Toxicological Risk Assessment of Coffee Oil (Coffee Seed Oil and Spent Coffee Grounds Oil) as a Novel Food with Focus on Cafestol
Abstract
1. Introduction
2. Literature Research
3. Compositional and Toxicological Data on Coffee Oil
3.1. General Composition of Coffee Oil
3.1.1. Triacylglycerols
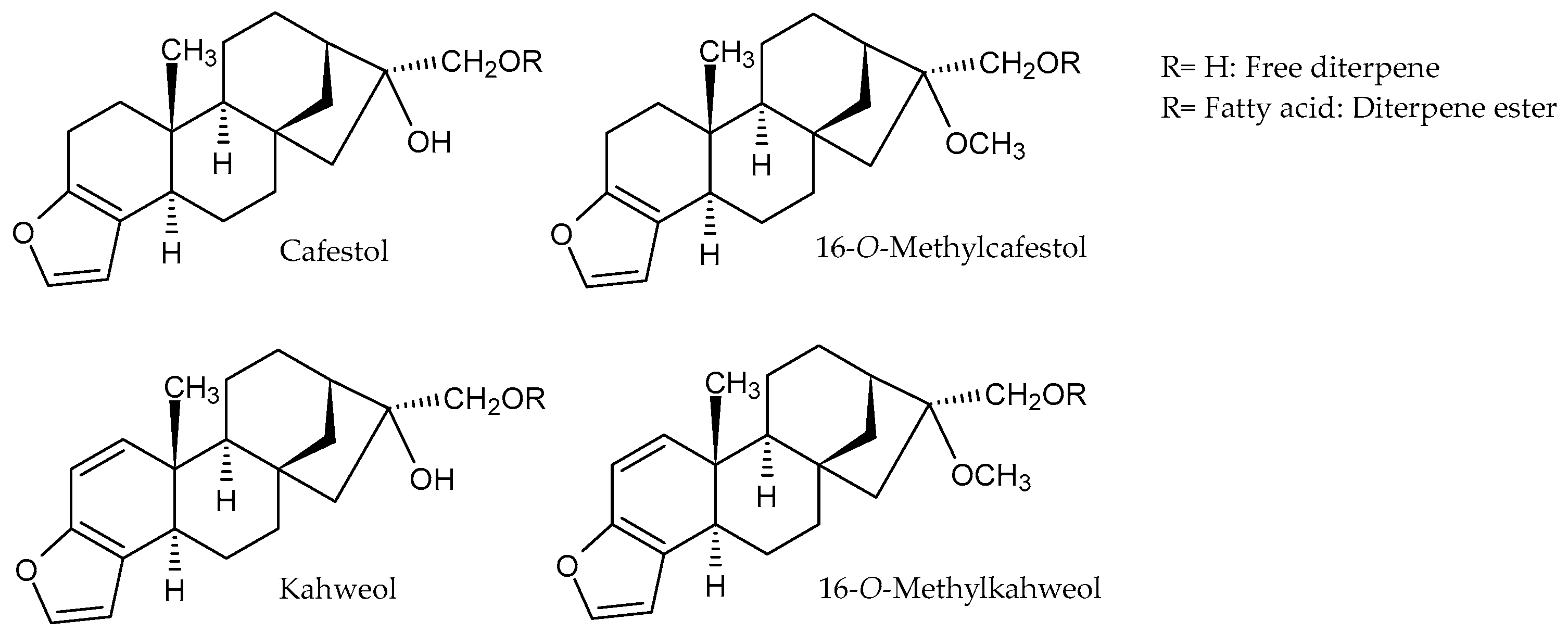
3.1.2. Diterpenes and Their Derivatives
3.1.3. Distribution of Diterpenes in Coffea arabica and Coffea canephora
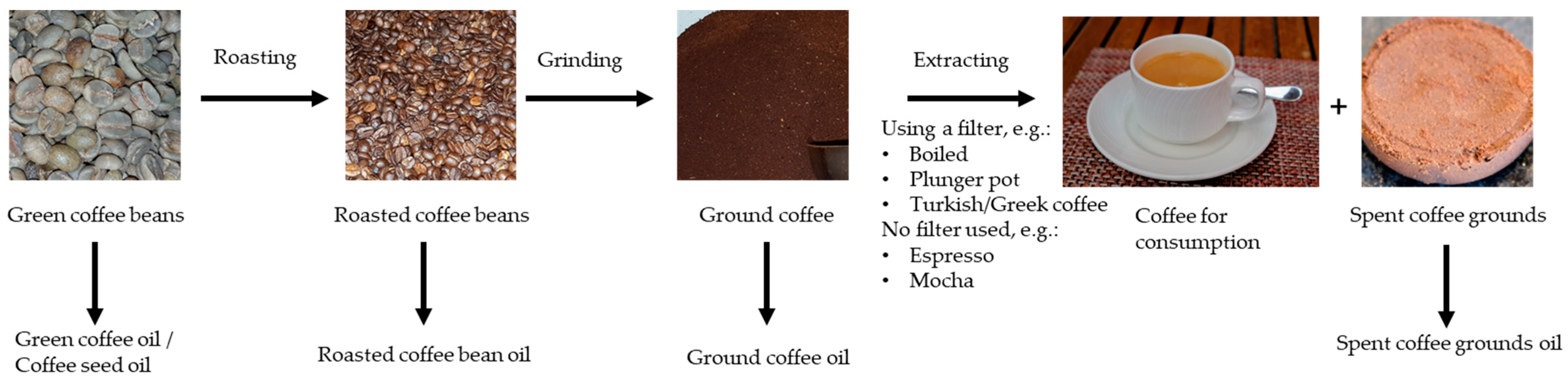
3.2. Coffee Oil Extraction
3.3. Potential Use of Coffee Oil in Food Products
3.4. Influence of the Brewing Technique on Coffee Oil in Coffee Beverages
3.5. Estimated Intake of Cafestol and Kahweol
3.6. Bioavailability of Cafestol
3.7. Human Studies
3.7.1. Epidemiology
3.7.2. Effect on Serum Lipids

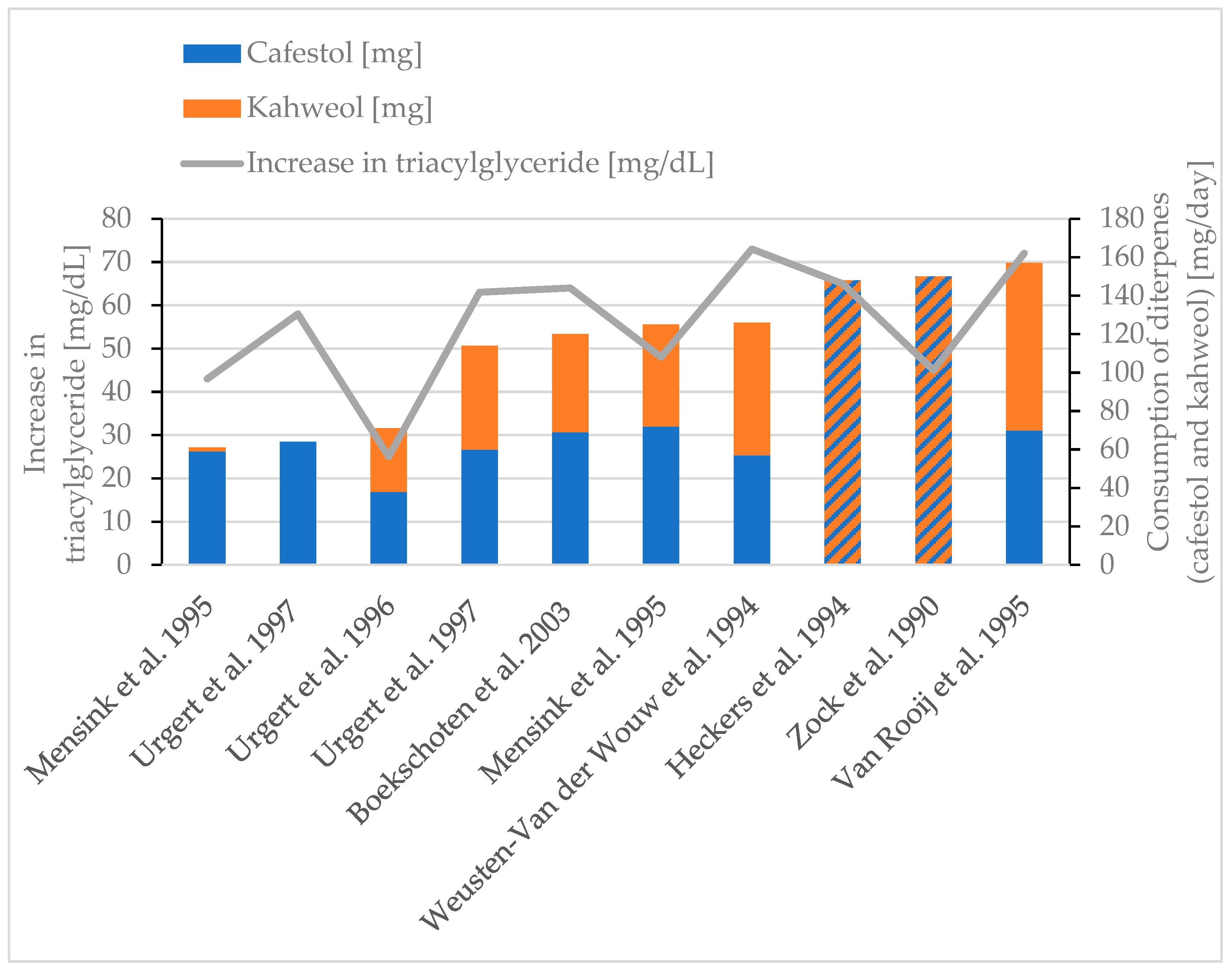
| Effect | Studies with Humans | Cafestol/Kahweol Content (per Day) | ∆ |
|---|---|---|---|
| Rise in total serum cholesterol | Boekschoten et al., 2003 [86] 2 mL coffee oil/day, 5 weeks | 69 mg cafestol and 51 mg kahweol | +43 mg/dL |
| Weusten-Van der Wouw et al., 1994 [38] 2 g coffee oil/day, 4 weeks | 57 mg cafestol and 69 mg kahweol | +49 mg/dL | |
| Heckers et al., 1994 [88] 148 mg diterpenes/day | N/A | +48 mg/dL | |
| Van Rooij et al., 1995 [30] 1 g coffee oil (Arabica/day), 6 weeks | 70 mg cafestol and 87 mg kahweol | +44 mg/dL | |
| Van Rooij et al., 1995 [30] 1 g coffee oil (Canephora/day), 6 weeks | 39 mg cafestol and 1 mg kahweol | +19 mg/dL | |
| Urgert et al., 1997 [85] 64 mg cafestol/day, 28 days | 64 mg cafestol | +31 mg/dL | |
| Urgert et al., 1997 [85] 114 mg diterpenes/day, 28 days | 60 mg cafestol and 54 mg kahweol | +36 mg/dL | |
| Urgert et al., 1996 [77] 71 mg diterpenes/day, 24 weeks | 38 mg cafestol and 33 mg kahweol | +21 mg/dL | |
| Mensink et al., 1995 [87] 2 g coffee oil/day, 3 weeks | 72 mg cafestol and 53 mg kahweol | +25 mg/dL | |
| Mensink et al., 1995 [87] 2 g coffee oil/day, 3 weeks | 59 mg cafestol (cafestol and O-methyl-cafestol) and 2 mg kahweol | +21 mg/dL | |
| Zock et al., 1990 [78] 1.3 g coffee lipids/day, 6 weeks | 150 mg unsaponifiable lipids | +41 mg/dL | |
| Rise in triacylglycerol | Boekschoten et al., 2003 [86] 2 mL coffee oil/day, 5 weeks | 69 mg cafestol and 51 mg kahweol | +64 mg/dL |
| Weusten-Van der Wouw et al., 1994 [38] 2 g coffee oil/day, 4 weeks | 57 mg cafestol and 69 mg kahweol | +73 mg/dL | |
| Van Rooij et al., 1995 [30] 1 g coffee oil (Arabica/day), 6 weeks | 70 mg cafestol and 87 mg kahweol | +72 mg/dL | |
| Urgert et al., 1997 [85] 64 mg cafestol/day, 28 days | 64 mg cafestol | +58 mg/dL | |
| Urgert et al., 1997 [85] 114 mg diterpenes/day, 28 days | 60 mg cafestol and 54 mg kahweol | +63 mg/dL | |
| Heckers et al., 1994 [88] 148 mg diterpenes/day | N/A | +65 mg/dL | |
| Urgert et al., 1996 [77] 71 mg diterpenes/day, 24 weeks | 38 mg cafestol and 33 mg kahweol | +25 mg/dL | |
| Mensink et al., 1995 [87] 2 g coffee oil/day, 3 weeks | 72 mg cafestol and 53 mg kahweol | +48 mg/dL | |
| Mensink et al., 1995 [87] 2 g coffee oil/day, 3 weeks | 59 mg cafestol (cafestol and O-methyl-cafestol) and 2 mg kahweol | +43 mg/dL | |
