Polymer-Based Mass Cytometry Reagents: Synthesis and Biomedical Applications
Abstract
1. Introduction
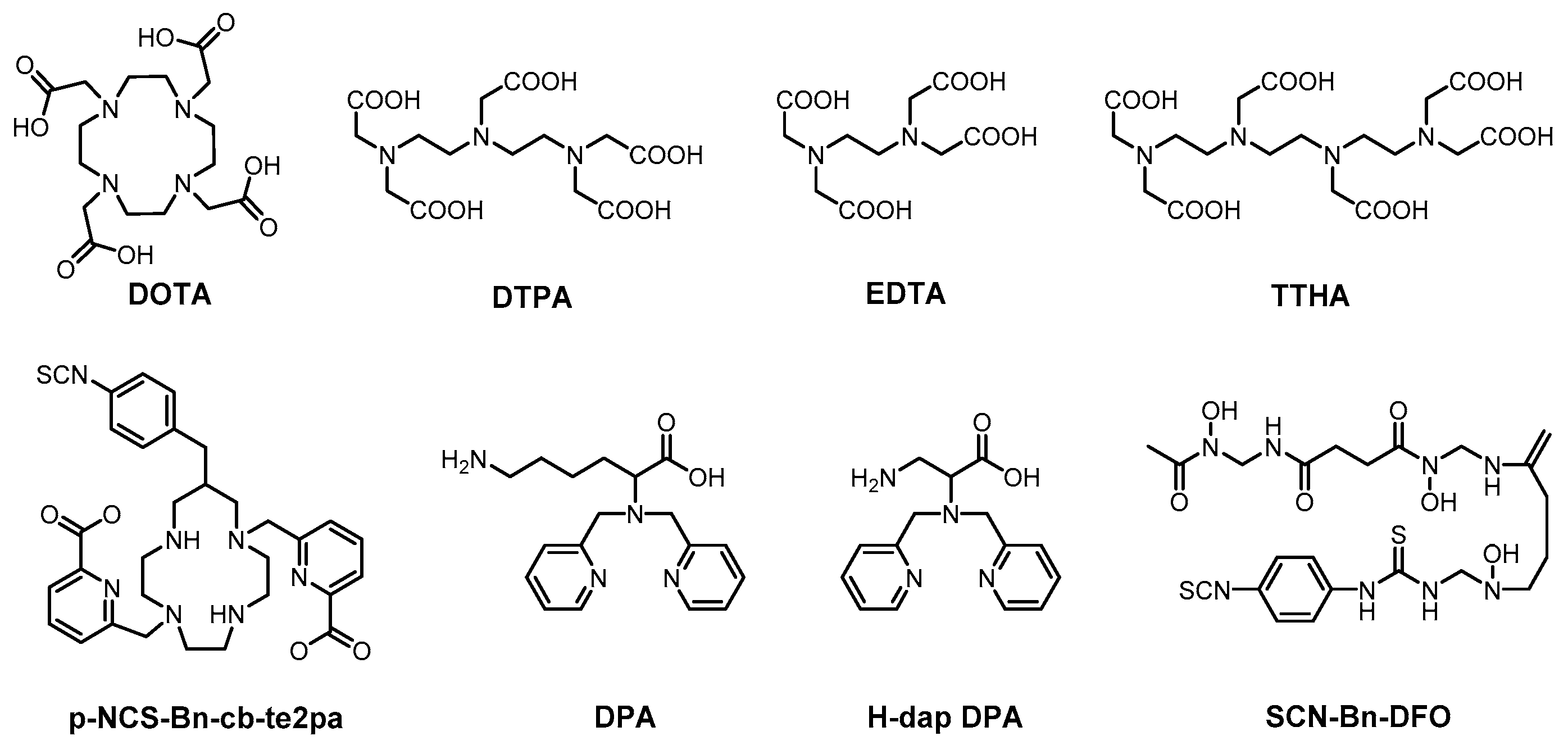
2. Metal-Chelating Polymers as Mass Cytometry Reagents
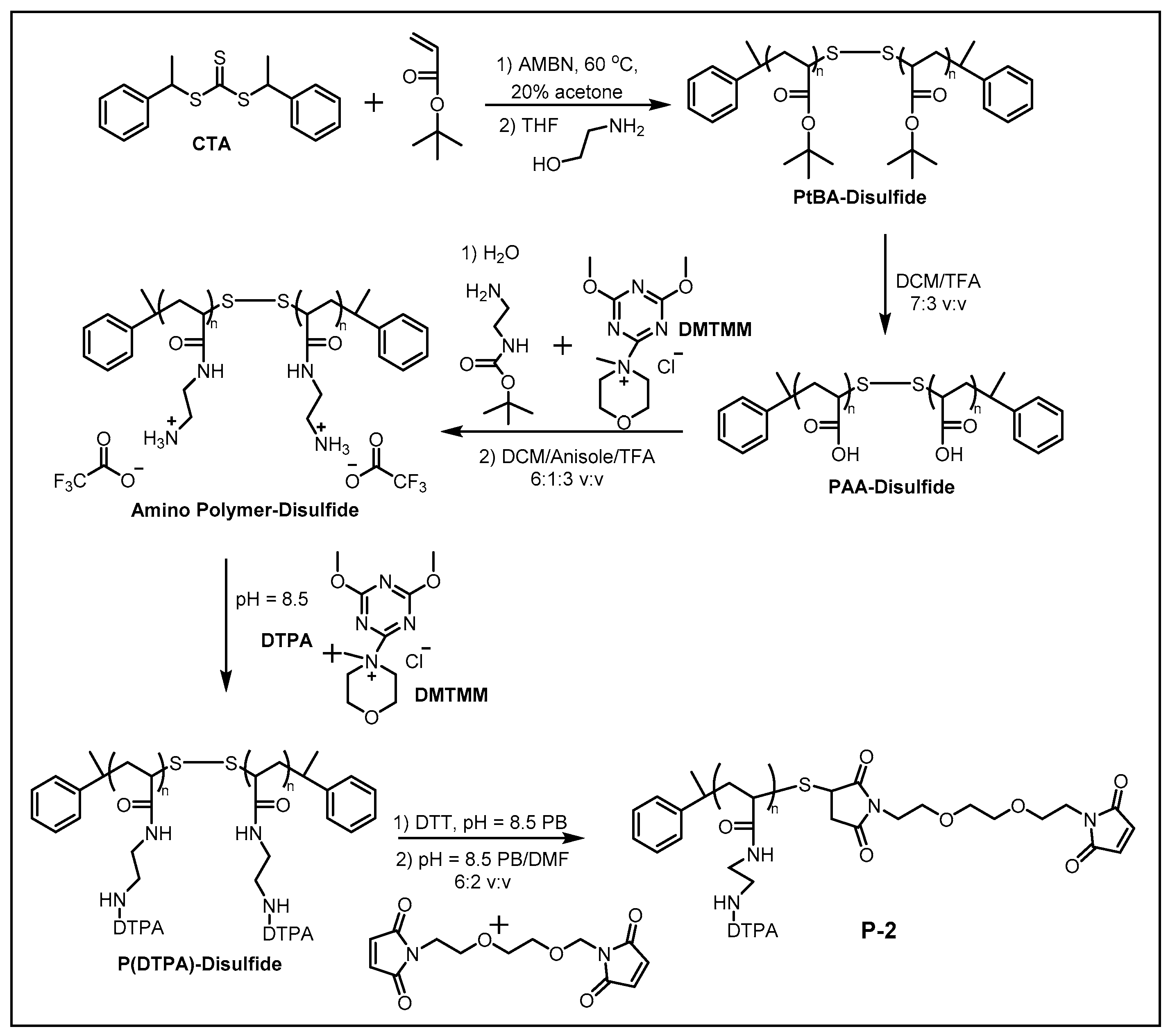
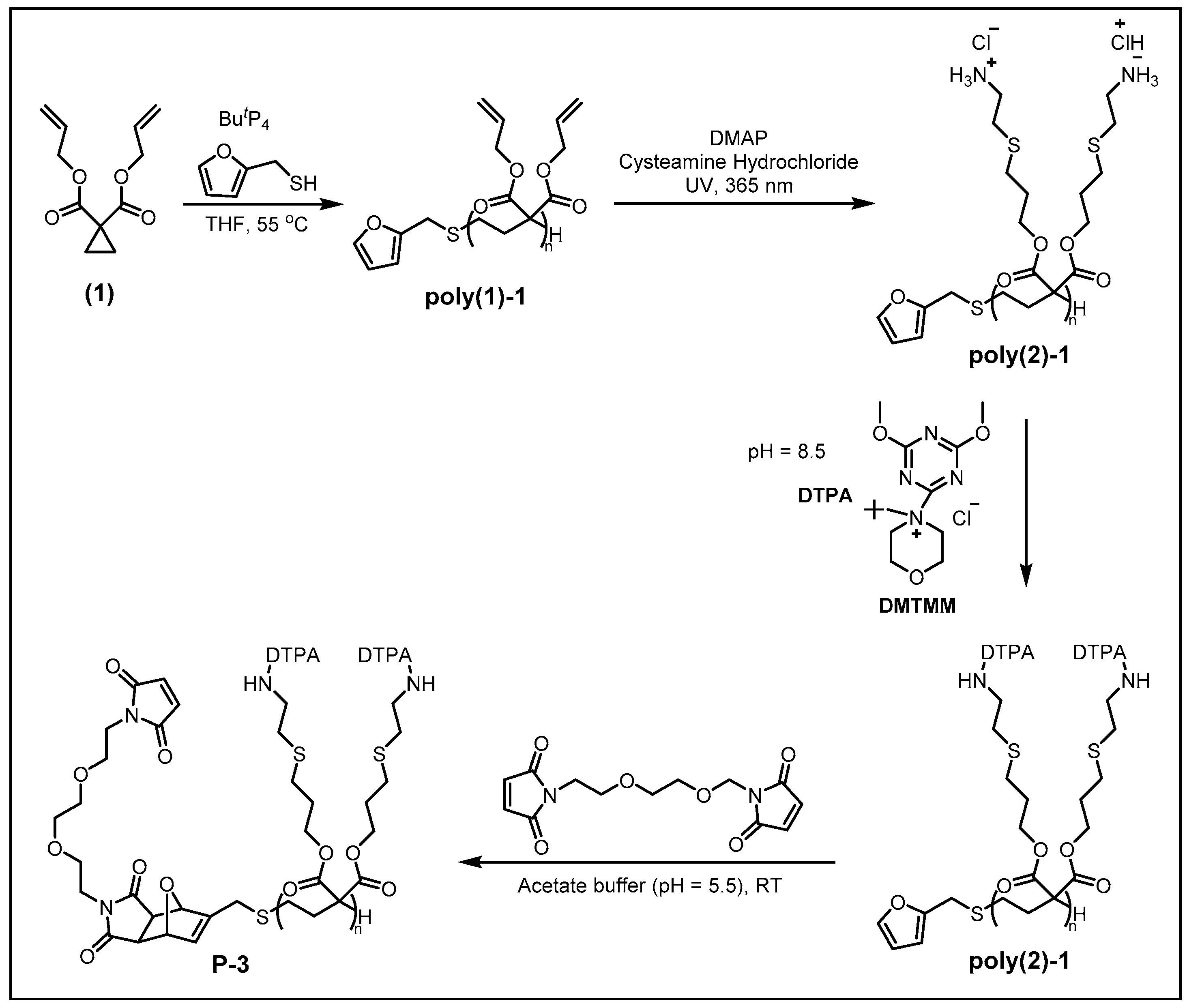
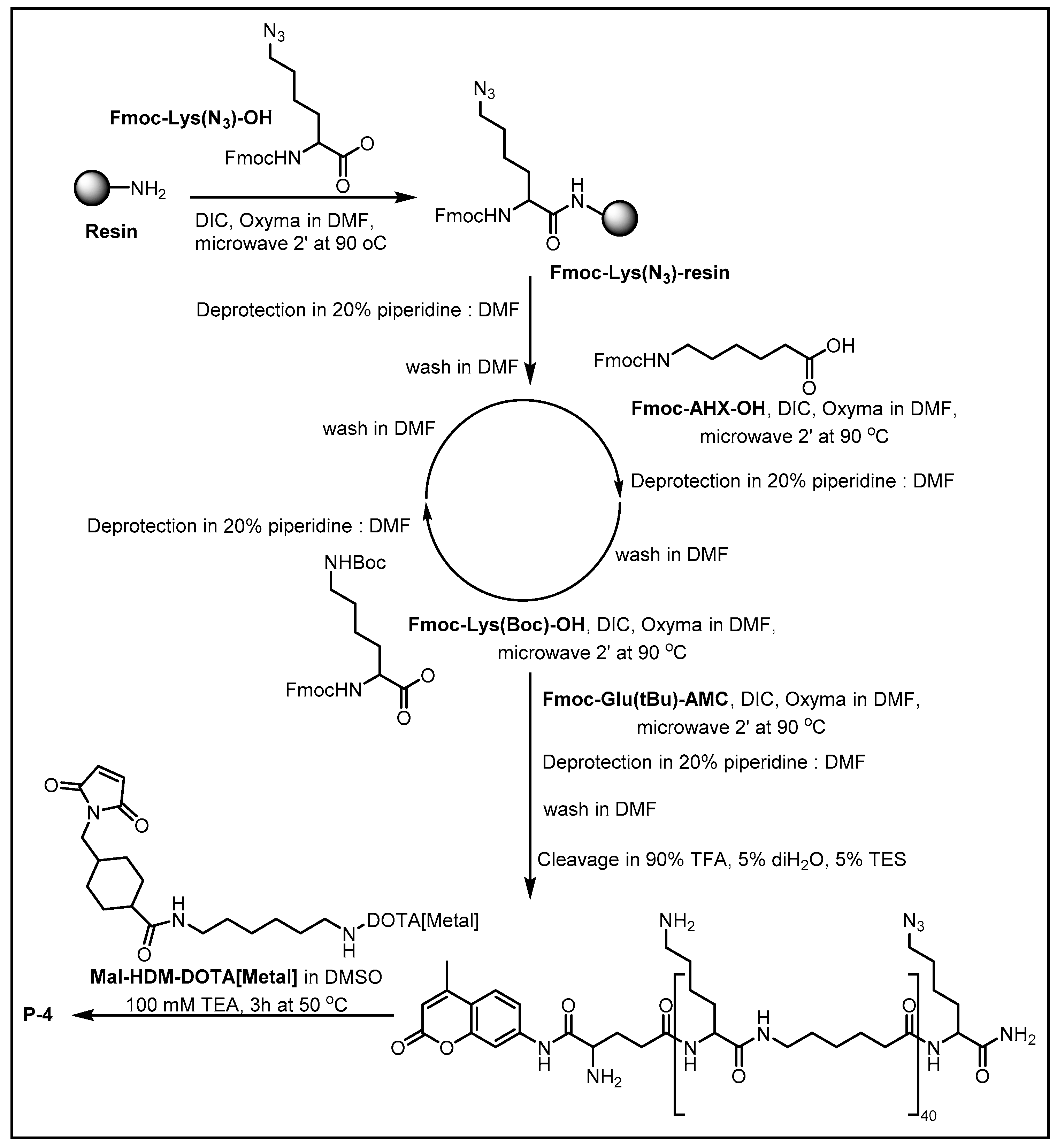
2.1. Synthesis of MCPs
2.2. Transition Metal-Chelating Polymers
2.3. Main-Group Metal-Chelating Polymers
3. Polymeric Particles as Mass Cytometry Regents
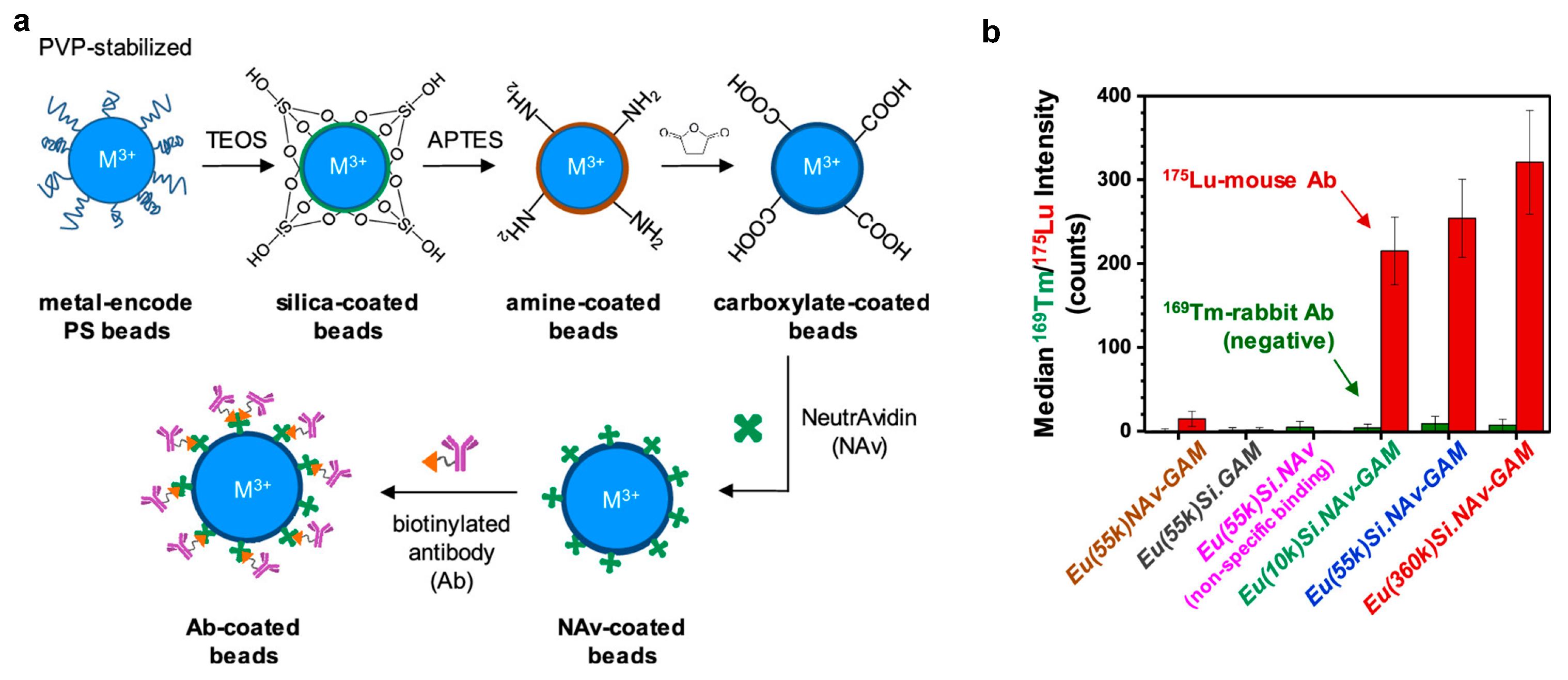
3.1. Polymer Microspheres
3.2. Polymer Dots
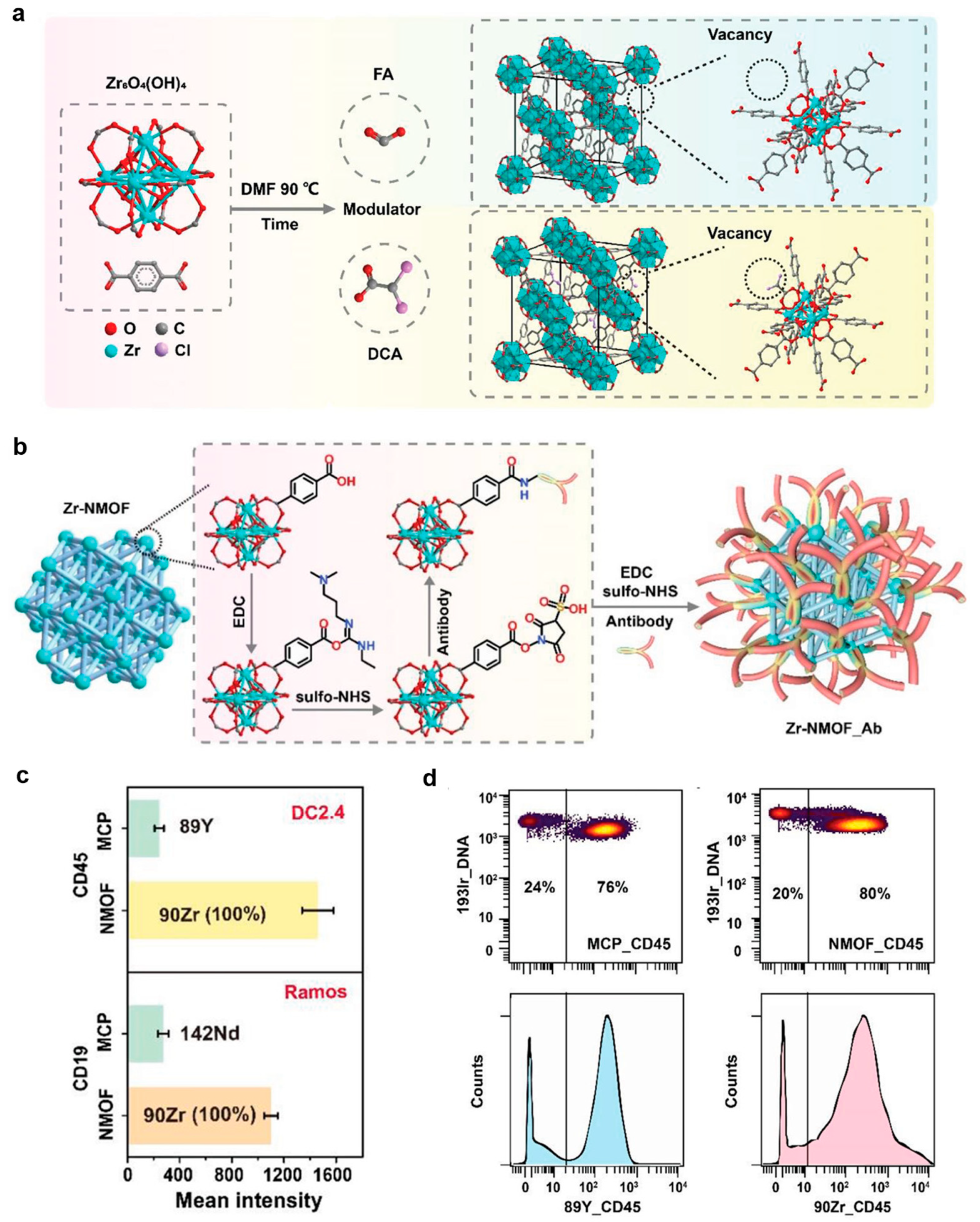
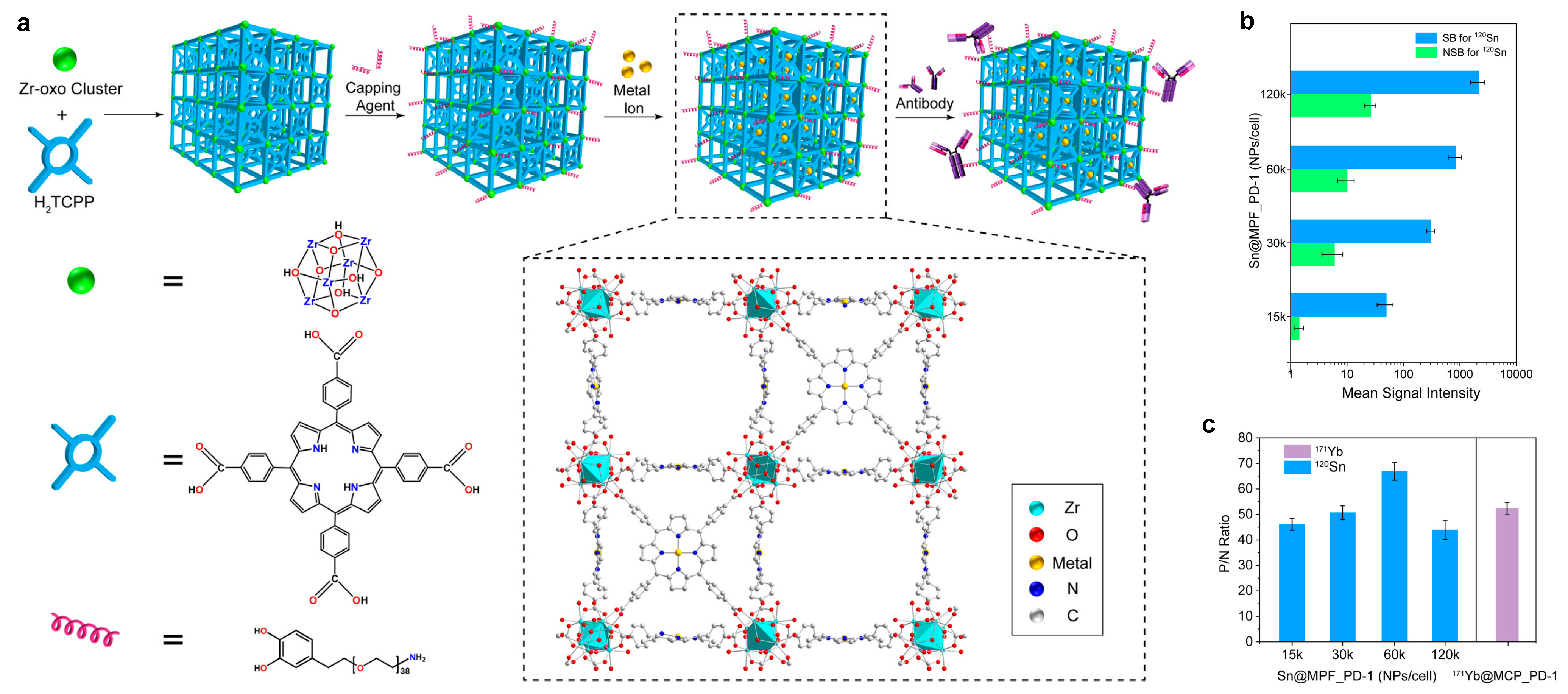
4. Metal–Organic Framework as Mass Cytometry Regents
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TOF-MS | Time-of-flight mass spectrometry |
| CyTOF | Time-of-flight cytometry |
| ICP-TOF-MS | Inductively coupled plasma time-of-flight mass spectrometry |
| MC | Mass cytometry |
| SMC | Suspension mass cytometry |
| IMC | Imaging mass cytometry |
| MCP | Metal-chelating polymer |
| m/z | Mass-to-charge ratio |
| MOF | Metal–organic framework |
| RAFT | Reversible addition–fragmentation chain transfer |
| Ln | Lanthanide |
| ITC | Isothermal titration calorimetry |
| DOTA | 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid |
| DTPA | Diethylenetriaminepentaacetic acid |
| TEPA | Tetraethylenepentamine |
| EDTA | Ethylenedia minetetraacetic acid |
| TTHA | Triethylenetetramine-N,N,N′,N′′,N′′′,N′′′-hexaacetic acid |
| DPA | 2,2′-Dipicolylamine |
| DFO | Deferoxamine |
| PtBA | Poly(t-butyl acrylate) |
| TFA | Trifluoroacetic acid |
| PAA | Poly(acrylic acid) |
| BOC | t-Butyloxy carbonyl |
| DTT | Dithiothreitol |
| SPPS | Solid phase peptide synthesis |
| TCO | Transcyclooctene |
| SPAAC | Strain-promoted alkyne-azide cycloaddition |
| DBCO | Dibenzocyclooctyne |
| MAP | Aptamer nanoprobe |
| PBMC | Peripheral blood mononuclear cell |
| NSB | Nonspecific binding |
| PEG | Polyethylene glycol |
| PSBMA | Poly(sulfobetaine methacrylate) |
| GSH | Glutathione |
| NK | Natural killer |
| NPs | Nanoparticles |
| Pdots | Polymer dots |
| PS | Polystyrene |
| PMMA | Poly(methyl methacrylate) |
| AA | Acrylic acid |
| AAEM | Acetoacetylethyl methacrylate |
| 2-DisP | Two-stage dispersion polymerization |
| P(S-MAA) | Poly(styrene-co-methacrylic acid) |
| MMA | Methacrylic acid |
| PVP | Polyvinylpyrrolidone |
| Ln-Pdots | Ln-coordinated Pdots |
| PC | Poly[(9,9-dioctylfluorenyl-2,7-diyl)-co-(1,4-benzo-{2,10,3}-thiadazole)] polymer |
| Zr-NMOFs | Zirconium-based nanoscale metal–organic frameworks |
| H2BDC | Terephthalic acid |
| ACQ | Aggregation-caused quenching |
| ROI | Region-of-interest |
| MPF | Mesoporous porphyrinic framework |
| APEB | 4-(Amino-polyethylene glycol-ethyl)benzene-1,2-diol |
References
