Chemical Composition, Cytotoxicity, and Encapsulation of Lavender Essential Oil (Lavandula angustifolia) in Alginate Hydrogel—Application and Therapeutic Effect on Animal Model
Abstract
1. Introduction
2. Results
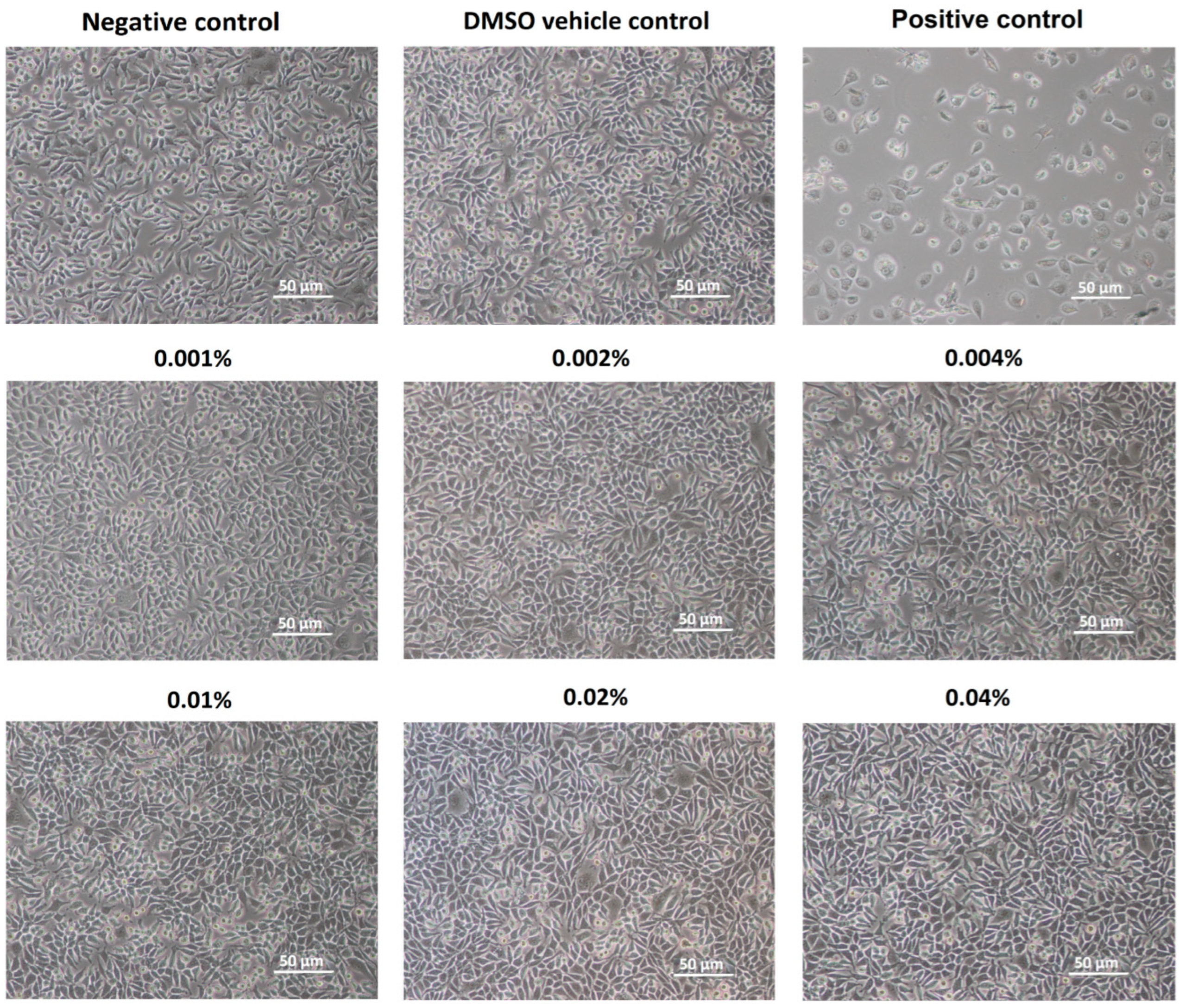
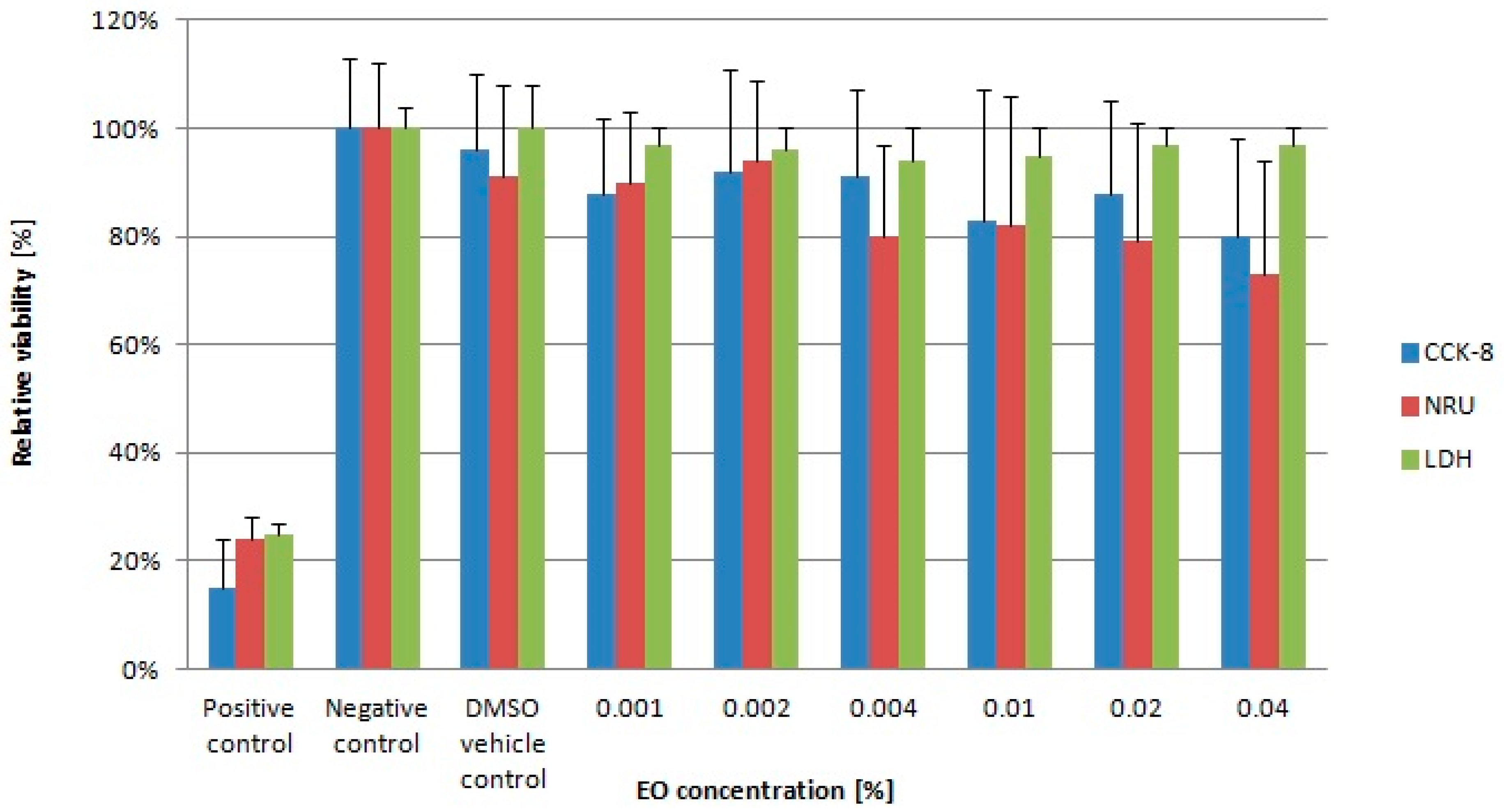
2.1. Essential Oil
2.2. Bird Trial
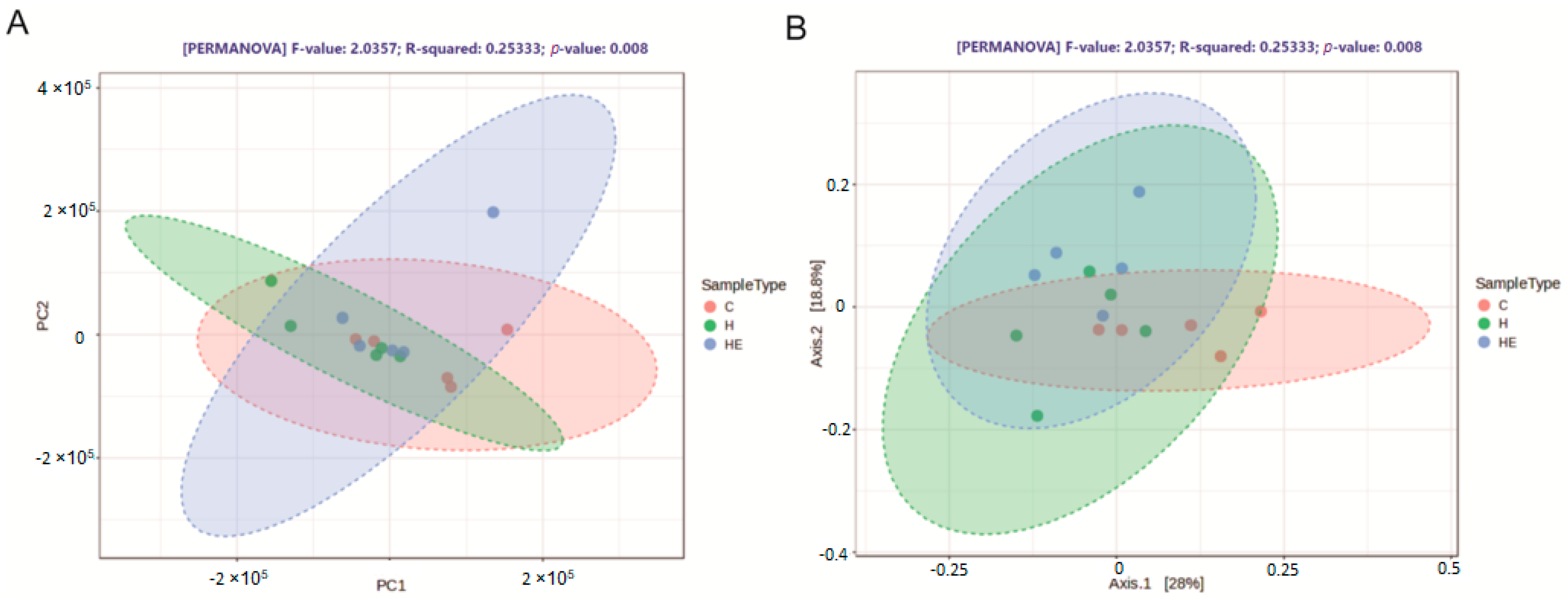
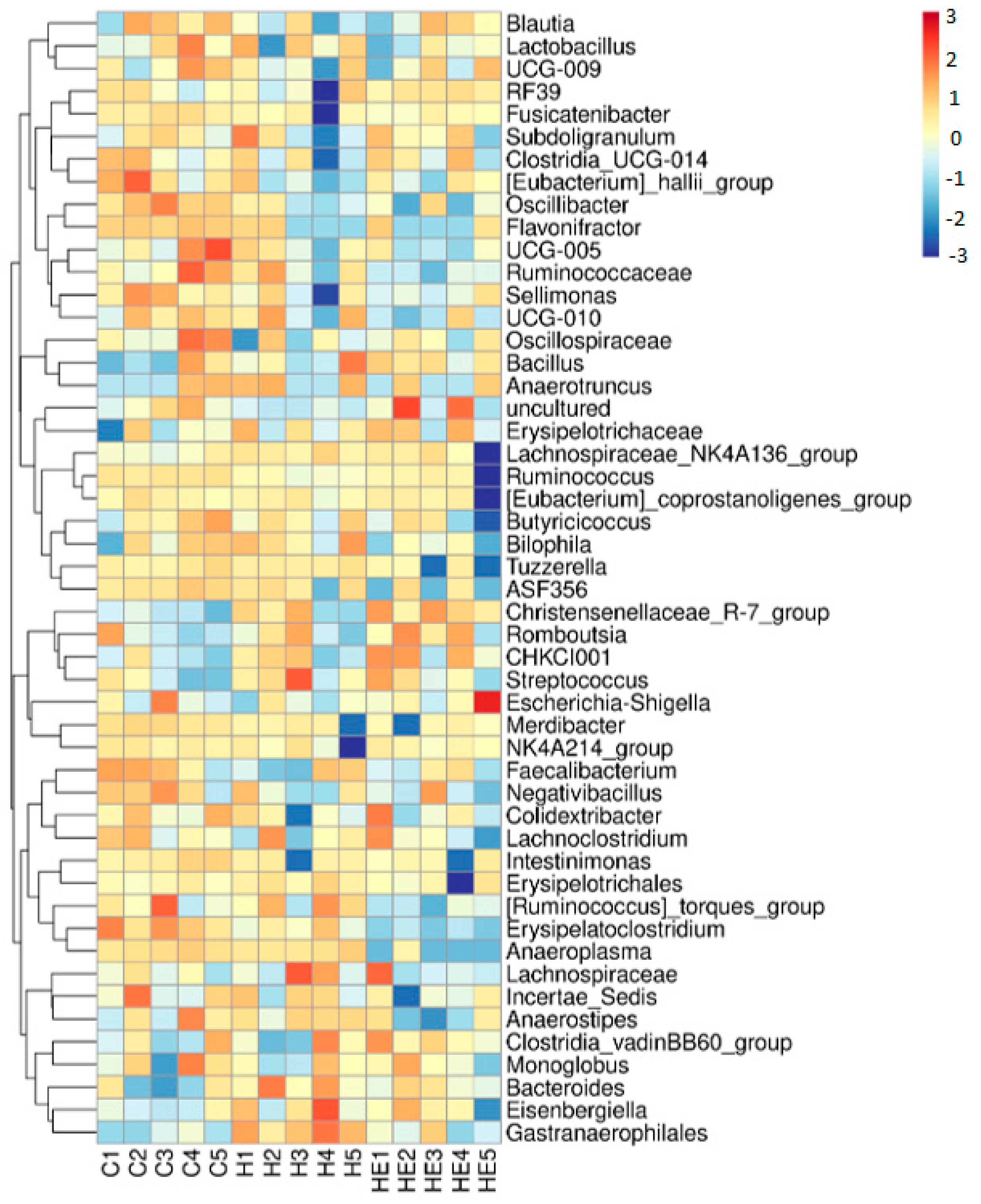
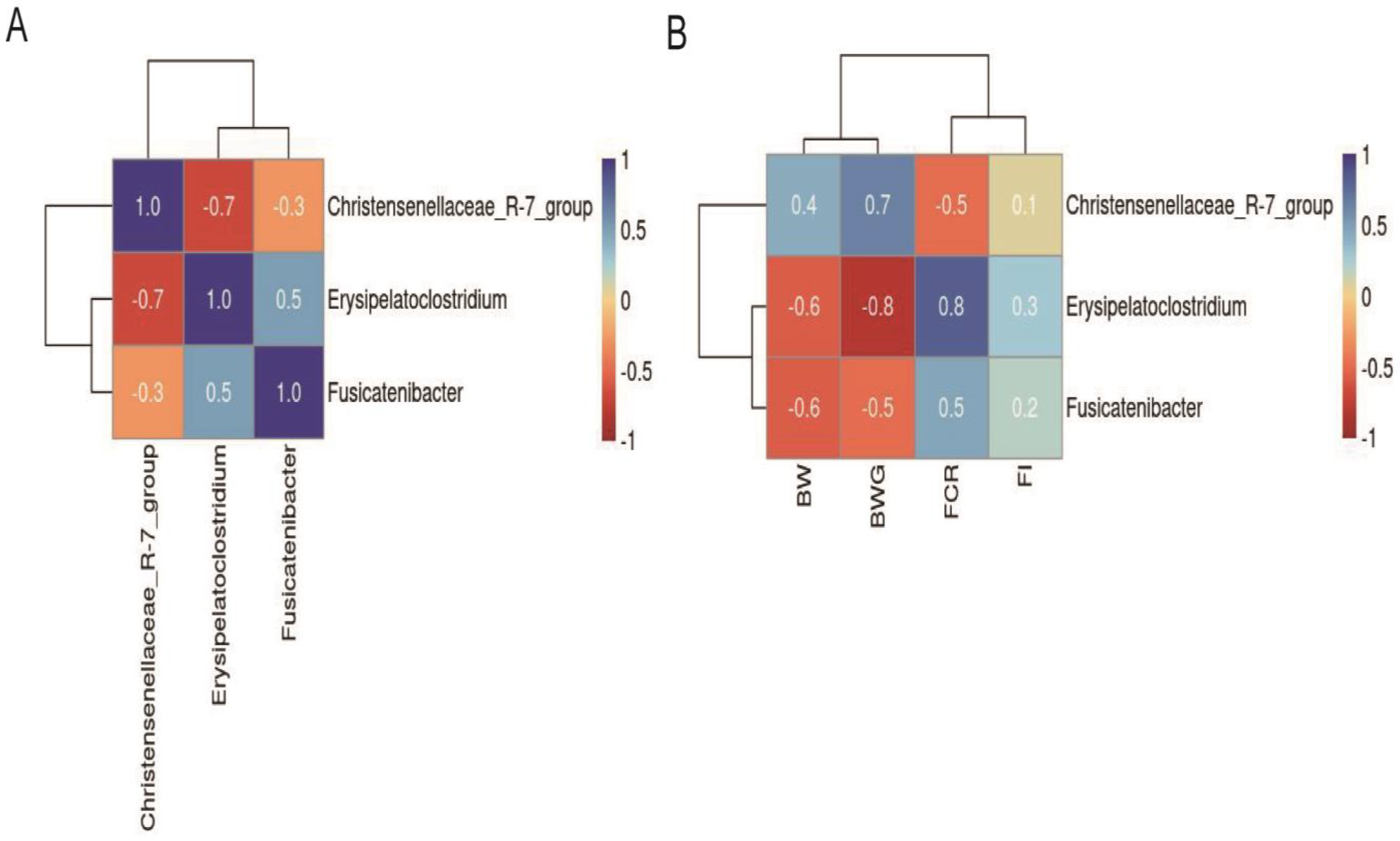
2.3. Effect of Supplementation on Cecal Microbiota
3. Discussion
4. Materials and Methods
4.1. Lavender Essential Oil and Alginate Hydrogels
4.2. Cytotoxicity of Lavender Essential Oil
4.3. Bird Trial
4.4. 16S rRNA Sequencing and Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BW | Body weight |
| BWG | Body weight gain |
| DMSO | Dimethylsulfoxide |
| Eos | Essential oils |
| FI | Feed Intake |
| FCR | Feed Conversion Ratio |
| LEO | Lavender essential oil |
| WI | Water Intake |
References
- Goh, C.H.; Heng, P.W.S.; Chan, L.W. Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohydr. Polym. 2012, 88, 1–12. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, J.; Ao, Q. Preparation of Alginate-Based Biomaterials and Their Applications in Biomedicine. Mar. Drugs 2021, 19, 264. [Google Scholar] [CrossRef] [PubMed]
- Farshidfar, N.; Iravani, S.; Varma, R.S. Alginate-Based Biomaterials in Tissue Engineering and Regenerative Medicine. Mar. Drugs 2023, 8, 189. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, A.; Aljabali, A.A.A.; Mishra, V.; Tambuwala, M.M.; Serrano-Aroca, Á. Alginate: Enhancement Strategies for Advanced Applications. Int. J. Mol. Sci. 2022, 23, 4486. