Elastocaloric Performance of Natural Rubber: The Role of Nanoclay Addition
Abstract
1. Introduction
2. Results and Discussion
2.1. Evaluation of the Density and Crosslinking Degree
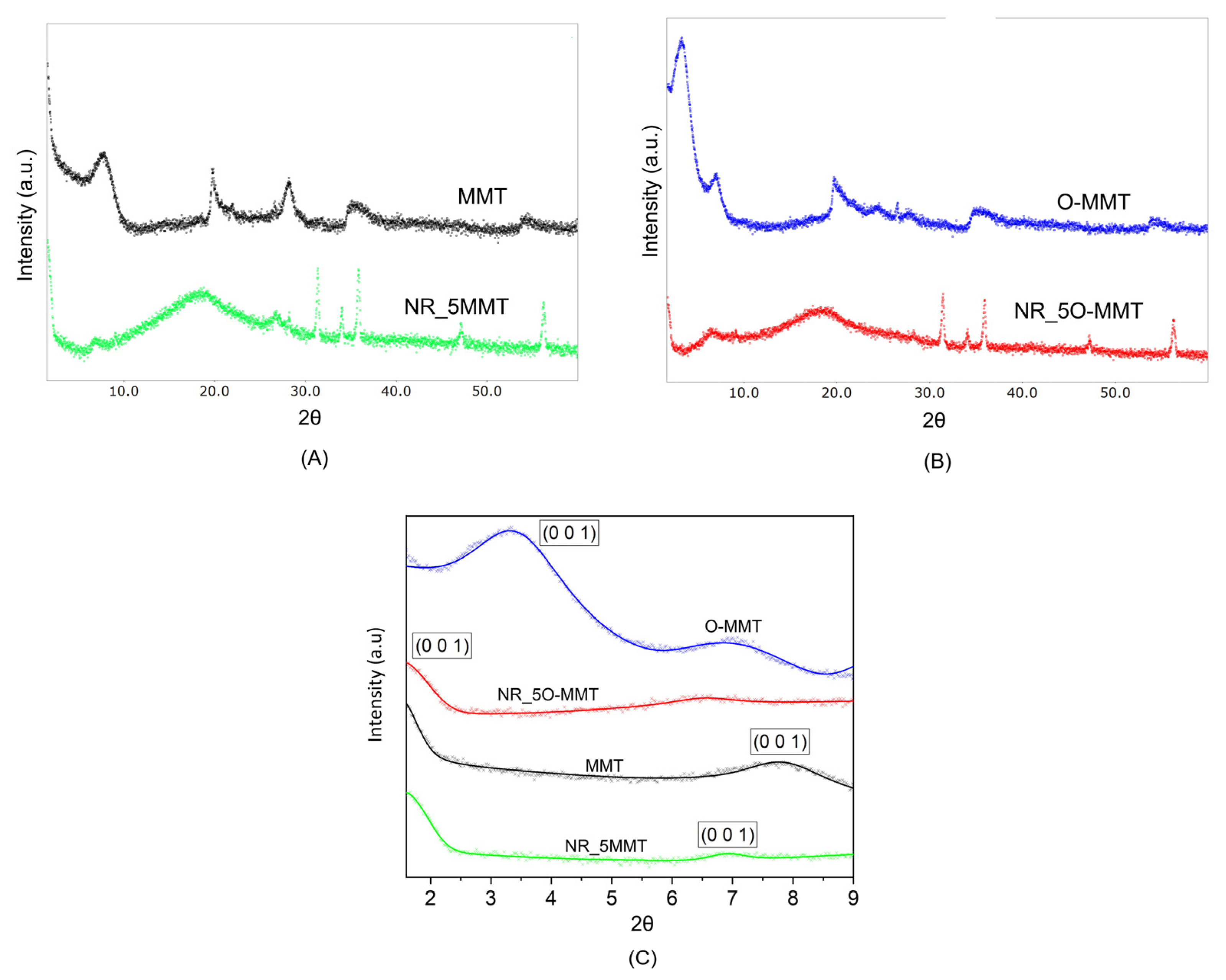
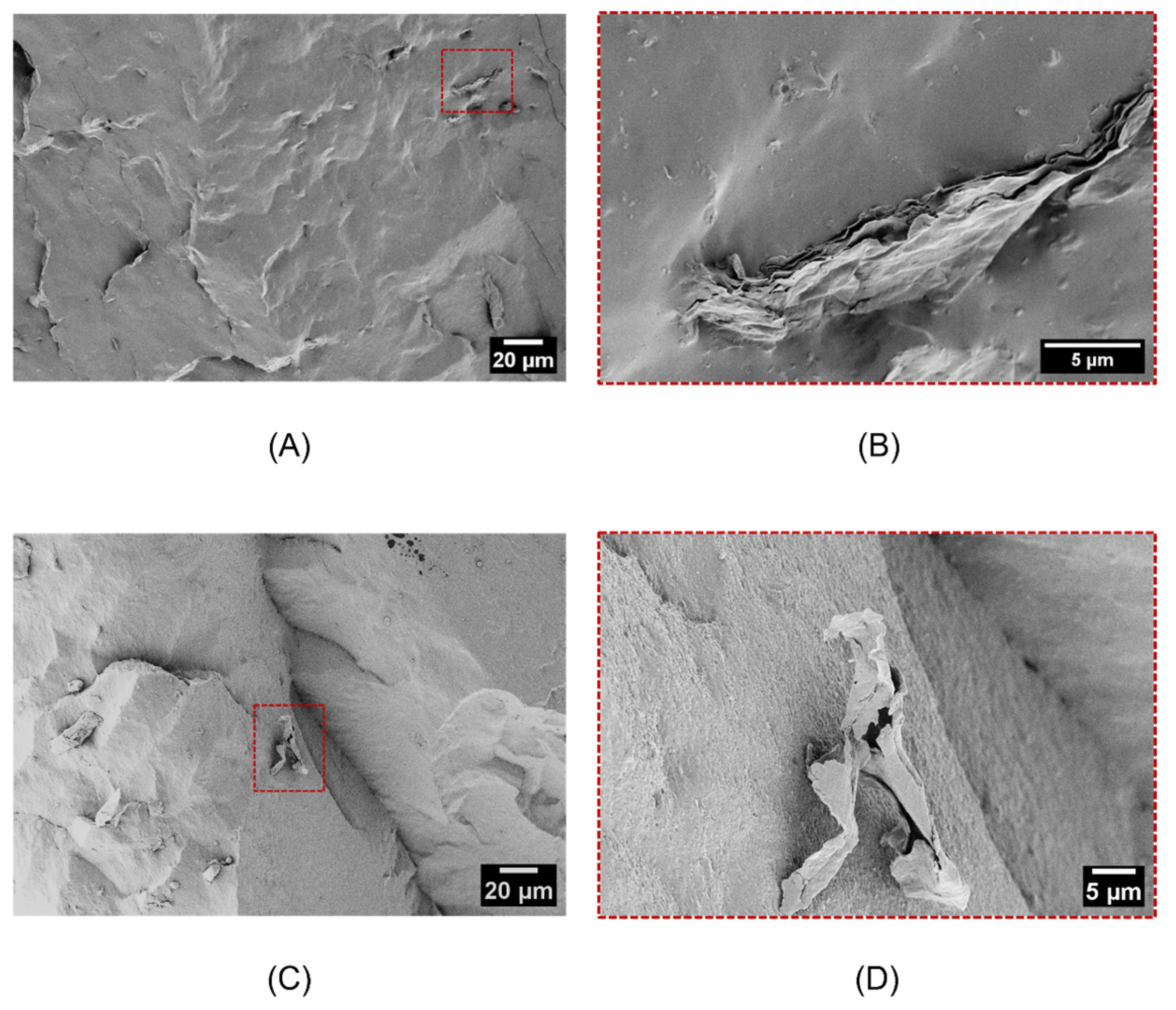
2.2. Structural and Morphological Analysis
2.3. Thermal Properties
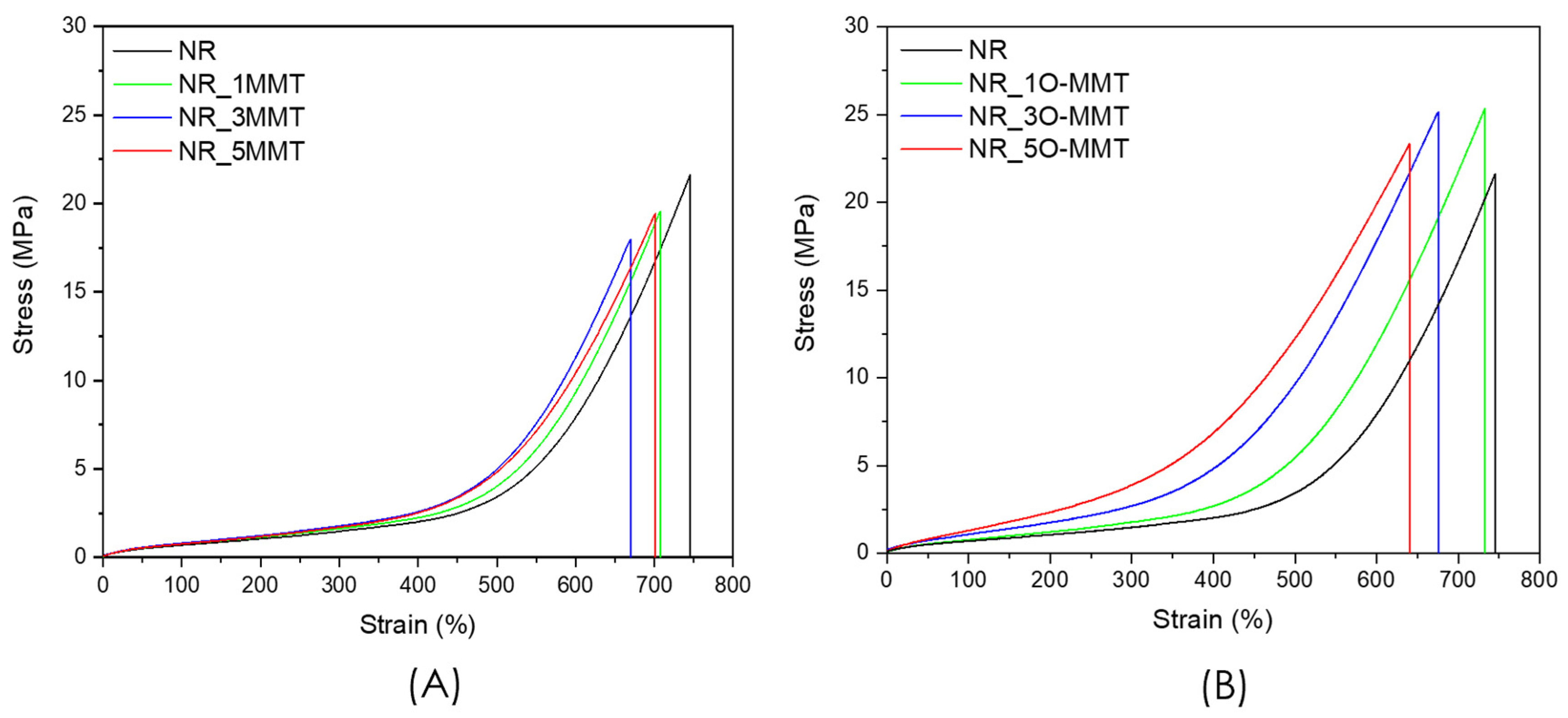
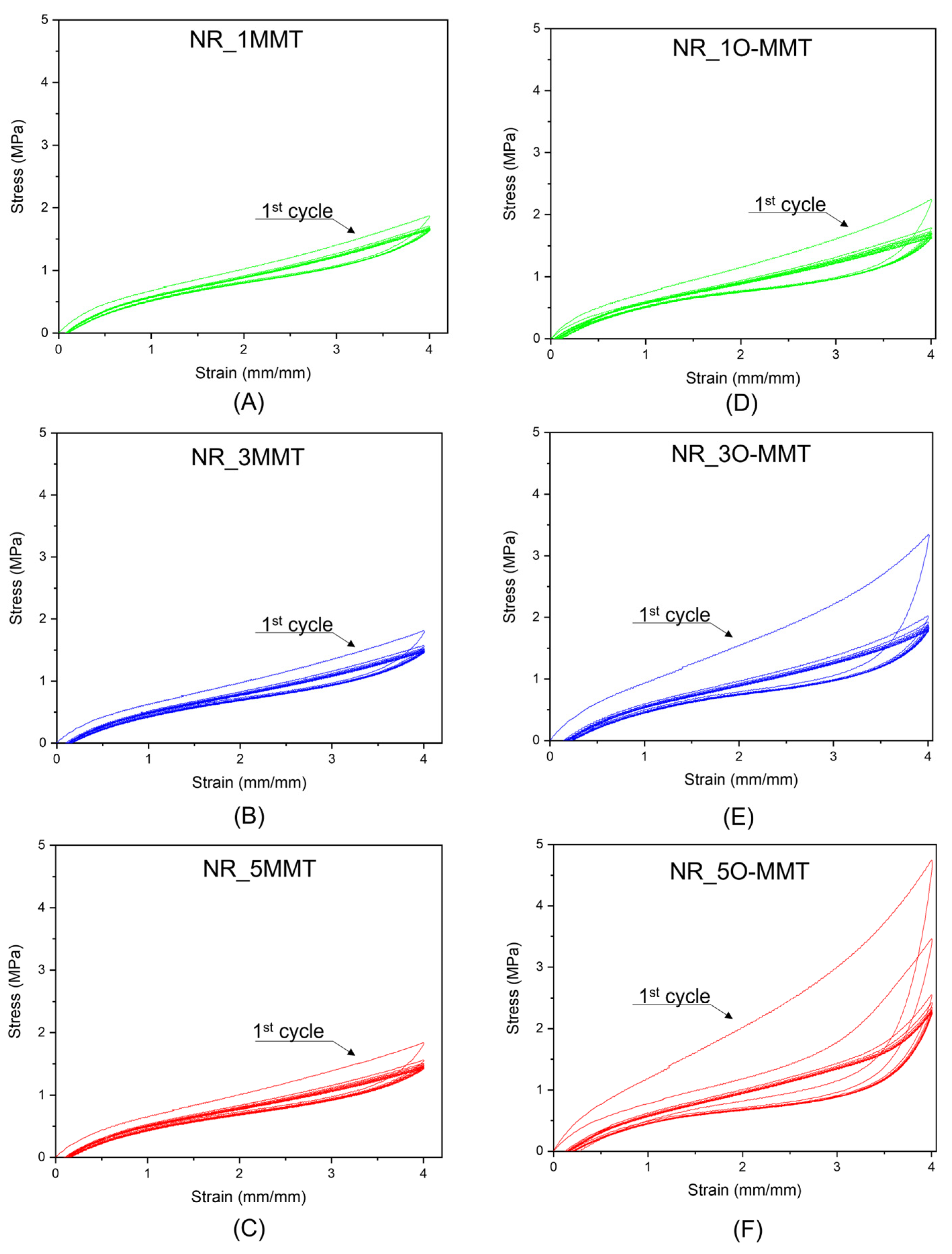
