Diastereoselective Synthesis and Biological Evaluation of Spiro[chromane-2,4′-pyrimidin]-2′(3′H)-ones as Novel Antimicrobial and Antioxidant Agents
Abstract
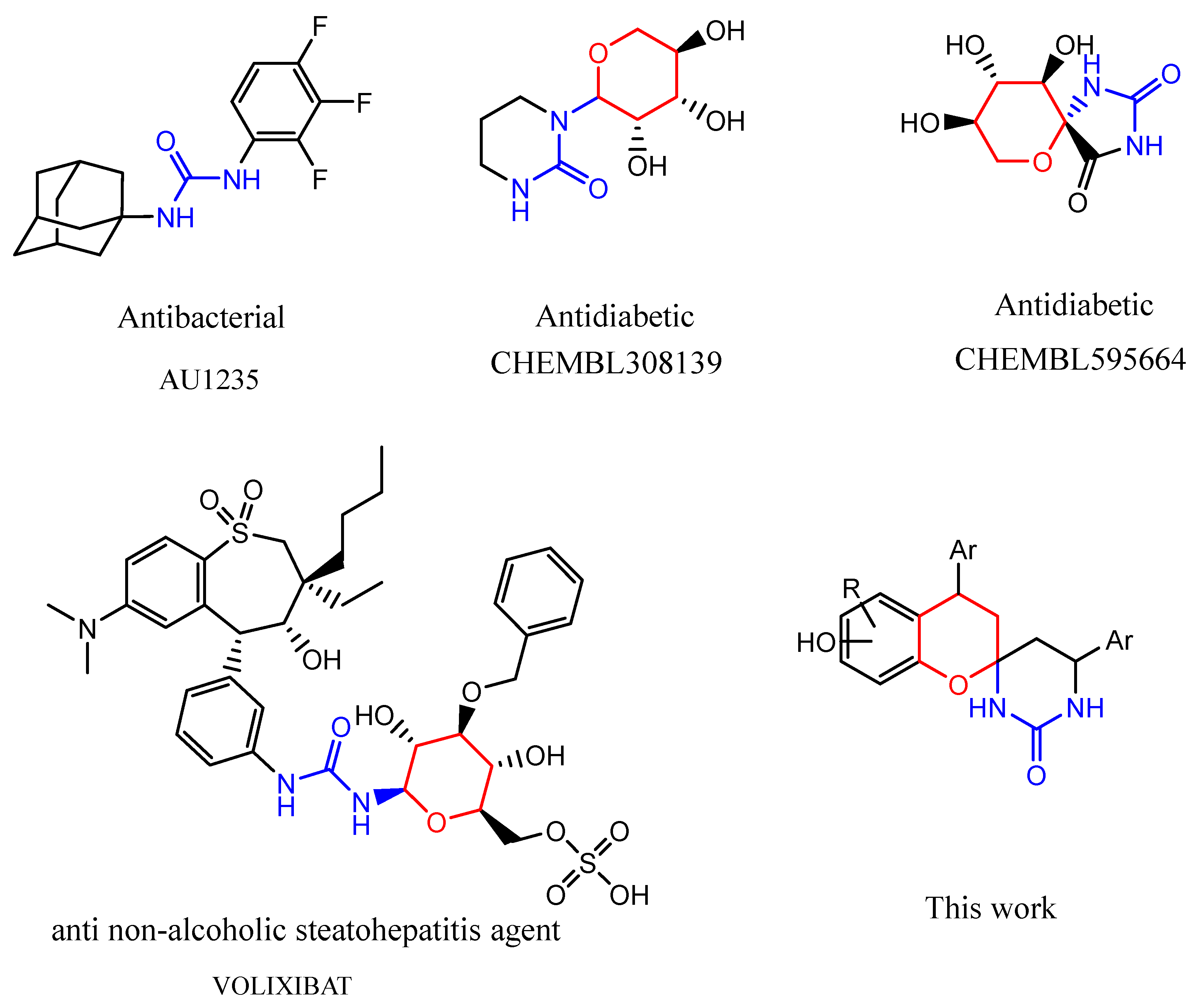
1. Introduction
2. Results and Discussion
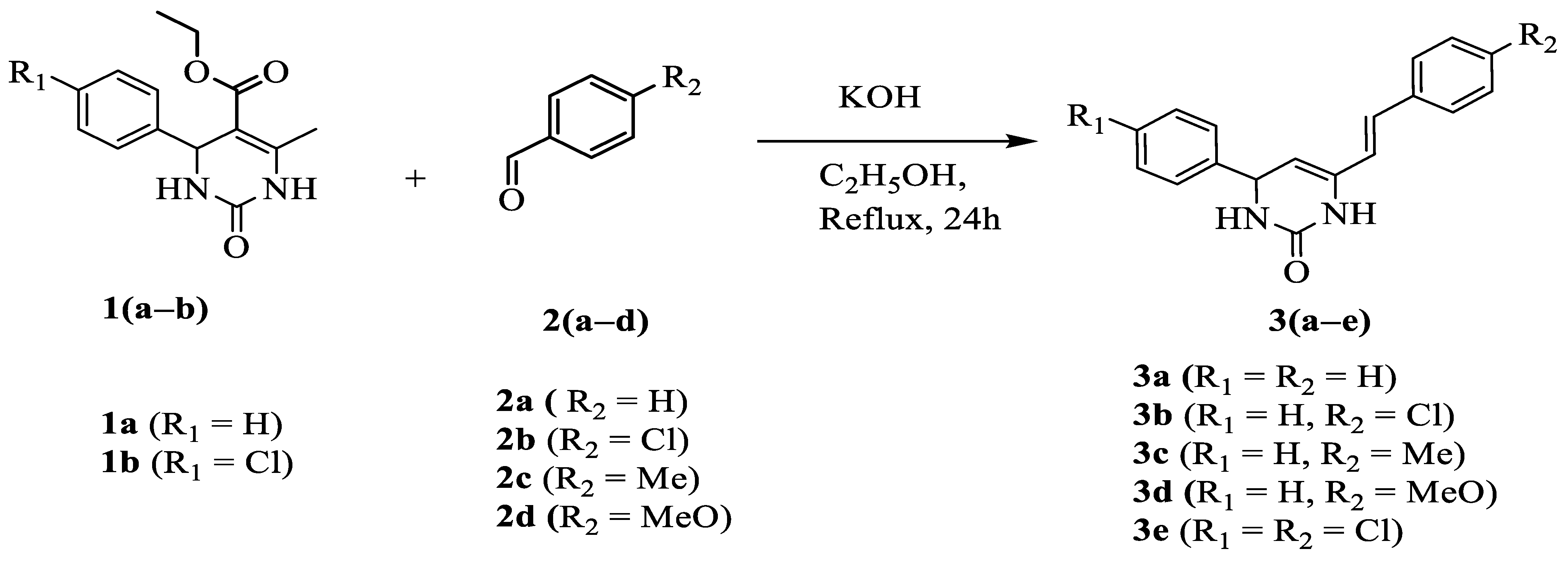
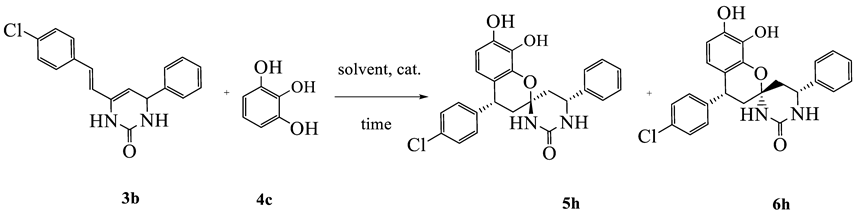
2.1. Chemistry
2.2. X-Ray Studies
2.3. Biological Studies
2.3.1. Minimal Inhibition Concentration (MIC)
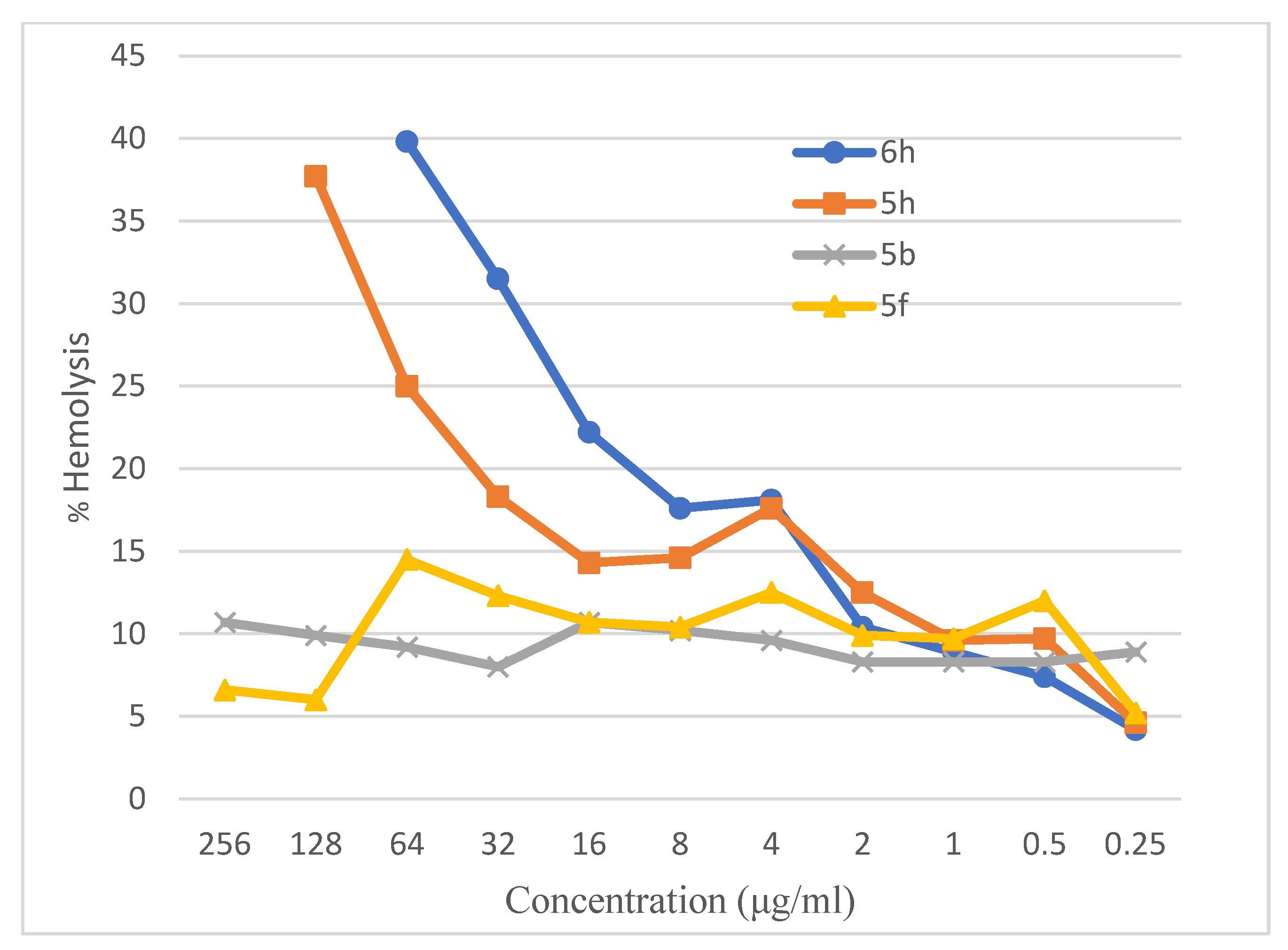
2.3.2. Hemolytic Activity
2.3.3. Antibiofilm Activity
2.3.4. Pharmacokinetics Analysis
2.4. Antioxidant Assay
3. Experimental
3.1. Materials and Methods
3.2. Synthesis and Characterization of 6-Stiryl-4-Aryldihydropyrimidin-2-Ones
3.3. Synthesis and Characterization of Spiro[chromane-2,4′-pyrimidin]-2′(3′H)-ones
3.4. Single-Crystal X-Ray Structure Determination
3.5. Biological Evaluation
3.5.1. In Vitro Antibacterial Activity
3.5.2. MIC Measurement
3.5.3. Hemolytic Activity Assay
3.5.4. Biofilm Inhibition Assay
3.6. Antioxidant Activity
3.7. Pharmacokinetic Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chakroborty, S.; Panda, P.; Ramirez-Lopez, S.C.; Garcia, E.M.A.; Yañez, E.C.; Strekowski, L.; Unnamatlad, M.V.B. Recent progress on synthesis of spirochromanone and spirochromanederivatievs. Heterocycles 2022, 104, 27–68. [Google Scholar] [CrossRef]
- Lattanzio, V. Phenolic compounds: Introduction 50. In Natural Products; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1543–1580. [Google Scholar] [CrossRef]
