Enhanced Heat Transfer of 1-Octadecanol Phase-Change Materials Using Carbon Nanotubes
Abstract
1. Introduction
2. Results and Discussion
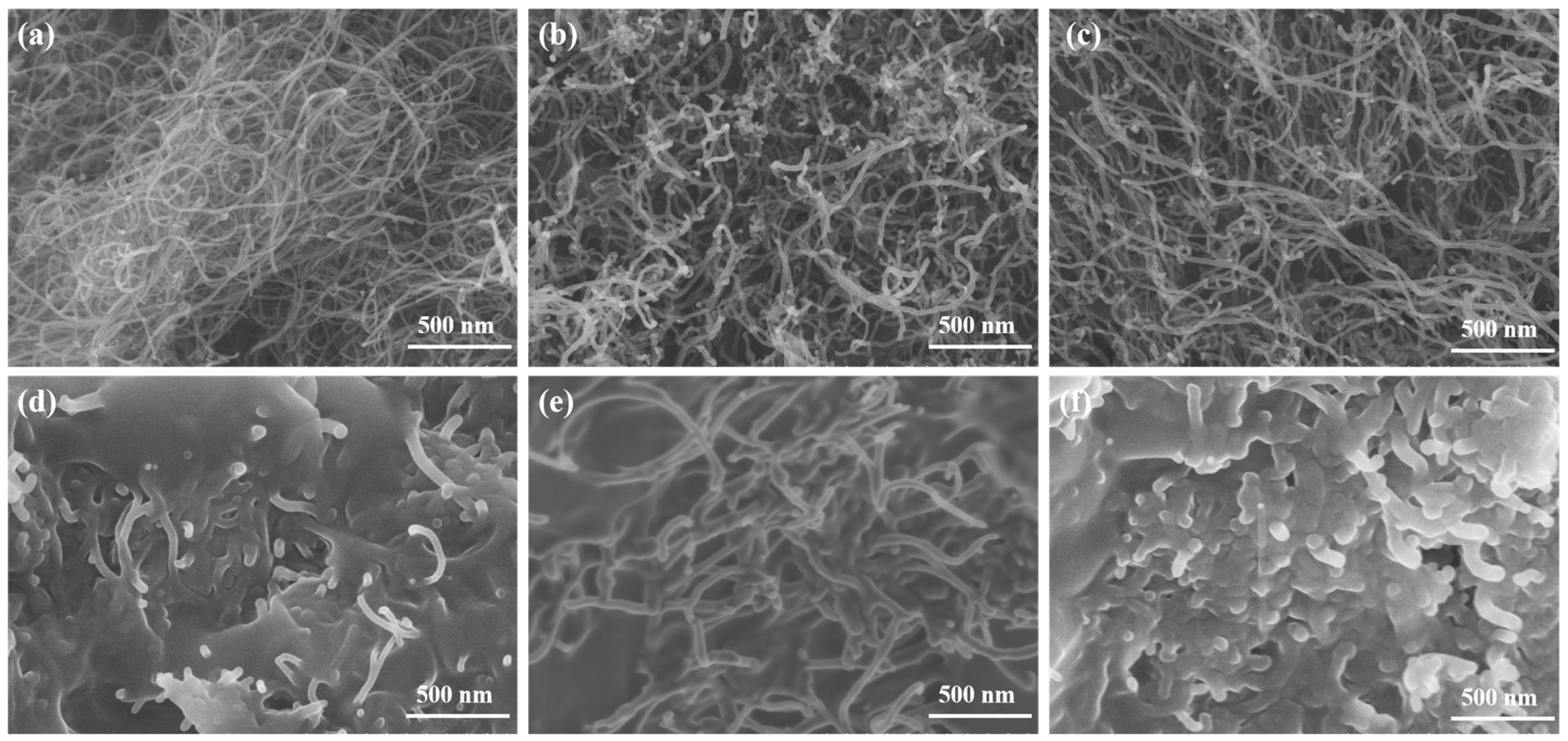
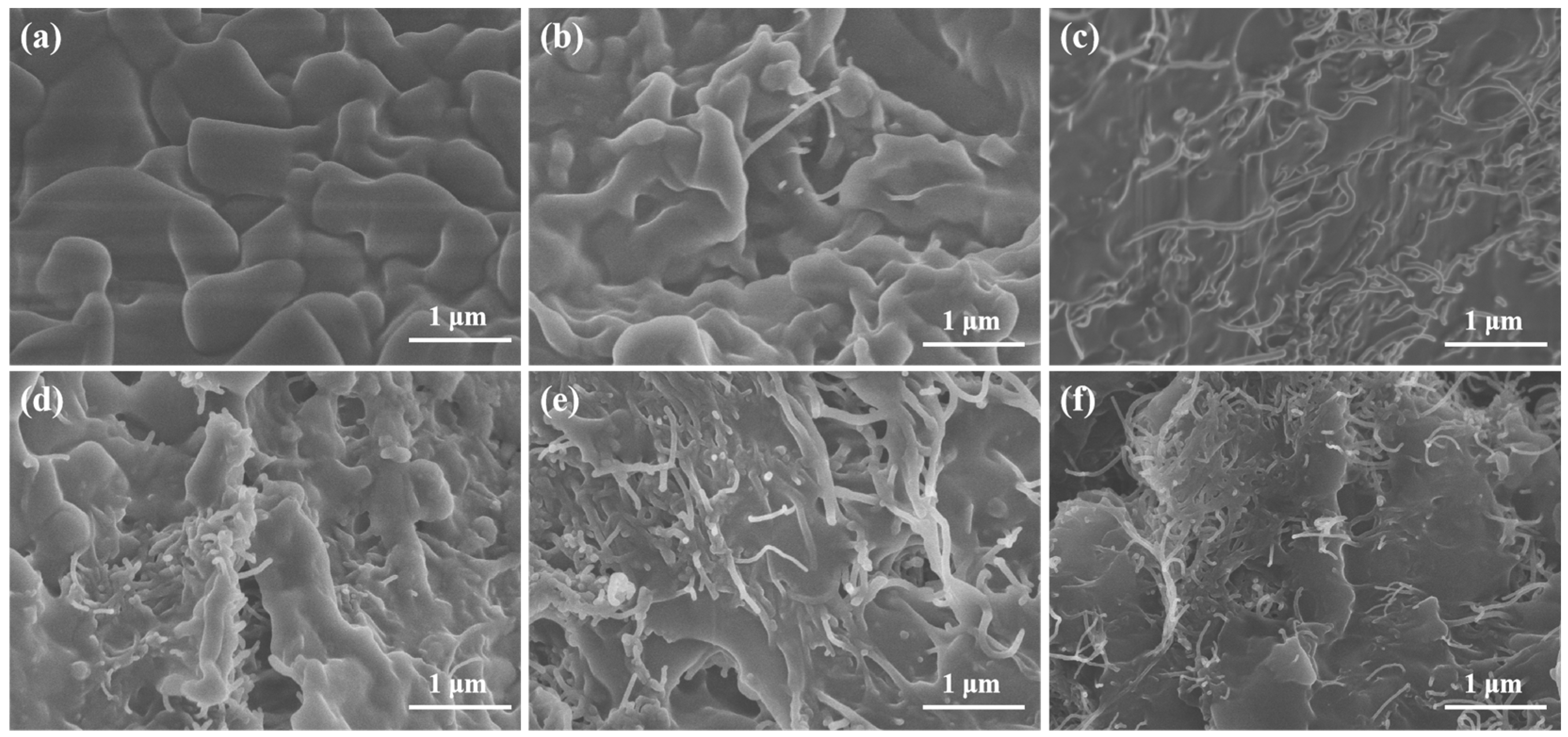
2.1. Microstructure
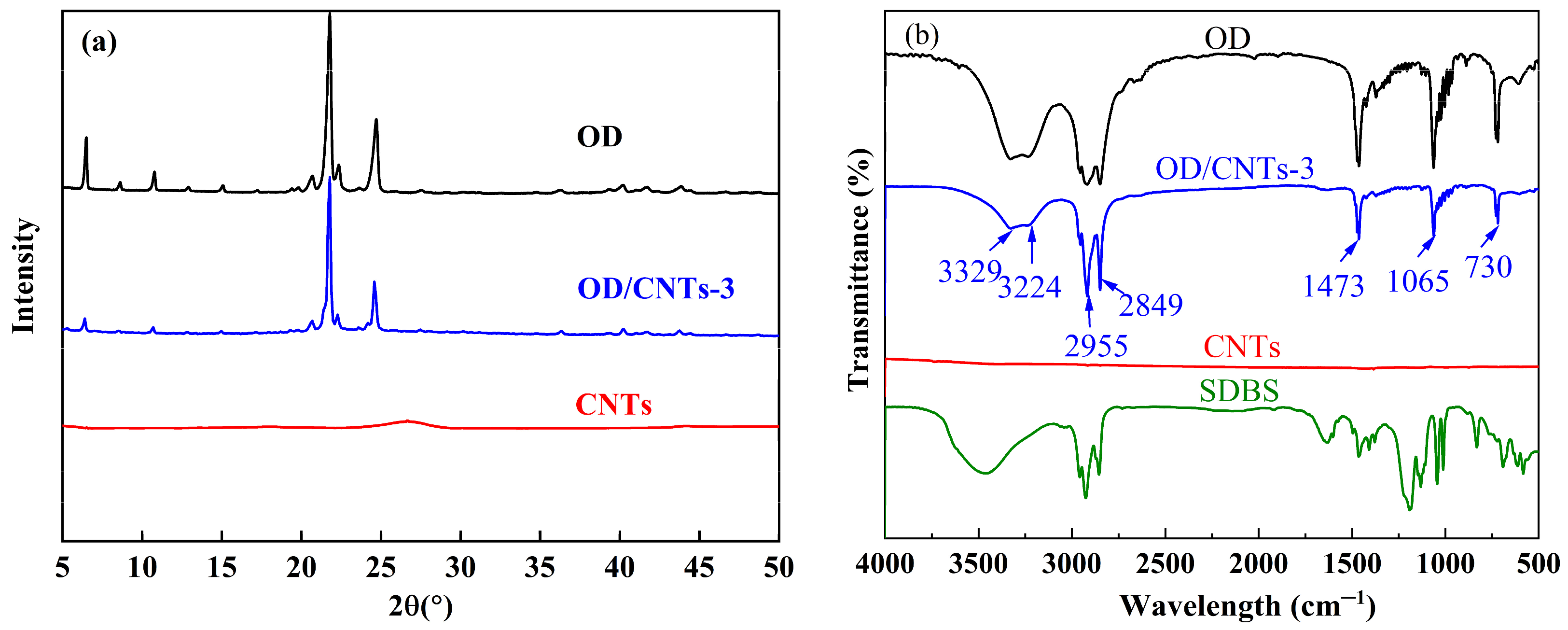
2.2. Component
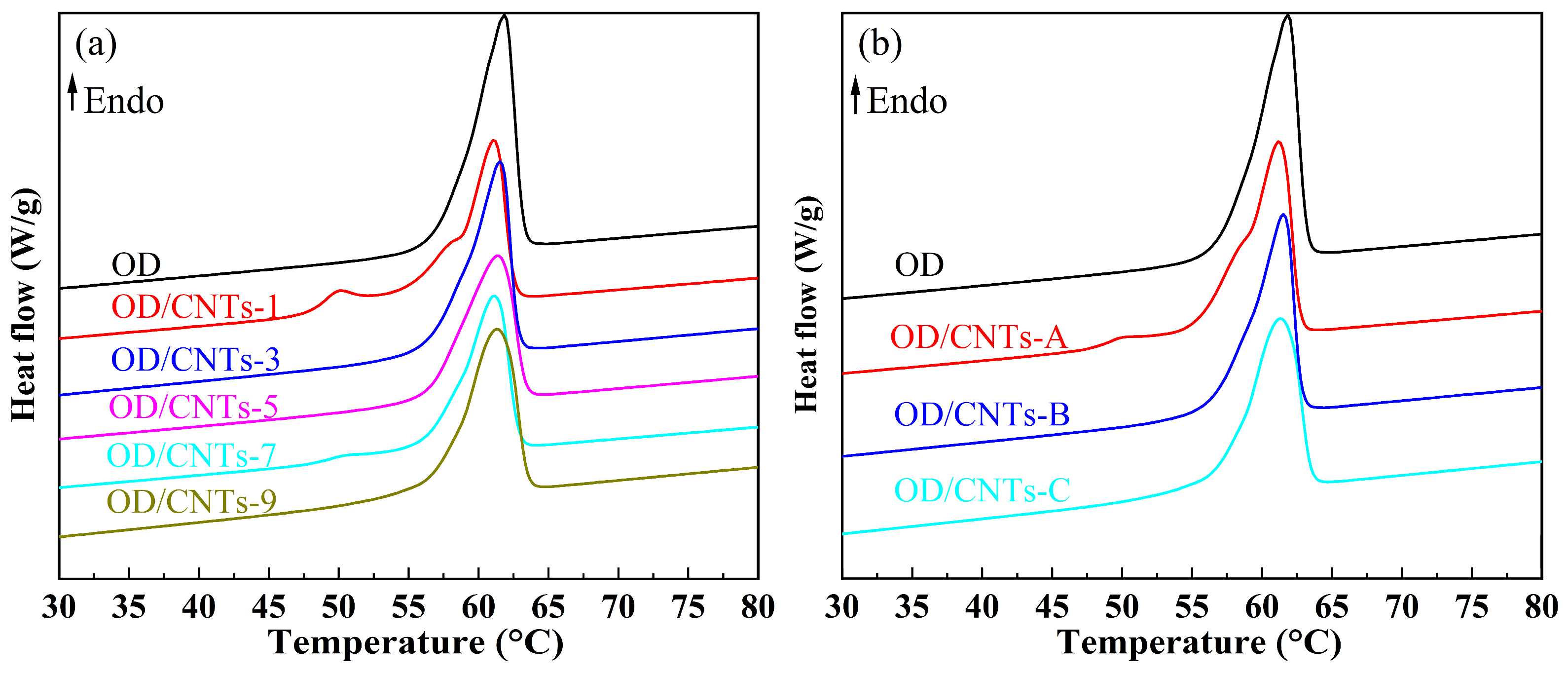
2.3. Thermal Storage Properties
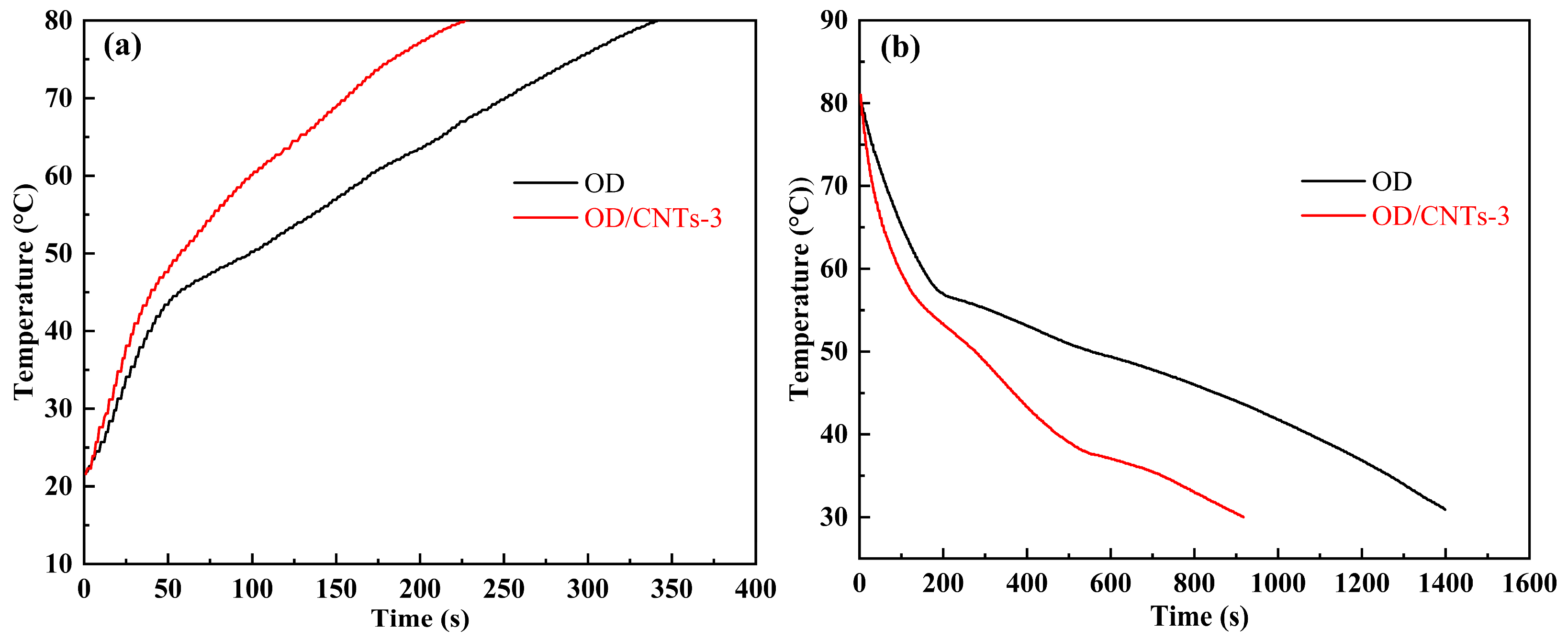
2.4. Heat Transfer Performance Analysis
