Abstract
Recently, the design and fabrication of novel electrode materials for electrochemical and electronic devices have received the widespread attention of the scientific community. In particular, electrochemical sensors and supercapacitors (SCs) involve the use of catalysts, which can enhance the electrochemical reactions at the surface of the electrode. Bismuth tungstate (Bi2WO6) is a cost-effective and efficient electrode material with decent optoelectronic properties and stability. The properties of Bi2WO6 can be improved by incorporating carbon-based materials, and the resulting composite may be a promising electrode material for electrochemical sensing and SCs. As per the available reports, Bi2WO6 has been combined with various nanostructured and conductive materials for electrochemical sensing and SC applications. This review discusses synthetic methods for the preparation of Bi2WO6. Progress in the construction of hybrid composites for electrochemical sensing and SC applications is reviewed. The Conclusion section discusses the role of electrode materials and their limitations with future perspectives for electrochemical sensing and SCs. It is believed that the present review may be useful for researchers working on Bi2WO6-based materials for electrochemical sensing and SC applications.
1. Introduction
Previously, nanostructured materials have received enormous interest for a variety of optoelectronic applications, such as electrochemical sensors [1], photocatalytic [2], optical [3], energy storage [4] and photovoltaics [5]. Nanostructured materials possess decent conductive properties, electrocatalytic properties, stability, and efficient specific surface area, which makes them desirable materials for such optoelectronic applications [6,7,8]. Semiconducting metal oxides are stable electrode materials for various electrochemical applications such as energy storage [9,10] and sensors/biosensors [11,12]. In particular, bismuth tungstate (Bi2WO6) is a semiconducting metal oxide and exhibits excellent physicochemical properties, such as photoluminescence [13,14], photocatalysis [15,16], pyroelectric [17], nonlinear dielectric induction [18], piezoelectric [19], and ferroelectric [20]. In previous years, various methods were utilized for the formation of Bi2WO6, including the solvothermal method [21,22], co-precipitation method [23], solid phase preparation method [24], ion exchange method [25], sol–gel method [26], ultrasonic spray pyrolysis method [27], hydrothermal method [28], ultrasonic chemical method [29], electro-spinning method [30], and microwave method [31]. It is well known that each method has its own limitations, disadvantages, and advantages. Bi2WO6 formed via different methods has different surface morphologies and particle sizes [32]. Thus, it is worth mentioning that the properties of Bi2WO6 can be tuned by employing a suitable synthetic method. In previous years, Bi2WO6 and its composites were explored in catalysis [33], energy conversion [34], gas sensors [35], dye degradation [36], hydrogen production [37], oxygen evolution reactions [38], CO oxidation [39], adsorption [40], and biomedical [41] applications. It is believed that Bi2WO6-based materials may be efficient electrode materials for the development of electrochemical sensors and energy storage devices.
At present, energy consumption has been significantly enhanced due to growth in the global population and the modernization of society [42]. Fossil fuels are extensively utilized to supply energy but have some limitations, such as limited abundance and the contribution of fossil fuels to environmental pollution [43]. Therefore, renewable energy-based technologies should be developed by exploring innovative approaches and strategies. In this regard, various energy technologies were developed, with supercapacitors (SCs) bridging the gap between batteries and traditional capacitors [44]. SCs have the potential to store and release energy within a short time, making them a desirable candidate for energy storage applications [45]. Numerous materials, such as transition metal oxides, polymers and semiconducting materials, are used for electrochemical applications [1,4]. It was observed that the utilization of new electrode materials for the fabrication of electrochemical devices is of particular importance. In this regard, Bi2WO6 emerged as a promising electrode material for sensing and SC applications. It is understood that Bi2WO6 offers several advantages, including environmental friendliness, decent stability, redox properties, and electrocatalytic properties. The incorporation of Bi2WO6 with other conductive materials also exhibits improved synergistic interactions and conductive properties, with more electrochemically active sites for redox reactions. Thus, it can be concluded that Bi2WO6 is a promising electrode material for electrochemical applications. Bi2WO6-based materials with semiconducting metal oxides, MXenes, LDH, rGO, etc., were utilized for electrochemical sensors and SC applications.
In this review, we report the progress in the design and construction of Bi2WO6-based materials with MXenes, semiconducting metal oxides, LDH, carbon nanotubes, rGO, etc., for sensing and SC applications. The examined literature on Bi2WO6-based materials for electrochemical sensing and SCs is shown in Chart 1a and Chart 1b, respectively. The reported literature on the synthesis of Bi2WO6 is summarized in Chart 1c. It can be observed that numerous reports are available on the synthesis of Bi2WO6, but limited reports are available on the fabrication of Bi2WO6-based electrochemical sensors and SCs.
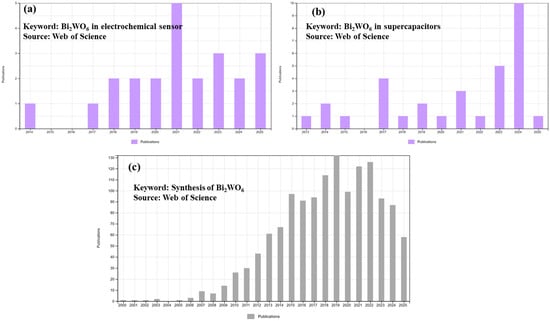
Chart 1.
Bar graph shows reports on Bi2WO6-based (a) electrochemical sensors and (b) SCs. (c) Bar graph shows reports on the synthesis of Bi2WO6. Source: Web of Science.
2. Synthesis Methods
In the last few years, various methods, including electrochemical, hydrothermal, solvothermal, microwave, and sol–gel, were utilized for the preparation of Bi2WO6 and its composites. In this section, we briefly discuss the synthesis of Bi2WO6-based materials using different methods.
2.1. Hydrothermal Method
The hydrothermal method needs an autoclave/sealed container to improve the reaction between tungsten and bismuth precursors using water as a solvent. In a previous report, flower-like Bi2WO6 was synthesized through the hydrothermal method [46]. The bismuth nitrate and sodium tungstate precursors were dissolved in Milli-Q water and transferred to the autoclave system. The autoclave reactor was tightly sealed and kept at 160 °C for 12 h in a furnace, which yielded flower-like Bi2WO6. In contrast, the authors also observed that hydrothermal treatment at 200 °C for 24 h yielded Bi2WO6 NPs. It is clear that the morphology of Bi2WO6 can be tuned by optimizing the temperature, pH and reaction time. In another study [47], nickel (Ni)-doped Bi2WO6 was prepared through the hydrothermal method, possessing a hydrangea-like surface structure. The hydrothermal treatment at 160 °C for 4 h yielded Ni-doped Bi2WO6. The effects of pH were also studied by Wang et al. [48] to obtain Bi2WO6 NPs. However, this study used a relatively higher temperature of 200 °C for 20 h. It is noted that the hydrothermal method may be considered ecofriendly if the reaction is carried out at less than 100 °C with reduced time. But the abovementioned study involved 200 °C for 20 h, which is the major limitation for practical applications and scalability. Li et al. [49] reported the synthesis of Bi2WO6 using the hydrothermal method, which involves a relatively low temperature of 150 °C (time = 6 h). Gao et al. [50] utilized the hydrothermal method for the preparation of copper (Cu)-doped Bi2WO6. The authors also optimized the pH of the reaction solution, and hydrothermally grown Cu-doped Bi2WO6 exhibited a flower-like surface morphology under optimized conditions. Metal precursors are dissolved in water, and the pressure in the hydrothermal reactor facilitates the dissolution of precursors in solvents and promotes the nucleation process of the preparation of Bi2WO6. The hydrothermal method has various advantages, such as tuning the reaction time, pH, concentration of precursors, and temperature, and the high pressure in the autoclave has a significant role in optimizing the surface morphology of Bi2WO6.
2.2. Solvothermal Method
The solvothermal method is a similar approach for the preparation of nanostructured materials, except for the use of different solvents. In hydrothermal methods, water is utilized as a solvent, whereas the solvothermal method involves the use of solvents such as ethylene glycol. In a published research article, the solvothermal method was explored for the fabrication of a titanium dioxide (TiO2) nanotube (NT)/Bi2WO6 composite [51]. Boron (B)-doped Bi2WO6 was obtained through the solvothermal method by utilizing ethylene glycol (EG) as a solvent [52]. The temperature for the solvothermal treatment was fixed at 433 K for 12 h. In another study [53], it was found that the solvothermal treatment of bismuth nitrate pentahydrate and sodium tungstate dehydrate in the presence of an EG solvent at 160 °C for 24 h yielded a hollow-shaped Bi2WO6 structure. Wu et al. [54] also explored novel strategies such as the microwave-assisted solvothermal approach towards the fabrication of Bi2WO6 nanosheets. The working principle for the solvothermal method is similar to the hydrothermal method. Thus, it is clear that the solvothermal method also offers several advantages, such as the optimization of pH, temperature, precursor concentration, and reaction time, to tune the surface morphology of the nanostructured materials.
2.3. Electrochemical Method
Electrodeposition, which is also called the electrochemical method, has been explored for the preparation of nanostructured materials and polymers. Zhu et al. [55] utilized the electrochemical method for the preparation of a Bi2WO6/rGO composite using a three-electrode assembly. The authors used a −1.2 V potential for the electrodeposition process. Indium tin oxide (ITO) was used as a working electrode substrate, whereas platinum foil was adopted as a counter electrode, with silver/silver chloride (Ag/AgCl) as a reference electrode. However, the calcination process involved a high sintering temperature of 420 °C for 1 h, which is a drawback for this study. The electrochemical method has various advantages, including low temperature, precise control over composition, formation of uniform films, cost-effectiveness, direct deposition on substrate, in situ oxidation and selective deposition. Orimolade et al. [56] proposed the electrodeposition method followed by a calcination process for the preparation of Bi2WO6. Therefore, future research may focus on the fabrication of Bi2WO6 films on conductive substrates through the electrochemical method by avoiding the calcination process.
2.4. Sol–Gel Method
Previously, Zhang et al. [57] adopted the sol–gel method for the synthesis of Bi2WO6. In brief, bismuth nitrate and citric acid were dissolved in distilled water, stirring the reaction solution at 80 °C, and ethylene diaminetetraacetic acid (EDTA) ammonia solution was added, followed by the addition of ammonium tungstate. The obtained colorless transparent aqueous solution was evaporated at 80 °C and formed dark-colored polymeric precursors/citric gels. The calcination at 350 °C for 4 h yielded a Bi2WO6 powder sample. A further study indicated that the sol–gel method can be used for the preparation of Bi2WO6/graphene thin films, which exhibited excellent catalytic properties for nitric oxide oxidation [58]. Mesoporous Bi2WO6 sheets were also prepared by Han et al. [59] by adopting the sol–gel method. The synthesized material showed a high surface area of 36.7 m2 g−1, with a pore volume of 0.07 cm3 g−1. Therefore, Bi2WO6 displayed higher catalytic activity for photocatalytic applications. It is also understood that the sol–gel method also has numerous advantages, including homogeneous mixing, controlled composition, large-scale production, scalability, cost-effectiveness and coating capability.
2.5. Microwave Method
In 2023, the microwave method was adopted for the preparation of Bi2WO6 [60]. The microwave method involves the use of microwave irradiation to heat the precursor solution. A flower-like Bi2WO6 microstructure was also prepared using the rapid microwave-assisted method [61]. The authors also prepared a Bi2O3/Bi2WO6 composite using the microwave method. A Bi2WO6/Bi2S3 heterojunction was also developed by Liu et al. [62] for the reduction of chromium ions using the photocatalytic method. They suggested that the microwave method can also be used for the preparation of hybrid composite materials. It is also worth mentioning that the microwave method has several advantages, such as a shorter reaction time, fast heating process, uniform heating, and reproducibility. However, the large-scale production of Bi2WO6 via the microwave method is limited.
