Novel Tetraphenolic Porphyrazine Capable of MRSA Photoeradication
Abstract
1. Introduction
2. Results
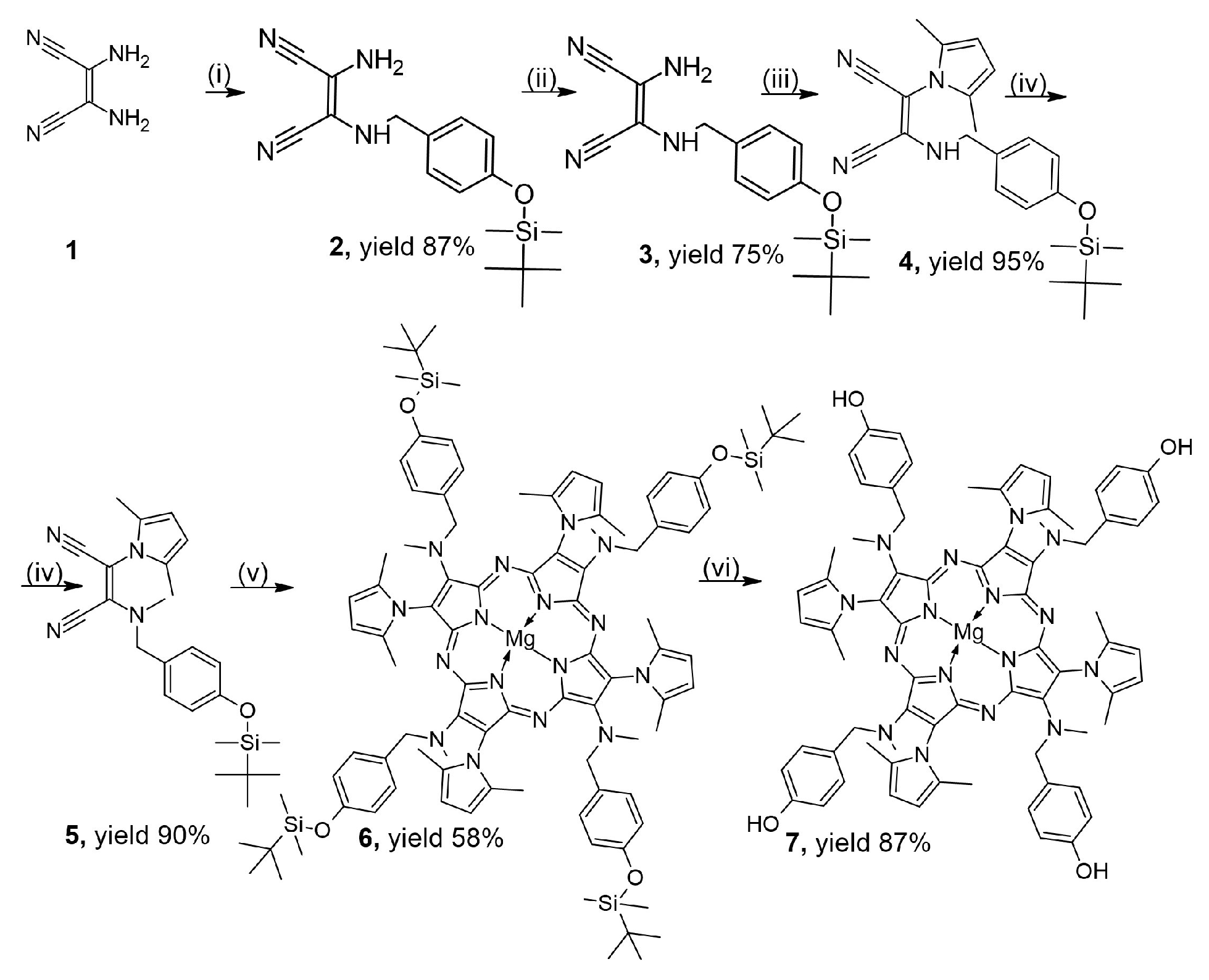
2.1. Synthesis and Characterization
2.2. Electrochemical and Spectroelectrochemical Studies
2.3. Absorption and Emission Properties
2.4. Singlet Oxygen-Generation Measurements
2.5. Acute Toxicity Assessment Using Microtox Test
2.6. Photodynamic Inactivation Studies
3. Materials and Methods
3.1. General Procedures
3.2. Synthesis of Derivatives 2–7
3.3. Electrochemical Measurements
3.4. Spectroelectrochemical Measurements
3.5. Fluorescence Measurements
3.6. Singlet Oxygen-Generation Quantum Yield Measurements
3.7. Microtox Acute Toxicity Assessment
3.8. Antimicrobial Assay
3.8.1. Determination of the Dark Toxicity
3.8.2. Photodynamic Inactivation of Microbial Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Youf, R.; Müller, M.; Balasini, A.; Thétiot, F.; Müller, M.; Hascoët, A.; Jonas, U.; Schönherr, H.; Lemercier, G.; Montier, T.; et al. Antimicrobial Photodynamic Therapy: Latest Developments with a Focus on Combinatory Strategies. Pharmaceutics 2021, 13, 1995. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, Present and Future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Dlugaszewska, J.; Szczolko, W.; Koczorowski, T.; Skupin-Mrugalska, P.; Teubert, A.; Konopka, K.; Kucinska, M.; Murias, M.; Düzgüneş, N.; Mielcarek, J.; et al. Antimicrobial and Anticancer Photodynamic Activity of a Phthalocyanine Photosensitizer with N -Methyl Morpholiniumethoxy Substituents in Non-Peripheral Positions. J. Inorg. Biochem. 2017, 172, 67–79. [Google Scholar] [CrossRef] [PubMed]
- De Annunzio, S.R.; De Freitas, L.M.; Blanco, A.L.; Da Costa, M.M.; Carmona-Vargas, C.C.; De Oliveira, K.T.; Fontana, C.R. Susceptibility of Enterococcus Faecalis and Propionibacterium Acnes to Antimicrobial Photodynamic Therapy. J. Photochem. Photobiol. B: Biol. 2018, 178, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Downie, G.H.; Cuenca, R.; Hu, X.-H.; Childs, C.J.; Sibata, C.H. Photosensitizers in Clinical PDT. Photodiagnosis Photodyn. Ther. 2004, 1, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Cortese, D.A.; Edell, E.S.; Kinsey, J.H. Photodynamic Therapy for Early Stage Squamous Cell Carcinoma of the Lung. Mayo Clin. Proc. 1997, 72, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Plaetzer, K.; Krammer, B.; Berlanda, J.; Berr, F.; Kiesslich, T. Photophysics and Photochemistry of Photodynamic Therapy: Fundamental Aspects. Lasers Med. Sci. 2009, 24, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Maisch, T.; Baier, J.; Franz, B.; Maier, M.; Landthaler, M.; Szeimies, R.-M.; Bäumler, W. The Role of Singlet Oxygen and Oxygen Concentration in Photodynamic Inactivation of Bacteria. Proc. Natl. Acad. Sci. USA 2007, 104, 7223–7228. [Google Scholar] [CrossRef] [PubMed]
