Recent Advances in Microtubule Targeting Agents for Cancer Therapy
Abstract
1. Introduction
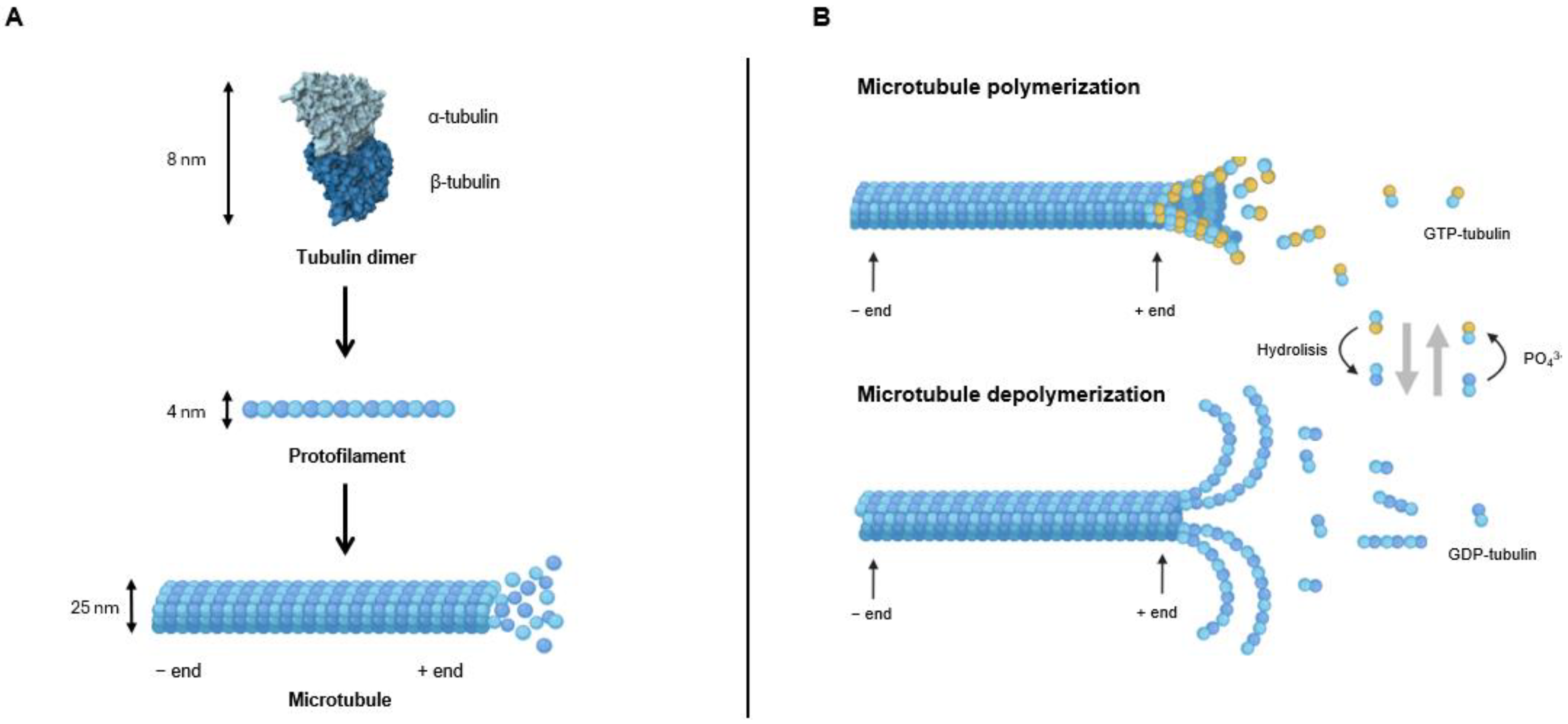
2. Microtubule’s Structure and Dynamics
3. Microtubules as a Prime Target in Cancer Therapeutics
4. Binding Site Landscape of MTAs
5. MTAs Resistance
6. Recent Advances in MTAs
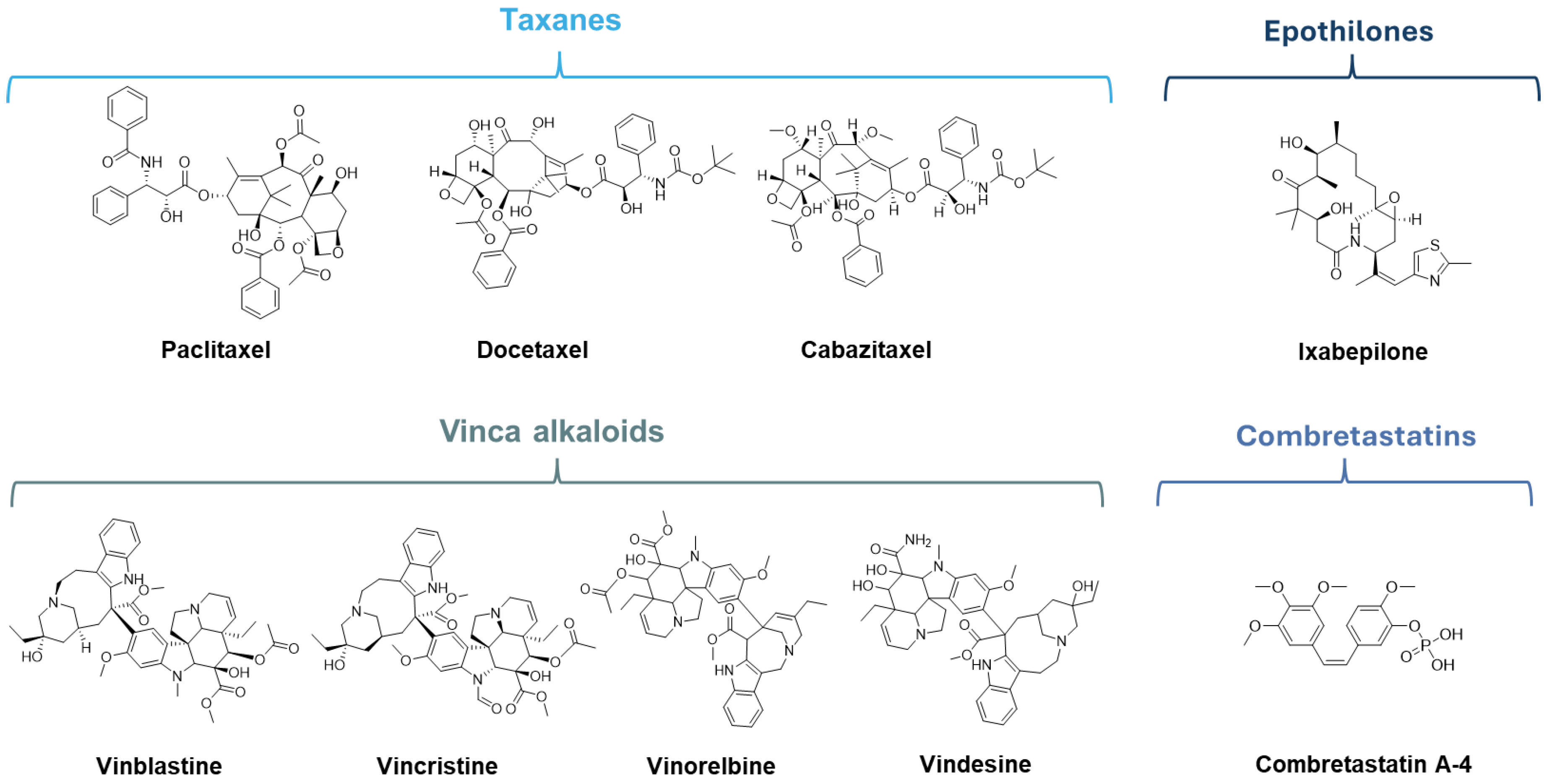
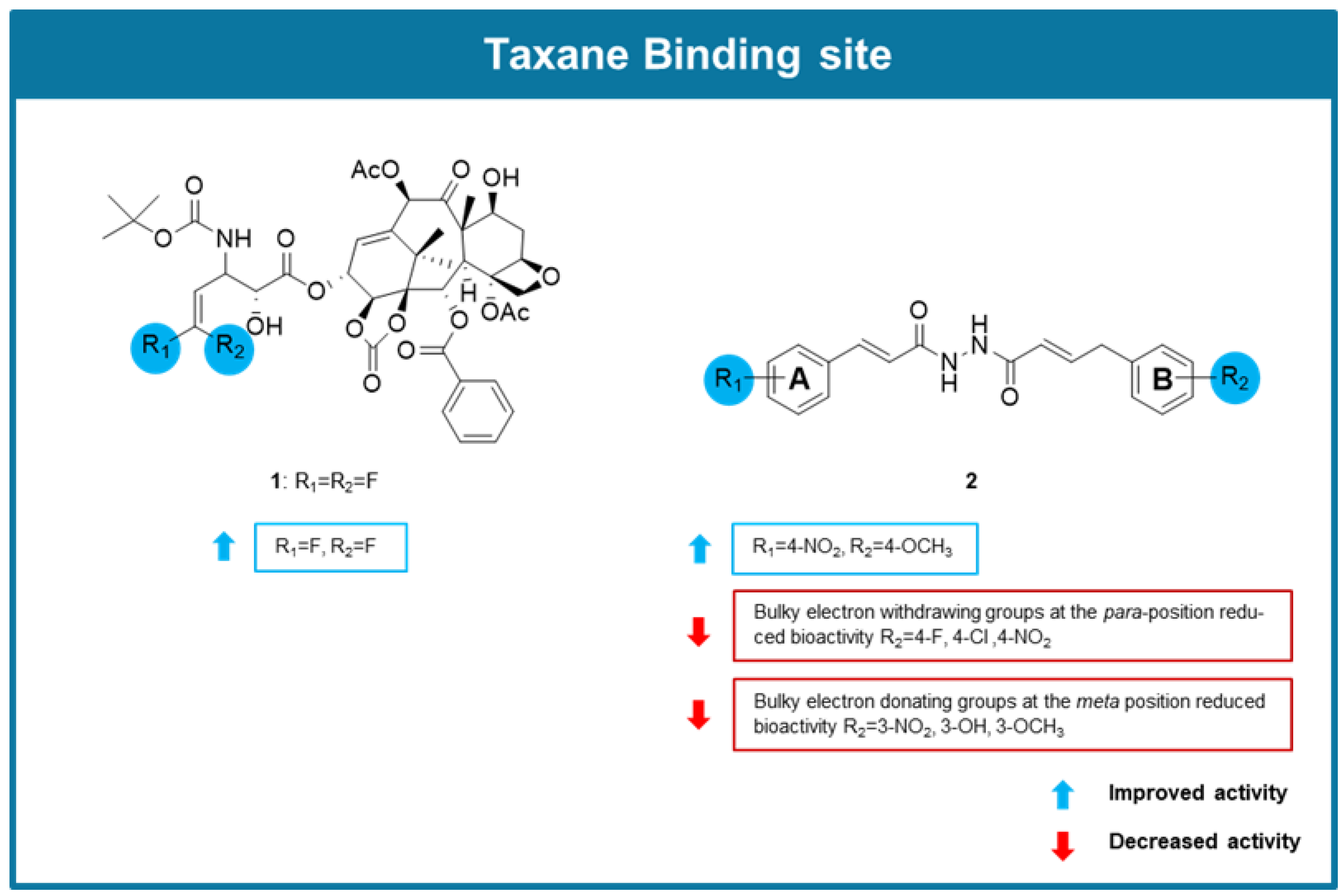
6.1. MTAs Targeting Taxane Binding Site
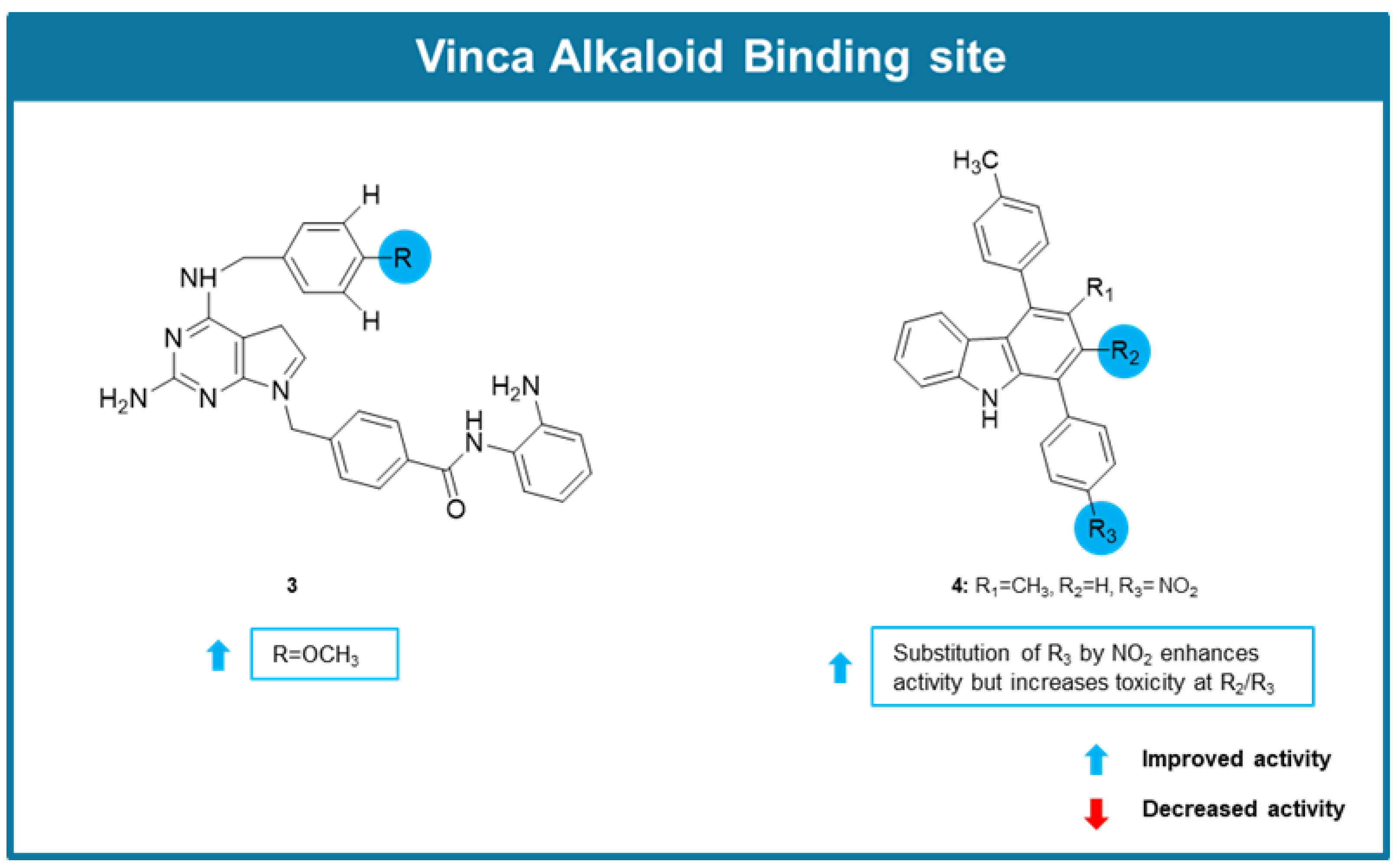
6.2. MTAs Targeting Vinca Alkaloids Binding Site
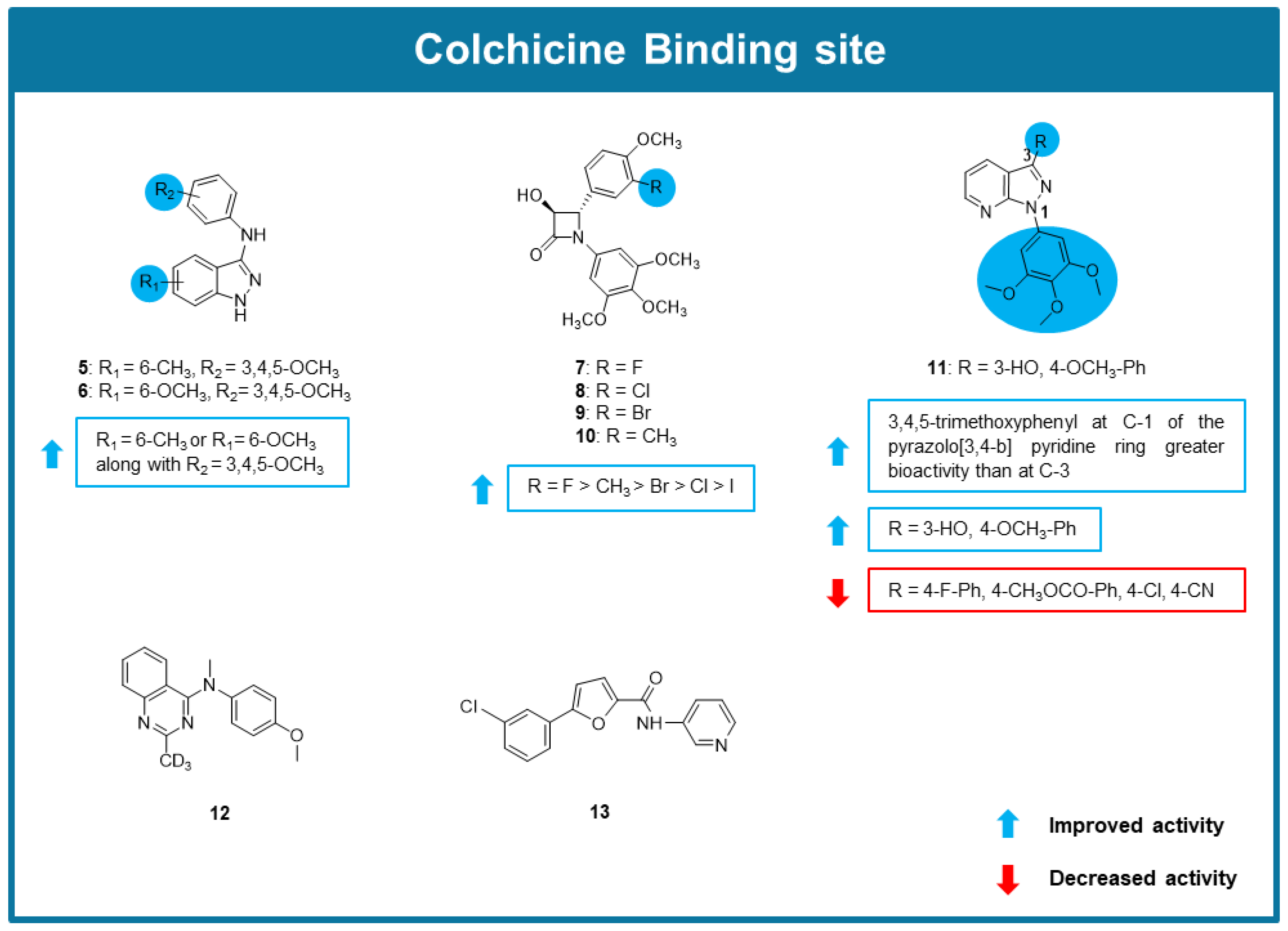
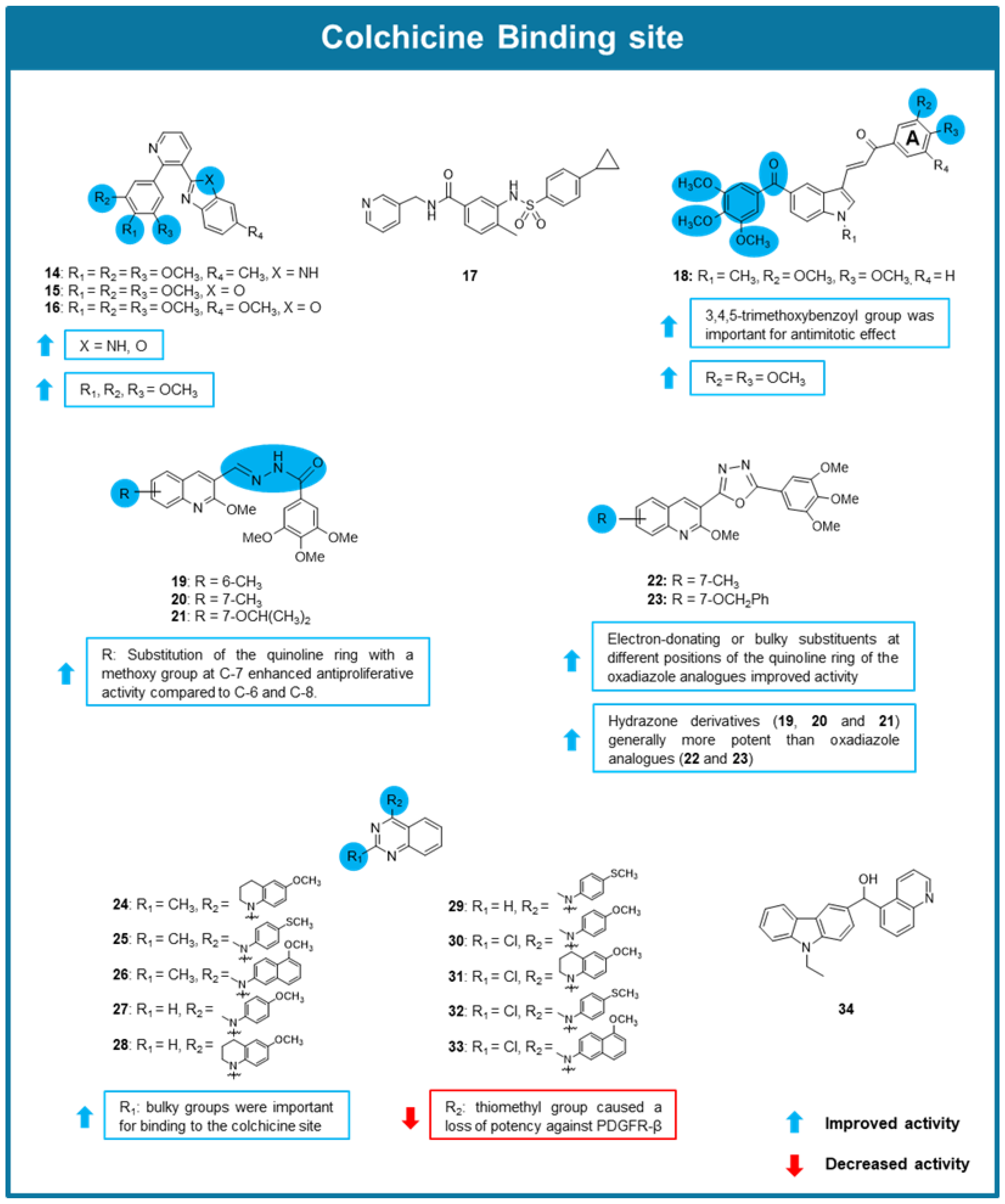
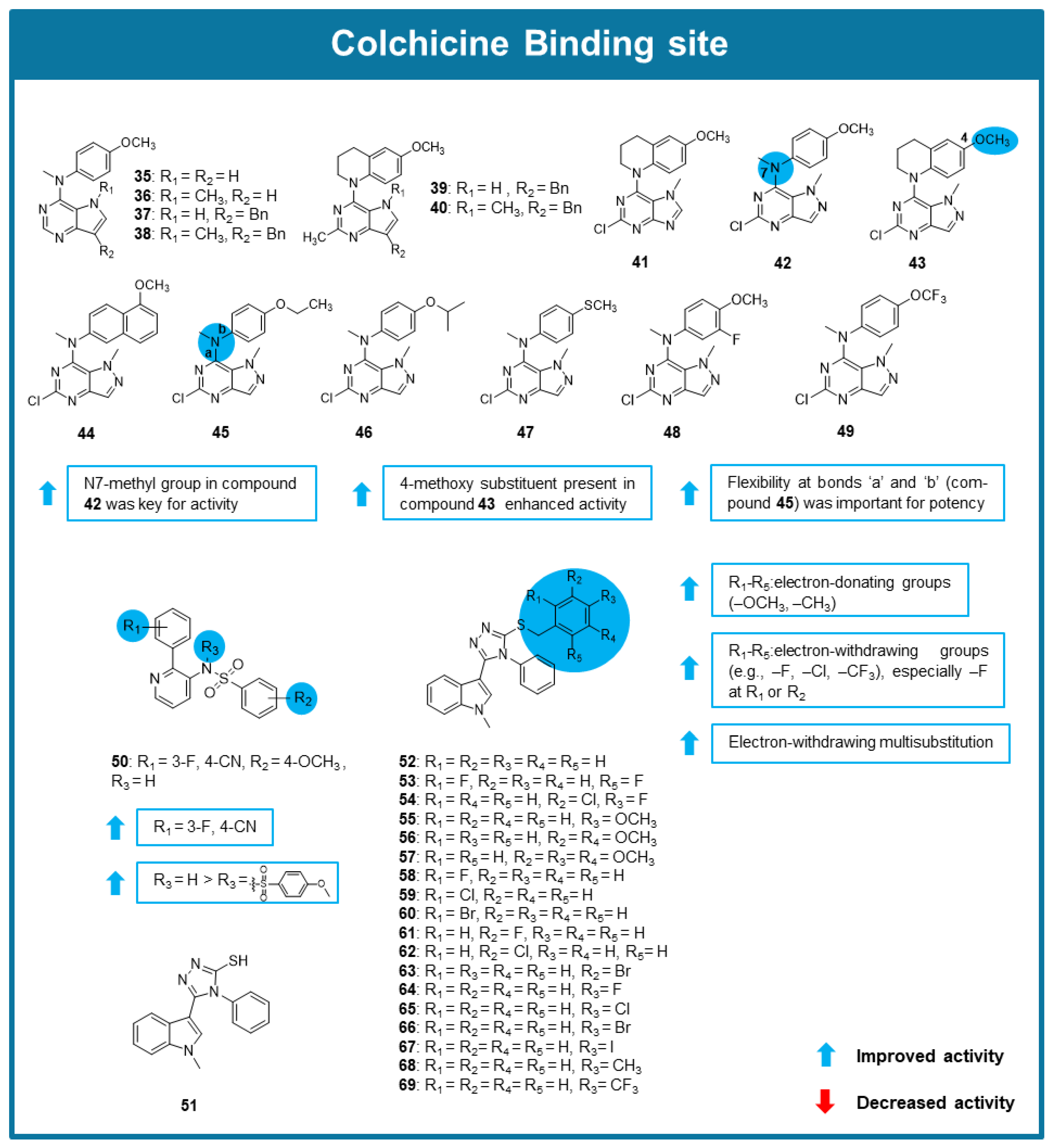
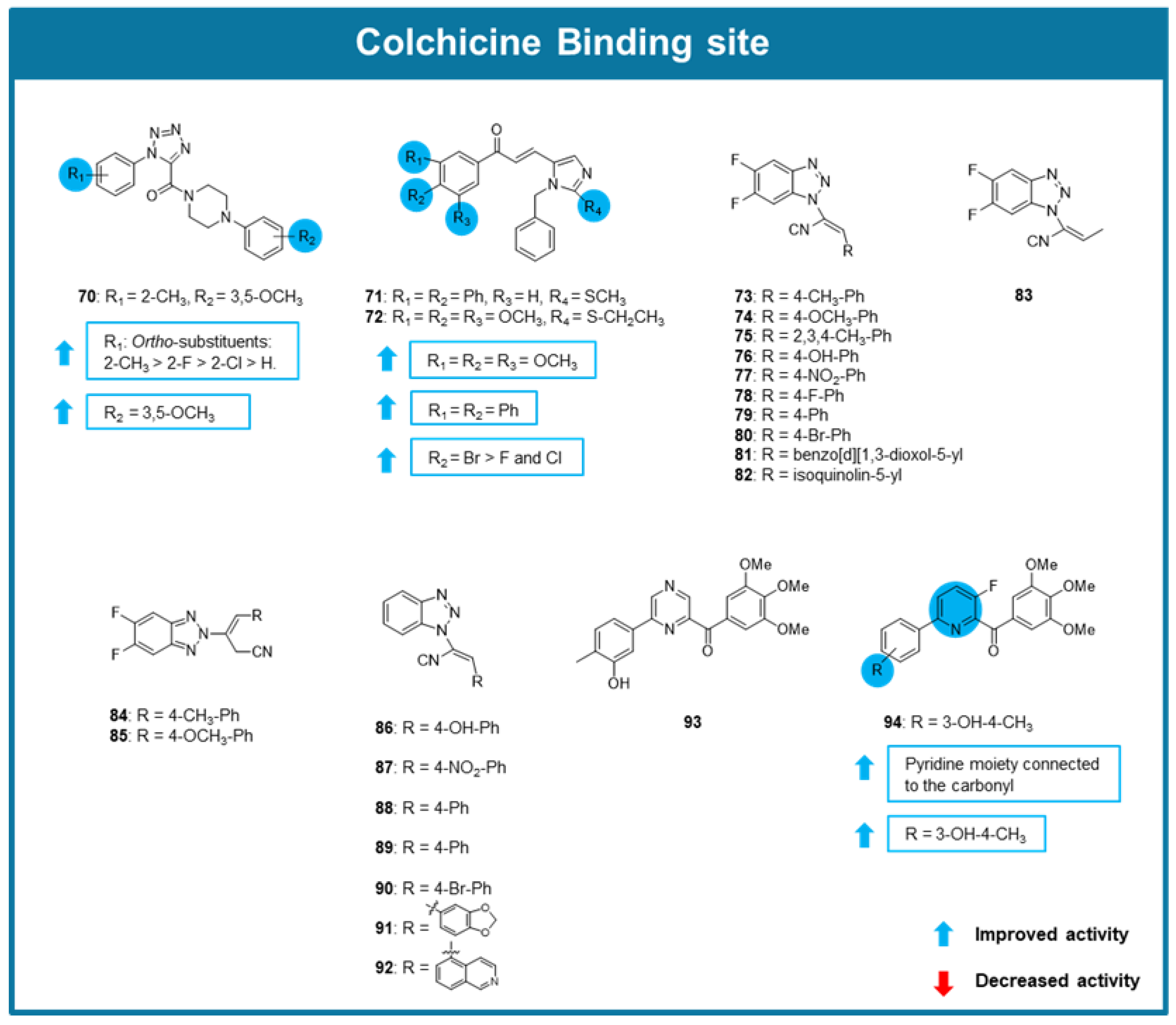
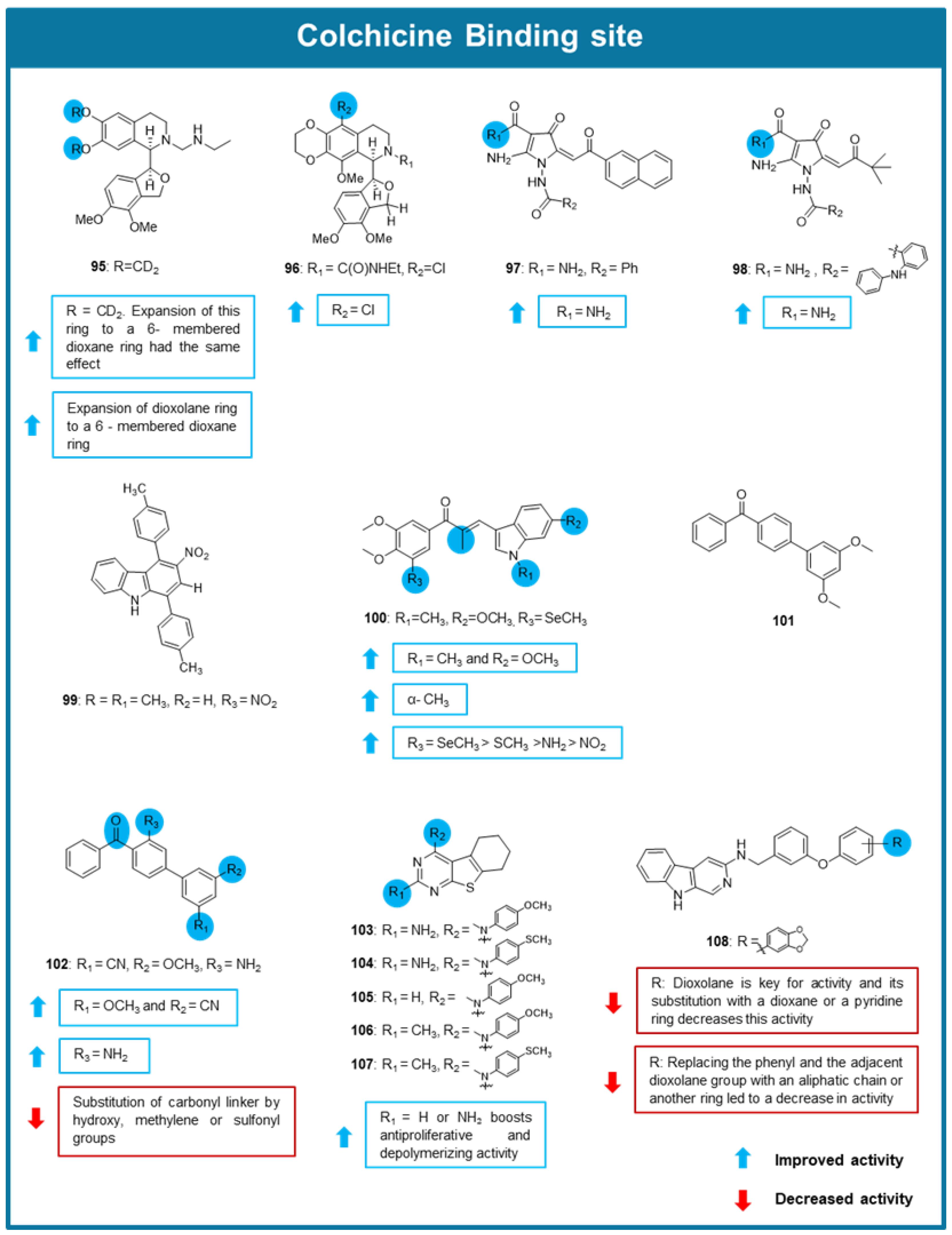
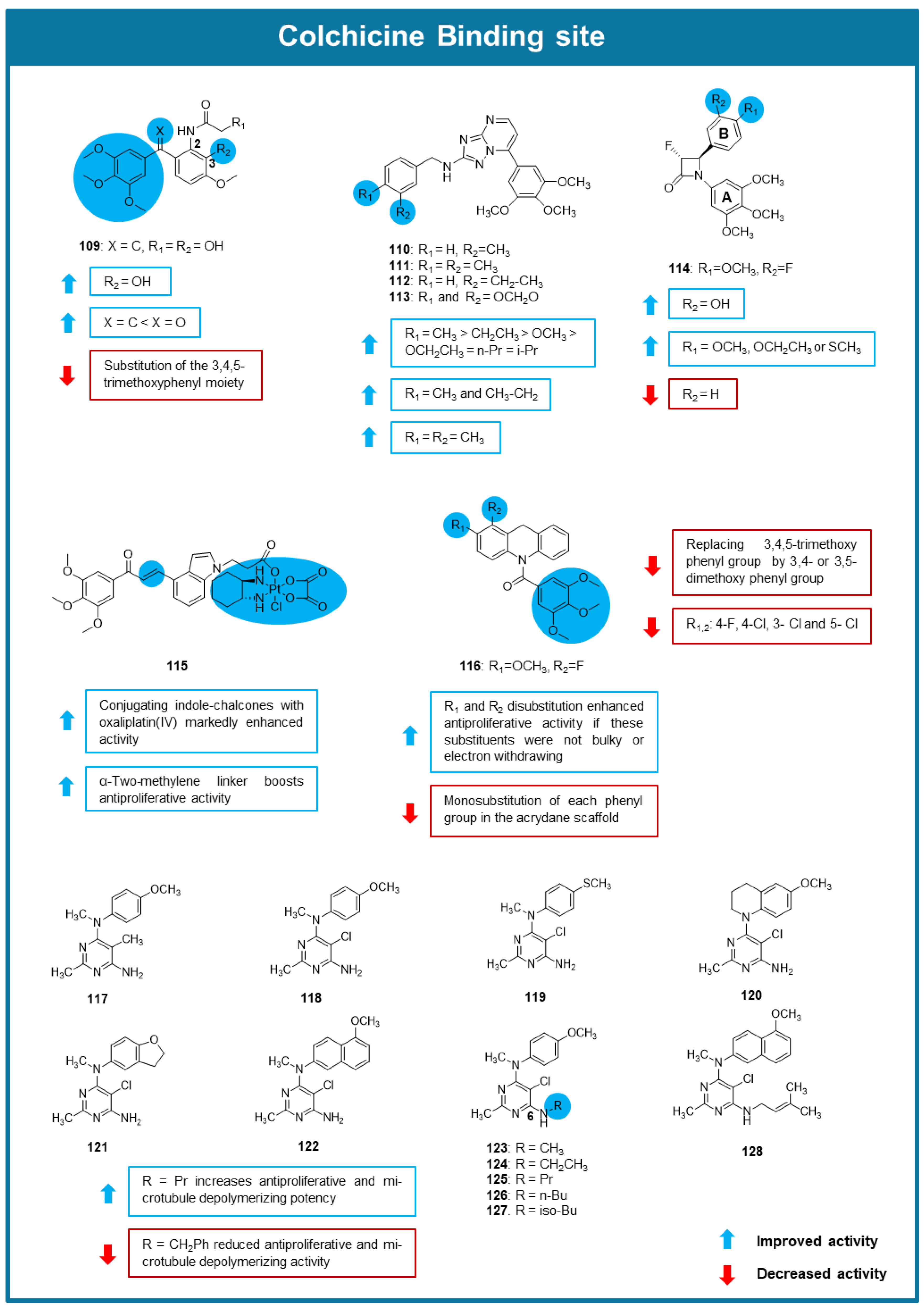
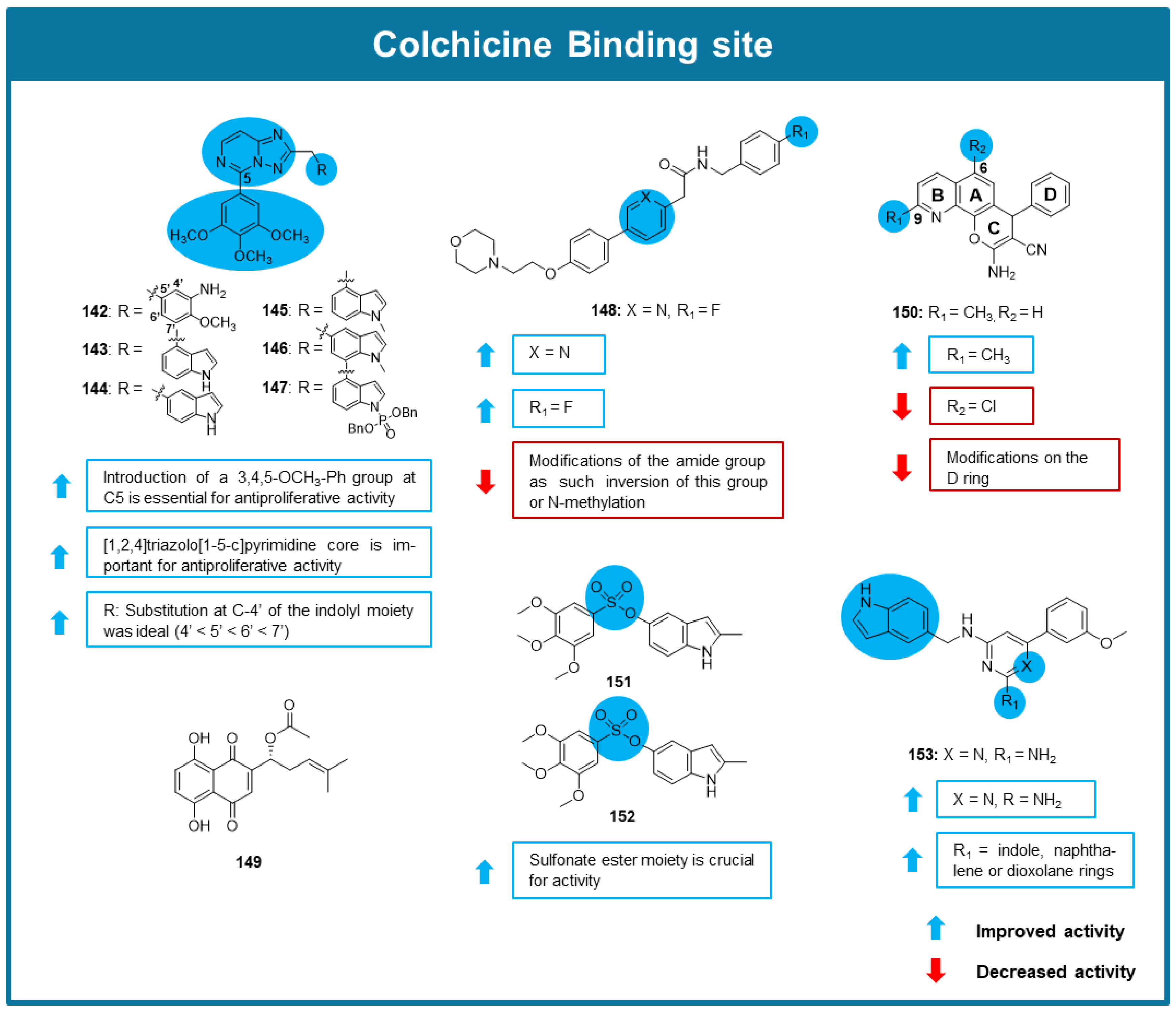
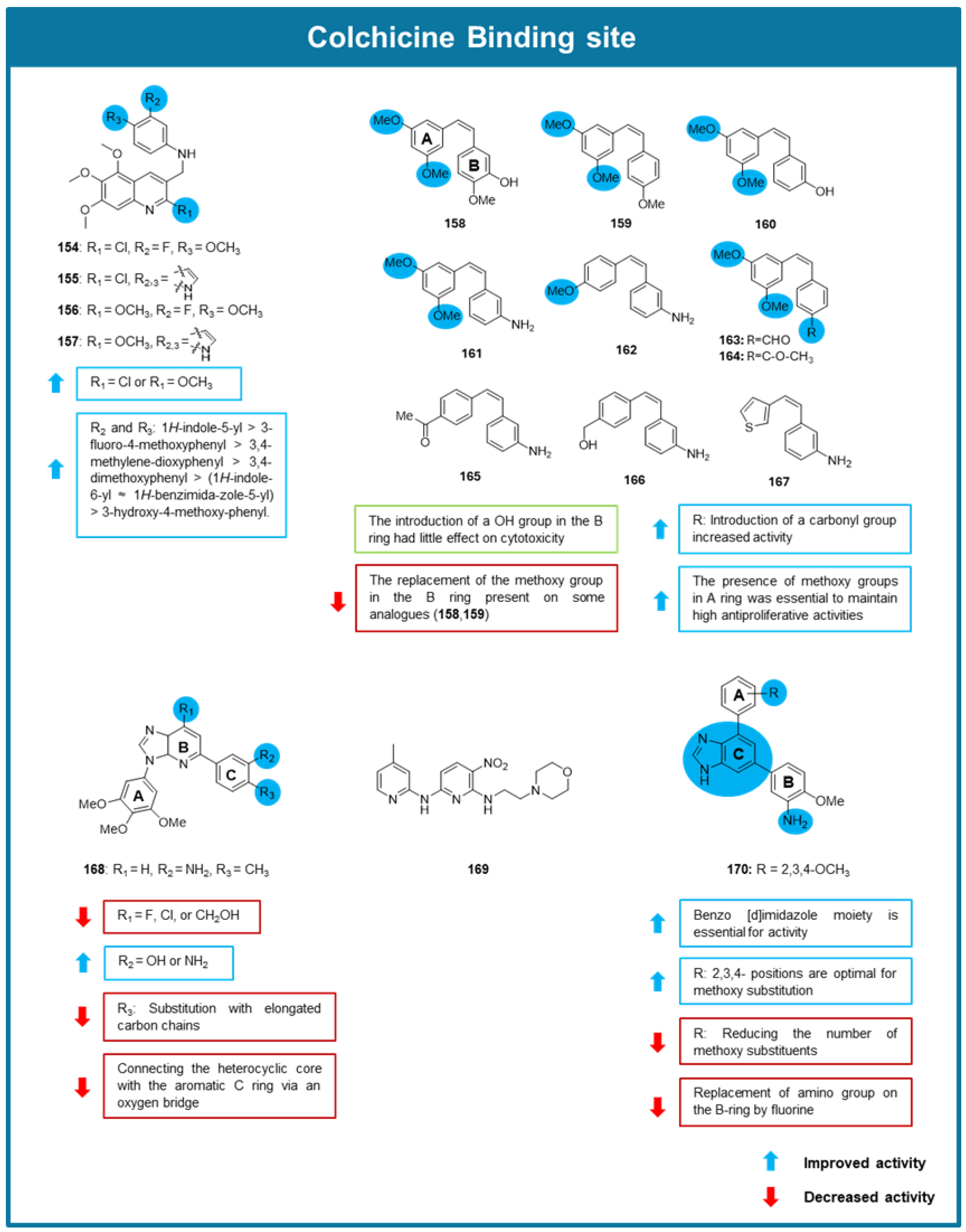
6.3. MTAs Targeting Colchicine Binding Site
6.4. MTAs Targeting Maytansine Binding Site
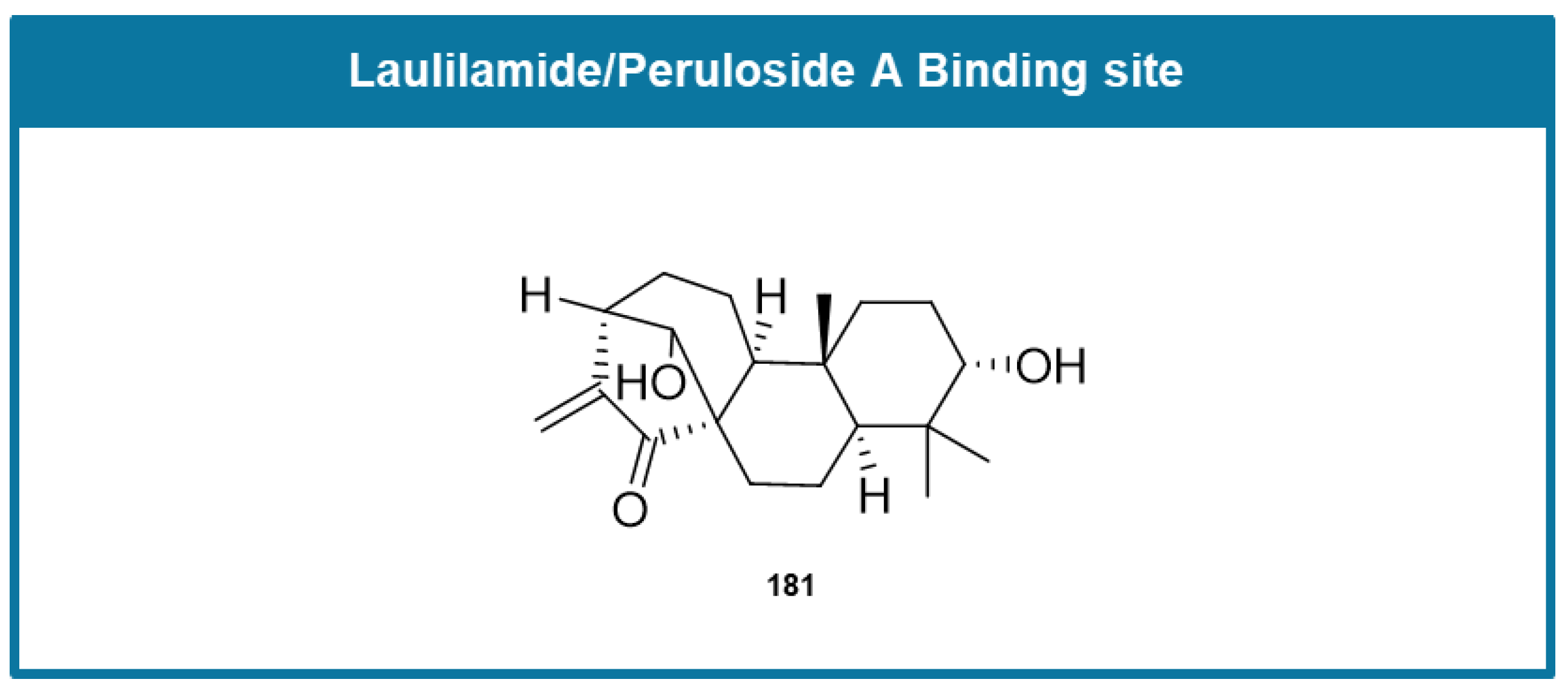
6.5. MTAs Targeting Laulimalide/Peloruside A Binding Site
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MTA | Microtubule-targeting agent |
| SAR | Structure–activity relationship |
| MDR | Multidrug-resistant |
| GTP | Guanosine triphosphate |
| MAPs | Microtubule-associated proteins |
| MTOC | Microtubule-organizing center |
| CBS | Colchicine binding site |
| P-pg | P-glycoprotein |
| MRP | Multidrug resistance-associated proteins |
| EBI | N,N-ethylenebis(iodoacetamide) |
References
- Kelling, J.; Sullivan, K.; Wilson, L.; Jordan, M.A. Suppression of centromere dynamics by Taxol in living osteosarcoma cells. Cancer Res. 2023, 63, 2794–2801. [Google Scholar]
- Barrales-Cureño, H.J. Pharmacological applications and in vitro biotechnological production of anticancer alkaloids of Catharanthus roseus. Biotecnol. Apl. 2015, 32, 1101–1110. [Google Scholar]
- Mosca, L.; Ilari, A.; Fazi, F.; Assaraf, Y.G.; Colotti, G. Taxanes in cancer treatment: Activity, chemoresistance and its overcoming. Drug Resist. Updat. 2021, 54, 100742. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Stanton, R.A.; Gernert, K.M.; Nettles, J.H.; Aneja, R. Drugs that target dynamic microtubules: A new molecular perspective. Med. Res. Rev. 2011, 31, 443–481. [Google Scholar] [CrossRef] [PubMed]
- Fojo, T.; Menefee, M. Mechanisms of multidrug resistance: The potential role of microtubule-stabilizing agents. Ann. Oncol. 2007, 18 (Suppl. S5), v3–v8. [Google Scholar] [CrossRef] [PubMed]
- Martino, E.; Casamassima, G.; Castiglione, S.; Cellupica, E.; Pantalone, S.; Papagni, F.; Rui, M.; Siciliano, A.M.; Collina, S. Vinca alkaloids and analogues as anti-cancer agents: Looking back, peering ahead. Bioorg. Med. Chem. Lett. 2018, 28, 2816–2826. [Google Scholar] [CrossRef]
- Marupudi, N.; Han, J.; Li, K.; Renard, V.; Tyler, B.; Brem, H. Paclitaxel: A review of adverse toxicities and novel delivery strategies. Expert Opin. Drug Saf. 2007, 6, 609–621. [Google Scholar] [CrossRef]
- Ebenezer, O.; Shapi, M.; Tuszynski, J.A. A Review of the Recent Developments of Molecular Hybrids Targeting Tubulin Polymerization. Int. J. Mol. Sci. 2022, 23, 4001. [Google Scholar] [CrossRef]
- Weng, H.; Li, J.; Zhu, H.; Carver Wong, K.F.; Zhu, Z.; Xu, J.A.-O. An update on the recent advances and discovery of novel tubulin colchicine binding inhibitors. Futur. Med. Chem. 2023, 15, 73–95. [Google Scholar] [CrossRef]
- Hong, Y.; Zhu, Y.Y.; He, Q.; Gu, S.X. Indole derivatives as tubulin polymerization inhibitors for the development of promising anticancer agents. Bioorg. Med. Chem. 2022, 55, 116597. [Google Scholar] [CrossRef]
- Pal, D.; Song, I.H.; Dashrath Warkad, S.; Song, K.S.; Seong Yeom, G.; Saha, S.; Shinde, P.B.; Balasaheb Nimse, S. Indazole-based microtubule-targeting agents as potential candidates for anticancer drugs discovery. Bioorg. Chem. 2022, 122, 105735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Kanakkanthara, A. Beyond the Paclitaxel and Vinca Alkaloids: Next Generation of Plant-Derived Microtubule-Targeting Agents with Potential Anticancer Activity. Cancers 2020, 12, 1721. [Google Scholar] [CrossRef]