- Russo, C.M.; Boyer, Z.W.; Scheunemann, K.; Farren, J.; Minich, A.; Wenthur, C.J.; O’Reilly, M.C. Evaluation of Antibacterial Functionalized Dihydropyrimidine Photoaffinity Probes Toward Mechanism of Action Studies. ACS Med. Chem. Lett. 2024, 15, 1094–1101. [Google Scholar] [CrossRef]
- Falcão-Silva, V.S.; Silva, D.A.; Souza, M.D.F.V.; Siqueira-Junior, J.P. Modulation of drug resistance in Staphylococcus aureus by a kaempferol glycoside from Herissantiatiubae (Malvaceae). Phytother. Res. 2009, 23, 1367–1370. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Tice, C.M.; Singh, S.B. The use of spirocyclic scaffolds in drug discovery. Bioorg. Med. Chem. Lett. 2014, 24, 3673–3682. [Google Scholar] [CrossRef] [PubMed]
- Ghatpande, N.G.; Jadhav, J.S.; Kaproormath, R.V.; Soliman, M.E.; Shaikh, M.M. A brief overview on recent advances in spiro[chromane-2,4′-piperidine]-4(3H)-one-functionalized compounds in medicinal chemistry research. Bioorg. Med. Chem. 2020, 28, 115813. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Anjum, S.; Bhatt, J.D.; Dixit, B.C.; Singh, A.; Fatima, S.; Sabiha khan; Patel, T.S.; Hoda, N. Sulfonamide based pyrimidine derivatives combating Plasmodium parasite by inhibiting falcipains-2 and falcipains-3 as antimalarial agents. RSC Adv. 2024, 14, 24725–24740. [Google Scholar] [CrossRef]
- Brindisi, M.; Gemma, S.; Kunjir, S.; Di Cerbo, L.; Brogi, S.; Parapini, S.; D’Alessandro, S.; Taramelli, D.; Habluetzel, A.; Tapanelli, S.; et al. Synthetic spirocyclicendoperoxides: New antimalarial scaffolds. MedChemComm 2015, 6, 357–362. [Google Scholar] [CrossRef]
