Essential Oil from the Aerial Parts of Artemisia serotina Bunge (Winter Wormwood) Growing in Kazakhstan—Phytochemical Profile and Bioactivity
Abstract
1. Introduction
2. Results
2.1. Chemical Composition
2.2. Antibacterial and Antifungal Activity
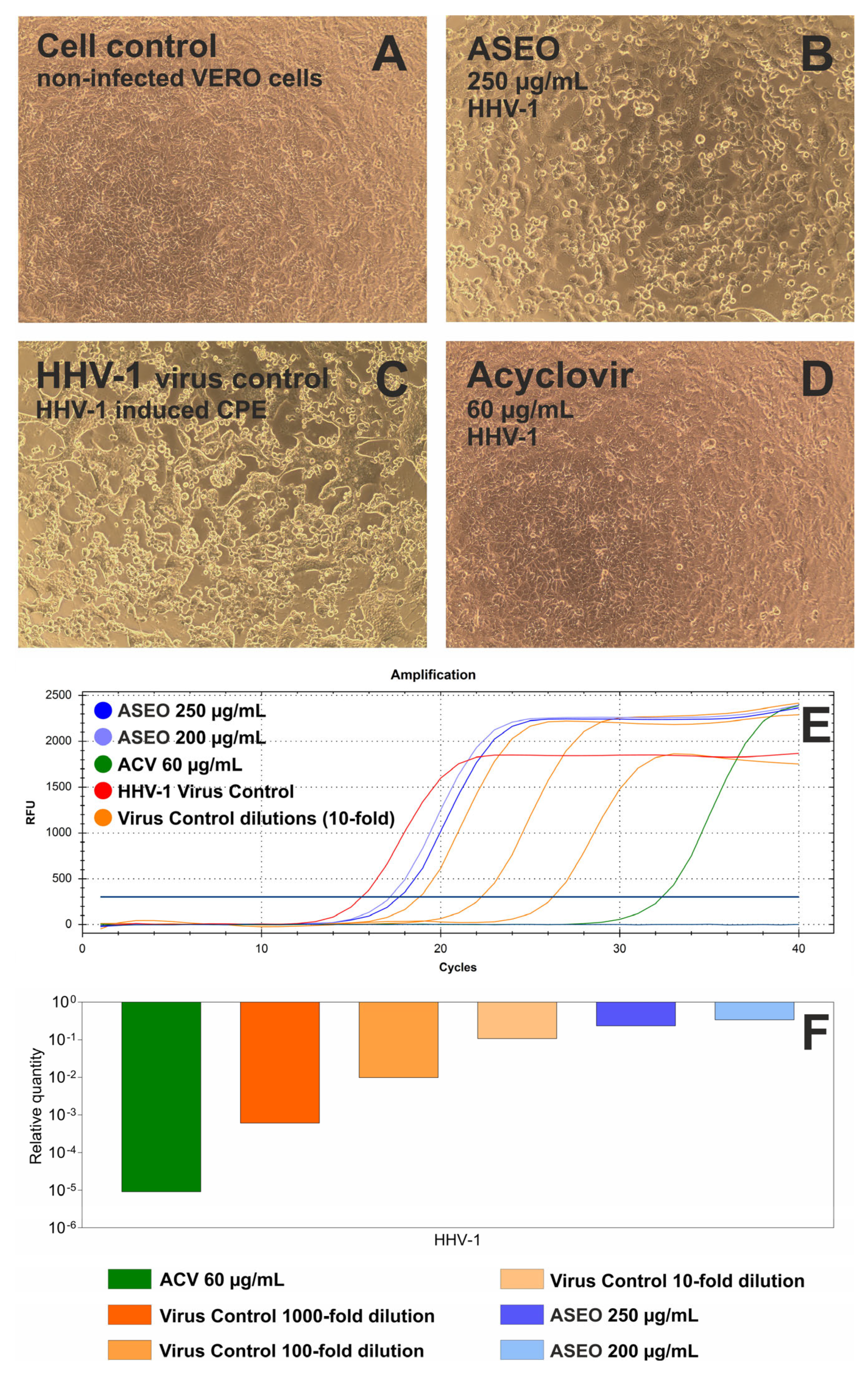
2.3. Antiviral Activity
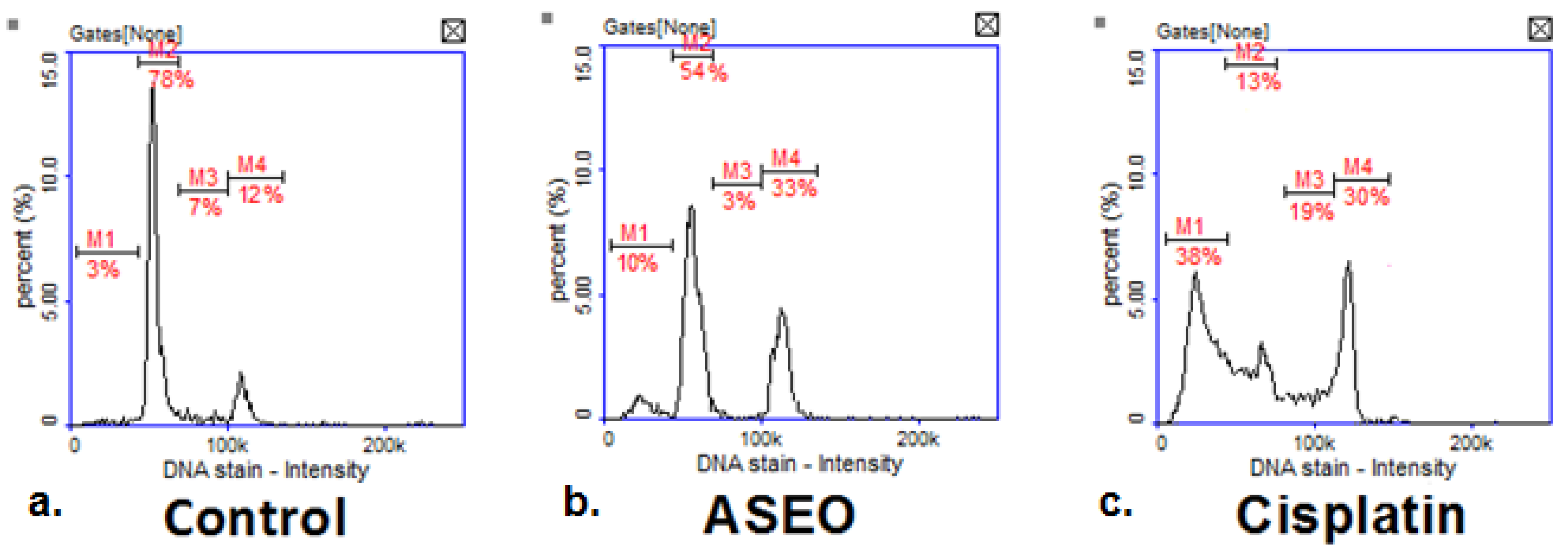
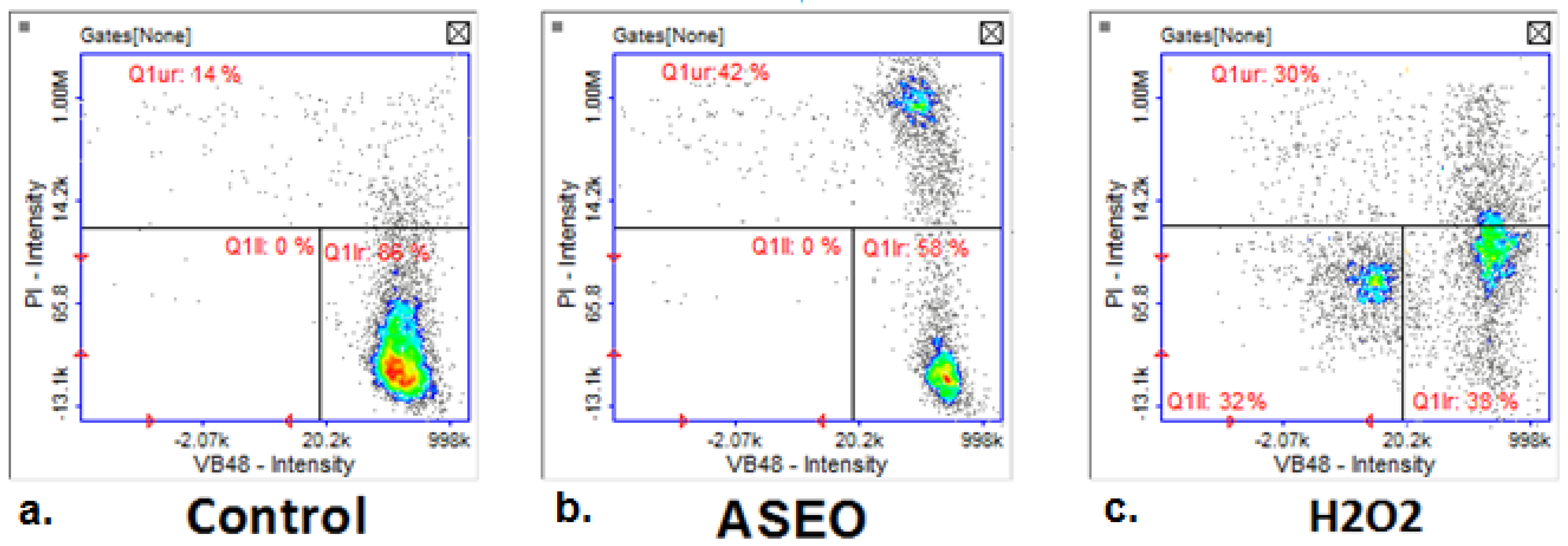
2.4. Anticancer Activity
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Method of Quantitative Isolation of Essential Oil from Plant Material
4.3. GC/MS Analysis
4.4. Antimicrobial Activity Assay
4.5. Determination of Antiviral Activity
4.5.1. Cell Maintenance and Cytotoxicity Testing
4.5.2. Antiviral Assays
4.6. Determination of Anticancer Activity
4.6.1. Cell Culturing and Treatment
4.6.2. Cytotoxic Evaluation
4.6.3. Cell Cycle Analysis
4.6.4. Analysis of Thiol Levels and Cell Vitality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bora, K.S.; Sharma, A. The Genus Artemisia: A Comprehensive Review. Pharm. Biol. 2011, 49, 101–109. [Google Scholar] [CrossRef]
- Hussain, M.; Thakur, R.K.; Khazir, J.; Ahmed, S.; Khan, M.I.; Rahi, P.; Peer, L.A.; Shanmugam, P.V.; Kaur, S.; Raina, S.N.; et al. Traditional Uses, Phytochemistry, Pharmacology, and Toxicology of the Genus artemisia L. (Asteraceae): A High-Value Medicinal Plant. Curr. Top. Med. Chem. 2024, 24, 301–342. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Herrera-Bravo, J.; Semwal, P.; Painuli, S.; Badoni, H.; Ezzat, S.M.; Farid, M.M.; Merghany, R.M.; Aborehab, N.M.; Salem, M.A.; et al. Artemisia spp.: An Update on Its Chemical Composition, Pharmacological and Toxicological Profiles. Oxidative Med. Cell. Longev. 2022, 2022, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Nurlybekova, A.; Kudaibergen, A.; Kazymbetova, A.; Amangeldi, M.; Baiseitova, A.; Ospanov, M.; Aisa, H.A.; Ye, Y.; Ibrahim, M.A.; Jenis, J. Traditional Use, Phytochemical Profiles and Pharmacological Properties of Artemisia Genus from Central Asia. Molecules 2022, 27, 5128. [Google Scholar] [CrossRef] [PubMed]