3. Progress in Electrochemical Sensors
Electrochemical sensors are the most promising next-generation devices, offering several advantages towards the monitoring of environmental pollutants and biomolecules. Electrochemical sensors can be utilized for environmental monitoring and clinical/healthcare systems. The selection of a suitable electrode modifier is of great significance for the development of highly sensitive and selective electrochemical sensors. In this context, a cost-effective and promising electrode material (Bi2WO6) was explored for electrochemical sensing applications. Peng et al. [63] explored the potential application of hydrothermally grown Bi2WO6 nanoplates (NPts) for the simultaneous detection of diethylstilbestrol (DES) and bisphenol A (BPA). The formation of the Bi2WO6 phase was authenticated using the X-ray diffraction (XRD) method, whereas its morphological features were evaluated via scanning electron microscopy (SEM). It is well understood that DES and BPA are environmental pollutants; therefore, the detection of DES and BPA is necessary. Electrochemical impedance spectroscopy (EIS)-based investigations revealed that a Bi2WO6 NPts-modified carbon paste electrode (CPE) exhibits a low charge transfer resistance (Rct) value compared to the bare CPE. It suggests that Bi2WO6 NPts-modified CPE possesses higher electrical conductivity and electrocatalytic properties, which may be ascribed to the presence of Bi2WO6 NPts on the CPE surface. The cyclic voltammetry (CV) results shows that diffusion plays a crucial role for the electrochemical determination of DES and BPA. Differential pulse voltametric (DPV) analysis further suggested that redox peak current responses for DES and BPA linearly increase with increasing concentration. Thus, these authors were able to obtain limits of detection (LOD) of 15 nM and 20 nM for the sensing of DES and BPA, respectively. The spike approach-based studies indicate that DES and BPA can be recovered from milk samples. Liu et al. [64] adopted the hydrothermal synthetic technique for the fabrication of three-dimensional (3D) Bi2WO6. The SEM results shows that the prepared Bi2WO6 is composed of flower-like microspheres (MSs), which were further used to immobilize horseradish peroxidase (HRP) for the construction of a hydrogen peroxide (H2O2) sensor. H2O2 is widely used in various industries, but its toxic effects on human health motivated the researcher to develop electrochemical sensors. The immobilization process is described in Figure 1a. The flower-like MSs structure of the prepared Bi2WO6 was confirmed through SEM analysis, as shown in Figure 1b. The immobilization of HRP with Bi2WO6 MSs was also confirmed, as seen in the SEM image (Figure 1c). The nafion/HRP/3D-Bi2WO6 was coated on a glassy carbon electrode (GCE) to determine the H2O2 using the amperometry (AMP) technique. The AMP response of the nafion/HRP/3D-Bi2WO6/GCE with the spike of different H2O2 concentrations is presented in Figure 1d. It was observed that the current response linearly increased with a spike in H2O2 (Figure 1e). The stability for 30 days was also checked for long-term applications, and further studies revealed good reproducibility for the detection of H2O2. The H2O2 recovery in real samples was also satisfactory, revealing the potential of the proposed nafion/HRP/3D-Bi2WO6/GCE for practical applications.
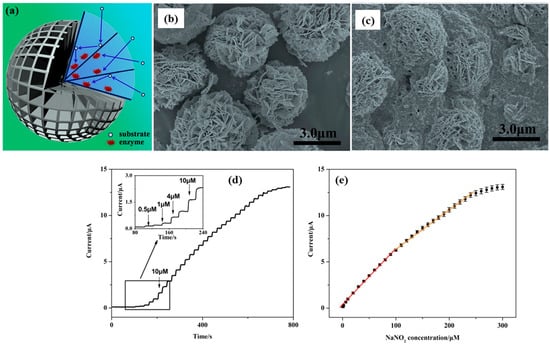
Figure 1.
(a) Schematic description for 3D-Bi2WO6 MSs immobilizing HRP. SEM image of (b) 3D-Bi2WO6 MSs and (c) Nafion/HRP/3D-Bi2WO6 MSs. AMP response of the modified electrode for H2O2 sensing (d) and corresponding linear calibration plot (applied potential = −0.35 V) (e). Reproduced with permission [64].
In other previous work [65], the voltametric determination of Sudan I was also reported using Bi2WO6 nanosheet (NS)-modified GCE. The formation of Bi2WO6 NS was checked using Raman spectroscopy, SEM, XRD, energy-dispersive X-ray spectroscopy (EDX) and Fourier transform infrared (FTIR) spectroscopy. The electrochemical oxidation of Sudan I involves a two-proton/two-electron transfer mechanism and diffusion-controlled process. The LOD of 2 nM, sensitivity of 3.0563 µA µM−1 and linear range (LR) of 0.02 µM to 114.6 µM were achieved for Sudan I sensing. The authors also recovered Sudan I from food samples, including chili powder and apple juice. The dual-mode electrochemical/photoelectrochemical detection platform for hydrogen sulfide (H2S) was explored by Xu et al. [66]. In this context, a titanium dioxide (TiO2)-modified Bi2WO6/silver (Ag) heterojunction was developed for the reduction of H2O2. The proposed electrode exhibited an LOD of 60 nM and LR of 0.5 µM to 300 µM, with reasonably good stability and reproducibility. This electrode was also efficient and selective for the determination of H2O2 in the presence of many interfering substances, including selectivity, Na2SO4, Na2S2O3, NaHSO4, Na2SO3, ascorbic acid (AA), l-Cys, dopamine (DA), and 30 μM H2S. The presence of synergistic interactions may be responsible for the improved selectivity and electrochemical performance of the proposed H2O2 sensor. Hu et al. [67] developed a sulfamethoxazole (SMX) electrochemical sensor by exploring Br-terminated 2D Bi2WO6 as a sensing layer. The authors proposed that the prepared Br-terminated 2D Bi2WO6 can be explored in electrochemical sensing and photochemical water treatments. Vitamins are organic compounds and are essential for the operation of the human body. Vitamin deficiency may cause diseases in the human body. Riboflavin (RF), which is also called vitamin B2 (RF-7, 8-dimethyl 10 ribityl-isoalloxazine), plays a vital role in the development of flavin mononucleotide (FMN) and flavin coenzyme, comprising flavin adenine dinucleotides (FADs), which are important for tissue aeration. It is well known that RF can facilitate the transformation of fats, proteins, and carbohydrates to ATP. RF deficiency may cause biological problems such as burning and scorching of the eyes. Therefore, monitoring the level of RF is important. In this regard, Bi2WO6 was prepared, and its morphological characteristics were optimized by using sodium hydroxide (NaOH), polyvinylpyrrolidone (PVP) and a mixture of ternary solvents. The hollow spheres of Bi2WO6 were obtained by employing PVP + NaOH as a solvent [68]. The Bi2WO6 hollow sphere-modified GCE exhibited improved electro-oxidation of RF using the DPV technique. The fabricated RF sensor displayed a reasonable LOD of 3.65 nM, sensitivity of 1.14 µA µM−1 cm−2 and LR of 0.03 µM to 457 µM for the reduction of RF, whereas the oxidation process showed an LOD of 8.9 nM with a sensitivity of 0.95 µA µM−1 cm−2. The presence of hollow spheres acted as a tunnel for the travel of electrons and ions, which enhanced the electrochemical sensing of RF. In another report [69], Bi2WO6 was decorated on a paraffin-deposited graphite electrode (PGE) for the electrochemical determination of AA. AA is an efficient antioxidant and plays a vital role in the human body. Accurately monitoring the level of AA is of great significance. Bi2WO6/PGE shows acceptable electrochemical performance for the detection of AA using CV and DPV techniques, and observations revealed that the fabricated electrode has higher electrochemical activity for AA detection at a pH of 7.0. The current response was also linearly increased, and an LOD of 77.85 mM was obtained, with a sensitivity of 0.26 mM/mA. The LOD of the Bi2WO6/PGE-based AA sensor needs to be further improved by introducing novel strategies or combining Bi2WO6 with other conductive and high-surface-area materials. The misuse of antibiotics such as ampicillin (AMP) is dangerous for humans as their residues can enter the human body through the ecological and food chains and can exert negative effects on human health. Tang et al. [70] reported the fabrication of a novel AMP sensor by exploring the electrochemical properties of a Bi2WO6-based composite. The hydrothermally prepared Bi2WO6 was combined with carboxylated multi-walled carbon nanotubes (MWCNTs-COOH) by employing the ultrasonication method. Other steps involved the immobilization of metal β-lactamases (MBLs) + chitosan (CS) to develop the MBLs/CS/Bi2WO6/MWCNTs-COOH/GCE for the determination of AMP (Figure 2). The constructed MBLs/CS/Bi2WO6/MWCNTs-COOH/GCE showed improved stability, reproducibility, repeatability, selectivity and acceptable recovery of AMP in real samples. The LOD of 0.17 nM and two LR of 0.0007 to 0.05 µM and 0.1 to 10 µM were observed for AMP detection. The sensing mechanism for the electrochemical detection of AMP is explained in Figure 2.
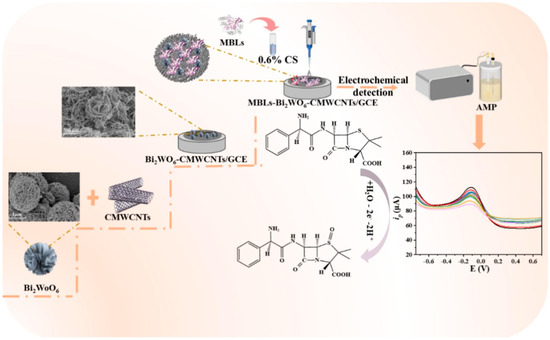
Figure 2.
Schematic graph shows the fabrication of MBLs/CS/Bi2WO6/MWCNTs-COOH/GCE towards the determination of AMP. Reproduced with permission [70].
Another study reported the benign wet-chemical co-precipitation method for the preparation of Bi2WO6 [71]. The prepared Bi2WO6 was characterized by XRD, which confirmed the formation of the desired product and matched with JCPDS number 73-1126. The authors observed that XRD analysis revealed the formation of orthorhombic Bi2WO6. The transmission electron microscopy (TEM) analysis indicated the presence of the polycrystalline nature of the prepared orthorhombic Bi2WO6. In further studies, it was observed that the Bi2WO6-modified electrode has relatively higher electrical conductivity compared to the bare electrode. The authors explored the potential of the Bi2WO6-modified electrode for the simultaneous determination of hydroquinone and resorcinol (HQ and RS) using the CV and DPV methods. The pH of the analyte solution was also optimized, and it was observed that the fabricated electrode is highly active at a pH of 7.0. In high basic or high acidic pH conditions, no significant peak was observed for the detection of HQ and RS. This sensor was also found to be highly stable, selective, and reproducible for the determination of HQ and RS. The real sample studies in tap water and ointment suggested its significance for the real-time monitoring of HQ and RS. Previously, a zinc vanadate-modified Bi2WO6 (Zn3(VO4)2/Bi2WO6) composite was fabricated using the hydrothermal method, as shown in Figure 3a [72]. The prepared Zn3(VO4)2/Bi2WO6 shows decent crystalline nature and was found to be in agreement with JCPDS number 24-1482 and 39-0256 for Zn3(VO4)2 and Bi2WO6, respectively. It was also observed from XRD analysis that Zn3(VO4)2 has a monoclinic phase, whereas Bi2WO6 possesses an orthorhombic crystal structure. The Zn3(VO4)2/Bi2WO6-modified electrode demonstrated an LOD of 1 pM and LR of 1 nM to 5 µM for the determination of cotinine. The proposed cotinine sensor also showed good replicability and stability, as shown in Figure 3b and Figure 3c, respectively.