- Janes, M.R.; Rommel, C. Next-generation flow cytometry. Nat. Biotechnol. 2011, 29, 602–604. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, M.H.; Nolan, G.P. Mass Cytometry: Single Cells, Many Features. Cell 2016, 165, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.A.; Vogel, C. New horizons in the stormy sea of multimodal single-cell data integration. Mol. Cell 2022, 82, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, F.J.; Bendall, S.C. Immune monitoring using mass cytometry and related high-dimensional imaging approaches. Nat. Rev. Rheumatol. 2019, 16, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Perfetto, S.P.; Chattopadhyay, P.K.; Roederer, M. Seventeen-colour flow cytometry: Unravelling the immune system. Nat. Rev. Immunol. 2004, 4, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Bendall, S.C.; Nolan, G.P. From single cells to deep phenotypes in cancer. Nat. Biotechnol. 2012, 30, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Bjornson, Z.B.; Nolan, G.P.; Fantl, W.J. Single-cell mass cytometry for analysis of immune system functional states. Curr. Opin. Immunol. 2013, 25, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Warden, A.R.; Li, Y.; Ding, X. Progress and applications of mass cytometry in sketching immune landscapes. Clin. Transl. Med. 2020, 10, e206. [Google Scholar] [CrossRef] [PubMed]
- Baranov, V.I.; Quinn, Z.; Bandura, D.R.; Tanner, S.D. A Sensitive and Quantitative Element-Tagged Immunoassay with ICPMS Detection. Anal. Chem. 2002, 74, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, F.; Zhang, Y.; Wang, X.; Zhang, X. A novel combination of immunoreaction and ICP-MS as a hyphenated technique for the determination of thyroid-stimulating hormone (TSH) in human serum. J. Anal. At. Spectrom. 2001, 16, 1393–1396. [Google Scholar] [CrossRef]
- Tanner, S.D.; Bandura, D.R.; Ornatsky, O.; Baranov, V.I.; Nitz, M.; Winnik, M.A. Flow cytometer with mass spectrometer detection for massively multiplexed single-cell biomarker assay. Pure Appl. Chem. 2008, 80, 2627–2641. [Google Scholar] [CrossRef]
- Bandura, D.R.; Baranov, V.I.; Ornatsky, O.I.; Antonov, A.; Kinach, R.; Lou, X.; Pavlov, S.; Vorobiev, S.; Dick, J.E.; Tanner, S.D. Mass Cytometry: Technique for Real Time Single Cell Multitarget Immunoassay Based on Inductively Coupled Plasma Time-of-Flight Mass Spectrometry. Anal. Chem. 2009, 81, 6813–6822. [Google Scholar] [CrossRef] [PubMed]
- Bendall, S.C.; Simonds, E.F.; Qiu, P.; Amir, E.D.; Krutzik, P.O.; Finck, R.; Bruggner, R.V.; Melamed, R.; Trejo, A.; Ornatsky, O.I.; et al. Single-Cell Mass Cytometry of Differential Immune and Drug Responses Across a Human Hematopoietic Continuum. Science 2011, 332, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Behbehani, G.K. Applications of Mass Cytometry in Clinical Medicine. Clin. Lab. Med. 2017, 37, 945–964. [Google Scholar] [CrossRef] [PubMed]
- Baca, Q.; Cosma, A.; Nolan, G.; Gaudilliere, B. The road ahead: Implementing mass cytometry in clinical studies, one cell at a time. Cytom. Part B 2017, 92, 10–11. [Google Scholar] [CrossRef]
