Design of Experiments Approach for Efficient Heavy Metals Stabilization Using Metakaolin-Based Geopolymers
Abstract
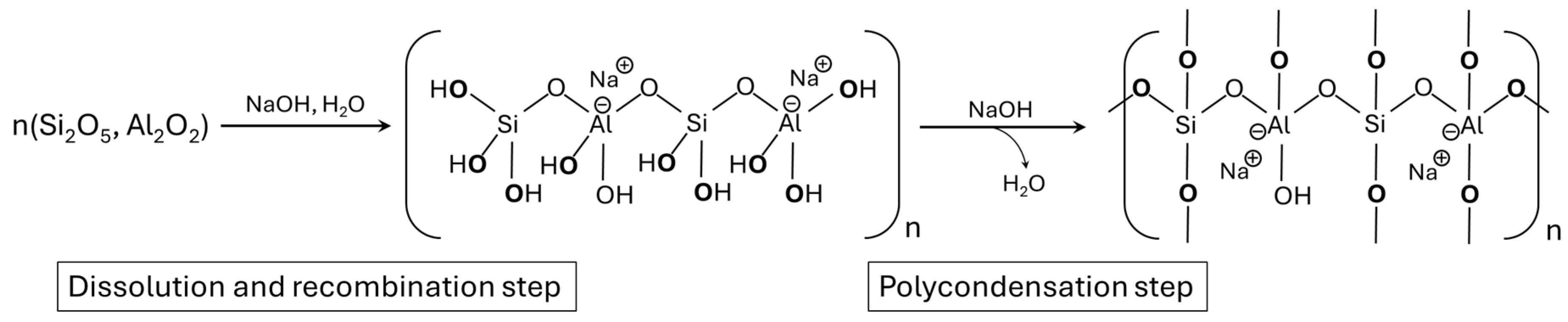
1. Introduction
2. Results and Discussion
2.1. Chromium and Nickel Quantification: Uptake and Leaching
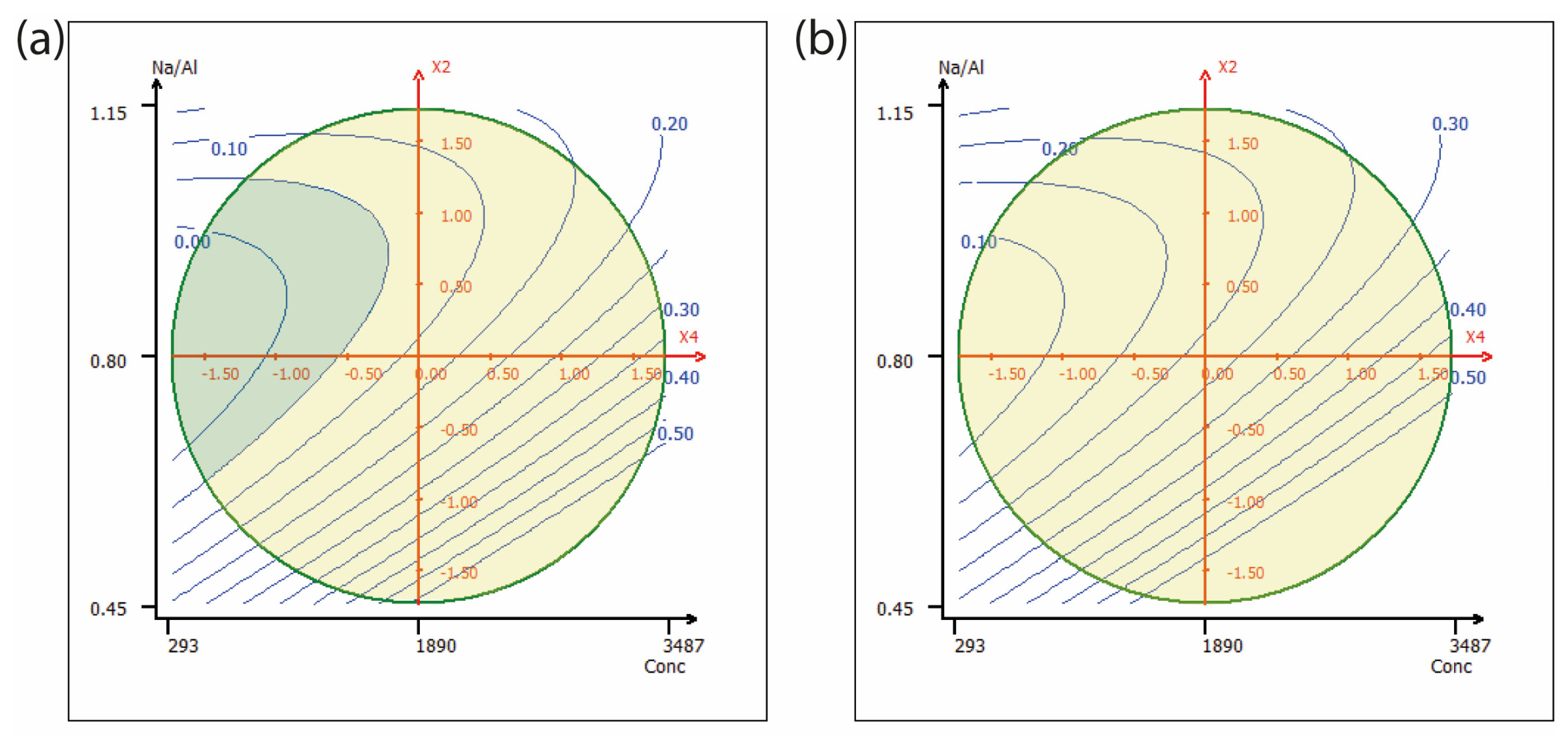
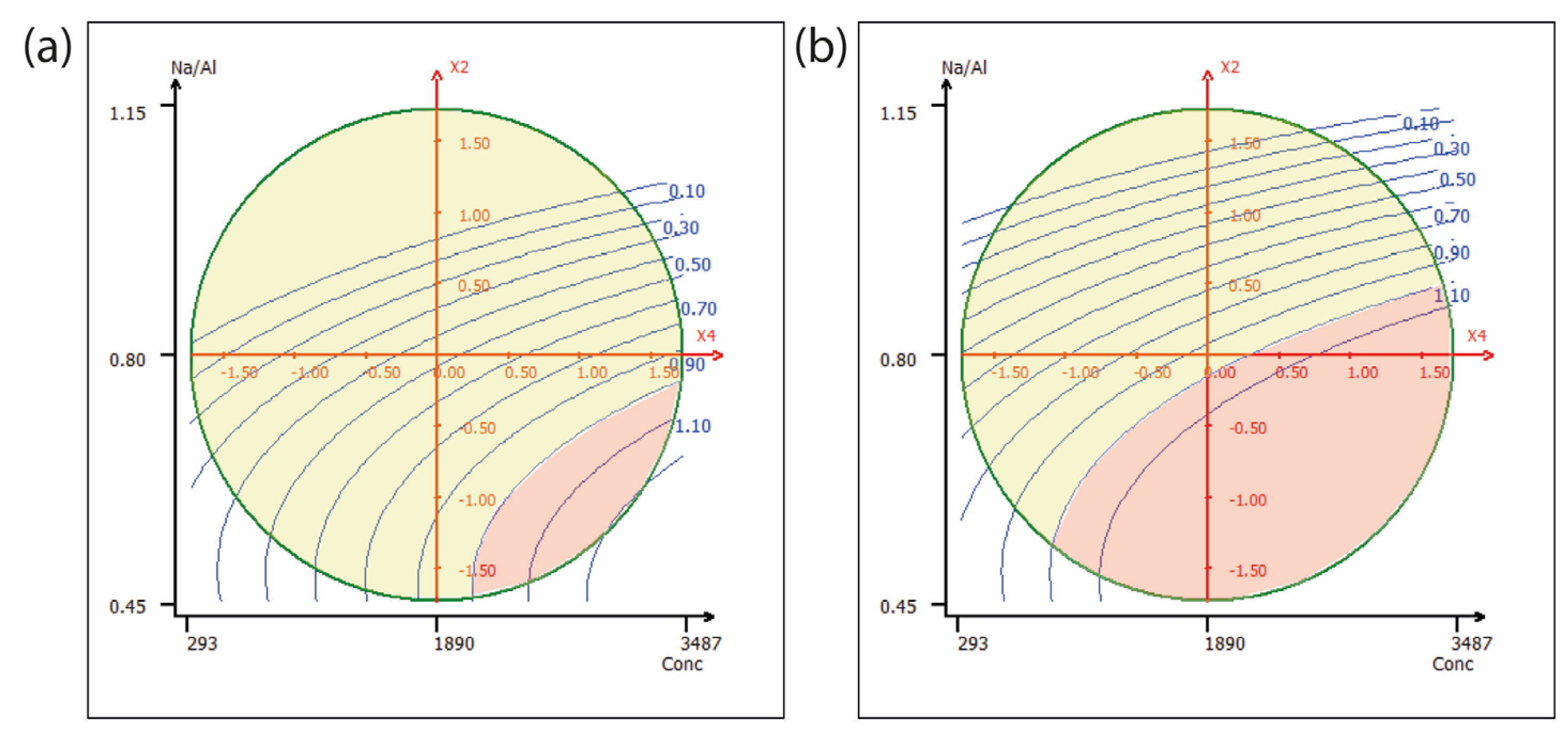
2.2. Nickel
2.3. Chrome
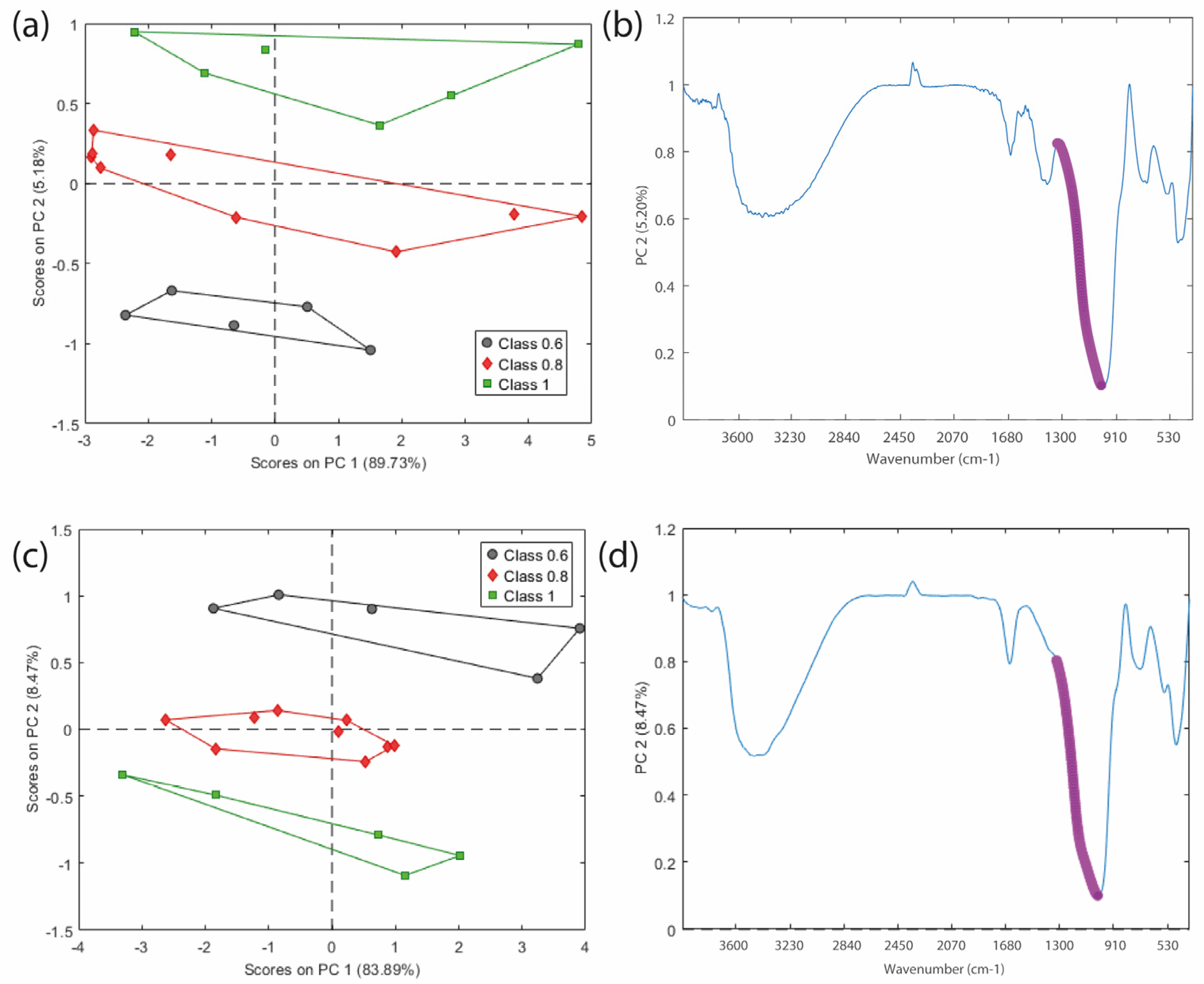
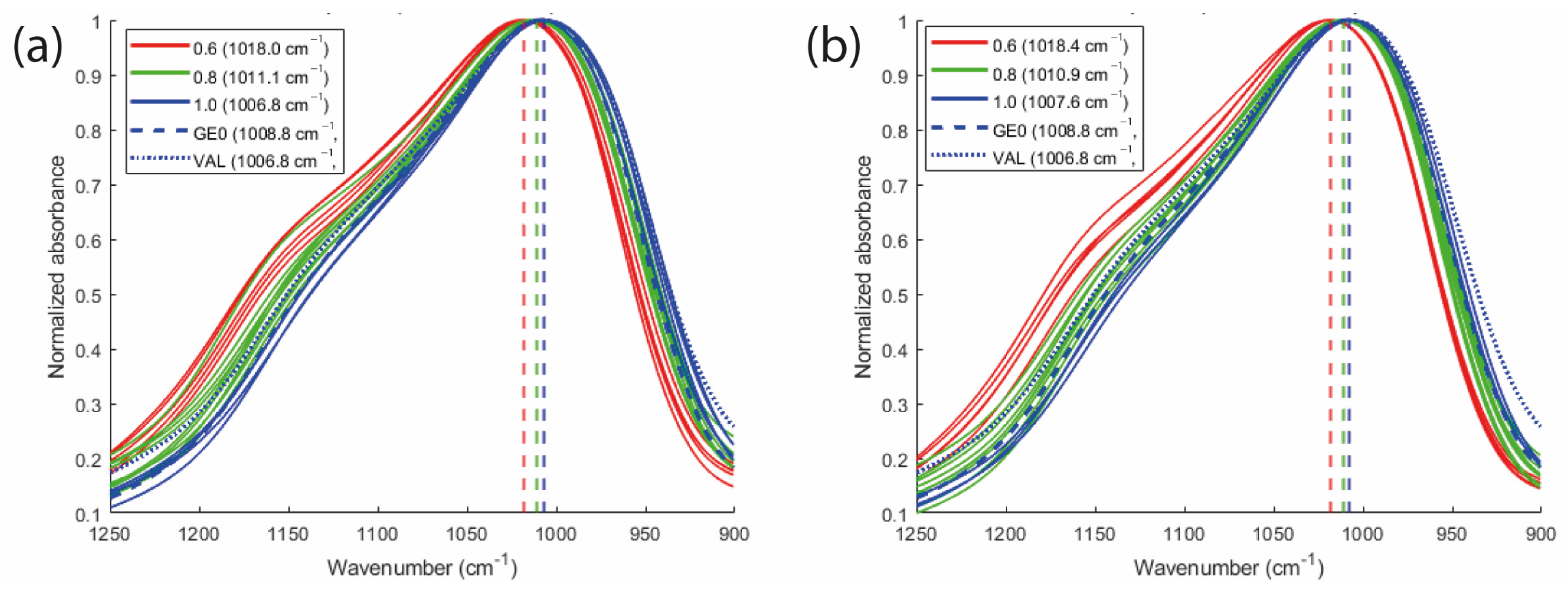
2.4. FTIR Analysis
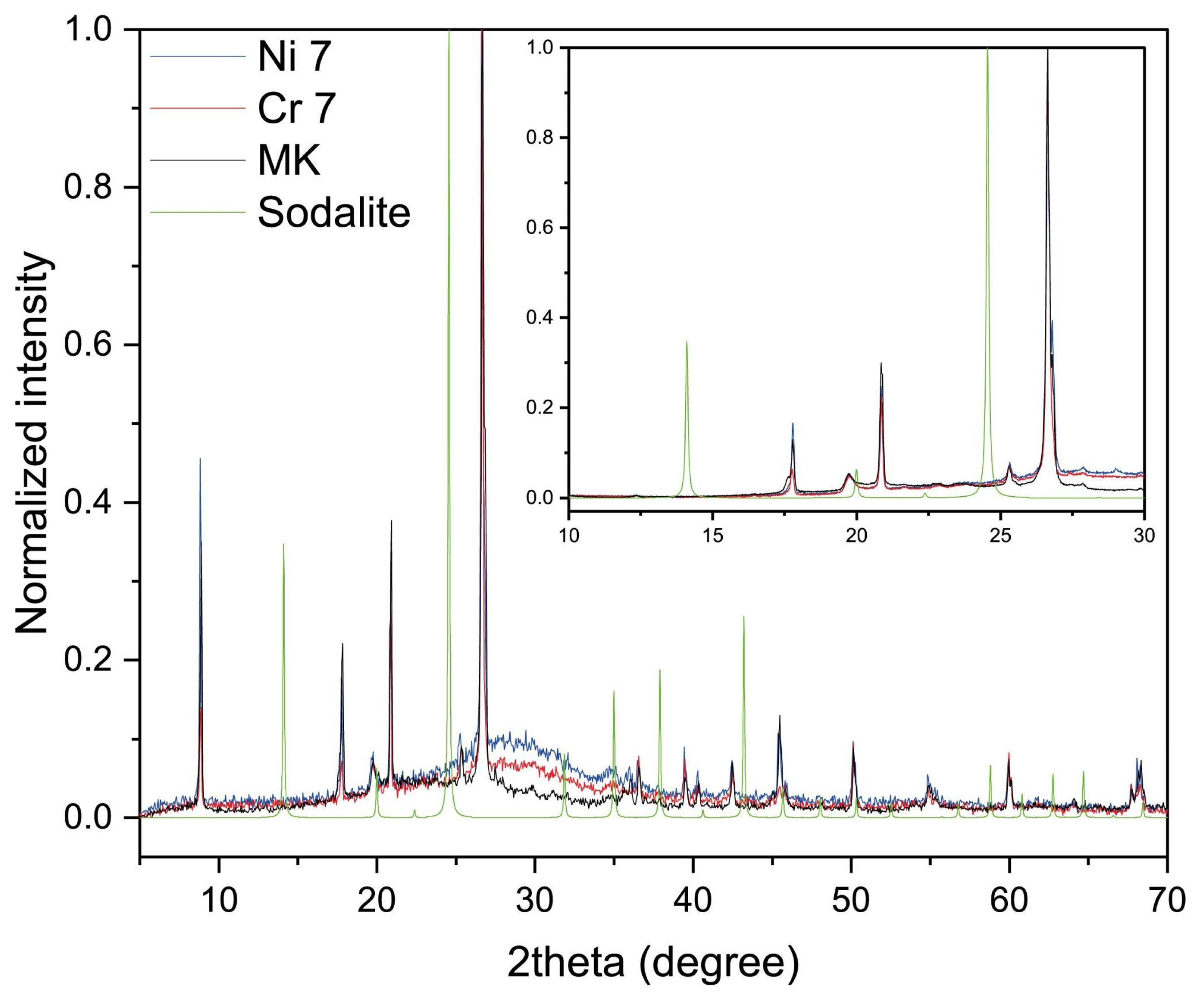
2.5. XRD Analysis
3. Materials and Methods
3.1. Reagents and Materials
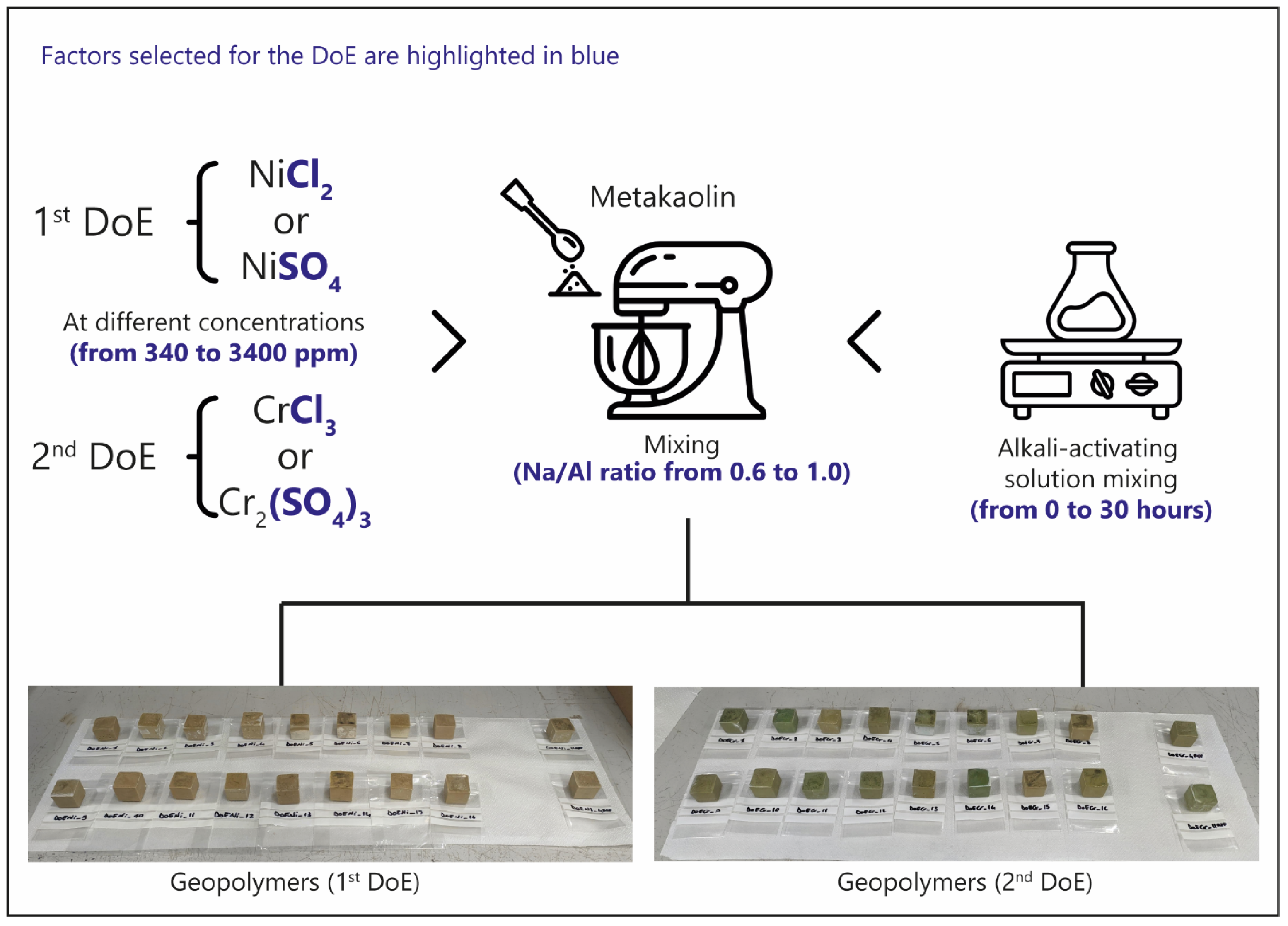
3.2. Geopolymers Formulation
3.3. Chemometric Approach
3.4. Geopolymer Digestion and Leaching Tests
3.5. Characterization of Geopolymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Provis, J.L.; Van Deventer, J.S.J. Geopolymers and Other Alkali-Activated Materials. In Lea’s Chemistry of Cement and Concrete; Elsevier: Amsterdam, The Netherlands, 2019; pp. 779–805. ISBN 9780081007730. [Google Scholar]
