A Supramolecular Extension of Mosher’s Method: Absolute Configuration Assignment of N-Amino Acid Derivatives via Bis-Thiourea Chiral Solvating Agent
Abstract
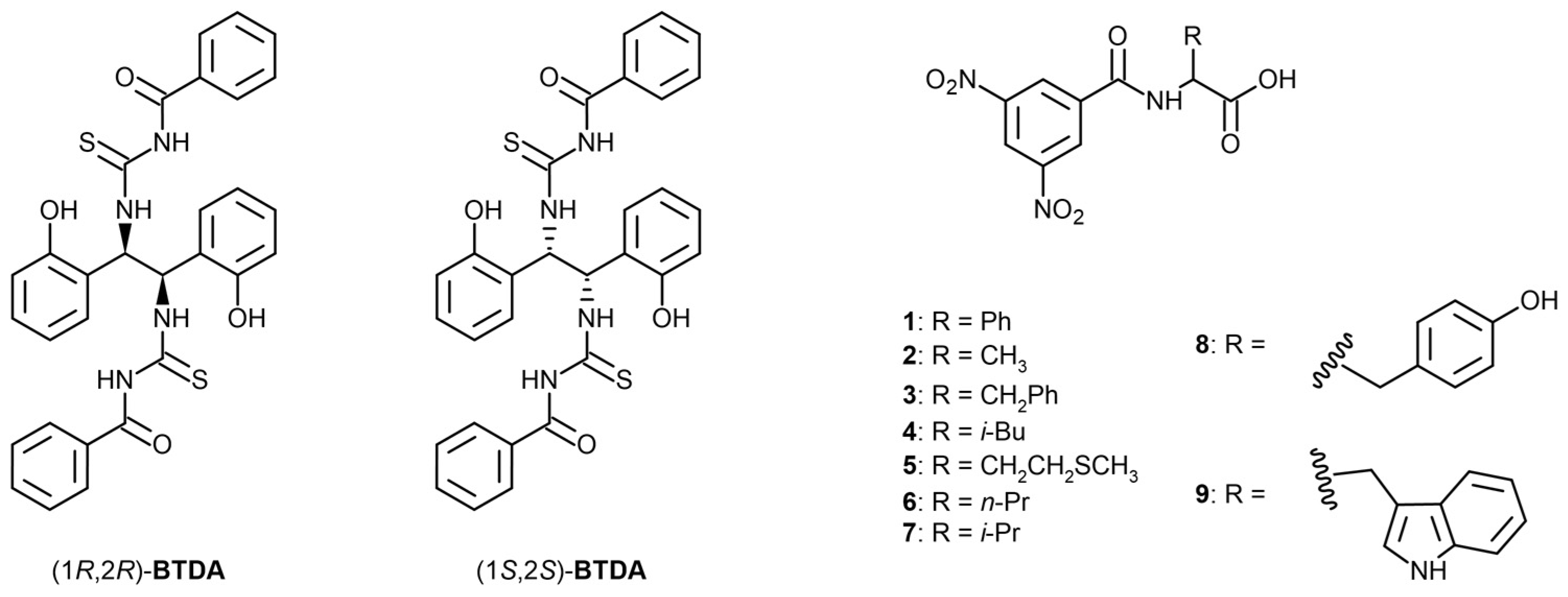
1. Introduction
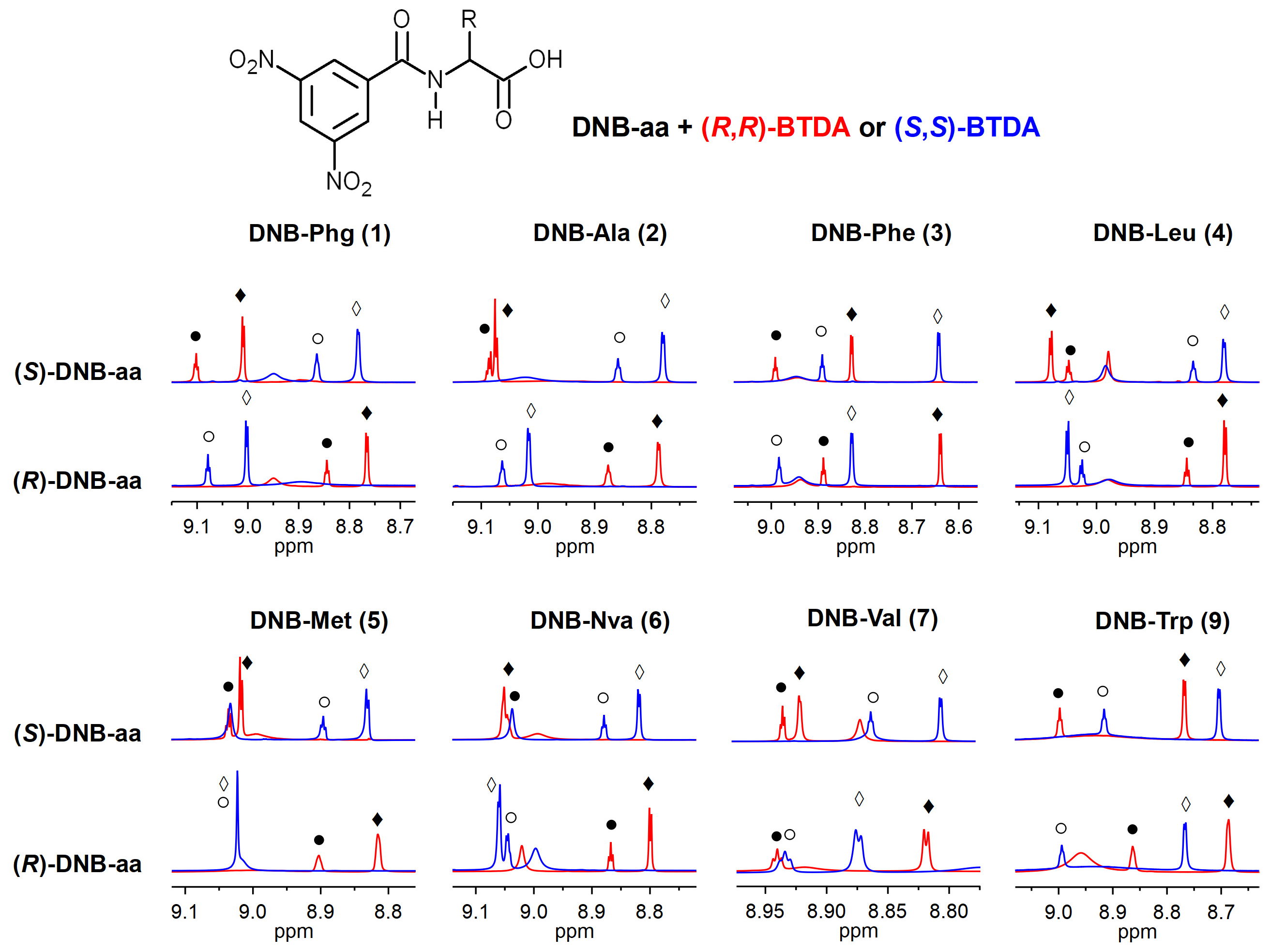
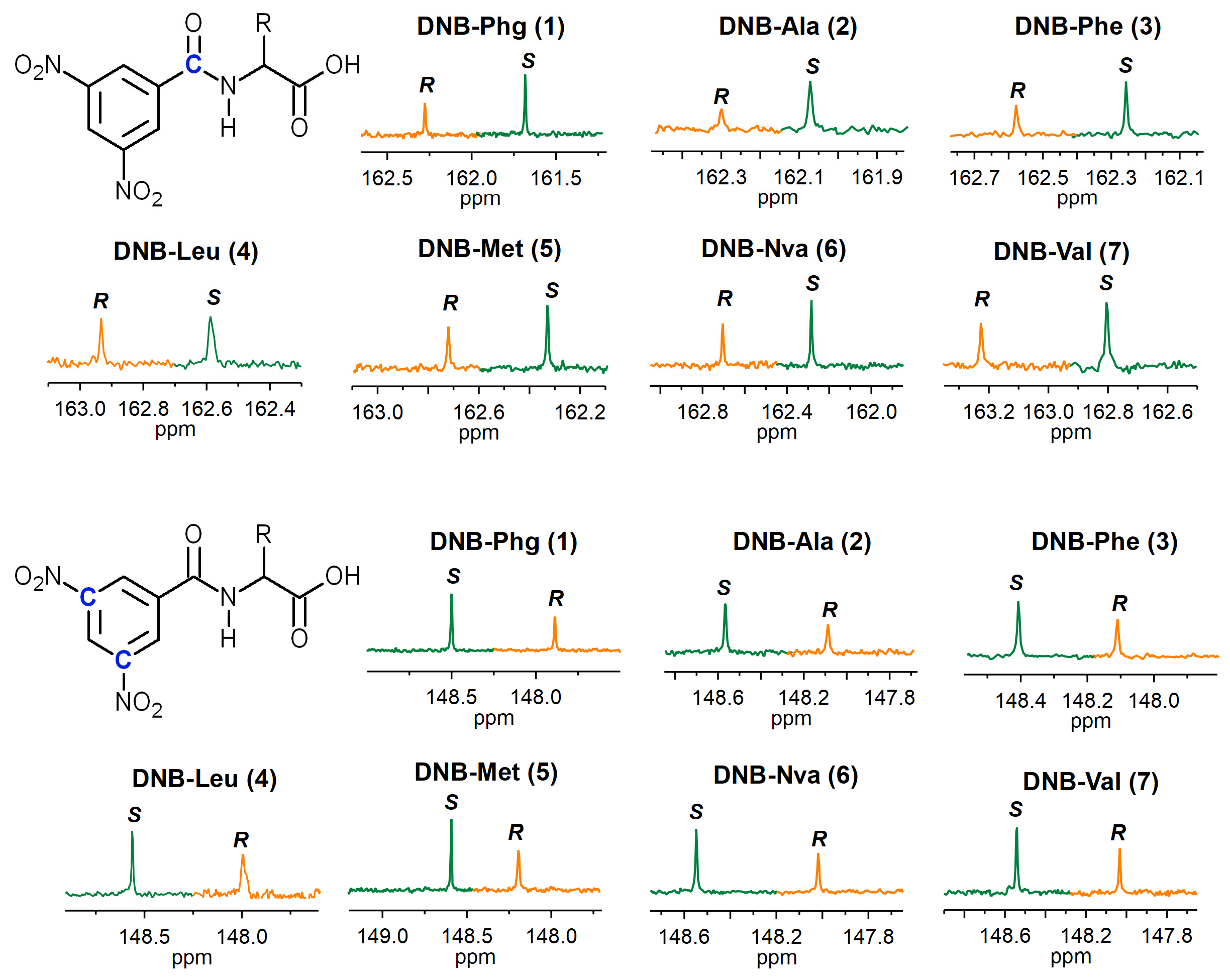
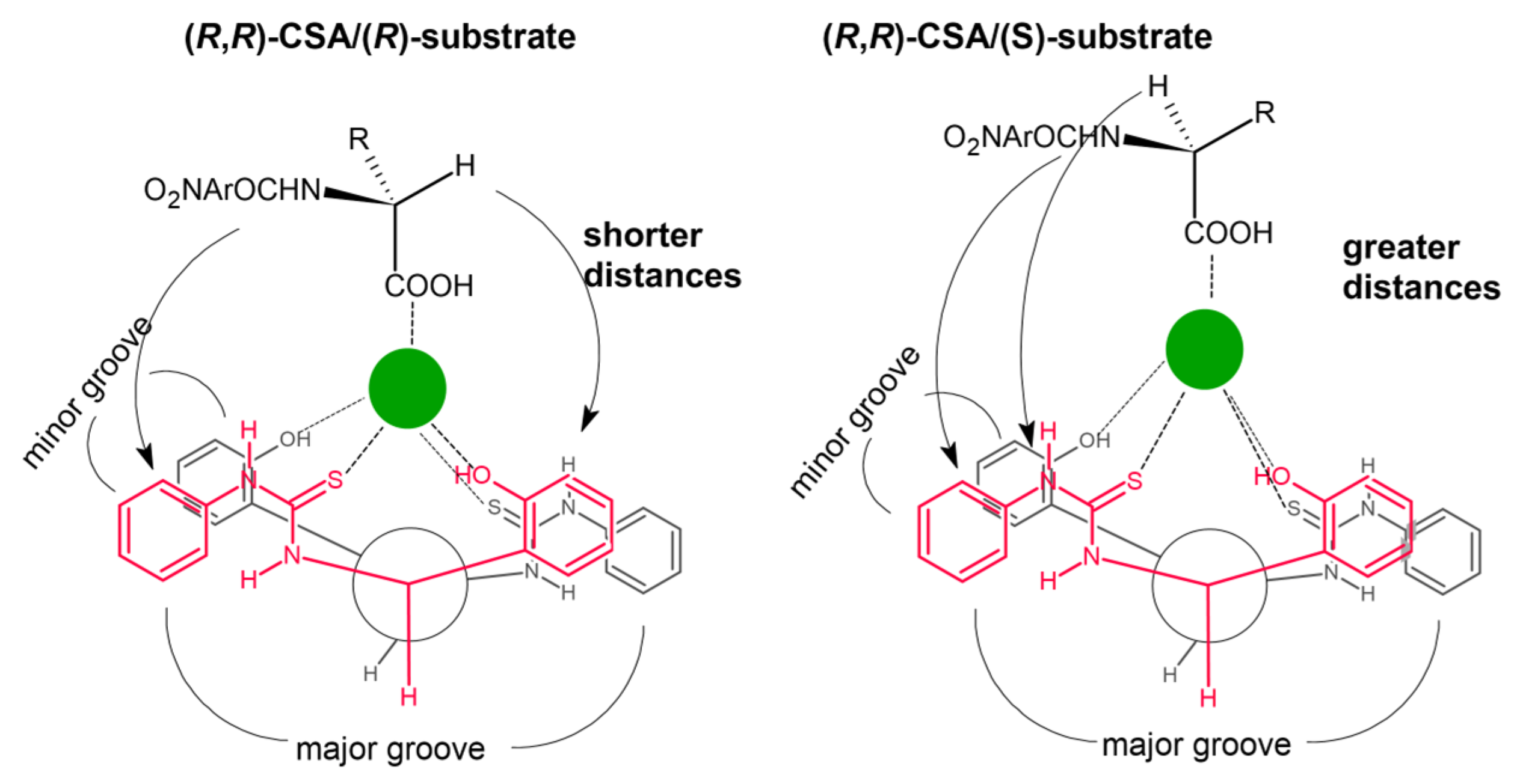
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Methods
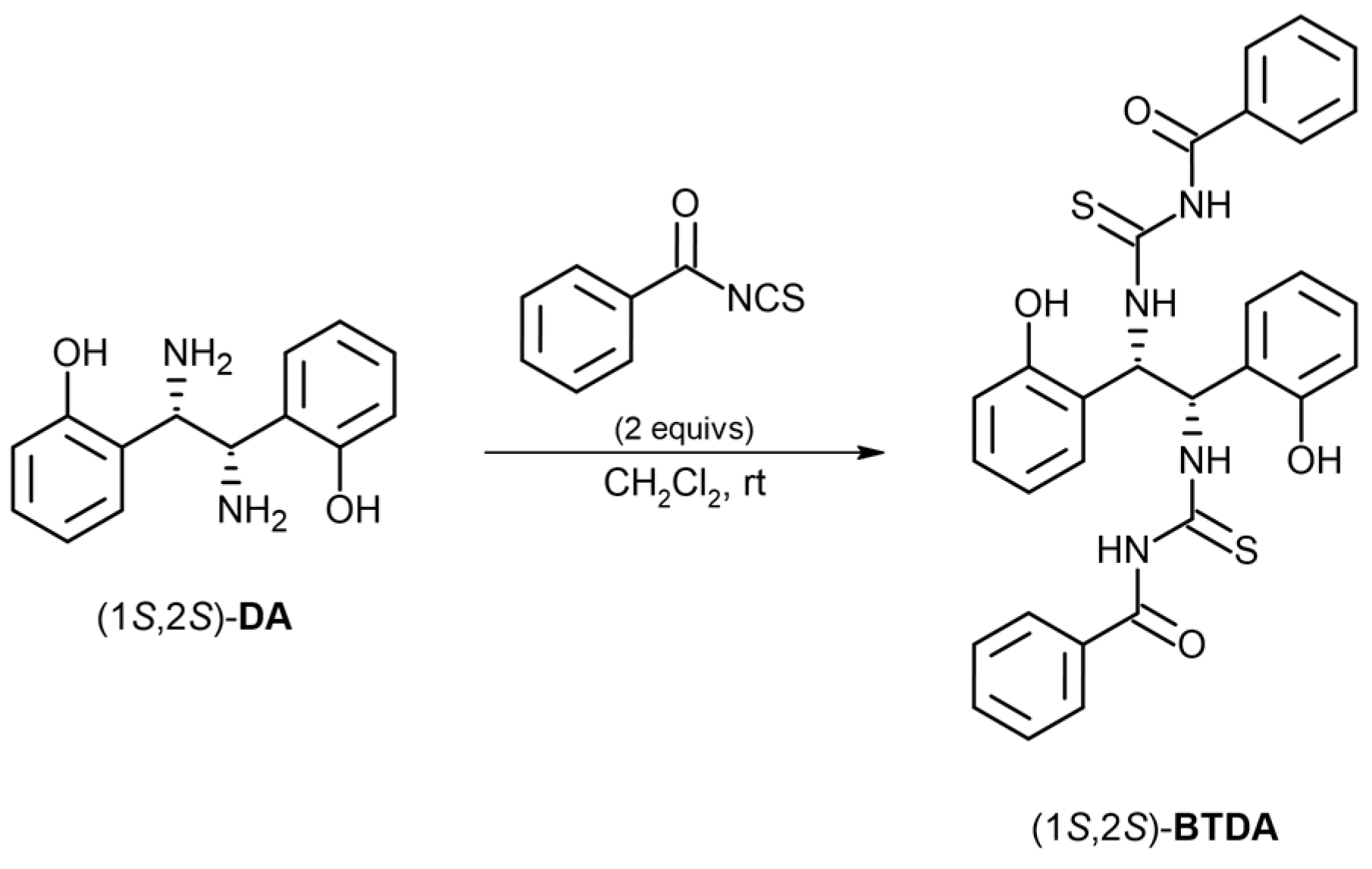
3.3. Synthesis of (S,S)-BTDA
3.4. Sample Preparation for the NMR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bada, J.L. Racemization of Amino Acids. In Chemistry and Biochemistry of the Amino Acids; Barrett, G.C., Ed.; Springer: Dordrecht, The Netherlands, 1985; pp. 399–414. ISBN 978-94-009-4832-7. [Google Scholar]
- Duengo, S.; Muhajir, M.I.; Hidayat, A.T.; Musa, W.J.A.; Maharani, R. Epimerisation in Peptide Synthesis. Molecules 2023, 28, 8017. [Google Scholar] [CrossRef] [PubMed]
- McCudden, C.R.; Kraus, V.B. Biochemistry of Amino Acid Racemization and Clinical Application to Musculoskeletal Disease. Clin. Biochem. 2006, 39, 1112–1130. [Google Scholar] [CrossRef]
- Bhushan, R. Enantioselective and Chemoselective Optical Detection of Chiral Organic Compounds without Resorting to Chromatography. Chem.-Asian J. 2023, 18, e202300825. [Google Scholar] [CrossRef]
- Peluso, P.; Chankvetadze, B. Recognition in the Domain of Molecular Chirality: From Noncovalent Interactions to Separation of Enantiomers. Chem. Rev. 2022, 122, 13235–13400. [Google Scholar] [CrossRef]
- Vargas-Caporali, J.; Juaristi, E. Fundamental Developments of Chiral Phase Chromatography in Connection with Enantioselective Synthesis of β-Amino Acids. Isr. J. Chem. 2017, 57, 896–912. [Google Scholar] [CrossRef]
