Synergistic Enhancement of Rhodamine B Adsorption by Coffee Shell Biochar Through High-Temperature Pyrolysis and Water Washing
Abstract
1. Introduction
2. Results and Discussion
2.1. Material Characterization
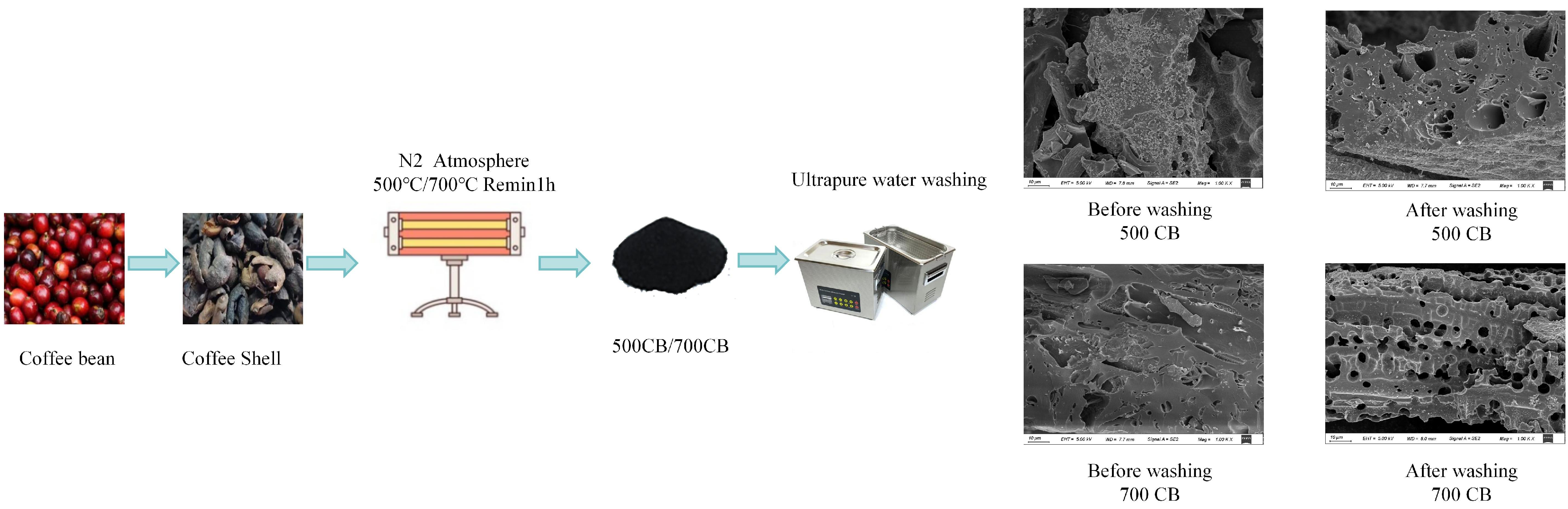
2.1.1. SEM Analysis
2.1.2. BET Surface Area and Pore Structure Analysis
2.1.3. XRD Analysis
2.1.4. FTIR Analysis
2.1.5. Chemical Composition: Ash, Volatile Matter, and Moisture Content
2.2. Adsorption Kinetics: Rate Analysis and Model Fitting
Adsorption Kinetics
2.3. Effects of Pyrolysis Temperature, Washing Treatment, and Solid-to-Liquid Ratio on Adsorption
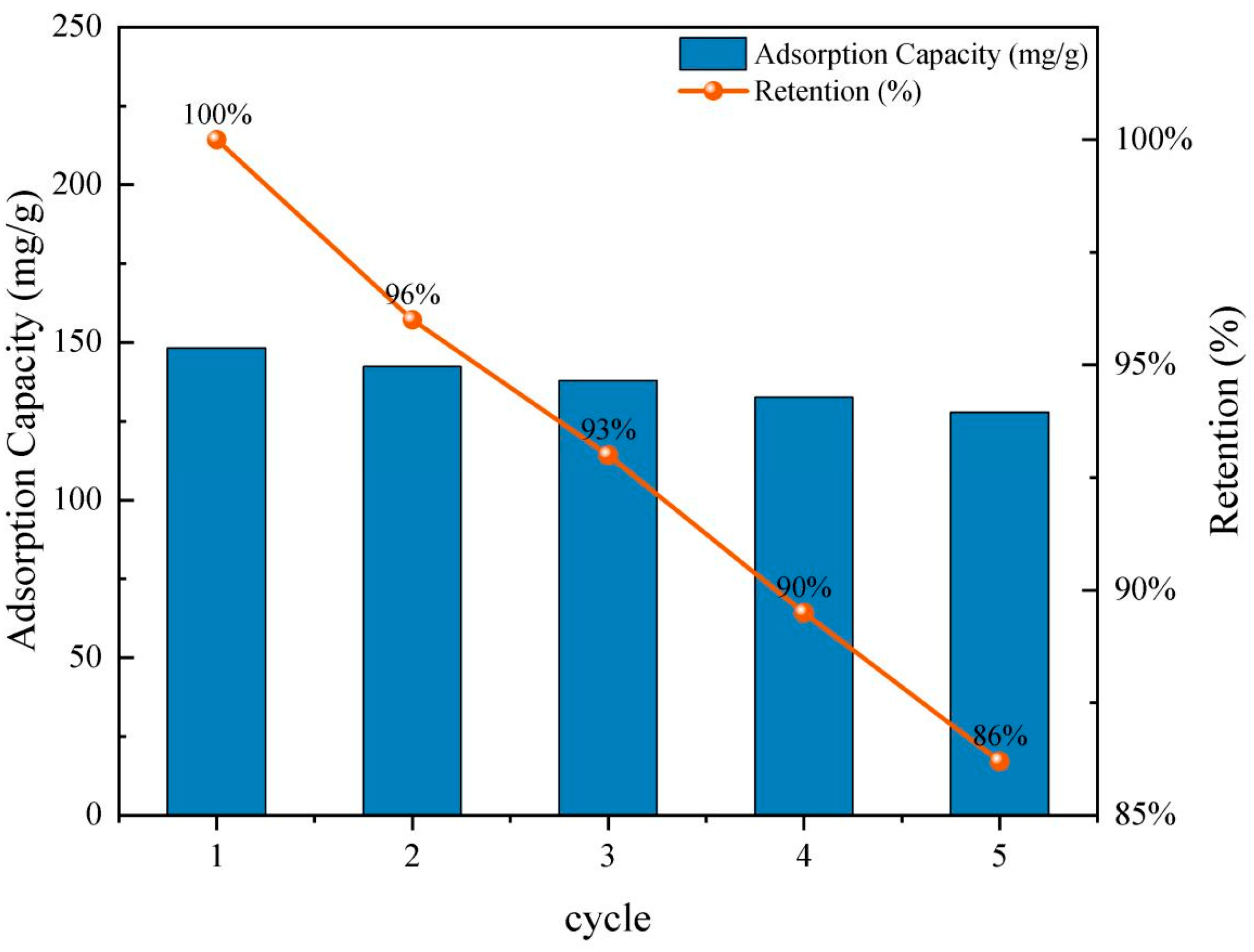
2.4. Regeneration and Reusability
2.5. Adsorption Mechanism
2.5.1. Surface Morphology and Porosity
2.5.2. Surface Functional Groups
2.5.3. Electrostatic Interactions
2.5.4. XRD Insights: Role of Residual Minerals
3. Materials and Methods
3.1. Material Preparation
3.2. Biochar Preparation Process
3.3. Material Characterization Methods
3.4. Adsorption Experiment
3.5. Adsorption Kinetics and Isotherm Models
3.6. Regeneration Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BET | Brunauer–Emmett–Teller method |
| CSB | Coffee shell-derived biochar |
| FTIR | Fourier transform infrared spectroscopy |
| RhB | Rhodamine B |
| SEM | Scanning electron microscopy |
| XRD | X-ray diffraction |
References
- Huang, D.; Pang, T.; Bai, X.; Chen, M.; Liu, J. Evaluating the surface water pollution risk of mineral resource exploitation via an improved approach: A case study in Liaoning Province, Northeastern China. Environ. Monit. Assess. 2024, 196, 750. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, Y.; Chen, L.; Zhao, D.; Yu, S.; Huang, L. Preparation of KHA/SA/MMT composites and their adsorption properties for Rhodamine B. Environ. Sci. Pollut. Res. 2024, 31, 24220–24234. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, H.; Li, X.; Liu, X.; Du, W.; Chen, J. Study on the Efficiency of Zero-Valent Iron Activated Persulfate Degradation of Rhodamine B and Citric Acid Enhancement. Water Air Soil Pollut. 2024, 235, 181. [Google Scholar] [CrossRef]
- Cardoso, R.; Muniz, D.; Matos, C.L.; Caliman, J.; Linares, J.J. The challenging removal of emerging pollutants: Electrochemical regeneration to recover the adsorption capacity of a caffeine-saturated activated carbon. Chem. Pap. 2023, 77, 4589–4598. [Google Scholar] [CrossRef]
- Ahmad, W.; Muhammad, T.; Ahmad, I.; Khan, M.; Nazneen, S. Adsorption of hydrocarbon pollutants from wastewater using Cu- and Zn-loaded activated carbon derived from waste tires. Environ. Prog. Sustain. Energy 2024, 43, e14360. [Google Scholar] [CrossRef]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A review of biochar and its use and function in soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar] [CrossRef]
- Wu, P.; Ata-Ul-Karim, S.T.; Singh, B.P.; Wang, H.; Wu, T.; Liu, C.; Fang, G.; Zhou, D.; Wang, Y.; Chen, W. A scientometric review of biochar research in the past 20 years (1998–2018). Biochar 2019, 1, 23–43. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Cui, G.; Wang, Z.; Liu, Y. Performance and Mechanism of a Green and Sustainable γ-nFe2O3-Based Magnetic Biochar for the Effective Adsorption of Cadmium. Arab. J. Sci. Eng. 2024, 49, 165–179. [Google Scholar] [CrossRef]
- Xie, H.; Ao, H.; Xu, L.; Ao, S.; Zhang, T.; Li, W.; Yang, Y. Quantum chemical DFT-based adsorption mechanism of Pb(II) on a modified biochar. Biomass-Convers. Biorefin. 2024, 14, 13547–13562. [Google Scholar] [CrossRef]
- Zhou, X.; Lu, L.; Li, X.; Meng, F.; Ding, Z.; Li, Z.; Chen, B.; Zhang, J. Mechanism and effect of magnetic biochar on the removal of norfloxacin from water by ozone peroxidation adsorption-coagulation process. Asian J. Surg. 2025, 69, 106655. [Google Scholar] [CrossRef]
