Modulatory Effect of Curcumin on Expression of Methyltransferase/Demethylase in Colon Cancer Cells: Impact on wt p53, mutp53 and c-Myc
Abstract
1. Introduction
2. Results
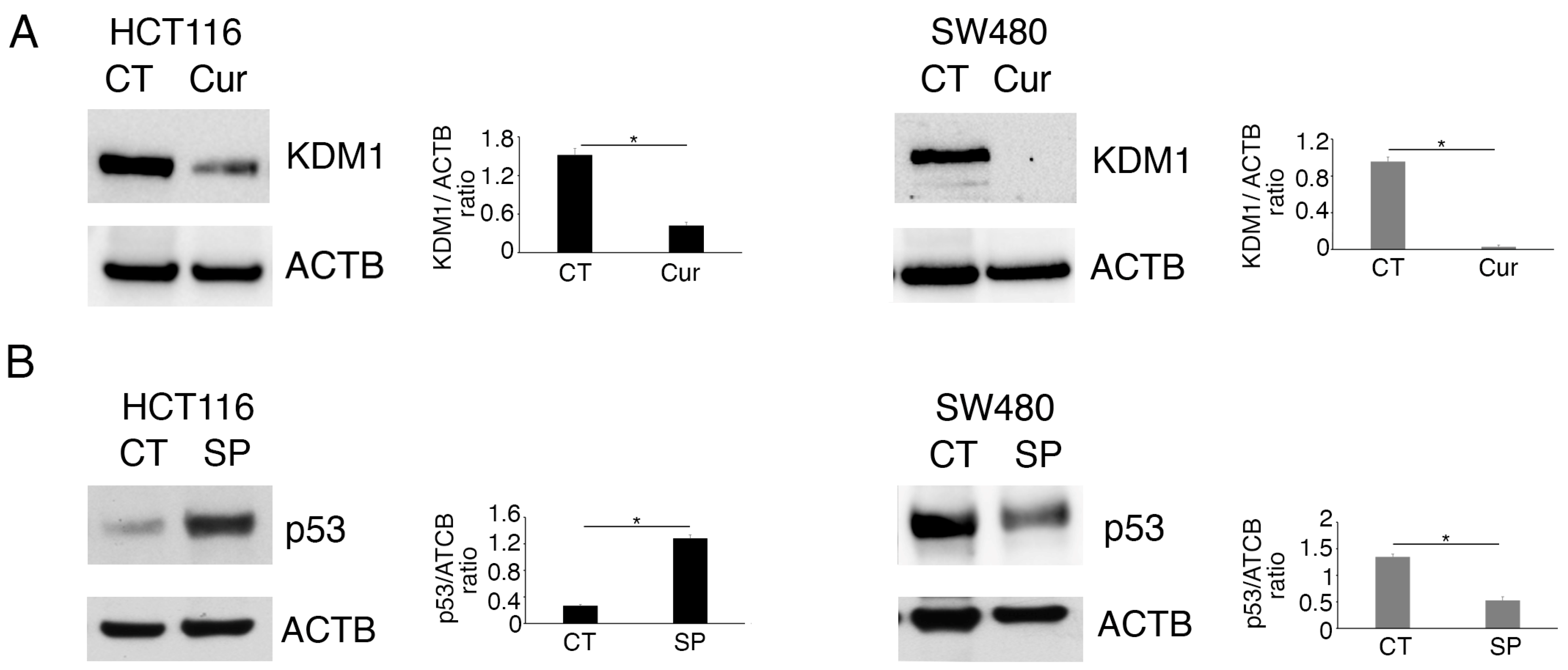
