Anti-Helicobacter pylori and Anti-Inflammatory Sesquiterpenoids from the Rhizoma of Atractylodes macrocephala
Abstract
1. Introduction
2. Results and Discussions
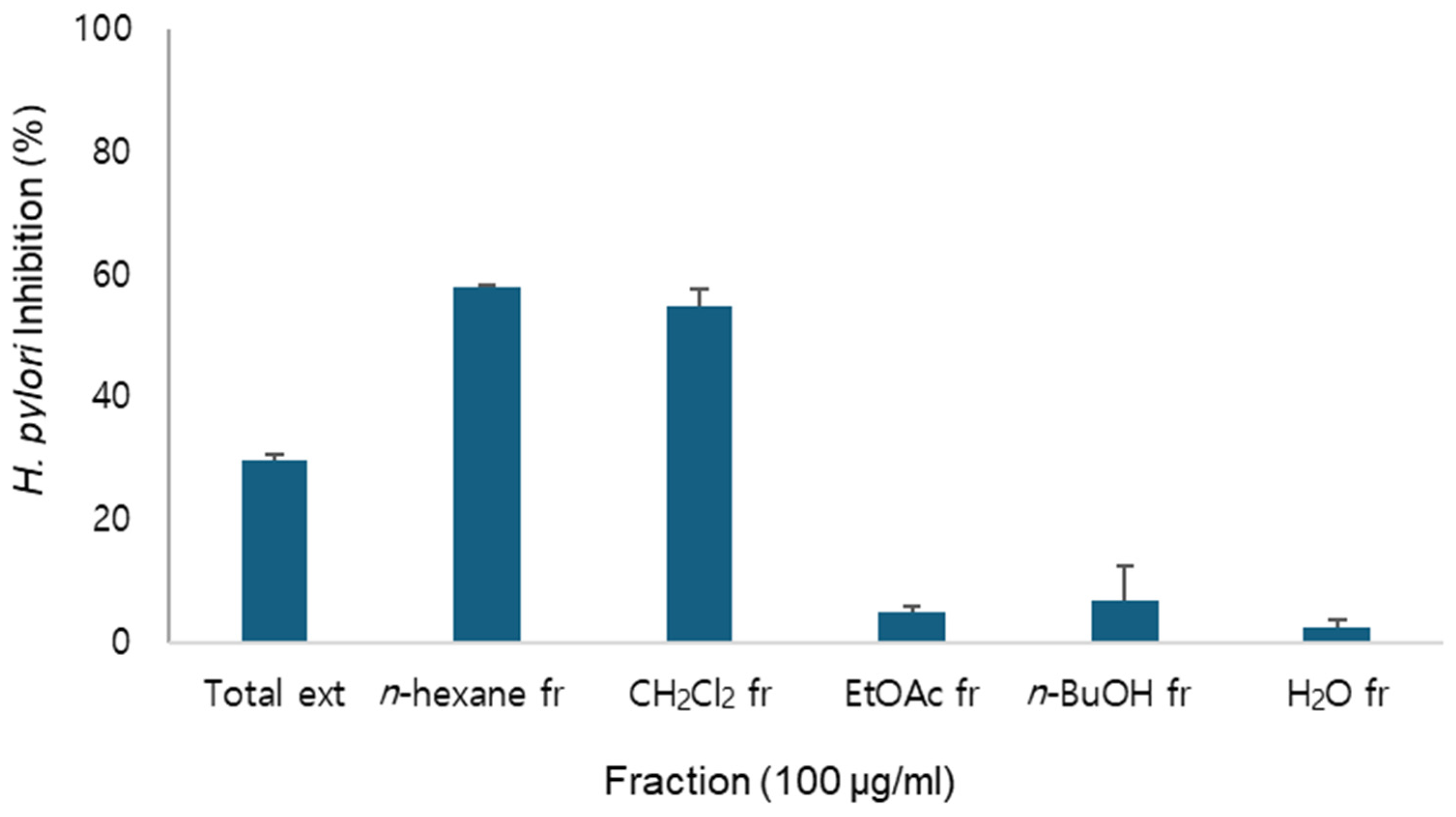
2.1. Anti-H.pylori Activity of A. macrocephala Extract and Fractions
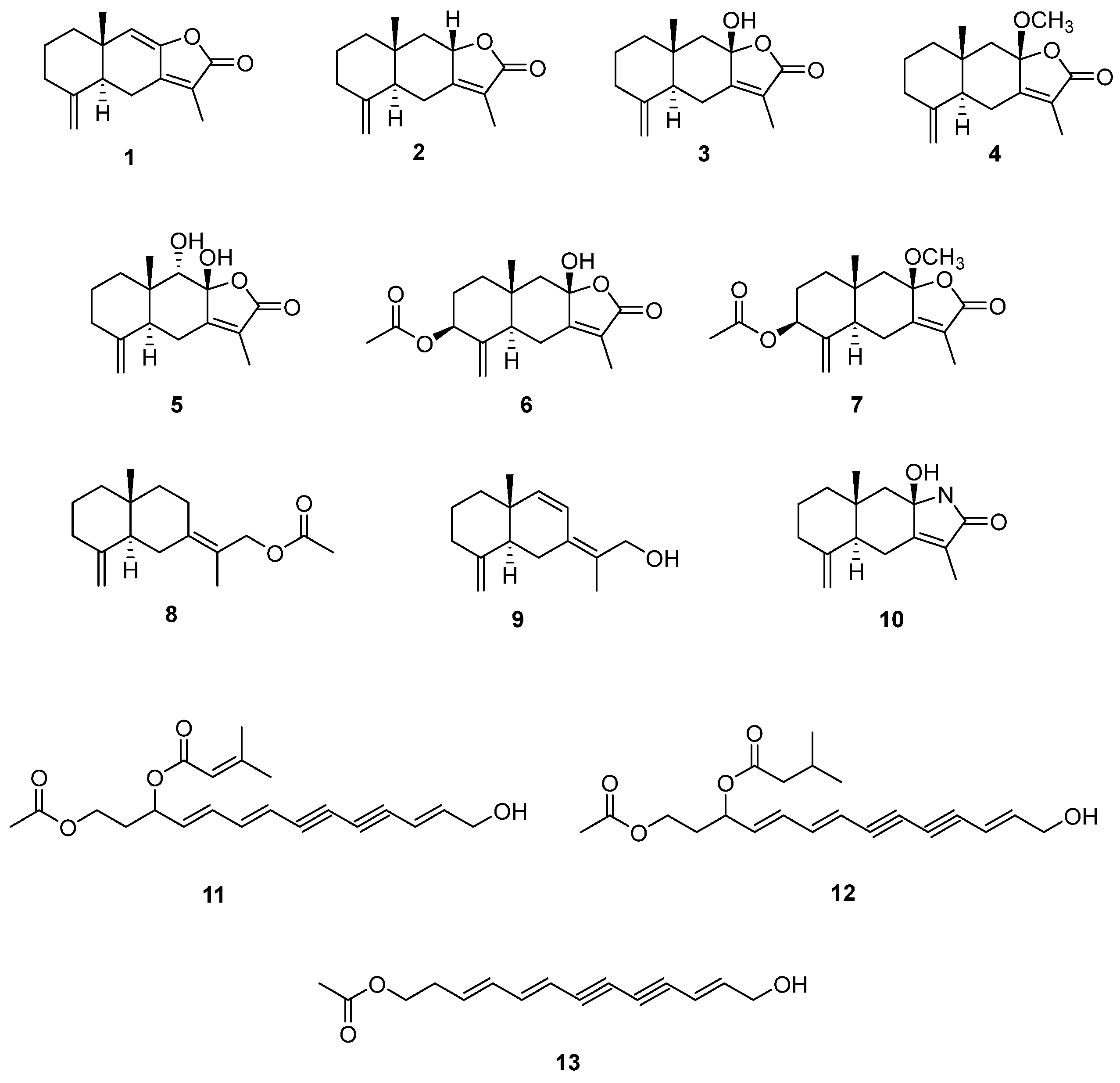
2.2. Isolation of Compounds from A. macrocephala
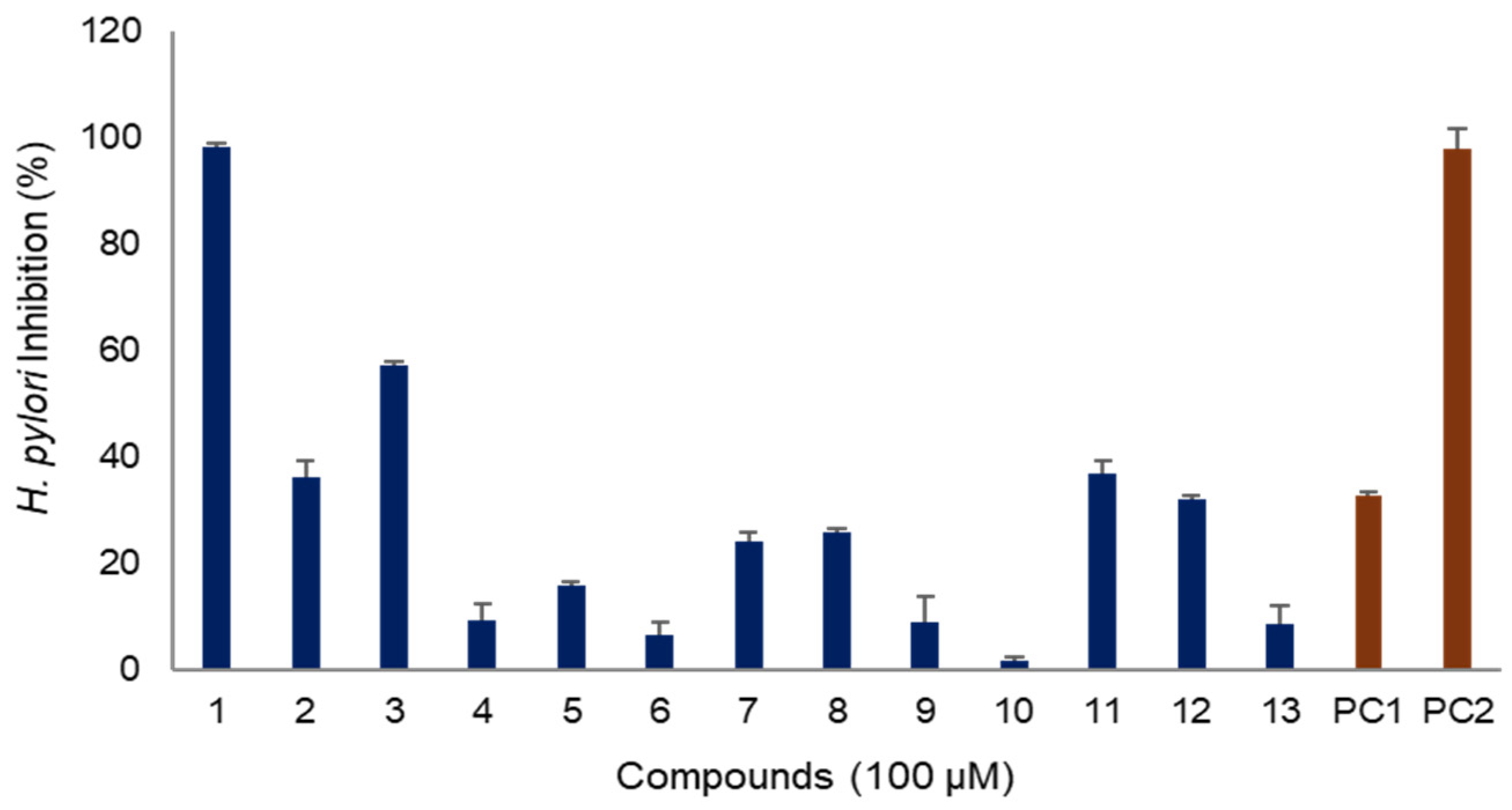
2.3. Anti-H. pylori Activity of Compounds of A. macrocephala Rhizoma
2.3.1. H. pylori Inhibitory Activity
2.3.2. Structure Activity Relationship for H. pylori Inhibitory Activity
2.3.3. Minimum Inhibitory Concentrations (MIC) Against Different H. pylori Strains
2.3.4. Urease Inhibitory Activity
2.4. Anti-Inflammatory Activity
2.5. Potential as Treatment for H. pylori Infection
3. Materials and Methods
3.1. Plant Material
3.2. General Experimental Procedure
3.3. Extraction and Fractionation
3.4. Isolation of Compounds
3.5. Identification of Structures
3.6. Helicobacter pylori Culture
3.7. MICs and MBC Determination
3.8. Urease Inhibitory Activity
3.9. Measurement of LPS-Induced NO Production
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uemura, N.; Okamoto, S.; Yamamoto, S.; Matsumura, N.; Yamaguchi, S.; Yamakido, M.; Taniyama, K.; Sasaki, N.; Schlemper, R.J. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001, 345, 784–789. [Google Scholar] [CrossRef]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology 2001, 153, 420–429. [Google Scholar] [CrossRef]
- Zamani, M.; Ebrahimtabar, F.; Zamani, V.; Miller, W.H.; Alizadeh-Navaei, R.; Shokri-Shirvani, J.; Derakhshan, M.H. Systematic review with meta-analysis: The worldwide prevalence of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2018, 47, 868–876. [Google Scholar] [CrossRef]
