Influence of Glutinous Rice Raw Material Characteristics on the Aroma Profile of Rice Wine
Abstract
1. Introduction
2. Results and Discussion
2.1. Glutinous Rice Parameter Analysis
2.2. Rice Wine Parameter Analysis
2.3. Results of GC–MS and GC-O
2.4. Quantification of Aroma Compounds
2.5. Partial Least Squares Discriminant Analysis (PLS-DA)
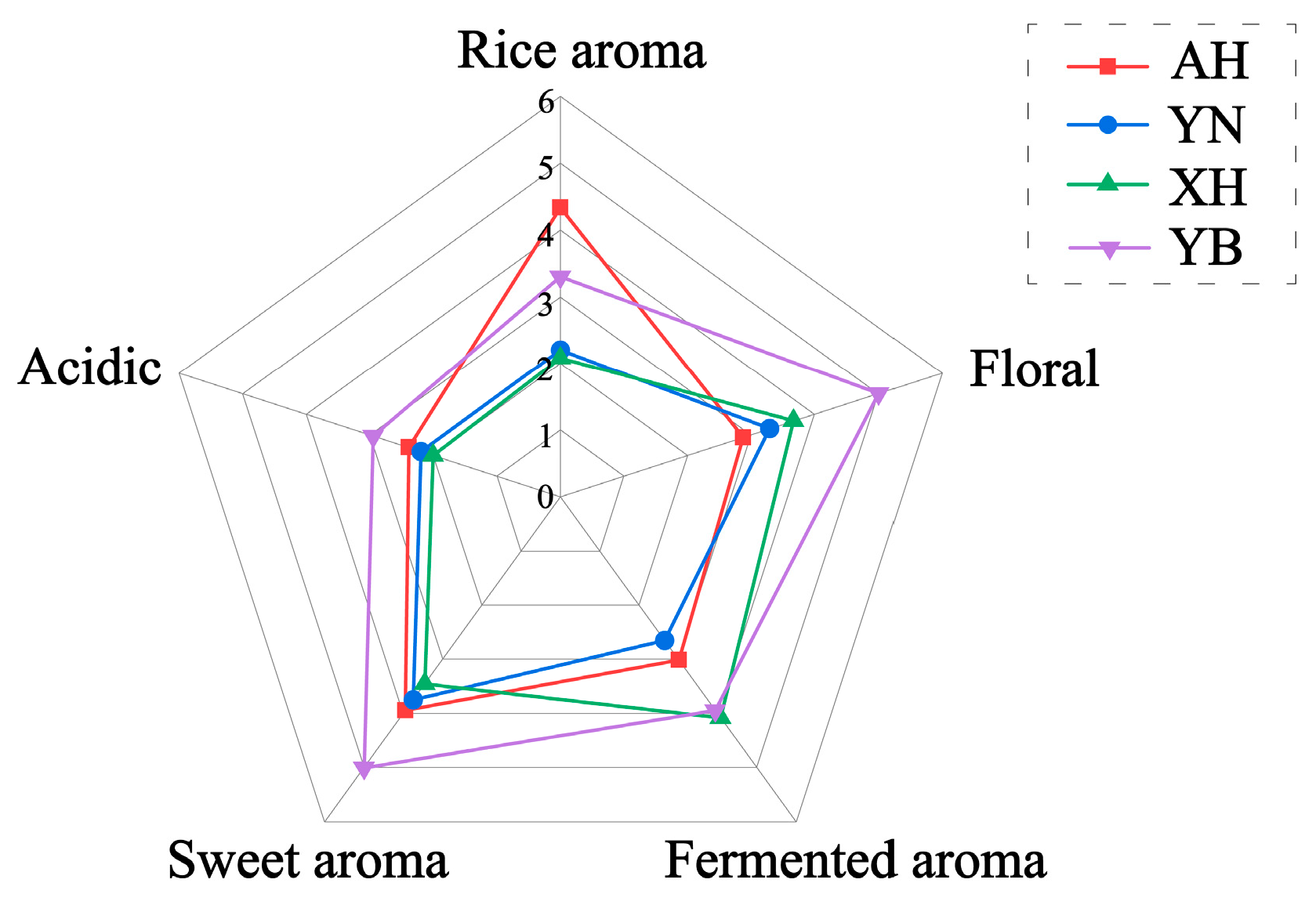
2.6. Sensory Quantitative Descriptive Analysis
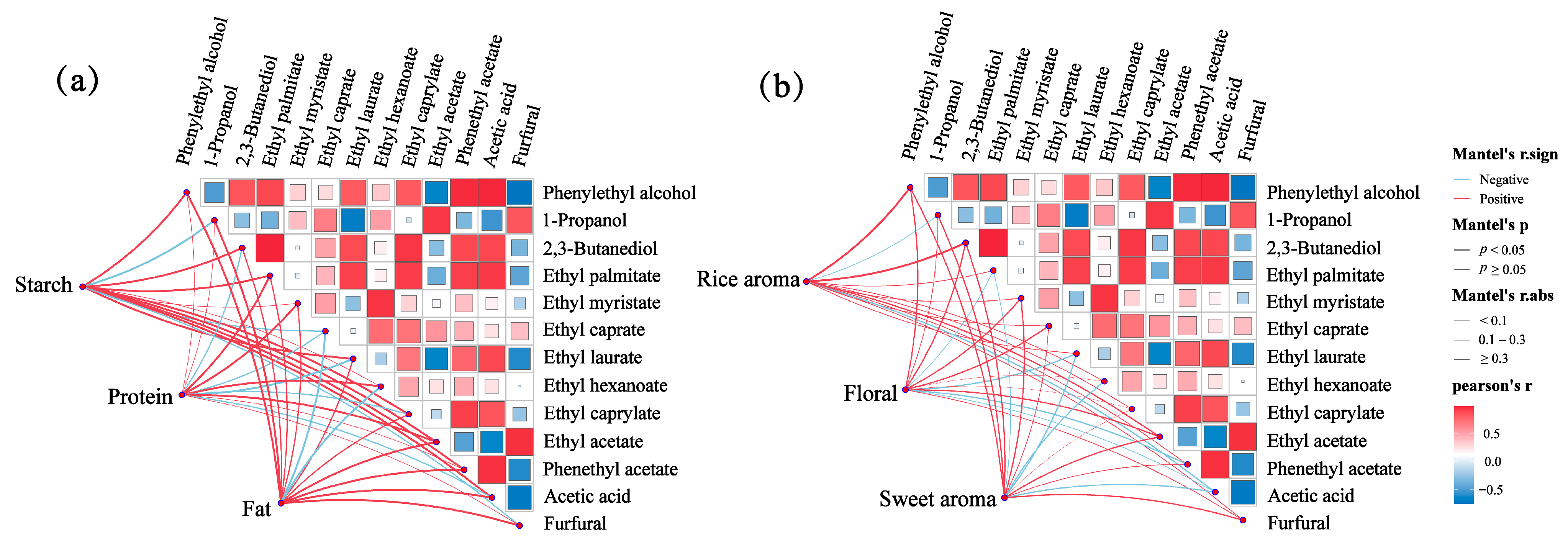
2.7. Relativity Analysis
3. Materials and Methods
3.1. Materials and Reagents
3.2. Determination of Chemical Properties of Glutinous Rice and Rice Wine
3.3. Rice Wine Brewing
3.4. GC–MS Analysis
3.5. Aroma Extract Dilution Analysis (AEDA) and GC-O Analysis
3.6. Qualitative and Quantitative Analysis of Volatile Compounds
3.7. Sensory Evaluation Panel
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, H.; Xie, T.; Xie, J.; Ai, L.; Tian, H. Characterization of key aroma compounds in Chinese rice wine using gas chromatography-mass spectrometry and gas chromatography-olfactometry. Food Chem. 2019, 293, 814. [Google Scholar] [CrossRef] [PubMed]
- Rao, W.J.; Li, X.; Ouyang, L.H.; Zhu, S.L.; Hu, S.; Zhou, J.Y. Characterization of key aroma compounds in Rice flavor baijiu from different rice raw materials by gas chromatography-ion mobility spectrometry and gas chromatography-olfactometry-quadrupole time of flight mass spectrometry. Food Biosci. 2023, 56, 103370. [Google Scholar] [CrossRef]
- Song, Y.R.; Lim, B.U.; Baik, S.H. Microbial Diversity and Volatile Flavor Changes during Gayangju Fermentation, a Traditional Korean House Rice Wine. Foods 2022, 11, 2604. [Google Scholar] [CrossRef]
- Pereira, C.; Mendes, D.; Dias, T.; Garcia, R.; da Silva, M.G.; Cabrita, M.J. Revealing the yeast modulation potential on amino acid composition and volatile profile of Arinto white wines by a combined chromatographic-based approach. J. Chromatogr. A 2021, 1641, 461991. [Google Scholar] [CrossRef]
- Bian, M.; Fang, Y.; Yang, S.; Yuan, T.; Zhou, X.; Xu, Q.; Jiang, D.; Han, B. Dynamic Changes in Physicochemical Properties, Amino Acid Content, and Flavour-Related Substances During Fortified Fermentation of Rice Wine With Saccharomycopsis fibuligera. J. Food Process. Preserv. 2024, 2024, 5581068. [Google Scholar] [CrossRef]
- Yang, Y.; Zhong, H.; Yang, N.; Zhu, D.; Li, J.; Yang, Z.; Yang, T. Effects of the proteins of indica rice and indica waxy rice on the formation of volatiles of sweet rice wine. Int. J. Food Sci. Technol. 2022, 57, 3604–3615. [Google Scholar] [CrossRef]
- Pan, T.Q.; Cheng, W.; Zhang, J.; Li, N.; Wu, L.H.; Han, X.; Xing, D.; Gong, Z.L. Contrast Analysis of Two Rice Wine Products Made by Different Raw Materials. Liquor.-Mak. Sci. Technol. 2018, 83–87. [Google Scholar] [CrossRef]