2.1. Methyltransferase/Demethylase Modulation Correlates with wtp53 Stabilization and mutp53 Downregulation in Colon Cancer Cells Treated by Curcumin
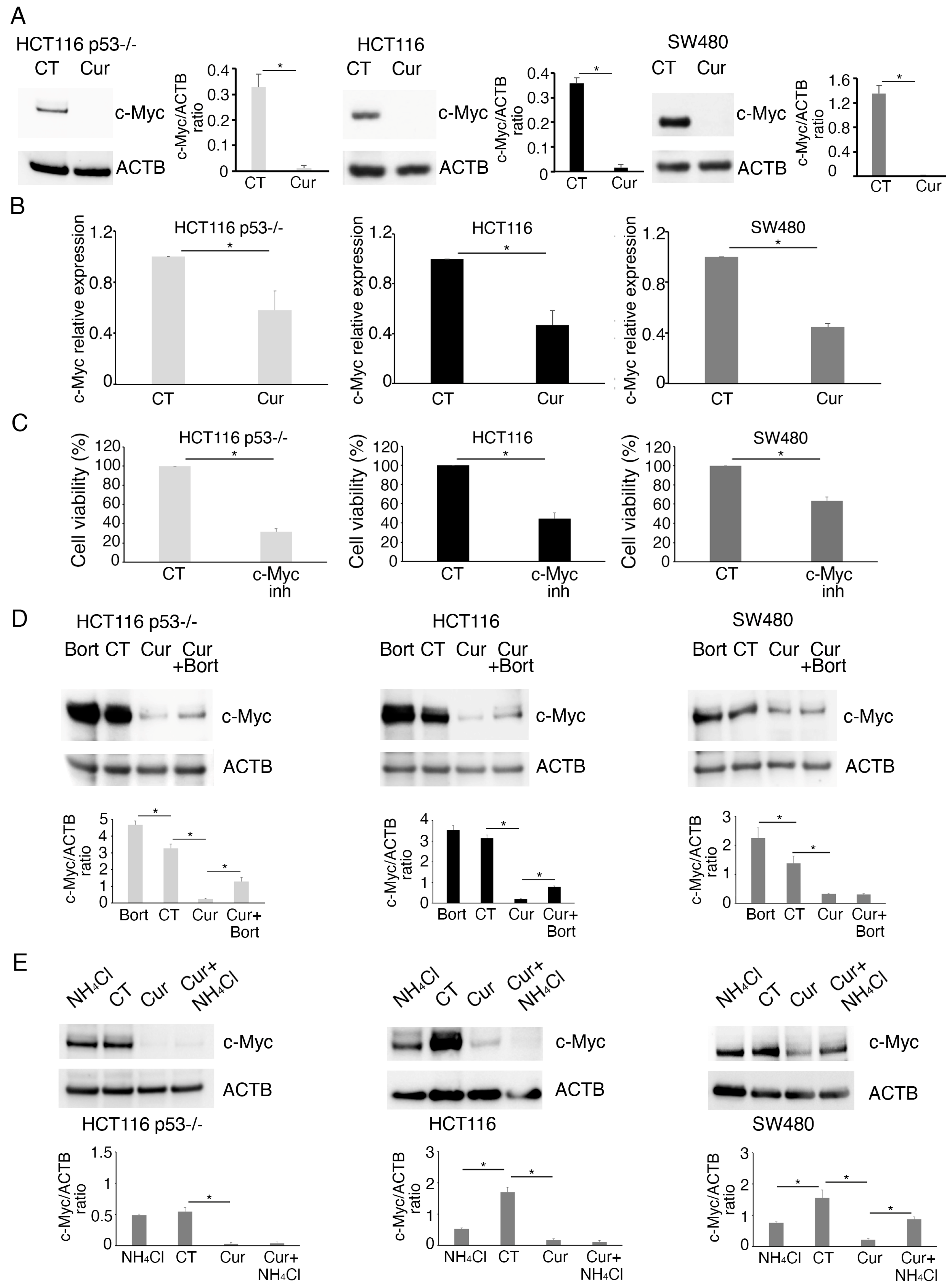
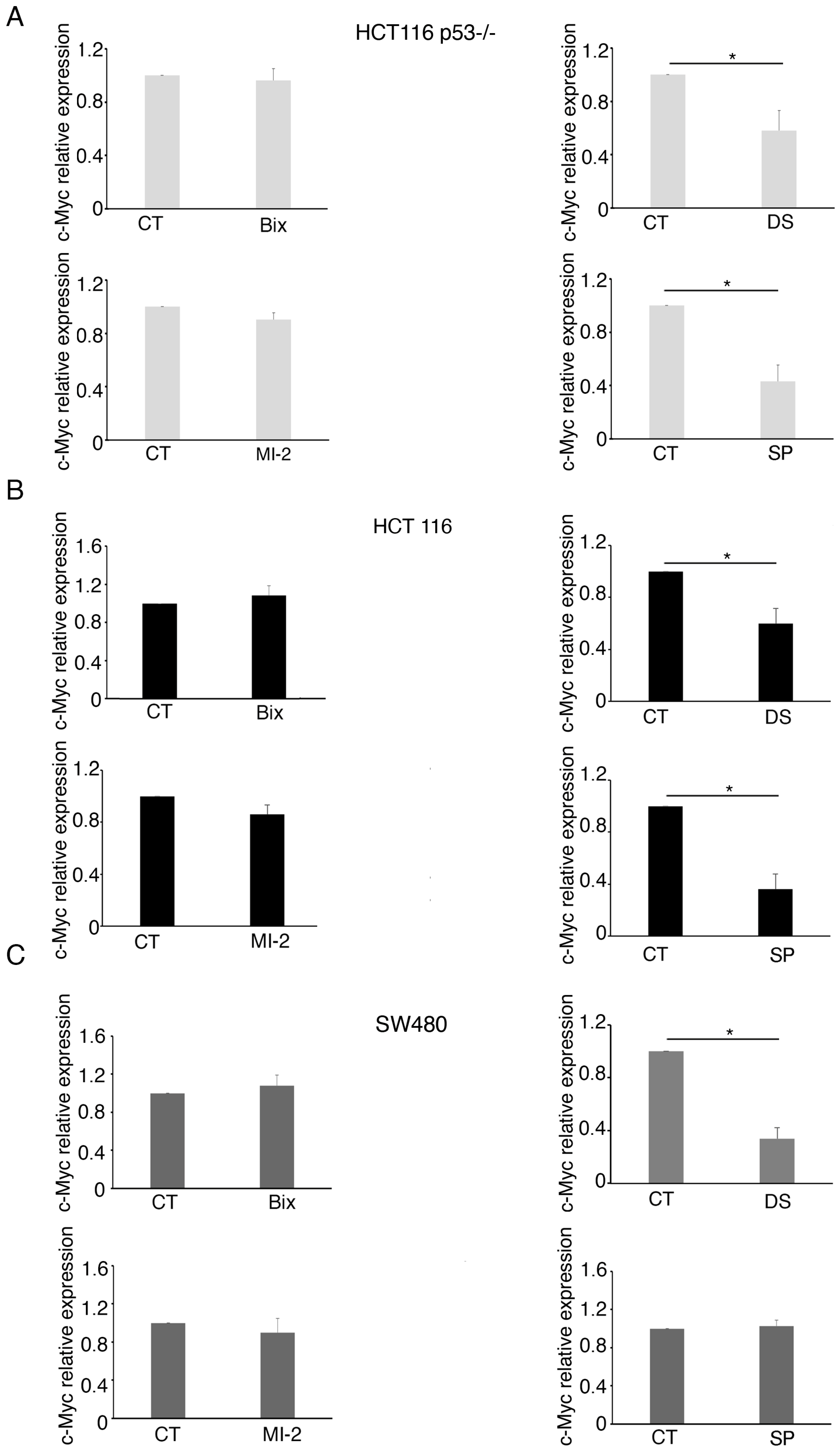
2.2. Curcumin Reduces the Expression of c-Myc in