Bivalent Inhibitors of Mannose-Specific Bacterial Adhesion: A Xylose-Based Conformational Switch to Control Glycoligand Distance †
Abstract
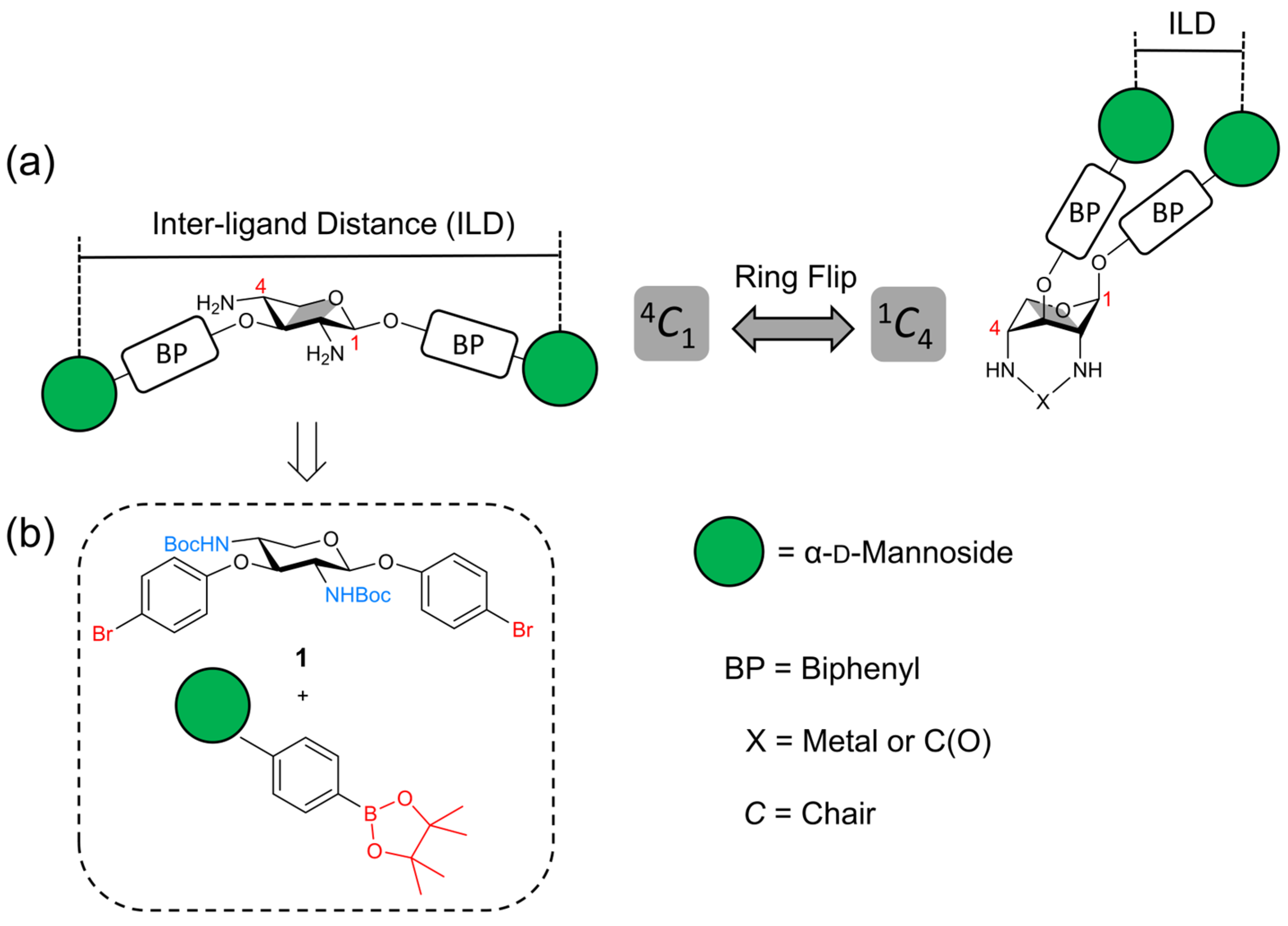
1. Introduction
2. Results
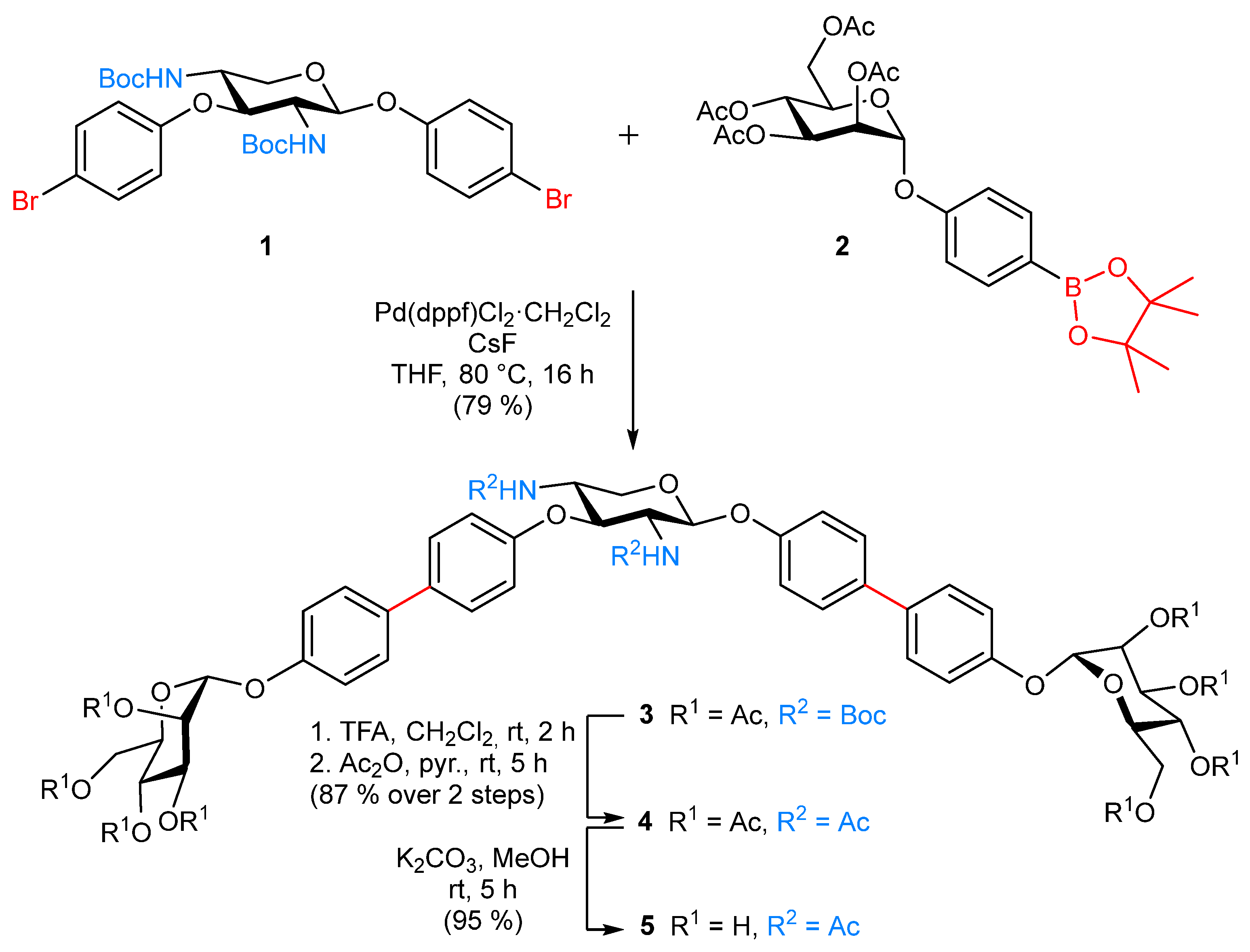
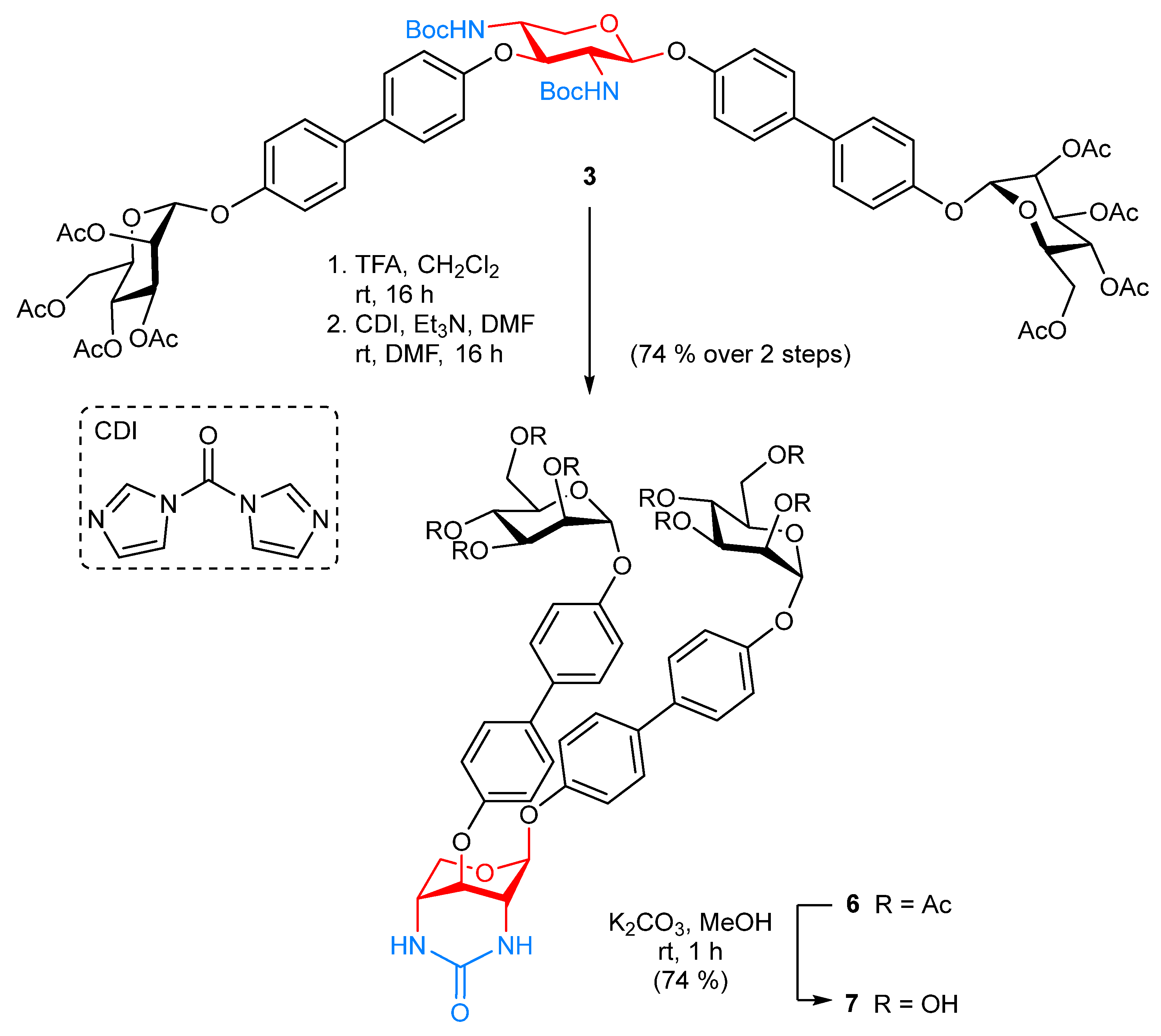
2.1. Synthesis
2.2. Molecular Dynamics
2.3. Biological Testing
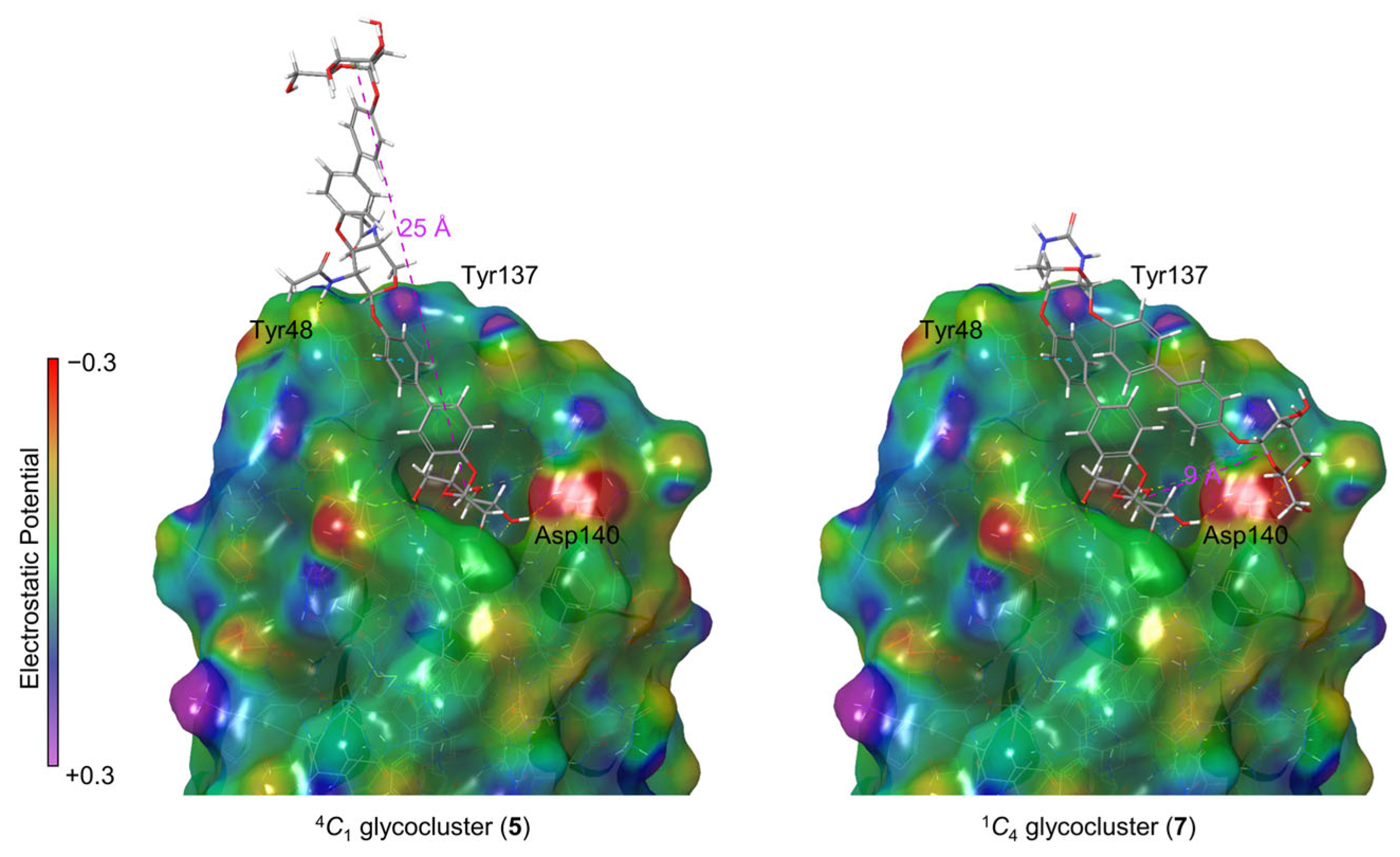
2.4. Molecular Modeling
3. Materials and Methods
3.1. General Information Regarding Synthesis and Spectroscopy
3.2. Synthesis
- 4′-[(1,1′-Biphenyl)-2,3,4,6-tetra-O-acetyl-α-d-mannopyranoside]-4-yl 2,4-dideoxy-2,4-N-Boc-3-O-{4′-[(1,1′-biphenyl)-2,3,4,6-tetra-O-acetyl-α-d-mannopyranoside]-4-yl}-β-d-xylopyranoside (3). The xyloside 1 [26] (60.0 mg, 87.2 µmol), the pinacoyl ester 2 [28] (144 mg, 261 µmol), CsF (79.4 mg, 523 µmol) and Pd(dppf)Cl2·CH2Cl2 (14.2 mg, 17.4 µmol) were dissolved in dry THF (6.00 mL) and the reaction mixture was heated to 80 °C and stirred for 16 h. After cooling to room temperature, it was diluted with ethyl acetate and the