- Benfodda, Z.; Bénimélis, D.; Jean, M.; Naubron, J.-V.; Rolland, V.; Meffre, P. Synthesis, Resolution, and Determination of Absolute Configuration of Protected α-Ethynylphenylalanine Enantiomers. Amino Acids 2015, 47, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Reason, A.J. Validation of Amino Acid Analysis Methods. In The Protein Protocols Handbook; Walker, J.M., Ed.; Springer Protocols Handbooks; Humana Press: Totowa, NJ, USA, 2009; pp. 1015–1028. ISBN 978-1-59745-198-7. [Google Scholar]
- Nazareth, P.M.P.; Antunes, O.A.C. Chiral Ligand Exchange Chromatography: Separation of Enantiomeric Mixtures of Underivatized α-Amino Acids under UV Detection. J. Braz. Chem. Soc. 2002, 13, 658–663. [Google Scholar] [CrossRef][Green Version]
- Yang, L.; Luo, F.; Wei, W. Simultaneous Determination of the Concentration and Enantiomeric Excess of Amino Acids with a Coumarin-Derived Achiral Probe. Anal. Methods 2021, 13, 1905–1910. [Google Scholar] [CrossRef]
- Hahn, D.; Wang, W.; Choi, H.; Kang, H. Determination of Sequence and Absolute Configuration of Peptide Amino Acids by HPLC–MS/CD-Based Detection of Liberated N-Terminus Phenylthiohydantoin Amino Acids. Sci. Rep. 2022, 12, 10285. [Google Scholar] [CrossRef]
- Nelson, E.; Formen, J.S.S.K.; Wolf, C. Rapid Organocatalytic Chirality Analysis of Amines, Amino Acids, Alcohols, Amino Alcohols and Diols with Achiral Iso(Thio)Cyanate Probes. Chem. Sci. 2021, 12, 8784–8790. [Google Scholar] [CrossRef]
- Jin, Q.; Wang, F.; Chen, S.; Zhou, L.; Jiang, H.; Zhang, L.; Liu, M. Circularly Polarized Luminescence of Aluminum Complexes for Chiral Sensing of Amino Acid and Amino Alcohol. Chem.-Asian J. 2020, 15, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.C.; Santos, Z.A.D.l.; Wolf, C. Chiroptical Sensing of Unprotected Amino Acids, Hydroxy Acids, Amino Alcohols, Amines and Carboxylic Acids with Metal Salts. Chem. Commun. 2019, 55, 6297–6300. [Google Scholar] [CrossRef]
