Rapid and One-Pot Synthesis of Aryl Ynamides from Aryl Alkynyl Acids by Metal-Free C-N Cleavage of Tertiary Amines
Abstract
1. Introduction
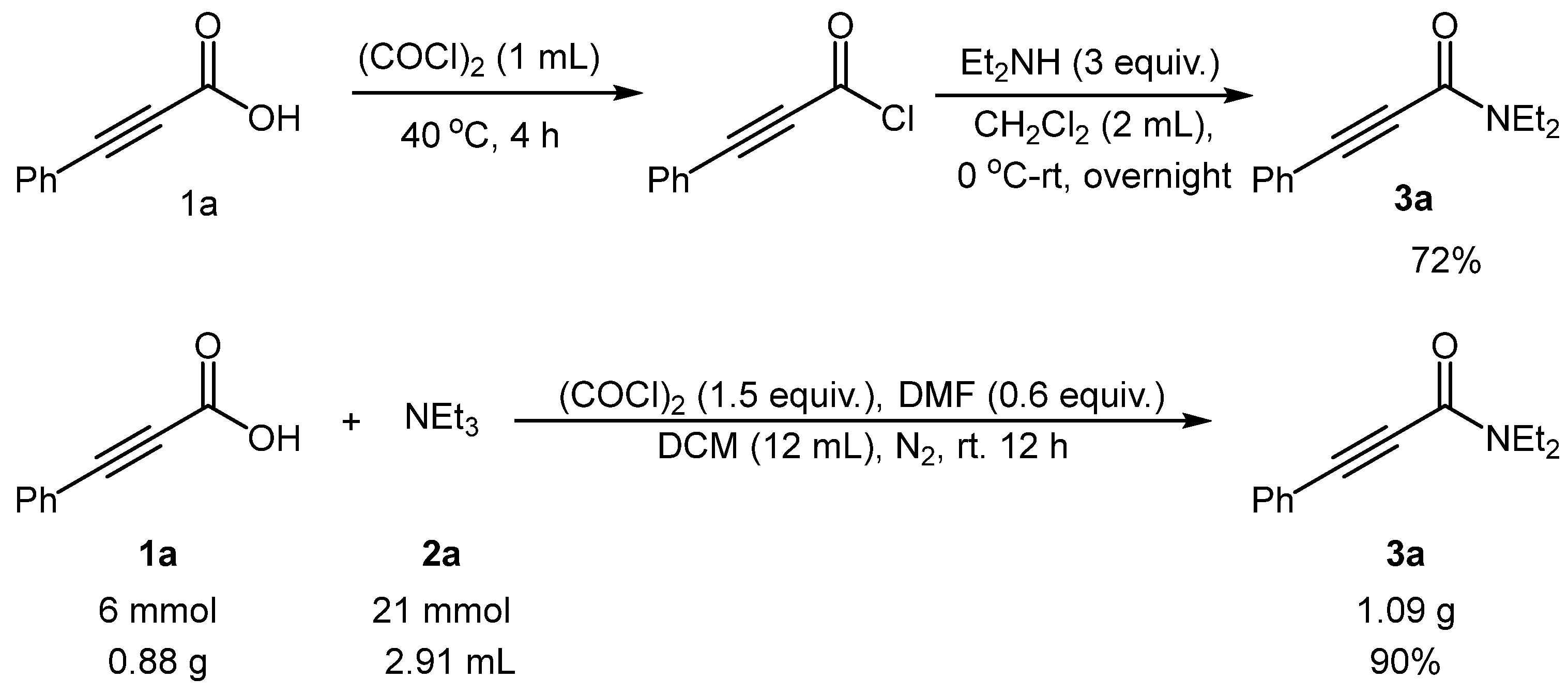
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Experimental Procedure for Ynamides
- N,N-Diethyl-3-phenylpropiolamide (3a): Yellow liquid and the yield is 94%; 1H NMR (400 MHz, CDCl3) δ 7.50–7.45 (m, 2H), 7.38–7.27 (m, 3H), 3.61 (q, J = 7.1 Hz, 2H), 3.42 (q, J = 7.1 Hz, 2H), 1.22 (t, J = 7.1 Hz, 3H), 1.12 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 153.71, 132.05, 129.66, 128.27, 120.49, 88.71, 81.73, 43.39, 39.10, 14.18, 12.64. HRMS: calcd for C13H16ON [M + H]+: 202.1226, found: 202.1222.
- N,N-Diethyl-3-(p-tolyl)propiolamide (3b): Yellow liquid and the yield is 84%; 1H NMR (400 MHz, CDCl3) δ 7.42 (d, J = 8.3 Hz, 2H), 7.15 (d, J = 7.8 Hz, 2H), 3.69–3.61 (m, 2H), 3.46 (q, J = 7.1 Hz, 2H), 2.36 (s, 3H), 1.27 (t, J = 7.1 Hz, 3H), 1.16 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 154.09, 140.29, 132.22, 129.20, 117.57, 89.34, 81.44, 43.54, 39.22, 21.58, 14.34, 12.83. HRMS: calcd for C14H18ON [M + H]+: 216.1383, found: 216.1329.