- Tanner, S.D.; Baranov, V.I.; Ornatsky, O.I.; Bandura, D.R.; George, T.C. An introduction to mass cytometry: Fundamentals and applications. Curr. Opin. Immunol. 2013, 62, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Bruckner, K.; Zitterbart, R.; Seitz, O.; Beck, S.; Linscheid, M.W. Solid phase synthesis of short peptide-based multimetal tags for biomolecule labeling. Bioconjug. Chem. 2014, 25, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Bendall, S.C.; Nolan, G.P.; Roederer, M.; Chattopadhyay, P.K. A deep profiler’s guide to cytometry. Trends Immunol. 2012, 33, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Arnett, L.P.; Rana, R.; Chung, W.W.; Li, X.; Abtahi, M.; Majonis, D.; Bassan, J.; Nitz, M.; Winnik, M.A. Reagents for Mass Cytometry. Chem. Rev. 2023, 123, 1166–1205. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Gonzalez, A.; Sanchez-Martin, R.M. Mass Cytometry Tags: Where Chemistry Meets Single-Cell Analysis. Anal. Chem. 2021, 93, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, S.; Wei, C.; Xing, Z.; Zhang, S.; Zhang, X. Metal Stable Isotope Tagging: Renaissance of Radioimmunoassay for Multiplex and Absolute Quantification of Biomolecules. Acc. Chem. Res. 2016, 49, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Tibbetts, I.; Kostakis, G. Recent Bio-Advances in Metal-Organic Frameworks. Molecules 2020, 25, 1291. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Shi, S.; Liu, B. Metal-Organic Framework-Based Colloidal Particle Synthesis, Assembly, and Application. ChemPlusChem 2023, 88, e202200396. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, P.; Cui, J.; Cui, X.; Xing, H. The Chemistry of Metal–Organic Frameworks for Multicomponent Gas Separation. Angew. Chem. Int. Ed. 2024, 63, e202414503. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Chen, J.; Luo, X.; Qu, Y.; Zhou, M.; Xia, R.; Wang, W.; Zheng, X. Porphyrin-engineered nanoscale metal-organic frameworks: Enhancing photodynamic therapy and ferroptosis in oncology. Front. Pharmacol. 2024, 15, 1481168. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; Zhang, G.; Herrera, I.; Kinach, R.; Ornatsky, O.; Baranov, V.; Nitz, M.; Winnik, M.A. Polymer-based elemental tags for sensitive bioassays. Angew. Chem. Int. Ed. 2007, 46, 6111–6114. [Google Scholar] [CrossRef] [PubMed]
- Viola-Villegas, N.; Doyle, R.P. The coordination chemistry of 1,4,7,10-tetraazacyclododecane-N,N′,N′′,N′′′-tetraacetic acid (H4DOTA): Structural overview and analyses on structure–stability relationships. Coord. Chem. Rev. 2009, 253, 1906–1925. [Google Scholar] [CrossRef]
- Pałasz, A.; Czekaj, P. Toxicological and cytophysiological aspects of lanthanides action. Acta Biochim. Pol. 2020, 47, 1107–1114. [Google Scholar] [CrossRef]
- Harriswangler, C.; Frías, J.C.; Albelda, M.T.; Valencia, L.; García-España, E.; Esteban-Gómez, D.; Platas-Iglesias, C. Donor Radii in Rare-Earth Complexes. Inorg. Chem. 2023, 62, 17030–17040. [Google Scholar] [CrossRef] [PubMed]
