Development and Validation of a Stability-Indicating RP-HPLC Method for Edaravone Quantification
Abstract
1. Introduction
2. Results and Discussion
2.1. HPLC Method Development
2.2. Specificity
2.3. Linearity and Sensitivity
2.4. Precision and Accuracy
3. Materials and Methods
3.1. Materials
3.2. HPLC Method Development and Final Chromatographic Conditions
3.3. Preparation of Calibration Standards for Optimised Method
3.4. Validation Methodology
3.4.1. Specificity
3.4.2. Linearity and Sensitivity
3.4.3. Precision and Accuracy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, C.; Li, M.; Lin, L.; Chen, S.; Chen, Y.; Hong, L. Clinical effects and safety of edaravone in treatment of acute ischaemic stroke: A meta-analysis of randomized controlled trials. J. Clin. Pharm. Ther. 2021, 46, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, J.D. Edaravone: A new drug approved for ALS. Cell 2017, 171, 725. [Google Scholar] [CrossRef] [PubMed]
- Genge, A.; Brooks, B.R.; Oskarsson, B.; Kalin, A.; Ji, M.; Apple, S.; Bower, L. Analysis of the US safety data for edaravone (Radicava®) from the third year after launch. Drugs R D 2022, 22, 205–211. [Google Scholar] [CrossRef] [PubMed]
- RADICAVA (Edaravone) Injection, for Intravenous use; RADICAVA ORS (Edaravone) Oral Suspension—accessdata.fda.gov. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215446s000lbl.pdf (accessed on 20 May 2025).
- FDA Approval of RADICAVA ORS® for the Treatment of ALS|News Releases|Mitsubishi Chemical Group Corporation. Available online: https://www.mcgc.com/english/news_release/01258.html (accessed on 18 September 2024).
- For the Treatment of ALS, Edaravone RADICUT® Oral Suspension 2.1% Now Available in Japan|News Releases|Mitsubishi Chemical Group Corporation. Available online: https://www.mcgc.com/english/news_release/01563.html (accessed on 21 May 2025).
- Parikh, A.; Kathawala, K.; Tan, C.C.; Garg, S.; Zhou, X.-F. Development of a novel oral delivery system of edaravone for enhancing bioavailability. Int. J. Pharm. 2016, 515, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Fanse, S.; Rajput, S. Development and validation of a simple UV spectrophometric and isocratic RP-HPLC method for estimation of edaravone in bulk and its injection formulation. Indo Am. J. Pharm. Res. 2015, 5, 584–592. [Google Scholar]
- Tanaka, M.; Sugimura, N.; Fujisawa, A.; Yamamoto, Y. Stabilizers of edaravone aqueous solution and their action mechanisms. 1. Sodium bisulfite. J. Clin. Biochem. Nutr. 2017, 61, 159–163. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zeng, J.; Ren, Y.; Zhou, C.; Yu, S.; Chen, W.-H. Preparation and physicochemical characteristics of the complex of edaravone with hydroxypropyl-β-cyclodextrin. Carbohydr. Polym. 2011, 83, 1101–1105. [Google Scholar] [CrossRef]
- Baghel, M.; Rajput, S.J. Stress degradation of edaravone: Separation, isolation and characterization of major degradation products. Biomed. Chromatogr. 2018, 32, e4146. [Google Scholar] [CrossRef] [PubMed]
- LB092-Analysis of Edaravone (Under the Condition of Japanese Pharmacopoeia)|Technical Information|GL Sciences. Available online: https://www.glsciences.com/technique/app/detail.php?data_number=LB092 (accessed on 10 March 2025).