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogels: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- El Sayed, M.M. Production of Polymer Hydrogel Composites and Their Applications. J. Polym. Environ. 2023, 31, 2855–2879. [Google Scholar] [CrossRef]
- Lai, J.; Azad, A.K.; Sulaiman, W.M.A.W.; Kumarasamy, V.; Subramaniyan, V.; Alshehade, S.A. Alginate-Based Encapsulation Fabrication Technique for Drug Delivery: An Updated Review of Particle Type, Formulation Technique, Pharmaceutical Ingredient, and Targeted Delivery System. Pharmaceutics 2024, 16, 370. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in Engineering Hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- O’Shea, G.M.; Sun, A.M. Encapsulation of rat islets of Langerhans prolongs xenograft survival in diabetic mice. Diabetes 1986, 35, 943–946. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for medical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Acevedo-Puello, V.; Figueroa-López, K.J.; Ortega-Toro, R. Gelatin-Based Hydrogels Containing Microcrystalline and Nanocrystalline Cellulose as Moisture Absorbers for Food Packaging Applications. J. Compos. Sci. 2023, 7, 337. [Google Scholar] [CrossRef]
- Alven, S.; Peter, S.; Aderibigbe, B.A. Polymer-Based Hydrogels Enriched with Essential Oils: A Promising Approach for the Treatment of Infected Wounds. Polymers 2022, 9, 3772. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From controlled release to pH- responsive drug delivery. Drug Discov. Ther. 2002, 10, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Adaszyńska-Swkirzynska, M.; Szczerbińska, D. Use of essential oils in broiler chicken production—A review. Ann. Anim. Sci. 2017, 17, 317–335. [Google Scholar] [CrossRef]