- Mukhtar, E.; Adhami, V.M.; Mukhtar, H. Targeting microtubules by natural agents for cancer therapy. Mol. Cancer Ther. 2014, 13, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Naaz, F.; Haider, M.R.; Shafi, S.; Yar, M.S. Anti-tubulin agents of natural origin: Targeting taxol, vinca, and colchicine binding domains. Eur. J. Med. Chem. 2019, 171, 310–331. [Google Scholar] [CrossRef]
- Chaaban, S.; Brouhard, G.J. A microtubule bestiary: Structural diversity in tubulin polymers. Mol. Biol. Cell 2017, 28, 2924–2931. [Google Scholar] [CrossRef]
- Goodson, H.V.; Jonasson, E.M. Microtubules and Microtubule-Associated Proteins. Cold Spring Harb. Perspect. Biol. 2018, 10, a022608. [Google Scholar] [CrossRef] [PubMed]
- Brouhard, G.J.; Rice, L.M. Microtubule dynamics: An interplay of biochemistry and mechanics. Nat. Rev. Mol. Cell Biol. 2018, 19, 451–463. [Google Scholar] [CrossRef]
- Dumontet, C.; Jordan, M.A. Microtubule-binding agents: A dynamic field of cancer therapeutics. Nat. Rev. Drug Discov. 2010, 9, 790–803. [Google Scholar] [CrossRef]
- Lafanechere, L. The microtubule cytoskeleton: An old validated target for novel therapeutic drugs. Front. Pharmacol. 2022, 13, 969183. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Huang, J.; Zhang, W.X.; Liu, Y.H.; Wang, X.; Song, J.; Jin, C.Y.; Zhang, S.Y. Tubulin degradation: Principles, agents, and applications. Bioorg. Chem. 2023, 139, 106684. [Google Scholar] [CrossRef]
- Tymon-Rosario, J.; Adjei, N.N.; Roque, D.M.; Santin, A.D. Microtubule-Interfering Drugs: Current and Future Roles in Epithelial Ovarian Cancer Treatment. Cancers 2021, 13, 6239. [Google Scholar] [CrossRef]
- Prota, A.E.; Bargsten, K.; Zurwerra, D.; Field, J.J.; Díaz, J.F.; Altmann, K.-H.; Steinmetz, M.O. Molecular Mechanism of Action of Microtubule-Stabilizing Anticancer Agents. Science 2013, 339, 587–590. [Google Scholar] [CrossRef]
- Jose, W.M. Taxanes—The Backbone of Medical Oncology. Indian J. Med. Paediatr. Oncol. 2021, 41, 221–234. [Google Scholar] [CrossRef]
- Trivedi, M.; Budihardjo, I.; Loureiro, K.; Reid, T.R.; Ma, J.D. Epothilones: A Novel Class of Microtubule-Stabilizing Drugs for the Treatment of Cancer. Futur. Oncol. 2008, 4, 483–500. [Google Scholar] [CrossRef]
- Gallego-Jara, J.; Lozano-Terol, G.; Sola-Martinez, R.A.; Canovas-Diaz, M.; de Diego Puente, T. A Compressive Review about Taxol((R)): History and Future Challenges. Molecules 2020, 25, 5986. [Google Scholar] [CrossRef] [PubMed]
- Preston, N.J. Paclitaxel (Taxol)—A guide to administration. Eur. J. Cancer Care 1996, 5, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Lippincott Williams & Wilkins, Inc. FDA Approves Cabazitaxel for Metastatic Hormone-Refractory Prostate Cancer. Oncol. Times 2010, 32, 10. [Google Scholar] [CrossRef]
- Villegas, C.; Gonzalez-Chavarria, I.; Burgos, V.; Iturra-Beiza, H.; Ulrich, H.; Paz, C. Epothilones as Natural Compounds for Novel Anticancer Drugs Development. Int. J. Mol. Sci. 2023, 24, 6063. [Google Scholar] [CrossRef]
- Cobham, M.V.; Donovan, D. Ixabepilone: A new treatment option for the management of taxane-resistant metastatic breast cancer. Cancer Manag. Res. 2009, 1, 69–77. [Google Scholar] [CrossRef]
- Laisne, M.-C.; Michallet, S.; Lafanechère, L. Characterization of Microtubule Destabilizing Drugs: A Quantitative Cell-Based Assay That Bridges the Gap between Tubulin Based- and Cytotoxicity Assays. Cancers 2021, 13, 5226. [Google Scholar] [CrossRef]
- Banyal, A.; Tiwari, S.; Sharma, A.; Chanana, I.; Patel, S.K.S.; Kulshrestha, S.; Kumar, P. Vinca alkaloids as a potential cancer therapeutics: Recent update and future challenges. 3 Biotech. 2023, 13, 211. [Google Scholar] [CrossRef] [PubMed]
- Avendaño, C.; Menéndez, J.C. Chapter 9—Anticancer drugs targeting tubulin and microtubules. In Medicinal Chemistry of Anticancer Drugs, 3rd ed.; Avendaño, C., Menéndez, J.C., Eds.; Elsevier: Boston, MA, USA, 2023; pp. 445–491. [Google Scholar]
- Edelman, M.J.; Rupard, E.J. TUMORS, MALIGNANT|Chemotherapeutic Agents. In Encyclopedia of Respiratory Medicine; Laurent, G.J., Shapiro, S.D., Eds.; Academic Press: Oxford, UK, 2006; pp. 332–338. [Google Scholar]