- Wiehe, A.; O’Brien, J.M.; Senge, M.O. Trends and Targets in Antiviral Phototherapy. Photochem. Photobiol. Sci. 2019, 18, 2565–2612. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, J.; Rahban, D.; Aghamiri, S.; Teymouri, A.; Bahador, A. Photosensitizers in Antibacterial Photodynamic Therapy: An Overview. Laser Ther. 2018, 27, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Jarvi, M.T.; Niedre, M.J.; Patterson, M.S.; Wilson, B.C. Singlet Oxygen Luminescence Dosimetry (SOLD) for Photodynamic Therapy: Current Status, Challenges and Future Prospects. Photochem. Photobiol. 2006, 82, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Baier, J. Time Dependence of Singlet Oxygen Luminescence Provides an Indication of Oxygen Concentration during Oxygen Consumption. J. Biomed. Opt. 2007, 12, 064008. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qin, R.; Zaat, A.J.S.; Breukink, E.; Heger, M. Antibacterial Photodynamic Therapy: Overview of a Promising Approach to Fight Antibiotic-Resistant Bacterial Infections. J. Clin. Transl. Res. 2015, 1, 140–167. [Google Scholar] [CrossRef] [PubMed]
- Law, S.K.; Leung, A.W.N.; Xu, C. Photodynamic Action of Curcumin and Methylene Blue against Bacteria and SARS-CoV-2—A Review. Pharmaceuticals 2023, 17, 34. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, I.D.P.; Pinto, J.G.; Souza, B.M.N.; Miñán, A.G.; Ferreira-Strixino, J. Antimicrobial Photodynamic Therapy with Curcumin on Methicillin-Resistant Staphylococcus Aureus Biofilm. Photodiagnosis Photodyn. Ther. 2022, 37, 102729. [Google Scholar] [CrossRef] [PubMed]
- Gourlot, C.; Gosset, A.; Glattard, E.; Aisenbrey, C.; Rangasamy, S.; Rabineau, M.; Ouk, T.-S.; Sol, V.; Lavalle, P.; Gourlaouen, C.; et al. Antibacterial Photodynamic Therapy in the Near-Infrared Region with a Targeting Antimicrobial Peptide Connected to a π-Extended Porphyrin. ACS Infect. Dis. 2022, 8, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial Photodynamic Therapy—What We Know and What We Don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [PubMed]
- Kolarikova, M.; Hosikova, B.; Dilenko, H.; Barton-Tomankova, K.; Valkova, L.; Bajgar, R.; Malina, L.; Kolarova, H. Photodynamic Therapy: Innovative Approaches for Antibacterial and Anticancer Treatments. Med. Res. Rev. 2023, 43, 717–774. [Google Scholar] [CrossRef] [PubMed]