- Anibogwu, R.; Jesus, K.D.; Pradhan, S.; Pashikanti, S.; Mateen, S.; Sharma, K. Extraction, Isolation and Characterization of Bioactive Compounds from Artemisia and Their Biological Significance: A Review. Molecules 2021, 26, 6995. [Google Scholar] [CrossRef] [PubMed]
- Bisht, D.; Kumar, D.; Kumar, D.; Dua, K.; Chellappan, D.K. Phytochemistry and Pharmacological Activity of the Genus Artemisia. Arch. Pharm. Res. 2021, 44, 439–474. [Google Scholar] [CrossRef]
- Ekiert, H.; Klimek-Szczykutowicz, M.; Rzepiela, A.; Klin, P.; Szopa, A. Artemisia Species with High Biological Values as a Potential Source of Medicinal and Cosmetic Raw Materials. Molecules 2022, 27, 6427. [Google Scholar] [CrossRef]
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. Genus: A Review of Bioactive Essential Oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef]
- Pandey, A.K.; Singh, P. The Genus Artemisia: A 2012–2017 Literature Review on Chemical Composition, Antimicrobial, Insecticidal and Antioxidant Activities of Essential Oils. Medicines 2017, 4, 68. [Google Scholar] [CrossRef]
- Nabi, N.; Singh, S.; Saffeullah, P. An Updated Review on Distribution, Biosynthesis and Pharmacological Effects of Artemisinin: A Wonder Drug. Phytochemistry 2023, 214, 113798. [Google Scholar] [CrossRef]
- Goryaev, M.; Satdarova, E. Analysis of the Essential Oil of Artemisia Serotina. Trudy Instituta Him. Nauk, Akademija Nauk Kazahskoj SSR 1959, 4, 37–43. [Google Scholar]
- Mukhamatkhanova, R.F.; Bobakulov, K.M.; Sham’ianov, I.D.; Abdullaev, N.D. [Terpenoids and other components of Artemisia sogdiana and A. serotina, growing in Uzbekistan] Терпенoиды и другие кoмпoненты Artemisia sogdiana и A. serotina, прoизрастающих в Узбекистане. Chem. Plant Raw Mater. 2016, 2, 133–136. [Google Scholar] [CrossRef]