organic layer was washed with H2O. The combined organic layers were dried over MgSO4, it was filtered and concentrated. Purification by column chromatography on silica gel (cyclohexane/ethyl acetate, 1:1) yielded 3 (93.0 mg, 79%) as a colorless solid; Rf = 0.22 (cyclohexane/ethyl acetate, 1:1); [α = +31.2 (c 0.02, acetone). 1H NMR (600 MHz, acetone-d6, 298 K, TMS): δ = 7.63–7.57 (m, 6H, 6 Harom), 7.52 (d, 3J = 8.7 Hz, 2H, Harom), 7.27–7.21 (m, 4H, 4 Harom), 7.20–7.11 (m, 4H, 4 Harom), 6.50 (d, 3JNH,2 = 8.6 Hz, 1H, NH), 6.33 (d, 3JNH,4 = 7.9 Hz, 1H, NH), 5.71 (d, 3J1,2 = 1.1 Hz, 1H, H-1Man), 5.70 (d, 3J1,2 = 1.1 Hz, 1H, H-1Man), 5.51–5.46 (m, 4H, 2 H-2Man, 2 H-3Man), 5.43 (d, 3J1,2 = 7.2 Hz, 1H, H-1Xyl), 5.34 (dd~t, 3J3,4 = 9.9 Hz, 3J4,5 = 9.9 Hz, 2H, 2 H-4Man), 4.93 (t, 3J3,4 = 9.2 Hz, 3J2,3 = 9.2 Hz, 1H, H-3Xyl), 4.25 (dd, 2J6a,6b = 12.0 Hz, 3J5,6a = 5.9 Hz, 1H, H-6aMan), 4.24 (dd, 2J6a,6b = 12.0 Hz, 3J5,6a = 5.9 Hz, 1H, H-6aMan), 4.20–4.15 (m, 2H, 2 H-5Man), 4.10–4.07 (m, 2H, 2 H-6bMan), 4.03 (dd, 2J5a,5b = 11.1 Hz, 3J5a,4 = 4.7 