A Review on the Recent Advancements of Ni-Based Sulfides and Mixed Sulfides for Supercapacitors and Electrocatalysis (Oxygen Evolution Reaction)
Abstract
1. Introduction
2. Brief Discussion on Energy Storage and Conversion
3. Synthetic Approaches
4. Energy Storage and Conversion Application of NS and NMS Materials
4.1. Supercapacitor (SC)
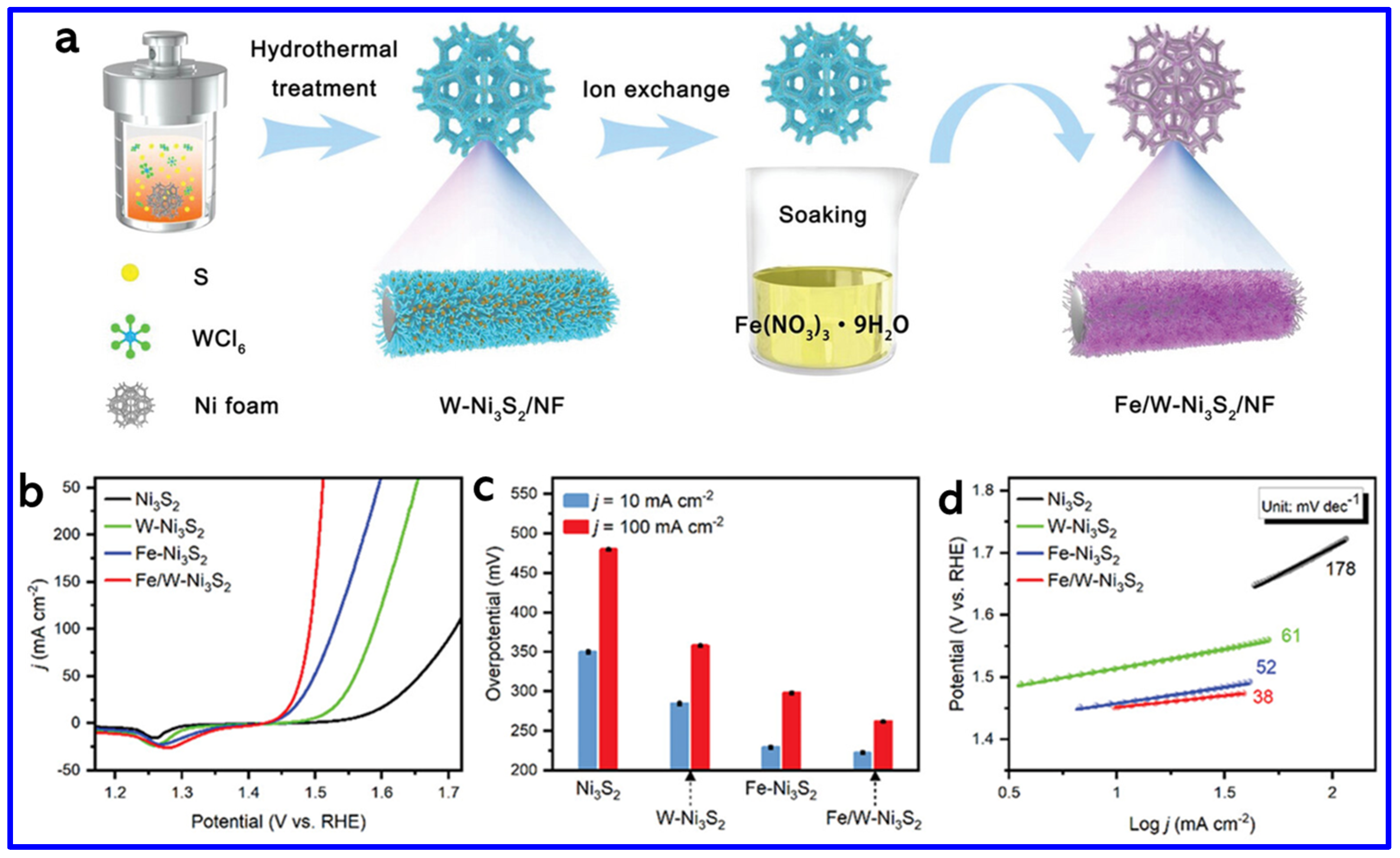
4.2. OER
5. Conclusions and Future Prospects
- The detailed charge storage mechanism of NMS materials has not been clearly explored yet. In this aspect, the suitable integration of theoretical calculations and experimental results is highly essential.
- The subsequent volume expansion and the fallout of the active SC electrode materials from the current collectors negatively affect the cycling performance and rate capability. To solve this issue, specifically designed electrode materials with high chemical stability are essential.
- All the relevant works reported the evaluation of the electrochemical performance of NS and NMS materials in KOH electrolyte. Further increment of electrochemical performance is possible through the addition of redox species in the electrolyte. However, in those cases, the cycling performance of the electrodes should be monitored carefully.
- The charge storage kinetics should also be evaluated thoroughly. In the case of OER activity, the NMS materials have the tendency to oxidize easily to their oxide and hydroxide counterparts, which sometimes harms the OER activities by deteriorating stability and catalytic activity. To solve this issue, the NMS materials have been combined with metal oxides and hydroxides and sometimes grown on conductive substrates. However, in those cases, the catalyst loading should be provided with accuracy.
- The in situ characterization techniques should be implemented to understand several factors, like the charge storage mechanism, the sources of enhanced catalytic activity, the interaction between the electrode and electrolytes, intermolecular interactions, and structural changes of the electrode during the repetitive electrochemical processes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pershaanaa, M.; Bashir, S.; Ramesh, S.; Ramesh, K. Every bite of Supercap: A brief review on construction and enhancement of supercapacitor. J. Energy Storage 2022, 50, 104599. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, S.; Pandey, R.; Joanni, E.; Yadav, R.M. Electromagnetic irradiation-assisted synthesis, exfoliation and modification of graphene-based materials for energy storage and sensing applications. Mater. Sci. Eng. R Rep. 2024, 161, 100860. [Google Scholar] [CrossRef]
- Sahoo, S.; Kumar, R.; Hussain, I.; Zhang, K. Heteroatom doping in 2D MXenes for energy storage/conversion applications. Adv. Powder Mater. 2024, 3, 100246. [Google Scholar] [CrossRef]
- Hemavathi, S.; Srirama, S.; Prakash, A. Present and future generation of secondary batteries: A review. ChemBioEng Rev. 2023, 10, 1123–1145. [Google Scholar] [CrossRef]
- Wu, F.; Liu, M.; Li, Y.; Feng, X.; Zhang, K.; Bai, Y.; Wang, X.; Wu, C. High-mass-loading electrodes for advanced secondary batteries and supercapacitors. Electrochem. Energy Rev. 2021, 4, 382–446. [Google Scholar] [CrossRef]
- Zubair, M.; Hassan, M.M.U.; Mehran, M.T.; Baig, M.M.; Hussain, S.; Shahzad, F. 2D MXenes and their heterostructures for HER, OER and overall water splitting: A review. Int. J. Hydrogen Energy 2022, 47, 2794–2818. [Google Scholar] [CrossRef]
- Qin, D.-D.; Tang, Y.; Ma, G.; Qin, L.; Tao, C.-L.; Zhang, X.; Tang, Z. Molecular metal nanoclusters for ORR, HER and OER: Achievements, opportunities and challenges. Int. J. Hydrogen Energy 2021, 46, 25771–25781. [Google Scholar] [CrossRef]
- Zhang, K.; Zou, R. Advanced transition metal-based OER electrocatalysts: Current status, opportunities, and challenges. Small 2021, 17, 2100129. [Google Scholar] [CrossRef]
- Hansen, J.N.; Prats, H.; Toudahl, K.K.; Mørch Secher, N.; Chan, K.; Kibsgaard, J.; Chorkendorff, I. Is there anything better than Pt for HER? ACS Energy Lett. 2021, 6, 1175–1180. [Google Scholar] [CrossRef]