- Nurlybekova, A.K.; Kudaibergen, A.A.; Dyusebaeva, M.A.; Ibrahim, M.A.; Jenis, J. Chemical Constituents of Artemisia Serotina. Rep. Natl. Acad. Sci. Repub. Kazakhstan 2021, 5, 158–165. [Google Scholar] [CrossRef]
- Mahboubi, M. Artemisia Sieberi Besser Essential Oil and Treatment of Fungal Infections. Biomed. Pharmacother. 2017, 89, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Masoudi, S.; Rustaiyan, A.; Vahedi, M. Volatile Oil Constituents of Different Parts of Artemisia Chamaemelifolia and the Composition and Antibacterial Activity of the Aerial Parts of A. Turcomanica from Iran. Nat. Prod. Commun. 2012, 7, 1519–1522. [Google Scholar] [CrossRef] [PubMed]
- Muselli, A.; Desjobert, J.-M.; Bernardini, A.-F.; Costa, J. Santolina Alcohol as Component of the Essential Oil of Achillea ageratum L. from Corsica Island. J. Essent. Oil Res. 2007, 19, 319–322. [Google Scholar] [CrossRef]
- Gou, J.; Lu, Y.; Xie, M.; Tang, X.; Chen, L.; Zhao, J.; Li, G.; Wang, H. Antimicrobial Activity in Asterceae: The Selected Genera Characterization and against Multidrug Resistance Bacteria. Heliyon 2023, 9, e14985. [Google Scholar] [CrossRef]
- El Rabey, H.A.; Almutairi, F.M. The Antioxidant, Antidiabetic, Antimicrobial and Anticancer Constituents of Artemisia Species. Nat. Prod. Res. 2025, 39, 1685–1695. [Google Scholar] [CrossRef]
- Umam, K.; Feng, C.-S.; Yang, G.; Tu, P.-C.; Lin, C.-Y.; Yang, M.-T.; Kuo, T.-F.; Yang, W.-C.; Tran Nguyen Minh, H. Phytochemistry, Pharmacology and Mode of Action of the Anti-Bacterial Artemisia Plants. Bioengineering 2023, 10, 633. [Google Scholar] [CrossRef]
- Bilia, A.R.; Santomauro, F.; Sacco, C.; Bergonzi, M.C.; Donato, R. Essential Oil of Artemisia annua L.: An Extraordinary Component with Numerous Antimicrobial Properties. Evid. Based Complement. Alternat. Med. 2014, 2014, 159819. [Google Scholar] [CrossRef]