- Thanzeel, F.Y.; Sripada, A.; Wolf, C. Quantitative Chiroptical Sensing of Free Amino Acids, Biothiols, Amines, and Amino Alcohols with an Aryl Fluoride Probe. J. Am. Chem. Soc. 2019, 141, 16382–16387. [Google Scholar] [CrossRef] [PubMed]
- Raikar, P.; Bannimath, G. Recent Trends in Chiral Separation-A Collective Paradigm of Selected Chiral Impurities. Curr. Pharm. Anal. 2020, 16, 456–473. [Google Scholar] [CrossRef]
- Gumustas, M.; Ozkan, S.A.; Chankvetadze, B. Analytical and Preparative Scale Separation of Enantiomers of Chiral Drugs by Chromatography and Related Methods. Curr. Med. Chem. 2018, 25, 4152–4188. [Google Scholar] [CrossRef]
- Tok, K.C.; Gumustas, M.; Jibuti, G.; Suzen, H.S.; Ozkan, S.A.; Chankvetadze, B. The Effect of Enantiomer Elution Order on the Determination of Minor Enantiomeric Impurity in Ketoprofen and Enantiomeric Purity Evaluation of Commercially Available Dexketoprofen Formulations. Molecules 2020, 25, 5865. [Google Scholar] [CrossRef] [PubMed]
- B’Hymer, C.; Montes-Bayon, M.; Caruso, J.A. Marfey’s Reagent: Past, Present, and Future Uses of 1-Fluoro-2,4-Dinitrophenyl-5-L-Alanine Amide. J. Sep. Sci. 2003, 26, 7–19. [Google Scholar] [CrossRef]
- Marfey, P. Determination of D-Amino Acids. II. Use of a Bifunctional Reagent, 1,5-Difluoro-2,4-Dinitrobenzene. Carlsberg Res. Commun. 1984, 49, 591. [Google Scholar] [CrossRef]
- Wenzel, T.J. Strategies for Using NMR Spectroscopy to Determine Absolute Configuration. Tetrahedron Asymmetry 2017, 28, 1212–1219. [Google Scholar] [CrossRef]