- Alvear-Daza, J.J.; Rengifo-Herrera, J.A.; Pizzio, L.R. Performance and optimization of diclofenac and ibuprofen adsorption onto activated carbon synthesized from sunflower seed shell (Helianthus annuus) in natural groundwater samples. Adsorption 2024, 30, 783–800. [Google Scholar] [CrossRef]
- Bilal, M.; Kaur, S.; Mushtaq, J. Adsorption investigation of physico-chemical characteristics of different water samples by coalesce Coconut shell. Int. J. Environ. Eng. 2023, 1. [Google Scholar] [CrossRef]
- Xu, S.; Bu, J.; Li, C.; Tiong, Y.W.; Sharma, P.; Liu, K.; Jin, C.; Ma, C.; Tong, Y.W. Biochar enhanced methane yield on anaerobic digestion of shell waste and the synergistic effects of anaerobic co-digestion of shell and food waste. Fuel 2024, 357, 129933. [Google Scholar] [CrossRef]
- Kumar, N.S.; Shaikh, H.M.; Asif, M.; Al-Ghurabi, E.H. Engineered biochar from wood apple shell waste for high-efficient removal of toxic phenolic compounds in wastewater. Sci. Rep. 2021, 11, 2586. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Zhu, W.; He, L.; Tan, F.; Zhu, N.; Zhou, Q.; He, M.; Hu, G. Calcium-rich biochar from crab shell: An unexpected super adsorbent for dye removal. Bioresour. Technol. 2018, 267, 510–516. [Google Scholar] [CrossRef]
- Zhou, H.; Guo, J.; Liu, H.; Wang, J.; Wang, Y. Effects of Biochar Pyrolysis Temperature and Application Rate on Saline Soil Quality and Maize Yield. Agronomy 2024, 14, 1529. [Google Scholar] [CrossRef]
- Yildirim, O.A.; Hasan, M.; Pehlivan, E. Synthesis and performance evaluation of biochar-supported ZTO nanocatalyst for photodegradation of Rhodamine B in water. J. Mater. Res. 2024, 39, 576–589. [Google Scholar] [CrossRef]
- Tomas, J.; Aura, M.; Dainius, V.P. The characteristics of sewage sludge pellet biochar prepared using two different pyrolysis methods. Biomass Convers. Biorefin. 2024, 14, 891–900. [Google Scholar]
- Yang, H.; Yang, J.; Liu, L.; Wang, B. Nano-biochar derived from bamboo biomass: A dual-functional material for electrochemical sensing of ferulic acid and adsorptive removal of surfactants in cosmetic wastewater. Int. J. Electrochem. Sci. 2025, 20, 100959. [Google Scholar] [CrossRef]
- Gokarn, A.N.; Yeole, A.A. Multi useful material biochar. Pestology 2024, 48, 15–21. [Google Scholar]
- Fahruddin, F.; Syahri, Y.F.; Fauziah, S.; Samawi, M.F.; Johannes, E.; Tambaru, E.; Tuwo, M.; Abdullah, A. Combining biochar with sediment in the treatment for the effectiveness of sulfate and heavy metal Pb reduction of acid mine drainage. J. Degraded Min. Lands Manag. 2024, 11, 6329–6335. [Google Scholar] [CrossRef]
- Mourgkogiannis, N.; Nikolopoulos, I.; Kordouli, E.; Lycourghiotis, A.; Kordulis, C.; Karapanagioti, H.K. The Influence of Biowaste Type on the Physicochemical and Sorptive Characteristics of Corresponding Biochar Used as Sustainable Sorbent. Sustainability 2024, 16, 2889. [Google Scholar] [CrossRef]
- Cao, D.; Niu, R.; Mo, G.; Deng, H.; Liu, R.; Liu, J.; Fan, J. Adsorption properties and competitive adsorption mechanism exhibited by carbon-nanotube-modified biochar for removal of crude oil and Ni(II) pollutants from water. Ecotoxicol. Environ. Saf. 2025, 290, 117557. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Hou, D.; Zhao, B.; Xu, W.; Ok, Y.S.; Bolan, N.S.; Alessi, D.S. Stability of heavy metals in soil washing residue with and without biochar addition under accelerated ageing. Sci. Total Environ. 2018, 619–620, 185–193. [Google Scholar] [CrossRef]
- Lopicic, Z.; Avdalovic, J.; Milojkovic, J.; Antanaskovic, A.; Ljesevic, M.; Lugonja, N.; Sostaric, T. Removal of diesel pollution by biochar—Support in water remediation. Chem. Ind. 2021, 75, 329–339. [Google Scholar] [CrossRef]