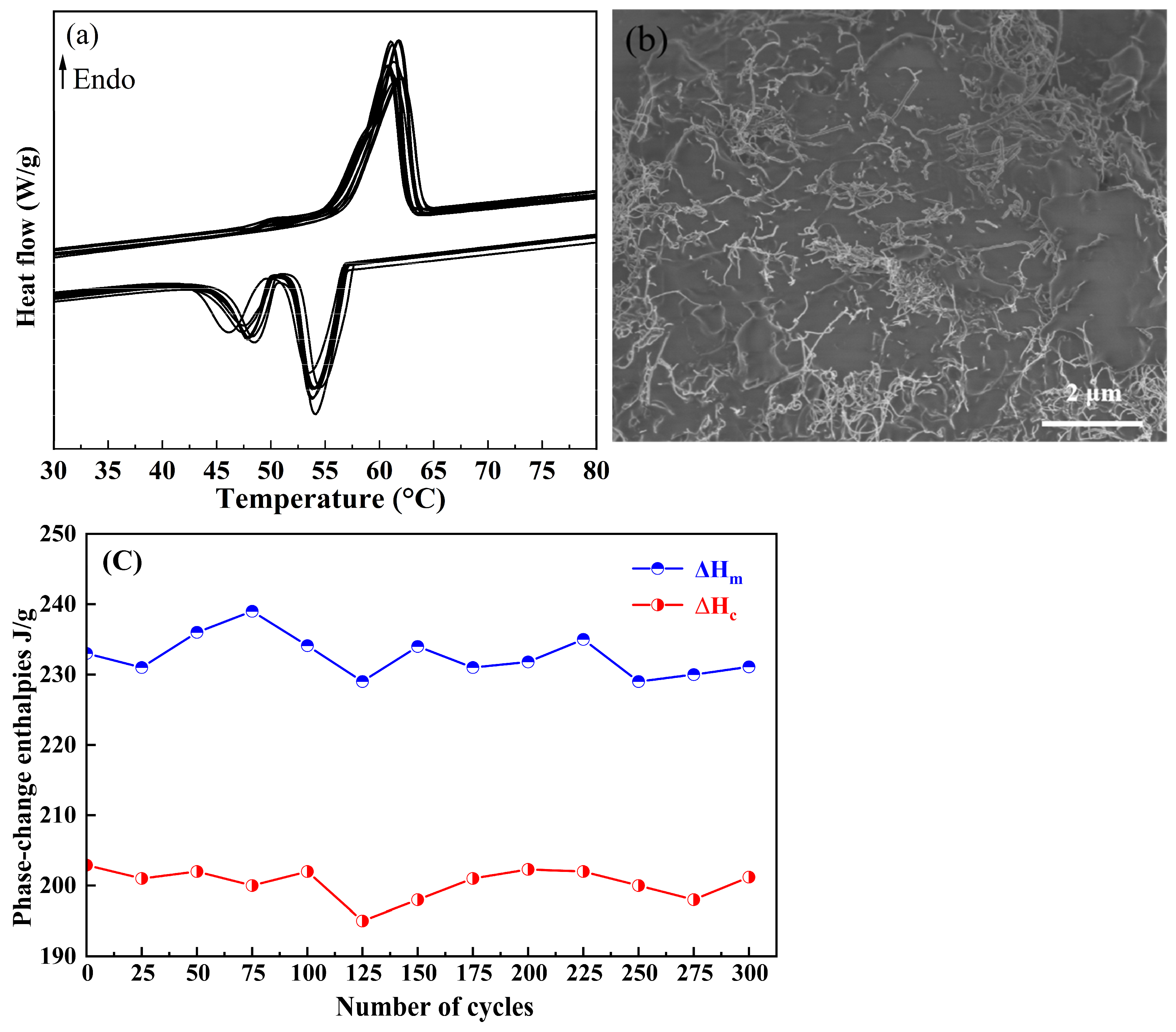
2.5. Reliability and Reversible Stability
2.6. Heat Storage and Release Performance
3. Materials and Methods
3.1. Materials
3.2. Preparation of OD/CNTs CPCMs
3.3. Characterization
4. Conclusions
- (1)
- The XRD pattern of the OD/CNTs simply represents a superposition of the individual diffraction patterns of CNTs and OD, demonstrating that only physical mixing occurs between the components without any chemical reaction.
- (2)
- The incorporation of CNTs showed negligible effects on the melting and solidification temperatures of OD. Notably, the OD/CNTs composites maintained high latent heat values for both phase transitions. Specifically, the OD/CNTs-3 composite exhibited melting and solidification temperatures of 56.9 °C and 57.2 °C, respectively, with corresponding latent heats of 233.0 J/g and 202.9 J/g. The OD/CNTs-3 CPCMs demonstrated significant thermal enhancement, exhibiting thermal diffusivity and conductivity values 1.2 times and 1.5 times higher than pure OD, respectively.
- (3)
- With increasing CNT loading, the OD/CNTs CPCMs exhibited a marginal reduction in heat storage capacity, while both thermal conductivity and thermal diffusivity demonstrated linear enhancement.
- (4)
- The composite showed remarkable improvements in thermal cycling efficiency, with heating time reduced by 39.7% and cooling time decreased by 43.1% compared to the OD. The CNTs formed an efficient thermal conduction network within the OD, significantly reducing both the heating and cooling durations of the PCMs.
- (1)
- For electronic thermal management, it serves as chip-level phase-change cooling modules capable of absorbing transient high heat flux.