- Suerbaum, S. The complex flagella of gastric Helicobacter species. Trends Microbiol. 1995, 3, 168–170. [Google Scholar] [CrossRef]
- Weeks, D.L.; Eskandari, S.; Scott, D.R.; Sachs, G.A. H+-gated urea channel: The link between Helicobacter pylori urease and gastric colonization. Science 2000, 287, 482–485. [Google Scholar] [CrossRef]
- Schoep, T.D.; Fulurija, A.; Good, F.; Lu, W.; Himbeck, R.P.; Schwan, C.; Choi, S.S.; Berg, D.E.; Mittl, P.R.; Benghezal, M.; et al. Surface properties of Helicobacter pylori urease complex are essential for persistence. PLoS ONE 2010, 5, e15042. [Google Scholar] [CrossRef]
- Amieva, M.; Peek, R.M., Jr. Pathobiology of Helicobacter pylori-induced gastric cancer. Gastroenterology. 2016, 150, 64–78. [Google Scholar] [CrossRef]
- Vita, N.A.; Anderson, S.M.; LaFleur, M.D.; Lee, R.E. Targeting Helicobacter pylori for antibacterial drug discovery with novel therapeutics. Curr. Opin. Microbiol. 2022, 70, 102203. [Google Scholar] [CrossRef]
- Di Lodovico, S.; Napoli, E.; Di Campli, E.; Di Fermo, P.; Gentile, D.; Ruberto, G.; Nostro, A.; Marini, E.; Cellini, L.; Di Giulio, M. Pistacia vera L. oleoresin and levofloxacin is a synergistic combination against resistant Helicobacter pylori strains. Sci. Rep. 2019, 9, 4646. [Google Scholar] [CrossRef]
- Sukri, A.; Lopes, B.S.; Hanafiah, A. The emergence of multidrug-resistant Helicobacter pylori in Southeast Asia: A systematic review on the trends and intervention strategies using antimicrobial peptides. Antibiotics 2021, 10, 1061. [Google Scholar] [CrossRef]
- Elbestawy, M.K.M.; El-Sherbiny, G.M.; Moghannem, S.A. Antibacterial, antibiofilm and anti-inflammatory activities of eugenol clove essential oil against resistant Helicobacter pylori. Molecules 2023, 28, 2448. [Google Scholar] [CrossRef]
