Cannabidiol as Modulator of Spontaneous Adipogenesis in Human Adipose-Derived Stem Cells
Abstract
1. Introduction
2. Results
2.1. Characterization of hASCs
2.2. Effects of Cannabidiol on hASC Number and Viability
2.3. Effects of Cannabidiol on hASC Proliferation and Metabolism
2.4. Effects of Cannabidiol on Spontaneous Adipogenic Commitment in hASCs
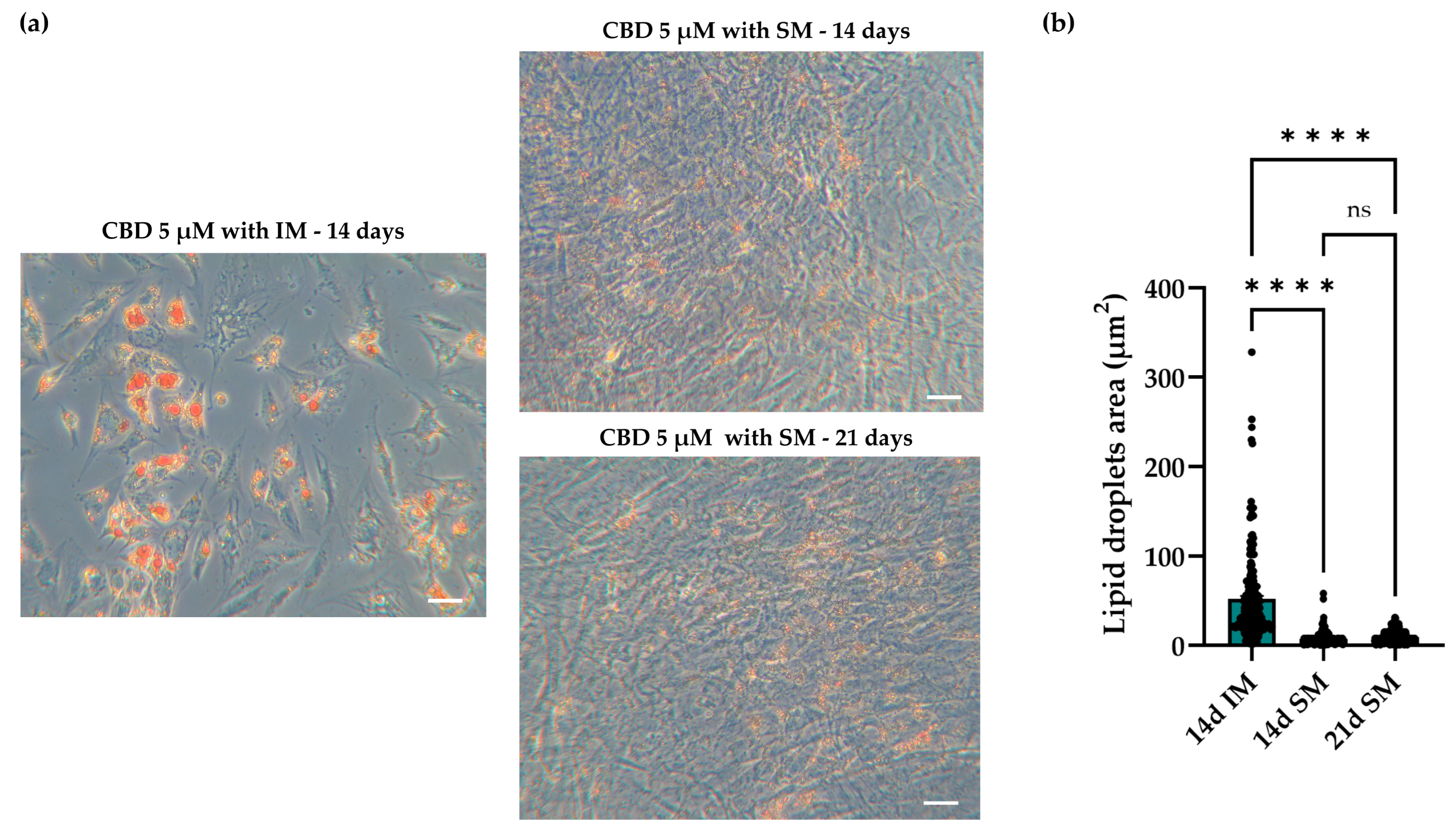
2.4.1. Oil Red O (O.R.O) Staining and Lipid Droplet Analysis
2.4.2. Gene Expression Analysis of Adipogenic and Osteogenic Markers
2.4.3. Spontaneous and Induced Adipogenic Commitment: Morphology and Dimension of Lipid Droplets in hASCs
3. Discussion
4. Materials and Methods
4.1. Harvesting and Culturing of hASCs
4.2. hASC Characterization
4.3. Cannabidiol Treatments
4.4. Cell Count and Viability
4.5. Annexin V Apoptosis Detection Assay
4.6. Bromodeoxyuridine (BrdU) Assay
4.7. Resazurin-Based Assay
4.8. O.R.O Staining and Analysis of Lipid Droplet Size
4.9. Staining and Quantification of Lipid Droplets and Actin
4.10. Gene Expression Analysis
4.10.1. RNA Extraction and RT-PCR
4.10.2. Real-Time PCR
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahla, R.S. Stem Cells Applications in Regenerative Medicine and Disease Therapeutics. Int. J. Cell Biol. 2016, 2016, 6940283. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.; Domingues, A.; Ratajczak, J.; Ratajczak, M.Z. Potential Clinical Applications of Stem Cells in Regenerative Medicine. Adv. Exp. Med. Biol. 2019, 1201, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Konno, M.; Hamabe, A.; Hasegawa, S.; Ogawa, H.; Fukusumi, T.; Nishikawa, S.; Ohta, K.; Kano, Y.; Ozaki, M.; Noguchi, Y.; et al. Adipose-derived mesenchymal stem cells and regenerative medicine. Dev. Growth Differ. 2013, 55, 309–318. [Google Scholar] [CrossRef]
- Câmara, D.A.D.; Shibli, J.A.; Müller, E.A.; De-Sá-Junior, P.L.; Porcacchia, A.S.; Blay, A.; Lizier, N.F. Adipose Tissue-Derived Stem Cells: The Biologic Basis and Future Directions for Tissue Engineering. Materials 2020, 13, 3210. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Gerini, G.; Megiorni, F.; Pontecorvi, P.; Messina, E.; Camero, S.; Anastasiadou, E.; Romano, E.; Onesti, M.G.; Napoli, C.; et al. Inhibiting DNA methylation as a strategy to enhance adipose-derived stem cells differentiation: Focus on the role of Akt/mTOR and Wnt/β-catenin pathways on adipogenesis. Front. Cell Dev. Biol. 2022, 10, 926180. [Google Scholar] [CrossRef]
