Abstract
This review focuses on the synthesis of spacer-armed phosphooligosaccharides structurally related to the capsular phosphoglycans of pathogenic bacteria, including the Haemophilus influenzae serotypes a, b, c, and f, Neisseria meningitidis serogroups a and x, the Streptococcus pneumoniae serotypes 6a, 6b, 6c, 6f, 19a, and 19f, and the Campylobacter jejuni serotype HS:53, strain RM1221, in which the phosphodiester linkage is a structural component of a phosphoglycan backbone. Also, in this review, we summarize the current knowledge on the preparation and immunogenicity of neoglycoconjugates based on synthetic phosphooligosaccharides. The discussed data helps evaluate the prospects for the development of conjugate vaccines on the basis of synthetic phosphooligosaccharide antigens.
1. Introduction
Among many smart tools for the colonization of human organs, pathogenic bacteria use a multilayer cell envelope [1,2], which is largely composed of glycans and their conjugates. The outermost thick layer found in the majority of Gram-negative and some Gram-positive bacteria is called a glycan capsule, the composition and structure of which depend on the particular bacterial species [3]. Most capsular glycans are long-chain linear negatively charged CPSs or phosphoglycans. In the course of infection, this part of the bacterial outer shell first comes into contact with the components of innate and adaptive immunity. The anionic glycans of pathogenic bacteria shield them from the action of the components of the complement system, phagocytes and cationic antimicrobial peptides, which are secreted by immune and epithelial cells and destabilize the membrane of pathogenic bacteria [4,5]. Thus, it was established [6] that the phosphoglycan capsule of Hib prevents phagocyte attachment and the subsequent uptake of these bacteria. Moreover, Hib can survive and multiply after having been engulfed by a phagocyte in the acidic medium of phagolysosomes [7]. On the one hand, these biopolymers act as both protective shields and adhesive agents, enabling the bacterial evasion of host immunity [8], and on the other hand, they represent a target for the host immune system.
It is known that a key step in the adaptive immune response is the production of antigen-specific antibodies, and the presence of IgG indicates the development of immunological memory to surface biopolymers [9]. Immunological memory enables the body to quickly recognize the antigen on the surface of the pathogen during the second and subsequent contact and more effectively activate the body’s defenses. This phenomenon encouraged the development of a whole range of antibacterial prophylactic vaccines. To date, the most effective type of vaccines for the prevention of bacterial infections are conjugate vaccines [10,11,12,13,14,15,16], in which a capsular glycan of the targeted pathogen is covalently linked to a protein carrier. The use of glycoconjugates helps circumvent the problem of the low immunogenicity of CPS and directs immunity along the T-dependent pathway [17,18,19,20].
The majority of commercial conjugate vaccines are currently manufactured on the basis of CPS produced in bacterial cell cultures. This approach has significant disadvantages, which are the laborious and operationally challenging steps of manufacturing, sophisticated quality control, and the high cost of producing pathogenic microbiological material. At present, an advanced approach to glycan conjugate vaccines is being developed, which employs synthetic OS antigens as an alternative to bacterial CPS [21,22,23,24,25,26].
In the first step of conjugate OS vaccine development, it is important to specify the particular bacterial glycoantigen to be mimicked. The probability factor speaks in favor of regular glycans, which have a repeating unit. These include the CPS of both Gram-positive and Gram-negative bacteria, lipopolysaccharide O-antigens of Gram-negative bacteria, and the cell-wall teichoic acids and lipoteichoic acids of Gram-positive bacteria. Today, commercial glycoconjugate vaccines incorporate bacterial glycan antigens exclusively.
In the second step, the optimum length of the OS, which is sufficient for the induction of protective immunity, has to be specified. On the one hand, the use of shorter OSs can significantly reduce production costs, and on the other hand, the OS chain has to be long enough to mimic the polymer and minimize the possible influence of the terminal monosaccharide residue. Identification of the minimum length of the OS antigen is performed in laboratory animals, which are immunized with the conjugates of synthetic oligosaccharides of different lengths, and the interaction of induced antibodies with bacterial antigens or directly with bacteria is investigated. It is commonly accepted [27] that the minimum length of an effective glycoantigen is 3–4 repeating units. A reliable algorithm that can predict the optimum length of OS antigens has not yet been developed. Today, the identification of protective glycotopes for conjugate vaccine candidates can be defined by the screening of glycan arrays, which encompass a series of synthetic OSs related to capsular glycans [28,29] in combination with conformational studies [29,30]. Alternatively, the development of the anti-Hib vaccine Quimi-Hib® (Centro de Ingeniería Genética y Biotecnología (CIGB), Republic of Cuba), which is a unique commercial synthetic OS-based conjugate vaccine, circumvented the problem of the choice of the optimum antigen length by the production of a protein-conjugated homologous phosphooligosaccharide obtained by oligomerization, with 7–8 repeating units as the average length of antigens [31]. The choice of the optimum structure of an OS antigen for a glycovaccine is complicated by the possibility of structural changes in a synthetic antigen after the injection of the vaccine into the recipient’s body. Partial lysis or migration of acetyl groups can occur in lymphs (pH 7.4–9.0) or endolysosomes of follicular B-lymphocytes [32,33,34], which are known to have an acidic environment.
In a number of human pathogens, a phosphodiester bond is involved in the formation of the main polymer chain. Phosphoglycans of this type were found, for example, in the cell wall of the human pathogens Hia, Hib, Hic, and Hif, the S. pneumonia serotypes 6A, 6B, 11A, 17F, 19A, and 19F, the N. meningitidis serogroups A and X, the Campylobacter jejuni serotypes HS53 (strain RM1221) and HS1 (strain ATCC 43429), and Escherichia coli K100 and K2 (Figure 1) [35,36,37,38]. Today, eleven bacterial pathogens of this type (Hib, Men A, and the S. pneumonia serotypes 6A, 6B, 10A, 11A, 15B, 18C, 19A, 19F, and 23F) are targeted by preventive vaccination, with commercial conjugate vaccines based on the corresponding capsular phosphooligosaccharides [21].
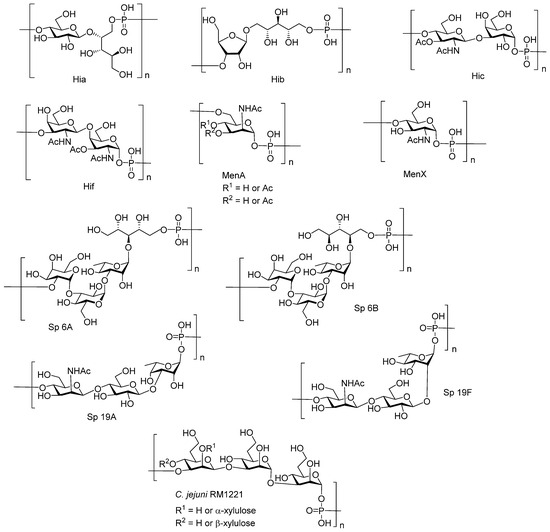
Figure 1.
Examples of bacterial capsular phosphoglycans.
In this review, we focus on the synthesis of spacer-armed mono-, di-, and oligomeric antigens structurally related to bacterial capsular phosphoglycans (Figure 1), in which the repeating units are connected via a phosphodiester bond, and consider the preparation and immunogenicity of neoglycoconjugates on the basis of these antigens.
Phosphoglycans are the common glycocalyx components of pathogenic Gram-negative and Gram-positive bacteria [35,36,39], yeast [39], and protozoan parasites [39,40]. In a living cell, phosphodiester-linked carbohydrates are arranged via the transfer of a hexose 1-phosphate to a glycan acceptor. In the presence of enzymes, which belong to the Stealth enzyme family, a hydroxyl group of a glycan acceptor attacks the phosphoester group in the nucleotide donor, and finally, a phosphodiester interglycosidic bridge unit is formed. It was established [41] (Figure 2) that in MenX bacteria, the hydroxyl group of N-acetyl glucosamine acceptor attacks the P-atom of uridine-5′-diphosphate-N-acetyl glucosamine to form an α-glycosyl phosphodiester, and the uridine monophosphate moiety serves as a leaving group.
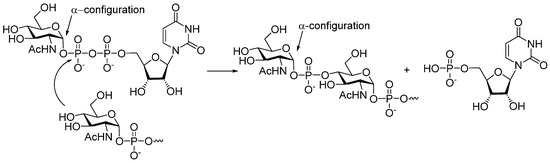
Figure 2.
Biosynthesis of MenX phosphoglycan involves nucleotide glycosyl donor uridine-5′-diphosphate-N-acetyl glucosamine and is catalyzed by hexose phosphotransferase [41].
A similar method of the establishment of a phosphodiester intersaccharide bridge is used in laboratory practice for the preparation of phosphooligosaccharides related to bacterial phosphoglycans. For the efficient and stereoselective formation of a phosphodiester linkage, a phosphodiester synthon is first introduced into one of the saccharide blocks, and the resulting product is reacted with a free hydroxyl group of another saccharide block.
As a rule, phosphodiesters, along with their diverse precursors (mono- and diphosphates, H-phosphonates, and phosphamidites; Figure 3), decompose under the conditions of a glycosylation reaction. Therefore, the retrosynthetic analysis of phosphoglycans suggests the formation of phosphodiester bridges within the latest steps of the synthetic route after the glycosidic linkages are already established. Usually, the preparation of phosphooligosaccharides related to natural biopolymers is a multi-step synthesis, which can be performed following a linear, convergent, or oligomerization pathway via the formation of an O-P-O tether between selectively protected and activated saccharide blocks.
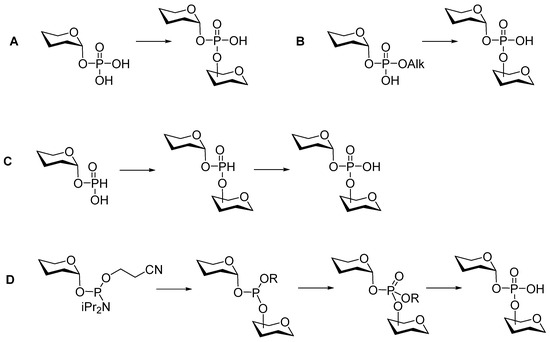
Figure 3.
Three main types of synthetic approaches used in the preparation of glycosyl phosphodiesters.
The conventional synthetic blocks for the preparation of glycosylphosphodiesters are monophosphates (Figure 3A), diphosphates (Figure 3B), H-phosphonates (Figure 3C), and phosphoramidites (Figure 3D). Previously developed methods of condensation involved phosphorus (V) chemistry (Figure 3A,B) and were promoted by N,N′-dicyclohexylcarbodiimide and 1-(2,4,6-triisopropylbenzenesulfonyl)-3-nitro-1H-1,2,4-triazole. In the late 1980s, methods A and B gave way to fast, efficient, and convenient techniques that employed H-phosphonates (Figure 3C) and phosphoramidites (Figure 3D).
The H-phosphonate condensation general procedure [42,43] was first proposed in the 1950s for oligonucleotide synthesis by Todd et al. [44], and it was further developed [45,46] and adapted for solid-phase synthesis [47] and customized to the needs of phosphoglycan chemistry [39,48]. The phosphoramidite method was first proposed by van Boom [49] and was later successfully applied in the solid-phase preparation of long-chain oligophosphodiesters [50] and the P-modified analogs of glycosylphosphate oligomers [51]. In addition to current methods, novel approaches are being actively developed, which are aimed at the preparation of glycosylphosphates with a predetermined anomeric configuration and the synthesis of the stabilized mimetics of phosphoglycans [52,53,54,55,56,57,58].
One of the key features of bacterial phosphoglycans and synthetic phosphooligosaccharides is their susceptibility to degradation via hydrolytic cleavage, transesterification, and rearrangement. Thus, PRP (Figure 1) was found to degrade spontaneously [59,60] in aqueous media. This molecule is destabilized by a hydroxyl group at C-2 (D-ribose), which is located in close proximity to the phosphodiester moiety and promotes the depolymerization and formation of cyclophosphate and phosphate monoester terminal groups [61]. In vaccine production, the inherent tendency of PRP to autolyze results in the loss of manufactured phosphoglycan and conjugate preparations [60]. Additionally, the low stability of PRP imposes significant limitations on the use of liquid Hib vaccines, especially in view of the acceleration of the degradation process in the presence of an alum adjuvant [62]. Stability studies conducted by Cintra et al. [60] showed that the rate of PRP depolymerization accelerates substantially with an increase in pH in the range 5.41–7.55 and with a rise in temperature in the interval of 28–40 °C. As a result, it may be assumed that in a host organism, PRP intensely degrades into fragments, which neutralize anti-PRP protective antibodies, thus hampering the immune response. In a similar way, partial PRP destruction after immunization with a conjugate Hib vaccine may result in the loss of Hib epitopes and reduce the level of protective anti-Hib antibodies. Two more factors that affect the stability of PRP are the presence of Na+ [60] and Ca2+ [59] cations. Similar to PRP, the capsular phosphoglycan of Hif (Figure 1) was shown to decompose under mild conditions [59].
MenA capsular phosphoglycan is especially susceptible to hydrolysis. It is assumed that the hydrolytic destruction of α-glycosylphosphodiester linkage occurs by two pathways. One of these includes the formation of an oxocarbenium ion, and within another pathway, the phosphodiester bond is cleaved with the assistance of the axial NAc group located at C-2 of the ManNAc, and a thermodynamically stable oxazoline is formed [63].
2. Synthesis of Pseudo-Oligosaccharides Structurally Related to PRP
Highly effective infant immunization with conjugate Hib vaccines, which are composed of a partially depolymerized Hib capsular phosphoglycan and a protein carrier (see review [15]), inspired researchers to develop conjugate vaccines using oligomer synthetic PRP fragments (Figure 4). Several series of PRP-related oligomers were synthesized, which were equipped with different types and locations of amino-spacers for convenient attachment to a protein carrier (compounds 1–15, Figure 4).
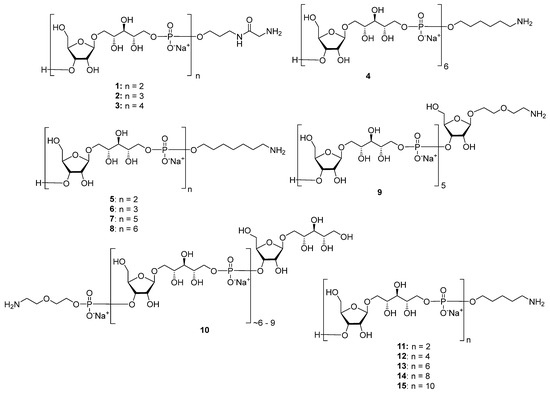
Figure 4.
Synthetic pseudo-oligosaccharides structurally related to PRP.
In pseudo-oligosaccharide 13 with a N-glycyl-aminopropyl spacer, which comprises two, three, or four residues of PRP repeating units [64], as well as in hexamer 4 [65] and oligomers 5–8 [66], the ω-aminospacer is linked to the D-ribose fragment via a phosphodiester bond. In pentamer 9 [67], the spacer is attached immediately to C-1 of a D-ribose residue as an aglycone. In the oligomeric mixture 10 [68] and in most of the representative oligomer series, which comprises the individual compounds 11–15 [69], the spacer is connected to a D-ribose via a phosphodiester bridge. In this review, basic synthetic blocks and strategies used in the preparation of compounds 1–15 are briefly considered, with a focus on the arrangement of a phosphodiester linkage and the introduction of a ω-aminospacer. A more detailed consideration of the syntheses of pseudo-oligosaccharides 1–15 was presented in our earlier review [15].
Between 1988 and 1992, van Boom and his research group at Leiden University synthesized spacer-armed PRP-related fragments for the first time. Dimer 1 [64,70], trimer 2 [64,70], and tetramer 3 [71] (Figure 4) were prepared by a sequential elongation of the oligomer chain from the non-reducing end, and the final equipment with an aminospacer. In brief, the phosphorylation of a free 3-OH group in the selectively protected riboside 16 with bis(benzotriazol-1-yl) (2-chlorophenyl) phosphate 17 produced the key activated phosphotriester building block 18 (Scheme 1). The condensation of compound 18 with the selectively protected disaccharide 19 in the presence of N-methylimidazole in Py, followed by the removal of the 1-O-propenyl group, was carried out in a sequential manner, one, two, or three times, followed by the final capping with phosphotriester 20. Total deprotection afforded the series of conjugation-ready compounds 1–3.
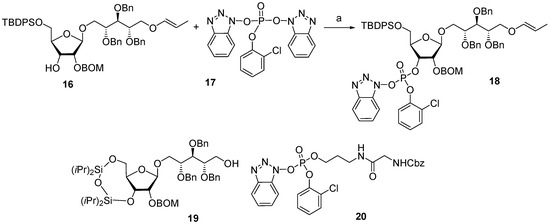
Scheme 1.
Key building blocks for the synthesis of spacer-armed PRP-related oligomers (1–3). Reagents and conditions: (a) Py/dioxane, r.t., 30 min [64,70,71].
The spacer-armed trimer 2 and tetramer 3 were conjugated to TT (Figure 5) to create the corresponding neoglycoconjugates 21 and 22. The antigenic properties of oligomers 2 and 3 as components of neoglycoconjugates 21 and 22 were examined [71] in competitive inhibition ELISA experiments. A conjugate of bacterial PRP and tyramine was used as a coating antigen, and normal human serum and bacterial PRP were used as positive controls.
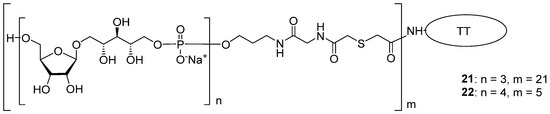
Figure 5.
Conjugates 21 and 22 obtained from PRP oligomers 2 and 3 with TT [71].
Total inhibition was observed when bacterial PRP and conjugate 22 (tetramer + TT, Figure 5) at a concentration of 25 μg/mL were used as inhibitors, and conjugate 21 with the trimeric PRP–antigen was less effective. Compared to conjugates, the inhibitory capacity of the unconjugated oligomers 2 and 3 was much lower, and at a concentration of 25 μg/mL, it did not reach 40%.
The immunogenicity of glycoconjugates 21 and 22 was studied [71] in mice at a dose of 1 µg of phosphoglycan per mouse. IgG antibodies in immune sera were analyzed in ELISA using a bacterial PRP–tyramine conjugate as a coating antigen and normal human serum as a positive control. Conjugate 21 with a trimeric antigen was found to be low immunogenic in a mouse model. In IgG ELISA, the mean optical density value for the sera of immunized mice was less than two times that of the corresponding value obtained for the sera of mice that received PBS.
For the immune sera from mice vaccinated with conjugate 22, which comprised tetrameric residues, the mean optical density value was three times that of the negative control, thus unambiguously evidencing the immunogenicity of conjugate 22. Immunization experiments were conducted using preparations formulated with AlPO4 or without an adjuvant. It was shown that the presence of AlPO4 had no effect on the result of immunization [71].
To date, there is no animal model that reliably predicts the immunogenicity of glycoconjugate preparations in humans, and the choice of experimental animals for the examination of the immune activity of Hib preparations is usually at the discretion of the researcher. In the blood sera of immunized mice and guinea pigs, the concentration of antibodies directed against Hib antigens does not reach 1 mg/mL, and these animals are not recommended for laboratory studies of conjugate vaccines [66].
As shown in Scheme 1, the key reaction in the assembly of the synthetic Hib phosphooligosaccharides 1–3 is the arrangement of the O-P-O linkage, which can be applied iteratively. As no new chiral centers or other types of isomerism are created, this reaction sequence can be fulfilled using a solid-phase approach. This approach significantly decreases the number of laborious steps of the chromatographic separation of the product. In 1989, van Boom synthesized the spacer-armed hexamer 4 using controlled-pore glass as a solid support [65]. First, the selectively protected pseudo-disaccharide 23 was phosphitylated with chlorophosphoramidite 24 (Scheme 2), and the resulting phosphoramidite 25 was used for a stepwise extension of the oligomer chain starting from ribosylribitol 26, which was immobilized on a glass support. After each step of chain elongation, the phosphorus atom was oxidized with iodine in an acetonitrile/water/collidine mixture to obtain the corresponding phosphotriester, and the terminal DMTr protective group was removed to provide a free hydroxyl group for the next phosphitylation step. After the sequential attachment of five repeating units and spacer 27 and the removal of the protective groups, the target spacer-armed hexamer 4 was obtained.
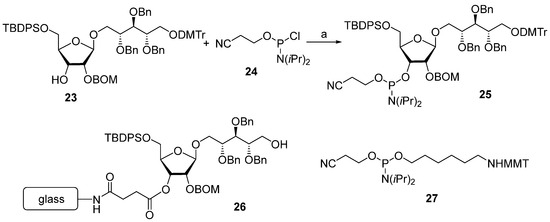
Scheme 2.
Key building blocks for the synthesis of hexamer 4. Reagents and conditions: (a) diisopropylethylamide/dichloromethane, 85% yield [65].
In 1992, Kandil et al. [66] synthesized the series of spacer-armed Hib oligomers 5–8 on a PEG support using a strategy similar to the synthesis of hexamer 4 (Scheme 3). In this synthesis, the tert-butyldiphenylsilyl and benzyloxymethyl protective groups in the starting pseudo-disaccharide 28 were replaced with easily removable benzyl groups. Thus, the sequential condensation of phosphoramidite 29 with PEG-immobilized pseudo-disaccharide 30 and detritylation was repeated five times. Next, the pre-spacer 31 was attached, and finally, total deprotection resulted in the formation of hexamer 6. The latter was conjugated to synthetic peptides structurally related to the Hib outer membrane protein, and one of these preparations showed immunogenicity comparable to that shown by the commercial Hib vaccine [72].
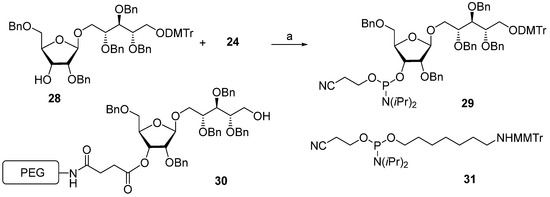
Scheme 3.
Key building blocks used in the synthesis of the spacer-armed oligomers 5–8. Reagents and conditions: (a) 1H-tetrazole/acetonitrile [66].
The same year, a group of Swedish researchers under the leadership of Norberg published [67] the synthesis of the Hib pentamer 9 on a polystyrene support (Scheme 4). In this work, the H-phosphonate method was used for the establishment of a phosphodiester bond, which subsequently became the most popular in the preparation of oligophosphoglycans related to the glycocalyx components of bacteria and parasites [39,56]. The selectively protected disaccharide 32 was reacted with 5,5-dimethyl-2-chloro-1,3,2-dioxaphosphorinane 2-oxide (compound 33) in Py and the presence of phosphonic acid to obtain H-phosphonate 34 with an 84% yield.
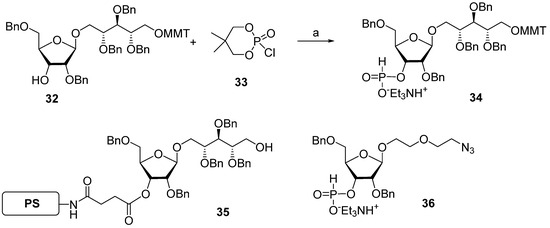
Scheme 4.
Key building blocks for the synthesis of pentamer 9 [67]. Reagents and conditions: (a) H3PO3, Py, yield of 84%.
The activation of H-phosphonates for subsequent attachment to alcohols can be achieved in the presence of the acyl chlorides of sterically hindered acids, e.g., PivCl or 1-adamantanecarbonyl chloride. In this reaction, the formation of by-products depends significantly on the order of the addition of reagents. For example, the activation of H-phosphonate by PivCl in Py in the absence of alcohol led to the formation of unwanted bis-acylated phosphites, and the use of a large excess of PivCl in solid-phase synthesis resulted in the pivaloylation of the support [67]. The search for the optimum conditions for the attachment of H-phosphonate 34 to be selectively protected and immobilized on the solid support monomer 35 resulted in the discovery of the most efficient proportion—5 eq. of PivCl and 5 eq. of H-phosphonate 34—that provided the adduct with a 95% yield. The authors noted [67] that the yield decreased with each chain extension step and dropped to 86% in the fourth cycle. The optimum sequence of reagent addition was found as follows: in the first step, H-phosphonate was activated by the addition of PivCl in Py, and then, the mixture was added to the immobilized alcohol. As a result, the yield of the pentamer increased to 96%. In the final step, the chain was capped with 2-(2-azidoethoxy) ethyl riboside 36, H-phosphonate was oxidized with iodine in 2% aqueous Py, and the protective groups were removed to obtain oligomer 9.
The multistep synthetic pathways discussed above are based on the linear sequential elongation of an oligomeric chain. As a rule, laboratory processes of this type cannot be scaled up to a commercial scale because of the high cost. For profitable manufacturing, a convergent synthetic scheme is needed. Under the leadership of Verez-Bencomo and Roy, a convergent synthesis of a mixture of homologous PRP oligomers (Figure 4, compound 10) was developed and then scaled up to the commercial production of the anti-Hib vaccine Quimi-Hib® (Heber Biotec, S.A., Republic of Cuba). In this synthesis, polycondensation of a bifunctional monomer is employed for the establishment of O-P-O linkages as a key step of oligomer synthesis in the place of stepwise elongation (Scheme 5).
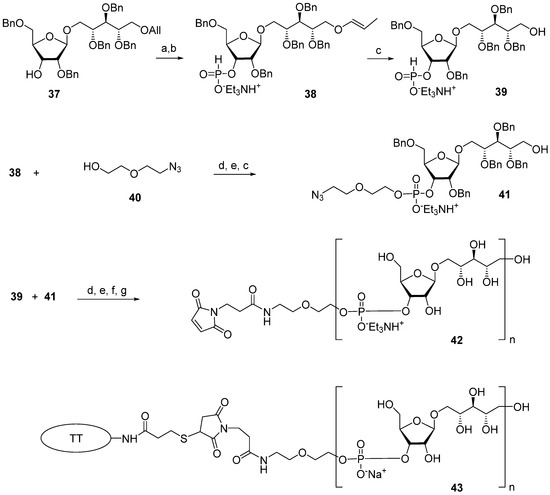
Scheme 5.
Key building blocks for preparation of oligomeric mixture 10 (n= 6–9) [68]. Reagents and conditions: (a) potassium tert-butoxide/DMSO, 100 °C; (b) PCl3, imidazole, Et3N; (c) CH3COOH/H2O, 80 °C; (d) PivCl/Py; (e) I2, Py/H2O; (f) H2, Pd/C, EtOH-H2O-EtOAc-AcOH at 1.5 atm; (g) SMP.
In brief, the allyl group of the selectively protected pseudo-disaccharide 37 is isomerized into the 1-O-propenyl group by the action of potassium tert-butoxide in DMSO. The resulting isomer is converted to H-phosphonate 38 by the action of PCl3 and imidazole, and finally, the 1-O-propenyl group is removed under acidic conditions to obtain the key heterobifunctional monomer 39. The interaction of 38 with the diethylene glycol spacer 40 in the presence of PivCl/Py, followed by oxidation with iodine in aqueous Py, and the further removal of the 1-O-propenyl group, creates alcohol 41, which serves as a terminal unit in the polycondensation. The polycondensation of 39 and 41 is carried out in the presence of PivCl in Py, followed by oxidation of the mixture of H-phosphonates into the corresponding phosphodiester mixture, reduction of the azido groups, N-acetylation, and total deprotection. The obtained mixture of the spacer-equipped oligomer 10 is activated by the action of SMP to obtain a conjugation-ready mixture of maleimide 42. The conjugation of the activated oligophosphodiesters with thiolated TT results in the production of a set of conjugate 43 with the average weight ratio PRP:TT of 1:2.6 [31,68].