- (2)
- In solar energy systems, it significantly enhances intermittent energy utilization efficiency when coupled with solar collectors for low-temperature thermal storage.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PCMs | Phase-change materials |
| CNTs | Carbon nanotubes |
| OD | 1-octadecanol |
| CPCMs | Composite phase-change materials |
| XRD | X-Ray diffraction |
| FTIR | Fourier-transform infrared spectroscopy |
| SEM | Scanning electron microscopy |
| DSC | Differential Scanning Calorimeter |
| SDBS | Sodium dodecyl benzene sulfonate |
| ΔHm | Melting enthalpies |
| ΔHs | Solidification enthalpies |
References
- Han, Z.; Zhou, Z.; Wang, C.; Zhao, Y.; Hu, S.; Nie, R. Research on thermal management and waste heat utilization of building distributed energy storage battery. J. Energy Storage 2025, 128, 117209. [Google Scholar] [CrossRef]
- Khan, J.; Singh, P. Review on phase change materials for spacecraft avionics thermal management. J. Energy Storage 2024, 87, 111369. [Google Scholar] [CrossRef]
- Zhou, C.; Zeng, S.; Liu, B.; Yu, T.; Qiu, J.; Du, J. Flexible HMF/HTC/PEG composite foam with photothermal conversion, energy storage, and shape memory for adaptive thermal management. Carbon 2025, 243, 120476. [Google Scholar] [CrossRef]
- Gao, J.; Zuo, H.; Lu, Y.; Xu, H.; Fang, Z.; Li, J.; Wang, X.; Zeng, K.; Yang, Y.; Yang, H. Development of cost-effective latent heat thermal energy storage based on growing fins. Appl. Therm. Eng. 2025, 269, 125995. [Google Scholar] [CrossRef]
- Zhang, N.; Yuan, Y.; Cao, X.; Du, Y.; Zhang, Z.; Gui, Y. Latent heat thermal energy storage systems with solid–liquid phase change materials: A review. Adv. Eng. Mater. 2018, 20, 1700753. [Google Scholar] [CrossRef]
- Burcu, Ç. A numerical comparison of the thermal performances of nano-PCM heat sinks with Fe3O4, MgO, ZnO and xGNP nanoparticles: Key role of increased thermal conductivity. Therm. Sci. Eng. Prog. 2025, 63, 103712. [Google Scholar]
- Radhakrishnan, N.; Sobhan, C. Thermophysical characterization and melting heat transfer analysis of an organic phase change material dispersed with GNP- Ag hybrid nanoparticles. Heat Mass Transf. 2022, 58, 1811–1828. [Google Scholar] [CrossRef]
- Lu, S.; Jia, Y.; Liang, B.; Wang, R.; Lin, Q.; He, Z. Optimal design and thermal performance study of a two-stage latent heat thermal energy storage technology for heating systems. Appl. Therm. Eng. 2023, 232, 121073. [Google Scholar] [CrossRef]
- Negi, A.; Ranakoti, L.; Verma, R.P.; Kumar, V.; Bhandari, P.; Khargotra, R.; Singh, T. Enhancing solar still productivity with organic phase change materials: A literature review. Energy Convers. Manag. X 2025, 26, 100984. [Google Scholar] [CrossRef]
- John Paul, K.; Kadirgama, M.; Samykano, A.K.; Pandey, V.V. A comprehensive review on thermophysical properties and solar thermal applications of organic nano composite phase change materials. J. Energy Storage 2022, 45, 103415. [Google Scholar] [CrossRef]
- Bilal, L.; Safae, E.M.; Tauseef-ur, R.; Tarik, K. Comprehensive analysis of waste heat recovery and thermal energy storage integration in air conditioning systems. Energy Convers. Manag. X 2024, 24, 100708. [Google Scholar] [CrossRef]
- Abolfazl, N.; Marcello, I.; Giuseppe, L.; Nicola, B. Enhancing PCMs thermal conductivity: A comparison among porous metal foams, nanoparticles and finned surfaces in triplex tube heat exchangers. Appl. Therm. Eng. 2022, 212, 118623. [Google Scholar] [CrossRef]
- Bahrami, H.R.; Ghaedi, M. Strategies for passive thermal management of lithium-ion batteries in microgravity: Combining PCMs, metal foams, fins, and nanoparticles. Case Stud. Therm. Eng. 2025, 71, 106247. [Google Scholar] [CrossRef]
- Latibari, S.; Sadrameli, S. Carbon based material included-shaped stabilized phase change materials for sunlight-driven energy conversion and storage: An extensive review. Sol. Energy 2018, 170, 1130–1161. [Google Scholar] [CrossRef]
- Kim, Y.U.; Yang, S.; Kim, S. Thermal characteristics of cementitious building materials with carbon and PCM for improved heat storage performance. Constr. Build. Mater. 2024, 449, 138550. [Google Scholar] [CrossRef]