- Chen, T.; Wu, F.; Guo, J.; Ye, M.; Hu, H.; Guo, J.; Liu, X. Effects of glutinous rice protein components on the volatile substances and sensory properties of Chinese rice wine. J. Sci. Food Agric. 2020, 100, 3297–3307. [Google Scholar] [CrossRef]
- Mao, X.; Yue, S.-J.; Xu, D.-Q.; Fu, R.-J.; Han, J.-Z.; Zhou, H.-M.; Tang, Y.-P. Research Progress on Flavor and Quality of Chinese Rice Wine in the Brewing Process. ACS Omega 2023, 8, 32311–32330. [Google Scholar] [CrossRef]
- Milled Rice. 2018, Volume 16. Available online: https://kns.cnki.net/kcms2/article/abstract?v=ywQ10qv17SF6qVnRjWw782AmlwHZO1JqB0QyBG9achOSDVkUyLWClXmESgOnnfOxTNPhGWT-VYlK8djbH3_WpBuYL0yHFPk9ClEOLE6DczmZnXZqifBY2aQDS9bT42gk84v5Mjhc_S83NnZUz2cEk_dozCCILoERz0fLD8YgWpkeCxGvfcc8GxJm8C3srEo7&uniplatform=NZKPT&language=CHS (accessed on 8 January 2025).
- Niu, J.; Shen, Y.; Zhang, G.H.; Cheng, W.; Li, H.H.; Zheng, F.P.; Sun, B.G. Research progress on the relationship between raw materials and Baijiu quality. Food Ferment. Ind. 2023, 49, 322–328. [Google Scholar] [CrossRef]
- Li, C.; Hu, Y.; Huang, T.; Gong, B.; Yu, W.-W. A combined action of amylose and amylopectin fine molecular structures in determining the starch pasting and retrogradation property. Int. J. Biol. Macromol. 2020, 164, 2717–2725. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Dang, K.; Gong, X.W.; Wang, H.L.; Zhang, S.H.; Ma, Y.F.; Guo, L.; Feng, B. Analysis on the physicochemical properties and Baijiu-making characteristics in grain of japonica sorghum and glutinous sorghum. China Brew. 2021, 40, 77–82. [Google Scholar] [CrossRef]
- Ma, Y.; Wei, Z.; Xiao, X.; Yu, K.; Huang, H.; Tan, J.; Wang, Y.; Du, Y.; Li, Y. Investigating the impact of various sorghum types on the key aroma compounds of Sichuan Xiaoqu Baijiu through application of the sensomics approach. Food Chem. X 2024, 22, 101367. [Google Scholar] [CrossRef]
- Yuan, G.Y.; He, Y.L.; Wang, C.X.; Qiu, S.Y. Research progress on the factors correlated with flavor quality in rice wine. Food Ferment. Ind. 2022, 48, 286–294. [Google Scholar] [CrossRef]
- Yan, H.W. Study on the Diversity of Fungi in Sweet Wine Koji and Analysis of Nutritional Components of Sweet Wine; Qufu Normal University: Qufu, China, 2015. [Google Scholar]
- Green Food-Rice Wine. 2017, Volume 7. Available online: https://kns.cnki.net/kcms2/article/abstract?v=GQPEaosfmU_C1I62M4Sl10wD8I5qbovMJi5jIMANTOJ9bwTNrpMuN5A1eq5C-XcTI8DutGIgbc_rRywzdyP8FkniDQF6-2U6aEkKgd8MwnZHtAWAlzPLCluqayqvxLDrIwLgwIfCfENbOMkJWtlaNGoanzjpME4W3NbUAXhIm015YfXBg0HRdxiWYLFLzgL5&uniplatform=NZKPT&language=CHS (accessed on 8 January 2025).