- Othman, D.I.; Hamdi, A.; Elhusseiny, W.M.; El-Azab, A.S.; Bakheit, A.H.; Hefnawy, M.; Alaa, A.M. Synthesis of novel spirochromane incorporating Schiff’s bases, potential antiproliferative activity, and dual EGFR/HER2 inhibition: Cell cycle analysis and in silico study. Saudi Pharm. J. 2023, 31, 101803. [Google Scholar] [CrossRef]
- Vali, Y.K.; Gundla, R.; Singh, O.V.; Tamboli, Y.; Manelli, L.D.C.; Ghelardini, C.; Al-Tamimi, A.-M.S.; Carta, F.; Angeli, A.; Supuran, C.T. Spirocyclic sulfonamides with carbonic anhydrase inhibitory and anti-neuropathic pain activity. Bioorg. Chem. 2019, 92, 103210. [Google Scholar] [CrossRef]
- Hiesinger, K.; Dar’in, D.; Proschak, E.; Krasavin, M. Spirocyclic scaffolds in medicinal chemistry. J. Med. Chem. 2020, 64, 150–183. [Google Scholar] [CrossRef]
- Talele, T.T. Opportunities for tapping into three-dimensional chemical space through a quaternary carbon. J. Med. Chem. 2020, 63, 13291–13315. [Google Scholar] [CrossRef]
- Chupakhin, E.; Babich, O.; Prosekov, A.; Asyakina, L.; Krasavin, M. Spirocyclic motifs in natural products. Molecules 2019, 24, 4165. [Google Scholar] [CrossRef] [PubMed]
- Lovering, F.; Bikker, J.; Humblet, C. Escape from flatland: Increasing saturation as an approach to improving clinical success. J. Med. Chem. 2009, 52, 6752–6756. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Li, Z.; Wu, Y.; Yang, X.; Li, L.; Chen, S.; Qi, B.; Wang, Y.; Li, C.; Zhao, Y. Exploring plant polyphenols as anti-allergic functional products to manage the growing incidence of food allergy. Front. Nutr. 2023, 10, 1102225. [Google Scholar] [CrossRef]