- González-Burgos, E.; Gómez-Serranillos, M.P. Chapter 4—Vinca Alkaloids as Chemotherapeutic Agents Against Breast Cancer. In Discovery and Development of Anti-Breast Cancer Agents from Natural Products; Brahmachari, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 69–101. [Google Scholar]
- Ehrhardt, H.; Pannert, L.; Pfeiffer, S.; Wachter, F.; Amtmann, E.; Jeremias, I. Enhanced anti-tumour effects of Vinca alkaloids given separately from cytostatic therapies. Br. J. Pharmacol. 2013, 168, 1558–1569. [Google Scholar] [CrossRef]
- Dhyani, P.; Quispe, C.; Sharma, E.; Bahukhandi, A.; Sati, P.; Attri, D.C.; Szopa, A.; Sharifi-Rad, J.; Docea, A.O.; Mardare, I.; et al. Anticancer potential of alkaloids: A key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Cancer Cell Int. 2022, 22, 206. [Google Scholar] [CrossRef]
- Fraga, A.G.M. Combretastatins and their Analogues: Nature as na Alternative Source for the Therapy of Cancer. Rev. Virtual De. Química 2015, 7, 765–790. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, M.; Wang, G.; Li, Y. Recent Progress on Microtubule Degradation Agents. J. Med. Chem. 2023, 66, 13354–13368. [Google Scholar] [CrossRef] [PubMed]
- Muhlethaler, T.; Gioia, D.; Prota, A.E.; Sharpe, M.E.; Cavalli, A.; Steinmetz, M.O. Comprehensive Analysis of Binding Sites in Tubulin. Angew. Chem. Int. Ed. Engl. 2021, 60, 13331–13342. [Google Scholar] [CrossRef] [PubMed]
- Matthew, S.; Chen, Q.Y.; Ratnayake, R.; Fermaintt, C.S.; Lucena-Agell, D.; Bonato, F.; Prota, A.E.; Lim, S.T.; Wang, X.; Diaz, J.F.; et al. Gatorbulin-1, a distinct cyclodepsipeptide chemotype, targets a seventh tubulin pharmacological site. Proc. Natl. Acad. Sci. USA 2021, 118, e2021847118. [Google Scholar] [CrossRef]
- Rao, S.; Horwitz, S.B.; Ringel, I. Direct photoaffinity labeling of tubulin with taxol. J. Natl. Cancer Inst. 1992, 84, 785–788. [Google Scholar] [CrossRef]
- Field, J.J.; Diaz, J.F.; Miller, J.H. The binding sites of microtubule-stabilizing agents. Chem. Biol. 2013, 20, 301–315. [Google Scholar] [CrossRef]
- Eli, S.; Castagna, R.; Mapelli, M.; Parisini, E. Recent Approaches to the Identification of Novel Microtubule-Targeting Agents. Front. Mol. Biosci. 2022, 9, 841777. [Google Scholar] [CrossRef]
- Gigant, B.; Wang, C.; Ravelli, R.B.; Roussi, F.; Steinmetz, M.O.; Curmi, P.A.; Sobel, A.; Knossow, M. Structural basis for the regulation of tubulin by vinblastine. Nature 2005, 435, 519–522. [Google Scholar] [CrossRef]
- Wang, X.; Gigant, B.; Zheng, X.; Chen, Q. Microtubule-targeting agents for cancer treatment: Seven binding sites and three strategies. MedComm-Oncology 2023, 2, e46. [Google Scholar] [CrossRef]
- Wang, J.; Miller, D.D.; Li, W. Molecular interactions at the colchicine binding site in tubulin: An X-ray crystallography perspective. Drug Discov. Today 2022, 27, 759–776. [Google Scholar] [CrossRef]
- Karamanou, M.; Tsoucalas, G.; Pantos, K.; Androutsos, G. Isolating Colchicine in 19th Century: An Old Drug Revisited. Curr. Pharm. Des. 2018, 24, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Massarotti, A.; Coluccia, A.; Silvestri, R.; Sorba, G.; Brancale, A. The tubulin colchicine domain: A molecular modeling perspective. ChemMedChem 2012, 7, 33–42. [Google Scholar] [CrossRef]
- Prota, A.E.; Bargsten, K.; Diaz, J.F.; Marsh, M.; Cuevas, C.; Liniger, M.; Neuhaus, C.; Andreu, J.M.; Altmann, K.H.; Steinmetz, M.O. A new tubulin-binding site and pharmacophore for microtubule-destabilizing anticancer drugs. Proc. Natl. Acad. Sci. USA 2014, 111, 13817–13821. [Google Scholar] [CrossRef] [PubMed]
- Kupchan, S.M.; Komoda, Y.; Court, W.A.; Thomas, G.J.; Smith, R.M.; Karim, A.; Gilmore, C.J.; Haltiwanger, R.C.; Bryan, R.F. Maytansine, a novel antileukemic ansa macrolide from Maytenus ovatus. J. Am. Chem. Soc. 1972, 94, 1354–1356. [Google Scholar] [CrossRef]
- Alpízar-Pedraza, D.; Veulens, A.d.l.N.; Araujo, E.C.; Piloto-Ferrer, J.; Sánchez-Lamar, Á. Microtubules destabilizing agents binding sites in tubulin. J. Mol. Struct. 2022, 1259, 132723. [Google Scholar] [CrossRef]
- Corley, D.G.; Herb, R.; Moore, R.E.; Scheuer, P.J.; Paul, V.J. Laulimalides. New potent cytotoxic macrolides from a marine sponge and a nudibranch predator. J. Org. Chem. 1988, 53, 3644–3646. [Google Scholar] [CrossRef]