- Kriven, W.M.; Leonelli, C.; Provis, J.L.; Boccaccini, A.R.; Attwell, C.; Ducman, V.S.; Ferone, C.; Rossignol, S.; Luukkonen, T.; van Deventer, J.S.J.; et al. Why Geopolymers and Alkali-Activated Materials Are Key Components of a Sustainable World: A Perspective Contribution. J. Am. Ceram. Soc. 2024, 107, 5159–5177. [Google Scholar] [CrossRef]
- Ji, Z.; Pei, Y. Bibliographic and Visualized Analysis of Geopolymer Research and Its Application in Heavy Metal Immobilization: A Review. J. Environ. Manag. 2019, 231, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, I.; Kamseu, E.; Michelazzi, M.; Barbieri, L.; Corradi, A.; Leonelli, C. Chemical Stability of Geopolymers Containing Municipal Solid Waste Incinerator Fly Ash. Waste Manag. 2010, 30, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Pei, Y. Immobilization Efficiency and Mechanism of Metal Cations (Cd2+, Pb2+ and Zn2+) and Anions (AsO43− and Cr2O72−) in Wastes-Based Geopolymer. J. Hazard. Mater. 2020, 384, 121290. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Y.; Zhang, W.; Ye, J.; Wang, R. Solidification Performance and Mechanism of Typical Radioactive Nuclear Waste by Geopolymers and Geopolymer Ceramics: A Review. Prog. Nucl. Energy 2024, 169, 105106. [Google Scholar] [CrossRef]
- Boldrini, G.; Sgarlata, C.; Lancellotti, I.; Barbieri, L.; Giorgetti, M.; Ciabocco, M.; Zamponi, S.; Berrettoni, M.; Leonelli, C. Efficient Chemical Stabilization of Tannery Wastewater Pollutants in a Single Step Process: Geopolymerization. Sustain. Environ. Res. 2021, 31, 33. [Google Scholar] [CrossRef]
- Tian, Q.; Bai, Y.; Pan, Y.; Chen, C.; Yao, S.; Sasaki, K.; Zhang, H. Application of Geopolymer in Stabilization/Solidification of Hazardous Pollutants: A Review. Molecules 2022, 27, 4570. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-Activated Binders: A Review. Part 1. Historical Background, Terminology, Reaction Mechanisms and Hydration Products. Constr. Build. Mater. 2008, 22, 1305–1314. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-Part Alkali-Activated Materials: A Review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J. Geopolymer Technology: The Current State of the Art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Valentini, L. Modeling Dissolution-Precipitation Kinetics of Alkali-Activated Metakaolin. ACS Omega 2018, 3, 18100–18108. [Google Scholar] [CrossRef]
- El-Eswed, B.I.; Aldagag, O.M.; Khalili, F.I. Efficiency and Mechanism of Stabilization/Solidification of Pb(II), Cd(II), Cu(II), Th(IV) and U(VI) in Metakaolin Based Geopolymers. Appl. Clay Sci. 2017, 140, 148–156. [Google Scholar] [CrossRef]
- Hou, D.; Zhang, J.; Pan, W.; Zhang, Y.; Zhang, Z. Nanoscale Mechanism of Ions Immobilized by the Geopolymer: A Molecular Dynamics Study. J. Nucl. Mater. 2020, 528, 151841. [Google Scholar] [CrossRef]
- Walkley, B.; San Nicolas, R.; Sani, M.A.; Rees, G.J.; Hanna, J.V.; van Deventer, J.S.J.; Provis, J.L. Phase Evolution of C-(N)-A-S-H/N-A-S-H Gel Blends Investigated via Alkali-Activation of Synthetic Calcium Aluminosilicate Precursors. Cem. Concr. Res. 2016, 89, 120–135. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer CHemistry and Applications, 5th ed.; Geopolymer Institute: Saint-Quentin, France, 2008. [Google Scholar]
- Giorgetti, M.; Berrettoni, M.; Aquilanti, G.; Boldrini, G.; Lancellotti, I.; Leonelli, C. The Coordination Core and Charge of Chromium in Metakaolin-Geopolymers as Revealed by X-Ray Absorption Spectroscopy. Mater. Lett. 2020, 270, 127741. [Google Scholar] [CrossRef]
- D’Angelo, A.; Vertuccio, L.; Leonelli, C.; Alzeer, M.I.M.; Catauro, M. Entrapment of Acridine Orange in Metakaolin-Based Geopolymer: A Feasibility Study. Polymers 2023, 15, 675. [Google Scholar] [CrossRef]
- Genua, F.; Lancellotti, I.; Leonelli, C. Geopolymer-Based Stabilization of Heavy Metals, the Role of Chemical Agents in Encapsulation and Adsorption: Review. Polymers 2025, 17, 670. [Google Scholar] [CrossRef]
- EPA/530/R-93/012; Technical Resource Document Solidification/Stabilization and Its Application to Waste Materials. Battelle: Columbus, OH, USA, 1993.