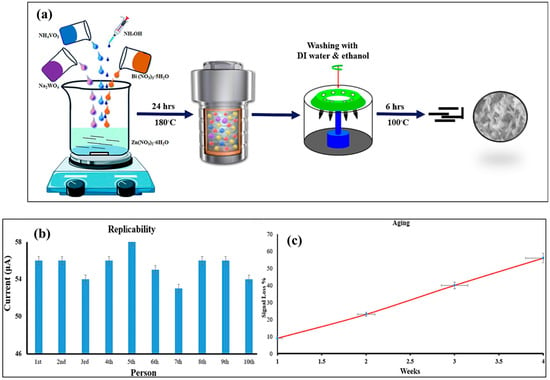
Figure 3.
(a) Schematic graph for the preparation of Zn3(VO4)2/Bi2WO6 composite. (b) Replicability and (c) stability of Zn3(VO4)2/Bi2WO6-based cotinine sensor. Reproduced with permission [72].
A single-step hydrothermal method was explored for the fabrication of a morphologically engineered biomimetic 3D biconcave-shaped Bi2WO6 [73]. The authors used aluminum (Al) foil as a substrate to develop the Bi2WO6-based nicotine electrochemical sensor. The Bi2WO6-modified Al foil-based nicotine sensor exhibited excellent selectivity, LOD of 0.9 nM, sensitivity of 83.23 μA/(μM cm2), and acceptable stability/reproducibility. Another study reported the fabrication of a photoelectrochemical DA sensor using the benign approach [74]. In this connection, a cobalt tetraaminophthalocyanine covalently linked graphene oxide (CoTAPc-GO) composite was fabricated using simple strategies. Furthermore, CoTAPc-GO and Cu-doped Bi2WO6 microflowers (MFs) were combined by employing the ultrasonication method. The obtained material (CoTAPc-GO/Cu-Bi2WO6) was coated on an indium tin oxide (ITO) surface, and its sensing activity for DA was evaluated. The authors obtained an LOD of 7.2 nM, with two LR of 0.05 µM to 5 µM and 5 µM to 250 µM for the detection of DA. Xu et al. [75] reported a photoelectrochemical immunosensor for the determination of amyloid beta (Aβ). The flower-like amorphous structure of Bi2WO6 was obtained, which was further combined with Mn2+-doped cadmium selenide (CdSe). The obtained results exhibited a reasonable LOD of 0.068 pg/mL with LR of 0.2 pg/mL to 50 ng/mL. The flower-like Bi2WO6 was decorated on graphene nanoribbons (GNRs) by employing the microwave synthesis method [76]. The fabrication procedure for Bi2WO6@GNRs is depicted in Figure 4a. The Raman and X-ray photoelectron spectroscopy (XPS) studies confirmed the presence of Bi2WO6 on the GNR surface. Further studies revealed that a Bi2WO6@GNR-modified screen-printed electrode (SPE) has a good selective nature for the determination of HQ and CC. The impedance studies indicate the presence of enhanced electrical conductivity of the constructed Bi2WO6@GNRs/SPE. Therefore, Bi2WO6@GNRs/SPE shows an LOD of 5.31 nM and 7.24 nM for CC and HQ detection, respectively. The proposed sensor was also effective for the detection of CC and HQ in a water sample and face cream sample, respectively. In addition, the proposed sensor exhibited decent selectivity for the determination of CC and HQ in the presence of various interfering substances (Ni+, Cu2+, Cr+, Na+, Zn2+, Cl−, Hg+, Cd+, DA, hydrazine (Hz), nitrobenzene (NBz), 4-nitrophenol (4-NP), 4-aminophenol (4-AP), 2-aminophenol (2-AP), glucose (glu), nitrite, resorcinol (RS), epinephrine, AA, vitamin B6 and vitamin B9). The enhanced performance of the Bi2WO6@GNRs/SPE was ascribed to the presence of synergistic interactions and the high surface area of the electrode material. Verma et al. [77] prepared a plate-like Bi2WO6-modified mesh-like graphitic carbon nitride (g-CN) composite, and rGO was loaded into this prepared hybrid material using the hydrothermal-assisted synthesis method. The authors tuned the percentage of g-CN, and the optimized sample BCN-200/rGO (Bi2WO6/g-CN-200 mg/rGO) exhibited enhanced catalytic properties. The formation of the BCN-200/rGO is shown in Figure 4b. The obtained Bi2WO6/g-CN/rGO composite was explored for the fabrication of an electrochemical sensor towards the detection of 4-NP. The CVs of the g-CN, Bi2WO6, BCN-200 and BCN-200/rGO-modified GCE are shown in Figure 4c.
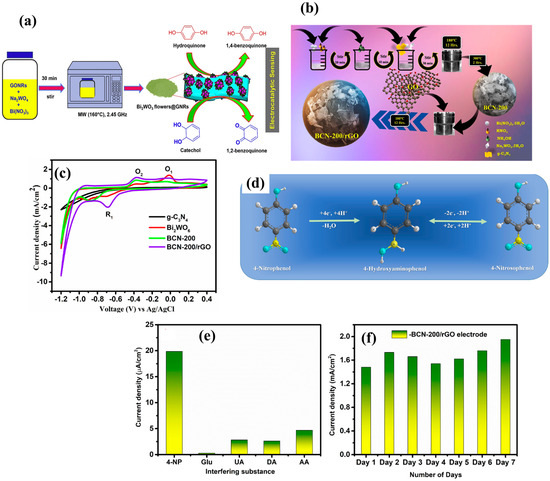
Figure 4.
(a) Schematic representation of the fabrication of Bi2WO6@GNRs for HQ and CC sensing. (b) Schematic representation of the fabrication of BCN-200/rGO. (c) CVs of g-CN, Bi2WO6, BCN-200 and BCN-200/rGO for 4-NP sensing. (d) Mechanism for 4-NP detection. (e) Selectivity and (f) stability test. Reproduced with permission [76,77].
It can be seen that BCN-200/rGO/GCE has a higher redox peak current response for the detection of 4-NP. The mechanism involved in the sensing of 4-NP is shown in Figure 4d. The BCN-200/rGO-modified GCE also showed excellent selectivity and stability for the sensing of 4-NP, as shown in Figure 4e and Figure 4f, respectively. The presence of synergy in the Bi2WO6, g-CN, and rGO enhanced the catalytic behavior of the BCN-200/rGO-modified GCE. Additionally, the fabricated 4-NP sensor delivered an LOD of 14 nM, LR of 0.2 μM to 100 μM and sensitivity of 12.86 μA.μM−1.cm−2. It is well known that 4-Nitroquinoline N-oxide (4-NQO) is an important tumorigenic organic compound, which has adverse effects on the human body. Thus, in a previous report, a Bi2WO6 nanosphere-decorated rGO composite was prepared using the sonication method [78]. The Bi2WO6/rGO composite was further deposited on the GCE surface and its electrochemical activities for the detection of 4-NQO using the AMP method. The proposed electrode shows an LOD of 6.11 nM and excellent detection of 4-NQO in urine and human blood samples. The plasmonic Ag-induced metallic Ag Bi2WO6/TiO2 composite was developed for oxygen/hydrogen evolution and DA electrochemical sensing applications [79]. The CV results show that the current response linearly increases with an increasing concentration of DA. Due to the multifunctional applications of the proposed material in a single report, the authors did not significantly focus on the sensing of DA. Thus, more in-depth studies are required to explore the potential of the above-proposed electrode material for DA sensing applications. In recent years, MXene-based materials have been widely used to enhance the physicochemical properties of seminconducting metal oxides and metal sulfides, etc. The incorporation of MXene materials enhanced the electrical conductivity of the resulting composite materials. In this context, niobium carbide (Nb4C3Tx) MXene was incorporated with Bi2WO6 (as first layer), and salmon ds-DNA was engineered as a second layer/biological recognition element on a conductive substrate [80]. Thus, these authors obtained a decent LOD of 2.8 nM, LR of 0.01 µM to 100 µM, and acceptable recovery of 98.7% to 103.6% for the determination of pemetrexed using Bi2WO6/Nb4C3Tx/ds-DNA/SPE. Another study [81] reported a novel sandwich-type SARS-CoV-2 (COVID-19) nucleocapsid protein immunosensor by employing simple strategies. The Bi2WO6/bismuth sulfide (Bi2S3) composite was utilized as an electrode platform, whereas g-CN/Au NPs/tungsten trioxide (WO3) was adopted for signal amplification. The proposed sensor delivered an LOD of fg/mL. Nitrite has a negative influence on human health and the environment due to its toxic properties. The determination of nitrite is necessary, and a previous report indicated that Co phthalocyanine (CoPc) NPs were covalently linked to Bi2WO6 and nickel tungstate (NiWO4), which can used as sensing layers for the determination of nitrite [82]. The authors observed that CoPc covalently linked to Bi2WO6-modified GCE shows better performance compared to the CoPc covalently linked to NiWO4-modified GCE. An interesting LOD of 0.063 µM to 1.7 µM was observed for the detection of nitrite. Kanamycin (KAN) is a well-known amino glycoside, which has serious negative impacts on human health. In this regard, it is useful to develop the KAN sensor by exploring Bi2WO6/Ti3C2 MXene QDs [83]. The electrostatically driven hydrothermal-assisted-formed Bi2WO6/Ti3C2 MXene QD composite was adopted as a sensing material. The proposed KAN sensor exhibited recoveries in the range of 94.2% to 101.3%, LOD of 0.025 nM, and LR of 0.01 nM to 50 nM. The dual 3D structural Bi2WO6/graphene hydrogel (GH)-modified molecularly imprinted polymer (MIP)-based electrode was developed for the detection of 4-NP in PM2.5 [84]. The fabricated MIPs/Bi2WO6@GH/ITO electrode had an LOD of 5.78 × 10−13 M and LR of 5.0 × 10−12 to 1.0 × 10−7 M for the determination of 4-NP. This enhanced electrochemical performance of the proposed electrode was attributed to the 3D flower-like morphology of Bi2WO6 and porous network of GH. It is proven that hexavalent chromium (Cr) has toxic effects, and exposure to Cr via ingestion or inhalation may cause allergies, affect internal organs, and contribute to cancer. Therefore, it is really of great significance to develop an electrochemical sensor for the monitoring of Cr. In this connection, the solvothermal method was adopted for the preparation of a β-Bi2O3/Bi2WO6/meso-tetraphenylporphyrin (H2TPP) composite on a fluorine-doped tin oxide (FTO) substrate [85]. It was observed that the presence of H2TPP enhances the active site density and provides an efficient surface area for effective adsorption by providing pyrrolic and pyridinic-N atoms to β-Bi2O3/Bi2WO6/H2TPP. The β-Bi2O3/Bi2WO6/5 wt % H2TPP exhibited excellent cyclic stability, sensitivity, reproducibility and an LOD of 8 nM. The electrochemical sensing performances of the various reported electrochemical sensors are summarized in Table 1.

Table 1.
Performance of some previously reported electrochemical sensors.
It can be summarized that the above-discussed electrochemical sensors demonstrated promising electrochemical performance for the determination of various environmental pollutants. It was observed that pristine Bi2WO6 exhibits moderate electrochemical performance in terms of sensitivity. In contrast, the electrochemical performance of pristine Bi2WO6 was significantly enhanced by combining it with conductive materials, such as rGO, MXenes (Nb4C3Tx, Ti3C2), MWCNTs, g-C3N4, NiO, or Ag NPs. It was observed that Bi2WO6/MWCNTs/MBLs/CS exhibits the lowest LOD of 0.17 nM for ampicillin detection due to the presence of synergistic interactions such as high surface area and more abundant active sites. On the other side, the Bi2WO6/g-C3N4/rGO composite-based electrode demonstrates enhanced sensitivity of 12.86 μA μM−1 cm−2 for 4-NP, which can be assigned to the high electron mobility and surface area of the electrode material. It can be understood that Bi2WO6-based hybrid composites with MXenes and carbonaceous materials (rGO or CNTs) are promising electrode materials compared to the other hybrid composites for electrochemical sensing applications.