- Bennett, M.J.; Barakat, K.; Huzil, J.T.; Tuszynski, J.; Schriemer, D.C. Discovery and characterization of the laulimalide-microtubule binding mode by mass shift perturbation mapping. Chem. Biol. 2010, 17, 725–734. [Google Scholar] [CrossRef]
- Churchill, C.D.; Klobukowski, M.; Tuszynski, J.A. The Unique Binding Mode of Laulimalide to Two Tubulin Protofilaments. Chem. Biol. Drug Des. 2015, 86, 190–199. [Google Scholar] [CrossRef]
- Coulup, S.K.; Georg, G.I. Revisiting microtubule targeting agents: Alpha-Tubulin and the pironetin binding site as unexplored targets for cancer therapeutics. Bioorg. Med. Chem. Lett. 2019, 29, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Wordeman, L.; Vicente, J.J. Microtubule Targeting Agents in Disease: Classic Drugs, Novel Roles. Cancers 2021, 13, 5650. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yu, Y.; Li, Y.; Yan, W.; Ye, H.; Niu, L.; Tang, M.; Wang, Z.; Yang, Z.; Pei, H.; et al. Cevipabulin-tubulin complex reveals a novel agent binding site on α-tubulin with tubulin degradation effect. Sci. Adv. 2021, 7, eabg4168. [Google Scholar] [CrossRef]
- Giannakakou, P.; Snyder, J.P. Resistance to Microtubule-Targeting Drugs; Humana Press: Totowa, NJ, USA, 2008; pp. 357–394. [Google Scholar]
- Mariani, M.; Karki, R.; Spennato, M.; Pandya, D.; He, S.; Andreoli, M.; Fiedler, P.; Ferlini, C. Class III beta-tubulin in normal and cancer tissues. Gene 2015, 563, 109–114. [Google Scholar] [CrossRef]
- Kanakkanthara, A.; Miller, J.H. βIII-tubulin overexpression in cancer: Causes, consequences, and potential therapies. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188607. [Google Scholar] [CrossRef]
- Stengel, C.; Newman, S.P.; Leese, M.P.; Potter, B.V.L.; Reed, M.J.; Purohit, A. Class III β-tubulin expression and in vitro resistance to microtubule targeting agents. Br. J. Cancer 2010, 102, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Kavallaris, M. Microtubules and resistance to tubulin-binding agents. Nat. Rev. Cancer 2010, 10, 194–204. [Google Scholar] [CrossRef]
- Szakács, G.; Homolya, L.; Sarkadi, B.; Váradi, A. MDR-ABC Transporters. In Encyclopedia of Molecular Pharmacology; Offermanns, S., Rosenthal, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 748–752. [Google Scholar]
- Dean, M.; Rzhetsky, A.; Allikmets, R. The Human ATP-Binding Cassette (ABC) Transporter Superfamily. Genome Res. 2001, 11, 1156–1166. [Google Scholar] [CrossRef]
- Ahmed, J., II; Abdul Hamid, A.A.; Abd Halim, K.B.; Che Has, A.T. P-glycoprotein: New insights into structure, physiological function, regulation and alterations in disease. Heliyon 2022, 8, e09777. [Google Scholar] [CrossRef] [PubMed]
- Krause, W. Resistance to anti-tubulin agents: From vinca alkaloids to epothilones. Cancer Drug Resist. 2019, 2, 82–106. [Google Scholar] [CrossRef]
- Lee, F.Y.; Smykla, R.; Johnston, K.; Menard, K.; McGlinchey, K.; Peterson, R.W.; Wiebesiek, A.; Vite, G.; Fairchild, C.R.; Kramer, R. Preclinical efficacy spectrum and pharmacokinetics of ixabepilone. Cancer Chemother. Pharmacol. 2009, 63, 201–212. [Google Scholar] [CrossRef]
- Sodani, K.; Patel, A.; Kathawala, R.J.; Chen, Z.-S. Multidrug resistance associated proteins in multidrug resistance. Chin. J. Cancer 2012, 31, 58–72. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.-H.; Guo, X. New insights into Vinca alkaloids resistance mechanism and circumvention in lung cancer. Biomed. Pharmacother. 2017, 96, 659–666. [Google Scholar] [CrossRef]
- Yu, C.-C.; Liu, S.-P.; Hsu, J.-L.; Hsu, J.T.; Kudryavtsev, K.V.; Guh, J.-H. KUD773, a phenylthiazole derivative, displays anticancer activity in human hormone-refractory prostate cancers through inhibition of tubulin polymerization and anti-Aurora A activity. J. Biomed. Sci. 2015, 22, 2. [Google Scholar] [CrossRef]
- Pandey, M.K.; Gowda, K.; Sung, S.-S.; Abraham, T.; Budak-Alpdogan, T.; Talamo, G.; Dovat, S.; Amin, S. A novel dual inhibitor of microtubule and Bruton’s tyrosine kinase inhibits survival of multiple myeloma and osteoclastogenesis. Exp. Hematol. 2017, 53, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Rong, D.; Wang, C.; Zhang, X.; Wei, Y.; Zhang, M.; Liu, D.; Farhan, H.; Momen Ali, S.A.; Liu, Y.; Taouil, A.; et al. A novel taxane, difluorovinyl-ortataxel, effectively overcomes paclitaxel-resistance in breast cancer cells. Cancer Lett. 2020, 491, 36–49. [Google Scholar] [CrossRef]