- Ambele, M.A.; Dhanraj, P.; Giles, R.; Pepper, M.S. Adipogenesis: A Complex Interplay of Multiple Molecular Determinants and Pathways. Int. J. Mol. Sci. 2020, 21, 4283. [Google Scholar] [CrossRef]
- Roxburgh, J.; Metcalfe, A.D.; Martin, Y.H. The effect of medium selection on adipose-derived stem cell expansion and differentiation: Implications for application in regenerative medicine. Cytotechnology 2016, 68, 957–967. [Google Scholar] [CrossRef]
- Shafaei, H.; Kalarestaghi, H. Adipose-derived stem cells: An appropriate selection for osteogenic differentiation. J. Cell. Physiol. 2020, 235, 8371–8386. [Google Scholar] [CrossRef]
- Ujváry, I.; Hanuš, L. Human Metabolites of Cannabidiol: A Review on Their Formation, Biological Activity, and Relevance in Therapy. Cannabis Cannabinoid Res. 2016, 1, 90–101. [Google Scholar] [CrossRef]
- Castillo-Arellano, J.; Canseco-Alba, A.; Cutler, S.J.; León, F. The Polypharmacological Effects of Cannabidiol. Molecules 2023, 28, 3271. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Liu, Y.; Xu, Y.; Yang, B.; Li, H.; Chen, L. An overview on synthetic and biological activities of cannabidiol (CBD) and its derivatives. Bioorg. Chem. 2023, 140, 106810. [Google Scholar] [CrossRef] [PubMed]
- Kicman, A.; Toczek, M. The Effects of Cannabidiol, a Non-Intoxicating Compound of Cannabis, on the Cardiovascular System in Health and Disease. Int. J. Mol. Sci. 2020, 21, 6740. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Fan, M.; An, C.; Ni, F.; Huang, W.; Luo, J. A narrative review of molecular mechanism and therapeutic effect of cannabidiol (CBD). Basic Clin. Pharmacol. Toxicol. 2022, 130, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Schmuhl, E.; Ramer, R.; Salamon, A.; Peters, K.; Hinz, B. Increase of mesenchymal stem cell migration by cannabidiol via activation of p42/44 MAPK. Biochem. Pharmacol. 2014, 87, 489–501. [Google Scholar] [CrossRef]
- Lanza Cariccio, V.; Scionti, D.; Raffa, A.; Iori, R.; Pollastro, F.; Diomede, F.; Bramanti, P.; Trubiani, O.; Mazzon, E. Treatment of Periodontal Ligament Stem Cells with MOR and CBD Promotes Cell Survival and Neuronal Differentiation via the PI3K/Akt/mTOR Pathway. Int. J. Mol. Sci. 2018, 19, 2341. [Google Scholar] [CrossRef]