- Ismail, S.A.; Prasher, S.; Chénier, M.; Patel, R. Evaluation of biochar soil amendments in reducing soil and water pollution from total and fecal coliforms in poultry manure. Can. Biosyst. Eng. 2016, 58, 1.21–1.31. [Google Scholar] [CrossRef]
- Handiso, B.; Pääkkönen, T.; Wilson, B.P. Effect of pyrolysis temperature on the physical and chemical characteristics of pine wood biochar. Waste Manag. Bull. 2024, 2, 281–287. [Google Scholar] [CrossRef]
- Saiyud, N.; Deethayat, T.; Asanakham, A.; Duongbia, N.; Kamopas, W.; Kiatsiriroat, T. Biochar production from co-pyrolysis of coffee ground and native microalgae consortium. Biomass Convers. Biorefin. 2024, 14, 6855–6863. [Google Scholar] [CrossRef]
- Pelagalli, V.; Langone, M.; Matassa, S.; Race, M.; Tuffi, R.; Papirio, S.; Lens, P.N.L.; Lazzazzara, M.; Frugis, A.; Petta, L.; et al. Pyrolysis of municipal sewage sludge: Challenges, opportunities and new valorization routes for biochar, bio-oil, and pyrolysis gas. Environ. Sci. Water Res. Technol. 2024, 10, 2282–2312. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, J.; Shen, C.; Li, J.; Wang, T.; Xue, Y. Effect of zinc chloride-modified biochar with varying pore structures on VOCs inhibition and pavement performance of asphalt. Constr. Build. Mater. 2025, 472, 140887. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, L.; Gao, B.; Xu, X.; Cao, X. The Interfacial Behavior between Biochar and Soil Minerals and Its Effect on Biochar Stability. Environ. Sci. Technol. 2016, 50, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, H. Pb(II) sorption from aqueous solution by novel biochar loaded with nano-particles. Chemosphere 2018, 192, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Rojith, G.; Bright, S.I.S. Cellulose Crystallinity Change Assessment of Biochar Produced by Pyrolysis of Coir Pith. Res. J. Recent Sci. 2013, 2, 98–101. [Google Scholar]
- Zhu, H.; Yan, S.; Wu, Y.; Xu, H.; Chen, H.; Zhang, H.; Guo, X.; Hu, X.; Zhang, S.; Gao, W. Effect of biochar structure on the selective adsorption of heavy components in bio-oil. Biofuels Bioprod. Biorefin. 2024, 18, 1280–1288. [Google Scholar] [CrossRef]
- Olusanya, S.O.; Ajayi, S.M.; Sodeinde, K.O.; Fapojuwo, D.P.; Atunde, M.O.; Diduyemi, A.E.; Olumayede, E.G.; Lawal, O.S. Hydrophobic modification of cellulose from oil palm waste in aqueous medium. Polym. Bull. 2024, 81, 1349–1371. [Google Scholar] [CrossRef]
- Fang, Q.; Chen, B.; Lin, Y.; Guan, Y. Aromatic and Hydrophobic Surfaces of Wood-derived Biochar Enhance Perchlorate Adsorption via Hydrogen Bonding to Oxygen-containing Organic Groups. Environ. Sci. Technol. 2014, 48, 279–288. [Google Scholar] [CrossRef]
- Ruan, Z.-H.; Wu, J.-H.; Huang, J.-F.; Lin, Z.-T.; Li, Y.-F.; Liu, Y.-L.; Cao, P.-Y.; Fang, Y.-P.; Xie, J.; Jiang, G.-B. Facile preparation of rosin-based biochar coated bentonite for supporting α-Fe2O3 nano-particles and its application for Cr(vi) adsorption. J. Mater. Chem. A 2015, 3, 4595–4603. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, Z.; Li, Y.; Ding, L.; Sun, D.; Dong, Z. Fabrication of novel Fe/Mn/N co-doped biochar and its enhanced adsorption for bisphenol a based on π–π electron donor–acceptor interaction. Bioresour. Technol. 2022, 364, 128018. [Google Scholar] [CrossRef]
- Li, X.; Shi, J.; Luo, X. Enhanced adsorption of rhodamine B from water by Fe-N co-modified biochar: Preparation, performance, mechanism and reusability. Bioresour. Technol. 2021, 343, 126103. [Google Scholar] [CrossRef]
- Chen, X.; Li, M.; He, J.; Wu, Y.; Sun, J.; Wen, X. Waste cotton-based activated carbon with excellent adsorption performance towards dyes and antibiotics. Chemosphere 2025, 376, 144292. [Google Scholar] [CrossRef]
- Gumus, H.; Büyükkıdan, B. A facile preparation of biochar-anchored magnetic photocatalytic PVDF composite for water remediation. Colloid Polym. Sci. 2024, 302, 103–115. [Google Scholar] [CrossRef]