- Tian, C.; Luo, Z.; Liu, L.; Liu, X.; Zhang, M.; Chen, M.; Hai, R. Solidification/Stabilization of Chromium in Red Mud-Based Geopolymer. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2024, 39, 819–830. [Google Scholar] [CrossRef]
- Rożek, P.; Król, M.; Mozgawa, W. Geopolymer-Zeolite Composites: A Review. J. Clean. Prod. 2019, 230, 557–579. [Google Scholar] [CrossRef]
- Rees, C.A.; Provis, J.L.; Lukey, G.C.; Van Deventer, J.S.J. Attenuated Total Reflectance Fourier Transform Infrared Analysis of Fly Ash Geopolymer Gel Aging. Langmuir 2007, 23, 8170–8179. [Google Scholar] [CrossRef]
- Greiser, S.; Sturm, P.; Gluth, G.J.G.; Hunger, M.; Jäger, C. Differentiation of the Solid-State NMR Signals of Gel, Zeolite Phases and Water Species in Geopolymer-Zeolite Composites. Ceram. Int. 2017, 43, 2202–2208. [Google Scholar] [CrossRef]
- Leardi, R. Experimental Design in Chemistry: A Tutorial. Anal. Chim. Acta 2009, 652, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi-Najafabadi, H.; Leardi, R.; Jalali-Heravi, M. Experimental Design in Analytical Chemistry-Part I: Theory. J. AOAC Int. 2014, 97, 3–11. [Google Scholar] [CrossRef] [PubMed]
- García-Mejía, T.A.; de Lourdes Chávez-García, M. Compressive Strength of Metakaolin-Based Geopolymers: Influence of KOH Concentration, Temperature, Time and Relative Humidity. Mater. Sci. Appl. 2016, 7, 772–791. [Google Scholar] [CrossRef]
- Al-Sughayer, R.; Alkhateb, H.; Yasarer, H.; Najjar, Y.; Al-Ostaz, A. Evaluation and Prediction of Slag-Based Geopolymer’s Compressive Strength Using Design of Experiment (DOE) Approach and Artificial Neural Network (ANN) Algorithms. Constr. Build. Mater. 2024, 440, 137322. [Google Scholar] [CrossRef]
- Muracchioli, M.; Menardi, G.; D’Agostini, M.; Franchin, G.; Colombo, P. Modeling the Compressive Strength of Metakaolin-Based Geopolymers Based on the Statistical Analysis of Experimental Data. Appl. Clay Sci. 2023, 242, 107020. [Google Scholar] [CrossRef]
- Venkatesan, M.; Zaib, Q.; Shah, I.H.; Park, H.S. Optimum Utilization of Waste Foundry Sand and Fly Ash for Geopolymer Concrete Synthesis Using D-Optimal Mixture Design of Experiments. Resour. Conserv. Recycl. 2019, 148, 114–123. [Google Scholar] [CrossRef]
- Driouich, A.; El Hassani, S.A.; Hamah Sor, N.; Zmirli, Z.; El Harfaoui, S.; Mydin, M.A.O.; Aziz, A.; Deifalla, A.F.; Chaair, H. Mix Design Optimization of Metakaolin-Slag-Based Geopolymer Concrete Synthesis Using RSM. Results Eng. 2023, 20, 101573. [Google Scholar] [CrossRef]
- Emdadi, Z.; Asim, N.; Amin, M.H.; Yarmo, M.A.; Maleki, A.; Azizi, M.; Sopian, K. Development of Green Geopolymer Using Agricultural and Industrial Waste Materials with High Water Absorbency. Appl. Sci. 2017, 7, 514. [Google Scholar] [CrossRef]
- Lo, K.W.; Lin, K.L.; Lin, Y.W.; Cheng, T.W. Identification of Main Factors Affecting Mechanical Characteristics of Silicon Carbide Sludge-Based Geopolymer via Experimental Design and Associated Statistical Analysis. Environ. Prog. Sustain. Energy 2023, 42, e14023. [Google Scholar] [CrossRef]
- Jiang, C.; Yang, Y.; Wang, M.; Xu, W.; Guo, Y.; Li, S.; Xu, S.; Liao, J. Optimization and Performance Prediction of Phosphate Tailing-Based Geopolymer Formulations Using D-Optimal Mixture Design and Multi-Algorithm Collaboration. Process Saf. Environ. Prot. 2025, 199, 107242. [Google Scholar] [CrossRef]
- Rossatto, D.L.; Netto, M.S.; Jahn, S.L.; Mallmann, E.S.; Dotto, G.L.; Foletto, E.L. Highly Efficient Adsorption Performance of a Novel Magnetic Geopolymer/Fe3O4 Composite towards Removal of Aqueous Acid Green 16 Dye. J. Environ. Chem. Eng. 2020, 8, 103804. [Google Scholar] [CrossRef]
- Ferreira, A.P.; Natal, A.P.S.; Baldo, A.P.; Silva, A.S.; Diaz de Tuesta, J.L.; Marin, P.; Peres, J.A.; Gomes, H.T. Response Surface Method-Driven Design of Experiments for the Synthesis of Fly Ash-Based Geopolymers in the Gallic Acid Optimized Removal from Wastewater. Chem. Eng. J. Adv. 2025, 21, 100703. [Google Scholar] [CrossRef]
- Genua, F.; Giovini, M.; Santoni, E.; Berrettoni, M.; Lancellotti, I.; Leonelli, C. Factors Affecting Consolidation in Geopolymers for Stabilization of Galvanic Sludge. Materials 2025, 18, 3015. [Google Scholar] [CrossRef]
- Gioia Lobbia, G.; Novara, G. References therein. Analisi Qualitativa e Complementi Di Chimica; Bulgarini: Loreto, Italy, 1994. [Google Scholar]
- Yuan, J.; Li, L.; He, P.; Chen, Z.; Lao, C.; Jia, D.; Zhou, Y. Effects of Kinds of Alkali-Activated Ions on Geopolymerization Process of Geopolymer Cement Pastes. Constr. Build. Mater. 2021, 293, 123536. [Google Scholar] [CrossRef]
- Chairunnisa, N.; Nurwidayati, R. The Effect of Natrium Hydroxide Molarity Variation and Alkali Ratio on the Compressive Strength of Geopolymer Paste and Mortar. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Banjarmasin, Indonesia, 23–25 October 2022; Institute of Physics: London, UK, 2023; Volume 1184. [Google Scholar]