- Shaklee, J.; Srivastava, K.; Brown, H.; Arriaga, E.A.; Pierre, V.C.; van Berlo, J.H. Development of a Click-Chemistry Reagent Compatible with Mass Cytometry. Sci. Rep. 2018, 8, 6657. [Google Scholar] [CrossRef] [PubMed]
- De León-Rodríguez, L.M.; Kovacs, Z. The Synthesis and Chelation Chemistry of DOTA-Peptide Conjugates. Bioconjug. Chem. 2008, 19, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Baranyai, Z.; Tircsó, G.; Rösch, F. The Use of the Macrocyclic Chelator DOTA in Radiochemical Separations. Eur. J. Inorg. Chem. 2019, 2020, 36–56. [Google Scholar] [CrossRef]
- Chilla, S.N.M.; Henoumont, C.; Elst, L.V.; Muller, R.N.; Laurent, S. Importance of DOTA derivatives in bimodal imaging. Isr. J. Chem. 2017, 57, 800–808. [Google Scholar] [CrossRef]
- Harriswangler, C.; Argibay-Otero, S.; Freire-García, A.; Albelda, M.T.; García-España, E.; Esteban-Gómez, D.; Frías, J.C.; Platas-Iglesias, C. Dynamics of Lanthanide(III) complexes from crystallographic data and computational studies. Inorg. Chem. Commun. 2024, 170, 113348. [Google Scholar] [CrossRef]
- Majonis, D.; Herrera, I.; Ornatsky, O.; Schulze, M.; Lou, X.; Soleimani, M.; Nitz, M.; Winnik, M.A. Synthesis of a Functional Metal-Chelating Polymer and Steps toward Quantitative Mass Cytometry Bioassays. Anal. Chem. 2010, 82, 8961–8969. [Google Scholar] [CrossRef] [PubMed]
- Illy, N.; Majonis, D.; Herrera, I.; Ornatsky, O.; Winnik, M.A. Metal-chelating polymers by anionic ring-opening polymerization and their use in quantitative mass cytometry. Biomacromolecules 2012, 13, 2359–2369. [Google Scholar] [CrossRef] [PubMed]
- Varnava, K.G.; Sarojini, V. Making Solid-Phase Peptide Synthesis Greener: A Review of the Literature. Chem. Asian J. 2019, 14, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Noki, S.; de la Torre, B.G.; Albericio, F. Safety-Catch Linkers for Solid-Phase Peptide Synthesis. Molecules 2024, 29, 1429. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Verma, S.; Chen, Y.-C. Peptide Dendrimer-Based Antibacterial Agents: Synthesis and Applications. ACS Infect. Dis. 2024, 10, 1034–1055. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Lubell, W.D. N-to C-Peptide Synthesis, Arguably the Future for Sustainable Production. J. Pept. Sci. 2025, 31, e70019. [Google Scholar] [CrossRef] [PubMed]
- Verhoeff, J.; van Asten, S.; Kuijper, L.; van den Braber, M.; Amstalden-van Hove, E.; Haselberg, R.; Kalay, H.; Garcia-Vallejo, J.J. A monodispersed metal-complexing peptide-based polymer for mass cytometry enabling spectral applications. New Biotechnol. 2024, 81, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Rosman, K.J.R.; Taylor, P.D.P. Isotopic compositions of the elements 1997 (Technical Report). Pure Appl. Chem. 1998, 70, 217–235. [Google Scholar] [CrossRef]