- 3-(4-(Tert-butyl)phenyl)-N,N-diethylpropiolamide (3c): Yellow liquid and the yield is 87%; 1H NMR (400 MHz, CDCl3) δ 7.46 (d, J = 8.3 Hz, 2H), 7.37 (d, J = 8.5 Hz, 2H), 3.65 (q, J = 7.1, 6.6 Hz, 2H), 3.46 (q, J = 7.1 Hz, 2H), 1.30 (s, 9H), 1.26 (t, J = 7.0 Hz, 3H), 1.17 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 154.05, 153.31, 132.05, 125.43, 117.58, 89.24, 81.41, 43.52, 39.22, 34.84, 30.97, 14.30, 12.79. HRMS: calcd for C17H24ON [M + H]+: 258.1852, found: 258.1845.
- N,N-Diethyl-3-(4-methoxyphenyl)propiolamide (3d): Yellow liquid and the yield is 95%; 1H NMR (400 MHz, CDCl3) δ 7.47 (d, J = 7.0 Hz, 2H), 6.86 (d, J = 6.9 Hz, 2H), 3.81 (s, 3H), 3.64 (q, J = 7.2 Hz, 2H), 3.45 (q, J = 7.1 Hz, 2H), 1.26 (t, J = 7.1 Hz, 3H), 1.16 (t, J = 8.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 160.82, 154.23, 134.02, 114.12, 112.59, 89.46, 81.12, 55.30, 43.52, 39.20, 14.33, 12.84. HRMS: calcd for C14H18O2N [M + H]+: 232.1332, found: 232.1328.
- 3-(4-Bromophenyl)-N,N-diethylpropiolamide (3e): Yellow liquid and the yield is 81%; 1H NMR (400 MHz, CDCl3) δ 7.49 (d, J = 8.3 Hz, 2H), 7.37 (d, J = 8.3 Hz, 2H), 3.63 (q, J = 7.2 Hz, 2H), 3.46 (q, J = 7.2 Hz, 2H), 1.26 (t, J = 7.2 Hz, 3H), 1.16 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 153.64, 133.60, 131.79, 124.41, 119.59, 87.76, 82.79, 43.54, 39.28, 14.36, 12.77. HRMS: calcd for C13H15BrON [M + H]+: 280.0332, found: 280.0329.
- 3-(4-Cyanophenyl)-N,N-diethylpropiolamide (3f): Yellow liquid and the yield is 38%; 1H NMR (400 MHz, CDCl3) δ 7.63 (q, J = 8.4, 7.9 Hz, 4H), 3.64 (q, J = 7.1 Hz, 2H), 3.47 (q, J = 7.0 Hz, 2H), 1.27 (t, J = 7.2 Hz, 3H), 1.18 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 153.11, 132.66, 132.12, 125.53, 117.95, 113.21, 86.53, 85.22, 43.58, 39.40, 14.39, 12.73. HRMS: calcd for C14H15ON2 [M + H]+: 227.1179, found: 227.1174.
- N,N-Diethyl-3-(4-formylphenyl)propiolamide (3g): Yellow liquid and the yield is 32%; 1H NMR (400 MHz, CDCl3) δ 10.03 (s, 1H), 7.88 (d, J = 8.5 Hz, 2H), 7.68 (d, J = 8.2 Hz, 2H), 3.66 (q, J = 7.1 Hz, 2H), 3.48 (q, J = 7.2 Hz, 2H), 1.29 (t, J = 7.2 Hz, 3H), 1.18 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 191.20, 153.34, 136.49, 132.76, 129.52, 126.73, 87.43, 84.83, 43.59, 39.37, 14.42, 12.78. HRMS: calcd for C14H16O2N [M + H]+: 230.2870, found: 230.2867.
- 3-(4-Acetylphenyl)-N,N-diethylpropiolamide (3h): Yellow liquid and the yield is 55%; 1H NMR (400 MHz, CDCl3) δ 7.93 (d, J = 8.4 Hz, 2H), 7.60 (d, J = 8.5 Hz, 2H), 3.65 (q, J = 7.0 Hz, 2H), 3.47 (q, J = 7.1 Hz, 2H), 2.60 (s, 3H), 1.27 (t, J = 7.2 Hz, 3H), 1.17 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 197.05, 153.44, 137.40, 132.35, 128.21, 125.34, 87.65, 84.31, 43.58, 39.33, 26.60, 14.36, 12.74. HRMS: calcd for C15H18O2N [M + H]+: 244.1332, found: 244.1328.