- Zhou, X.; Fu, Y.H.; Zou, Y.Y.; Meng, J.; Ou-Yang, G.P.; Ge, Q.S.; Wang, Z.C. Discovery of Simple Diacylhydrazine-Functionalized Cinnamic Acid Derivatives as Potential Microtubule Stabilizers. Int. J. Mol. Sci. 2022, 23, 12365. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Chang, T.Y.; Kaur, N.; Hsu, K.C.; Yen, Y.; Lin, T.E.; Lai, M.J.; Lee, S.B.; Liou, J.P. CAP rigidification of MS-275 and chidamide leads to enhanced antiproliferative effects mediated through HDAC1, 2 and tubulin polymerization inhibition. Eur. J. Med. Chem. 2021, 215, 113169. [Google Scholar] [CrossRef]
- Sinicropi, M.S.; Tavani, C.; Rosano, C.; Ceramella, J.; Iacopetta, D.; Barbarossa, A.; Bianchi, L.; Benzi, A.; Maccagno, M.; Ponassi, M.; et al. A Nitrocarbazole as a New Microtubule-Targeting Agent in Breast Cancer Treatment. Appl. Sci. 2021, 11, 9139. [Google Scholar] [CrossRef]
- Cui, Y.J.; Ma, C.C.; Zhang, C.M.; Tang, L.Q.; Liu, Z.P. The discovery of novel indazole derivatives as tubulin colchicine site binding agents that displayed potent antitumor activity both in vitro and in vivo. Eur. J. Med. Chem. 2020, 187, 111968. [Google Scholar] [CrossRef] [PubMed]
- Malebari, A.M.; Fayne, D.; Nathwani, S.M.; O’Connell, F.; Noorani, S.; Twamley, B.; O’Boyle, N.M.; O’Sullivan, J.; Zisterer, D.M.; Meegan, M.J. beta-Lactams with antiproliferative and antiapoptotic activity in breast and chemoresistant colon cancer cells. Eur. J. Med. Chem. 2020, 189, 112050. [Google Scholar] [CrossRef]
- Jian, X.E.; Yang, F.; Jiang, C.S.; You, W.W.; Zhao, P.L. Synthesis and biological evaluation of novel pyrazolo[3,4-b]pyridines as cis-restricted combretastatin A-4 analogues. Bioorg. Med. Chem. Lett. 2020, 30, 127025. [Google Scholar] [CrossRef]
- Lin, B.Y.; Liu, W.L.; Huang, H.; Hu, Y.G.; Gong, S.; Meng, Y.H.; Yan, J.; Lu, Y.Z.; Chen, H.L. AQ-4, a deuterium-containing molecule, acts as a microtubule-targeting agent for cancer treatment. Eur. J. Pharmacol. 2020, 877, 173093. [Google Scholar] [CrossRef]
- Han, H.J.; Park, C.; Hwang, J.; N R, T.; Kim, S.O.; Han, J.; Woo, M.; B, S.; Ryoo, I.J.; Lee, K.H.; et al. CPPF, A Novel Microtubule Targeting Anticancer Agent, Inhibits the Growth of a Wide Variety of Cancers. Int. J. Mol. Sci. 2020, 21, 4800. [Google Scholar] [CrossRef]
- He, J.; Zhang, M.; Tang, L.; Liu, J.; Zhong, J.; Wang, W.; Xu, J.P.; Wang, H.T.; Li, X.F.; Zhou, Z.Z. Synthesis, Biological Evaluation, and Molecular Docking of Arylpyridines as Antiproliferative Agent Targeting Tubulin. ACS Med. Chem. Lett. 2020, 11, 1611–1619. [Google Scholar] [CrossRef]
- Du, T.; Lin, S.; Ji, M.; Xue, N.; Liu, Y.; Zhang, Z.; Zhang, K.; Zhang, J.; Zhang, Y.; Wang, Q.; et al. A novel orally active microtubule destabilizing agent S-40 targets the colchicine-binding site and shows potent antitumor activity. Cancer Lett. 2020, 495, 22–32. [Google Scholar] [CrossRef]
- Kode, J.; Kovvuri, J.; Nagaraju, B.; Jadhav, S.; Barkume, M.; Sen, S.; Kasinathan, N.K.; Chaudhari, P.; Mohanty, B.S.; Gour, J.; et al. Synthesis, biological evaluation, and molecular docking analysis of phenstatin based indole linked chalcones as anticancer agents and tubulin polymerization inhibitors. Bioorg. Chem. 2020, 105, 104447. [Google Scholar] [CrossRef]
- Ibrahim, T.S.; Hawwas, M.M.; Malebari, A.M.; Taher, E.S.; Omar, A.M.; O’Boyle, N.M.; McLoughlin, E.; Abdel-Samii, Z.K.; Elshaier, Y. Potent Quinoline—Containing Combretastatin A—4 Analogues: Design, Synthesis, Antiproliferative, and Anti-Tubulin Activity. Pharmaceuticals 2020, 13, 393. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Doshi, A.; Luckett-Chastain, L.; Ihnat, M.; Hamel, E.; Mooberry, S.L.; Gangjee, A. Potential of substituted quinazolines to interact with multiple targets in the treatment of cancer. Bioorg. Med. Chem. 2021, 35, 116061. [Google Scholar] [CrossRef] [PubMed]
- Horne, E.A.; Diaz, P.; Cimino, P.J.; Jung, E.; Xu, C.; Hamel, E.; Wagenbach, M.; Kumasaka, D.; Wageling, N.B.; Azorin, D.D.; et al. A brain-penetrant microtubule-targeting agent that disrupts hallmarks of glioma tumorigenesis. Neurooncol Adv. 2021, 3, vdaa165. [Google Scholar] [CrossRef]
