell, C.R.; et al. The Biosynthesis of Thymol, Carvacrol, and Thymohydroquinone in Lamiaceae Proceeds via Cytochrome P450s and a Short-Chain Dehydrogenase. Proc. Natl. Acad. Sci. USA 2021, 118, e2110092118. [Google Scholar] [CrossRef]
- Kachur, K.; Suntres, Z. The Antibacterial Properties of Phenolic Isomers, Carvacrol and Thymol. Crit. Rev. Food Sci. Nutr. 2020, 60, 3042–3053. [Google Scholar] [CrossRef]
- Yanishlieva, N.V.; Marinova, E.M.; Gordon, M.H.; Raneva, V.G. Antioxidant Activity and Mechanism of Action of Thymol and Carvacrol in Two Lipid Systems. Food Chem. 1999, 64, 59–66. [Google Scholar] [CrossRef]
- Mastelić, J.; Jerković, I.; Blažević, I.; Poljak-Blaži, M.; Borović, S.; Ivancić-Baće, I.; Smrečki, V.; Zarković, N.; Brčić-Kostić, K.; Vikić-Topić, D.; et al. Comparative Study on the Antioxidant and Biological Activities of Carvacrol, Thymol, and Eugenol Derivatives. J. Agric. Food Chem. 2008, 56, 3989–3996. [Google Scholar] [CrossRef]
- Olszowy, M.; Dawidowicz, A.L. Essential Oils as Antioxidants: Their Evaluation by DPPH, ABTS, FRAP, CUPRAC, and β-Carotene Bleaching Methods. Monatsh. Chem. 2016, 147, 2083–2091. [Google Scholar] [CrossRef]
- Ebhohimen, I.E.; Okolie, N.P.; Okpeku, M.; Unweator, M.; Adeleke, V.T.; Edemhanria, L. Evaluation of the Antioxidant Properties of Carvacrol as a Prospective Replacement for Crude Essential Oils and Synthetic Antioxidants in Food Storage. Molecules 2023, 28, 1315. [Google Scholar] [CrossRef] [PubMed]
- M100 Ed34; Performance Standards for Antimicrobial Susceptibility Testing, 34th Ed. Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2024.
- Longbottom, C.J.; Carson, C.F.; Hammer, K.A.; Mee, B.J.; Riley, T.V. Tolerance of Pseudomonas aeruginosa to Melaleuca alternifolia (Tea Tree) Oil Is Associated with the Outer Membrane and Energy-Dependent Cellular Processes. J. Antimicrob. Chemother. 2004, 54, 386–392. [Google Scholar] [CrossRef]
- Gavaric, N.; Smole Mozina, S.; Kladar, N.V.; Bozin, B. Chemical Profile, Antioxidant and Antibacterial Activity of Thyme and Oregano Essential Oils, Thymol and Carvacrol and Their Possible Synergism. J. Essent. Oil Bear. Plants 2015, 18, 1013–1021. [Google Scholar] [CrossRef]
- Soulaimani, B. Comprehensive Review of the Combined Antimicrobial Activity of Essential Oil Mixtures and Synergism with Conventional Antimicrobials. Nat. Prod. Commun. 2025, 20, 1–22. [Google Scholar] [CrossRef]