- Adaszyńska-Skwirzyńska, M.; Szczerbińska, D. The effect of lavender (Lavandula angustifolia) essential oil as a drinking water supplement on the production performance, blood biochemical parameters, and ileal microflora in broiler chickens. Poult. Sci. 2019, 98, 358–365. [Google Scholar] [CrossRef]
- Adaszyńska-Skwirzyńska, M.; Dzięcioł, M.; Bucław, M.; Majewska, D.; Szczerbińska, D. Determination of Sesquiterpenic Acids with Sedative Properties in Extracts of Medicinal Lavender (Lavandula angustifolia Mill.). Appl. Sci. 2024, 14, 554. [Google Scholar] [CrossRef]
- Adaszyńska-Skwirzyńska, M.; Szczerbińska, D.; Zych, S. The Use of Lavender (Lavandula angustifolia) Essential Oil as an Additive to Drinking Water for Broiler Chickens and Its In Vitro Reaction with Enrofloxacin. Animals 2021, 11, 1535. [Google Scholar] [CrossRef]
- Yarmohammadi Barbarestani, S.; Jazi, V.; Mohebodini, H.; Ashayerizadeh, A.; Shabani, A.; Toghyani, M. Effects of dietary lavender essential oil on growth performance, intestinal function, and antioxidant status of broiler chickens. Livest. Sci. 2020, 2223, 103958. [Google Scholar] [CrossRef]
- Salinas-Chavira, J.; Barrios-García, H.B. Essential Oils, Chemical Compounds, and Their Effects on the Gut Microorganisms and Broiler Chicken Production: Review. Agriculture 2024, 14, 1864. [Google Scholar] [CrossRef]
- European Pharmacopeia 11th Edition. Available online: https://pheur.edqm.eu/subhome/11-7 (accessed on 20 May 2025).