- N,N-Diethyl-3-(o-tolyl)propiolamide (3i): Yellow liquid and the yield is 86%; 1H NMR (400 MHz, CDCl3) δ 7.50 (d, J = 7.8 Hz, 1H), 7.28 (t, J = 7.5 Hz, 1H), 7.21 (d, J = 7.6 Hz, 1H), 7.16 (t, J = 7.5 Hz, 1H), 3.66 (q, J = 6.7 Hz, 2H), 3.47 (q, J = 7.1, 6.6 Hz, 2H), 2.46 (s, 3H), 1.26 (t, J = 7.2 Hz, 3H), 1.17 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 154.03, 141.06, 132.88, 129.81, 129.57, 125.68, 120.52, 87.98, 85.67, 43.52, 39.28, 20.58, 14.40, 12.81. HRMS: calcd for C14H18ON [M + H]+: 216.1383, found: 216.1328.
- N,N-Diethyl-3-(2-methoxyphenyl)propiolamide (3j): Yellow liquid and the yield is 90%; 1H NMR (400 MHz, CDCl3) δ 7.48 (d, J = 7.6 Hz, 1H), 7.35 (t, J = 7.6 Hz, 1H), 6.91 (t, J = 7.6 Hz, 1H), 6.87 (d, J = 8.4 Hz, 1H), 3.85 (s, 3H), 3.70 (q, J = 7.1 Hz, 2H), 3.46 (q, J = 7.1 Hz, 2H), 1.26 (t, J = 7.1 Hz, 3H), 1.15 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 161.08, 154.17, 134.13, 131.41, 120.41, 110.57, 109.96, 86.00, 85.53, 55.62, 43.50, 39.17, 14.28, 12.83. HRMS: calcd for C14H18O2N [M + H]+: 232.1332, found: 232,1328.
- 3-(2-Chlorophenyl)-N,N-diethylpropiolamide (3k): Yellow liquid and the yield is 87%; 1H NMR (400 MHz, CDCl3) δ 7.59 (d, J = 7.4 Hz, 1H), 7.40 (t, J = 5.6 Hz, 1H), 7.32 (t, J = 7.4 Hz, 1H), 7.25 (d, J = 7.4 Hz, 1H), 3.71 (q, J = 7.0 Hz, 2H), 3.46 (q, J = 7.1 Hz, 2H), 1.26 (t, J = 7.1 Hz, 3H), 1.26 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 153.61, 136.67, 134.42, 130.89, 129.35, 126.65, 120.88, 86.44, 85.13, 43.56, 39.34, 14.45, 12.78. HRMS: calcd for C13H15ClON [M + H]+: 236.0837, found: 286.0834.
- N,N-Diethyl-3-(naphthalen-2-yl)propiolamide (3l): Yellow liquid and the yield is 70%; 1H NMR (400 MHz, CDCl3) δ 8.08 (s, 1H), 7.81 (dd, J = 7.3, 4.4 Hz, 3H), 7.56–7.48 (m, 3H), 3.70 (q, J = 7.1 Hz, 2H), 3.49 (q, J = 7.2 Hz, 2H), 1.31 (t, J = 7.1 Hz, 3H), 1.19 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 153.94, 133.39, 132.99, 132.60, 128.20, 128.05, 127.92, 127.76, 127.46, 126.81, 117.87, 89.37, 82.06, 43.59, 39.27, 14.40, 12.82. HRMS: calcd for C17H18ON [M + H]+: 252.3370, found: 252.3368.
- N,N-Diethyl-3-(thiophen-2-yl)propiolamide (3m): Yellow liquid and the yield is 83%; 1H NMR (400 MHz, CDCl3) δ 7.38 (t, J = 3.4 Hz, 2H), 7.02 (t, J = 4.3 Hz, 1H), 3.61 (q, J = 7.2 Hz, 2H), 3.45 (q, J = 7.2 Hz, 2H), 1.25 (t, J = 7.1 Hz, 3H), 1.15 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 153.70, 134.79, 129.72, 127.30, 120.36, 85.83, 82.69, 43.47, 39.21, 14.34, 12.79. HRMS: calcd for C11H14OSN [M + H]+: 208.2990, found: 208.2986.
- 3-Phenyl-N,N-dipropylpropiolamide (3q): Yellow liquid and the yield is 73%; 1H NMR (400 MHz, CDCl3) δ 7.52 (d, J = 7.3 Hz, 2H), 7.42–7.33 (m, 3H), 3.56 (t, J = 7.4 Hz, 2H), 3.36 (t, J = 7.4 Hz, 2H), 1.73–1.66 (m, 2H),1.63–1.56 (m, 2H), 0.96 (t, J = 7.4 Hz, 3H), 0.92 (t, J = 7.4 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 154.53, 132.25, 129.82, 128.46, 120.78, 89.26, 82.11, 50.84, 46.50, 22.17, 20.69, 11.32, 11.23. HRMS: calcd for C15H20ON [M + H]+: 230.1539, found: 230.1536.