On the basis of the mixture of conjugate 43, an anti-Hib vaccine, Quimi-Hib®, was developed, which successfully passed all the required preclinical [73] and clinical trials [31,74]. The antigenicity of synthetic PRP oligomer 10 was compared to the antigenicity of bacterial PRP in ELISA experiments [31]. A conjugate of a mixture of the spacer-equipped oligomer 10 with HSA (10-HSA), prepared similarly to conjugate 43, and a conjugate of partially depolymerized bacterial PRP (PRPDp30) with HSA were used as coating antigens. The PRPDp30-HSA conjugate was obtained by the reductive amination of the mixture of bacterial PRP depolymerized via periodate oxidation to a length of ~30 monomeric units and conjugation with HSA [68]. Standard rabbit anti-PRP antibodies were obtained by the immunization of animals with two licensed commercial conjugate vaccines based on bacterial PRP. These were Hiberix® (PRP-TT), which contains PRP activated by cyanogen bromide and conjugated to TT via an adipic acid dihydrazide linker, and Vaxem-Hib® (PRP-CRM197), which is a product of the conjugation of partially depolymerized bacterial PRP with an avDP10 repeating unit and the protein carrier CRM197 via an adipic acid dihydrazide spacer. The correlation coefficient for the titers obtained in the ELISA experiments with standard rabbit sera and the coating antigens 10-HSA and PRPDp30-HSA was in the high-value range (0.972–0.978), indicating the similarity of the antigenic properties of the synthetic oligosaccharide 10 and Hib CPS [75].
For the preliminary evaluation of the immunological activity of the conjugate 43 in vivo, rats, mice, and rabbits were chosen as experimental animals. Rats were immunized subcutaneously twice at an interval of 4 weeks with a dose of conjugate 43 containing 2 µg of the ligand 10 [75]. Mice were immunized intraperitoneally three times at an interval of 2 weeks with a dose of conjugate 43 containing 2.5 µg of ligand 10. Rabbits were immunized with conjugate 43 using both two-step and three-step regimens at a dose of 5 µg of ligand 10 per animal. The efficiency of immunization was assessed by the evaluation of IgG titers, which were calculated as the logarithm of the highest dilution at which the light absorbance of the diluted serum sample is twice as high as that of the negative control. For the negative control, the animals were injected with PBS. A PRP-HSA conjugate was used as a coating antigen in the ELISA experiments. The study of the PRP-specific antibodies showed that the immune response to conjugate 43 in rodents was weak and inconsistent. In contrast, the immunization of rabbits with conjugate 43 efficiently evoked PRP-specific antibodies, as shown in the inhibition ELISA experiments with bacterial PRP as the inhibitor [75].
In a phase 1 clinical trial [31], more than 100 children aged 4 to 5 years were immunized with a single dose of a vaccine preparation formulated on the basis of conjugate 43 without an adjuvant. Comparative studies of the immunogenicity of synthetic antigens in conjugate 43 and partly depolymerized bacterial PRP in Vaxem-Hib® showed that the average anti-PRP IgG titers were similar. It was found that PRP-specific IgG antibodies induced in children by immunization with conjugate 43 had bactericidal activity comparable to that stimulated by the action of Vaxem-Hib®. In the course of phase 2 clinical trials [31], 1141 infants were immunized three times at 2, 4, and 6 months of age with or without AlPO4, and the positive control group was immunized with Vaxem-Hib®. Antibody tests showed that 99.7% of infants had serum anti-PRP IgG concentrations >1 µg/mL, which is known to provide prolonged protection against Hib infection [76], and the mean concentration of anti-PRP IgG was as high as 27.4 µg/mL.
It is noteworthy that in Quimi-Hib®, different-sized oligomeric antigens were present, thus circumventing the problem of the elucidation of the optimal length of an oligomeric PRP antigen. The success of the development and application of the cost-effective vaccine Quimi-Hib® with a synthetic Hib-antigen inspired scientists to search for the shortest protective Hib-antigen, which is likely to be in the range of chain lengths that are obtained during the polycondensation of 39.
One of the novel and efficient approaches to the preparation of PRP-related oligomeric phosphoglycans suggested by Seeberger et al. [69] involved the elongation of the chain by two PRP repeating units at once (Scheme 6). The applied strategy effectively shortened the task of the oligomer assembly and raised the degree of convergence.
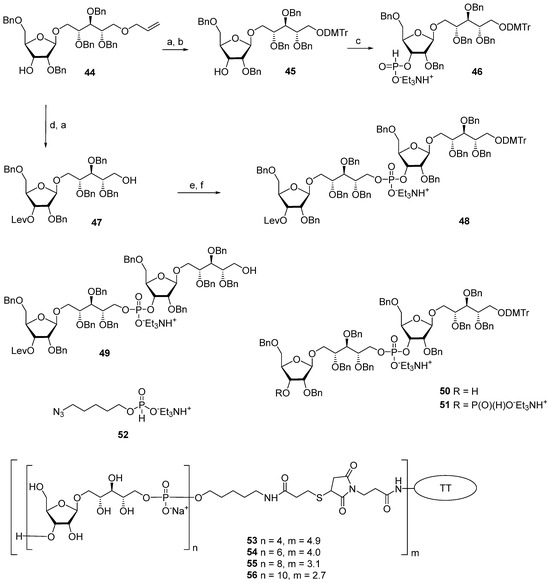
Scheme 6.
Key building blocks for preparation of oligomers 12–15 [69]. Reagents and conditions: (a) Pd(PPh3)4, 1,3-dimethylbarbituric acid, methanol, 75%; (b) DMTCl, DMAP, CH2Cl2, 93%; (c) PCl3, Et3N, imidazole, CH2Cl2, 0 °C, 85%; (d) levulinic acid, DMAP, CH2Cl2, 93%; (e) 46, PivCl, Py, 0 °C; (f) I2, Py/H2O, 85%.
For the preparation of a selectively protected and activated bifunctional dimeric key unit, the universal precursor 44 was deallylated, and the resulting diol was regioselectively 4,4′-dimethoxytritylated at the primary hydroxyl group to obtain compound 45, which was then converted to H-phosphonate 46 by the action of PCl3, Et3N, and imidazole. For the preparation of alcohol 47, the universal precursor 44 was levulinoylated and deallylated. Alcohol 47 was reacted with H-phosphonate 46 in PivCl/Py to obtain the key dimeric precursor 48 with readily removable orthogonal levulinoyl and dimethoxytrityl protective groups. The de-4,4′-dimethoxytritylation of pseudo-tetrasaccharide 48 in the presence of trichloroacetic acid afforded alcohol 49 with a free terminal hydroxyl group in the ribitol residue. The delevulinoylation of universal precursor 48 by the action of hydrazinium acetate resulted in the formation of alcohol 50, which was converted into H-phosphonate 51. The combination of H-phosphonate 51 and primary alcohol 49, followed by oxidation, yielded a tetramer, which was, in turn, de-4,4′-dimethoxytritylated and reacted with H-phosphonate 46 for the elongation of the chain or with the 5-azidopentyl derivative 52 for chain termination. Using this elegant strategy, PRP-related tetramer (85%), hexamer (85%), octamer (83%), and decamer (80%) were prepared, which were subjected to sequential detritylation, the attachment of a 5-azidopentyl spacer under the action of H-phosphonate 52, the transformation of the azido group into an amino group, and total deprotection to obtain the spacer-armed oligomers 12–15 (Figure 4). After activation, oligomers 12–15 were reacted with TT to obtain conjugates 53–56 [69].
The antigenicity of oligomers 11–15 (Figure 4) was examined using a glycan microarray. Compounds 11–15 were immobilized on a N-hydroxysuccinimide hydrogel surface on glass slides and reacted with polyclonal anti-Hib hyperimmune rabbit sera or standard human sera, which were mixed with different concentrations of the WHO PRP standard as an inhibitor. The subsequent addition of a fluorescent secondary antibody revealed the dependence of the fluorescence intensity on the PRP concentration, thus proving the presence of antibodies specific to the synthetic PRP-oligomers 11–15. Hexamer 13, octamer 14, and decamer 15 interacted with standard human sera in a similar way, binding with tetramer 12 was weaker, and in the case of dimer 11, the adsorption of the antibodies was even less effective. In contrast, rabbit hyperimmune sera interacted with all five oligomers equally well [69].
The immunogenicity of conjugates 53–56 was studied using an animal model. Rabbits were immunized with these conjugates at a dose of 5 μg of an oligomer without an adjuvant, and the positive control group received ActHib® (PRP-TT). Bacterial PRP was used as a coating antigen. Immunization with conjugates 53 and 55, based on tetramer 12 and octamer 14 ligands, respectively, resulted in a high level of PRP-specific antibodies. Conjugates 54 and 56, which comprised hexamer 13 and decamer 15, induced substantially lower levels of PRP-specific antibodies [69]. Therefore, in a series of conjugates equipped with antigens with an even number of repeating units, the tetramer was the most promising synthetic antigen candidate for the design of an anti-Hib vaccine.
In a continuation of this work, Seeberger et al. synthesized [77] a series of mimetics of synthetic PRP-related dimer, tetramer, hexamer, and octamer compounds (11–14), which comprised 2-deoxy-, 2-deoxy-2-fluoro-, 2-deoxy-2-(N,N-dimethyl)-carbamoyloxy-, and 2-O-methylated ribose residues in different combinations. This research was aimed at the preparation of analogs of PRP antigens with higher hydrolytic stability. The study of the conjugates of these antigens with the protein carrier CRM197 showed that the most promising are mimetics, in which ribose residues are methylated at the position O-2. Conjugates of these mimetics with CRM197 stimulated Hib-specific immune responses in both animals and humans, which confirms the possibility of their commercial use in anti-Hib vaccines. The replacement of the 2-OH group, which promotes the hydrolysis of the phosphodiester bond, with a methoxy group allowed for a significant increase in the integrity of the antigen [77].
3. Synthesis of Oligomers Structurally Related to Capsular Phosphoglycans of Hia, Hic, and Hif
Hib bacteria are the common and most dangerous serotype of the H. influenzae species, which comprises at least five more encapsulated serotypes and numerous non-capsulated (non-typeable) variants. The introduction of anti-Hib conjugate vaccines [13,15,21,23] in national immunization schedules in a number of European countries, North and South America countries, Australia, and the South African Republic significantly contributed to a reduction in invasive diseases caused by Hib-infection [78,79]. At the same time, along with the expansion of anti-Hib vaccination programs, the spread of other serotypes of H. influenzae was reported [80,81,82,83,84,85,86,87,88,89]. As a consequence, after more than thirty years’ use of anti-Hib conjugate vaccines, an “antigenic shift” is observed, which is expressed in the increased incidence of invasive diseases, including meningitis, meningoencephalitis, and septicemia caused by encapsulated H. influenzae serotypes different from b [82,90].
In European countries, the number of invasive diseases caused by Hia is also growing. For example, in England, in 2022, Hia was responsible for 19% of all invasive disease cases caused by encapsulated H. influenzae. Before 2017, only sporadic cases occurred [80], and the contribution of Hif and Hie is also growing [90]. In South Africa, the rise of Hie is observed [82]. In American countries, H. influenzae diseases are often caused by Hif [82,91]. Also, an increase in invasive diseases caused by Hia [85,89,92,93] and Hic [91] was reported.
In the early 1990s, in the northern regions of Canada and Alaska, a routine anti-Hib immunization schedule was introduced, and in the late 1990s, a growing number of cases of Hia infection were registered. The majority of cases (more than 60%) were reported in children under five years of age. As a result, special research is being conducted in Canada aimed at the development of a vaccine for the prevention of diseases caused by Hia [84]. Considering that invasive diseases caused by encapsulated H. influenzae serotypes different from serotype b remain relatively rare and are often localized in specific areas, corresponding vaccines will have limited use according to epidemic indications. In this situation, conjugate vaccines with synthetic antigens structurally related to H. influenzae capsular glycans may be considered as a drug of choice.
Inspired by the success of the Quimi-Hib® vaccine, which comprises synthetic oligomeric phosphoglycans structurally related to Hib capsular phosphoglycan, researchers synthesized [94,95] the series of oligomers 57–61 with one, two, three, four, and five repeating units of Hia capsular phosphoglycans and 3-aminopropyl linkers (Figure 6). In another recent study, the series of fragments of Hia capsular phosphoglycans 62–64 equipped with a 66 diethyleneglycol linker was obtained [96] (Figure 6). Syntheses of aminoalkyl glycosides related to the Hic phosphoglycan (compounds 65 and 66) and related to the Hif phosphoglycan (compounds 67 and 68) were performed by Oscarson et al. [97].
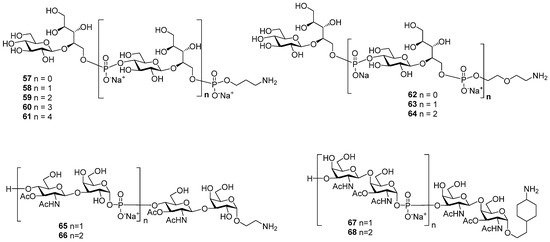
Figure 6.
Synthetic oligomers 57–61 [94] and 62–64 related to Hia capsular phosphoglycans [96], oligomers 65 and 66 related to Hic capsular phosphoglycans, and oligomers 67 and 68 related to Hif capsular phosphoglycans [97].
In the two series of the spacer-armed oligomers 57–61 [94] and 62–64 [96] structurally related to Hia capsular phosphoglycans, the linker is connected to the glycan antigen via a phosphodiester bridge. Compounds in both series were prepared using a convergent synthetic strategy with iterative elongation of the linear structure starting from the non-reducing end, and finally, the spacer was added (Scheme 7 and Scheme 8). The major difference between the syntheses of these two series is the type of universal, selectively protected bifunctional synthon. In the preparation of oligomers 58–61 [94], a monomer block with a phosphoramide group at C-4 (Glc) was used, and for compounds 63–64 [96], the synthetic pathway involved the use of a non-phosphorus monomer.
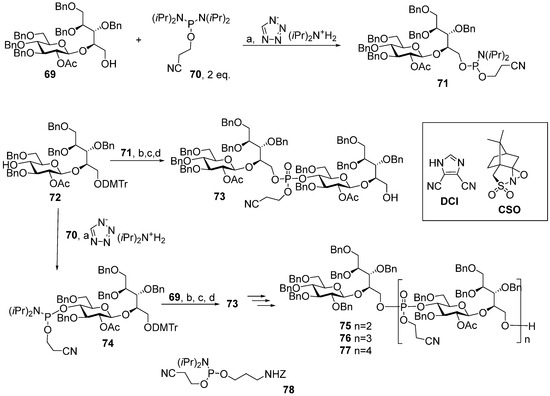
Scheme 7.
Key building blocks for the synthesis [94] of oligomers structurally related to Hia capsular phosphoglycans. Reagents and conditions: (a) CH2Cl2, r.t., 98% for compound 71 and 95% for compound 73; (b) DCI, CH2Cl2, 4Å molecular sieves; (c) CSO; (d) 0.18M TCA in CH2Cl2, 79% over 3 steps.
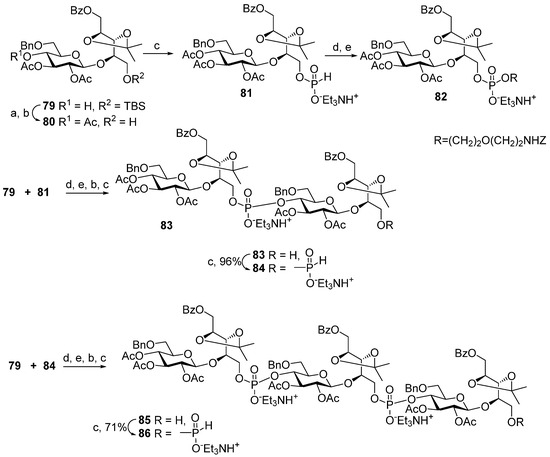
Scheme 8.
Key blocks for synthesis of oligomers structurally related to Hia phosphoglycans [96]. Reagents and conditions: (a) acetic anhydride, DMAP, Py; (b) HF (aq.), Py, 60 °C, 97% for two stages; (c) H3PO3, PivCl, Py, 74%; (d) ZNH(CH2)2O(CH2)2OH, PivCl, Py; (e) I2, Py/H2O.
For the choice of the optimum strategy for the assembly of oligomers 57–61 (Scheme 7) [95], two alternative ways were considered. The first strategy involved pseudo-disaccharide 69 with a free hydroxyl group in the ribitol residue, which was reacted with 2-cyanoethyl N,N,N′,N′-tetraisopropylphosphordiamidite (compound 70) in the presence of diispropylammonium tetrazolide to obtain the selectively protected phosphoramidite 71 as a universal precursor with a 95% yield. The following condensation of phosphoramidite 71 and glycoside 72 with a free hydroxyl group at C-4 (Glc), oxidation of a phosphite into a phosphodiester, and detritylation created the conjugation-ready dimer 73 with a 73% yield over three steps.
Another way of preparing phosphotriester 73 (Scheme 7) employed phosphoramidite 74 with a pro-phosphodiester group located at C-4 (Glc). The sequential combination of phosphoramidite 74 with alcohol 69, oxidation, and detritylation resulted in the formation of the phosphodiester block 73 with a 79% yield over three steps [94,95]. Therefore, both assembly strategies were equally effective. Starting with phosphoramidite 74, protected pseudo-oligosaccharides 75–77 were obtained and then converted to ligands 58–61 by interaction with the pre-spacer phosphoramidite 78 and total deprotection. The authors pointed out that in comparison to the H-phosphonate approach, the phosphoramidite protocol was more efficient for the construction of longer phosphodiester-linked chains. Oligomers 57–61 were N-acylated with a di(N-hydroxysuccinimidyl) adipate linker, and the activated esters were conjugated to CRM197. The resulting conjugates contained 13–25 copies of the oligomeric antigen per CRM197 unit. Rats were immunized with three doses, which contained 2 μg of the glycan antigen, and the IgG antibodies were analyzed in the sera using ELISA. Conjugates of trimer 59 and pentamer 61 with HSA were used as coating antigens. All the conjugates were immunogenic and induced similar levels of IgG antibodies, which recognized immobilized synthetic antigens. The researchers noted that the immunogenicity data for the CRM197-based conjugates of oligomers 57–61 was independent of the length of the oligomeric antigen [95].
Compounds 62–64 [96] were prepared (Scheme 8) starting from the universal precursor pseudo-disaccharide 79. The acetylation of compound 79 resulted in acetate 80, which was phosphitylated to obtain key monomeric H-phosphonate 81 with a 97% yield. The condensation of H-phosphonate 81 with a pre-spacer N-carbamoyl aminopropanol, as shown in Scheme 8, created phosphodiester 82 (yield: 34%), which, after the removal of the protective groups, was transformed into the spacer-armed target monomer 62 (Figure 6). To obtain dimer 83, alcohol 79 was condensed with H-phosphonate 81 with a 77% yield and then oxidized. Phosphodiester 83 was, in turn, converted into H-phosphonate 84. The interaction of H-phosphonate 84 with alcohol 79 produced phosphodiester 85, which was converted into H-phosphonate 86. Spacer-armed derivatives were obtained by the interaction of H-phosphonates 84 and 86 with N-carbamoyl aminopropanol to obtain, after total deprotection, the spacer-armed dimer 63 and trimer 64, respectively (Figure 6). It is interesting to note that the efficiency of condensation with N-protected aminopropanol increased in the series 81 > 84 > 86 with the rise in the number of phosphodiester fragments present in these H-phosphonates, whereas numerous experimental data suggest the opposite [39].
In 2001, Oscarson et al. synthesized the amino spacer-armed oligomeric fragments of Hic (compounds 65 and 66) and Hif (compounds 67 and 68) phosphoglycans [97] (Figure 6), in which the repeating disaccharide units are linked via a phosphodiester bridge. Similar to the block syntheses of Hib and Hia fragments described above, the establishment of a phosphodiester linkage between selectively protected oligosaccharide blocks was used for the chain elongation. However, unlike Hib and Hia, in Hic and Hif capsular phosphoglycans, the anomeric carbons in hexoses are involved in the formation of a phosphodiester bridge. Therefore, the development of a synthetic strategy for the preparation of Hic and Hif fragments has to be developed with respect to the possibility of the formation of an unwanted anomer. At the step of chain assembly, it can be particularly difficult to provide stereocontrol in the formation of a C-1-O-P bond. Instability of the anomeric phosphodiester linkage also has to be considered, which makes it possible to use anomeric phosphodiesters as glycosyl donors in the glycosylation reaction. A convenient strategy for the preparation of the desired anomer includes the initial stereocontrolled glycosylation of a phosphodiester synthon, which provides an intermediate product with the target anomeric configuration, and the subsequent coupling of this compound in mild conditions for the prevention of anomerization. These conditions are met in the H-phosphonate protocol, as hexose hemiacetals retain the anomeric configuration, while they are transformed into H-phosphonates upon the action of triimidazolyl phosphine prepared in situ from PCl3 and imidazole in the presence of Et3N [39].
For the preparation of the spacer-armed oligomers 65 and 66 (Figure 6) [97], which are structurally related to Hic phosphoglycans, hemiacetal 87 with an axial hydroxyl group at C-1 was converted into the key α-H-phosphonate 88 (yield: 89%), which was condensed with the selectively protected alcohol 89 and oxidized with I2 (Scheme 9A) to provide phosphodiester 90 (yield: 71%). The desilylation of phosphodiester 90 produced alcohol 91, which was readily condensed with α-H-phosphonate 88, and the phosphite group was oxidized to produce phosphate 92 in a moderate yield (36%). After total deprotection, the reduction of the azido group, and the N-acetylation of compounds 91 and 92, the target spacer-armed phosphooligosaccharides 65 and 66 were obtained.
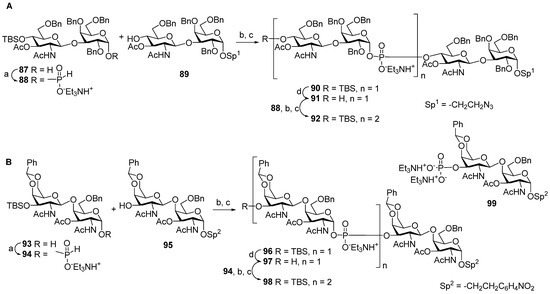
Scheme 9.
(A) Key blocks 87–92 for the synthesis of oligomers [97] 65 and 66 related to Hic phosphoglycans; (B) key blocks 93–98 for the synthesis of oligomers 67 and 68 related to Hif phosphoglycans. Reagents and conditions: (a) PivCl, Py; (b) PCl3, imidazole, Et3N, 85%; (c) I2, Py/H2O, -40 °C; (d) Et3N(HF)3.
Analogously, the favorable α-configuration of the GalNAc hemiacetal residue in disaccharide 93 (Scheme 9B) paved the way to the preparation of the spacer-armed phosphooligosaccharides 67 and 68 related to Hif phosphoglycans [97]. Upon action with PCl3 and imidazole, hemiacetal 93 was transformed into α-H-phosphonate 94 with a retention of configuration (yield: 87%). The condensation of α-H-phosphonate 94 with disaccharide 95 and the subsequent oxidation resulted in the efficient formation of phosphodiester 96 (yield: 81%), which was desilylated to obtain alcohol 97. Similar to the condensation of compounds 91 and 88, the interaction of 97 and 94 was less efficient, and phosphodiester 98 was obtained with a 37% yield. Most likely, low yield in this reaction is associated with the high lability of the phosphodiester group in acceptors 91 and 97 in the conditions of oxidation with I2 [39]. The authors [97] observed the formation of a significant amount (up to 40%) of 3′-O-phosphate 99, which indicates the lability of a glycosyl-O-phosphodiester tether under oxidation conditions. The low yields of attachment of the second glycosyl-phosphodiester residue for variants A and B, shown in Scheme 9, suggest that the stability of a phosphodiester bond is largely determined by the condensation and oxidation conditions and is less dependent on the structure of disaccharide residues. The target spacer-armed phosphooligosaccharides 67 and 68 were obtained after complete deblocking of the compounds and the reduction of the nitrophenyl residue into an aniline residue.
In view of the development of an anti-Hia vaccine, two aspects have to be addressed. Similar immunogenic properties of synthetic Hia antigens from monomer to pentamer can be connected with the low stability of phosphodiester linkages in aqueous solutions. In this case, the design of a vaccine may require a mimetic structure for the antigen. Also, it is advisable to use a phosphoramidite-based strategy for the preparation of phosphooligosaccharide antigens for industrial production, as the considered examples demonstrate the advantage of the phosphoramidite-based method over the H-phosphonate procedure. Meanwhile, anti-Hic and anti-Hif conjugate vaccines are not considered to be of high importance in contemporary glycoscience, as researchers have not referred to this topic for more than 20 years.
4. Synthesis of Phosphooligomers Related to Capsular Phosphoglycan of MenA
In 2010, a conjugate polysaccharide meningococcal monovalent vaccine, MenAfriVac® (MenA-TT), was licensed [98,99]. In contrast to meningococcal polysaccharide vaccines, the conjugate preparation was found to be highly effective. For example, the results of mass vaccination campaigns in African meningitis belt countries that were carried out in 2010–2015 showed more than a 99% decrease in the incidence of MenA-associated meningitis [100]. Today, MenA phosphoglycan is a component of a number of polyvalent conjugate meningococcal vaccines, including Menactra® (A-meningococcal component MenA-diphtheria toxoid), Menveo® (A-meningococcal component MenA-CRM197), and Nimerix® (A-meningococcal component MenA-TT) [14].
Polymer chains of MenA phosphoglycan are highly labile in aqueous media [63]. This intrinsic property of MenA phosphoglycan creates the need for the cold-chain transport of conjugate anti-MenA vaccines [101], which significantly increases the cost per dose and, in some cases, is an insurmountable obstacle to the use of this type of preparation [102]. Also, this feature imposes additional requirements on the production of both monovalent vaccines and polyvalent vaccines in a convenient liquid form. In this regard, considerable research efforts have been directed towards the synthesis of oligomeric antigens structurally related to MenA CPS fragments or corresponding mimetics with a view to preparing commercial conjugate vaccines. The use of anti-MenA conjugate preparations with a synthetic oligoside antigen, the structure of which fully corresponds to MenA CPS fragments, could allow the monitoring of the structural integrity of this preparation during storage and transportation, and the design of an antigen using ManNAc phosphate mimetics obtained by replacing the oxygen atom with the methylene group in the pyranose ring with (carba-analogs) or by replacing the anomeric oxygen atom with a methylene group (C-phosphonates) will make the antigen structure resistant to hydrolysis.
It is important to emphasize that in MenA bacterial phosphoglycans, ~80% of 3-OH groups and ~10% of 4-OH groups in ManNAc residues are acetylated [103]. A study of phosphoglycan structures present on the surface of a living MenA bacterial cell [104], which was conducted using high-resolution magic-angle spinning, showed the presence of acetyl substituents at 50–60% of 3-OH groups and 25–30% of 4-OH groups. The acetylation of MenA phosphoglycans is known to be an important antigenicity factor [105,106].