- dos Santos Barbosa, C.R.; Scherf, J.R.; de Freitas, T.S.; de Oliveira, D.R.; de Oliveira, C.A.; Poser, G.L.; Micke, G.A. Effect of Carvacrol and Thymol on NorA Efflux Pump Inhibition in Multidrug-Resistant (MDR) Staphylococcus aureus Strains. J. Bioenerg. Biomembr. 2021, 53, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Hamoud, R.; Zimmermann, S.; Reichling, J.; Wink, M. Synergistic Interactions in Two-Drug and Three-Drug Combinations (Thymol, EDTA and Vancomycin) Against Multidrug-Resistant Bacteria Including E. coli. Phytomedicine 2014, 21, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Wijesundara, N.; Lee, S.; Cheng, Z.; Davidson, R.; Rupasinghe, H. Carvacrol Exhibits Rapid Bactericidal Activity Against Streptococcus pyogenes Through Cell Membrane Damage. Sci. Rep. 2021, 11, 409. [Google Scholar] [CrossRef]
- Gan, C.; Langa, E.; Valenzuela, A.; Ballestero, D.; Pino-Otín, M. Synergistic Activity of Thymol with Commercial Antibiotics Against Critical and High WHO Priority Pathogenic Bacteria. Plants 2023, 12, 1868. [Google Scholar] [CrossRef] [PubMed]
- Aleksic Sabo, V.; Nikolic, I.; Mimica-Dukic, N.; Knezevic, P. Anti-Acinetobacter baumannii Activity of Selected Phytochemicals Alone, in Binary Combinations and in Combinations with Conventional Antibiotics. Nat. Prod. Res. 2021, 35, 5964–5967. [Google Scholar] [CrossRef]
- Bisso Ndezo, B.; Tokam Kuate, C.R.; Dzoyem, J.P. Synergistic Antibiofilm Efficacy of Thymol and Piperine in Combination with Three Aminoglycoside Antibiotics Against Klebsiella pneumoniae Biofilms. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 7029944. [Google Scholar] [CrossRef]
- Palaniappan, K.; Holley, R.A. Use of Natural Antimicrobials to Increase Antibiotic Susceptibility of Drug Resistant Bacteria. Int. J. Food Microbiol. 2010, 140, 164–168. [Google Scholar] [CrossRef]
- Gan, C.; Langa, E.; Wang, G.; Valenzuela, A.; Ballestero, D.; Pino-Otín, M. Mechanisms of Action and Resistance Prevention of Synergistic Thymol and Carvacrol Combinations with Antibiotics in Staphylococcus aureus and Acinetobacter baumannii. Nat. Prod. Bioprospect. 2025, 15, 36. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, K.; Rajabi, E.; Varpaei, H.A.; Iranzadasl, M.; Khodaparast, S.; Salehi, M. Thymol and Carvacrol Against Klebsiella: Anti-Bacterial, Anti-Biofilm, and Synergistic Activities—A Systematic Review. Front. Pharmacol. 2024, 15, 1487083. [Google Scholar] [CrossRef]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.H.; Mohamed, M.S.M.; Khalil, M.S.; Azmy, M.; Mabrouk, M.I. Combination of Essential Oil and Ciprofloxacin to Inhibit/Eradicate Biofilms in Multidrug-Resistant Klebsiella pneumoniae. J. Appl. Microbiol. 2018, 125, 84–95. [Google Scholar] [CrossRef]
