Essential Per- and Polyfluoroalkyl Substances (PFAS) in Our Society of the Future
Abstract
1. Introduction
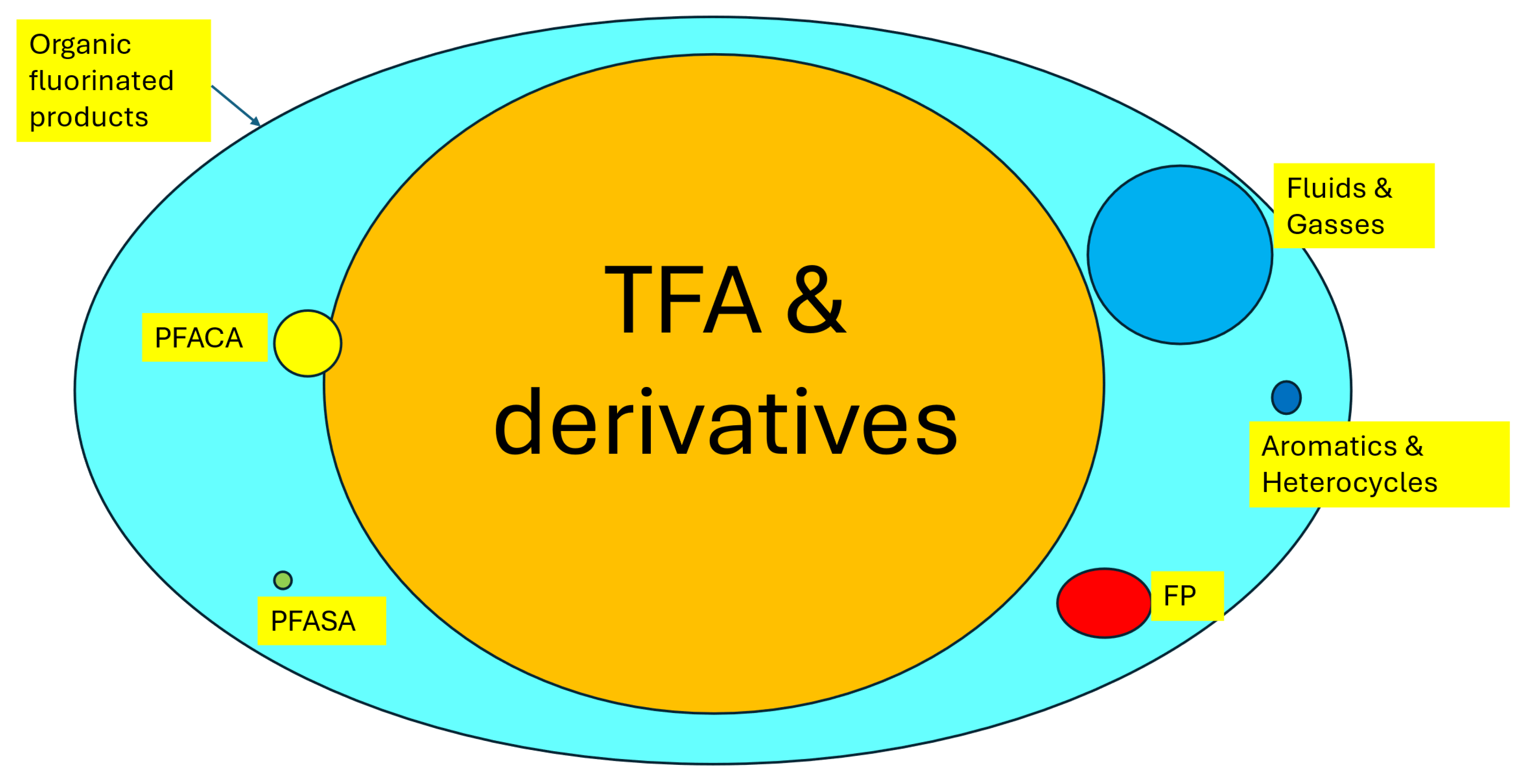
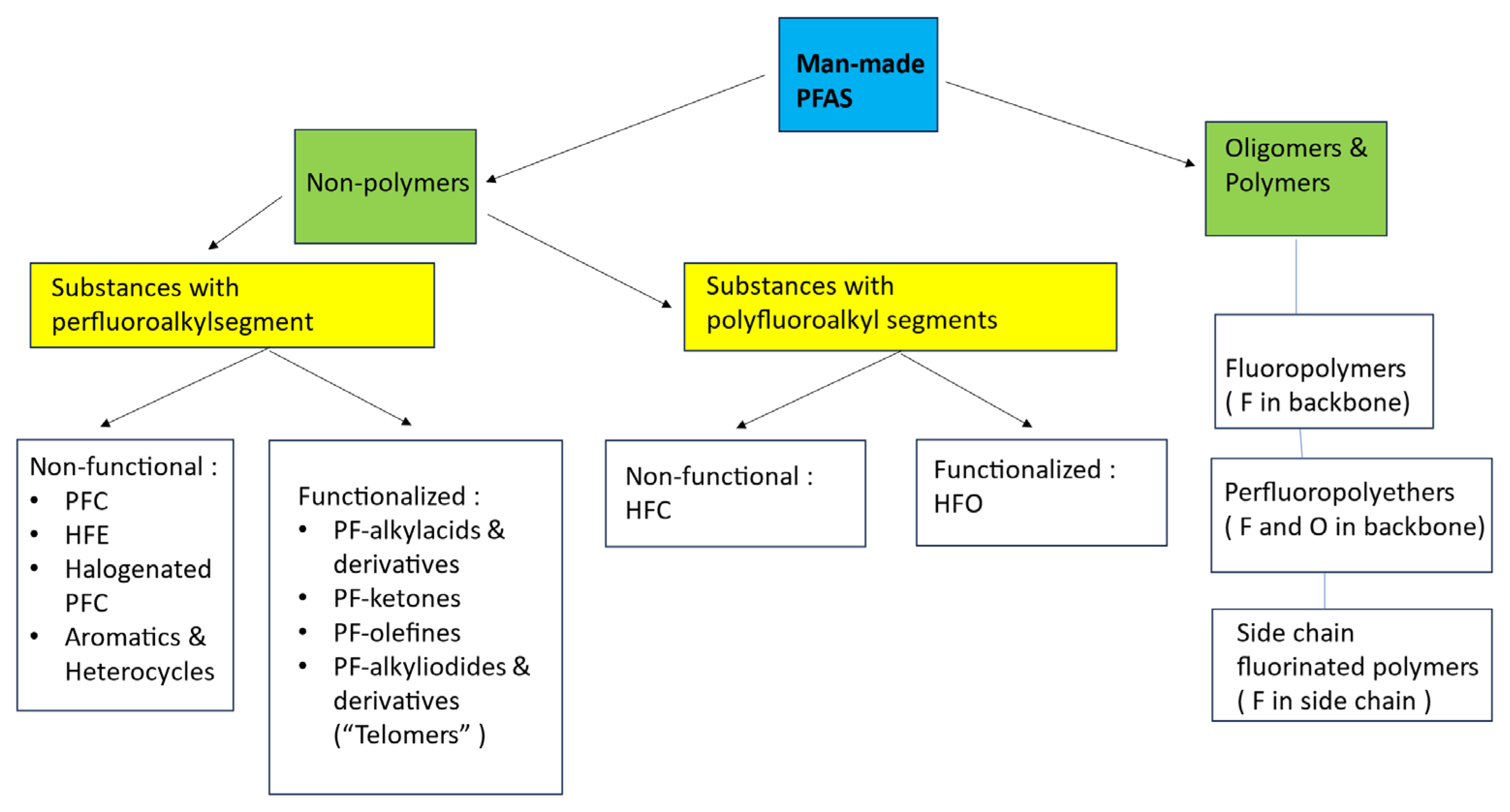
2. The PFAS Family
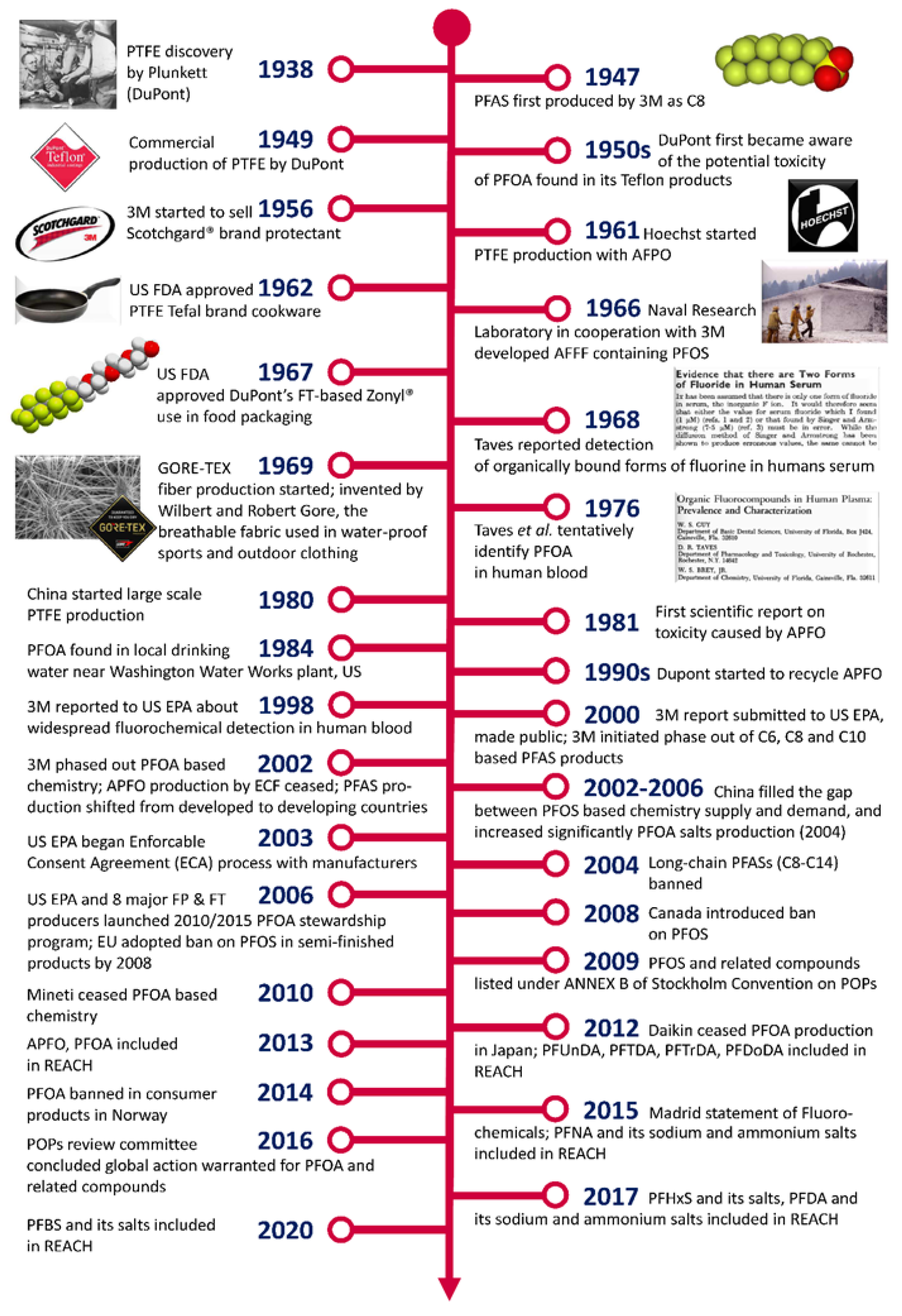
3. PFAS History
4. Why Fluorine and Organofluorine Substances?
- Low heat of vaporization for refrigerants,
- Low surface tension for surfactants,
- Low surface energy for oil, water, stain and dirt repellents for soft and hard surfaces,
- Low refractive index for optical applications,
- High solubility for oxygen for organ preservation and cancer therapy,
- High anti-stick and anti-friction properties for lubricants and release agents,
- Biocompatibility for medical devices and pharmaceuticals,
- UV-stability for many fluoropolymers.
5. Consumer Uses and Industrial Applications
5.1. Healthcare Applications
5.2. Food Security
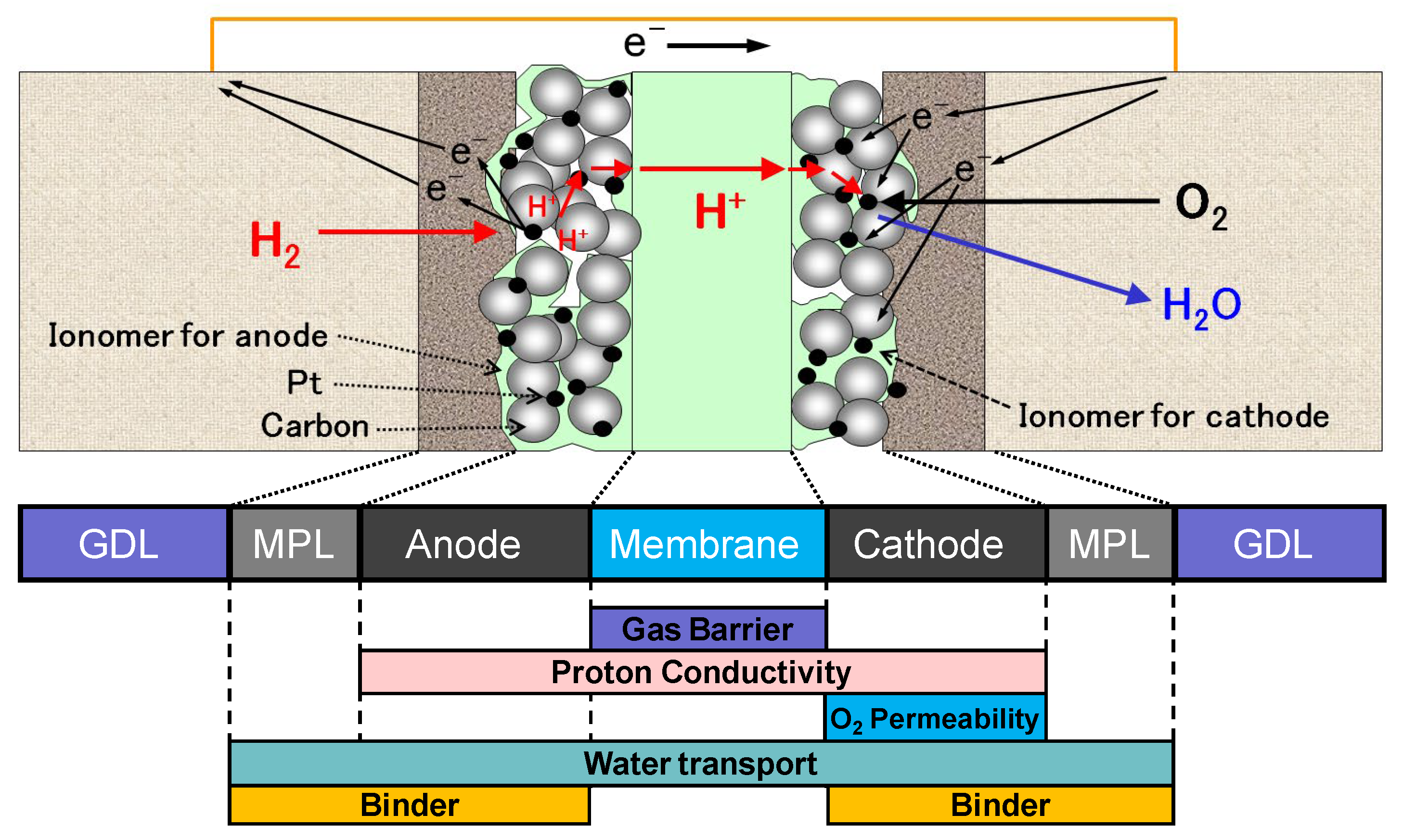
5.3. Energy
5.4. Materials
5.4.1. Fluoropolymers and Oligomers
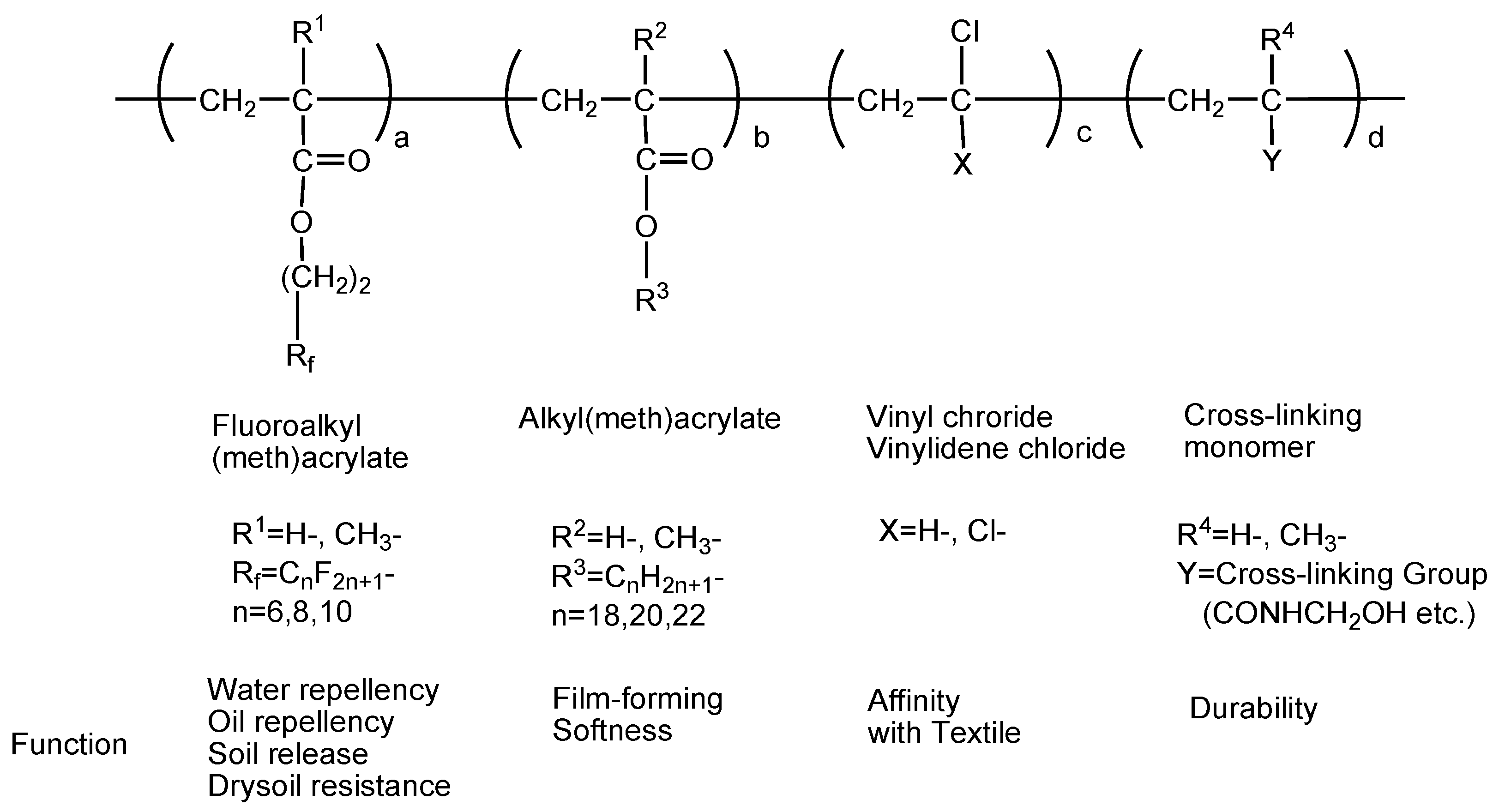
5.4.2. Perfluoroalkyl (Meth)Acrylates
5.4.3. Perfluoroalkyl-Derived Surfactants and Emulsifiers
5.4.4. Fluorinated Gasses and Fluids
6. Impact of PFASs on the Planet and Society
6.1. Unique Properties Leading to Environmental, Health and Safety (EHS) Issues