Spacer-armed synthetic antigens structurally related to the MenA capsular phosphoglycan and the corresponding phosphono- and carba-analogs are attractive synthetic compounds considering their potential use as ligands in marketed meningococcal vaccines. Since the first time a phosphodiester bridge was arranged between two αManNAc residues (Figure 7, compounds 100 and 101) in 1993 by the Shibaev group [107], a vast number of MenA-related mono-, di-, and oligosaccharides 102–109 (Figure 7) have been synthesized [108,109,110,111,112]. However, the preparation of αManNAc anomeric phosphodiesters remains a challenge, as these compounds comprise a highly labile linkage between C-1 and O-1 of αManNAc, which is, in addition, destabilized by the presence of the NHAc group at C-2 of αManNAc. As a result, researchers have focused significant efforts on the design of hydrolytically stable phosphono-mimetics (compounds 111–115) [113,114,115] and carba-mimetics (compounds 116–127) [116,117,118]. However, new challenges are emerging, which are associated with the establishment of C-C bonds and the stereoselective formation of new chiral centers. The majority of publications that describe the preparation of oligomeric antigens related to MenA phosphoglycans concern nonacetylated molecules, whereas bacterial MenA phosphoglycans comprise the acetyl groups at O-3/O-4 (Figure 6), which are important for the antigenic properties of the polymer [105,106]. Additionally, a number of synthetic MenA-related antigens were reported (compounds 107–109 and 127), which are totally or partially acetylated at O-3 of αManNAc, with a view to bringing their antigenic properties closer to those of the bacterial antigen. Synthetic fragments of MenA phosphoglycans with 3,4-di-O-acetylated αGManNAc residues are not considered in this review.
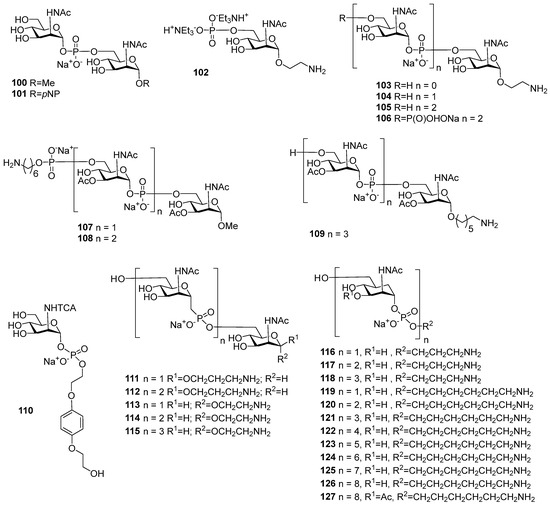
Figure 7.
Synthetic oligosaccharides structurally related to MenA phosphoglycans.
As the capsular MenA glycan is an αManNAc(1→(-PO3)→6) polymer, the H-phosphonate method is applicable, provided that 1-OH has the axial orientation in the selectively protected H-phosphonate ManNAc or 2-deoxy-2-azido mannose precursor, as described above for oligomers related to Hic and Hif phosphoglycans (Scheme 9). A number of convenient and efficient procedures have been developed for the preparation of this type of compound. The common protocol suggests phosphitylation with tri(1-imidazolyl) phosphine (Figure 8, compound 128), which is obtained in situ by the interaction of PCl3 and imidazole in the presence of Et3N and salicylchlorophosphite 129 (2-chloro-4H-1,3,2-benzodioxaphosphin-4-one; Figure 8). Alternatively, within a phosphoramidite protocol, chloroanhydrides 130 and 131 (Figure 8) were especially designed for automated synthesis to produce stable phosphoramidites as synthons of α-mannosamine phosphodiesters [108].
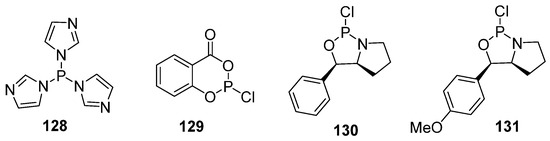
Figure 8.
Reagents used for synthesis of H-phosphonates.
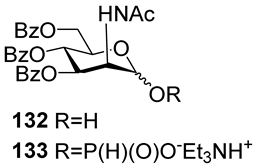
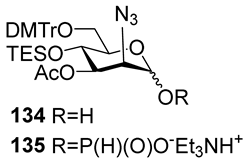
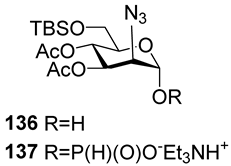
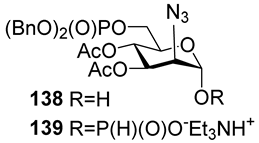
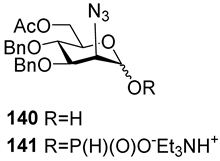
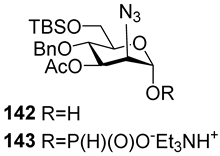
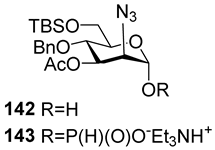
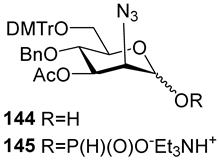
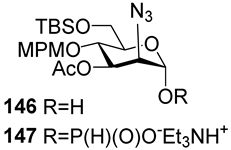
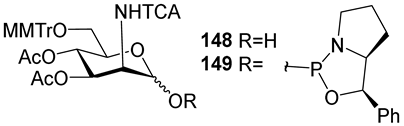
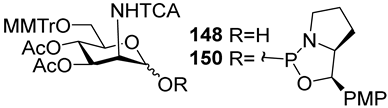
For the PCl3/imidazole protocol, the key prerequisite for the preparation of ManNAc or 2-deoxy-2-azido mannose α-H-phosphonates is the α-configuration of the 1-OH group in the corresponding selectively protected hemiacetal. This feature imposes certain restrictions on the use of the H-phosphonate protocol. Hemiacetal 132 (Table 1) with a 5:1 ratio of α/β anomers was quantitatively converted into the 5:1 mixture of α- and β-isomeric H-phosphonate 133 upon the action of PCl3 and imidazole in acetonitrile at 0 °C, followed by hydrolysis with an aqueous solution of Et3NH∙HCO3 at 20 °C (Table 1, entry 1) [107]. In a similar way, phosphitylation of the mixture of hemiacetal 134 with a 3.5:1 ratio of α/β anomers in analogous conditions proceeded with the retention of the configuration of the anomeric center and resulted in the formation of the mixture of H-phosphonate 135, with the α:β ratio 3.5:1 and an 88% yield (Table 1, entry 2) [110]. In similar conditions, the interaction of the α-anomer of hemiacetal 136 (Table 1, entry 3) [111] afforded α-H-phosphonate 137 with a 97% yield, and hemiacetal 138 with a dibenzyl phosphate group at C-6 was converted into α-H-phosphonate 139 (Table 1, entry 4) [111]. The phosphitylation of hemiacetal 140 (Table 1) with an α-configuration of the anomeric center upon the action of (PhO)2P(O)H in Py, followed by hydrolysis, resulted in a mixture of α- and β-isomer 141 in a ratio of 93:3 (Table 1, entry 5) [112]. Similarly, the phosphitylation of α-hemiacetal 142 in these conditions formed α-H-phosphonate 143 with a 78% yield (Table 1, entry 6) [109]. Anomeric mixtures of hemiacetals, which contain considerable quantities of β-isomers, can be efficiently converted into α-H-phosphonates with chlorophosphite 129 (Figure 8) [110,119]. For example, the phosphitylation of the anomeric mixture 144, with a ratio of α- and β-anomers of 4.5:1, was transformed into α-H-phosphonate 145 by the action of chlorophosphite 129 in a mixture of dioxane and triethylamine with an 88% yield (Table 1, entry 8) [110]. Similarly, in these conditions, hemiacetals 142 and 146 were converted into the corresponding α-H-phosphonates 143 and 147 at 91 and 90% yields, respectively (Table 1, entries 7 and 9) [110,119]. Unlike H-phosphonates, phosphoramidites are rarely used as synthetic blocks for the establishment of phosphodiester bonds between α-ManNAc residues because of their utmost lability. Two successful examples of ManNAc phosphoramidites are compounds 149 and 150, which are stabilized by rigid oxazaphospholidine substituents. These compounds were obtained from the anomeric mixture of hemiacetal 148 by the action of tricyclic chlorophosphoroamidites 130 and 131 at yields of 36 and 39% (Table 1, entries 10 and 11), respectively [108]. The generation of α-H-phosphonates and the anomerization of the α/β mixtures of hemiacetals or the stereoselective cleavage of the unwanted β-isomer in the presence of H3PO3 [107] or AgOTf [110] were ineffective.

Table 1.
Synthesis of H-phosphonates and phosphoramidites from selectively protected 2-deoxy-2-azido-D-mannose and ManNAc hemiacetals.
The majority of syntheses of the spacer-armed oligomers related to MenA phosphoglycans (Figure 7) were performed using a straightforward and efficient protocol suggested by Van Boom [120], which constitutes the condensation of H-phosphonates with alcohols, with the subsequent oxidation of phosphites into phosphodiesters. In the first step, the dissolved alcohol is activated by the addition of sterically hindered chloroanhydride, e.g., PivCl, followed by the addition of H-phosphonate in Py and stirring for 5–30 min. In the second step, the intermediate products are subjected to oxidation with a 0.5 M solution of I2 in a Py:H2O mixture within the temperature interval from −40 °C to 0 °C for 30 min. The first syntheses of the non-acetylated fragments of MenA phosphoglycans as methyl glycoside 100 and nitrophenyl ether 101 were accomplished by the Shibaev group [107] using the H-phosphonate protocol (Figure 7). Then, monomers 102 and 103, dimer 104, and trimer 105 (Figure 7), related to MenA phosphoglycans and equipped with a spacer carrying a primary amino group for conjugation with protein carriers, were synthesized by Pozsgay et al. [112]. The condensation of H-phosphonate 141 with alcohol 151, with subsequent oxidation, resulted in the formation of phosphodiester 152 with a yield of 95% over two steps (Scheme 10). The successive reduction of an azido group into the amino group and N-acetylation yielded mannosaminyl phosphodiester 153, which was converted into alcohol 154 by chemoselective 6-O′-deacetylation. The condensation of H-phosphonate 141 and alcohol 154 was less effective (a yield of 74% over two steps) in connection with the destruction of the phosphodiester bond in the reaction conditions. The authors noted that attempts at further elongation of the chain were unsuccessful. Phosphoester 102 and phosphodiesters 103, 104, and 105 were converted into conjugates with HSA. The antigenic properties of the conjugates were confirmed by a double immunodiffusion assay with the MenA phosphoglycan as a positive reference and chemically modified HSA as a negative reference, and anti-MenA horse serum [112].
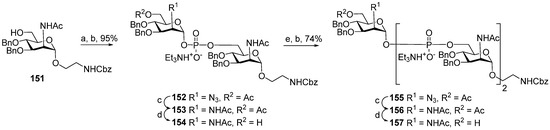
Scheme 10.
Key building blocks for the synthesis of oligomers 103, 104, and 105 related to MenA phosphoglycans [112]. Reagents and conditions: (a) 141 (0.8 eq.), PivCl (2.2 eq.), 23 °C, 30 min. Py; (b) I2 (2.2 eq.), Py:H2O 95:1, 0 °C, 30 min, 95% yield over 2 steps; (c) NiCl2∙6H2O (4.8 eq.), NaBH4 (8.0 eq.), methanol, 0 °C → 10 °C, 30 min; (d) NaOMe/MeOH; (e) 141 (1 eq.), PivCl (1.5 eq.), Py, 23 °C, 5 min.
In 2005, the non-acetylated spacer-armed MenA phosphoglycan-related oligomer 105 (Figure 7), which comprises three ManNAc residues connected by phosphodiester bridges and trimer 106 (Figure 7), composed of three non-acetylated MenA repeating units, was synthesized by the Oscarson group [111]. In contrast to the abovementioned synthetic strategy designed by the Pozsgay group, which suggested the reduction of the azido group and N-acetylation after each chain elongation step (Scheme 10), 2-azido glycoside 158 was used as a starting unit for further chain elongation, and 2-azido H-phosphonate 137 (Table 1, entry 3) was applied as a repeating unit synthon without intermediate N3→NHAc transformation (Scheme 11). The condensation of H-phosphonate 137 and acceptor 158 with a free hydroxyl group at C-6, followed by oxidation, resulted in the efficient formation of phosphodiester 159 (yield: 96%), which was then 6′-O-desilylated to create a new active site in acceptor 160. However, the attachment of the next monomer unit 137 to phosphodiester 160 was less effective and, after oxidation, formed the corresponding phosphodiester 161 with a 62% yield. With a view to synthesizing trimer 106 with a phosphoester substituent at C-6, acceptor 160 was reacted with phosphodiester 139 (Table 1, entry 4) and a dibenzylphosphate group at C-6 to obtain the trimeric precursor 162 with a 59% yield. A reduction in all azido groups, total N-acetylation, and deprotection in compounds 161 and 162 afforded the spacer-armed conjugation-ready antigens 105 and 106 [111].
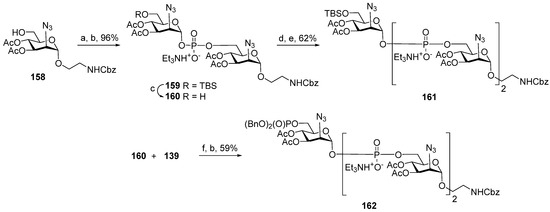
Scheme 11.
Key building blocks for synthesis of spacer-armed antigens 105 and 106 related to MenA phosphoglycans [111]. Reagents and conditions: (a) 137 (1.3 eq.), PivCl (3.8 eq.), Py, r.t., 60 min; (b) I2 (1.2 eq.), Py:H2O 49:1, −40 °C → 0 °C, 30 min, 96% yield over 2 steps; (c) Et3N∙3HF (5 eq.), THF, 91% yield; (d) 137 (1.3 eq.), PivCl (2.5 eq.), r.t., 120 min. Py; (e) I2 (1.2 eq.), Py:H2O 49:1, −40 °C → −10 °C., 62% yield over 2 steps; (f) PivCl, Py.
3-O-Acetylated dimer 107 and trimer 108 related to MenA capsular phosphoglycans were synthesized as methyl glycosides (Figure 7) [110]. The phosphitylation of acceptors 163, 164, and 165, which comprised one, two, or three 2-deoxy-2-azido mannose residues with H-phosphonate 145, followed by oxidation and detritylation, afforded phosphodiesters 164, 165, and 166 with 88%, 74%, and 68% yields, respectively. The alcohols obtained were subjected to phosphitylation with H-phosphonate 167 and subsequent oxidation (Scheme 12). The connection of H-phosphonate 167 to the 6-OH of the terminal monosaccharide was effective only for the shorter alcohols 164 and 165 (88% over two steps) and resulted in the preparation of protected di- and trimers 168 and 169 (Scheme 12). The transformation of azido groups into amino groups and the total N-acetylation and debenzylation of compounds 168 and 169 formed the spacer-armed dimer 107 and trimer 108, which carry acetyl groups at O-3 of each ManNAc residue, as they do in bacterial MenA phosphoglycans. In the homologous series 164–166, attempts to phosphitylate the longest alcohol 166 with H-phosphonate 145 in order to obtain a tetramer or with the pre-spacer H-phosphonate 167 were not successful, in contrast to compounds 164 and 165.
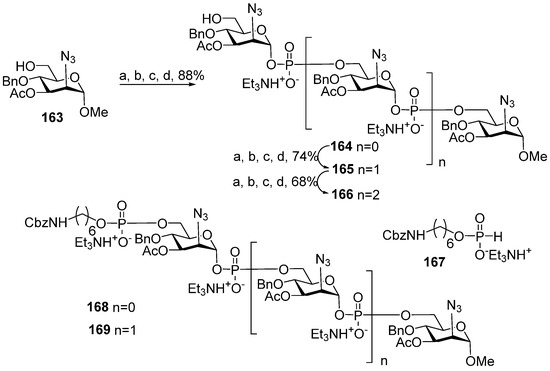
Scheme 12.
Key building blocks for synthesis of spacer-armed oligomers 107 and 108 [110] related to MenA capsular phosphoglycans. Reagents and conditions: (a) 145 (1.2 eq.), PivCl (1.8 eq.), Py, 20 °C, 30 min; (b) Et3N (4.8 eq.), −40 °C, Py:H2O 95:5; (c) −40 °C, I2 (2.4 eq.), 30 min; (d) 10 °C, 1% TFA in dichloromethane.
In general, the examples of the synthesis of oligomers related to MenA capsular phosphoglycans discussed above are in line with the tendency outlined in an earlier review [39], which discussed the decrease in the efficiency of phosphitylation with H-phosphonates with the increase in the number of already formed phosphodiester bonds and suggested the lability of phosphodiester fragments in the conditions of the H-phosphonate protocol. However, in 2017, a group of Indian researchers synthesized an aminohexyl glycoside of oligomer 109 related to capsular phosphoglycans, which contained four 3-O-acetylated ManNAc residues, using a linear, synthetic strategy (Scheme 13) [109]. In a sequence of phosphitylation steps, H-phosphonate 143 was used as a universal monomer (Table 1, entry 6). The establishment of the first phosphodiester linkage between alcohol 170 and H-phosphonate 143 afforded compound 171 (yield: 92%), which was, in turn, desilylated to form acceptor 172. The phosphitylation of alcohol 172 with H-phosphonate 143, followed by oxidation, resulted in the formation of compound 173 with two phosphodiester bridges (yield: 89%). The desilylation of compound 173 formed acceptor 174, which was phosphitylated with H-phosphonate 143 and, after oxidation, compound 175 with three phosphodiester bridges was obtained with a 76% yield.
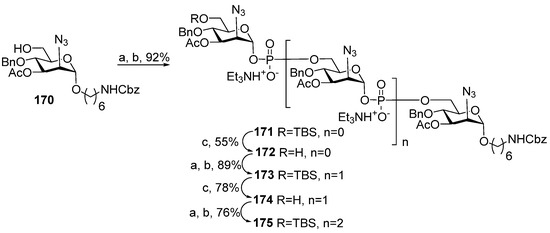
Scheme 13.
Key building blocks for the synthesis of oligomer 109 structurally related to MenA phosphoglycans [109]. Reagents and conditions: (a) 143 (2 eq.), PivCl (3 eq.), Py, 20 °C, 90 min; (b) −40 °C, solution of I2 (5 eq.) in Py/H2O 20:1, 150 min, −40 °C → −10 °C, 30 min; (c) Et3N∙3HF (5 eq.), THF.
After the transformation of azido groups into NHAc groups and the desilylation, debenzylation, and deprotection of the spacer amino group, the spacer-armed ligand 109 was conjugated to TT. The antigenic properties of oligomer 109 were evaluated in a competitive ELISA experiment. Both oligomer 109 and its conjugate with TT in the concentration range 12.5–400 μg glycan/mL were found to neutralize anti-MenA rabbit antiserum and inhibit the binding of antibodies to the bacterial MenA phosphoglycan used as a coating antigen. In comparison to the conjugate, oligomer 109 showed lower inhibition [109]. It can be concluded that the improvement of synthetic protocols made it possible to obtain oligomeric antigens related to MenA capsular phosphoglycans, which contain up to four ManNAc residues. However, for the efficient application of antigens of this type in immunodiagnostic tests and vaccine production, the hydrolytic lability issue has to be addressed.
5. Synthesis of Glycomimetics of MenA Capsular Phosphoglycans
Today, all anti-MenA conjugate vaccines include a lyophilized bacterial MenA component except for the fully liquid commercial vaccine preparation Menactra®. The presence of the MenA antigen in the dissolved form substantially shortens the shelf life for Menactra® to 18 months at 2–8 °C compared to the 4-year shelf life of MenQuadfi® and the 3-year shelf life of Menjugate® and MenAfriVac® in these conditions. With a view to preparing hydrolytically stable MenA antigens, a number of oligomeric analogs were designed, in which NHAc groups were replaced with trichloroacetamide groups as they are not likely to undergo transformation into oxazolines. Another type of mimetics is compounds with the isosteric replacement of one of the hemiacetal oxygens with a methylene group (phosphono- and carba-analogs).
A comparative conformational analysis of a hexapyranose ring in 2-deoxy-2-acetamido mannohexapyranosyl phosphate 176 and its phosphono-analog 177 and carba-analog 178 (Figure 9) in a study of conformations in combination with NMR experiments showed that for compound 176, 4C1 was almost the only populated conformer, whereas for phosphonate 177, the proportion of pyranose ring conformers other than 4C1 was 4%, and for the carba-analog 178, this proportion rose to 7% [121]. The most populated 4C1 conformer for compound 178 was confirmed by quantum mechanics and molecular dynamics calculations [122]. The molecular dynamics calculations performed for a decamer of a MenA capsular phosphoglycan repeating unit and its carba-analog showed that, despite a number of conformational and dynamic variations, the carba-mimetics of the fragments of MenA capsular phosphoglycans can be considered candidate antigens for the construction of anti-MenA vaccine preparations [123].
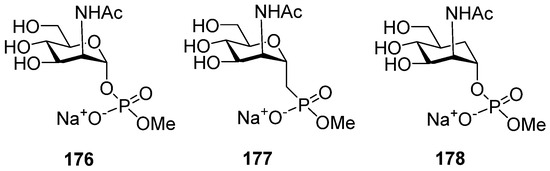
Figure 9.
Sodium salt phosphodiesters of α-ManNAc (176) and its phosphono-analog (177) and carba-analog (178) as model compounds for comparative conformational analysis.
As mentioned previously, the axial NHAc group at C-2 of the ManNAc residue contributes largely to the lability of MenA capsular phosphoglycans and their fragments via neighboring group participation. One of the possible ways to circumvent this obstacle is the replacement [55] of the acetamide group at C-2 with the trichloroacetamide group, which is not likely to form oxazolines. To study the possibility of the preparation of the N-trichloroacetamide mimetics of ManNHAc, oxazaphospholidines 149 and 150 (Table 1) were obtained in a solid-phase synthesis. Bis(2-hydroxyethyl) hydroquinone 179 (Scheme 14) [108] was immobilized on a glass support and phosphitylated with phosphoramidites 149 and 150. The phosphitylation of hydroquinone 179 with phosphoramidite 149 in the presence of 1-(cyanomethyl) pyrrolidinium trifluoromethanesulfonate (compound 180) resulted in the formation of phosphite 181, which carried a 2-phenylpyrrolydine residue as a result of the cycle opening. Phosphotriester 181 was subjected to mild oxidation with DCSO (compound 182) and desilylated with TFA/TES to produce phosphotriester 183, with a free 6-OH group for the following elongation of the oligomeric chain. Finally, the cleavage of the 2-phenylpyrrolydine ether was fulfilled in the presence of a base, followed by deacetylation and removal of the solid support by the action of MeONa/MeOH.
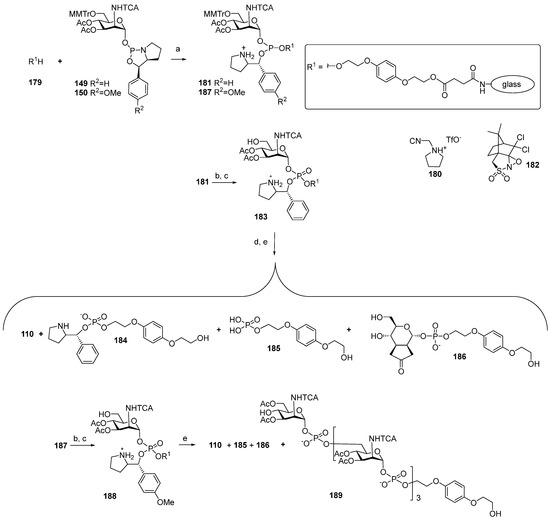
Scheme 14.
Key building blocks for the solid-phase synthesis of a monomer of MenA capsular phosphoglycans, 110, in which the NHAc moiety has been substituted for trichloroacetamide [108]. Reagents and conditions: (a) 180, r.t., acetonitrile; (b) 182, acetonitrile; (c) 1% THF, CH2Cl2, TES, r.t., 1 min., 1 min.; (d) 2,6-lutidine, acetonitrile; (e) NaOMe/MeOH.
However, researchers faced considerable difficulties at the step of the cleavage of the 2-phenylpyrrolydine ether. After the support was removed, analysis of the products showed that the use of DBU for the cleavage of the ether did not provide target phosphodiester 110, and the identified products 184–186 did not contain a ManNAc residue. When the weaker bases of Et3N or 2,6-lutidine were used as basic catalysts, the target product 110 was obtained with a moderate yield (17%) and low conversion.
In order to replace the step of the basic hydrolysis of the O(P)-protective group for acidic hydrolysis, phosphoramidite 150 with a p-MeO-Ph residue in the place of the Ph residue, as in compound 149, was used. Phosphoramidite 150 was attached to a solid support, and phosphite 187 was oxidized with DCSO (compound 182) to yield phosphotriester 188, followed by the simultaneous acid hydrolysis of the trityl group and removal of the 2-(p-methoxybenzyl) pyrrolidine moiety. However, after deacetylation and removal of the solid support with 50 mM NaOMe/MeOH, the unwanted products 186 and 187 were detected. Without taking into account inefficient deprotection, this method provided the preparation of tetramer 189, which was O-acetylated and N-trichloroacetylated. The authors noted that the developed method in the current state is not suitable for the synthesis of oligomers [108].
In 2005, the preparation of the first isosteric hydrolysis-resistant phosphono-analog 111 structurally related to MenA phosphoglycans was published by Lay et al. [115]. The interaction of iodide 190, in which the iodomethylene group is arranged axially, with trimethylphosphite (Scheme 15) afforded phosphonodiester 191, which was further converted to ester 192 by the action of triethylamine and thiophenol.
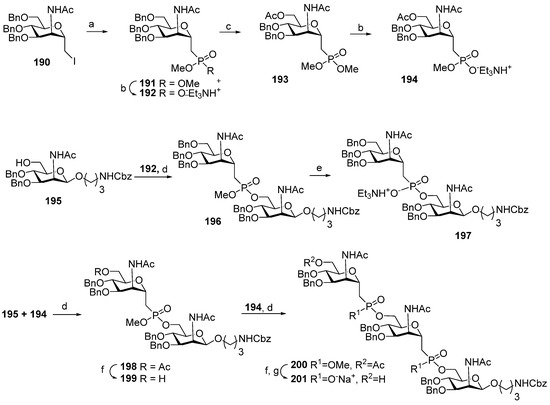
Scheme 15.
Key blocks for the synthesis of spacer-armed phosphono-analogs of MenA phosphoglycans, 111 and 112 (Figure 6) [113,115]. Reagents and conditions: (a) P(OMe)3, 100 °C, vacuum; (b) Et3N, PhSH, THF, r.t., 62%; (c) ZnCl2, Ac2O:AcOH 2:1, r.t., 16 h, 92%; (d) Ph3P, DIAD, THF, 0 °C, 24 h, yield 97% for 197, 90% for 198, 83% for 200; (e) Et3N, PhSH, toluene, 110 °C, 36 h, Amberlite IR120 (Na+) 95%; (f) NaOMe/MeOH; (g) DBU, PhSH, THF, r.t., 8 h, Amberlite IR120 (Na+), 95%.