- Lobiuc, A.; Pavăl, N.E.; Mangalagiu, I.I.; Gheorghiță, R.; Teliban, G.C.; Amăriucăi-Mantu, D.; Stoleru, V. Future antimicrobials: Natural and functionalized phenolics. Molecules 2023, 28, 114. [Google Scholar] [CrossRef] [PubMed]
- Kauffmann, A.C.; Castro, V.S. Phenolic compounds in bacterial inactivation: A perspective from Brazil. Antibiotics 2023, 12, 645. [Google Scholar] [CrossRef]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications. Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef]
- Malik, S.S.; Mundra, S. Increasing consumption of antibiotics during the COVID-19 pandemic: Implications for patient health and emerging anti-microbial resistance. Antibiotics 2022, 12, 45. [Google Scholar] [CrossRef]
- De Rossi, L.; Rocchetti, G.; Lucini, L.; Rebecchi, A. Antimicrobial Potential of Polyphenols: Mechanisms of Action and Microbial Responses—A Narrative Review. Antioxidants 2025, 14, 200. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, J.; Xiao, A.; Liu, L. Antibacterial activity of polyphenols: Structure-activity relationship and influence of hyperglycemic condition. Molecules 2017, 22, 1913. [Google Scholar] [CrossRef]
- Asano, S.; Morii, M.; Takeguchi, N. Molecular and cellular regulation of the gastric proton pump. Biol. Pharm. Bull. 2004, 27, 1–12. [Google Scholar] [CrossRef]
- Hassett, D.J.; Lamkin, T.J.; Panmanee, W.; Taylor, D.E.; Shea, C.J.A. Multi-Tiered, High Through-Put Screen for Compounds Effective Against Bacterial Biofilm Compounds Effective for Inhibiting and Eradicating Bacterial Biofilm. U.S. Patent 20160024551A1, 28 January 2016. Available online: https://pubchem.ncbi.nlm.nih.gov/patent/US-2016024551-A1 (accessed on 29 April 2025).