- N,N-Dihexyl-3-phenylpropiolamide (3r): Yellow liquid and the yield is 94%; 1H NMR (400 MHz, CDCl3) δ 7.52 (d, J = 7.0 Hz, 2H), 7.42–7.33 (m, 3H), 3.58 (t, J = 7.5 Hz, 2H), 3.38 (t, J = 7.5 Hz, 2H), 1.69–1.61 (m, 2H), 1.60–1.53 (m, 2H), 1.37–1.25 (m, 12H), 0.90–0.84 (m, 6H). 13C NMR (101 MHz, CDCl3) δ 154.33, 132.21, 129.76, 128.42, 120.79, 89.13, 82.14, 54.09, 49.11, 44.81, 31.78, 31.53, 28.82, 27.41, 26.59, 26.38, 22.51, 13.96, 13.90. HRMS: calcd for C21H32ON [M + H]+: 314.2478, found: 314.2474.
- N,N-Dioctyl-3-phenylpropiolamide (3s): Yellow liquid and the yield is 80%; 1H NMR (400 MHz, CDCl3) δ 7.52 (d, J = 7.3 Hz, 2H), 7.41–7.41 (m, 3H), 3.58 (t, J = 7.5 Hz, 2H), 3.38 (t, J = 7.5 Hz, 2H), 1.65 (t, J = 7.3 Hz, 2H), 1.56 (t, J = 7.4 Hz, 2H), 1.34–1.23 (m, 20H), 0.89–0.83 (m, 6H). 13C NMR (101 MHz, CDCl3) δ 154.40, 132.26, 129.80, 128.46, 120.83, 89.22, 82.17, 53.91, 49.18, 44.87, 31.77, 31.74, 29.34, 29.26, 29.20, 28.89, 27.48, 26.98, 26.75, 22.61, 22.58, 14.05, 14.03. HRMS: calcd for C25H40ON [M + H]+: 370.3104, found: 370.3100.
- N,N-Dibenzyl-3-phenylpropiolamide (3t): Yellow liquid and the yield is 33%; 1H NMR (400 MHz, CDCl3) δ 7.52 (s, 1H), 7.50 (s, 1H), 7.43–7.24 (m, 13H), 4.76 (s, 2H), 4.57 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 154.98, 136.19, 136.03, 132.41, 130.09, 128.85, 128.66, 128.47, 128.43, 127.93, 127.69, 127.62, 120.31, 90.80, 81.60, 51.41, 46.34. HRMS: calcd for C23H20ON [M + H]+: 326.1539, found: 326.1534.
- N,N-Diallyl-3-phenylpropiolamide (3u): Yellow liquid and the yield is 68%; 1H NMR (400 MHz, CDCl3) δ 7.53 (d, J = 7.2 Hz, 2H), 7.44–7.32 (m, 3H), 5.89–5.71 (m, 2H), 5.27–5.15 (m, 4H), 4.22 (d, J = 5.8 Hz, 2H), 4.06 (d, J = 6.0 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 154.41, 132.69, 132.33, 132.09, 129.99, 128.45, 120.43, 118.07, 117.94, 89.87, 81.47, 50.72, 46.37. HRMS: calcd for C15H16ON [M + H]+: 226.1226, found: 226.1221.
- N,N-Dimethyl-3-phenylpropiolamide (3v): Yellow liquid and the yield is 87%; 1H NMR (400 MHz, CDCl3) δ 7.53 (d, J = 8.0 Hz, 2H), 7.43–7.32 (m, 3H), 3.28 (s, 3H), 3.02 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 154.59, 132.28, 129.91, 128.45, 120.56, 90.12, 81.53, 38.34, 34.13. HRMS: calcd for C11H12ON [M + H]+: 174.0913, found: 174.0908.
- N-Ethyl-N-isopropyl-3-phenylpropiolamide (3w): Yellow liquid and the yield is 95%; 1H NMR (400 MHz, CDCl3) δ 7.52 (d, J = 6.8 Hz, 2H), 7.53–7.32 (m, 3H), 4.74–4.65 (m, 1H), 3.55 (q, J = 7.2 Hz, 1H), 3.34 (q, J = 7.0 Hz, 1H), 1.32 (t, J = 7.4 Hz, 1H), 1.25 (d, J = 7.3 Hz, 4H), 1.19 (d, J = 7.2 Hz, 4H). 13C NMR (101 MHz, CDCl3) δ 154.35, 153.85, 132.20, 132.18, 129.75, 129.70, 128.40, 128.37, 89.35, 88.53, 82.54, 81.91, 50.52, 45.33, 39.22, 35.36, 21.21, 20.30, 16.58, 14.45. HRMS: calcd for C14H18ON [M + H]+: 216.1383, found: 216.1379.