4. Progress in SCs
The design and fabrication of novel electrode materials are of great importance for SCs. Bi2WO6-based composites were significantly explored for the construction of SCs. Herein, we compile the progress in the preparation of Bi2WO6-based materials for the development of next-generation SCs.
4.1. Bi2WO6-Based Materials for SCs
In a previous report, Bi2WO6 NPs were synthesized via the sonochemical approach to explore an ecofriendly and cost-effective SC device [86]. The electrochemical performance of the Bi2WO6 NPs was evaluated in different aqueous electrolytes (1 M NaOH, 1 M sodium sulfate (Na2SO4), and 1 M lithium hydroxide (LiOH)). It was revealed that the KOH electrolyte was effective compared to the other electrolytes. Thus, the authors also used 1 M and 6 M KOH solutions for further electrochemical studies with higher ionic mobility, smaller hydration sphere radius and lower equivalent series resistance. The galvanostatic charge–discharge (GCD) studies show a specific capacitance of 608 F/g, which can be obtained for Bi2WO6 in 1 M KOH at a current density of 0.5 mA/cm2. The specific capacitance of 304 F/g at 3 mA/cm2 was also observed in a 1 M KOH electrolyte system. This study suggested that Bi2WO6 NPs can be explored as negative electrode modifiers for SCs in a 1 M KOH electrolyte. In another study [87], Bi2WO6 was also synthesized using the hydrothermal method. The authors used different times (3, 6, 12 and 24 h) for the preparation of Bi2WO6. The synthesized Bi2WO6 samples at 3, 6, 12 and 24 h were named BWO1, BWO2, BWO3 and BWO4, respectively. The optimized studies show that Bi2WO6-based SCs exhibit a specific capacitance of 363 F/g in neutral electrolytes and 5000 cyclic stabilities. The 3D Bi2WO6 globules were vertically grown in nickel foam (NF) electrodes using benign conditions [88]. The 3D Bi2WO6-modified NF electrode was utilized as a negative electrode, and areal capacitance of 1.120 F/cm3 was observed at a current density of 1.5 mA/cm2 with excellent cyclic stability of 1000. Shembade et al. [89] reported the hydrothermal preparation of mesoporous spherical nanoflower-like Bi2WO6. The Bi2WO6 was deposited on NF, and its electrochemical performance for SCs was examined using CV and GCD. It was found that Bi2WO6 NFs exhibit the presence of an orthorhombic phase in which atoms are present in a regular arrangement. The optimized electrode (Bi2WO6/NF-2 = BWO-2) showed a specific capacitance of 647 F/g in a 1 M KOH electrolyte, including stability of 5000 cycles. In another study, various nanostructures of Bi2WO6 were obtained by exploring hydrothermal treatment under various pH conditions [90]. pH 1-, 7- and 11-based synthesis exhibits the formation of Bi2WO6 with flower-like hierarchical structures (average diameter = 7 µm), irregular flake-like structures (average thickness = 90 nm) and uniform spherical structures (average size = 85 nm), respectively. The flower-like hierarchical, flake-like and spherical-shaped Bi2WO6 demonstrated specific capacitance of 255 F/g, 214 F/g, and 412 F/g, respectively. It can be noted that Bi2WO6 spheres exhibited the highest electrochemical performance for SCs. Karnan et al. [91] pioneered one-pot preparation of Bi2WO6 nanostructures for SCs. A benign ultrasonic-assisted synthesis method was adopted to fabricate bare Bi2WO6 (BWO-bare). The authors also heated Bi2WO6 at 600 °C, which can be labeled as BWO-600. Figure 5 illustrates the formation of BWO-600, and the obtained material was characterized by XRD and SEM to investigate its phase purity and morphological features. The SEM analysis revealed that BWO-600 consists of a nanostructure with a diameter of 50 to 310 nm. In contrast, BWO-bare does not show evident clusters. Under a 6 M KOH electrolyte system, the BWO-600-based electrode shows specific capacitance of 614 C/g in a three-electrode system, whereas the specific capacity of 376 C/g was observed in asymmetric SCs.
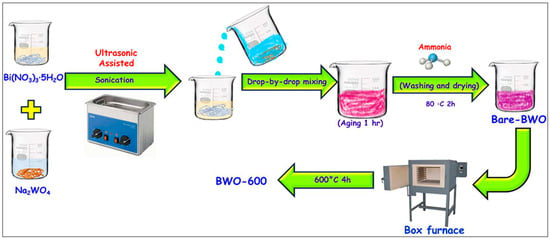
Figure 5.
Schematic graph presenting synthetic procedure for BWO-600. Reproduced with permission under license CC-BY-NC-ND 4.0 [91].
Other work adopted the solution combustion method for the preparation of Bi2WO6 NPs [92]. The authors used jackfruit extract as fuel in the synthesis of Bi2WO6 NPs. Nithya et al. [93] proposed the fabrication of the polyvinylpyrrolidone (PVP)-assisted synthesis of Bi2WO6 NPs through the sonochemical method. The concentration of the PVP was tuned in the range of 0.05 to 0.2 g. The optimized results for PVP-assisted Bi2WO6 demonstrated an acceptable specific capacitance value of 462 F/g, which is higher than pure Bi2WO6 (304 F/g) at a current density of 3 mA/cm2. In another study, Nithya et al. [94] also proposed the preparation of the surfactant-assisted sonochemical synthesis of Bi2WO6 NPs, which exhibited specific capacitance of 708 F/g under the optimized conditions.
4.2. Bi2WO6-Based Compoiste Materials for SCs
The supercritical water method was also adopted for the fabrication of a Bi2WO6/rGO hierarchical composite [95]. The supercritical water approach requires much less time to complete the reaction for the preparation of the Bi2WO6/rGO composite. The proposed electrode material exhibited specific capacity of 1158 C/g at 3 A/g in a 6 M KOH electrolyte. A novel electrode material, i.e., a bismuth vanadate (BiVO4)/Bi2WO6 microsphere composite, was also prepared using the microwave-assisted hydrothermal method [96]. It was stated that decoration of the BiVO4 NPs onto the Bi2WO6 NSs may prevent the restacking of Bi2WO6 NSs in a disorganized manner and facilitate the formation of a mesoporous BiVO4/Bi2WO6 hierarchical microsphere composite. It was also found that the prepared BiVO4/Bi2WO6 hierarchical microsphere composite has higher structural stability, larger surface area and enhanced electron/ion migration. Thus, due to the above promising properties, an interesting specific capacitance of 616.8 F/g was obtained at 1 A/g and a stability of 4000 cycles with 87% retention using BiVO4/Bi2WO6 hierarchical microspheres as an electrode material. The synthesis of the Bi2WO6/ZnO/rGO composite is described in Figure 6 [97]. The Bi2WO6/ZnO/rGO composite was prepared via multiple steps. It was believed that the proposed ternary material may be a promising electrode modifier for SC applications. The ratio of the rGO was optimized to enhance the electrochemical activity of the Bi2WO6/ZnO/rGO composite for SC applications. The authors achieved a specific capacitance of 254.34 F/g at 2 A/g under optimized conditions. The presence of decent electrical conductivity, improved electrochemical activity and synergistic interactions enhanced the performance of the Bi2WO6/ZnO/rGO composite-based SCs.
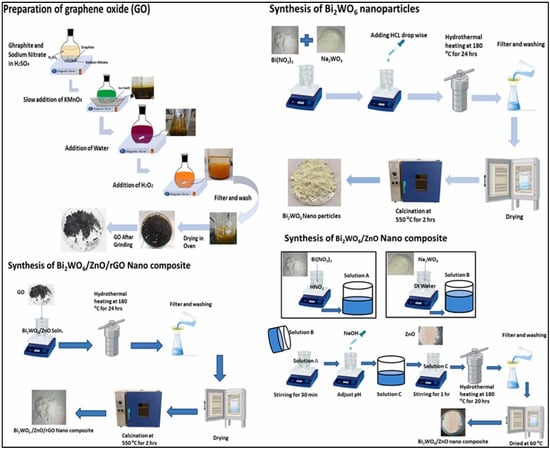
Figure 6.
Schematic representation of the formation of the proposed Bi2WO6/ZnO/rGO composite. Reproduced with permission [97].
Qadeer et al. [98] proposed that the electrochemical properties of the pristine Bi2WO6 can be further improved by combining it with titanium disulfide (TiS2). Therefore, the Bi2WO6/TiS2 composite was obtained via the hydrothermal method. The morphological characteristic properties of the Bi2WO6/TiS2 composite were checked using SEM technique, which revealed that TiS2 nanoplates are uniformly dispersed across the hierarchically opened surface of Bi2WO6. Thus, it was observed that the resulting Bi2WO6/TiS2 composite exhibited improved porosity and specific surface area, which may facilitate the electron and ion transport. The GCD studies displayed specific capacitance of 453.3 F/g, 523.7 F/g and 1153.3 F/g for TiS2, Bi2WO6, and Bi2WO6/TiS2 composites, respectively. Another previous study proposed the incorporation of tin oxide (SnO2) to the Bi2WO6 nanoflowers (NFs) using simple strategies, as shown in Figure 7 [99].
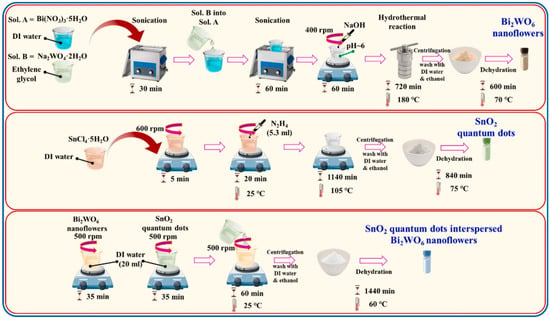
Figure 7.
Schematic graph shows the fabrication of SnO2-Bi2WO6 NFs. Reproduced with permission [99].
The SnO2 QD-interspersed Bi2WO6 NFs were utilized as an electrode material, with a specific capacitance of 812 and 741 F/g at 7 A/g for Bi2WO6/SnO2 QDs and Bi2WO6-based electrodes, respectively. The improved specific capacitance for the Bi2WO6/SnO2 QD-based electrode may be ascribed to the quantum dot size of SnO2 and the presence of synergistic interactions. This material offers great potential for SC applications. Nickel oxide (NiO) is a promising semiconducting metal oxide, with reasonably good electrochemical properties. The Bi2WO6/NiO composite was adopted as an electrode modifier for SCs [100]. The capacitive diffusion and Nyquist plots of Bi2WO6, NiO and Bi2WO6/NiO composite-based electrodes are shown in Figure 8a and Figure 8b, respectively. It can be seen that the Bi2WO6/NiO composite has decent electrical conductivity. The GCD curves of the Bi2WO6, NiO and Bi2WO6/NiO composite at different current densities are depicted in Figure 8c, Figure 8d and Figure 8e, respectively. It can be observed that the prepared materials have higher specific capacity at 2 A/g. The highest specific capacitance for the Bi2WO6/NiO composite-based electrode was attributed to the presence of synergism in the hybrid composite material. The capacitive retention/coulombic efficiency of the Bi2WO6/NiO composite is shown in Figure 8f. Thus, it is also suggested that the Bi2WO6/NiO composite has decent cyclic stability of 2000 for SCs.
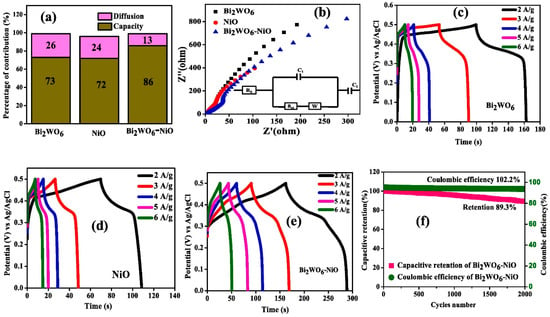
Figure 8.
