Synthesis and Molecular Modeling of Antioxidant and Anti-Inflammatory Five-Membered Heterocycle–Cinnamic Acid Hybrids
Abstract
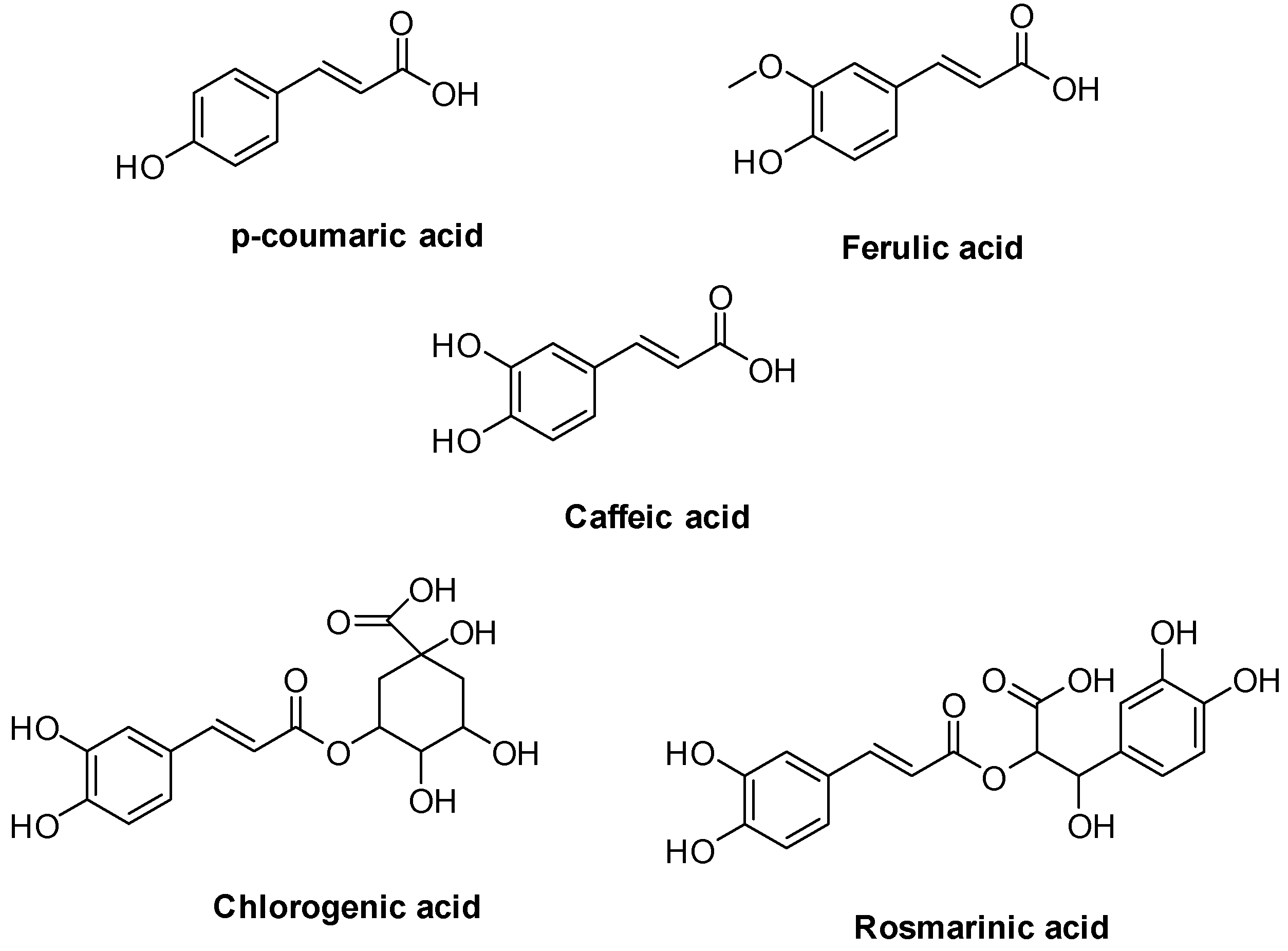
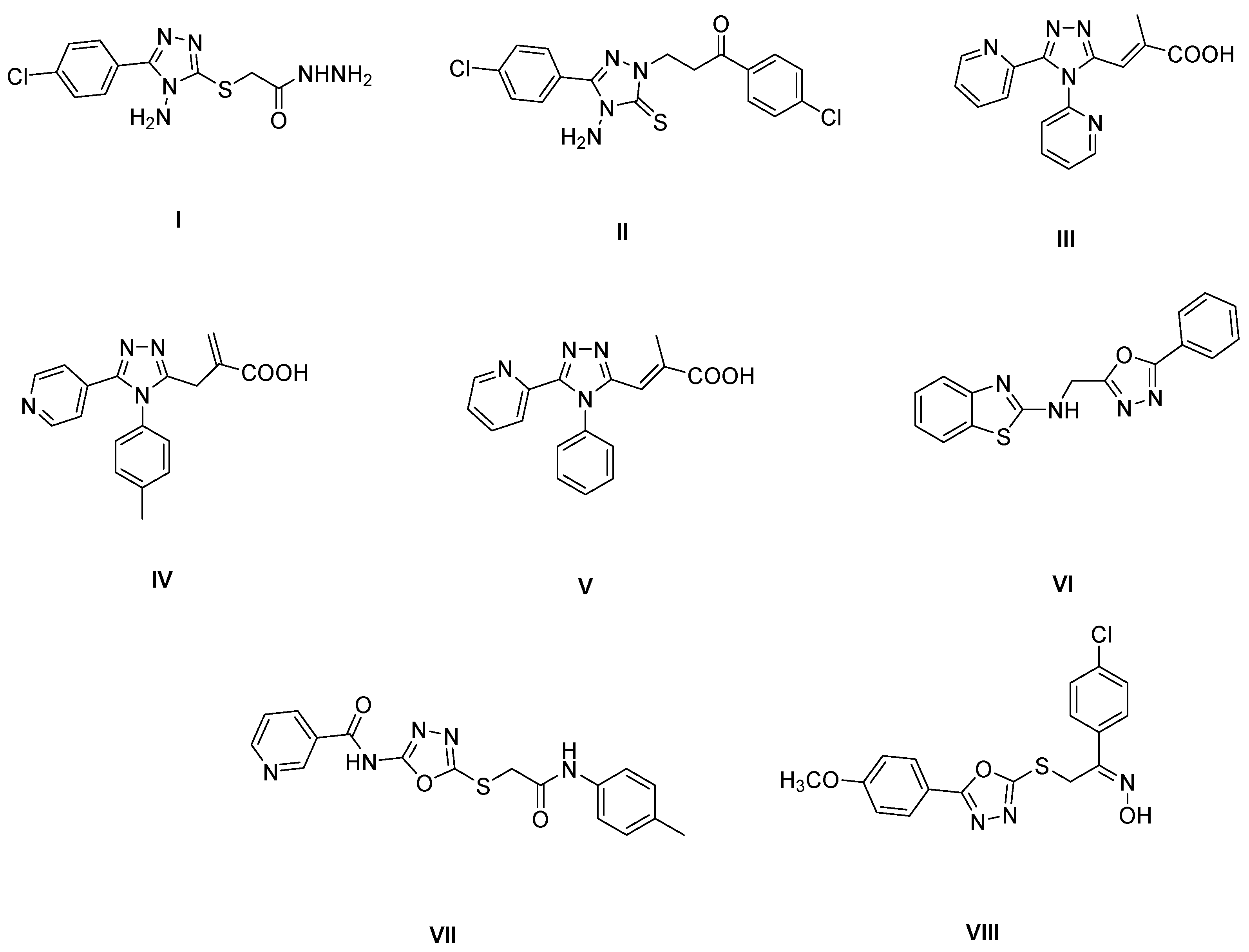
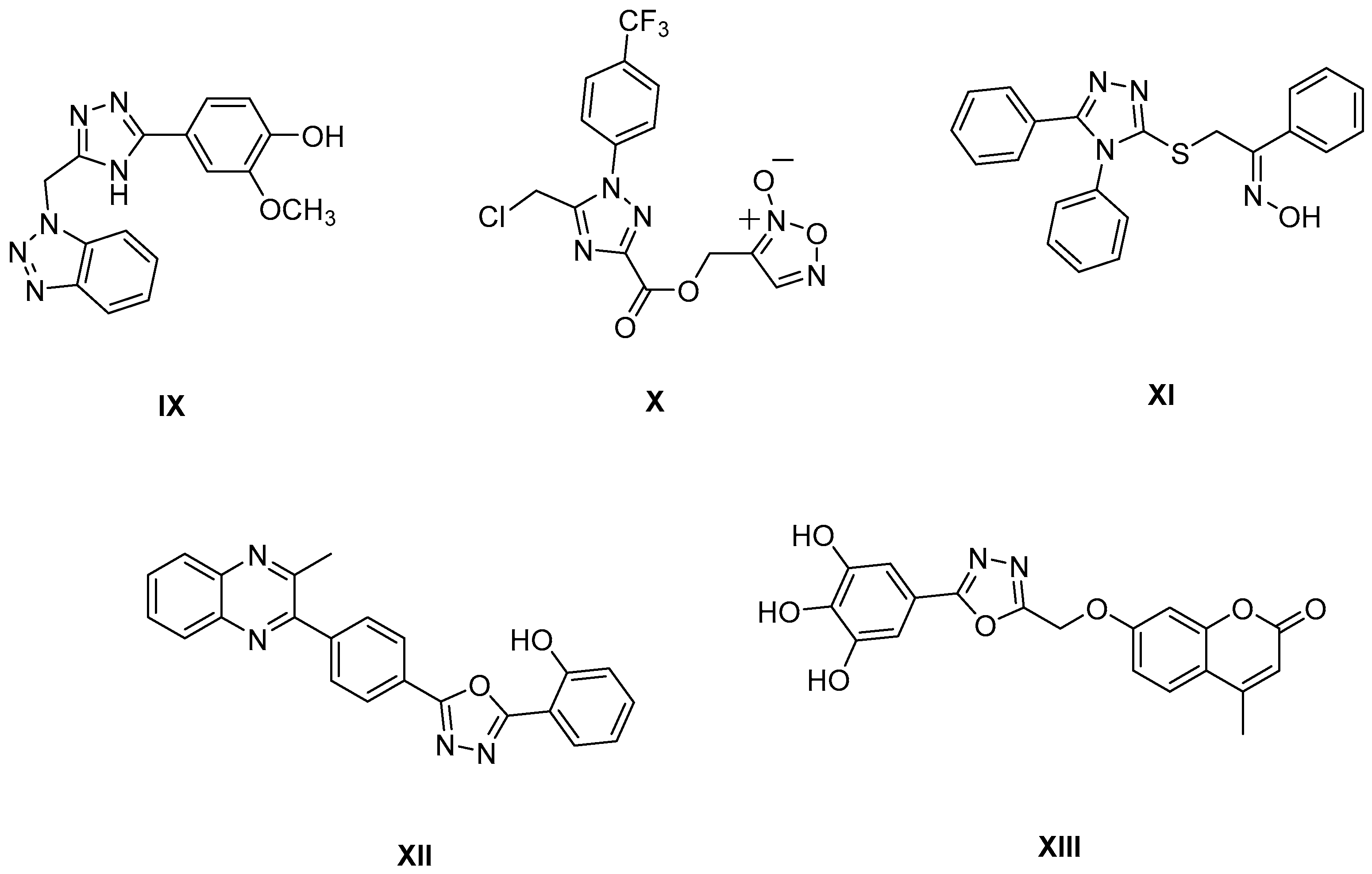
1. Introduction
2. Results
2.1. Chemistry
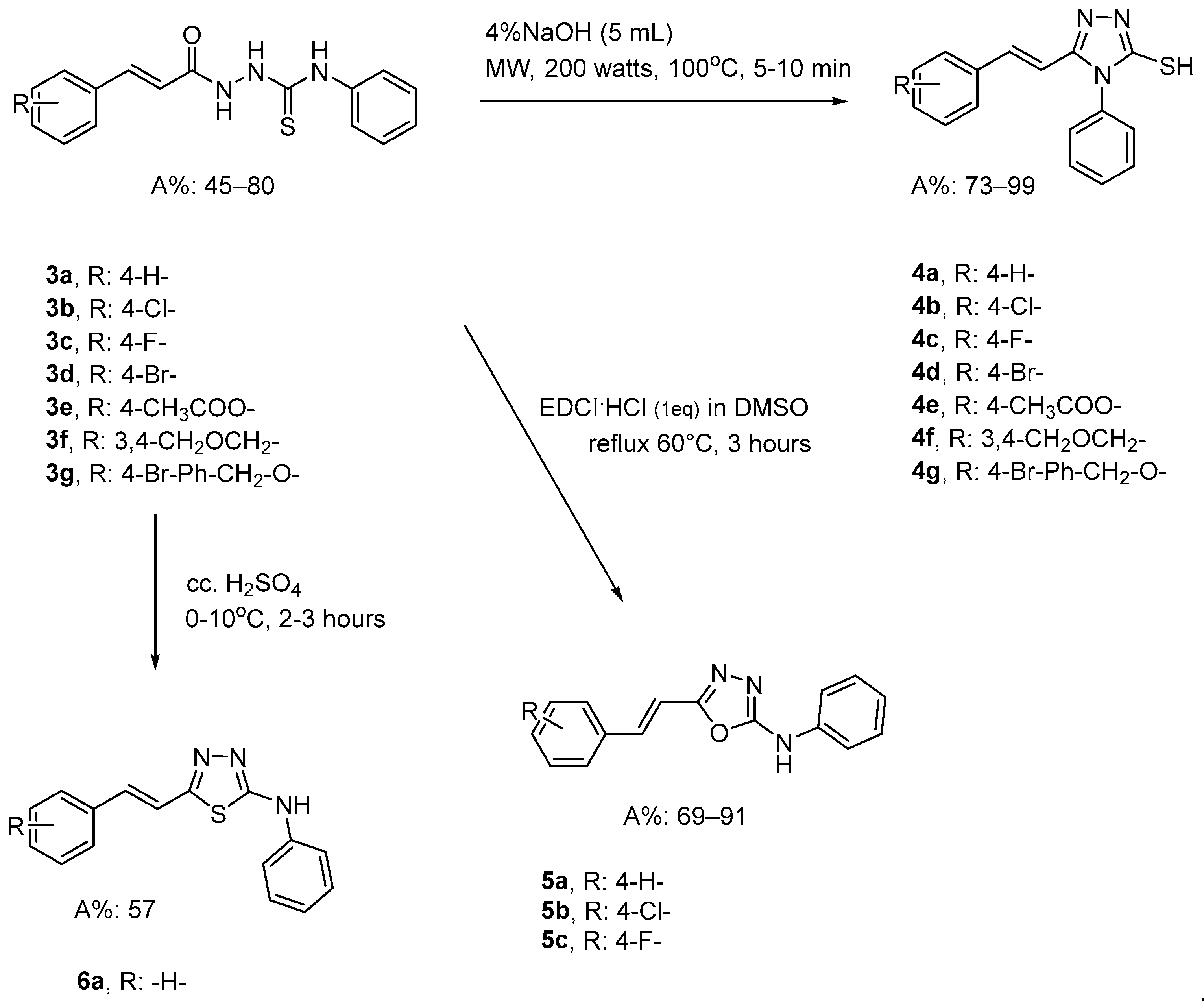
2.1.1. Synthesis of Key Intermediates