- Islam, F.; Quadery, T.M.; Bai, R.; Luckett-Chastain, L.R.; Hamel, E.; Ihnat, M.A.; Gangjee, A. Novel pyrazolo[4,3-d]pyrimidine microtubule targeting agents (MTAs): Synthesis, structure-activity relationship, in vitro and in vivo evaluation as antitumor agents. Bioorg. Med. Chem. Lett. 2021, 41, 127923. [Google Scholar] [CrossRef]
- Zhu, H.; Ying, S.; Zhou, B.; Liang, X.; He, Q.; Song, P.; Hu, X.; Shi, K.; Xiong, M.; Jin, H.; et al. Discovery of novel 2-aryl-3-sulfonamido-pyridines (HoAns) as microtubule polymerization inhibitors with potent antitumor activities. Eur. J. Med. Chem. 2021, 211, 113117. [Google Scholar] [CrossRef]
- Wu, M.K.; Man, R.J.; Liao, Y.J.; Zhu, H.L.; Zhou, Z.G. Discovery of novel indole-1,2,4-triazole derivatives as tubulin polymerization inhibitors. Drug Dev. Res. 2021, 82, 1008–1020. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Liu, Z.; Wang, Z.; Liu, Z.; Man, S.; Zhang, Y.; Bao, K.; Wu, Y.; Guan, Q.; et al. Design, synthesis and biological evaluation of 1-Aryl-5-(4-arylpiperazine-1-carbonyl)-1H-tetrazols as novel microtubule destabilizers. J. Enzym. Inhib. Med. Chem. 2021, 36, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadeh Oskuei, S.; Mirzaei, S.; Reza Jafari-Nik, M.; Hadizadeh, F.; Eisvand, F.; Mosaffa, F.; Ghodsi, R. Design, synthesis and biological evaluation of novel imidazole-chalcone derivatives as potential anticancer agents and tubulin polymerization inhibitors. Bioorg. Chem. 2021, 112, 104904. [Google Scholar] [CrossRef] [PubMed]
- Riu, F.; Sanna, L.; Ibba, R.; Piras, S.; Bordoni, V.; Scorciapino, M.A.; Lai, M.; Sestito, S.; Bagella, L.; Carta, A. A comprehensive assessment of a new series of 5’,6’-difluorobenzotriazole-acrylonitrile derivatives as microtubule targeting agents (MTAs). Eur. J. Med. Chem. 2021, 222, 113590. [Google Scholar] [CrossRef]
- Chen, H.; Deng, S.; Albadari, N.; Yun, M.K.; Zhang, S.; Li, Y.; Ma, D.; Parke, D.N.; Yang, L.; Seagroves, T.N.; et al. Design, Synthesis, and Biological Evaluation of Stable Colchicine-Binding Site Tubulin Inhibitors 6-Aryl-2-benzoyl-pyridines as Potential Anticancer Agents. J. Med. Chem. 2021, 64, 12049–12074. [Google Scholar] [CrossRef]
- Yong, C.; Devine, S.M.; Abel, A.C.; Tomlins, S.D.; Muthiah, D.; Gao, X.; Callaghan, R.; Steinmetz, M.O.; Prota, A.E.; Capuano, B.; et al. 1,3-Benzodioxole-Modified Noscapine Analogues: Synthesis, Antiproliferative Activity, and Tubulin-Bound Structure. ChemMedChem 2021, 16, 2882–2894. [Google Scholar] [CrossRef]
- Boichuk, S.; Galembikova, A.; Syuzov, K.; Dunaev, P.; Bikinieva, F.; Aukhadieva, A.; Zykova, S.; Igidov, N.; Gankova, K.; Novikova, M.; et al. The Design, Synthesis, and Biological Activities of Pyrrole-Based Carboxamides: The Novel Tubulin Inhibitors Targeting the Colchicine-Binding Site. Molecules 2021, 26, 5780. [Google Scholar] [CrossRef]
- Yan, J.; Xu, Y.; Jin, X.; Zhang, Q.; Ouyang, F.; Han, L.; Zhan, M.; Li, X.; Liang, B.; Huang, X. Structure modification and biological evaluation of indole-chalcone derivatives as anti-tumor agents through dual targeting tubulin and TrxR. Eur. J. Med. Chem. 2022, 227, 113897. [Google Scholar] [CrossRef]
- Cheng, B.; Zhu, G.; Meng, L.; Wu, G.; Chen, Q.; Ma, S. Identification and optimization of biphenyl derivatives as novel tubulin inhibitors targeting colchicine-binding site overcoming multidrug resistance. Eur. J. Med. Chem. 2022, 228, 113930. [Google Scholar] [CrossRef]
- Islam, F.; Doshi, A.; Robles, A.J.; Quadery, T.M.; Zhang, X.; Zhou, X.; Hamel, E.; Mooberry, S.L.; Gangjee, A. Design, Synthesis, and Biological Evaluation of 5,6,7,8-Tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidines as Microtubule Targeting Agents. Molecules 2022, 27, 321. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Zhu, Z.; Yan, W.; Zhang, C.; Yang, Z.; Bai, P.; Tang, M.; Shi, M.; He, W.; et al. Structure-Based Design and Synthesis of N-Substituted 3-Amino-beta-Carboline Derivatives as Potent alphabeta-Tubulin Degradation Agents. J. Med. Chem. 2022, 65, 2675–2693. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Pang, Y.; Zhang, W.; OuYang, F.; Lin, H.; Li, X.; Yan, J. Discovery of a Novel Stilbene Derivative as a Microtubule Targeting Agent Capable of Inducing Cell Ferroptosis. J. Med. Chem. 2022, 65, 4687–4708. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, R.; Oliva, P.; Prencipe, F.; Manfredini, S.; Budassi, F.; Brancale, A.; Ferla, S.; Hamel, E.; Corallo, D.; Aveic, S.; et al. Design, Synthesis and Biological Investigation of 2-Anilino Triazolopyrimidines as Tubulin Polymerization Inhibitors with Anticancer Activities. Pharmaceuticals 2022, 15, 1031. [Google Scholar] [CrossRef] [PubMed]