Hz, 1H, H-5aXyl), 3.96–3.90 (m, 1H, H 4Xyl), 3.84–3.78 (m, 1H, H-2Xyl), 3.73 (t, 2J5a,5b = 10.9 Hz, 3J5a,4 = 10.9 Hz, 1H, H-5bXyl), 2.16, 2.06, 1.99, 1.95, 1.94 (each s, 24H, 8 C(O)CH3), 1.33, 1.31 (each s, 18H, 2 C(CH3)3) ppm. 13C NMR (125 MHz, acetone-d6, 298 K, TMS): δ = 170.62, 170.40, 170.37, 170.28 (8C, C(O)CH3), 160.33 (NH-C(O)), 158.03 (NH-C(O)), 155.91 (Cquart), 155.75 (Cquart), 138.50 (2 Cquart), 136.50 (Cquart), 136.54 (Cquart), 136.27 (Cquart), 135.53 (Cquart), 128.61 (2 CHarom), 128.57 (2 CHarom), 128.47 (2 CHarom), 128.24 (2 CHarom), 118.24 (2 CHarom), 118.23 (2 CHarom), 118.15 (2 CHarom), 118.12 (2 CHarom), 100.54 (C-1Xyl), 96.97 (2 C-1Man), 79.06 (C-3Xyl), 70.28 (2 C-5Man), 69.88 (2 C-2Man), 69.80 (2 C-3Man), 66.61 (2 C-4Man), 64.64 (C-5Xyl), 62.94 (2 C-6Man), 57.89 (C-2Xyl), 53.26 (C-4Xyl), 28.55 (3C, C(CH3)3), 28.53 (3C, C(CH3)3), 21.71, 20.65, 20.61, 20.59 (8C, C(O)CH3) ppm. IR (ATR) νmax/cm−1 = 3309, 1750, 1683, 1497, 1367, 1220, 1039, 824, 601; ESI-HRMS m/z = 1362.5278 [M + NH4]+, calcd for [M + NH4]+ 1362.5292.