- Zeng, F.; Mebrahtu, C.; Liao, L.; Beine, A.K.; Palkovits, R. Stability and deactivation of OER electrocatalysts: A review. J. Energy Chem. 2022, 69, 301–329. [Google Scholar] [CrossRef]
- Liang, R.; Du, Y.; Xiao, P.; Cheng, J.; Yuan, S.; Chen, Y.; Yuan, J.; Chen, J. Transition metal oxide electrode materials for supercapacitors: A review of recent developments. Nanomaterials 2021, 11, 1248. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Wickramathilaka, K.Y.; Njeri, E.; Silva, D.; Suib, S.L. A review on transition metal oxides in catalysis. Front. Chem. 2024, 12, 1374878. [Google Scholar] [CrossRef] [PubMed]
- Deka, S. Nanostructured mixed transition metal oxide spinels for supercapacitor applications. Dalton Trans. 2023, 52, 839–856. [Google Scholar] [CrossRef] [PubMed]
- Pathak, M.; Jeong, S.M.; Rout, C.S. Spinel NiCo2O4 based hybrid materials for supercapacitors: Recent developments and future perspectives. J. Energy Storage 2023, 73, 108881. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, Y.; Chen, Y.; Zhang, M.; Wang, Y.; Liu, B.; Zhang, X.; Zhang, Y.; Qiu, R.; Yan, K. Facile synthesis of NiCo2O4 nanosheets efficient for electrocatalysis of water. Appl. Surf. Sci. 2024, 677, 161013. [Google Scholar] [CrossRef]
- Perović, I.M.; Mitrović, S.D.; Brković, S.M.; Pašti, I.A. Advances in Nickel-Based Catalysts for Alkaline Water Electrolysis: Comprehensive Review of Current Research Direction for HER and OER Applications. Chem. Rec. 2025, e202500049. [Google Scholar] [CrossRef]
- Wang, Y.-T.; He, X.-F.; He, G.-Y.; Meng, C.; Chen, X.-M.; Li, F.-T.; Zhou, Y. A critical review on nickel sulfide-based electrode materials for supercapacitors. Crit. Rev. Solid State Mater. Sci. 2023, 48, 502–518. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, Y.; Cui, Z.; Huang, H.; Cai, D.; Zhang, J.; Zhou, Y.; Xu, M.; Tong, R. Nickel sulfide-based electrocatalysts for overall water splitting. Int. J. Hydrogen Energy 2023, 48, 27992–28017. [Google Scholar] [CrossRef]
- Qi, Y.; Qiu, L.; Ma, X.; Wu, J.; Xiang, J.; Guo, C.; Yu, J.; Li, K.; Tao, Z.; Lv, Y. Progress in electrocatalytic materials of nickel-based sulfur complexes for HER and OER. Int. J. Hydrogen Energy 2024, 83, 520–544. [Google Scholar] [CrossRef]
- He, R.; Huang, X.; Feng, L. Recent progress in transition-metal sulfide catalyst regulation for improved oxygen evolution reaction. Energy Fuels 2022, 36, 6675–6694. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, L. Review on recent advances in nanostructured transition-metal-sulfide-based electrode materials for cathode materials of asymmetric supercapacitors. Chem. Eng. J. 2022, 430, 132745. [Google Scholar] [CrossRef]
- Li, J.; Dong, Z.; Chen, R.; Wu, Q.; Zan, G. Advanced nickel-based composite materials for supercapacitor electrodes. Ionics 2024, 30, 1833–1855. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Q.; Bai, T.; Wang, W.; He, F.; Ye, M. Nickel and cobalt sulfide-based nanostructured materials for electrochemical energy storage devices. Chem. Eng. J. 2021, 409, 127237. [Google Scholar] [CrossRef]
- Shahroudi, A.; Esfandiari, M.; Habibzadeh, S. Nickel sulfide and phosphide electrocatalysts for hydrogen evolution reaction: Challenges and future perspectives. RSC Adv. 2022, 12, 29440–29468. [Google Scholar] [CrossRef]
- Sahoo, S.; Kumar, R.; Joanni, E.; Singh, R.K.; Shim, J.-J. Advances in pseudocapacitive and battery-like electrode materials for high performance supercapacitors. J. Mater. Chem. A 2022, 10, 13190–13240. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Narenthiran, B.; Sivanantham, A.; Bhatlu, L.D.; Maridurai, T. Supercapacitor: Evolution and review. Mater. Today Proc. 2021, 46, 3984–3988. [Google Scholar] [CrossRef]
- Kanwade, A.; Shirage, P.M. A review on synergy of transition metal oxide nanostructured materials: Effective and coherent choice for supercapacitor electrodes. J. Energy Storage 2022, 55, 105692. [Google Scholar]