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data 2011, 40, 1–47. [Google Scholar] [CrossRef]
- Amer, S.A.; Abdel-Wareth, A.A.A.; Gouda, A.; Saleh, G.K.; Nassar, A.H.; Sherief, W.R.I.A.; Albogami, S.; Shalaby, S.I.; Abdelazim, A.M.; Abomughaid, M.M. Impact of Dietary Lavender Essential Oil on the Growth and Fatty Acid Profile of Breast Muscles, Antioxidant Activity, and Inflammatory Responses in Broiler Chickens. Antioxidants 2022, 11, 1798. [Google Scholar] [CrossRef] [PubMed]
- Amer, S.A.; Tolba, S.A.; AlSadek, D.M.; Abdel Fattah, D.M.; Hassan, A.M.; Metwally, A.E. Effect of supplemental glycerol monolaurate and oregano essential oil blend on the growth performance, intestinal morphology, and amino acid digestibility of broiler chickens. BMC Vet. Res. 2021, 17, 312. [Google Scholar] [CrossRef] [PubMed]
- Adaszyńska-Skwirzyńska, M.; Zych, S.; Bucław, M.; Majewska, D.; Dzięcioł, M.; Szczerbińska, D. Evaluation of the Antibacterial Activity of Gentamicin in Combination with Essential Oils Isolated from Different Cultivars and Morphological Parts of Lavender (Lavandula angustifolia Mill.) against Selected Bacterial Strains. Molecules 2023, 28, 5781. [Google Scholar] [CrossRef] [PubMed]
- Caputo, L.; Souza, L.F.; Alloisio, S.; Cornara, L.; De Feo, V. Coriandrum sativum and Lavandula angustifolia Essential Oils: Chemical Composition and Activity on Central Nervous System. Int. J. Mol. Sci. 2016, 17, 1999. [Google Scholar] [CrossRef]
- ISO 3515:2002; Oil of Lavender (Lavandula angustifolia Mill.). Edition 3; ISO: Geneva, Switzerland, 2002. Available online: https://www.iso.org/standard/36253.html (accessed on 20 May 2025).