- 4′-[(1,1′-Biphenyl)-2,3,4,6-tetra-O-acetyl-α-d-mannopyranoside]-4-yl 2,4-dideoxy-2,4-N-acetyl-3-O-{4′-[(1,1′-biphenyl)-2,3,4,6-tetra-O-acetyl-α-d-mannopyranoside]-4-yl}-β-d-xylopyranoside (4). The N-Boc-protected glycocluster 3 (20.0 mg, 14.9 µmol) was dissolved in CH2Cl2 (1.00 mL), TFA (200 µL) was added and the reaction mixture stirred for 2 h at room temperature. Then all volatiles were removed in vacuo and the residue co-evaporated with toluene (3×). The crude product was dissolved in dry pyridine (2.00 mL), Ac2O (500 µL) was added and the reaction mixture stirred at room temperature for 5 h. Then it was diluted with ethyl acetate and the organic layer was subsequently washed with 1N HCl, sodium bicarbonate and brine. The organic layer was dried over MgSO4, its was filtered and concentrated. Purification by column chromatography on silica (toluene/acetone, 1:1) gave the title compound 4 (16.0 mg, 87%) as a colorless solid; Rf = 0.32 (toluene/acetone, 1:1); [α = +16.6 (c 0.02, acetone). 1H NMR (500 MHz, acetone-d6, 298 K, TMS): δ = 7.63–7.59 (m, 4H, 4 Harom), 7.58–7.54 (m, 4H, 4 Harom), 7.51 (d, 3JNH,2 = 8.6 Hz, 1H, NH), 7.36 (d, 3JNH,4 = 8.4 Hz, 1H, NH), 7.27–7.22 (m, 4H, 4 Harom), 7.19 (d, 3J = 8.9 Hz, 2H, Harom), 7.11 (d, 3J = 8.8 Hz, 2H, Harom), 5.71–5.69 (m, 2H, 2 H-1Man), 5.57 (d, 3J1,2 = 6.5 Hz, 1H, H-1Xyl), 5.50-5.46 (m, 4H, 2 H-2Man, 2 H-3Man), 5.33 (dd~t, 3J3,4 = 9.9 Hz, 3J4,5 = 9.9 Hz, 2H, 2 H-4Man), 4.99 (t, 3J3,4 = 7.9 Hz, 3J2,3 = 7.9 Hz, 1H, H-3Xyl), 4.23 (dd, 2J6a,6b = 12.0 Hz, 3J5,6a = 5.9 Hz, 2H, 2 H-6aMan), 4.21–4.10 (m, 4H, 2 H-5Man, H-5aXyl, H-4Xyl), 4.08 (dd, 2J6a,6b = 12.0 Hz, 3J5,6b = 2.3 Hz, 2H, 2 H-6bMan), 4.05–3.99 (m, 1H, H-2Xyl), 3.73 (dd, 2J5a,5b = 11.6 Hz, 3J5a,4 = 8.4 Hz, 1H, H-5bXyl), 2.16, 2.05, 1.99, 1.94 (each s, 24H, 8 C(O)CH3), 1.80, 1.75 (each s, 6H, NHC(O)CH3) ppm. 13C NMR (125 MHz, acetone-d6, 298 K, TMS): δ = 170.65, 170.43, 170.40, 170.31 (8C, C(O)CH3), 170.54, 170.34 (2C, NHC(O)CH3), 159.70 (Cquart), 159.62 (Cquart), 155.94 (Cquart), 155.80 (Cquart), 136.45 (Cquart), 136.31 (Cquart), 134.33 (Cquart), 128.63 (2 CHarom), 128.59 (2 CHarom), 128.49 (2 CHarom), 128.23 (2 CHarom), 118.28 (4C, CHarom), 118.10 (2 CHarom), 117.97 (2 CHarom), 99.43 (C-1Xyl), 97.01 (2 C-1Man), 77.09 (C-3Xyl), 70.31 (2 C-5Man), 69.9 2 (2 C-2Man), 69.83 (2 C-3Man), 66.64 (2 C-4Man), 63.38 (C-5Xyl), 62.97 (2 C-6Man), 55.57 (C-2Xyl), 50.93 (C-4Xyl), 23.09, 23.00 (2C, NHC(O)CH3), 21.74, 20.68, 20.64, 20.61 (8C, C(O)CH3) ppm. IR (ATR) νmax/cm−1 2298, 1750, 1662, 1496, 1370, 1221, 1039, 825, 600. ESI-HRMS m/z = 1246.4428 [M + NH4]+, calcd for [M + NH4]+ = 1246.4449.
- 4′-[(1,1′-Biphenyl)-α-d-mannopyranosyloxy]-4-yl 2,4-dideoxy-2,4-N-acetyl-3-O-{4′-[(1,1′-biphenyl)-α-d-mannopyranosyloxy]-4-yl}-β-d-xylopyranoside (5). The protected glycocluster 4 (13.0 mg, 10.6 µmol) was dissolved in MeOH (1.00 mL), K2CO3 (414 µg, 3.00 µmol) was added and it was stirred for 5 h at room temperature. It was neutralized with Amberlite® IR-120, filtered and concentrated to dryness. Co-evaporation with toluene and CH2Cl2 yielded 5 (9.00 mg, 95%) as a colorless solid; [α = +26.3 (c 0.02, MeOH). 1H NMR (600 MHz, MeOD-d3, 298 K, TMS): δ = 7.53–7.47 (m, 8H, 8 Harom), 7.24–7.05 (m, 8H, 8 Harom), 5.51 (s, 2H, 2 H-1Man), 5.35 (d, 3J1,2 = 7.7 Hz, 1H, H-1Xyl), 4.85–4.82 (m, 1H, H-3Xyl), 4.24–4.18 (m, 1H, H-4Xyl), 4.06–4.00 (m, 4H, H-2Xyl, H-5aXyl, 2 H-2Man), 3.92 (dd, 3J3,4 = 8.3 Hz, 3J2,3 = 4.0 Hz, 2H, 2 H-3Man), 3.80–3.70 (m, 6H, 2 H-4Man, 2 H-6aMan, 2 H-6bMan), 3.66–3.59 (m, 3H, 2 H-5Man, H-5bXyl), 1.81, 1.76 (each s, 6H, NHC(O)CH3) ppm. 13C NMR (125 MHz, MeOD-d3, 298 K, TMS): δ = 173.68, 173.53 (2C, NHC(O)CH3), 159.96 (Cquart), 157.96 (Cquart), 157.23 (Cquart), 157.14 (Cquart), 136.70 (Cquart), 136.11 (Cquart), 136.06 (Cquart), 135.69 (Cquart), 129.22 (2 CHarom), 128.77 (2 CHarom), 128.64 (2 CHarom), 128.61 (2 CHarom), 118.21 (4C, CHarom), 118.11 (4C, CHarom), 100.47 (C-1Xyl), 100.23 (2 C-1Man), 78.89 (C-3Xyl), 75.40 (2 C-5Man), 72.43 (2 C-2Man), 72.03 (2 C-3Man), 68.36 (2 C-4Man), 64.40 (C-5Xyl), 62.70 (2 C-6Man), 57.20 (C-2Xyl), 52.18 (C-4Xyl), 22.74, 22.66 (2C, NHC(O)CH3) ppm. IR (ATR) νmax/cm−1 3364, 2467, 1494, 1220, 999, 824, 677, 575. ESI-HRMS m/z = 910.3593 [M + NH4]+, calcd for [M + NH4]+ 910.3604.