- N-Isopropyl-N-methyl-3-phenylpropiolamide (3x): Yellow liquid and the yield is 23%; 1H NMR (400 MHz, CDCl3) δ 7.53 (d, J = 6.7 Hz, 2H), 7.42–7.33 (m,3H), 4.89–4.71 (m, 1H), 3.12 (s, 1H), 2.86 (s, 2H), 1.24 (d, J = 6.7 Hz, 4H), 1.15 (d, J = 6.8 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 154.31, 154.09, 132.28, 129.83, 128.44, 120.72, 90.03, 89.95, 82.16, 81.50, 50.09, 43.96, 29.79, 25.57, 20.36, 19.22. HRMS: calcd for C13H16ON [M + H]+: 202.1226, found: 202.1222.
- 3-Phenyl-1-(piperidin-1-yl)prop-2-yn-1-one (3aa): Yellow liquid and the yield is 68%; 1H NMR (400 MHz, CDCl3) δ 7.54 (d, J = 7.4 Hz, 2H), 7.42–7.33 (m, 3H), 3.77 (t, J = 5.1 Hz, 2H), 3.62 (t, J = 5.5 Hz, 2H), 1.66 (t, J = 7.4 Hz, 4H), 1.61–1.55 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 152.89, 132.26, 129.81, 128.42, 120.68, 90.20, 81.41, 48.17, 42.32, 26.40, 25.34, 24.48. HRMS: calcd for C14H16ON [M + H]+: 214.1226, found: 214.1223.
- 1-(4-Chloropiperidin-1-yl)-3-phenylprop-2-yn-1-one (3ab): Yellow liquid and the yield is 67%; 1H NMR (400 MHz, CDCl3)δ7.56–7.51 (m, 2H), 7.45–7.33 (m, 3H), 4.36–4.31 (m, 1H), 4.07–3.98 (m, 1H), 3.88–3.79 (m, 2H), 3.77–3.69 (m, 1H), 2.18–2.02 (m, 2H), 1.99–1.83 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 152.94, 132.32, 130.07, 128.49, 120.32, 90.81, 80.89, 56.17, 44.01, 38.23, 35.12, 34.14. HRMS: calcd for C14H15ClON [M + H]+: 248.0837, found: 248.0836.
- 3-Phenyl-1-(pyrrolidin-1-yl)prop-2-yn-1-one (3ac): Yellow liquid and the yield is 93%; 1H NMR (400 MHz, CDCl3) δ 7.52 (d, J = 8.3 Hz, 2H), 7.41–7.32 (m, 3H), 3.71 (t, J = 6.5 Hz, 2H), 3.51 (t, J = 6.4 Hz, 2H), 1.99–1.90 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 152.65, 132.31, 129.85, 128.41, 120.56, 88.61, 82.59, 48.08, 45.29, 25.31, 24.65. HRMS: calcd for C13H14ON [M + H]+: 200.1070, found: 200.1068.
- 1-Morpholino-3-phenylprop-2-yn-1-one (3ad): Yellow liquid and the yield is 91%; 1H NMR (400 MHz, CDCl3) δ 7.52 (d, J = 7.6 Hz, 2H), 7.43–7.33 (m, 3H), 3.82 (t, J = 4.6 Hz, 2H), 3.73 (t, J = 4.5 Hz, 2H), 3.68 (s, 4H). 13C NMR (101 MHz, CDCl3) δ 153.13, 132.30, 130.12, 128.49, 120.19, 91.11, 80.68, 66.82, 66.41, 47.24, 41.90. HRMS: calcd for C13H14O2N [M + H]+: 216.1016, found: 216.1018.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonald, I.M.; Mate, R.A.; Zusi, F.C.; Huang, H.; Post Munson, D.J.; Ferrante, M.A.; Gallagher, L.; Bertekap, R.L., Jr.; Knox, R.J.; Robertson, B.J.; et al. Discovery of a novel series of quinolone α7 nicotinic acetylcholine receptor agonists. Bioorg. Med. Chem. Lett. 2013, 23, 1684–1688. [Google Scholar] [CrossRef] [PubMed]
- Eibl, C.; Tomassoli, I.; Munoz, L.; Stokes, C.; Papke, R.L.; Gundisch, D. The 3,7-diazabicyclo [3.3.1]nonane scaffold for subtype selective nicotinic acetylcholine receptor ligands. Part 2: Carboxamide derivatives with different spacer motifs. Bioorg. Med. Chem. 2013, 21, 7309–7329. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.; Neuville, L.; Retailleau, P.; Zhu, J. Synthesis of 3-(Diarylmethylenyl)oxindole by a Palladium-Catalyzed Domino Carbopalladation/C−H Activation/C−C Bond-Forming Process. Org. Lett. 2006, 8, 4927–4930. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.; Stang, P. Synthesis of Bicyclic Enediynes from Bis[phenyl[(trifluoromethanesulfonyl)oxy]iodo]acetylene: A Tandem Diels−Alder/Palladium(II)- and Copper(I)-Cocatalyzed Cross-Coupling Approach. J. Org. Chem. 1996, 61, 6162–6165. [Google Scholar] [CrossRef]