- Ma, L.; Wei, L.; Chen, X.; Wang, W.; Lu, J.; Li, Y.; Yao, L. Chemical Composition, Antioxidative and Antimicrobial Activities of Essential Oil of Wild Artemisia Annua from Ningxia, China. Nat. Prod. Res. 2024, 38, 4340–4346. [Google Scholar] [CrossRef] [PubMed]
- Abu-Darwish, M.S.; Cabral, C.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Zulfiqar, A.; Khan, I.A.; Efferth, T.; Salgueiro, L. Chemical Composition and Biological Activities of Artemisia Judaica Essential Oil from Southern Desert of Jordan. J. Ethnopharmacol. 2016, 191, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Deyno, S.; Mtewa, A.G.; Abebe, A.; Hymete, A.; Makonnen, E.; Bazira, J.; Alele, P.E. Essential Oils as Topical Anti-Infective Agents: A Systematic Review and Meta-Analysis. Complement. Ther. Med. 2019, 47, 102224. [Google Scholar] [CrossRef]
- Contaldo, M.; Di Stasio, D.; Romano, A.; Fiori, F.; Della Vella, F.; Rupe, C.; Lajolo, C.; Petruzzi, M.; Serpico, R.; Lucchese, A. Oral Candidiasis and Novel Therapeutic Strategies: Antifungals, Phytotherapy, Probiotics, and Photodynamic Therapy. Curr. Drug Deliv. 2023, 20, 441–456. [Google Scholar] [CrossRef]
- Kshirsagar, S.G.; Rao, R.V. Antiviral and Immunomodulation Effects of Artemisia. Med. Kaunas Lith. 2021, 57, 217. [Google Scholar] [CrossRef]
- Davison, A.J. Herpesvirus Systematics. Vet. Microbiol. 2010, 143, 52–69. [Google Scholar] [CrossRef]
- Saddi, M.; Sanna, A.; Cottiglia, F.; Chisu, L.; Casu, L.; Bonsignore, L.; De Logu, A. Antiherpevirus Activity of Artemisia Arborescens Essential Oil and Inhibition of Lateral Diffusion in Vero Cells. Ann. Clin. Microbiol. Antimicrob. 2007, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Sinico, C.; De Logu, A.; Zaru, M.; Müller, R.H.; Fadda, A.M. SLN as a Topical Delivery System for Artemisia Arborescens Essential Oil: In Vitro Antiviral Activity and Skin Permeation Study. Int. J. Nanomed. 2007, 2, 419–425. [Google Scholar]
