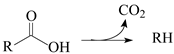1. Introduction
Intensive global efforts have been devoted to developing hydrocarbon biofuels, which offer a compelling alternative for diversifying the world’s energy matrix and making it more sustainable. Hydrocarbon biofuels exhibit properties and performance akin to those of petroleum fuels, can utilize existing supply infrastructure (tanks, pipelines, pumps, etc.), can be used without engine modification, and can be blended with conventional fuels in any proportion, thus complying with the “drop-in” concept. These issues are particularly relevant for producing SAF (sustainable aviation fuel), given the risks inherent to the aviation sector. However, diesel-like hydrocarbon production has also increased [
1,
2,
3].
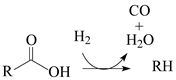
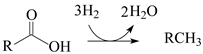
Currently, HEFA (hydroprocessing of esters and fatty acids) is the most advanced technology available to produce hydrocarbon biofuels. The key step in HEFA is the deoxygenation of fatty chains resulting from the split of acylglycerides that constitute lipid feedstocks (vegetable, animal, and algal oils and fats). This is achieved through a process similar to that employed in the hydroprocessing of petroleum and its derivatives. i.e., thermal treatment at temperatures between around 300 and 400 °C, under elevated H
2 pressure (~20–100 bar), and in the presence of a heterogeneous catalyst [
1,
2,
3,
4,
5,
6]. This procedure is usually called HDO (hydrodeoxygenation). The possibility of retrofitting existing petroleum refinery facilities to perform HEFA is a major advantage of this process.
The HDO of lipid feedstocks and other O-rich mixtures (e.g., lignocellulose-derived bio-oils) can be carried out in the presence of the same catalysts employed in the hydroprocessing of petroleum and its derivatives, i.e., sulfides of group 6 transition metals (Mo or W), promoted by a transition metal with a higher number of d electrons (Ni or Co), deposited on porous oxide supports (mainly γ-Al
2O
3) [
4,
5,
6,
7,
8,
9]. However, some published research has stressed that these catalysts are not ideal for hydroprocessing O-rich mixtures. From a support perspective, challenges are presented by the fact that inorganic materials such as γ-Al
2O
3 are susceptible to hydrolysis by the abundant water formed during the process, which can lead to catalyst deactivation [
10]. In turn, from the perspective of the metal-containing phase, the fact that the formed water (and possibly other oxygenated molecules such as CO
2) causes sulfur replacement by oxygen, which gradually reduces the HDO catalyst activity [
4,
11,
12,
13,
14,
15,
16], presents an additional challenge. To overcome this problem, some authors have added sulfiding agents like dimethyl disulfide (DMDS) or H
2S [
4,
11,
12,
13,
14,
15,
16,
17] alongside the main feed. Another reported issue with using sulfided catalysts in HDO is their potential to contaminate the produced biofuel with sulfur. This contamination can originate from catalyst leaching or the co-fed sulfiding agents [
13,
14,
15,
18]. This is particularly concerning given that, unlike petroleum derivatives, biofuels are naturally nearly sulfur-free.
In this scenario, the present work aimed to develop sulfur-free heterogeneous catalysts with improved activity and stability for the HDO of lipid feedstocks. To reach this goal, metallic Pt was used as the active phase, supported by homemade activated carbons (ACs).
The choice to use ACs as a support was mainly justified by their high thermal and chemical stability in both acidic and basic media, including resistance to hydrolysis [
18,
19,
20]. Furthermore, ACs exhibit low acidity, which contributes to reduce hydrocracking and coke formation [
19,
21,
22]; present larger surfaces areas than those of inorganic supports; display a pore size distribution and surface chemistry that can be tailored according to the envisaged application [
23,
24,
25,
26]; can be obtained from cheap and abundant precursors such as biomass residues, coal, and petroleum coke; facilitate the recovery of the metallic phase from spent catalysts by burning the carbon [
18,
19]; can be produced in various forms, such as powder, grains, fibers, pellets and monoliths, providing great versatility [
26,
27,
28]; and present good mechanical properties, depending on the precursor and the employed activation methodology and conditions [
25,
26,
29].