- Su, A.; Zhang, C.; Liu, C.; Ge, X.; Xu, M.; Yao, L. Quality comparison of glutinous rice fermented wine and waxy maize fermented wine. Sci. Technol. Food Ind. 2022, 43, 26–32. [Google Scholar] [CrossRef]
- Yang, K. Effects of Different Raw Materials and Roasting Processes on the Quality of Fermented Grains and Analysis; Shaanxi Normal University: Xi’an, China, 2020. [Google Scholar] [CrossRef]
- Li, T.; Deng, M.S.; Li, S.; Lei, Y.; Li, D.; Li, K. Revealing differences in flavor compounds during plum wine fermentation using single and mixed yeast strains through metabolomic analysis. Food Chem. X 2025, 25, 102100. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Su, W.; Mu, Y.; Jiang, L.; Mu, Y. Correlations between microbiota with physicochemical properties and volatile flavor components in black glutinous rice wine fermentation. Food Res. Int. 2020, 138, 109800. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, Y.; Qian, M.C. Aroma characterization of chinese rice wine by gas chromatography-olfactometry, chemical quantitative analysis and aroma reconstitution. J. Agric. Food Chem. 2013, 61, 11295–11302. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Chen, X.; Wang, C.; Liu, Y.; Li, M.; Pan, X.; Chang, X. Microbial communities and correlation between microbiota and volatile compounds in fermentation starters of Chinese sweet rice wine from different regions. Foods 2023, 12, 2932. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lei, Y.; Li, D.; Li, J.Y.; Jin, L. Impact of Sequential Fermentation with Different Non-Saccharomyces Yeasts and Saccharomyces cerevisiae on the Aroma of Yinhong Plum Wine. Food Sci. 2023, 44, 179–187. [Google Scholar] [CrossRef]
- Zheng, R.L.; Yang, C.J.; Niu, C.T.; Zheng, F.Y.; Wang, J.J.; Li, Q.; Liu, C.F. Screening of Saccharomyces cerevisiae with rice wine by liquid-state fermentation with non-Miqu. J. Northeast. Agric. Univ. 2022, 53, 51–62. [Google Scholar] [CrossRef]
- Mimura, N.; Isogai, A.; Iwashita, K.; Bamba, T.; Fukusaki, E. Gas chromatography/mass spectrometry based component profiling and quality prediction for Japanese sake. J. Biosci. Bioeng. 2014, 118, 406–414. [Google Scholar] [CrossRef]
- Lei, Y.; Gong, Y.L.; Tang, H.H.; Wang, Z.N.; Li, Z.J.; Zhu, S.S. Evaluation and analysis of brewing characteristics and flavor compounds of different glutinous rice varieties. Food Sci. Technol. 2024, 49, 90–97. [Google Scholar] [CrossRef]
- Wang, N.; Chen, S.; Zhou, Z. Characterization of volatile organic compounds as potential aging markers in Chinese rice wine using multivariable statistics. J. Sci. Food Agric. 2019, 99, 6444–6454. [Google Scholar] [CrossRef] [PubMed]
- Su, J.J. Study on Preparation Technology and Quality Characteristics of Brown Rice Wine. Master’s Thesis, Jiangnan University, Wuxi, China, 2020. [Google Scholar] [CrossRef]
- Fan, S.H.; Li, Y.X.; Bai, B.Q. Determination of 5 higher alcohols in light-flavor Baijiu by vortex-assisted dispersion liquid-liquid microextraction-gas chromatography. China Brew. 2020, 39, 194–200. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Zhi, H.X.; Liang, A.J.; Zhu, C.L.; Tang, J.N. Optimization of Fermentation Process and Comparative Analysis of Flavor Components of Rice Wine Fermented by Different Regions of Sweet Wine Koji. Mod. Food Sci. Technol. 2025, 41, 1–9. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Y.J.; Yong, Y.Y.; Wen, R.X.; Kong, B.H. Research Progress on Relationship and Mechanism between MicrobialDiversity and Flavor Development in Traditional Fermented Foods. Sci. Technol. Food Ind. 2021, 42, 412–419. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, Y.; Wang, G.; Tao, L.; Yu, J.; Ai, L. Effects of boiling, ultra-high temperature and high hydrostatic pressure on free amino acids, flavor characteristics and sensory profiles in Chinese rice wine. Food Chem. 2019, 275, 407–416. [Google Scholar] [CrossRef]
- Gong, X.H.; Zhang, D.Y.; Xie, L.; Chen, C. Research Progress on the Relationship between Microbial Community and Flavor Quality Formation in Rice Wine. Food Sci. 2024, 45, 358–366. [Google Scholar] [CrossRef]
- Xu, J.-Z.; Zhang, Y.-Y.; Zhang, W.-G. Correlation between changes in flavor compounds and microbial community ecological succession in the liquid fermentation of rice wine. World J. Microbiol. Biotechnol. 2024, 40, 17. [Google Scholar] [CrossRef]
- Chen, S.; Wang, C.; Qian, M.; Li, Z.; Xu, Y. Characterization of the Key Aroma Compounds in Aged Chinese Rice Wine by Comparative Aroma Extract Dilution Analysis, Quantitative Measurements, Aroma Recombination, and Omission Studies. J. Agric. Food Chem. 2019, 67, 4876–4884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Zhang, W.G.; Xu, J.Z.; Qian, H.; Ma, X.C.; Zhu, X.W. Changes of physicochemical indices and flavor substances in rice wine during liquid fermentation. J. Northeast. Agric. Univ. 2020, 51, 79–89. [Google Scholar] [CrossRef]
- Su, Y.T. Study on the Flavor Formation and Characteristics of Sweet Rice Wine. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2015. [Google Scholar] [CrossRef]
- Zhao, Y.; Gu, M.; Jiang, P.; Fang, S.; Yan, N.; Kong, F.; Ma, D.; Ren, D.; Pang, X.; Qiu, J. Characterisation of aroma compounds, sensory characteristics, and bioactive components of a new type of huangjiu fermented with Chinese wild rice (Zizania latifolia). Food Chem. 2024, 452, 139524. [Google Scholar] [CrossRef]
- Determination of Moisture in Foods. 2016, Volume 12. Available online: https://kns.cnki.net/kcms2/article/abstract?v=6TwuVQQ8bf_a8JVOHg32jN8wxQVJcscMyPXi1zAQyTS7LWOtMSVmwCfSJo3WBW8zUSDU_t7GeOHq_2X7TGGnkVe82vY5dXqQD3Oz65Z-pT5YprMeT52R6A-_sxN9O2CzwwiyXQHt7byKlfSXSdDBwZtFQiqht5HjHuwy7fmjXhZeC7TfIJxrR7AW9ebD0yJx&uniplatform=NZKPT&language=CHS (accessed on 8 January 2025).