- Shao, Z.; Shuangbao; Wu, S.; Gao, Y.; Liu, X.; Dai, Y. Two-step pyrolytic preparation of biochar for the adsorption study of tetracycline in water. Environ. Res. 2024, 242, 117566. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Zhou, Q.; Jin, M.; Fu, L.; Wu, W. Preparation of biochar-supported nanoscale zero-valent iron (nZVI@BC) and its adsorption and degradation of chlortetracycline in water and soil. Mater. Jan. 2024, 29, e20240425. [Google Scholar] [CrossRef]
- Crombie, K.; Mašek, O.; Sohi, S.P.; Brownsort, P.; Cross, A. The effect of pyrolysis conditions on biochar stability as determined by three methods. GCB Bioenergy 2013, 5, 122–131. [Google Scholar] [CrossRef]
- Das, S.; Goud, V.V. Biochar-assisted upgradation of pyrolytic oil via slow pyrolysis of rice husk under a carbon dioxide environment. Biomass Convers. Biorefin. 2024, 14, 13807–13819. [Google Scholar] [CrossRef]
- Mahmoud, E.R.I.; Aly, H.M.; Hassan, N.A.; Aljabri, A.; Khan, A.L.; El-Labban, H.F. Biochar from Date Palm Waste via Two-Step Pyrolysis: A Modified Approach for Cu (II) Removal from Aqueous Solutions. Processes 2024, 12, 1189. [Google Scholar] [CrossRef]
- Kujawska, J.; Wojtaś, E.; Charmas, B. Biochar Derived from Sewage Sludge: The Impact of Pyrolysis Temperature on Chemical Properties and Agronomic Potential. Sustainability 2024, 16, 8225. [Google Scholar] [CrossRef]
- Khater, E.-S.; Bahnasawy, A.; Hamouda, R.; Sabahy, A.; Abbas, W.; Morsy, O.M. Biochar production under different pyrolysis temperatures with different types of agricultural wastes. Sci. Rep. 2024, 14, 2625. [Google Scholar] [CrossRef]
- Adesina, A.A.; Makanjuola, F.O.; Salami, Q.O.; Akinbomi, J.G. Design of an Anaerobic Biofilter Using Biochar from Agricultural Waste and Its Application for Safe Water Discharge from the Food Industry in Developing Countries. In Proceedings of the International Conference on Innovation, Sustainability, and Applied Sciences, Dubai, United Arab Emirates, 22–23 February 2025; Springer: Cham, Switzerland, 2025. [Google Scholar]
- Luo, J.; Chen, Y.; Huang, H.; Ma, R.; Ma, N.; Yan, F.; Xu, J.; Zhang, J.; Chen, J.; Sun, S. Microwave-coordinated KOH directionally modulated N/O co-doped porous biochar from Enteromorpha and its structure–effect relationships in efficient CO2 capture. Chem. Eng. J. 2023, 473, 145279. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; You, X.; Wu, D.; Ma, C.; Li, N.; Li, Y.; Fan, X.; Zhang, F.; Peng, W. High-Density Single-Atomic Mn–N–C Site in Hierarchical Porous Biochar for Superoxide Radical-Dominated Ozonation. Chem. Mater. 2023, 35, 3640–3651. [Google Scholar] [CrossRef]
- Zhou, Y.; Wei, Z.; Yao, S.; Li, Z.; Zhang, Z.; Ji, L.; Jing, H. Activated biochar derived from Enteromorpha with high specific surface area for efficient removal of phenanthrene: Experiments, mechanism and DFT calculations. Environ. Pollut. 2024, 340, 122709. [Google Scholar] [CrossRef] [PubMed]
- Hadjittofi, L.; Pashalidis, I. Uranium sorption from aqueous solutions by activated biochar fibres investigated by FTIR spectroscopy and batch experiments. J. Radioanal. Nucl. Chem. 2015, 304, 897–904. [Google Scholar] [CrossRef]
- Kaal, J.; Cortizas, A.M.; Reyes, O.; Soliño, M. Molecular characterization of Ulex europaeus biochar obtained from laboratory heat treatment experiments—A pyrolysis–GC/MS study. J. Anal. Appl. Pyrolysis 2012, 95, 205–212. [Google Scholar] [CrossRef]
- Pitoyo, J.; Jamilatun, S. Scanning Electron Microscope-Energy Dispersive X-Ray (SEM-EDX) Study of Fe-C Catalyst from Iron Sand and Biochar for Advance Oxidation Process (AOPs). Int. J. Renew. Energy Res. 2024, 14, 804–813. [Google Scholar]
- ASTM D1762-84; Standard Test Method for Chemical Analysis of Wood Charcoal. ASTM International: West Conshohocken, PA, USA, 2021.