- Mlynarczyk, D.T.; Dlugaszewska, J.; Falkowski, M.; Popenda, L.; Kryjewski, M.; Szczolko, W.; Jurga, S.; Mielcarek, J.; Goslinski, T. Tribenzoporphyrazines with Dendrimeric Peripheral Substituents and Their Promising Photocytotoxic Activity against Staphylococcus Aureus. J. Photochem. Photobiol. B Biol. 2020, 204, 111803. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morgade, M.S.; Stuzhin, P.A. The Chemistry of Porphyrazines: An Overview. J. Porphyr. Phthalocyanines 2004, 08, 1129–1165. [Google Scholar] [CrossRef]
- Goslinski, T.; Zhong, C.; Fuchter, M.J.; White, A.J.P.; Barrett, A.G.M.; Hoffman, B.M. Serendipitous Synthesis of Trimetallic Porphyrazine Triads. Tetrahedron Lett. 2009, 50, 5178–5181. [Google Scholar] [CrossRef]
- Sobotta, L.; Dlugaszewska, J.; Ziental, D.; Szczolko, W.; Koczorowski, T.; Goslinski, T.; Mielcarek, J. Optical Properties of a Series of Pyrrolyl-Substituted Porphyrazines and Their Photoinactivation Potential against Enterococcus Faecalis after Incorporation into Liposomes. J. Photochem. Photobiol. A Chem. 2019, 368, 104–109. [Google Scholar] [CrossRef]
- Tuncer, S.; Koca, A.; Gül, A.; Avcıata, U. Synthesis, Characterization, Electrochemistry and Spectroelectrochemistry of Novel Soluble Porphyrazines Bearing Unsaturated Functional Groups. Dye. Pigment. 2012, 92, 610–618. [Google Scholar] [CrossRef]
- Koczorowski, T.; Rębiś, T. The Influence of an Extended π Electron System on the Electrochemical Properties and Oxidizing Activity of a Series of Iron(III) Porphyrazines with Bulky Pyrrolyl Substituents. Molecules 2023, 28, 7214. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, E.; Mlynarczyk, D.T.; Kucinska, M.; Dlugaszewska, J.; Piskorz, J.; Popenda, L.; Szczolko, W.; Jurga, S.; Murias, M.; Mielcarek, J.; et al. Photophysical Properties and Photocytotoxicity of Free and Liposome-Entrapped Diazepinoporphyrazines on LNCaP Cells under Normoxic and Hypoxic Conditions. Eur. J. Med. Chem. 2018, 150, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Skupin-Mrugalska, P.; Piskorz, J.; Goslinski, T.; Mielcarek, J.; Konopka, K.; Düzgüneş, N. Current Status of Liposomal Porphyrinoid Photosensitizers. Drug Discov. Today 2013, 18, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Koczorowski, T.; Szczolko, W.; Burda, K.; Nowak, M.; Dawidowska, M.; Teubert, A.; Sobotta, L.; Gdaniec, M.; Korecki, J.; Mielcarek, J.; et al. Influence of Bulky Pyrrolyl Substitent on the Physicochemical Properties of Porphyrazines. Dye. Pigment. 2015, 112, 138–144. [Google Scholar] [CrossRef]
- Koczorowski, T.; Ber, J.; Sokolnicki, T.; Teubert, A.; Szczolko, W.; Goslinski, T. Electrochemical and Catalytic Assessment of Peripheral Bromoaryl-Substituted Manganese and Iron Porphyrazines. Dye. Pigment. 2020, 178, 108370. [Google Scholar] [CrossRef]
