Beer-Derived (Poly)phenol Metabolism in Individuals With and Without Metabolic Syndrome: A Comparative Dietary Intervention
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Subjects
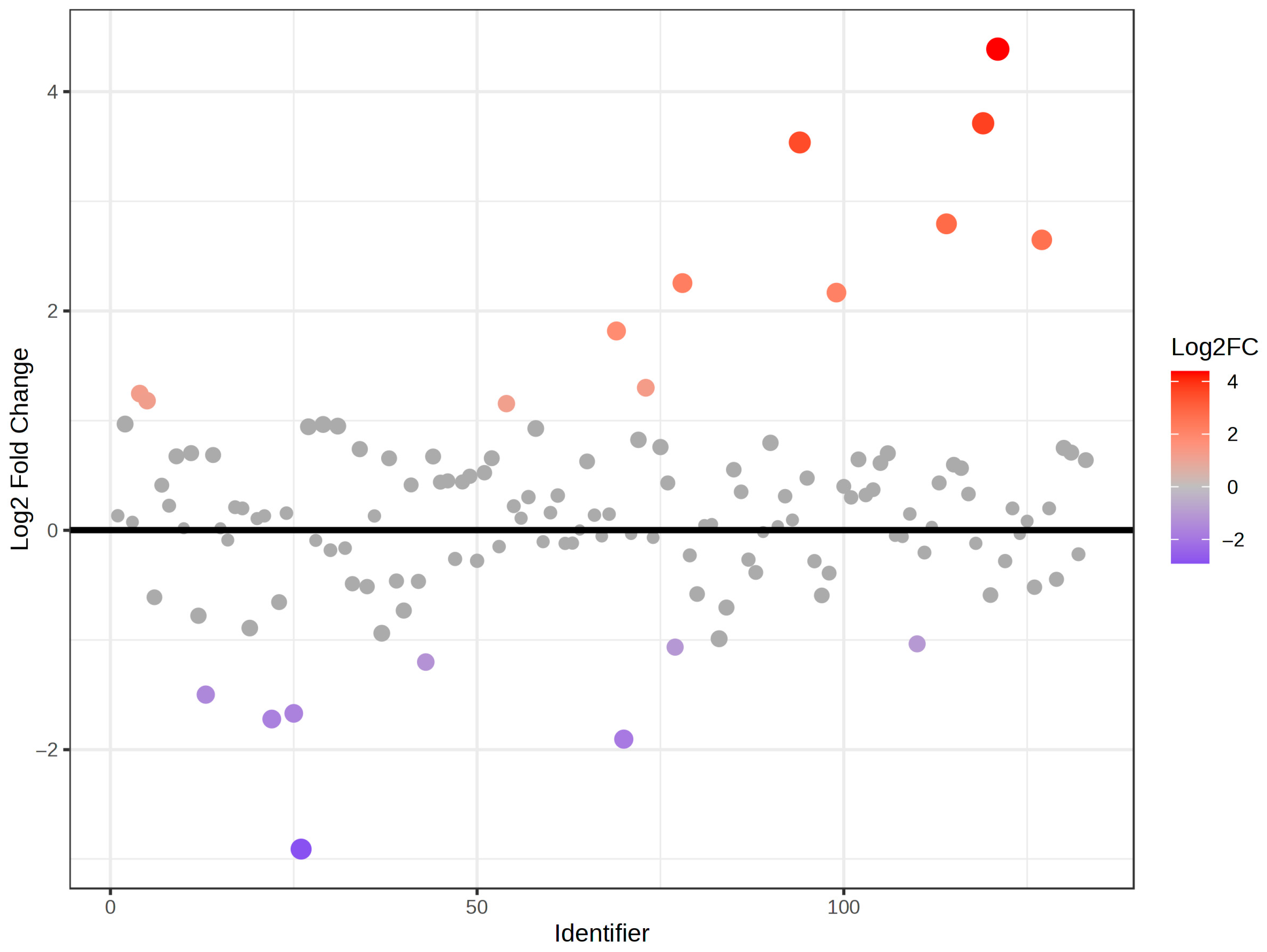
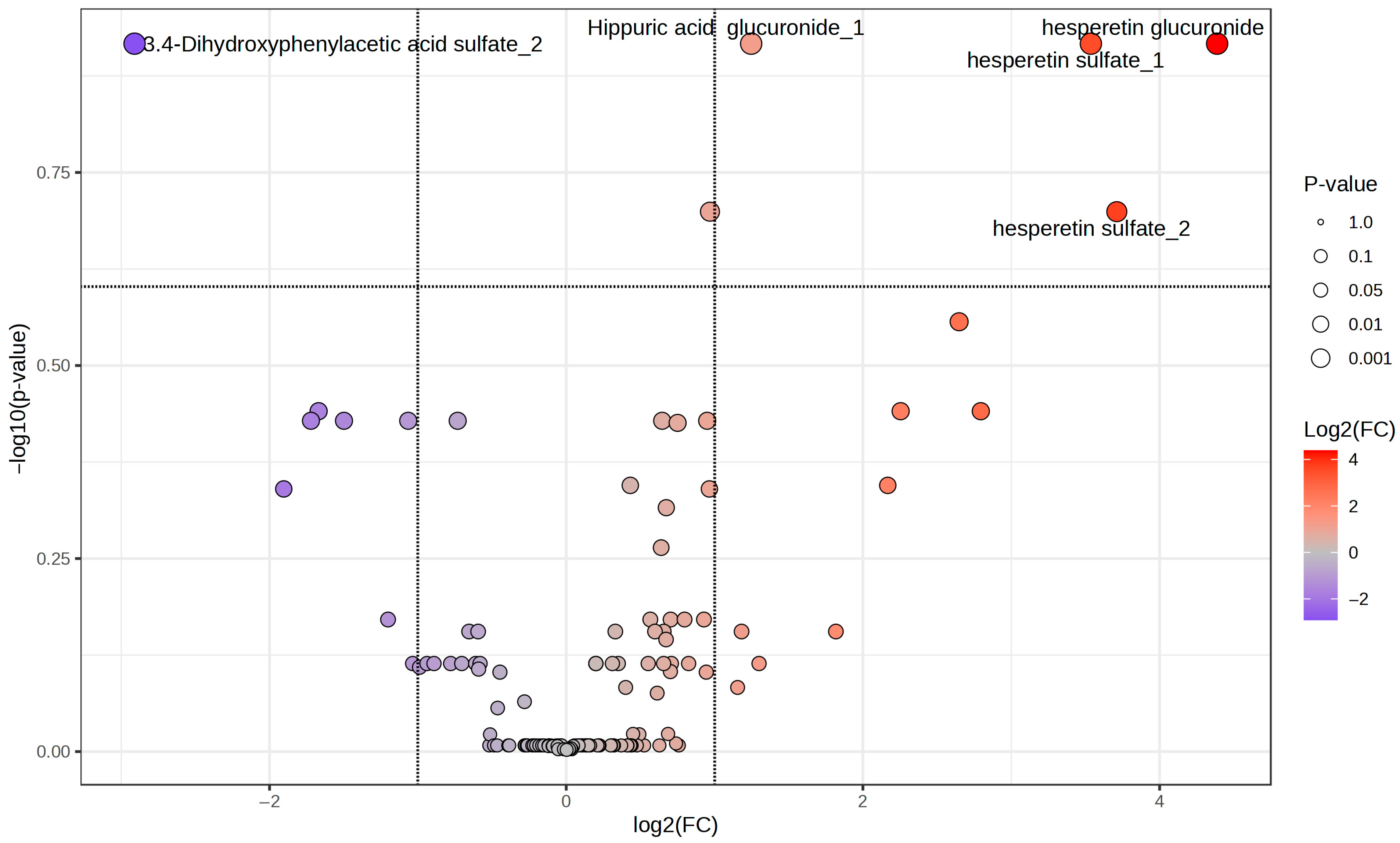
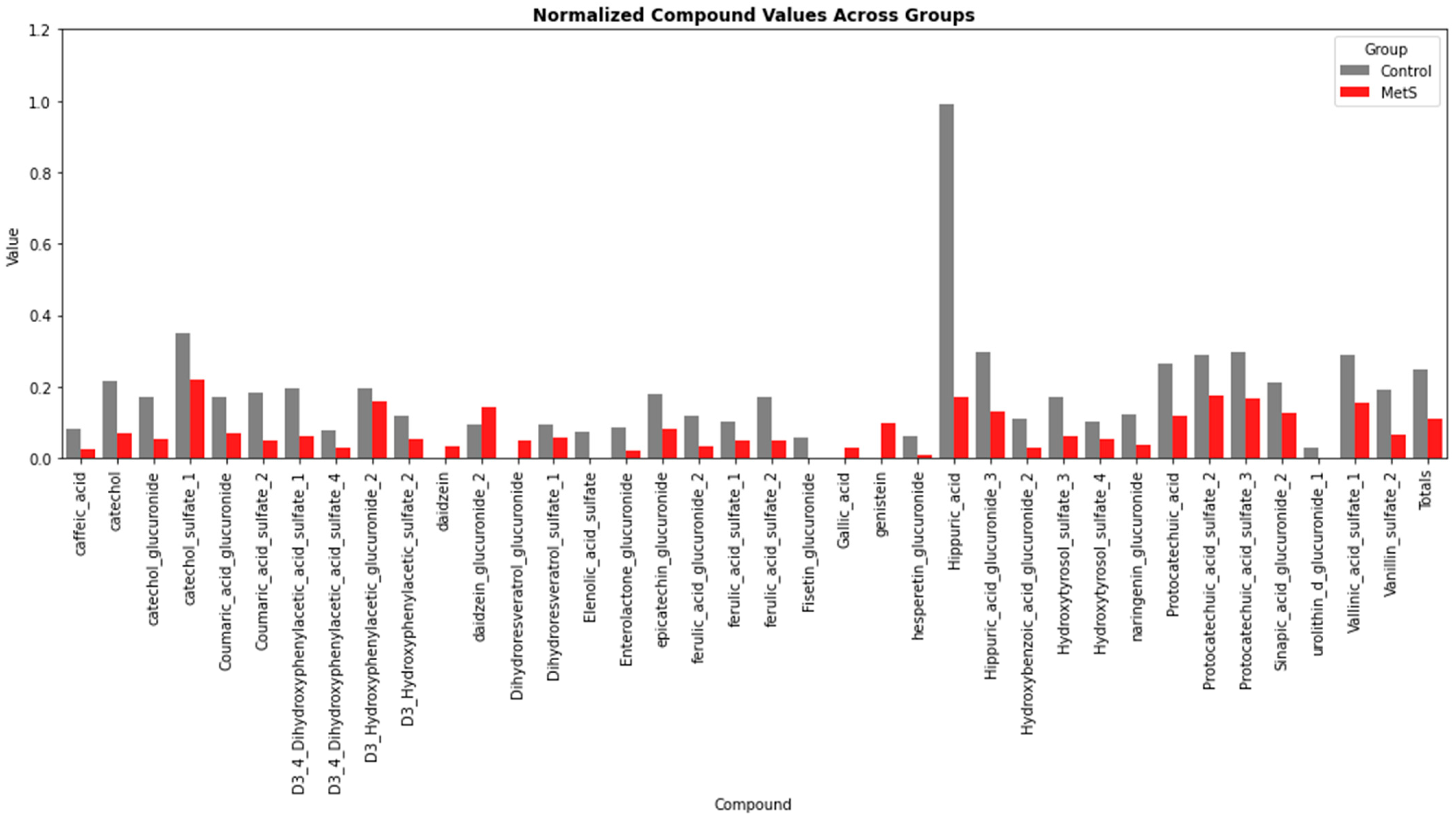
2.2. Phenolic Profiles of the Whole Population During the Intervention
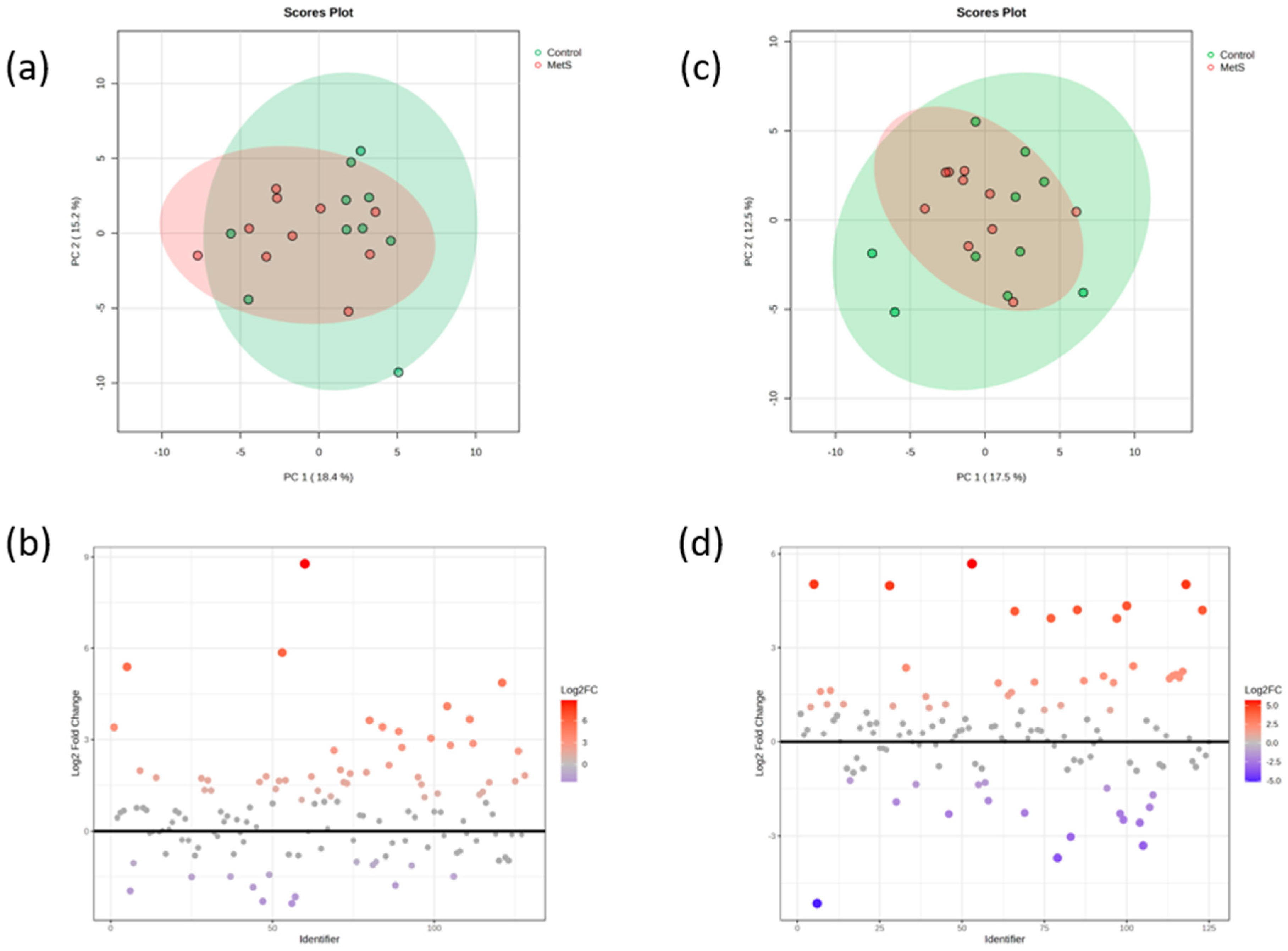
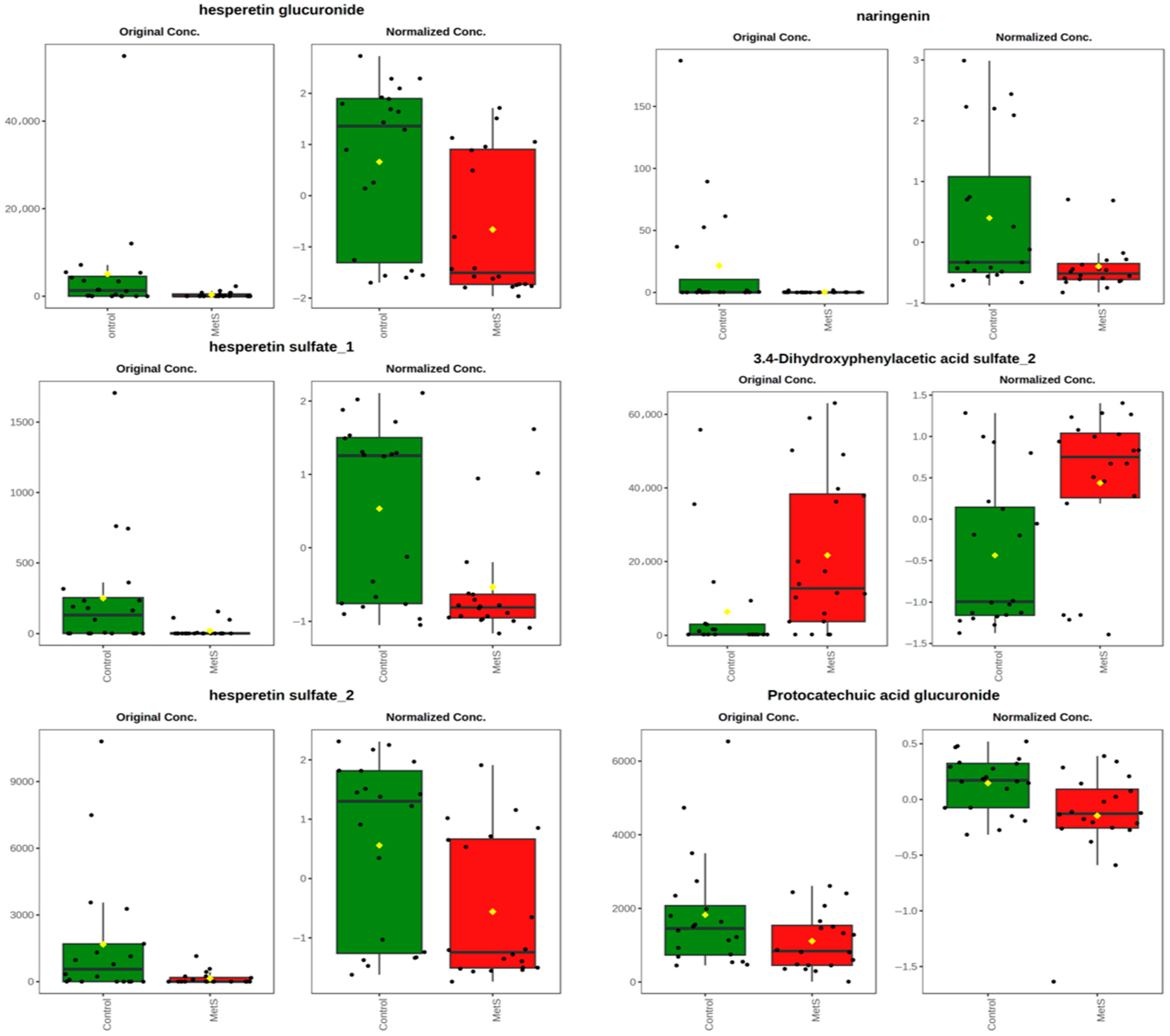
2.3. Phenolic Profiles According to the Metabolic Status of the Participants
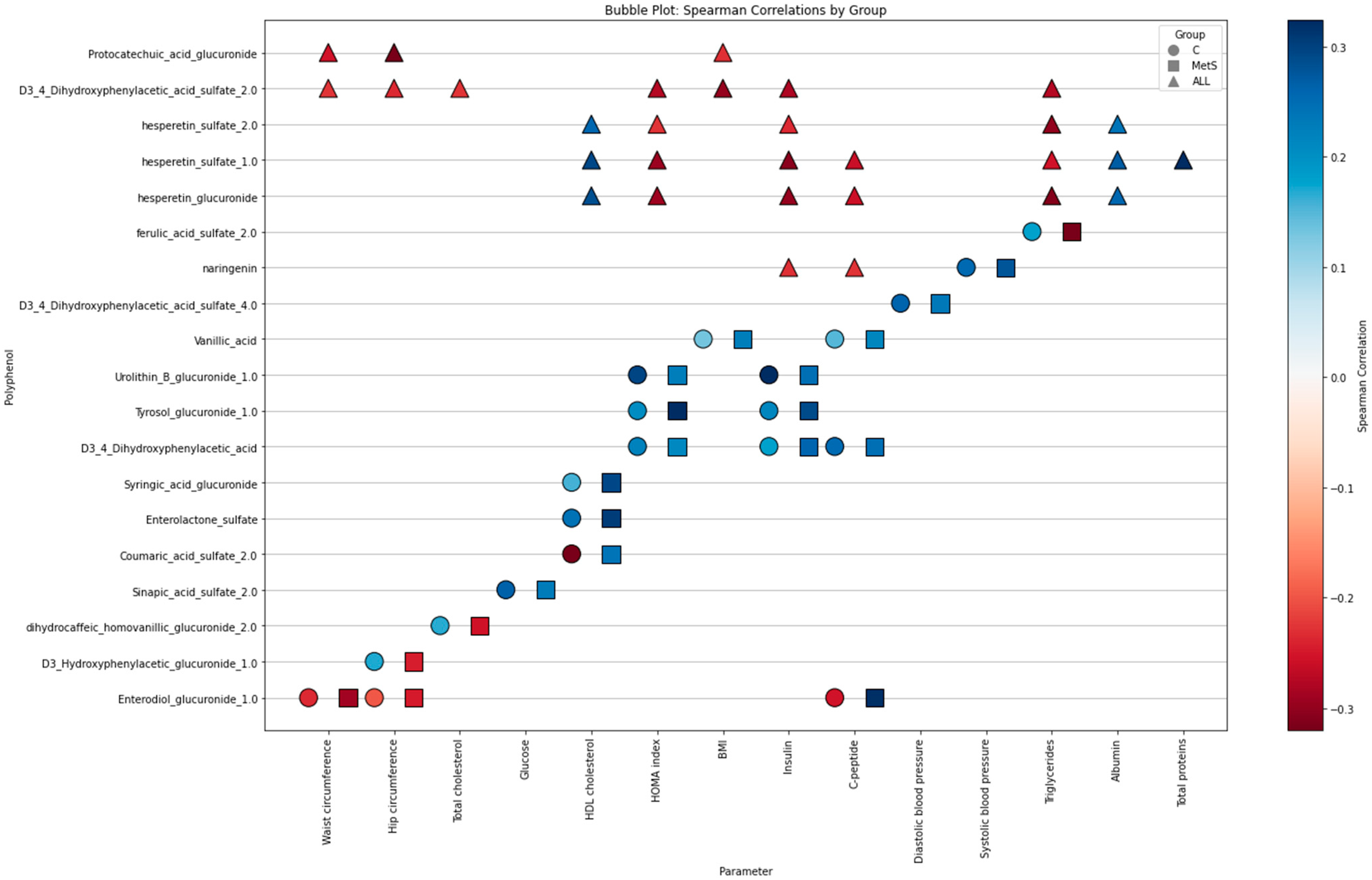
2.4. Relationships Between Biochemical Phenolic Profiles According to the Metabolic Status of the Participants
3. Discussion
4. Materials and Methods
4.1. Intervention Design and Sample Processing
4.2. Phenolic Compound Analysis
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Åberg, F.; Byrne, C.D.; Pirola, C.J.; Männistö, V.; Sookoian, S. Alcohol Consumption and Metabolic Syndrome: Clinical and Epidemiological Impact on Liver Disease. J. Hepatol. 2023, 78, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, H.; Li, C. Dietary Regulation of Oxidative Stress in Chronic Metabolic Diseases. Foods 2021, 10, 1854. [Google Scholar] [CrossRef] [PubMed]
- Borsoi, F.T.; Neri-Numa, I.A.; De Oliveira, W.Q.; De Araújo, F.F.; Pastore, G.M. Dietary Polyphenols and Their Relationship to the Modulation of Non-Communicable Chronic Diseases and Epigenetic Mechanisms: A Mini-Review. Food Chem. Mol. Sci. 2023, 6, 100155. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, M.; Aradwad, P.; Sanwal, N.; Sahu, J.K.; Kumar, V.; Naik, S.N. Fermented Foods in Health and Disease Prevention. In Microbes in the Food Industry; Chhikara, N., Panghal, A., Chaudhary, G., Eds.; Wiley: Hoboken, NJ, USA, 2023; pp. 39–85. ISBN 978-1-119-77558-4. [Google Scholar]
- Kang, Q.; Sun, J.; Wang, B.; Sun, B. Wine, Beer and Chinese Baijiu in Relation to Cardiovascular Health: The Impact of Moderate Drinking. Food Sci. Hum. Wellness 2023, 12, 1–13. [Google Scholar] [CrossRef]
- Lopes, A.C.A.; Eda, S.H.; Andrade, R.P.; Amorim, J.C.; Duarte, W.F. New Alcoholic Fermented Beverages—Potentials and Challenges. In Fermented Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 577–603. ISBN 978-0-12-815271-3. [Google Scholar]
- Ambra, R.; Pastore, G.; Lucchetti, S. The Role of Bioactive Phenolic Compounds on the Impact of Beer on Health. Molecules 2021, 26, 486. [Google Scholar] [CrossRef]
- Martínez-Montoro, J.I.; Quesada-Molina, M.; Gutiérrez-Repiso, C.; Ruiz-Limón, P.; Subiri-Verdugo, A.; Tinahones, F.J.; Moreno-Indias, I. Effect of Moderate Consumption of Different Phenolic-Content Beers on the Human Gut Microbiota Composition: A Randomized Crossover Trial. Antioxidants 2022, 11, 696. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Badimon, L. Effects of Polyphenol Intake on Metabolic Syndrome: Current Evidences from Human Trials. Oxidative Med. Cell. Longev. 2017, 2017, e5812401. [Google Scholar] [CrossRef]
- Manach, C.; Morand, C.; Gil-Izquierdo, A.; Bouteloup-Demange, C.; Rémésy, C. Bioavailability in Humans of the Flavanones Hesperidin and Narirutin after the Ingestion of Two Doses of Orange Juice. Eur. J. Clin. Nutr. 2003, 57, 235–242. [Google Scholar] [CrossRef]