- Zhai, Z.; Zhang, L.; Du, T.; Ren, B.; Xu, Y.; Wang, S.; Miao, J.; Liu, Z. A review of carbon materials for supercapacitors. Mater. Des. 2022, 221, 111017. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Hou, H.; Xu, W.; Duan, G.; He, S.; Liu, K.; Jiang, S. Recent progress in carbon-based materials for supercapacitor electrodes: A review. J. Mater. Sci. 2021, 56, 173–200. [Google Scholar] [CrossRef]
- Anil Kumar, Y.; Koyyada, G.; Ramachandran, T.; Kim, J.H.; Sajid, S.; Moniruzzaman, M.; Alzahmi, S.; Obaidat, I.M. Carbon materials as a conductive skeleton for supercapacitor electrode applications: A review. Nanomaterials 2023, 13, 1049. [Google Scholar] [CrossRef]
- Tadesse, M.G.; Ahmmed, A.S.; Lübben, J.F. Review on conductive polymer composites for supercapacitor applications. J. Compos. Sci. 2024, 8, 53. [Google Scholar] [CrossRef]
- Iqbal, M.F.; Ashiq, M.N.; Zhang, M. Design of metals sulfides with carbon materials for supercapacitor applications: A review. Energy Technol. 2021, 9, 2000987. [Google Scholar] [CrossRef]
- Gaikwad, P.; Tiwari, N.; Kamat, R.; Mane, S.M.; Kulkarni, S.B. A comprehensive review on the progress of transition metal oxides materials as a supercapacitor electrode. Mater. Sci. Eng. B 2024, 307, 117544. [Google Scholar] [CrossRef]
- Shaheen, I.; Hussain, I.; Zahra, T.; Javed, M.S.; Shah, S.S.A.; Khan, K.; Hanif, M.B.; Assiri, M.A.; Said, Z.; Arifeen, W.U. Recent advancements in metal oxides for energy storage materials: Design, classification, and electrodes configuration of supercapacitor. J. Energy Storage 2023, 72, 108719. [Google Scholar] [CrossRef]
- Sahoo, S.; Shim, J.-J. Facile synthesis of three-dimensional ternary ZnCo2O4/reduced graphene oxide/NiO composite film on nickel foam for next generation supercapacitor electrodes. ACS Sustain. Chem. Eng. 2017, 5, 241–251. [Google Scholar] [CrossRef]
- Ding, H.; Liu, H.; Chu, W.; Wu, C.; Xie, Y. Structural transformation of heterogeneous materials for electrocatalytic oxygen evolution reaction. Chem. Rev. 2021, 121, 13174–13212. [Google Scholar] [CrossRef]
- Xiong, Y.; He, P. A review on electrocatalysis for alkaline oxygen evolution reaction (OER) by Fe-based catalysts. J. Mater. Sci. 2023, 58, 2041–2067. [Google Scholar] [CrossRef]
- Li, H.; Lin, Y.; Duan, J.; Wen, Q.; Liu, Y.; Zhai, T. Stability of electrocatalytic OER: From principle to application. Chem. Soc. Rev. 2024, 53, 10709–10740. [Google Scholar] [CrossRef]
- Yan, H.; Zhu, K.; Liu, X.; Wang, Y.; Wang, Y.; Zhang, D.; Lu, Y.; Peng, T.; Liu, Y.; Luo, Y. Ultra-thin NiS nanosheets as advanced electrode for high energy density supercapacitors. RSC Adv. 2020, 10, 8760–8765. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Luo, C.; Zhang, Z.; Sun, H.; Xu, C.; Chen, H. Hydrothermal synthesis of β-NiS nanoparticles and their applications in high-performance hybrid supercapacitors. Nanomaterials 2024, 14, 1299. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Fu, N.; Tian, D.; Zheng, Y.; Wang, F.; Liu, C.; Wang, X.; Zhou, Z.; Niu, Y. MOF-derived carbon-coated NiS/NiS2 yolk-shell spheres as a satisfactory positive electrode material for hybrid supercapacitors. Adv. Compos. Hybrid Mater. 2025, 8, 1–12. [Google Scholar] [CrossRef]
- Das, A.; Maitra, A.; Mondal, A.; De, A.; Maity, P.; Khatua, B.B. Hydrothermal synthesis of Cu2S/NiS/Ni3S4 as high performance supercapacitor application. J. Energy Storage 2024, 92, 112293. [Google Scholar] [CrossRef]
- Tan, X.; Feng, Z.; Yang, W.; Zou, H.; Chen, S. Flower-like heterogeneous phosphorus-doped Co3S4@ Ni3S4 nanoparticles as a binder-free electrode for asymmetric all-solid-state supercapacitors. ACS Appl. Energy Mater. 2023, 6, 702–713. [Google Scholar] [CrossRef]
- Wei, W.; Guo, Z.; Qin, X.; Mi, L. Innovative solvent-free compound-direct synthesis of defect-rich ultra-thin NiS nanosheets for high-performance supercapacitors. Nanoscale 2024, 16, 2522–2530. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, D.; Yang, B.; Shi, H.; Wang, K.; Han, L.; Wang, S.; Wang, Y. Targeted synthesis of NiS and NiS2 nanoparticles for high–performance hybrid supercapacitor via a facile green solid–phase synthesis route. J. Energy Storage 2020, 32, 101852. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Liang, M.; He, Z.; Li, K.; Song, W.; Tian, S.; Duan, W.; Zhao, Y.; Miao, Z. Novel dealloying-fabricated NiS/NiO nanoparticles with superior cycling stability for supercapacitors. ACS Omega 2021, 6, 17999–18007. [Google Scholar] [CrossRef] [PubMed]