- Flores-Treviño, S.; Mendoza-Olazarán, S.; Bocanegra-Ibarias, P.; Maldonado-Garza, H.J.; Garza-González, E. Helicobacter pylori drug resistance: Therapy changes and challenges. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 819–827. [Google Scholar] [CrossRef]
- Krzyżek, P.; Junka, A.; Słupski, W.; Dołowacka-Jóźwiak, A.; Płachno, B.J.; Sobiecka, A.; Matkowski, A.; Chodaczek, G.; Płusa, T.; Gościniak, G.; et al. Antibiofilm and antimicrobial-enhancing activity of Chelidonium majus and Corydalis cheilanthifolia extracts against multidrug-resistant Helicobacter pylori. Pathogens 2021, 10, 1033. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/0981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Al-Rajhi, A.M.H.; Qanash, H.; Almashjary, M.N.; Hazzazi, M.S.; Felemban, H.R.; Abdelghany, T.M. Anti-Helicobacter pylori, antioxidant, antidiabetic and anti-Alzheimer’s activities of laurel leaf extract treated by moist heat and molecular docking of its flavonoid constituent, naringenin, against acetylcholinesterase and butyrylcholinesterase. Life 2023, 13, 1512. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, Q.L.; Hua, J.W.; Cheng, W.L.; Qin, L.P. The traditional uses, phytochemistry, and pharmacology of Atractylodes macrocephala Koidz.: A review. J. Ethnopharmacol. 2018, 226, 143–167. [Google Scholar] [CrossRef]
- Yang, L.; Yu, H.; Hou, A.; Man, W.; Wang, S.; Zhang, J.; Wang, X.; Zheng, S.; Jiang, H.; Kuang, H. A Review of the ethnopharmacology, phytochemistry, pharmacology, application, quality control, processing, toxicology, and pharmacokinetics of the dried rhizome of Atractylodes macrocephala. Front. Pharmacol. 2021, 12, 727154. [Google Scholar] [CrossRef]