- Fellous, T.; De Maio, F.; Kalkan, H.; Carannante, B.; Boccella, S.; Petrosino, S.; Maione, S.; Di Marzo, V.; Iannotti, F.A. Phytocannabinoids promote viability and functional adipogenesis of bone marrow-derived mesenchymal stem cells through different molecular targets. Biochem. Pharmacol. 2020, 175, 113859. [Google Scholar] [CrossRef]
- Hou, M.; Wang, S.; Yu, D.; Lu, X.; Zhao, X.; Chen, Z.; Yan, C. Cannabidiol prevents depressive-like behaviors through the modulation of neural stem cell differentiation. Front. Med. 2022, 16, 227–239. [Google Scholar] [CrossRef]
- Chang, R.C.; Thangavelu, C.S.; Joloya, E.M.; Kuo, A.; Li, Z.; Blumberg, B. Cannabidiol promotes adipogenesis of human and mouse mesenchymal stem cells via PPARγ by inducing lipogenesis but not lipolysis. Biochem. Pharmacol. 2022, 197, 114910. [Google Scholar] [CrossRef]
- Yu, L.; Zeng, L.; Zhang, Z.; Zhu, G.; Xu, Z.; Xia, J.; Weng, J.; Li, J.; Pathak, J.L. Cannabidiol Rescues TNF-α-Inhibited Proliferation, Migration, and Osteogenic/Odontogenic Differentiation of Dental Pulp Stem Cells. Biomolecules 2023, 13, 118. [Google Scholar] [CrossRef]
- Kowalczuk, A.; Marycz, K.; Kornicka-Garbowska, K.; Kornicka, J.; Bujalska-Zadrożny, M.; Groborz, S. Cannabidiol (CBD) Protects Adipose-Derived Mesenchymal Stem Cells (ASCs) against Endoplasmic Reticulum Stress Development and Its Complications. Int. J. Environ. Res. Public Health 2022, 19, 10864. [Google Scholar] [CrossRef]
- Kariminekoo, S.; Movassaghpour, A.; Rahimzadeh, A.; Talebi, M.; Shamsasenjan, K.; Akbarzadeh, A. Implications of mesenchymal stem cells in regenerative medicine. Artif. Cells Nanomed. Biotechnol. 2016, 44, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Margiana, R.; Markov, A.; Zekiy, A.O.; Hamza, M.U.; Al-Dabbagh, K.A.; Al-Zubaidi, S.H.; Hameed, N.M.; Ahmad, I.; Sivaraman, R.; Kzar, H.H.; et al. Clinical application of mesenchymal stem cell in regenerative medicine: A narrative review. Stem Cell Res. Ther. 2022, 13, 366. [Google Scholar] [CrossRef] [PubMed]
- Harasymiak-Krzyżanowska, I.; Niedojadło, A.; Karwat, J.; Kotuła, L.; Gil-Kulik, P.; Sawiuk, M.; Kocki, J. Adipose tissue-derived stem cells show considerable promise for regenerative medicine applications. Cell. Mol. Biol. Lett. 2013, 18, 479–493. [Google Scholar] [CrossRef]