- Hassett, D.J.; Lamkin, T.J.; Panmanee, W.; Taylor, D.E.; Shea, C.J. Multi-Tiered High Through-Put Screen for Compounds Effective Against Bacterial Biofilm and Compounds Effective for Inhibiting and Eradicating Bacterial Biofilm. U.S. Patent 20190127776A1, 2 May 2019. Available online: https://pubchem.ncbi.nlm.nih.gov/patent/US-2019127776-A1 (accessed on 29 April 2025).
- Lukin, A.; Komarova, K.; Vinogradova, L.; Rogacheva, E.; Kraeva, L.; Dogonadze, M.; Vinogradova, T.; Krasavin, M. Urea derivatives of spirocyclicpiperidines endowed with antibacterial activity. Mendeleev Commun. 2023, 33, 109–111. [Google Scholar] [CrossRef]
- Kelley, J.A.; Driscoll, J.S.; McCormack, J.J.; Roth, J.S.; Marquez, V.E. Furanose-pyranose isomerization of reduced pyrimidine and cyclic urea ribosides. J. Med. Chem. 1986, 29, 2351–2358. [Google Scholar] [CrossRef] [PubMed]
- Benltifa, M.; Hayes, J.M.; Vidal, S.; Gueyrard, D.; Goekjian, P.G.; Praly, J.-P.; Kizilis, G.; Tiraidis, C.; Alexacou, K.-M.; Chrysina, E.D.; et al. Glucose-based spiro-isoxazolines: A new family of potent glycogen phosphorylase inhibitors. Bioorg. Med. Chem. 2009, 17, 7368–7380. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A. New Therapies on the Horizon for Primary Biliary Cholangitis. Drugs 2024, 84, 1–15. [Google Scholar] [CrossRef]
- McNeil, M.B.; O’Malley, T.; Dennison, D.; Shelton, C.D.; Sunde, B.; Parish, T. Multiple mutations in Mycobacterium tuberculosis MmpL3 increase resistance to MmpL3 inhibitors. mSphere 2020, 5, e00985-20. [Google Scholar] [CrossRef]
- Grzegorzewicz, A.E.; Pham, H.; Gundi, V.A.; Scherman, M.S.; North, E.J.; Hess, T.; Jackson, M. Inhibition of mycolic acid transport across the Mycobacterium tuberculosis plasma membrane. Nat. Chem. Biol. 2012, 8, 334–341. [Google Scholar] [CrossRef]
- Zigeuner, G.; Swoboda, R. ÜberHeterocyclen, 13. Mitt.:ZurStruktur der Spiro-(2-oxo-bzw.2-thionohexahydropyrimidin-4, 2′-chromane). Monatsh. Chem. 1966, 97, 1422–1427. [Google Scholar] [CrossRef]
- Zigeuner, G.; Frank, A.; Dujmovits, H.; Adam, W. Dihydro-2(1H)-pyrimidinethiones (Heterocycles, XXI) ÜberHeterocyclen, 21. Mitt. Monatsh. Chem. 1970, 101, 1415–1430. [Google Scholar] [CrossRef]
- Korsatko, W.; Knopp, C.; Fuchsgruber, A.; Zigeuner, G. On the structure of tetrahydro-4-phenylspiro([1]benzopyran-2,4′-(1′H)-pyrimidin)-2′(3′H)-ones and -thiones. Monatsh. Chem. 1976, 107, 745–755. [Google Scholar] [CrossRef]
- Filimonov, S.I.; Vaganova, E.I. Synthesis of Substituted Hydroxyspiro([1]benzopyran-2,4′(1′H)-pyrimidine)-2′(3′H)thiones and Hydroxyspiro-([1]benzopyran-2,4′(1′H)-pyrimidin)-2′(3′H) ones. Chem. Heterocycl. Compd. 2003, 39, 233–237. [Google Scholar] [CrossRef]
- Filimonov, S.I.; Filimonova, S.A.; Shashkov, A.S.; Firgang, S.I.; Stashina, G.A. Synthesis of substituted octahydrochromeno [3, 2-i]quinazoline-2(1H)-thiones. Russ. Chem. Bull. 2005, 54, 1500–1504. [Google Scholar] [CrossRef]