2.1.2. Synthesis of Target Molecules
2.2. Physicochemical Studies
2.2.1. Determination of Lipophilicity
2.2.2. Theoretical Calculation of Physicochemical Properties
2.3. Biological Evaluation
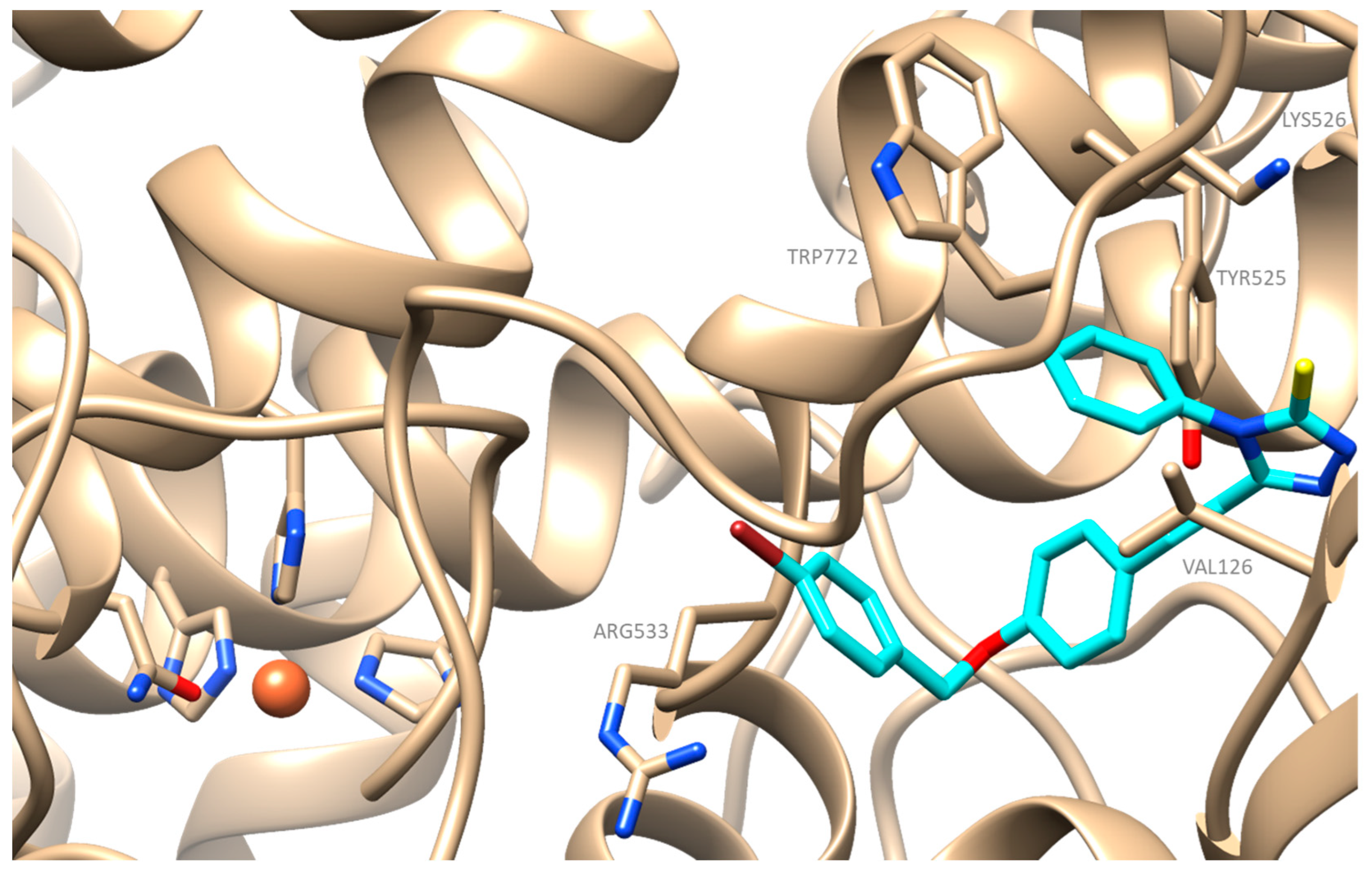
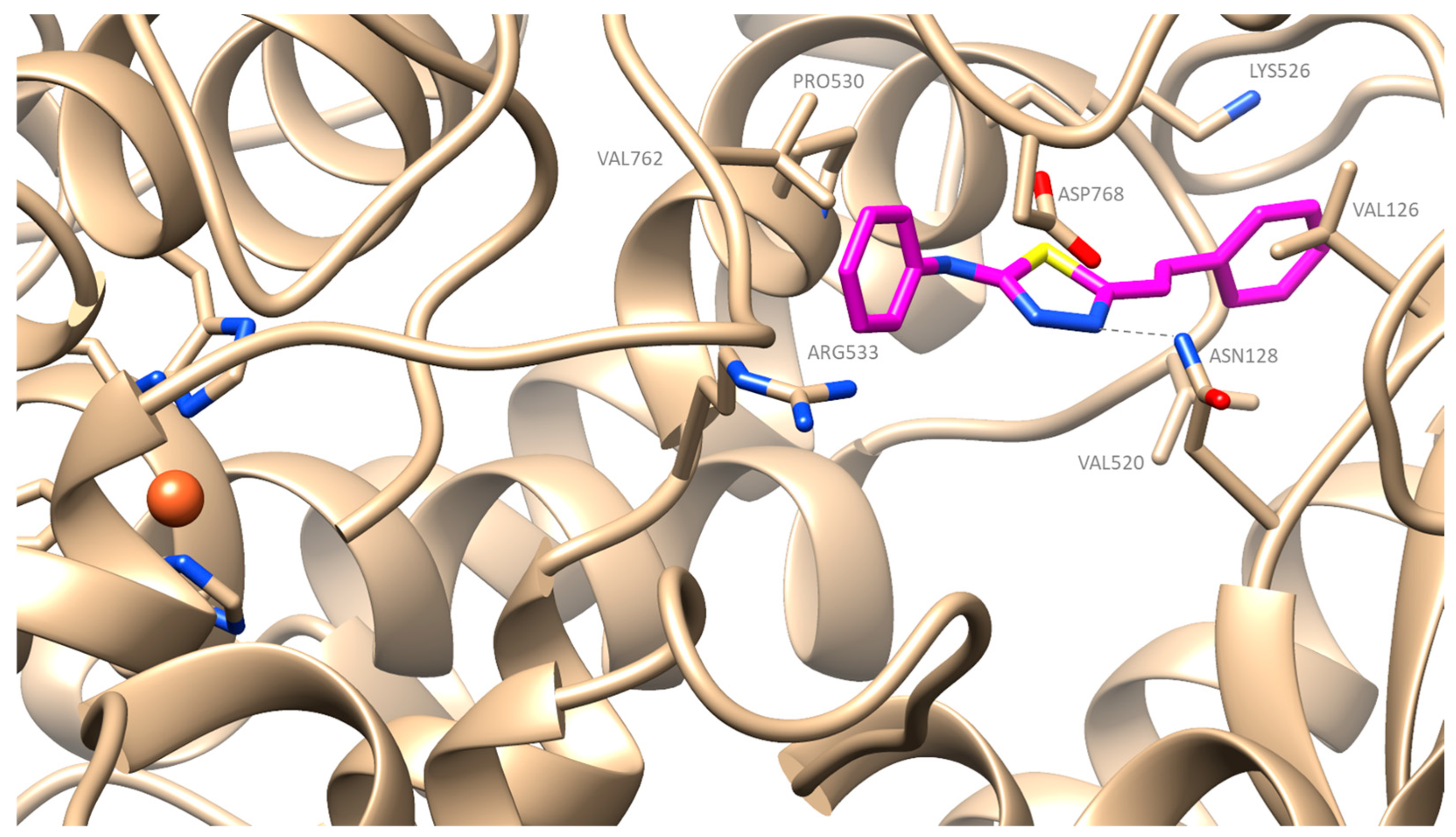
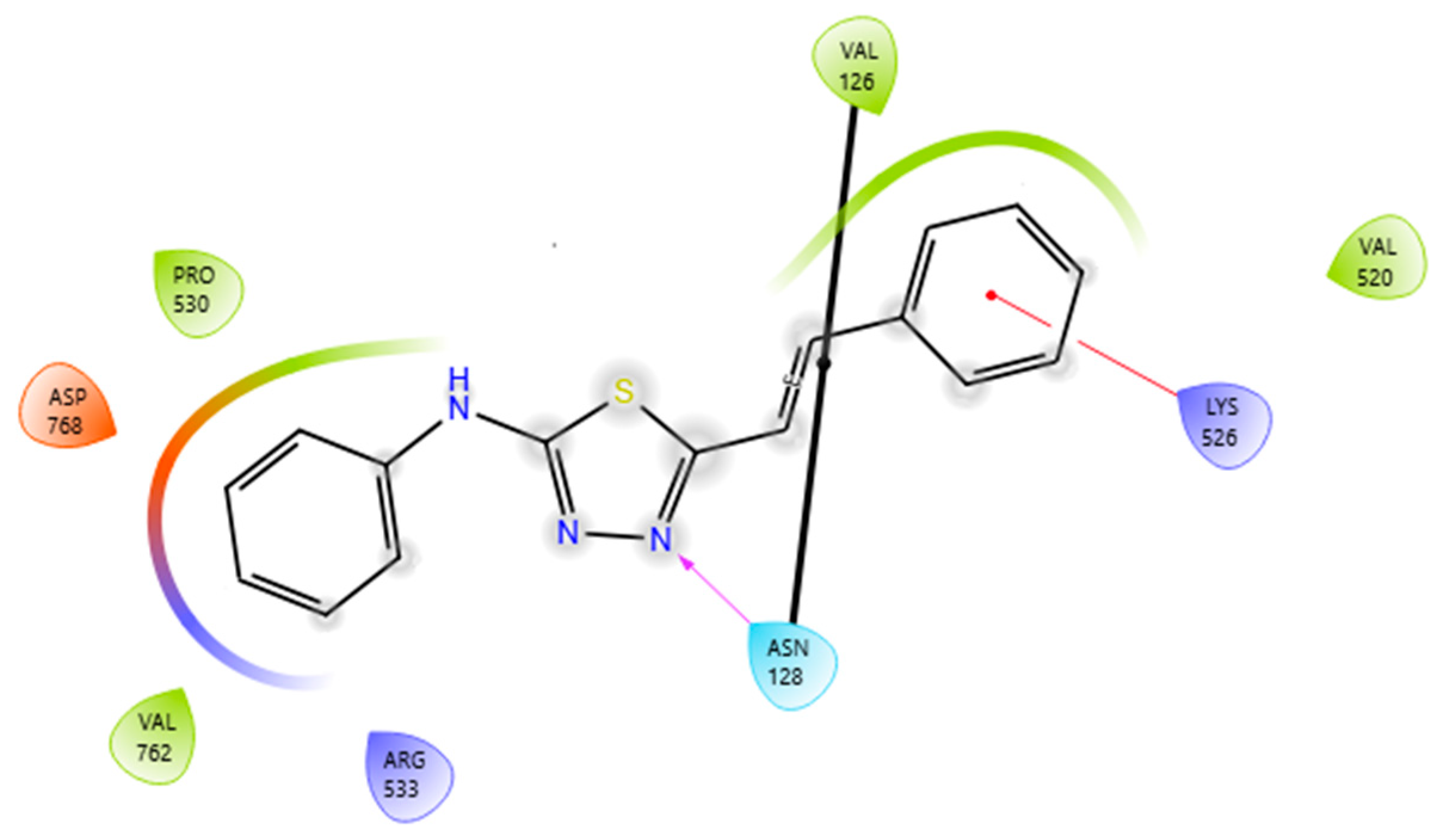
2.4. Docking Simulation Soybean Lipoxygenase Studies
3. Experimental Section
3.1. Materials and Instruments
3.2. Chemistry General Procedures and Characterization Data
3.2.1. Synthesis of α,β- Unsaturated Carboxylic Acids (1a–1g)
3.2.2. General Method for the Synthesis of Cinnamoyl-Thiosemicarbazide Derivatives (3a–3g)
- 2-Cinnamoyl-N-phenylhydrazine-1-carbothioamide (3a): The spectral data were in agreement with the literature data [55].