- Shombe, G.B.; Khan, M.D.; Zequine, C.; Zhao, C.; Gupta, R.K.; Revaprasadu, N. Direct solvent free synthesis of bare α-NiS, β-NiS and α-β-NiS composite as excellent electrocatalysts: Effect of self-capping on supercapacitance and overall water splitting activity. Sci. Rep. 2020, 10, 3260. [Google Scholar] [CrossRef]
- Mohapatra, S.; Das, H.T.; Tripathy, B.C.; Das, N. Architecture of Binder-Free Positrodes for Advance Supercapacitors: A Electrodeposited Battery-Type Ternary Cobalt–Nickel–Copper Sulfide. ACS Appl. Energy Mater. 2025, 8, 1369–1378. [Google Scholar] [CrossRef]
- Karmakar, D.A.; Mondal, A.S.; Patra, A.K.; Bhattacharya, S. An Advanced Asymmetric Supercapacitor Electrode Material Based on Mg(OH)2-Ni3s4 Nanocomposite. J. Energy Storage 2025, 114, 115732. [Google Scholar]
- Wang, S.; Zhang, P.; Liu, C. Synthesis of hierarchical bimetallic sulfide NiCo2S4 for high-performance supercapacitors. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126334. [Google Scholar] [CrossRef]
- Amedzo-Adore, M.; Han, J.-I. Surfactant-Assisted NiCo2S4 for Redox Supercapacitors. Batteries 2024, 10, 360. [Google Scholar] [CrossRef]
- Shwetha, K.; Manjunatha, C.; Kamath, M.S.; Rastogi, C.K.; Chaudhary, V.; Maurya, G.; Athreya, Y.; Shivaraj, B.; Khosla, A. Fabrication of super-high energy density asymmetric supercapacitor prototype device employing NiCo2S4@ f-MWCNT nanocomposite. J. Energy Storage 2023, 72, 108657. [Google Scholar] [CrossRef]
- He, D.; Li, F.; Xiao, Y.; Chen, S.; Zhu, Z.; Chen, H.; Hu, X.; Peng, W.; Xin, S.; Bai, Y. Electrostatic self-assembly assisted hydrothermal synthesis of bimetallic NiCo2S4@ N, S co-doped graphene for high performance asymmetric supercapacitors. Electrochim. Acta 2022, 404, 139751. [Google Scholar] [CrossRef]
- Tue, L.N.M.; Sahoo, S.; Dhakal, G.; Nguyen, V.H.; Lee, J.; Lee, Y.R.; Shim, J.-J. NiCo2S4/MoS2 nanocomposites for long-life high-performance hybrid supercapacitors. Nanomaterials 2023, 13, 689. [Google Scholar] [CrossRef]
- Van Hoa, N.; Dat, P.A.; Van Hieu, N.; Le, T.N.; Minh, N.C.; Van Tang, N.; Nga, D.T.; Ngoc, T.Q. Rapid and efficient synthesis of high-porous reduced graphene oxide/NiCo2S4 nanocomposites for supercapacitor application. Diam. Relat. Mater. 2020, 106, 107850. [Google Scholar] [CrossRef]
- Chen, R.; Xue, J.; Gong, Y.; Yu, C.; Hui, Z.; Xu, H.; Sun, Y.; Zhao, X.; An, J.; Zhou, J. Mesh-like vertical structures enable both high areal capacity and excellent rate capability. J. Energy Chem. 2021, 53, 226–233. [Google Scholar] [CrossRef]
- Min, X.; Bo, Z.; Xu, Z.; Feng, J.; Lin, X.; Ni, Y. Porous nanosheet–nanosphere@ nanosheet FeNi2-LDH@ FeNi2S4 core–shell heterostructures for asymmetric supercapacitors. Dalton Trans. 2023, 52, 12119–12129. [Google Scholar] [CrossRef]
- Guo, Y.; Hao, C.; Yang, Y.; Wu, X.; Ni, C.; Wang, X.; Wang, X. Convenient synthesis of Ni-Mn-S@ rGO composite with enhanced performance for advanced energy storage applications. Ceram. Int. 2022, 48, 9558–9568. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, C.; Zhao, L.; Yu, H.; Wang, J.; Zhang, Z. Preparation of rGO/Ni2ZnS4 and high-performance asymmetric supercapacitors with GO/ZIF-8 template. Diam. Relat. Mater. 2022, 125, 108965. [Google Scholar] [CrossRef]
- Min, X.; Wen, B.; Feng, J.; Lin, X. Nanosheet@ nanosheet NiZn2O4@NiZn2S4 core-shell mesoporous heterostructure as an electrode material for aqueous hybrid supercapacitors. J. Energy Storage 2024, 81, 110410. [Google Scholar] [CrossRef]
- Xu, G.; Xu, L.; Zhang, F.; Yu, C.; Song, Y. Facile synthesis of NiS1.03@Ni7S6/carbon composites with the assistance of biomass resource for supercapacitors with superior electrochemical performance. Chem. Phys. Lett. 2024, 834, 140969. [Google Scholar] [CrossRef]
- Shwetha, K.; Kamath, M.S.; Athreya, Y.N.; Rastogi, C.K.; Nagaraju, K.; Khosla, A.; Manjunatha, C. Development of NiS@f-MWCNT nanocomposite-based high-performance supercapacitor coin cell prototype device. J. Energy Storage 2024, 75, 109404. [Google Scholar] [CrossRef]