- Li, L.; He, Y.; Wang, N.; Li, Y.; Du, Y.; He, N.; Wang, B.; Zhang, T. Atractylone in the Atractylodes macrocephala rhizoma essential oil and its anti-inflammatory activity. Molecules 2023, 28, 7340. [Google Scholar] [CrossRef]
- Liu, C.; Wang, S.; Xiang, Z.; Xu, T.; He, M.; Xue, Q.; Song, H.; Gao, P.; Cong, Z. The chemistry and efficacy benefits of polysaccharides from Atractylodes macrocephala Koidz. Front. Pharmacol. 2022, 13, 952061. [Google Scholar] [CrossRef]
- Dong, H.; He, L.; Huang, M.; Dong, Y. Anti-inflammatory components isolated from Atractylodes macrocephala Koidz. J. Nat. Prod. Res. 2008, 22, 418–1427. [Google Scholar]
- Duan, J.A.; Wang, L.; Qian, S.; Su, S.; Tang, Y. A new cytotoxic prenylated dihydrobenzofuran derivative and other chemical constituents from the rhizomes of Atractylodes lancea DC. Arch. Pharm. Res. 2008, 31, 965–969. [Google Scholar] [CrossRef]
- Zhu, L.P.; Li, Y.; Yang, J.Z.; Zuo, L.; Zhang, D.M. Two new sesquiterpene lactones from Sarcandra glabra. J. Asian Nat. Prod. Res. 2008, 10, 541–545. [Google Scholar] [CrossRef]
- Cheng, Y.B.; Chen, C.Y.; Kuo, Y.H.; Shen, Y.C. New nitrogen-containing sesquiterpenoids from the taiwanese soft coral Cespitularia taeniata May. Chem. Biodivers. 2009, 6, 1266–1272. [Google Scholar] [CrossRef]
- Chen, L.G.; Jan, Y.S.; Tsai, P.W.; Norimoto, H.; Michihara, S.; Murayama, C.; Wang, C.C. Anti-inflammatory and antinociceptive constituents of Atractylodes japonica Koidzumi. J. Agric. Food Chem. 2016, 64, 2254–2262. [Google Scholar] [CrossRef]