- Patel, D.A.; Raj, H.A.; Patel, R. Development and validation of stability indicating reversed phase HPLC method for estimation of edaravone and argatroban in synthetic mixture. Int. J. Appl. Med. Sci. 2015, 1, 51–69. [Google Scholar]
- Yu, Y.-Y.; Zheng, X.-X.; Bian, T.-T.; Li, Y.-J.; Wu, X.-W.; Yang, D.-Z.; Jiang, S.-S.; Tang, D.-Q. Development and application of a LC-MS/MS assay for the simultaneous quantification of edaravone and taurine in beagle plasma. J. Sep. Sci. 2013, 36, 3837–3844. [Google Scholar] [CrossRef] [PubMed]
- Baghel, M.; Rajput, S. Development of stability indicating TLC-densitometry method of edaravone using QbD approach: Degradation kinetic study. Indian J. Pharm. Educ. Res. 2017, 51, S61–S68. [Google Scholar] [CrossRef]
- CAPCELL PAK ADME-HR Type|HPLC Columns|Products|HPLC|Osaka Soda. Available online: https://sub.osaka-soda.co.jp/HPLC/e/column/cpadme.html (accessed on 16 June 2025).
- ICH Q2(R2) Guideline on Validation of Analytical Procedures. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q2r2-guideline-validation-analytical-procedures-step-5-revision-1_en.pdf (accessed on 26 September 2024).
- Tanaka, M.; Motomiya, S.; Fujisawa, A.; Yamamoto, Y. Stabilizers of edaravone aqueous solution and their action mechanisms. 2. Glutathione. J. Clin. Biochem. Nutr. 2017, 61, 164–168. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bailly, C.; Hecquet, P.E.; Kouach, M.; Thuru, X.; Goossens, J.F. Chemical reactivity and uses of 1-phenyl-3-methyl-5-pyrazolone (PMP), also known as edaravone. Bioorg. Med. Chem. 2020, 28, 115463. [Google Scholar] [CrossRef] [PubMed]

| Reference | Fanse & Rajput [8] | Tanaka et al. [9] | Zeng et al. [10] | Baghel & Rajput [11] | JP [12] | Patel et al. [13] |
|---|---|---|---|---|---|---|
| Equipment | Shimadzu LC system; Shimadzu LC-20AT pump, Shimadzu SPD-20AV UV detector, Rheodyne 7725 injector, Spinchrome software | Not reported | Shimadzu HPLC system | Waters Acquity; PDA UV detector, Empower-2 software | LC800 HPLC system | Shimadzu LC system; Shimadzu LC-10 single pump, Shimadzu SPD-10A UV detector, Winchrome software |