- 4′-[(1,1′-Biphenyl)-2,3,4,6-tetra-O-acetyl-α-d-mannopyranoside]-4-yl 2,4-N-carbonyl-2,4-dideoxy-3-O-{4′-[(1,1′-biphenyl)-2,3,4,6-tetra-O-acetyl-α-d-mannopyranoside]-4-yl}-β-d-xylopyranoside (6). The glycocluster 3 (20.0 mg, 14.9 µmol) was dissolved in CH2Cl2 (1.00 mL), TFA (200 µL) was added and it was stirred for 2 h at room temperature. Then all volatiles were removed under reduced pressure and the residue was co-evaporated with toluene (3×). The crude product was dissolved in DMF (150 µL) and N,N-carbonyldiimidazole (2.90 mg, 17.9 µmol) was added, followed by dropwise addition of triethylamine (10.4 µL, 74.5 µmol). The reaction mixture was stirred for 16 h at room temperature, then it was diluted with ethyl acetate and washed with 1N HCl, sodium bicarbonate and brine. The combined organic layer were dried over MgSO4, it was filtered and concentrated. Purification by column chromatography on silica gel (toluene/acetone, 4:6) gave the title compound 6 (13.0 mg, 74%) as a colorless solid; Rf 0.15 (toluene/acetone, 4:6); [α = −27.3 (c 0.02, acetone). 1H NMR (600 MHz, acetone-d6, 298 K, TMS): δ = 7.68 (d, 3J = 8.7 Hz, 2H, 2 CHarom), 7.65 (d, 3J = 8.7 Hz, 2H, 2 CHarom), 7.59 (d, 3J = 8.7 Hz, 2H, 2 CHarom), 7.55 (d, 3J = 8.7 Hz, 2H, 2 CHarom), 7.29 (d, 3J = 8.7 Hz, 2H, 2 CHarom), 7.26 (d, 3J = 8.7 Hz, 2H, 2 CHarom), 7.23 (d, 3J = 8.7 Hz, 2H, 2 CHarom), 7.09 (d, 3J = 8.7 Hz, 2H, 2 CHarom), 6.17 (d, 3JNH,2 = 4.2 Hz, 1H, NH), 6.07 (d, 3JNH,4 = 4.6 Hz, 1H, NH), 5.72–5.68 (m, 2H, 2 H-1Man), 5.52 (s, 1H, H-1Xyl), 5.51–5.45 (m, 4H, 2 H-2Man, 2 H-3Man), 5.33 (dd~t, 3J3,4 = 10.0 Hz, 3J4,5 = 10.0 Hz, 2H, 2 H-4Man), 5.06 (t, 3J3,4 = 3.1 Hz, 3J2,3 = 3.1 Hz, 1H, H-3Xyl), 4.45 (d, 2J5a,5b = 11.4 Hz, 1H, H-5aXyl), 4.23 (dd, 2J6a,6b = 11.9 Hz, 3J5,6a = 5.9 Hz, 2H, 2 H-6aMan), 4.20–4.14 (m, 2H, 2 H-5Man), 4.08 (dd, 2J6a,6b = 12.0 Hz, 3J5,6b = 2.2 Hz, 2H, 2 H-6bMan), 3.95 (s, 1H, H-4Xyl), 3.69 (s, 1H, H-2Xyl), 3.60 (d, 2J5a,5b = 12.0 Hz, 1H, H-5bXyl), 2.16, 2.16, 2.06, 2.04, 1.99, 1.99, 1.95, 1.93 (each s, 24H, 8 C(O)CH3) ppm. 13C NMR (125 MHz, acetone-d6, 298 K, TMS): δ = 170.66, 170.43, 170.40, 170.31 (8 C(O)CH3), 157.37, 157.22, 155.86, 155.82, 136.35, 136.31, 134.81, 134.59 (8 Cquart), 128.71, 128.53, 128.52, 128.49, 118.27, 118.23, 117.57, 117.32 (16 CHarom), 99.31 (C-1Xyl), 96.98, 96.96 (2 C-1Man), 70.26 (2 C-5Man), 69.88 (2 C-2Man), 69.80 (2 C-3Man), 68.88 (C-3Xyl), 66.60 (2 C-4Man), 62.94 (2 C-6Man), 61.98 (C-5Xyl), 48.57 (C-2Xyl), 47.85 (C-4Xyl), 21.71, 20.65, 20.61, 20.59 (8 C(O)CH3) ppm. IR (ATR) νmax/cm−1 2925, 1749, 1607, 1496, 1369, 1217, 1039, 825, 598. ESI-HRMS m/z = 1188.4013 [M + NH4]+, calcd for [M + NH4]+ 1188.4031.