- Hassan, W.U.; Greiser, U.; Wang, W. Role of adipose-derived stem cells in wound healing. Wound Repair Regen. 2014, 22, 313–325. [Google Scholar] [CrossRef]
- Sheykhhasan, M.; Wong, J.K.L.; Seifalian, A.M. Human Adipose-Derived Stem Cells with Great Therapeutic Potential. Curr. Stem Cell Res. Ther. 2019, 14, 532–548. [Google Scholar] [CrossRef]
- Tan, S.S.; Loh, W. The utility of adipose-derived stem cells and stromal vascular fraction for oncologic soft tissue reconstruction: Is it safe? A matter for debate. Surgeon 2017, 15, 186–189. [Google Scholar] [CrossRef]
- Thakkar, K.; Ruan, C.H.; Ruan, K.H. Recent advances of cannabidiol studies in medicinal chemistry, pharmacology, and therapeutics. Future Med. Chem. 2021, 13, 1935–1937. [Google Scholar] [CrossRef]
- Stella, N. THC and CBD: Similarities and differences between siblings. Neuron 2023, 111, 302–327. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Feng, J.; Sun, L.; Xuan, Y.W.; Wen, L.; Li, Y.X.; Yang, S.; Zhu, B.; Tian, X.Y.; Li, S.; et al. Cannabidiol Promotes Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells in the Inflammatory Microenvironment via the CB2-dependent p38 MAPK Signaling Pathway. Int. J. Stem Cells 2022, 15, 405–414. [Google Scholar] [CrossRef]
- Scott, M.A.; Nguyen, V.T.; Levi, B.; James, A.W. Current methods of adipogenic differentiation of mesenchymal stem cells. Stem Cells Dev. 2011, 20, 1793–1804. [Google Scholar] [CrossRef]
- Ailhaud, G. Adipose cell differentiation in culture. Mol. Cell. Biochem. 1982, 49, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Li, Y.B.; Wang, Y.S. Dexamethasone-induced adipogenesis in primary marrow stromal cell cultures: Mechanism of steroid-induced osteonecrosis. Chin. Med. J. 2006, 119, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kwon, J.S.; Hong, M.H.; Kim, J.; Song, C.H.; Jeong, M.H.; Cho, J.G.; Park, J.C.; Kang, J.C.; Ahn, Y. Promigratory activity of oxytocin on umbilical cord blood-derived mesenchymal stem cells. Artif. Organs 2010, 34, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Umek, R.M.; McKnight, S.L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991, 5, 1538–1552. [Google Scholar] [CrossRef]
- Almousa, A.S.; Subash-Babu, P.; Alanazi, I.O.; Alshatwi, A.A.; Alkhalaf, H.; Bahattab, E.; Alsiyah, A.; Alzahrani, M. Hemp Seed Oil Inhibits the Adipogenicity of the Differentiation-Induced Human Mesenchymal Stem Cells through Suppressing the Cannabinoid Type 1 (CB1). Molecules 2024, 29, 1568. [Google Scholar] [CrossRef]
