Preliminary Evaluation of the Nutraceutical Properties in Monovarietal Extra-Virgin Olive Oils and Monitoring Their Stability During Storage
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Chemical Parameters in Different EVOO Cultivars
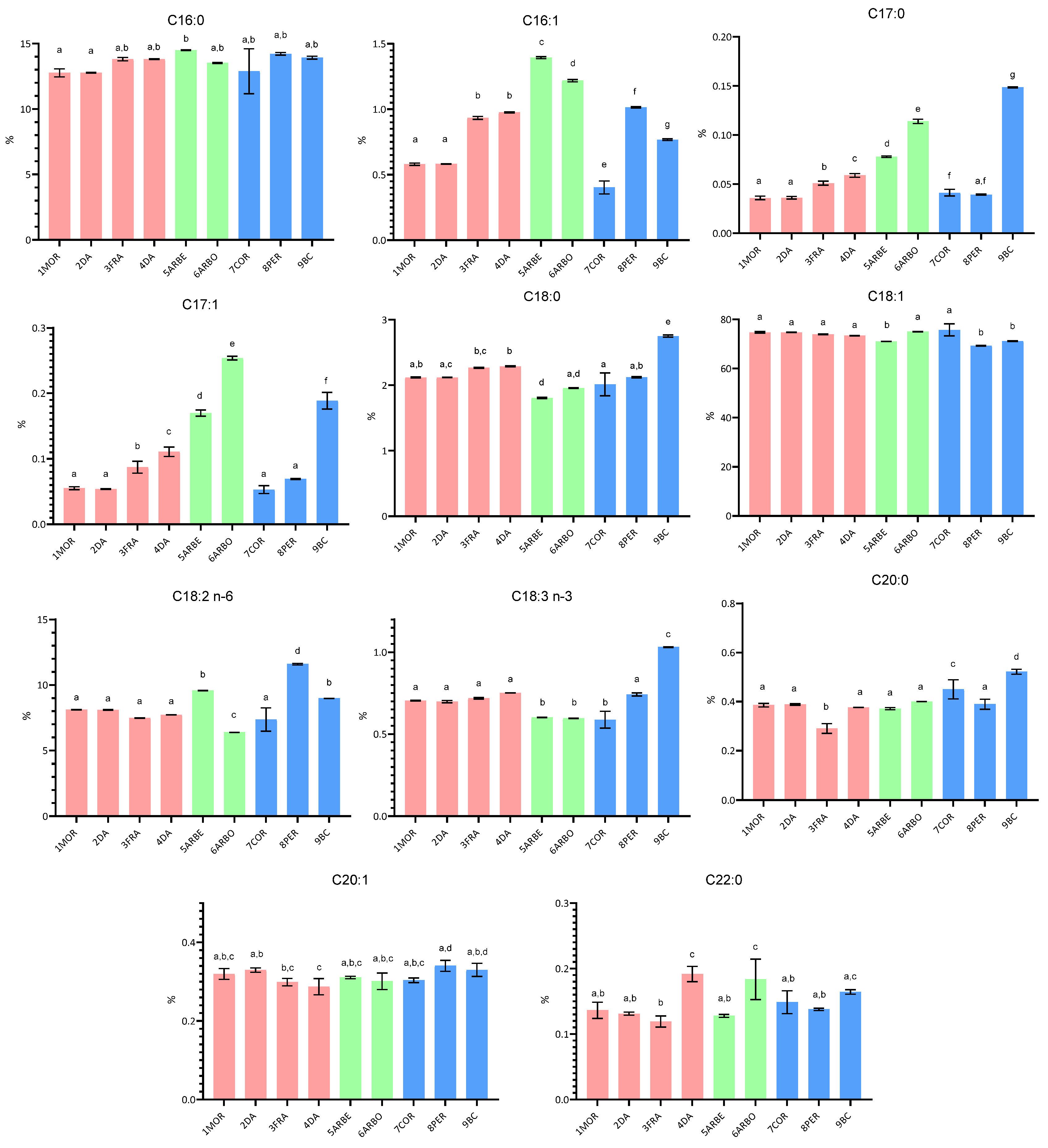
2.1.1. Fatty Acid Composition
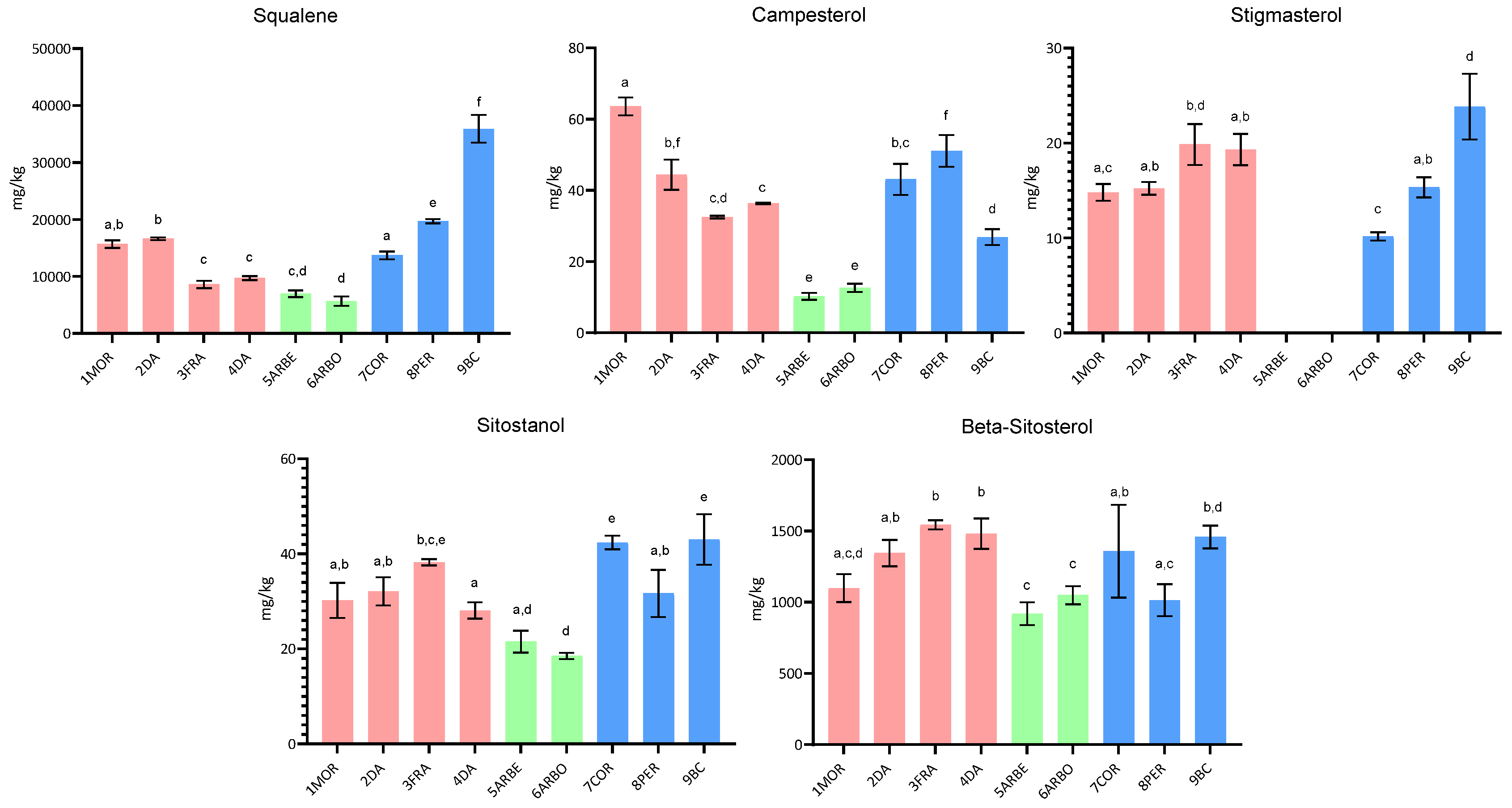
2.1.2. Squalene and Sterol Content
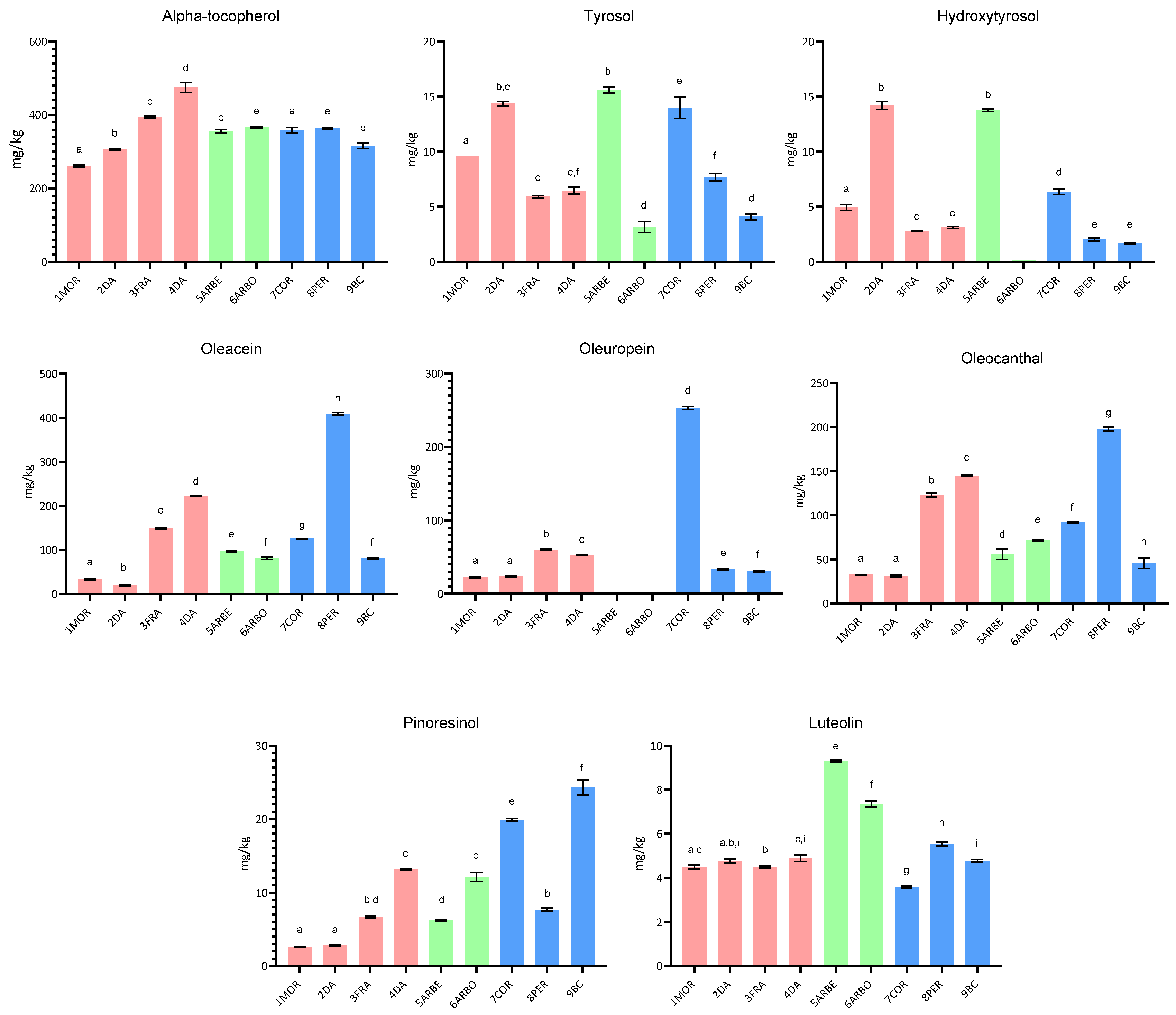
2.1.3. Content of α-Tocopherol and Phenolic Compounds
2.1.4. Principal Components Analysis and Linear Discriminant Analysis
2.2. Sensorial Analysis and Phenolic Content
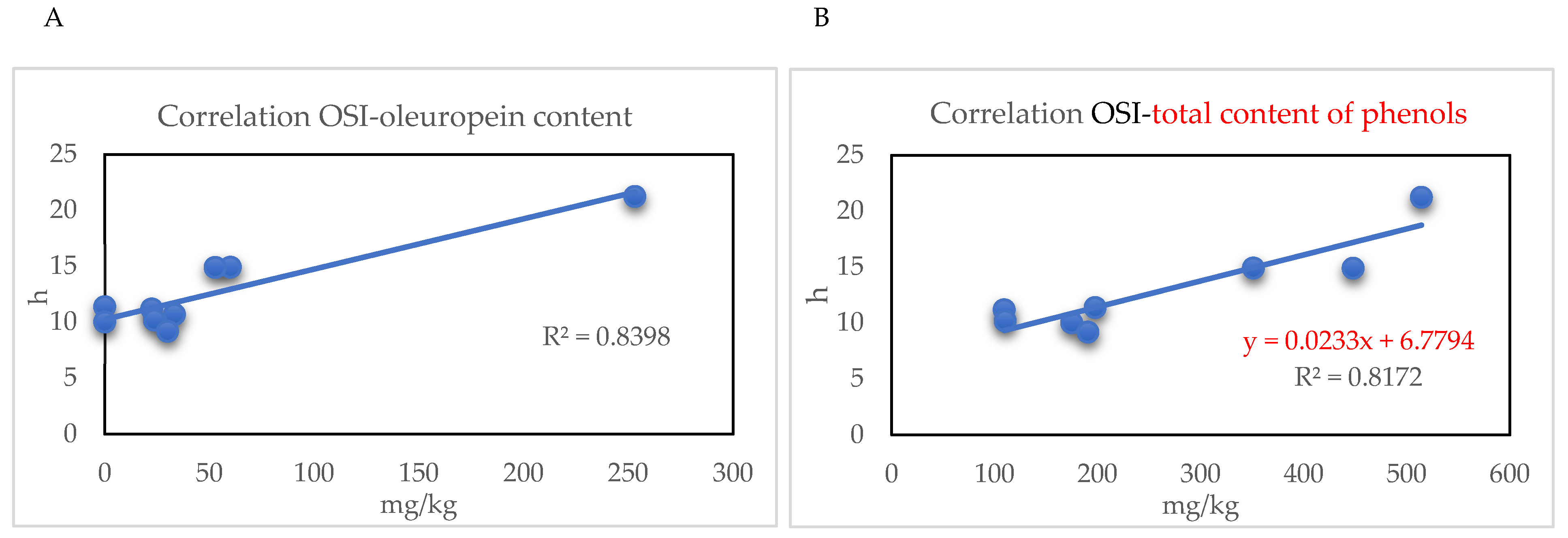
2.3. Oxidative Stability Index and Antioxidant Content
2.4. Evaluation of Changes in the Content of EVOO Bioactive Compounds During Storage
3. Materials and Methods
3.1. Reagents and Solvents
3.2. Samples and Storage Conditions
3.3. Sensory Profile Evaluation