| Zock et al., 1990 [78] 1.3 g coffee lipids/day, 6 weeks | 150 mg unsaponifiable lipids | +45 mg/dL | |
| No significant effect on triacylglycerol | Van Rooij et al., 1995 [30] 1 g coffee oil (Canephora/day), 6 weeks | 39 mg cafestol and 1 mg kahweol | No significant change |
| Decrease in HDL cholesterol | Urgert et al., 1997 [85] 64 mg cafestol/day, 28 days | 64 mg cafestol | −2 mg/dL |
| Urgert et al., 1997 [85] 114 mg diterpenes/day, 28 days | 60 mg cafestol and 54 mg kahweol | −3 mg/dL | |
| Zock et al., 1990 [78] 1.3 g coffee lipids/day, 6 weeks | 150 mg unsaponifiable lipids | −0.8 mg/dL (nearly unchanged) | |
| No significant effect on HDL cholesterol | Van Rooij et al., 1995 [30] 1 g coffee oil (Arabica/day), 6 weeks | 70 mg cafestol and 87 mg kahweol | No significant change |
| Van Rooij et al., 1995 [30] 1 g coffee oil (Canephora/day), 6 weeks | 39 mg cafestol and 1 mg kahweol | No significant change | |
| Boekschoten et al., 2005 [90] 2 mL coffee oil/day, 5 weeks | 69 mg cafestol and 51 mg kahweol | No significant change | |
| Weusten-Van der Wouw et al., 1994 [38] 2 g coffee oil/day, 4 weeks | 57 mg cafestol and 69 mg kahweol | No significant change | |
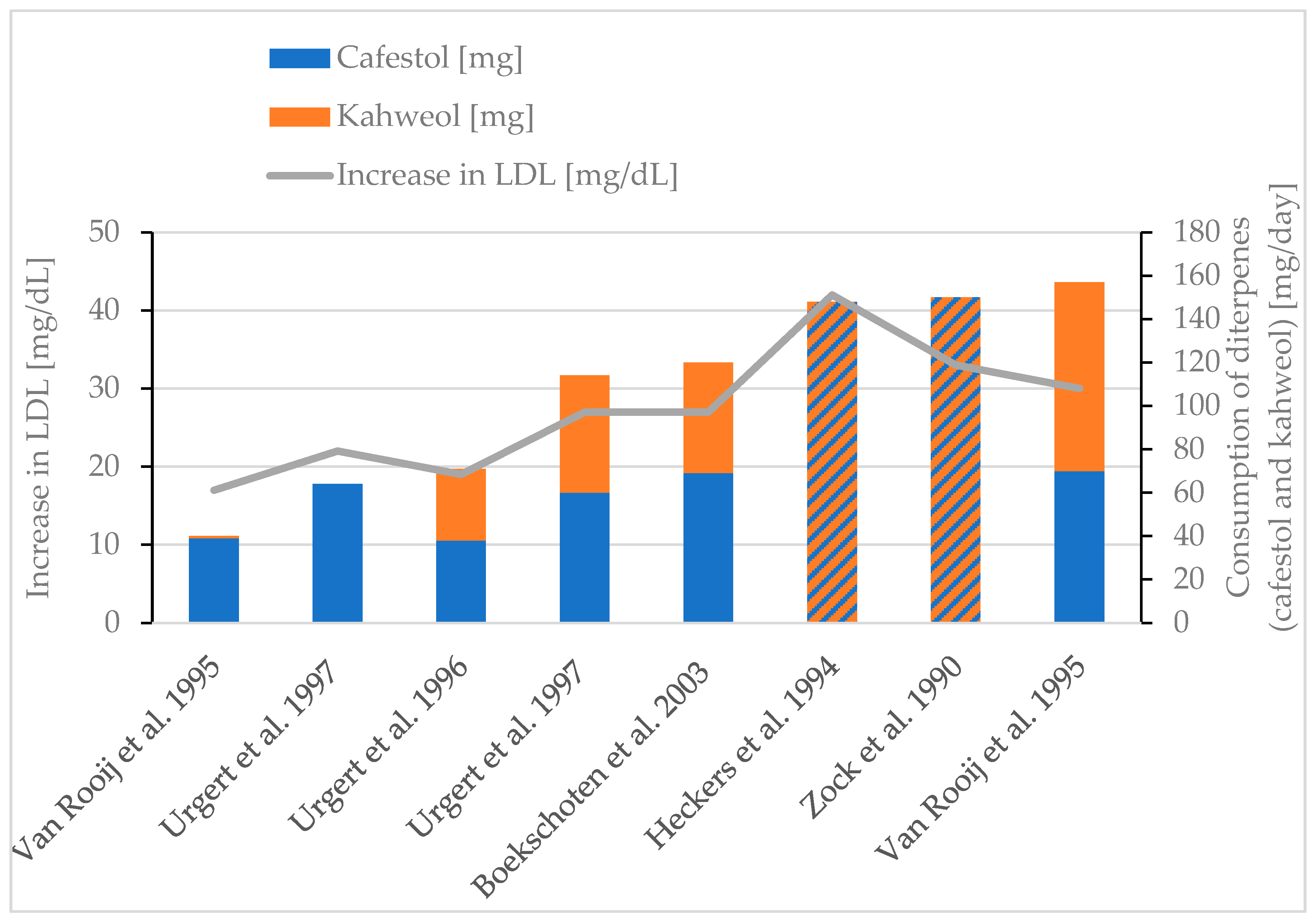
| Rise in LDL cholesterol | Boekschoten et al., 2003 [86] 2 mL coffee oil/day, 5 weeks | 69 mg cafestol and 51 mg kahweol | +27 mg/dL |
| Weusten-Van der Wouw et al., 1994 [38] 2 g coffee oil/day, 4 weeks | 57 mg cafestol and 69 mg kahweol | Increased (not further defined) | |
| Heckers et al., 1994 [88] 148 mg diterpenes/day | N/A | +42 mg/dL | |
| Urgert et al., 1997 [85] 64 mg cafestol/day, 28 days | 64 mg cafestol | +22 mg/dL | |
| Urgert et al., 1997 [85] 114 diterpenes/day, 28 days | 60 mg cafestol and 54 mg kahweol | +27 mg/dL | |
| Urgert et al., 1996 [77] 71 mg diterpenes/day, 24 weeks | 38 mg cafestol and 33 mg kahweol | +19 mg/dL | |
| Van Rooij et al., 1995 [30] 1 g coffee oil (Arabica/day), 6 weeks | 70 mg cafestol and 87 mg kahweol | +30 mg/dL | |
| Van Rooij et al., 1995 [30] 1 g coffee oil (Canephora/day), 6 weeks | 39 mg cafestol and 1 mg kahweol | +17 mg/dL | |
| Zock et al., 1990 [78] 1.3 g coffee lipids/day, 6 weeks | 150 mg unsaponifiable lipids | +33 mg/dL |
3.7.3. Effects on Liver Enzymes
3.7.4. Effects on Other Parameters
3.8. Proposed Mechanism of Action of Cafestol
3.9. Potential Health Benefits of Cafestol and Kahweol
3.10. Acute or Subacute Toxicity
3.11. Further Remarks
4. Discussion
4.1. Comparison of Blood Parameter Changes Due to Coffee Oil with Other Foods
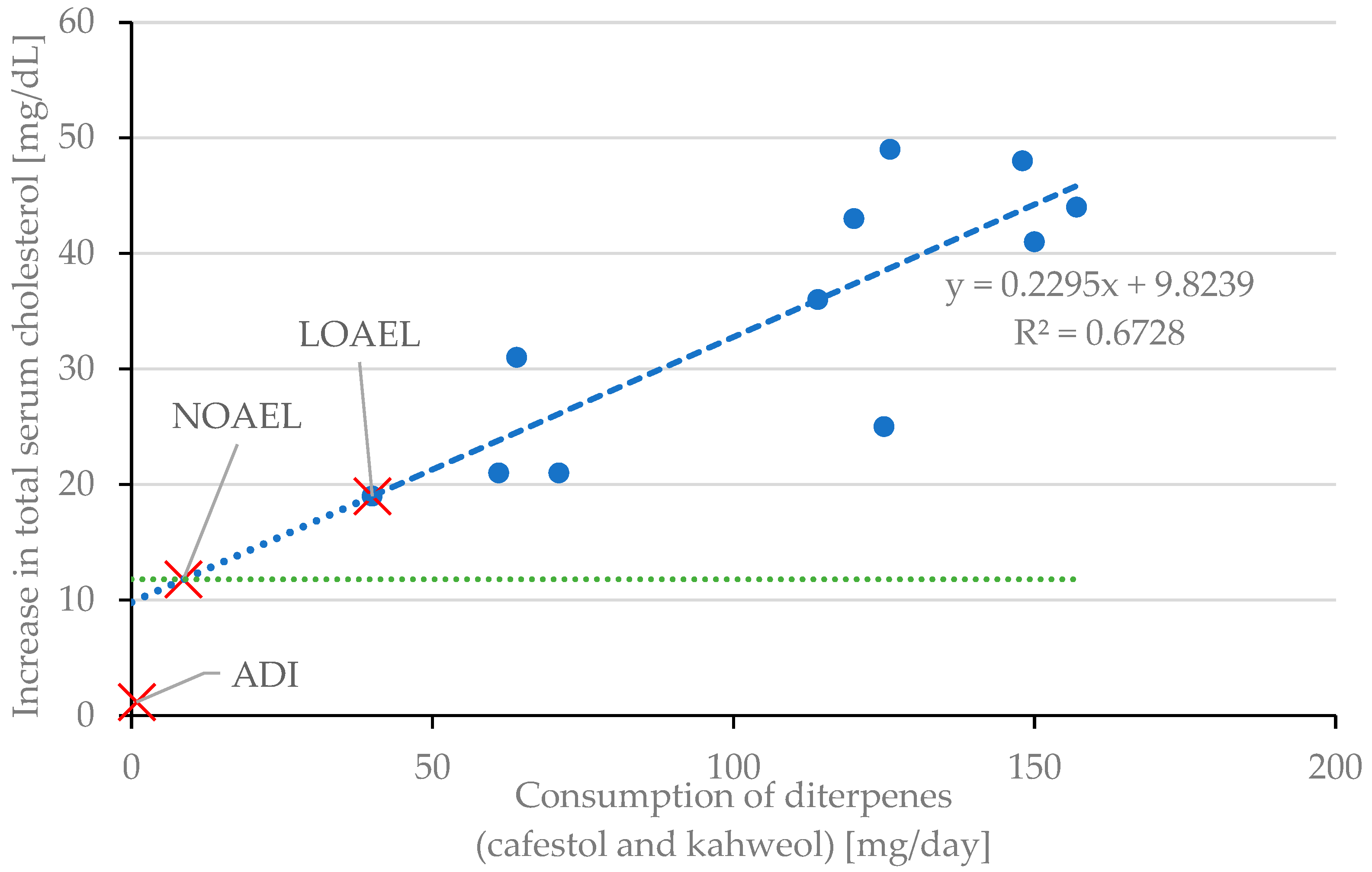
4.2. Determining LOAEL, NOAEL, and ADI of Cafestol and Kahweol
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maurin, O.; Davis, A.P.; Chester, M.; Mvungi, E.F.; Jaufeerally-Fakim, Y.; Fay, M.F. Towards a Phylogeny for Coffea (Rubiaceae): Identifying well-supported lineages based on nuclear and plastid DNA sequences. Ann. Bot. 2007, 100, 1565–1583. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.P.; Tosh, J.; Ruch, N.; Fay, M.F. Growing coffee: Psilanthus (Rubiaceae) subsumed on the basis of molecular and morphological data; implications for the size, morphology, distribution and evolutionary history of Coffea. Bot. J. Linn. 2011, 167, 357–377. [Google Scholar] [CrossRef]