mutp53, wtp53, and p53-/- Colon Cancer Cells
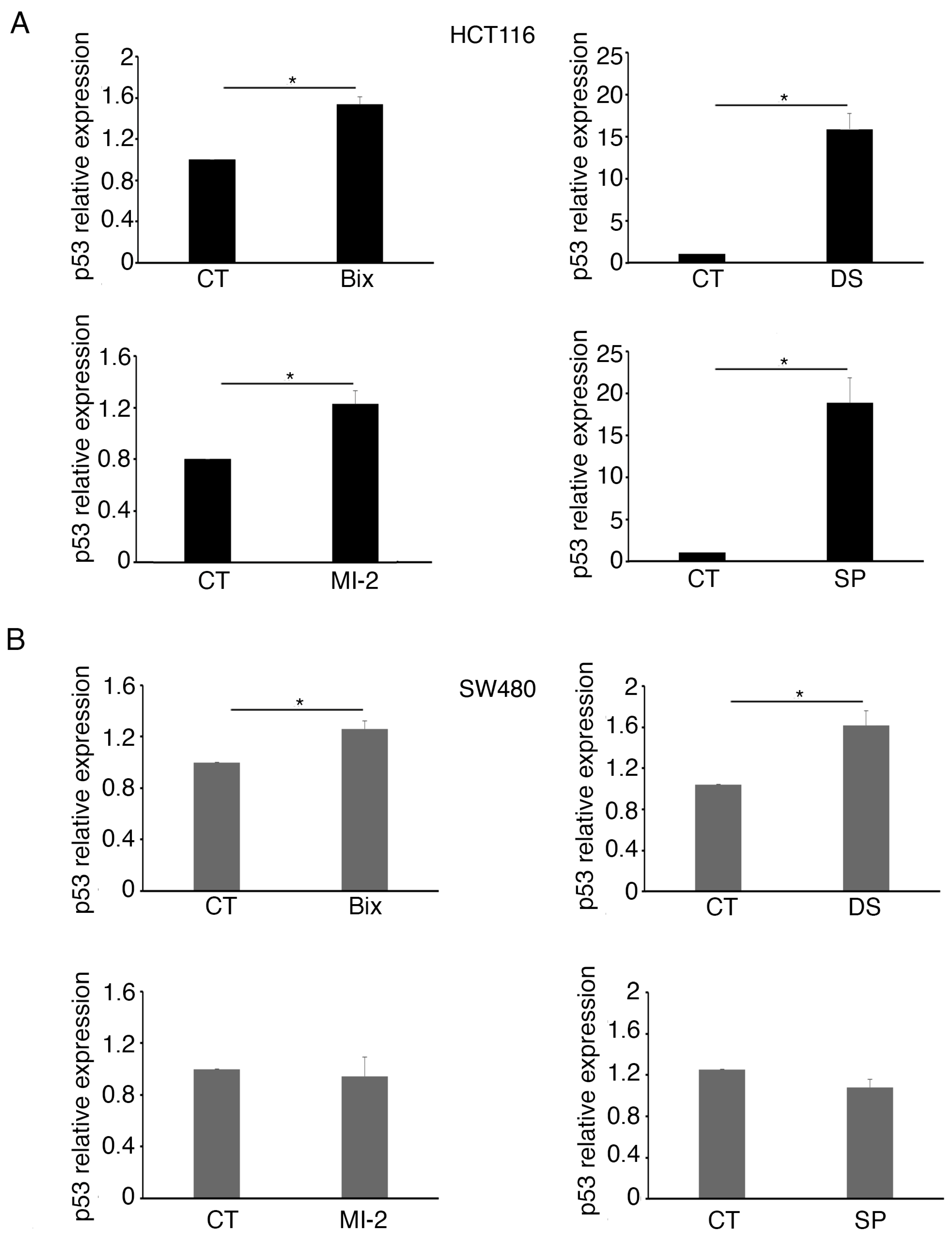
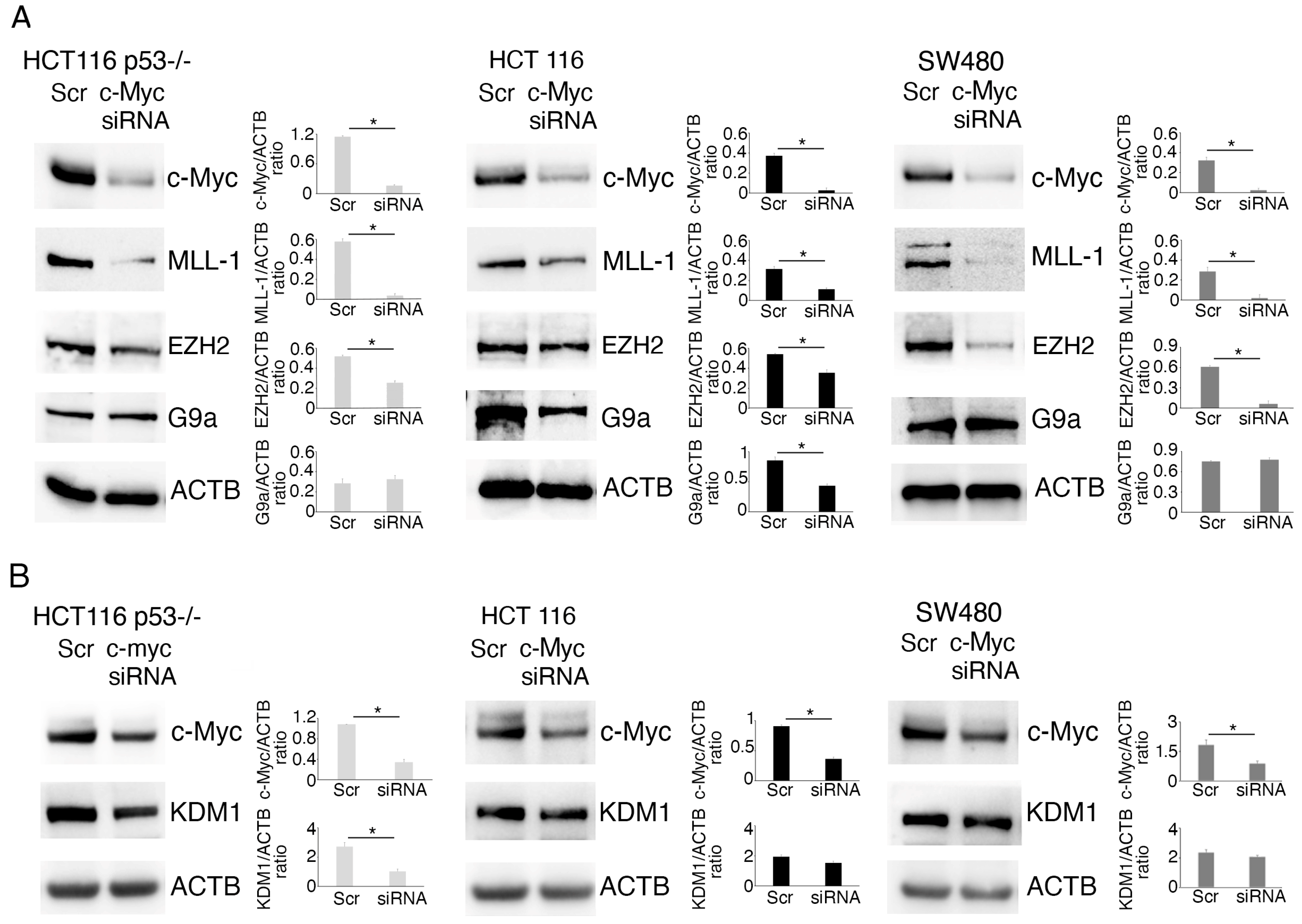
2.3. MLL1 and KDM1 Sustain c-Myc Expression in Colon Cancer Cells
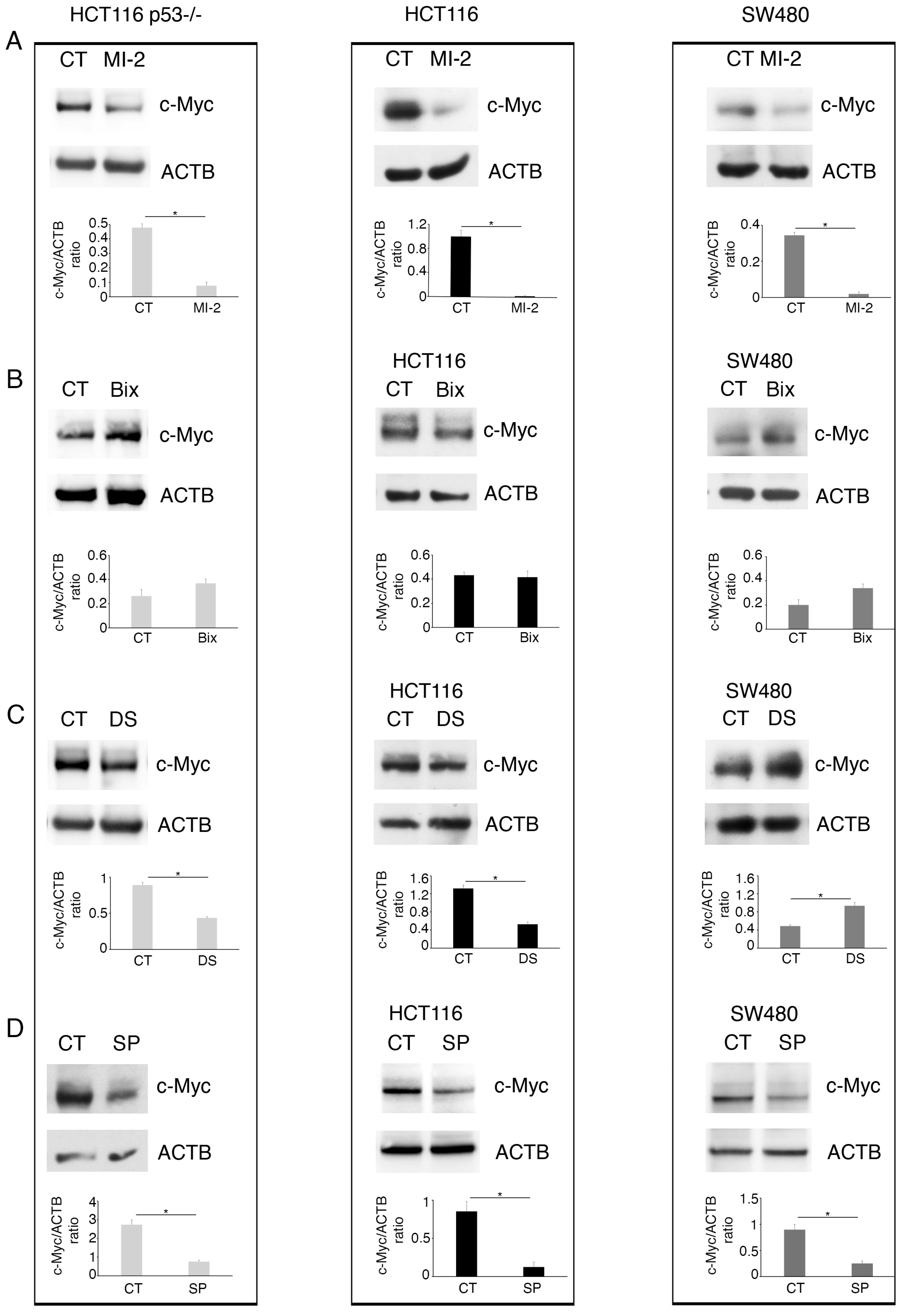