- Abdel-Baki, A.-A.S.; Aboelhadid, S.M.; Al-Quraishy, S.; Hassan, A.O.; Daferera, D.; Sokmen, A.; Kamel, A.A. Cytotoxic, Scolicidal, and Insecticidal Activities of Lavandula stoechas Essential Oil. Separations 2023, 10, 100. [Google Scholar] [CrossRef]
- Lupoae, S.D.R.; Mihalcea, L.; Aprodu, I.; Socaci, S.A.; Cotârleț, M.; Enachi, E.; Crăciunescu, O.; Barbu, V.; Oancea, A.; Dulf, F.V.; et al. Fostering lavender as a source for valuable bioactives for food and pharmaceutical applications through extraction and microencapsulation. Molecules 2020, 25, 5001. [Google Scholar] [CrossRef]
- Tayarani-Najaran, Z.; Amiri, A.; Karimi, G.; Emami, S.A.; Asili, J.; Mousavi, S.H. Comparative studies of cytotoxic and apoptotic properties of different extracts and the essential oil of Lavandula angustifolia on malignant and normal cells. Nutr Cancer. 2014, 66, 424–434. [Google Scholar] [CrossRef]
- Gavanji, S.; Mohammadi, E.; Larki, B.; Bakhtari, A. Antimicrobial and cytotoxic evaluation of some herbal essential oils in comparison with common antibiotics in bioassay condition. Integr. Med. Res. 2014, 3, 142–152. [Google Scholar] [CrossRef]
- Georgantopoulos, A.; Vougioukas, A.; Kalousi, F.D.; Tsialtas, I.; Psarra, A.G. Comparative studies on the anti-inflammatory and apoptotic activities of four greek essential oils: Involvement in the regulation of NF-κΒ and steroid receptor signaling. Life 2023, 13, 1534. [Google Scholar] [CrossRef]
- Rios-Estepa, R.; Turner, G.W.; Lee, J.M.; Croteau, R.B.; Lange, B.M. A systems biology approach identifies the biochemical mechanisms regulating monoterpenoid essential oil composition in peppermint. Proc. Natl. Acad. Sci. USA 2008, 105, 2818–2823. [Google Scholar] [CrossRef]
- Marzec, M.; Polakowski, C.; Chilczuk, R.; Kołodziej, B. Evaluation of essential oil content, its chemical composition and price of thyme (Thymus vulgaris L.) raw material available in Poland. Herba Pol. 2010, 56, 37–52. [Google Scholar]
- Venskutonis, P.R. Effect of drying on the volatile constituents of thyme (Thymus vulgaris L.) and sage (Salvia officinals L.). Food Chem. 1997, 2, 219–227. [Google Scholar]
- Balladin, D.A.; Headley, O. Evaluation of solar dried thyme (Thymus vulgaris Linné) herbs. Renew. Energy 1999, 17, 523–531. [Google Scholar] [CrossRef]
- Fijałkowska, A.; Wesołowska, A.; Rakoczy, R.; Jedrzejczak-Silicka, M. A comparative study of thyme (Thymus vulgaris L.) essential oils and thymol–differences in chemical composition and cytotoxicity. Chem. Process Eng. New Front. 2024, 45, e55. [Google Scholar] [CrossRef]
- Vlaicu, P.A.; Untea, A.E.; Panaite, T.D.; Saracila, M.; Turcu, R.P.; Dumitru, M. Effect of Basil, Thyme and Sage Essential Oils as Phytogenic Feed Additives on Production Performances, Meat Quality and Intestinal Microbiota in Broiler Chickens. Agriculture 2023, 13, 874. [Google Scholar] [CrossRef]
- Puvača, N.; Tufarelli, V.; Giannenas, I. Essential Oils in Broiler Chicken Production, Immunity and Meat Quality: Review of Thymus vulgaris, Origanum vulgare, and Rosmarinus officinalis. Agriculture 2022, 12, 874. [Google Scholar] [CrossRef]
- Elbaz, A.M.; Ashmawy, E.S.; Salama, A.A.; Abdel-Moneim, A.M.; Badri, F.B.; Thabet, H.A. Effects of garlic and lemon essential oils on performance, digestibility, plasma metabolite, and intestinal health in broilers under environmental heat stress. BMC Vet. Res. 2022, 18, 430. [Google Scholar] [CrossRef]
- Gopi, M.; Karthik, K.; Manjunathachar, H.V.; Tamilmahan, P.; Kesavan, M.; Dash-Prakash, M.; Balaraju, B.L.; Purushothaman, M.R. Essential oils as a feed additive in poultry nutrition. Adv. Anim. Vet. Sci. 2014, 1, 1–7. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, S.; Wang, H.; Paio, X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: A review. J. Anim. Sci. Biotechnol. 2015, 6, 7–15. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Peng, Q.Y.; Liu, Y.R.; Ma, Q.G.; Zhang, J.Y.; Guo, Y.P.; Xue, Z.; Zhao, L.H. Effects of Oregano Essential Oil as an Antibiotic Growth Promoter Alternative on Growth Performance, Antioxidant Status, and Intestinal Health of Broilers. Poult. Sci. 2021, 100, 101163. [Google Scholar] [CrossRef]
- Ruan, D.; Fan, Q.; Fouad, A.M.; Sun, Y.; Huang, S.; Wu, A.; Lin, C.; Kuang, Z.; Zhang, C.; Jiang, S. Effects of Dietary Oregano Essential Oil Supplementation on Growth Performance, Intestinal Antioxidative Capacity, Immunity, and Intestinal Microbiota in Yellow-Feathered Chickens. J. Anim. Sci. 2021, 99, skab033. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, L.; Guo, F.; Huang, J.; Qiao, J.; Bi, R.; Huang, J.; Zhang, K.; Guo, Y.; Wang, Z. Dietary Supplemental Coated Essential Oils and Organic Acids Mixture Improves Growth Performance and Gut Health along with Reduces Salmonella Load of Broiler Chickens Infected with Salmonella Enteritidis. J. Anim. Sci. Biotechnol. 2023, 14, 95. [Google Scholar] [CrossRef] [PubMed]
- Lowman, Z.; Parkhurst, C. The effect of feeding Hydrogel-95 to emu chicks at hatch. J. Appl. Poult. Res. 2014, 23, 129–131. [Google Scholar] [CrossRef]