- Alasmar, K.M.; Zeid, I.; Al-Attar, A.M. Medicinal Properties of Arabica coffee (Coffea arabica) Oil: An Overview. Adv. Life Sci. 2020, 8, 20–29. [Google Scholar] [CrossRef]
- International Coffee Organization. Coffee Market Report April 2025. Available online: https://www.ico.org/documents/cy2024-25/cmr-0425-e.pdf (accessed on 29 May 2025).
- Kusolwa, P.M.; Makwinja, F.; Nashon, J.; Marianna, M.; Kibola, A. Morphological Diversity of Wild Coffee (Coffea kihansiensis) a Potential Coffee Species for Genetic Improvement. Tanz. J. Sci. 2019, 45, 629–649. [Google Scholar]
- United States Department of Agriculture Foreign Agricultural Service. Coffee: World Markets and Trade. Available online: https://fas.usda.gov/sites/default/files/2024-06/coffee.pdf (accessed on 29 May 2025).
- Eurostat. Happy International Coffee Day! Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/w/edn-20241001-1 (accessed on 29 May 2025).
- Calligaris, S.; Munari, M.; Arrighetti, G.; Barba, L. Insights into the physicochemical properties of coffee oil. Eur. J. Lipid Sci. Technol. 2009, 111, 1270–1277. [Google Scholar] [CrossRef]
- Speer, K.; Kölling-Speer, I. The lipid fraction of the coffee bean. Braz. J. Plant Physiol. 2006, 18, 201–216. [Google Scholar] [CrossRef]
- Folmer, B. The Craft and Science of Coffee; Academic Press/Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780128035580. [Google Scholar]
- Khan, N.A.; Brown, J.B. The Composition of Coffee Oil and Its Component Fatty Acids. J. Am. Oil Chem. Soc. 1953, 30, 606–609. [Google Scholar] [CrossRef]
- Ribeiro, R.C.; Mota, M.F.S.; Silva, R.M.V.; Silva, D.C.; Novaes, F.J.M.; da Veiga, V.F.; Bizzo, H.R.; Teixeira, R.S.S.; Rezende, C.M. Coffee Oil Extraction Methods: A Review. Foods 2024, 13, 2601. [Google Scholar] [CrossRef]
- Ravindranath, R.; Khan, R.Y.A.; Obi Reddy, T.; Thirumala Rao, S.D.; Reddy, B.R. Composition and characteristics of Indian coffee bean, spent grounds and oil. J. Sci. Food Agric. 1972, 23, 307–310. [Google Scholar] [CrossRef]
- Clarke, R.J.; Vitzthum, O.G. Coffee: Recent Developments; Blackwell Science: Oxford, UK, 2001. [Google Scholar] [CrossRef]
- Denmark, Danish Veterinary and Food Administration (DVFA). Consultation on the Determination of the Status of a Novel Food under Article 4 (2) of Regulation (EU) 2015/2283. Available online: https://food.ec.europa.eu/document/download/4cd74eda-5681-430f-aafc-fb5735781e0e_en?filename=novel-food_consult-status_coffee-grounds.pdf (accessed on 16 June 2024).
- Czech Republic, Ministry of Agriculture, Food Safety Department Consultation. Request to Determine the Novel Food Status of Coffee Grounds Oil Extract. Available online: https://food.ec.europa.eu/document/download/98c97f9a-5501-4e2a-bb99-4ce55f217597_en?filename=novel-food_consult-status_coffee-grounds-oil-extract.pdf (accessed on 19 February 2025).
- Germany. Consultation on the Determination of the Status of a Novel Food Under Article 4 (2) of Regulation (EU) 2015/2283. Available online: https://food.ec.europa.eu/document/download/03b97969-b53f-4745-a66f-fbf1eaabe167_en?filename=novel-food_consult-status_2022-4778355.pdf (accessed on 15 January 2025).