- Leipold, M.D.; Herrera, I.; Ornatsky, O.; Baranov, V.; Nitz, M. ICP-MS-Based Multiplex Profiling of Glycoproteins Using Lectins Conjugated to Lanthanide-Chelating Polymers. J. Proteome Res. 2009, 8, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Leipold, M.D.; Ornatsky, O.; Baranov, V.; Whitfield, C.; Nitz, M. Development of mass cytometry methods for bacterial discrimination. Anal. Biochem. 2011, 419, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Allo, B.; Lou, X.; Bouzekri, A.; Ornatsky, O. Clickable and High-Sensitivity Metal-Containing Tags for Mass Cytometry. Bioconjug. Chem. 2018, 29, 2028–2038. [Google Scholar] [CrossRef] [PubMed]
- Majonis, D.; Ornatsky, O.; Kinach, R.; Winnik, M.A. Curious results with palladium- and platinum-carrying polymers in mass cytometry bioassays and an unexpected application as a dead cell stain. Biomacromolecules 2011, 12, 3997–4010. [Google Scholar] [CrossRef] [PubMed]
- Muftuoglu, M.; Li, L.; Liang, S.; Mak, D.; Lin, A.J.; Fang, J.; Burks, J.K.; Chen, K.; Andreeff, M. Extended live-cell barcoding approach for multiplexed mass cytometry. Sci. Rep. 2021, 11, 12388. [Google Scholar] [CrossRef] [PubMed]
- Majonis, D.; Ornatsky, O.; Weinrich, D.; Winnik, M.A. Dual-purpose polymer labels for fluorescent and mass cytometric affinity bioassays. Biomacromolecules 2013, 14, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
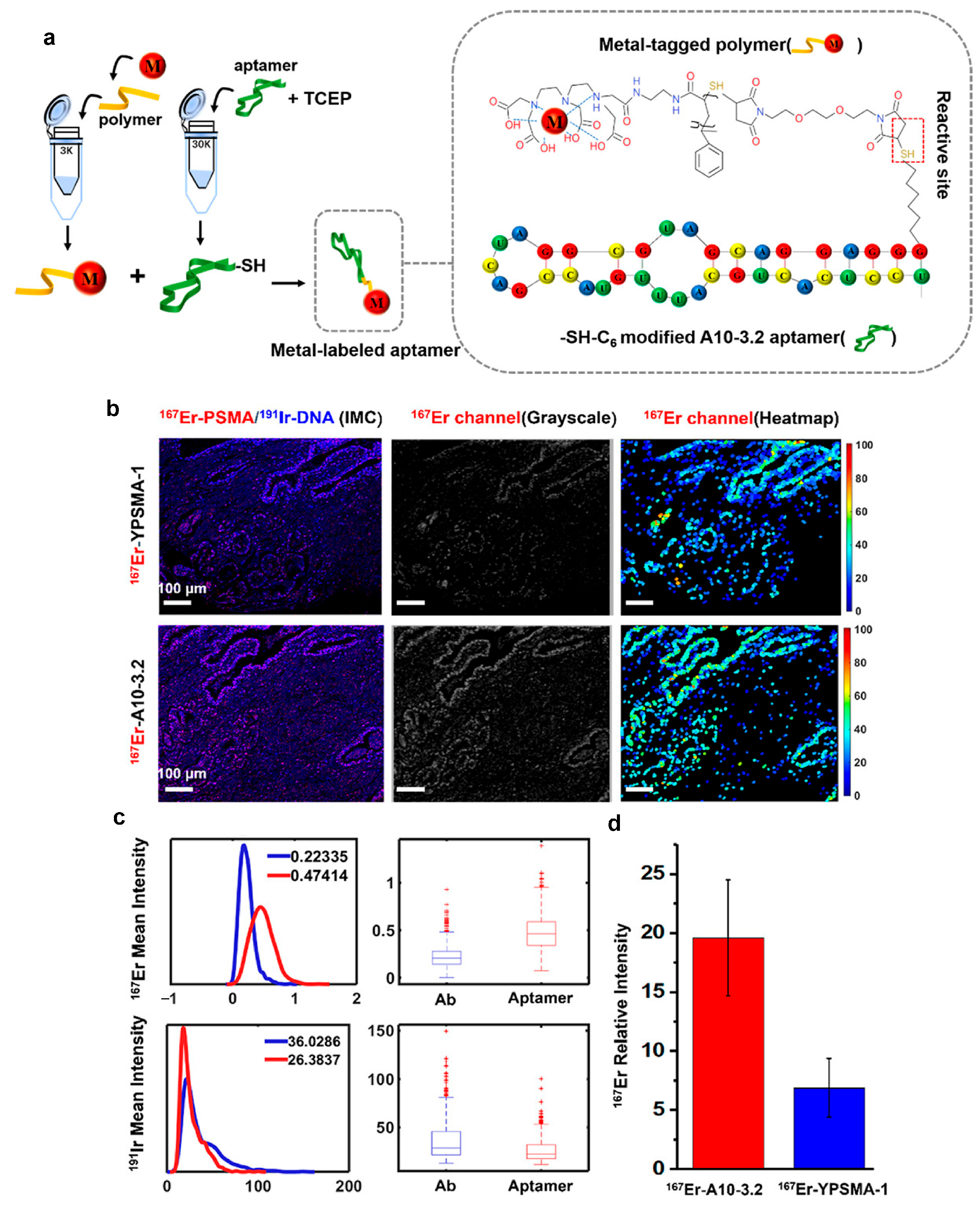
- Yu, Y.; Dang, J.; Liu, X.; Wang, L.; Li, S.; Zhang, T.; Ding, X. Metal-Labeled Aptamers as Novel Nanoprobes for Imaging Mass Cytometry Analysis. Anal. Chem. 2020, 92, 6312–6320. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Liu, P.; Pichaandi, J.; Closson, T.L.L.; Majonis, D.; Leighton, P.L.A.; Swanson, E.; Ornatsky, O.; Baranov, V.; Winnik, M.A. A metal-chelating polymer for chelating zirconium and its use in mass cytometry. Eur. Polym. J. 2019, 120, 109175. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, P.; Majonis, D.; Winnik, M.A. Polymeric dipicolylamine based mass tags for mass cytometry. Chem. Sci. 2022, 13, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.C.N.; Zhang, Y.; Yang, T.; Liu, Y.; Abtahi, M.; Chen, X.; Ajayi, A.J.; Li, X.; Majonis, D.; Winnik, M.A. Optimizing the Structure of a Pt Metal-Chelating Polymer to Reduce Nonspecific Binding for Mass Cytometry. Biomacromolecules 2024, 25, 6716–6726. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.C.N.; Yang, T.; Zhang, Y.; Liu, Y.; Majonis, D.; Abtahi, M.; Chen, X.; Li, X.; Hawrysh, P.; Closson, T.; et al. A platinum polymer probe for multiplexed single cell suspension mass cytometry and imaging mass cytometry assays. Chem. Eng. J. 2025, 505, 159745. [Google Scholar] [CrossRef]
- Han, G.; Chen, S.Y.; Gonzalez, V.D.; Zunder, E.R.; Fantl, W.J.; Nolan, G.P. Atomic mass tag of bismuth-209 for increasing the immunoassay multiplexing capacity of mass cytometry. Cytom. A 2017, 91, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Spitzer, M.H.; Bendall, S.C.; Fantl, W.J.; Nolan, G.P. Metal-isotope-tagged monoclonal antibodies for high-dimensional mass cytometry. Nat. Protoc. 2018, 13, 2121–2148. [Google Scholar] [CrossRef] [PubMed]