- Van der Pol, C.W.; Maatjens, C.; Aalbers, G.; Van Roovert-Reijrink, I. Effect of feed and water access on hatchling body weight changes between hatch and pull. In Proceedings of the 20th European Symposium on Poultry Nutrition 2015, Prague, Czech Republic, 24–27 August 2015. [Google Scholar]
- Areaaer, A.H.; Almrsomi, T.S.; Hammod, A.J. Effect of Fasting and Early Feeding by Using Hydro-Gel 95 after Hatching on the Growth Parameters of Broiler Chicks. Sci. J. King Faisal Univ. 2020, 21, 50–54. [Google Scholar] [CrossRef]
- Souza da Silva, C.; Molenaar, R.; Giersberg, M.F.; Rodenburg, T.B.; van Riel, J.W.; De Baere, K.; Van Dosselaer, I.; Kemp, B.; van den Brand, H.; Jong, I.C. Day-old chicken quality and performance of broiler chickens from 3 different hatching systems. Poult. Sci. 2021, 100, 100953. [Google Scholar] [CrossRef]
- Maman, A.H.; Özlü, S.; Ucar, A.; Elibol, O. Effect of chick body temperature during post-hatch handling on broiler live performance. Poult. Sci. 2019, 98, 244–250. [Google Scholar] [CrossRef]
- Özlü, S.; Erkuş, T.; Kamanl, S.; Nicholson, A.D.; Elibol, O. Influence of the preplacement holding time and feeding hydration supplementation before placement on yolk sac utilization, the crop filling rate, feeding behavior and first-week broiler performance. Poult. Sci. 2022, 101, 102056. [Google Scholar] [CrossRef]
- Willemsen, H.; Debonne, M.; Swennen, Q.; Everaert, N.; Careghi, C.; Han, H.; Bruggeman, V.; Tona, K.; Decuypere, E. Delay in feed access and spread of hatch: Importance of early nutrition. World’s Poult. Sci. J. 2010, 66, 177–188. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils-present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Abers, M.; Schroeder, S.; Goelz, L.; Sulser, A.; St Rose, T.; Puchalski, K.; Langland, J. Antimicrobial activity of the volatile substances from essential oils. BMC Complement Med. Ther. 2021, 21, 124. [Google Scholar] [CrossRef]
- Mancianti, F.; Ebani, V.V. Biological Activity of Essential Oils. Molecules 2020, 25, 678. [Google Scholar] [CrossRef]
- Díaz-Sánchez, S.; Perrotta, A.R.; Rockafellow, I.; Alm, E.J.; Okimoto, R.; Hawken, R.; Hanning, I. Using fecal microbiota as biomarkers for predictions of performance in the selective breeding process of pedigree broiler breeders. PLoS ONE 2019, 14, e0216080. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Hu, Y.; Yao, X.; He, Y.; Chen, J.; Wu, J.; Wu, S.; Zhang, H.; He, X.; Song, Z. Dietary essential oils improves the growth performance, antioxidant properties and intestinal permeability by inhibiting bacterial proliferation, and altering the gut microbiota of yellow-feather broilers. Poult. Sci. 2022, 101, 102087. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.Y.; Yu, Y.H. Effect of Bacillus species–fermented products and essential oils on growth performance, gut morphology, cecal short-chain fatty acid levels, and microbiota community in broilers. Poult. Sci. 2022, 101, 101970. [Google Scholar] [CrossRef] [PubMed]
- Brandes, A.; Dunning, M.; Langland, J. Antimicrobial Activity of Individual Volatile Compounds from Various Essential Oils. Molecules 2024, 29, 1811. [Google Scholar] [CrossRef]
- Adaszyńska-Skwirzyńska, M.; Szczerbińska, D. The antimicrobial activity of lavender essential oil (Lavandula angustifolia) and its influence on the production performance of broiler chickens. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1020–1025. [Google Scholar] [CrossRef]
- Li, F.; Chen, X.; Xu, X.; Wang, L.; Yan, J.; Yu, Y.; Shan, X.; Zhang, R.; Xing, H.; Zhang, T.; et al. Alterations of intestinal mucosal barrier, cecal microbiota diversity, composition, and metabolites of yellow-feathered broilers under chronic corticosterone-induced stress: A possible mechanism underlying the anti-growth performance and glycolipid metabolism disorder. Microbiol. Spectr. 2024, 12, e0347323. [Google Scholar] [CrossRef]
- Ruan, D.; Jiang, J.; Huang, W.; Fouad, A.M.; El-Senousey, H.K.; Lin, X.; Zhang, S.; Sun, L.; Yan, S.; Jiang, Z.; et al. Integrated metabolomics and microbiome analysis reveal blended oil diet improves meat quality of broiler chickens by modulating flavor and gut microbiota. Anim. Nutr. 2014, 19, 453–465. [Google Scholar] [CrossRef]
- Waters, J.L.; Ley, R.E. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 2019, 17, 83. [Google Scholar] [CrossRef]
- Tavella, T.; Rampelli, S.; Guidarelli, G.; Bazzocchi, A.; Gasperini, C.; Pujos-Guillot, E.; Comte, B.; Barone, M.; Biagi, E.; Candela, M.; et al. Elevated gut microbiome abundance of Christensenellaceae, Porphyromonadaceae and Rikenellaceae is associated with reduced visceral adipose tissue and healthier metabolic profile in Italian elderly. Gut Microbes 2021, 13, 1–19. [Google Scholar] [CrossRef]
- Ma, Y.; Deng, X.; Yang, X.; Wang, J.; Li, T.; Hua, G.; Han, D.; Da, L.; Li, R.; Rong, W.; et al. Characteristics of bacterial microbiota in different intestinal segments of Aohan fine-wool sheep. Front. Microbiol. 2022, 13, 874536. [Google Scholar] [CrossRef]