- Lorbeer, L.; Schwarz, S.; Franke, H.; Lachenmeier, D.W. Toxicological Assessment of Roasted Coffee Silver Skin (Testa of Coffea sp.) as Novel Food Ingredient. Molecules 2022, 27, 6839. [Google Scholar] [CrossRef]
- Turck, D.; Bohn, T.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pentieva, K.; et al. Guidance on the scientific requirements for an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283. EFSA J. 2024, 22, e8961. [Google Scholar] [CrossRef] [PubMed]
- Klingel, T.; Kremer, J.I.; Gottstein, V.; Rajcic de Rezende, T.; Schwarz, S.; Lachenmeier, D.W. A Review of Coffee By-Products Including Leaf, Flower, Cherry, Husk, Silver Skin, and Spent Grounds as Novel Foods within the European Union. Foods 2020, 9, 665. [Google Scholar] [CrossRef]
- al-Kanhal, M.A.; Ahmed, F.; Arif, Z. Effect of coffee oil and its unsaponifiable fraction on plasma cholesterol level in female rats. Int. J. Food Sci. Nutr. 1999, 50, 99–103. [Google Scholar] [CrossRef]
- al Kanhal, M.A. Lipid analysis of Coffea arabica Linn. beans and their possible hypercholesterolemic effects. Int. J. Food Sci. Nutr. 1997, 48, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Firestone, D. (Ed.) Physical and Chemical Characteristics of Oils, Fats, and Waxes, 2nd ed.; AOCS Press: Champaign, Ill, USA, 2006; ISBN 9781893997998. [Google Scholar]
- D’Amelio, N.; De Angelis, E.; Navarini, L.; Schievano, E.; Mammi, S. Green coffee oil analysis by high-resolution nuclear magnetic resonance spectroscopy. Talanta 2013, 110, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.S.; Franca, A.S.; Mendonça, J.C.; Barros-Júnior, M.C. Proximate composition and fatty acids profile of green and roasted defective coffee beans. LWT 2006, 39, 235–239. [Google Scholar] [CrossRef]
- Antoine, G.; Vaissayre, V.; Meile, J.-C.; Payet, J.; Conéjéro, G.; Costet, L.; Fock-Bastide, I.; Joët, T.; Dussert, S. Diterpenes of Coffea seeds show antifungal and anti-insect activities and are transferred from the endosperm to the seedling after germination. Plant Physiol. Biochem. 2023, 194, 627–637. [Google Scholar] [CrossRef]
- Cavin, C.; Holzhaeuser, D.; Scharf, G.; Constable, A.; Huber, W.W.; Schilter, B. Cafestol and kahweol, two coffee specific diterpenes with anticarcinogenic activity. Food Chem. Toxicol. 2002, 40, 1155–1163. [Google Scholar] [CrossRef]
- Speer, K. 16-O-Methylcafestol—Ein neues Diterpen im Kaffee Methoden zur Bestimmung des 16-O-Methylcafestols in Rohkaffees und in behandelten Kaffees. Eur. Food Res. Technol. 1989, 189, 326–330. [Google Scholar] [CrossRef]
- Estévez-Sánchez, K.H.; Ochoa-Velasco, C.E.; Ruiz-Espinosa, H.; Ruiz-López, I.I. Kahweol and Cafestol. In A Centum of Valuable Plant Bioactives; Mushtaq, M., Anwar, F., Eds.; Elsevier Science & Technology: San Diego, CA, USA, 2021; pp. 159–192. [Google Scholar] [CrossRef]
- van Rooij, J.; van der Stegen, G.H.; Schoemaker, R.C.; Kroon, C.; Burggraaf, J.; Hollaar, L.; Vroon, T.F.; Smelt, A.H.; Cohen, A.F. A placebo-controlled parallel study of the effect of two types of coffee oil on serum lipids and transaminases: Identification of chemical substances involved in the cholesterol-raising effect of coffee. Am. J. Clin. Nutr. 1995, 61, 1277–1283. [Google Scholar] [CrossRef]
- Urgert, R.; Katan, M.B. The cholesterol-raising factor from coffee beans. Annu. Rev. Nutr. 1997, 17, 305–324. [Google Scholar] [CrossRef]
- Gunning, Y.; Defernez, M.; Watson, A.D.; Beadman, N.; Colquhoun, I.J.; Le Gall, G.; Philo, M.; Garwood, H.; Williamson, D.; Davis, A.P.; et al. 16-O-methylcafestol is present in ground roast Arabica coffees: Implications for authenticity testing. Food Chem. 2018, 248, 52–60. [Google Scholar] [CrossRef]
- Burton, I.W.; Martinez Farina, C.F.; Ragupathy, S.; Arunachalam, T.; Newmaster, S.; Berrué, F. Quantitative NMR Methodology for the Authentication of Roasted Coffee and Prediction of Blends. J. Agric. Food Chem. 2020, 68, 14643–14651. [Google Scholar] [CrossRef] [PubMed]
- Guercia, E.; Colomban, S.; Navarini, L. 16-O-Methylated diterpenes in green Coffea arabica: Ultra-high-performance liquid chromatography-tandem mass spectrometry method optimization and validation. J. Mass Spectrom. 2020, 55, e4636. [Google Scholar] [CrossRef] [PubMed]
- Okaru, A.O.; Scharinger, A.; Rajcic de Rezende, T.; Teipel, J.; Kuballa, T.; Walch, S.G.; Lachenmeier, D.W. Validation of a Quantitative Proton Nuclear Magnetic Resonance Spectroscopic Screening Method for Coffee Quality and Authenticity (NMR Coffee Screener). Foods 2020, 9, 47. [Google Scholar] [CrossRef]
- Speer, K.; Tewis, R.; Montag, A. 16-O-Methylcafestol-Ein neues Diterpen im Kaffee. Eur. Food Res. Technol. 1991, 192, 451–454. [Google Scholar] [CrossRef]
- Kölling-Speer, I.; Strohschneider, S.; Speer, K. Determination of Free Diterpenes in Green and Roasted Coffees. J. High Resol. Chromatogr. 1999, 22, 43–46. [Google Scholar] [CrossRef]
- Weusten-Van der Wouw, M.P.; Katan, M.B.; Viani, R.; Huggett, A.C.; Liardon, R.; Lund-Larsen, P.G.; Thelle, D.S.; Ahola, I.; Aro, A. Identity of the cholesterol-raising factor from boiled coffee and its effects on liver function enzymes. J. Lipid Res. 1994, 35, 721–733. [Google Scholar] [CrossRef]
- Dias, L.D.; Carbinatto, F.M.; Da Almeida, I.S.; Blanco, K.C.; Marquele-Oliveira, F.; Munari, C.C.; Bagnato, V.S. Eco-Friendly Extraction of Green Coffee Oil for Industrial Applications: Its Antioxidant, Cytotoxic, Clonogenic, and Wound Healing Properties. Fermentation 2023, 9, 370. [Google Scholar] [CrossRef]
- Vardanega, R.; Osorio-Tobón, J.F.; Duba, K. Contributions of supercritical fluid extraction to sustainable development goal 9 in South America: Industry, innovation, and infrastructure. J. Supercrit. Fluids. 2022, 188, 105681. [Google Scholar] [CrossRef]
- Duba, K.S.; Fiori, L. Extraction of bioactives from food processing residues using techniques performed at high pressures. Curr. Opin. Food Sci. 2015, 5, 14–22. [Google Scholar] [CrossRef]
- Franca, A.S.; Oliveira, L.S. Potential Uses of Spent Coffee Grounds in the Food Industry. Foods 2022, 11, 2064. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Petrik, S.; Benesova, P.; Svoboda, Z.; Eremka, L.; Marova, I. Utilization of oil extracted from spent coffee grounds for sustainable production of polyhydroxyalkanoates. Appl. Microbiol. Biotechnol. 2014, 98, 5883–5890. [Google Scholar] [CrossRef]
- Carisano, A.; Gariboldi, L. Gas chromatographic examination of the fatty acids of coffee oil. J. Sci. Food Agric 1964, 15, 619–622. [Google Scholar] [CrossRef]
- Castro, A.C.C.M.; Oda, F.B.; Almeida-Cincotto, M.G.J.; Davanço, M.G.; Chiari-Andréo, B.G.; Cicarelli, R.M.B.; Peccinini, R.G.; Zocolo, G.J.; Ribeiro, P.R.V.; Corrêa, M.A.; et al. Green coffee seed residue: A sustainable source of antioxidant compounds. Food Chem. 2018, 246, 48–57. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.A.; Oomah, B.D. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Battista, F.; Zanzoni, S.; Strazzera, G.; Andreolli, M.; Bolzonella, D. The cascade biorefinery approach for the valorization of the spent coffee grounds. Renew. Energy 2020, 157, 1203–1211. [Google Scholar] [CrossRef]