- Tissandié, L.; Brevard, H.; Belhassen, E.; Alberola, M.; Meierhenrich, U.; Filipp, J.J. Integrated comprehensive two-dimensional gas-chromatographic and spectroscopic characterization of vetiveryl acetates: Molecular identifications, quantification of constituents, regulatory and olfactory considerations. J. Chromatogr. A 2018, 1573, 125–150. [Google Scholar] [CrossRef]
- Wang, S.Y.; Dinga, L.F.; Sub, J.; Pengb, L.Y.; Song, L.D.; Wub, X.D. Atractylmacrols A-E, sesquiterpenes from the rhizomes of Atractylodes macrocephala. Phytochem. Lett. 2018, 23, 127–131. [Google Scholar] [CrossRef]
- Eaton, K.A.; Brooks, C.; Morgan, D.; Krakowka, S. Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect. Immun. 1991, 59, 2470–2475. [Google Scholar] [CrossRef]
- Ibrar, A.; Khan, I.; Abbas, N. Structurally diversified heterocycles and related privileged scaffolds as potential urease inhibitors: A brief overview. Arch. Pharm. 2013, 346, 423–446. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, K.; Wang, Y.; Ye, M.; Zhang, Y.; Xu, W.; Chen, L.; Li, H. The rhizome of Atractylodes macrocephala Koidz.: A comprehensive review on the traditional uses, phytochemistry and pharmacology. Chem. Biodivers. 2024, 2024, e202401879. [Google Scholar] [CrossRef]
- Roszczenko-Jasińska, P.; Wojtyś, M.I.; Jagusztyn-Krynicka, E.K. Helicobacter pylori treatment in the post-antibiotics era-searching for new drug targets. Appl. Microbiol. Biotechnol. 2020, 104, 9891–9905. [Google Scholar] [CrossRef]
- Zhou, W.; Zheng, X.; Wang, X.; Tian, Y.; Wen, Y.; Tu, Y.; Lei, J.; Cheng, H.; Yu, J. Bioassay-guided isolation of antibacterial and anti-inflammatory components from Atractylodes lancea. Phytochemistry 2024, 227, 114232. [Google Scholar] [CrossRef]
- Jeong, S.I.; Kim, S.Y.; Kim, S.J.; Hwang, B.S.; Kwon, T.H.; Yu, K.Y.; Hang, S.H.; Suzuki, K.; Kim, K.J. Antibacterial activity of phytochemicals isolated from Atractylodes japonica against methicillin-resistant Staphylococcus aureus. Molecules 2010, 15, 7395–7402. [Google Scholar] [CrossRef]