Concerning the metallic phase, Pt is well-known for its ability to activate a wide range of reactions, including hydrogenation, dehydrogenation, hydrogenolysis, and oxygen electroreduction. Pt supported on the surface of acidic porous solids has been employed to promote the hydroisomerization of
n-alkanes into iso-alkanes [
30,
31,
32,
33], which allows for improvement in the cold properties of hydrocarbon fuels, making them more appropriate for use in the aviation sector [
1]. Furthermore, Pt deposited on different inorganic supports (e.g., γ-Al
2O
3, SiO
2, Nb
2O
5, ZrO
2, TiO
2, and several zeolites) has been used to promote HDO, as reported in several reviews [
2,
3,
4,
5,
6,
11,
12,
13,
34,
35,
36]. Notably, Chen et al. [
37] employed Pt deposited on SAPO-11 zeolite to promote the hydroconversion of Jatropha oil into a hydrocarbon mixture with a high iso-alkane content (HDO and hydroisomerization took place in a one-step process).
Nevertheless, the literature contains few studies regarding the use of Pt supported on ACs (Pt/ACs) in the HDO of lipid feedstocks. Among these few works, Jin et al. [
38] reported a high conversion of palm oil into alkanes (>90%) during hydroprocessing in a fixed-bed reactor (T = 300 °C, 30 bar of H
2, and LHSV = 1.5 h
−1) over Pt (1 wt%) supported on the surface of a N-doped AC. In addition to excellent performance, the catalyst showed notable stability. The authors concluded that the nitrogen incorporated into the carbon favored the interaction with carbonyls, thereby boosting decarboxylation reactions. In turn, Makcharoen et al. [
39] investigated the effects of various operating parameters on the hydroprocessing of crude palm kernel oil (CPKO) over an AC-supported Pt catalyst (5 wt% of Pt) in a fixed-bed reactor. The best result, with a yield of 58.3% in biojet fuel, was obtained at operating conditions of 420 °C, 34.5 bar, with a H
2 flow of 17.5 mL min
−1, a CPKO flow of 0.02 mL min
−1, and a H
2-to-oil molar ratio of 28.0.
At this point, it is worth mentioning that the high cost of Pt demands the optimization of its catalytic activity. However, despite extensive research on the use of Pt/AC catalysts across a large range of applications, there are currently no definitive conclusions regarding the methods and conditions that yield optimal catalytic activity, which can be attributed to the complexity of the system and the large number of variables to be considered. In general, high catalyst activity is considered to be directly related to a high dispersion of the metallic phase on the surface of a solid with appropriate porosity. The dispersion depends on factors such as the properties of the support (surface area and surface chemistry), the nature of the metal precursor, the impregnation procedure employed, the polarity of the solvent, and the pH of the impregnating solution. In turn, appropriate porosity results in a pore size distribution that not only provides a high surface area for metal dispersion but also ensures the accessibility of the reactants and products to and from the catalytic active sites within the pore network.
The described scenario sparked our interest in further investigating the use of Pt/AC catalysts in the HDO of lipid feedstocks, with a particular focus on identifying the optimal conditions for obtaining catalysts with improved performance. We investigated the influence of the support surface chemistry (ACs with varying contents of oxygenated functional groups were synthesized and employed), the impregnation methodology (incipient wetness impregnation versus wet impregnation), and the acidification of the impregnating solution with HCl.