- Bashir, S.; Salam, A.; Chhajro, M.A.; Fu, Q.; Khan, M.J.; Zhu, J.; Shaaban, M.; Kubar, K.A.; Ali, U.; Hu, H. Comparative efficiency of rice husk-derived biochar (RHB) and steel slag (SS) on cadmium (Cd) mobility and its uptake by Chinese cabbage in highly contaminated soil. Int. J. Phytoremediat. 2018, 20, 1221–1228. [Google Scholar] [CrossRef]
- Dai, Y.; Yin, H.; Zhao, J.; Zhu, P.; Suo, Z. Preparation of Biochar from Straw in Northeast China to Assist in Carbon Neutrality:Data Visualization and Comprehensive Evaluation. Water Air Soil Pollut. 2025, 236, 324. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, R.; Liu, R. Characterization of biochar from fast pyrolysis and its effect on chemical properties of the tea garden soil. J. Anal. Appl. Pyrolysis 2014, 110, 375–381. [Google Scholar] [CrossRef]
- Chen, L.; Guo, L.; Ali, A.; Zhou, Q.; Liu, M.; Zhan, S.; Pan, X.; Zeng, Y. Effect of biochar on the form transformation of heavy metals in paddy soil under different water regimes. Arch. Agron. Soil Sci. 2023, 69, 387–398. [Google Scholar] [CrossRef]
- Kastono, D.; Suryanto, P.; Rogomulyo, R.; Handayani, S.; Supriyanta, S.; Widyawan, M.H.; Alam, T. Differences in Biochar Sources for Controlled Nitrogen Loss in a Hybrid Maize Agroforestry System with Melaleuca cajuputi. J. Eng. Technol. Sci. 2022, 54, 220109. [Google Scholar] [CrossRef]
- Zhai, S.; Jin, R.; Zhang, Y.; Liu, G.; Qi, D. Enhancement of adsorption and regeneration ability for biochar aerogel over in situ supported FeOOH/Cu2O particles. Biomass Convers. Biorefin. 2024, 14, 14053–14063. [Google Scholar] [CrossRef]
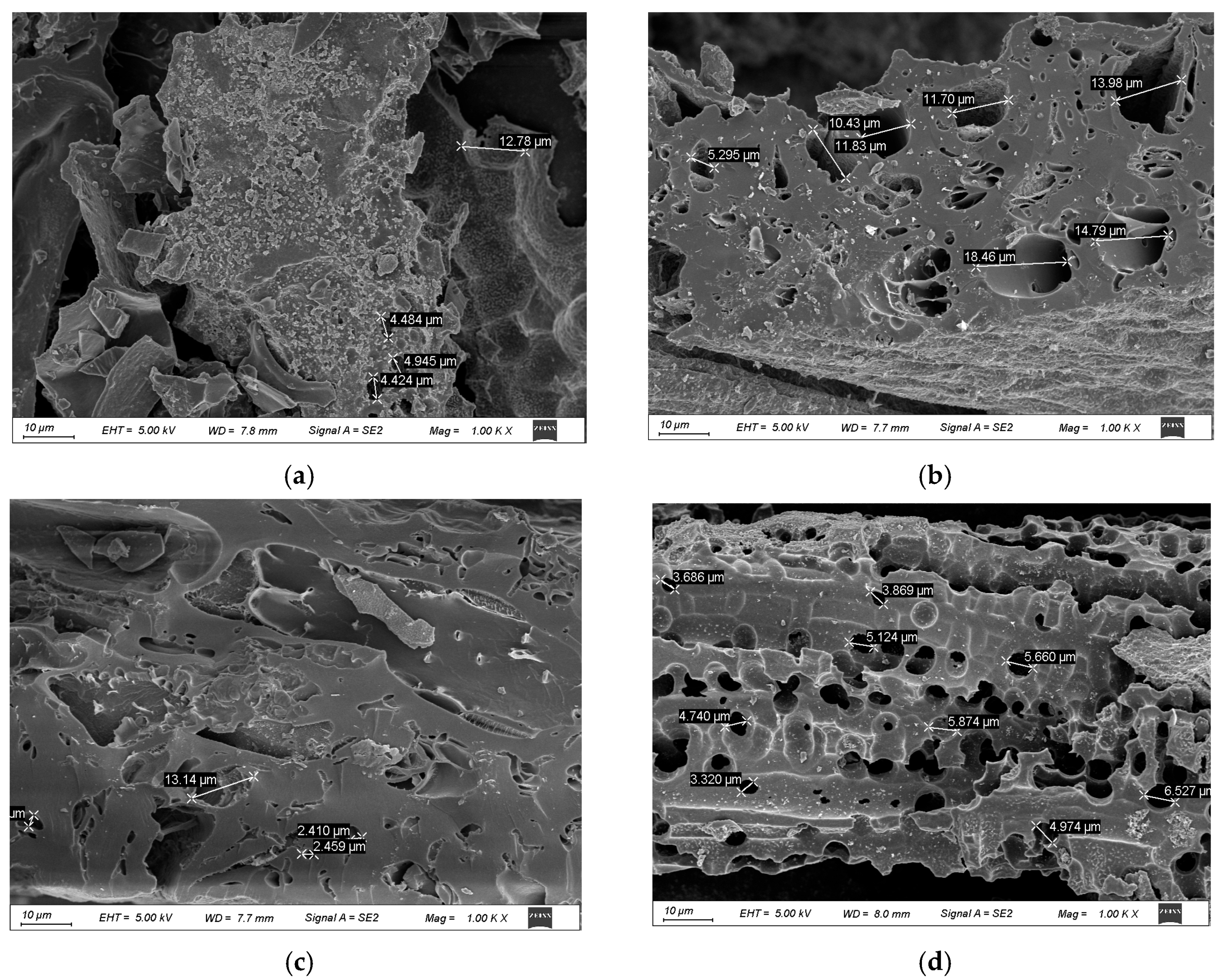
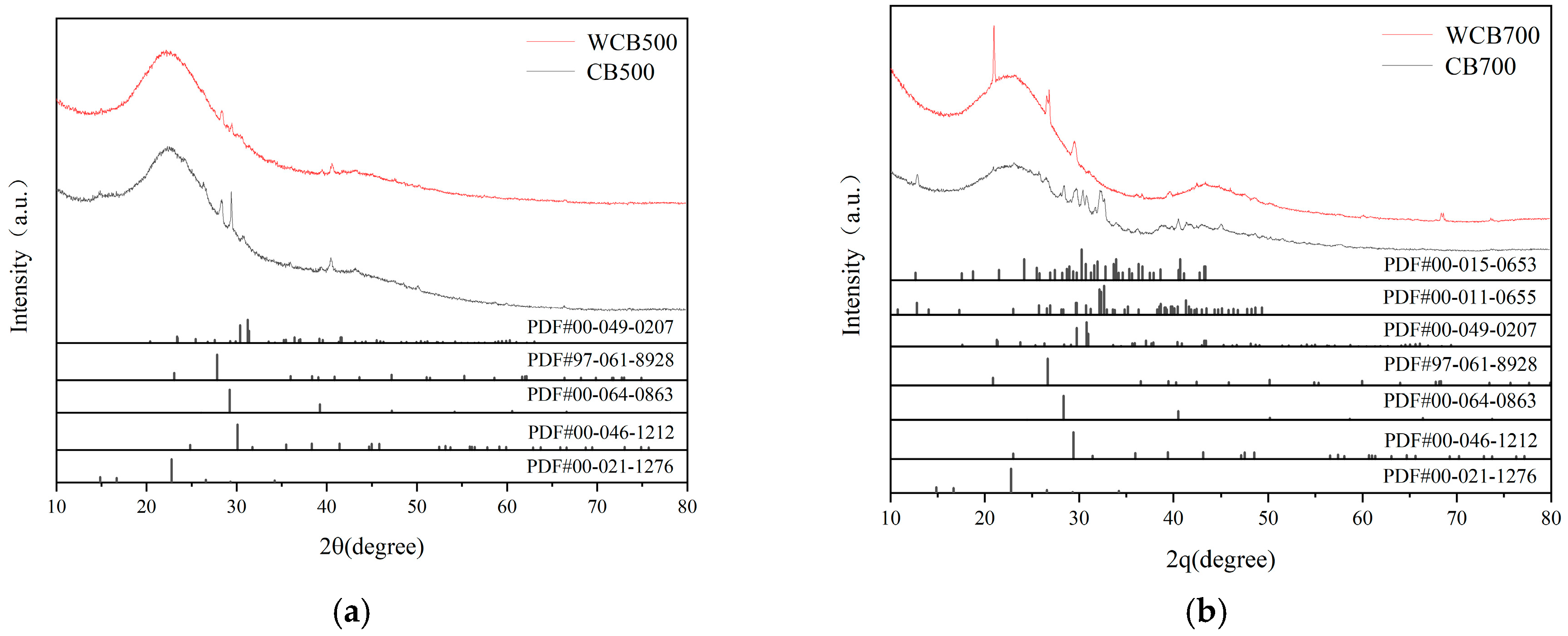

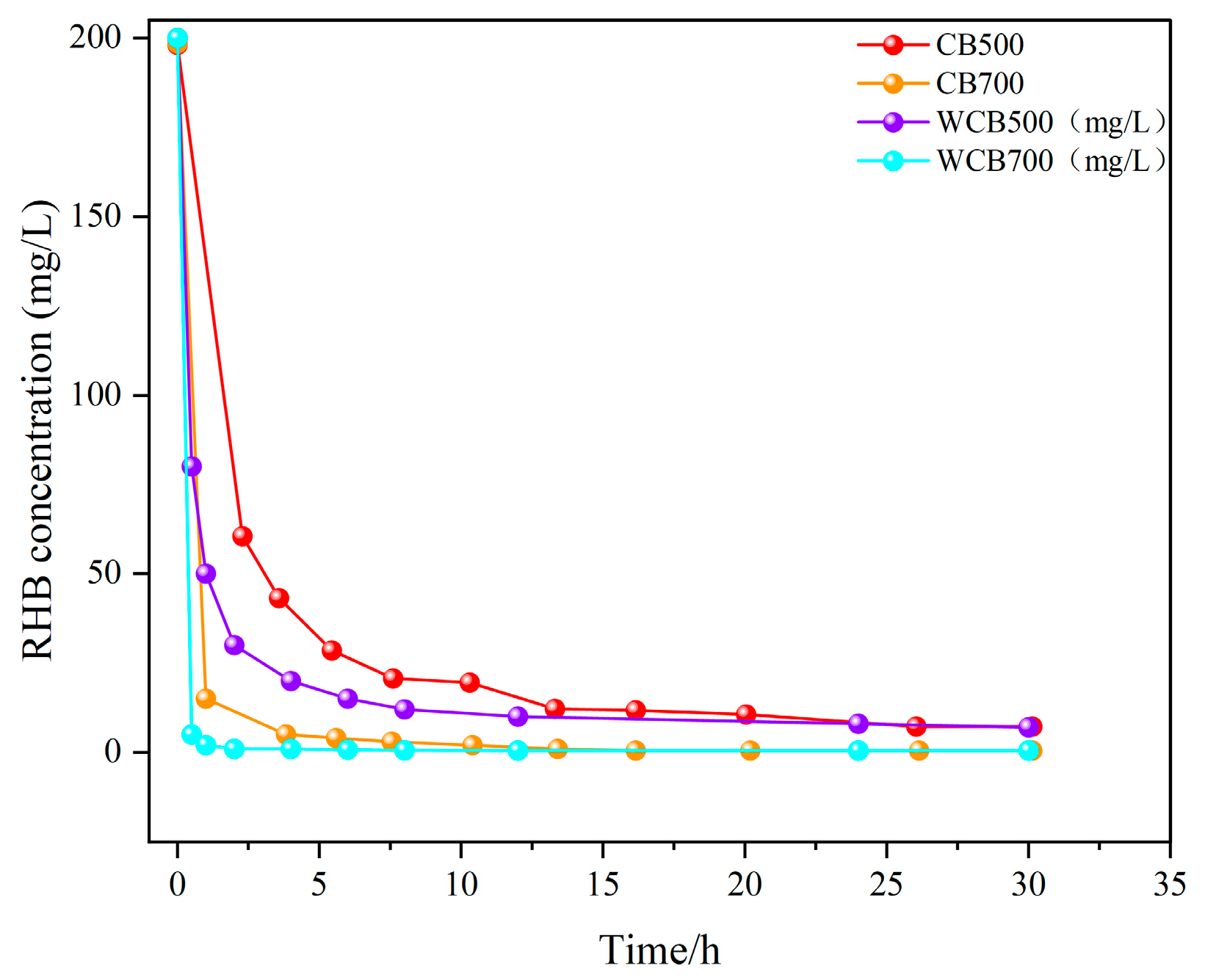
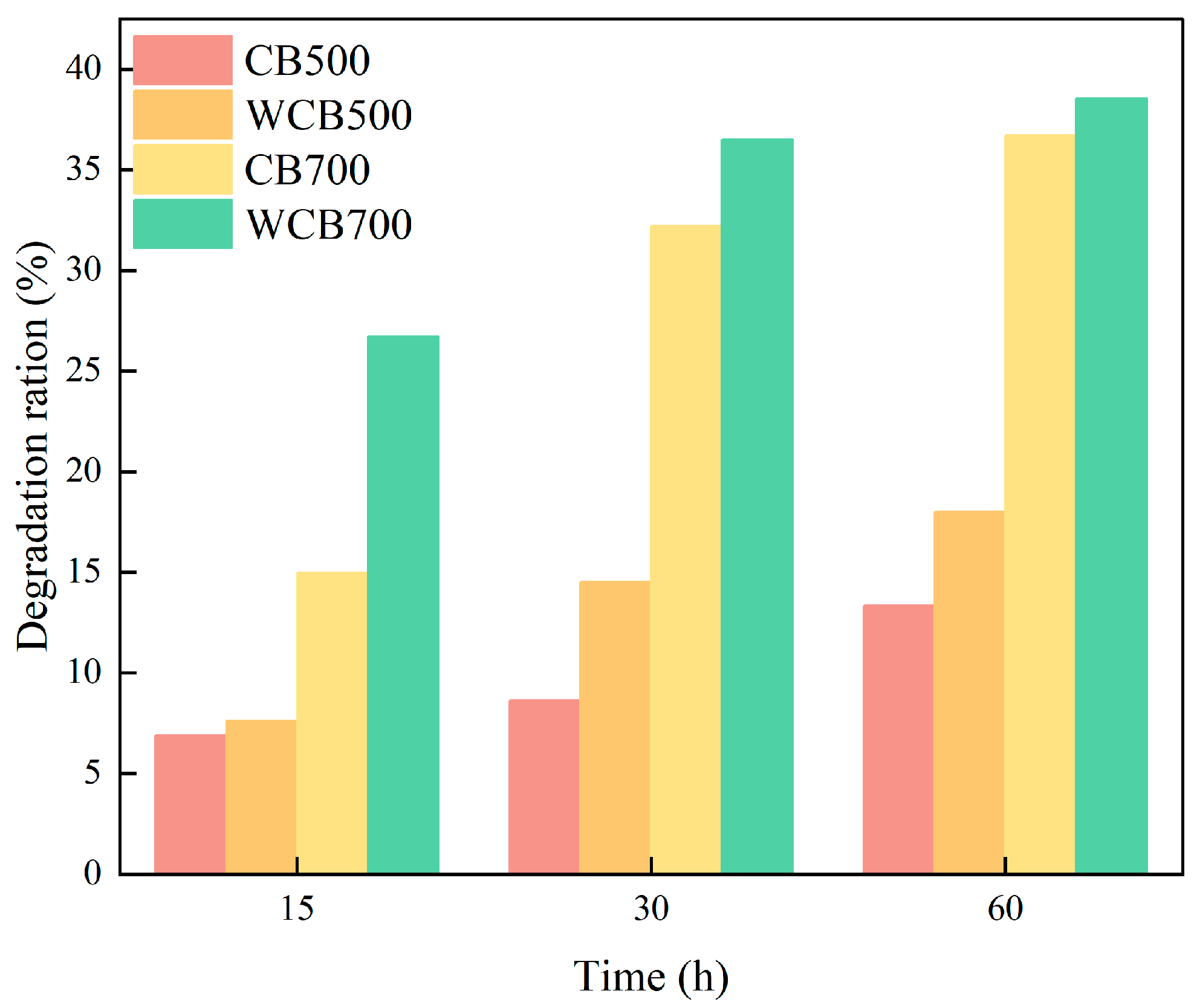

| Sample | Temperature (℃) | Washing | BET Surface Area (m3/g) | Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|---|---|