3.4. Determination of Peroxide Value
3.5. Determination of Free Fatty Acids
3.6. Determination of α-Tocopherol
3.7. Determination of Phenolic Compounds
3.8. Determination of Fatty Acid Methyl Esters (FAMEs) Profiles
3.9. Analysis of Squalene and Sterols
3.10. Determination of Oxidative Stability Index (OSI)
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flynn, M.M.; Tierney, A.; Itsiopoulos, C. Is Extra Virgin Olive Oil the Critical Ingredient Driving the Health Benefits of a Mediterranean Diet? A Narrative Review. Nutrients 2023, 15, 2916. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, J.; Xin, Q.; Yuan, R.; Miao, Y.; Yang, M.; Mo, H.; Chen, K.; Cong, W. Protective Effects of Oleic Acid and Polyphenols in Extra Virgin Olive Oil on Cardiovascular Diseases. Food Sci. Hum. Wellness 2024, 13, 529–540. [Google Scholar] [CrossRef]
- Seidita, A.; Soresi, M.; Giannitrapani, L.; Di Stefano, V.; Citarrella, R.; Mirarchi, L.; Cusimano, A.; Augello, G.; Carroccio, A.; Iovanna, J.L.; et al. The Clinical Impact of an Extra Virgin Olive Oil Enriched Mediterranean Diet on Metabolic Syndrome: Lights and Shadows of a Nutraceutical Approach. Front. Nutr. 2022, 9, 980429. [Google Scholar] [CrossRef]
- Alkhalifa, A.E.; Al-Ghraiybah, N.F.; Kaddoumi, A. Extra-Virgin Olive Oil in Alzheimer’s Disease: A Comprehensive Review of Cellular, Animal, and Clinical Studies. Int. J. Mol. Sci. 2024, 25, 1914. [Google Scholar] [CrossRef]
- Markellos, C.; Ourailidou, M.E.; Gavriatopoulou, M.; Halvatsiotis, P.; Sergentanis, T.N.; Psaltopoulou, T. Olive Oil Intake and Cancer Risk: A Systematic Review and Meta-analysis. PLoS ONE 2022, 17, e0261649. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, A.; Martínez-Ortega, A.J.; Remón-Ruiz, P.J.; Piñar-Gutiérrez, A.; Pereira-Cunill, J.L.; García-Luna, P.P. Therapeutic Properties and Use of Extra Virgin Olive Oil in Clinical Nutrition: A Narrative Review and Literature Update. Nutrients 2022, 14, 1440. [Google Scholar] [CrossRef]
- Gagour, J.; Hallouch, O.; Asbbane, A.; Bijla, L.; Laknifli, A.; Lee, L.H.; Zengin, G.; Bouyahya, A.; Sakar, E.H.; Gharby, S. A Review of Recent Progresses on Olive Oil Chemical Profiling, Extraction Technology, Shelf-life, and Quality Control. Chem. Biodivers. 2024, 21, e202301697. [Google Scholar] [CrossRef]
- European Commission. Commission Delegated Regulation (EU) 2022/2104 of 29 July 2022 supplementing Regulation (EU) No 1308/2013 of the European Parliament and of the Council as Regards Marketing Standards for Olive Oil, and Repealing Commission Regulation (EEC) No 2568/91 and Commission Implementing Regulation (EU) No 29/2012. Off. J. Eur. Union. 2022, L284, 1–22. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32022R2104 (accessed on 31 March 2025).