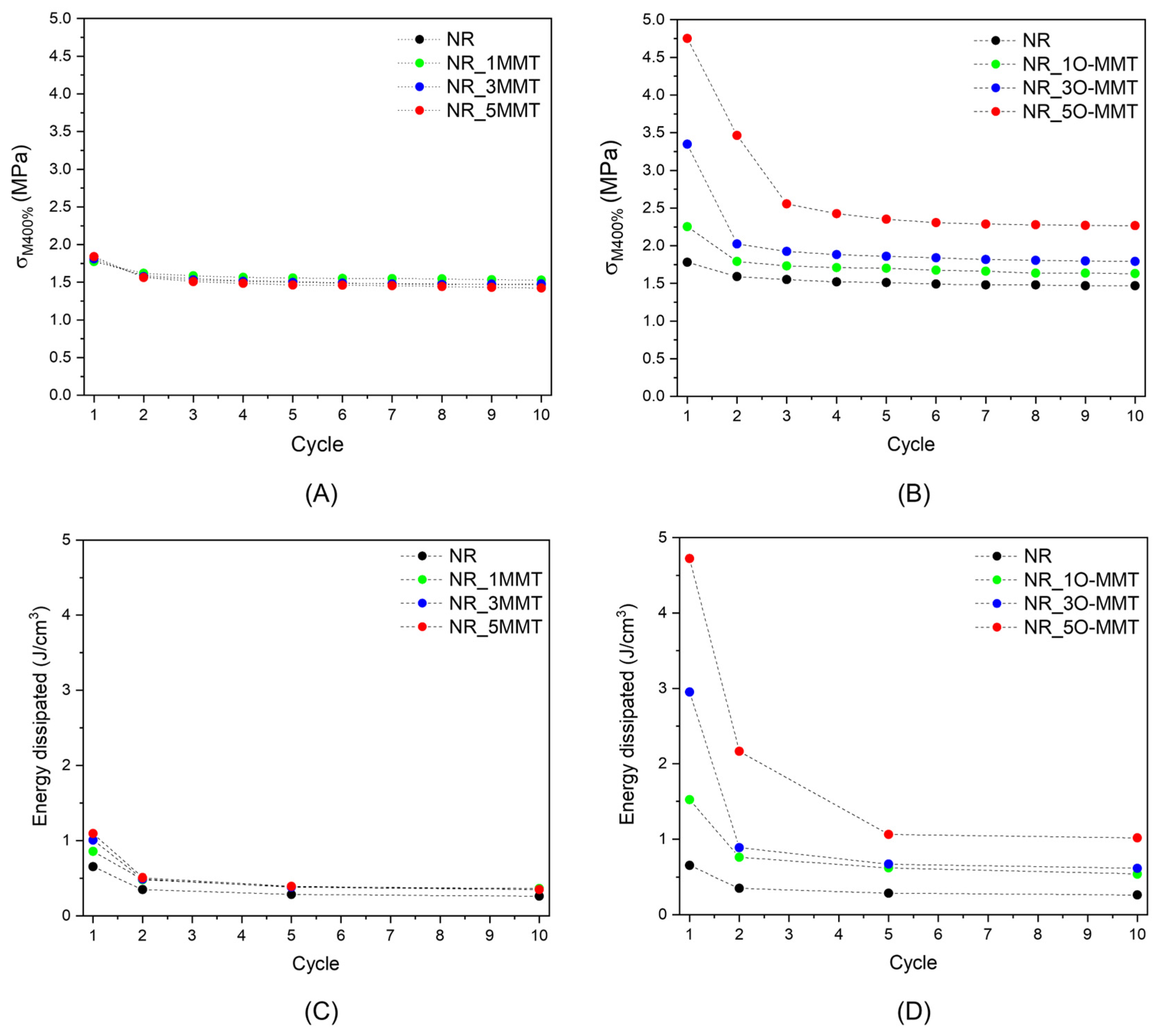
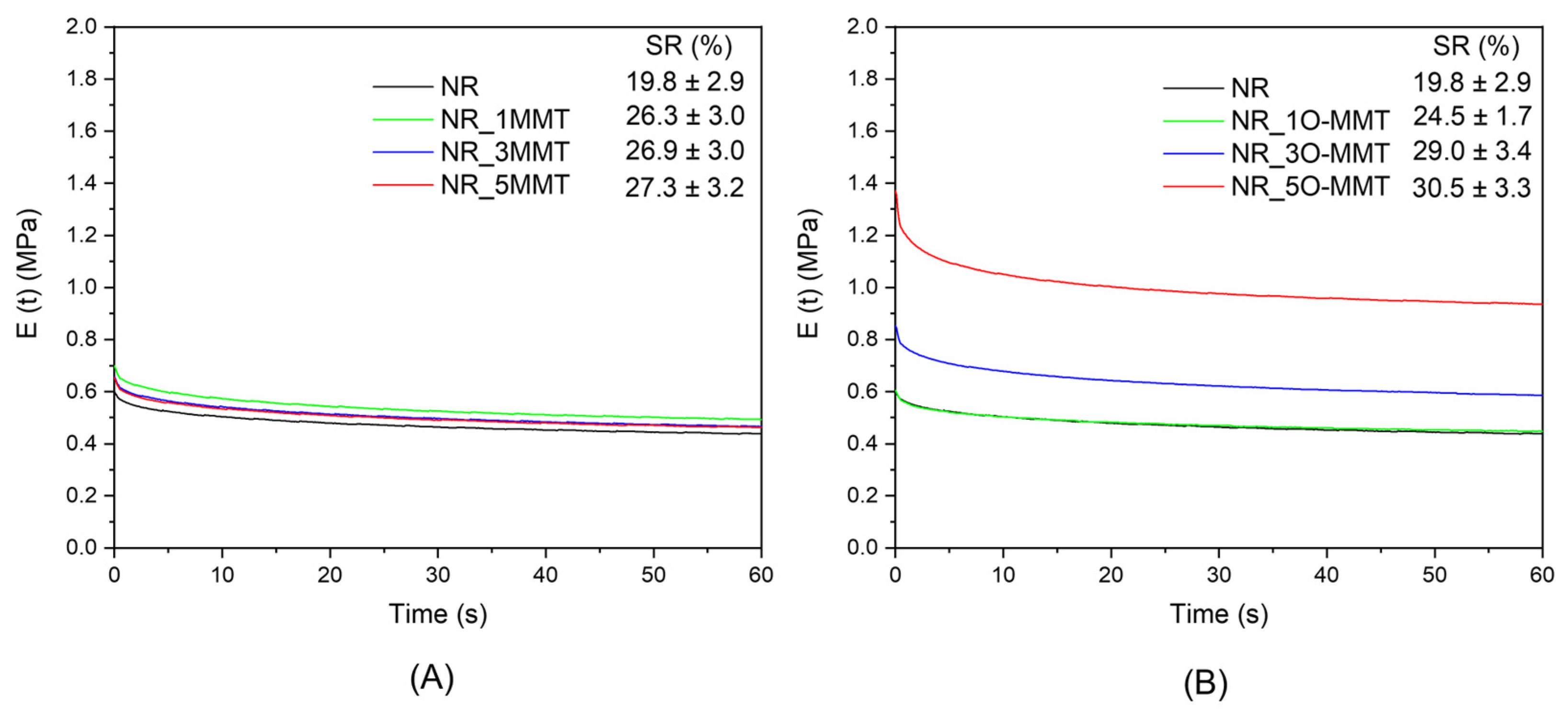
2.4. Mechanical Properties
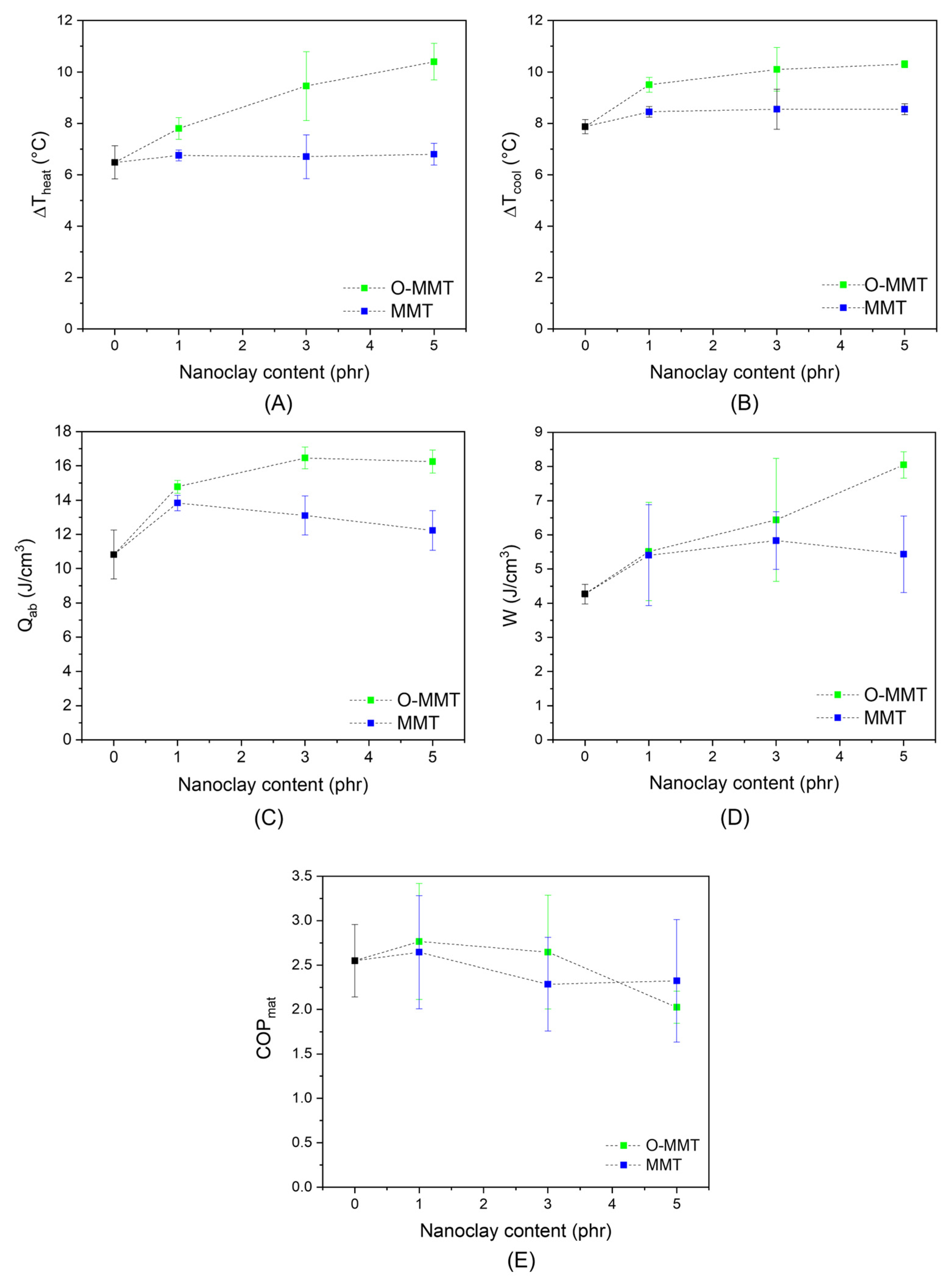
2.5. Evaluation of the Elastocaloric Effect
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation
3.3. Characterization
3.3.1. Evaluation of the Density and Crosslinking Degree
3.3.2. Structural and Morphological Analysis
3.3.3. Thermal Properties
3.3.4. Mechanical Properties