- Hay, L.; Koenig, T.; Ginah, F.; Copp, J.; Mitchell, D. Palladium-Catalyzed Hydroarylation of Propiolamides. A Regio- and Stereocontrolled Method for Preparing 3,3-Diarylacrylamides. J. Org. Chem. 1998, 63, 5050–5058. [Google Scholar] [CrossRef]
- Xie, X.; Lu, X.; Liu, Y.; Xu, W. Palladium(II)-Catalyzed Synthesis of α-Alkylidene-γ-butyrolactams from N-Allylic 2-Alkynamides. Total Synthesis of (±)-Isocynodine and (±)-Isocynometrine. J. Org. Chem. 2001, 66, 6545–6550. [Google Scholar] [CrossRef]
- Peng, H.; Liu, G. Palladium-Catalyzed Tandem Fluorination and Cyclization of Enynes. Org. Lett. 2011, 13, 772–775. [Google Scholar] [CrossRef]
- Donets, P.A.; Sharma, N. Efficient Synthesis of the 3-Benzazepine Framework via Intramolecular Heck Reductive Cyclization. Org. Lett. 2007, 9, 3017–3020. [Google Scholar] [CrossRef]
- Zhu, H.; Sun, D.; Zhang, S.; Chen, J.; Xu, Z. Rhodium-Catalyzed Pyridylation of Alkynamides with Pyridylboronic Acids: A Route to Functionalized Enamides. Chem. Eur. J. 2024, 30, e202401830. [Google Scholar] [CrossRef]
- Bowman, W.R.; Heaney, H.; Jordan, B.M. Synthesis of oxindoles by radical cyclization. Tetrahedron. Lett. 1988, 29, 6657–6660. [Google Scholar] [CrossRef]
- Halluli, L.; Sokolov, V.A.; Byvsheva, S.V.; Ipatova, E.V.; Boyarskaya, I.A.; Vasilyev, A.V. Reactions of 3-Arylpopynoic Acid Amides with Arenes in Trifluoromethanesulfonic Acid. Russ. J. Gen. Chem. 2025, 95, 1–8. [Google Scholar] [CrossRef]
- Zhao, S.; Ding, L.; Sun, Y.; Wang, M.; Zhao, D. Synergistic Palladium/Lewis Acid-Catalyzed Regio- and Stereo divergent Bissilylation of Alkynoates: Scope, Mechanism, and Origin of Selectivity. Angew. Chem. Int. Ed. 2023, 62, e202309169. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Jiang, K.; Zhu, H.; Yin, B. CuCl2-catalyzed highly stereoselective and chemoselective reduction of alkynyl amides into α, β- unsaturated amides using silanes as hydrogen donors. Org. Biomol. Chem. 2021, 19, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Tohda, Y.; Sonogashira, K.; Hagihara, N. A Convenient Synthesis of 1-Alkynyl Ketones and 2-Alkynamides. Synthesis 1977, 1977, 777–778. [Google Scholar] [CrossRef]
- Suda, T.; Noguchi, K.; Hirano, M.; Tanaka, K. Highly Enantioselective Synthesis of N,N-Dialkylbenzamides with Aryl–Carbonyl Axial Chirality by Rhodium-Catalyzed [2+2+2] Cycloaddition. Chem. Eur. J. 2008, 14, 6593–6596. [Google Scholar] [CrossRef]
- Lee, Y.; Motoyama, Y.; Tsuji, K.; Yoon, S.H.; Mochida, I.; Nagashima, H. (Z)-Selective Partial Hydrogenation of Internal Alkynes by Using Palladium Nanoparticles Supported on Nitrogen-Doped Carbon Nanofiber. ChemCatChem 2012, 4, 778–781. [Google Scholar] [CrossRef]
- Rosenberg, S.H.; Rapoport, H. Intramolecular Michael reactions. Addition to the .alpha.-carbon of ynamides. J. Org. Chem. 1985, 50, 3979–3982. [Google Scholar] [CrossRef]
- Xu, W.; Kong, A.; Lu, X. Palladium(II)-Catalyzed Asymmetric Synthesis of (Z)-α-Alkylidene-γ-butyrolactams from (Z)-N-Allylic 2-Alkynamides. Total Synthesis of (−)-Isocynometrine. J. Org. Chem. 2006, 71, 3854–3858. [Google Scholar] [CrossRef]
- Donets, P.A.; Van Hecke, K.; Van Meervelt, L.; Van der Eycken, E.V. Efficient Synthesis of the Indoloazocine Framework via Intramolecular Alkyne Carbocyclization. Org. Lett. 2009, 11, 3618–3621. [Google Scholar] [CrossRef]
- Li, H.; Pan, C.; Cheng, Y.; Zhu, C. Copper-catalyzed oxidative coupling of carboxylic acids with formamides for the synthesis of α,β-unsaturated amides. Tetrahedron Lett. 2013, 54, 6679–6681. [Google Scholar] [CrossRef]