- Jin Cho, E.; Gyo Lee, Y.; Song, Y.; Nguyen, D.-T.; Bae, H.-J. An integrated process for conversion of spent coffee grounds into value-added materials. Bioresour. Technol. 2022, 346, 126618. [Google Scholar] [CrossRef]
- Deotale, S.M.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Coffee oil as a natural surfactant. Food Chem. 2019, 295, 180–188. [Google Scholar] [CrossRef]
- Meerasri, J.; Sothornvit, R. Novel development of coffee oil extracted from spent coffee grounds as a butter substitute in bakery products. J. Food Process. Preserv. 2022, 46, e16687. [Google Scholar] [CrossRef]
- Bijla, L.; Hmitti, A.; Fadda, A.; Oubannin, S.; Gagour, J.; Aissa, R.; Laknifli, A.; Sakar, E.H.; Gharby, S. Valorization of spent coffee ground as a natural antioxidant and its use for sunflower oil shelf-Life extension. Eur. J. Lipid Sci. Technol. 2024, 126, e2300115. [Google Scholar] [CrossRef]
- De Oliveira, W.Q.; Wurlitzer, N.J.; de Oliveira Araújo, A.W.; Comunian, T.A.; Bastos, M.D.S.R.; de Oliveira, A.L.; Magalhães, H.C.R.; Ribeiro, H.L.; de Figueiredo, R.W.; de Sousa, P.H.M. Complex coacervates of cashew gum and gelatin as carriers of green coffee oil: The effect of microcapsule application on the rheological and sensorial quality of a fruit juice. Food Res. Int. 2020, 131, 109047. [Google Scholar] [CrossRef]
- Frascareli, E.C.; Silva, V.M.; Tonon, R.V.; Hubinger, M.D. Effect of process conditions on the microencapsulation of coffee oil by spray drying. Food Bioprod. Process. 2012, 90, 413–424. [Google Scholar] [CrossRef]
- Hu, X.; Meng, Z. An overview of edible foams in food and modern cuisine: Destabilization and stabilization mechanisms and applications. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13284. [Google Scholar] [CrossRef]
- Urgert, R.; Katan, M.B. The cholesterol-raising factor from coffee beans. J. R. Soc. Med. 1996, 89, 618–623. [Google Scholar] [CrossRef]
- Ahola, I.; Jauhiainen, M.; Aro, A. The hypercholesterolaemic factor in boiled coffee is retained by a paper filter. J. Intern. Med. 1991, 230, 293–297. [Google Scholar] [CrossRef]
- Buchmann, S.; Zahm, A.; Kölling-Speer, I.; Speer, K. Lipids in coffee brews-Impact of grind size, water temperature, and coffee/water ratio on cafestol and the carboxylic acid-5-hydroxytryptamides. Colloq. Sci. Int. Sur Le Cafe 2010, 16, 101–109. [Google Scholar]
- Moeenfard, M.; Alves, A. New trends in coffee diterpenes research from technological to health aspects. Food Res. Int. 2020, 134, 109207. [Google Scholar] [CrossRef] [PubMed]
- Lachenmeier, D.W.; Rajcic de Rezende, T.; Schwarz, S. An Update on Sustainable Valorization of Coffee By-Products as Novel Foods within the European Union. Biol. Life Sci. Forum 2021, 6, 37. [Google Scholar] [CrossRef]
- Urgert, R.; Schulz, A.G.; Kata, M.B. Effects of cafestol and kahweol from coffee grounds on serum lipids and serum liver enzymes in humans. Am. J. Clin. Nutr. 1995, 61, 149–154. [Google Scholar] [CrossRef]
- Ratnayake, W.M.; Hollywood, R.; O’Grady, E.; Stavric, B. Lipid content and composition of coffee brews prepared by different methods. Food Chem. Toxicol. 1993, 31, 263–269. [Google Scholar] [CrossRef]
- Ranheim, T.; Halvorsen, B. Coffee consumption and human health--beneficial or detrimental?--Mechanisms for effects of coffee consumption on different risk factors for cardiovascular disease and type 2 diabetes mellitus. Mol. Nutr. Food Res. 2005, 49, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Brand, A.; Silva, A.; Andriolo, C.; Mellinger, C.; Uekane, T.; Garrett, R.; Rezende, C. Bioaccessibility of Cafestol from Coffee Brew: A Metabolic Study Employing an In Vitro Digestion Model and LC-HRMS. J. Agric. Food Chem. 2024, 72, 27876–27883. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, N.; Franke, H.; Schwarz, S.; Lachenmeier, D.W. Risk Assessment of Trigonelline in Coffee and Coffee By-Products. Molecules 2023, 28, 3460. [Google Scholar] [CrossRef]
- Babylon, L.; Meißner, J.; Eckert, G.P. Combination of Secondary Plant Metabolites and Micronutrients Improves Mitochondrial Function in a Cell Model of Early Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 10029. [Google Scholar] [CrossRef]
- De Roos, B.; Meyboom, S.; Kosmeijer-Schuil, T.G.; Katan, M.B. Absorption and urinary excretion of the coffee diterpenes cafestol and kahweol in healthy ileostomy volunteers. J. Intern. Med. 1998, 244, 451–460. [Google Scholar] [CrossRef]
- Socała, K.; Szopa, A.; Serefko, A.; Poleszak, E.; Wlaź, P. Neuroprotective Effects of Coffee Bioactive Compounds: A Review. Int. J. Mol. Sci. 2020, 22, 107. [Google Scholar] [CrossRef] [PubMed]
- Iriondo-DeHond, A.; Cornejo, F.S.; Fernandez-Gomez, B.; Vera, G.; Guisantes-Batan, E.; Alonso, S.G.; Andres, M.I.S.; Sanchez-Fortun, S.; Lopez-Gomez, L.; Uranga, J.A.; et al. Bioaccesibility, Metabolism, and Excretion of Lipids Composing Spent Coffee Grounds. Nutrients 2019, 11, 1411. [Google Scholar] [CrossRef]
- van Cruchten, S.T.J.; De Waart, D.R.; Kunne, C.; Hooiveld, G.J.E.J.; Boekschoten, M.V.; Katan, M.B.; Elferink, R.P.J.O.; Witkamp, R.F. Absorption, distribution, and biliary excretion of cafestol, a potent cholesterol-elevating compound in unfiltered coffees, in mice. Drug Metab. Dispos. 2010, 38, 635–640. [Google Scholar] [CrossRef]
- Post, S.M.; de Roos, B.; Vermeulen, M.; Afman, L.; Jong, M.C.; Dahlmans, V.; Havekes, L.M.; Stellaard, F.; Katan, M.B.; Princen, H. Cafestol Increases Serum Cholesterol Levels in Apolipoprotein E*3-Leiden Transgenic Mice by Suppression of Bile Acid Synthesis. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1551–1556. [Google Scholar] [CrossRef]
- van Cruchten, S.T.J.; de Haan, L.H.J.; Mulder, P.P.J.; Kunne, C.; Boekschoten, M.V.; Katan, M.B.; Aarts, J.M.M.J.G.; Witkamp, R.F. The role of epoxidation and electrophile-responsive element-regulated gene transcription in the potentially beneficial and harmful effects of the coffee components cafestol and kahweol. J. Nutr. Biochem. 2010, 21, 757–763. [Google Scholar] [CrossRef]
- Førde, O.H.; Knutsen, S.F.; Arnesen, E.; Thelle, D.S. The Tromsø heart study: Coffee consumption and serum lipid concentrations in men with hypercholesterolaemia: An randomised intervention study. Br. Med. J. (Clin Res. Ed) 1985, 290, 893–895. [Google Scholar] [CrossRef] [PubMed]
- Aro, A.; Tuomilehto, J.; Kostiainen, E.; Uusitalo, U.; Pietinen, P. Boiled coffee increases serum low density lipoprotein concentration. Metabolism 1987, 36, 1027–1030. [Google Scholar] [CrossRef] [PubMed]
- Bønaa, K.; Arnesen, E.; Thelle, D.S.; Førde, O.H. Coffee and cholesterol: Is it all in the brewing? The Tromsø Study. BMJ 1988, 297, 1103–1104. [Google Scholar] [CrossRef]
- Bak, A.A.; Grobbee, D.E. The effect on serum cholesterol levels of coffee brewed by filtering or boiling. N. Engl. J. Med. 1989, 321, 1432–1437. [Google Scholar] [CrossRef] [PubMed]