3.3.5. Evaluation of the Elastocaloric Effect
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NR | Natural rubber |
| ACs | Air conditioners |
| SIC | Strain-induced crystallization |
| MMT | Montmorillonite |
| COP | Coefficient of performance |
| O-MMT | Organo-modified montmorillonite |
| OM | Organo-modified |
| XRD | X-ray diffraction |
| LFA | Laser flash analysis |
| DSC | Differential scanning calorimetry |
References
- Russo, S.; Sillmann, J.; Sterl, A. Humid heat waves at different warming levels. Sci. Rep. 2017, 7, 7477. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, J.W.; Dessy, J.B.; Vecchi, G.A.; Oppenheimer, M. Temporally Compound Heat Wave Events and Global Warming: An Emerging Hazard. Earths Future. 2019, 7, 411–427. [Google Scholar] [CrossRef]
- Alamgir, W.; Shan, H. The Multifaceted Consequences of Climate Change on Human Health. Life Sci. 2023, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Birol, D.F. The Future of Cooling. Opportunities for Energy-Efficient Air Conditioners. International Energy Agency. 2018. Available online: http://www.iea.org/reports/the-future-of-cooling (accessed on 1 April 2025).
- ilPost. Il Problema coi Condizionatori. 2018. Available online: https://www.ilpost.it/2018/09/01/condizionatori-ambiente/ (accessed on 16 July 2025).