- 4′-[(1,1′-Biphenyl)-α-d-mannopyranoside]-4-yl 2,4-N-carbonyl-2,4-dideoxy-3-O-{4′-[(1,1′-biphenyl)-α-d-mannopyranoside]-4-yl}-β-d-xylopyranoside (7). The protected glycocluster 6 (10.0 mg, 8.54 µmol) was dissolved in MeOH (1.00 mL), K2CO3 (354 µg, 2.56 µmol) was added and the reaction mixture was stirred for 4 h at room temperature. It was neutralized with Amberlite® IR-120 (Sigma-Aldrich, St. Louis, MO, USA), filtered and concentrated to dryness. Co-evaporation with toluene and CH2Cl2 gave 7 (5.30 mg, 74%) as a colorless solid; [α = +36.2 (c 0.02, MeOH). 1H NMR (600 MHz, MeOD-d3, 298 K, TMS): δ = 7.63–7.46 (m, 8H, 8 Harom), 7.25–7.01 (m, 8H, 8 Harom), 5.52 (s, 1H, H-1Man), 5.50 (s, 1H, H-1Man), 5.43 (s, 1H, H-1Xyl), 5.01 (t, 3J3,4 = 3.7 Hz, 3J2,3 = 3.7 Hz, 1H, H-3Xyl), 4.50 (dd, 2J5a,5b = 11.5 Hz, 3J4,5a = 11.5 Hz, H, H-5aXyl), 4.04-4.01 (m, 2H, 2 H-2Man), 3.94-3.90 (m, 2H, 2 H-3Man), 3.89 (s, 1H, H-2Xyl), 3.80-3.57 (m, 10H, 2 H-4Man, 2 H-5Man, 2 H-6aMan, 2 H-6bMan, H-4Xyl, H-5bXyl), ppm. 13C NMR (125 MHz, MeOD-d3, 298 K, TMS): δ = 160.15 (NHC(O)NH), 157.41 (Cquart), 157.36 (Cquart), 157.19 (Cquart), 157.14 (Cquart), 136.18 (Cquart), 136.15 (Cquart), 136.00 (Cquart), 135.85 (Cquart), 128.96 (2 CHarom), 128.75 (2 CHarom), 128.72 (2 CHarom), 128.68 (2 CHarom), 118.14 (2C, CHarom), 118.08 (2C, CHarom), 117.79 (2C, CHarom), 117.46 (2C, CHarom), 99.64 (C-1Xyl), 100.24 (2 C-1Man), 75.39(2 C-5Man), 72.43 (2 C-3Man), 72.03 (2 C-2Man), 68.68 (C-3Xyl), 68.36 (2 C-4Man), 62.69 (2 C-6Man), 61.94 (C-5Xyl), 52.79 (C-4Xyl), 47.85 (C-2Xyl) ppm. IR (ATR) νmax/cm−1 3251, 2013, 1495, 1220, 999, 825, 674, 584. ESI-HRMS m/z = 852.3178 [M + NH4]+, calcd for [M + NH4]+ 852.3185.
3.3. Cultivation of Bacteria
3.4. Adhesion–Inhibition Assay with GFP-PKL1162 E. coli Bacteria
3.5. Molecular Dynamics
3.6. Molecular Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mydock-McGrane, L.K.; Hannan, T.J.; Janetka, J.W. Rational Design Strategies for FimH Antagonists: New Drugs on the Horizon for Urinary Tract Infection and Crohn’s Disease. Expert Opin. Drug Discov. 2017, 12, 711–731. [Google Scholar] [CrossRef] [PubMed]
- Sarshar, M.; Behzadi, P.; Ambrosi, C.; Zagaglia, C.; Palamara, A.T.; Scribano, D. FimH and Anti-Adhesive Therapeutics: A Disarming Strategy Against Uropathogens. Antibiotics 2020, 9, 397. [Google Scholar] [CrossRef] [PubMed]
- Fares, M.; Imberty, A.; Titz, A. Bacterial Lectins: Multifunctional Tools in Pathogenesis and Possible Drug Targets. Trends Microbiol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Despras, G.; Lindhorst, T.K. Organizing Multivalency in Carbohydrate Recognition. Chem. Soc. Rev. 2016, 45, 3275–3302. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, G.; Reise, F.; Schuler, M.; Nehmé, R.; Despras, G.; Brekalo, J.; Morin, P.; Renard, P.-Y.; Lindhorst, T.K.; Tatibouët, A. Bifunctional Mannoside–Glucosinolate Glycoconjugates as Enzymatically Triggered Isothiocyanates and FimH Ligands. Org. Biomol. Chem. 2018, 16, 4900–4913. [Google Scholar] [CrossRef] [PubMed]
- Tamburrini, A.; Colombo, C.; Bernardi, A. Design and Synthesis of Glycomimetics: Recent Advances. Med. Res. Rev. 2020, 40, 495–531. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, L.M.; Lindhorst, T.K. Orthogonal Photoswitching of Heterobivalent Azobenzene Glycoclusters: The Effect of Glycoligand Orientation in Bacterial Adhesion. Beilstein J. Org. Chem. 2025, 21, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Sauer, M.M.; Jakob, R.P.; Eras, J.; Baday, S.; Eriş, D.; Navarra, G.; Bernèche, S.; Ernst, B.; Maier, T.; Glockshuber, R. Catch-Bond Mechanism of the Bacterial Adhesin FimH. Nat. Commun. 2016, 7, 10738. [Google Scholar] [CrossRef] [PubMed]
- Sharon, N. Carbohydrates as Future Anti-Adhesion Drugs for Infectious Diseases. Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Ernst, B.; Magnani, J.L. From Carbohydrate Leads to Glycomimetic Drugs. Nat. Rev. Drug Discov. 2009, 8, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Lindhorst, T.K. The Bacterial Lectin FimH, a Target for Drug Discovery—Carbohydrate Inhibitors of Type 1 Fimbriae-Mediated Bacterial Adhesion. Eur. J. Org. Chem. 2011, 2011, 3583–3609. [Google Scholar] [CrossRef]
- Leusmann, S.; Ménová, P.; Shanin, E.; Titz, A.; Rademacher, C. Glycomimetics for the Inhibition and Modulation of Lectins. Chem. Soc. Rev. 2023, 52, 3663–3740. [Google Scholar] [CrossRef] [PubMed]
- Fiege, B.; Rabbani, S.; Preston, R.C.; Jakob, R.P.; Zihlmann, P.; Schwardt, O.; Jiang, X.; Maier, T.; Ernst, B. The Tyrosine Gate of the Bacterial Lectin FimH: A Conformational Analysis by NMR Spectroscopy and X-Ray Crystallography. ChemBioChem 2015, 16, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Firon, N.; Ashkenazi, S.; Mirelman, D.; Ofek, I.; Sharon, N. Aromatic Alpha-Glycosides of Mannose Are Powerful Inhibitors of the Adherence of Type 1 Fimbriated Escherichia coli to Yeast and Intestinal Epithelial Cells. Infect. Immun. 1987, 55, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Chalopin, T.; Brissonnet, Y.; Sivignon, A.; Deniaud, D.; Cremet, L.; Barnich, N.; Bouckaert, J.; Gouin, S.G. Inhibition Profiles of Mono- and Polyvalent FimH Antagonists against 10 Different Escherichia coli Strains. Org. Biomol. Chem. 2015, 13, 11369–11375. [Google Scholar] [CrossRef] [PubMed]