| CB500 | 500 | No | 56.3 | 0.039 | 2.77 |
| WCB500 | 500 | Yes | 69.5 | 0.046 | 2.65 |
| CB700 | 700 | No | 333 | 0.187 | 2.25 |
| WCB700 | 700 | Yes | 378.3 | 0.205 | 2.16 |
| Sample | Temperature (°C) | Ash Content (%) | Volatile Matter (%) | Moisture Content |
|---|---|---|---|---|
| Coffee Shell | - | 10.5 | 70.1 | 5.0 |
| CB500 | 500 | 12.3 | 64.2 | 4.7 |
| CB700 | 500 | 9.1 | 67.5 | 3.9 |
| WCB500 | 700 | 14.8 | 60.1 | 4.4 |
| WCB700 | 700 | 7.2 | 62.3 | 3.5 |
| Sample | Model | qe (mg/g) | k (Rate Constant) | R2 |
|---|---|---|---|---|
| CB500 | Pseudo-first-order | 85.4 | 0.092 h−1 | 0.882 |
| CB500 | Pseudo-second-order | 93.7 | 0.0047 g·mg−1·h−1 | 0.983 |
| CB700 | Pseudo-first-order | 121.1 | 0.121 h−1 | 0.897 |
| CB700 | Pseudo-second-order | 133.2 | 0.0118 g·mg−1·h−1 | 0.996 |
| WCB500 | Pseudo-first-order | 103.3 | 0.101 h−1 | 0.913 |
| WCB500 | Pseudo-second-order | 115.9 | 0.0075 g·mg−1·h−1 | 0.99 |
| WCB700 | Pseudo-first-order | 138.8 | 0.143 h−1 | 0.921 |
| WCB700 | Pseudo-second-order | 147.6 | 0.0171 g·mg−1·h−1 | 0.998 |
| Contrast Dimensions | This Study | [39] | [40] |
|---|---|---|---|
| Raw materials and preparation methods | Low-cost recycling of agricultural waste (coffee shell); only pyrolysis (500/700 °C) + water washing, no chemical activator, and an environmentally friendly process. | Coconut shell requires Fe/N co-doping (urea+FeSO4), complex chemical modification, and potential secondary contamination risk. | Waste cotton needs KOH high-temperature activation (800 °C), high energy consumption, large KOH consumption (mass ratio 1:5), and high cost. |
| Adsorption capacity; loading capacity | 193.5 mg/g (RhB) to meet the actual wastewater treatment needs and balance the adsorption performance and cost. | 12.41 mg/g (RhB), with low capacity, requiring multiple adsorptions or increased dosage. | Ultra-high capacity (7265 mg/g CR), but requires very high pollutant concentration (700 mg/L), limited practical application. |