- Cazorla, C. Novel mechanocaloric materials for solid-state cooling applications. Appl. Phys. Rev. 2019, 6, 041316. [Google Scholar] [CrossRef]
- Mañosa, L.; Planes, A. Solid-state cooling by stress: A perspective. Appl. Phys. Lett. 2020, 116, 050501. [Google Scholar] [CrossRef]
- Chauhan, A.; Patel, S.; Vaish, R.; Bowen, C.R. A review and analysis of the elasto-caloric effect for solidstate refrigeration devices: Challenges and opportunities. MRS. Energy. Sustain. 2015, 2, 16. [Google Scholar] [CrossRef]
- Xie, Z.; Sebald, G.; Guyomar, D. Comparison of elastocaloric effect of natural rubber with other caloric effects on different-scale cooling application cases. Appl. Therm. Eng. 2017, 111, 914–926. [Google Scholar] [CrossRef]
- Bianchi, M.; Fambri, L.; Fredi, G.; Pegoretti, A.; Dorigato, A. Elastocaloric Performance of Natural Rubber in Solid State Cooling: Evaluation of the Effect of Crosslinking Density. Appl. Sci. 2024, 14, 10525. [Google Scholar] [CrossRef]
- Candau, N.; Zimny, A.; Vives, E.; Maspoch, M.L. Elastocaloric Waste/Natural Rubber Materials with Various Crosslink Densities. Polymers 2023, 15, 2566. [Google Scholar] [CrossRef] [PubMed]
- Guyomar, D.; Li, Y.; Sebald, G.; Cottinet, P.J.; Ducharne, B.; Capsal, J.F. Elastocaloric modeling of natural rubber. Appl. Therm. Eng. 2013, 57, 33–38. [Google Scholar] [CrossRef]
- Davies, B. Natural rubber—Its engineering characteristics. Mater. Des. 1986, 7, 68–74. [Google Scholar] [CrossRef]
- Bennacer, R.; Liu, B.; Yang, M.; Chen, A. Refrigeration performance and the elastocaloric effect in natural and synthetic rubbers. Appl. Therm. Eng. 2022, 204, 117938. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Y.; Zhu, S.; Theodorakis, P.; Song, F.; Rachid, B.; Chen, K. A Lower Temperature Difference of The Elastocaloric Effect by Natural Rubber. Int. J. Refrig. 2023, 155, 163–172. [Google Scholar] [CrossRef]
- Yukihiro, Y. Effet Élastocalorique Dans le Caoutchouc Naturel et le Terpolymère: Mécanismes Responsables de la Variation de Température et Bilan Énergétique sous Déformation. Ph.D. Thesis, Universitè de Lion, Lyon, France, 2016. [Google Scholar]
- Candau, N.; Vives, E.; Fernández, A.I.; Maspoch, M.L. Elastocaloric effect in vulcanized natural rubber and natural/wastes rubber blends. Polymer 2021, 236, 124309. [Google Scholar] [CrossRef]
- Le Cam, J.B.; Albouy, P.A.; Charlès, S. Comparison between x-ray diffraction and quantitative surface calorimetry based on infrared thermography to evaluate strain-induced crystallinity in natural rubber. Rev. Sci. Instrum. 2020, 91, 044902. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.C.; Meier, D.J. Rapid stress-induced crystallization in natural rubber. J. Polym. Sci. Part A-2 Polym. Phys. 1968, 6, 1689–1703. [Google Scholar] [CrossRef]
- Haissoune, H.; Chenal, J.M.; Chazeau, L.; Sebald, G.; Morfin, I.; Lebrun, L.; Dalmas, F.; Coativy, G. Elastocaloric effect: Impact of heat transfer on strain-induced crystallization kinetics of natural rubber. Polymer 2022, 263, 125506. [Google Scholar] [CrossRef]
- Carretero-González, J.; Retsos, H.; Verdejo, R.; Toki, S.; Hsiao, B.S.; Giannelis, E.P.; López-Manchado, M.A. Effect of Nanoclay on Natural Rubber Microstructure. Macromolecules 2008, 41, 6763–6772. [Google Scholar] [CrossRef]
- Carretero-Gonzalez, J.; Verdejo, R.; Toki, S.; Hsiao, B.S.; Giannelis, E.P.; López-Manchado, M.A. Real-Time Crystallization of Organoclay Nanoparticle Filled Natural Rubber under Stretching. Macromolecules 2008, 41, 2295–2298. [Google Scholar] [CrossRef]
- Nie, Y.; Huang, G.; Qu, L.; Wang, X.; Weng, G.; Wu, J. New insights into thermodynamic description of strain-induced crystallization of peroxide cross-linked natural rubber filled with clay by tube model. Polymer 2011, 52, 3234–3242. [Google Scholar] [CrossRef]
- Qu, L.; Huang, G.; Liu, Z.; Zhang, P.; Weng, G.; Nie, Y. Remarkable reinforcement of natural rubber by deformation-induced crystallization in the presence of organophilic montmorillonite. Acta Mater. 2009, 57, 5053–5060. [Google Scholar] [CrossRef]