(a) Capacitive diffusion and (b) Nyquist plots. GCD curves of (c) Bi2WO6, NiO (d) and (e) Bi2WO6/NiO composite. (f) Capacitive retention/coulombic efficiency of Bi2WO6/NiO composite. Reproduced with permission [100].
Zheng et al. [101] proposed a benign one-pot hydrothermal method for the fabrication of a 3D porous Bi2WO6/rGO hydrogel composite. The electrochemical studies revealed that the 3D porous Bi2WO6/rGO hydrogel composite has higher capacitive properties and demonstrates specific capacitance of 268.7 F/g at an applied current density of 0.75 A/g. This material also displayed robust stability of 1000 cycles at 3 A/g, with capacitance retention of 81%. This improved performance may be due to the synergistic interactions, unique 3D porous rGO hydrogel-supported Bi2WO6 architecture, which provided good electrical conductivity and more channels for ion diffusion. Zhang et al. [102] also adopted a nano Bi2WO6-decorated rGO composite via the hydrothermal method. The proposed Bi2WO6/rGO composite shows improved electrical conductivity and increased charge transfer channels. Thus, it is expected that the presence of these properties may facilitate the electron transfer, and Bi (III) may oxidize to Bi (IV), which provide higher specific capacitance and stability. Therefore, the authors achieved a specific capacitance of 922 F/g at 3 A/g, which is higher than pristine Bi2WO6 (574 F/g at 2 A/g). The Al-based MOF (Materiaux De Lavoisier = MIL)-53 was prepared via the solvothermal method [103]. It is observed from these studies that MIL-53 (Al) has low conductivity, which restricts its potential applications in energy storage systems. Therefore, MIL-53 (Al) was incorporated with Bi2WO6, and its properties were evaluated via the CV, EIS and GCD methods. The MIL-53 (Al)/Bi2WO6-based electrode displayed a larger surface area and improved conductive nature. Therefore, an enhanced specific capacitance value of 220 F/g was observed at 0.5 A/g in the 3 M KOH electrolyte with excellent cyclic stability (5000 cycles; retention capacitance = 78%). Sheoran et al. [104] reported a novel electrode modifier comprising a carbon black (CB)-anchored bismuth tungstate-aniline complex composite ((Bi2(WO4)3/Aniline/CB) (BTACB)). The authors reported a specific capacitance of 306 F/g at 1 A/g, which can be attributed to the synergism between aniline complex, CB and bismuth tungstate, which provide larger surface area and acceptable conductivity. A sandwich-like heterostructure of the Bi2WO6/g-CN composite was developed using benign approaches [105]. This fabricated Bi2WO6/g-CN composite was adopted as an electrode modifier for energy storage applications, and the obtained results showed specific capacitance of 158 mF/g at 1 mA/cm2. It is well understood that 3D porous structures may promote the electron transport process and improve the electrochemical capacitive properties of the SCs. In this regard, the 3D aerogel/poriferous structure of Bi2WO6 sheets was combined with graphene NSs via benign hydrothermal treatment [106]. It was stated that the 3D multi-hole structure of the prepared hybrid aerogel may provide high surface area and promote electron transport and ion transmission. This may decrease the internal electrical resistance and enhance the capacitive properties of the fabricated electrode. The Bi2WO6/graphene aerogel composite demonstrated specific capacitance of 714 F/g at 4 A/g. In another previous report [107], MIL-53 (Al) and Bi2WO6 were obtained via the hydrothermal route, whereas the MIL-53 (Al)/Bi2WO6 composite was fabricated using the impregnation method. Due to the lower equivalent series resistance and synergistic interactions, the MIL-53 (Al)/Bi2WO6 composite demonstrated specific capacitance of 554 F/g at 0.5 A/g. Due to the high surface area and high porosity, copper MOF (Cu-MOF) has been utilized in the fabrication of hybrid materials for SCs. In this connection, a Cu-MOF/Bi2WO6 binary hybrid composite was developed through the solvothermal method [108]. The weight percentage was varied to optimize the electrochemical performance of the Cu-MOF/Bi2WO6 binary hybrid composite for SCs. The samples with different weight percentages were labeled as CuBW20, CuBW50 and CuBW80. 3 M KOH was explored as a suitable electrolyte, and CuBW80 demonstrated excellent specific capacitance of 1137 F/g, specific power of 4000 W/kg, specific energy of 11 Wh/kg at a current density of 0.5 A/g. This optimized electrode material was also suitable for stability of 1000 cycles. In another study [109], Bi2O3/Bi2WO6 (BO/BWO) and Au-decorated Bi2O3/Bi2WO6 (Au-BO/BWO) composites were developed through the hydrothermal method. The electrochemical performance of BO/BWO and Au-BO/BWO was evaluated via CV analysis, as shown in Figure 9a. It can be seen that A-BO/BWO displayed higher electrochemical activity compared to BO/BWO. Similarly, A-BO/BWO showed improved capacitance and suggested its potential for SCs (Figure 9b). The specific capacitance versus scan rate graph is shown in Figure 9c, whereas the specific capacitance versus current density graph is depicted in Figure 9d. The BO/BWO-based electrode demonstrated specific capacitance of 148.81 F/g, whereas the highest specific capacity of 495.05 F/g for the A-BO/BWO-based electrode was obtained at 1 A/g in a 1 M KOH electrolyte system. The improved specific capacity of the A-BO/BWO-based electrode may be attributed to the presence of Au NPs, which improved electrical conductivity and decent electrical contacts between the electrolyte and electrode, which may have improved the effective surface area. The multi-functional polyaniline (PANI)-wrapped Bi2WO6 2D nanoplate/BiOCl (BW-BIC-PANI) composite was also prepared by Kavinkumar et al. [110].
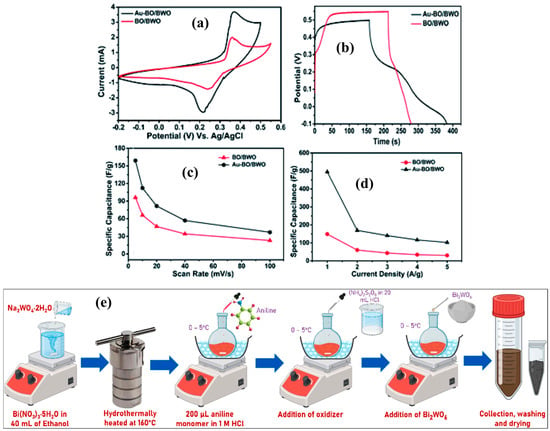
Figure 9.
(a) CV curves of BO/BWO and Au-BO/BWO at scan rate of 10 mV/s. (b) GCE curves of BO/BWO and Au-BO/BWO at 1 A/g. (c) Specific capacitance versus scan rate and (d) specific capacitance versus current density graphs. Reproduced with permission [109]. (e) Schematic picture shows the preparation of BW-BIC-PANI composite. Reproduced with permission [110].
The fabrication of the BW-BIC-PANI composite via the hydrothermal method followed by the chemical oxidative polymerization method is shown in Figure 9e. The authors proposed that a specific capacitance of 156 F/g can be obtained at 1 A/g by employing a BW-BIC-PANI200-based electrode. The presence of synergistic interactions enhances the electrochemical capacitive properties of the BW-BIC-PANI200 for SCs. The hydrothermal method was also utilized for the preparation of Ag-doped Bi2WO6 (ABW) [111]. Furthermore, ABW was incorporated with MXene to fabricate the ABW@MXene composite via the sonication method. The specific capacitances of 870 F/g and 1420 F/g for the ABW@MXene-based electrode were observed using CV and GCD, respectively. In contrast, MXene exhibited a specific capacitance value of 200 F/g and 413 F/g using CV and GCD, respectively. The improved performance for ABW@MXene was attributed to the presence of synergistic interactions between ABW and MXene. Liu et al. [112] prepared Bi2S3 NFs, which comprised 1D NRs. The Bi2S3 NFs were obtained through a topotactic transformation approach. The Bi2WO6 NFs were used as a precursor template for the preparation of Bi2S3 NFs. The Bi2WO6-derived Bi2S3 NFs exhibited decent specific capacitance and electrochemical stability for SCs. The electrochemical capacitive performance of the Bi2WO6-based materials is summarized in Table 2.

Table 2.
Electrochemical performance of previously reported SCs based on Bi2WO6 and its hybrid composites as electrode material.
5. Conclusions, Limitations and Perspectives
It is concluded that Bi2WO6 has been widely used for the fabrication of working electrodes for electrochemical sensing and SC applications. Bi2WO6 has low conductive properties, which can be enhanced by preparing Bi2WO6-based hybrid composites with conductive materials, such as carbon-based materials or MXenes. The Bi2WO6-based composites exhibited excellent sensing performance for the detection of various analytes, including DES, H2O2, H2S, Sudan I, ampicillin, 4-NP, pemetrexed, etc. It was suggested in previous reports that DPV is the most widely used sensing technique for the determination of environmental pollutants. Bi2WO6 may exhibit a smaller hydration sphere radius, higher ionic mobility, and lower equivalent series resistance, which benefit researchers by producing a higher specific capacity for SCs. The presence of Bi3+ with 6s2 lone pair electrons may improve the abundant active sites and electronic conductivity. Thus, Bi2WO6-based composites also exhibited excellent specific capacitance for SC applications. Despite several advantages, some challenges exist, which are mentioned below.
- Pristine Bi2WO6 suffers from low conductivity, which needs to be addressed.
- Although several efforts were made to enhance the conductivity of Bi2WO6 by combining it with conductive supports such as MXene or rGO, synthetic procedures are not cost-effective for practical applications.
- DPV is a more sensitive technique, but, in some cases, the presence of similar interfering substances affects the selectivity of electrochemical sensors.
- Real sample studies are conducted using the standard addition method, which exhibits accepted recovery. However, we believe that such methods are not reliable for real sample analysis. Thus, new methods should be developed.
- The depth mechanism for sensing applications should be studied.
- For SCs, stability is the major concern, which needs to be properly studied in depth.
We believe that future studies may involve the following key points for further research.
- Green and cost-effective methods need to be developed for scalability and practical applications.
- Electrochemical sensors can be combined with machine learning technology for the accurate analysis of environmental pollutants.
- Electrochemical sensors can be explored in smartphone-based devices for environmental monitoring.
- Flexible and wearable electrochemical sensors can be developed for environmental monitoring.
- MXene is a new material, and its potential is not fully explored. Thus, Bi2WO6 and MXene-based materials should be studied in depth, and a cost-effective and acid etching-free method should be developed for the transformation of MAX to MXene.
Author Contributions
Conceptualization, K.A.; writing—original draft preparation, K.A.; writing—review and editing, D.K.; and T.H.O.; supervision, T.H.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by project number RS-2025-02317758.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were generated. Authors are unable to provide data.