- Lima, Y.R.; Peglow, T.J.; Nobre, P.C.; Campos, P.T.; Perin, G.; Lenardão, E.J.; Silva, M.S. Chalcogen-Containing Diols: A Novel Chiral Derivatizing Agent for 77Se and 125Te NMR Chiral Recognition of Primary Amines. Chem. Select 2019, 4, 4797–4803. [Google Scholar] [CrossRef]
- Yang, S.; Bian, G.; Sa, R.; Song, L. Assigning the Absolute Configurations of Chiral Primary Amines Based on Experimental and DFT-Calculated 19F Nuclear Magnetic Resonance. Front. Chem. 2019, 7, 318. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Xu, L.; Wang, N.; Ying, J.; Zhao, Y.; Huang, S. Trans-4-Fluoro-l-Proline: A Sensitive 19F NMR Probe for the Rapid Simultaneous Enantiomeric Analysis of Multicomponent Amines. Anal. Chem. 2022, 94, 1867–1873. [Google Scholar] [CrossRef]
- Kriegelstein, M.; Profous, D.; Přibylka, A.; Lyčka, A.; Cankař, P. Limitations of Trifluoromethylbenzoimidazolylbenzoic Acid as a Chiral Derivatizing Agent to Assign Absolute Configuration for β-Chiral Aminoalcohols. ACS Omega 2022, 7, 12734–12746. [Google Scholar] [CrossRef]
- Rithchumpon, P.; Khamto, N.; Kongsak, C.; Saema, W.; Duangdesh, S.; Choommongkol, V.; Meepowpan, P. Application of Methyl Itaconate–Anthracene Adducts (MIAs) in Configuration Assignment of Chiral Primary Amines by Means of 1H NMR. Tetrahedron 2023, 143, 133545. [Google Scholar] [CrossRef]
- Torales, E.; Fomine, S.; Cárdenas, J. Assignment of the Absolute Configuration of Secondary Alcohols by 13C NMR and Its Correlation with Methyl-1-(Chloromethyl)-Oxopyrrolidine-2-Carboxylate and Quantum-Mechanical GIAO Calculations. New J. Chem. 2023, 47, 9222–9228. [Google Scholar] [CrossRef]
- Lesot, P.; Gil, R.R. Anisotropic One-Dimensional/Two-Dimensional NMR in Molecular Analysis. In Two-Dimensional (2D) NMR Methods; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2023; pp. 209–296. ISBN 978-1-119-80672-1. [Google Scholar]