- Grao-Cruces, E.M.; Montserrat-de La Paz, S.; Martin, M.E. Moderate Beer Consumption and Metabolic Health: A Comprehensive Review from the Lipoprotein Perspective. J. Funct. Foods 2022, 95, 105188. [Google Scholar] [CrossRef]
- Kováčová, J.; Lehotay, J.; Űrgeová, E.; Mocák, J.; Čižmárik, J. Determination of Selected Flavonoids in Hop Extract by HPLC. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 329–340. [Google Scholar] [CrossRef]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The Gut Microbiota: A Key Factor in the Therapeutic Effects of (Poly)Phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Kerimi, A.; Williamson, G. Bioavailability and Metabolism of Chlorogenic Acids (Acyl-quinic Acids) in Humans. Comp. Rev. Food Sci. Food Safe 2020, 19, 1299–1352. [Google Scholar] [CrossRef]
- Chen, W.; Liu, R.; Zhu, X.; Lu, Q.; Yang, H.; Liu, R. Microbial Phenolic Metabolites 3-(3’,4’-Dihydroxyphenyl)Propanoic Acid and 3’,4’-Dihydroxyphenylacetic Acid Prevent Obesity in Mice Fed High-Fat Diet. Food Sci. Hum. Wellness 2024, 13, 327–338. [Google Scholar] [CrossRef]
- Jakobek, L.; Blesso, C. Beneficial Effects of Phenolic Compounds: Native Phenolic Compounds vs. Metabolites and Catabolites. Crit. Rev. Food Sci. Nutr. 2024, 64, 9113–9131. [Google Scholar] [CrossRef]
- Pallister, T.; Jackson, M.A.; Martin, T.C.; Zierer, J.; Jennings, A.; Mohney, R.P.; MacGregor, A.; Steves, C.J.; Cassidy, A.; Spector, T.D.; et al. Hippurate as a Metabolomic Marker of Gut Microbiome Diversity: Modulation by Diet and Relationship to Metabolic Syndrome. Sci. Rep. 2017, 7, 13670. [Google Scholar] [CrossRef]