- Hekimoğlu, G.; Sarı, A.; Gencel, O.; Tyagi, V.V.; Sharma, R.K. Activated carbon/expanded graphite hybrid structure for development of nonadecane based composite PCM with excellent shape stability, enhanced thermal conductivity and heat charging-discharging performance. Therm. Sci. Eng. Prog. 2023, 44, 102081. [Google Scholar] [CrossRef]
- Alexandr, V.S.; Aleksei, V.S.; Vladimir, V.K.; Pablo, I.; Maxim, A.C. Advances in Electrically and Thermally Conductive Functional Nanocomposites Based on Carbon Nanotubes. Polymers 2024, 17, 71. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Xu, S.; Robera, D. Experimental investigation of thermal performance for pulsating flow in a microchannel heat sink filled with PCM (paraffin/CNT composite). Energy Convers. Manag. 2021, 236, 114071. [Google Scholar] [CrossRef]
- Abdelrazik, A.S.; Saidur, R.; Al-Sulaiman, F.A.; Al-Ahmed, A.; Rached, B. Multiwalled CNT and graphene nanoplatelets based nano-enhanced PCMs: Evaluation for the thermal performance and its implications on the performance of hybrid PV/thermal systems. Mater. Today Commun. 2022, 31, 103618. [Google Scholar] [CrossRef]
- Han, Z.; Du, D.; Zhang, F. CNTs composite aerogel incorporating phase-change microcapsules for solar-thermal conversion and energy storage. Carbon 2025, 237, 120125. [Google Scholar] [CrossRef]
- Wu, X.; Wang, C.; Wang, Y.; Zhu, Y. Experimental study of thermo-physical properties and application of paraffin-carbon nanotubes composite phase change materials. Int. J. Heat Mass Transf. 2019, 140, 671–677. [Google Scholar] [CrossRef]
- Ali, K.; Alper, B.; Ahmet, S.; Vineet, V.T. Thermal characteristics of expanded perlite/paraffin composite phase change material with enhanced thermal conductivity using carbon nanotubes. Energy Convers. Manag. 2017, 134, 373–381. [Google Scholar] [CrossRef]
- Lin, J.; Liu, D.; Liu, X.; Liu, M.; Cui, Y. CNT@MXene porous composite PCM based thermal management for lithium-ion battery system. Appl. Therm. Eng. 2025, 262, 125240. [Google Scholar] [CrossRef]
- Du, Y.; Zhou, T.; Zhao, C.; Ding, Y. Molecular dynamics simulation on thermal enhancement for carbon nano tubes (CNTs) based phase change materials (PCMs). Int. J. Heat Mass Transf. 2022, 182, 122017. [Google Scholar] [CrossRef]
- Feng, D.; Zhao, Z.; Li, P.; Li, Y.; Zha, J.; Hu, J.; Zhang, Y.; Feng, Y. Multifunctional performance of carbon nanotubes in thermal energy storage materials. Mater. Today 2024, 75, 285–308. [Google Scholar] [CrossRef]
- Yuan, M.; Zhang, Y.; Xie, F.; Yang, H.; Carla, B.; Rony, S.; Li, W. Nano copper-modified GO and CNTs for enhanced the epoxy resin composite thermal properties. Appl. Surf. Sci. 2025, 690, 162616. [Google Scholar] [CrossRef]
- Tuan, H.; Tam, L.; Nguyen, G. Methylated mesoporous silica loaded with 1-octadecanol as a new shape-stabilized phase change material for enhanced thermal energy storage efficiency. Can. J. Chem. 2023, 101, 235–241. [Google Scholar] [CrossRef]
- Wang, X.; Huang, Y.; Shi, L.; Zhang, S.; Li, W. Enhanced thermal performance of phase change mortar using multi-scale carbon-based materials. J. Build. Eng. 2024, 98, 111259. [Google Scholar] [CrossRef]
- Xu, J.; Cheng, X.; Li, Y. Preparation and properties of l-octadecanol/l,3:2,4-di-(3,4-dimethyl) benzylidene sorbitol/expanded graphite form-stable composite phase change material. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2019, 34, 728–735. [Google Scholar] [CrossRef]
- Gong, S.; Cheng, X.; Li, Y.; Wang, X.; Wang, Y.; Zhong, H. Effect of nano-SiC on thermal properties of expanded graphite/1-octadecanol composite materials for thermal energy storage. Powder Technol. 2020, 367, 32–39. [Google Scholar] [CrossRef]
- Hamza, A.; Veerakumar, C.; Myeongjae, S.; Honghyun, C. Investigation of 1-tetradecanol with functionalized multi-walled carbon nanotubes as PCM for high-density thermal energy storage. J. Mater. Res. Technol. 2023, 27, 4005–4011. [Google Scholar]
- Dimberu, G.A.; Beom, Y.; Seunghwan, W.; Yujin, K.; Sumin, K. A comparative analysis of biochar, activated carbon, expanded graphite, and multi-walled carbon nanotubes with respect to PCM loading and energy-storage capacities. Environ. Res. 2021, 195, 110853. [Google Scholar]