- Aroulanda, C.; Lesot, P. Molecular Enantiodiscrimination by NMR Spectroscopy in Chiral Oriented Systems: Concept, Tools, and Applications. Chirality 2022, 34, 182–244. [Google Scholar] [CrossRef]
- Uccello Barretta, G.; Wenzel, T.J.; Balzano, F. Spectroscopic Analysis: NMR and Shift Reagents. In Comprehensive Chirality, 2nd ed.; Cossy, J., Ed.; Academic Press: Oxford, UK, 2024; pp. 560–592. ISBN 978-0-323-90645-6. [Google Scholar]
- Balzano, F.; Uccello-Barretta, G.; Aiello, F. Chiral Analysis by NMR Spectroscopy: Chiral Solvating Agents. In Chiral Analysis, 2nd ed.; Polavarapu, P.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 367–427. ISBN 978-0-444-64027-7. [Google Scholar]
- Wenzel, T.J.; Wilcox, J.D. Chiral Reagents for the Determination of Enantiomeric Excess and Absolute Configuration Using NMR Spectroscopy. Chirality 2003, 15, 256–270. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Park, H.; Duong, Q.H.; Kwahk, E.-J.; Kim, H. Determining the Enantiomeric Excess and Absolute Configuration of In Situ Fluorine-Labeled Amines and Alcohols by 19F NMR Spectroscopy. Anal. Chem. 2022, 94, 1441–1446. [Google Scholar] [CrossRef]
- Ritter, K.; Jork, N.; Unmüßig, A.-S.; Köhn, M.; Jessen, H.J. Assigning the Absolute Configuration of Inositol Poly- and Pyrophosphates by NMR Using a Single Chiral Solvating Agent. Biomolecules 2023, 13, 1150. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, H.; Yang, C.; Fang, L.; Zheng, L.; Lv, H.; Stavropoulos, P.; Ai, L.; Zhang, J. Chiral Recognition of Chiral (Hetero)Cyclic Derivatives Probed by Tetraaza Macrocyclic Chiral Solvating Agents via 1H NMR Spectroscopy. Anal. Chem. 2024, 96, 5188–5194. [Google Scholar] [CrossRef]