- Determination of Fat in Foods. 2016, Volume 16. Available online: https://kns.cnki.net/kcms2/article/abstract?v=6TwuVQQ8bf_o9WM3N4Yh79bmythHeRqYLOALIUx_ZiGv56icB0e8IY0n0zMQT4AQ7f6ZRTffKUuyCj9OdTa5cYuCPQ85ZujfK-Ecqeb9kEJPcg5ZTrSCT_v3-xmFeASIKBjOlipSDwPH9aS1fBdo6cImE0b-JRWn--c03EdH3jNHdqE4kthW1ku4geUku6wg&uniplatform=NZKPT&language=CHS (accessed on 8 January 2025).
- Determination of Starch in Foods. 2016, Volume 16. Available online: https://kns.cnki.net/kcms2/article/abstract?v=6TwuVQQ8bf_j2VRr6gQ0-lKeZ7TAxQq3xBkZ986C1h_etZy79Yx8ynr6B3WnNwOgb9adGodp6b_fGYx5vmzuXrFB_OeI8vUfl2uEjS2H2PCyiM9_rf1g4g8OsDcXpZXww1-dfR-w9JzKjrNATOGNjvCAHbuK3PGn0AUz2duiJvqZwKMjVr5EUZRKxY8GLLvR&uniplatform=NZKPT&language=CHS (accessed on 8 January 2025).
- Determination of Protein in Foods. 2016, Volume 12. Available online: https://kns.cnki.net/kcms2/article/abstract?v=6TwuVQQ8bf-T9E0tYLTKDTvSMGnACFLEITmFC8GflBDQkTb_RW6-tRsSrCPMrXrurejle2dQbELWHtODaVpZR_79m3nG8AS5ASlKVAU4sdSbtvO5CVAQ0XaYsoIvdXZZqtqSUiXdHs-v8y4OjWWFkrPPv_XqDMtc2UeYlE06QugpS7ubn9p_uV4qPuadi5mT&uniplatform=NZKPT&language=CHS (accessed on 8 January 2025).
- Determination of Amylose in Rice. 2008, Volume 16. Available online: https://kns.cnki.net/kcms2/article/abstract?v=6TwuVQQ8bf_IZU02Qioj-vu8-vxciEq4GUoj5lD7pq_gnTQtZm06CCqrzpnuddXnD8MelYQ8Ko-ktqPGnlnNBF0CY6POoHK-CYhUdi43EkrKKp-bK0fvjEeZWXkEGyPcLts6EKTm3BT5kiPR5R_XdtYzRb4YzgQA4VVjtFpTcD_sIZpOsf4Nao5bgg3ImiUe&uniplatform=NZKPT&language=CHS (accessed on 8 January 2025).
- Huangjiu. 2018, Volume 28. Available online: https://kns.cnki.net/kcms2/article/abstract?v=GQPEaosfmU_yuZU-eWLtF6qU-JAyTzftkaHdex57tkIG5A9DqJwgc0EV0qC_m7v881P1T0eSCzZV3PucqQeAI8fMpf2H4cpGMuUEe-apGeq4_qIbfADzYjFsrRDavtpza75UcxqcHt-voafxYjD8vj-IVblmt5zHKqgjk4sdvAj9BXKAixZ2n3aMkwiUks6B&uniplatform=NZKPT&language=CHS (accessed on 8 January 2025).