- Romaldini, A.; Mastrogiacomo, M.; Cancedda, R.; Descalzi, F. Platelet Lysate Activates Human Subcutaneous Adipose Tissue Cells by Promoting Cell Proliferation and Their Paracrine Activity Toward Epidermal Keratinocytes. Front. Bioeng. Biotechnol. 2018, 6, 203. [Google Scholar] [CrossRef]
- Yang, Y.K.; Ogando, C.R.; Wang See, C.; Chang, T.Y.; Barabino, G.A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 2018, 9, 131. [Google Scholar] [CrossRef]
- Kamali, A.; Oryan, A.; Hosseini, S.; Ghanian, M.H.; Alizadeh, M.; Baghaban Eslaminejad, M.; Baharvand, H. Cannabidiol-loaded microspheres incorporated into osteoconductive scaffold enhance mesenchymal stem cell recruitment and regeneration of critical-sized bone defects. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 101, 64–75. [Google Scholar] [CrossRef]
- Ihejirika-Lomedico, R.; Patel, K.; Buchalter, D.B.; Kirby, D.J.; Mehta, D.; Dankert, J.F.; Muiños-López, E.; Ihejirika, Y.; Leucht, P. Non-psychoactive Cannabidiol Prevents Osteoporosis in an Animal Model and Increases Cell Viability, Proliferation, and Osteogenic Gene Expression in Human Skeletal Stem and Progenitor Cells. Calcif. Tissue Int. 2023, 112, 716–726. [Google Scholar] [CrossRef]
- Saidova, A.A.; Vorobjev, I.A. Lineage Commitment, Signaling Pathways, and the Cytoskeleton Systems in Mesenchymal Stem Cells. Tissue Eng. Part B Rev. 2000, 26, 13–25. [Google Scholar] [CrossRef]
- Rodriguez, J.P.; Gonzalez, M.; Rios, S.; Cambiazo, V. Cytoskeletal organization of human mesenchymal stem cells (MSC) changes during their osteogenic differentiation. J. Cell. Biochem. 2004, 93, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, P.S.; Loboa, E.G. Cytoskeletal and focal adhesion influences on mesenchymal stem cell shape, mechanical properties, and differentiation down osteogenic, adipogenic, and chondrogenic pathways. Tissue Eng. Part B Rev. 2012, 18, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Docheva, D.; Padula, D.; Popov, C.; Mutschler, W.; Clausen-Schaumann, H.; Schieker, M. Researching into the cellular shape, volume and elasticity of mesenchymal stem cells, osteoblasts and osteosarcoma cells by atomic force microscopy. J. Cell. Mol. Med. 2008, 12, 537–552. [Google Scholar] [CrossRef]