- Hatton, N.E.; Nabarro, J.; Yates, N.D.J.; Parkin, A.; Wilson, L.G.; Baumann, C.G.; Fascione, M.A. Mannose-Presenting “Glyco-Colicins” Convert the Bacterial Cell Surface into a Multivalent Adsorption Site for Adherent Bacteria. JACS Au 2024, 4, 2122–2129. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Das, S.K.; Santoyo-González, F.; Hernández-Mateo, F.; Dam, T.K.; Brewer, C.F. Synthesis of “Sugar-Rods” with Phytohemagglutinin Cross-Linking Properties by Using the Palladium-Catalyzed Sonogashira Reaction. Chem. Eur. J. 2000, 6, 1757–1762. [Google Scholar] [CrossRef]
- Roy, R.; Trono, M.C.; Giguère, D. Effects of Linker Rigidity and Orientation of Mannoside Cluster for Multivalent Interactions with Proteins. In Glycomimetics: Modern Synthetic Methodologies; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2005; Volume 896, pp. 137–150. ISBN 978-0-8412-3880-0. [Google Scholar]
- Bergeron-Brlek, M.; Shiao, T.C.; Trono, M.C.; Roy, R. Synthesis of a Small Library of Bivalent α-D-Mannopyranosides for Lectin Cross-Linking. Carbohydr. Res. 2011, 346, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Varki, A.; Cummings, R.D.; Aebi, M.; Packer, N.H.; Seeberger, P.H.; Esko, J.D.; Stanley, P.; Hart, G.; Darvill, A.; Kinoshita, T.; et al. Symbol Nomenclature for Graphical Representations of Glycans. Glycobiology 2015, 25, 1323–1324. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, H.; Hashimoto, H. Bending Trisaccharides by a Chelation-Induced Ring Flip of a Hinge-Like Monosaccharide Unit. J. Am. Chem. Soc. 1999, 121, 5089–5090. [Google Scholar] [CrossRef]
- Yuasa, H.; Miyagawa, N.; Izumi, T.; Nakatani, M.; Izumi, M.; Hashimoto, H. Hinge Sugar as a Movable Component of an Excimer Fluorescence Sensor. Org. Lett. 2004, 6, 1489–1492. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, J.; Ohkubo, A.; Yuasa, H. A Ring-Flippable Sugar as a Stimuli-Responsive Component of Liposomes. Chem. Asian J. 2015, 10, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, S.O.; Lindhorst, T.K. Versatile Synthesis of Diaminoxylosides via Iodosulfonamidation of Xylal Derivatives. Eur. J. Org. Chem. 2021, 2021, 6312–6318. [Google Scholar] [CrossRef]
- Hung, C.-S.; Bouckaert, J.; Hung, D.; Pinkner, J.; Widberg, C.; DeFusco, A.; Auguste, C.G.; Strouse, R.; Langermann, S.; Waksman, G.; et al. Structural Basis of Tropism of Escherichia coli to the Bladder during Urinary Tract Infection. Mol. Microbiol. 2002, 44, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.; Eris, D.; Schwardt, O.; Sager, C.P.; Rabbani, S.; Kleeb, S.; Ernst, B. Urinary Tract Infection: Which Conformation of the Bacterial Lectin FimH Is Therapeutically Relevant? J. Med. Chem. 2017, 60, 5646–5662. [Google Scholar] [CrossRef] [PubMed]
- Miyaura, N.; Yanagi, T.; Suzuki, A. The Palladium-Catalyzed Cross-Coupling Reaction of Phenylboronic Acid with Haloarenes in the Presence of Bases. Synth. Commun. 1981, 11, 513–519. [Google Scholar] [CrossRef]
- Schwizer, D.; Gäthje, H.; Kelm, S.; Porro, M.; Schwardt, O.; Ernst, B. Antagonists of the Myelin-Associated Glycoprotein: A New Class of Tetrasaccharide Mimics. Bioorg. Med. Chem. 2006, 14, 4944–4957. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Pinkner, J.S.; Ford, B.; Chorell, E.; Crowley, J.M.; Cusumano, C.K.; Campbell, S.; Henderson, J.P.; Hultgren, S.J.; Janetka, J.W. Lead Optimization Studies on FimH Antagonists: Discovery of Potent and Orally Bioavailable Ortho-Substituted Biphenyl Mannosides. J. Med. Chem. 2012, 55, 3945–3959. [Google Scholar] [CrossRef] [PubMed]
- Amatore, C.; Jutand, A.; Le Duc, G. The Triple Role of Fluoride Ions in Palladium-Catalyzed Suzuki–Miyaura Reactions: Unprecedented Transmetalation from [ArPdFL2] Complexes. Angew. Chem. Int. Ed. 2012, 51, 1379–1382. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Hiromoto, K.; Kagechika, H. Development of a Library of 6-Arylcoumarins as Candidate Fluorescent Sensors. Org. Lett. 2007, 9, 1315–1318. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, S.O.; Lindhorst, T.K.; Auer, A. Between Two Chairs: Combination of Theory and Experiment for the Determination of the Conformational Dynamics of Xylosides. Chem. Eur. J. 2022, 28, e202201544. [Google Scholar] [CrossRef] [PubMed]
- Zemplén, G.; Pacsu, E. Über Die Verseifung Acetylierter Zucker Und Verwandter Substanzen. Berichte Dtsch. Chem. Ges. B Ser. 1929, 62, 1613–1614. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Pyplo-Schnieders, J.; Redlich, H.; Luftmann, H.; Fröhlich, R. Preparation of 2-Deoxystreptamine Derivatives with All-Axial Substituents for Desymmetrization. Tetrahedron Lett. 2007, 48, 8145–8148. [Google Scholar] [CrossRef]
- Schrödinger Release 2021-1: Desmond Molecular Dynamics System; D. E. Shaw Research: New York, NY, USA, 2021.
- Schrödinger Release 2021-1: Maestro-Desmond Interoperability Tools; Schrödinger: New York, NY, USA, 2021.
- Schrödinger Release 2021-1: Maestro; Schrödinger LLC: New York, NY, USA, 2021.