- Xu, Y.; Jin, Y.; Su, J.; Yang, N.; Xu, X.; Jin, Z.; Cui, B.; Wu, F. Changes in the nutritional value, flavor, and antioxidant activity of brown glutinous rice during fermentation. Food Biosci. 2021, 43, 101273. [Google Scholar] [CrossRef]
- Zhou, W.J.; Zhang, F.; Wang, P.; Zhan, P.; Tian, H.L. GC-MS/GC-O Combined with Chemometrics for the Screening and Identification of Aroma Characteristics of Korla Pear Wine. Food Sci. 2018, 39, 222–227. [Google Scholar] [CrossRef]
- Huang, L. The Distinct Flavor of Regional Vidal Icewines Andeffects of Nonvolatile Contents on Aromas. Master’s Thesis, Jiangnan University, Wuxi, China, 2018. [Google Scholar]
- Zhang, Q.A.; Xu, B.W.; Chen, B.Y.; Zhang, B.S.; Cheng, S. Changes of Wine Flavor Properties from the Decreased Higher Alcohols Induced by Ultrasound Irradiation. Sci. Agric. Sin. 2021, 54, 1772–1786. [Google Scholar] [CrossRef]
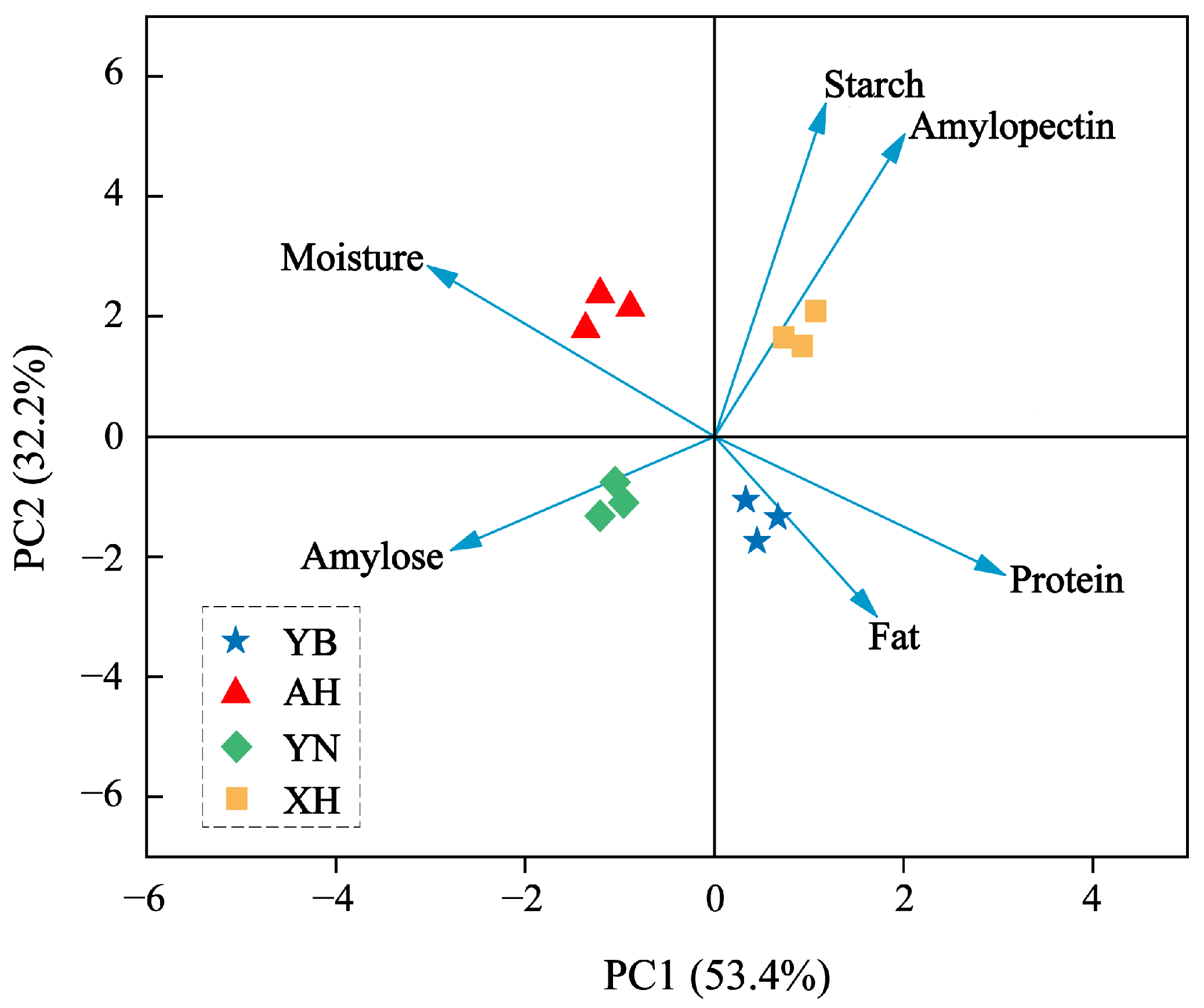
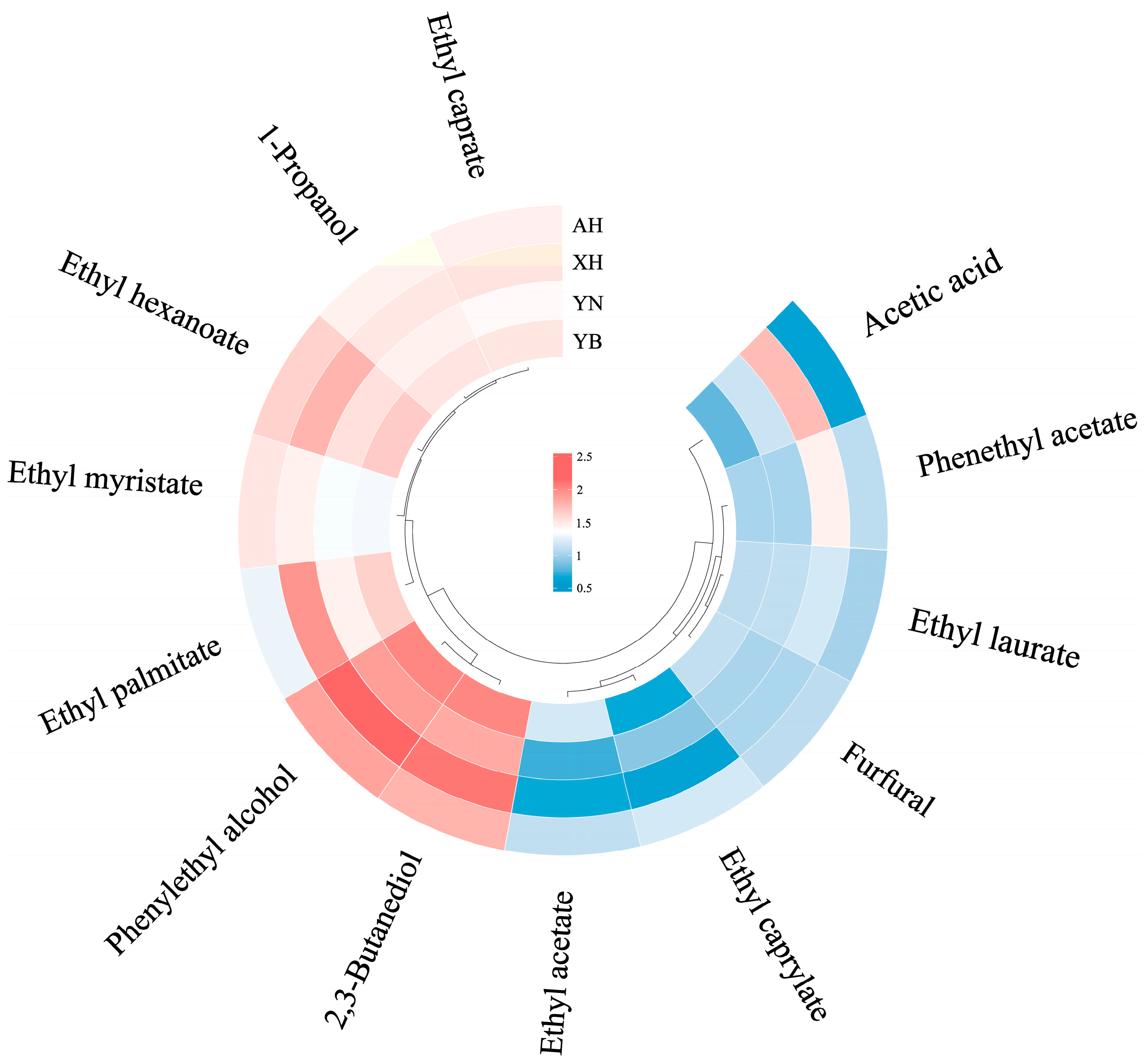
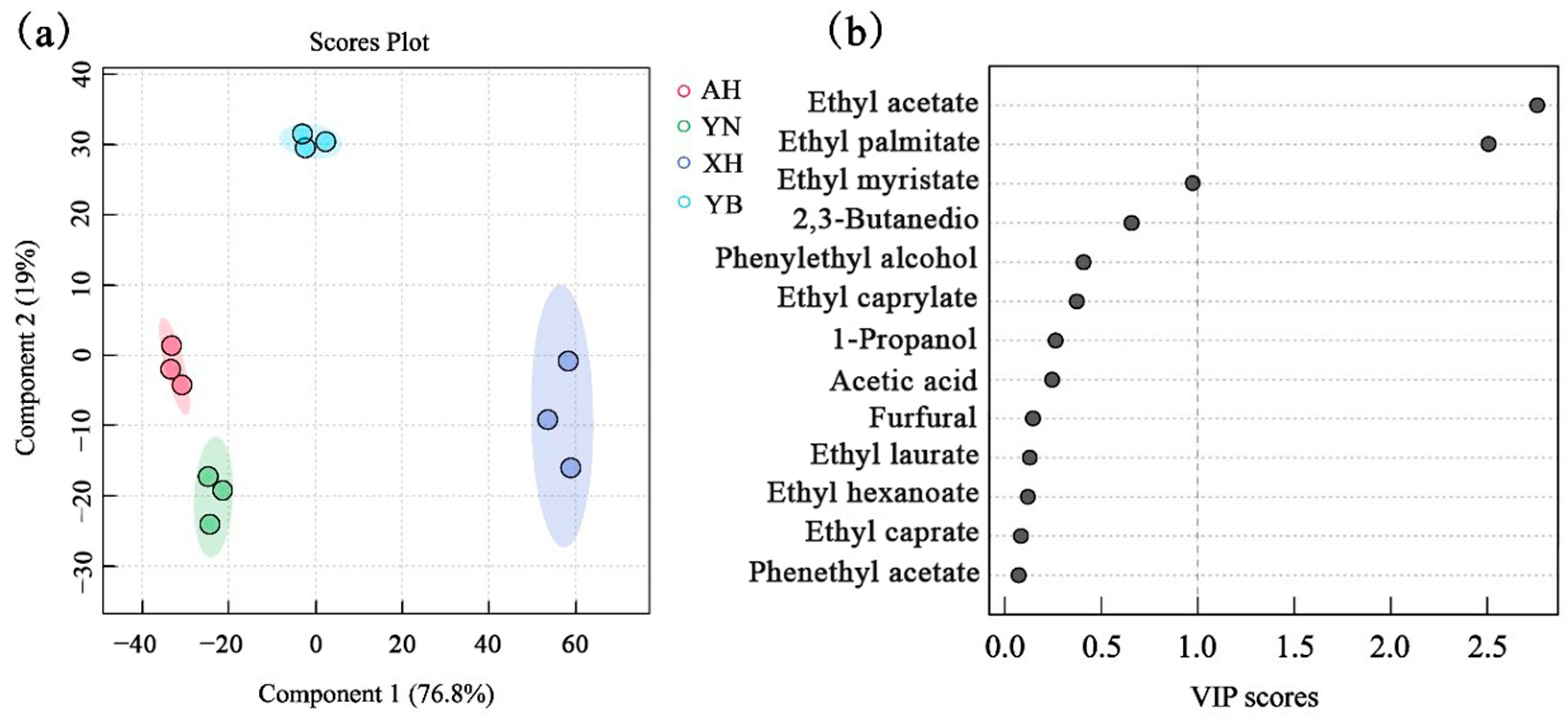
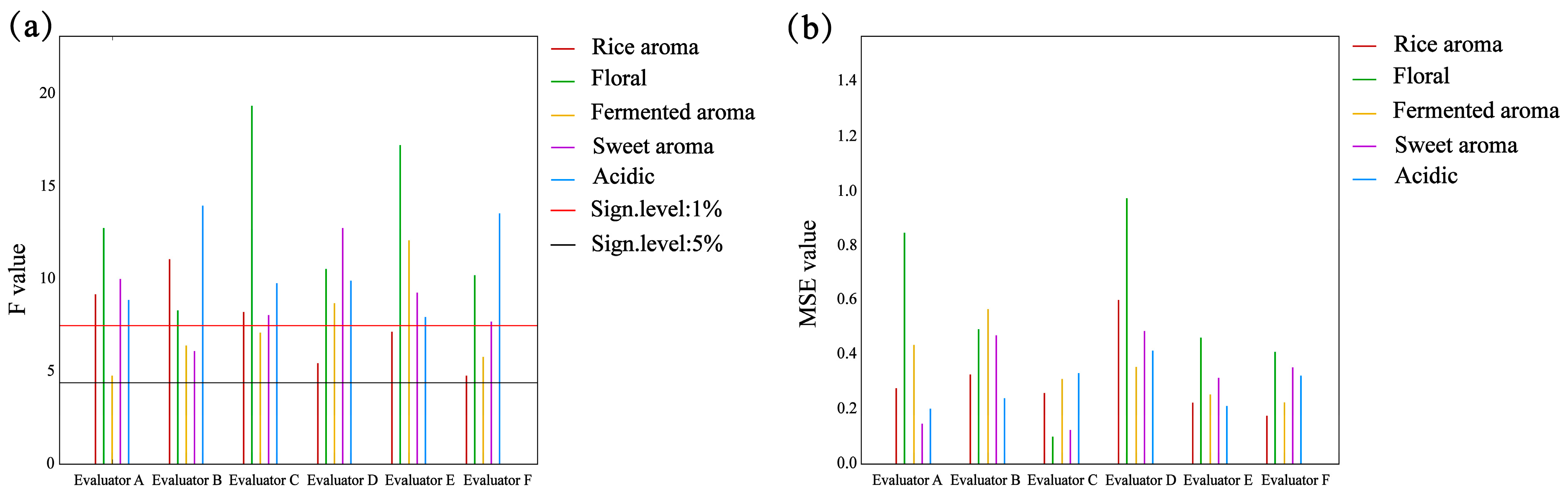
| Sample | Moisture | Starch | Amylose | Amylopectin | Protein | Fat |
|---|---|---|---|---|---|---|
| YN | 13.54 ± 0.02 b | 70.62 ± 0.46 c | 2.15 ± 0.08 a | 68.33 ± 0.26 c | 7.58 ± 0.09 b | 0.87 ± 0.03 ab |
| AH | 15.02 ± 0.22 a | 75.83 ± 0.06 b | 1.48 ± 0.07 b | 74.35 ± 0.10 b | 7.53 ± 0.26 b | 0.64 ± 0.02 c |
| XH | 10.77 ± 0.12 c | 78.32 ± 0.04 a | 1.26 ± 0.08 c | 77.05 ± 0.11 a | 8.36 ± 0.09 a | 0.92 ± 0.05 a |
| YB | 10.51 ± 0.08 d | 69.50 ± 0.02 d | 1.27 ± 0.07 c | 68.23 ± 0.08 c | 8.58 ± 0.04 a | 0.82 ± 0.07 b |
| Sample | Alcohol Content (%) | Soluble Solids (g/100 g) | Total Sugar (mg/mL) | pH |
|---|---|---|---|---|
| YN | 12.31 ± 0.04 c | 5.02 ± 0.01 a | 21.32 ± 0.47 c | 3.51 ± 0.13 a |
| AH | 13.05 ± 0.12 b | 4.57 ± 0.35 b | 28.04 ± 0.98 b | 3.38 ± 0.03 a |
| XH | 13.21 ± 0.08 a | 5.01 ± 0.01 a | 28.95 ± 0.62 b | 3.54 ± 0.09 a |
| YB | 12.13 ± 0.04 d | 5.05 ± 0.03 a | 31.43 ± 0.35 a | 3.45 ± 0.04 a |