- Neeland, I.J.; Lim, S.; Tchernof, A.; Gastaldelli, A.; Rangaswami, J.; Ndumele, C.E.; Powell-Wiley, T.M.; Després, J.-P. Metabolic Syndrome. Nat. Rev. Dis. Primers 2024, 10, 77. [Google Scholar] [CrossRef]
- Zugravu, C.-A.; Medar, C.; Manolescu, L.S.C.; Constantin, C. Beer and Microbiota: Pathways for a Positive and Healthy Interaction. Nutrients 2023, 15, 844. [Google Scholar] [CrossRef]
- García-Conesa, M.-T. Dietary Polyphenols against Metabolic Disorders: How Far Have We Progressed in the Understanding of the Molecular Mechanisms of Action of These Compounds? Crit. Rev. Food Sci. Nutr. 2015, 57, 1769–1786. [Google Scholar] [CrossRef]
- De Simone, G.; Balducci, C.; Forloni, G.; Pastorelli, R.; Brunelli, L. Hippuric Acid: Could Became a Barometer for Frailty and Geriatric Syndromes? Ageing Res. Rev. 2021, 72, 101466. [Google Scholar] [CrossRef] [PubMed]
- Lord, R.S.; Bralley, J.A. Clinical Applications of Urinary Organic Acids. Part 2. Dysbiosis Markers. Altern. Med. Rev. 2008, 13, 292–306. [Google Scholar]
- Pan, L.; Ye, H.; Pi, X.; Liu, W.; Wang, Z.; Zhang, Y.; Zheng, J. Effects of Several Flavonoids on Human Gut Microbiota and Its Metabolism by in Vitro Simulated Fermentation. Front. Microbiol. 2023, 14, 1092729. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; González-Sarrías, A.; Vallejo, F.; Espín, J.C.; Tomás-Barberán, F.A. Hesperetin and Its Sulfate and Glucuronide Metabolites Inhibit TNF-α Induced Human Aortic Endothelial Cell Migration and Decrease Plasminogen Activator Inhibitor-1 (PAI-1) Levels. Food Funct. 