- Kwon, S.; Lee, Y.; Jung, Y.; Kim, J.H.; Baek, B.; Lim, B.; Lee, J.; Kim, I.; Lee, J. Mitochondria-Targeting Indolizino[3,2-c]Quinolines as Novel Class of Photosensitizers for Photodynamic Anticancer Activity. Eur. J. Med. Chem. 2018, 148, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Goslinski, T.; Tykarska, E.; Szczolko, W.; Osmalek, T.; Smigielska, A.; Walorczyk, S.; Zong, H.; Gdaniec, M.; Hoffman, B.M.; Mielcarek, J.; et al. Synthesis and Characterization of Periphery-Functionalized Porphyrazines Containing Mixed Pyrrolyl and Pyridylmethylamino Groups. J. Porphyr. Phthalocyanines 2009, 13, 223–234. [Google Scholar] [CrossRef]
- Tillo, A.; Kryjewski, M.; Bendzińska-Berus, W.; Langer, D.; Rebis, T.; Popenda, L.; Jurga, S.; Mielcarek, J.; Goslinski, T.; Tykarska, E. Tetrapyrazinoporphyrazine with Eight Peripheral Adamantanylsulfanyl Units—Synthesis and Physicochemical Study. Synth. Met. 2018, 244, 66–72. [Google Scholar] [CrossRef]
- Schwartz, Z.; Theisen, P.; Bjornstal, O.; Rodebaugh, M.; Jemal, M.; Lee, D.; Shelton, S.; Zhao, Z.; Du, L.; Kerwin, S. Scalable Synthesis and Cancer Cell Cytotoxicity of Rooperol and Analogues. Molecules 2022, 27, 1792. [Google Scholar] [CrossRef] [PubMed]
- Szczolko, W.; Sobotta, L.; Fita, P.; Koczorowski, T.; Mikus, M.; Gdaniec, M.; Orzechowska, A.; Burda, K.; Sobiak, S.; Wierzchowski, M.; et al. Synthesis, Characteristics and Photochemical Studies of Novel Porphyrazines Possessing Peripheral 2,5-Dimethylpyrrol-1-Yl and Dimethylamino Groups. Tetrahedron Lett. 2012, 53, 2040–2044. [Google Scholar] [CrossRef]
- Szczolko, W.; Koczorowski, T.; Wicher, B.; Kryjewski, M.; Krakowska, Z.; Tykarska, E.; Goslinski, T. Porphyrazines with Bulky Peripheral Pyrrolyl Substituents—Synthesis via Microwave-Assisted Suzuki-Miyaura Cross-Coupling Reaction, Optical and Electrochemical Properties. Dye. Pigment. 2022, 206, 110607. [Google Scholar] [CrossRef]
- Szczolko, W.; Kryjewski, M.; Koczorowski, T.; Zuchowska, E.; Popenda, L.; Mlynarczyk, D.T. Pyrrole Dicarboxylate Substituted Porphyrazine, Microwave Assisted Synthesis and Properties. Sci. Rep. 2025, 15, 16668. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Brown, D.P.; Wang, Y.-J.; Chen, Z.-S. New Phenstatin–Fatty Acid Conjugates: Synthesis and Evaluation. Bioorganic Med. Chem. Lett. 2013, 23, 5119–5122. [Google Scholar] [CrossRef] [PubMed]
- Iida, N.; Tanaka, K.; Tokunaga, E.; Takahashi, H.; Shibata, N. Regioisomer-Free C4h β-Tetrakis(Tert-butyl)Metallo-phthalocyanines: Regioselective Synthesis and Spectral Investigations. ChemistryOpen 2015, 4, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Szczolko, W.; Wzgarda, A.; Koczorowski, T.; Wicher, B.; Sobotta, L.; Gdaniec, Z.; Gdaniec, M.; Mielcarek, J.; Tykarska, E.; Goslinski, T. The Suzuki Cross-Coupling Reaction for the Synthesis of Porphyrazine Possessing Bulky 2,5-(Biphenyl-4-Yl)Pyrrol-1-Yl Substituents in the Periphery. Polyhedron 2015, 102, 462–468. [Google Scholar] [CrossRef]