Similarly, 6-O-acetylated phosphonodiester 193 was converted to the corresponding ester 194 by partial hydrolysis. Mitsunobu reaction conditions were used for the condensation of ester 192 with the selectively protected ManNAc 195, which formed phosphonodiester 196 (yield: 97%) [113]. Partial hydrolysis of phosphonodiester 196 into phosphonoester 197 was also efficient. 6′-O-Acetylated phosphonodiester 198 was synthesized by the condensation of 195 with phosphonoester 194, with a 90% yield. By deacetylation, phosphonodiester 198 was converted into alcohol 199 and condensed with phosphonoester 194 to form compound 200 with two phosphonodiester bridges and an 83% yield. Chemoselective hydrolysis of the methyl phosphonate moieties in compound 200 formed phosphonoester 201. Total deprotection of phosphonoester 197 with two ManNAc residues and 201 with three ManNAc residues resulted in phosphono-analogs 111 and 112 (Figure 6) [113].
A similar strategy in combination with Mitsunobu reaction modification, where Ph3P is replaced with tris(4-chlorophenyl) phosphine, was used for the synthesis of the series of phosphono-analogs 113–115 of MenA phosphoglycans (Scheme 16) [114]. The 6″-O-deacetylation of phosphonodiester 202 resulted in alcohol 203, which was then reacted with universal monosaccharide block 194 in modified Mitsunobu conditions to obtain the phosphono-analog 204 of MenA phosphoglycans with three phosphonodiester-bridged fragments (yield: 87%). Partial hydrolysis of diester moieties afforded compound 205 (yield: 75%), and after total deprotection, phosphono-analog 115 was obtained with three pseudo-ManNAc residues.
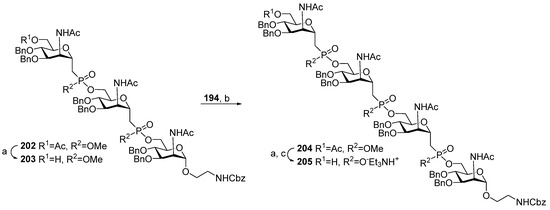
Scheme 16.
Key blocks for the synthesis of phospho-analog 115 (Figure 6) related to MenA phosphoglycans [114]. Reagents and conditions: (a) 1 M KOH/MeOH, 84%; (b) (p-ClC6H4)3P, DIAD, THF, 0 °C, 30 min, 87%; (c) DBU, PhSH, acetonitrile, r.t., 75%.
The antigenic properties of ligands 111 and 112 [113] were studied in competitive ELISA experiments with anti-MenA human antisera. Native MenA phosphoglycans were used as a coating antigen and positive control, and MenY phosphoglycans were used as a negative control. For both ligands, the EC50 was about 10−3 mg/mL, which is three orders higher than the EC50 of 6.6 × 10−6 mg/mL for MenA phosphoglycans.
Aminopropyl glycosides 111 and 112 and 3-aminopropyl β-D-ManNAc were transformed into conjugates with HSA using the squarate procedure [124] (Figure 10). One series consisted of conjugates 206–208 (Figure 10) with the maximum saccharide/protein molar ratio, and another series of conjugates was composed of compounds 209–211 (Figure 10) with the saccharide/protein molar ratio being half of the value achieved for conjugates 206–208. Competitive ELISA experiments with the mouse polyclonal anti-MenA antisera and MenA capsular phosphoglycans as a coating antigen showed that at an inhibitor concentration of 1 mg/mL, inhibition using the fully loaded conjugate 206 was 55%. For conjugates 207 and 208, it reached 65%, whereas MenA capsular phosphoglycans provided 100% inhibition. Inhibition with the half-loaded conjugates 209–211 was 5–15% lower than for the fully loaded conjugates with the same antigen type [124]. Conjugates 206–211 were used for the immunization of mice at a dose of 2 μg/mouse and efficiently evoked IgG antibodies, which is a reliable marker of the induction of thymus-dependent immune responses. Quantitative elucidation of the level of anti-MenA IgG antibodies developed against conjugates 206–211 showed that immunization with the half-loaded conjugates 209–211 was more efficient compared to the fully loaded conjugates 206–208. It is important to note that the level of induced anti-MenA antibodies was similar for the half-loaded conjugates 209–211, regardless of the length of the pseudo-oligosaccharide antigen. The authors concluded that this result indicated the antibodies’ recognition of the ManNAc epitope [124].
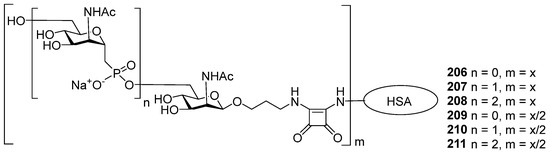
Figure 10.
Conjugates of amino-spacered C-phosphono analogs of MenA capsular phosphoglycans [124].
In contrast to C-phosphono mimetics, in which the methylene group stands in the place of anomeric oxygen, in carba-analogs, the decrease in electrophilicity of the carbonyl carbon, along with the increase in the stability of the phosphodiester linkage, is achieved by the replacement of the ring hemiacetal oxygen of ManNAc with a methylene group [125].
Synthesis of the series of carba-analogs 116–118 (Figure 7) of aminopropyl glycosides of a monomer, a dimer, and a trimer of MenA capsular phosphoglycans, and the advanced series of carba-analogs 119–126, from monomers to octamers, as aminohexyl glycosides, was performed by the Lay group [116,117,118]. Carba-analogs 116–118 [118] were obtained by the sequential elongation of a pseudo-oligosaccharide chain, starting from the spacer-equipped monomer (Scheme 17). For the preparation of monomer 116, the universal orthogonally protected precursor 212 was transformed into alcohol 213, which was phosphitylated with H-phosphonate 214 and oxidized to obtain the spacer-armed phosphodiester 215 with an 81% yield.
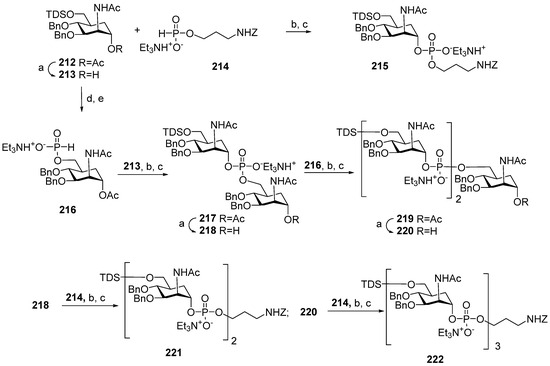
Scheme 17.
Key blocks for preparation of spacer-armed carba-analogs of oligomers 116–118 related to MenA CPS [118]. Reagents and conditions: (a) NaOMe/MeOH, r.t., 4 h, yield of compound 213—84%, 218—87%, 220—70%; (b) Py, PivCl, r.t., 45 min; (c) I2, Py -H2O 19:1, r.t., 15 min, yield for compound 215—81%, 217—82%, 219—81%, 221—85%, 222—57%; (d) Bu4NF, THF, r.t., 3 h; (e)–129, CH3CN-Py 3:1, r.t., 45 min, yield 82%.
For the preparation of oligomers 117 and 118, the universal precursor 212 was desilylated and converted into H-phosphonate 216, which was used as a universal monomer block for chain elongation. The interaction of H-phosphonate 216 with alcohol 213 and the subsequent oxidation formed phosphodiester 217 (yield: 82%), which was deacetylated to obtain alcohol 218. The condensation of alcohol 218 with H-phosphonate 216 resulted in pseudo-trisaccharide 219 (yield: 81%), which was deacetylated to produce alcohol 220. Finally, pre-spacer 214 was introduced into alcohols 218 and 220 to obtain the spacer-armed, protected dimers 221 and 222 with yields of 85% and 57%, respectively. The high efficiency of the phosphitylation of alcohol 218, which already includes a phosphodiester bridge with H-phosphonates 214 and 216, evidences the increase in stability of the phosphodiester linkage in protected carba-analogs compared to phosphodiester-linked oligosaccharides. However, the phosphitylation of 220, which already comprised two phosphodiester moieties, was less efficient, indicating the limitations of the application of the H-phosphonate procedure to the linear synthesis of longer oligomers. The target carba-analogs 116–118 of monomers, dimers, and trimers related to MenA phosphoglycans were obtained after the total deprotection of compounds 215, 221, and 222 [118].
For the synthesis of the advanced series of carba-analogs 119–126, from monomers to octamers, the Lay group used the strategy of a step-by-step chain extension using the spacer-equipped monomer as a starting compound and phosphitylation with a selectively protected monomeric phosphoramidite as the key step (Scheme 18) [116]. Alcohol 223 was converted into the universal phosphoramidite block 224 under the action of chlorophosphoroamidite 24. The interaction of alcohol 223 with the phosphoramidite pre-spacer 225 in the presence of DCI, followed by oxidation with DCSO and detritylation, yielded phosphotriester 226 with a free hydroxyl group for further phosphitylation, which was used as a starting compound for chain elongation. The sequential execution of phosphitylation with the universal monomer block 224, oxidation, and detritylation afforded the protected compounds 227–233 with excellent yields. After total deblocking, the spacer-armed carba-analogs 119–126, structurally related to MenA capsular phosphoglycans, were obtained. For better resemblance of the octamer antigen to the natural structure, octamer 126 was N-Boc-protected, subjected to random monoacetylation, and N-deblocked, and the mixture of oligoacetates 234 was obtained, which contained a certain amount of oligomer 127 related to MenA capsular phosphoglycans [116].
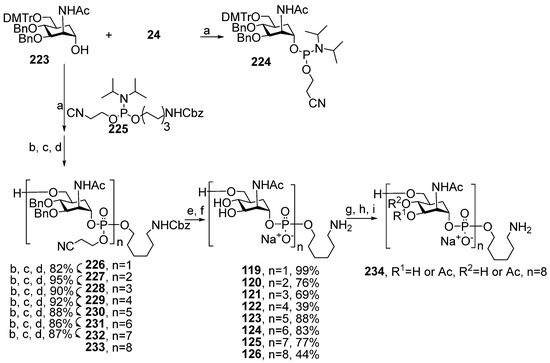
Scheme 18.
Key blocks for synthesis of spacer-armed carba-analogs 119–126 related to MenA capsular phosphoglycans [116]. Reagents and conditions: (a) DIPEA, CH2Cl2, r.t., 94%; (b) DCI, MeCN; (c) DCSO, MeCN; (d) TCA, CH2Cl2, H2O, 94% over 3 steps; (e) NH4OH, H2O, dioxane; (f) H2, Pd, H2O, AcOH, 44%; (g) (Boc)2O, NaHCO3, r.t. 16 h; (h) Ac2O/imidazole, 9 d.; (i) TFA, r.t., 1 h.
The stability of compound 126 was studied using an accelerated stability test and fragments of natural and deacetylated MenA phosphoglycans, with avDP15 as a reference compound. During four weeks of keeping these samples in buffered 5 mM sodium acetate at pH 7 and 37 °C, a drastic decrease in chain length for the deacetylated bacterial oligosaccharide was observed, and the natural sample with acetyl groups was subjected to partial depolymerization, and for compound 126, no trace of decomposition was detected [116].
Competitive ELISA experiments with polyclonal immune mouse anti-MenA sera and MenA capsular phosphoglycans as a coating antigen were used for the assessment of the antigenic properties of the mono-, di-, and trimeric pseudo-oligosaccharides 116–118 [118]. MenA capsular phosphoglycans and their derivatives MenA avDP15 and MenA avDP3 were used as positive controls. The highest inhibition rate was found for MenA capsular phosphoglycans and MenA avDP15, with an IC50 of 5.15 × 10−6 and 4.3 × 10−3 mM, respectively. For the carba-dimer 117, the IC50 was about 0.16–0.091 mM, which is less than the IC50 (4.3 × 10−2) found for MenA avDP3. For the carba-monomer 116 and carba-trimer 118, only 30% inhibition was achieved [118]. The synthetic carba-analogs 116–118 were converted into conjugates with CRM197 (compounds 235–237, Figure 11) and HSA (compounds 238–240, Figure 11). Mice were immunized three times with conjugates 235–237, 241, and 242, which comprised MenA avDP5 and MenA avDP15 antigens, respectively, at a dose of 2 μg of antigen per mouse. The induced antibodies were analyzed in ELISA experiments using the HSA-based conjugates 238–240 as coating antigens and MenA capsular phosphoglycans. The immunogenicity of conjugates 235, 236, and 237 was evaluated using the HSA-conjugated coating antigens 238, 239, and 240, respectively. In this experiment, the immunogenicity of conjugate 238, which comprised the monomeric antigen 116, was somewhat lower compared to conjugates 239 and 240 with dimeric and trimeric antigens, respectively. In ELISA experiments with MenA capsular phosphoglycans as a coating antigen, it was found that only conjugate 240 with a trimeric antigen evoked antibodies specific to the natural phosphoglycans, and conjugates 238 and 239 did not induce anti-MenA immunity. However, the level of IgG antibodies induced by immunization with 240 was 2–3 orders lower than that induced by conjugates 241 and 242, which contained fragments of natural phosphoglycans with intact acetyl substituents. These data are in line with the results of an in vitro bactericidal assay, which showed the intensity of the complement-mediated lysis of N. meningitidis [118].
Figure 11.
Neoglycoconjugates of mono- and oligomeric carba-analogs and partially fragmented MenA CPS with protein carrier CRM197 and HSA [116].
The carbocyclic hexamer 124 and the non-acetylated and acetylated octamers 126 and 234 were conjugated to CRM197 to form compounds 243, 244, and 245, respectively (Figure 11). Conjugates 243–245 were used for the immunization of mice, and another group of mice was immunized with a conjugate composed of partially depolymerized MenA phosphoglycans as a positive control. ELISA analysis of IgG antibodies on plates coated with MenA capsular phosphoglycans showed that immunization with conjugate 245, based on the acetylated carba-octamer, efficiently induced anti-MenA immunity. For this conjugate, the IgG titers were comparable to the titers registered for conjugate 242 based on fragmented bacterial antigens, whereas the titers measured in animals immunized with conjugates 243 and 244, which carried non-acetylated synthetic antigens, were two orders lower [116].
The urgency of the development of anti-MenA conjugate vaccines is evidenced by the considerable efforts of several research groups aimed toward antigenic and non-hydrolyzable synthetic phosphooligosaccharides. However, the antigenicity studies of phosphono- and carba-mimetics show that these oligomers do not induce anti-MenA immunity and, therefore, are unable to prevent MenA infection. Another obstacle in the way of the design of the semisynthetic anti-MenA vaccine is the importance of acetyl groups at the O3/O4 of ManNAc for the protective properties of the vaccines. The introduction of these substituents increases production costs.
To date, synthetic MenA antigens cannot compete with multiple conjugate anti-MenA vaccine preparations with a bacterial antigen. However, the repeating unit of MenA phosphoglycans is a simple monosaccharide, and it is a suitable target for production on automated synthesis platforms. We expect that the development of a stable mimetic with high antigenicity, in combination with automated synthesis technology, will help create an affordable anti-MenA semisynthetic conjugate vaccine.
6. Synthesis of Phosphooligomers Related to MenX Capsular Phosphoglycans
High anti-MenA vaccination coverage in the African meningitis belt in 2010–2015 resulted in a significant reduction of MenA-associated invasive diseases [100,126]. At the same time, outbreaks of invasive diseases caused by MenX were registered in Sub-Saharan Africa [127,128]. The clinical and epidemiological characteristics of diseases associated with MenX are similar to those of MenA. Over 90% of cases are registered during the dry season, the median age of patients is 9.2 years, and the mortality rate is 11.9% [129]. As a result, the development of a conjugated anti-MenX vaccine became an urgent issue [130].
For the elucidation of the minimal immunogenic epitope of MenX capsular phosphoglycans, a bactericidal mAb, MenX.01, was obtained, which induced bactericidal killing at a physiological concentration of 1 μg/mL in the first step. The interaction of this antibody with fragments of the natural MenX capsular phosphoglycans of different lengths showed that the minimal immunogenic epitope comprises 5–6 repeating units [131].
With a view to developing a conjugated anti-MenX glycovaccine with a fully synthetic glycan epitope, phosphodiester 246 [132] and the series of spacer-armed mono-, di-, and trimeric phosphoglycans 247–249 [129,133], were synthesized. In addition, a mixture of oligomeric phosphoglycan 250, with an average length of 12 repeating units, was prepared using a combined chemo-enzymatic protocol, and pseudo-tetrasaccharide 251 was used as a glycoside with an aminohexyl spacer attached as an aglycon (Figure 12) [134].
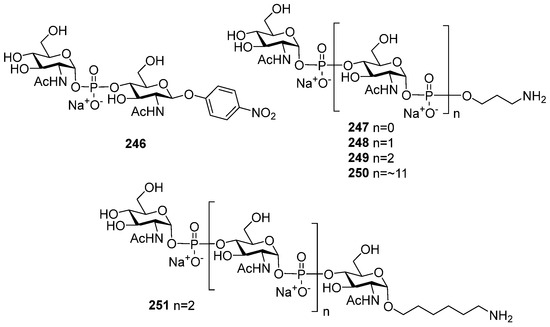
Figure 12.
Synthetic phosphooligosaccharides 246–251 structurally related to MenX capsular phosphoglycans.
The preparation of the synthetic oligosaccharide fragments of MenX capsular phosphoglycans was most commonly performed using an H-phosphonate procedure for the establishment of the phosphodiester linkage. In MenX phosphoglycans, the GlcNAc residue was incorporated in the polymer chain as an α-anomer. In synthetic fragments, the anomeric configuration of GlcNAc was determined with the configuration of the H-phosphonate, which, in turn, depends on the configuration of C-1 in the starting hemiacetal.
In 1991, pseudo-disaccharide 246 was synthesized for the first time by the Shibaev group (Scheme 19) [132]. Hemiacetal 252 with the NHAc group at C-2 was transformed into α-H-phosphonate 253 with a 95% yield under the action of in situ generated tris(imidazol-1-yl) phosphine. The condensation of H-phosphonate 253 and alcohol 254, followed by oxidation, afforded phosphodiester 255 (yield: 72%), which was deblocked to obtain pseudo-disaccharide 246 (Figure 12).
Scheme 19.
Key blocks for the synthesis of pseudo-disaccharide fragment 246 structurally related to MenX capsular phosphoglycans [132]. Reagents and conditions: (a) PCl3 (14 eq.), imidazole (4.3 eq.), Et3N (15 eq.), acetonitrile, 0 °C, 30 min, 95%; (b) PivCl (2.5 eq.), Py, 20 °C, 30 min. 72%; (c) Py:H2O 95:5; I2 (2.0 eq.), 20 °C, 10 min; (d) MeONa/MeOH, 71%.
In 2013, the series of spacer-armed oligomers 247 –250 was synthesized by the Lay group [133]. Unsuccessfully, 2-deoxy-2 azido hemiacetals 256 and 257, intended for the preparation of the corresponding H-phosphonates 258 and 259, were obtained as anomeric mixtures, thus challenging the preparation of H-phosphonates 258 and 259 as α-anomers. In order to provide the α-stereoselectivity of phosphonylation, the reaction of hemiacetals 256 and 257 with chlorophosphite 129 was conducted in the presence of phosphoric acid, which is known to destroy the β-anomer [39] (Scheme 20). The reaction took more than a week and afforded α-H-phosphonates 258 and 259 with 41% and 52% yields, respectively.
Scheme 20.
Key building blocks for the synthesis of pseudo-oligosaccharides 247–249 structurally related to MenX capsular phosphoglycans [129,133]. Reagents and conditions: (a) 129, 2 M H3PO3/Py, 0 °C → 40 °C, 8–9 days, yield for 258— 41% (+45% of starting material 256), yield for 259—52% (+38% of starting 257); (b) HOCH2CH2CH2CH2NHCbz, PivCl (2.5 eq), Py, 45 min, followed by 0.5 M I2 in Py:H2O 19:1 (2.5 eq.), −40 °C, 10 min, yield over 2 steps for compound 260—62%, for compound 261—64%; (c) MeONa/MeOH, (d) PivCl (2.5 eq.), Py, 45 min, followed by 0.5 M I2 in Py:H2O 19:1 (2.5 eq.); 1 M triethylammonium bicarbonate buffer; (e) 129, 1M triethylammonium bicarbonate buffer, batch 62% yield, microreactor 76% yield.
The phosphitylation of benzyl N-(3-hydroxypropyl) carbamate with benzylidenated H-phosphonate 258, followed by oxidation, resulted in phosphodiester 260 (yield: 62%), and the condensation of this alcohol with 4-O-acetylated H-phosphonate 259 formed phosphodiester 262 (yield: 64%), which was deacetylated to afford acceptor 262. The condensation of acceptor 262 with 4,6-O-benzylidenated H-phosphonate 258 and 4-O-acetylated H-phosphonate 259 was conducted with a moderate yield of phosphodiester 263 (45%) and 264 (40%). After deacetylation, pseudo-disaccharide 264 was converted into alcohol 265, which was reacted with 4,6-O-benzylidenated H-phosphonate 258 to afford trimer 266 with a 33% yield. The authors attribute the low efficiency of the phosphodiester linkage formation in trimer 266 to the presence of the azido group at C-2 of the hexapyranose ring. Total deprotection of compounds 260, 263, and 266 and transformation of the azido group into NHAc produced the target spacer-armed mono-, di-, and trimeric phosphodiesters 247, 248, and 249, structurally related to MenX capsular phosphooligoglycan [133].
Unlike 2-deoxy-2-azido hemiacetals 256 and 257, hemiacetal 267 with an NHAc group at C-2 exists as an α-anomer and is readily converted into α-H-phosphonate 268 under the action of chlorophosphite 129 (Scheme 20). Further improvement was achieved when the process was conducted in a microreactor, which provided a reduction in the reaction time to 30 min. and an increase in efficiency of the formation of α-H-phosphonate 268 to 76% [129].
In the synthesis of a mixture of the spacer-armed oligomer 250 with avDP12, the trimeric phosphoglycan 249 was extended using an enzymatic method. The mixture of trimer 249 and a nucleotide GlcNAc-UDP was passed through a HiTrap® column with immobilized recombinant capsule polymerase CsxA [135], followed by fractionation of the resulting oligomers.
An advanced method of phosphonylation [134] of an anomeric mixture of hemiacetal 259 with chlorophosphite 129 was used by Indian researchers for the preparation of pseudo-tetrasaccharide 251 as an aminohexyl glycoside (Figure 12). Interaction of the hemiacetal mixture 259 with a phosphonylating agent (PhO)2POH in Py in the presence of Et3N, followed by treatment with H3PO3 for 4 days, afforded the desired α-H-phosphonate 261 with a 65% yield [134].
In the first step of the assembly of pseudo-tetrasaccharide 251, the selectively protected aminohexyl glycoside 269 was condensed with H-phosphonate 259, and after oxidation, the pseudo-tetrasaccharide 270 was formed. By sequential deacetylation, condensation with H-phosphonate 259, and oxidation, the protected pseudo-tetrasaccharide 271 was obtained and further deblocked to yield the target pseudo-tetrasaccharide 271 (Scheme 21). In the course of the synthesis, the efficiency of the formation of the phosphodiester linkage decreased in each subsequent step (yields of 86% > 60% > 53%) [134], reflecting the tendency of the phosphodiester bond to be destroyed in the conditions of the H-phosphonate procedure.
Scheme 21.
Key building blocks for the synthesis of pseudo-tetrasaccharide 251 (Figure 12) structurally related to MenX capsular phosphoglycans [134]. Reagents and conditions: (a) PivCl (2–3 eq. relative to 259), Py, 45 min; (b) 0.5 M I2 in Py:H2O 9.75:0.25; (c) MeONa/MeOH.
The spacer-armed pseudo-saccharides 247–251 (Figure 12) were conjugated to protein carriers, and the immunogenic and antigenic properties of the resulting neoglycoconjugates were studied. Oligomers 247–249 were attached to CRM197 to yield conjugates 273–275 (Figure 13) and the corresponding conjugates 277–279 with HSA (Figure 13). Conjugates 273–275 were used for the immunization of mice at a dose of 0.3 μg of glycan per mouse. In addition, conjugate 275 was used at a dose of 1 μg per mouse. For positive controls, a group of mice was immunized with a conjugate of the partially fragmented MenX phosphoglycan avDP15 with CRM197 (MenXDP15-CRM197) at a dose of 0.3 μg and 1 μg of phosphoglycans, and bacterial MenX phosphoglycans were used as a coating antigen in ELISA experiments.
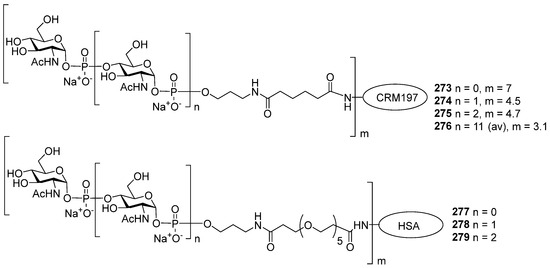
Figure 13.
Neoglycoconjugates 273–275 prepared using synthetic MenX-related antigens 247–249 and CRM197 protein carrier and corresponding neoglycoconjugates 277–279 with HSA protein carrier [129].
Conjugates 273 and 274 did not induce anti-MenX IgG antibodies. Immunization with conjugate 275 with trimeric antigens and MenXDP15-CRM197 efficiently elicited anti-MenX IgG antibodies, yet the intensity of the specific immune response for conjugate 275 was significantly weaker. For these conjugates, the immune response did not depend on the dose [133].
In the ELISA experiments, the immunogenic capacity of conjugates 273–275 was assessed using HSA conjugates 277–279 as coating antigens. It was found that the hyperimmune antisera of mice immunized with conjugates 273 (monomeric ligand) and 274 (dimeric ligand) contained negligible amounts of IgG antibodies to synthetic antigens. In contrast, immunization with conjugate 279 elicited anti-trimer IgG antibodies. An assessment of bactericidal activity in the rSBA tests showed that the pooled mouse sera obtained from animals immunized with conjugates 273 and 274 revealed that these conjugates did not elicit bactericidal antibodies. For conjugate 276, rSBA titers were 16 times lower compared to MenXDP15-CRM197. Therefore, the length of the synthetic oligomers 247–250 (Figure 12), which are incorporated into conjugates 273–275, was too small to effectively induce an anti-MenX immune response [133].
The mixture of the spacer-armed oligomer 250 with avDP12 was conjugated with the protein carrier CRM197. The resulting conjugate 276 was used for the immunization of mice, and the group of mice immunized with MenXDP15-CRM197 was used as a positive control. The antigenicity of conjugates 276 and MenXDP15-CRM197 was assessed in ELISA experiments with MenX capsular phosphoglycans as a coating antigen. It was found that conjugates 276 and MenXDP15-CRM197 demonstrate similar antigenic properties. However, SBA titers evidence the slightly greater bactericidal activity of antibodies elicited by MenXDP15-CRM197 [135].
7. Synthesis of Pseudo-Oligosaccharides Structurally Related to Phosphoglycans of S. Pneumonia
On a global scale, one in five infants under 1 year of age has had pneumonia, and ¾ of these pneumonia cases are caused by bacteria belonging to the S. pneumoniae species [136]. The prevention of community-acquired diseases associated with the most virulent S. pneumoniae serotypes is provided by a number of polyvalent conjugated pneumococcal vaccines, which comprise a number of pneumococcal capsular glycans conjugated to protein carriers.