- Mark, P.; Nilsson, L. Structure and Dynamics of the TIP3P, SPC, and SPC/E Water Models at 298 K. J. Phys. Chem. A 2001, 105, 9954–9960. [Google Scholar] [CrossRef]
- Reisner, A.; Haagensen, J.A.J.; Schembri, M.A.; Zechner, E.L.; Molin, S. Development and Maturation of Escherichia coli K-12 Biofilms. Mol. Microbiol. 2003, 48, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Horst, A.K.; Klemm, P.; Lindhorst, T.K. A Kit for the Investigation of Live Escherichia coli Cell Adhesion to Glycosylated Surfaces. Chem. Commun. 2010, 46, 330–332. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Abgottspon, D.; Wittwer, M.; Rabbani, S.; Herold, J.; Jiang, X.; Kleeb, S.; Lüthi, C.; Scharenberg, M.; Bezençon, J.; et al. FimH Antagonists for the Oral Treatment of Urinary Tract Infections: From Design and Synthesis to in Vitro and in Vivo Evaluation. J. Med. Chem. 2010, 53, 8627–8641. [Google Scholar] [CrossRef] [PubMed]
- Sperling, O.; Fuchs, A.; Lindhorst, T.K. Evaluation of the Carbohydrate Recognition Domain of the Bacterial Adhesin FimH: Design, Synthesis and Binding Properties of Mannoside Ligands. Org. Biomol. Chem. 2006, 4, 3913–3922. [Google Scholar] [CrossRef] [PubMed]
- Sauer, M.M.; Jakob, R.P.; Luber, T.; Canonica, F.; Navarra, G.; Ernst, B.; Unverzagt, C.; Maier, T.; Glockshuber, R. Binding of the Bacterial Adhesin FimH to Its Natural, Multivalent High-Mannose Type Glycan Targets. J. Am. Chem. Soc. 2019, 141, 936–944. [Google Scholar] [CrossRef] [PubMed]
- von der Lieth, C.-W.; Frank, M.; Lindhorst, T.K. Molecular Dynamics Simulations of Glycoclusters and Glycodendrimers. Rev. Mol. Biotechnol. 2002, 90, 311–337. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger Release 2024-2: Glide; Schrödinger LLC: New York, NY, USA, 2024.
- Schrödinger Release 2024-2: Maestro; Schrödinger LLC: New York, NY, USA, 2024.
- Bouckaert, J.; Berglund, J.; Schembri, M.; Genst, E.D.; Cools, L.; Wuhrer, M.; Hung, C.-S.; Pinkner, J.; Slättegård, R.; Zavialov, A.; et al. Receptor Binding Studies Disclose a Novel Class of High-Affinity Inhibitors of the Escherichia coli FimH Adhesin. Mol. Microbiol. 2005, 55, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Wellens, A.; Garofalo, C.; Nguyen, H.; Gerven, N.V.; Slättegård, R.; Hernalsteens, J.-P.; Wyns, L.; Oscarson, S.; Greve, H.D.; Hultgren, S.; et al. Intervening with Urinary Tract Infections Using Anti-Adhesives Based on the Crystal Structure of the FimH–Oligomannose-3 Complex. PLoS ONE 2008, 3, e2040. [Google Scholar] [CrossRef]
- Wellens, A.; Lahmann, M.; Touaibia, M.; Vaucher, J.; Oscarson, S.; Roy, R.; Remaut, H.; Bouckaert, J. The Tyrosine Gate as a Potential Entropic Lever in the Receptor-Binding Site of the Bacterial Adhesin FimH. Biochemistry 2012, 51, 4790–4799. [Google Scholar] [CrossRef] [PubMed]
- Schönemann, W.; Cramer, J.; Mühlethaler, T.; Fiege, B.; Silbermann, M.; Rabbani, S.; Dätwyler, P.; Zihlmann, P.; Jakob, R.P.; Sager, C.P.; et al. Improvement of Aglycone π-Stacking Yields Nanomolar to Sub-Nanomolar FimH Antagonists. ChemMedChem 2019, 14, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger Release 2024-2: Prime; Schrödinger LLC: New York, NY, USA, 2024.
- Despras, G.; Poonthiyil, V.; Lindhorst, T.K. Photochromic Carbohydrate Conjugates. In Molecular Photoswitches; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2022; pp. 1015–1045. ISBN 978-3-527-82762-6. [Google Scholar]
- Friedrich, L.M.; Hartke, B.; Lindhorst, T.K. Advancing Optoglycomics: Two Orthogonal Azobenzene Glycoside Antennas in One Glycocluster—Synthesis, Switching Cycles, Kinetics and Molecular Dynamics. Chem. Eur. J. 2024, 30, e202402125. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Barbarroja, G.; Maisonneuve, S.; Xie, J.; García Fernández, J.M.; Ortiz Mellet, C. Light-Responsive Glycosidase Inhibitors: Tuning Enzyme Selectivity and Switching Factors through Integrated Chemical and Optoglycomic Strategies. Bioorg. Chem. 2025, 162, 108575. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wu, C.; Ghoreishi, D.; Chen, W.; Wang, L.; Damm, W.; Ross, G.A.; Dahlgren, M.K.; Russell, E.; Von Bargen, C.D.; et al. OPLS4: Improving Force Field Accuracy on Challenging Regimes of Chemical Space. J. Chem. Theory Comput. 2021, 17, 4291–4300. [Google Scholar] [CrossRef] [PubMed]

| Glycocluster | IC50 a [µmol] | IC50 MeMan b [mmol] | RIP c | Average RIP d |
|---|---|---|---|---|
| 5 4C1 conformation of the xyloside scaffold | 15.2 (±1.5) | 7.08 (±0.44) | 466 (±76) | 410 (±68) |
| 15.7 (±1.3) | 5.34 (±0.48) | 339 (±60) | ||
| 7 1C4 conformation of the xyloside scaffold | 63.9 (±14.1) | 7.08 (±0.44) | 111 (±31) | 157 (±45) |
| 36.0 (±11.1) | 5.34 (±0.48) | 148 (±59) |
| Glycocluster (RIP) | Glide Score | Binding Energy [kcal mol−1] |
|---|---|---|
| 4C1 glycocluster 5 (410 ± 68) | −10.468 | −83.02 |
| 1C4 glycocluster 7 (157 ± 45) | −10.581 | −67.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaeschke, S.O.; vom Sondern, I.; Lindhorst, T.K. Bivalent Inhibitors of Mannose-Specific Bacterial Adhesion: A Xylose-Based Conformational Switch to Control Glycoligand Distance. Molecules 2025, 30, 3074. https://doi.org/10.3390/molecules30153074
Jaeschke SO, vom Sondern I, Lindhorst TK. Bivalent Inhibitors of Mannose-Specific Bacterial Adhesion: A Xylose-Based Conformational Switch to Control Glycoligand Distance. Molecules. 2025; 30(15):3074. https://doi.org/10.3390/molecules30153074
Chicago/Turabian StyleJaeschke, Sven Ole, Ingo vom Sondern, and Thisbe K. Lindhorst. 2025. "Bivalent Inhibitors of Mannose-Specific Bacterial Adhesion: A Xylose-Based Conformational Switch to Control Glycoligand Distance" Molecules 30, no. 15: 3074. https://doi.org/10.3390/molecules30153074
APA StyleJaeschke, S. O., vom Sondern, I., & Lindhorst, T. K. (2025). Bivalent Inhibitors of Mannose-Specific Bacterial Adhesion: A Xylose-Based Conformational Switch to Control Glycoligand Distance. Molecules, 30(15), 3074. https://doi.org/10.3390/molecules30153074