- (E)-2-(3-(4-Chlorophenyl)acryloyl)-N-phenylhydrazine-1-carbothioamide (3b): Yield: 74%, yellow solid, m.p.: 208–209 °C (recrystallization from C2H5OH). IR (KBr, cm−1): 3282 (N-H), 3227 (N-H), 3088 (N-H), 1651 (C=O), 1624 (C=C), 1362. 1H NMR (500 MHz, DMSO-d6): δ 10.24 (s, 1H, -NH), 9.76 (brs, 2H, -NH-NH-), 7.64 (d, J = 8.5 Hz, 2H, ArH), 7.56 (d, J = 15.8 Hz, 1H, -CH=CH-), 7.51 (d, J = 8.4 Hz, 2H, -ArH), 7.48–7.40 (m, 2H, ArH), 7.33 (t, J = 7.7 Hz, 2H, ArH), 7.15 (t, J = 7.4 Hz, 1H, ArH), 6.66 (d, J = 15.8 Hz, 1H, -CH=CH-). 13C NMR: (126 MHz, DMSO): δ 180.8, 139.2, 138.6, 134.3, 133.6, 129.4, 129.2, 128.1, 126.2, 125.4, 120.7.
- (E)-2-(3-(4-Fluorophenyl)acryloyl)-N-phenylhydrazine-1-carbothioamide (3c): Yield: 45%, yellow solid, m.p.: 202–203 °C (recrystallization from C2H5OH). IR (KBr, cm−1): 3287 (N-H), 3223 (N-H), 2981 (N-H), 1652 (C=O), 1626 (C=C), 1363. 1H NMR (500 MHz, DMSO-d6): δ 10.22 (s, 1H, NH), 9.78 (s, 1H, -NH-NH-), 9.72 (s, 1H, -NHNH-), 7.68 (dd, J = 8.5 Hz, 2H, ArH), 7.55 (d, J = 15.9 Hz, 1H, -CH=CH-), 7.50–7.40 (m, 2H, ArH), 7.29 (dt, J = 8.3 Hz, 4H, ArH), 7.15 (t, J = 7.3 Hz, 1H, ArH), 6.61 (d, J = 15.9 Hz, 1H, -CH=CH-). 13C NMR: (126 MHz, DMSO): δ 181.0, 164.0, 162.1, 139.3, 139.0, 131.4, 130.1, 128.3, 126.1, 125.2, 119.9, 116.2.
- (E)-2-(3-(4-Bromophenyl)acryloyl)-N-phenylhydrazine-1-carbothioamide (3d): Yield: 79%, yellow solid, m.p.: 209–210 °C (recrystallization from C2H5OH). IR (KBr, cm−1): 3282 (N-H), 3232 (N-H), 2981 (N-H), 1652 (C=O), 1623 (C=C), 1362. 1H NMR (500 MHz, DMSO-d6): δ 10.24 (s, 1H, NH), 9.77 (brs, 2H, -NH-NH-), 7.70–7.56 (m, 4H, ArH), 7.54 (d, J = 16.1 Hz, 1H, -CH=CH-), 7.45 (d, J = 7.7, 2H, ArH), 7.32 (t, J = 7.7 Hz, 2H, ArH), 7.15 (t, J = 7.3 Hz, 1H, ArH), 6.67 (d, J = 16.2 Hz, 1H, -CH=CH-). 13C NMR: (126 MHz, DMSO): δ 180.8, 180.4, 139.2, 138.7, 133.9, 132.1, 129.6, 128.1, 125.9, 1250, 123.1, 120.8.
- (E)-4-(3-Oxo-3-(2-(phenylcarbamothioyl)hydrazineyl)prop-1-en-1-yl)phenyl acetate (3e): Yield: 46%, yellow solid, m.p.: 183–184 °C (recrystallization from C2H5OH). IR (KBr, cm−1): 3280 (N-H), 3219 (N-H), 2981 (N-H), 1650 (C=O), 1623 (C=C), 1366. 1H NMR (500 MHz, DMSO-d6): δ 10.22 (s, 1H, NH), 9.77 (s, 1H, -NH-NH-), 9.72 (s, 1H, -NHNH-), 7.66 (d, J = 8.3 Hz, 2H, ArH), 7.57 (d, J = 15.9 Hz, 1H, -CH=CH-), 7.46 (d, J = 7.3 Hz, 2H, ArH), 7.33 (t, J = 7.8 Hz, 2H, ArH), 7.21 (d, J = 8.3 Hz, 2H, ArH), 7.15 (t, J = 7.5 Hz, 1H, ArH), 6.63 (d, J = 15.9 Hz, 1H, -CH=CH-), 2.28 (s, 3H, CH3). 13C NMR: (126 MHz, DMSO): δ 180.8, 169.1, 151.5, 139.5, 139.2, 139.0, 132.3, 128.9, 128.1, 125.8, 125.0, 122.6, 120.0, 20.9.
- (E)-2-(3-(Benzo[d][1,3]dioxol-5-yl)acryloyl)-N-phenylhydrazine-1-carbothioamide (3f): Yield: 45%, yellow solid, m.p.: 183–184 °C (recrystallization from C2H5OH). IR (KBr, cm−1): 3283 (N-H), 3227 (N-H), 2981 (N-H), 1650 (C=O), 1625 (C=C), 1356. 1H NMR (500 MHz, DMSO-d6): δ 10.10 (s, 1H, NH), 9.74 (s, 1H, -NH-NH-), 9.71 (s, 1H, -NHNH-), 7.47 (t, J = 15.4, 8.7 Hz, 3H, -CH=CH-, ArH), 7.32 (t, J = 7.7 Hz, 2H, ArH), 7.19 (s, 1H, ArH), 7.14 (dd, J = 7.8 Hz, 2H, ArH), 6.98 (d, J = 7.9 Hz, 1H, ArH), 6.49 (d, J = 15.8 Hz, 1H, -CH=CH-), 6.08 (s, 2H, -CH2). 13C NMR: (126 MHz, DMSO): δ 180.8, 148.8, 148.0, 139.8, 139.2, 129.0, 128.0, 125.9, 125.0, 123.6, 117.9, 108.7, 106.3, 101.6.
- (E)-2-(3-(4-((4-Bromobenzyl)oxy)phenyl)acryloyl)-N-phenylhydrazine-1-carbothioamide (3g): Yield: 78%, yellow solid, m.p.: 207–208 °C (recrystallization from C2H5OH). IR (KBr, cm−1): 3248 (N-H), 2981 (N-H), 2160, 1591 (C=O), 1361. 1H NMR (500 MHz, DMSO-d6): δ 10.11 (s, 1H, NH), 9.74 (s, 1H, -NH-NH-), 9.71 (s, 1H, -NHNH-), 7.60 (d, J = 8.1 Hz, 2H, ArH), 7.57 (d, J = 8.4 Hz, 2H, ArH), 7.51 (d, J = 15.9 Hz, 1H, -CH=CH-), 7.44 (dd, J = 8.1, 7.5 Hz, 4H, ArH), 7.32 (t, J = 7.7 Hz, 2H, ArH), 7.15 (t, J = 7.4 Hz, 1H, ArH), 7.07 (d, J = 8.4 Hz, 2H, ArH), 6.51 (d, J = 15.8 Hz, 1H, -CH=CH-), 5.14 (s, 2H, CH2). 13C NMR: (126 MHz, DMSO): δ 180.8, 159.5, 139.6, 139.2, 136.3, 131.4, 129.9, 129.4, 128.0, 127.5, 125.9, 124.9, 121.1, 117.6, 115.4, 68.5.