2016, 7, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, A.; Mujawdiya, P.K.; Noor, S.; Lysiuk, R.; Darmohray, R.; Piscopo, S.; Lenchyk, L.; Antonyak, H.; Dehtiarova, K.; Shanaida, M.; et al. Polyphenols in Metabolic Diseases. Molecules 2022, 27, 6280. [Google Scholar] [CrossRef]
- Sluik, D.; Atkinson, F.S.; Brand-Miller, J.C.; Fogelholm, M.; Raben, A.; Feskens, E.J.M. Contributors to Dietary Glycaemic Index and Glycaemic Load in the Netherlands: The Role of Beer. Br. J. Nutr. 2016, 115, 1218–1225. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Magraner, E.; Condines, X.; Valderas-Martínez, P.; Roth, I.; Arranz, S.; Casas, R.; Navarro, M.; Hervas, A.; Sisó, A.; et al. Effects of Alcohol and Polyphenols from Beer on Atherosclerotic Biomarkers in High Cardiovascular Risk Men: A Randomized Feeding Trial. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 36–45. [Google Scholar] [CrossRef]
- López-Yerena, A.; Muñoz-García, N.; De Santisteban Villaplana, V.; Padro, T.; Badimon, L. Effect of Moderate Beer Intake on the Lipid Composition of Human Red Blood Cell Membranes. Nutrients 2024, 16, 3541. [Google Scholar] [CrossRef]
- Laveriano-Santos, E.P.; Quifer-Rada, P.; Marhuenda-Muñoz, M.; Arancibia-Riveros, C.; Vallverdú-Queralt, A.; Tresserra-Rimbau, A.; Ruiz-León, A.M.; Casas, R.; Estruch, R.; Bodega, P.; et al. Microbial Phenolic Metabolites in Urine Are Inversely Linked to Certain Features of Metabolic Syndrome in Spanish Adolescents. Antioxidants 2022, 11, 2191. [Google Scholar] [CrossRef]