- persistence (long environmental and atmospheric lifetime),
- bioaccumulation potential in plants, animals and humans,
- potential for adverse effects on living organisms,
- mobility in aqueous systems and the atmosphere over a long range,
- absorption on soil,
- high GWP.
6.2. Environmental Aspects—Atmospheric Impact
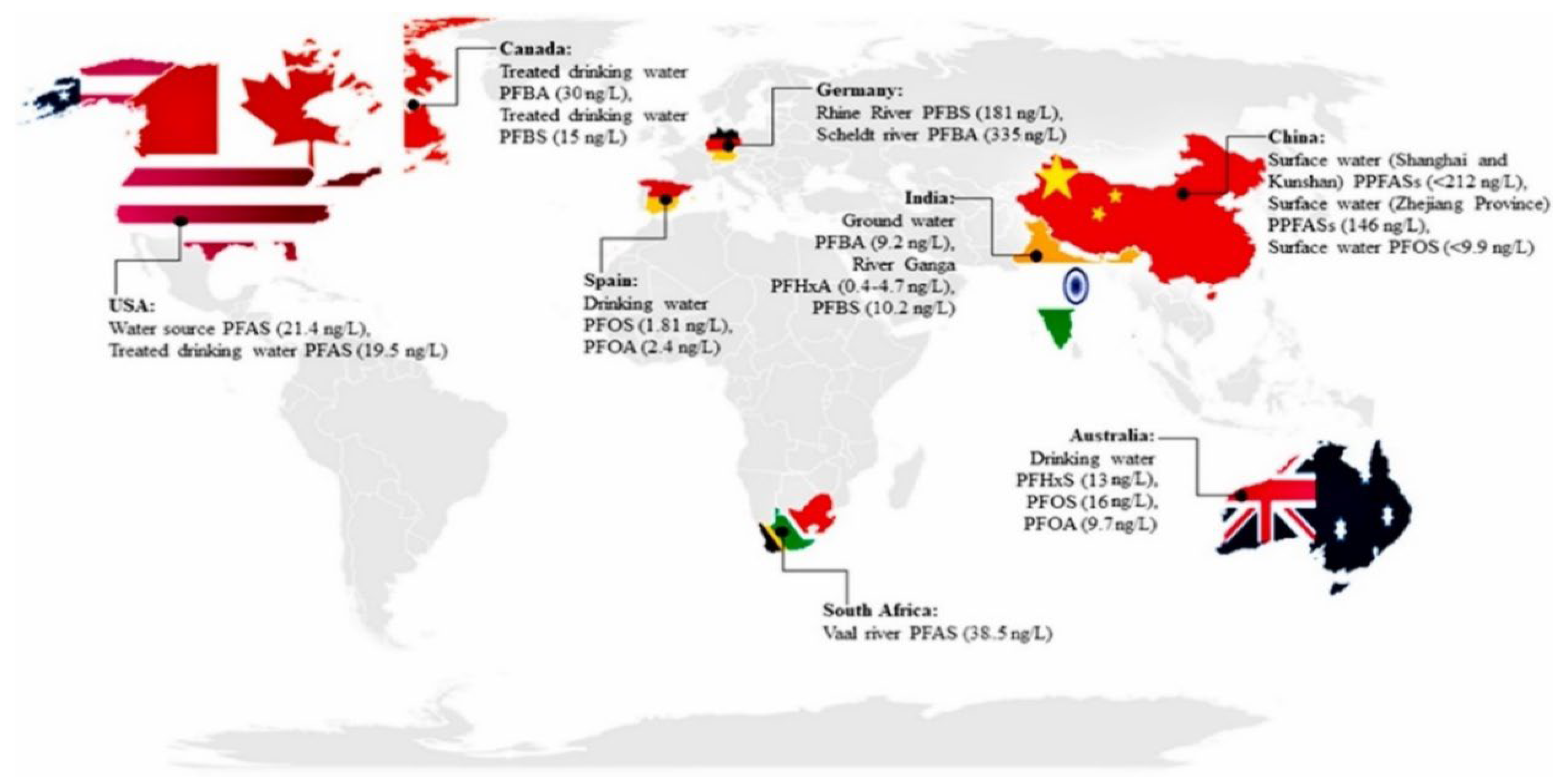
6.3. Environmental Aspects—Impact on Water and Soil
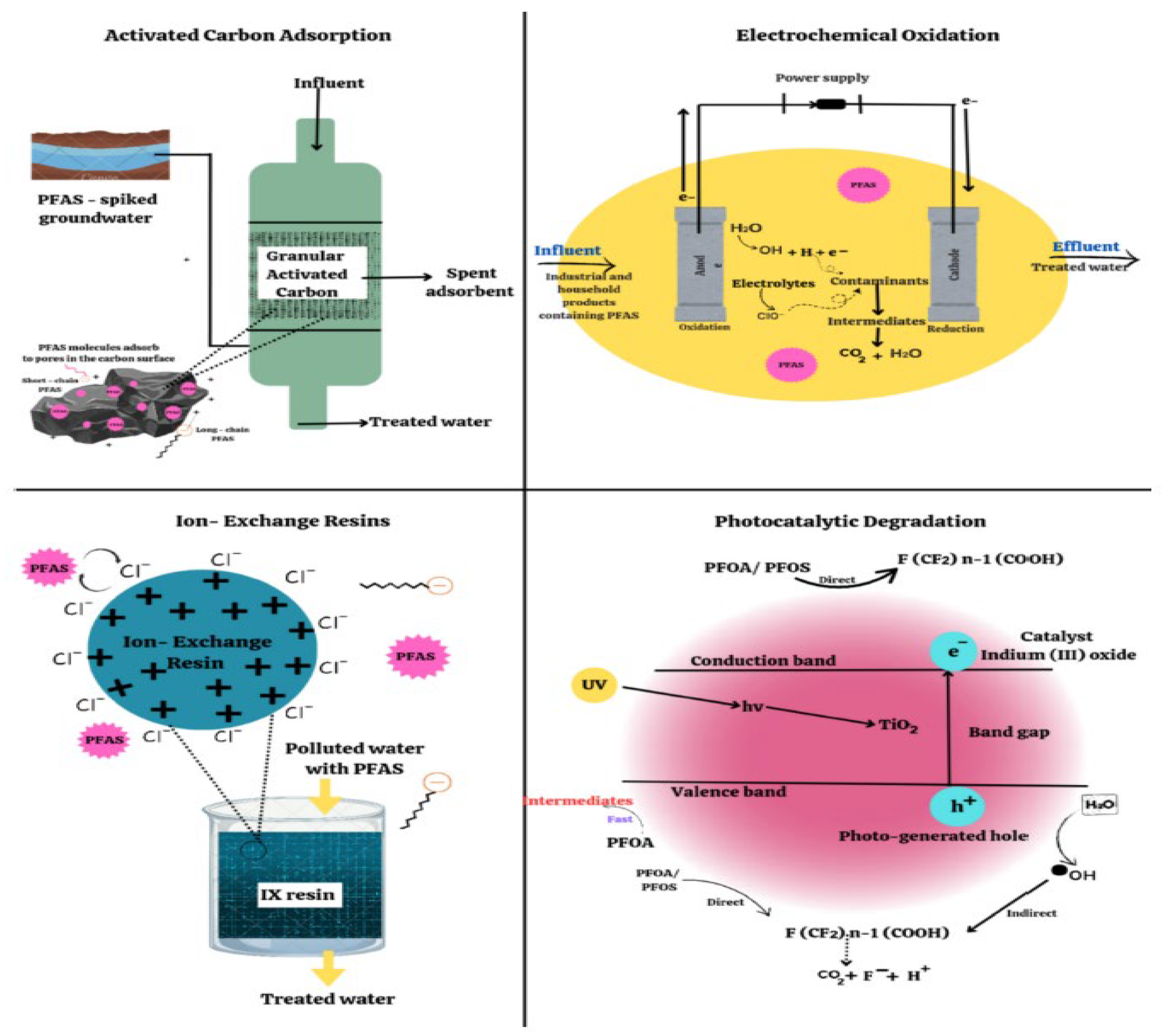
6.4. Remediation Technologies
6.5. Reduction in Impact by Industry
- Worldwide emissions to air and water by fluoropolymer production [26,90,91] were significant in the past decades. In the 1990s, Du Pont and Hoechst started recycling APFO, the ammonium salt of PFOA, used as an emulsifier in fluoropolymer production, and replaced it initially with other non-bioaccumulative fluorosurfactants [26] and later by fluorine-free polymerization aids [59]; the recycling and re-use of fluoropolymers took off [26,91]. Air emissions have been drastically reduced by many producers, but further worldwide reductions are mandatory [88,92].
- 2000: 3M initiated the voluntary phase-out of C6, C7, C8 and C10-based PFAS products and their manufacturing and the company announced it will stop PFAS manufacturing by the end of 2025 [93].