- Wu, W.; Yan, Y.; Wang, X.; Wei, C.; Xu, T.; Li, X. Interface construction of CuCoSe@ NiS based on an ultrathin nanosheet for high-performance supercapacitors. J. Mater. Chem. A 2024, 12, 13818–13829. [Google Scholar] [CrossRef]
- Darsara, S.A.; Seifi, M.; Askari, M.B.; Osquian, M. Hierarchical 3D starfish-like Ni3S4–NiS on reduced graphene oxide for high-performance supercapacitors. Ceram. Int. 2021, 47, 20992–20998. [Google Scholar] [CrossRef]
- Guo, Y.; Chang, J.; Hu, L.; Lu, Y.; Yao, S.; Su, X.; Zhang, X.; Zhang, H.; Feng, J. Hollow Bowl NiS2@ polyaniline Conductive Linker/Graphene Conductive Network: A Triple Composite for High-Performance Supercapacitor Applications. ChemSusChem 2024, 17, e202301148. [Google Scholar] [CrossRef]
- Godlaveeti, S.K.; El-marghany, A.; Nagireddy, R.R.; Gedi, S.; Chintaparty, R. Comparative study of electrochemical supercapacitor performance among various nickel phases: Hydroxide, oxide, and sulfide. Ceram. Int. 2025, 51, 20620–20627. [Google Scholar] [CrossRef]
- Narthana, K.; Durai, G.; Kuppusami, P.; Theerthagiri, J.; Sujatha, S.; Lee, S.J.; Choi, M.Y. One-step synthesis of hierarchical structured nickel copper sulfide nanorods with improved electrochemical supercapacitor properties. Int. J. Energy Res. 2021, 45, 9983–9998. [Google Scholar] [CrossRef]
- Alitabar, K.; Zardkhoshoui, A.M.; Hosseiny Davarani, S.S. One-Step Synthesis of Porous Ni–Co–Fe–S Nanosheet Arrays as an Efficient Battery-Type Electrode Material for Hybrid Supercapacitors. Batter. Supercaps 2020, 3, 1311–1320. [Google Scholar] [CrossRef]
- Jinlong, L.; Tongxiang, L.; Meng, Y.; Ken, S.; Hideo, M. Performance comparison of NiCo2O4 and NiCo2S4 formed on Ni foam for supercapacitor. Compos. Part B Eng. 2017, 123, 28–33. [Google Scholar] [CrossRef]
- Meng, S.; Wang, Y.; He, J.; Quan, W.; Gao, M.; Jiang, D.; Chen, M. Nanowire-assembled Co3O4@NiS core–shell hierarchical with enhanced electrochemical performance for asymmetric supercapacitors. Nanotechnology 2020, 31, 295403. [Google Scholar] [CrossRef]
- Sahoo, S.; Dhakal, G.; Kim, W.K.; Shim, J.-J. Ternary nanohybrid of Ni3S2/CoMoS4/MnO2 on nickel foam for aqueous and solid-state high-performance supercapacitors. Nanomaterials 2022, 12, 1945. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.A.K.; Han, H.; Kim, K.C.; Bae, S. Heterostructured NiCo2S4@ SnS2 hybrid for all-solid-state supercapacitor applications: Delocalized charges on Co–S heterojunction improved electrochemical kinetics. ACS Appl. Energy Mater. 2022, 5, 13751–13762. [Google Scholar] [CrossRef]
- Hu, J.; Pan, Y.; Zhang, Q.; Dong, Z.; Han, S. Constructing flower-shaped NiCo2S4@CoAl-LDH heterojunction nanosheets exhibits extraordinary electrochemical behavior for a light-assisted asymmetric supercapacitor. Energy Fuels 2024, 38, 6459–6470. [Google Scholar] [CrossRef]
- Cheng, S.; Du, K.; Wang, X.; Han, Y.; Li, L.; Wen, G. Fabrication of Hierarchical MOF-Derived NiCo2S4@ Mo-Doped Co-LDH Arrays for High-Energy-Density Asymmetric Supercapacitors. Nanomaterials 2023, 13, 2663. [Google Scholar] [CrossRef]
- Cao, J.; Hu, Y.; Zhu, Y.; Cao, H.; Fan, M.; Huang, C.; Shu, K.; He, M.; Chen, H.C. Synthesis of mesoporous nickel-cobalt-manganese sulfides as electroactive materials for hybrid supercapacitors. Chem. Eng. J. 2021, 405, 126928. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, L.; Lei, X.; Huang, H.; Guo, W.; Wang, S. Hierarchical ternary Zn-Ni-Co sulfide/oxide heterostructure for high specific energy hybrid supercapacitor. J. Alloys Compd. 2023, 962, 171201. [Google Scholar] [CrossRef]
- Ni, S.; Qu, H.; Xing, H.; Xu, Z.; Zhu, X.; Yuan, M.; Rong, M.; Wang, L.; Yu, J.; Li, Y. Interfacial engineering of transition-metal sulfides heterostructures with built-in electric-field effects for enhanced oxygen evolution reaction. Chin. J. Chem. Eng. 2022, 41, 320–328. [Google Scholar] [CrossRef]