- National Cholesterol Education Program (US). Expert Panel on Detection; Treatment of High Blood Cholesterol in Adults. In Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III); The Program; National Heart, Lung, and Blood Institute: Bethesda, MD, USA, 2002; ISBN 1-58808-003-X. [Google Scholar]
- Showing Report on Alcoholic Beverages—Phenol-Explorer. Available online: http://phenol-explorer.eu/reports/38#beertext (accessed on 7 July 2025).
- Marhuenda-Muñoz, M.; Laveriano-Santos, E.P.; Tresserra-Rimbau, A.; Lamuela-Raventós, R.M.; Martínez-Huélamo, M.; Vallverdú-Queralt, A. Microbial Phenolic Metabolites: Which Molecules Actually Have an Effect on Human Health? Nutrients 2019, 11, 2725. [Google Scholar] [CrossRef]
- Campins-Machado, F.M.; Casas, R.; Lamuela-Raventós, R.M.; Galkina, P.; Martínez-González, M.Á.; Fitó, M.; Ros, E.; Estruch, R.; Domínguez-López, I.; Pérez, M. Microbiota-Derived Resveratrol Metabolites: New Biomarkers of Red Wine Consumption Are Inversely Associated with Inflammation in a Longitudinal Study of a Mediterranean Population. J. Nutr. Health Aging 2025, 29, 100542. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a Unified Platform for Metabolomics Data Processing, Analysis and Interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef] [PubMed]

| Parameters (Control n = 10/MetS n = 10) | Control—Baseline | Control—End Time | MetS—Baseline | MetS—End Time |
|---|---|---|---|---|
| Weight (kg) a,b | 65.6 ± 7.8 | 65.4 ± 7.4 | 93.7 ± 14.4 | 93.1 ± 15.1 |
| BMI (kg/m2) a,b | 22.9 ± 2.5 | 22.8 ± 2.4 | 34.6 ± 4.8 | 34.3 ± 4.9 |