- During the last two decades, more than 100 fluorine-free alternatives for oil and water repellents, AFFFs, paint and coating additives, cosmetic additives and food packaging have been developed and marketed [94], although not always successfully, since most replacements are not drop-in solutions abd are still in the R&D stage. Their supply chain is not secured yet or cannot match the performances and cost of their PFAS counterparts [34,35].
6.6. Toxicological Aspects
6.7. The Analytical Dilemma
7. Closing the Fluorine Loop and Circularity
- (i)
- Focus on essential PFAS uses [37,122] and applications and phase out all others as soon as possible. By “essential uses”, it is meant those applications which are critical to our society and for which no viable and equally performing substitute exists.Examples of non-essential uses therefore include, for example, the packaging of popcorn and fast food, cosmetics and skin care, ski waxes, oil and water repellents for carpet, textiles, paper, home fabrics and the like, frying and cooking pans, window cleaners and paint leveling agents. On the other hand, examples of essential uses [122] where no adequate alternatives are available [35,36] for the time being, are some anesthetics, hydraulic fluids, optical fibers, lithium or sodium battery electrolytes and binders of cathodes, coatings and specific items for the nuclear and chemical industries, bridge and building bearings, semiconductor and chip manufacturing, high-temperature sealing applications, medical devices (implants, catheters, tubings, wound dressings and propellants for inhalers), insulations for cables, high-voltage switch gears, fire extinguishing for aviation, ships and military, fire regulated air conditioning, specialty refrigeration, professional clothing for army, police and firefighters and metal plating surfactants.
- (ii)
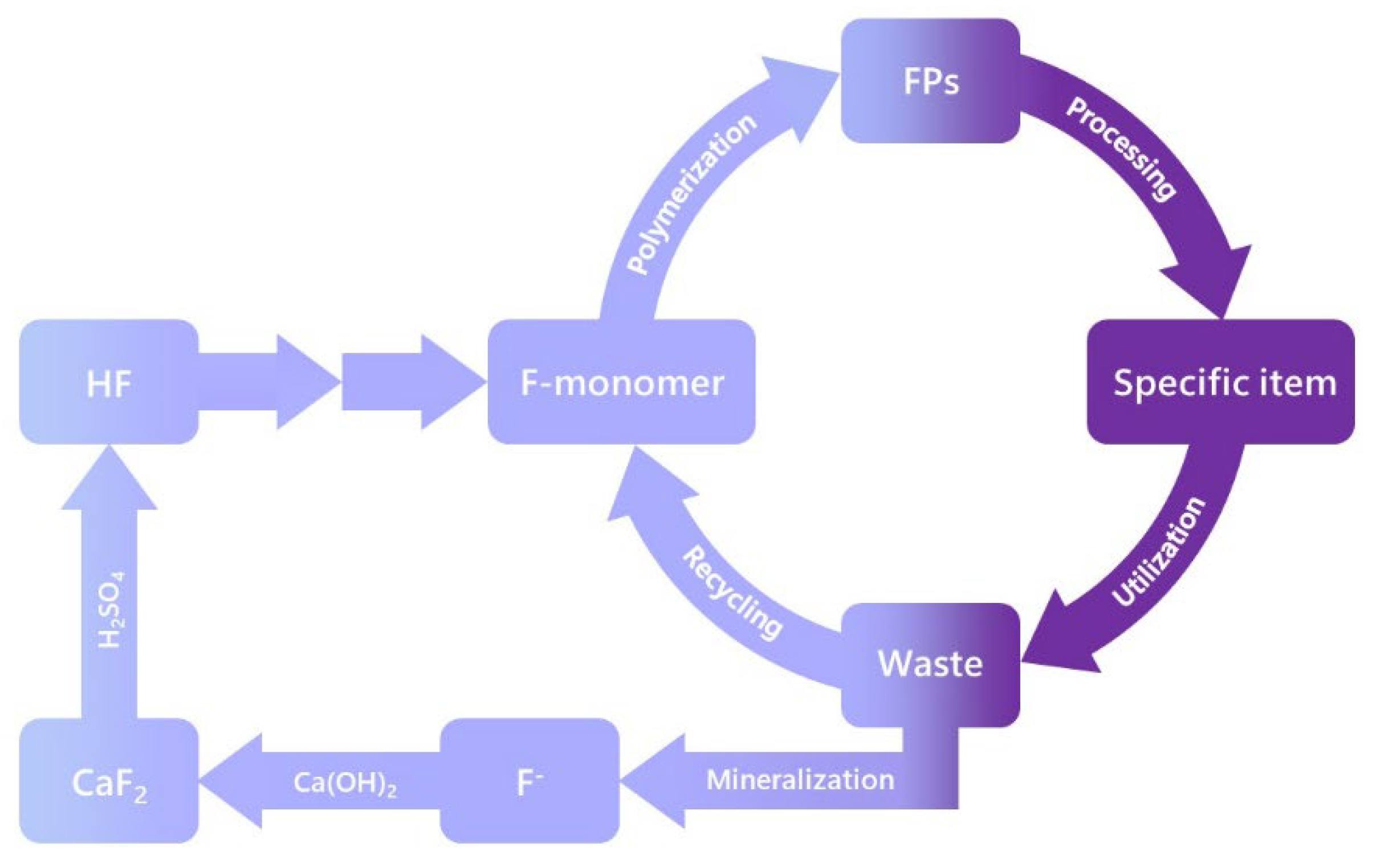
- Implement circularity [26,88,89,90,91,92,93,94,95,96,97,98,99] and closed applications for these essential uses, without burdening the environment (Figure 9). Environmental awareness requires considering the complete life cycle of products and consequently recycling PFASs, as exemplified for fluoropolymers by Dams and Hintzer [26] and in Figure 9.A key action will be to enforce new business models such as the licensing or leasing of PFASs (and chemicals in general), so producers stay accountable for their products.