- Pegoretti, A.; Dorigato, A.; Penati, A. Tensile mechanical response of polyethylene – clay nanocomposites. Express Polym. Lett. 2007, 1, 123–131. [Google Scholar] [CrossRef]
- Pegoretti, A.; Dorigato, A.; Brugnara, M.; Penati, A. Contact angle measurements as a tool to investigate the filler–matrix interactions in polyurethane–clay nanocomposites from blocked prepolymer. Eur. Polym. J. 2008, 44, 1662–1672. [Google Scholar] [CrossRef]
- Dorigato, A.; Pegoretti, A.; Penati, A. Effect of the polymer—Filler interaction on the thermo-mechanical response of polyurethane-clay nanocomposites from blocked prepolymer. J. Reinf. Plast. Compos. 2011, 30, 325–335. [Google Scholar] [CrossRef]
- Dorigato, A.; Pegoretti, A.; Quaresimin, M. Thermo-mechanical characterization of epoxy/clay nanocomposites as matrices for carbon/nanoclay/epoxy laminates. Mater. Sci. Eng. A 2011, 528, 6324–6333. [Google Scholar] [CrossRef]
- Dorigato, A.; Morandi, S.; Pegoretti, A. Effect of nanoclay addition on the fiber/matrix adhesion in epoxy/glass composites. J. Compos. Mater. 2012, 46, 1439–1451. [Google Scholar] [CrossRef]
- Vishvanathperumal, S.; Gopalakannan, S. Effects of the Nanoclay and Crosslinking Systems on the Mechanical Properties of Ethylene-propylene-diene Monomer/styrene Butadiene Rubber Blends Nanocomposite. Silicon 2019, 11, 117–135. [Google Scholar] [CrossRef]
- Kathuria, A.; Zhang, S. Sustainable and Repulpable Barrier Coatings for Fiber-Based Materials for Food Packaging: A Review. Front. Mater. 2022, 9, 929501. [Google Scholar] [CrossRef]
- Pusch, R. Is Montmorillonite-Rich Clay of MX-80 Type the Ideal Buffer for Isolation of HLW? Technical Report TR-99-33; Swedish Nuclear Fuel and Waste Management Company: Stockholm, Sweden, 1999. [Google Scholar]
- Diani, J.; Fayolle, B.; Gilormini, P. A review on the Mullins effect. Eur. Polym. J. 2009, 45, 601–612. [Google Scholar] [CrossRef]
- Derham, C.J. Creep and stress relaxation of rubbers? the effects of stress history and temperature changes. J. Mater. Sci. 1973, 8, 1023–1029. [Google Scholar] [CrossRef]
- Meera, A.P.; Said, S.; Grohens, Y.; Luyt, A.S.; Thomas, S. Tensile Stress Relaxation Studies of TiO2 and Nanosilica Filled Natural Rubber Composites. Ind. Eng. Chem. Res. 2009, 48, 3410–3416. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Q.; Li, C.; Fu, Y.; Zhang, H.; Ye, Z.; Zhou, X.; Li, Q.; Wang, T.; Wang, S.; et al. Solid-state cooling by elastocaloric polymer with uniform chain-lengths. Nat. Commun. 2022, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- BYK. CLOISITE-20 A. Available online: https://www.byk.com/en/products/additive-guide/cloisite-20-a (accessed on 1 November 2023).
- Carlo Erba Reagents. Metanolo-Product Sheet. Available online: https://www.carloerbareagents.com/cerstorefront/cer-it/p/111500DD14150/000000000000309004/downloadVariant (accessed on 1 November 2023).
- Carlo Erba Reagents. Acetone-Product Sheet. Available online: https://www.carloerbareagents.com/cerstorefront/cer-it/p/002100DD14150/000000000000508200/downloadVariant (accessed on 1 November 2023).
- Carlo Erba Reagents. Toluene-Product Sheet. Available online: https://www.carloerbareagents.com/cerstorefront/cer-it/p/225800DD14150/000000000000386002/downloadVariant (accessed on 1 November 2023).
- D792-20; Standard Test Methods for Density and Specific Gravity (Relative Density) of Plastics by Displacement. ASTM International: West Conshohocken, PA, USA, 2020.
- Fu, W.; Wang, L.; Huang, J.; Liu, C.; Peng, W.; Xiao, H.; Li, S. Mechanical Properties and Mullins Effect in Natural Rubber Reinforced by Grafted Carbon Black. Adv. Polym. Technol. 2019, 1–11. [Google Scholar] [CrossRef]
- Qian, M.; Zou, B.; Chen, Z.; Huang, W.; Wang, X.; Tang, B.; Liu, Q.; Zhu, Y. The Influence of Filler Size and Crosslinking Degree of Polymers on Mullins Effect in Filled NR/BR Composites. Polymers 2021, 13, 2284. [Google Scholar] [CrossRef] [PubMed]

| Sample | Density (g/cm3) | Crosslinking Density (mol·10−4/cm3) |
|---|---|---|
| NR | 0.958 ± 0.006 | 2.94 ± 0.21 |
| NR_1MMT | 0.960 ± 0.003 | 3.02 ± 0.01 |