- Cabral, T.L.G.; Dal Poggetto, G.; Brussolo da Silva, J.P.; Nilsson, M.; Tormena, C.F. Determining the Absolute Configuration of Small Molecules by Diffusion NMR Experiments. Angew. Chem. Int. Ed. 2025, 64, e202418508. [Google Scholar] [CrossRef]
- Shi, S.-H.; Wang, X.-J.; Gao, Y.-Y.; Wang, T.; Wang, C.-Y.; Wang, Z.; Wang, Q.; Li, F.; Li, G.-W. DACH-Based Chiral Sensing Platforms as Tunable Benzamide-Chiral Solvating Agents for NMR Enantioselective Discrimination. Anal. Chem. 2025, 97, 1900–1908. [Google Scholar] [CrossRef] [PubMed]
- Spiaggia, F.; Uccello Barretta, G.; Iuliano, A.; Baldassari, C.; Aiello, F.; Balzano, F. A Squaramide-Based Organocatalyst as a Novel Versatile Chiral Solvating Agent for Carboxylic Acids. Molecules 2024, 29, 2389. [Google Scholar] [CrossRef] [PubMed]
- Recchimurzo, A.; Maccabruni, F.; Uccello Barretta, G.; Balzano, F. Quinine as a Highly Responsive Chiral Sensor for the 1H and 19F NMR Enantiodiscrimination of N-Trifluoroacetyl Amino Acids with Free Carboxyl Functions. Analyst 2022, 147, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Cefalì, F.; Iuliano, A.; Balzano, F.; Uccello Barretta, G.; Zullo, V.; Baldassari, C. Isohexide-Based Tunable Chiral Platforms as Amide- and Thiourea-Chiral Solvating Agents for the NMR Enantiodiscrimination of Derivatized Amino Acids. Molecules 2024, 29, 1307. [Google Scholar] [CrossRef]
- Chen, Z.; Fan, H.; Yang, S.; Bian, G.; Song, L. Chiral Sensors for Determining the Absolute Configurations of α-Amino Acid Derivatives. Org. Biomol. Chem. 2018, 16, 8311–8317. [Google Scholar] [CrossRef]
- Recchimurzo, A.; Micheletti, C.; Uccello-Barretta, G.; Balzano, F. Thiourea Derivative of 2-[(1R)-1-Aminoethyl]Phenol: A Flexible Pocket-like Chiral Solvating Agent (CSA) for the Enantiodifferentiation of Amino Acid Derivatives by NMR Spectroscopy. J. Org. Chem. 2020, 85, 5342–5350. [Google Scholar] [CrossRef]