- Grenier, L.; Beyler, M.; Closson, T.; Zabinyakov, N.; Bouzekri, A.; Zhang, Y.; Pichaandi, J.M.; Winnik, M.A.; Liu, P.; Ornatsky, O.I.; et al. Enabling Indium Channels for Mass Cytometry by Using Reinforced Cyclam-Based Chelating Polylysine. Bioconjug. Chem. 2020, 31, 2103–2115. [Google Scholar] [CrossRef] [PubMed]
- Grenier, L.; Beyler, M.; Platas-Iglesias, C.; Closson, T.; Gómez, D.E.; Seferos, D.S.; Liu, P.; Ornatsky, O.I.; Baranov, V.; Tripier, R. Highly Stable and Inert Complexation of Indium(III) by Reinforced Cyclam Dipicolinate and a Bifunctional Derivative for Bead Encoding in Mass Cytometry. Chem. Eur. J. 2019, 25, 15387–15400. [Google Scholar] [CrossRef] [PubMed]
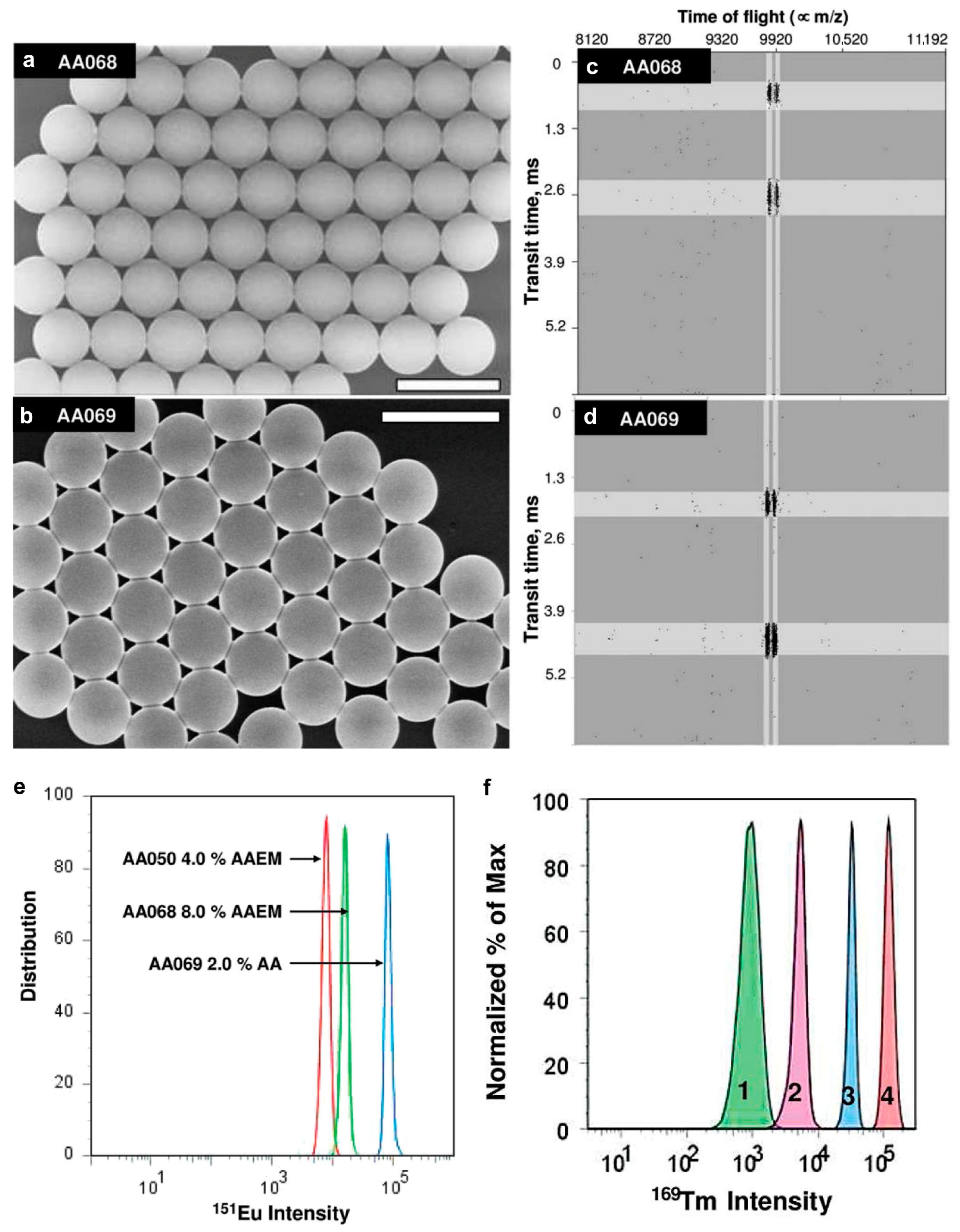
- Abdelrahman, A.I.; Dai, S.; Thickett, S.C.; Ornatsky, O.; Bandura, D.; Baranov, V.; Winnik, M.A. Lanthanide-Containing Polymer Microspheres by Multiple-Stage Dispersion Polymerization for Highly Multiplexed Bioassays. J. Am. Chem. Soc. 2009, 131, 15276–15283. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Abdelrahman, A.I.; Baranov, V.; Winnik, M.A. The synthesis and characterization of lanthanide-encoded poly(styrene-co-methacrylic acid) microspheres. Polymer 2011, 52, 5040–5052. [Google Scholar] [CrossRef]
- Liang, Y.; Abdelrahman, A.I.; Baranov, V.; Winnik, M.A. The release and extraction of lanthanide ions from metal-encoded poly (styrene-co-methacrylic acid) microspheres. Polymer 2012, 53, 998–1004. [Google Scholar] [CrossRef]
- Liu, J.; Wong, E.C.N.; Lu, E.; Jarzabek, J.; Majonis, D.; Winnik, M.A. Control of Metal Content in Polystyrene Microbeads Prepared with Metal Complexes of DTPA Derivatives. Chem. Mater. 2021, 33, 3802–3813. [Google Scholar] [CrossRef]
- Liu, J.; Jarzabek, J.; Roberts, M.; Majonis, D.; Winnik, M.A. A Silica Coating Approach to Enhance Bioconjugation on Metal-Encoded Polystyrene Microbeads for Bead-Based Assays in Mass Cytometry. Langmuir 2021, 37, 8240–8252. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Allo, B.; Winnik, M.A. Development of Multiplexed Bead-Based Immunoassays for Profiling Soluble Cytokines and CD163 Using Mass Cytometry. ACS Meas. Sci. Au 2022, 2, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Budzinski, L.; Schulz, A.R.; Baumgart, S.; Burns, T.; Rose, T.; Hirseland, H.; Mei, H.E. Osmium-Labeled Microspheres for Bead-Based Assays in Mass Cytometry. J. Immunol. 2019, 202, 3103–3112. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Zhao, G.; Zeng, Z.; Winnik, M.A. PMMA Microspheres with Embedded Lanthanide Nanoparticles by Photoinitiated Dispersion Polymerization with a Carboxy-Functional Macro-RAFT Agent. Macromolecules 2015, 48, 3629–3640. [Google Scholar] [CrossRef]