- IOC International Olive Council. Trade Standard Applying to Olive Oils and Olive Pomace Oils. COI/T.15/NC No 3/Rev. 17. Madrid, Spain. 2021. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2021/11/COI-T15-NC3-REV-17_ENK.pdf (accessed on 31 March 2025).
- Farhoosh, R.; Hoseini-Yazdi, S.Z. Shelf-Life Prediction of Olive Oils Using Empirical Models Developed at Low and High Temperatures. Food Chem. 2013, 141, 557–565. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.C. Shelf Life of Extra Virgin Olive Oil and Its Prediction Models. J. Food Qual. 2018, 2018, 639260. [Google Scholar] [CrossRef]
- Mancebo-Campos, V.; Fregapane, G.; Salvador, M.D. Kinetic Study for the Development of an Accelerated Oxidative Stability Test to Estimate Virgin Olive Oil Potential Shelf Life. Eur. J. Lipid Sci. Technol. 2008, 110, 969–976. [Google Scholar] [CrossRef]
- Mancebo-Campos, V.; Salvador, M.D.; Fregapane, G. Modelling Virgin Olive Oil Potential Shelf-Life from Antioxidants and Lipid Oxidation Progress. Antioxidants 2022, 11, 539. [Google Scholar] [CrossRef]
- Calligaris, S.; Lucci, P.; Milani, A.; Rovellini, P.; Lagazio, C.; Conte, L.; Nicoli, M.C. Application of Accelerated Shelf-Life Test (ASLT) Procedure for the Estimation of the Shelf-Life of Extra Virgin Olive Oils: A Validation Study. Food Packag. Shelf Life 2022, 34, 100990. [Google Scholar] [CrossRef]
- Rastrelli, L.; Passi, S.; Ippolito, F.; Vacca, G.; De Simone, F. Rate of Degradation of α-Tocopherol, Squalene, Phenolics, and Polyunsaturated Fatty Acids in Olive Oil during Different Storage Conditions. J. Agric. Food Chem. 2002, 50, 5566–5570. [Google Scholar] [CrossRef]
- Castillo-Luna, A.; Criado-Navarro, I.; Ledesma-Escobar, C.A.; López-Bascón, M.A.; Priego-Capote, F. The Decrease in the Health Benefits of Extra Virgin Olive Oil during Storage is Conditioned by the Initial Phenolic Profile. Food Chem. 2021, 336, 127730. [Google Scholar] [CrossRef]
- Gagour, J.; Oubannin, S.; Bouzid, H.A.; Bijla, L.; Moudden, H.E.; Sakar, E.H.; Koubachi, J.; Laknifli, A.; Gharby, S. Physicochemical Characterization, Kinetic Parameters, Shelf Life and Its Prediction Models of Virgin Olive Oil from Two Cultivars (“Arbequina” and “Moroccan Picholine”) Grown in Morocco. OCL Oils Fat Crop Li. 2022, 29, 39. [Google Scholar] [CrossRef]
- Hrncirik, K.; Fritsche, S. Relation Between the Endogenous Antioxidant System and the Quality of Extra Virgin Olive Oil under Accelerated Storage Conditions. J. Agric. Food Chem. 2005, 53, 2103–2110. [Google Scholar] [CrossRef]
- Gargouri, B.; Zribi, A.; Bouaziz, M. Effect of Containers on the Quality of Chemlali Olive Oil during Storage. J. Food Sci. Technol. 2015, 52, 1948–1959. [Google Scholar] [CrossRef]
- Aparicio, R.; Luna, G. Characterisation of Monovarietal Virgin Olive Oils. Eur. J. Lipid Sci. Technol. 2002, 104, 614–627. [Google Scholar] [CrossRef]
- Lombardo, N.; Marone, E.; Alessandrino, M.; Godino, G.; Madeo, A.; Fiorino, P. Influence of Growing Season Temperatures in the Fatty Acids (FAs) of Triacylglycerols (TAGs) Composition in Italian Cultivars of Olea europaea. Adv. Hortic. Sci. 2008, 22, 49–53. Available online: http://www.jstor.org/stable/42882611 (accessed on 9 June 2025).