- Shao, T.; Shao, L.; Li, H.; Xie, Z.; He, Z.; Wen, C. Combined signature of the fecal microbiome and metabolome in patients with gout. Front. Microbiol. 2017, 8, 268. [Google Scholar] [CrossRef] [PubMed]
- Zakham, F.; Pillonel, T.; Brunel, A.S.; Zambelli, P.Y.; Greub, G.; Croxatto, A.; Bertelli, C. Molecular diagnosis and enrichment culture identified a septic pseudoarthrosis due to an infection with Erysipelatoclostridium ramosum. Int. J. Infect Dis. 2019, 81, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; DiBaise, J.K.; Zuccolo, A.; Kudrna, D.; Braidotti, M.; Yu, Y.; Parameswaran, P.; Crowell, M.D.; Wing, R.; Rittmann, B.E.; et al. Human gut microbiota in obesity and after gastric bypass. Proc. Natl. Acad. Sci. USA 2009, 106, 2365–2370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, Y.; Mo, Q.; Kulyar, M.F.; He, Y.; Yao, W.; Quan, C.; Gong, S.; Li, F.; Fu, Y.; et al. Sodium butyrate ameliorates thiram-induced tibial dyschondroplasia and gut microbial dysbiosis in broiler chickens. Ecotoxicol. Environ. Saf. 2022, 245, 114134. [Google Scholar] [CrossRef]
- Chen, P.; Lv, H.; Du, M.; Liu, W.; Che, C.; Zhao, J.; Liu, H. Bacillus subtilis HW2 enhances growth performance and alleviates gut injury via attenuation of endoplasmic reticulum stress and regulation of gut microbiota in broilers under necrotic enteritis challenge. Poult. Sci. 2024, 103, 103661. [Google Scholar] [CrossRef]
- Xiao, G.; Zheng, L.; Yan, X.; Gong, L.; Yang, Y.; Qi, Q.; Zhang, X.; Zhang, H. Effects of Dietary Essential Oils Supplementation on Egg Quality, Biochemical Parameters, and Gut Microbiota of Late-Laying Hens. Animals 2022, 12, 2561. [Google Scholar] [CrossRef]
- Mokhtari, S.; Rahati, M.; Seidavi, A.; Haq, Q.M.I.; Kadim, I.; Laudadio, V.; Tufarelli, V. Effects of feed supplementation with lavender (Lavandula angustifolia) essence on growth performance, carcass traits, blood constituents and caecal microbiota of broiler chickens. Eur. Poult. Sci. 2018, 82, 1–11. [Google Scholar] [CrossRef]
- Özbilgin, A.; Mogulkoç, M.; Kara, K.; Urçar Gelen, S.; Karataş, Ö.; Ülger Özbek, D. Effects of Lavender (Lavandula angustifolia) Essential Oil on Fattening Performance, Meat Quality, Serum Antioxidant Enzymes, Gut Microbiota and Intestinal Histomorphology in Japanese Quails. Braz. J. Poult. Sci. 2023, 25, 1–16. [Google Scholar] [CrossRef]
- Jedrzejczak-Silicka, M.; Kordas, M.; Konopacki, M.; Rakoczy, R. The cell type-dependent response to the rotating magnetic field (RMF)—An in vitro wound healing study. In Chemical and Process Engineering for Environment and Health; Sosnowski, T.R., Szwast, M., Eds.; Publishing House of Lukasiewicz—Institute of Substantial Technology: Radom, Poland, 2020; pp. 122–133. [Google Scholar]
- Aleksandrzak, M.; Jedrzejczak-Silicka, M.; Sielicki, K.; Piotrowska, K.; Mijowska, E. Size-Dependent in vitro biocompatibility and uptake process of polymeric carbon nitride. ACS Appl. Mater. Interfaces 2019, 11, 47739–47749. [Google Scholar] [CrossRef]
- Verrax, J.; Buc, C.P. Comparison between the TC10™ automated cell counter and the lactate dehydrogenase (LDH) assay to assess cellular toxicity in vitro. Life Sci. Group 2011, 6087, 11-01230911. [Google Scholar]
- Aviagen, R. Ross Broiler Management Handbook; Aviagen Group: Huntsville, AL, USA, 2019. [Google Scholar]

| Compound | RI 1 (RI Ref) 2 | Percentage (Relative Peak Area) ± SD 3 |
|---|---|---|
| α-Pinene | 934 (935) | 0.81 ± 0.09 |
| Camphene | 946 (950) | 0.45 ± 0.05 |
| β-Pinene | 976 (977) | 0.65 ± 0.08 |
| β-Myrcene | 989 (989) | 0.58 ± 0.01 |
| Hexyl acetate | 1008 (1010) | 0.61 ± 0.06 |
| δ-3-Carene | 1010 (1011) | 0.20 ± 0.01 |
| p-Cymene | 1022 (1024) | 0.85 ± 0.05 |
| Limonene | 1028 (1029) | 0.15 ± 0.06 |
| Eucalyptol | 1031 (1031) | 1.75 ± 0.08 |
| (Z)-β-Ocimene | 1037 (1038) | 2.70 ± 0.21 |
| (E)-β-Ocimene | 1047 (1048) | 0.10 ± 0.01 |
| Linalool oxide | 1074 (1075) | 1.14 ± 0.01 |
| Linalool | 1101 (1099) | 31.55 ± 0.91 |
| Camphor | 1145 (1144) | 0.35 ± 0.02 |
| Borneol | 1165 (1166) | 1.85 ± 0.10 |
| 4-Terpineol | 1177 (1177) | 2.05 ± 0.05 |
| p-Cymen-8-ol | 1183 (1184) | 0.50 ± 0.01 |
| α-Terpineol | 1191 (1191) | 0.35 ± 0.02 |
| Linalyl acetate | 1256 (1256) | 41.20 ± 1.01 |
| Bornyl acetate | 1280 (1282) | 0.10 ± 0.01 |
| Lavandulyl acetate | 1289 (1291) | 2.22 ± 0.02 |
| Cuminic alcohol | 1293 (1295) | 0.10 ± 0.01 |
| Neryl acetate | 1362 (1364) | 0.80 ± 0.09 |
| Geranyl acetate | 1379 (1380) | 0.19 ± 0.01 |
| α-Copaene | 1381 (1381) | 0.14 ± 0.04 |
| β-Caryophyllene | 1419 (1420) | 2.59 ± 0.10 |
| (E)-β-Farnesene | 1455 (1456) | 0.15 ± 0.01 |
| Caryophyllene oxide | 1577 (1577) | 2.15 ± 0.11 |
| Epi-bicyclosesquiphellandrene | 1641 (1642) | 0.81 ± 0.15 |
| Day 3 | Group 1 | SEM 4 | p-Value 2 | ||||
|---|---|---|---|---|---|---|---|
| C | H | HE | C vs. H | H vs. HE | HE vs. C | ||
| BW (g/bird) | |||||||
| 1 | 43.31 | 43.15 | 43.26 | 0.07 | 1.000 | 1.000 | 1.000 |
| 10 | 279.10 | 275.55 | 293.12 | 5.14 | 0.55 | 0.023 | 0.001 |
| 35 | 2119.63 | 2121.20 | 2250.51 | 14.61 | 0.67 | 0.041 | 0.001 |
| BWG (g/bird) | |||||||
| 1–10 | 235.79 | 232.40 | 250.0 | 4.15 | 0.662 | 0.031 | 0.038 |