- Xie, Y.X.; Song, R.J.; Yang, X.H.; Xiang, J.N.; Li, J.H. Copper-Catalyzed Amidation of Acids Using Formamides as the Amine Source. Eur. J. Org. Chem. 2013, 2013, 5737–5742. [Google Scholar] [CrossRef]
- Wu, J.J.; Li, Y.; Zhou, H.Y.; Wen, A.H.; Lun, C.C.; Yao, S.Y.; Ke, Z.; Ye, B.H. Copper-Catalyzed Carbamoylation of Terminal Alkynes with Formamides via Cross-Dehydrogenative Coupling. ACS Catal. 2016, 6, 1263–1267. [Google Scholar] [CrossRef]
- Hwang, J.; Choi, J.; Park, K.; Kim, W.Y.; Song, K.H.; Lee, S. Palladium-Catalyzed Oxidative Aminocarbonylation by Decarboxylative Coupling: Synthesis of Alkynyl Amides. Eur. J. Org. Chem. 2015, 2015, 2235–2243. [Google Scholar] [CrossRef]
- Wang, L.F.; Qiu, J.H.; Zhang, B.H.; Chen, M.Q.; Wang, H.X.; Miao, X.H.; Wu, Z.Y.; Zhao, M.Q.; Shen, H.T.; Lai, M.; et al. Nickel-Catalyzed Amidation of Aryl Alkynyl Acids with Tetraalkylthiuram Disulfides: A Facile Synthesis of Aryl Alkynyl Amides. ACS Omega 2023, 8, 7699–7713. [Google Scholar] [CrossRef]
- Uenoyama, Y.; Fukuyama, T.; Nobuta, O.; Matsubara, H.; Ryu, I. Alkyne Carbonylation by Radicals: Tin-Radical-Catalyzed Synthesis of α-Methylene Amides from 1-Alkynes, Carbon Monoxide, and Amines. Angew. Chem. Int. Ed. Engl. 2005, 44, 1075–1078. [Google Scholar] [CrossRef]
- Dhawan, R.; Dghaym, R.D.; St. Cyr, D.J.; Arndtsen, B.A. Direct, Palladium-Catalyzed, Multicomponent Synthesis of β-Lactams from Imines, Acid Chloride, and Carbon Monoxide. Org. Lett. 2006, 8, 3927–3930. [Google Scholar] [CrossRef]
- Khedkar, M.V.; Sasaki, T.; Bhanage, B.M. Immobilized palladium metal-containing ionic liquid-catalyzed alkoxycarbonylation, phenoxycarbonylation, and aminocarbonylation reactions. ACS Catal. 2013, 3, 287. [Google Scholar] [CrossRef]
- Gautam, P.; Bhanage, B.M. Palladacycle-Catalyzed Carbonylative Suzuki–Miyaura Coupling with High Turnover Number and Turnover Frequency. J. Org. Chem. 2015, 80, 7810–7815. [Google Scholar] [CrossRef]
- Mane, R.S.; Bhanage, B.M. Pd/C-catalyzed facile synthesis of primary aromatic amides by aminocarbonylation of aryl iodides using ammonia surrogates. RSC Adv. 2015, 5, 76122–76127. [Google Scholar] [CrossRef]
- Mane, R.S.; Sasaki, T.; Bhanage, B.M. Silica supported palladium-phosphine as a reusable catalyst for alkoxycarbonylation and aminocarbonylation of aryl and heteroaryl iodides. RSC Adv. 2015, 5, 94776–94785. [Google Scholar] [CrossRef]
- Guo, S.; Qian, B.; Xie, Y.; Xia, C.; Huang, H. Copper-Catalyzed Oxidative Amination of Benzoxazoles via C−H and C−N Bond Activation: A New Strategy for Using Tertiary Amines as Nitrogen Group Sources. Org. Lett. 2011, 13, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Su, Y.; Li, L.; Huang, H. Transition-metal catalysed C-N bond activation. Chem. Soc. Rev. 2016, 45, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, B.; Gao, B.; Zhang, T.; Huang, H. Triple-Bond Insertion Triggers Highly Regioselective 1,4-Aminomethylamination of 1,3-Enynes with Aminals Enabled by Pd-Catalyzed C-N Bond Activation. Org. Lett. 2019, 21, 535–539. [Google Scholar] [CrossRef]
- Ouyang, K.; Hao, W.; Zhang, W.; Xi, Z. Transition-Metal-Catalyzed Cleavage of C-N Single Bonds. Chem. Rev. 2015, 115, 12045–12090. [Google Scholar] [CrossRef] [PubMed]
- Mane, R.S.; Bhanage, B.M. Palladium-Catalyzed Oxidative N-Dealkylation/Carbonylation of Tertiary Amines with Alkynes to α,β-Alkynylamides. J. Org. Chem. 2016, 81, 4974–4980. [Google Scholar] [CrossRef]
- Idris, M.A.; Kim, M.; Kim, J.G.; Lee, S. Palladium-catalyzed decarboxylative aminocarbonylation with alkynoic acid and tertiary amine for the synthesis of alkynyl amide. Tetrahedron 2019, 75, 4130–4137. [Google Scholar] [CrossRef]