- Zhou, N.; Liu, R.; Wu, X.; Ding, Y.; Zhang, X.; Liang, S.; Deng, C.; Qin, G.; Huang, Z.; Chen, B. One-spot autogenous formation of crystalline-amorphous Ni3S2/NiFeOxHy heterostructure nanosheets array for synergistically boosted oxygen evolution reaction. J. Power Sources 2023, 574, 233163. [Google Scholar] [CrossRef]
- Shi, Z.; Mao, C.; Zhong, L.; Peng, J.; Liu, M.; Li, H.; Huang, J. Mo-doped Ni3S4 nanosheets grown on carbonized wood as highly efficient and durable electrocatalysts for water splitting. Appl. Catal. B Environ. 2023, 339, 123123. [Google Scholar] [CrossRef]
- Prakash, C.; Sahoo, P.; Yadav, R.; Pandey, A.; Singh, V.K.; Dixit, A. Nanoengineered Zn-modified Nickel Sulfide (NiS) as a bifunctional electrocatalyst for overall water splitting. Int. J. Hydrogen Energy 2023, 48, 21969–21980. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, D.; Sun, S.; Huang, S.; Wu, Y.; Zhang, L.; Dou, S.X.; Liu, H.K.; Dou, Y.; Xu, J. Iron, Tungsten Dual-Doped Nickel Sulfide as Efficient Bifunctional Catalyst for Overall Water Splitting. Small 2024, 20, 2311770. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.-F.; Yang, M.; Hou, S.; Dong, B.; Chen, T.-S.; Ma, X.; Xie, J.-Y.; Zhou, Y.-N.; Nan, J.; Chai, Y.-M. Copper and cobalt co-doped Ni3S2 grown on nickel foam for highly efficient oxygen evolution reaction. Appl. Surf. Sci. 2020, 502, 144172. [Google Scholar] [CrossRef]
- Mao, X.; Liu, Y.; Chen, Z.; Fan, Y.; Shen, P. Fe and Co dual-doped Ni3S4 nanosheet with enriched high-valence Ni sites for efficient oxygen evolution reaction. Chem. Eng. J. 2022, 427, 130742. [Google Scholar] [CrossRef]
- Manjunatha, C.; Srinivasa, N.; Chaitra, S.; Sudeep, M.; Kumar, R.C.; Ashoka, S. Controlled synthesis of nickel sulfide polymorphs: Studies on the effect of morphology and crystal structure on OER performance. Mater. Today Energy 2020, 16, 100414. [Google Scholar] [CrossRef]
- Majeed, M.; Alrowaily, A.W.; Alotaibi, B.; Alyousef, H.A.; Alotiby, M.F.; Khan, M.J.; Hussein, K.I.; Saleem, M.I. Effective OER activity of rhombohedral nickel sulphide nanoparticles supported via reduced graphene oxide. Diam. Relat. Mater. 2024, 148, 111354. [Google Scholar] [CrossRef]
- Li, B.; Li, Z.; Pang, Q.; Zhang, J.Z. Core/shell cable-like Ni3S2 nanowires/N-doped graphene-like carbon layers as composite electrocatalyst for overall electrocatalytic water splitting. Chem. Eng. J. 2020, 401, 126045. [Google Scholar] [CrossRef]
- Li, T.; Lu, T.; Li, Y.; Yin, J.; Tang, Y.; Zhang, M.; Pang, H.; Xu, L.; Yang, J.; Zhang, Y. Interfacial engineering-induced electronic regulation drastically enhances the electrocatalytic oxygen evolution: Immobilization of Janus-structured NiS/NiO nanoparticles onto carbon nanotubes/nanofiber-integrated superstructures. Chem. Eng. J. 2022, 428, 131094. [Google Scholar] [CrossRef]
- Yang, X.; Liang, S.; Wang, G.; Zhou, B.; Duan, Z.; Xie, Z.; Hu, Y. Synergistic effect of nanocrystalline NiCo2S4 and NiCo alloy embedded in N-doped carbon fibers towards high-performance electrocatalysts for oxygen evolution reaction. J. Alloys Compd. 2024, 1007, 176441. [Google Scholar] [CrossRef]
- Lv, Y.; Duan, S.; Zhu, Y.; Yin, P.; Wang, R. Enhanced OER performances of Au@NiCo2S4 core-shell heterostructure. Nanomaterials 2020, 10, 611. [Google Scholar] [CrossRef]
- Khalid, H.D.; Bilal, A.; Javed, M.; Amjad, A.; Ali, A.; Bahadur, A.; Iqbal, S.; Mahmood, S.; Saleh, T.A.; Rana, A. Sustainable energy generation: High-performance NiCo2S4@ Sg-C3N4 bifunctional electrocatalyst advances water splitting efficiency. Int. J. Hydrogen Energy 2024, 68, 128–138. [Google Scholar] [CrossRef]
- Ke, W.; Zhang, Y.; Imbault, A.L.; Li, Y. Metal-organic framework derived iron-nickel sulfide nanorods for oxygen evolution reaction. Int. J. Hydrogen Energy 2021, 46, 20941–20949. [Google Scholar] [CrossRef]
- Xu, Y.; Sumboja, A.; Groves, A.; Ashton, T.; Zong, Y.; Darr, J.A. Enhancing bifunctional catalytic activity of cobalt–nickel sulfide spinel nanocatalysts through transition metal doping and its application in secondary zinc–air batteries. RSC Adv. 2020, 10, 41871–41882. [Google Scholar] [CrossRef]