- (iii)
- Strive for global zero-emissions in local manufacturing and applications, resulting in 100% mass balances.Key actions will be to implement remediation strategies for historical contaminations in water and soil as well as the complete mineralization of all emissions to soil, water and air and recycling the released fluorides [88,89]. Furthermore, creating a worldwide equilibrium between observed acute and long-term environmental and toxicological effects, analytical detection limits, global standards for contaminations, food and drinking water guidelines and, last but not least, overall norms and standards for industrial activities is crucial.
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFFF | aqueous film-forming foam |
| ATC | alcohol type concentrate |
| CAGR | aompound Annual Growth Rate |
| CFC | chlorofluorocarbon |
| CTFE | chlorotrifluoroethylene |
| ECF | electrochemical Fluorination |
| EOF | extractable organic fluorine |
| GAC | granulated activated carbon |
| GWP | global warming potential |
| HCFC | hydrochlorofluorocarbon |
| HFA | hydrofluoric acid |
| HFC | hydrofluorocarbon |
| HFE | hydrofluoroether |
| HFO | hydrofluoroolefin |
| HFP | hexafluoropropylene |
| HFPO | hexafluoropropylene oxide |
| NoEL | no effect level |
| ODP | ozone depletion potential |
| PFAS | poly or perfluorinated alkyl/aryl substances |
| PFBA | perfluorobutanoic acid |
| PFBS | perfluorobutyl sulphonic acid |
| PFC | perfluorocarbon |
| PFCA | perfluoroalkyl carboxylic acid |
| PFNA | perfluorononanoic acid |
| PFOA | perfluorooctanoic acid |
| PFOS | perfluorooctanesulphonic acid |
| PFSA | perfluoroalkyl sulphonic acid |
| PTFE | polytetrafluoroethylene |
| PVDF | polyvinylidene fluoride |
| RO | reverse osmosis |
| TFA | trifluoroacetic acid |
| TFE | tetrafluoroethylene |
| VDF | 1,1-difluoroethene (or vinylidene fluoride) |
References
- Buck, R.; Franklin, J.; Berger, V.; Cader, J.; Cousins, I.; de Voogt, P.; Jensen, A.; Kamman, A.; Mobury, S.; Van Leeuwen, S. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef]
- European Chemicals Agency (ECHA). Substances Information: PFAS. 2023. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.308.021 (accessed on 7 December 2023).
- Secundo, L.; Metrangolo, P.; Dichiarante, V. Current Approaches in the Classification of PFAS: An Overview. Chem. Asian J. 2025, 20, e202500127. [Google Scholar] [CrossRef] [PubMed]
- Dalmijn, J.; Glüge, J.; Scheringer, M.; Cousins, I.T. Emission inventory of PFASs and other fluorinated organic substances for the fluoropolymer production industry in Europe. Environ. Sci. Proc. Impacts 2024, 26, 269–287. [Google Scholar] [CrossRef]
- Scott, B.; MacDonald, R.; Kannan, K.; Fisk, A.; Witter, A.; Durham, L.; Yamashita, N.; Spencer, C.; Muir, D. Trifluoroacetate profiles in the Arctic, Atlantic and Pacific Oceans. Environ. Sci. Technol. 2005, 39, 6555–6560. [Google Scholar] [CrossRef]
- Joudan, S.; DeSilva, A.; Young, C. Insufficient evidence for the existence of natural trifluoroacetic acid. Environ. Sci. Process Impacts 2021, 23, 1641–1649. [Google Scholar] [CrossRef]
- Scheurer, M.; Freeling, F.; Janda, J.; Happel, O.; Riegel, M.; Muller, V.; Storck, F.; Fleig, M.; Lange, F.; Brunsch, A.; et al. Small, mobile, persistent: Trifluoroacetate in the water cycle. Water Res. 2017, 126, 460–471. [Google Scholar] [CrossRef]
- Solomon, K.; Velders, G.; Wilson, S.; Matronich, S.; Longstreth, J.; Aucamp, J.; Bomman, P. Sources, Faith, Toxicity and Risks of Trifluoroacetic Acid and Its Salts. 2016. Available online: https://www.tandfonline.com/doi/full/10.1080/10937404.2016.1175981 (accessed on 5 May 2025).
- Velders, G.; Danich, J.; Montzka, S.; Vimont, I.; Rigby, M.; Krummel, P.; Muehle, J.; Prinn, S.O.R.; Weiss, R.; Young, D. Projections of HFC emissions and the resulting global warming based on recent trends in observed abundances and current policies. Atmos. Chem. Phys. 2022, 22, 6087–6101. [Google Scholar] [CrossRef]
- Ohkura, M.; Morizawa, Y. Fluoroplastics and Fluoroelastomers-Basic Chemistry and High-Performance Applications. In Fluorinated Polymers, Volume 2 Applications; Ameduri, B., Sawada, H., Eds.; Royal Society of Chemistry: London, UK, 2017; Chapter 4. [Google Scholar]
- Henry, B.; Carlin, J.; Hammerschmidt, J.; Buck, R.; Buxton, L.; Fiedler, L.; Seed, J.; Hernandez, O. A critical review of the application of polymer of low concern and regulatory criteria for fluoropolymers. Integr. Environ. Assess. Manag. 2018, 14, 316–334. [Google Scholar] [CrossRef]
- Korzeniowski, S.; Buck, R.; Newkold, R.; El Kassmi, A.; Laganis, E.; Matsuoka, Y.; Dinelli, B.; Beauchet, S.; Adamsky, F.; Weilandt, K. Critical review of the application of polymer of low concern regulatory criteria to Fluoropolymers II: Fluoroplastics and Fluoroelastomers. Integr. Environ. Assess. Manag. 2023, 19, 326–354. [Google Scholar] [CrossRef]
- Walkowiak-Kulikowska, J. Poly/perfluoroalkyl Substances (PFAS)-Synthetic Methods, Properties and Applications. In Perfluorinated Substances: Synthesis, Applications, Challenges and Regulations; Ameduri, B., Ed.; Royal Society of Chemistry: London, UK, 2022; Chapter 2; pp. 22–65. [Google Scholar]
- Elliot, A. Chlorofluorocarbons. In Organofluorine Chemistry, Principles and Commercial Applications; Banks, R., Smart, B., Tatlow, J., Eds.; Plenum Press: New York, NY, USA, 1994; Chapter 6. [Google Scholar]
- Okazoe, T. Overview of the history of organofluorine chemistry from the viewpoint of materials industry. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2009, 85, 276–289. [Google Scholar] [CrossRef]
- Dreveton, A. Overview of the fluorochemicals industrial sectors. Procedia Eng. 2016, 138, 240–247. [Google Scholar] [CrossRef]
- Sicard, A.; Baker, R. Fluorocarbon refrigerants and their synthesis: Past to present. Chem. Rev. 2024, 120, 9164–9303. [Google Scholar] [CrossRef]
- Booten, C.; Nicholson, S.; Mann, M.; Abdelaziz, O. Refrigerants: Market Trends and Supply Chain Assessment. 2020. Available online: https://www.osti.gov/scitech (accessed on 5 May 2025).
- Xi, Y.X.; Gao, S.; Pan, S.; Jiao, Z. A review on the application of fluorinated greenhouse gases measurement technologies. Appl. Ecol. Environ. Res. 2025, 23, 1985–1998. [Google Scholar] [CrossRef]
- Schöffler, F.; Scherer, O. German, “Verfahren zur Darstellung von Polymerisationsprodukten”. Patent DE 1939/677071, 17 June 1939. [Google Scholar]
- Coldwhite, H. The Manhattan Project, Fluorine—The First 100 Years (1886–1986); Banks, R., Sharp, D., Tatlow, J., Eds.; Elsevier: New York, NY, USA, 1986; Chapter 5. [Google Scholar]
- Dieslin, A.; Kauck, E.; Simons, J. Fluorocarbon Acids and Derivatives. U.S. Patent 1951/2,567,011, 10 January 1949. [Google Scholar]
- Alsmeyer, Y.; Childs, W.; Flynn, R.; Moore, G.; Smeltzer, J. Electrochemical Fluorination and Its Applications. In Organofluorine Chemistry, Principles and Commercial Applications; Banks, R., Smart, B., Tatlow, J., Eds.; Plenum Press: New York, NY, USA, 1994; Chapter 5. [Google Scholar]
- Yamamoto, I. Fluoroalkyl Acrylate Polymers and Their Applications. In Fluorinated Polymers, Volume 2 Applications; Ameduri, B., Sawada, H., Eds.; Royal Society of Chemistry: London, UK, 2017; Chapter 2; pp. 32–53. [Google Scholar]
- Ameduri, B.; Boutevin, B. Telomerisation Reactions of Fluorinated Alkenes. In Well-Architectured Fluoropolymers: Synthesis, Properties and Applications; Elsevier Ltd.: Oxford, UK, 2004; Chapter 1; pp. 1–99. [Google Scholar]
- Dams, R.; Hintzer, K. Industrial Aspects of Fluorinated Oligomers and Polymers. In Fluorinated Polymers, Volume 2 Applications; Ameduri, B., Sawada, H., Eds.; Royal Society of Chemistry: London, UK, 2017; Chapter 1. [Google Scholar]
- Friesen, C.; Ameduri, B. Outstanding Telechelic Perfluoroalkylethers and their Applications. Progr. Polym. Sci. 2018, 81, 238. [Google Scholar] [CrossRef]
- Molina, M.; Rowland, F. Stratospheric sink for chlorofluoromethanes: Chlorine atom-catalyzed destruction of ozone. Nature 1974, 249, 810. [Google Scholar] [CrossRef]
- Fluorinated Gases and Ozone-Depleting Substances: Council Greenlights New Rules to Reduce Harmful Emissions. Available online: https://www.consilium.europa.eu/en/press/press-releases/2024/01/29/fluorinated-gases-and-ozone-depleting-substances-council-greenlights-new-rules-to-reduce-harmful-emissions/ (accessed on 1 November 2024).