| Waist (cm) a,b | 80.3 ± 8.9 | 79.6 ± 7.5 | 109.1 ± 10.2 | 104.9 ± 8.5 |
| Hip (cm) a,b | 97.0 ± 6.4 | 97.0 ± 6.6 | 114.9 ± 12.8 | 112.6 ± 12.4 |
| Systolic blood pressure (mmHg) a,b | 114.0 ± 14.5 | 112.1 ± 12.4 | 137.5 ± 13.2 | 129.4 ± 12.1 |
| Diastolic blood pressure (mmHg) a,b | 69.4 ± 8.3 | 71.1 ± 10.1 | 88.8 ± 10.3 | 86.7 ± 6.7 |
| Glucose (mg/dL) a,b | 82.6 ± 6.8 | 88.5 ± 5.8 | 114.7 ± 36.2 | 119.0 ± 24.6 |
| Creatinine (mg/dL) | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.7 ± 0.2 | 0.8 ± 0.2 |
| Uric Acid (mg/dL) b | 4.2 ± 0.8 | 4.5 ± 1.1 | 5.3 ± 1.4 | 5.9 ± 1.3 |
| Na (mEq/L) | 140.0 ± 1.3 | 140.5 ± 2.2 | 140.3 ± 2.2 | 139.0 ± 3.7 |
| Fe (µg/dL) | 77.5 ± 20.4 | 75.6 ± 24.2 | 71.1 ± 34.4 | 62.1 ± 27.6 |
| Cholesterol (mg/dL) | 192.5 ± 31.0 | 190.1 ± 30.3 | 208.6 ± 43.1 | 214.4 ± 45.4 |
| HDL (mg/dL) a,b | 64.0 ± 12.6 | 65.9 ± 12.4 | 48.7 ± 11.7 | 47.9 ± 12.1 |
| LDL (mg/dL) | 113.6 ± 28.6 | 109.6 ± 27.8 | 129.6 ± 31.2 | 127.9 ± 39.3 |
| Triglycerides (mg/dL) a,b | 74.6 ± 38.6 | 73.1 ± 33.5 | 151.7 ± 89.2 | 206.4 ± 168.2 |
| Energy (kcal) | 1610.1 ± 291.6 | 1704.3 ± 203.3 | 1617.2 ± 549.3 | 1697.6 ± 297.8 |
| Total Protein (g) | 65.7 ± 15.2 | 68.0 ± 13.0 | 71.0 ± 34.0 | 74.1 ± 14.0 |
| Total Fat (g) | 81.7 ± 24.4 | 79.6 ± 16.8 | 82.4 ± 38.7 | 89.9 ± 22.0 |
| Total Carbohydrates (g) | 155.3 ± 26.8 | 178.4 ± 27.6 | 147.8 ± 44.8 | 147.3 ± 29.3 |