Today, the pharmaceutical industry provides a whole range of anti-pneumococcal conjugated vaccines, including PCV7 (the S. pneumoniae serotypes 4, 6B, 9V, 14, 19F, 18C, and 23F), PCV10 (the S. pneumoniae serotypes 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F) and (the S. pneumoniae serotypes 1, 5, 6A, 6B, 7F, 9V, 14, 19A, 19F, and 23F), PCV13 (the S. pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 19A, 19F, 18C, and 23F), PCV15 (the S. pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F), and PCV20 (S. pneumoniae 1, 3, 4, 5, 6A, 6B, 7F, 8, 9V, 10A, 11A, 12F, 14, 15B, 18C, 19A, 19F, 22F, 23F, and 33F). Among the capsular glycan antigens used in these vaccines, five biopolymers, in particular S. pneumoniae 6A, S. pneumoniae 6B, S. pneumoniae 10A, S. pneumoniae 19A, and S. pneumoniae 19F, comprise phosphodiester bonds in the main chain. S. pneumoniae 6A and S. pneumoniae 6B phosphoglycans are built of pseudo-tetrasaccharide repeating units connected by phosphodiester linkage. The repeating unit of S. pneumoniae 10A phosphoglycans is a pseudo-heptaglycosyl phosphate, and the phosphoglycans of S. pneumoniae 19A and S. pneumoniae 19F are composed of trisaccharide phosphate and hexasaccharide phosphate repeating units [137].
Although the anti-pneumococcal vaccines for the prevention of S. pneumoniae 6A, S. pneumoniae 6B, S. pneumoniae 19A, and S. pneumoniae 19F have been available for more than 10 years, these serotypes are still frequently identified as the causative agents in pneumococcal pneumonia [136] and meningitis [138] and colonize the upper respiratory tract as opportunistic pathogens [139]. One of the factors hindering the broad coverage of people with anti-pneumococcal vaccination programs is high costs [9], which can be reduced by the replacement of bacterial antigens with their synthetic analogs [22].
The S. pneumoniae strains of serotype 6 are the common causative agents of invasive disease. The group is subdivided into serotypes 6A and 6B, which both have a capsule composed of phosphoglycans with Rha–ribitol–Gal–Glu subunits, which differ in the type of Rha–ribitol linkage. In serotype 6A, it is Rha1→3ribitol, and in serotype 6B, it is Rha1→4ribitol. It was reported that point mutation between S. pneumoniae 6A and S. pneumoniae 6B, as well as recombination, can compromise the linkage specificity and mediate serotype change [140]. As a result, it is reasonable to include both glycan antigens in combined vaccines. The surveillance of invasive pneumococcal disease in different countries evidences the immense importance of pneumococcal conjugated vaccines in the prevention of S. pneumoniae 6a- and S. pneumoniae 6b-associated invasive diseases [139,141,142]. In contrast, in regions with low immunization coverage [143], this pathogen makes a substantial contribution to the burden of invasive pneumonia.
Recently, syntheses of the spacer-armed pseudo-oligosaccharides 280–286 [144,145] structurally related to S. pneumoniae 6A phosphoglycans (Figure 14) have been reported. For the construction of a phosphodiester bond in compound 280, Nifantiev et al. [144] used the H-phosphonate procedure. Glycoside 287 (Scheme 22) was converted into H-phosphonate 288 by treatment with H3PO3 in Py and the presence of PivCl. The condensation of H-phosphonate 288 with the primary alcohol 289, followed by oxidation, afforded phosphodiester 290, which, after removal of the protective groups, was converted into aminoethyl glycoside 280.
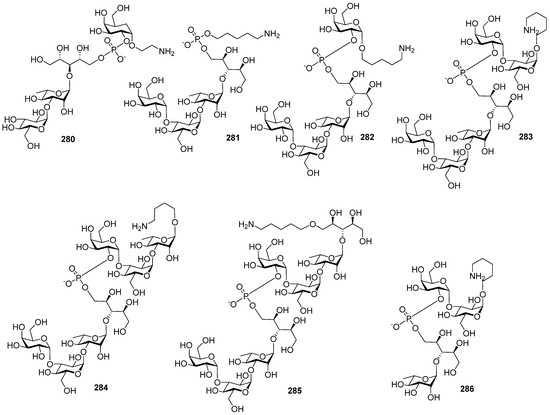
Figure 14.
Synthetic pseudo-tetrasaccharide 280 [144] and the series of pseudo-oligosaccharides 281–286 [145] structurally related to S. pneumoniae 6A capsular phosphoglycans.
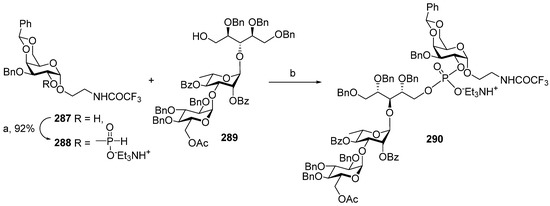
Scheme 22.
Key building blocks for the synthesis of pseudo-oligosaccharide 280 structurally related to S. pneumoniae 6A capsular phosphoglycans [144]. Reagents and conditions: (a) PivCl, Py, H3PO3, 92%; (b) PivCl (4.8 eq), Py, 3 h, followed by 0.5 M I2 in Py:H2O 2:1, yield over 2 steps at 73%.
Pseudo-oligosaccharides 281–286 were assembled by Taiwan scientists [145] using a phosphoramidite procedure for the establishment of phosphodiester linkage (Scheme 23). The selectively protected pseudo-tetrasaccharide 291 with a primary hydroxyl group in the ribitol residue was transformed into phosphoramidite 292 by the action of diamidite 70 and diisopropylammonium tetrazolide. In similar conditions, pseudo-disaccharide 293 was converted into phosphoramidite 294.
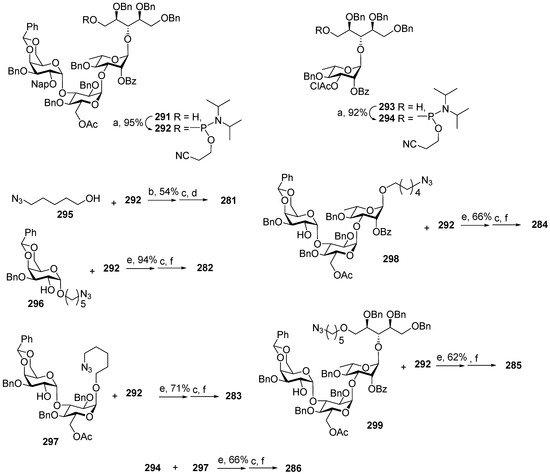
Scheme 23.
Key building blocks for the synthesis of pseudo-oligosaccharides 281–286 structurally related to S. pneumoniae 6A capsular phosphoglycans [145]. Reagents and conditions: (a) diamidite 70, diisopropylammonium tetrazolide; (b) 1H-tetrazole, CH2Cl2, r.t., 1 h, followed by mCPBA, −20 °C, 30 min; (c) Bu4NOH, CH2Cl2, H2O, r.t., 4 h; (d) Li/NH3, THF, −78 °C, 1 h, followed by MeOH, 16 h; (e) 5-ethylthio-1H-tetrazole, 3 Å MS, CH3CN, r.t., 1 h, followed by H2O, I2/THF, r.t., 2 h; (f) 0.2 M NaOMe/MeOH, followed by Pd(OH)2/C, H2, MeOH, H2O, AcOH.
The interaction of the key phosphoramidite 292 with 5-azidopentanol (compound 295) in the presence of 1H-tetrazole, followed by oxidation with mCPBA, the removal of the protective groups, and the reduction of the azido group, resulted in pseudo-tetrasaccharide 281 (Scheme 23). The condensation of phosphoramidite 292 with alcohols 296–299 was carried out in the presence of 5-ethylthio-1H-tetrazole, followed by oxidation of a phosphite group to a phosphotriester with I2 in THF. The efficiency of the formation of the phosphotriester linkage decreased in the sequence of monosaccharide 296 (94%), disaccharide 297 (71%), trisaccharide 298 (66%), and pseudo-tetrasaccharide 299 (62%), along with an increase in the steric hindrance of the hydroxyl group involved in condensation. The reduction and deblocking of the obtained phosphotriesters afforded the amino-spacered derivatives 282–285. The condensation of phosphoramidite 294 with disaccharide 297, followed by oxidation, the reduction of the azido group, and deblocking, formed pseudo-tetrasaccharide 286 [145].
Due to interest in the development of vaccine preparations based on the synthetic phosphooligosaccharides related to S. pneumoniae 6B, extensive libraries of the spacer-armed pseudo-oligosaccharides 300–314 [145,146,147,148] (Figure 15) related to S. pneumoniae 6B CPS fragments have been created. Despite their structural diversity, these compounds comprise only one phosphodiester linkage that is connected with the synthetic complexity of the target molecules.
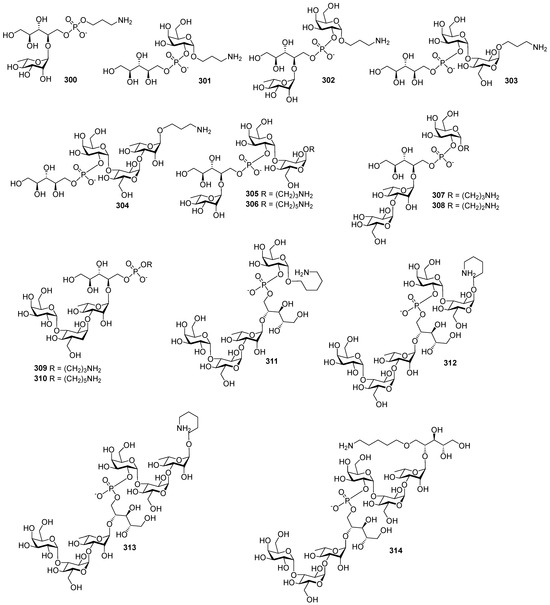
Figure 15.
Synthetic oligosaccharides 300–303 [146], 304, 305, 307, 309, and 309 [147], 308 [148], and 306 and 310–314 [145] related to S. pneumoniae 6B capsular phosphoglycans.
The assembly of the spacer-armed pseudo-disaccharides 300 and 301 and pseudo-trisaccharides 302 and 303 was carried out by Vliegenthart [146] using the H-phosphonate method. For the preparation of pseudo-disaccharide 300, the starting pseudo-disaccharide 315 was converted to the key H-phosphonate 316 by the action of chlorophosphite 129 (Scheme 24). H-phosphonate 316 was condensed with N-protected aminopropanol in the presence of PivCl in Py, the product was oxidized, and a phosphodiester 317 was obtained with a 43% yield. The deprotection of phosphodiester 317 yielded the target spacer-armed pseudo-disaccharide 300.
Scheme 24.
Key building blocks for the synthesis of pseudo-oligosaccharides 300–304, 305, and 307 structurally related to S. pneumoniae 6B capsular phosphoglycans [146,147]. Reagents and conditions: (a) 129, Py, 24 h; (b) HOCH2CH2CH2CH2NHCbz, PivCl, Py, followed by 0.5 M I2 in Py:H2O 95:5; 20 min; (c) PivCl, Py, followed by 0.5 M I2 in Py:H2O 95:5, 20 min; (d) 214, PivCl, Py, followed by 0.5 M I2 in Py:H2O 95:5, 20 min.
Under similar conditions, alcohol 318 (Scheme 25) was transformed into H-phosphonate 319, and the phosphonylation of alcohol 320 produced H-phosphonate 321 with an excellent yield. Phosphodiester 322 was prepared via two alternative routes. The condensation of the secondary hydroxyl group in galactoside 318 with H-phosphonate 321, followed by oxidation, formed 322 with a 40% yield, and the interaction of a primary hydroxyl group in the selectively protected ribitol 320 with H-phosphonate 319 and oxidation of the intermediate phosphonate afforded pseudo-disaccharide 322 with a 38% yield. By the removal of the benzyl and Cbz groups, compound 322 was converted into the target pseudo-disaccharide 301 (Figure 15) [146].
Scheme 25.
Key building blocks for the synthesis of pseudo-oligosaccharide 308 structurally related to S. pneumoniae 6B phosphoglycans [148]. Reagents and conditions: (a) 129, Py, 1 h; (b) PivCl, Py, 2 h, followed by I2 in Py:H2O 2:1, 4 h.
Unlike the synthesis of pseudo-disaccharide 322, the phosphitylation of alcohol 315 with H-phosphonate 319 was more efficient and readily formed pseudo-trisaccharide 323 (yield: 89%) [147], which was deprotected to yield the target spacer-armed pseudo-trisaccharide 302 (Scheme 24) [146]. The substantial difference in the yields for pseudo-disaccharide 322 (from 319 and 320) and pseudo-trisaccharide 323 may be related to the easier accessibility of a less sterically hindered phosphodiester bond in pseudo-disaccharide 322 for the I2-induced rapid rupture of the bridging P-O bonds. The total deprotection of pseudo-trisaccharide 323 resulted in the formation of the target spacer-armed pseudo-trisaccharide 302 (Figure 15) [147].
The condensation of alcohol 324 with H-phosphonate 321 (Scheme 24), followed by oxidation, afforded pseudo-tetrasaccharide 325 with a 77% yield [147]. Similar to the phosphitylation of alcohols 315 and 320 with H-phosphonate 319, the improved efficiency of preparation of more sterically hindered pseudo-tetrasaccharide 325 compared to 322 (from 318 and 321, 40%) [146] can be attributed to the easier destruction with I2 during the oxidation step. By deprotection, pseudo-trisaccharide 325 was converted into the target spacer-armed pseudo-trisaccharide 303 (Figure 15) [147].
The interaction of H-phosphonate 321 with the trisaccharide alcohol 326 (Scheme 24), followed by oxidation, formed pseudo-tetrasaccharide 327 (78%) with a phosphodiester bond between a bulky trisaccharide and a flexible ribitol residue. On the contrary, the efficiency of the formation of pseudo-tetrasaccharide 328 (from disaccharide 324) and pseudo-tetrasaccharide 330 (from pseudo-tetrasaccharide 329), which comprise bulky protected glycoside residues on both sides of a phosphate moiety, was much lower, and the yields did not exceed 50%. The total deprotection of pseudo-tetrasaccharides 328 and 329 resulted in the target spacer-armed compounds 304 and 305, respectively [147].
The condensation of pseudo-tetrasaccharide 331 with H-phosphonate 214, followed by oxidation, provided the introduction of a pre-spacer group, which is connected to the pseudo-tetrasaccharide via a phosphodiester bridge. The moderate yield of compound 332 (53%) can be attributed to the conformational flexibility of the pre-spacer group and the easier availability of the phosphate group for the action of I2. Compounds 331 and 332 were deblocked to yield the target spacer-armed pseudo-tetrasaccharides 307 and 309, respectively (Scheme 24) [147].
For the assembly of pseudo-tetrasaccharide 308, Nifantiev et al. [148] investigated two alternative pathways according to the schemes [3 + 1], which differed in the position of the H-phosphonate group on one or the other condensing part (Scheme 25). For this purpose, alcohol 333 was converted into H-phosphonate 334 by the action of chlorophosphite 129, and galactoside 335 was converted into H-phosphonate 336 under similar conditions. The condensation of H-phosphonate 336 with the primary hydroxyl group of pseudo-tetrasaccharide acceptor 333, followed by oxidation, produced phosphodiester 337 in a high yield (85%), whereas the interaction of H-phosphonate 334 with the secondary hydroxyl group in galactoside 335 proceeded with low efficiency (the yield of pseudo-tetrasaccharide 337 was 22%).
For the preparation of pseudo-oligosaccharides 306 and 310–314 [145], Taiwan researchers used the universal pseudo-tetrasaccharide block 338 (Scheme 26), which was readily converted into the corresponding phosphoramidite 339 by the action of diamidite 70 in the presence of diisopropylammonium tetrazolide. In similar conditions, pseudo-disaccharide 340 was converted into phosphoramidite 341. The condensation of alcohols 295–298 and 342 with compound 339 added a repeating unit to the chains. Thus, the phosphitylation of the primary hydroxyl group in alcohol 295 with phosphoramidite 339 in the presence of 1H-tetrazole, followed by the oxidation of phosphite to phosphate and the removal of the 2-naphthylmethyl protective group, afforded alcohol 342, and the total deprotection and reduction of the azido group resulted in the target spacer-armed pseudo-tetrasaccharide 310, with the spacer group connected to the pseudo-tetrasaccharide by a phosphodiester bridge.
Scheme 26.
Key building blocks for the synthesis of pseudo-oligosaccharides 281–286 structurally related to S. pneumoniae 6B phosphoglycans [145]. Reagents and conditions: (a) diamidite 70, diisopropylammonium tetrazolide; (b) 1H-tetrazole, CH2Cl2, r.t., 1.5 h, followed by mCPBA, −20 °C, 30 min; (c) Bu4NOH, CH2Cl2, H2O, r.t.; (d) Li/NH3, THF, −78 °C, 1 h, followed by MeOH, 16 h; (e) 5-ethylthio-1H-tetrazole, molecular sieves 3Å, CH3CN, r.t., 1 h, followed by H2O, I2/THF, r.t., 2 h; (f) 0.2 M NaOMe/MeOH, followed by Pd(OH)2/C, H2, MeOH, H2O, AcOH.
In a similar way, phosphoramidite 339 readily phosphitylated secondary hydroxyl groups in monosaccharide 296, disaccharide 297, and trisaccharide 298 (Scheme 26). The resulting phosphites were oxidized and deprotected to obtain target compounds 311–313. The condensation of two pseudo-tetrasaccharides 342 and 339, followed by oxidation, afforded a pseudo-octasaccharide (87%), with two bulky fragments connected by a phosphodiester bridge. After the deprotection and reduction of the azido group, the spacer-armed pseudo-octasaccharide 314 was obtained. Disaccharide 297 was phosphitylated with phosphoramidite 341. The oxidation of the intermediate phosphite, removal of the protective groups, and reduction of the azido group afforded pseudo-tetrasaccharide 306. It can be concluded that the use of phosphoramidites as phosphitylating agents in the preparation of compounds 306 and 310–314 was efficient regardless of the steric demands of the secondary hydroxyl group in the acceptor molecule. This result shows that the phosphoramidite method is preferred for the synthesis of larger structures.
The phosphoglycan structure of S. pneumoniae 6C differs from S. pneumoniae 6A in the orientation of a single hydroxyl group in one of the monosaccharide blocks (Glc in S. pneumoniae 6C vs. Gal in S. pneumoniae 6A), and a similar structural difference is observed between S. pneumoniae 6D and S. pneumoniae 6B (Figure 16). It is generally accepted that a close structural resemblance gives rise to similar antigenic properties, and one could expect cross-reactivity between all serotypes in group S. pneumoniae 6. However, surveillance data and clinical efficacy studies for subtypes of S. pneumoniae 6 obtained in connection with the use of pneumococcal vaccines are controversial. Generally, cross-protection was observed from the S. pneumoniae 6B conjugate in PCV7 and PCV10 against S. pneumoniae 6A but not against S. pneumoniae 6C and S. pneumoniae 6D. However, it was shown that PCV13, which comprises the S. pneumoniae 6A antigen, was effective against serotypes 6C and 6D invasive pneumococcal disease and microbial carriage [149,150]. The challenges associated with the assessment of the cross-reactivity and cross-protection of S. pneumoniae group 6 could be met via the identification of a glycotope for each S. pneumoniae 6 subtype. To this end, the series of oligosaccharides 343 and 344, structurally related to S. pneumoniae 6C, and 345 and 346, structurally related to S. pneumoniae 6D, were synthesized (Figure 16) [151]. The preparation of pseudo-tetrasaccharides 343 and 344, which comprise phosphodiester linkages, was performed using phosphoramidite chemistry. To obtain pseudo-oligosaccharide 343 with an interglycosidic phosphodiester bridge, disaccharide 347 was reacted with phosphoramidite 294 in acetonitrile in the presence of 5-ethylthio-1H-tetrazole (Scheme 27). The reaction mixture was oxidized under the action of iodine in THF with the addition of water, the protective groups were removed, and the N3 group was reduced to NH2. The phosphitylation of the primary OH-group in pseudo-tetrasaccharide 348 with diamidite 70 resulted in the efficient formation of phosphoramidite 349. The condensation of phosphoramidite 349 with alcohol 295, followed by the reduction of the N3 group and total deprotection, afforded pseudo-tetrasaccharide 344, in which a phosphodiester linkage connects the ribitol part and a spacer [151].
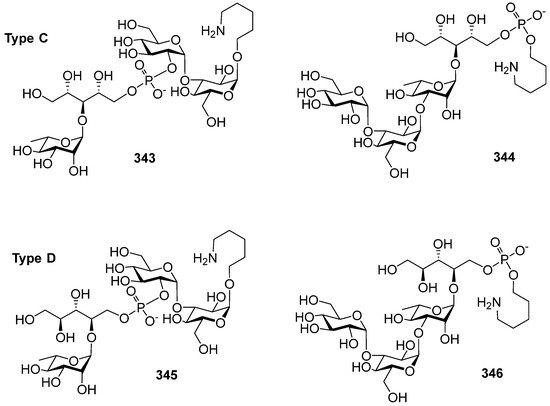
Figure 16.
Synthetic phosphooligosaccharides related to S. pneumoniae 6C (compounds 343 and 344) and S. pneumoniae 6D (compounds 345 and 346) capsular phosphoglycans [151].
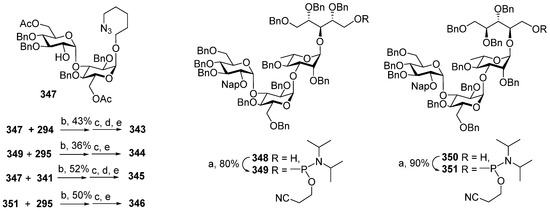
Scheme 27.
Key building blocks for the synthesis of pseudo-oligosaccharides 343 and 344, structurally related to S. pneumoniae 6C phosphoglycans, and pseudo-oligosaccharides 345 and 346, structurally related to S. pneumoniae 6D phosphoglycans [151]. Reagents and conditions: (a) diamidite 70, diisopropylammonium tetrazolide, 1 h; (b) 5-ethylthio-1H-tetrazole, molecular sieves 3Å, CH3CN, r.t., 1 h, followed by H2O, I2/THF, r.t., 2 h; (c) Bu4NOH, CH2Cl2, H2O, r.t.; (d) followed by 0,3 M NaOMe, MeOH, CH2Cl2, 2 h; (e) Pd(OH)2/C, H2, MeOH, H2O, AcOH, 36 h.
In a similar way, pseudo-tetrasaccharide 345 with an interglycosidic phosphodiester bridge was obtained by the condensation of disaccharide 247 and phosphoramidite 341, oxidation, reduction of the N3 group, and deprotection. For the preparation of phosphodiester 346, pseudo-tetrasaccharide 350 was transformed into phosphoramidite 351, which was then condensed with alcohol 295. After oxidation, the condensation product was reduced and deprotected to yield pseudo-tetrasaccharide 346, with a phosphodiester bridge between ribitol and a spacer. Notably, in the preparation of compounds 343–346, the corresponding protected phosphodiesters were obtained in moderate yields regardless of the position of the phosphodiester bridge in the molecule. It can be concluded that the used method is not sensitive to steric factors and can be applied for the condensation of bulky counterparts.
The cross-reactivity of S. pneumoniae 6A and S. pneumoniae 6B was evaluated in an immunological study of pseudo-tetrasaccharides 280 [144] related to S. pneumoniae 6A (Figure 14) and 308 [148] (Figure 15) related to S. pneumoniae 6B. Conjugates of these compounds with BSA (280-BSA and 308-BSA, respectively) were used for the immunization of mice at a dose of 20 μg/mouse on days 0 and 14. The hyperimmune serum obtained after immunization with 280-BSA and 308-BSA was analyzed in ELISA experiments using the corresponding N-biotinylated conjugates 352 and 353 on streptavidin-coated plates. It was shown that the antibodies induced by the S. pneumoniae 6A-related conjugate 280-BSA recognized not only the S. pneumoniae 6A-related N-biotinylated conjugate 352 but also the S. pneumoniae 6B-related N-biotinylated conjugate 353. Inversely, the specificity of the serum obtained from mice immunized with the S. pneumoniae 6B-related conjugate 308-BSA to the S. pneumoniae 6A-related conjugate 352 was detected. These results evidence the cross-reactivity of pseudo-tetrasaccharides 280 and 308 and indicate the presence of a common epitope in these antigens.
The immunogenic properties of synthetic phosphooligosaccharides 285 and 286 related to S. pneumoniae 6A phosphoglycans (Figure 14) and phosphooligosaccharides 306 and 314 related to S. pneumoniae 6B phosphoglycans were studied. The conjugates of these compounds with CRM197 were obtained and used for the immunization of mice at a dose of 2.2 μg of glycan per mouse, with three shots in two-week intervals. The glycan-specific IgG in the serum was analyzed using a microarray with a number of synthetic glycan antigens [145]. Mice in the negative control group were immunized with CRM197. It was found that conjugates 286-CRM197 and 306-CRM197, which contain pseudo-tetrasaccharides, effectively induced IgG antibodies against the majority of other synthetic antigens. On the contrary, conjugates 285-CRM197 and 314-CRM197 showed low immunogenicity [145].
The antigenic properties of S. pneumoniae 6B-related pseudo-disaccharide 301, pseudo-trisaccharide 303, and pseudo-tetrasaccharide 305 were investigated using their conjugates 301-KLH–305-KLH with the highly immunogenic protein KLH (Figure 17) [152]. The neoglycoconjugates 301-KLH–305-KLH and a conjugate obtained by the condensation of bacterial S. pneumoniae 6B phosphoglycans and KLH (S. pneumoniae 6B-KLH) were used for the immunization of mice at a dose of 2.5 μg and rabbits at a dose of 10 μg. The hyperimmune sera were analyzed in ELISA experiments with bacterial S. pneumoniae 6B and S. pneumoniae 6A phosphoglycans. Human serum samples were obtained by pooling infant antisera obtained by vaccination with PCV7-CRM197 at a dose of 4 mg of 6B PS.
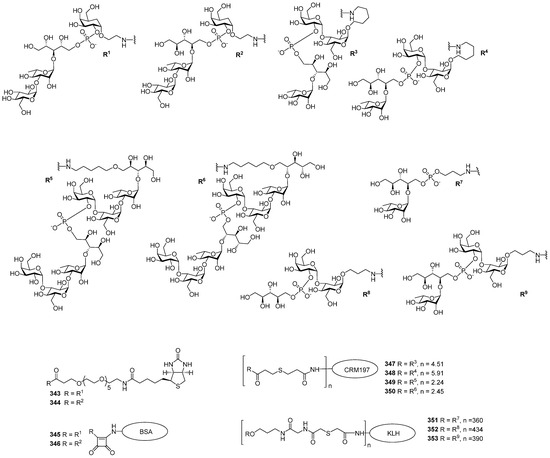
Figure 17.
Neoglycoconjugates 343–353 obtained on the basis of synthetic pseudo-oligosaccharides structurally related to S. pneumoniae 6A and S. pneumoniae 6B capsular phosphoglycans.
It was shown that in mice conjugates, 301-KLH with pseudo-disaccharide antigens and 303-KLH with pseudo-trisaccharide antigens were poorly immunogenic. On the contrary, the conjugate 305-KLH was more immunogenic than S. pneumoniae 6B-KLH. ELISA analysis of the rabbit hyperimmune antisera using bacterial S. pneumoniae 6A phosphoglycans as a coating antigen showed that conjugates 303-KLH and 305-KLH were able to induce anti-S. pneumoniae 6A antibodies. Antibody specificity was confirmed in ELISA inhibition and phagocytosis experiments. The binding of human PCV7-CRM197 antisera to the conjugates 301-KLH–305-KLH was inhibited by bacterial S. pneumoniae 6A and S. pneumoniae 6B phosphooligosaccharides.