3. Materials and Methods
3.1. P54 Preparation
A dried endocarp of coconut shell (
Cocos nucifera L.) originating from the Federal District region of Brazil, was used as raw material for synthesis of the AC P54. The endocarp was crushed and sieved, and the fraction in the range of 40–80 mesh (0.177–0.400 mm) was chemically activated with H
3PO
4 (85%; Vetec, Speyer, Germany), following a procedure described elsewhere [
40]. Briefly, the raw material was impregnated with an aqueous solution containing the desired amount of H
3PO
4 and heated to 450 °C under an inert atmosphere (N
2). Afterwards, the carbonized material was washed with deionized water to remove the chemicals.
3.2. P54 Modification
P54 was treated with a 1.0 mol L−1 HNO3 solution. For this purpose, 20 g of P54 were added to a beaker jar containing 200 mL of the acidic solution. The mixture was kept under reflux (~75 °C) and magnetic stirring for 1 h. After cooling, the solid was separated by filtration, washed with deionized water until a stable pH of approximately 6 was reached, and then dried at 110 °C overnight.
P54 was also treated up to 800 °C (2 h; 5 °C min−1) under a H2 atmosphere (~100 mL min−1) in a horizontal tubular furnace.
3.3. Preparation of the Pt Catalysts
Pt was deposited on the ACs from an aqueous solution of hexachloroplatinic acid (H2PtCl6 · 6H2O; ≥99.9%; Sigma Aldrich, St. Louis, MI, USA) via two different methodologies: incipient wetness impregnation and wet impregnation. In the former, the impregnating solution was added dropwise over the AC, so that it diffused through the pores by capillarity. The solution volume (determined through preliminary tests on aliquot samples) corresponded to the maximum volume that could be added before external wetness was observed on the material surface. Afterwards, the material was dried overnight at 110 °C. In the case of wet impregnation, an excess of impregnating solution was used (around 13 mL per gram of AC). The system was closed and kept under stirring for 48 h at 50 °C. After that, the excess water was evaporated by opening the vessel. Finally, the material was dried overnight at 110 °C.
The employed impregnating solutions contained H2PtCl6 in a concentration corresponding to 1.0 wt% Pt relative to the AC support. In some tests, the solution was acidified with HCl in an amount equivalent to eight times the molar amount of Pt.
After impregnation, the material was reduced under a H2 atmosphere (~100 mL min−1) at 400 °C (2 h).
3.4. Characterization of ACs and Catalysts
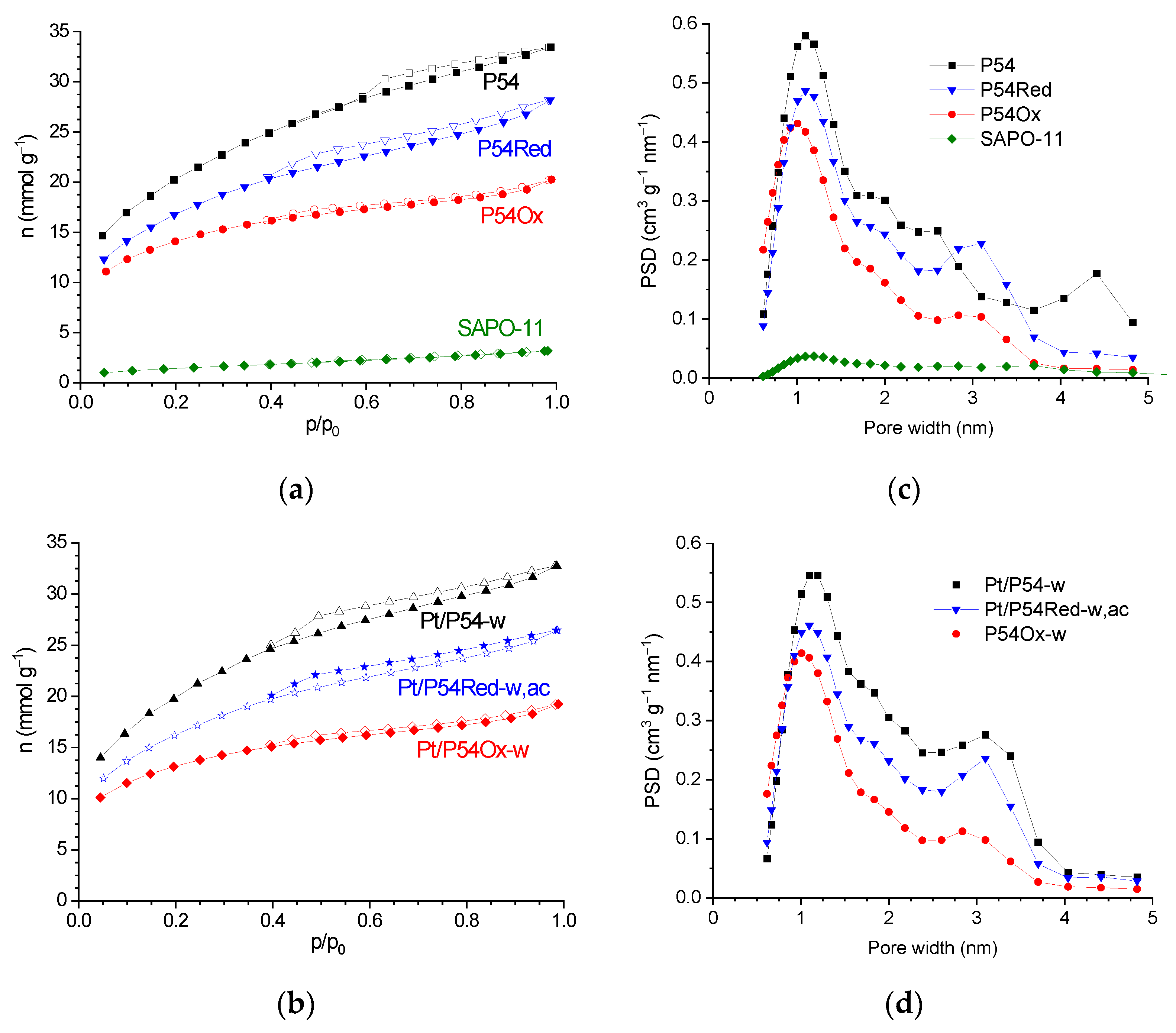
The textural properties were characterized by adsorption/desorption isotherms of N2 (−196 °C) obtained on a Quantachrome NovaWin 2200e volumetric automatic system (Boynton Beach, FL, USA). Specific surface area and Vmic were determined by applying the Brunauer–Emmett–Teller (BET) and Dubinin–Radushkevich (DR) equations, respectively. The volume of liquid N2 adsorbed at p/p0 0.95 was termed V0.95, which was considered the sum of Vmic and Vmes. Therefore, Vmes was determined by subtracting Vmic from V0.95. The PSD curves were obtained using AsiQwin software (version 4.0, Quantachrome Instruments, Boynton Beach, FL, USA), applying the quenched solid density functional theory (QSDFT) to the N2 adsorption data.
The C, H, and N content of the ACs was determined by EA using a Parkin Elmer analyzer model EA 2400 Series II equipped with an AD6 ultra-microbalance (Waltham, MA, USA). The ash content was determined on a dry basis by heating the material at 600 °C for 6 h in a muffle furnace open to ambient air. The bulk content of Pt was determined by ICP/OES analyses carried out on an Agilent 5100 device (Santa Clara, CA, USA).