| NR_3MMsT | 0.973 ± 0.001 | 3.04 ± 0.09 |
| NR_5MMT | 0.981 ± 0.005 | 2.94 ± 0.16 |
| NR_1O-MMT | 0.959 ± 0.001 | 3.32 ± 0.02 |
| NR_3O-MMT | 0.966 ± 0.001 | 3.73 ± 0.03 |
| NR_5O-MMT | 0.971 ± 0.003 | 3.68 ± 0.04 |
| Sample | d001 Spacing [Å] |
|---|---|
| MMT | 11.40 |
| NR_5MMT | 12.68 |
| O-MMT | 26.08 |
| NR_5O-MMT | >51.70 |
| Sample | Specific Heat Capacity (J/g·K) | Thermal Diffusivity (mm2/s) | Thermal Conductivity (W/m·K) |
|---|---|---|---|
| NR | 1.70 ± 0.05 | 0.081 ± 0.001 | 0.132 ± 0.003 |
| NR_1MMT | 1.87 ± 0.08 | 0.085 ± 0.001 | 0.153 ± 0.006 |
| NR_3MMT | 1.82 ± 0.03 | 0.086 ± 0.001 | 0.151 ± 0.002 |
| NR_5MMT | 1.75 ± 0.03 | 0.088 ± 0.001 | 0.150 ± 0.003 |
| NR_1O-MMT | 1.82 ± 0.05 | 0.081 ± 0.002 | 0.141 ± 0.001 |
| NR_3O-MMT | 1.82 ± 0.04 | 0.081 ± 0.001 | 0.144 ± 0.002 |
| NR_5O-MMT | 1.75 ± 0.02 | 0.084 ± 0.001 | 0.142 ± 0.004 |
| Sample | E100–200 (MPa) | σ200% (MPa) | σ400% (MPa) | σb (MPa) | εb (mm/mm) | Eb (J/cm3) | Shore A |
|---|---|---|---|---|---|---|---|
| NR | 0.35 ± 0.08 | 1.08 ± 0.13 | 2.10 ± 0.23 | 21.28 ± 3.73 | 730 ± 14 | 32.2 ± 6.4 | 38.0 ± 0.8 |
| NR_1MMT | 0.37 ± 0.02 | 1.12 ± 0.04 | 2.11 ± 0.15 | 19.45 ± 4.97 | 701 ± 58 | 28.5 ± 9.9 | 40.4 ± 0.5 |
| NR_3MMT | 0.42 ± 0.04 | 1.22 ± 0.10 | 2.48 ± 0.29 | 19.55 ± 3.05 | 686 ± 32 | 29.2 ± 6.8 | 40.7 ± 1.0 |
| NR_5MMT | 0.42 ± 0.06 | 1.20 ± 0.03 | 2.56 ± 0.07 | 18.96 ± 3.34 | 698 ± 30 | 30.2 ± 6.1 | 41.6 ± 0.4 |
| NR_1O-MMT | 0.44 ± 0.02 | 1.19 ± 0.07 | 2.62 ± 0.33 | 26.08 ± 2.94 | 754 ± 41 | 44.7 ± 12.1 | 42.0 ± 0.2 |
| NR_3O-MMT | 0.57 ± 0.09 | 1.54 ± 0.19 | 4.22 ± 0.46 | 25.39 ± 0.58 | 653 ± 34 | 42.6 ± 3.3 | 45.9 ± 0.2 |
| NR_5O-MMT | 0.77 ± 0.20 | 1.91 ± 0.33 | 5.36 ± 0.94 | 20.05 ± 3.55 | 630 ± 72 | 35.6 ± 10.9 | 48.0 ± 0.5 |
| Mechanism | Stretching Phase | Retraction Phase |
|---|---|---|
| Thermoelastic effect | ↑ | ↓ |
| SIC | ↑ | \ |
| Internal friction macromolecules-macromolecules and macromolecules-nanoclays | ↑ | ↑ |
| Melting of the crystallites | \ | ↓ |
| Sample * | τc,stretching (s) | τc,retraction (s) |
|---|---|---|
| NR natural convection | 22.3 ± 4.6 | 54.1 ± 2.3 |
| NR forced convection | 13.4 ± 3.4 | 18.8 ± 2.2 |
| NR_1MMT | 5.7 ± 0.5 | 17.3 ± 0.8 |
| NR_3MMT | 6.0 ± 0.7 | 17.7 ± 0.1 |
| NR_5MMT | 7.5 ± 3.9 | 20.2 ± 2.0 |
| NR_1O-MMT | 8.8 ± 0.8 | 19.8 ± 0.4 |
| NR_3O-MMT | 6.7 ± 0.5 | 15.4 ± 0.6 |
| NR_5O-MMT | 4.8 ± 1.7 | 16.8 ± 0.2 |
| Trade Name | Code | Organic Modifier | Density (g/cm3) | d001 Spacing (nm) |
|---|---|---|---|---|
| Cloisite® Na+ | MMT | None | 2.86 | 1.17 |
| Cloisite® 20A | O-MMT |  | 1.80 | 2.70 |
| Sample | SMR 10 (phr) | Sulfur (phr) | ZnO (phr) | Stearic Acid (phr) | ZDBC (phr) | MMT (phr) | O-MMT (phr) |
|---|---|---|---|---|---|---|---|
| NR | 100.0 | 1.5 | 5.0 | 2.0 | 0.7 | - | - |
| NR_1MMT | 100.0 | 1.5 | 5.0 | 2.0 | 0.7 | 1.0 | - |
| NR_3MMT | 100.0 | 1.5 | 5.0 | 2.0 | 0.7 | 3.0 | - |
| NR_5MMT | 100.0 | 1.5 | 5.0 | 2.0 | 0.7 | 5.0 | - |
| NR_1O-MMT | 100.0 | 1.5 | 5.0 | 2.0 | 0.7 | - | 1.0 |
| NR_3O-MMT | 100.0 | 1.5 | 5.0 | 2.0 | 0.7 | - | 3.0 |
| NR_5O-MMT | 100.0 | 1.5 | 5.0 | 2.0 | 0.7 | - | 5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchi, M.; Fambri, L.; Bortolotti, M.; Pegoretti, A.; Dorigato, A. Elastocaloric Performance of Natural Rubber: The Role of Nanoclay Addition. Molecules 2025, 30, 3035. https://doi.org/10.3390/molecules30143035
Bianchi M, Fambri L, Bortolotti M, Pegoretti A, Dorigato A. Elastocaloric Performance of Natural Rubber: The Role of Nanoclay Addition. Molecules. 2025; 30(14):3035. https://doi.org/10.3390/molecules30143035
Chicago/Turabian StyleBianchi, Marica, Luca Fambri, Mauro Bortolotti, Alessandro Pegoretti, and Andrea Dorigato. 2025. "Elastocaloric Performance of Natural Rubber: The Role of Nanoclay Addition" Molecules 30, no. 14: 3035. https://doi.org/10.3390/molecules30143035
APA StyleBianchi, M., Fambri, L., Bortolotti, M., Pegoretti, A., & Dorigato, A. (2025). Elastocaloric Performance of Natural Rubber: The Role of Nanoclay Addition. Molecules, 30(14), 3035. https://doi.org/10.3390/molecules30143035