| Stationary phase | Kromasil C18 (250 × 4.6 mm, 5 µm) | CAPCELL PAK ADME (250 × 4.6 mm, 5 µm) | Hedera ODS C18 (250 × 4.6 mm, 5 µm) | Thermo Scientific BDS Hypersil RP-C18 (250 × 4.6 mm, 5 µm) | Inertsil ODS-3 C18 (150 × 4.6 mm, 5 µm) | Inert cell C18 (250 × 4.6 mm, 5 µm) |
| Mobile phase | Isocratic: 55% water, 45% ACN | Isocratic: 60% MeOH, 40% NaHPO4 (aqueous; 40 mM) | Isocratic: 50% MeOH, 50% KH2PO4 (aqueous; 0.05 M; pH 3.5) | Gradient (time (min)/%B): 0.01/05, 04/15, 10/25, 12/45, 14/25, 16/15, 17/05, 20/05 A: Ammonium acetate buffer (10 mM; pH 5.8) B: 90:10 ACN:MeOH | Isocratic: 50% MeOH, 50% Acetic acid (1% in water) | Isocratic: 35% formic acid (0.1% in water), 33% ACN, 32% MeOH |
| Injection volume (µL) | 20 | Not reported | 20 | 20 | 10 | 20 |
| Flow rate (mL/min) | 1 | 0.5 | 1 | 0.8 | 0.95 | 1 |
| Detection wavelength (nm) | 243 | 295 | 240 | 244 | 240 | 260 |
| Temperature (°C) | Ambient | Not reported | 35 | 34 | 40 | Ambient |
| Description of peak | Chromatogram showed a sharp and symmetrical peak | Chromatogram not provided | Chromatogram not provided | Chromatogram showed a sharp and symmetrical peak | Chromatogram showed a sharp and symmetrical peak | Chromatogram showed a sharp and symmetrical peak |
| Edaravone RT (min) | 4.25 | Not reported | 8.5 | Approx. 11.6, estimated from published chromatogram | Approx. 4, estimated from published chromatogram | 4.13 |
| Linearity concentration range (µg/mL) | 10–80 | Not reported | 10.15–101.5 | 10–300 | Not reported | 120–360 |
| DL (µg/mL) | 0.3566 | Not reported | Not reported | 0.5341 | Not reported | 0.88 |
| QL (µg/mL) | 1.08 | Not reported | Not reported | 1.6186 | Not reported | 2.66 |
| Precision (RSD) | Intraday: 0.1378% (n = 3) Interday: 1.0239% (n = 9) | Not reported | > 5% RSD | Intraday: 0.2011% Interday: 0.3035% | Not reported | Intraday: 0.032–0.049% (n = 9) Interday: 0.086–0.094% (n = 9) |
| Accuracy (% drug recovery) | 99–101% (n = 9) | Not reported | Not reported | > 99% | Not reported | 100.01–100.19% (n = 9) |
| Specificity | Complete separation of the drug peak from peaks of unnamed excipients | Not reported | Not reported | Complete separation of the drug peak from its degradation products | Not reported | Complete separation of the drug peak from its degradation products and from argatroban |