- Malebari, A.M.; Duffy Morales, G.; Twamley, B.; Fayne, D.; Khan, M.F.; McLoughlin, E.C.; O’Boyle, N.M.; Zisterer, D.M.; Meegan, M.J. Synthesis, Characterisation and Mechanism of Action of Anticancer 3-Fluoroazetidin-2-ones. Pharmaceuticals 2022, 15, 1044. [Google Scholar] [CrossRef]
- Cao, X.; Li, R.; Wang, H.; Guo, C.; Wang, S.; Chen, X.; Zhao, R. Novel indole–chalcone platinum(IV) complexes as tubulin polymerization inhibitors to overcome oxaliplatin resistance in colorectal cancer. J. Mol. Struct. 2023, 1272, 134169. [Google Scholar] [CrossRef]
- Peng, X.; Ren, Y.; Pan, W.; Liu, J.; Chen, J. Discovery of Novel Acridane-Based Tubulin Polymerization Inhibitors with Anticancer and Potential Immunomodulatory Effects. J. Med. Chem. 2023, 66, 627–640. [Google Scholar] [CrossRef]
- Choudhary, S.; Kaku, K.; Robles, A.J.; Hamel, E.; Mooberry, S.L.; Gangjee, A. Simple monocyclic pyrimidine analogs as microtubule targeting agents binding to the colchicine site. Bioorg. Med. Chem. 2023, 82, 117217. [Google Scholar] [CrossRef]
- Pochampally, S.; Hartman, K.L.; Wang, R.; Wang, J.; Yun, M.K.; Parmar, K.; Park, H.; Meibohm, B.; White, S.W.; Li, W.; et al. Design, Synthesis, and Biological Evaluation of Pyrimidine Dihydroquinoxalinone Derivatives as Tubulin Colchicine Site-Binding Agents That Displayed Potent Anticancer Activity Both In Vitro and In Vivo. ACS Pharmacol. Transl. Sci. 2023, 6, 526–545. [Google Scholar] [CrossRef]
- Song, J.; Wang, S.Y.; Wang, X.; Jia, M.Q.; Tian, X.Y.; Fu, X.J.; Jin, C.Y.; Zhang, S.Y. Discovery of a novel Coumarin-Dihydroquinoxalone derivative MY-673 as a tubulin polymerization inhibitor capable of inhibiting the ERK pathway with potent anti-gastric cancer activities. Bioorg. Chem. 2023, 137, 106580. [Google Scholar] [CrossRef] [PubMed]
- Graff, B.T.; Palanivel, C.; Jenkins, C.B.; Baranowska-Kortylewicz, J.; Yan, Y. Benzimidazole carbamate induces cytotoxicity in breast cancer cells via two distinct cell death mechanisms. Cell Death Discov. 2023, 9, 162. [Google Scholar] [CrossRef]
- Song, I.H.; Park, S.J.; Yeom, G.S.; Song, K.S.; Kim, T.; Nimse, S.B. Not all benzimidazole derivatives are microtubule destabilizing agents. Biomed. Pharmacother. 2023, 164, 114977. [Google Scholar] [CrossRef]
- Song, J.; Liu, S.; Ren, Y.; Zhang, X.; Zhao, B.; Wang, X.; Li, Y. Organotin benzohydroxamate derivatives (OTBH) target colchicine-binding site exerting potent antitumor activity both in vitro and vivo revealed by quantitative proteomic analysis. Eur. J. Pharm. Sci. 2023, 187, 106488. [Google Scholar] [CrossRef]
- Dong, H.; Lu, L.; Song, X.; Li, Y.; Zhou, J.; Xu, Y.; Zhang, Y.; Qi, J.; Liang, T.; Wang, J. Design, synthesis and biological evaluation of tetrahydroquinoxaline sulfonamide derivatives as colchicine binding site inhibitors. RSC Adv. 2023, 13, 30202–30216. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.J.; Zhou, Y.; Zhang, X.W.; Dou, B.K.; Ma, C.C.; Zhang, J. The discovery of water-soluble indazole derivatives as potent microtubule polymerization inhibitors. Eur. J. Med. Chem. 2023, 262, 115870. [Google Scholar] [CrossRef]
- Chen, L.; Hu, Y.; Lu, Z.; Lin, Z.; Li, L.; Wu, J.Q.; Yu, Z.L.; Wang, C.; Chen, W.H.; Hu, J. Design, Synthesis, and Antitumor Efficacy of Substituted 2-Amino[1,2,4]triazolopyrimidines and Related Heterocycles as Dual Inhibitors for Microtubule Polymerization and Janus Kinase 2. J. Med. Chem. 2023, 66, 15006–15024. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kang, M.; Lim, A.; Cho, K.J.; Chae, C.H.; Koh, B.; Jeon, H. Synthesis and evaluation of tirbanibulin derivatives: A detailed exploration of the structure-activity relationship for anticancer activity. RSC Adv. 2023, 13, 35583–35591. [Google Scholar] [CrossRef]