3.2.3. General Method for the Synthesis of Triazole-Cinnamic Acid Derivatives (4a–4g)
- (E)-4-Phenyl-5-styryl-4H-1,2,4-triazole-3-thiol (4a): Yield: 89%, white crystal solid, m.p.: 259–260 °C (recrystallization from C2H5OH). IR (KBr, cm−1): 2690 (S-H), 1634 (N=C), 1544, 1339, 1295 (C-N). 1H NMR (500 MHz, DMSO-d6): δ 14.04 (s, 1H, SH), 7.65–7.54 (m, 3H, ArH), 7.48–7.41 (m, 4H, ArH), 7.35 (d, J = 8.0 Hz, 2H, ArH), 7.31 (d, J = 16.4 Hz, 2H, -CH=CH-, ArH), 6.46 (d, J = 16.4 Hz, 1H, -CH=CH-). 13C NMR (126 MHz, DMSO-d6): δ 167.8, 149.1, 136.2, 134.8, 133.6, 129.6, 129.6, 129.5, 128.9, 128.5, 127.2, 111.0.
- (E)-5-(4-Chlorostyryl)-4-phenyl-4H-1,2,4-triazole-3-thiol (4b): Yield: 99%, white solid, m.p.: 267–268 °C (recrystallization from C2H5OH). IR (KBr, cm−1): 2638 (S-H), 1591(N=C), 1547, 1290 (C-N). 1H NMR (500 MHz, DMSO-d6): δ 13.22 (s, 1H, SH), 6.76 (dq, J = 7.1 Hz, 3H, ArH), 6.63 (dd, J = 7.5 Hz, 4H, ArH), 6.56 (d, J = 8.2 Hz, 2H, ArH), 6.49 (d, J = 16.4 Hz, 1H, -CH=CH-), 5.64 (d, J = 16.4 Hz, 1H, -CH=CH-).13C NMR (126 MHz, DMSO-d6): δ 167.9, 149.0, 134.8, 133.9, 133.7, 133.5, 129.6, 129.6, 129.0, 128.9, 128.5, 111.8.
- (E)-5-(4-Fluorostyryl)-4-phenyl-4H-1,2,4-triazole-3-thiol (4c): Yield: 99%, white solid, m.p.: 240–241 °C (recrystallization from C2H5OH). IR (KBr, cm−1): 2615 (S-H), 1636 (N=C), 1546, 1297 (C-N). 1H NMR (500 MHz, DMSO-d6): δ 13.19 (s, 1H, SH), 6.78–6.74 (m, 1H, ArH), 6.74–6.71 (m, 2H, ArH), 6.67 (dd, J = 7.7 Hz, 2H, ArH), 6.60 (dd, J = 6.6 Hz, 2H, ArH), 6.48 (d, J = 16.4 Hz, 1H, -CH=CH-), 6.32 (t, J = 8.9 Hz, 2H, ArH), 5.56 (d, J = 16.4 Hz, 1H, -CH=CH-).13C NMR (126 MHz, DMSO-d6): δ 167.8, 163.7, 161.7, 149.2, 135.0, 133.5, 131.5, 129.6, 128.5, 116.0, 115.8, 110.9.
- (E)-5-(4-Bromostyryl)-4-phenyl-4H-1,2,4-triazole-3-thiol (4d): The spectral data were in agreement with the literature data [54].
- (E)-4-(2-(5-Mercapto-4-phenyl-4H-1,2,4-triazol-3-yl)vinyl)phenyl acetate (4e): Yield: 91%, white solid, m.p.: 265–266 °C (recrystallization from C2H5OH). IR (KBr, cm−1): 2658 (S-H), 1635 (N=C), 1543, 1299 (C-N). 1H NMR (500 MHz, DMSO-d6): δ 13.93 (s, 1H, SH), 9.85 (dd, J = 8.2 Hz, 1H), 7.59 (dq, J = 7.9 Hz, 2H, ArH), 7.43 (dq, J = 7.8 Hz, 2H, ArH), 7.32–7.16 (m, 2H, ArH), 6.72 (tt, J = 13.6, 7.1 Hz, 2H, ArH -CH=CH-), 6.25–6.13 (m, 2H, ArH, -CH=CH-), 2.47 (s, 3H, CH3). 13C NMR (126 MHz, DMSO-d6): δ 167.6, 159.0, 149.7, 136.3, 133.7, 129.6, 129.6, 129.0, 128.6, 125.9, 115.8, 107.3, 6.5.
- (E)-5-(2-(Benzo[d][1,3]dioxol-5-yl)vinyl)-4-phenyl-4H-1,2,4-triazole-3-thiol (4f): Yield: 99%, white solid, m.p.: 283–284 °C (recrystallization from C2H5OH). IR (KBr, cm−1): 2701 (S-H), 1633 (N=C), 1293 (C-N). 1H NMR (500 MHz, DMSO-d6): δ 13.98 (s, 1H, SH), 7.64–7.53 (m, 3H, ArH), 7.46–7.40 (m, 2H, ArH), 7.22 (d, J = 16.3 Hz, 1H, -CH=CH-), 7.14 (s, 1H, ArH), 6.91 (dd, J = 8.1, 1H, ArH), 6.88 (d, J = 8.0 Hz, 1H, ArH), 6.33 (d, J = 16.3 Hz, 1H, -CH=CH-), 6.02 (s, 2H, CH2). 13C NMR (126 MHz, DMSO-d6): δ 167.7, 149.5, 148.5, 148.0, 136.1, 133.6, 129.6, 129.3, 128.5, 123.3, 109.2, 108.6, 106.0, 101.5.
- (E)-5-(4-((4-Bromobenzyl)oxy)styryl)-4-phenyl-4H-1,2,4-triazole-3-thiol (4g): Yield: 73%, yellow solid, m.p.: 278–279 °C (recrystallization from C2H5OH). IR (KBr, cm−1): 2659 (S-H), 1603 (N=C), 1545, 1298 (C-N). 1H NMR (500 MHz, DMSO-d6): δ 13.99 (s, 1H, SH), 7.64–7.54 (m, 5H, ArH), 7.46–7.42 (m, 2H, ArH), 7.39 (dd, J = 8.5 Hz, 4H, ArH), 7.27 (d, J = 16.3 Hz, 1H, -CH=CH-), 7.00–6.94 (m, 2H, ArH), 6.29 (d, J = 16.3 Hz, 1H, -CH=CH-), 5.09 (s, 2H, CH2). 13C NMR (126 MHz, DMSO-d6): δ 167.6, 159.2, 149.5, 136.2, 135.8, 133.6, 131.4, 129.9, 129.6, 128.9, 128.5, 127.8, 121.0, 115.3, 108.7, 68.5.
3.2.4. General Method for the Synthesis of Oxadiazole-Cinnamic Acid Derivatives (5a–5c)
- (E)-N-Phenyl-5-styryl-1,3,4-oxadiazol-2-amine (5a): The spectral data were in agreement with the literature data [53].
- (E)-5-(4-Chlorostyryl)-N-phenyl-1,3,4-oxadiazol-2-amine (5b): Yield: 91%, solid, m.p.: 244–246 °C. IR (KBr, cm−1): 3201.8, 3021.3 (NH), 1628.3 (C=C). 1H NMR: (500 MHz, DMSO): δ 10.66 (s, 1H, -NH), 7.73 (d, J = 8.2 Hz, 2H, ArH), 7.59 (d, J = 8.0 Hz, 2H, ArH), 7.44 (d, J = 8.2 Hz, 2H, ArH), 7.34–7.24 (m, 4H, ArH, -CH=CH-), 6.98 (t, J = 7.3 Hz, 1H, ArH). 13C NMR: (126 MHz, DMSO): δ 159.4, 157.9, 138.5, 134.1, 134, 133.9, 129.2, 129.1, 128.9, 122.00, 117.2, 111.3.
- (E)-5-(4-Fluorostyryl)-N-phenyl-1,3,4-oxadiazol-2-amine (5c): Yield: 78%, solid, m.p.: 213–215 °C. IR (KBr, cm−1): 3221.6, 3023.7 (NH), 1701.7, 1685.8 (C=C). 1H NMR: (500 MHz, DMSO): δ 10.66 (s, 1H, -NH), 7.82–7.79 (m, 2H, ArH), 7.61–7.59 (m, 2H, ArH), 7.37–7.34 (m, 2H, ArH), 7.28–7.19 (m, 4H, ArH, -CH=CH-), 7.01 (t, J = 7.3 Hz, 1H, ArH). 13C NMR: (126 MHz, DMSO): δ 159.7, 158.3, 147.9, 139, 134.7, 130.1, 129.5, 125.1, 122.3, 117.5, 116.3, 110.8.
3.2.5. General Method for the Synthesis of Thiadiazole-Cinnamic Acid Derivative (6a)
- (E)-N-Phenyl-5-styryl-1,3,4-thiadiazol-2-amine (6a): The spectral data were in agreement with the literature data [52].