Acknowledgments
This research was supported by the Korea Basic Science Institute (National research Facilities and Equipment Center), grant funded by the Ministry of Education (RS-2025-02317758).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Al-Kadhi, N.S.; Hefnawy, M.A.; Alamro, F.S.; Pashameah, R.A.; Ahmed, H.A.; Medany, S.S. Polyaniline-Supported Nickel Oxide Flower for Efficient Nitrite Electrochemical Detection in Water. Polymers 2023, 15, 1804. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, F.; Zhong, Y.; Xiao, T.; Yu, Q.; Zhu, X.; Feng, W.; Qi, Z. Preparation and Photocatalytic Performance of In2O3/Bi2WO6 Type II Heterojunction Composite Materials. Molecules 2024, 29, 4911. [Google Scholar] [CrossRef]
- Yoon, J.; Hong, W.-K.; Kim, Y.; Park, S.-Y. Nanostructured Vanadium Dioxide Materials for Optical Sensing Applications. Sensors 2023, 23, 6715. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, L. Nanostructured Materials for Energy Storage and Conversion. Nanomaterials 2022, 12, 1583. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K. Nanostructured Materials for Solar Cell Applications. Nanomaterials 2022, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Harish, V.; Tewari, D.; Gaur, M.; Yadav, A.B.; Swaroop, S.; Bechelany, M.; Barhoum, A. Review on Nanoparticles and Nanostructured Materials: Bioimaging, Biosensing, Drug Delivery, Tissue Engineering, Antimicrobial, and Agro-Food Applications. Nanomaterials 2022, 12, 457. [Google Scholar] [CrossRef]
- Panžić, I.; Bafti, A.; Radovanović-Perić, F.; Gašparić, D.; Shi, Z.; Borenstein, A.; Mandić, V. Advancements in Nanostructured Functional Constituent Materials for Gas Sensing Applications: A Comprehensive Review. Appl. Sci. 2025, 15, 2522. [Google Scholar] [CrossRef]
- Barhoum, A.; García-Betancourt, M.L.; Jeevanandam, J.; Hussien, E.A.; Mekkawy, S.A.; Mostafa, M.; Omran, M.M.; Abdalla, M.S.; Bechelany, M. Review on Natural, Incidental, Bioinspired, and Engineered Nanomaterials: History, Definitions, Classifications, Synthesis, Properties, Market, Toxicities, Risks, and Regulations. Nanomaterials 2022, 12, 177. [Google Scholar] [CrossRef]
- Bandas, C.; Orha, C.; Nicolaescu, M.; Morariu, M.-I.; Lăzău, C. 2D and 3D Nanostructured Metal Oxide Composites as Promising Materials for Electrochemical Energy Storage Techniques: Synthesis Methods and Properties. Int. J. Mol. Sci. 2024, 25, 12521. [Google Scholar] [CrossRef]
- Das, A.; Peu, S.D.; Hossain, M.S.; Akanda, M.A.M.; Salah, M.M.; Akanda, M.M.H.; Rahman, M.; Das, B.K. Metal Oxide Nanosheet: Synthesis Approaches and Applications in Energy Storage Devices (Batteries, Fuel Cells, and Supercapacitors). Nanomaterials 2023, 13, 1066. [Google Scholar] [CrossRef]
- He, Y.; Jiao, M. A Mini-Review on Metal Oxide Semiconductor Gas Sensors for Carbon Monoxide Detection at Room Temperature. Chemosensors 2024, 12, 55. [Google Scholar] [CrossRef]
- Subhan, M.A.; Neogi, N.; Choudhury, K.P.; Rahman, M.M. Advances in Biosensor Applications of Metal/Metal-Oxide Nanoscale Materials. Chemosensors 2025, 13, 49. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, Z.; Wang, M.; Ge, X. Gamma ray radiation effect on Bi2WO6 photocatalyst. Chin. J. Chem. Phys. 2018, 31, 701–706. [Google Scholar] [CrossRef]
- El Aouni, A.; El Ouardi, M.; Arab, M.; Saadi, M.; Haspel, H.; Kónya, Z.; Ben Ali, A.; Jada, A.; BaQais, A.; Ait Ahsaine, H. Design of Bismuth Tungstate Bi2WO6 Photocatalyst for Enhanced and Environmentally Friendly Organic Pollutant Degradation. Materials 2024, 17, 1029. [Google Scholar] [CrossRef]
- Kovalevskiy, N.; Cherepanova, S.; Gerasimov, E.; Lyulyukin, M.; Solovyeva, M.; Prosvirin, I.; Kozlov, D.; Selishchev, D. Enhanced Photocatalytic Activity and Stability of Bi2WO6–TiO2-N Nanocomposites in the Oxidation of Volatile Pollutants. Nanomaterials 2022, 12, 359. [Google Scholar] [CrossRef]
- Jiang, H.; He, J.; Deng, C.; Hong, X.; Liang, B. Advances in Bi2WO6-Based Photocatalysts for Degradation of Organic Pollutants. Molecules 2022, 27, 8698. [Google Scholar] [CrossRef]
- Kudo, A.; Hijii, S. H2 or O2 evolution from aqueous solutions on layered oxide photocatalysts consisting of Bi3+ with 6s2 configuration and d0 transition metal ions. Chem. Lett. 1999, 28, 1103–1104. [Google Scholar] [CrossRef]
- Wang, D.; Zhen, Y.; Xue, G.; Fu, G.; Lin, X.; Li, D. Synthesis of mesoporous Bi2WO6 architectures and their gas sensitivity to ethanol. J. Mater. Chem. C 2013, 1, 4153–4162. [Google Scholar] [CrossRef]
- Zeng, T.; Yu, X.; Hui, S.; Zhou, Z.; Dong, X. Structural and electrical properties of Bi2WO6 piezoceramics prepared by solid state reaction method. Mater. Res. Bull. 2015, 68, 271–275. [Google Scholar] [CrossRef]
- Ma̧czka, M.; Macalik, L.; Hermanowicz, K.; Kȩpiński, L.; Tomaszewski, P. Phonon properties of nanosized bismuth layered ferroelectric material—Bi2WO6. J. Raman Spectrosc. 2009, 41, 1059–1066. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, J.G.; Bian, Z.F. Solvothermal synthesis of highly active Bi2WO6 visible photocatalyst. Res. Chem. Intermed. 2009, 35, 799–806. [Google Scholar] [CrossRef]
- Gao, Y.; Shan, X.; Song, D.; Gulnigar, E.; Wang, Y.; Yang, W.; Chen, Y. One-step solvothermal synthesis of hollow Bi2WO6 photocatalyst. Can. J. Chem. Eng. 2019, 97, 2440–2446. [Google Scholar] [CrossRef]
- Jonjana, S.; Phuruangrat, A.; Thongtem, S.; Thongtem, T. Synthesis, characterization and photocatalysis of heterostructure AgBr/Bi2WO6 nanocomposites. Mater. Lett. 2018, 216, 92–96. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Wang, W.Z.; Shang, M.; Yin, W.Z. Low-temperature combustion synthesis of Bi2WO6 nanoparticles as a visible-light-driven photocatalyst. J. Hazard. Mater. 2010, 177, 1013–1018. [Google Scholar] [CrossRef]
- Xu, F.; Xu, C.; Chen, H.; Wu, D.; Gao, Z.; Ma, X.; Zhang, Q.; Jiang, K. The synthesis of Bi2S3/2D-Bi2WO6 composite materials with enhanced photocatalytic activities. J. Alloys Compd. 2019, 780, 634–642. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, H.; Hu, J.; Li, Z. Synthesis and characterization of Bi2WO6 nanoplates using egg white as a biotemplate through sol-gel method. Mater. Lett. 2015, 139, 401–404. [Google Scholar] [CrossRef]
- Huang, Y.; Ai, Z.; Ho, W.; Chen, M.; Lee, S. Ultrasonic spray pyrolysis synthesis of porous Bi2WO6 microspheres and their visible-light-induced photocatalytic removal of NO. J. Phys. Chem. C 2010, 114, 6342–6349. [Google Scholar] [CrossRef]
- Wang, W.; Guo, M.; Lu, D.; Wang, W.; Fu, Z. Effect of HNO3 concentration on the morphologies and properties of Bi2WO6 photocatalyst synthesized by a hydrothermal method. Crystals 2016, 6, 75. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, Z.; Chai, Q.; Li, J. Facile Synthesis of a Bi2WO6/BiO2−x Heterojunction for Efficient Photocatalytic Degradation of Ciprofloxacin under Visible Light Irradiation. Catalysts 2023, 13, 469. [Google Scholar] [CrossRef]
- Liu, Z.; Shen, L.; He, X.; Pun, E.Y.B.; Lin, H. Rare-earth functioned Bi2WO6 nanofibers via electrospinning: Boosted catalytic performance and contact-free temperature monitoring on degradation process. Colloid Interface Sci. Commun. 2021, 44, 100494. [Google Scholar] [CrossRef]
- Dumrongrojthanath, P.; Phuruangrat, A.; Doungarno, K.; Thongtem, T.; Patiphatpanya, P.; Thongtem, S. Microwave-hydrothermal synthesis of BiOBr/Bi2WO6 nanocomposites for enhanced photocatalytic performance. Ceram. Int. 2018, 44, S148–S151. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, Y.; Liu, F. Preparation, crystallization and properties of Bi2WO6 nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2020, 590, 124493. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, M.; Zhang, S.; Yue, F.; Yang, C.; Meng, Y.; Li, W.; Li, C.; Berrettoni, M.; Zamponi, S.; et al. Preparation of Bi2WO6/MXene(Ti3C2Tx) Composite Material and Its Photothermal Catalytic Reduction of CO2 in Air. Catalysts 2024, 14, 859. [Google Scholar] [CrossRef]
- Luo, D.; Chen, Q.; Qiu, Y.; Liu, B.; Zhang, M. Carbon Dots-Decorated Bi2WO6 in an Inverse Opal Film as a Photoanode for Photoelectrochemical Solar Energy Conversion under Visible-Light Irradiation. Materials 2019, 12, 1713. [Google Scholar] [CrossRef]
- El-Muraikhi, M.D.; Ayesh, A.I.; Mirzaei, A. Review of nanostructured Bi2O3, Bi2WO6, and BiVO4 as resistive gas sensors. Surf. Interfaces 2025, 60, 106003. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Fei, Y.; Cheng, H.; Pan, H.; Wu, C. Ag3PO4/Bi2WO6 Heterojunction Photocatalyst with Remarkable Visible-Light-Driven Catalytic Activity. Crystals 2023, 13, 1531. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Yang, C.; Gan, L.H. Preparation of direct Z-scheme Bi2WO6/TiO2 heterojunction by one-step solvothermal method and enhancement mechanism of photocatalytic H2 production. Int. J. Hydrogen Energy 2023, 48, 19372–19384. [Google Scholar] [CrossRef]
- Mandari, K.K.; Son, N.; Kang, M. Ag3PO4–Bi2WO6–TiO2 as a high performance electrocatalyst for oxygen evolution reaction. Appl. Surf. Sci. 2021, 566, 150681. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, B.; Zhang, S.; Huang, W. Flower-Like Au–CuO/Bi2WO6 Microsphere Catalysts: Synthesis, Characterization, and Their Catalytic Performances for CO Oxidation. Catalysts 2017, 7, 266. [Google Scholar] [CrossRef]
- Zhou, Y.; Lv, P.; Meng, X.; Tang, Y.; Huang, P.; Chen, X.; Shen, X.; Zeng, X. CTAB-Assisted Fabrication of Bi2WO6 Thin Nanoplates with High Adsorption and Enhanced Visible Light-Driven Photocatalytic Performance. Molecules 2017, 22, 859. [Google Scholar] [CrossRef]