S. pneumoniae 19F is one of the most virulent pneumococcal serotypes. Similarly to S. pneumoniae 6A and S. pneumoniae 6B, the diseases caused by S. pneumoniae 19F infection are associated with increased mortality and morbidity [153] despite the fact that the S. pneumoniae 19F capsular glycan is a component of many pneumococcal conjugated commercial vaccines. In light of this, it could be useful to develop an anti-S. pneumoniae 19F conjugate vaccine with a synthetic glycan antigen structurally related to the capsular glycan of S. pneumoniae 19F. N-trifuoroacetamidopropyl glycoside 357, which comprises a pseudo-hexasaccharide fragment of the S. pneumoniae 19F capsular polysaccharide, was obtained as a mixture of isomers. In the first step, the selectively protected trisaccharide 354 with a free hydroxyl group at C-4 (ManNAc) was converted into H-phosphonate 355 under the action of chlorophosphite 129 in Py [154] (Scheme 28). The condensation of H-phosphonate 355 with hemiacetal 356, followed by oxidation and deprotection, resulted in the formation of a diastereomeric mixture of pseudo-hexasaccharides, which contained the spacer-armed phosphooligosaccharide 357.
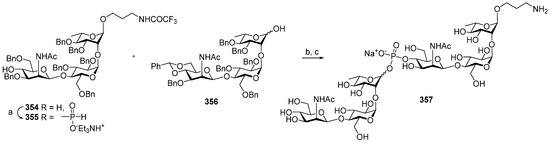
Scheme 28.
Key building blocks for the synthesis of pseudo-oligosaccharide 357 structurally related to S. pneumoniae 19F capsular glycan [154]. Reagents and conditions: (a) 129, acetonitrile, Py, 1 h.; (b) PivCl, Py, then I2 B Py:H2O 19:1, yield 61% over 3 steps; (c) H2, Pd(OH)2/C, MeOH–H2O.
The structure of the repeating unit of S. pneumoniae 19A is close to that of 19F, with the only difference being the type of linkage between Glc and Rha monosaccharide residues, which is α-D-Glc-(1→3)-Rha in S. pneumoniae 19A phosphoglycans and α-D-Glc-(1→2)-Rha in S. pneumoniae 19F phosphoglycans. Whereas S. pneumoniae 19F phosphoglycans are a component of all commercial PCV compositions, S. pneumoniae 19A phosphoglycans were not included in PCV7 and PCV10. After the introduction of PCV7, serotype replacement resulted in an increase in the number of invasive pneumococcal diseases caused by S. pneumoniae 19A [155,156]. This observation shows the absence of cross-protection between serotypes 19A and 19F. Nevertheless, the synthetic complexity of the repeating units of S. pneumoniae 19A and S. pneumoniae 19F phosphoglycans, which share a common structure, stimulated attempts to develop a universal synthetic glycoantigen for the induction of protective antibodies to both serotypes.
With a view to investigating the ability of a neoglycoconjugate with a chimeric synthetic antigen to induce a protective immune response, the Seeberger group prepared [157] phosphohexasaccharide 358, which is a combination of S. pneumoniae 19A and S. pneumoniae 19F phosphoglycan repeating units in one molecule and the spacer-armed phosphotrisaccharides 359 and 360 related to repeating units of S. pneumoniae 19F and S. pneumoniae 19A, respectively (Figure 18).
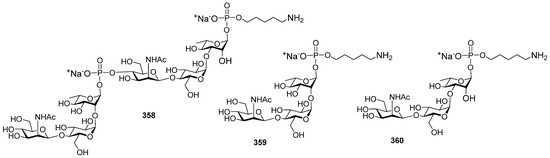
Figure 18.
Synthetic phosphohexasaccharide hybrid antigen 358, which comprises repeating units of S. pneumoniae 19F and S. pneumoniae 19A and phosphotrisaccharides related to S. pneumoniae 19F (compound 359) and S. pneumoniae 19A capsular glycan (compound 360) [157].
Hemiacetal 361 was readily transformed into the corresponding α-H-phosphonate 362 under the action of chlorophosphite 129 and H3PO3 in Py (Scheme 29). The authors suggest that α-stereoselectivity was attained due to thermodynamic reaction control, which favored α-H-phosphonate 362 in the conditions of SN2 displacement with H3PO3 at the anomeric center. In a similar manner, hemiacetal 363 was stereoselectively converted into α-H-phosphonate 364 as a precursor of the S. pneumoniae 19A repeating unit. α-H-Phosphonates 362 and 364 were coupled with alcohol 295, oxidized, reduced, and deprotected to yield the spacer-armed glycoantigens 359 and 360, which correspond to repeating units of S. pneumoniae 19F and S. pneumoniae 19A phosphoglycans [157].
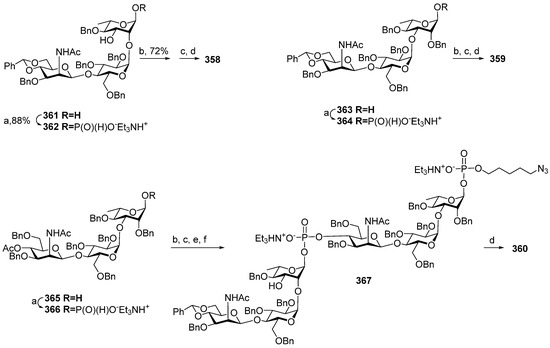
Scheme 29.
Key building blocks for the synthesis of pseudo-oligosaccharides 358–360 [157]. Reagents and conditions: (a) chlorophosphite 129, H3PO3, Py, r.t, 2 h then 50 °C, 36 h; (b) alcohol 295, PivCl, pyridine, r.t., 1.5 h; (c) I2, Py:H2O (18:2), −40 °C, 30 min, then Et3NHBr; (d) H2, Pd/C, EtOAc:MeOH:H2O, 3 days, then Dowex 50W X4, Na+; (e) 0.5 M NaOMe, MeOH, r.t., 3 h; (f) α-H-phosphonate 362, PivCl, Py, r.t., 2 h.
For the preparation of the hybrid antigen 358, another precursor of the S. pneumoniae 19A repeating unit, hemiacetal 365 with an acetyl protecting group at O-4 (MaNAc) was phosphonylated to obtain α-H-phosphonate 366, which was then condensed with alcohol 295 and oxidized. The following 4-O-deacetylation and phosphitylation with α-H-phosphonate 362 and oxidation yielded the protected phosphodiester 367. The deblocking and reduction of the azido group in phosphodiester 367 afforded the conjugation-ready chimeric phosphodiester 358 [157]. Phosphooligosaccharides 358–361 were conjugated to CRM197 via a succinimidyl adipate linker, and the resulting conjugates 358-CRM197 (average glycan loading: 5), 359-CRM197 (average glycan loading: 5), and 360-CRM197 (average glycan loading: 7) were adsorbed on alum and used for the vaccination of rabbits on day 0 and boosted on days 14, 28, and 133. IgG antibodies were raised in response to immunization with the semisynthetic neoglycoconjugates 358-CRM197, 359-CRM197, 360-CRM197, and Prevnar 13® (PCV13).
Oligosaccharide-specific antibody titers were analyzed by glycan microarray with specific immobilized oligosaccharides. Chimeric 358-CRM197 generated antibodies that recognized trisaccharide antigens 359 and 360 in quantities exceeding those generated by Prevnar 13®. At the same time, ELISA experiments showed that antibodies produced by vaccination with Prevnar 13® more efficiently recognized S. pneumoniae 19A and S. pneumoniae 19F phosphoglycans compared to vaccine preparations with synthetic antigens. This observation reveals that upon immunization with Prevnar 13®, the antibodies are elicited to larger glycotopes. Immunization with chimeric 358-CRM197 produced antibodies that efficiently recognized phosphotrisaccharide antigens 359 and 360 and S. pneumoniae 19A and S. pneumoniae 19F phosphoglycans, whereas antibodies elicited by 359-CRM197 and 360-CRM197 did not recognize S. pneumoniae 19A phosphoglycans, and only moderate interaction of 359-CRM197-induced antibodies and S. pneumoniae 19F phosphoglycans was detected. Bactericidal properties of the obtained hyperimmune sera were studied in the opsonophagocytic killing assay. Chimeric 358-CRM197-induced opsonic antibodies were able to kill both S. pneumoniae 19A and S. pneumoniae 19F. The opsonic activity of 359-CRM197-induced antibodies was very weak, and antibodies induced by the ST19A conjugate were not bactericidal [157].
As previously mentioned, clinically significant cross-protection between S. pneumoniae 19A and S. pneumoniae 19F serotypes has not been reported. One of the possible reasons for weak antibody cross-protection is the conformational difference between these two phosphoglycans [158]. In shorter oligosaccharides, the chain exists as another ensemble of conformers, which may be able to induce antibodies to phosphoglycans of both serotypes. The capsular phosphoglycans S. pneumoniae 19A and S. pneumoniae 19F share a common disaccharide structural element: P(1→4)ManNAc-β-(1→4)-Glc. With a view to studying the universal glycotope for these serotypes, a series of short glycans 368–370 (Figure 19) were prepared, which comprise the basic structural elements of the common disaccharide.
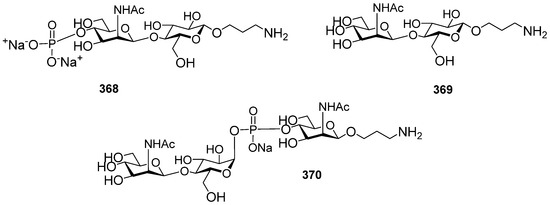
Figure 19.
Synthetic glycans related to phosphodisaccharide P(1→4)ManNAc-β-(1→4)-Glc, which is a common structural element of S. pneumoniae 19A and S. pneumoniae 19F phosphoglycans.
The phosphoramidite approach was applied for the construction of the phosphodiester bridge in pseudotrisaccharide 370 [159]. The selectively protected glycoside 371 with a free hydroxyl group at C-4 (ManNAc) was transformed into phosphamidite 372 under the action of chlorophosphoramidite 24 (Scheme 30). The condensation of phosphamidite 372 with hemiacetal 373 resulted in the formation of a mixture of α- and β-glucopyranosides (α:β 55:45). The α-isomer was oxidized, decyanoethylated, and deprotected to obtain phosphodiester 370.
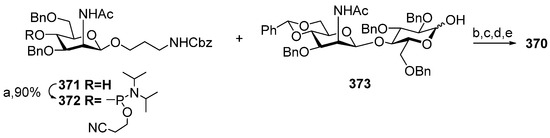
Scheme 30.
Key building blocks for the synthesis of pseudo-trisaccharide 370 [159]. Reagents and conditions: (a) 24, DIPEA, CH2Cl2, 90%; (b) DCI, CH2Cl2, 95%; (c) tBuOOH, CH3CN, 0 °C to r.t., 73% (55% α); (d) Et3N, DCM, 4 days; (e) Pd/C, H2, MeOH/H2O, 90%.
Compounds 368–370 were printed on epoxysilane-coated slides together with bacterial S. pneumoniae 19A and S. pneumoniae 19F phosphoglycans as controls. A glycan microarray was arranged, and the glycan antigens interacted with the hyperimmune sera of rabbits immunized with whole bacteria. The group’s 19 sera were bound to S. pneumoniae 19A and S. pneumoniae 19F and phosphodisaccharide 368, and the interaction with disaccharide 369 and the pseudotrisaccharide was weak. The interaction of synthetic and bacterial glycans was studied with factor reference antisera obtained by vaccination with whole cell bacteria S. pneumoniae 19A or S. pneumoniae 19F and cleared of the antibodies recognizing the common epitopes. Reference antisera to S. pneumoniae 19A readily bound to phosphate 368 and did not interact with compounds 369 and 370. Reference antisera to S. pneumoniae 19F showed moderate binding to compounds 368–370.
Two considered approaches to the search for a common epitope in S. pneumoniae 19A and S. pneumoniae 19F phosphoglycans open new horizons in the development of semisynthetic conjugated glycovaccines. The design of a universal glycotope for introduction in neoglycoconjugate vaccines is aimed at a decrease in protein loading of the vaccination dose and reduction of the production costs.
8. Synthesis of Pseudo-Oligosaccharides Structurally Related to Phosphoglycans of C. Jejuni
C. jejuni enteritis is one of the most common causes of intestinal infection and traveler’s diarrhea. It is associated with many severe sequelae, including Guillain–Barré syndrome. Penner serotyping in combination with PCR multiplex-based typing revealed 35 capsular types [160]. Until now, no commercial vaccines for the prevention of C. jejuni enteritis have been developed either for humans or for cattle and poultry, which are the frequent source of human campylobacteriosis. Whole-cell vaccines are not applicable, as a number of C. jejuni strains present sialylated LOS that induce antibodies to the gangliosides β-D-GalNAc-(1→4)-[α-Neu5Ac-(2→3)]-β-D-Gal-(1→4)-β-D-Glc-(1→1)-N-octadecanoyl sphingosine and α-Neu5Ac-(2→3)-β-D-Gal-(1→4)-β-D-Glc-(1→1)-N-octadecanoyl sphingosine, which are associated with the onset of Guillain–Barré syndrome [161]. Despite recent advances in the understanding of the pathogenesis of C. jejuni, the development of subunit or glycoconjugate preventive vaccines still remains a challenge [162]. Current knowledge on C. jejuni infection has revealed that the key structural feature is the O-methylphosphoramidate modification of capsular glycan, which is involved in both C. jejuni pathogenesis and immunogenicity. In the context of the development of conjugate vaccines on the basis of synthetic glycan antigens, O-methylphosphoramidate is regarded as the immunodominant epitope [163]. However, it should be noted that this moiety is easily hydrolyzed and can be lost during the conjugation process [160].
Up to now, only one synthesis of a P-linked pseudo-oligosaccharide related to C. jejuni phosphoglycans has been described. Therefore, the selectively protected hemiacetal 374 was converted into α-H-phosphonate 375 under the action of chlorophosphite 129. The condensation of phosphonate 375 with the heptabioside block 376 in the presence of PivCl/Py, followed by oxidation, afforded a phosphodiester, which was then transformed into the target pseudo-trisaccharide 377 corresponding to a repeating unit of the capsular glycan of a clinically important strain RM1221 of C. jejuni serotype HS:53 (Scheme 31) [164]. The prospects of conjugated vaccines for the prevention of campilobacterioses are outlined in a recent review [165].
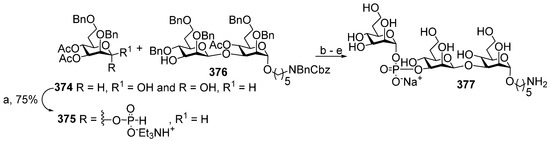
Scheme 31.
Key building blocks for the synthesis of capsular glucan of C. jejuni serotype HS:53, strain RM1221 [164]. Reagents and conditions: (a) chlorophosphite 129, Py, MeCN, 0 °C → r.t.; (b) PivCl, Py, 1 h. 74%; (c) I2, Py/H2O, 0 °C. 0,5 h., 86%; (d) 1.2 M MeONa/MeOH; (e) H2/Pd-C, 48 h.
9. Conclusions
In this review, studies published over the last 20 years on the methods of construction of interglycosidic phosphodiester linkages used for the preparation of spacer-armed phosphooligosaccharides structurally related to the capsular phosphoglycans of the pathogenic bacteria H. influenzae serotypes a, b, c, and f, N. meningitidis serogroups a and x, S. pneumoniae serotypes 6a, 6b, 6c, 6f, 19a, and 19f, and the C. jejuni serotype HS:53, strain RM1221 are summarized.
As shown, in the syntheses of phosphoglycans, two types of intermediate compounds are widely employed: H-phosphonates and phosphoramidites. Both methods provide an effective tool for the establishment of phosphodiester bridges between different types of saccharide blocks. Due to high yields and simple procedures, these methods can be scaled up and form the basis for the manufacturing process. The H-phosphonate-based method is a one-pot polycondensation procedure used in the industrial-scale synthesis of Hib oligosaccharides for the manufacturing of the Quimi-Hib® vaccine. However, the low stability of phosphodiesters in the conditions of the oxidation of interglycosidic phosphonates poses limitations on the preparation of oligomers with two or more phosphodiester bridges. The application of phosphoramidites is, in general, more efficient and allows the preparation of longer oligomers.
The majority of the spacer-armed synthetic fragments of phosphoglycans described in this paper were converted into neoglycoconjugates, which are shown to be safe and immunogenic in laboratory animals. For Hib, the combination of surface plasmon resonance, saturation transfer difference nanomagnetic resonance, and X-ray crystallography evidences that the minimal epitope consists of only two repeating units [29]. In the research conducted by Seeberger et al. [69], the conjugate with a tetramer Hib antigen (the shortest of 4, 6, 8, and 10-mers) was shown to be the most immunogenic. For Hia, the CRM197 conjugates of 1, 2, 3, and 4-mers were almost equally immunogenic. Immunogenicity with the conjugates of the phosphono-mimetics of MenA was independent of the length of the pseudo-oligosaccharide antigens, and non-acetylated carba-mimetics of MenA failed to evoke anti-MenA immunity. For MenX, immunization with conjugates of mono-, di-, and trimer with CRM197 was inefficient. For phosphooligosaccharides related to S. pneumoniae capsular phosphoglycans, the correlation between the length and immunity was not analyzed, as the majority of synthetic antigens comprised only one repeating unit. It can be concluded that longer oligomers are needed for a more profound study of immunogenicity, antigenicity, and protective immunity of neoglycoconjugates on the basis of synthetic phosphooligosaccharides.
Although the first examples of polysaccharide assembly via polycondensation (for example see [166]) were published almost 40 years ago, and in the last decade, synthesis of a high-molecular weight glycan containing 25-mer related to arabinogalactan [167] and a rhamnomannan consisting of 256 monosaccharide units [168], as well as an automated glycan assembly of a 151-mer polymannoside [169] and 15-mer phosphoglycan [50] related to the LPG of Leishmania donovani were reported, long oligosaccharides, which relate to bacterial phosphoglycans, are yet unattainable. However, the synthetic approaches described above permit the preparation of sufficiently long oligosaccharides of this type, including spacer-armed molecules, which are applicable as indispensable tools in any glycobiology studies and glycotechnology developments.
As described in this paper, a number of Hib and MenA oligomers were synthesized using a solid support method. This method has since evolved into a flexible and universal strategy based on the use of automated synthesis platforms. In glycochemistry, the Seeberger group [170] has been developing this approach for almost 30 years, and, finally, the automated glycan assembly technology has been created, which provides quick access to homogeneous oligomers and polymers starting from the properly protected monomer blocks [171]. Recent advances in automated glycan assembly [50,172,173] offer a powerful synthetic technique for the accomplishment of key steps in oligosaccharide synthesis. Protected monomer blocks required for automated glycan assembly can be attained through HPLC-based automated synthetic facilities [174,175,176].
As mentioned, MenA and Hib phosphoglycans are extremely susceptible to hydrolytic cleavage. For the preparation of stable MenA antigens, C-phosphono-mimetics were synthesized as non-acetylated compounds, and carba-mimetics were synthesized in both non-acetylated and acetylated variants. Immunization with corresponding protein conjugates was not efficient. It can be concluded that the problem of the preparation of a stable MenA antigen still waits to be resolved. On the contrary, Seeberger et. al. found that the introduction of a substituent, e.g., a methyl group, at O-2 provides a stable hydrolysis-resistant synthetic polyribosylribitolphosphate, which can be used in anti-Hib vaccines [77]. Also, the stability of an interglycosidic phosphodiester linkage can be achieved by the replacement of a non-bridging oxygen atom with a borano group [177].
To date, Quimi-Hib® remains the only commercial vaccine with a fully synthetic phosphoglycan antigen. However, recent developments in glycobiology and glycochemistry provide new ways to the fundamental knowledge, which is pivotal for the design of new vaccine preparations on the basis of synthetic phosphooligosaccharides.
Author Contributions
Writing—original draft preparation, E.A.K., A.A.K., D.V.Y. and N.E.N.; writing—review and editing, E.A.K., D.V.Y. and N.E.N.; funding acquisition, N.E.N. All authors have read and agreed to the published version of the manuscript.