- D’Imperio, M.; Dugo, G.; Alfa, M.; Mannina, L.; Segre, A.L. Statistical Analysis on Sicilian Olive Oils. Food Chem. 2007, 102, 956–965. [Google Scholar] [CrossRef]
- El Riachy, M.; Hamade, A.; Ayoub, R.; Dandachi, F.; Chalak, L. Oil Content, Fatty Acid and Phenolic Profiles of Some Olive Varieties Growing in Lebanon. Front. Nutr. 2019, 6, 94. [Google Scholar] [CrossRef]
- Montaño, A.; Hernández, M.; Garrido, I.; Llerena, J.; Espinosa, F. Fatty Acid and Phenolic Compound Concentrations in Eight Different Monovarietal Virgin Olive Oils from Extremadura and the Relationship with Oxidative Stability. Int. J. Mol. Sci. 2016, 17, 11960. [Google Scholar] [CrossRef]
- Consonni, R.; Cagliani, L.R. NMR Studies on Italian PDO Olive Oils and their Potential in Olive-Tree-Derived Products Characterization. Eur. J. Lipid Sci. Technol. 2019, 121, 1800174. [Google Scholar] [CrossRef]
- Aresta, A.; Damascelli, A.; De Vietro, N.; Zambonin, C. Measurement of Squalene in Olive Oil by Fractional Crystallization or Headspace Solid Phase Microextraction Coupled with Gas Chromatography. Int. J. Food Prop. 2020, 23, 1845–1853. [Google Scholar] [CrossRef]
- Beltrán, G.; Bucheli, M.E.; Aguilera, M.P.; Belaj, A.; Jimenez, A. Squalene in Virgin Olive Oil: Screening of Variability in Olive Cultivars: Squalene in Monovarietal Virgin Olive Oils. Eur. J. Lipid Sci. Technol. 2016, 118, 1250–1253. [Google Scholar] [CrossRef]
- Boskou, D. Olive Oil: Chemistry and Technology, 2nd ed.; AOCS Publishing: New York, NY, USA, 2006; ISBN 978-1-893997-88-2. [Google Scholar]
- Torres, M.; Pierantozzi, P.; Contreras, C.; Stanzione, V.; Tivani, M.; Mastio, V.; Gentili, L.; Searles, P.; Brizuela, M.; Fernández, F.; et al. Thermal Regime and Cultivar Effects on Squalene and Sterol Contents in Olive Fruits: Results from a Field Network in Different Argentinian Environments. Sci. Hortic. 2022, 303, 111230. [Google Scholar] [CrossRef]
- Yu, L.; Wang, Y.; Wu, G.; Jin, J.; Jin, Q.; Wang, X. Quality and Composition of Virgin Olive Oils from Indigenous and European Cultivars Grown in China. J. Am. Oil Chem. Soc. 2019, 97, 341–353. [Google Scholar] [CrossRef]
- Franco, M.N.; Galeano-Diaz, T.; Sanchez, J.; De Miguel, C.; Martin-Vertedor, D. Total Phenolic Compounds and Tocopherols Profiles of Seven Olive Oil Varieties Grown in the South-West of Spain. J. Oleo Sci. 2014, 63, 115–125. [Google Scholar] [CrossRef]
- Ninfali, P.; Bacchiocca, M.; Biagiotti, E.; Esposto, S.; Servili, M.; Rosati, A.; Montedoro, G. A 3-Year Study on Quality, Nutritional and Organoleptic Evaluation of Organic and Conventional Extra-Virgin Olive Oils. J. Am. Oil Chem. Soc. 2008, 85, 151–158. [Google Scholar] [CrossRef]
- Baiano, A.; Terracone, C.; Viggiani, I.; Del Nobile, M.A. Changes Produced in Extra-Virgin Olive Oils from cv. Coratina during a Prolonged Storage Treatment. Czech, J. Food Sci. 2014, 32, 1–9. [Google Scholar] [CrossRef]
- Esposito Salsano, J.; Digiacomo, M.; Cuffaro, D.; Bertini, S.; Macchia, M. Content Variations in Oleocanthalic Acid and Other Phenolic Compounds in Extra-Virgin Olive Oil during Storage. Foods 2022, 11, 1354. [Google Scholar] [CrossRef]