- Perná, I.; Hanzlíček, T.; Šupová, M. The Identification of Geopolymer Affinity in Specific Cases of Clay Materials. Appl. Clay Sci. 2014, 102, 213–219. [Google Scholar] [CrossRef]
- Baykara, H.; Cornejo, M.H.; Espinoza, A.; García, E.; Ulloa, N. Preparation, Characterization, and Evaluation of Compressive Strength of Polypropylene Fiber Reinforced Geopolymer Mortars. Heliyon 2020, 6, e03755. [Google Scholar] [CrossRef]
- Finocchiaro, C.; Barone, G.; Mazzoleni, P.; Leonelli, C.; Gharzouni, A.; Rossignol, S. FT-IR Study of Early Stages of Alkali Activated Materials Based on Pyroclastic Deposits (Mt. Etna, Sicily, Italy) Using Two Different Alkaline Solutions. Constr. Build. Mater. 2020, 262, 120095. [Google Scholar] [CrossRef]
- Tippayasam, C.; Balyore, P.; Thavorniti, P.; Kamseu, E.; Leonelli, C.; Chindaprasirt, P.; Chaysuwan, D. Potassium Alkali Concentration and Heat Treatment Affected Metakaolin-Based Geopolymer. Constr. Build. Mater. 2016, 104, 293–297. [Google Scholar] [CrossRef]
- Hassan, B.I.; Grundy, H.D. The Crystal Structures of Sodalite-Group Minerals. Acta Crystallogr. 1984, B40, 6–13. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Mid-Infrared Spectroscopic Studies of Alkali-Activated FLy Ash Structure. Microporous Mesoporous Mater. 2005, 86, 207–214. [Google Scholar] [CrossRef]
- Zaharaki, D.; Komnitsas, K.; Perdikatsis, V. Use of Analytical Techniques for Identification of Inorganic Polymer Gel Composition. J. Mater. Sci. 2010, 45, 2715–2724. [Google Scholar] [CrossRef]
- Król, M.; Rożek, P.; Mozgawa, W. Synthesis of the Sodalite by Geopolymerization Process Using Coal Fly Ash. Pol. J. Environ. Stud. 2017, 26, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhang, H.; Yang, J. Hydrothermal Synthesis of Sodalite on Alkali-Activated Coal Fly Ash for Removal of Lead Ions. Procedia Environ. Sci. 2016, 31, 605–614. [Google Scholar] [CrossRef]
- Nath, S.K.; Maitra, S.; Mukherjee, S.; Kumar, S. Microstructural and Morphological Evolution of Fly Ash Based Geopolymers. Constr. Build. Mater. 2016, 111, 758–765. [Google Scholar] [CrossRef]
- Ponzoni, C.; Lancellotti, I.; Barbieri, L.; Spinella, A.; Saladino, M.L.; Martino, D.C.; Caponetti, E.; Armetta, F.; Leonelli, C. Chromium Liquid Waste Inertization in an Inorganic Alkali Activated Matrix: Leaching and NMR Multinuclear Approach. J. Hazard. Mater. 2015, 286, 474–483. [Google Scholar] [CrossRef]
- Castillo, H.; Collado, H.; Droguett, T.; Sánchez, S.; Vesely, M.; Garrido, P.; Palma, S. Factors Affecting the Compressive Strength of Geopolymers: A Review. Minerals 2021, 11, 1317. [Google Scholar] [CrossRef]
- Wang, H.; Wu, H.; Xing, Z.; Wang, R.; Dai, S. The Effect of Various Si/al, Na/al Molar Ratios and Free Water on Micromorphology and Macro-Strength of Metakaolin-Based Geopolymer. Materials 2021, 14, 3845. [Google Scholar] [CrossRef]
- Segura, I.P.; Jensen, P.A.; Damø, A.J.; Ranjbar, N.; Jensen, L.S.; Canut, M. Influence of Sodium-Based Activators and Water Content on the Fresh and Hardened Properties of Metakaolin Geopolymers. SN Appl. Sci. 2022, 4, 283. [Google Scholar] [CrossRef]
- Leardi, R. D-Optimal Designs. In Encyclopedia of Analytical Chemistry; Wiley: Hoboken, NJ, USA, 2018; pp. 1–11. [Google Scholar]
- Mathieu, D.; Nony, J.; Phan-Than-Lu, R.; Beal, A. NemrodW®, Version 2017; L.P.R.A.I: Marseille, France, 2017.
- EN 12457-2:2002; Characterisation of Waste-Leaching-Compliance test For Leaching of Granular Waste Materials and Sludges. Available online: https://standards.iteh.ai/catalog/standards/cen/db6fbdf3-1de7-457c-a506-46c4898e3f09/en-12457-2-2002?srsltid=AfmBOoptwNem32D3vh9zVledcN87KpXcDIdAT8G5vO6xXRY9MM8ynmwJ (accessed on 17 June 2025).
- Directive (EU) 2018/850 of the European Parliament and of the Council of 30 May 2018 Amending Directive 1999/31/EC on the Landfill of Waste. Available online: https://eur-lex.europa.eu/eli/dir/2018/850/oj/eng (accessed on 17 June 2025).
- PLS_toolbox, Version 9.5; Eigenvector Research, Inc.: Manson, WA, USA, 2024.
- The MathWorks Inc. MATLAB, Version: 24.2.0 (R2024b); The MathWorks Inc.: Natick, MA, USA, 2024. [Google Scholar]
| n°Exp | Run | Anion | Na/Al (mol) | Aging Time (h) | Metal (ppm) | Effective Metal (Cr) (ppm) | Effective Metal (Ni) (ppm) | Chromium Leaching (ppm) | Nickel Leaching (ppm) | Immobilization Rate (Cr) (%) | Immobilization Rate (Ni) (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | Chloride | 0.6 | 6 | 970 | 975 | 1015 | 1.15 | 0.21 | 98.8 | 99.8 |
| 2 | 9 | Chloride | 1.0 | 6 | 970 | 1133 | 1057 | 0.14 | 0.07 | 99.9 | 99.9 |
| 3 | 13 | Chloride | 1.0 | 24 | 970 | 1127 | 1117 | 0.14 | 0.08 | 99.9 | 99.9 |
| 4 | 2 | Chloride | 1.0 | 6 | 2800 | 3193 | 3367 | 1.51 | 0.28 | 99.5 | 99.9 |