| Adsorption mechanism | Comprehensive mechanism analysis (π-π stacking, hydrogen bonding, electrostatic, pore filling), through multiple characterization verification, is highly scientific. | Dependent on Fe/N doping to enhance chemical adsorption, but the mechanism validation, that is, the Fe-N bond role, has not been thoroughly explored. | Depends on physical adsorption (pore filling + van der Waals force) with weak chemical action and limited applicability to complex systems. |
| Regenerative performance | After five cycles, 85% of the capacity is retained, only NaOH/ethanol desorption is required, the cost of regeneration is low, and stability is excellent. | After five cycles, the capacity is reduced to 43.8%, combining the H2O2 oxidation recovery performance (complex operation and may destroy the structure). | Mass loss of 87% (50 mg; 6.63 mg) after three cycles, calcination + KOH for regeneration, high energy consumption, and not sustainable. |
| Theoretical contribution | To reveal the synergistic effect of pyrolysis temperature and washing and to provide a universality strategy for chemical optimization of biochar surface. | The Fe-N co-modification improves the adsorption capacity, but it is limited to a single RhB pollutant and lacks universality. | Activated carbon with a high specific surface area was developed, but the problem of regeneration was not solved, and its practical application value is limited. |
| Environmental protection and economy | Entirely based on waste + no chemical activation, in accordance with the circular economy; simple process, easy to large-scale production. | Relying on chemical modification (FeSO4/urea), Fe/N residue may be introduced in wastewater treatment. | KOH activation requires a large amount of strong alkali, high cost + high environmental burden, and low feasibility of industrialization. |