2.4. c-Myc Alters Methyltransferase/Demethylase Expression in Colon Cancer Cells
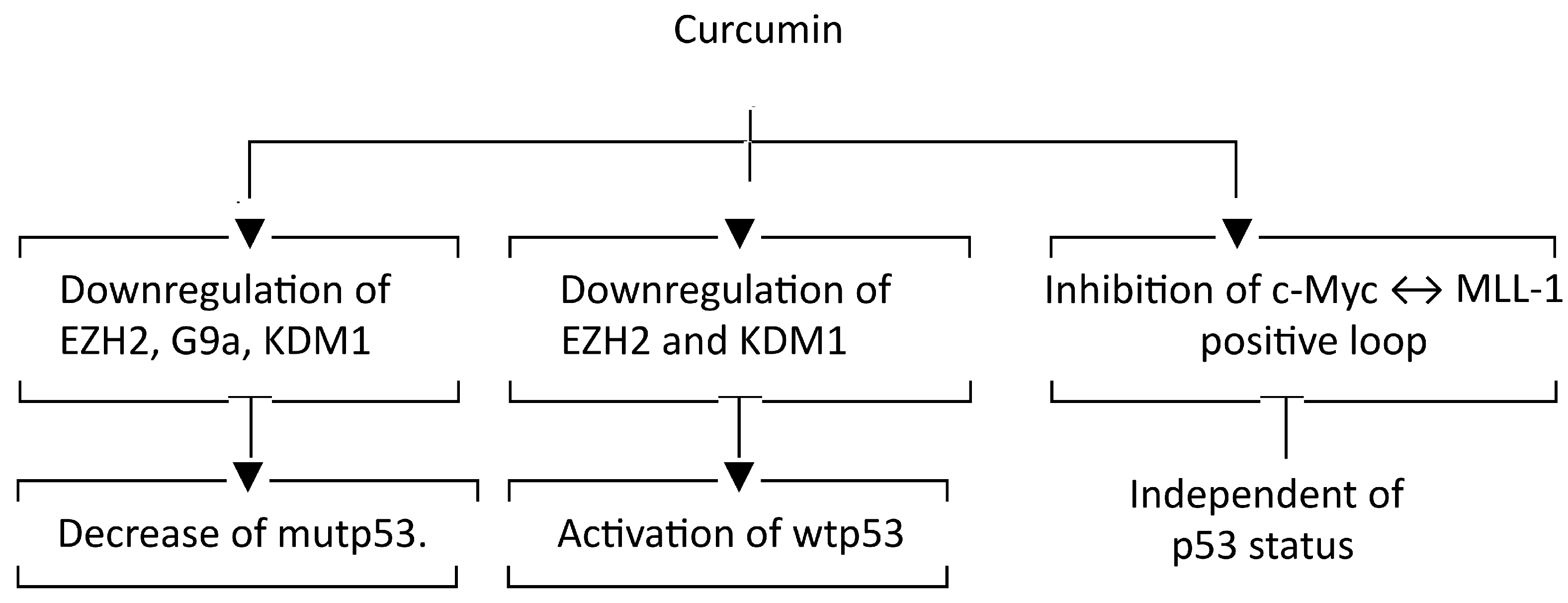
3. Discussion
4. Materials and Methods
4.1. Cell Cultures, Reagents, and Treatments
- -
- Curcumin (Cur), (C1386, Sigma Aldrich, St. Louis, MO, USA), 25 µM;
- -
- G9a inhibitor Bix-01294 (Bix), (HY-10587, MedChemExpress Monmouth Junction, NJ 08852, USA), 3 µM;
- -
- EZH2 inhibitor DS-3201 (DS, Valemetostat, Selleckchem, Cologne, Germany),
- -
- 5 µM;
- -
- Menin inhibitor MI-2 (HY-15222, MedChemExpress Monmouth Junction, NJ 08852, USA), 2.5 µM;
- -
- KDM1 inhibitor SP2503 (SP) (HY-12635, MedChemExpress Monmouth Junction, NJ 08852, USA), 1.5 µM;
- -
- c-Myc inhibitor, (475956, Sigma Aldrich, St. Louis, MO, USA), 65 µM.