| Stability indicating? | Not reported | Not reported | Not reported | Yes; the drug was subjected to acid, base, oxidative, thermal, photolytic, and high-humidity degradation conditions | Not reported | Yes; the drug was subjected to acid, base, oxidative, hydrolytic, thermal, and photolytic degradation conditions |
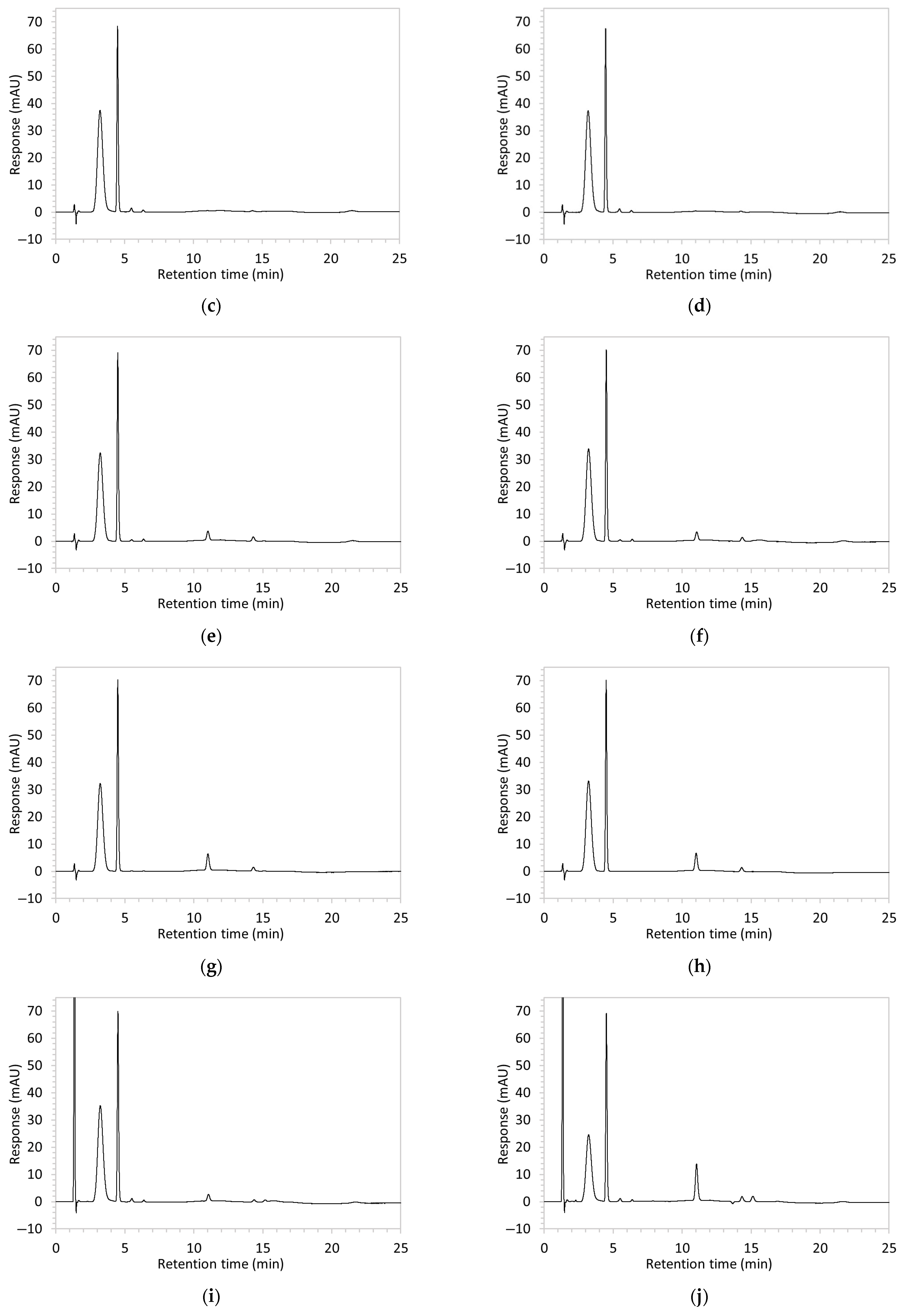
| Method | Chromatogram | Results | Changes Made to Resolve Issues, Leading to Next Method |
| 1 * | 100 µg/mL edaravone: | No apparent drug peak observed. | Mobile phase composition was modified. |
| 2 * | 100 µg/mL edaravone: | Drug peak observed, but peak was broad (1.9 min wide). | Increased the proportion of organic solvent in mobile phase to facilitate faster elution of edaravone. |
| 3 | 100 µg/mL edaravone: | No drug peak observed; a new peak appeared at RT 3.1 min, also present in the blank. | Changed isocratic method to gradient elution to improve control over edaravone elution. |
| 4 | 100 µg/mL edaravone: | No drug peak observed; peak present at RT 3.7 was also present in the blank. | Modified both mobile phase and the sample solvent. Reverted to isocratic elution. |
| 5 * | 100 µg/mL edaravone: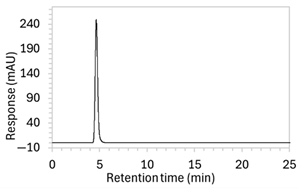 | Drug peak detected but significant tailing resulted in increased peak width (2.1 min wide, including tail end). | Mobile phase composition was adjusted. |
| 6 * | 100 µg/mL edaravone: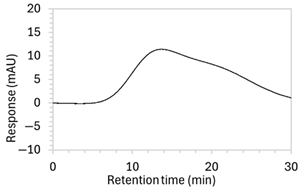 | No drug peak observed. | Further modifications to the mobile phase. |
| 7 * | 100 µg/mL edaravone: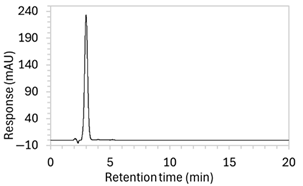 | Drug peak was sharp and symmetrical (1.2 min wide) but eluted too early, resulting in poor resolution from void signal. | Decreased the organic solvent percentage in the mobile phase to slow edaravone elution. Injection volume was reduced to counteract peak broadening. |
| 8 |
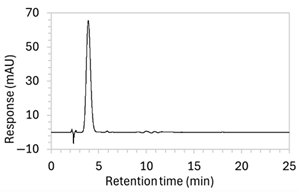
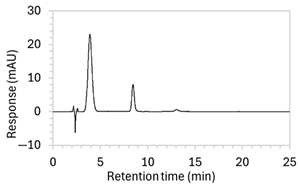 |