- Del Coco, L.; de Pascali, S.; Fanizzi, F. 1H NMR Spectroscopy and Multivariate Analysis of Monovarietal EVOOs as a Tool for Modulating Coratina-Based Blends. Foods 2014, 3, 238–249. [Google Scholar] [CrossRef]
- Mansouri, F.; Ben Moumen, A.; Aazza, S.; Belhaj, K.; Fauconnier, M.L.; Sindic, M.; Serghini Caid, H.; Elamrani, A. Quality and Chemical Profiles of Virgin Olive Oils of three European Cultivars Suitable for Super-High-Density Planting Conditions in Eastern Morocco. Mater. Today Proc. 2019, 13, 998–1007. [Google Scholar] [CrossRef]
- Cecchi, L.; Migliorini, M.; Mulinacci, N. Virgin Olive Oil Volatile Compounds: Composition, Sensory Characteristics, Analytical Approaches, Quality Control, and Authentication. J. Agric. Food Chem. 2021, 69, 2013–2040. [Google Scholar] [CrossRef]
- Redondo-Cuevas, L.; Castellano, G.; Torrens, F.; Raikos, V. Revealing the Relationship Between Vegetable Oil Composition and Oxidative Stability: A Multifactorial Approach. J. Food Compos. Anal. 2018, 66, 221–229. [Google Scholar] [CrossRef]
- Gallina-Toschi, T.; Cerretani, L.; Bendini, A.; Bonoli-Carbognin, M.; Lercker, G. Oxidative Stability and Phenolic Content of Virgin Olive Oil: An Analytical Approach by Traditional and High Resolution Techniques. J. Sep. Sci. 2005, 28, 859–870. [Google Scholar] [CrossRef]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Lercker, G. Phenolic Molecules in Virgin Olive Oils: A Survey of their Sensory Properties, Health Effects, Antioxidant Activity and Analytical Methods: An Overview of the Last Decade. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef]
- Del Carlo, M.; Sacchetti, G.; Di Mattia, C.; Compagnone, D.; Mastrocola, D.; Liberatore, L.; Cichelli, A. Contribution of the Phenolic Fraction to the Antioxidant Activity and Oxidative Stability of Olive Oil. J. Agric. Food Chem. 2004, 52, 4072–4079. [Google Scholar] [CrossRef]
- Esposto, S.; Taticchi, A.; Servili, M.; Urbani, S.; Sordini, B.; Veneziani, G.; Daidone, L.; Selvaggini, R. Overall Quality Evolution of Extra Virgin Olive Oil Exposed to Light for 10 Months in Different Containers. Food Chem. 2021, 351, 129297. [Google Scholar] [CrossRef]
- Botosoa, E.P.; Chèné, C.; Karoui, R. Front Face Fluorescence Spectroscopy Combined with PLS-DA Allows to Monitor Chemical Changes of Edible Vegetable Oils during Storage at 60 °C. Eur. J. Lipid Sci. Technol. 2021, 123, 2000088. [Google Scholar] [CrossRef]
- International Olive Council. COI/T.20/Doc. No 15/Rev. 11. Sensory Analysis of Olive Oil-Method for the Organoleptic Assessment of Virgin Olive Oil. International Olive Council Website. 2024. Available online: https://www.internationaloliveoil.org/what-we-do/chemistry-standardisation-unit/standards-and-methods/ (accessed on 10 April 2025).
- International Olive Council. COI/T.20/Doc. No. 35/Rev. 1—Determination of Peroxide Value—Decision DEC-III-12/106-VI/2017. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2022/10/12-DEC-MET-35-Peroxydes-Eng.pdf (accessed on 10 April 2025).
- International Olive Council. COI/T.20/Doc. No 34//Rev.1/2017—Determination of Free Fatty Acids. Cold Method. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2022/10/12-DEC-MET-35-Peroxydes-Eng.pdf (accessed on 10 April 2025).