- Polito, F.; Papaianni, M.; Woo, S.L.; Malaspina, P.; Cornara, L.; De Feo, V. Artemisia arborescens (Vaill.) L.: Micromorphology, Essential Oil Composition, and Its Potential as an Alternative Biocontrol Product. Plants 2024, 13, 825. [Google Scholar] [CrossRef]
- Kamarya, Y.; Xia, L.; Li, J. Chemical Constituents and Antitumor Mechanisms of Artemisia. Anticancer Agents Med. Chem. 2022, 22, 1838–1844. [Google Scholar] [CrossRef]
- Cherfi, I.; Nasma, M.; Hasan, G.G.; Benaissa, A.; Benaissa, Y.; Laouini, S.E.; Bouafia, A.; Alharthi, F.; Emran, T.B.; Mallick, J. Therapeutic Potential of Artemisia campestris Essential Oil: Antioxidant, Anti-Inflammatory, and Anticancer Insights from In Silico Analysis. Biomed. Chromatogr. 2025, 39, e70012. [Google Scholar] [CrossRef] [PubMed]
- Break, M.K.B.; Hussein, W.; Huwaimel, B.; Alafnan, A.; Almansour, K.; Alafnan, D.; Alshammari, A.S.; Alanazi, I.A.; Alshammari, D.S.; Alanzi, F.S.; et al. Artemisia Sieberi Besser Essential Oil Inhibits the Growth and Migration of Breast Cancer Cells via Induction of S-Phase Arrest, Caspase-Independent Cell Death and Downregulation of ERK. J. Ethnopharmacol. 2023, 312, 116492. [Google Scholar] [CrossRef]
- Singla, R.K.; Wang, X.; Gundamaraju, R.; Joon, S.; Tsagkaris, C.; Behzad, S.; Khan, J.; Gautam, R.; Goyal, R.; Rakmai, J.; et al. Natural Products Derived from Medicinal Plants and Microbes Might Act as a Game-Changer in Breast Cancer: A Comprehensive Review of Preclinical and Clinical Studies. Crit. Rev. Food Sci. Nutr. 2023, 63, 11880–11924. [Google Scholar] [CrossRef]
- State Pharmacopoeia of the Republic of Kazakhstan, 2nd ed.; Zhibek Zholy: Almaty, Republic of Kazakhstan, 2015; Volume I.