| No. | CAS | Compound 1 | Aroma Description | RI 2 | Identification 3 | FD factor 4 | |||
|---|---|---|---|---|---|---|---|---|---|
| XH | AH | YN | YB | ||||||
| Esters | |||||||||
| 1 | 628-97-7 | Ethyl palmitate | Faintly waxed | 2262 | MS, O, RI | >256 | 64 | 128 | 128 |
| 2 | 124-06-1 | Ethyl myristate | Sour | 2042 | MS, O, RI | 128 | >64 | >64 | >64 |
| 3 | 110-38-3 | Ethyl caprate | Brandy, Pear | 1392 | MS, O, RI, | 64 | 64 | 64 | 64 |
| 4 | 106-33-2 | Ethyl laurate | Floral, Fruit | 1835 | MS, O, RI | >32 | >32 | >32 | >32 |
| 5 | 627-90-7 | Ethyl undecanoate | Coconut, Fruit | 1652 | MS, O, RI | 8 | 8 | 8 | 16 |
| 6 | 123-66-0 | Ethyl hexanoate | Fruit, Brandy | 1241 | MS, O, RI | 128 | >64 | >64 | >64 |
| 7 | 106-32-1 | Ethyl caprylate | Fruit, Floral, Fat | 1425 | MS, O, RI | 32 | >32 | 32 | 32 |
| 8 | 141-78-6 | Ethyl acetate | Aromatic, Grape | 906 | MS, O, RI | >32 | >32 | >32 | 64 |
| 9 | 54515-77-4 | 2-Octyl acetate | Fruit, Jasmine-like | 1250 | MS, O, RI | 8 | 8 | 8 | 16 |
| 10 | 123-92-2 | Isoamyl acetate | Pear, Apple | 1065 | MS, O, RI | 4 | 4 | 4 | 4 |
| 11 | 103-45-7 | Phenethyl acetate | Honey, Rose, Floral, | 1940 | MS, O, RI | 64 | 32 | 32 | 32 |
| 12 | 28598-81-4 | Ethyl 9-bromononanoate | Fruit, Rose | 1900 | MS, O, RI | 4 | 4 | 4 | 8 |
| 13 | 6114-18-7 | Ethyl elaidate; | Floral, Fruit | 2345 | MS, O, RI | 8 | 8 | 8 | 8 |
| 14 | 123-25-1 | Diethyl succinate | Cotton, Fabric | 1673 | MS, O, RI | 4 | 4 | 4 | 4 |
| 15 | 54546-22-4 | Ethyl 9-hexadecenoate | -- | 2167 | MS, O, RI | -- | -- | -- | -- |
| 16 | 111-62-6 | Ethyl oleate | Dairy | 1988 | MS, O, RI | -- | -- | 2 | |
| Alcohols | |||||||||
| 17 | 60-12-8 | Phenylethyl alcohol | Sweet, Rose | 1936 | MS, O, RI | >256 | >256 | >256 | >256 |
| 18 | 98-00-0 | Furfuryl alcohol | Caramel | 900 | MS, O, RI | 4 | 4 | 4 | 2 |
| 19 | 78-83-1 | Isobutanol | Apple, Bitter | 1095 | MS, O, RI | 32 | 32 | 32 | 64 |
| 20 | 71-23-8 | 1-Propanol | Candy, Alcohol | 1023 | MS, O, RI | 128 | 128 | 128 | 128 |