| 5 | 6 | Chloride | 0.6 | 15 | 1890 | 2200 | 2183 | 1.67 | 0.59 | 99.2 | 99.7 |
| 6 | 1 | Chloride | 0.8 | 30 | 1890 | 1690 | 2113 | 1.04 | 0.10 | 99.4 | 100.0 |
| 7 | 14 | Chloride | 0.8 | 15 | 3440 | 3398 | 3094 | 1.37 | 0.39 | 99.6 | 99.9 |
| 8 | 11 | Chloride | 0.8 | 15 | 1890 | 2106 | 2106 | 0.36 | 0.10 | 99.8 | 100.0 |
| 9 | rep11 | Chloride | 0.8 | 15 | 1890 | 1946 | 1936 | 0.94 | 0.32 | 99.5 | 99.8 |
| 10 | 15 | Sulfate | 0.6 | 24 | 970 | 728 | 1085 | 0.19 | 0.03 | 99.7 | 100.0 |
| 11 | 7 | Sulfate | 0.6 | 6 | 2800 | 1887 | 3636 | 0.34 | 0.48 | 99.8 | 99.9 |
| 12 | 5 | Sulfate | 0.6 | 24 | 2800 | 2169 | 3418 | 1.22 | 0.81 | 99.4 | 99.8 |
| 13 | 12 | Sulfate | 1.0 | 24 | 2800 | 1764 | 3046 | 0.19 | 0.11 | 99.9 | 100.0 |
| 14 | 16 | Sulfate | 0.8 | 15 | 1890 | 1236 | 2084 | 0.29 | 0.06 | 99.8 | 100.0 |
| 15 | 10 | Sulfate | 0.8 | 0 | 1890 | 1262 | 2304 | 0.29 | 0.07 | 99.8 | 100.0 |
| 16 | 8 | Sulfate | 0.8 | 15 | 340 | 456 | 516 | 0.15 | 0.03 | 99.7 | 99.9 |
| 17 | 4 | Sulfate | 0.8 | 15 | 1890 | 1179 | 1904 | 0.73 | 0.25 | 99.4 | 99.9 |
| 18 | rep4 | Sulfate | 0.8 | 15 | 1890 | 1251 | 1936 | 0.20 | 0.07 | 99.8 | 100.0 |
| Test point validation | 17 | Chloride | 1.0 | 24 | 1890 | 2175 | 2320 | 0.51 | 0.03 | 99.8 | 100.0 |
| Geopolymer homogeneity analysis (ICP-OES) | |||||||||||
| 9 (rep 11) | Top | Chloride | 0.8 | 15 | 1890 | 1920 | 1892 | 0.98 | 0.33 | ||
| 9 (rep 11) | Bottom | Chloride | 0.8 | 15 | 1890 | 1972 | 1980 | 0.90 | 0.31 | ||
| Factors | Terms | Coefficient | VIF | Standard Deviation | t Exp. | p Value |
|---|---|---|---|---|---|---|
| Intercept | b0 | 0.168 | 0.052 | 3.59 | 0.013 | |
| Anion | b1 | 0.052 | 1.20 | 0.033 | 1.74 | 0.152 |
| Na/Al | b2 | −0.125 | 1.16 | 0.047 | −2.98 | 0.028 |
| Aging Time (Ag.) | b3 | −0.002 | 1.15 | 0.038 | −0.05 | 0.965 |
| Conc | b4 | 0.125 | 1.36 | 0.037 | 3.80 | 0.010 |
| Na/Al2 | b22 | 0.078 | 1.50 | 0.075 | 1.16 | 0.317 |
| Ag. Time2 | b33 | −0.049 | 1.05 | 0.036 | −1.51 | 0.204 |
| Conc2 | b44 | 0.017 | 2.03 | 0.040 | 0.46 | 0.683 |
| Na/Al × Time | b23 | −0.037 | 1.01 | 0.046 | −0.89 | 0.440 |
| Na/Al × Conc | b24 | −0.064 | 1.16 | 0.038 | −1.90 | 0.122 |
| Ag. Time × Conc | b34 | 0.047 | 1.19 | 0.037 | 1.41 | 0.234 |
| Factor | Term | Coefficient | VIF | Standard Deviation | t Exp. | p Value |
|---|---|---|---|---|---|---|
| Intercept | b0 | 0.801 | 0.150 | 5.35 | 0.003 | |
| Anion | b1 | 0.236 | 1.28 | 0.069 | 3.43 | 0.019 |
| Na/Al | b2 | −0.456 | 2.44 | 0.136 | −3.37 | 0.020 |
| Aging (Ag.) Time | b3 | 0.311 | 3.10 | 0.120 | 2.60 | 0.048 |
| Conc | b4 | 0.228 | 1.51 | 0.099 | 2.30 | 0.070 |
| Na/Al2 | b22 | −0.140 | 1.23 | 0.134 | −1.04 | 0.345 |
| Ag.Time2 | b33 | −0.089 | 1.30 | 0.076 | −1.16 | 0.297 |
| Conc2 | b44 | −0.013 | 1.56 | 0.093 | −0.14 | 0.897 |
| Na/Al × Time | b23 | 0.026 | 1.21 | 0.101 | 0.26 | 0.807 |
| Na/Al × Conc | b24 | −0.022 | 2.55 | 0.189 | −0.12 | 0.910 |
| Ag. Time × Conc | b34 | 0.462 | 3.14 | 0.183 | 2.52 | 0.053 |
| Factor | Coded Variable | Type of Variable | Levels | ||||
|---|---|---|---|---|---|---|---|
| −α | −1 | 0 | 1 | α | |||
| Anion | x1 | Discrete qualitative | Sulfate | Chloride | |||
| Na/Al (molar ratio) | x2 | Continuous quantitative | 0.6 | 0.8 | 1 | ||
| Aging time (h) | x3 | Continuous quantitative | 0 | 6 | 15 | 24 | 30 |
| Conc. of heavy metal (ppm) | x4 | Continuous quantitative | 340 | 970 | 1890 | 2800 | 3440 |
| α is an axian level also known as the “star point”, which is specific to composite center design (α = 1.68) | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, R.E.; Santoni, E.; Fattobene, M.; Giovini, M.; Genua, F.; Leonelli, C.; Lancellotti, I.; Herrero, A.; Berrettoni, M. Design of Experiments Approach for Efficient Heavy Metals Stabilization Using Metakaolin-Based Geopolymers. Molecules 2025, 30, 3235. https://doi.org/10.3390/molecules30153235
Russo RE, Santoni E, Fattobene M, Giovini M, Genua F, Leonelli C, Lancellotti I, Herrero A, Berrettoni M. Design of Experiments Approach for Efficient Heavy Metals Stabilization Using Metakaolin-Based Geopolymers. Molecules. 2025; 30(15):3235. https://doi.org/10.3390/molecules30153235
Chicago/Turabian StyleRusso, Raffaele Emanuele, Elisa Santoni, Martina Fattobene, Mattia Giovini, Francesco Genua, Cristina Leonelli, Isabella Lancellotti, Ana Herrero, and Mario Berrettoni. 2025. "Design of Experiments Approach for Efficient Heavy Metals Stabilization Using Metakaolin-Based Geopolymers" Molecules 30, no. 15: 3235. https://doi.org/10.3390/molecules30153235
APA StyleRusso, R. E., Santoni, E., Fattobene, M., Giovini, M., Genua, F., Leonelli, C., Lancellotti, I., Herrero, A., & Berrettoni, M. (2025). Design of Experiments Approach for Efficient Heavy Metals Stabilization Using Metakaolin-Based Geopolymers. Molecules, 30(15), 3235. https://doi.org/10.3390/molecules30153235