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST). Determination of Minimum Inhibitory Concentrations (MICs) of Antibacterial Agents by Broth Dilution. Clin. Microbiol. Infect. 2003, 9, ix–xv. [Google Scholar] [CrossRef]
- CLSI Standard M27; CLSI Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. 4th ed. CLSI: Wayne, PA, USA, 2017.
- Biernasiuk, A.; Berecka-Rycerz, A.; Gumieniczek, A.; Malm, M.; Łączkowski, K.Z.; Szymańska, J.; Malm, A. The Newly Synthesized Thiazole Derivatives as Potential Antifungal Compounds against Candida Albicans. Appl. Microbiol. Biotechnol. 2021, 105, 6355–6367. [Google Scholar] [CrossRef]
- Pecio, Ł.; Kozachok, S.; Saber, F.R.; Garcia-Marti, M.; El-Amier, Y.; Mahrous, E.A.; Świątek, Ł.; Boguszewska, A.; Skiba, A.; Elosaily, A.H.; et al. Metabolic Profiling of Ochradenus Baccatus Delile. Utilizing UHPLC-HRESIMS in Relation to the In Vitro Biological Investigations. Food Chem. 2023, 412, 135587. [Google Scholar] [CrossRef]
- Mukhamedsadykova, A.Z.; Kasela, M.; Kozhanova, K.K.; Sakipova, Z.B.; Kukuła-Koch, W.; Józefczyk, A.; Świątek, Ł.; Rajtar, B.; Iwan, M.; Kołodziej, P.; et al. Anthelminthic and Antimicrobial Effects of Hedge Woundwort (Stachys sylvatica L.) Growing in Southern Kazakhstan. Front. Pharmacol. 2024, 15, 1386509. [Google Scholar] [CrossRef] [PubMed]
- Korga-Plewko, A.; Michalczyk, M.; Adamczuk, G.; Humeniuk, E.; Ostrowska-Lesko, M.; Jozefczyk, A.; Iwan, M.; Wojcik, M.; Dudka, J. Apigenin and Hesperidin Downregulate DNA Repair Genes in MCF-7 Breast Cancer Cells and Augment Doxorubicin Toxicity. Molecules 2020, 25, 4421. [Google Scholar] [CrossRef]
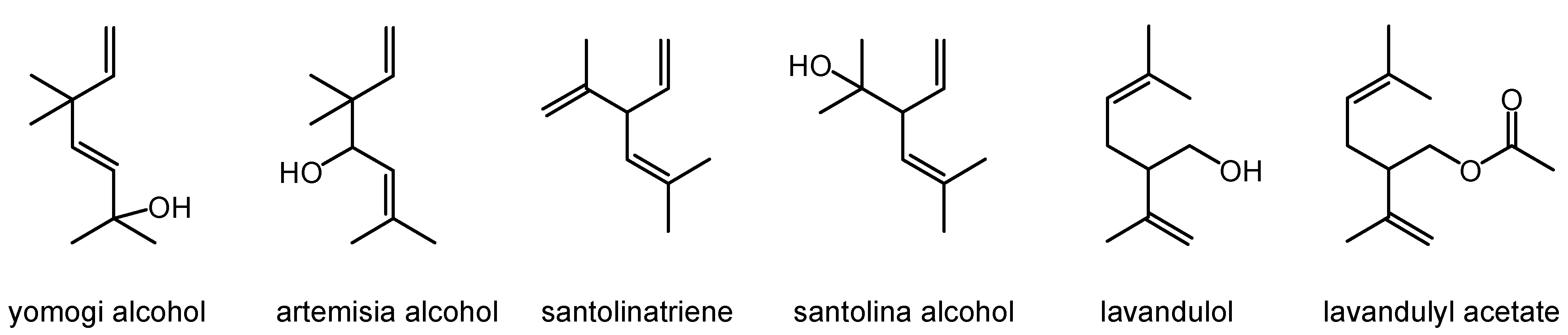
| No. | Compound | RIexp | RIlit | Relative Percentage |
|---|---|---|---|---|
| 1 | Santolinatriene | 906 | 909 | 0.2 |
| 2 | Camphene | 954 | 950 | 0.2 |
| 3 | 1-Octen-3-ol | 984 | 962 | 0.2 |
| 4 | Yomogi alcohol | 998 | 991 | 1.1 |
| 5 | [M+] 168(1), 43(100), 81(55), 95(50) | 1030 | - | 2.1 |
| 6 | Santolina alcohol | 1036 | 1029 | 34.6 |
| 7 | Artemisia alcohol | 1084 | 1073 | 0.6 |
| 8 | trans-p-Menth-2-en-1-ol | 1117 | 1116 | 3.0 |
| 9 | Camphor | 1156 | 1132 | 7.6 |
| 10 | [M+] 166(1), 79(100), 43(85), 93(80), 121(60) | 1159 | - | 2.3 |