- Wang, T.; Huang, B.; Wang, Y.Q. Enantioselective Synthesis of Spiro Chroman-Isoindolinones via Formal (4+2) Cycloaddition of In Situ-Generated ortho-Quinone Methides with 3-Methylene Isoindolinones. Adv. Synth. Catal. 2022, 364, 2596–2605. [Google Scholar] [CrossRef]
- Yoshida, K.; Inoue, H.; Oji, Y.; Suzuki, H.; Takao, K.I. Enantioselective organocatalytic construction of spirochroman derivatives. J. Org. Chem. 2020, 85, 10189–10197. [Google Scholar] [CrossRef]
- Kar, S.; Ramamoorthy, G.; Mitra, K.; Shivalingegowda, N.; Mavileti, S.K.; Krishnappagowda, L.N.; Doble, M.; Golakoti, N.R. Synthesis of novel spirobibenzopyrans as potent anticancer leads inducing apoptosis in HeLa cells. Bioorg. Med. Chem. Lett. 2020, 30, 127199. [Google Scholar] [CrossRef] [PubMed]
- Filimonov, S.I.; Savinsky, N.G.; Evstigneeva, E.M. Reactions of resorcinols with ketones. Mendeleev Commun. 2003, 13, 194–197. [Google Scholar] [CrossRef]
- Shutalev, A.D.; Aksenov, A.N. Simple synthesis of 4-aryl-6-styryl-1,2,3,4-tetrahydropyrimidin-2-ones by the alkaline hydrolysis of Biginelli compounds. Mendeleev Commun. 2005, 15, 73–75. [Google Scholar] [CrossRef]
- Mondal, S.; Mondal, M.A. Synthesis of 3,4-dihydropyrimidin-2(1H)-one via Retro-Biginelli reaction. J. Het. Chem. 2020, 57, 4175–4180. [Google Scholar] [CrossRef]
- Milović, E.; Janković, N.; Bogdanović, G.A.; Petronijević, J.; Joksimović, N. On water synthesis of the novel 2-oxo-1,2,3,-tetrahydropyrimidines. Tetrahedron 2021, 78, 131790. [Google Scholar] [CrossRef]
- Karandeeva, A.S.; Uryadova, A.M.; Makarova, E.S.; Kabanova, M.V.; Filimonov, S.I.; Chirkova, Z.V.; Suponitsky, K.Y. Synthesis of spiro[chromane-2,4′-pyrimidines] by condensation of 6-styryldihydropyrimidin-2-ones with resorcinols. Mendeleev Commun. 2023, 33, 779–781. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard, 9th ed.; CLSI Document M07-A9; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J Food Sci Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- APEX2 and SAINT; Bruker AXS Inc.: Madison, WI, USA, 2014.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Cryst. Struct. Commun. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Shetnev, A.; Baykov, S.; Kalinin, S.; Belova, A.; Sharoyko, V.; Rozhkov, A.; Krasavin, M. 1,2,4-Oxadiazole/2-imidazoline hybrids: Multi-target-directed compounds for the treatment of infectious diseases and cancer. Int. J. Mol. Sci. 2019, 20, 1699. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, S.; Ahmad, S.; Rehman Khan, K.u.; Tabassum, F.; Khursheed, A.; Zaman, Q.u.; Bukhari, N.A.; Alfagham, A.; Hatamleh, A.A.; Chen, Y. Phytochemical Profiling, Antioxidant, Anti-Inflammatory, Thrombolytic, Hemolytic Activity In Vitro and In Silico Potential of Portulacariaafra. Molecules 2022, 27, 2377. [Google Scholar] [CrossRef]
- Singh, H.; Gahane, A.; Singh, V.; Ghosh, S.; Thakur, A. Antibiofilm activity of Fmoc-phenylalanine against Gram-positive and Gram-negative bacterial biofilms. J. Antibiot. 2021, 74, 407–416. [Google Scholar] [CrossRef]