| 11–35 | 1840.53 | 1845.65 | 1957.39 | 15.8 | 0.891 | 0.036 | 0.021 |
| 1–35 | 2076.32 | 2078.05 | 2207.25 | 21.51 | 0.910 | 0.001 | 0.001 |
| FI (g/bird) | |||||||
| 1–10 | 264.30 | 262.9 | 265.50 | 1.89 | 0.922 | 0.988 | 0.942 |
| 11–35 | 2613.5 | 2599.60 | 2609.20 | 9.21 | 0.923 | 0.989 | 0.983 |
| 1–35 | 2877.8 | 2862.5 | 2874.7 | 13.41 | 0.899 | 0.933 | 0.934 |
| FCR (g/g) | |||||||
| 1–10 | 1.12 | 1.13 | 1.06 | 0.03 | 1.000 | 0.047 | 0.046 |
| 11–35 | 1.42 | 1.41 | 1.33 | 0.04 | 1.000 | 0.021 | 0.020 |
| 1–35 | 1.39 | 1.38 | 1.30 | 0.05 | 1.000 | 0.034 | 0.031 |
| WI (mL/bird) | |||||||
| 1–10 | 684.70 | 665.80 | 660.43 | 7.56 | 0.049 | 0.56 | 0.039 |
| 11–35 | 5329.3 | 5369.7 | 5383.38 | 18.55 | 0.892 | 0.864 | 0.877 |
| 1–35 | 6021.0 | 6035.5 | 6033.81 | 20.87 | 0.874 | 0.799 | 0.768 |
| p-Value 2 | Effect Size | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | C 1 | H | HE | SEM 3 | C vs. H | H vs. HE | HE vs. C | |
| Chao1 | 62.0 | 60.0 | 55.8 | 0.93 | 0.856 | 0.170 | 0.023 | 0.43 |
| Fisher alpha | 8.0 | 7.9 | 7.3 | 0.11 | 0.947 | 0.178 | 0.025 | 0.39 |
| Shannon | 3.0 | 2.8 | 2.8 | 0.05 | 0.178 | 0.947 | 0.116 | 0.24 |
| Simpson | 0.9 | 0.9 | 0.9 | 0.01 | 0.363 | 0.861 | 0.363 | 0.06 |
| Group 1 | p-Value 2 | Effect Size | ||||||
|---|---|---|---|---|---|---|---|---|
| Item | C | H | HE | SEM 3 | C vs. H | H vs. HE | HE vs. C | |
| Phylum | ||||||||
| Firmcutes | 75.7 | 66.5 | 67.9 | 2.28 | 0.363 | 0.947 | 0.615 | −0.01 |
| Bacteroidota | 17.9 | 28.4 | 22.8 | 2.01 | 0.363 | 0.363 | 0.363 | 0.11 |
| Proteobacteria | 4.3 | 2.3 | 7.8 | 1.81 | 0.745 | 0.484 | 0.861 | −0.05 |
| Desulfobacterota | 1.8 | 1.9 | 1.1 | 0.20 | 0.947 | 0.178 | 0.363 | 0.13 |
| Cyanobacteria | 0.3 | 0.8 | 0.4 | 0.08 | 0.025 | 0.043 | 0.484 | 0.61 |
| Genus | ||||||||
| Bacteroides | 17.9 | 28.4 | 22.8 | 2.01 | 0.363 | 0.363 | 0.363 | 0.11 |
| Faecalibacterium | 15.6 | 12.1 | 11.8 | 0.98 | 0.60 | 0.994 | 0.363 | 0.08 |
| RF39 | 7.1 | 5.8 | 8.2 | 0.60 | 0.861 | 0.260 | 0.615 | 0.04 |
| Lactobacillus | 7.4 | 7.1 | 4.8 | 0.77 | 0.947 | 0.484 | 0.484 | −0.01 |
| Clostridia_vadinBB60_group | 4.8 | 4.8 | 5.9 | 0.46 | 0.947 | 0.484 | 0.484 | −0.01 |
| Lachnospiraceae | 4.3 | 5.9 | 4.7 | 0.65 | 0.947 | 0.484 | 0.615 | −0.05 |
| Escherichia-Shigella | 4.3 | 2.3 | 7.8 | 1.81 | 0.745 | 0.484 | 0.861 | −0.01 |
| Clostridia_UCG-014 | 4.9 | 3.9 | 4.6 | 0.33 | 0.363 | 0.615 | 0.947 | 0.23 |
| Blautia | 3.2 | 1.6 | 2.4 | 0.36 | 0.260 | 0.484 | 0.615 | 0.10 |
| CHKCI001 | 1.0 | 2.1 | 4.0 | 0.58 | 0.745 | 0.484 | 0.178 | 0.12 |
| Christensenellaceae_R-7_group | 0.9 | 2.4 | 3.8 | 0.47 | 0.615 | 0.363 | 0.025 | 0.38 |
| Butyricicoccus | 2.7 | 2.3 | 1.6 | 0.28 | 0.745 | 0.484 | 0.484 | 0.01 |
| Bilophila | 1.6 | 1.8 | 1.0 | 0.18 | 0.861 | 0.116 | 0.363 | 0.18 |
| UCG-005 | 1.8 | 1.2 | 1.0 | 0.19 | 0.745 | 0.260 | 0.615 | 0.06 |
| Ruminococcus_torques_group | 1.4 | 1.5 | 0.7 | 0.18 | 0.861 | 0.178 | 0.072 | 0.29 |
| Erysipelatoclostridium | 1.8 | 1.2 | 0.7 | 0.14 | 0.116 | 0.025 | 0.025 | 0.76 |
| Fusicatenibacter | 2.3 | 0.6 | 0.5 | 0.30 | 0.025 | 0.861 | 0.025 | 0.63 |
| Incertae_Sedis | 1.0 | 0.9 | 0.7 | 0.08 | 0.947 | 0.615 | 0.861 | −0.11 |
| Bacillus | 0.7 | 0.9 | 0.9 | 0.18 | 0.745 | 0.484 | 0.615 | −0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adaszyńska-Skwirzyńska, M.; Yu, Y.-H.; Konieczka, P.; Kozłowski, K.; Witkowska, D.; Dybus, A.; Hukowska-Szematowicz, B.; Jędrzejczak-Silicka, M.; Bucław, M.; Bartkowiak, A. Chemical Composition, Cytotoxicity, and Encapsulation of Lavender Essential Oil (Lavandula angustifolia) in Alginate Hydrogel—Application and Therapeutic Effect on Animal Model. Molecules 2025, 30, 2931. https://doi.org/10.3390/molecules30142931
Adaszyńska-Skwirzyńska M, Yu Y-H, Konieczka P, Kozłowski K, Witkowska D, Dybus A, Hukowska-Szematowicz B, Jędrzejczak-Silicka M, Bucław M, Bartkowiak A. Chemical Composition, Cytotoxicity, and Encapsulation of Lavender Essential Oil (Lavandula angustifolia) in Alginate Hydrogel—Application and Therapeutic Effect on Animal Model. Molecules. 2025; 30(14):2931. https://doi.org/10.3390/molecules30142931
Chicago/Turabian StyleAdaszyńska-Skwirzyńska, Michalina, Yu-Hsiang Yu, Paweł Konieczka, Krzysztof Kozłowski, Dorota Witkowska, Andrzej Dybus, Beata Hukowska-Szematowicz, Magdalena Jędrzejczak-Silicka, Mateusz Bucław, and Artur Bartkowiak. 2025. "Chemical Composition, Cytotoxicity, and Encapsulation of Lavender Essential Oil (Lavandula angustifolia) in Alginate Hydrogel—Application and Therapeutic Effect on Animal Model" Molecules 30, no. 14: 2931. https://doi.org/10.3390/molecules30142931
APA StyleAdaszyńska-Skwirzyńska, M., Yu, Y.-H., Konieczka, P., Kozłowski, K., Witkowska, D., Dybus, A., Hukowska-Szematowicz, B., Jędrzejczak-Silicka, M., Bucław, M., & Bartkowiak, A. (2025). Chemical Composition, Cytotoxicity, and Encapsulation of Lavender Essential Oil (Lavandula angustifolia) in Alginate Hydrogel—Application and Therapeutic Effect on Animal Model. Molecules, 30(14), 2931. https://doi.org/10.3390/molecules30142931