| 11 | [M+] 152(2), 43(100), 95(55), 59(25), 138(15) | 1164 | - | 27.0 |
| 12 | Lavandulol | 1169 | 1150 | 0.7 |
| 13 | Borneol | 1183 | 1159 | 0.3 |
| 14 | Terpinen-4-ol | 1189 | 1164 | 0.2 |
| 15 | a-Terpineol | 1204 | 1176 | 0.3 |
| 16 | [M+] 168 (2), 82(100), 67(60), 110(15) | 1249 | - | 7.1 |
| 17 | Carvone | 1253 | 1214 | 0.2 |
| 18 | Lavandulyl acetate | 1284 | 1275 | 0.2 |
| 19 | (Z)-Jasmone | 1401 | 1381 | 0.2 |
| 20 | Germacrene D | 1496 | 1476 | 0.3 |
| 21 | Bicyclogermacrene | 1510 | 1494 | 0.2 |
| 22 | Spathulenol | 1596 | 1572 | 0.7 |
| 23 | Globulol | 1681 | 1659 | 0.2 |
| Total | 89.5 | |||
| Irregular monoterpenes | 37.4 | |||
| Regular monoterpenes | 11.8 | |||
| Sesquiterpenes | 1.4 | |||
| Unidentified compounds | 38.5 | |||
| Other | 0.4 | |||
| Bacterial Species | MIC [mg/mL] | MBC [mg/mL] | MBC/MIC | MIC for Ciprofloxacin or Vancomycin * [μg/mL] |
|---|---|---|---|---|
| Gram-positive bacteria | ||||
| Staphylococcus aureus ATCC 29213 | 8 | 16 | 2 | 0.48 |
| Staphylococcus aureus ATCC 43300 | 8 | 16 | 2 | 0.24 |
| Staphylococcus epidermidis ATCC 12228 | 8 | 16 | 2 | 0.12 |
| Enterococcus faecalis ATCC 29212 | 8 | 16 | 2 | 0.98 * |
| Micrococcus luteus ATCC 10240 | 4 | 8 | 2 | 0.98 |
| Bacillus subtilis ATCC 6633 | 8 | 16 | 2 | 0.03 |
| Bacillus cereus ATCC 10876 | 16 | 16 | 1 | 0.06 |
| Gram-negative bacteria | ||||
| Salmonella Typhimurium ATCC 14028 | 16 | >16 | >1 | 0.06 |
| Escherichia coli ATCC 25922 | 8 | 16 | 2 | 0.004 |
| Pseudomonas aeruginosa ATCC 27853 | 8 | 16 | 2 | 0.48 |
| Fungal (yeast) species | MIC [mg/mL] | MFC [mg/mL] | MFC/MIC | MIC for nystatin [μg/mL] |
| Candida albicans ATCC 10231 | 2 | 4 | 2 | 0.48 |
| Cancer Cell Line | IC50 [µg/mL] | IC50 [µg/mL] |
|---|---|---|
| 24 h | 48 h | |
| G-361 (melanoma) | 273.22 ± 2.58 | 252.51 ± 3.45 |
| HeLa (cervical carcinoma) | >500 | >500 |
| PANC-1 (pancreatic carcinoma) | 473.21 ± 4.74 | 412.42 ± 4.32 |
| MCF-7 (breast carcinoma) | >500 | 491.76 ± 2.42 |
| T47-D (breast carcinoma) | 40.81 ± 4.21 | 33.17 ± 2.11 |
| PC-3 (prostate carcinoma) | >500 | >500 |
| DU-145 (prostate carcinoma) | >500 | >500 |
| LNCaP (prostate carcinoma) | >500 | 433.28 ± 5.63 |
| A-549 (lung carcinoma) | >500 | >500 |
| NCI-H1563 (lung carcinoma) | 258.43 ± 6.43 | 234.13 ± 2.86 |