- Touati, A.; Mairi, A.; Ibrahim, N.A.; Idres, T. Essential Oils for Biofilm Control: Mechanisms, Synergies, and Translational Challenges in the Era of Antimicrobial Resistance. Antibiotics 2025, 14, 503. [Google Scholar] [CrossRef]
- Jayakumar, D.; Mini, M.; Kumar, P.; Vaikkathillam, P.; Mohan, A.; Khan, S. Synergistic Effect of Thymol-Ciprofloxacin Combination on Planktonic Cells and Biofilm of Pseudomonas aeruginosa. Curr. Microbiol. 2023, 81, 1. [Google Scholar] [CrossRef] [PubMed]
- Miladi, H.; Zmantar, T.; Kouidhi, B.; Chaabouni, Y.; Mandouani, K.; Bakhrouf, A.; Mahdouani, K. Use of Carvacrol, Thymol, and Eugenol for Biofilm Eradication and Resistance Modifying Susceptibility of Salmonella enterica Serovar Typhimurium Strains to Nalidixic Acid. Microb. Pathog. 2017, 104, 56–63. [Google Scholar] [CrossRef]
- Santos, M.I.S.; Marques, C.; Mota, J.; Pedroso, L.; Lima, A. Applications of Essential Oils as Antibacterial Agents in Minimally Processed Fruits and Vegetables—A Review. Microorganisms 2022, 10, 760. [Google Scholar] [CrossRef] [PubMed]
- Holley, R.A.; Patel, D. Improvement in Shelf-Life and Safety of Perishable Foods by Plant Essential Oils and Smoke Antimicrobials. Food Microbiol. 2005, 22, 273–292. [Google Scholar] [CrossRef]
- Kabir, M.N.; Aras, S.; Allison, A.; Adhikari, J.; Chowdhury, S.; Fouladkhah, A. Interactions of Carvacrol, Caprylic Acid, Habituation, and Mild Heat for Pressure-Based Inactivation of O157 and Non-O157 Serogroups of Shiga Toxin-Producing Escherichia coli in Acidic Environment. Microorganisms 2019, 7, 145. [Google Scholar] [CrossRef] [PubMed]
- Zaharioudakis, K.; Salmas, C.E.; Andritsos, N.D.; Kollia, E.; Leontiou, A.; Karabagias, V.K.; Karydis-Messinis, A.; Moschovas, D.; Zafeiropoulos, N.E.; Avgeropoulos, A.; et al. Carvacrol, Citral, Eugenol and Cinnamaldehyde Casein-Based Edible Nanoemulsions as Novel Sustainable Active Coatings for Fresh Pork Tenderloin Meat Preservation. Front. Food Sci. Technol. 2024, 4, 1400224. [Google Scholar] [CrossRef]