| 21 | 513-85-9 | 2,3-Butanediol | Butter, Sour | 976 | MS, O, RI | >256 | 256 | 256 | 256 |
| 22 | 123-51-3 | Isoamylol | Burnt, Floral, Malt | 1204 | MS, O, RI | 8 | 8 | 8 | 8 |
| 23 | 57-55-6 | Propylene glycol | -- | 950 | MS, O, RI | -- | -- | -- | -- |
| 24 | 764-48-7 | 2-(Vinyloxy)ethanol | -- | 1053 | MS, O, RI | -- | -- | -- | -- |
| 25 | 149-32-6 | Erythritol | Sweet | 2328 | MS, O, RI | 8 | 8 | 8 | 16 |
| 26 | 111-27-3 | 1-Hexanol | Woody, Herbaceous, Sweet | 1365 | MS, O, RI | 8 | 4 | 8 | 8 |
| 27 | 540-51-2 | 2-Bromoethanol | -- | 954 | MS, O, RI | -- | -- | -- | -- |
| 28 | 598-75-4 | 3-Methyl-2-Butanol | Alcohol | 1156 | MS, O, RI | 4 | 4 | 4 | 4 |
| 29 | 15356-70-4 | Menthol | Peppermint fragrance | 1247 | MS, O, Std | 2 | -- | -- | 2 |
| Acids | |||||||||
| 30 | 64-19-7 | Acetic acid | Vinegar | 1455 | MS, O, RI | 128 | 64 | 64 | 64 |
| 31 | 124-07-2 | Octanoic acid | Cheese | 2132 | MS, O, RI | 2 | 2 | -- | 2 |
| Aldehydes | |||||||||
| 32 | 124-19-6 | Nonanal | Fat, Herb, Floral | 1394 | MS, O, RI | 8 | 4 | 4 | 8 |
| 33 | 98-01-1 | Furfural | Almond, Burnt sugar | 1482 | MS, O, RI | >32 | >32 | >32 | >32 |
| 34 | 75-07-0 | Acetaldehyde | Fruit | 744 | MS, O, RI | 16 | 16 | 8 | 16 |
| 35 | 620-02-0 | 5-Methyl furfural | Burnt sugar | 1589 | MS, O, RI | 2 | -- | 2 | -- |
| Ketones | |||||||||
| 36 | 116-09-6 | Acetol | Herb, Malt | 1281 | MS, O, RI | 2 | 4 | 2 | -- |
| 37 | 111-13-7 | 2-Octanone | Floral, Bitter, Fruity | 1295 | MS, O, RI | -- | 2 | 2 | -- |
| 38 | 5878-19-3 | Methoxyacetone | -- | 965 | MS, O, RI | -- | -- | -- | -- |
| No. | Compound | Slope | Intercept | R2 |
|---|---|---|---|---|
| 1 | Ethyl palmitate | 164.22 | −0.1089 | 0.9953 |
| 2 | Ethyl myristate | 94.06 | 0.3133 | 0.9964 |
| 3 | Ethyl caprate | 85.61 | 0.0118 | 0.9947 |
| 4 | Ethyl laurate | 124.47 | 0.0718 | 0.9945 |
| 5 | Ethyl hexanoate | 115.25 | −0.0064 | 0.9905 |
| 6 | Ethyl caprylate | 96.87 | −0.0142 | 0.9940 |
| 7 | Ethyl acetate | 51.10 | −0.0032 | 0.9957 |