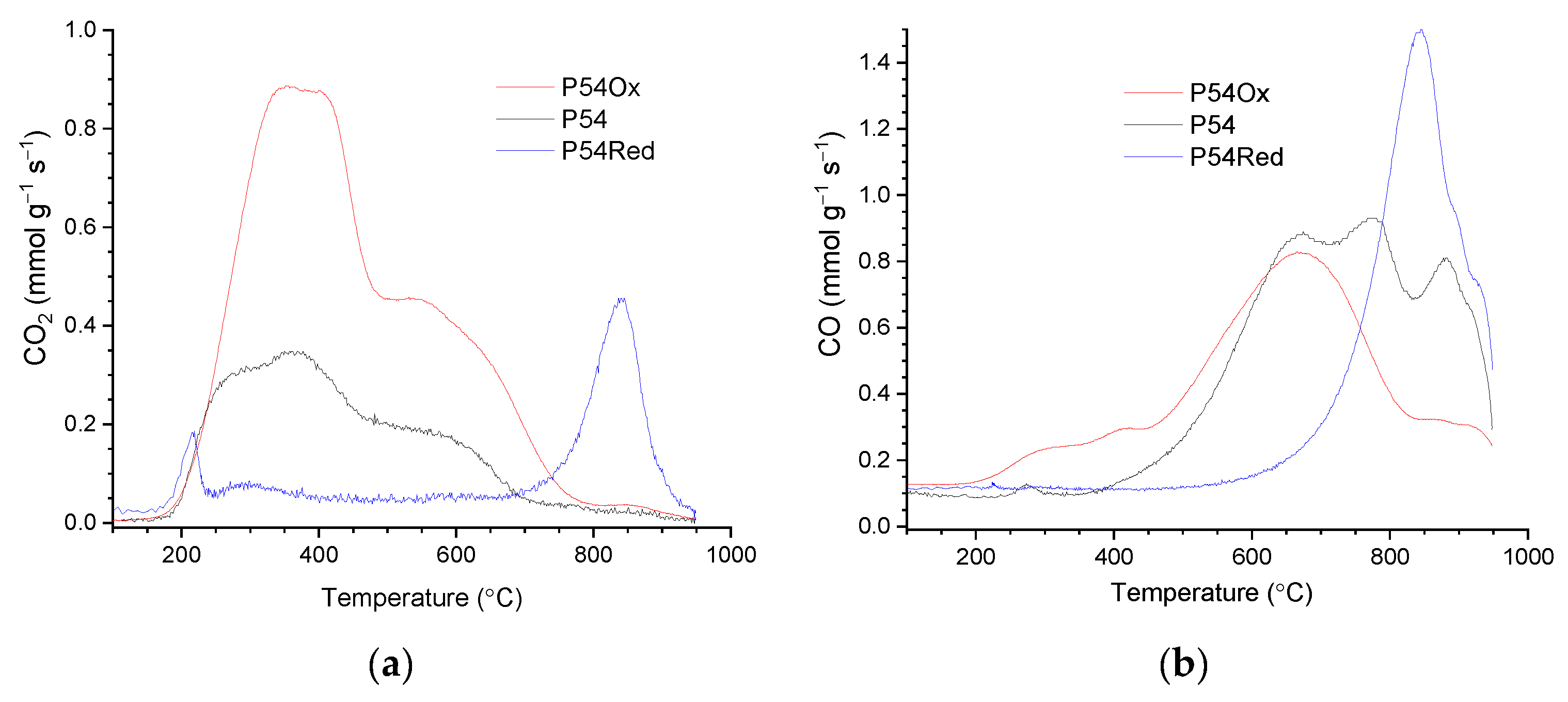
TPD/MS analyses were carried out on an automated reaction system model AMI-90R coupled to a Dymaxion quadrupole mass spectrometer (Altamira Instruments, Pittsburgh, PA, USA). The sample (~100 mg) was placed in a U-tube of quartz under Ar flow (10 cm
3 min
−1; atmospheric pressure). The system was first heated to 110 °C (10 °C min
−1; 30 min) to release the physically adsorbed molecules. The temperature was then raised to 950 °C (10 °C min
−1). The emitted gases were monitored with the mass spectrometer. The CO and CO
2-profiles were fitted assuming a Gaussian distribution for each peak. The peak areas were correlated to the amount of gas present through a routine daily calibration that consisted of the injection of a known volume of pure gas using a calibrated loop [
62].