Funding
The study was performed with financial support from the Russian Science Foundation (project 19-73-30017-P).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| avDP | average degree of polymerization |
| Ac2O | acetic anhydride |
| Boc | tert-butyloxycarbonyl |
| BOM | benzyloxymethyl |
| CA | chloroacetyl |
| Cbz | benzyloxycarbonyl |
| CPS | capsular polysaccharide |
| CRM197 | cross-reacting material 197, a non-toxic mutant of the diphtheria toxin |
| DBU | 1,8-diazabicyclo(5.4.0)undec-7-ene |
| DCI | 4,5-dicyanoimidazole |
| DCSO | (+)-(10-camphorsulfonyl)oxaziridine |
| DIAD | diisopropyl azodicarboxylate |
| DIC | 1,3-diisopropylcarbodiimide |
| DIPEA | N,N-diisopropylethylamine |
| DMTr | bis(4-methoxyphenyl)-phenylmethyl |
| DMAP | 4-dimethylaminopyridine |
| DMSO | dimetylsulfoxide |
| DMTr | 4,4-dimethoxytrityl |
| EC50 | half maximal effective concentration |
| ELISA | enzyme-linked immunosorbent assay |
| EtOAc | ethyl acetate |
| EtOH | ethanol |
| HSA | human serum albumin |
| IC | inhibitory concentration |
| IgA | immunoglobulin A |
| IgG | immunoglobulin G |
| IgM | immunoglobulin M |
| KLH | keyhole limpet haemocyanin |
| mCPBA | 3-chloroperbenzoic acid |
| MenA | Neisseria meningitidis serogroup a |
| MenX | Neisseria meningitidis serogroup x |
| MMTr | monomethoxytrityl |
| MPM | 4-methoxybenzyl |
| Nap | 2-naphthylmethyl |
| MeOPN | O-methylphosphoramidate |
| OS | oligosaccharide |
| PBS | phosphate buffer saline |
| PCV | pneumococcal conjugated vaccines |
| PEG | polyethylene glycol |
| PivCl | pivaloyl chloride |
| Ph3P | triphenylphosphine |
| PhSH | thiophenol |
| PMP | 4-methoxyphenyl |
| pNP | 4-nitrophenyl |
| PRP | poly-3-β-D-ribosyl-(1→1)-D-ribitol-5-phosphate |
| Py | pyridine |
| rSBA | bactericidal assay (SBA) titers using rabbit serum |
| SMP | succinimidyl-3-maleimidopropionate |
| TBDPS | tert-butyldiphenylsilyl |
| TDS | thexyldimethylsilyl |
| TCA | trichloroacetic acid |
| TES | triethylsilane |
| TFA | trifluoroacetic acid |
| THF | tetrahydrofuran |
| TT | tetanus toxoid |
References
- Fivenson, E.M.; Dubois, L.; Bernhardt, T.G. Co-Ordinated Assembly of the Multilayered Cell Envelope of Gram-Negative Bacteria. Curr. Opin. Microbiol. 2024, 79, 102479. [Google Scholar] [CrossRef] [PubMed]
- Ribet, D.; Cossart, P. How Bacterial Pathogens Colonize Their Hosts and Invade Deeper Tissues. Microbes Infect. 2015, 17, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Cescutti, P. Bacterial Capsular Polysaccharides and Exopolysaccharides. In Microbial Glycobiology; Holst, O., Brennan, P.J., von Itzstein, M., Moran, A.P., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 93–108. ISBN 978-0-12-374546-0. [Google Scholar]
- Gao, S.; Jin, W.; Quan, Y.; Li, Y.; Shen, Y.; Yuan, S.; Yi, L.; Wang, Y.; Wang, Y. Bacterial Capsules: Occurrence, Mechanism, and Function. npj Biofilms Microbiomes 2024, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Willis, L.M.; Whitfield, C. Structure, Biosynthesis, and Function of Bacterial Capsular Polysaccharides Synthesized by ABC Transporter-Dependent Pathways. Carbohydr. Res. 2013, 378, 35–44. [Google Scholar] [CrossRef]
- Noel, G.J.; Hoiseth, S.K.; Edelson, P.J. Type b Capsule Inhibits Ingestion of Haemophilus Influenzae by Murine Macrophages: Studies with Isogenic Encapsulated and Unencapsulated Strains. J. Infect. Dis. 1992, 166, 178–182. [Google Scholar] [CrossRef]
- Williams, A.E.; Maskell, D.J.; Moxon, E.R. Relationship between Intracellular Survival in Macrophages and Virulence of Haemophilus Influenzae Type b. J. Infect. Dis. 1991, 163, 1366–1369. [Google Scholar] [CrossRef]
- Cress, B.F.; Englaender, J.A.; He, W.; Kasper, D.; Linhardt, R.J.; Koffas, M.A.G. Masquerading Microbial Pathogens: Capsular Polysaccharides Mimic Host-Tissue Molecules. FEMS Microbiol. Rev. 2014, 38, 660–697. [Google Scholar] [CrossRef]
- Cyster, J.G.; Allen, C.D.C. B Cell Responses: Cell Interaction Dynamics and Decisions. Cell 2019, 177, 524–540. [Google Scholar] [CrossRef]
- van der Put, R.M.F.; Metz, B.; Pieters, R.J. Carriers and Antigens: New Developments in Glycoconjugate Vaccines. Vaccines 2023, 11, 219. [Google Scholar] [CrossRef]
- Rohokale, R.; Guo, Z. Development in the Concept of Bacterial Polysaccharide Repeating Unit-Based Antibacterial Conjugate Vaccines. ACS Infect. Dis. 2023, 9, 178–212. [Google Scholar] [CrossRef]
- Sorieul, C.; Dolce, M.; Romano, M.R.; Codée, J.; Adamo, R. Glycoconjugate Vaccines against Antimicrobial Resistant Pathogens. Expert Rev. Vaccines 2023, 22, 1055–1078. [Google Scholar] [CrossRef]
- Anderluh, M.; Berti, F.; Bzducha-Wróbel, A.; Chiodo, F.; Colombo, C.; Compostella, F.; Durlik, K.; Ferhati, X.; Holmdahl, R.; Jovanovic, D.; et al. Recent Advances on Smart Glycoconjugate Vaccines in Infections and Cancer. FEBS J. 2022, 289, 4251–4303. [Google Scholar] [CrossRef]
- Berti, F.; Romano, M.R.; Micoli, F.; Adamo, R. Carbohydrate Based Meningococcal Vaccines: Past and Present Overview. Glycoconj. J. 2021, 38, 401–409. [Google Scholar] [CrossRef]
- Khatuntseva, E.A.; Nifantiev, N.E. Glycoconjugate Vaccines for Prevention of Haemophilus Influenzae Type b Diseases. Russ. J. Bioorganic Chem. 2021, 47, 26–52. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Bagnoli, F.; Rappuoli, R.; Serruto, D. The Role of Vaccines in Combatting Antimicrobial Resistance. Nat. Rev. Microbiol. 2021, 19, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Stefanetti, G.; Borriello, F.; Richichi, B.; Zanoni, I.; Lay, L. Immunobiology of Carbohydrates: Implications for Novel Vaccine and Adjuvant Design Against Infectious Diseases. Front. Cell. Infect. Microbiol. 2022, 11, 808005. [Google Scholar] [CrossRef] [PubMed]
- Khatun, F.; Toth, I.; Stephenson, R.J. Immunology of Carbohydrate-Based Vaccines. Adv. Drug Deliv. Rev. 2020, 165–166, 117–126. [Google Scholar] [CrossRef]
- Avci, F.; Berti, F.; Dull, P.; Hennessey, J.; Pavliak, V.; Prasad, A.K.; Vann, W.; Wacker, M.; Marcq, O. Glycoconjugates: What It Would Take to Master These Well-Known yet Little-Understood Immunogens for Vaccine Development. mSphere 2019, 4, e00520-19. [Google Scholar] [CrossRef]
- Rappuoli, R. Glycoconjugate Vaccines: Principles and Mechanisms. Sci. Transl. Med. 2018, 10, eaat4615. [Google Scholar] [CrossRef]
- Del Bino, L.; Østerlid, K.E.; Wu, D.-Y.; Nonne, F.; Romano, M.R.; Codée, J.; Adamo, R. Synthetic Glycans to Improve Current Glycoconjugate Vaccines and Fight Antimicrobial Resistance. Chem. Rev. 2022, 122, 15672–15716. [Google Scholar] [CrossRef]
- Gening, M.L.; Kurbatova, E.A.; Nifantiev, N.E. Synthetic Analogs of Streptococcus Pneumoniae Capsular Polysaccharides and Immunogenic Activities of Glycoconjugates. Russ. J. Bioorg. Chem. 2021, 47, 1–25. [Google Scholar] [CrossRef]
- Seeberger, P.H. Discovery of Semi- and Fully-Synthetic Carbohydrate Vaccines Against Bacterial Infections Using a Medicinal Chemistry Approach. Chem. Rev. 2021, 121, 3598–3626. [Google Scholar] [CrossRef]
- Zasłona, M.E.; Downey, A.M.; Seeberger, P.H.; Moscovitz, O. Semi- and Fully Synthetic Carbohydrate Vaccines against Pathogenic Bacteria: Recent Developments. Biochem. Soc. Trans. 2021, 49, 2411–2429. [Google Scholar] [CrossRef]
- Mettu, R.; Chen, C.-Y.; Wu, C.-Y. Synthetic Carbohydrate-Based Vaccines: Challenges and Opportunities. J. Biomed. Sci. 2020, 27, 9. [Google Scholar] [CrossRef]
- Gening, M.L.; Kurbatova, E.A.; Tsvetkov, Y.E.; Nifantiev, N.E. Development of Approaches to a Conjugated Carbohydrate Vaccine of the Third Generation against Streptococcus Pneumoniae: The Search for Optimal Oligosaccharide Ligands. Russ. Chem. Rev. 2015, 84, 1100–1113. [Google Scholar] [CrossRef]
- Anish, C.; Beurret, M.; Poolman, J. Combined Effects of Glycan Chain Length and Linkage Type on the Immunogenicity of Glycoconjugate Vaccines. npj Vaccines 2021, 6, 150. [Google Scholar] [CrossRef]
- Kaplonek, P.; Seeberger, P.H. Glycan Microarrays Containing Synthetic Streptococcus Pneumoniae CPS Fragments and Their Application to Vaccine Development. Methods Mol. Biol. 2022, 2460, 193–206. [Google Scholar] [CrossRef]
- Nonne, F.; Iacono, L.D.; Bertuzzi, S.; Unione, L.; Proietti, D.; Norais, N.; Margarit, I.; Adamo, R.; Jiménez-Barbero, J.; Carboni, F.; et al. A Multidisciplinary Structural Approach to the Identification of the Haemophilus Influenzae Type b Capsular Polysaccharide Protective Epitope. ACS Cent. Sci. 2024, 10, 978–987. [Google Scholar] [CrossRef]
- Gerbst, A.G.; Nikolaev, A.V.; Yashunsky, D.V.; Shashkov, A.S.; Dmitrenok, A.S.; Nifantiev, N.E. Theoretical and NMR-Based Conformational Analysis of Phosphodiester-Linked Disaccharides. Sci. Rep. 2017, 7, 8934. [Google Scholar] [CrossRef]
- Verez-Bencomo, V.; Fernández-Santana, V.; Hardy, E.; Toledo, M.E.; Rodríguez, M.C.; Heynngnezz, L.; Rodriguez, A.; Baly, A.; Herrera, L.; Izquierdo, M.; et al. A Synthetic Conjugate Polysaccharide Vaccine against Haemophilus Influenzae Type b. Science 2004, 305, 522–525. [Google Scholar] [CrossRef]
- Rastogi, I.; Jeon, D.; Moseman, J.E.; Muralidhar, A.; Potluri, H.K.; McNeel, D.G. Role of B Cells as Antigen Presenting Cells. Front. Immunol. 2022, 13, 954936. [Google Scholar] [CrossRef]
- Inpanathan, S.; Botelho, R.J. The Lysosome Signaling Platform: Adapting With the Times. Front. Cell Dev. Biol. 2019, 7, 113. [Google Scholar] [CrossRef]
- Avalos, A.M.; Ploegh, H.L. Early BCR Events and Antigen Capture, Processing, and Loading on MHC Class II on B Cells. Front. Immunol. 2014, 5, 92. [Google Scholar] [CrossRef]
- Knirel, Y.A.; Van Calsteren, M.-R. Bacterial Exopolysaccharides. In Comprehensive Glycoscience, 2nd ed.; Barchi, J.J., Ed.; Elsevier: Oxford, UK, 2021; pp. 21–95. ISBN 978-0-12-822244-7. [Google Scholar]
- Whitfield, C. Structure and Assembly of Escherichia Coli Capsules. EcoSal Plus 2009, 3, 10–128. [Google Scholar] [CrossRef]
- Tsui, F.P.; Egan, W.; Summers, M.F.; Byrd, R.A.; Schneerson, R.; Robbins, J.B. Determination of the Structure of the Escherichia Coli K100 Capsular Polysaccharide, Cross-Reactive with the Capsule from Type b Haemophilus Influenzae. Carbohydr. Res. 1988, 173, 65–74. [Google Scholar] [CrossRef]
- Jann, K.; Jann, B.; Schmidt, M.A.; Vann, W.F. Structure of the Escherichia Coli K2 Capsular Antigen, a Teichoic Acid-like Polymer. J. Bacteriol. 1980, 143, 1108–1115. [Google Scholar] [CrossRef]
- Nikolaev, A.V.; Botvinko, I.V.; Ross, A.J. Natural Phosphoglycans Containing Glycosyl Phosphate Units: Structural Diversity and Chemical Synthesis. Carbohydr. Res. 2007, 342, 297–344. [Google Scholar] [CrossRef]
- Teufel, L.U.; Joosten, L.A.B.; dos Santos, J.C. Differential Structure and Immunomodulatory Functions of Lipophosphoglycan between Leishmania spp. Immunol. Lett. 2024, 268, 106885. [Google Scholar] [CrossRef]
- Mikkola, S. Nucleotide Sugars in Chemistry and Biology. Molecules 2020, 25, 5755. [Google Scholar] [CrossRef]
- Sobkowski, M.; Kraszewski, A.; Stawinski, J. Recent Advances in H-Phosphonate Chemistry. Part 1. H-Phosphonate Esters: Synthesis and Basic Reactions. Top. Curr. Chem. 2015, 361, 137–177. [Google Scholar] [CrossRef]
- Kraszewski, A.; Stawinski, J. H-Phosphonates: Versatile Synthetic Precursors to Biologically Active Phosphorus Compounds. Pure Appl. Chem. 2007, 79, 2217–2227. [Google Scholar] [CrossRef]
- Hall, R.H.; Alexander Todd, R.F. Webb 644. Nucleotides. Part Xli. Mixed Anhydrides as Intermediates in the Synthesis of Dinucleoside Phosphates. J. Chem. Soc. 1957, 3291–3296. [Google Scholar] [CrossRef]
- Aviñó, A.; Fàbrega, C.; Eritja, R. Nucleic Acids Chemistry: Modifications and Conjugates for Biomedicine and Nanotechnology. In Methods for the Synthesis of Oligonucleotides; De Gruyter: Berlin, Germany, 2021; pp. 1–44. ISBN 3-11-063579-8. [Google Scholar]
- Garegg, P.J.; Lindh, I.; Regberg, T.; Stawinski, J.; Strömberg, R.; Henrichson, C. Nucleoside H-Phosphonates. III. Chemical Synthesis of Oligodeoxyribonucleotides by the Hydrogenphosphonate Approach. Tetrahedron Lett. 1986, 34, 4051–4054. [Google Scholar] [CrossRef]
- Garegg, P.J.; Lindh, I.; Regberg, T.; Stawinski, J.; Strömberg, R.; Henrichson, C. Nucleoside H-Phosphonates. IV. Automated Solid Phase Synthesis of Oligoribonucleotides by the Hydrogenphosphonate Approach. Tetrahedron Lett. 1986, 27, 4055–4058. [Google Scholar] [CrossRef]
- Nikolaev, A.V.; Shibaev, V.N.; Kochetkov, N.K. Glycosyl Phosphosugars via Glycosyl Hydrogenphosphonates. Bioorg. Khim. 1987, 13, 1591–1593. [Google Scholar]
- Marugg, J.E.; Burik, A.; Tromp, M.; van der Marel, G.A.; van Boom, J.H. A New and Versatile Approach to the Preparation of Valuable Deoxynucleoside 3′-Phosphite Intermediates. Tetrahedron Lett. 1986, 27, 2271–2274. [Google Scholar] [CrossRef]
- Kawato, K.; Sato, K.; Wada, T. α-Selective Solid-Phase Synthesis of Glycosyl Phosphate Repeating Structure Via the Phosphoramidite Method. Chem. Eur. J. 2024, 30, e202401226. [Google Scholar] [CrossRef]
- Sato, K.; Hagio, T.; Sano, M.; Muramoto, K.; Yaoita, A.; Noro, M.; Hara, R.I.; Wada, T. Solid-Phase Stereocontrolled Synthesis of Oligomeric P-Modified Glycosyl Phosphate Derivatives Using the Oxazaphospholidine Method. ACS Omega 2021, 6, 20026–20041. [Google Scholar] [CrossRef]
- Ma, Z.; Ensley, H.E.; Lowman, D.W.; Kruppa, M.D.; Williams, D.L. Recent Advances in Chemical Synthesis of Phosphodiester Linkages Found in Fungal Mannans. Carbohydr. Res. 2025, 547, 109325. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Ding, J.; Zhao, Y.; Zhang, H.; Zhu, Y. Stereoselective Gold(I)-Catalyzed Approach to the Synthesis of Complex α-Glycosyl Phosphosaccharides. Nat. Commun. 2022, 13, 421. [Google Scholar] [CrossRef]
- Yang, G.; Mei, G.; Shen, P.; Hong, H.; Wu, Z. Rapid Assembly of Phosphate-Bridged Tetra-Mannose by Ionic Liquid-Supported Oligosaccharide Synthesis. Carbohydr. Res. 2021, 500, 108209. [Google Scholar] [CrossRef] [PubMed]
- Hara, R.I.; Kobayashi, S.; Noro, M.; Sato, K.; Wada, T. Synthesis and Properties of 2-Deoxy-2-Fluoromannosyl Phosphate Derivatives. Tetrahedron 2017, 73, 4560–4565. [Google Scholar] [CrossRef]
- Oka, N.; Sato, K.; Wada, T. Recent Progress in the Synthesis of Glycosyl Phosphate Derivatives. Trends Glycosci. Glycotechnol. 2012, 24, 152–168. [Google Scholar] [CrossRef]
- Fujita, S.; Oka, N.; Matsumura, F.; Wada, T. Synthesis of Oligo(α-D-Glycosyl Phosphate) Derivatives by a Phosphoramidite Method via Boranophosphate Intermediates. J. Org. Chem. 2011, 76, 2648–2659. [Google Scholar] [CrossRef]
- Zamyatina, A.; Kosma, P. Synthesis of Anomeric Phosphates of Aldoses and 2-Ulosonic Acids. In Carbohydrate Chemistry; Academic Press: London, UK, 2009; Volume 35, pp. 71–98. ISBN 978-1-84755-970-8. [Google Scholar]
- Egan, W.; Schneerson, R.; Werner, K.E.; Zon, G. Structural Studies and Chemistry of Bacterial Capsular Polysaccharides. Investigations of Phosphodiester-Linked Capsular Polysaccharides Isolated from Haemophilus Influenzae Types a, b, c, and f: NMR Spectroscopic Identification and Chemical Modification of End Groups and the Nature of Base-Catalyzed Hydrolytic Depolymerization. J. Am. Chem. Soc. 1982, 104, 2898–2910. [Google Scholar] [CrossRef]
- de Oliveira Cintra, F.; Takagi, M. Study of the Chemical Stability of the Capsular Polysaccharide Produced by Haemophilus Influenzae Type b. Carbohydr. Polym. 2015, 116, 167–172. [Google Scholar] [CrossRef]
- Lemercinier, X.; Jones, C. An NMR Spectroscopic Identity Test for the Control of the Capsular Polysaccharide from Haemophilus Influenzae Type b. Biologicals 2000, 28, 175–183. [Google Scholar] [CrossRef]
- Sturgess, A.W.; Rush, K.; Charbonneau, R.J.; Lee, J.I.; West, D.J.; Sitrin, R.D.; Hennessy, J.P. Haemophilus Influenzae Type b Conjugate Vaccine Stability: Catalytic Depolymerization of PRP in the Presence of Aluminum Hydroxide. Vaccine 1999, 17, 1169–1178. [Google Scholar] [CrossRef]
- Berti, F.; Romano, M.R.; Micoli, F.; Pinto, V.; Cappelletti, E.; Gavini, M.; Proietti, D.; Pluschke, G.; MacLennan, C.A.; Costantino, P. Relative Stability of Meningococcal Serogroup A and X Polysaccharides. Vaccine 2012, 30, 6409–6415. [Google Scholar] [CrossRef]
- Hoogerhout, P.; Funke, C.W.; Mellema, J.-R.; Wagenaars, G.N.; Van Boeckel, C.A.A.; Evenberg, D.; Poolman, J.T.; Lefeber, A.W.M.; Van Der Marel, G.A.; Van Booma, J.H. Synthesis of Fragments of the Capsular Polysaccharide of Haemophilus Influnzae Type B Part II. Preparation and Structural Analysis of Fragments Comprising Two and Three Repeating Units. J. Carbohydr. Chem. 1988, 7, 399–416. [Google Scholar] [CrossRef]
- Elie, C.J.J.; Muntendam, H.J.; van den Elst, H.; van der Marel, G.A.; van Boom, J.H.; Hoogerhout, P. Synthesis of Fragments of the Capsular Polysaccharide of Haemophilus Influenzae Type b: Part IIII-3. A Solid-phase Synthesis of a Spacer-containing Ribosylribitol Phosphate Hexamer. Recl. Trav. Chim. Pays-Bas. 1989, 108, 219–223. [Google Scholar] [CrossRef]
- Kandil, A.A.; Chan, N.; Chong, P.; Klein, M. Synthesis of Fragments of the Capsular Polysaccharide of Haemophilus Influenzae Type b on Soluble Polymeric Support. Synlett 1992, 7, 555–557. [Google Scholar] [CrossRef]
- Nilsson, S.; Bengtsson, M.; Norberg, T. Solid-Phase Synthesis of a Fragment of the Capsular Polysaccharide of Haemophilus Influenzae Type B Using H-Phosphonate Intermediates. J. Carbohydr. Chem. 1991, 11, 265–285. [Google Scholar] [CrossRef]
- Fernandez Santana, V.; Peña Icart, L.; Beurret, M.; Costa, L.; Verez Bencomo, V. Glycoconjugate Vaccines against Haemophilus Influenzae Type b. Methods Enzymol. 2006, 415, 153–163. [Google Scholar] [CrossRef]
- Baek, J.Y.; Geissner, A.; Rathwell, D.C.K.; Meierhofer, D.; Pereira, C.L.; Seeberger, P.H. A Modular Synthetic Route to Size-Defined Immunogenic Haemophilus Influenzae b Antigens Is Key to the Identification of an Octasaccharide Lead Vaccine Candidate. Chem. Sci. 2018, 9, 1279–1288. [Google Scholar] [CrossRef]
- Hoogerhout, P.; Evenberg, D.; van Boeckel, C.A.A.; Poolman, J.T.; Beuvery, E.C.; van der Marel, G.A.; van Boom, J.H. Synthesis of Fragments of the Capsular Polysaccharide of Haemophilus Influenzae Type B, Comprising Two or Three Repeating Units. Tetrahedron Lett. 1987, 28, 1553–1556. [Google Scholar] [CrossRef]
- Peeters, C.C.; Evenberg, D.; Hoogerhout, P.; Käyhty, H.; Saarinen, L.; van Boeckel, C.A.; van der Marel, G.A.; van Boom, J.H.; Poolman, J.T. Synthetic Trimer and Tetramer of 3-Beta-D-Ribose-(1-1)-D-Ribitol-5-Phosphate Conjugated to Protein Induce Antibody Responses to Haemophilus Influenzae Type b Capsular Polysaccharide in Mice and Monkeys. Infect. Immun. 1992, 60, 1826–1833. [Google Scholar] [CrossRef]
- Kandil, A.A.; Chan, N.; Klein, M.; Chong, P. Chemical Synthesis of Haemophilus Influenzae Glycopeptide Conjugates. Glycoconj. J. 1997, 14, 13–17. [Google Scholar] [CrossRef]
- Bacardí, D.; Cosme, K.; Aldana, L.; Merino, N.; Suárez, J.; Mosqueda, O.; Urquiza, D.; Romero, J.; Madrigal, R.; Amaya, R.; et al. Preclinical Safety Testing of the Quimi-Hib® Vaccine Adjuvanted with Aluminum Phosphate during Product Development. Biotecnol. Apl. 2013, 30, 118–124. [Google Scholar]
- Toraño, G.; Toledo, M.E.; Baly, A.; Fernandez-Santana, V.; Rodriguez, F.; Alvarez, Y.; Serrano, T.; Musachio, A.; Hernandez, I.; Hardy, E.; et al. Phase I Clinical Evaluation of a Synthetic Oligosaccharide-Protein Conjugate Vaccine against Haemophilus Influenzae Type b in Human Adult Volunteers. Clin. Vaccine Immunol. 2006, 13, 1052–1056. [Google Scholar] [CrossRef]
- Fernández-Santana, V.; Cardoso, F.; Rodriguez, A.; Carmenate, T.; Peña, L.; Valdés, Y.; Hardy, E.; Mawas, F.; Heynngnezz, L.; Rodríguez, M.C.; et al. Antigenicity and Immunogenicity of a Synthetic Oligosaccharide-Protein Conjugate Vaccine against Haemophilus Influenzae Type b. Infect. Immun. 2004, 72, 7115–7123. [Google Scholar] [CrossRef]
- Käyhty, H.; Peltola, H.; Karanko, V.; Mäkelä, P.H. The Protective Level of Serum Antibodies to the Capsular Polysaccharide of Haemophilus Influenzae Type b. J. Infect. Dis. 1983, 147, 1100. [Google Scholar] [CrossRef] [PubMed]
- Seeberger, P.H.; Pereira, C. Stable Hydrolysis-Resistant Synthetic Polyribosylribitolphosphate Derivatives as Vaccines Against Haemophilus Influenzae Type B. WO/2018/020046, 2 January 2018. Available online: https://patentscope.wipo.int/search/en/WO2018020046 (accessed on 25 June 2025).
- Gilsdorf, J.R. Hib Vaccines: Their Impact on Haemophilus Influenzae Type b Disease. J. Infect. Dis. 2021, 224, S321–S330. [Google Scholar] [CrossRef] [PubMed]
- Peltola, H. Worldwide Haemophilus Influenzae Type b Disease at the Beginning of the 21st Century: Global Analysis of the Disease Burden 25 Years after the Use of the Polysaccharide Vaccine and a Decade after the Advent of Conjugates. Clin. Microbiol. Rev. 2000, 13, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Bertran, M.; D’Aeth, J.C.; Hani, E.; Amin-Chowdhury, Z.; Fry, N.K.; Ramsay, M.E.; Litt, D.J.; Ladhani, S.N. Trends in Invasive Haemophilus Influenzae Serotype a Disease in England from 2008-09 to 2021-22: A Prospective National Surveillance Study. Lancet Infect. Dis. 2023, 23, 1197–1206. [Google Scholar] [CrossRef]
- Shuel, M.; Knox, N.; Tsang, R.S.W. Global Population Structure of Haemophilus Influenzae Serotype a (Hia) and Emergence of Invasive Hia Disease: Capsule Switching or Capsule Replacement? Can. J. Microbiol. 2021, 67, 875–884. [Google Scholar] [CrossRef]
- Slack, M.P.E.; Cripps, A.W.; Grimwood, K.; Mackenzie, G.A.; Ulanova, M. Invasive Haemophilus Influenzae Infections after 3 Decades of Hib Protein Conjugate Vaccine Use. Clin. Microbiol. Rev. 2021, 34, e0002821. [Google Scholar] [CrossRef]
- Tsang, R.S.W.; Proulx, J.-F.; Hayden, K.; Shuel, M.; Lefebvre, B.; Boisvert, A.-A.; Moore, D. Characteristics of Invasive Haemophilus Influenzae Serotype a (Hia) from Nunavik, Canada and Comparison with Hia Strains in Other North American Arctic Regions. Int. J. Infect. Dis. 2017, 57, 104–107. [Google Scholar] [CrossRef]
- Cox, A.D.; Barreto, L.; Ulanova, M.; Bruce, M.G.; Tsang, R. Conference contributors Developing a Vaccine for Haemophilus Influenzae Serotype a: Proceedings of a Workshop. Can. Commun. Dis. Rep. 2017, 43, 89–95. [Google Scholar] [CrossRef]
- Boisvert, A.-A.; Moore, D. Invasive Disease Due to Haemophilus Influenzae Type A in Children in Canada’s North: A Priority for Prevention. Can. J. Infect. Dis. Med. Microbiol. 2015, 26, 291–292. [Google Scholar] [CrossRef]
- Ulanova, M.; Tsang, R.S.W. Haemophilus Influenzae Serotype a as a Cause of Serious Invasive Infections. Lancet Infect. Dis. 2014, 14, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Resman, F.; Ristovski, M.; Ahl, J.; Forsgren, A.; Gilsdorf, J.R.; Jasir, A.; Kaijser, B.; Kronvall, G.; Riesbeck, K. Invasive Disease Caused by Haemophilus Influenzae in Sweden 1997-2009; Evidence of Increasing Incidence and Clinical Burden of Non-Type b Strains. Clin. Microbiol. Infect. 2011, 17, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.; Román, F.; Pérez-Vázquez, M.; Oteo, J.; Aracil, B.; Cercenado, E. Spanish Study Group for Haemophilus influenzae Type E Infections Due to Haemophilus Influenzae Serotype E: Microbiological, Clinical, and Epidemiological Features. Clin. Infect. Dis. 2003, 37, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, G.S.; Reis, J.N.; Cordeiro, S.M.; Lima, J.B.T.; Gouveia, E.L.; Petersen, M.; Salgado, K.; Silva, H.R.; Zanella, R.C.; Almeida, S.C.G.; et al. Prevention of Haemophilus Influenzae Type b (Hib) Meningitis and Emergence of Serotype Replacement with Type a Strains after Introduction of Hib Immunization in Brazil. J. Infect. Dis. 2003, 187, 109–116. [Google Scholar] [CrossRef]
- Whittaker, R.; Economopoulou, A.; Dias, J.G.; Bancroft, E.; Ramliden, M.; Celentano, L.P. European Centre for Disease Prevention and Control Country Experts for Invasive Haemophilus influenzae Disease Epidemiology of Invasive Haemophilus Influenzae Disease, Europe, 2007–2014. Emerg. Infect. Dis. 2017, 23, 396–404. [Google Scholar] [CrossRef]
- MacNeil, J.R.; Cohn, A.C.; Farley, M.; Mair, R.; Baumbach, J.; Bennett, N.; Gershman, K.; Harrison, L.H.; Lynfield, R.; Petit, S.; et al. Current Epidemiology and Trends in Invasive Haemophilus Influenzae Disease—United States, 1989–2008. Clin. Infect. Dis. 2011, 53, 1230–1236. [Google Scholar] [CrossRef]
- Soeters, H.M.; Blain, A.; Pondo, T.; Doman, B.; Farley, M.M.; Harrison, L.H.; Lynfield, R.; Miller, L.; Petit, S.; Reingold, A.; et al. Current Epidemiology and Trends in Invasive Haemophilus Influenzae Disease-United States, 2009–2015. Clin. Infect. Dis. 2018, 67, 881–889. [Google Scholar] [CrossRef]
- Jin, Z.; Romero-Steiner, S.; Carlone, G.M.; Robbins, J.B.; Schneerson, R. Haemophilus Influenzae Type a Infection and Its Prevention. Infect. Immun. 2007, 75, 2650–2654. [Google Scholar] [CrossRef]
- Kohout, C.V. Synthesis of Haemophilus Influenzae Type a Oligosaccharides for Vaccine Development. Doctoral Thesis, Università degli Studi di Milano, Milan, Italy, 2021. [Google Scholar]
- Kohout, C.V.; Del Bino, L.; Petrosilli, L.; D’Orazio, G.; Romano, M.R.; Codée, J.D.C.; Adamo, R.; Lay, L. Semisynthetic Glycoconjugates as Potential Vaccine Candidates Against Haemophilus Influenzae Type a. Chem. Eur. J. 2024, 30, e202401695. [Google Scholar] [CrossRef]
- Kamneva, A.A.; Yashunsky, D.V.; Khatuntseva, E.A.; Nifantiev, N.E. Synthesis of Pseudooligosaccharides Related to the Capsular Phosphoglycan of Haemophilus Influenzae Type a. Molecules 2023, 28, 5688. [Google Scholar] [CrossRef]
- Hansson, J.; Garegg, P.J.; Oscarson, S. Syntheses of Anomerically Phosphodiester-Linked Oligomers of the Repeating Units of the Haemophilus Influenzae Types c and f Capsular Polysaccharides. J. Org. Chem. 2001, 66, 6234–6243. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L. Vaccine Introduction. The Beginning of the End for Africa’s Devastating Meningitis Outbreaks? Science 2010, 330, 1466–1467. [Google Scholar] [CrossRef] [PubMed]
- Frasch, C.E.; Preziosi, M.-P.; LaForce, F.M. Development of a Group A Meningococcal Conjugate Vaccine, MenAfriVac(TM). Hum. Vaccines Immunother. 2012, 8, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Trotter, C.L.; Lingani, C.; Fernandez, K.; Cooper, L.V.; Bita, A.; Tevi-Benissan, C.; Ronveaux, O.; Préziosi, M.-P.; Stuart, J.M. Impact of MenAfriVac in Nine Countries of the African Meningitis Belt, 2010–2015: An Analysis of Surveillance Data. Lancet Infect. Dis. 2017, 17, 867–872. [Google Scholar] [CrossRef]
- WHO. WHO/IVB/13.04 Use of MenAfriVac™ (Meningitis A Vaccine) in a Controlled Temperature Chain (CTC) During Campaigns: Guidance for Immunization Programme Decision-Makers and Managers; WHO Press: Geneva, Switzerland, 2013.