- Kırmusaoğlu, S. (Ed.) Antimicrobials, Antibiotic Resistance, Antibiofilm Strategies and Activity Methods; BoD–Books on Demand; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Borba, J.V.; Alves, V.M.; Braga, R.C.; Korn, D.R.; Overdahl, K.; Silva, A.C.; Tropsha, A. STopTox: An in silico alternative to animal testing for acute systemic and topical toxicity. Environ. Health Perspect. 2022, 130, 027012. [Google Scholar] [CrossRef]


| Isolated Yield of Compounds 5 and 6, % | ||
|---|---|---|
| R1, R2, R3 | Isomer 5 | Isomer 6 |
| a: R1 = R2 = R3 = H | 17 | 25 |
| b: R1 = H, R2 = Cl, R3 = H | 18 | 52 |
| c: R1 = H, R2 = Me, R3 = H | - | 36 |
| d: R1 = H, R2 = OMe, R3 = H | - | 18 |
| e: R1 = H, R2 = Cl, R3 = 2-Me | 14 | - |
| f: R1 = R2 = Cl, R3 = H | 25 | - |
| g: R1 = R2 = H, R3 = 2-OH | 32 | 29 |
| h: R1 = H, R2 = Cl, R3 = 2-OH | 37 | 38 |
| i: R1 = H, R2 = Me, R3 = 2-OH | 16 | - |
| j: R1 = H, R2 = MeO, R3 = 2-OH | 17 | - |
| k: R1 = R2 = Cl, R3 = 2-OH | 34 | 28 |
 | |||||
| Entry | Catalyst (mmol) | Promoter (mmol) | Time, h | Isolated Yield, % | Ratio 5h/6h, % |
|---|---|---|---|---|---|
| 1 | CH3SO3H (0.48) | AcOH (27.2) | 3 | 47 | 50:50 |
| 2 | CH3SO3H (1.2) | AcOH (27.2) | 3 | 53 | 50:50 |
| 3 | CH3SO3H (2.4) | AcOH (27.2) | 3 | 75 | 50:50 |
| 4 | CH3SO3H (3.6) | AcOH (27.2) | 3 | 60 | 50:50 |
| 5 | CH3SO3H (4.8) | AcOH (27.2) | 3 | 43 | 50:50 |
| 6 | CH3SO3H (7.2) | AcOH (27.2) | 3 | 26 | 50:50 |
| 7 | CH3SO3H (2.4) | - | 3 | 42 | 94:6 |
| 8 | CH3SO3H (2.4) | AcOH (6.8) | 3 | 47 | 80:20 |
| 9 | CH3SO3H (2.4) | AcOH (13.6) | 3 | 55 | 63:37 |
| 10 | CH3SO3H (2.4) | AcOH (40.8) | 3 | 50 | 11:89 |
| 11 | CH3SO3H (2.4) | AcOH (54.4) | 3 | 44 | 7:93 |
| 12 | CH3SO3H (2.4) | AcOH (54.4) | 1 | 11 | 100:0 |
| 13 | TsOH∙H2O (0.3) | AcOH (27.2) | 3 | - | - |
| 14 | CH3SO3H (1.2), TsOH∙H2O (1.2) | - | 3 | 32 | 0:100 |
| 15 | TsOH∙H2O (0.3) | - | 3 | - | - |
| ID | Bacterial Strain | Fungi | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Gram “+” | Gram “−” | ||||||||
| Staphylococcus aureus, clinical Isolate | Staphylococcus aureus ATCC-25923, мкг/мл | Bacillus cereus IP 5832, | Enterococcus faecium K-1 | Micrococcus luteus 2665 | Escherichia coli C1, | Escherichia coli ADH52REF | Pseudomonas fluorescens A1 | Candida albicans ATCC 10231, | |
| 5a | >256 | 32 | 16 | 256 | 16 | >256 | 2 | >256 | 64 |
| 6a | >256 | >256 | >256 | >256 | >256 | >256 | >256 | >256 | >256 |
| 5b | 128 | 8 | 16 | 16 | 16 | >256 | 8 | 8 | 32 |
| 6b | >256 | >256 | >256 | >256 | >256 | >256 | >256 | >256 | >256 |
| 6c | >256 | >256 | >256 | >256 | >256 | >256 | 2 | >256 | >256 |
| 6d | >256 | >256 | >256 | >256 | >256 | >256 | >256 | >256 | >256 |
| 5e | 256 | 64 | 64 | 256 | 64 | >256 | 2 | >256 | 32 |
| 5f | ≥256 | 4 | 4 | ≥256 | 2 | ≥256 | 4 | 4 | 4 |
| 5g | >256 | 32 | 16 | 256 | 16 | >256 | 2 | >256 | 64 |
| 6g | 128 | 32 | 128 | 128 | 128 | >256 | 128 | 128 | 128 |