3.3. Biological in Vitro Assays
3.3.1. Determination of the Reducing Activity of the Stable Radical DPPH
3.3.2. Inhibition of AAPH-Induced Linoleic Acid Peroxidation
3.3.3. Inhibition of Soybean Lipoxygenase
3.4. Computational Methods
3.4.1. Molecular Docking Studies on Soybean Lipoxygenase
3.4.2. In Silico Determination of Drug-likeness and Lipophilicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glomb, T.; Minta, J.; Nowosadko, M.; Radzikowska, J.; Świątek, P. Search for New Compounds with Anti-Inflammatory Activity Among 1,2,4-Triazole Derivatives. Molecules 2024, 29, 6036. [Google Scholar] [CrossRef]
- Paprocka, R.; Wiese, M.; Eljaszewicz, A.; Helmin-Basa, A.; Gzella, A.; Modzelewska-Banachiewicz, B.; Michalkiewicz, J. Synthesis and anti-inflammatory activity of new 1,2,4-triazole derivatives. Bioorg. Med. Chem. Lett. 2015, 25, 2664–2667. [Google Scholar] [CrossRef]
- Paprocka, R.; Kołodziej, P.; Wiese-Szadkowska, M.; Helmin-Basa, A.; Bogucka-Kocka, A. Evaluation of Anthelmintic and Anti-Inflammatory Activity of 1,2,4-Triazole Derivatives. Molecules 2022, 27, 4488. [Google Scholar] [CrossRef]
- El-Serwy, W.S.; Mohamed, N.A.; Abbas, E.M.; Abdel-Rahman, R.F. Synthesis and anti-inflammatory properties of novel 1,2,4-triazole derivatives. Res. Chem. Intermed. 2013, 39, 2543–2554. [Google Scholar] [CrossRef]
- Matore, B.W.; Banjare, P.; Guria, T.; Roy, P.P.; Singh, J. Oxadiazole derivatives: Histone deacetylase inhibitors in anticancer therapy and drug discovery. Eur. J. Med. Chem. Rep. 2022, 5, 100058. [Google Scholar] [CrossRef]
- Iyer, V.B.; Gurupadayya, B.; Koganti, V.S.; Inturi, B.; Chandan, R.S. Design, synthesis and biological evaluation of 1,3,4-oxadiazoles as promising anti-inflammatory agents. Med. Chem. Res. 2017, 26, 190–204. [Google Scholar] [CrossRef]
- Hamoud, M.M.S.; Osman, N.A.; Rezq, S.; Abd El-wahab, A.A.H.; Hassan, A.E.A.; Hassan, A.; Abdel-Fattah, H.A.; Romero, D.G.; Ghanim, A.M. Design and Synthesis of Novel 1,3,4-Oxadiazole and 1,2,4-Triazole Derivatives as Cyclooxygenase-2 Inhibitors with Anti-inflammatory and Antioxidant activity in LPS-stimulated RAW264.7 Macrophages. Bioorg. Chem. 2022, 124, 105808. [Google Scholar] [CrossRef]
- Almasirad, A.; Mousavi, Z.; Tajik, M.; Assarzadeh, M.J.; Shafiee, A. Synthesis, analgesic and anti-inflammatory activities of new methyl-imidazolyl-1,3,4-oxadiazoles and 1,2,4-triazoles. DARU J. Pharm. Sci. 2014, 22, 22. [Google Scholar] [CrossRef]
- Abd-Ellah, H.S.; Abdel-Aziz, M.; Shoman, M.E.; Beshr, E.A.M.; Kaoud, T.S.; Ahmed, A.-S.F.F. New 1,3,4-oxadiazole/oxime hybrids: Design, synthesis, anti-inflammatory, COX inhibitory activities and ulcerogenic liability. Bioorg. Chem. 2017, 74, 15–29. [Google Scholar] [CrossRef]
- Ruwizhi, N.; Aderibigbe, B.A. Cinnamic Acid Derivatives and Their Biological Efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef]
- Gabriele, E.; Brambilla, D.; Ricci, C.; Regazzoni, L.; Taguchi, K.; Ferri, N.; Asai, A.; Sparatore, A. New sulfurated derivatives of cinnamic acids and rosmaricine as inhibitors of STAT3 and NF-κB transcription factors. J. Enzym. Inhib. Med. Chem. 2017, 32, 1012–1028. [Google Scholar] [CrossRef]
- Ruan, B.-F.; Ge, W.-W.; Cheng, H.-J.; Xu, H.-J.; Li, Q.-S.; Liu, X.-H. Resveratrol-based cinnamic ester hybrids: Synthesis, characterization, and anti-inflammatory activity. J. Enzym. Inhib. Med. Chem. 2017, 32, 1282–1290. [Google Scholar] [CrossRef]
- Song, F.; Li, H.; Sun, J.; Wang, S. Protective effects of cinnamic acid and cinnamic aldehyde on isoproterenol-induced acute myocardial ischemia in rats. J. Ethnopharmacol. 2013, 150, 125–130. [Google Scholar] [CrossRef]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Ou, S.; Kwok, K. Ferulic acid: Pharmaceutical functions, preparation and applications in foods. J. Sci. Food Agric. 2004, 84, 1261–1269. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef]
- Petersen, M. Rosmarinic acid: New aspects. Phytochem. Rev. 2013, 12, 207–227. [Google Scholar] [CrossRef]
- Pragasam, S.J.; Venkatesan, V.; Rasool, M. Immunomodulatory and Anti-inflammatory Effect of p-Coumaric Acid, a Common Dietary Polyphenol on Experimental Inflammation in Rats. Inflammation 2013, 36, 169–176. [Google Scholar] [CrossRef]
- da Cunha, F.M.; Duma, D.; Assreuy, J.; Buzzi, F.C.; Niero, R.; Campos, M.M.; Calixto, J.B. Caffeic Acid Derivatives: In Vitro and In Vivo Anti-inflammatory Properties. Free Radic. Res. 2004, 38, 1241–1253. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, L.; Qiu, W.; Shi, Y. Ferulic acid exhibits anti-inflammatory effects by inducing autophagy and blocking NLRP3 inflammasome activation. Mol. Cell. Toxicol. 2022, 18, 509–519. [Google Scholar] [CrossRef]
- Ahmed, A.U. An overview of inflammation: Mechanism and consequences. Front. Biol. 2011, 6, 274. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Van Dyke, T.E.; Kornman, K.S. Inflammation and Factors That May Regulate Inflammatory Response. J. Periodontol. 2008, 79, 1503–1507. [Google Scholar] [CrossRef]
- Sugimoto, M.A.; Sousa, L.P.; Pinho, V.; Perretti, M.; Teixeira, M.M. Resolution of Inflammation: What Controls Its Onset? Front. Immunol. 2016, 7, 160. [Google Scholar] [CrossRef]
- Varela, M.L.; Mogildea, M.; Moreno, I.; Lopes, A. Acute Inflammation and Metabolism. Inflammation 2018, 41, 1115–1127. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Cumpstey, A.; Feelisch, M. Free Radicals in Inflammation. In Inflammation—From Molecular and Cellular Mechanisms to the Clinic; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; pp. 695–726. [Google Scholar]
- Sánchez, A.; Calpena, A.; Clares, B. Evaluating the Oxidative Stress in Inflammation: Role of Melatonin. Int. J. Mol. Sci. 2015, 16, 16981–17004. [Google Scholar] [CrossRef]
- Wisastra, R.; Dekker, F. Inflammation, Cancer and Oxidative Lipoxygenase Activity are Intimately Linked. Cancers 2014, 6, 1500–1521. [Google Scholar] [CrossRef]
- Pratt, C.L.; Brown, C.R. The role of eicosanoids in experimental Lyme arthritis. Front. Cell. Infect. Microbiol. 2014, 4, 69. [Google Scholar] [CrossRef]
- Ackermann, J.A.; Hofheinz, K.; Zaiss, M.M.; Krönke, G. The double-edged role of 12/15-lipoxygenase during inflammation and immunity. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2017, 1862, 371–381. [Google Scholar] [CrossRef]
- Hu, C.; Ma, S. Recent development of lipoxygenase inhibitors as anti-inflammatory agents. Medchemcomm 2018, 9, 212–225. [Google Scholar] [CrossRef]