4.2. c-Myc Silencing
4.3. Cell Viability
4.4. Western Blotting
4.5. Antibodies
4.6. Densitometric Analysis
4.7. RNA Extraction, Reverse Transcription, and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MSI-H | microsatellite instability-high |
| EZH2 | enhancer of Zeste 2 Polycomb repressive complex 2 Subunit |
| MLL-1 | mixed lineage leukemia protein-1 |
| KMD1 | Lysine-Specific Histone Demethylase 1 (KMD1/LSD1) |
| KMTs | Histone Lysine Methyltransferases |
References
- Islam, M.R.; Rauf, A.; Akash, S.; Trisha, S.I.; Nasim, A.H.; Akter, M.; Dhar, P.S.; Ogaly, H.A.; Hemeg, H.A.; Wilairatana, P.; et al. Targeted therapies of curcumin focus on its therapeutic benefits in cancers and human health: Molecular signaling pathway-based approaches and future perspectives. Biomed. Pharmacother. 2024, 170, 116034. [Google Scholar] [CrossRef] [PubMed]
- Ameer, S.F.; Mohamed, M.Y.; Elzubair, Q.A.; Sharif, E.A.M.; Ibrahim, W.N. Curcumin as a novel therapeutic candidate for cancer: Can this natural compound revolutionize cancer treatment? Front. Oncol. 2024, 14, 1438040. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.U.; Rehman, M.S.; Khan, M.S.; Ali, M.A.; Javed, A.; Nawaz, A.; Yang, C. Curcumin as an Alternative Epigenetic Modulator: Mechanism of Action and Potential Effects. Front. Genet. 2019, 10, 514. [Google Scholar] [CrossRef] [PubMed]
- Tahghighi, A.; Seyedhashemi, E.; Mohammadi, J.; Moradi, A.; Esmaeili, A.; Pornour, M.; Jafarifar, K.; Ganji, S.M. Epigenetic marvels: Exploring the landscape of colorectal cancer treatment through cutting-edge epigenetic-based drug strategies. Clin. Epigenet. 2025, 17, 34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Guo, Y.; Qiu, B.; Dai, X.; Wang, Y.; Cao, X. Global, regional, and national trends in colorectal cancer burden from 1990 to 2021 and projections to 2040. Front. Oncol. 2024, 14, 1466159. [Google Scholar] [CrossRef] [PubMed]
- Grady, W.M.; Markowitz, S.D. Genetic and epigenetic alterations in colon cancer. Annu. Rev. Genom. Hum. Genet. 2002, 3, 101–128. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.; Hernandez-Illan, E.; Moreira, L.; Balaguer, F.; Goel, A. Epigenetics of colorectal cancer: Biomarker and therapeutic potential. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 111–130. [Google Scholar] [CrossRef] [PubMed]
- Quan, S.; Huang, H. Epigenetic contribution to cancer. Int. Rev. Cell Mol. Biol. 2024, 387, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Tian, Y.; Deng, Z.; Yang, F.; Chen, E. Epigenetic Alteration in Colorectal Cancer: Potential Diagnostic and Prognostic Implications. Int. J. Mol. Sci. 2024, 25, 3358. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, R.; Curra, P.; Di Crosta, M.; Gonnella, R.; Gilardini Montani, M.S.; Cirone, M. Changes in Lysine Methylation Contribute to the Cytotoxicity of Curcumin in Colon Cancer Cells. Molecules 2025, 30, 335. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Sengupta, R.; Espejo, A.B.; Lee, M.G.; Dorsey, J.A.; Richter, M.; Opravil, S.; Shiekhattar, R.; Bedford, M.T.; Jenuwein, T.; et al. p53 is regulated by the lysine demethylase LSD1. Nature 2007, 449, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Saeed, H.; Leibowitz, B.J.; Zhang, L.; Yu, J. Targeting Myc-driven stress addiction in colorectal cancer. Drug Resist. Updates 2023, 69, 100963. [Google Scholar] [CrossRef] [PubMed]
- Modlhammer, A.; Pfurtscheller, S.; Feichtner, A.; Hartl, M.; Schneider, R. The Diarylheptanoid Curcumin Induces MYC Inhibition and Cross-Links This Oncoprotein to the Coactivator TRRAP. Front. Oncol. 2021, 11, 660481. [Google Scholar] [CrossRef] [PubMed]
- Granato, M.; Rizzello, C.; Romeo, M.A.; Yadav, S.; Santarelli, R.; D’Orazi, G.; Faggioni, A.; Cirone, M. Concomitant reduction of c-Myc expression and PI3K/AKT/mTOR signaling by quercetin induces a strong cytotoxic effect against Burkitt’s lymphoma. Int. J. Biochem. Cell Biol. 2016, 79, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Moya Ramírez, M.A.; Sánchez Martín, V.; Herrera Merchán, A.; Medina Vico, P.; Cuadros Celorrio, M. Choosing the right cell line for rectal cancer research. Arch. Med. Univ. 2015, 3, 17–20. [Google Scholar]