| NCI-H2170 (lung carcinoma) | >500 | 463.75 ± 4.32 |
| Hep-G2 (hepatocellular carcinoma) | 450.38 ± 5.21 | 411.23 ± 5.61 |
| AGS (gastric adenocarcinoma) | 471.90 ± 24.69 | 364.20 ± 5.89 * |
| FaDu (hypopharyngeal squamous-cell carcinoma) | 393.88 ± 18.19 | 230.58 ± 19.01 * |
| RKO (colon cancer) | 346.15 ± 29.35 | 161.95 ± 6.15 * |
| Normal cell line | ||
| MCF-10 (epithelial cells from the mammary gland) | >500 | 465.62 ± 3.86 |
| HUVEC (endothelial cells from veins) | >500 | 451.45 ± 1.78 |
| BJ (fibroblasts from skin) | 475.32 ± 3.86 | 429 ± 2.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadyrbay, A.; Ibragimova, L.N.; Iwan, M.; Ludwiczuk, A.; Biernasiuk, A.; Sakipova, Z.B.; Świątek, Ł.; Salwa, K.; Korga-Plewko, A.; Zhaparkulova, K.A.; et al. Essential Oil from the Aerial Parts of Artemisia serotina Bunge (Winter Wormwood) Growing in Kazakhstan—Phytochemical Profile and Bioactivity. Molecules 2025, 30, 2956. https://doi.org/10.3390/molecules30142956
Kadyrbay A, Ibragimova LN, Iwan M, Ludwiczuk A, Biernasiuk A, Sakipova ZB, Świątek Ł, Salwa K, Korga-Plewko A, Zhaparkulova KA, et al. Essential Oil from the Aerial Parts of Artemisia serotina Bunge (Winter Wormwood) Growing in Kazakhstan—Phytochemical Profile and Bioactivity. Molecules. 2025; 30(14):2956. https://doi.org/10.3390/molecules30142956
Chicago/Turabian StyleKadyrbay, Arshyn, Liliya N. Ibragimova, Magdalena Iwan, Agnieszka Ludwiczuk, Anna Biernasiuk, Zuriyadda B. Sakipova, Łukasz Świątek, Kinga Salwa, Agnieszka Korga-Plewko, Karlygash A. Zhaparkulova, and et al. 2025. "Essential Oil from the Aerial Parts of Artemisia serotina Bunge (Winter Wormwood) Growing in Kazakhstan—Phytochemical Profile and Bioactivity" Molecules 30, no. 14: 2956. https://doi.org/10.3390/molecules30142956
APA StyleKadyrbay, A., Ibragimova, L. N., Iwan, M., Ludwiczuk, A., Biernasiuk, A., Sakipova, Z. B., Świątek, Ł., Salwa, K., Korga-Plewko, A., Zhaparkulova, K. A., Bekezhanova, T. S., Józefczyk, A., Szymańska, J., & Malm, A. (2025). Essential Oil from the Aerial Parts of Artemisia serotina Bunge (Winter Wormwood) Growing in Kazakhstan—Phytochemical Profile and Bioactivity. Molecules, 30(14), 2956. https://doi.org/10.3390/molecules30142956