| The percentage of organic solvent in the mobile phase was decreased to slow elution and enhance separation from void signals. To counteract peak broadening due to slower elution, a shorter HPLC column was used to moderate elution rate. |
| 9 |
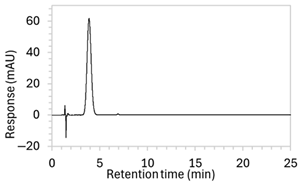
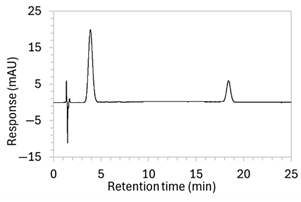 |
| Changed to gradient elution method to retain separation between the drug and void signals while ensuring complete elution of all degradants. The detection wavelength was changed from 243 to 250 nm to enhance peak visibility. Internal standard was included. |
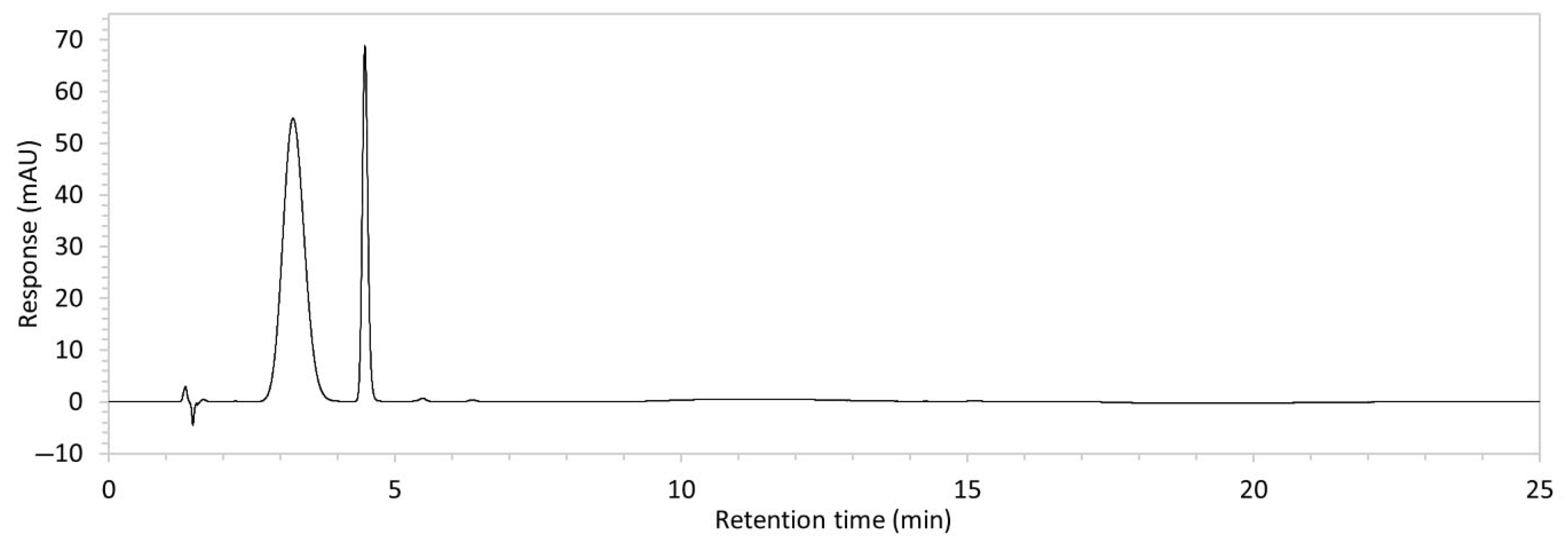
| 10 | See Figure 1 and Figure 2. | Optimised conditions achieved. Drug peak was sharp (1.5 min wide) with complete separation from all degradants. | - |
| Edaravone Peak | IS (MHB) 5 Peak | Degradant Peaks | ||||||
|---|---|---|---|---|---|---|---|---|
| Peak RT (min) | 3.2 | 4.5 | 2.2 | 11.0 | 13.9 | 14.4 | 15.6 | |
| Forced Degradation Condition | Drug Content (% of Water-Exposed Edaravone) | Peak AUC | ||||||
| Water | 100 | 1055.5 | 468.4 | - | - | - | 3.9 | - |
| Water + heating 1 | 99.1 | 1045.6 | 459.8 | - | 1.6 | - | 5.4 | - |
| Acid 2 | 83.4 | 880.5 | 471.2 | - | 43.2 | - | 20.5 | - |
| Acid + heating | 87.8 | 926.7 | 482.1 | - | 39.5 | - | 18.6 | - |
| Base 3 | 83.9 | 885.7 | 478.0 | - | 76.7 | - | 18.1 | - |
| Base + heating | 86.3 | 910.5 | 479.8 | - | 81.4 | - | 19.7 | - |
| Peroxide 4 | 94.5 | 997.0 | 482.4 | 0.8 | 31.9 | - | 11.1 | 8.2 |
| Peroxide + heating | 67.0 | 707.2 | 476.4 | 4.6 | 170.1 | 5.5 | 24.5 | 27.6 |
| Run | Linearity Range (µg/mL) | Regression Equation | Coefficient of Correlation (R) | Slope (Average) | Y-Intercept (SD) | DL (µg/mL) | QL (µg/mL) |
|---|---|---|---|---|---|---|---|
| 1 | 0–90.9 | y = 15.6013x − 1.5570 | 0.999981 | 15.5859 | 1.6041 | 0.340 | 1.03 |
| 2 | 0–90.9 | y = 15.7426x − 0.4545 | 0.999940 | ||||
| 3 | 0–90.9 | y = 15.4136x − 1.6134 | 0.999988 |
| Run | Linearity Range (µg/mL) | Regression Equation | Coefficient of Correlation (R) | Slope (Average) | Y-Intercept (SD) | DL (µg/mL) | QL (µg/mL) |
|---|---|---|---|---|---|---|---|
| 1 | 0–90.9 | y = 0.0338x − 0.0022 | 0.999941 | 0.0333 | 0.0036 | 0.352 | 1.07 |