- Yang, Y.; Meng, H.; Kong, C.; Yan, S.; Ma, W.; Zhu, H.; Ma, F.; Wang, C.; Hu, Z. Heterogeneous Ni3S2@ FeNi2S4@ NF nanosheet arrays directly used as high efficiency bifunctional electrocatalyst for water decomposition. J. Colloid Interface Sci. 2021, 599, 300–312. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, M.; Cao, Z.; Zhu, P.; Cao, Y.; Xu, X.; Xu, C.; Yin, Z. Heterogeneous bimetallic sulfides based seawater electrolysis towards stable industrial-level large current density. Appl. Catal. B Environ. 2021, 291, 120071. [Google Scholar] [CrossRef]
- He, J.-Z.; Niu, W.-J.; Wang, Y.-P.; Sun, Q.-Q.; Liu, M.-J.; Wang, K.; Liu, W.-W.; Liu, M.-C.; Yu, F.-C.; Chueh, Y.-L. In-situ synthesis of hybrid nickel cobalt sulfide/carbon nitrogen nanosheet composites as highly efficient bifunctional oxygen electrocatalyst for rechargeable Zn-air batteries. Electrochim. Acta 2020, 362, 136968. [Google Scholar] [CrossRef]
- Xu, Y.; Sumboja, A.; Zong, Y.; Darr, J.A. Bifunctionally active nanosized spinel cobalt nickel sulfides for sustainable secondary zinc–air batteries: Examining the effects of compositional tuning on OER and ORR activity. Catal. Sci. Technol. 2020, 10, 2173–2182. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Chen, S.; He, P.; Xu, Y.; Jia, L.; Yang, D.; He, X.; Deng, H.; Jia, B. Facile one-pot synthesis of binder-free nano/micro structured dendritic cobalt activated nickel sulfide: A highly efficient electrocatalyst for oxygen evolution reaction. Int. J. Hydrogen Energy 2020, 45, 19304–19312. [Google Scholar] [CrossRef]
- Chinnadurai, D.; Rajendiran, R.; Kandasamy, P. Bimetallic copper nickel sulfide electrocatalyst by one step chemical bath deposition for efficient and stable overall water splitting applications. J. Colloid Interface Sci. 2022, 606, 101–112. [Google Scholar] [CrossRef]
- Ehsan, M.A.; Khan, A.; Zafar, M.N.; Akber, U.A.; Hakeem, A.S.; Nazar, M.F. Aerosol-assisted chemical vapor deposition of nickel sulfide nanowires for electrochemical water oxidation. Int. J. Hydrogen Energy 2022, 47, 42001–42012. [Google Scholar] [CrossRef]
- Li, T.; Jiang, K.; Li, Y.; Luo, H.; Wang, Z.; Liu, Y.-Q. Crystalline nickel sulfide integrated with amorphous cobalt sulfide as an efficient bifunctional electrocatalyst for water splitting. Int. J. Hydrogen Energy 2023, 48, 7337–7345. [Google Scholar] [CrossRef]
- Dong, W.; Zhou, H.; Mao, B.; Zhang, Z.; Liu, Y.; Liu, Y.; Li, F.; Zhang, D.; Zhang, D.; Shi, W. Efficient MOF-derived V–Ni3S2 nanosheet arrays for electrocatalytic overall water splitting in alkali. Int. J. Hydrogen Energy 2021, 46, 10773–10782. [Google Scholar] [CrossRef]
- Ji, X.; Xu, B.; Zhang, H.; Zhang, X.; Yang, P. NiS2 nanoparticles anchored on Co-carbon nanotubes for supercapacitor and overall water splitting. J. Alloys Compd. 2023, 968, 172192. [Google Scholar] [CrossRef]
- Zhou, B.; Li, J.; Zhang, X.; Guo, J. Engineering P-doped Ni3S2-NiS hybrid nanorod arrays for efficient overall water electrolysis. J. Alloys Compd. 2021, 862, 158391. [Google Scholar] [CrossRef]
- Tong, X.; Li, Y.; Pang, N.; Qu, Y.; Yan, C.; Xiong, D.; Xu, S.; Wang, L.; Chu, P.K. Co-doped Ni3S2 porous nanocones as high-performance bifunctional electrocatalysts in water splitting. Chem. Eng. J. 2021, 425, 130455. [Google Scholar] [CrossRef]

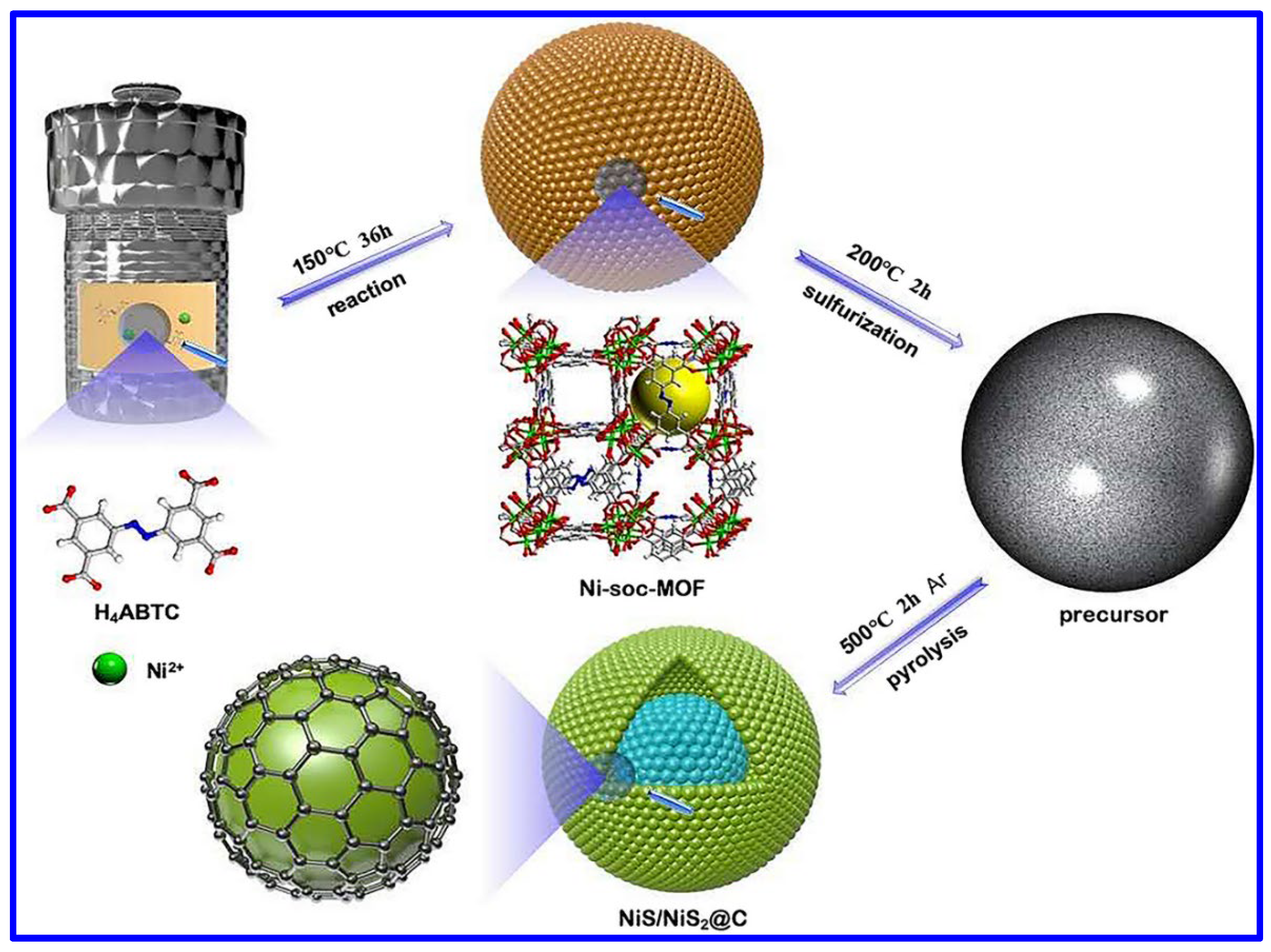
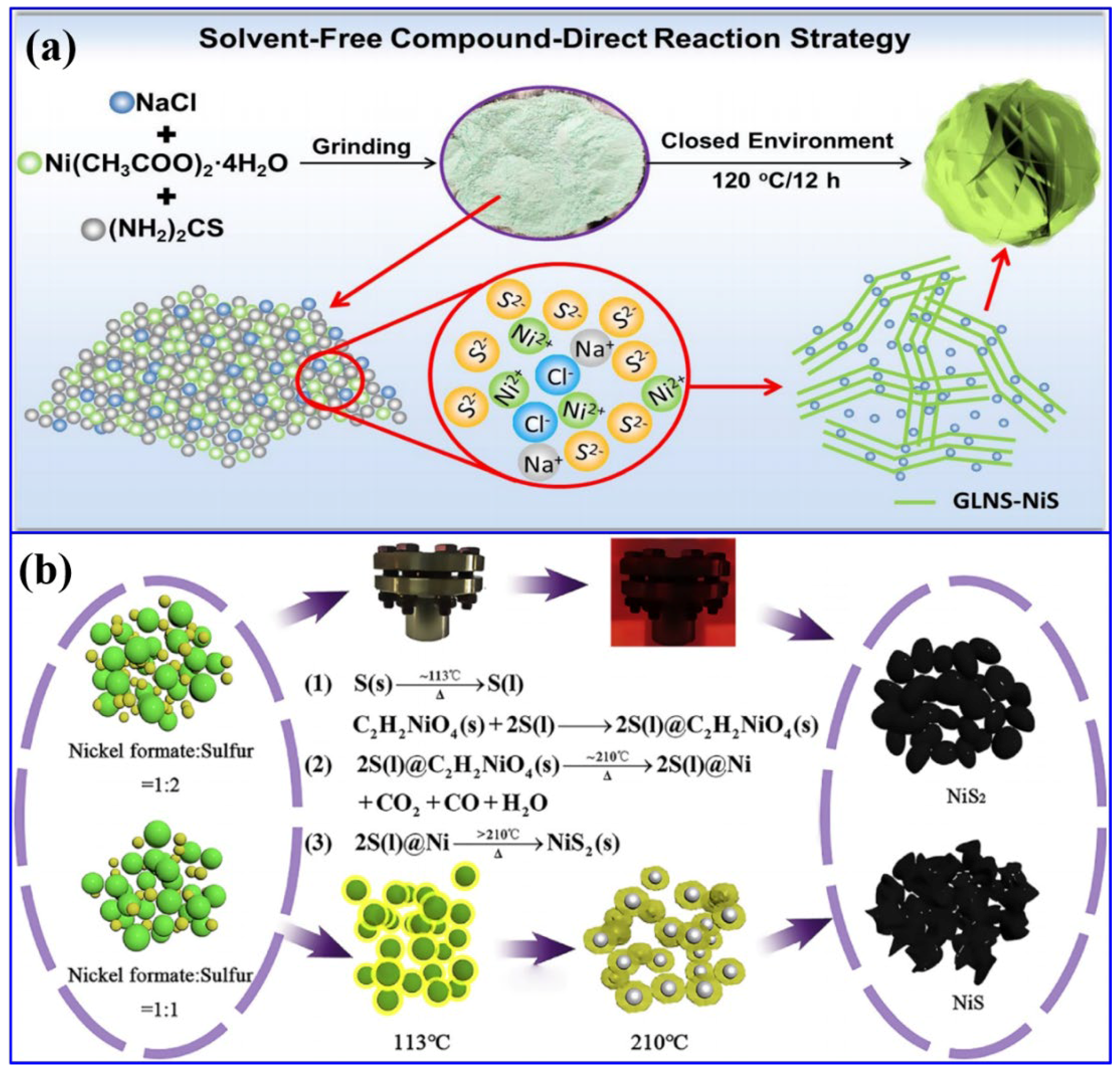
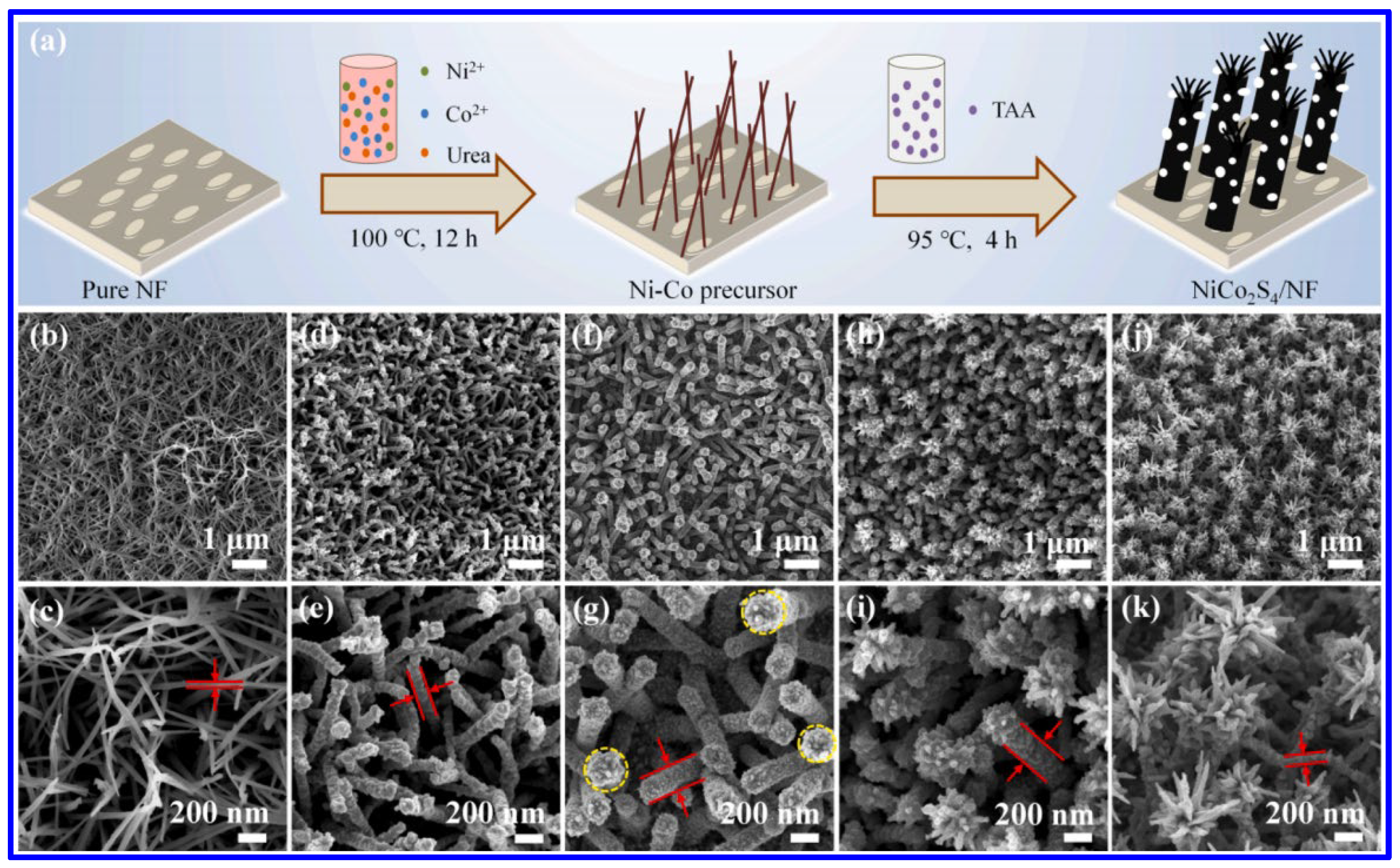

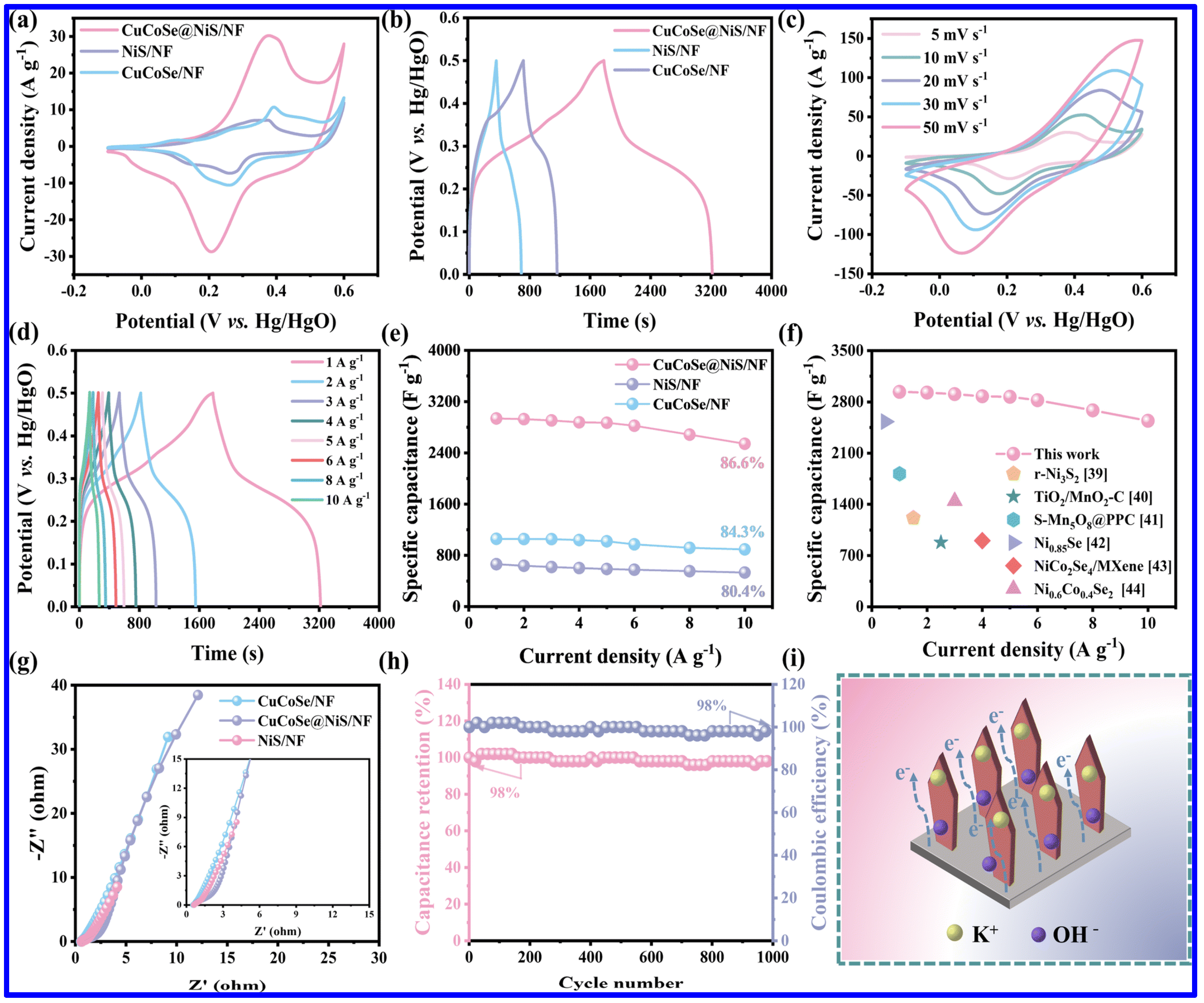
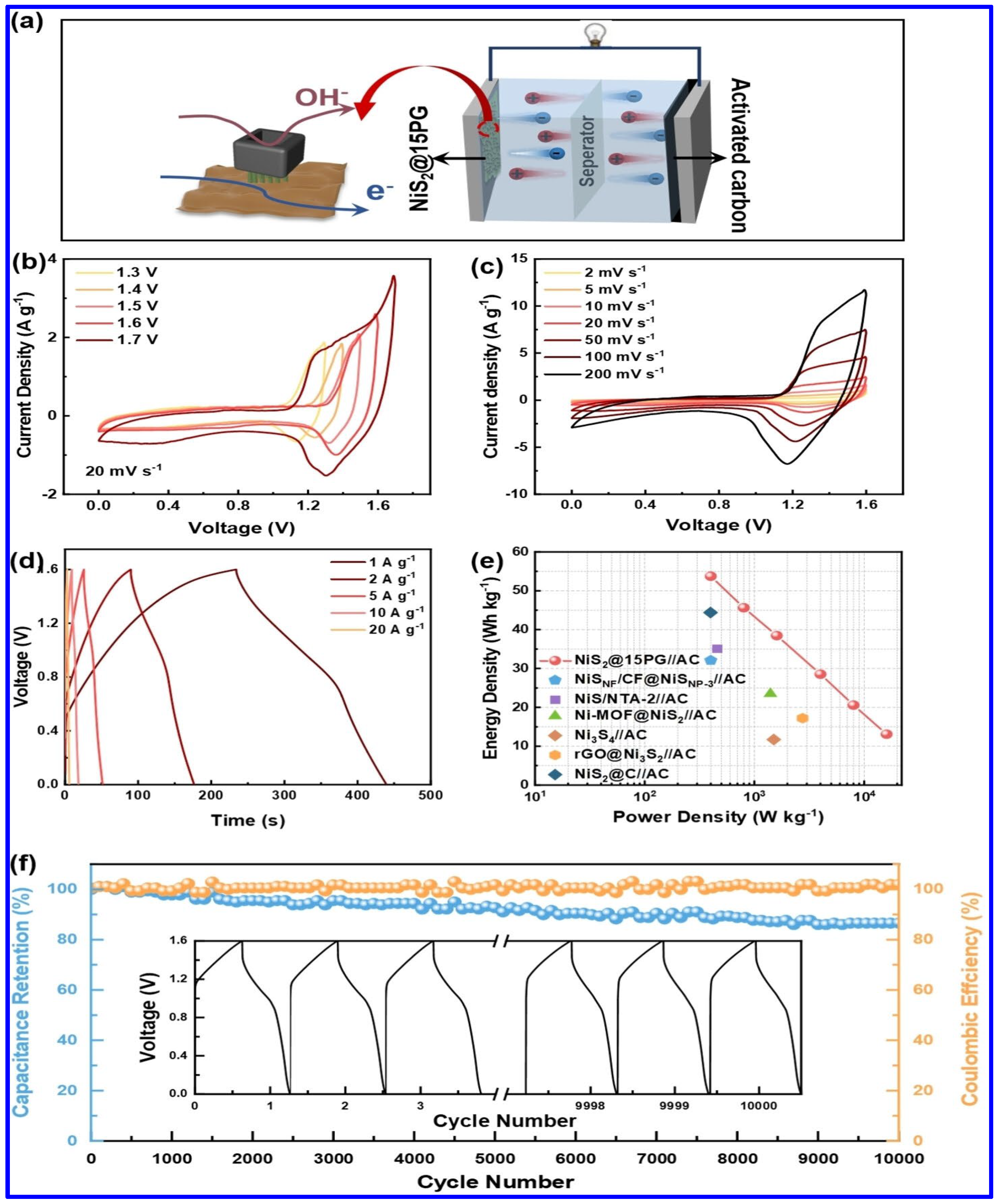
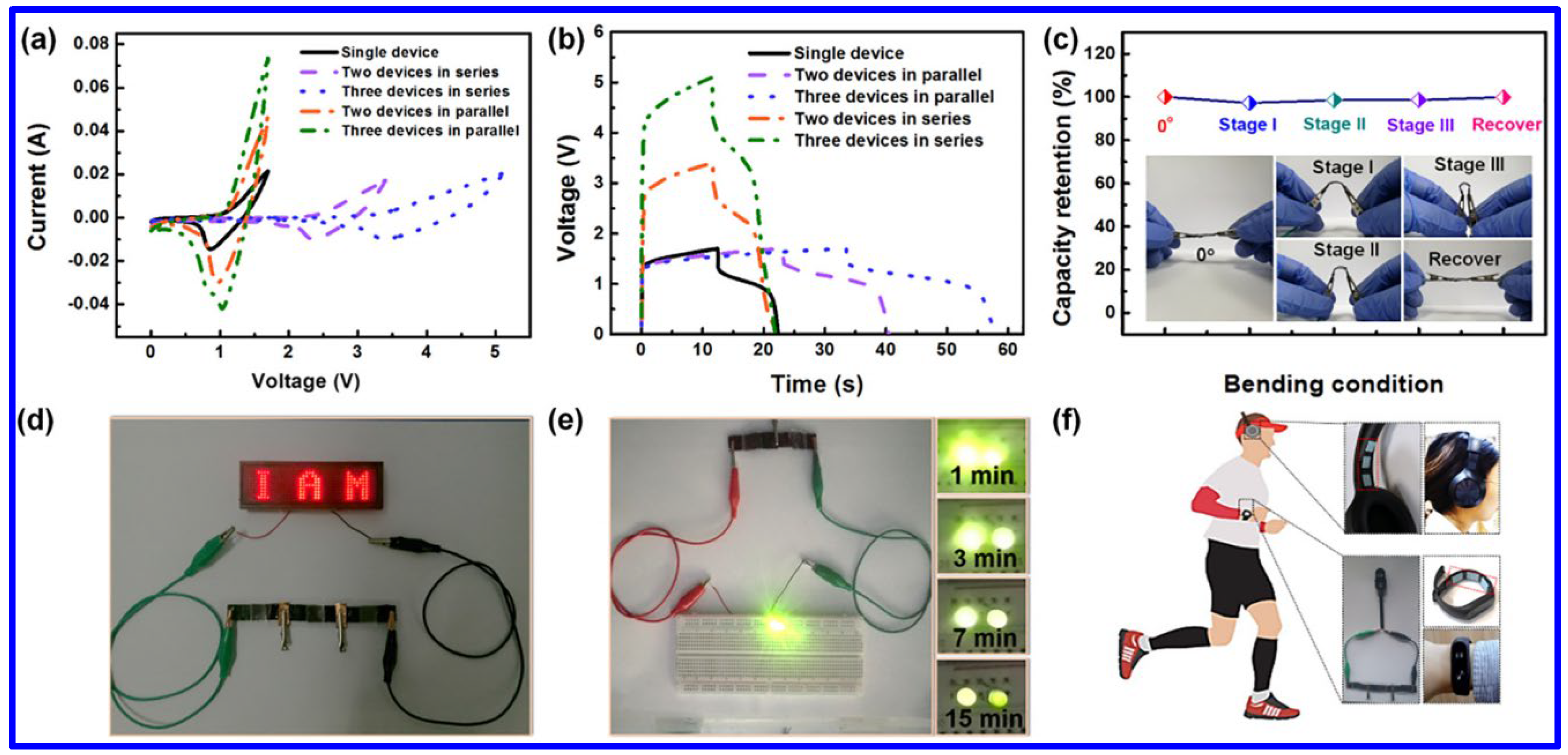
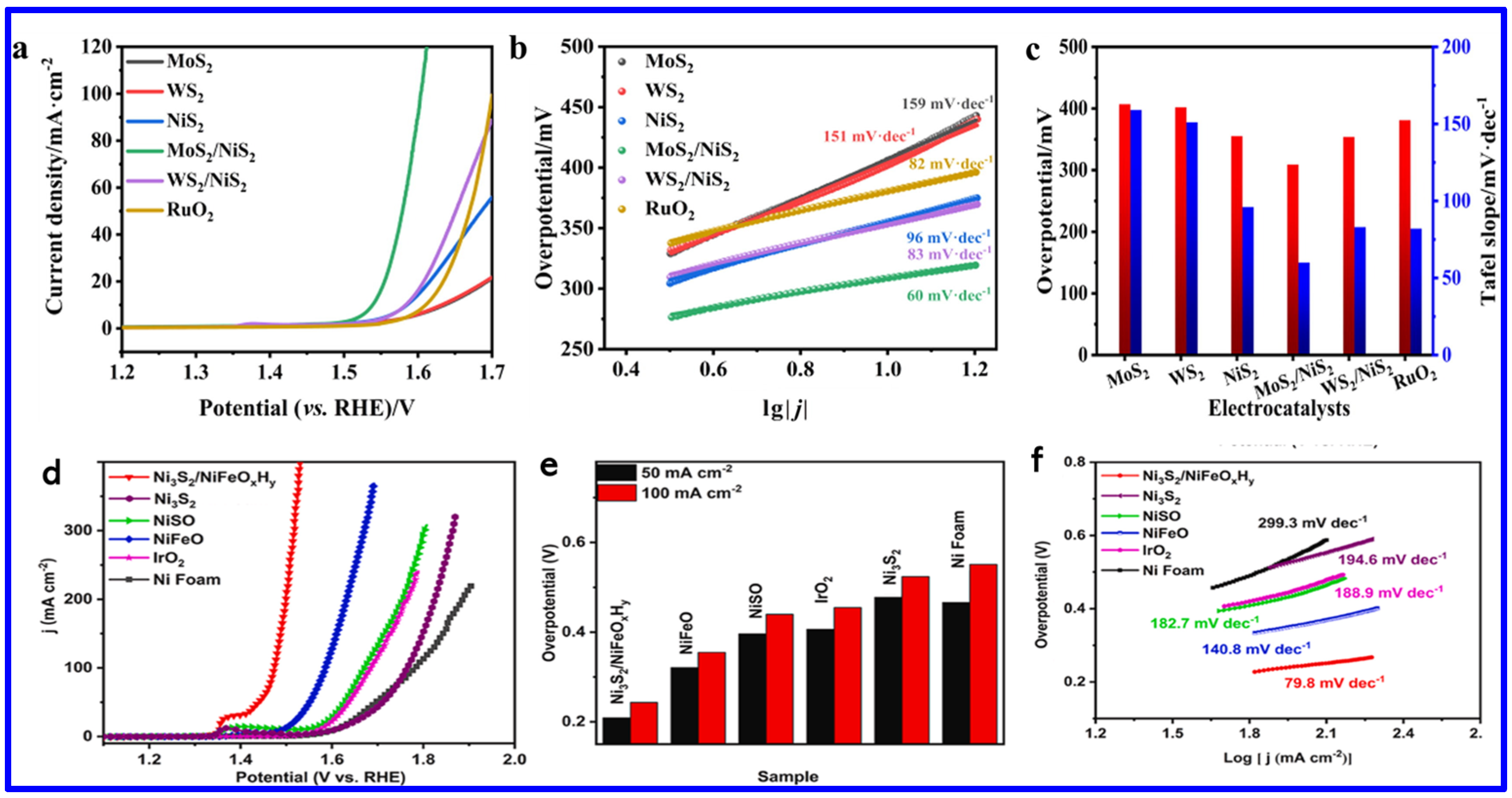
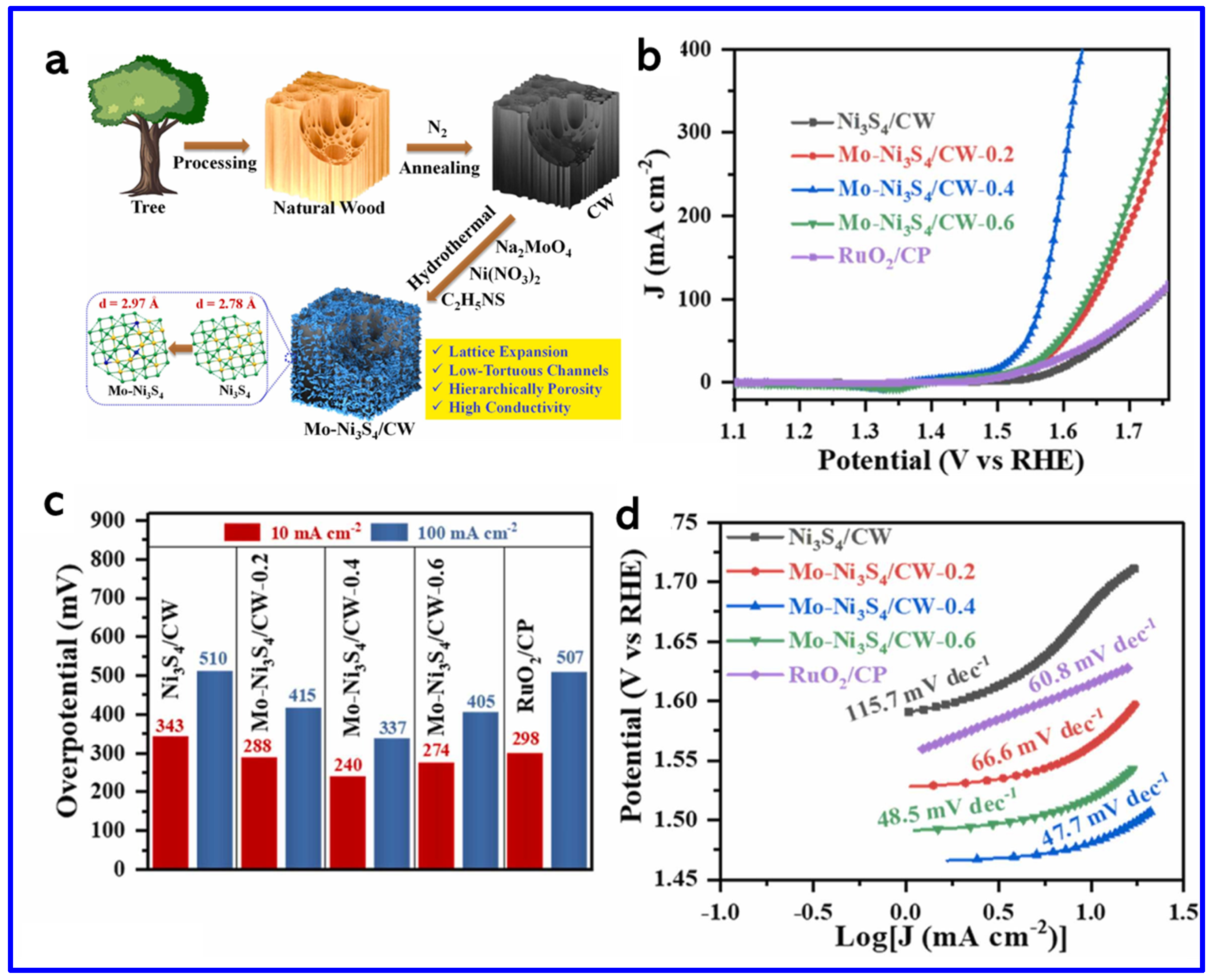
| Electrode | Electrolyte (Conc. of KOH) | Specific Capacitance (F g−1) or Specific Capacity (C g−1) | Cycling Stability (%) | Ref. |
|---|---|---|---|---|
| β-NiS | 2 M | 638.34 C g−1 at 1 A g−1 | - | [40] |
| NiS/NF | 3 M | 2587 F g−1 at 0.2 A g−1 | 95.8 after 4000 cycles | [39] |
| α-NiS/β-NiS | 3 M | 2250 F g−1 at 2 mV s−1 | - | [47] |
| NiS1.03/Ni7S6/Carbon | 6 M | 1554.6 F g−1 at 1 A g−1 | 80.4 after 5000 cycles | [61] |
| NiS/functionalized-MWCNT | 6 M | 1966 F g−1 at 1 A g−1 | 86.2 after 10,000 cycles | [62] |
| Co3O4/NiS/NF | 6 M | 1395.3 F g−1 at 1 A g−1 | 89.9 after 5000 cycles | [70] |