| 5h | 16 | 16 | 16 | 8 | 8 | >256 | 16 | 16 | 32 |
| 6h | 64 | 16 | 8 | 16 | 8 | >256 | 8 | 8 | 16 |
| 5i | ≥256 | 8 | 8 | ≥256 | 16 | ≥256 | 4 | 4 | 4 |
| 5j | 128 | 128 | 128 | 128 | 128 | >256 | 16 | >256 | 128 |
| 5k | ≥256 | ≥256 | ≥256 | ≥256 | 4 | >256 | 4 | 4 | 4 |
| 6k | ≥256 | ≥256 | ≥256 | ≥256 | 16 | >256 | 16 | 16 | 4 |
| Pef. ** | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 |
| No. | Compound | Hemolytic Activity (%) | p-Value |
|---|---|---|---|
| 1 | 5b | 8.01 ± 0.16 | <0.05 |
| 2 | 5f | 12.31 ± 0.16 | <0.05 |
| 3 | 5h | 18.3 ± 0.10 | <0.05 |
| 4 | 6h | 31.51 ± 0.11 | <0.05 |
| 5 | Triton X-100 (Control) | 95.59 ± 0.26 | <0.05 |
| Compd. | GI Absorption | Skin Permeation (cm/s) | BBB Permeation | P-gp Substrate | CYP1A2 Inhibitor | CYP2C19 Inhibitor | CYP2C9 Inhibitor | CYP2D6 Inhibitor | CYP3A4 Inhibitor |
|---|---|---|---|---|---|---|---|---|---|
| 5b | High | −6.21 | ✓ | ✓ | × | ✓ | ✓ | ✓ | ✓ |
| 5f | High | −6.55 | ✓ | ✓ | × | ✓ | ✓ | ✓ | × |
| 5h | High | × | ✓ | × | ✓ | ✓ | × | × | |
| 6h | High | −6.38 | × | ✓ | × | ✓ | ✓ | × | ✓ |
| No. | Compound | IC50 (μg /mL) |
|---|---|---|
| 1 | 5b | >400 |
| 2 | 5f | >400 |
| 3 | 5h | 12.5 ± 0.2 |
| 4 | 6h | 12.5 ± 0.2 |
| 5 | Ascorbic acid | 12.5 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karandeeva, A.S.; Bogdanova, N.A.; Kabanova, M.V.; Filimonov, S.I.; Chirkova, Z.V.; Romanycheva, A.A.; Panova, V.A.; Shetnev, A.A.; Togyzbayeva, N.A.; Kanzhar, S.A.; et al. Diastereoselective Synthesis and Biological Evaluation of Spiro[chromane-2,4′-pyrimidin]-2′(3′H)-ones as Novel Antimicrobial and Antioxidant Agents. Molecules 2025, 30, 2954. https://doi.org/10.3390/molecules30142954
Karandeeva AS, Bogdanova NA, Kabanova MV, Filimonov SI, Chirkova ZV, Romanycheva AA, Panova VA, Shetnev AA, Togyzbayeva NA, Kanzhar SA, et al. Diastereoselective Synthesis and Biological Evaluation of Spiro[chromane-2,4′-pyrimidin]-2′(3′H)-ones as Novel Antimicrobial and Antioxidant Agents. Molecules. 2025; 30(14):2954. https://doi.org/10.3390/molecules30142954
Chicago/Turabian StyleKarandeeva, Alena S., Natalia A. Bogdanova, Mariya V. Kabanova, Sergey I. Filimonov, Zhanna V. Chirkova, Anna A. Romanycheva, Valeria A. Panova, Anton A. Shetnev, Nurila A. Togyzbayeva, Saken A. Kanzhar, and et al. 2025. "Diastereoselective Synthesis and Biological Evaluation of Spiro[chromane-2,4′-pyrimidin]-2′(3′H)-ones as Novel Antimicrobial and Antioxidant Agents" Molecules 30, no. 14: 2954. https://doi.org/10.3390/molecules30142954
APA StyleKarandeeva, A. S., Bogdanova, N. A., Kabanova, M. V., Filimonov, S. I., Chirkova, Z. V., Romanycheva, A. A., Panova, V. A., Shetnev, A. A., Togyzbayeva, N. A., Kanzhar, S. A., Appazov, N. O., & Suponitsky, K. Y. (2025). Diastereoselective Synthesis and Biological Evaluation of Spiro[chromane-2,4′-pyrimidin]-2′(3′H)-ones as Novel Antimicrobial and Antioxidant Agents. Molecules, 30(14), 2954. https://doi.org/10.3390/molecules30142954