- de Souza, R.L.; Dantas, A.G.B.; de Oliveira Melo, C.; Felício, I.M.; Oliveira, E.E. Nanotechnology as a Tool to Improve the Biological Activity of Carvacrol: A Review. J. Drug Deliv. Sci. Technol. 2022, 76, 103834. [Google Scholar] [CrossRef]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed). Scientific opinion on the safety and efficacy of polyoxyethylene (20) sorbitan monooleate as a feed additive for all animal species. EFSA J. 2016, 14, 4443. [Google Scholar] [CrossRef]
- McClements, D.J.; Rao, J. Food-Grade Nanoemulsions: Formulation, Fabrication, Properties, Performance, Biological Fate, and Potential Toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef]
- Campolo, O.; Giunti, G.; Laigle, M.; Michel, T.; Palmeri, V. Essential Oil-Based Nano-Emulsions: Effect of Different Surfactants, Sonication and Plant Species on Physicochemical Characteristics. Ind. Crops Prod. 2020, 157, 112935. [Google Scholar] [CrossRef]
- Al-Asmari, F.; Koirala, P.; Rathod, N.B.; Alnemr, T.M.; Asiri, S.A.; Babeker, M.Y.; Li, L.; Nirmal, N.P. Antimicrobial Activity of Formulated Origanum and Thyme Essential Oil Nanoemulsions—A Comparative Study. Curr. Nutr. Food Sci. 2024, 20, e140923221094. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Chow, A.H.; Ren, K.; Gong, T.; Zhang, Z.; Zheng, Y. Self-Nanoemulsifying Drug Delivery System (SNEDDS) for Oral Delivery of Zedoary Essential Oil: Formulation and Bioavailability Studies. Int. J. Pharm. 2010, 383, 170–177. [Google Scholar] [CrossRef]
- Tan, T.B.; Yussof, N.S.; Abas, F.; Mirhosseini, H.; Nehdi, I.A.; Tan, C.P. Stability Evaluation of Lutein Nanodispersions Prepared via Solvent Displacement Method: The Effect of Emulsifiers with Different Stabilizing Mechanisms. Food Chem. 2016, 205, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Tibaldi, G.; Fontana, E.; Nicola, S. Growing Conditions and Postharvest Management Can Affect the Essential Oil of Origanum vulgare L. ssp. hirtum (Link) Ietswaart. Ind. Crops Prod. 2011, 34, 1516–1522. [Google Scholar] [CrossRef]
- Dodoš, T.; Novaković, J.; Vujisić, L.; Marin, P.D.; Rajčević, N. Geographic Variability of Winter Savory Essential Oil. Ind. Crops Prod. 2024, 210, 118167. [Google Scholar] [CrossRef]
- The European Directorate for the Quality of Medicines & HealthCare. European Pharmacopoeia, 10th ed.; Method 2.8.12—Essential Oils in Herbal Drugs; Council of Europe: Strasbourg, France, 2020; pp. 307–308. [Google Scholar]