- Hu, S.; Li, Y.; Zhou, J.; Xu, K.; Pang, Y.; Weiskirchen, R.; Ocker, M.; Ouyang, F. Identification of acetylshikonin as a novel tubulin polymerization inhibitor with antitumor activity in human hepatocellular carcinoma cells. J. Gastrointest. Oncol. 2023, 14, 2574–2586. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, D.; Yuan, Z.; Qiao, H.; Xia, Z.; Cao, F.; Lu, Y.; Jiang, F. Novel 4-Aryl-4H-chromene derivative displayed excellent in vivo anti-glioblastoma efficacy as the microtubule-targeting agent. Eur. J. Med. Chem. 2024, 267, 116205. [Google Scholar] [CrossRef]
- Homer, J.A.; Koelln, R.A.; Barrow, A.S.; Gialelis, T.L.; Boiarska, Z.; Steinohrt, N.S.; Lee, E.F.; Yang, W.H.; Johnson, R.M.; Chung, T.; et al. Modular synthesis of functional libraries by accelerated SuFEx click chemistry. Chem. Sci. 2024, 15, 3879–3892. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, W.; Liu, Y.; Tang, M.; Teng, Y.; Wang, F.; Hu, X.; Zhao, M.; Yang, J.; Li, Y. Structure-based design and synthesis of BML284 derivatives: A novel class of colchicine-site noncovalent tubulin degradation agents. Eur. J. Med. Chem. 2024, 268, 116265. [Google Scholar] [CrossRef]
- Doan, N.Q.H.; Tran, H.N.; Nguyen, N.T.M.; Nguyen, K.D.T.; Tao, V.M.; Lai, N.N.; Tran, H.T.T.; Luu, P.H.T. Design, synthesis, and evaluation of anti-breast cancer activity of colchicine—Combretastatin A—4 analogues containing quinoline as microtubule-targeting agents. J. Mol. Struct. 2024, 1312, 138465. [Google Scholar] [CrossRef]
- Silva, W.P.d.; Caiana, R.R.A.; Barros, M.E.S.B.; Freitas, J.C.R.; da Silva, P.B.N.; Militão, G.G.C.; Oliveira, R.A.; Menezes, P.H. Design, stereoselective synthesis, and antitumoral activity of combretastatin A-4 analogs. Results Chem. 2024, 7, 101539. [Google Scholar] [CrossRef]
- Jiang, F.; Yu, M.; Liang, Y.; Ding, K.; Wang, Y. Discovery of Novel Diaryl-Substituted Fused Heterocycles Targeting Katanin and Tubulin with Potent Antitumor and Antimultidrug Resistance Efficacy. J. Med. Chem. 2024, 67, 12118–12142. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.; Vanstreels, E.; Bardiot, D.; Prota, A.E.; Gaillard, N.; Gao, L.J.; Vercruysse, T.; Persoons, L.; Daems, T.; Waer, M.; et al. 3-nitropyridine analogues as novel microtubule-targeting agents. PLoS ONE 2024, 19, e0307153. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Yu, M.; Wang, Y. Design, synthesis and biological evaluation of novel diaryl-substituted fused nitrogen heterocycles as tubulin polymerization inhibitors to overcome multidrug resistance in vitro and in vivo. Eur. J. Med. Chem. 2025, 283, 117130. [Google Scholar] [CrossRef]
- Marzullo, P.; Boiarska, Z.; Perez-Pena, H.; Abel, A.C.; Alvarez-Bernad, B.; Lucena-Agell, D.; Vasile, F.; Sironi, M.; Altmann, K.H.; Prota, A.E.; et al. Maytansinol Derivatives: Side Reactions as a Chance for New Tubulin Binders. Chemistry 2022, 28, e202103520. [Google Scholar] [CrossRef]
- Yang, M.H.; Mao, J.; Zhu, J.H.; Zhang, H.; Ding, L. Wangzaozin A, a potent novel microtubule stabilizer, targets both the taxane and laulimalide sites on beta-tubulin through molecular dynamics simulations. Life Sci. 2022, 301, 120583. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assunção, H.C.; Silva, P.M.A.; Bousbaa, H.; Cidade, H. Recent Advances in Microtubule Targeting Agents for Cancer Therapy. Molecules 2025, 30, 3314. https://doi.org/10.3390/molecules30163314
Assunção HC, Silva PMA, Bousbaa H, Cidade H. Recent Advances in Microtubule Targeting Agents for Cancer Therapy. Molecules. 2025; 30(16):3314. https://doi.org/10.3390/molecules30163314
Chicago/Turabian StyleAssunção, Henrique C., Patrícia M. A. Silva, Hassan Bousbaa, and Honorina Cidade. 2025. "Recent Advances in Microtubule Targeting Agents for Cancer Therapy" Molecules 30, no. 16: 3314. https://doi.org/10.3390/molecules30163314
APA StyleAssunção, H. C., Silva, P. M. A., Bousbaa, H., & Cidade, H. (2025). Recent Advances in Microtubule Targeting Agents for Cancer Therapy. Molecules, 30(16), 3314. https://doi.org/10.3390/molecules30163314