- van Dusseldorp, M.; Katan, M.B.; van Vliet, T.; Demacker, P.N.; Stalenhoef, A.F. Cholesterol-raising factor from boiled coffee does not pass a paper filter. Arterioscler. Thromb. 1991, 11, 586–593. [Google Scholar] [CrossRef]
- Urgert, R.; Meyboom, S.; Kuilman, M.; Rexwinkel, H.; Vissers, M.N.; Klerk, M.; Katan, M.B. Comparison of effect of cafetière and filtered coffee on serum concentrations of liver aminotransferases and lipids: Six month randomised controlled trial. BMJ 1996, 313, 1362–1366. [Google Scholar] [CrossRef]
- Zock, P.L.; Katan, M.B.; Merkus, M.P.; van Dusseldorp, M.; Harryvan, J.L. Effect of a lipid-rich fraction from boiled coffee on serum cholesterol. Lancet 1990, 335, 1235–1237. [Google Scholar] [CrossRef]
- Moeenfard, M.; Erny, G.L.; Alves, A. Variability of some diterpene esters in coffee beverages as influenced by brewing procedures. J. Food Sci. Technol. 2016, 53, 3916–3927. [Google Scholar] [CrossRef]
- van Tol, A.; Urgert, R.; de Jong-Caesar, R.; van Gent, T.; Scheek, L.M.; de Roos, B.; Katan, M.B. The cholesterol-raising diterpenes from coffee beans increase serum lipid transfer protein activity levels in humans. Atherosclerosis 1997, 132, 251–254. [Google Scholar] [CrossRef]
- Tverdal, A.; Selmer, R.; Cohen, J.M.; Thelle, D.S. Coffee consumption and mortality from cardiovascular diseases and total mortality: Does the brewing method matter? Eur. J. Prev. Cardiol. 2020, 27, 1986–1993. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, T.A.F.; Rogero, M.M.; Mioto, B.M.; Tarasoutchi, D.; Tuda, V.L.; César, L.A.M.; Torres, E.A.F.S. Paper-filtered coffee increases cholesterol and inflammation biomarkers independent of roasting degree: A clinical trial. Nutrition 2013, 29, 977–981. [Google Scholar] [CrossRef]
- Butt, M.S.; Sultan, M.T. Coffee and its consumption: Benefits and risks. Crit. Rev. Food Sci. Nutr. 2011, 51, 363–373. [Google Scholar] [CrossRef]
- Larsson, S.C.; Wolk, A.; Håkansson, N.; Bäck, M. Coffee consumption and risk of aortic valve stenosis: A prospective study. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Urgert, R.; Essed, N.; van der Weg, G.; Kosmeijer-Schuil, T.G.; Katan, M.B. Separate effects of the coffee diterpenes cafestol and kahweol on serum lipids and liver aminotransferases. Am. J. Clin. Nutr. 1997, 65, 519–524. [Google Scholar] [CrossRef]
- Boekschoten, M.V.; Engberink, M.F.; Katan, M.B.; Schouten, E.G. Reproducibility of the serum lipid response to coffee oil in healthy volunteers. Nutr. J. 2003, 2, 8. [Google Scholar] [CrossRef][Green Version]
- Mensink, R.P.; Lebbink, W.J.; Lobbezoo, I.E.; Weusten-Van der Wouw, M.P.; Zock, P.L.; Katan, M.B. Diterpene composition of oils from Arabica and Robusta coffee beans and their effects on serum lipids in man. J. Intern. Med. 1995, 237, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Heckers, H.; Göbel, U.; Kleppel, U. End of the coffee mystery: Diterpene alcohols raise serum low-density lipoprotein cholesterol and triglyceride levels. J. Intern. Med. 1994, 235, 192–193. [Google Scholar] [CrossRef]
- Gressner, A.M.; Arndt, T. (Eds.) Lexikon der Medizinischen Laboratoriumsdiagnostik; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Boekschoten, M.V.; Hofman, M.K.; Buytenhek, R.; Schouten, E.G.; Princen, H.; Katan, M.B. Coffee oil consumption increases plasma levels of 7alpha-hydroxy-4-cholesten-3-one in humans. J. Nutr. 2005, 135, 785–789. [Google Scholar] [CrossRef]
- Jee, S.H.; He, J.; Appel, L.J.; Whelton, P.K.; Suh, I.; Klag, M.J. Coffee consumption and serum lipids: A meta-analysis of randomized controlled clinical trials. Am. J. Epidemiol. 2001, 153, 353–362. [Google Scholar] [CrossRef]
- Arnold, D.R.; Kwiterovich, P.O. Cholesterol Absorption, Function, and Metabolism. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 1226–1237. [Google Scholar] [CrossRef]
- Urgert, R.; Weusten-Van der Wouw, M.P.; Hovenier, R.; Meyboom, S.; Beynen, A.C.; Katan, M.B. Diterpenes from coffee beans decrease serum levels of lipoprotein(a) in humans: Results from four randomised controlled trials. Eur. J. Clin. Nutr. 1997, 51, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Guercia, E.; Berti, F.; De Zorzi, R.; Navarini, L.; Geremia, S.; Medagli, B.; De Conto, M.; Cassetta, A.; Forzato, C. On the Cholesterol Raising Effect of Coffee Diterpenes Cafestol and 16-O-Methylcafestol: Interaction with Farnesoid X Receptor. Int. J. Mol. Sci. 2024, 25, 6096. [Google Scholar] [CrossRef]
- Ricketts, M.-L.; Boekschoten, M.V.; Kreeft, A.J.; Hooiveld, G.J.E.J.; Moen, C.J.A.; Müller, M.; Frants, R.R.; Kasanmoentalib, S.; Post, S.M.; Princen, H.M.G.; et al. The cholesterol-raising factor from coffee beans, cafestol, as an agonist ligand for the farnesoid and pregnane X receptors. Mol. Endocrinol. 2007, 21, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- De Roos, B.; Katan, M.B. Possible mechanisms underlying the cholesterol-raising effect of the coffee diterpene cafestol. Curr. Opin. Lipidol. 1999, 10, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Post, S.M.; de Wit, E.C.; Princen, H.M. Cafestol, the cholesterol-raising factor in boiled coffee, suppresses bile acid synthesis by downregulation of cholesterol 7 alpha-hydroxylase and sterol 27-hydroxylase in rat hepatocytes. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 3064–3070. [Google Scholar] [CrossRef]
- Andriolo, C.V.; Novaes, F.J.M.; Pereira, H.M.G.; Sardela, V.F.; Rezende, C.M. Metabolic study of cafestol using in silico approach, zebrafish water tank experiments and liquid chromatography high-resolution mass spectrometry analyses. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2021, 1186, 123028. [Google Scholar] [CrossRef]
- Berti, F.; Navarini, L.; Guercia, E.; Oreški, A.; Gasparini, A.; Scoltock, J.; Forzato, C. Interaction of the Coffee Diterpenes Cafestol and 16-O-Methyl-Cafestol Palmitates with Serum Albumins. Int. J. Mol. Sci. 2020, 21, 1823. [Google Scholar] [CrossRef]
- Eldesouki, S.; Qadri, R.; Abu Helwa, R.; Barqawi, H.; Bustanji, Y.; Abu-Gharbieh, E.; El-Huneidi, W. Recent Updates on the Functional Impact of Kahweol and Cafestol on Cancer. Molecules 2022, 27, 7332. [Google Scholar] [CrossRef]
- Muriel, P.; Arauz, J. Coffee and liver diseases. Fitoterapia 2010, 81, 297–305. [Google Scholar] [CrossRef]
- Kim, H.G.; Hwang, Y.P.; Han, E.H.; Choi, J.H.; Kwon, K.; Chung, Y.C.; Jeong, M.H.; Jeong, T.C.; Kang, W.; Jeong, H.G. The coffee diterpene kahweol inhibits metastasis by modulating expressions of MMPs and VEGF via STAT3 inactivation. Food Chem. 2012, 133, 1521–1529. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jung, K.S.; Jeong, H.G. Suppressive effects of the kahweol and cafestol on cyclooxygenase-2 expression in macrophages. FEBS Lett. 2004, 569, 321–326. [Google Scholar] [CrossRef]
- Lee, K.-A.; Chae, J.-I.; Shim, J.-H. Natural diterpenes from coffee, cafestol and kahweol induce apoptosis through regulation of specificity protein 1 expression in human malignant pleural mesothelioma. J. Biomed. Sci. 2012, 19, 60. [Google Scholar] [CrossRef]