- Tura, D.; Gigliotti, C.; Pedò, S.; Failla, O.; Bassi, D.; Serraiocco, A. Influence of Cultivar and Site of Cultivation on Levels of Lipophilic and Hydrophilic Antioxidants in Virgin Olive Oils (Olea europaea L.) and Correlations with Oxidative Stability. Sci. Hortic. 2007, 112, 108–119. [Google Scholar] [CrossRef]
- International Olive Council. COI/T.20/Doc. No 29/Rev.2/2022—Determination of Phenolic Compounds. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2022/10/12-DEC-MET-35-Peroxydes-Eng.pdf (accessed on 10 April 2025).
- International Olive Council. COI/T.20/DOC. 33/Rev. 1/2017—Determination of Fatty Acid Methyl Esters by Gas Chromatography. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2022/10/12-DEC-MET-35-Peroxydes-Eng.pdf (accessed on 10 April 2025).
- Fernández-Cuesta, A.; León, L.; Velasco, L.; De la Rosa, R. Changes in Squalene and Sterols Associated with Olive Maturation. Food Res. Int. 2013, 54, 1885–1889. [Google Scholar] [CrossRef]
- Mousavi, S.; Stanzione, V.; Mencuccini, M.; Baldoni, L.; Bufacchi, M.; Mariotti, R. Biochemical and Molecular Profiling of Unknown Olive Genotypes from Central Italy: Determination of Major and Minor Components. Eur. Food Res. Technol. 2019, 245, 83–94. [Google Scholar] [CrossRef]
- Oxidative Stability of Oil and Fats—Rancimat Method; Metrohm Application Bulletin No. 204/1e; Metrohm AG: Herisau, Switzerland, 1993.






| Fruitiness | Bitterness | Pungency | |
|---|---|---|---|
| 1MOR | 3.00 ± 0.52 a | 1.60 ± 0.15 a | 2.74 ± 0.44 a,b |
| 2DA | 1.88 ± 0.23 b | 1.58 ± 0.37 a | 1.74 ± 0.35 a |
| 3FRA | 4.68 ± 0.66 c | 4.31 ± 0.78 b,c | 3.88 ± 1.30 b,c |
| 4DA | 3.79 ± 0.45 a,d | 3.94 ± 1.11 b,d | 4.14 ± 0.69 c |
| 5ARBE | 1.99 ± 0.36 b | 1.71 ± 0.38 a | 1.91 ± 0.67 a |
| 6ARBO | 3.15 ± 0.75 a | 2.21 ± 0.54 a | 2.33 ± 0.59 a |
| 7COR | 4.84 ± 0.66 c | 5.08 ± 0.32 c | 4.83 ± 0.93 c,d |
| 8PER | 4.26 ± 0.63 c,d | 4.19 ± 0.58 b,d | 4.03 ± 0.77 c |
| 9BC | 5.05 ± 0.57 c | 4.11 ± 0.53 b,d | 4.18 ± 0.62 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cossignani, L.; Calderini, O.; Marinotti, A.; Orrico, E.; Domesi, A.; Massaccesi, L.; Cucina, M.; Bufacchi, M. Preliminary Evaluation of the Nutraceutical Properties in Monovarietal Extra-Virgin Olive Oils and Monitoring Their Stability During Storage. Molecules 2025, 30, 3143. https://doi.org/10.3390/molecules30153143
Cossignani L, Calderini O, Marinotti A, Orrico E, Domesi A, Massaccesi L, Cucina M, Bufacchi M. Preliminary Evaluation of the Nutraceutical Properties in Monovarietal Extra-Virgin Olive Oils and Monitoring Their Stability During Storage. Molecules. 2025; 30(15):3143. https://doi.org/10.3390/molecules30153143
Chicago/Turabian StyleCossignani, Lina, Ornella Calderini, Antonello Marinotti, Emiliano Orrico, Andrea Domesi, Luisa Massaccesi, Mirko Cucina, and Marina Bufacchi. 2025. "Preliminary Evaluation of the Nutraceutical Properties in Monovarietal Extra-Virgin Olive Oils and Monitoring Their Stability During Storage" Molecules 30, no. 15: 3143. https://doi.org/10.3390/molecules30153143
APA StyleCossignani, L., Calderini, O., Marinotti, A., Orrico, E., Domesi, A., Massaccesi, L., Cucina, M., & Bufacchi, M. (2025). Preliminary Evaluation of the Nutraceutical Properties in Monovarietal Extra-Virgin Olive Oils and Monitoring Their Stability During Storage. Molecules, 30(15), 3143. https://doi.org/10.3390/molecules30153143