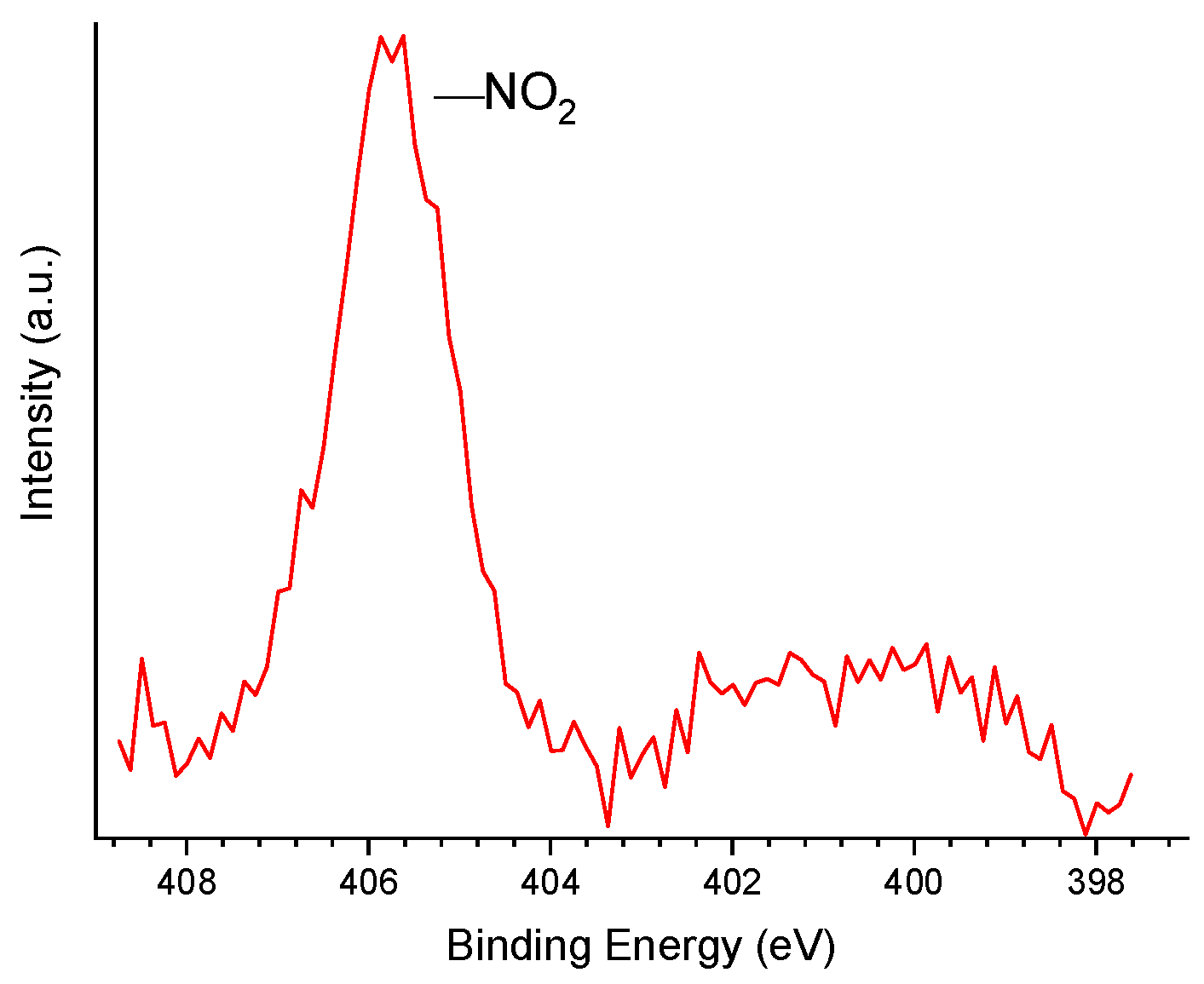
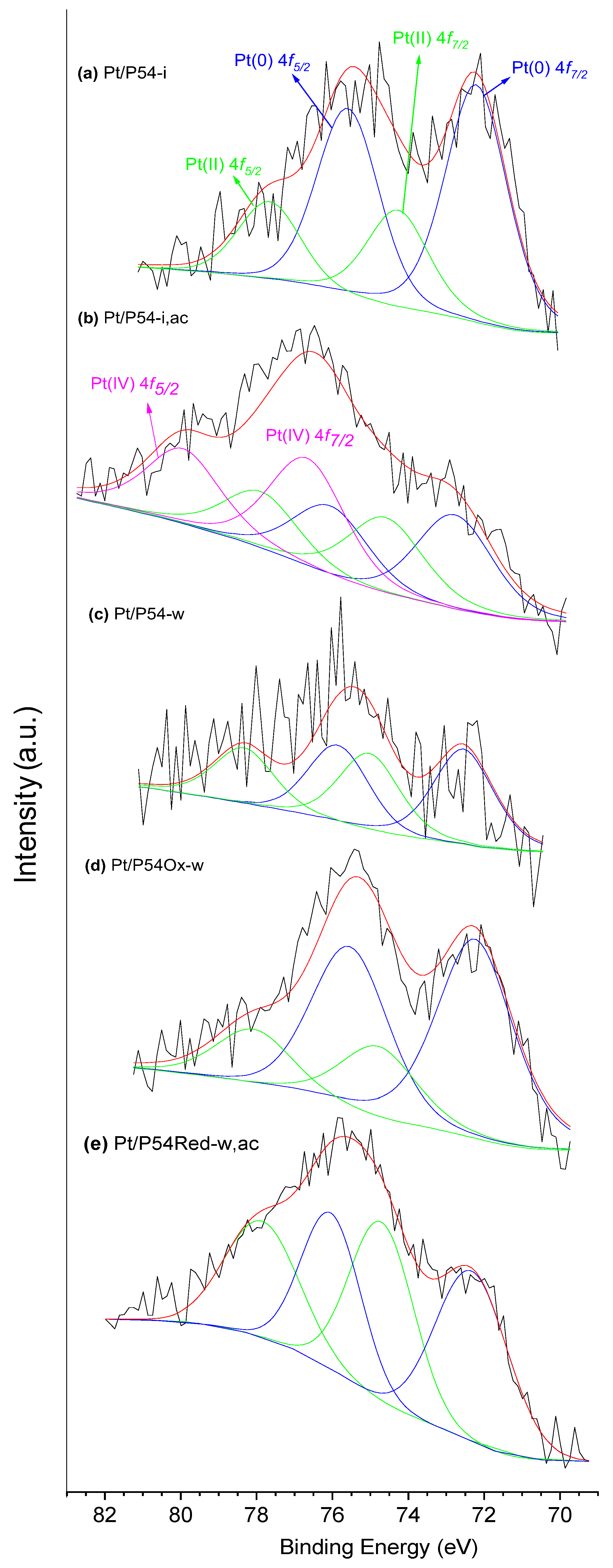
XPS measurements were performed on a Physical Electronics 5700 spectrometer using the Mg-Kα line (1253.6 eV) generated by a PHI model 04-548 Dual Anode X-rays Source (Chanhassen, MN, USA) operating at 300 W (10 keV and 30 mA). The pressure inside the vacuum chamber was 5 × 10−7 Pa. A Perkin Elmer 10-360 hemispherical analyzer (Eden Prairie, MN, USA) with a multi-channel detector was employed. The samples were analyzed at an incidence angle of 45° relative to the surface plane. The BEs were referred to the C 1s line of adventitious carbon at 284.8 eV and determined with the resolution of ±0.1 eV. The spectra were fitted assuming a Gaussian–Lorentzian distribution for each peak.