- Recchimurzo, A.; Micheletti, C.; Uccello-Barretta, G.; Balzano, F. A Dimeric Thiourea CSA for the Enantiodiscrimination of Amino Acid Derivatives by NMR Spectroscopy. J. Org. Chem. 2021, 86, 7381–7389. [Google Scholar] [CrossRef]
- Dale, J.A.; Mosher, H.S. Nuclear Magnetic Resonance Enantiomer Regents. Configurational Correlations via Nuclear Magnetic Resonance Chemical Shifts of Diastereomeric Mandelate, O-Methylmandelate, and MethoxyTrifluoromethylphenylacetate (MTPA) Esters. J. Am. Chem. Soc. 1973, 95, 512–519. [Google Scholar] [CrossRef]
- Aiello, F.; Recchimurzo, A.; Balzano, F.; Uccello Barretta, G.; Cefalì, F. A Thiourea Derivative of 2-[(1R)-1-Aminoethyl]Phenol as a Chiral Sensor for the Determination of the Absolute Configuration of N-3,5-Dinitrobenzoyl Derivatives of Amino Acids. Molecules 2024, 29, 1319. [Google Scholar] [CrossRef]
- Uccello-Barretta, G.; Balzano, F.; Caporusso, A.M.; Iodice, A.; Salvadori, P. Permethylated β-Cyclodextrin as Chiral Solvating Agent for the NMR Assignment of the Absolute Configuration of Chiral Trisubstituted Allenes. J. Org. Chem. 1995, 60, 2227–2231. [Google Scholar] [CrossRef]


| Substrate | p-DNB | o-DNB | CH-α | Substrate | p-DNB | o-DNB | CH-α |
|---|---|---|---|---|---|---|---|
| (R)-1 | −0.234 | −0.236 | 0.071 | (S)-1 | 0.238 | 0.227 | −0.097 |
| (R)-2 | −0.186 | −0.292 | 0.115 | (S)-2 | 0.212 | 0.275 | −0.107 |
| (R)-3 | −0.095 | −0.189 | 0.052 | (S)-3 | 0.101 | 0.186 | −0.053 |
| (R)-4 | −0.181 | −0.271 | 0.076 | (S)-4 | 0.215 | 0.299 | −0.032 |
| (R)-5 | −0.118 | −0.206 | 0.043 | (S)-5 | 0.138 | 0.184 | −0.090 |
| (R)-6 | −0.177 | −0.261 | 0.080 | (S)-6 | 0.165 | 0.236 | −0.097 |