- Vancaeyzeele, C.; Ornatsky, O.; Baranov, V.; Shen, L.; Abdelrahman, A.; Winnik, M.A. Lanthanide-Containing Polymer Nanoparticles for Biological Tagging Applications: Nonspecific Endocytosis and Cell Adhesion. J. Am. Chem. Soc. 2007, 129, 13653–13660. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Gonzalez, A.; Laz-Ruiz, J.A.; Cano-Cortes, M.V.; Huang, Y.-W.; Gonzalez, V.D.; Diaz-Mochon, J.J.; Fantl, W.J.; Sanchez-Martin, R.M. Hybrid Fluorescent Mass-Tag Nanotrackers as Universal Reagents for Long-Term Live-Cell Barcoding. Anal. Chem. 2022, 94, 10626–10635. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, Y.; Zhao, X.; Wang, T.; He, L.; Nan, X.; Vidović, D.; Bai, P. A universal mass tag based on polystyrene nanoparticles for single-cell multiplexing with mass cytometry. J. Colloid Interface Sci. 2023, 639, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; DeGottardi, Q.; Wu, I.C.; Yu, J.; Wu, L.; Ye, F.; Kuo, C.T.; Kwok, W.W.; Chiu, D.T. Lanthanide-Coordinated Semiconducting Polymer Dots Used for Flow Cytometry and Mass Cytometry. Angew. Chem. Int. Ed. 2017, 56, 14908–14912. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; DeGottardi, Q.; Wu, I.C.; Wu, L.; Yu, J.; Kwok, W.W.; Chiu, D.T. Ratiometric Barcoding for Mass Cytometry. Anal. Chem. 2018, 90, 10688–10694. [Google Scholar] [CrossRef]
- Zhu, Y.; Xin, N.; Qiao, Z.; Chen, S.; Zeng, L.; Zhang, Y.; Wei, D.; Sun, J.; Fan, H. Bioactive MOFs Based Theranostic Agent for Highly Effective Combination of Multimodal Imaging and Chemo-Phototherapy. Adv. Healthc. Mater. 2020, 9, 2000205. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal–Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, 1707365. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, T.; He, L.; Nan, X.; Sun, X.; Bai, P. A double emission turn-on Eu-MOF-based luminescent sensor towards an anthrax biomarker. Microchem. J. 2022, 180, 107618. [Google Scholar] [CrossRef]
- Lu, W.; Wei, Z.; Gu, Z.-Y.; Liu, T.-F.; Park, J.; Park, J.; Tian, J.; Zhang, M.; Zhang, Q.; Gentle Iii, T.; et al. Tuning the structure and function of metal–organic frameworks via linker design. Chem. Soc. Rev. 2014, 43, 5561–5593. [Google Scholar] [CrossRef] [PubMed]
- Kirchon, A.; Feng, L.; Drake, H.F.; Joseph, E.A.; Zhou, H.-C. From fundamentals to applications: A toolbox for robust and multifunctional MOF materials. Chem. Soc. Rev. 2018, 47, 8611–8638. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.; Li, H.; Zhang, L.; Li, S.; Zhang, T.; Huang, S.; Li, Y.; Huang, C.; Ke, Y.; Shen, G.; et al. New Structure Mass Tag based on Zr-NMOF for Multiparameter and Sensitive Single-Cell Interrogating in Mass Cytometry. Adv. Mater. 2021, 33, 2008297. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ye, T.; Wang, X.; Li, K.; Li, Y.; Jiang, L.; Ding, X. Luminescent Metal–Organic Framework Probes with Metallic and Fluorescent Dual-Properties for Mass Cytometry and Imaging. Anal. Chem. 2025, 97, 7906–7918. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, G.; Wang, P.; Liu, J.; Shi, H.; Zhao, J.; Zeng, X.; Luo, Y. Metal-Chelatable Porphyrinic Frameworks for Single-Cell Multiplexing with Mass Cytometry. Angew. Chem. Int. Ed. 2022, 61, e202208640. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, L.; Nan, X.; Zhao, X.; He, L.; Wang, T.; Bai, P. New perspective of non-specific binding: A powerful mass cytometry barcoding strategy based on UIO-66(Hf/Zr) typed MOF utilizing its strong positive charge. Chem. Eng. J. 2024, 496, 154118. [Google Scholar] [CrossRef]