- Szczolko, W.; Koczorowski, T.; Bakun, P.; Kryjewski, M.; Grajewski, M.; Popenda, L.; Goslinski, T. Magnesium(II) Porphyrazine with Peripherally Overloaded Pyrrolyl Substituents—Synthesis, Optical and Electrochemical Characterization. J. Mol. Struct. 2024, 1318, 139356. [Google Scholar] [CrossRef]
- Koza, P.; Koczorowski, T.; Mlynarczyk, D.T.; Goslinski, T. Zinc(II) Sulfanyltribenzoporphyrazines with Bulky Peripheral Substituents—Synthesis, Photophysical Characterization, and Potential Photocytotoxicity. Appl. Sci. 2022, 12, 6825. [Google Scholar] [CrossRef]
- Johnson, B.T. Microtox® Acute Toxicity Test. In Small-Scale Freshwater Toxicity Investigations; Blaise, C., Férard, J.-F., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 69–105. ISBN 978-1-4020-3119-9. [Google Scholar]
- Siedlecka, A.; Wolf, M.; Trusz, A. Ecotoxicity of Liners Impregnated with Resisns—A Microtox Bioassay. Inż. Ekolog. 2018, 19, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Burillo, A.; Bouza, E. Community-Acquired Methicillin-Resistant Staphylococcus Aureus: Is It Still a Significant Pathogen for Skin and Soft Tissue Infections? A 30-Year Overview. Curr. Opin. Infect. Dis. 2025, 38, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Borg, M.A.; Camilleri, L. What Is Driving the Epidemiology of Methicillin-Resistant Staphylococcus aureus Infections in Europe? Microb. Drug Resist. 2021, 27, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable Deaths and Disability-Adjusted Life-Years Caused by Infections with Antibiotic-Resistant Bacteria in the EU and the European Economic Area in 2015: A Population-Level Modelling Analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Bakun, P.; Wysocki, M.; Stachowiak, M.; Musielak, M.; Dlugaszewska, J.; Mlynarczyk, D.T.; Sobotta, L.; Suchorska, W.M.; Goslinski, T. Quaternized Curcumin Derivative—Synthesis, Physicochemical Characteristics, and Photocytotoxicity, Including Antibacterial Activity after Irradiation with Blue Light. Molecules 2024, 29, 4536. [Google Scholar] [CrossRef] [PubMed]
- Scherer, K.M.; Bisby, R.H.; Botchway, S.W.; Parker, A.W. New Approaches to Photodynamic Therapy from Types I, II and III to Type IV Using One or More Photons. Anti-Cancer Agents Med. Chem. 2017, 17, 171–189. [Google Scholar] [CrossRef] [PubMed]
- Batishchev, O.V.; Kalutskii, M.A.; Varlamova, E.A.; Konstantinova, A.N.; Makrinsky, K.I.; Ermakov, Y.A.; Meshkov, I.N.; Sokolov, V.S.; Gorbunova, Y.G. Antimicrobial Activity of Photosensitizers: Arrangement in Bacterial Membrane Matters. Front. Mol. Biosci. 2023, 10, 1192794. [Google Scholar] [CrossRef] [PubMed]