- Park, K.; Palani, T.; Pyo, A.; Lee, S. Synthesis of aryl alkynyl carboxylic acids and aryl alkynes from propiolic acid and aryl halides by site selective coupling and decarboxylation. Tetrahedron Lett. 2012, 53, 733–737. [Google Scholar] [CrossRef]
- Xie, Y.J.; Hu, J.H.; Wang, Y.Y.; Xia, C.G.; Huang, H.M. Palladium-Catalyzed Vinylation of Aminals with Simple Alkenes: A New Strategy To Construct Allylamines. J. Am. Chem. Soc. 2012, 134, 20613–20616. [Google Scholar] [CrossRef]
- Li, M.B.; Wang, Y.; Tian, S.K. Regioselective and Stereospecific Cross-Coupling of Primary Allylic Amines with Boronic Acids and Boronates through Palladium-Catalyzed C-N Bond Cleavage. Angew. Chem. Int. Ed. 2012, 51, 2968–2971. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, B.; Deng, C.L.; Tang, R.Y.; Zhang, X.G.; Li, J.H. Palladium-Catalyzed Oxidative Coupling of Trialkylamines with Aryl Iodides Leading to Alkyl Aryl Ketones. Org. Lett. 2011, 13, 2184–2187. [Google Scholar] [CrossRef]
- Shi, R.; Lu, L.; Zhang, H.; Chen, B.; Sha, Y.; Liu, C.; Lei, A. Palladium/Copper-Catalyzed Oxidative C–H Alkenylation/N-Dealkylative Carbonylation of Tertiary Anilines. Angew. Chem. Int. Ed. 2013, 52, 10582. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Gao, X.H.; Tang, R.Y.; Zhang, X.G.; Deng, C.L. A novel Pd-catalyzed N-dealkylative carbonylation of tertiary amines for the preparation of amides. Chem. Commun. 2014, 50, 14775–14777. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Zhang, H.; Lu, L.; Gan, P.; Sha, Y.; Zhang, H.; Liu, Q.; Beller, M.; Lei, A. (E)-α,β-unsaturated amides from tertiary amines, olefins and CO via Pd/Cu-catalyzed aerobic oxidative N-dealkylation. Chem. Commun. 2015, 51, 3247–3250. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, G.; Liu, Z.J.; Huang, H. Palladium-catalyzed carbonylation of allylamines via C–N bond activation leading to β,γ-unsaturated amides. RSC Adv. 2014, 4, 64235–64237. [Google Scholar] [CrossRef]




 | ||
|---|---|---|
| Entry | Variation from Standard Conditions | Yield b (%) |
| 1 | none | 94 |
| 2 | MeCN as a solvent | 84 |
| 3 | Toluene as a solvent | 52 |
| 4 | Dioxane as a solvent | 27 |
| 5 | DMSO as a solvent | NR |
| 6 | MeOH as a solvent | NR |
| 7 | without DMF | 66 |
| 8 | DMF (0.2 equiv.) | 78 |
| 9 | DMF (0.4 equiv.) | 87 |
| 10 | DMF (0.8 equiv.) | 90 |
| 11 | 0.5 h | 47 |
| 12 | 1.0 h | 75 |
| 13 | 1.5 h | 86 |
| 14 | 2.5 h | 93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, X.; Li, H.; Guo, S. Rapid and One-Pot Synthesis of Aryl Ynamides from Aryl Alkynyl Acids by Metal-Free C-N Cleavage of Tertiary Amines. Molecules 2025, 30, 2955. https://doi.org/10.3390/molecules30142955
Liu Y, Liu X, Li H, Guo S. Rapid and One-Pot Synthesis of Aryl Ynamides from Aryl Alkynyl Acids by Metal-Free C-N Cleavage of Tertiary Amines. Molecules. 2025; 30(14):2955. https://doi.org/10.3390/molecules30142955
Chicago/Turabian StyleLiu, Yong, Xiaoyong Liu, Hongwei Li, and Shengmei Guo. 2025. "Rapid and One-Pot Synthesis of Aryl Ynamides from Aryl Alkynyl Acids by Metal-Free C-N Cleavage of Tertiary Amines" Molecules 30, no. 14: 2955. https://doi.org/10.3390/molecules30142955
APA StyleLiu, Y., Liu, X., Li, H., & Guo, S. (2025). Rapid and One-Pot Synthesis of Aryl Ynamides from Aryl Alkynyl Acids by Metal-Free C-N Cleavage of Tertiary Amines. Molecules, 30(14), 2955. https://doi.org/10.3390/molecules30142955