- Subramanian, P.; Mendez, E.F.; Becerra, S.P. A Novel Inhibitor of 5-Lipoxygenase (5-LOX) Prevents Oxidative Stress–Induced Cell Death of Retinal Pigment Epithelium (RPE) Cells. Investig. Opthalmol. Vis. Sci. 2016, 57, 4581. [Google Scholar] [CrossRef]
- Bosquesi, P.L.; Melo, T.R.F.; Vizioli, E.O.; dos Santos, J.L.; Chung, M.C. Anti-Inflammatory Drug Design Using a Molecular Hybridization Approach. Pharmaceuticals 2011, 4, 1450–1474. [Google Scholar] [CrossRef]
- Bérubé, G. An overview of molecular hybrids in drug discovery. Expert Opin. Drug Discov. 2016, 11, 281–305. [Google Scholar] [CrossRef]
- Sampath Kumar, H.M.; Herrmann, L.; Tsogoeva, S.B. Structural hybridization as a facile approach to new drug candidates. Bioorg. Med. Chem. Lett. 2020, 30, 127514. [Google Scholar] [CrossRef]
- Chand, M.; Kaushik, R.; Chand Jain, S. Synthesis and antimicrobial and antioxidant activities of hybrid molecules containing benzotriazole and 1,2,4-triazole. Turk. J. Chem. 2018, 42, 1663–1677. [Google Scholar] [CrossRef]
- Li, S.-M.; Chou, J.-Y.; Tsai, S.-E.; Tseng, C.-C.; Chung, C.-Y.; Zeng, W.-Z.; Hu, Y.-P.; Uramaru, N.; Huang, G.-J.; Wong, F.-F. Synthesis and anti-inflammatory activity evaluation of NO-releasing furoxan/1,2,4-triazole hybrid derivatives. Eur. J. Med. Chem. 2023, 257, 115496. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.; Abuo-Rahma, G.E.-D.A.A.; Beshr, E.A.M.; Ali, T.F.S. New nitric oxide donating 1,2,4-triazole/oxime hybrids: Synthesis, investigation of anti-inflammatory, ulceroginic liability and antiproliferative activities. Bioorg. Med. Chem. 2013, 21, 3839–3849. [Google Scholar] [CrossRef]
- Rupapara, M.; Chauhan, M.; Sangani, C.B.; Afzal, M.; Alarifi, A.; Christy, M.; Kapadiya, K. Synthesis of oxadiazole-thiadiazole fluorochromane hybrids and their antioxidant activity. J. Fluor. Chem. 2023, 270, 110171. [Google Scholar] [CrossRef]
- Dewangan, D.; Nakhate, K.T.; Verma, V.S.; Nagori, K.; Badwaik, H.; Nair, N.; Tripathi, D.K.; Mishra, A. Synthesis and Molecular Docking Study of Novel Hybrids of 1,3,4-Oxadiazoles and Quinoxaline as a Potential Analgesic and Anti-Inflammatory Agents. J. Heterocycl. Chem. 2018, 55, 2901–2910. [Google Scholar] [CrossRef]
- George, N.; Al Sabahi, B.; AbuKhader, M.; Al Balushi, K.; Akhtar, M.J.; Khan, S.A. Design, synthesis and in vitro biological activities of coumarin linked 1,3,4-oxadiazole hybrids as potential multi-target directed anti-Alzheimer agents. J. King Saud Univ. Sci. 2022, 34, 101977. [Google Scholar] [CrossRef]
- Pawar, H.S.; Wagh, A.S.; Lali, A.M. Triethylamine: A potential N-base surrogate for pyridine in Knoevenagel condensation of aromatic aldehydes and malonic acid. New J. Chem. 2016, 40, 4962–4968. [Google Scholar] [CrossRef]
- Hadjipavlou-Litina, D.; Pontiki, E. Aryl-Acetic and Cinnamic Acids as Lipoxygenase Inhibitors with Antioxidant, Anti-inflammatory, and Anticancer Activity. Methods Mol. Biol. 2015, 1208, 361–377. [Google Scholar]
- Tsakos, M.; Schaffert, E.S.; Clement, L.L.; Villadsen, N.L.; Poulsen, T.B. Ester coupling reactions—An enduring challenge in the chemical synthesis of bioactive natural products. Nat. Prod. Rep. 2015, 32, 605–632. [Google Scholar] [CrossRef]
- Zhang, X.; Breslav, M.; Grimm, J.; Guan, K.; Huang, A.; Liu, F.; Maryanoff, C.A.; Palmer, D.; Patel, M.; Qian, Y.; et al. A New Procedure for Preparation of Carboxylic Acid Hydrazides. J. Org. Chem. 2002, 67, 9471–9474. [Google Scholar] [CrossRef]
- Kumar Sigalapalli, D.; Kadagathur, M.; Sujat Shaikh, A.; Jadhav, G.S.; Bakchi, B.; Nagendra Babu, B.; Tangellamudi, N.D. Microwave-Assisted TBHP-Mediated Synthesis of 2-Amino-1,3,4-oxadiazoles in Water. ChemistrySelect 2020, 5, 13248–13258. [Google Scholar] [CrossRef]
- El-Ashry, E.S.H.; Rashed, N.; Awad, L.F.; Ramadan, E.S.; Abdel-Maggeed, S.M.; Rezki, N. Synthesis of 5-Aryl-3-Glycosylthio-4-Phenyl-4H-1,2,4-Triazoles and Their Acyclic Analogs Under Conventional and Microwave Conditions. J. Carbohydr. Chem. 2008, 27, 70–85. [Google Scholar] [CrossRef]
- Mobinikhaledi, A.; Foroughifar, N.; Kalhor, M.; Ebrahimi, S.; Fard, M.A.B. Synthesis of Some Symmetrical Novel Bis-thiosemicarbazides, 1,2,4-Triazoles, 1,3,4-Thiadiazoles, and Their Derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2010, 186, 67–73. [Google Scholar] [CrossRef]
- Amir, M.; Saifullah, K.; Akhter, W. Design, synthesis and pharmacological evaluation of novel azole derivatives of aryl acetic acid as anti-inflammatory and analgesic agents. J. Enzyme Inhib. Med. Chem. 2011, 26, 141–148. [Google Scholar] [CrossRef]
- Thorat, S.M.; Bhowmick, A.; Sambasivan, S.; Bhat, R.G. Visible-Light Photoredox Catalyzed, Facile Synthesis of 2-Amino-1,3,4-Thiadiazoles from Aldehydes and Thiosemicarbazides. ChemRxiv 2025. [Google Scholar] [CrossRef]
- Asghar, S.F.; Yasin, K.A.; Habib-ur-Rehman Aziz, S. Synthesis and cyclisation of 1,4-disubstituted semicarbazides. Nat. Prod. Res. 2010, 24, 315–325. [Google Scholar] [CrossRef]
- Polucci, P.; Magnaghi, P.; Angiolini, M.; Asa, D.; Avanzi, N.; Badari, A.; Bertrand, J.; Casale, E.; Cauteruccio, S.; Cirla, A.; et al. Alkylsulfanyl-1,2,4-triazoles, a New Class of Allosteric Valosine Containing Protein Inhibitors. Synthesis and Structure–Activity Relationships. J. Med. Chem. 2013, 56, 437–450. [Google Scholar] [CrossRef]
- Quraishi, M.A.; Sardar, R.; Khan, S. An investigation of the inhibitive capability of synthesized thiosemicarbazides on the corrosion of carbon steel in acid solutions. Anti-Corros. Methods Mater. 2008, 55, 60–65. [Google Scholar] [CrossRef]
- Bate-Smith, E.C.; Westall, R.G. Chromatographic behaviour and chemical structure I. Some naturally occuring phenolic substances. Biochim. Biophys. Acta 1950, 4, 427–440. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef]
- Bariamis, S.E.; Magoulas, G.E.; Grafanaki, K.; Pontiki, E.; Tsegenidis, T.; Athanassopoulos, C.M.; Maroulis, G.; Papaioannou, D.; Hadjipavlou-Litina, D. Synthesis and biological evaluation of new C-10 substituted dithranol pleiotropic hybrids. Bioorg. Med. Chem. 2015, 23, 7251–7263. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D. Multi-Target Cinnamic Acids for Oxidative Stress and Inflammation: Design, Synthesis, Biological Evaluation and Modeling Studies. Molecules 2018, 24, 12. [Google Scholar] [CrossRef]
- Maccarrone, M.; Melino, G.; Finazzi-Agro, A. Lipoxygenases and their involvement in programmed cell death. Cell Death Differ. 2001, 8, 776–784. [Google Scholar] [CrossRef]
- Dixon, R.A.; Jones, R.E.; Diehl, R.E.; Bennett, C.D.; Kargman, S.; Rouzer, C.A. Cloning of the cDNA for human 5-lipoxygenase. Proc. Natl. Acad. Sci. USA 1988, 85, 416–420. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D. Lipoxygenase inhibitors: A comparative QSAR study review and evaluation of new QSARs. Med. Res. Rev. 2008, 28, 39–117. [Google Scholar] [CrossRef]