- Tsai, Y.-T.; Sung, L.-C.; Haw, W.-R.; Chen, C.-C.; Huang, S.-F.; Liu, J.-C.; Cheng, T.-H.; Chen, P.-Y.; Loh, S.-H.; Tsai, C.-S. Cafestol, a coffee diterpene, inhibits urotensin II-induced interleukin-8 expression in human umbilical vein endothelial cells. Eur. J. Pharmacol. 2018, 820, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Agustini, D.; Vernadesly, L.; Theodorus, D. The Potential of Robusta Coffee (Coffea canephora) as a Colorectal Cancer Therapy Modality: An In Silico Study. Asian J. Pharm. Clin. Res. 2021, 14, 83–87. [Google Scholar] [CrossRef]
- Lima, C.S.; Spindola, D.G.; Bechara, A.; Garcia, D.M.; Palmeira-dos-Santos, C.; Peixoto-da-Silva, J.; Erustes, A.G.; Michelin, L.; Pereira, G.; Smaili, S.S.; et al. Cafestol, a diterpene molecule found in coffee, induces leukemia cell death. Biomed. Pharmacother. 2017, 92, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Kotowski, U.; Heiduschka, G.; Seemann, R.; Eckl-Dorna, J.; Schmid, R.; Kranebitter, V.; Stanisz, I.; Brunner, M.; Lill, C.; Thurnher, D. Effect of the coffee ingredient cafestol on head and neck squamous cell carcinoma cell lines. Strahlenther. Onkol. 2015, 191, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Huber, W.W.; McDaniel, L.P.; Kaderlik, K.R.; Teitel, C.H.; Lang, N.P.; Kadlubar, F.F. Chemoprotection against the formation of colon DNA adducts from the food-borne carcinogen 2-amino-1-methyl-6-phenylimidazo4,5-bpyridine (PhIP) in the rat. Mutat. Res. 1997, 376, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Izumi, K.; Hiratsuka, K.; Kano, H.; Shimada, T.; Nakano, T.; Kadomoto, S.; Naito, R.; Iwamoto, H.; Yaegashi, H.; et al. Anti-proliferative and anti-migratory properties of coffee diterpenes kahweol acetate and cafestol in human renal cancer cells. Sci. Rep. 2021, 11, 675. [Google Scholar] [CrossRef]
- Heise, N.V.; Kozubek, M.; Hoenke, S.; Ludwig, S.; Deigner, H.-P.; Al-Harrasi, A.; Csuk, R. Towards Cytotoxic Derivatives of Cafestol. Molecules 2025, 30, 2291. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yoon, Y.C.; Sung, M.-J.; Hur, H.-J.; Park, J.-H. Antiangiogenic properties of cafestol, a coffee diterpene, in human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 2012, 421, 567–571. [Google Scholar] [CrossRef]
- Lee, K.J.; Jeong, H.G. Protective effects of kahweol and cafestol against hydrogen peroxide-induced oxidative stress and DNA damage. Toxicol. Lett. 2007, 173, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.L.; Choi, J.H.; Jeong, H.G. Hepatoprotective and antioxidant effects of the coffee diterpenes kahweol and cafestol on carbon tetrachloride-induced liver damage in mice. Food Chem. Toxicol. 2007, 45, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- Grubben, M.J.; van den Braak, C.C.; Broekhuizen, R.; De Jong, R.; van Rijt, L.; De Ruijter, E.; Peters, W.H.; Katan, M.B.; Nagengast, F.M. The effect of unfiltered coffee on potential biomarkers for colonic cancer risk in healthy volunteers: A randomized trial. Aliment. Pharmacol. Ther. 2000, 14, 1181–1190. [Google Scholar] [CrossRef]
- Cavin, C.; Mace, K.; Offord, E.A.; Schilter, B. Protective effects of coffee diterpenes against aflatoxin B1-induced genotoxicity: Mechanisms in rat and human cells. Food Chem. Toxicol. 2001, 39, 549–556. [Google Scholar] [CrossRef]
- Estévez-Sánchez, K.H.; Ochoa-Velasco, C.E.; Ruiz-Espinosa, H.; Ruiz-López, I.I. (Eds.) A Centum of Valuable Plant Bioactives; Elsevier Science & Technology: San Diego, CA, USA, 2021. [Google Scholar] [CrossRef]
- Açıkalın, B.; Sanlier, N. Coffee and its effects on the immune system. Trends Food Sci. Technol. 2021, 114, 625–632. [Google Scholar] [CrossRef]
- Schilter, B.; Perrin, I.; Cavin, C.; Huggett, A.C. Placental glutathione S-transferase (GST-P) induction as a potential mechanism for the anti-carcinogenic effect of the coffee-specific components cafestol and kahweol. Carcinogenesis 1996, 17, 2377–2384. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lam, L.K.; Sparnins, V.L.; Wattenberg, L.W. Effects of derivatives of kahweol and cafestol on the activity of glutathione S-transferase in mice. J. Med. Chem. 1987, 30, 1399–1403. [Google Scholar] [CrossRef]
- De Oliveira, N.A.; Sandini, T.M.; Cornelio-Santiago, H.P.; Martinelli, E.C.L.; Raspantini, L.E.R.; Raspantini, P.C.; Momo, C.; de Oliveira, A.L.; Fukumasu, H. Acute and subacute (28 days) toxicity of green coffee oil enriched with diterpenes cafestol and kahweol in rats. Regul. Toxicol. Pharmacol. 2020, 110, 104517. [Google Scholar] [CrossRef]
- Engel, S.; Tholstrup, T. Butter increased total and LDL cholesterol compared with olive oil but resulted in higher HDL cholesterol compared with a habitual diet. Am. J. Clin. Nutr. 2015, 102, 309–315. [Google Scholar] [CrossRef]
- Mensink, R.P.; Zock, P.L.; Katan, M.B.; Beynen, A.C. Boiled coffee does not increase serum cholesterol in gerbils and hamsters. Z. Ernahrungswiss. 1992, 31, 82–85. [Google Scholar] [CrossRef]
- Terpstra, A.; Katan, M.; Weusten-van der Wouw, M.; De Roos, B.; Beynen, A. The hypercholesterolemic effect of cafestol in coffee oil in gerbils and rats. J. Nutr. Biochem. 2000, 11, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Warnick, G.R.; Kimberly, M.M.; Waymack, P.P.; Leary, E.T.; Myers, G.L. Standardization of Measurements for Cholesterol, Triglycerides, and Major Lipoproteins. Lab. Med. 2008, 39, 481–490. [Google Scholar] [CrossRef]
| Compounds | % Dry Matter [9] |
|---|---|
| Triacylglycerols | 75.2 |
| Esters of diterpene alcohols and fatty acids | 18.5 |
| Diterpene alcohols | 0.4 |
| Esters of sterols and fatty acids | 3.2 |
| Sterols | 2.2 |
| Tocopherols | 0.04–0.06 |
| Phosphatides | 0.1–0.5 |
| Tryptamine derivatives | 0.6–1.0 |
| Cafestol (mg/100 mL) | Kahweol (mg/100 mL) | |
|---|---|---|
| Scandinavian boiled | 0.5–8 | 0.7–10 |
| Turkish/Greek | 0.3–6.7 | 0.1–7.1 |
| French press | 1.5–3.7 | 1.7–5.3 |
| Espresso | 0.1–1.9 | 0.1–2.6 |
| Filter | 0–0.1 | 0–0.1 |
| Adult | |
|---|---|
| Maximal daily intake of coffee [mL coffee/day] | 1914 |
| Maximal diterpene content of coffee [mg diterpenes/100 mL coffee] | 18 |
| Diterpene intake based on the max. coffee intake [mg diterpenes per day] | 345 1 |
| Diterpene intake based on the max. coffee intake 2 [mg diterpenes/kg bw/day] | 6 |
| Effect | Studies with Humans | Cafestol/Kahweol Content (per Day) | ∆ |
|---|---|---|---|
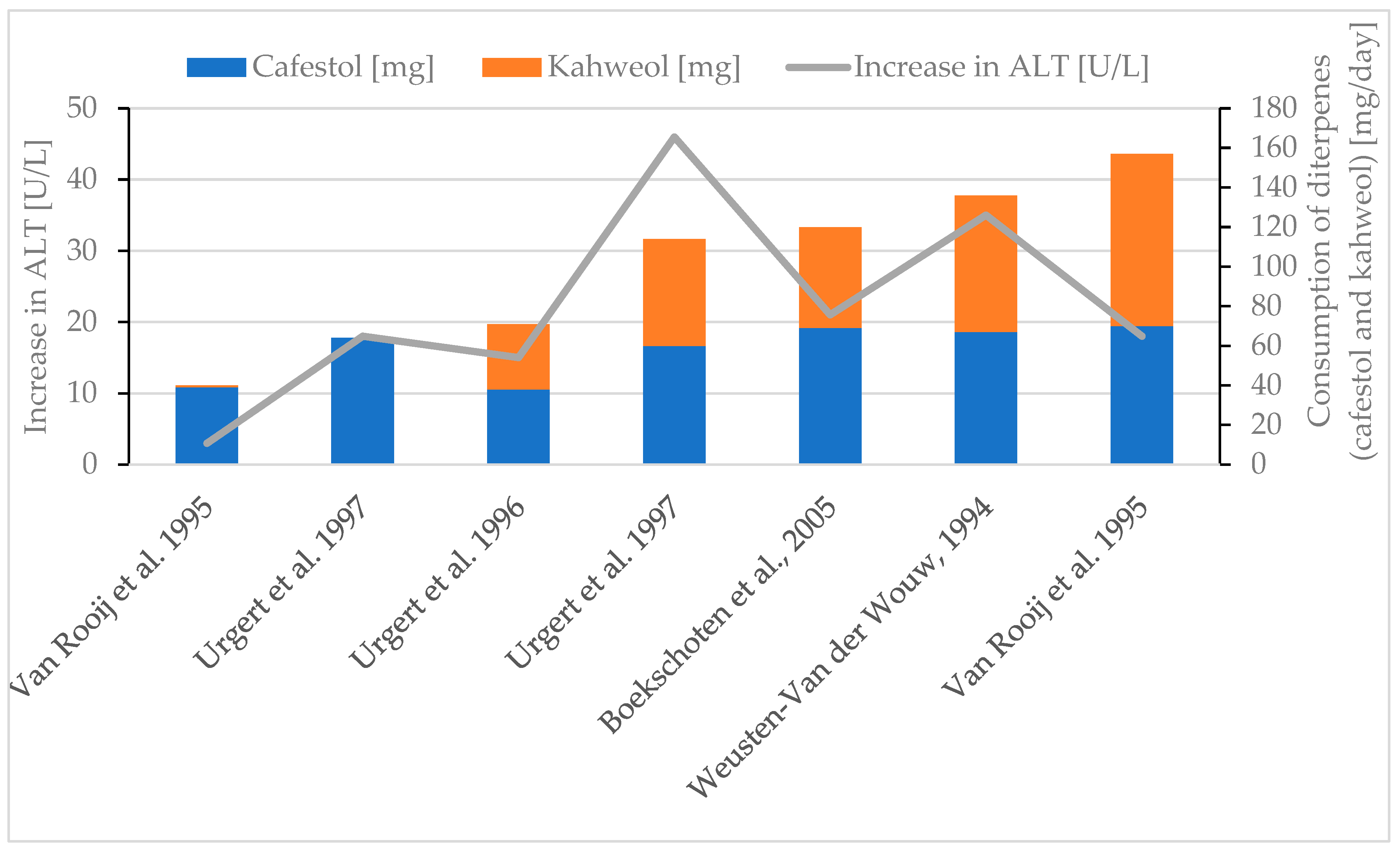
| Rise in ALT | Boekschoten et al., 2005 [90] 2 mL coffee oil/day, 5 weeks | 69 mg cafestol and 51 mg kahweol | +21 U/L |
| Weusten-Van der Wouw, 1994 [38] 2 g coffee oil/day, 4 weeks | 57 mg cafestol and 69 mg kahweol | +35 U/L | |
| Van Rooij et al., 1995 [30] 1 g coffee oil (Arabica/day), 6 weeks | 70 mg cafestol and 87 mg kahweol | +18 U/L | |
| Van Rooij et al., 1995 [30] 1 g coffee oil (Canephora/day), 6 weeks | 39 mg cafestol and 1 mg kahweol | +3 U/L | |
| Urgert et al., 1997 [85] 64 mg cafestol/day, 28 days | 64 mg cafestol | +18 U/L | |
| Urgert et al., 1997 [85] 114 diterpenes/day, 28 days | 60 mg cafestol and 54 mg kahweol | +46 U/L | |
| Urgert et al., 1996 [77] 71 mg diterpenes/day, 24 weeks | 38 mg cafestol and 33 mg kahweol | +15 U/L | |