- Park, W.S.; Bae, J.-Y.; Kim, H.J.; Kim, M.G.; Lee, W.-K.; Kang, H.-L.; Baik, S.-C.; Lim, K.M.; Lee, M.K.; Ahn, M.-J. Anti-Helicobacter pylori compounds from Maackia amurensis. Nat. Prod. Sci. 2015, 21, 49–53. [Google Scholar]
- Lee, S.; Kim, T.W.; Lee, Y.H.; Kang, D.M.; Ryoo, R.; Ko, Y.J.; Ahn, M.J.; Kim, K.H. Two new fatty acid derivatives, omphalotols A and B and anti-Helicobacter pylori fatty acid derivatives from poisonous mushroom Omphalotus japonicus. Pharmaceuticals 2022, 15, 139. [Google Scholar] [CrossRef]
- Kang, D.M.; Khalil, A.A.K.; Park, W.S.; Kim, H.J.; Akter, K.M.; Bae, J.Y.; Mehtap, B.S.; Kim, J.H.; Kang, K.K.; Ahn, M.J. Anti-Helicobacter pylori activity of six major compounds isolated from Rumex acetosa. ACS Omega 2023, 8, 42548–42554. [Google Scholar] [CrossRef]
- Vu, N.K.; Le, T.T.; Tran, T.T.; Ha, M.T.; Kim, J.A.; Min, B.S. Anti-inflammatory constituents from Artemisia iwayomogi Kitamura: A bioassay-guided fractionation study. Nat. Prod. Sci. 2025, 31, 43–48. [Google Scholar] [CrossRef]
| Strains | MIC (μg/mL) | Atractylenolide I (1) | Atractylenolide III (3) | PC1 | PC2 |
|---|---|---|---|---|---|
| 51 | MIC | 3.13 | 12.5 | 3.13 | 50 |
| MIC50 | 27.3 | 89.9 | 14.9 | >100 | |
| MIC90 | 45.8 | >100 | 49.8 | >100 | |
| MBC | 54.9 | >100 | 59.7 | >100 | |
| 26695 | MIC | 6.25 | 25 | 3.13 | 50 |
| MIC50 | 43.3 | >100 | 20.9 | >100 | |
| MIC90 | 79.1 | >100 | 42.1 | >100 | |
| MBC | 91.9 | >100 | 53.1 | >100 | |
| 43504 | MIC | 12.5 | 100 | 3.13 | 50 |
| MIC50 | 48.6 | >100 | 28.3 | >100 | |
| MIC90 | 87.2 | >100 | 62.1 | >100 | |