- Van den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas–Liquid Partition Chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Massada, Y. Analysis of Essential Oils by Gas Chromatography and Mass Spectrometry; Halsted Press: John Wiley & Sons: New York, NY, USA, 1976. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Puškárová, A.; Bučková, M.; Kraková, L.; Pangallo, D.; Kozics, K. The Antibacterial and Antifungal Activity of Six Essential Oils and Their Cyto/Genotoxicity to Human HEL 12469 Cells. Sci. Rep. 2017, 7, 8211. [Google Scholar] [CrossRef] [PubMed]
- Bodea, I.M.; Cătunescu, G.M.; Palop, A.; Fernandez, P.S.; Garre, A. Washing Solutions Containing Nanoemulsified Essential Oils as a Potential Substitute of Commercial Washes for the Decontamination of Escherichia coli O157:H7 on Cherry Tomatoes. LWT 2023, 190, 115549. [Google Scholar] [CrossRef]
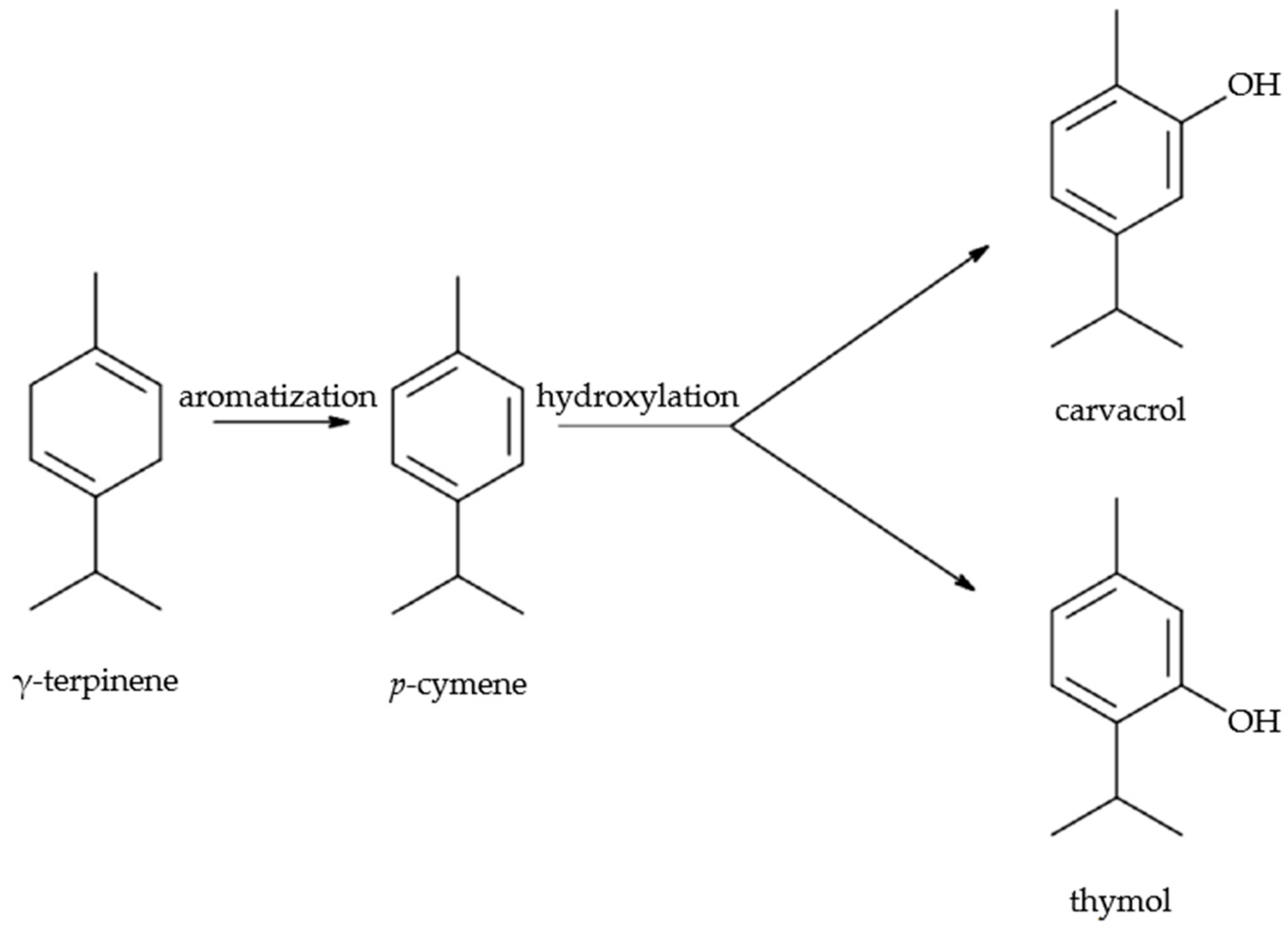
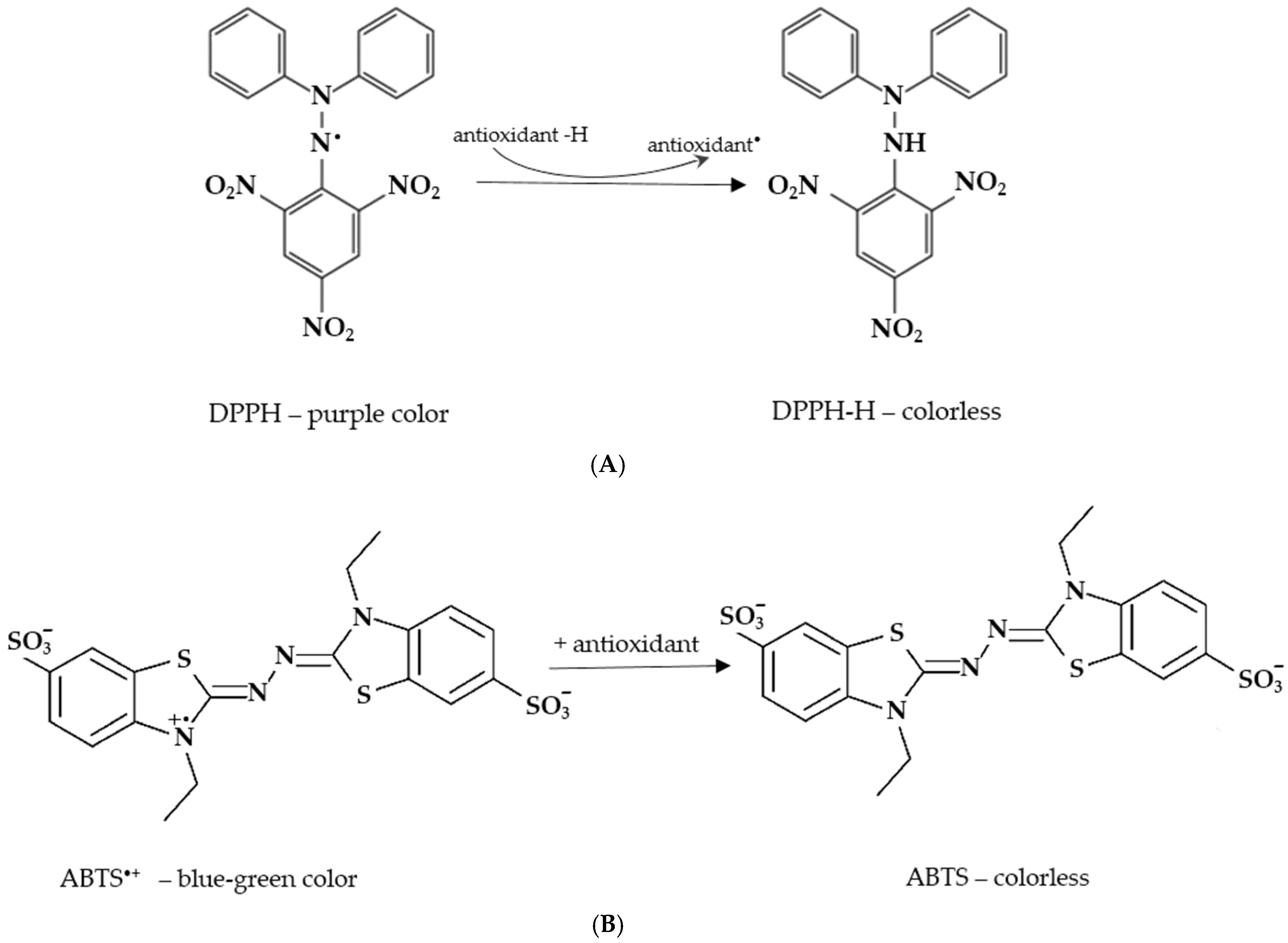
| Nr. | Species | Plant Code | Yield of Aerial Parts (% *) | Yield (% *) of Leaves and Flowers Without Stems |
|---|---|---|---|---|
| 1. | Origanum vulgare subsp. hirtum | OV-L | 4.06 | 6.19 |
| 2. | OV-P | 3.83 | 6.11 | |
| 3. | Thymbra capitata | TC-M | 1.66 | 3.00 |
| 4. | TC-L | 0.75 | 2.30 | |
| 5. | Satureja montana | SM-B | 0.39 | 0.80 |
| 6. | SM-D | 0.73 | 0.79 |
| Compounds a | AI b | OV-P (% c) | OV-L (% c) | ID d |
|---|---|---|---|---|
| α-Thujene | 924 | 1.16 | 0.97 | AI, MS |
| α-Pinene | 931 | 0.95 | 0.75 | AI, MS, Co-GC |
| Camphene | 945 | 0.54 | 0.66 | AI, MS |
| 1-Octen-3-ol | 983 | 0.6 | 0.6 | AI, MS |
| β-Myrcene | 990 | 1.5 | 1.5 | AI, MS, Co-GC |
| δ-2-Carene | 1003 | 0.2 | 0.14 | AI, MS |
| α-Phellandrene | 1005 | 0.2 | 0.2 | AI, MS |
| α-Terpinene | 1016 | 0.98 | 1.03 | AI, MS |
| p-Cymene | 1025 | 3.8 | 3.04 | AI, MS, Co-GC |
| Sylvestrene | 1029 | 0.3 | 0.26 | AI, MS |
| Eucalyptol | 1030 | 0.36 | nd | AI, MS |
| trans-Ocimene | 1038 | 0.1 | 0.08 | AI, MS |
| cis-Ocimene | 1050 | 0.1 | 0.06 | AI, MS |
| γ-Terpinene | 1059 | 5.8 | 5.4 | AI, MS, Co-GC |
| trans-Sabinenehydrate | 1070 | 0.46 | 0.34 | AI, MS |
| Terpinolene | 1085 | 0.1 | 0.07 | AI, MS |
| Linalool | 1102 | 0.1 | nd | AI, MS, Co-GC |
| α-Thujone | 1104 | 0.1 | 0.07 | AI, MS |
| Camphor | 1143 | 0.15 | 0.13 | AI, MS |
| Terpinen-4-ol | 1181 | 0.15 | 0.09 | AI, MS, Co-GC |
| Thymol | 1294 | 2.32 | 0.23 | AI, MS, Co-GC |
| Carvacrol | 1304 | 74.6 | 79.8 | AI, MS |
| α-Ylangene | 1371 | 0.1 | 0.05 | AI, MS |
| β-Caryophyllene | 1417 | 1.1 | 1.2 | AI, MS, Co-GC |
| α-Humulene | 1454 | 0.16 | 0.2 | AI, MS, Co-GC |
| Caryophyllene oxide | 1582 | 0.75 | 0.5 | AI, MS, Co-GC |
| Total | 96.68 | 97.37 |