| 2 | 0–90.9 | y = 0.0333x − 0.0012 | 0.999958 | ||||
| 3 | 0–90.9 | y = 0.0328x − 0.0050 | 0.999988 |
| Nominal Edaravone Concentration (µg/mL) | Measured Edaravone Concentrations (µg/mL) | Accuracy (Mean Measured Drug Recovery ± SD (%)) | Precision (RSD (%)) | |||
|---|---|---|---|---|---|---|
| Without IS | With IS | Without IS | With IS | Without IS | With IS | |
| 1.8 | 1.76 1.76 1.73 | 1.80 1.77 1.76 | 95.7 ± 0.8 | 97.2 ± 1.1 | 0.9 | 1.2 |
| 6.8 | 6.931 6.945 6.970 | 6.971 6.883 6.953 | 101.4 ± 0.3 | 101.2 ± 0.7 | 0.3 | 0.7 |
| 22.7 | 23.37 23.26 23.31 | 23.60 23.47 23.70 | 102.1 ± 0.2 | 103.3 ± 0.5 | 0.2 | 0.5 |
| 68.2 | 70.97 70.94 70.93 | 71.48 72.21 72.61 | 103.5 ± 0.0 | 105.2 ± 0.8 | 0.0 | 0.8 |
| 113.6 | 119.7 119.7 119.4 | 120.7 120.3 118.6 | 104.7 ± 0.1 | 105.0 ± 1.0 | 0.1 | 0.9 |
| Nominal Edaravone Concentration (µg/mL) | Measured Edaravone Concentrations (µg/mL) | Mean Measured Drug Recovery ± SD (%) | Precision (RSD (%)) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Without IS | With IS | Without IS | With IS | Without IS | With IS | |||||
| Day 1 | Day 2 | Day 3 | Day 1 | Day 2 | Day 3 | |||||
| 1.8 | 1.8 1.8 1.7 | 2.0 2.0 2.0 | 1.9 1.9 1.9 | 1.8 1.8 1.8 | 2.0 2.0 2.0 | 2.1 2.1 2.0 | 100.7 ± 3.9 | 101.7 ± 3.5 | 3.9 | 3.4 |
| 6.8 | 6.9 6.9 7.0 | 7.4 7.5 7.4 | 7.2 7.3 7.1 | 7.0 6.9 7.0 | 7.5 7.4 7.5 | 7.4 7.4 7.5 | 102.3 ± 1.1 | 102.8 ± 1.5 | 1.0 | 1.4 |
| 22.7 | 23.4 23.3 23.3 | 25.3 25.2 25.2 | 23.5 24.0 23.4 | 23.6 23.5 23.7 | 25.2 25.0 25.0 | 24.3 24.4 23.9 | 102.7 ± 1.8 | 102.9 ± 1.4 | 1.7 | 1.4 |
| 68.2 | 71.0 70.9 70.9 | 77.3 76.7 75.5 | 71.8 73.4 71.9 | 71.5 72.2 72.6 | 77.7 77.9 78.0 | 74.2 73.3 74.3 | 104.3 ± 1.5 | 105.5 ± 2.0 | 1.4 | 1.9 |
| 113.6 | 119.7 119.7 119.4 | 126.6 128.7 129.0 | 122.6 122.2 125.0 | 120.7 120.3 118.6 | 129.7 129.8 130.3 | 127.0 126.0 127.3 | 105.6 ± 1.1 | 106.2 ± 1.5 | 1.0 | 1.4 |
| Method | Column | Mobile Phase | Injection Volume (µL) | Flow Rate (mL/min) | Detection Wavelength (nm) | Calibration Standard Solution |
|---|---|---|---|---|---|---|