| 8 | Phenethyl acetate | 35.88 | 0.0008 | 0.9976 |
| 9 | Phenylethyl alcohol | 51.78 | 0.0207 | 0.9944 |
| 10 | 1-Propanol | 45.61 | 1.5385 | 0.9972 |
| 11 | 2,3-Butanediol | 94.81 | 0.9001 | 0.9947 |
| 12 | Acetic acid | 75.98 | −0.0041 | 0.9963 |
| 13 | Furfural | 21.96 | −0.0003 | 0.9993 |
| Sensory Descriptor | Sensory Description | Reference Standard |
|---|---|---|
| Rice aroma | Rice-like grain aroma | 100 mL of 1% aqueous ethanol containing 5 g of rice |
| Floral | Aroma similar to that of roses, moonflowers | 1 mg/L aqueous solution of phenethyl alcohol |
| Fermentation aroma | Aroma similar to fermented foods such as kimchi and soy sauce | 100 mL of a 10% aqueous ethanol solution of 1 mg/L 2-butanol |
| Sweet | Aroma like honey, mash | 100 mL of a 10% aqueous ethanol solution of 200 g/L honey |
| Acidic | The aroma of balsamic vinegar | 100 mL of a 10% ethanol aqueous solution of 1 mg/L acetic acid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yu, K.; Xiao, X.; Tan, J.; Liao, R.; Li, C.; Li, S.; Liu, N.; Ma, Y. Influence of Glutinous Rice Raw Material Characteristics on the Aroma Profile of Rice Wine. Molecules 2025, 30, 3315. https://doi.org/10.3390/molecules30163315
Wang Y, Yu K, Xiao X, Tan J, Liao R, Li C, Li S, Liu N, Ma Y. Influence of Glutinous Rice Raw Material Characteristics on the Aroma Profile of Rice Wine. Molecules. 2025; 30(16):3315. https://doi.org/10.3390/molecules30163315
Chicago/Turabian StyleWang, Yue, Kangjie Yu, Xiongjun Xiao, Jianxia Tan, Rui Liao, Cong Li, Siyu Li, Nian Liu, and Yi Ma. 2025. "Influence of Glutinous Rice Raw Material Characteristics on the Aroma Profile of Rice Wine" Molecules 30, no. 16: 3315. https://doi.org/10.3390/molecules30163315
APA StyleWang, Y., Yu, K., Xiao, X., Tan, J., Liao, R., Li, C., Li, S., Liu, N., & Ma, Y. (2025). Influence of Glutinous Rice Raw Material Characteristics on the Aroma Profile of Rice Wine. Molecules, 30(16), 3315. https://doi.org/10.3390/molecules30163315