- Lydon, P.; Zipursky, S.; Tevi-Benissan, C.; Djingarey, M.H.; Gbedonou, P.; Youssouf, B.O.; Zaffran, M. Economic Benefits of Keeping Vaccines at Ambient Temperature during Mass Vaccination: The Case of Meningitis A Vaccine in Chad. Bull. World Health Organ. 2014, 92, 86–92. [Google Scholar] [CrossRef]
- Lemercinier, X.; Jones, C. Full 1H NMR Assignment and Detailed O-Acetylation Patterns of Capsular Polysaccharides from Neisseria Meningitidis Used in Vaccine Production. Carbohydr. Res. 1996, 296, 83–96. [Google Scholar] [CrossRef]
- Gudlavalleti, S.K.; Szymanski, C.M.; Jarrell, H.C.; Stephens, D.S. In Vivo Determination of Neisseria Meningitidis Serogroup A Capsular Polysaccharide by Whole Cell High-Resolution Magic Angle Spinning NMR Spectroscopy. Carbohydr. Res. 2006, 341, 557–562. [Google Scholar] [CrossRef]
- Berti, F.; De Ricco, R.; Rappuoli, R. Role of O-Acetylation in the Immunogenicity of Bacterial Polysaccharide Vaccines. Molecules 2018, 23, 1340. [Google Scholar] [CrossRef]
- Berry, D.S.; Lynn, F.; Lee, C.-H.; Frasch, C.E.; Bash, M.C. Effect of O Acetylation of Neisseria Meningitidis Serogroup A Capsular Polysaccharide on Development of Functional Immune Responses. Infect. Immun. 2002, 70, 3707–3713. [Google Scholar] [CrossRef]
- Utkina, N.S.; Eliseeva, G.I.; Nikolaev, A.V.; Shibaev, V.N. Fragmenty biopolimerov, soderzhashchikh ostatki glikozilfosfatov. 12. Sintez glikozilfosfosakharov, soderzhashchikh ostatki 2-acetamido-2-dezoksi-alpha-D-mannopiranozilfosfata, v tom chisle fragmenta kapsul’nogoantigena Neisseria meningitidis A [Biopolymer fragments, containing glycosylphosphate residues. 12. Synthesis of glycosylphosphosugars, containing 2-acetamido-2-deoxy-alpha-D-mannopyranosylphosphate residues including a fragment of the Neisseria meningitidis A capsule antigen]. Bioorg. Khim. 1993, 19, 228–235. [Google Scholar]
- Sato, K.; Chiba, A.; Shiraishi, T.; Ogawa, Y.; Hara, R.I.; Wada, T. Solid-Phase Synthesis of N-Trichloroacetyl Mannosamine 1-Phosphate Repeating Units Mimicking Capsular Polysaccharide Derived from Neisseria Meningitidis Serotype, A. Carbohydr. Res. 2022, 518, 108585. [Google Scholar] [CrossRef]
- Harale, K.R.; Rout, J.K.; Chhikara, M.K.; Gill, D.S.; Misra, A.K. Synthesis and Immunochemical Evaluation of a Novel Neisseria Meningitidis Serogroup A Tetrasaccharide and Its Conjugate. Org. Chem. Front. 2017, 4, 2348–2357. [Google Scholar] [CrossRef]
- Black, A. Synthesis of Fragments of the Capsular Polysaccharide of Neisseria Meningitidis (Serogroup A) Suitable for Bioconjugation. Doctoral Thesis, University of Dundee, Dundee, Scotland, 2014. [Google Scholar]
- Slättegård, R.; Teodorovic, P.; Kinfe, H.H.; Ravenscroft, N.; Gammon, D.W.; Oscarson, S. Synthesis of Structures Corresponding to the Capsular Polysaccharide of Neisseria Meningitidis Group, A. Org. Biomol. Chem. 2005, 3, 3782–3787. [Google Scholar] [CrossRef] [PubMed]
- Berkin, A.; Coxon, B.; Pozsgay, V. Towards a Synthetic Glycoconjugate Vaccine against Neisseria Meningitidis, A. Chem. Eur. J. 2002, 8, 4424–4433. [Google Scholar] [CrossRef] [PubMed]
- Torres-Sanchez, M.I.; Zaccaria, C.; Buzzi, B.; Miglio, G.; Lombardi, G.; Polito, L.; Russo, G.; Lay, L. Synthesis and Biological Evaluation of Phosphono Analogues of Capsular Polysaccharide Fragments from Neisseria Meningitidis, A. Chem. Eur. J. 2007, 13, 6623–6635. [Google Scholar] [CrossRef]
- Teodorović, P.; Slättegård, R.; Oscarson, S. Synthesis of Stable C-Phosphonate Analogues of Neisseria Meningitidis Group A Capsular Polysaccharide Structures Using Modified Mitsunobu Reaction Conditions. Org. Biomol. Chem. 2006, 4, 4485–4490. [Google Scholar] [CrossRef]
- Torres-Sanchez, M.I.; Draghetti, V.; Panza, L.; Lay, L.; Russo, G. Synthesis of the Phosphono Analogue of the Dimeric Subunit of Neisseria Meningitidis Type A Capsular Polysaccharide. Synlett 2005, 2005, 1147–1151. [Google Scholar] [CrossRef]
- Enotarpi, J.; Tontini, M.; Balocchi, C.; van der Es, D.; Auberger, L.; Balducci, E.; Carboni, F.; Proietti, D.; Casini, D.; Filippov, D.V.; et al. A Stabilized Glycomimetic Conjugate Vaccine Inducing Protective Antibodies against Neisseria Meningitidis Serogroup, A. Nat. Commun. 2020, 11, 4434. [Google Scholar] [CrossRef]
- Gao, Q.; Tontini, M.; Brogioni, G.; Nilo, A.; Filippini, S.; Harfouche, C.; Polito, L.; Romano, M.R.; Costantino, P.; Berti, F.; et al. Immunoactivity of Protein Conjugates of Carba Analogues from Neisseria Meningitidis a Capsular Polysaccharide. ACS Chem. Biol. 2013, 8, 2561–2567. [Google Scholar] [CrossRef]
- Gao, Q.; Zaccaria, C.; Tontini, M.; Poletti, L.; Costantino, P.; Lay, L. Synthesis and Preliminary Biological Evaluation of Carba Analogues from Neisseria Meningitidis A Capsular Polysaccharide. Org. Biomol. Chem. 2012, 10, 6673–6681. [Google Scholar] [CrossRef]
- Fiebig, T.; Freiberger, F.; Pinto, V.; Romano, M.R.; Black, A.; Litschko, C.; Bethe, A.; Yashunsky, D.; Adamo, R.; Nikolaev, A.; et al. Molecular Cloning and Functional Characterization of Components of the Capsule Biosynthesis Complex of Neisseria Meningitidis Serogroup A: Toward in Vitro Vaccine Production. J. Biol. Chem. 2014, 289, 19395–19407. [Google Scholar] [CrossRef]
- Westerduin, P.; Veeneman, G.H.; van der Marel, G.A.; van Boom, J.H. Synthesis of the Fragment GlcNAc-α(1→p→6)-GlcNAc of the Cell Wall Polymer of Staphylococcus Lactis Having Repeating N-Acetyl-D-Glucosamine Phosphate Units. Tetrahedron Lett. 1986, 27, 6271–6274. [Google Scholar] [CrossRef]
- Toma, L.; Legnani, L.; Rencurosi, A.; Poletti, L.; Lay, L.; Russo, G. Modeling of Synthetic Phosphono and Carba Analogues of N-Acetyl-Alpha-D-Mannosamine 1-Phosphate, the Repeating Unit of the Capsular Polysaccharide from Neisseria Meningitidis Serovar, A. Org. Biomol. Chem. 2009, 7, 3734–3740. [Google Scholar] [CrossRef] [PubMed]
- Calloni, I.; Unione, L.; Jiménez-Osés, G.; Corzana, F.; Del Bino, L.; Corrado, A.; Pitirollo, O.; Colombo, C.; Lay, L.; Adamo, R.; et al. The Conformation of the Mannopyranosyl Phosphate Repeating Unit of the Capsular Polysaccharide of Neisseria Meningitidis Serogroup a and Its Carba-Mimetic. Eur. J. Org. Chem. 2018, 2018, 4548–4555. [Google Scholar] [CrossRef] [PubMed]
- Hlozek, J.; Ravenscroft, N.; Kuttel, M.M. Modeling the Conformations of Neisseria Meningitidis Serogroup a CPS and a Carba-Analogue: Implications for Vaccine Development. Carbohydr. Res. 2019, 486, 107838. [Google Scholar] [CrossRef]
- Fallarini, S.; Buzzi, B.; Giovarruscio, S.; Polito, L.; Brogioni, G.; Tontini, M.; Berti, F.; Adamo, R.; Lay, L.; Lombardi, G. A Synthetic Disaccharide Analogue from Neisseria Meningitidis A Capsular Polysaccharide Stimulates Immune Cell Responses and Induces Immunoglobulin G (IgG) Production in Mice When Protein-Conjugated. ACS Infect. Dis. 2015, 1, 487–496. [Google Scholar] [CrossRef]
- Arjona, O.; Gómez, A.M.; López, J.C.; Plumet, J. Synthesis and Conformational and Biological Aspects of Carbasugars. Chem. Rev. 2007, 107, 1919–2036. [Google Scholar] [CrossRef]
- Balmer, P.; Burman, C.; Serra, L.; York, L.J. Impact of Meningococcal Vaccination on Carriage and Disease Transmission: A Review of the Literature. Hum. Vaccines Immunother. 2018, 14, 1118–1130. [Google Scholar] [CrossRef]
- Xie, O.; Pollard, A.J.; Mueller, J.E.; Norheim, G. Emergence of Serogroup X Meningococcal Disease in Africa: Need for a Vaccine. Vaccine 2013, 31, 2852–2861. [Google Scholar] [CrossRef]
- Soumahoro, L.; Abitbol, V.; Vicic, N.; Bekkat-Berkani, R.; Safadi, M.A.P. Meningococcal Disease Outbreaks: A Moving Target and a Case for Routine Preventative Vaccination. Infect. Dis. Ther. 2021, 10, 1949–1988. [Google Scholar] [CrossRef]
- Morelli, L.; Cancogni, D.; Tontini, M.; Nilo, A.; Filippini, S.; Costantino, P.; Romano, M.R.; Berti, F.; Adamo, R.; Lay, L. Synthesis and Immunological Evaluation of Protein Conjugates of Neisseria Meningitidis X Capsular Polysaccharide Fragments. Beilstein J. Org. Chem. 2014, 10, 2367–2376. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Romano, M.R.; Tontini, M.; Cappelletti, E.; Gavini, M.; Proietti, D.; Rondini, S.; Swennen, E.; Santini, L.; Filippini, S.; et al. Development of a Glycoconjugate Vaccine to Prevent Meningitis in Africa Caused by Meningococcal Serogroup, X. Proc. Natl. Acad. Sci. USA 2013, 110, 19077–19082. [Google Scholar] [CrossRef] [PubMed]
- Pietri, G.P.; Tontini, M.; Brogioni, B.; Oldrini, D.; Robakiewicz, S.; Henriques, P.; Calloni, I.; Abramova, V.; Santini, L.; Malić, S.; et al. Elucidating the Structural and Minimal Protective Epitope of the Serogroup X Meningococcal Capsular Polysaccharide. Front. Mol. Biosci. 2021, 8, 745360. [Google Scholar] [CrossRef] [PubMed]
- Eliseeva, G.I.; Ivanova, I.A.; Nikolaev, A.V.; Shibaev, V.N. Fragments of Biopolymers Containing Glycosyl-Phosphate Residues. 8. Hydrogenphosphonate Synthesis of Glycosyl-Phosphosugars Containing Alpha-d-Glucopyranosyl Phosphate and 2-Acetamido-2-Deoxy-Alpha-d-Glucopyranosyl Phosphate Residues, Fragments of Bacteria Capsular Antigens and of Glycoproteins. Bioorg. Khim. 1991, 17, 1401–1411. [Google Scholar]
- Morelli, L.; Lay, L. Synthesis of Neisseria Meningitidis X Capsular Polysaccharide Fragments. Arkivoc 2012, 166–184. [Google Scholar] [CrossRef]
- Harale, K.R.; Dumare, N.B.; Singh, D.; Misra, A.K.; Chhikara, M.K. Synthesis of a Tetrasaccharide and Its Glycoconjugate Corresponding to the Capsular Polysaccharide of Neisseria Meningitidis Serogroup X and Its Immunochemical Studies. RSC Adv. 2015, 5, 41332–41340. [Google Scholar] [CrossRef]
- Oldrini, D.; Fiebig, T.; Romano, M.R.; Proietti, D.; Berger, M.; Tontini, M.; De Ricco, R.; Santini, L.; Morelli, L.; Lay, L.; et al. Combined Chemical Synthesis and Tailored Enzymatic Elongation Provide Fully Synthetic and Conjugation-Ready Neisseria Meningitidis Serogroup X Vaccine Antigens. ACS Chem. Biol. 2018, 13, 984–994. [Google Scholar] [CrossRef]
- Brooks, L.R.K.; Mias, G.I. Streptococcus Pneumoniae’s Virulence and Host Immunity: Aging, Diagnostics, and Prevention. Front. Immunol. 2018, 9, 1366. [Google Scholar] [CrossRef]
- Javed; Mandal, P.K. Bacterial Surface Capsular Polysaccharides from Streptococcus Pneumoniae: A Systematic Review on Structures, Syntheses, and Glycoconjugate Vaccines. Carbohydr. Res. 2021, 502, 108277. [Google Scholar] [CrossRef]
- Garcia Quesada, M.; Yang, Y.; Bennett, J.C.; Hayford, K.; Zeger, S.L.; Feikin, D.R.; Peterson, M.E.; Cohen, A.L.; Almeida, S.C.G.; Ampofo, K.; et al. Serotype Distribution of Remaining Pneumococcal Meningitis in the Mature PCV10/13 Period: Findings from the PSERENADE Project. Microorganisms 2021, 9, 738. [Google Scholar] [CrossRef]
- Cleary, D.W.; Jones, J.; Gladstone, R.A.; Osman, K.L.; Devine, V.T.; Jefferies, J.M.; Bentley, S.D.; Faust, S.N.; Clarke, S.C. Changes in Serotype Prevalence of Streptococcus Pneumoniae in Southampton, UK between 2006 and 2018. Sci. Rep. 2022, 12, 13332. [Google Scholar] [CrossRef]
- Sheppard, C.L.; Pichon, B.; George, R.C.; Hall, L.M.C. Streptococcus Pneumoniae Isolates Expressing a Capsule with Epitopes of Both Serotypes 6A and 6B. Clin. Vaccine Immunol. 2010, 17, 1820–1822. [Google Scholar] [CrossRef]
- Pérez-García, C.; Sempere, J.; de Miguel, S.; Hita, S.; Úbeda, A.; Vidal, E.J.; Llorente, J.; Limia, A.; de Miguel, A.G.; Sanz, J.C.; et al. Surveillance of Invasive Pneumococcal Disease in Spain Exploring the Impact of the COVID-19 Pandemic (2019-2023). J. Infect. 2024, 89, 106204. [Google Scholar] [CrossRef]
- von Gottberg, A.; Kleynhans, J.; de Gouveia, L.; Tempia, S.; Meiring, S.; Quan, V.; du Plessis, M.; von Mollendorf, C.; Crowther-Gibson, P.; Avenant, T.; et al. Long-Term Effect of Pneumococcal Conjugate Vaccines on Invasive Pneumococcal Disease Incidence among People of All Ages from National, Active, Laboratory-Based Surveillance in South Africa, 2005–2019: A Cohort Observational Study. Lancet Glob. Health 2024, 12, e1470–e1484. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Sundaresan, S.; Manoharan, A.; Shet, A. Serotype Distribution and Antimicrobial Susceptibility Pattern in Children≤5years with Invasive Pneumococcal Disease in India—A Systematic Review. Vaccine 2017, 35, 4501–4509. [Google Scholar] [CrossRef] [PubMed]
- Sukhova, E.V.; Yashunsky, D.V.; Kurbatova, E.A.; Akhmatova, E.A.; Tsvetkov, Y.E.; Nifantiev, N.E. Synthesis and Preliminary Immunological Evaluation of a Pseudotetrasaccharide Related to a Repeating Unit of the Streptococcus Pneumoniae Serotype 6A Capsular Polysaccharide. Front. Mol. Biosci. 2021, 8, 754753. [Google Scholar] [CrossRef]
- Mettu, R.; Lih, Y.-H.; Vulupala, H.R.; Chen, C.-Y.; Hsu, M.-H.; Lo, H.-J.; Liao, K.-S.; Cheng, Y.-Y.; Chiu, C.-H.; Wu, C.-Y. Synthetic Library of Oligosaccharides Derived from the Capsular Polysaccharide of Streptococcus Pneumoniae Serotypes 6A and 6B and Their Immunological Studies. ACS Infect. Dis. 2022, 8, 626–634. [Google Scholar] [CrossRef]
- Thijssen, M.J.; van Rijswijk, M.N.; Kamerling, J.P.; Vliegenthart, J.F. Synthesis of Spacer-Containing Di- and Tri-Saccharides That Represent Parts of the Capsular Polysaccharide of Streptococcus Pneumoniae Type 6B. Carbohydr. Res. 1998, 306, 93–109. [Google Scholar] [CrossRef]
- Thijssen, M.J.; Bijkerk, M.H.; Kamerling, J.P.; Vliegenthart, J.F. Synthesis of Four Spacer-Containing “tetrasaccharides” That Represent Four Possible Repeating Units of the Capsular Polysaccharide of Streptococcus Pneumoniae Type 6B. Carbohydr. Res. 1998, 306, 111–125. [Google Scholar] [CrossRef]
- Sukhova, E.V.; Yashunsky, D.V.; Kurbatova, E.A.; Tsvetkov, Y.E.; Nikolay, E.N. Synthesis of a Pseudotetrasaccharide Corresponding to a Repeating Unit of the Streptococcus Pneumoniae Type 6B Capsular Polysaccharide*. J. Carbohydr. Chem. 2018, 37, 1–17. [Google Scholar] [CrossRef]
- Grant, L.R.; Hanquet, G.; Sepúlveda-Pachón, I.T.; Theilacker, C.; Baay, M.; Slack, M.P.E.; Jodar, L.; Gessner, B.D. Effects of PCV10 and PCV13 on Pneumococcal Serotype 6C Disease, Carriage, and Antimicrobial Resistance. Vaccine 2024, 42, 2983–2993. [Google Scholar] [CrossRef]
- Feemster, K.; Hausdorff, W.P.; Banniettis, N.; Platt, H.; Velentgas, P.; Esteves-Jaramillo, A.; Burton, R.L.; Nahm, M.H.; Buchwald, U.K. Implications of Cross-Reactivity and Cross-Protection for Pneumococcal Vaccine Development. Vaccines 2024, 12, 974. [Google Scholar] [CrossRef]
- Mettu, R.; Cheng, Y.-Y.; Vulupala, H.R.; Lih, Y.-H.; Chen, C.-Y.; Hsu, M.-H.; Lo, H.-J.; Liao, K.-S.; Chiu, C.-H.; Wu, C.-Y. Chemical Synthesis of Truncated Capsular Oligosaccharide of Serotypes 6C and 6D of Streptococcus Pneumoniae with Their Immunological Studies. ACS Infect. Dis. 2024, 10, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Jansen, W.T.; Hogenboom, S.; Thijssen, M.J.; Kamerling, J.P.; Vliegenthart, J.F.; Verhoef, J.; Snippe, H.; Verheul, A.F. Synthetic 6B Di-, Tri-, and Tetrasaccharide-Protein Conjugates Contain Pneumococcal Type 6A and 6B Common and 6B-Specific Epitopes That Elicit Protective Antibodies in Mice. Infect. Immun. 2001, 69, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, D.M.; Harboe, Z.B.; Sanders, E.A.M.; Ndiritu, M.; Klugman, K.P.; Rückinger, S.; Dagan, R.; Adegbola, R.; Cutts, F.; Johnson, H.L.; et al. Association of Serotype with Risk of Death Due to Pneumococcal Pneumonia: A Meta-Analysis. Clin. Infect. Dis. 2010, 51, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Soliveri, G.; Bertolotti, A.; Panza, L.; Poletti, L.; Lay, L.; Jones, C. Synthesis of Phosphorylated Fragments of Streptococcus Pneumoniae Type 19F Capsular Polysaccharide. J. Chem. Soc. Perkin 1 2002, 2174–2181. [Google Scholar] [CrossRef]
- Desmet, S.; Theeten, H.; Laenen, L.; Cuypers, L.; Maes, P.; Bossuyt, W.; Van Heirstraeten, L.; Peetermans, W.E.; Lagrou, K. Characterization of Emerging Serotype 19A Pneumococcal Strains in Invasive Disease and Carriage, Belgium. Emerg. Infect. Dis. 2022, 28, 1606–1614. [Google Scholar] [CrossRef]
- Isturiz, R.; Sings, H.L.; Hilton, B.; Arguedas, A.; Reinert, R.-R.; Jodar, L. Streptococcus Pneumoniae Serotype 19A: Worldwide Epidemiology. Expert Rev. Vaccines 2017, 16, 1007–1027. [Google Scholar] [CrossRef]
- Sanapala, S.R.; Seco, B.M.S.; Baek, J.Y.; Awan, S.I.; Pereira, C.L.; Seeberger, P.H. Chimeric Oligosaccharide Conjugate Induces Opsonic Antibodies against Streptococcus Pneumoniae Serotypes 19A and 19F. Chem. Sci. 2020, 11, 7401–7407. [Google Scholar] [CrossRef]
- Kuttel, M.M.; Jackson, G.E.; Mafata, M.; Ravenscroft, N. Capsular Polysaccharide Conformations in Pneumococcal Serotypes 19F and 19A. Carbohydr. Res. 2015, 406, 27–33. [Google Scholar] [CrossRef]
- Morelli, L.; Lay, L.; Santana-Mederos, D.; Valdes-Balbin, Y.; Verez Bencomo, V.; van Diepen, A.; Hokke, C.H.; Chiodo, F.; Compostella, F. Glycan Array Evaluation of Synthetic Epitopes between the Capsular Polysaccharides from Streptococcus Pneumoniae 19F and 19A. ACS Chem. Biol. 2021, 16, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Tikhomirova, A.; McNabb, E.R.; Petterlin, L.; Bellamy, G.L.; Lin, K.H.; Santoso, C.A.; Daye, E.S.; Alhaddad, F.M.; Lee, K.P.; Roujeinikova, A. Campylobacter Jejuni Virulence Factors: Update on Emerging Issues and Trends. J. Biomed. Sci. 2024, 31, 45. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Li, K.; Cui, T.; Yan, Y. Detection of Anti-Ganglioside Antibodies in Guillain-Barré Syndrome. Ann. Transl. Med. 2023, 11, 289. [Google Scholar] [CrossRef] [PubMed]
- Poly, F.; Noll, A.J.; Riddle, M.S.; Porter, C.K. Update on Campylobacter Vaccine Development. Hum. Vaccines Immunother. 2019, 15, 1389–1400. [Google Scholar] [CrossRef]
- Thota, V.N.; Lowary, T.L. Synthesis of 6-Deoxy-d-Ido-Heptopyranose-Containing Fragments of the Campylobacter Jejuni Strain CG8486 Capsular Polysaccharide. Carbohydr. Res. 2024, 536, 109058. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Wang, J.; Yang, Y. Total Synthesis of the Trisaccharide Antigen of the Campylobacter Jejuni RM1221 Capsular Polysaccharide via de Novo Synthesis of the 6-Deoxy-d- Manno-Heptose Building Blocks. J. Org. Chem. 2019, 84, 2393–2403. [Google Scholar] [CrossRef]
- Cloutier, M.; Gauthier, C. Progress toward the Development of Glycan-Based Vaccines against Campylobacteriosis. ACS Infect. Dis. 2021, 7, 969–986. [Google Scholar] [CrossRef]
- Kochetkov, N.K.; Nifant’ev, N.E.; Backinowsky, L.V. Synthesis of the Capsular Polysaccharide of Streptococcus Pneumoniae Type 14. Tetrahedron 1987, 43, 3109–3121. [Google Scholar] [CrossRef]
- Pasari, S.; Manmode, S.; Walke, G.; Hotha, S. A Versatile Synthesis of Pentacosafuranoside Subunit Reminiscent of Mycobacterial Arabinogalactan Employing One Strategic Glycosidation Protocol. Chem. Eur. J. 2018, 24, 1128–1139. [Google Scholar] [CrossRef]
- Zhu, Q.; Shen, Z.; Chiodo, F.; Nicolardi, S.; Molinaro, A.; Silipo, A.; Yu, B. Chemical Synthesis of Glycans up to a 128-Mer Relevant to the O-Antigen of Bacteroides Vulgatus. Nat. Commun. 2020, 11, 4142. [Google Scholar] [CrossRef]
- Joseph, A.A.; Pardo-Vargas, A.; Seeberger, P.H. Total Synthesis of Polysaccharides by Automated Glycan Assembly. J. Am. Chem. Soc. 2020, 142, 8561–8564. [Google Scholar] [CrossRef] [PubMed]
- Seeberger, P.H. Automated Carbohydrate Synthesis as Platform to Address Fundamental Aspects of Glycobiology--Current Status and Future Challenges. Carbohydr. Res. 2008, 343, 1889–1896. [Google Scholar] [CrossRef] [PubMed]
- Danglad-Flores, J.; Sletten, E.T.; Reuber, E.E.; Bienert, K.; Riegler, H.; Seeberger, P.H. Optimized Platform for Automated Glycan Assembly. Device 2024, 2, 100499. [Google Scholar] [CrossRef]
- Guberman, M.; Seeberger, P.H. Automated Glycan Assembly: A Perspective. J. Am. Chem. Soc. 2019, 141, 5581–5592. [Google Scholar] [CrossRef] [PubMed]
- Guberman, M.; Bräutigam, M.; Seeberger, P.H. Automated Glycan Assembly of Lewis Type I and II Oligosaccharide Antigens. Chem. Sci. 2019, 10, 5634–5640. [Google Scholar] [CrossRef]
- Kashiwagi, G.A.; Petrosilli, L.; Escopy, S.; Lay, L.; Stine, K.J.; De Meo, C.; Demchenko, A.V. HPLC-Based Automated Synthesis and Purification of Carbohydrates. Chemistry 2024, 30, e202401214. [Google Scholar] [CrossRef]
- Lin, M.-H.; Wolf, J.B.; Sletten, E.T.; Cambié, D.; Danglad-Flores, J.; Seeberger, P.H. Enabling Technologies in Carbohydrate Chemistry: Automated Glycan Assembly, Flow Chemistry and Data Science. Chembiochem 2023, 24, e202200607. [Google Scholar] [CrossRef]
- Escopy, S.; Singh, Y.; Stine, K.J.; Demchenko, A.V. HPLC-Based Automated Synthesis of Glycans in Solution. Chem. Eur. J. 2022, 28, e202201180. [Google Scholar] [CrossRef]
- Sato, K.; Muramoto, K.; Hagio, T.; Hara, R.I.; Wada, T. Solid-Phase Synthesis of Glycosyl Phosphate Repeating Units via Glycosyl Boranophosphates as Stable Intermediates. Org. Lett. 2023, 25, 3927–3931. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).