| MBC | 94.3 | >100 | 81.5 | >100 |
| Urease Inhibition (%) | |||
|---|---|---|---|
| 250 μM | 500 μM | 1000 μM | |
| atractylenolide I (1) | 0.1 ± 4.4 | 5.8 ± 5.3 | 5.7 ± 3.2 |
| atractylenolide III (3) | 1.9 ± 5.0 | 2.2 ± 3.4 | 3.2 ± 5.1 |
| Inhibition on NO Production (%) | IC50 (μM) | |||
|---|---|---|---|---|
| 10 μM | 25 μM | 50 μM | ||
| atractylenolide I (1) | 29.6 ± 1.66 | 54.0 ± 3.00 | 89.9 ±0.99 | 23.3 |
| atractylenolide III (3) | 23.0 ± 3.30 | 43.6 ± 4.11 | 71.7 ± 4.33 | 31.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.Y.; Kang, D.-M.; Kim, H.-J.; Yeon, S.W.; Lee, H.H.; Kim, M.H.; Hwang, B.Y.; Ahn, M.-J.; Lee, M.K. Anti-Helicobacter pylori and Anti-Inflammatory Sesquiterpenoids from the Rhizoma of Atractylodes macrocephala. Molecules 2025, 30, 3142. https://doi.org/10.3390/molecules30153142
Jeong SY, Kang D-M, Kim H-J, Yeon SW, Lee HH, Kim MH, Hwang BY, Ahn M-J, Lee MK. Anti-Helicobacter pylori and Anti-Inflammatory Sesquiterpenoids from the Rhizoma of Atractylodes macrocephala. Molecules. 2025; 30(15):3142. https://doi.org/10.3390/molecules30153142
Chicago/Turabian StyleJeong, So Yeong, Dong-Min Kang, Hyun-Jun Kim, Sang Won Yeon, Hak Hyun Lee, Min Hee Kim, Bang Yeon Hwang, Mi-Jeong Ahn, and Mi Kyeong Lee. 2025. "Anti-Helicobacter pylori and Anti-Inflammatory Sesquiterpenoids from the Rhizoma of Atractylodes macrocephala" Molecules 30, no. 15: 3142. https://doi.org/10.3390/molecules30153142
APA StyleJeong, S. Y., Kang, D.-M., Kim, H.-J., Yeon, S. W., Lee, H. H., Kim, M. H., Hwang, B. Y., Ahn, M.-J., & Lee, M. K. (2025). Anti-Helicobacter pylori and Anti-Inflammatory Sesquiterpenoids from the Rhizoma of Atractylodes macrocephala. Molecules, 30(15), 3142. https://doi.org/10.3390/molecules30153142