| Rise in AST | Boekschoten et al., 2005 [90] 2 mL coffee oil/day, 5 weeks | 69 mg cafestol and 51 mg kahweol | +8.5 U/L |
| Van Rooij et al., 1995 [30] 1 g coffee oil (Arabica/day), 6 weeks | 70 mg cafestol and 87 mg kahweol | +3 U/L | |
| Van Rooij et al., 1995 [30] 1 g coffee oil (Canephora/day), 6 weeks | 39 mg cafestol and 1 mg kahweol | +1 U/L | |
| Urgert et al., 1997 [85] 64 mg cafestol/day, 28 days | 64 mg cafestol | +5 U/L | |
| Urgert et al., 1997 [85] 114 mg diterpenes/day, 28 days | 60 mg cafestol and 54 mg kahweol | +12 U/L | |
| Urgert et al., 1996 [77] 71 mg diterpenes/day, 24 weeks | 38 mg cafestol and 33 mg kahweol | Marginally increased (not further defined) | |
| Decrease in GGT | Weusten-Van der Wouw, 1994 [38] 2 g coffee oil/day, 4 weeks | 57 mg cafestol and 69 mg kahweol | Decreased (not further defined) |
| Urgert et al., 1997 [85] 64 mg cafestol/day, 28 days | 64 mg cafestol | −1 U/L | |
| Urgert et al., 1997 [85] 114 mg diterpenes/day, 28 days | 60 mg cafestol and 54 mg kahweol | −3 U/L | |
| Urgert et al., 1996 [77] 71 mg diterpenes/day, 24 weeks | 38 mg cafestol and 33 mg kahweol | Decreased (not further defined) | |
| Boekschoten et al., 2005 [90] 2 ml coffee oil/day, 5 weeks | 69 mg cafestol and 51 mg kahweol | −1 U/L | |
| Decrease in ALP | Urgert et al., 1997 [85] 64 mg cafestol/day, 28 days | 64 mg cafestol | −1 U/L |
| Urgert et al., 1997 [85] 114 mg diterpenes/day, 28 days | 60 mg cafestol and 54 mg kahweol | −5 U/L | |
| Urgert et al., 1996 [77] 71 mg diterpenes/day, 24 weeks | 38 mg cafestol and 33 mg kahweol. Maximum values from the 24-week study used | Decreased (not further defined) | |
| Boekschoten et al., 2005 [90] 2 mL coffee oil/day, 5 weeks | 69 mg cafestol and 51 mg kahweol | −7 U/L | |
| No effect on GGT | Van Rooij et al., 1995 [30] 1 g coffee oil (Arabica/day), 6 weeks | 70 mg cafestol and 87 mg kahweol | No significant change |
| Van Rooij et al., 1995 [30] 1 g coffee oil (Canephora/day), 6 weeks | 39 mg cafestol and 1 mg kahweol | No significant change | |
| Decrease in creatinine | Weusten-Van der Wouw, 1994 [38] 2 g coffee oil/day, 4 weeks | 57 mg cafestol and 69 mg kahweol | Decreased (not further defined) |
| Urgert et al., 1997 [85] 64 mg cafestol/day, 28 days | 64 mg cafestol | −3 µmol/L | |
| Urgert et al., 1997 [85] 114 mg diterpenes/day, 28 days | 60 mg cafestol and 54 mg kahweol | −8 µmol/L |
| Effect | Studies with Humans | Cafestol/Kahweol Content (per day) | ∆ |
|---|---|---|---|
| Decrease in Lipoprotein(a) level | Urgert, 1997 [93] coffee oil daily, 4 weeks | 85 mg cafestol and 103 mg kahweol | −4.8 mg/dL |
| Rise in 7a-Hydroxy-4-cholesten-3-one level | Boekschoten et al., 2005 [90] 2 mL coffee oil/day, 5 weeks | 69 mg cafestol and 51 mg kahweol | +2.7 µg/L |
| No effect on APO A-I | Van Dusseldorp 1991 [76] (0.9 L unfiltered boiled coffee/day, 14 weeks) | N/A | No significant change |
| Ahola et al., 1991 [56] (1 L unfiltered boiled coffee/day, 4 weeks) | N/A | No significant change | |
| Van Rooij et al., 1995 [30] 1 g coffee oil (Arabica/day), 6 weeks | 70 mg cafestol and 87 mg kahweol | No significant change | |
| Van Rooij et al., 1995 [30] 1 g coffee oil (Canephora/day), 6 weeks | 39 mg cafestol and 1 mg kahweol | No significant change | |
| No effect on bile acid | Boekschoten et al., 2005 [90] 2 mL coffee oil/day, 5 weeks | 69 mg cafestol and 51 mg kahweol | No significant change |
| No effect on bilirubin | Boekschoten et al., 2005 [90] 2 mL coffee oil/day, 5 weeks | 69 mg cafestol and 51 mg kahweol | No significant change |
| Decrease in amylase | Boekschoten et al., 2005 [90] 2 mL coffee oil/day, 5 weeks | 69 mg cafestol and 51 mg kahweol | −0.5 U/L |
| Increase in APO B | Van Dusseldorp 1991 [76] 0.9 L unfiltered boiled coffee/day, 14 weeks | N/A | +8.6 mmol/L +4412 g/L |
| Ahola et al., 1991 [56] 1 L unfiltered boiled coffee/day, 4 weeks | N/A | +0.0001 mmol/L +0.05 g/L | |
| Van Rooij et al., 1995 [30] 1 g coffee oil (Arabica/day), 6 weeks | 70 mg cafestol and 87 mg kahweol | +0.35 g/L | |
| Van Rooij et al., 1995 [30] 1 g coffee oil (Canephora/day), 6 weeks | 39 mg cafestol and 1 mg kahweol | +0.17 g/L | |
| No effect on serum campesterol level | Van Dusseldorp 1991 [76] 0.9 L unfiltered boiled coffee/day, 14 weeks | N/A | No significant change |
| Increase in serum lathosterol level | Van Dusseldorp 1991 [76] 0.9 L unfiltered boiled coffee/day, 14 weeks | N/A | N/A |
| No effect on LDH | Van Rooij et al., 1995 [30] 1 g coffee oil (Arabica/day), 6 weeks | 70 mg cafestol and 87 mg kahweol | No significant change |
| Van Rooij et al., 1995 [30] 1 g coffee oil (Canephora/day), 6 weeks | 39 mg cafestol and 1 mg kahweol | No significant change | |
| Boekschoten et al., 2005 [90] 2 mL coffee oil/day, 5 weeks | 69 mg cafestol and 51 mg kahweol | No significant change | |
| No effect on T3, T4, and TSH | Mensink et al., 1995 [87] 2 g coffee oil /day, 3 weeks | 72 mg cafestol and 53 mg kahweol | No significant change |
| Mensink et al., 1995 [87] 2 g coffee oil /day, 3 weeks | 59 mg cafestol (cafestol and O-methyl-cafestol) and 2 mg kahweol | No significant change | |
| No effect on body weight | Van Rooij et al., 1995 [30] 1 g coffee oil (Arabica/day), 6 weeks | 70 mg cafestol and 87 mg kahweol | No significant change |
| Van Rooij et al., 1995 [30] 1 g coffee oil (Canephora/day), 6 weeks | 39 mg cafestol and 1 mg kahweol | No significant change | |
| Urgert et al., 1996 [77] 71 mg diterpenes/day, 24 weeks | 38 mg cafestol and 33 mg kahweol. Maximum values from the 24-week study used | No significant change (less than 0.5 kg/m2) | |
| Boekschoten et al., 2003 [86] 2 mL coffee oil/day, 5 weeks | 69 mg cafestol and 51 mg kahweol | No significant change |
| Toxicological Threshold | Diterpene Intake (mg/day) | Coffee Oil Intake (mg/day) 1 |
|---|---|---|
| LOAEL | 40 | 10,000 |
| NOAEL | 9 | 2250 |
| ADI | 0.9 | 225 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maier, B.; Franke, H.; Schwarz, S.; Lachenmeier, D.W. Toxicological Risk Assessment of Coffee Oil (Coffee Seed Oil and Spent Coffee Grounds Oil) as a Novel Food with Focus on Cafestol. Molecules 2025, 30, 2951. https://doi.org/10.3390/molecules30142951
Maier B, Franke H, Schwarz S, Lachenmeier DW. Toxicological Risk Assessment of Coffee Oil (Coffee Seed Oil and Spent Coffee Grounds Oil) as a Novel Food with Focus on Cafestol. Molecules. 2025; 30(14):2951. https://doi.org/10.3390/molecules30142951
Chicago/Turabian StyleMaier, Bernadette, Heike Franke, Steffen Schwarz, and Dirk W. Lachenmeier. 2025. "Toxicological Risk Assessment of Coffee Oil (Coffee Seed Oil and Spent Coffee Grounds Oil) as a Novel Food with Focus on Cafestol" Molecules 30, no. 14: 2951. https://doi.org/10.3390/molecules30142951
APA StyleMaier, B., Franke, H., Schwarz, S., & Lachenmeier, D. W. (2025). Toxicological Risk Assessment of Coffee Oil (Coffee Seed Oil and Spent Coffee Grounds Oil) as a Novel Food with Focus on Cafestol. Molecules, 30(14), 2951. https://doi.org/10.3390/molecules30142951