- Available online: https://treaties.un.org/pages/viewdetails.aspx?src=treaty&mtdsg_no=xxvii-7-a&chapter=27&clang=_en (accessed on 3 April 2025).
- Sheldon, D.; Crimmin, M. Repurposing of F-gasses: Challenges and opportunities in fluorine chemistry. Chem. Soc. Rev. 2022, 51, 4977–4995. [Google Scholar] [CrossRef] [PubMed]
- Koulini, G.V.; Vinayagam, V.; Nambi, I.M.; Ravi Krishna, R. Per- and polyfluoroalkyl substances (PFAS) in Indian environment: Prevalence, impacts and solutions. J. Water Proc. Eng. 2024, 66, 105988. [Google Scholar] [CrossRef]
- Lindley, A. An inventory of fluorspar production, industrial use and emissions of trifluoroacetic acid (TFA). J. Geosci. Environ. Prot. 2023, 11, 1–16. [Google Scholar] [CrossRef]
- Figuière, R.; Miaz, L.; Savidou, E.; Cousins, I. An overview of potential alternatives for the multiple uses of per- and polyfluoroalkyl substances. Environ. Sci. Technol. 2025, 59, 2031–2042. [Google Scholar] [CrossRef]
- Lang-Koetz, C.; Hutschek, U. PFAS: Applications, Technical Functions and Substitution Possibilities in the Industry. 2024. Available online: https://www.thinktank-irs.de/wp-content/uploads/2024/02/RZ_THINKTANK_PFAS_A4_EN_web.pdf (accessed on 5 May 2025).
- Jacobs David, S.; Kosson, S. Assessment of Fluoropolymer Production and Use with Analysis of Alternative Replacement Materials, 2024 SRNL-STI-2023-00587. Available online: https://www.osti.gov/servlets/purl/2370520 (accessed on 11 April 2025).
- Glüge, J.; Scheiringer, M.; Cousins, I.; De Witt, J.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.; Trier, X.; Wang, Z. An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Proc. Impacts 2020, 22, 2345–2373. [Google Scholar] [CrossRef]
- Schlipf, M. Fluoropolymers–Indispensable in key technologies. In Proceedings of the CRC 1349 Fluorine-Specific Interactions: Fundamentals and Applications, Berlin, Germany, 7–8 April 2025. [Google Scholar]
- Inoue, M.; Sumii, Y.; Shibata, N. Contribution of Organofluorine Compounds to Pharmaceuticals. ACS Omega 2020, 5, 10633–10640. [Google Scholar] [CrossRef]
- Zheng, S.; Sarker, P.; Gursoy, D.; Wei, T.; Hsiao, B.S. Molecular Mechanisms of Perfluoroalkyl Substances Integration into Phospholipid Membranes. Langmuir 2025, 41, 9369–9376. [Google Scholar] [CrossRef]
- Lv, J.; Cheng, Y. Fluoropolymers in biomedical applications: State-of-the-art and future perspectives. Chem. Soc. Rev. 2021, 50, 5435–5467. [Google Scholar] [PubMed]
- Roina, Y.; Auber; Hocquet, F.; Herlem, D. ePTFE functionalization for medical applications. Mater. Today Chem. 2021, 20, 100412. [Google Scholar] [CrossRef]
- Koguchi, R.; Jankova, K.; Tanaka, M. Fluorine-containing bio-inert polymers: Roles of intermediate water. Acta Biomater. 2022, 138, 34–56. [Google Scholar] [CrossRef] [PubMed]
- Halpern, D. Fluorinated Inhalation Anesthetics. In Organofluorine Chemistry: Principles and Commercial Applications; Plenum Press: New York, NY, USA, 1994; Chapter 25. [Google Scholar]
- Krafft, M.P. From fluorine’s position in the periodic table to PFAS environmental issues. CR Acad. Des Sci. 2025, 28, 423–438. [Google Scholar] [CrossRef]
- Holman, R.; Lorton, O.; Guillemin, P.C.; Desgranges, S.; Contino-Pépin, C.; Salomir, R. Perfluorocarbon Emulsion Contrast Agents: A Mini Review. Front. Chem. 2022, 9, 810029. [Google Scholar] [CrossRef]
- Ogawa, Y.; Tokunaga, E.; Kobayashi, O.; Hirai, K.; Shibata, N. Current contributions of organofluorine compounds to the Agrochemical Industry. iScience 2020, 23, 101467. [Google Scholar] [CrossRef]
- Available online: https://legislature.maine.gov/legis/bills/getTestimonyDoc.asp?id=186013 (accessed on 15 May 2025).
- Hirai, T.; Morizawa, Y. Fluorinated Ionomers and Ionomer Membranes: Monomer and Polymer Synthesis and Applications. In Fluorinated Polymers, Volume 2 Applications; Ameduri, B., Sawada, H., Eds.; Royal Society of Chemistry: London, UK, 2017; Chapter 19. [Google Scholar]
- Liu, R.-T.; Xu, Z.-L.; Li, F.-M.; Chen, F.-Y.; Yu, J.-Y.; Yan, Y.; Chen, Y.; Xia, B.Y. Recent advances in proton exchange membrane water electrolysis. Chem. Soc. Rev. 2023, 52, 5652–5683. [Google Scholar] [CrossRef]
- Goldbach, J.; Amin-Sanayei, R.; He, W.; Henry, J.; Kosar, W.; Lefebvre, A.; O’Brien, G.; Vaesen, D.; Wood, K.; Zerafati, S. Commercial Synthesis of and Applications of Poly(Vinylidene Fluoride). In Fluorinated Polymers, Volume 2 Applications; Ameduri, B., Sawada, H., Eds.; Royal Society of Chemistry: London, UK, 2017; Chapter 6. [Google Scholar]
- Sherrell, P.C.; Šutka, A.; Timusk, M.; Šutka, A. Alternatives to Fluoropolymers for Motion-Based Energy Harvesting: Perspectives on Piezoelectricity, Triboelectricity, Ferroelectrets, and Flexoelectricity. Small 2024, 20, 2311570. [Google Scholar] [CrossRef]
- Savidou, E.; Rensmo, A.; Benskin, J.; Schellenberger, S.; Hu, X.; Well, M. PFAS-free Energy Storage. Investig. Altern. Lithium-Ion Batter. 2024, 58, 21908–21917. [Google Scholar]
- Ameduri, B.; Fomin, S. Fascinating Fluoropolymers; Elsevier: Oxford, UK, 2020; Volume 2. [Google Scholar]
- Available online: https://www.futuremarketinsights.com/reports/fluoropolymers-market (accessed on 14 June 2024).
- Lohmann, R.; Letcher, R.J. The universe of fluorinated polymers and polymeric substances and potential environmental impacts and concerns. Curr. Opin. Green Sustain. Chem. 2023, 41, 100795. [Google Scholar] [CrossRef]
- Ameduri, B. Fluorinated Elastomers. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2023. [Google Scholar] [CrossRef]
- Kissa, E. Fluorinated Surfactants and Repellents; Surfactant Science Series; Marcel Dekker: New York, NY, USA, 1994. [Google Scholar]
- Ameduri, B.; Sales, J.; Schlipf, M. Developments in fluoropolymer manufacturing technology to remove intentional use of PFAS as polymerization aids. ITRC Intern. Chem. Regul. Law Rev. 2023, 6, 18–28. [Google Scholar]
- Hill, C.; Czajka, A.; Hazell, G.; Grillo, I.; Rogers, S.; Skoda, M.; Goslin, N.; Payne, J.; Eastoe, J. Surface and bulk properties used in fire-fighting. J. Colloid Interf. Sci. 2018, 530, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Tickner, J.; Eliason, P.; Harriman, E.; Onasch, J. Guidance for Evaluating the Performance of Alternatives: Fit-for-Purpose Performance. 2022. Available online: https://apps.dtic.mil/sti/html/trecms/AD1217175/index.html (accessed on 5 June 2024).