| Compounds a | AI b | TC-M (% c) | TC-L (% c) | ID d |
|---|---|---|---|---|
| α-Pinene | 931 | 1.3 | 1.3 | AI, MS, Co-GC |
| β-Pinene | 973 | 0.2 | 0.4 | AI, MS, Co-GC |
| Octen-3-ol | 983 | 0.2 | 0.8 | AI, MS |
| β-Myrcene | 992 | 0.1 | 1.3 | AI, MS, Co-GC |
| α-terpinene | 931 | 0.9 | 1.0 | AI, MS, Co-GC |
| p-Cymene | 1024 | 4.4 | 4.56 | AI, MS, Co-GC |
| γ-terpinene | 1055 | 2.8 | 3.94 | AI, MS, Co-GC |
| cis-Sabinenehydrate | 1067 | 0.4 | 0.2 | AI, MS |
| Linalool | 1101 | 0.9 | 0.5 | AI, MS, Co-GC |
| Borneol | 1164 | 0.9 | 0.7 | AI, MS, Co-GC |
| 4-carvomenthenol | 1185 | 0.7 | 0.5 | AI, MS, Co-GC |
| o-cymen-5-ol | 1280 | 0.2 | 0.2 | AI, MS, Co-GC |
| 2-isopropyl-5-methyl-phenol | 1295 | 0.3 | 0.2 | AI, MS, Co-GC |
| Carvacrol | 1304 | 77.7 | 72.8 | AI, MS |
| 5-isopropyl-2-methyl phenol | 1358 | nd | 0.3 | AI, MS |
| 2-isopropyl-5-methyl-phenyl acetate | 1377 | 0.1 | nd | AI, MS |
| Caryophyllene | 1419 | 1.95 | 2.2 | AI, MS, Co-GC |
| Spathulenol | 1578 | 0.1 | 0.2 | AI, MS |
| Carryophyllene oxide | 1583 | 0.5 | 0.5 | AI, MS, Co-GC |
| Total | 93.65 | 91.6 |
| Compounds a | AI b | SM-B (% c) | SM-D (% c) | ID d |
|---|---|---|---|---|
| α-Thujene | 926 | 1.3 | 1.4 | AI, MS |
| α-Pinene | 931 | 0.9 | 0.7 | AI, MS, Co-GC |
| Camphene | 945 | 0.1 | 0.8 | AI, MS |
| β-Pinene | 973 | 0.1 | 0.1 | AI, MS, Co-GC |
| Octen-3-ol | 983 | nd | 0.2 | AI, MS |
| β-Myrcene | 992 | 1.4 | 1.0 | AI, MS, Co-GC |
| α-Phellandrene | 1003 | 1.03 | 0.9 | AI, MS |
| δ-2-Carene | 1008 | 0.2 | 0.3 | AI, MS |
| δ-3-Carene | 1015 | 1.3 | 1.4 | AI, MS, Co-GC |
| p-Cymene | 1024 | 8.9 | 11.8 | AI, MS, Co-GC |
| Limonene | 1027 | 0.6 | 0.9 | AI, MS |
| Eucalyptol | 1029 | 0.3 | 0.4 | AI, MS |
| trans-Ocimene | 1040 | 0.3 | 0.8 | AI, MS |
| cis-Ocimene | 1050 | 0.13 | 0.2 | AI, MS |
| γ-Terpinene | 1059 | 4.7 | 5.4 | AI, MS, Co-GC |
| cis-Sabinenehydrate | 1067 | 1.5 | 4.2 | AI, MS |
| Terpinolene | 1087 | 0.2 | 0.3 | AI, MS |
| trans-Sabinenehydrate | 1098 | 1.4 | 0.1 | AI, MS |
| Linalool | 1101 | 3.3 | 0.5 | AI, MS, Co-GC |
| α-Thujone | 1104 | 0.94 | 0.1 | AI, MS |
| β-Thujone | 1116 | 0.04 | tr | AI, MS |
| cis-p-Menth-2-en-1-ol | 1122 | 0.2 | tr | AI, MS |
| Camphor | 1143 | 0.3 | 0.3 | AI, MS |
| Borneol | 1164 | 2.4 | 2.8 | AI, MS, Co-GC |
| δ-Terpineol | 1169 | nd | 0.7 | AI, MS |
| Terpinene-4-ol | 1176 | 1.98 | 3.2 | AI, MS, Co-GC |
| p-Cymen-8-ol | 1187 | 0.3 | 0.1 | AI, MS |
| α-Terpineol | 1191 | 0.04 | 0.2 | AI, MS |