| Application potential | Suitable for medium and low concentrations of dye wastewater (with the initial RhB concentration of 200 mg/L), with stable regeneration performance, and suitable for long-term recycling use. | Magnetic separation is suitable for heavy metal or complex ion systems but has a low adsorption capacity and narrow application scenarios. | Only applicable to ultra-high concentration wastewater (such as industrial dye waste liquid), high regeneration cost is difficult to popularize. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kan, X.; Suo, Y.; Shi, B.; Zheng, Y.; Liu, Z.; Ma, W.; Li, X.; Zhang, J. Synergistic Enhancement of Rhodamine B Adsorption by Coffee Shell Biochar Through High-Temperature Pyrolysis and Water Washing. Molecules 2025, 30, 2769. https://doi.org/10.3390/molecules30132769
Kan X, Suo Y, Shi B, Zheng Y, Liu Z, Ma W, Li X, Zhang J. Synergistic Enhancement of Rhodamine B Adsorption by Coffee Shell Biochar Through High-Temperature Pyrolysis and Water Washing. Molecules. 2025; 30(13):2769. https://doi.org/10.3390/molecules30132769
Chicago/Turabian StyleKan, Xurundong, Yao Suo, Bingfei Shi, Yan Zheng, Zaiqiong Liu, Wenhui Ma, Xianghong Li, and Jianqiang Zhang. 2025. "Synergistic Enhancement of Rhodamine B Adsorption by Coffee Shell Biochar Through High-Temperature Pyrolysis and Water Washing" Molecules 30, no. 13: 2769. https://doi.org/10.3390/molecules30132769
APA StyleKan, X., Suo, Y., Shi, B., Zheng, Y., Liu, Z., Ma, W., Li, X., & Zhang, J. (2025). Synergistic Enhancement of Rhodamine B Adsorption by Coffee Shell Biochar Through High-Temperature Pyrolysis and Water Washing. Molecules, 30(13), 2769. https://doi.org/10.3390/molecules30132769