- Sha, L.; Ayoub, A.; Cho, U.S.; Dou, Y. Insights on the regulation of the MLL/SET1 family histone methyltransferases. Biochim. Biophys. Acta Gene Regul. Mech. 2020, 1863, 194561. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Yang, J.; Ge, S.; Chai, P.; Fan, J.; Jia, R. Novel insights into histone lysine methyltransferases in cancer therapy: From epigenetic regulation to selective drugs. J. Pharm. Anal. 2023, 13, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zhang, S.; Liu, H.M.; Zhang, Y.B.; Blair, C.A.; Mercola, D.; Sassone-Corsi, P.; Zi, X. Histone lysine-specific methyltransferases and demethylases in carcinogenesis: New targets for cancer therapy and prevention. Curr. Cancer Drug Targets 2013, 13, 558–579. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Karuniawati, H.; Jairoun, A.A.; Urbi, Z.; Ooi, J.; John, A.; Lim, Y.C.; Kibria, K.M.K.; Mohiuddin, A.K.M.; Ming, L.C.; et al. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers 2022, 14, 1732. [Google Scholar] [CrossRef] [PubMed]
- Kuismanen, S.A.; Holmberg, M.T.; Salovaara, R.; de la Chapelle, A.; Peltomaki, P. Genetic and epigenetic modification of MLH1 accounts for a major share of microsatellite-unstable colorectal cancers. Am. J. Pathol. 2000, 156, 1773–1779. [Google Scholar] [CrossRef] [PubMed]
- Kuser-Abali, G.; Gong, L.; Yan, J.; Liu, Q.; Zeng, W.; Williamson, A.; Lim, C.B.; Molloy, M.E.; Little, J.B.; Huang, L.; et al. An EZH2-mediated epigenetic mechanism behind p53-dependent tissue sensitivity to DNA damage. Proc. Natl. Acad. Sci. USA 2018, 115, 3452–3457. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ding, L.; Wang, D.; Ye, Z.; He, Y.; Ma, L.; Zhu, R.; Pan, Y.; Wu, Q.; Pang, K.; et al. EZH2 cooperates with gain-of-function p53 mutants to promote cancer growth and metastasis. EMBO J. 2019, 38, e99599. [Google Scholar] [CrossRef] [PubMed]
- Gonnella, R.; Collura, F.; Corrado, V.; Di Crosta, M.; Santarelli, R.; Cirone, M. EZH2 Inhibition by DS3201 Triggers the Kaposi’s Sarcoma-Associated Herpesvirus Lytic Cycle and Potentiates the Effects Induced by SAHA in Primary Effusion Lymphoma Cells. Viruses 2024, 16, 1490. [Google Scholar] [CrossRef] [PubMed]
- Rada, M.; Vasileva, E.; Lezina, L.; Marouco, D.; Antonov, A.V.; Macip, S.; Melino, G.; Barlev, N.A. Human EHMT2/G9a activates p53 through methylation-independent mechanism. Oncogene 2017, 36, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Sareddy, G.R.; Nair, B.C.; Krishnan, S.K.; Gonugunta, V.K.; Zhang, Q.G.; Suzuki, T.; Miyata, N.; Brenner, A.J.; Brann, D.W.; Vadlamudi, R.K. KDM1 is a novel therapeutic target for the treatment of gliomas. Oncotarget 2013, 4, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Yuan, M.; Shen, S.; Ma, X.; Fang, J.; Zhu, L.; Sun, L.; Liu, Z.; He, X.; Huang, D.; et al. Menin enhances c-Myc-mediated transcription to promote cancer progression. Nat. Commun. 2017, 8, 15278. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shah, K.; Busby, T.; Giles, K.; Khodadadi-Jamayran, A.; Li, W.; Jiang, H. Hijacking a key chromatin modulator creates epigenetic vulnerability for MYC-driven cancer. J. Clin. Investig. 2018, 128, 3605–3618. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santarelli, R.; Di Dio, C.; Di Crosta, M.; Currà, P.; Gonnella, R.; Cirone, M. Modulatory Effect of Curcumin on Expression of Methyltransferase/Demethylase in Colon Cancer Cells: Impact on wt p53, mutp53 and c-Myc. Molecules 2025, 30, 3054. https://doi.org/10.3390/molecules30153054
Santarelli R, Di Dio C, Di Crosta M, Currà P, Gonnella R, Cirone M. Modulatory Effect of Curcumin on Expression of Methyltransferase/Demethylase in Colon Cancer Cells: Impact on wt p53, mutp53 and c-Myc. Molecules. 2025; 30(15):3054. https://doi.org/10.3390/molecules30153054
Chicago/Turabian StyleSantarelli, Roberta, Claudia Di Dio, Michele Di Crosta, Paola Currà, Roberta Gonnella, and Mara Cirone. 2025. "Modulatory Effect of Curcumin on Expression of Methyltransferase/Demethylase in Colon Cancer Cells: Impact on wt p53, mutp53 and c-Myc" Molecules 30, no. 15: 3054. https://doi.org/10.3390/molecules30153054
APA StyleSantarelli, R., Di Dio, C., Di Crosta, M., Currà, P., Gonnella, R., & Cirone, M. (2025). Modulatory Effect of Curcumin on Expression of Methyltransferase/Demethylase in Colon Cancer Cells: Impact on wt p53, mutp53 and c-Myc. Molecules, 30(15), 3054. https://doi.org/10.3390/molecules30153054