- Döğüşcü, D.K.; Güler, O.; Hekimoğlu, G.; Sarı, A.; Gencel, O. High-performance CNT-integrated PolyHIPE networks enabling efficient PCM encapsulation via emulsion templating for advanced thermal energy storage. J. Energy Storage 2025, 130, 117465. [Google Scholar] [CrossRef]
- Luo, W.; Luo, L.; Ma, Y.; Liu, Y.; Xie, Y.; Hu, X.; Chen, W.; Jiang, X. Highly thermal conductive phase change materials enabled by CNTs-modified PVA aerogel for solar energy storage and thermal management of electronic components. J. Energy Storage 2024, 88, 111583. [Google Scholar] [CrossRef]
- Guo, J.; Han, X.; Ma, S.; Sun, Y.; Li, C.; Li, R.; Li, C. Form-Stable phase change composites with high thermal conductivity and enthalpy enabled by Graphene/Carbon nanotubes aerogel skeleton for thermal energy storage. Appl. Therm. Eng. 2024, 255, 123954. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, Y.; Liu, X.; Fu, M.; Luo, S.; Li, Y.; Cheng, X. Synergistically enhanced NF/CNTs@ PDA/PEG composite phase change materials for high-efficiency solar-thermal energy storage and conversion. Sol. Energy Mater. Sol. Cells 2025, 290, 113714. [Google Scholar] [CrossRef]
- Pin, J.; Hui, Y.S.; Wang, S.; Warintorn, T.; Zhang, X.; Kong, J.; Kai, D.; Beng, H.; Pei, W.; Qu, Z.; et al. Recent advances in enhanced thermal property in phase change materials using carbon nanotubes: A review. Sol. Energy Mater. Sol. Cells 2025, 279, 113228. [Google Scholar]
- Li, S.; Yan, T.; Huo, Y.; Pan, W. Carbon nanotube/carbon foam thermal-bridge enhancing solar energy conversion and storage of phase change materials. Mater. Today Sustain. 2024, 28, 100986. [Google Scholar] [CrossRef]



| Samples | Melting | Solidifying | ||
|---|---|---|---|---|
| Tm (°C) | ΔHm (J/g) | Ts (°C) | ΔHs (J/g) | |
| OD | 57.7 | 242.2 | 56.7 | 210.1 |
| OD/CNTs-1 | 57.1 | 238.8 | 56.9 | 207.0 |
| OD/CNTs-3 | 56.9 | 233.0 | 57.2 | 202.9 |
| OD/CNTs-5 | 57.3 | 227.1 | 57.0 | 198.6 |
| OD/CNTs-7 | 57.0 | 224.9 | 57.2 | 193.2 |
| OD/CNTs-9 | 56.8 | 218.5 | 57.1 | 190.1 |
| Samples | Melting | Solidifying | ||
|---|---|---|---|---|
| Tm (°C) | ΔHm (J/g) | Ts (°C) | ΔHs (J/g) | |
| OD/CNTs-A | 56.6 | 234.6 | 55.3 | 203.1 |
| OD/CNTs-B | 56.9 | 233.0 | 57.2 | 202.9 |
| OD/CNTs-C | 56.0 | 231.5 | 55.5 | 201.6 |
| Samples | Melting | Solidifying | K (W/m·K) | ||
|---|---|---|---|---|---|
| Tm (°C) | ΔHm (J/g) | Ts (°C) | ΔHs (J/g) | ||
| OD/CNTs-3(0) | 56.9 ± 0.3 | 233.0 | 57.2 ± 0.1 | 202.9 | 0.36 |
| OD/CNTs-3(100) | 56.6 ± 0.2 | 234.1 | 57.1 ± 0.2 | 202.0 | 0.36 |
| OD/CNTs-3(200) | 57.1 ± 0.1 | 231.8 | 56.8 ± 0.3 | 202.3 | 0.35 |
| OD/CNTs-3(300) | 56.8 ± 0.2 | 231.1 | 56.6 ± 0.2 | 201.2 | 0.35 |
| Samples | OD/g | Diameter of CNTs/nm | CNTs/g | SDBS/g |
|---|---|---|---|---|
| OD/CNTs-1 | 10 | 10–20 | 0.1 | 0.1 |
| OD/CNTs-3 | 10 | 10–20 | 0.3 | 0.3 |
| OD/CNTs-5 | 10 | 10–20 | 0.5 | 0.5 |
| OD/CNTs-7 | 10 | 10–20 | 0.7 | 0.7 |
| OD/CNTs-9 | 10 | 10–20 | 0.9 | 0.9 |
| OD/CNTs-A | 10 | <8 | 0.3 | 0.3 |
| OD/CNTs-B | 10 | 10–20 | 0.3 | 0.3 |
| OD/CNTs-C | 10 | 20–30 | 0.3 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wang, Q.; Cheng, X.; Yang, Y.; Chen, X.; Cheng, Q. Enhanced Heat Transfer of 1-Octadecanol Phase-Change Materials Using Carbon Nanotubes. Molecules 2025, 30, 3075. https://doi.org/10.3390/molecules30153075
Wang X, Wang Q, Cheng X, Yang Y, Chen X, Cheng Q. Enhanced Heat Transfer of 1-Octadecanol Phase-Change Materials Using Carbon Nanotubes. Molecules. 2025; 30(15):3075. https://doi.org/10.3390/molecules30153075
Chicago/Turabian StyleWang, Xiuli, Qingmeng Wang, Xiaomin Cheng, Yi Yang, Xiaolan Chen, and Qianju Cheng. 2025. "Enhanced Heat Transfer of 1-Octadecanol Phase-Change Materials Using Carbon Nanotubes" Molecules 30, no. 15: 3075. https://doi.org/10.3390/molecules30153075
APA StyleWang, X., Wang, Q., Cheng, X., Yang, Y., Chen, X., & Cheng, Q. (2025). Enhanced Heat Transfer of 1-Octadecanol Phase-Change Materials Using Carbon Nanotubes. Molecules, 30(15), 3075. https://doi.org/10.3390/molecules30153075