- Sonowal, H.; Kumar, A.; Bhattacharyya, J.; Gogoi, P.K.; Jaganathan, B.G. Inhibition of actin polymerization decreases osteogeneic differentiation of mesenchymal stem cells through p38 MAPK pathway. J. Biomed. Sci. 2013, 20, 71. [Google Scholar] [CrossRef]
- Chen, L.; Hu, H.; Qiu, W.; Shi, K.; Kassem, M. Actin depolymerization enhances adipogenic differentiation in human stromal stem cells. Stem Cell Res. 2018, 29, 76–83. [Google Scholar] [CrossRef]
- Petrocelli, G.; Abruzzo, P.M.; Pampanella, L.; Tassinari, R.; Marini, S.; Zamagni, E.; Ventura, C.; Facchin, F.; Canaider, S. Oxytocin Modulates Osteogenic Commitment in Human Adipose-Derived Stem Cells. Int. J. Mol. Sci. 2023, 24, 10813. [Google Scholar] [CrossRef]
- Pampanella, L.; Abruzzo, P.M.; Tassinari, R.; Alessandrini, A.; Petrocelli, G.; Ragazzini, G.; Cavallini, C.; Pizzuti, V.; Collura, N.; Canaider, S.; et al. Cytochalasin B Influences Cytoskeletal Organization and Osteogenic Potential of Human Wharton’s Jelly Mesenchymal Stem Cells. Pharmaceuticals 2023, 16, 289. [Google Scholar] [CrossRef]
- Bianconi, E.; Tassinari, R.; Alessandrini, A.; Ragazzini, G.; Cavallini, C.; Abruzzo, P.M.; Petrocelli, G.; Pampanella, L.; Casadei, R.; Maioli, M.; et al. Cytochalasin B Modulates Nanomechanical Patterning and Fate in Human Adipose-Derived Stem Cells. Cells 2022, 11, 1629. [Google Scholar] [CrossRef]
- Präbst, K.; Engelhardt, H.; Ringgeler, S.; Hübner, H. Basic Colorimetric Proliferation Assays: MTT, WST, and Resazurin. Methods Mol. Biol. 2017, 1601, 1–17. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Facchin, F.; Vitale, L.; Bianconi, E.; Piva, F.; Frabetti, F.; Strippoli, P.; Casadei, R.; Pelleri, M.C.; Piovesan, A.; Canaider, S. Complexity of bidirectional transcription and alternative splicing at human RCAN3 locus. PLoS ONE 2011, 6, e24508. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007, 8, R19. [Google Scholar] [CrossRef]
- Arya, M.; Shergill, I.S.; Williamson, M.; Gommersall, L.; Arya, N.; Patel, H.R. Basic principles of real-time quantitative PCR. Expert. Rev. Mol. Diagn. 2005, 5, 209–219. [Google Scholar] [CrossRef]

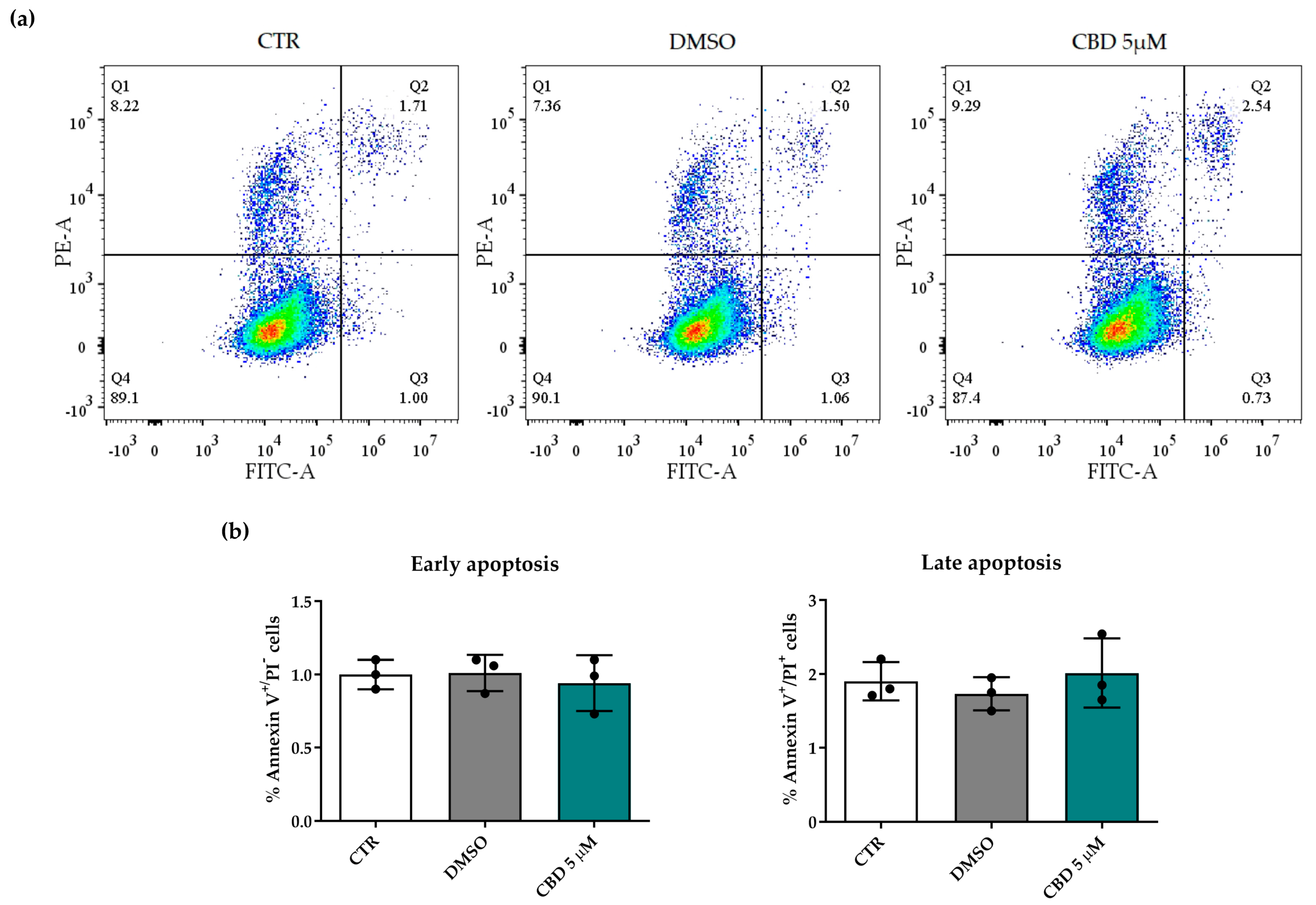



| Sample | % of Living Cells |
|---|---|
| CTR | 96.9 ± 0.4 |
| DMSO | 98.2 ± 0.8 |
| CBD 0.1 µM | 96.6 ± 0.3 |
| CBD 0.5 µM | 95.6 ± 0.8 |
| CBD 2.5 µM | 97.0 ± 0.5 |