- Patiny, L.; Borel, A. ChemCalc: A Building Block for Tomorrow’s Chemical Infrastructure. J. Chem. Inf. Model. 2013, 53, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef] [PubMed]
- Ogunsipe, A.; Maree, D.; Nyokong, T. Solvent Effects on the Photochemical and Fluorescence Properties of Zinc Phthalocyanine Derivatives. J. Mol. Struct. 2003, 650, 131–140. [Google Scholar] [CrossRef]
- Sobotta, L.; Fita, P.; Szczolko, W.; Wrotynski, M.; Wierzchowski, M.; Goslinski, T.; Mielcarek, J. Functional Singlet Oxygen Generators Based on Porphyrazines with Peripheral 2,5-Dimethylpyrrol-1-Yl and Dimethylamino Groups. J. Photochem. Photobiol. A Chem. 2013, 269, 9–16. [Google Scholar] [CrossRef]








| Comp. | Red(Pz−1/Pz) | Oxd1(Pz/Pz+1) | Oxd2(Pz+1/Pz+2) | Oxd3(Pz+2/Pz+3) |
|---|---|---|---|---|
| 6 | −1.96 | −0.32 | 0.17 | 0.34 |
| 7 | −1.94 | −0.19 | 0.03 | 0.70 |
| Compound | Pz 6 | Pz 7 | ||
|---|---|---|---|---|
| Solvent | DMF | DMSO | DMF | DMSO |
| ΦΔ | 0.05 | 0.06 | 0.02 | 0.02 |
| ΦF | 0.004 | 0.002 | <0.001 | <0.001 |
| absorption maxima λmax [nm], (molar absorption coefficients—log ε [M−1·cm−1]) | 725 (4.75); 351 (4.89) | 731 (4.65); 351 (4.81) | 718 (4.10); 350 (4.39) | 723 (4.11); 350 (4.41) |
| emission maximum λF [nm] | 742 | 751 | 744 | 750 |
| Stokes shift [cm−1] | 316 | 364 | 487 | 498 |
| Compound | Pz 6 | Pz 7 | Light Fluence [J/cm2] | |
|---|---|---|---|---|
| Conditions | Irradiation Time | Log Reduction in Bacterial Growth | ||
| Light | 60 min | −0.01 ± 0.09 | >5.68 ± 0.05 | 10.8 |
| 90 min | 0.01 ± 0.05 | >5.66 ± 0.05 | 16.2 | |
| Dark | 60 min | −0.01 ± 0.07 | 0.05 ± 0.03 | 10.8 |
| 90 min | 0.07 ± 0.04 | 0.09 ± 0.02 | 16.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczolko, W.; Zuchowska, E.; Koczorowski, T.; Kryjewski, M.; Dlugaszewska, J.; Mlynarczyk, D.T. Novel Tetraphenolic Porphyrazine Capable of MRSA Photoeradication. Molecules 2025, 30, 3069. https://doi.org/10.3390/molecules30153069
Szczolko W, Zuchowska E, Koczorowski T, Kryjewski M, Dlugaszewska J, Mlynarczyk DT. Novel Tetraphenolic Porphyrazine Capable of MRSA Photoeradication. Molecules. 2025; 30(15):3069. https://doi.org/10.3390/molecules30153069
Chicago/Turabian StyleSzczolko, Wojciech, Eunice Zuchowska, Tomasz Koczorowski, Michal Kryjewski, Jolanta Dlugaszewska, and Dariusz T. Mlynarczyk. 2025. "Novel Tetraphenolic Porphyrazine Capable of MRSA Photoeradication" Molecules 30, no. 15: 3069. https://doi.org/10.3390/molecules30153069
APA StyleSzczolko, W., Zuchowska, E., Koczorowski, T., Kryjewski, M., Dlugaszewska, J., & Mlynarczyk, D. T. (2025). Novel Tetraphenolic Porphyrazine Capable of MRSA Photoeradication. Molecules, 30(15), 3069. https://doi.org/10.3390/molecules30153069