- Krafft, M.-P.; Riess, J.G. Per- and polyfluorinated substances (PFASs): Environmental challenges. Curr. Opin. Colloid Interf. Sci. 2015, 20, 192–212. [Google Scholar] [CrossRef]
- Overview of Greenhouse Gases. Available online: https://www.epa.gov/ghgemissions/overview-greenhouse-gases (accessed on 5 June 2024).
- Wikipedia. Fluorinated Gasses. Available online: https://en.wikipedia.org/wiki/Fluorinated_gasses (accessed on 20 May 2024).
- Glüge, J.; Breuer, K.; Hafner, A.; Vering, C.; Muller, D.; Cousins, I.; Lohmann, R.; Goldenman, G.; Scheringer, M. Finding non-fluorinated alternatives to fluorinated gasses used as refrigerants. Environ. Sci. Process Impacts 2024, 26, 1955–1974. [Google Scholar] [CrossRef]
- Zhang, W.; Pang, S.; Lin, Z.; Mishra, S.; Bhatt, P.; Chen, S. Biotransformation of perfluoroalkylacid precursors from various environmental systems: Advances and perspectives. Environ. Pollut. 2021, 272, 115908. [Google Scholar] [CrossRef] [PubMed]
- Berhanu, A.; Mutanda, I.; Taolin, J.; Quaria, M.; Yang, B.; Zhu, D. A review of microbial degradation of per- and polyfluoroalkyl substances (PFAS): Biotransformation routes and enzymes. Sci. Total Environ. 2023, 859, 160010. [Google Scholar] [CrossRef]
- Zhang, Z.; Sarkar, D.; Biswas, J.; Datta, R. Biodegradation of per- and polyfluoroalkyl substances (PFAS): A review. Bioresour. Technol. 2024, 344, 126223. [Google Scholar] [CrossRef]
- Rhoads, K.; Janssen, E.; Luthy, R.; Craddle, C. Aerobic biotransformation and fate of N-ethyl perfluorooctane sulfonamidoethanol in activated sludge. Environ. Sci. Technol. 2008, 42, 2873–2878. [Google Scholar] [CrossRef]
- Avendano, A.; Liu, J. Production of PFOS from aerobic soil transformation of two perfluoroalkylsulfonamid derivatives. Chemosphere 2015, 119, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Dasu, K.; Lee, L.; Turco, R.; Nies, L. Aerobic biodegradation of 8:2 stearate monoester and 8:2 citrate triester in forest soil. Chemosphere 2013, 91, 399–405. [Google Scholar] [CrossRef]
- Liu, W.; Wu, J.; He, W.; Xu, F. A review on perfluoroalkylacids: Environmental behaviour, toxic effects and health risks. Ecosyst. Health Sustain. 2019, 5, 1–19. [Google Scholar] [CrossRef]
- DiStefano, R.; Feliciano, T.; Minna, R.; Redding, A. Thermal destruction of PFAS during full-scale reactivation of PFAS-laden granular activated carbon. Remediation 2022, 32, 231–238. [Google Scholar] [CrossRef]
- Liu, Y.; Li, T.; Hu, X.; Zhao, X.; Shao, L.; Li, C.; Lu, M. A review of treatment techniques for short-chain perfluoroalkyl substances. Appl. Sci. 2022, 12, 1941. [Google Scholar] [CrossRef]
- Chambial, P.; Thakur, N.; Kushawaha, J.; Kumar, R. Per- and polyfluoroalkyl substances in environment and potential health impacts: Sources, remediation treatment and management, policy guidelines, destructive technologies, and techno-economic analysis. Sci. Total Environ. 2025, 969, 178803. [Google Scholar]
- Hurst, J.; Hale, S.; Miles, J.; Dal, E.; Gevaerts, W.; Burdich, J. PFAS Soil Treatment Processes—A Review of Operating Ranges and Constraints. Report 8/24. 2024. Available online: www.concawe.eu (accessed on 5 June 2024).
- Long, A. Estimated cost to remove PFAS from the environment at current emission rates. Sci. Total Environ. 2024, 918, 170647. [Google Scholar] [CrossRef] [PubMed]
- Verna, S.; Lee, T.; Sahle-Demessie, E.; Ateia, M.; Nadagouda, M. Recent advances on PFAS degradation via thermal and nonthermal methods. Chem. Eng. J. Adv. 2023, 13, 100421. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Z.; He, X.; Song, M.; Westerhoff, P.; Doudrick, K.; Hanigan, D. Critical Review of Thermal Decomposition of per- and polyfluoroalkyl substances: Mechanism and implications for thermal treatment processes. Environ. Sci. Technol. 2022, 56, 5355–5370. [Google Scholar] [CrossRef] [PubMed]
- Gehrmann, H.-J.; Philip, T.; Aleksandrov, K.; Bergdolt, P.; Bologa, A.; Blye, D.; Dalal, P.; Gunasekar, P.; Herremanns, S.; Kapoor, D.; et al. Mineralization of fluoropolymers from combustion in a pilot plant under representative European municipal and hazardous waste combustor conditions. Chemosphere 2024, 365, 143403. [Google Scholar] [CrossRef]
- Verna, S.; Varma, R.; Nadagouda, M. Remediation and mineralization processes for per- and polyfluoroalkyl substances in water: A review. Sci. Total Environ. 2021, 794, 148987. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Mora, R.; Huang, Q.; Casson, R.; Wang, Y.; Woodard, S.; Anderson, H. Field demonstration of coupling ion-exchange resin with electrochemical oxidation for enhanced treatment of per- and polyfluoroalkyl substances in groundwater. Chem. Engin. J. Adv. 2022, 9, 100216. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.-X.; Qu, J.-P.; Kang, Y.-B. Photocatalytic low-temperature defluorination of PFASs. Nature 2024, 635, 610–617. [Google Scholar] [CrossRef]
- Pinkard, B.; Smith, S.; Vorarath, P.; Smrz, T.; Schmick, S.; Dressel, L.; Bryan, C.; Czerki, M.; de Marne, A.; Halevi, A.; et al. Degradation and defluorination of ultra-, short-, and long-chain PFAS in High Total Dissolved Solutions by Hydrothermal Alkaline Treatment-Closing the Fluorine Mass Balance. ACS Environ. Sci. Technol. Eng. 2024, 4, 2810–2818. [Google Scholar]
- Sabba, F.; Kassar, C.; Zeng, T.; Mallick, S.; Downing, L.; McNamara, P. PFAS in landfill leachate: Practical considerations for treatment and characterization. J. Hazard. Mat. 2025, 481, 136685. [Google Scholar] [CrossRef]
- Pilli, S.; Pandey, A.K.; Pandey, V.; Pandey, K.; Muddam, T.; Thirunagari, B.K.; Thota, S.T.; Varjani, S.; Tyagi, R.D. Detection and removal of poly and perfluoroalkyl polluting substances for sustainable environment. J. Environ. Manag. 2021, 297, 113336. [Google Scholar] [CrossRef]
- Alnaimat, S.; Mohsen, O.; Elnakar, H. Perfluorooctanoic Acids (PFOA) removal using electrochemical oxidation: A machine learning approach. J. Environ. Manag. 2024, 370, 122857. [Google Scholar] [CrossRef]
- Ameduri, B.; Hori, H. Recycling and End of Life assessment of Fluoropolymers: Recent developments. Chem. Soc. Rev. 2023, 52, 4208–4247. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, Z.; Goult, C.A.; Schlatzer, T.; Paton, R.S.; Gouverneur, V. Phosphate-enabled mechanochemical PFAS destruction for fluoride reuse. Nature 2025, 640, 100–106. [Google Scholar] [CrossRef]
- Dauchy, X. Evidence of large-scale deposition of airborne emissions of per- and polyfluoroalkyl substances (PFASs) near a fluoropolymer production plant in an urban area. Chemosphere 2023, 337, 139407. [Google Scholar] [CrossRef]
- Gebbink, W.; Van Leeuwen, S. Environmental contamination and human exposure to PFAS near a fluorochemical production plant: Review of historic and current PFOA and GenX contamination in the Netherlands. Environ. Int. 2020, 137, 105583. [Google Scholar] [CrossRef]
- Ameduri, B. Fluoropolymers as unique and irreplaceable materials: Challenges and future trends in these specific per-and polyfluoroalkyl substances. Molecules 2023, 28, 7654. [Google Scholar] [CrossRef]
- 3M to Exit PFAS Manufacturing by the End of 2025. Available online: https://news.3m.com (accessed on 2 March 2024).