| (R)-7 | −0.192 | −0.117 | 0.080 | (S)-7 | 0.241 | 0.150 | −0.072 |
| (R)-9 | −0.132 | −0.080 | 0.130 | (S)-9 | 0.082 | 0.064 | −0.070 |
| Substrate | KR | KS |
|---|---|---|
| 1 | 297 ± 13 1 | 54 ± 3 1 |
| 2 | 161 ± 6 | 72 ± 11 |
| 3 | 114 ± 3 | 35 ± 1 |
| 4 | 266 ± 19 | 10 ± 2 |
| 5 | 100 ± 8 | 58 ± 6 |
| 6 | 137 ± 7 | 7 ± 1 |
| 7 | 227 ± 5 | 58 ± 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rondinini, V.; Aiello, F.; Cefalì, F.; Recchimurzo, A.; Uccello Barretta, G.; Balzano, F. A Supramolecular Extension of Mosher’s Method: Absolute Configuration Assignment of N-Amino Acid Derivatives via Bis-Thiourea Chiral Solvating Agent. Molecules 2025, 30, 2930. https://doi.org/10.3390/molecules30142930
Rondinini V, Aiello F, Cefalì F, Recchimurzo A, Uccello Barretta G, Balzano F. A Supramolecular Extension of Mosher’s Method: Absolute Configuration Assignment of N-Amino Acid Derivatives via Bis-Thiourea Chiral Solvating Agent. Molecules. 2025; 30(14):2930. https://doi.org/10.3390/molecules30142930
Chicago/Turabian StyleRondinini, Virginia, Federica Aiello, Federica Cefalì, Alessandra Recchimurzo, Gloria Uccello Barretta, and Federica Balzano. 2025. "A Supramolecular Extension of Mosher’s Method: Absolute Configuration Assignment of N-Amino Acid Derivatives via Bis-Thiourea Chiral Solvating Agent" Molecules 30, no. 14: 2930. https://doi.org/10.3390/molecules30142930
APA StyleRondinini, V., Aiello, F., Cefalì, F., Recchimurzo, A., Uccello Barretta, G., & Balzano, F. (2025). A Supramolecular Extension of Mosher’s Method: Absolute Configuration Assignment of N-Amino Acid Derivatives via Bis-Thiourea Chiral Solvating Agent. Molecules, 30(14), 2930. https://doi.org/10.3390/molecules30142930