| CuCoSe/NiS/NF | 3 M | 2937.6 F g−1 at 1 A g−1 | 98 after 1000 cycles | [63] |
| Ni3S4/NiS/RGO | 2 M | 1578 F g−1 at 0.5 A g−1 | 91 after 5000 cycles | [64] |
| NiS2/PANI/graphene | 1 M | 536.13 C g−1 at 1 A g−1 | 80.5 after 5000 cycles | [65] |
| P-doped Co3S4/Ni3S4/NF | 2 M | 3614 F g−1 at 1 A g−1 | 73 after 3000 cycles | [43] |
| Ni3S2/CoMoS4/MnO2/NF | 1 M | 2021 F g−1 at 1 A g−1 | 90 after 4000 cycles | [71] |
| Mg(OH)2/Ni3S4 | 1 M | 3316.7 F g−1 at 1 A g−1 | - | [49] |
| Cu2S/NiS/Ni3S4 | 6 M | 363.05 mAh g−1 at 0.5 A g−1 | 94.8 after 8000 cycles | [42] |
| Co9S8/NiS2/Cu2S/NF | 3 M | 460.15 C g−1 at 1 A g−1 | - | [48] |
| NiCo2S4 | 3 M | 3506 F g−1 at 2 A g−1 | 90 after 5000 cycles | [51] |
| NiCo2S4/functionalized MWCNT | 6 M | 1360 F g−1 at 1 A g−1 | 80.6 after 10,000 cycles | [52] |
| NiCo2S4/MoS2 | 3 M | 2594 F g−1 at 0.8 A g−1 | 192 after 45,000 cycles | [54] |
| NiCo2S4/SnS2 | 1 M | 329.22 mAh g−1 at 2 A g−1 | 76.87 after 10,000 cycles | [72] |
| NiCo2S4/CoAl-LDH | 3 M | 2120 F g−1 at 1 A g−1 | 98.96 after 10,000 cycles | [73] |
| NiCo2S4/Mo-doped Co-LDH/carbon cloth | 3 M | 3049.3 F g−1 at 1 A g−1 | 91 after 10,000 cycles | [74] |
| FeNi2-LDH/FeNi2S4 | 1 M | 806 C g−1 at 1 A g−1 | 92.3 after 5000 cycles | [57] |
| Ni-Co-Mn-S | 6 M | 661 C g−1 at 1 A g−1 | - | [75] |
| Zn-Ni-Co-S/Zn-Ni-Co-O | 6 M | 1445 C g−1 at 1 A g−1 | 86.1 after 3000 cycles | [76] |
| NiZn2O4/NiZn2S4/NF | 1 M | 1516 C g−1 at 1 A g−1 | 86.9 after 10,000 cycles | [60] |
| Ni1.43Fe0.5Co0.5S0.97/NF | 6 M | 1156 C g−1 at 2 A g−1 | 92.9 after 10,000 cycles | [68] |
| Ni2ZnS4/RGO | 6 M | 1150 F g−1 at 1 A g−1 | 59.7 after 2000 cycles | [59] |
| Ni-Mn-S/RGO | 2 M | 2042.22 F g−1 at 1 A g−1 | 77.78 after 5000 cycles | [58] |
| Sl. N. | Materials | Overpotential (mV) at 10 mA cm−2 | Tafel Slope (mV dec−1) | Ref. |
|---|---|---|---|---|
| 1. | NiCo2S4/Carbon Nitrogen nanosheets | 360 | 76 | [95] |
| 2. | Zn-doped NiS | 320 (at 50 mA cm−2) | 36 | [80] |
| 3. | Ni1.5Co1.5S4 | 360 | 61 | [96] |
| 4. | Ni1.29Co1.49Mn0.22S4 | 348 | 65 | [92] |
| 5. | NiS | 362 (at 20 mA cm−2) | 65 | [84] |
| 6. | Co-Ni3S2/NF | 274 | 199 | [97] |
| 7. | Fe/W-doped Ni3S2 | 222 | 38 | [81] |
| 8. | Fe0.75Ni0.25S2/FeNiOOH | 247 | 47.6 | [91] |
| 9. | CuNiS | 337 | 43 | [98] |
| 10. | NiS | 210 | 60 | [99] |
| 11. | Ni3S2/CoSx | 226 | 56 | [100] |
| 12. | NiS/rGO | 162 | 32 | [85] |
| 13. | Ni3S2/FeNi2S4/NF | 235 | 92 | [93] |
| 14. | FeCO/Ni3S4 | 230 (at 20 mA cm−2) | 63.2 | [83] |
| 15. | V-doped Ni3S2/NF | 268 | 99 | [101] |
| 16. | N-doped CNTs/NiS2 | 235 | 88 | [102] |
| 17. | Mo-Ni3S4/Carbonized wood | 240 | 47.7 | [79] |
| 18. | P-doped Ni3S2/NiS/NF | 178 | 37 | [103] |
| 19. | Co-doped Ni3S2/NF | 297 (at 20 mA cm−2) | 50.3 | [104] |
| 20. | NiCo2S4/NiCo alloys/N-doped carbon fibers | 291 | 51.17 | [88] |
| 21. | Au/NiCo2S4 | 299 | 44.5 | [89] |
| 22. | NiCo2S4/S-doped g-C3N4 | 370 | 99.2 | [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhakal, G.; Sahoo, S.; Sharma, K.P.; Zhao, G.-L. A Review on the Recent Advancements of Ni-Based Sulfides and Mixed Sulfides for Supercapacitors and Electrocatalysis (Oxygen Evolution Reaction). Molecules 2025, 30, 2877. https://doi.org/10.3390/molecules30132877
Dhakal G, Sahoo S, Sharma KP, Zhao G-L. A Review on the Recent Advancements of Ni-Based Sulfides and Mixed Sulfides for Supercapacitors and Electrocatalysis (Oxygen Evolution Reaction). Molecules. 2025; 30(13):2877. https://doi.org/10.3390/molecules30132877
Chicago/Turabian StyleDhakal, Ganesh, Sumanta Sahoo, Krishna Prasad Sharma, and Guang-Lin Zhao. 2025. "A Review on the Recent Advancements of Ni-Based Sulfides and Mixed Sulfides for Supercapacitors and Electrocatalysis (Oxygen Evolution Reaction)" Molecules 30, no. 13: 2877. https://doi.org/10.3390/molecules30132877
APA StyleDhakal, G., Sahoo, S., Sharma, K. P., & Zhao, G.-L. (2025). A Review on the Recent Advancements of Ni-Based Sulfides and Mixed Sulfides for Supercapacitors and Electrocatalysis (Oxygen Evolution Reaction). Molecules, 30(13), 2877. https://doi.org/10.3390/molecules30132877