| CBD 5 µM | 97.7 ± 0.9 |
| CBD 10 µM | 88.8 ± 0.5 *** |
| Gene | Entrez Gene ID | Left Primer | Right Primer | Bio-Rad Unique Assay ID | A.L. (bp) |
|---|---|---|---|---|---|
| HSP70 (promoter) | 3303 | CGCCATGGAGACCAACACCC | GCGGTTCCCTGCTCTCTGTC | - | 500 |
| Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) | 2597 | - | - | qHsaCED0038674 | 117 |
| TATA box binding protein (TBP) | 6908 | - | - | qHsaCID0007122 | 120 |
| Hypoxanthine phosphoribosyl transferase 1(HPRT1) | 3251 | - | - | qHsaCID0016375 | 90 |
| Peroxisome proliferator-activated receptor gamma (PPARγ) | 5468 | TTGCAGTGGGGATGTCTCAT | TTTCCTGTCAAGATCGCCCT | - | 208 |
| CCAAT enhancer binding protein Alpha (CEBPα) | 1050 | GCAAACTCACCGCTCCAATG | TTCTCTCATGGGGGTCTGCT | - | 113 |
| Fatty acid binding protein 4 (FABP4) | 2167 | GATAAACTGGTGGTGGAATGCG | ATGCGAACTTCAGTCCAGGT | - | 100 |
| RUNX family transcription factor 2 (RUNX2) | 860 | CTCCCTGAACTCTGCACCAA | TAGAGTGGATGGACGGGGAC | - | 149 |
| Secreted phosphoprotein 1 (SPP1) | 6696 | ACTGATTTTCCCACGGACCT | CTCCTCGCTTTCCATGTGTG | - | 192 |
| Collagen type I alpha 1 chain (COL1A1) | 1277 | TGAAGGGACACAGAGGTTTCAG | GTAGCACCATCATTTCCACGA | - | 193 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrocelli, G.; Pampanella, L.; Abruzzo, P.M.; Cruciani, S.; Ventura, C.; Canaider, S.; Facchin, F. Cannabidiol as Modulator of Spontaneous Adipogenesis in Human Adipose-Derived Stem Cells. Molecules 2025, 30, 2367. https://doi.org/10.3390/molecules30112367
Petrocelli G, Pampanella L, Abruzzo PM, Cruciani S, Ventura C, Canaider S, Facchin F. Cannabidiol as Modulator of Spontaneous Adipogenesis in Human Adipose-Derived Stem Cells. Molecules. 2025; 30(11):2367. https://doi.org/10.3390/molecules30112367
Chicago/Turabian StylePetrocelli, Giovannamaria, Luca Pampanella, Provvidenza Maria Abruzzo, Sara Cruciani, Carlo Ventura, Silvia Canaider, and Federica Facchin. 2025. "Cannabidiol as Modulator of Spontaneous Adipogenesis in Human Adipose-Derived Stem Cells" Molecules 30, no. 11: 2367. https://doi.org/10.3390/molecules30112367
APA StylePetrocelli, G., Pampanella, L., Abruzzo, P. M., Cruciani, S., Ventura, C., Canaider, S., & Facchin, F. (2025). Cannabidiol as Modulator of Spontaneous Adipogenesis in Human Adipose-Derived Stem Cells. Molecules, 30(11), 2367. https://doi.org/10.3390/molecules30112367