| Thymol methyl ether | 1236 | 1.98 | 0.1 | AI, MS |
| Carvacrol methyl ether | 1244 | 5.2 | 5.5 | AI, MS |
| Bornyl acetate | 1286 | 0.04 | nd | AI, MS, Co-GC |
| Thymol | 1294 | 52.8 | 28.5 | AI, MS, Co-GC |
| Carvacrol | 1304 | 2.5 | 1.2 | AI, MS |
| Thymyl acetate | 1356 | 0.45 | 0.5 | AI, MS |
| α-Copaene | 1375 | 0.1 | 0.1 | AI, MS |
| β-Burbonene | 1384 | 0.1 | 0.2 | AI, MS |
| β-Caryophyllene | 1419 | nd | 2.3 | AI, MS, Co-GC |
| β-Copaene | 1428 | nd | 0.2 | AI, MS |
| γ-Elemene | 1434 | nd | 0.6 | AI, MS |
| Aromadendrene | 1438 | nd | 0.5 | AI, MS |
| Myltayl-4(12)-ene | 1443 | nd | nd | AI, MS |
| α-Carryophyllene | 1453 | 0.6 | 0.2 | AI, MS, Co-GC |
| Allo-Aromadendrene | 1460 | 0.7 | 0.2 | AI, MS |
| Dauca-5,8-diene | 1474 | nd | 0.55 | AI, MS |
| γ-Muurolene | 1477 | nd | 0.25 | AI, MS |
| Spathulenol | 1578 | nd | 0.13 | AI, MS |
| Carryophyllene oxide | 1583 | nd | 1.0 | AI, MS, Co-GC |
| Total | 98.33 | 80.83 |
| Species | EOs from Samples | DPPH μg/mL | ABTS μg/mL |
|---|---|---|---|
| Origanum vulgare subsp. hirtum | OV-L | 530 ± 8 | 110 ± 10 |
| OV-P | 600 ± 12 | 120 ± 8 | |
| Thymbra capitata | TC-M | 530 ± 9 | 180 ± 9 |
| TC-L | 570 ± 8 | 220 ±13 | |
| Satureja montana | SM-B | 1200 ± 5 | 460 ± 27 |
| SM-D | 820 ± 4 | 500 ± 14 |
| Species | EO from Sample | MIC (mg/mL) | |||||
|---|---|---|---|---|---|---|---|
| E. coli ATCC 10535 | S. Enteritidis ATCC 49223 | P. aeruginosa ATCC 9027 | M. luteus ATCC 10240 | S. maltophilia ATCC 13637 | C. albicans ATCC 10231 | ||
| Origanum vulgare subsp. hirtum | OV-L | 0.312 | 1.250 | 1.250 | 0.312 | 0.156 | 0.312 |
| OV-P | 0.625 | 0.625 | 1.250 | 0.625 | 0.156 | 0.312 | |
| Thymbra capitata | TC-M | 0.312 | 0.625 | 2.5 | 0.625 | 0.156 | 0.156 |
| TC-L | 0.625 | 1.250 | NO MIC | 0.625 | 0.312 | 0.156 | |
| Satureja montana | SM-B | 0.625 | 1.250 | NO MIC | 0.625 | 1.250 | 0.312 |
| SM-D | 1.250 | 2.5 | 2.5 | 0.625 | 0.625 | 0.625 | |
| Species | EO from Sample | Yield (% a) | Key Active Components | Antioxidant Activity (DPPH/ABTS, μg/mL b) | Antimicrobial Spectrum c |
|---|---|---|---|---|---|
| Origanum vulgare subsp. hirtum | OV-L | 4.06 | Carvacrol (74.6%), γ-Terpinene | 530/110 | Broad, strong |
| OV-P | 3.83 | Carvacrol (79.8%), γ-Terpinene | 600/120 | Broad, strong | |
| Thymbra capitata | TC-M | 1.66 | Carvacrol (77.7%), p-cymene | 530/180 | Broad, strong |
| TC-L | 0.75 | Carvacrol (72.8%), p-cymene | 570/220 | Moderate, inactive against P. aeruginosa | |