- He, Y.; Gao, H.; Liu, J. A Visible-Light-Active CuS/MoS2/Bi2WO6 Aptamer Sensitively Detects the Non-Steroidal Anti-Inflammatory Drug Diclofenac. Nanomaterials 2022, 12, 2834. [Google Scholar] [CrossRef]
- Ahmed, M.; Huan, W.; Ali, N.; Shafi, A.; Ehsan, M.; Abdelrahman, K.; Khan, A.A.; Abbasi, S.S.; Fnais, M.S. The Effect of Energy Consumption, Income, and Population Growth on CO2 Emissions: Evidence from NARDL and Machine Learning Models. Sustainability 2023, 15, 11956. [Google Scholar] [CrossRef]
- Ben-Salha, O.; Hakimi, A.; Zaghdoudi, T.; Soltani, H.; Nsaibi, M. Assessing the Impact of Fossil Fuel Prices on Renewable Energy in China Using the Novel Dynamic ARDL Simulations Approach. Sustainability 2022, 14, 10439. [Google Scholar] [CrossRef]
- Yassine, M.; Fabris, D. Performance of Commercially Available Supercapacitors. Energies 2017, 10, 1340. [Google Scholar] [CrossRef]
- Lakshmi, K.C.S.; Vedhanarayanan, B. High-Performance Supercapacitors: A Comprehensive Review on Paradigm Shift of Conventional Energy Storage Devices. Batteries 2023, 9, 202. [Google Scholar] [CrossRef]
- Karbasi, M.; Karimzadeh, F.; Raeissi, K.; Rtimi, S.; Kiwi, J.; Giannakis, S.; Pulgarin, C. Insights into the Photocatalytic Bacterial Inactivation by Flower-Like Bi2WO6 under Solar or Visible Light, Through in Situ Monitoring and Determination of Reactive Oxygen Species (ROS). Water 2020, 12, 1099. [Google Scholar] [CrossRef]
- Guo, W.; Jian, L.; Wang, X.; Zeng, W. Hydrothermal synthesis of Ni-doped hydrangea-like Bi2WO6 and the enhanced gas sensing property to n-butanol. Sens. Actuators B Chem. 2022, 357, 131396. [Google Scholar] [CrossRef]
- Wang, L.; Guo, C.; Chen, F.; Ning, J.; Zhong, Y.; Hu, Y. pH-induced hydrothermal synthesis of Bi2WO6 nanoplates with controlled crystal facets for switching bifunctional photocatalytic water oxidation/reduction activity. J. Colloid Interface Sci. 2021, 602, 868–879. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Luo, M.; Li, B.; Lin, Q.; Liao, X.; Yu, H.; Yu, C. 3-D hierarchical micro/nano-structures of porous Bi2WO6: Controlled hydrothermal synthesis and enhanced photocatalytic performances. Microporous Mesoporous Mater. 2021, 313, 110830. [Google Scholar] [CrossRef]
- Gao, X.; Fei, J.; Dai, Y.; Fu, F. Hydrothermal synthesis of series Cu-doped Bi2WO6 and its application in photo-degradative removal of phenol in wastewater with enhanced efficiency. J. Mol. Liq. 2018, 256, 267–276. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, J.; Xu, J.; Dan, X.; Guo, F.; Zhang, B.; Zhang, L.; Wang, Q.; Hou, J. Engineering Bi2WO6-loaded TiO2 nanotubes for enhanced photocatalytic organic wastewater degradation and photoelectric conversion. Environ. Res. 2025, 282, 122089. [Google Scholar] [CrossRef]
- Kuo, C.-Y.; Wu, C.-H.; Hsu, M.-J. Single-step solvothermal synthesis of boron-doped Bi2WO6 visible-light-induced photocatalyst and determination of surface characteristics and photocatalytic activities. Desalination Water Treat. 2017, 81, 209–215. [Google Scholar] [CrossRef]
- Hu, S.-P.; Xu, C.-Y.; Zhen, L.-Z. Solvothermal synthesis of Bi2WO6 hollow structures with excellent visible-light photocatalytic properties. Mater. Lett. 2013, 95, 117–120. [Google Scholar] [CrossRef]
- Wu, L.; Bi, J.; Li, Z.; Wang, X.; Fu, X. Rapid preparation of Bi2WO6 photocatalyst with nanosheet morphology via microwave-assisted solvothermal synthesis. Catal. Today 2008, 131, 15–20. [Google Scholar] [CrossRef]
- Zhu, Z.; Ren, Y.; Li, Q.; Liu, H.; Weng, H. One-pot electrodeposition synthesis of Bi2WO6/graphene composites for photocatalytic applications under visible light irradiation. Ceram. Int. 2018, 44, 3511–3516. [Google Scholar] [CrossRef]
- Orimolade, B.O.; Idris, A.O.; Feleni, U.; Mamba, B. Peroxymonosulfate assisted photoelectrocatalytic degradation of pharmaceuticals at a FTO-Bi2WO6 electrode: Mechanism and kinetics studies. Catal. Commun. 2022, 169, 106481. [Google Scholar] [CrossRef]
- Zhang, G.; Lü, F.; Li, M.; Yang, J.; Zhang, X.; Huang, B. Synthesis of nanometer Bi2WO6 synthesized by sol–gel method and its visible-light photocatalytic activity for degradation of 4BS. J. Phys. Chem. Solids 2010, 71, 579–582. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Y.; Su, Q. Sol-gel synthesis of Bi2WO6/graphene thin films with enhanced photocatalytic performance for nitric monoxide oxidation under visible light irradiation. Chem. Phys. Lett. 2018, 702, 49–56. [Google Scholar] [CrossRef]
- Han, T.; Wang, X.; Ma, Y.; Shao, G.; Dong, X.; Yu, C. Mesoporous Bi2WO6 sheets synthesized via a sol–gel freeze-drying method with excellent photocatalytic performance. J. Sol.-Gel. Sci.Technol. 2017, 82, 101–108. [Google Scholar] [CrossRef]
- Liu, T.; Xue, F.; Wang, B.; Wang, R.; Cao, W.; Zhao, X.; Xia, Y.; Jin, W.; Zhang, Y.; Lin, H.; et al. Rapid microwave synthesis of Bi2WO6 for C=C bonds oxidative cleavage to ketones with visible light irradiation in aerobic micellar medium. J. Catal. 2023, 417, 41–51. [Google Scholar] [CrossRef]
- Li, Z.-Q.; Chen, X.-T.; Xue, Z.-L. Microwave-assisted synthesis and photocatalytic properties of flower-like Bi2WO6 and Bi2O3–Bi2WO6 composite. J. Colloid Interface Sci. 2013, 394, 69–77. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, L.; Srinivasakannan, C.; Qu, W.; Lu, J.; Liu, Q. Low-temperature microwave-assisted synthesis of Bi2WO6/Bi2S3 heterojunction for photocatalytic reduction of Cr(VI) in industrial wastewater. J. Alloys Compd. 2024, 997, 174842. [Google Scholar] [CrossRef]
- Peng, L.; Dong, S.; Xie, H.; Gu, G.; He, Z.; Lu, J.; Huang, T. Sensitive simultaneous determination of diethylstilbestrol and bisphenol A based on Bi2WO6 nanoplates modified carbon paste electrode. J. Electroanal. Chem. 2014, 726, 15–20. [Google Scholar] [CrossRef]
- Liu, H.; Guo, K.; Duan, C.; Chen, X.; Zhu, Z. A novel biosensor based on the direct electrochemistry of horseradish peroxidase immobilized in the three-dimensional flower-like Bi2WO6 microspheres. Mater. Sci. Eng. C 2016, 64, 243–248. [Google Scholar] [CrossRef]
- Vinothkumar, V.; Sangili, A.; Chen, S.-M.; Chen, T.-W.; Abinaya, M.; Sethupathi, V. Voltammetric determination of Sudan I by using Bi2WO6 nanosheets modified glassy carbon electrode. Int. J. Electrochem. Sci. 2020, 15, 2414–2429. [Google Scholar] [CrossRef]
- Xu, H.; Shang, H.; Liu, Q.; Wang, C.; Di, J.; Chen, C.; Jin, L.; Du, Y. Dual mode electrochemical-photoelectrochemical sensing platform for hydrogen sulfide detection based on the inhibition effect of titanium dioxide/bismuth tungstate/silver heterojunction. J. Colloid Interface Sci. 2021, 581, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Fei, Q.; Li, Y.; Wang, B.; Yu, Y. Br-terminated 2D Bi2WO6 nanosheets as a sensitive light-regenerated electrochemical sensor for detecting sulfamethoxazole antibiotic. Surf. Interfaces 2021, 25, 101302. [Google Scholar] [CrossRef]
- Amalraj, A.J.J.; Wang, S.-F. An effective morphology controlled hydrothermal synthesis of Bi2WO6 and its application in riboflavin electrochemical sensor. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129183. [Google Scholar] [CrossRef]
- Alanazi, A.K.; Kumar, P.S.; Ramya, M.; Abo-Dief, H.M.; Rangasamy, G. Bifunctional electrode of bismuth tungsten for electrochemical sensing applications. Chemosphere 2023, 334, 139014. [Google Scholar] [CrossRef]
- Tang, N.; Shi, S.; Zhou, C.; Ding, J.; Chen, A.; He, Q.; Wang, W. Electrochemically ultrasensitive detection of ampicillin using enzyme-mediated metallo-β-lactamase/CS/Bi2WO6/MWCNTs-COOH biosensor. Microchem. J. 2024, 207, 112294. [Google Scholar] [CrossRef]
- Thenrajan, T.; Malar, M.M.; Kumaravel, S.; Rajaram, R.; Kundu, S.; Wilson, J. Bismuth tungstate nanocomposites for simultaneous detection of hydroquinone and resorcinol. Mater. Adv. 2024, 5, 1691–1701. [Google Scholar] [CrossRef]
- Shad, N.A.; Rakha, A.; Qayyum, M.A.; Siddiqi, M.H.; Mahmood, Z.; Nazir, S.; Khan, M.F.; Sajid, M.M.; Rehman, R.A.; Riaz, A.; et al. Zn3(VO4)2/Bi2WO6 composite based versatile platform for cotinine sensing and latent fingerprints development by using multiple modalities. Mater. Sci. Eng. B 2024, 301, 117203. [Google Scholar] [CrossRef]
- Veeralingam, S.; Badhulika, S. Biconcave Bi2WO6 nanoparticles for UV light-activated detection of nicotine in human sweat and cigarette samples. ACS Appl. Nano Mater. 2020, 3, 12250–12259. [Google Scholar] [CrossRef]
- Peng, J.; Zhuge, W.; Liu, Y.; Zhang, C.; Yang, W.; Huang, Y. Photoelectrochemical dopamine sensor based on Cu-doped Bi2WO6 micro-flowers sensitized cobalt tetraaminophthalocyanine functionalized graphene oxide. J. Electrochem. Soc. 2019, 166, B1612. [Google Scholar] [CrossRef]
- Xu, R.; Wei, D.; Du, B.; Cao, W.; Fan, D.; Zhang, Y.; Wei, Q.; Ju, H. A photoelectrochemical sensor for highly sensitive detection of amyloid beta based on sensitization of Mn:CdSe to Bi2WO6/CdS. Biosens. Bioelectron. 2018, 122, 37–42. [Google Scholar] [CrossRef]
- Rajaji, U.; Govindasamy, M.; Chen, S.-M.; Chen, T.-W.; Liu, X.; Chinnapaiyan, S. Microwave-assisted synthesis of Bi2WO6 flowers decorated graphene nanoribbon composite for electrocatalytic sensing of hazardous dihydroxybenzene isomers. Compos. Part B Eng. 2018, 152, 220–230. [Google Scholar] [CrossRef]
- Verma, A.; Kumar, S.; Fu, Y.-P. A ternary-hybrid as efficiently photocatalytic antibiotic degradation and electrochemical pollutant detection. Chem. Eng. J. 2021, 408, 127290. [Google Scholar] [CrossRef]