| Dietary Fiber (g) | 16.6 ± 6.4 | 16.1 ± 12.2 | 14.6 ± 5.0 | 14.3 ± 5.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hinojosa-Nogueira, D.; Díaz-Perdigones, C.M.; García-López, M.J.; Marcos, A.; Portillo, M.P.; Lamuela-Raventós, R.M.; Subiri-Verdugo, A.; Nova, E.; Milton-Laskibar, I.; Galkina, P.; et al. Beer-Derived (Poly)phenol Metabolism in Individuals With and Without Metabolic Syndrome: A Comparative Dietary Intervention. Molecules 2025, 30, 2932. https://doi.org/10.3390/molecules30142932
Hinojosa-Nogueira D, Díaz-Perdigones CM, García-López MJ, Marcos A, Portillo MP, Lamuela-Raventós RM, Subiri-Verdugo A, Nova E, Milton-Laskibar I, Galkina P, et al. Beer-Derived (Poly)phenol Metabolism in Individuals With and Without Metabolic Syndrome: A Comparative Dietary Intervention. Molecules. 2025; 30(14):2932. https://doi.org/10.3390/molecules30142932
Chicago/Turabian StyleHinojosa-Nogueira, Daniel, Cristina María Díaz-Perdigones, María José García-López, Ascensión Marcos, María P. Portillo, Rosa María Lamuela-Raventós, Alba Subiri-Verdugo, Esther Nova, Iñaki Milton-Laskibar, Polina Galkina, and et al. 2025. "Beer-Derived (Poly)phenol Metabolism in Individuals With and Without Metabolic Syndrome: A Comparative Dietary Intervention" Molecules 30, no. 14: 2932. https://doi.org/10.3390/molecules30142932
APA StyleHinojosa-Nogueira, D., Díaz-Perdigones, C. M., García-López, M. J., Marcos, A., Portillo, M. P., Lamuela-Raventós, R. M., Subiri-Verdugo, A., Nova, E., Milton-Laskibar, I., Galkina, P., Tinahones, F. J., & Moreno-Indias, I. (2025). Beer-Derived (Poly)phenol Metabolism in Individuals With and Without Metabolic Syndrome: A Comparative Dietary Intervention. Molecules, 30(14), 2932. https://doi.org/10.3390/molecules30142932