| Satureja montana | SM-B | 0.39 | Thymol (52.8%), p-Cymene | 1200/460 | Weak, inactive against P. aeruginosa |
| SM-D | 0.73 | Thymol (28.5%), p-Cymene | 820/500 | Weakest overall |
| Nanoemulsion of EO from Sample | Particle Size (nm a) | PDI | Z-Potential (mV b) |
|---|---|---|---|
| OV-L | 132.4 ±15.3 | 0.152 ±0.126 | −11.2 ± 2.8 |
| TC-M | 191.8± 17.2 | 0.076 ±0.09 | −9.6 ± 0.5 |
| Nr. | Species | Location | Plant Code | Collection Date | Altitude * (m.a.s.l.) | Coordinates | |
|---|---|---|---|---|---|---|---|
| Latitude (N) | Longitude (E) | ||||||
| 1. | Origanum vulgare subsp. hirtum | Lukovë | OV-L | 7 August 2023 | ~55 | 39°97′88″ | 19°91′29″ |
| 2. | Qafë Pishë | OV-P | 4 August 2023 | ~1200 | 40°25′92″ | 19°79′11″ | |
| 3. | Thymbra capitata | Mallakastër | TC-M | 4 August 2023 | ~54 | 41°58′47″ | 20°13′12″ |
| 4. | Lukovë | TC-L | 13 August 2023 | ~179 | 39°98′19″ | 19°91′71″ | |
| 5. | Satureja montana | Bego Mauntain | SM-B | 4 August 2023 | ~1500 | 40°25′44″ | 19°78′86″ |
| 6. | Dajti Mountain | SM-D | 19 August 2023 | ~724 | 41°36′90″ | 19°94′20″ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basha, E.; Mamoçi, E.; Sharma, A.; Hodaj-Çeliku, E.; Zejnelhoxha, S.; Medeleanu, M.L.; Socaci, S.A.; Bisha, B. Essential Oils from Wild Albanian Lamiaceae: GC-MS Profiling, Biological Activity, and Enhanced Delivery via Nanoencapsulation. Molecules 2025, 30, 3329. https://doi.org/10.3390/molecules30163329
Basha E, Mamoçi E, Sharma A, Hodaj-Çeliku E, Zejnelhoxha S, Medeleanu ML, Socaci SA, Bisha B. Essential Oils from Wild Albanian Lamiaceae: GC-MS Profiling, Biological Activity, and Enhanced Delivery via Nanoencapsulation. Molecules. 2025; 30(16):3329. https://doi.org/10.3390/molecules30163329
Chicago/Turabian StyleBasha, Elton, Erjon Mamoçi, Aniket Sharma, Entela Hodaj-Çeliku, Sanije Zejnelhoxha, Mădălina L. Medeleanu, Sonia A. Socaci, and Bledar Bisha. 2025. "Essential Oils from Wild Albanian Lamiaceae: GC-MS Profiling, Biological Activity, and Enhanced Delivery via Nanoencapsulation" Molecules 30, no. 16: 3329. https://doi.org/10.3390/molecules30163329
APA StyleBasha, E., Mamoçi, E., Sharma, A., Hodaj-Çeliku, E., Zejnelhoxha, S., Medeleanu, M. L., Socaci, S. A., & Bisha, B. (2025). Essential Oils from Wild Albanian Lamiaceae: GC-MS Profiling, Biological Activity, and Enhanced Delivery via Nanoencapsulation. Molecules, 30(16), 3329. https://doi.org/10.3390/molecules30163329