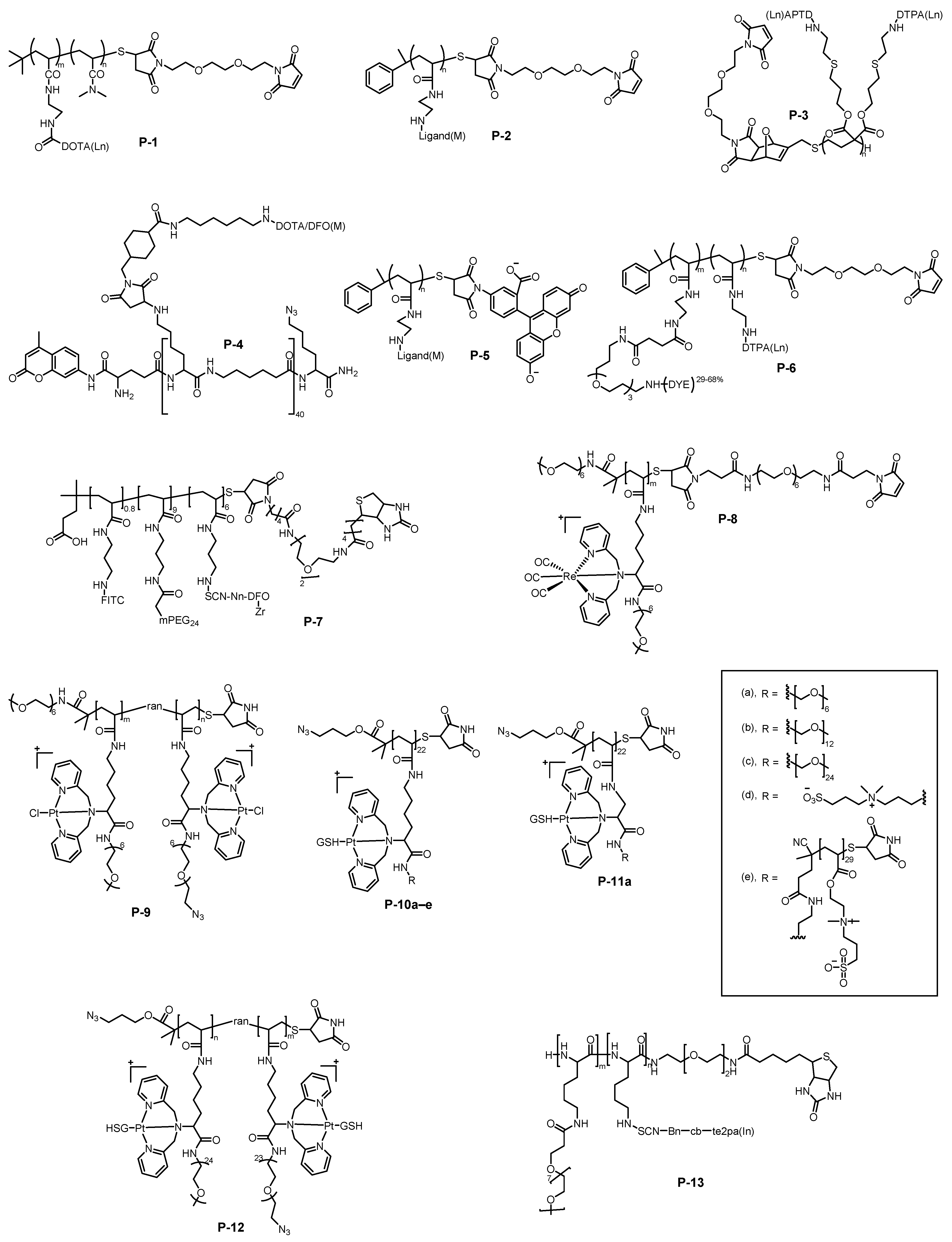
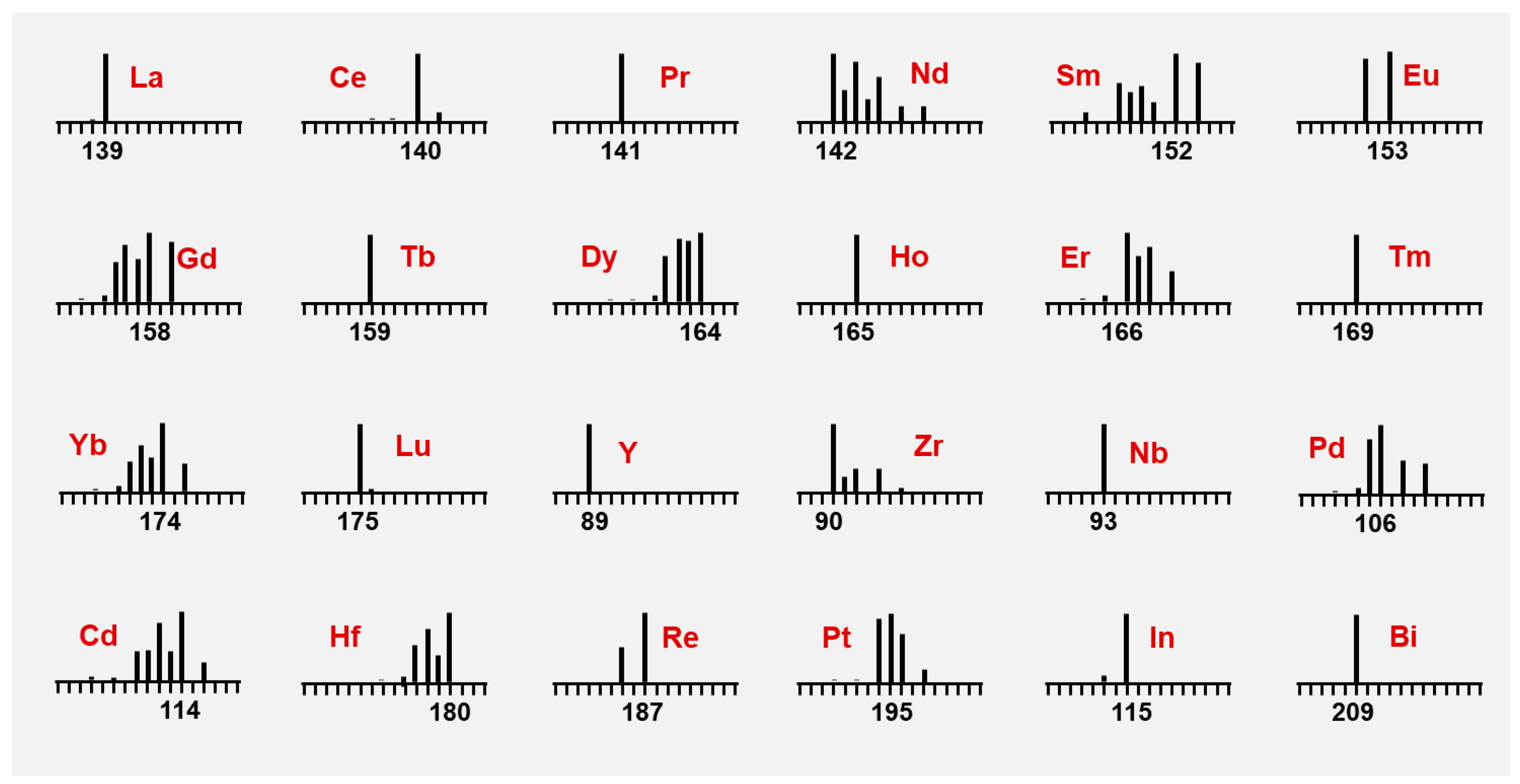


| Element | Isotope | Abundance (%) | Element | Isotope | Abundance (%) |
|---|---|---|---|---|---|
| Lanthanum | 138La | 0.09 | Ytterbium | 168Yb | 0.13 |
| 139La | 99.91 | 170Yb | 3.04 | ||
| Cerium | 136Ce | 0.185 | 171Yb | 14.28 | |
| 138Ce | 0.251 | 172Yb | 21.83 | ||
| 140Ce | 88.450 | 173Yb | 16.13 | ||
| 142Ce | 11.114 | 174Yb | 31.83 | ||
| Praseodymium | 141Pr | 100 | 176Yb | 12.76 | |
| Neodymium | 142Nd | 27.2 | Lutetium | 175Lu | 97.41 |
| 143Nd | 12.2 | 176Lu | 2.59 | ||
| 144Nd | 23.8 | Yttrium | 89Y | 100 | |
| 145Nd | 8.3 | Zirconium | 90Zr | 51.45 | |
| 146Nd | 17.2 | 91Zr | 11.22 | ||
| 148Nd | 5.7 | 92Zr | 17.15 | ||
| 150Nd | 5.6 | 94Zr | 17.38 | ||
| Samarium | 144Sm | 3.07 | 96Zr | 2.80 | |
| 147Sm | 14.99 | Niobium | 93Nb | 100 | |
| 148Sm | 11.24 | Palladium | 102Pd | 1.02 | |
| 149Sm | 13.82 | 104Pd | 11.14 | ||
| 150Sm | 7.38 | 105Pd | 22.33 | ||
| 152Sm | 26.75 | 106Pd | 27.33 | ||
| 154Sm | 22.75 | 108Pd | 26.46 | ||
| Europium | 151Eu | 47.81 | 110Pd | 11.72 | |
| 153Eu | 52.19 | Cadmium | 106Cd | 1.25 | |
| Gadolinium | 152Gd | 0.20 | 108Cd | 0.89 | |
| 154Gd | 2.18 | 110Cd | 12.49 | ||
| 155Gd | 14.80 | 111Cd | 12.80 | ||
| 156Gd | 20.47 | 112Cd | 24.13 | ||
| 157Gd | 15.65 | 113Cd | 12.22 | ||
| 158Gd | 24.84 | 114Cd | 28.73 | ||
| 160Gd | 21.86 | 116Cd | 7.49 | ||
| Terbium | 159Tb | 100 | Hafnium | 174Hf | 0.16 |
| Dysprosium | 156Dy | 0.06 | 176Hf | 5.26 | |
| 158Dy | 0.10 | 177Hf | 18.60 | ||
| 160Dy | 2.34 | 178Hf | 27.28 | ||
| 161Dy | 18.91 | 179Hf | 13.62 | ||
| 162Dy | 25.51 | 180Hf | 35.08 | ||
| 163Dy | 24.90 | Rhenium | 185Re | 37.40 | |
| 164Dy | 28.18 | 187Re | 62.60 | ||
| Holmium | 165Ho | 100 | Platinum | 190Pt | 0.014 |
| Erbium | 162Er | 0.14 | 192Pt | 0.782 | |
| 164Er | 1.61 | 194Pt | 32.967 | ||
| 166Er | 33.61 | 195Pt | 33.832 | ||
| 167Er | 22.93 | 196Pt | 25.242 | ||
| 168Er | 26.78 | 198Pt | 7.163 | ||
| 170Er | 14.93 | Indium | 113In | 4.29 | |
| Thulium | 169Tm | 100 | 115In | 95.71 | |
| Bismuth | 209Bi | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-F.; Wu, W.; Ge, Y.-H. Polymer-Based Mass Cytometry Reagents: Synthesis and Biomedical Applications. Molecules 2025, 30, 3034. https://doi.org/10.3390/molecules30143034
Wang Y-F, Wu W, Ge Y-H. Polymer-Based Mass Cytometry Reagents: Synthesis and Biomedical Applications. Molecules. 2025; 30(14):3034. https://doi.org/10.3390/molecules30143034
Chicago/Turabian StyleWang, Yin-Feng, Wenying Wu, and Ya-Hui Ge. 2025. "Polymer-Based Mass Cytometry Reagents: Synthesis and Biomedical Applications" Molecules 30, no. 14: 3034. https://doi.org/10.3390/molecules30143034
APA StyleWang, Y.-F., Wu, W., & Ge, Y.-H. (2025). Polymer-Based Mass Cytometry Reagents: Synthesis and Biomedical Applications. Molecules, 30(14), 3034. https://doi.org/10.3390/molecules30143034