- Kostopoulou, I.; Tzani, A.; Polyzos, N.-I.; Karadendrou, M.-A.; Kritsi, E.; Pontiki, E.; Liargkova, T.; Hadjipavlou-Litina, D.; Zoumpoulakis, P.; Detsi, A. Exploring the 2′-Hydroxy-Chalcone Framework for the Development of Dual Antioxidant and Soybean Lipoxygenase Inhibitory Agents. Molecules 2021, 26, 2777. [Google Scholar] [CrossRef]
- El Khatabi, K.; El-Mernissi, R.; Aanouz, I.; Ajana, M.A.; Lakhlifi, T.; Khan, A.; Wei, D.-Q.; Bouachrine, M. Identification of novel acetylcholinesterase inhibitors through 3D-QSAR, molecular docking, and molecular dynamics simulation targeting Alzheimer’s disease. J. Mol. Model. 2021, 27, 302. [Google Scholar] [CrossRef]
- Kostopoulou, I.; Diassakou, A.; Kavetsou, E.; Kritsi, E.; Zoumpoulakis, P.; Pontiki, E.; Hadjipavlou-Litina, D.; Detsi, A. Novel quinolinone–pyrazoline hybrids: Synthesis and evaluation of antioxidant and lipoxygenase inhibitory activity. Mol. Divers. 2021, 25, 723–740. [Google Scholar] [CrossRef]
- Mavridis, E.; Bermperoglou, E.; Pontiki, E.; Hadjipavlou-Litina, D. 5-(4H)-Oxazolones and Their Benzamides as Potential Bioactive Small Molecules. Molecules 2020, 25, 3173. [Google Scholar] [CrossRef]
- Maestro. Free Maestro Academic Licence—Schrödinger 14.4 Release 2025-2; Schrödinger LLC: New York, NY, USA, 2025. [Google Scholar]
- Kouzi, O.; Pontiki, E.; Hadjipavlou-Litina, D. 2-Arylidene-1-indandiones as Pleiotropic Agents with Antioxidant and Inhibitory Enzymes Activities. Molecules 2019, 24, 4411. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Fiser, A.; Šali, A. Modeller: Generation and Refinement of Homology-Based Protein Structure Models. Methods Enzymol. 2003, 374, 461–491. [Google Scholar]
- Case, D.A.; Aktulga, H.M.; Belfon, K.; Cerutti, D.S.; Cisneros, G.A.; Cruzeiro, V.W.D.; Forouzesh, N.; Giese, T.J.; Götz, A.W.; Gohlke, H.; et al. AmberTools. J. Chem. Inf. Model. 2023, 63, 6183–6191. [Google Scholar] [CrossRef]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.L.; Dror, R.O.; Shaw, D.E. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins Struct. Funct. Bioinform. 2010, 78, 1950–1958. [Google Scholar] [CrossRef]
- Li, P.; Roberts, B.P.; Chakravorty, D.K.; Merz, K.M. Rational Design of Particle Mesh Ewald Compatible Lennard-Jones Parameters for +2 Metal Cations in Explicit Solvent. J. Chem. Theory Comput. 2013, 9, 2733–2748. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Halgren, T.A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Sousa da Silva, A.W.; Vranken, W.F. ACPYPE—AnteChamber PYthon Parser Interface. BMC Res. Notes 2012, 5, 367. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol. Graph. Model. 2006, 25, 247–260. [Google Scholar] [CrossRef]
- Hess, B.; Kutzner, C.; van der Spoel, D.; Lindahl, E. GROMACS 4: Algorithms for Highly Efficient, Load-Balanced, and Scalable Molecular Simulation. J. Chem. Theory Comput. 2008, 4, 435–447. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Chem-Bio Informatics and Comparative QSAR. 2002. Available online: http://biobyte.com/bb/prod/bioloom.html (accessed on 24 July 2025).





| Compound | Milog P a | TPSA b | No. of Atoms | No. of O and N c | No. of OH and NH d | No. of Violations | No. of Rotational Bonds e | Volume f | MW g | Clog P h |
|---|---|---|---|---|---|---|---|---|---|---|
| 3a | 2.78 | 53.15 | 21 | 4 | 3 | 0 | 6 | 266.92 | 297.38 | 2.39 |
| 3b | 3.46 | 53.15 | 22 | 4 | 3 | 0 | 6 | 280.46 | 331.83 | 3.10 |
| 3c | 2.94 | 53.15 | 22 | 4 | 3 | 0 | 6 | 271.85 | 315.37 | 2.53 |
| 3d | 3.59 | 53.15 | 22 | 4 | 3 | 0 | 6 | 284.81 | 376.28 | 3.25 |
| 3e | 2.33 | 79.46 | 25 | 6 | 3 | 0 | 8 | 311.45 | 355.42 | 1.74 |
| 3f | 2.67 | 71.62 | 24 | 6 | 3 | 0 | 6 | 290.85 | 341.39 | 2.36 |
| 3g | 5.24 | 62.39 | 30 | 5 | 3 | 1 | 9 | 382.00 | 482.40 | 4.94 |
| 4a | 3.93 | 30.72 | 20 | 3 | 0 | 0 | 3 | 248.99 | 279.37 | 4.53 |
| 4b | 4.61 | 30.72 | 21 | 3 | 0 | 0 | 3 | 262.53 | 313.81 | 5.24 |
| 4c | 4.09 | 30.72 | 21 | 3 | 0 | 0 | 3 | 253.92 | 297.36 | 5.39 |
| 4d | 4.74 | 30.72 | 21 | 3 | 0 | 0 | 3 | 266.88 | 358.26 | 4.67 |
| 4e | 3.48 | 57.02 | 24 | 5 | 0 | 0 | 5 | 293.52 | 337.40 | 3.88 |
| 4f | 3.82 | 49.19 | 23 | 5 | 0 | 0 | 3 | 272.92 | 323.28 | 4.5 |
| 4g | 6.39 | 39.95 | 29 | 4 | 0 | 1 | 6 | 364.07 | 464.39 | 7.08 |
| 5a | 3.94 | 50.95 | 20 | 4 | 1 | 0 | 4 | 239.94 | 263.30 | 3.93 |
| 5b | 4.61 | 50.95 | 21 | 4 | 1 | 0 | 4 | 253.47 | 297.75 | 4.64 |
| 5c | 4.10 | 50.95 | 21 | 4 | 1 | 0 | 4 | 244.87 | 281.29 | 4.08 |
| 6a | 4.76 | 37.81 | 20 | 3 | 1 | 0 | 4 | 249.08 | 279.37 | 4.76 |
| Compound | RA % 100 μM 20 min | RA % 100 μM 60 min | AAPH % Inhibition 100 μM | LOX % Inhibition 100 μM or IC50 (μM) |
|---|---|---|---|---|
| 3a | 61 | 92 | 67 | 8.5 μM |
| 3b | 90 | 83 | 37 | 49% |
| 3c | 90 | 91 | 12 | 45% |
| 3d | 29 | 97 | 33 | 8 μM |
| 3e | 83 | 21 | 47 | 9.75 μM |
| 3f | 70 | 89 | 58 | 35 μΜ |
| 3g | 98 | 87 | 91 | 10 μM |
| 4a | 74 | 93 | 26 | 7.5 μM |
| 4b | 33 | 88 | 34 | 4.5 μM |
| 4c | 82 | 93 | 94 | 35% |
| 4d | 68 | 57 | 20 | 97.5 μM |
| 4e | 96 | 93 | 87 | 5.5 μM |
| 4f | 87 | 76 | 71 | 7.5 μM |
| 4g | 97 | 92 | 50 | 4.5 μM |
| 5a | n.a. | 7 | 17 | 32.5 μM |
| 5b | 65 | 72 | n.a. | 18 μM |
| 5c | 92 | 82 | 63 | 17 μM |
| 6a | 98 | 96 | 60 | 5 μM |
| NDGA | 87 | 93 | - | 93 (0.45 μM) |
| Trolox | - | - | 92 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theodoridis, K.; Charissopoulos, E.; Tsioumela, D.; Pontiki, E. Synthesis and Molecular Modeling of Antioxidant and Anti-Inflammatory Five-Membered Heterocycle–Cinnamic Acid Hybrids. Molecules 2025, 30, 3148. https://doi.org/10.3390/molecules30153148
Theodoridis K, Charissopoulos E, Tsioumela D, Pontiki E. Synthesis and Molecular Modeling of Antioxidant and Anti-Inflammatory Five-Membered Heterocycle–Cinnamic Acid Hybrids. Molecules. 2025; 30(15):3148. https://doi.org/10.3390/molecules30153148
Chicago/Turabian StyleTheodoridis, Konstantinos, Eleftherios Charissopoulos, Dimitra Tsioumela, and Eleni Pontiki. 2025. "Synthesis and Molecular Modeling of Antioxidant and Anti-Inflammatory Five-Membered Heterocycle–Cinnamic Acid Hybrids" Molecules 30, no. 15: 3148. https://doi.org/10.3390/molecules30153148
APA StyleTheodoridis, K., Charissopoulos, E., Tsioumela, D., & Pontiki, E. (2025). Synthesis and Molecular Modeling of Antioxidant and Anti-Inflammatory Five-Membered Heterocycle–Cinnamic Acid Hybrids. Molecules, 30(15), 3148. https://doi.org/10.3390/molecules30153148