| 1 | Agilent ZORBAX Extend-C18 column (250 × 4.6 mm, 5 µm) | Adapted from method of Baghel & Rajput [11] Gradient (time (min)/%B): 0.01/05, 04/15, 10/25, 12/45, 14/25, 16/15, 17/05, 20/05 A: Ammonium acetate buffer (10 mM; pH 5.8) B: 90:10 ACN:MeOH | 20 [8,10,11,13] | 1 [8,10,13] | 243 [8] | Edaravone in ACN [8] |
| 2 | Adapted from method of Fanse & Rajput [8] Isocratic: 55% water 45% ACN | |||||
| 3 | Isocratic: 25% water 75% ACN | |||||
| 4 | Gradient (time (min)/B%): 0/25, 7/25, 12/100, 18/100, 20/25, 25/25 A: water B: ACN | |||||
| 5 | Adapted from method of Tanaka et al. [9] Isocratic: 50% MeOH 50% Na2HPO4 (aqueous; 10 mM; pH 5) | Edaravone in MeOH | ||||
| 6 | Adapted from JP method [12] Isocratic: 25% MeOH 75% Acetic acid (0.1% in water; to pH 5.5 with aqueous NH3) | |||||
| 7 | Adapted from method of Patel et al. [13] Isocratic: 35% formic acid (0.1% in water) 65% 50:50 ACN:MeOH | |||||
| 8 | Isocratic: 50% formic acid (0.1% in water) 50% 50:50 ACN:MeOH | 5 | ||||
| 9 | Agilent ZORBAX Extend-C18 column (150 × 4.6 mm, 5 µm) | Isocratic: 65% formic acid (0.1% in water) 35% 50:50 ACN:MeOH | ||||
| 10 | Gradient (time (min)/B%): 0/40, 7/40, 11/50, 16/50, 20/40, 25/40 A: formic acid (0.1% in water) B: 50:50 ACN:MeOH | 250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Neill, R.; Yoo, O.; Burcham, P.; Nguyen, M.; Lim, L.Y. Development and Validation of a Stability-Indicating RP-HPLC Method for Edaravone Quantification. Molecules 2025, 30, 2866. https://doi.org/10.3390/molecules30132866
O’Neill R, Yoo O, Burcham P, Nguyen M, Lim LY. Development and Validation of a Stability-Indicating RP-HPLC Method for Edaravone Quantification. Molecules. 2025; 30(13):2866. https://doi.org/10.3390/molecules30132866
Chicago/Turabian StyleO’Neill, Riuna, Okhee Yoo, Philip Burcham, Minh Nguyen, and Lee Yong Lim. 2025. "Development and Validation of a Stability-Indicating RP-HPLC Method for Edaravone Quantification" Molecules 30, no. 13: 2866. https://doi.org/10.3390/molecules30132866
APA StyleO’Neill, R., Yoo, O., Burcham, P., Nguyen, M., & Lim, L. Y. (2025). Development and Validation of a Stability-Indicating RP-HPLC Method for Edaravone Quantification. Molecules, 30(13), 2866. https://doi.org/10.3390/molecules30132866