- Muthumariyappan, A.; Rajaji, U.; Chen, S.-M.; Chen, T.-W.; Li, Y.-L.; Ramalingam, R.J. One-pot sonochemical synthesis of Bi2WO6 nanospheres with multilayer reduced graphene nanosheets modified electrode as rapid electrochemical sensing platform for high sensitive detection of oxidative stress biomarker in biological sample. Ultrason. Sonochem. 2019, 57, 233–241. [Google Scholar] [CrossRef]
- Kavinkumar, V.; Verma, A.; Uma, K.; Moscow, S.; Jothivenkatachalam, K.; Fu, Y.-P. Plasmonic metallic silver induced Bi2WO6/TiO2 ternary junction towards the photocatalytic, electrochemical OER/HER, antibacterial and sensing applications. Appl. Surf. Sci. 2021, 569, 150918. [Google Scholar] [CrossRef]
- Zare, N.; Karimi-Maleh, H.; Darabi, R.; Fu, L.; Ghalkhani, M.; Karaman, O. Bi2WO6/Nb4C3Tx/dsDNA bio-nano-engineered composite as a powerful biosensor component to diagnose and monitor pemetrexed in pharmaceutical and environmental fluids. Adv. Compos. Hybrid Mater. 2025, 8, 88. [Google Scholar] [CrossRef]
- Karaman, C.; Yola, B.B.; Karaman, O.; Atar, N.; Polat, İ.; Yola, M.L. Sensitive sandwich-type electrochemical SARS-CoV-2 nucleocapsid protein immunosensor. Microchim. Acta 2021, 188, 425. [Google Scholar] [CrossRef] [PubMed]
- Ndebele, N.; Mgidlana, S.; Nyokong, T. Electrochemical detection of nitrite using an asymmetrically substituted cobalt phthalocyanine conjugated to metal tungstate nanoparticles. Electroanalysis 2022, 34, 1348. [Google Scholar] [CrossRef]
- Liu, C.; Ye, C. Bi2WO6/Ti3C2 MXene quantum dots heterostructure-based label-free photoelectrochemical aptamer sensor for ultrasensitive kanamycin monitoring. J. Electrochem. Soc. 2023, 170, 107502. [Google Scholar] [CrossRef]
- Yu, L.; Li, J.; Zhang, J.; Chen, X.; Yuan, M.; Zhang, Q.; Xu, B.; Yao, Z.; Xu, Q. Dual 3D structural Bi2WO6@graphene hydrogel composites modified molecularly imprinted polymers: Application for sensitive photoelectrochemical sensing of 4-nitrophenol in PM2.5. J. Appl. Polym. Sci. 2025, 142, e56699. [Google Scholar] [CrossRef]
- Pal, S.; Mahamiya, V.; Ray, P.; Sarkar, A.; Sultana, F.; Adhikary, B.; Chakraborty, B.; Show, B. β-Bi2O3–Bi2WO6 nanocomposite ornated with meso-tetraphenylporphyrin: Interfacial electrochemistry and photoresponsive detection of nanomolar hexavalent Cr. Inorg. Chem. 2023, 62, 21201–21223. [Google Scholar] [CrossRef]
- Nithya, V.D.; Selvan, R.K.; Kalpana, D.; Vasylechko, L.; Sanjeeviraja, C. Synthesis of Bi2WO6 nanoparticles and its electrochemical properties in different electrolytes for pseudocapacitor electrodes. Electrochim. Acta 2013, 109, 720–731. [Google Scholar] [CrossRef]
- Jalalah, M.; Sasmal, A.; Nayak, A.K.; Harraz, F.A. Rapid, external acid-free synthesis of Bi2WO6 nanocomposite for efficient supercapacitor application. J. Taiwan Inst. Chem. Eng. 2023, 143, 104697. [Google Scholar] [CrossRef]
- Kumar, S.A.; Sindhuja, V.; Gowdhaman, A.; Balaji, C.; Ramesh, R.; Anbarasan, P.M. Construction of vertically aligned MnCo2O4 needles and Bi2WO6 globules as optimal electrode materials for hybrid supercapacitor. Electrochim. Acta 2023, 459, 142545. [Google Scholar] [CrossRef]
- Shembade, U.V.; Gurav, S.R.; Gurav, A.N.; Wategaonkar, S.B.; Padalkar, N.S.; Sonkawade, R.G.; Park, J.P.; Moholkar, A.V. Exploring the effect of concentration on hydrothermally synthesized mesoporous spherical nanoflowers of bismuth tungstate for hybrid supercapacitor and water-splitting applications. J. Energy Storage 2024, 89, 111679. [Google Scholar] [CrossRef]
- Wang, F.; Yang, H.; Zhang, H.; Su, J.; Wang, X. Electrochemical performance of morphologically different Bi2WO6 nanostructures synthesized via a hydrothermal route. J. Electron. Mater. 2017, 46, 182–187. [Google Scholar] [CrossRef]
- Karnan, M.; Lolupiman, K.; Okhawilai, M.; Pattananuwat, P.; Shetty, M.; Venkateshalu, S.; Chen, J.S.; Tan, P.; Qin, J. Nanoarchitectonic marvels: Pioneering one-pot synthesis of Bi2WO6 nanostructures for breakthrough in symmetric supercapacitor innovation. ACS Appl. Energy Mater. 2024, 7, 5490–5500. [Google Scholar] [CrossRef]
- Pramila, S.; Ranganatha, V.L.; Soundarya, T.L.; Ramu, R.; Nagaraju, G.; Mallikarjunaswamy, C. Eco-mediated synthesis of visible active Bi2WO6 nanoparticles and its performance towards photocatalyst, supercapacitor, biosensor, and antioxidant activity. J. Clust. Sci. 2022, 33, 2233–2248. [Google Scholar] [CrossRef]
- Nithya, V.D.; Selvan, R.K.; Vasylechko, L.; Sanjeeviraja, C. Effect of carbon coating on the electrochemical properties of Bi2WO6 nanoparticles by PVP-assisted sonochemical method. J. Appl. Electrochem. 2015, 45, 473–485. [Google Scholar] [CrossRef]
- Nithya, V.D.; Selvan, R.K.; Vasylechko, L.; Sanjeeviraja, C. Surfactant assisted sonochemical synthesis of Bi2WO6 nanoparticles and their improved electrochemical properties for use in pseudocapacitors. RSC Adv. 2014, 4, 4343–4352. [Google Scholar] [CrossRef]
- Shetty, M.; Manickavasakam, K.; Sabbanahalli, C.; Bekal, C.; Misnon, I.I.; Subrahmanya, A.P.; Roy, K.; Shivaramu, P.D.; Shenoy, S.B.; Rangappa, D. Rapid single pot synthesis of hierarchical Bi2WO6 microspheres/RGO nanocomposite and its application in energy storage: A supercritical water approach. J. Energy Storage 2023, 72, 108116. [Google Scholar] [CrossRef]
- Lee, K.-C.; Huang, J.-H.; Pang, W.K.; Wang, K.-S.; Hsu, S.-C.; Weng, H.C.; Liu, T.-Y. Construction of hierarchical flower-like BiVO4/Bi2WO6 microspheres with enhanced electrochemical performance for supercapacitors and lithium ion batteries. J. Energy Storage 2024, 101, 113865. [Google Scholar] [CrossRef]
- Yousaf, M.I.; Hameed, S.; Raza, M.A. Synthesis and application of nanorod Bi2WO6/ZnO/rGO composite as a latent high-performance supercapacitor electrode. J. Alloys Compd. 2024, 1004, 175834. [Google Scholar] [CrossRef]
- Qadeer, M.A.; Ali, A.R.; Tanveer, M.; Yasmeen, S.; Nabi, G.; Cheema, H.H.; Alshammari, R.H.; Sakr, H.A. Bi2WO6 and TiS2 composite nanostructures displaying synergetic boosted energy storage in supercapacitor. Ceram. Int. 2024, 50, 43477–43489. [Google Scholar] [CrossRef]
- Akkinepally, B.; Nadar, N.R.; Reddy, I.N.; Rao, H.J.; Nisar, K.S.; Shim, J. Unlocking enhanced electrochemical performance of SnO2–Bi2WO6 nanoflowers for advanced supercapacitor device. J. Alloys Compd. 2024, 970, 172677. [Google Scholar] [CrossRef]
- Chelladurai, A.; Veerasingam, M.; Kesavan, T.; Alotaibi, N.H.; Sangaraju, S.; Muthusamy, K.K.; Sonaimuthu, M.; Sakkarapani, S. Facile synthesis of Bi2WO6–NiO nanocomposite for supercapacitor application. Mater. Sci. Eng. B 2025, 313, 117939. [Google Scholar] [CrossRef]
- Zheng, H.; Yang, G.; Chen, S.; Jia, Y. Hydrothermal synthesis of 3D porous structure Bi2WO6/reduced graphene oxide hydrogels for enhancing supercapacitor performance. ChemElectroChem 2017, 4, 577–584. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, P.; Zhang, Y.; Xu, G.; Lu, Z.; Wang, X.; Wang, Y.; Yang, L.; Tao, X.; Wang, H.; et al. Enhanced performance of nano-Bi2WO6–graphene as pseudocapacitor electrodes by charge transfer channel. Sci. Rep. 2015, 5, 8624. [Google Scholar] [CrossRef]
- Tomar, S.; Singh, V.K. Facile synthesis of bismuth tungstate modified MIL-53(Al) and its application for electrochemical energy storage device. J. Indian Chem. Soc. 2023, 100, 101021. [Google Scholar] [CrossRef]
- Sheoran, K.; Devi, N.; Alsanie, W.F.; Siwal, S.S.; Thakur, V.K. An aniline-complexed bismuth tungstate nanocomposite anchored on carbon black as an electrode material for supercapacitor applications. ChemistrySelect 2023, 8, e202301878. [Google Scholar] [CrossRef]
- Manikandan, V.S.; George, K.; Shimna, M.; Thirumurugan, A.; Harish, S.; Navaneethan, M. Electrochemical performance of Bi2WO6/g-C3N4 (2D–2D) hybrid heterostructure as negative electrode materials for supercapacitor application. J. Mater. Sci. Mater. Electron. 2024, 35, 51. [Google Scholar] [CrossRef]
- Xu, X.; Ming, F.; Hong, J.; Wang, Z. Three-dimensional Bi2WO6/graphene aerogel electrode for high-performance supercapacitor. Adv. Mater. Lett. 2018, 9, 188–191. [Google Scholar] [CrossRef]
- Tomar, S.; Singh, V.K. MIL-53(Al)/Bi2WO6 composite for asymmetric supercapacitor application. Bull. Mater. Sci. 2024, 47, 72. [Google Scholar] [CrossRef]
- Tomar, S.; Singh, V. Preparation of Cu-MOF/Bi2WO6 binary composites by the solvothermal method: Its characterization and application as supercapacitor electrode materials. Energy Storage 2024, 6, e70045. [Google Scholar] [CrossRef]
- Gote, G.H.; Pathak, M.; More, M.A.; Late, D.J.; Rout, C.S. Development of pristine and Au-decorated Bi2O3/Bi2WO6 nanocomposites for supercapacitor electrodes. RSC Adv. 2019, 9, 32573–32580. [Google Scholar] [CrossRef] [PubMed]
- Kavinkumar, V.; Verma, A.; Jothivenkatachalam, K.; Fu, Y.-P. A multifunctional PANI-wrapped Bi2WO6 2D-nanoplates with in-situ grown BiOCl nanocomposite for electrochemical seawater splitting, supercapacitor, and photocatalytic applications. Appl. Surf. Sci. 2025, 696, 162979. [Google Scholar] [CrossRef]
- Akbar, S.; Irshad, A.; Zulfiqar, S.; ALOthman, Z.A.; Shakir, I.; Warsi, M.F.; Cochran, E.W. Rationally designed silver doped bismuth tungstate dispersed on multifunctional MXene sheets for enhanced supercapacitor applications. J. Sol-Gel Sci. Technol. 2024, 102, 1–15. [Google Scholar] [CrossRef]
- Liu, K.L.; Chen, F.; Liu, Y.; Li, D.; Shi, W.D. Synthesis of hierarchical Bi2S3 nanoflowers via a topotactic transformation from hierarchical Bi2WO6 nanoflowers and their supercapacitor performance. CrystEngComm 2017, 19, 570–575. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).