- ChemSec. PFAS Alternatives. Available online: https://chemsec.org/with-over-100-new-alternatives-to-pfas-chemsec-marketplace-becomes-key-industry-resource/ (accessed on 29 June 2025).
- Wee, S.; Aris, A.Z. Revisiting the “forever chemicals”, PFOA and PFOS in drinking water. npj Clean Water 2023, 6, 57. [Google Scholar] [CrossRef]
- Spyrakis, F.; Dragani, T. The EU’s Per- and perfluoroalkyl Substances (PFAS) Ban: A case of policy over Science. Toxics 2023, 11, 721. [Google Scholar] [CrossRef]
- Perez, F.; Nadal, M.; Ortega, A.; Fabrega, F.; Domingo, J.; Barcelo, D.; Farré, M. Accumulation of perfluoroalkylsubstances in human tissues. Environ. Int. 2013, 59, 354–362. [Google Scholar] [CrossRef]
- Crisalli, A.; Cai, A.; Cho, B.P. Probing the interactions of perfluorocarboxylic acids of various chain lengths with human serum albumin. Chem. Res. Toxicol. 2023, 36, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Gui, S.-Y.; Qiao, J.-C.; Xu, K.-X.; Li, Z.-L.; Chen, Y.-N.; Wu, K.-J.; Jiang, Z.-X.; Hu, C.-Y. Association between per- and polyfluoroalkyl substances exposure and risk of diabetes: A systematic review and meta-analysis. Expos. Sci. Environ. Epidem. 2023, 33, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Pizzurro, D.; Seeley, M.; Kerper, L.; Beck, B. Interspecies differences in perfluoroalkyl substances (PFAS) Toxicokinetics and Application of health-based criteria. Regul. Toxicol. Pharmacol. 2019, 106, 239–250. [Google Scholar] [CrossRef]
- Tsuda, S. Differential toxicity between perfluorooctane sulfonate and perfluorooctanoic acid. J. Toxicol. Sci. 2016, 41, 27–36. [Google Scholar] [CrossRef]
- Fenton, S.; Pucatman, A.; Boobis, A.; De Witt, J.; Lau, C.; Ng, C.; Smith, J.; Roberts, S. Per-and polyfluoroalkyl Substance Toxicity and Human Health Review: Current state of knowledge and strategies for future research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef]
- Mancini, F.R.; Cano-Sancho, G.; Gambaretti, J.; Marchand, P.; Boutron-Ruault, M.-C.; Severi, G.; Arveux, P.; Antignac, J.-P.; Kvaskoff, M. Perfluorinated alkylated substances serum concentration and breast cancer risk: Evidence from a nested case-control study in the French E3N cohort. Int. J. Cancer 2020, 146, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Zahm, S.; Bonde, J.P.; Chiu, W.A.; Hoppin, J. Carninogenicity of perfluorooctanoic acid and perfluorooctane sulphonic acid. Lancet 2024, 25, 16–17. [Google Scholar] [CrossRef] [PubMed]
- Hammarstrand, S.; Andersson, E.M.; Andersson, E.; Larsson, K.; Xu, Y.; Li, Y.; Jakobsson, K. The impact of high exposure to perfluoroalkyl substances and risk for hormone receptor-positive breast cancer—A Swedish cohort study. Environ. Int. 2024, 193, 109140. [Google Scholar] [CrossRef]
- Barry, V.; Winquist, A.; Steenland, K. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ. Health Persp. 2013, 121, 1313–1318. [Google Scholar] [CrossRef]
- Nicole, W. PFOA and cancer in a highly exposed community: New findings from the C8 science panel. Environ. Health Persp. 2013, 121, A340. [Google Scholar] [CrossRef]
- Biggeri, A.; Faccido, L. All-cause, cardiovascular disease and cancer mortality in the population of a large Italian area contaminated by PFAS (1980–2018). Environ. Health 2024, 23, 42. [Google Scholar] [CrossRef]
- Sacher, F. PFAS from a Drinking Water Supplier’s Perspective. In Proceedings of the CRC 1349 Fluorine-Specific Interactions: Fundamentals and Applications, Berlin, Germany, 7–8 April 2025. [Google Scholar]
- Lee, J.C.; Smaoui, S.; Duffill, J.; Marandi, B.; Varzakas, T. Research Progress in Current and Emerging Issues of PFASs’ Global Impact: Long-Term Health Effects and Governance of Food Systems. Food 2025, 14, 958. [Google Scholar] [CrossRef]
- Research Report Flanders. Available online: https://www.vlaanderen.be/pfas-vervuiling/pfas-aanpak-regio-zwijndrecht (accessed on 23 February 2024).
- Hamscher, G. Significance of Perfluoroalkyl Substances (PFAS) in Food and Their Current Legal Regulation. In Proceedings of the CRC 1349 Fluorine-Specific Interactions: Fundamentals and Applications, Berlin, Germany, 7–8 April 2025. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain (EFSA CONTAM Panel); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.C.; et al. Risk to human health related to the presence of perfluoroalkyl substances in food. EFSA J. 2020, 18, e06223. [Google Scholar] [CrossRef]
- Bouomet, J.; Bingham, P.; Calamari, D.; de Rooij, C.; Franklin, J.; Kawano, T.; Libre, J.; Odom, J.; Rusch, G.; Smythe, K.; et al. Environmental risk assessment of trifluoroacetic acid. Hum. Ecol. Risk Assess. 1999, 5, 59–124. [Google Scholar]
- Arp, H.; Gredelj, A.; Glüge, J.; Scheringer, M.; Cousins, I. The global Threat from the Irreversible Accumulation of Trifluoroacetic Acid (TFA). Environ. Sci. Technol. 2024, 58, 19925–19935. [Google Scholar] [CrossRef] [PubMed]
- Ateia, M.; Maroli, A.; Tharaji, N.; Karinfol, T. The overlooked short- and ultrashort chain perfluorinated substances: A review. Chemosphere 2019, 220, 866–882. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://echa.europa.eu/fr/registration-dossier/-/registered-dossier/5203/7/9/3 (accessed on 4 April 2024).
- Vogel, C.; Slee, D. Analytical Techniques for Per- and Polyfluoroalkyl Substances (PFAS). In Per- and Polyfluoroalkyl Substances, Occurrence, Toxicity and Remediation of PFAS; Naidu, R., Megharaj, M., Liu, Y., Umeh, A., Eds.; Walter de Gruyter GmbH: Berlin, Germany, 2024; Chapter 5. [Google Scholar]
- Naftalovich, R.; Naftalovich, D.; Greenway, F.L. Polytetrafluoroethylene Ingestion as a Way to Increase Food Volume and Hence Satiety Without Increasing Calorie Content. J. Diabetes Sci. Technol. 2016, 10, 971–976. [Google Scholar] [CrossRef]
- Ameduri, B. What do we know about PFAS? Macromolecules 2025, 58, 2781–2791. [Google Scholar] [CrossRef]
- Pantanowitz, J. Fluoropolymers in Focus: A Case Study on Industry Applications, Regulatory Frameworks, and Compliance Challenges; PFAS Summit: Brussels, Belgium, 14–15 May 2025. [Google Scholar]
- Cousins, I.; Goldenman, G.; Herzke, D.; Lohmann, R.; Miller, M.; Ng, C.; Patton, S.; Sheringer, M.; Trier, X.; Vieske, L.; et al. The concept of essential use for determining when uses of PFAS can be phased out. Environ. Sci. Process. Impacts 2019, 21, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Bartl, A. The Future of the Proposed PFAS Restriction Dossier: New EU Policy Shifts and Regulatory Impacts; PFAS Summit: Brussels, Belgium, 14–15 May 2025. [Google Scholar]
- Available online: https://echa.europa.eu/-/echa-and-five-european-countries-issue-progress-update-on-pfas-restriction (accessed on 22 November 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dams, R.; Ameduri, B. Essential Per- and Polyfluoroalkyl Substances (PFAS) in Our Society of the Future. Molecules 2025, 30, 3220. https://doi.org/10.3390/molecules30153220
Dams R, Ameduri B. Essential Per- and Polyfluoroalkyl Substances (PFAS) in Our Society of the Future. Molecules. 2025; 30(15):3220. https://doi.org/10.3390/molecules30153220
Chicago/Turabian StyleDams, Rudy, and Bruno Ameduri. 2025. "Essential Per- and Polyfluoroalkyl Substances (PFAS) in Our Society of the Future" Molecules 30, no. 15: 3220. https://doi.org/10.3390/molecules30153220
APA StyleDams, R., & Ameduri, B. (2025). Essential Per- and Polyfluoroalkyl Substances (PFAS) in Our Society of the Future. Molecules, 30(15), 3220. https://doi.org/10.3390/molecules30153220