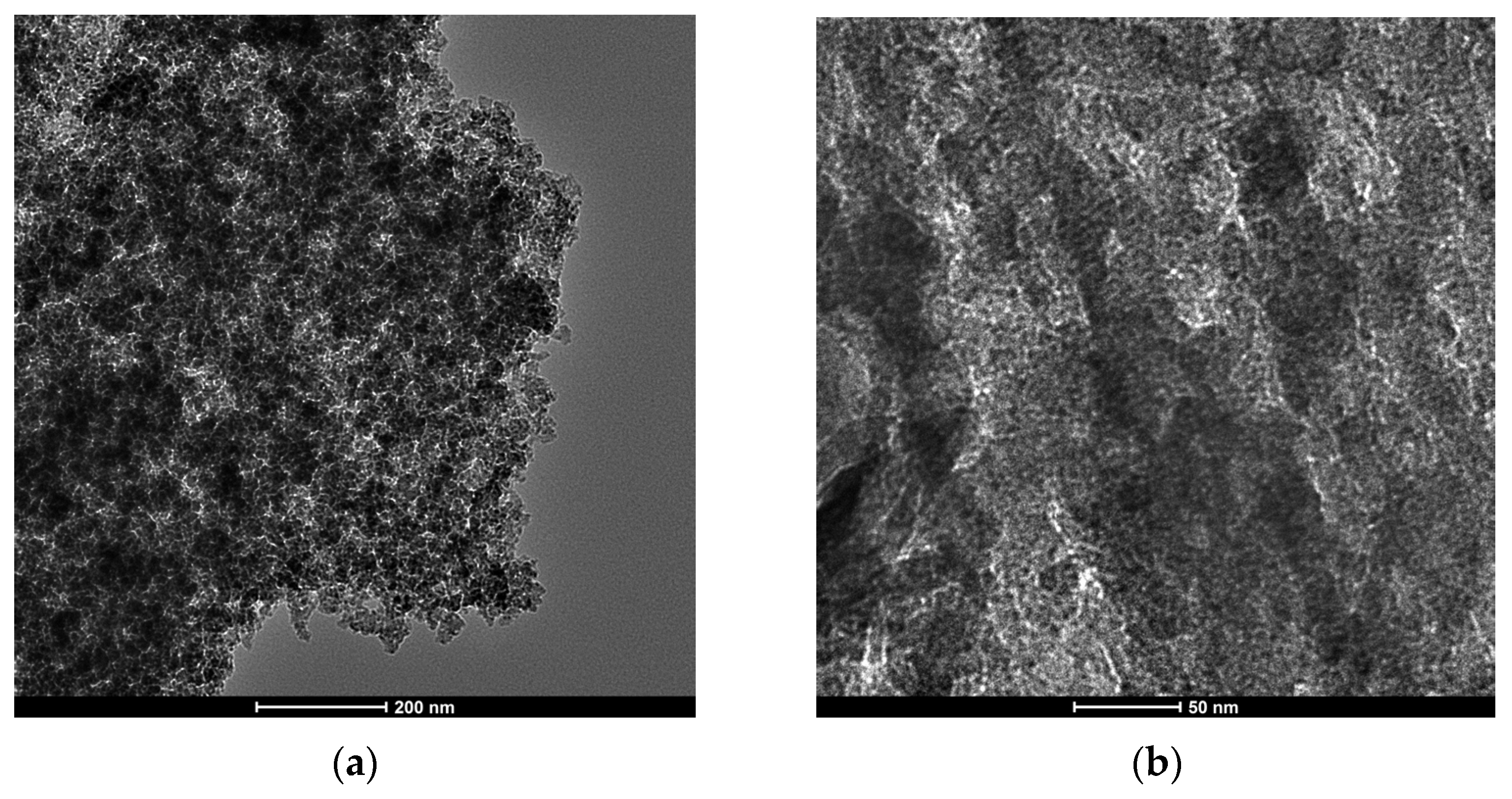
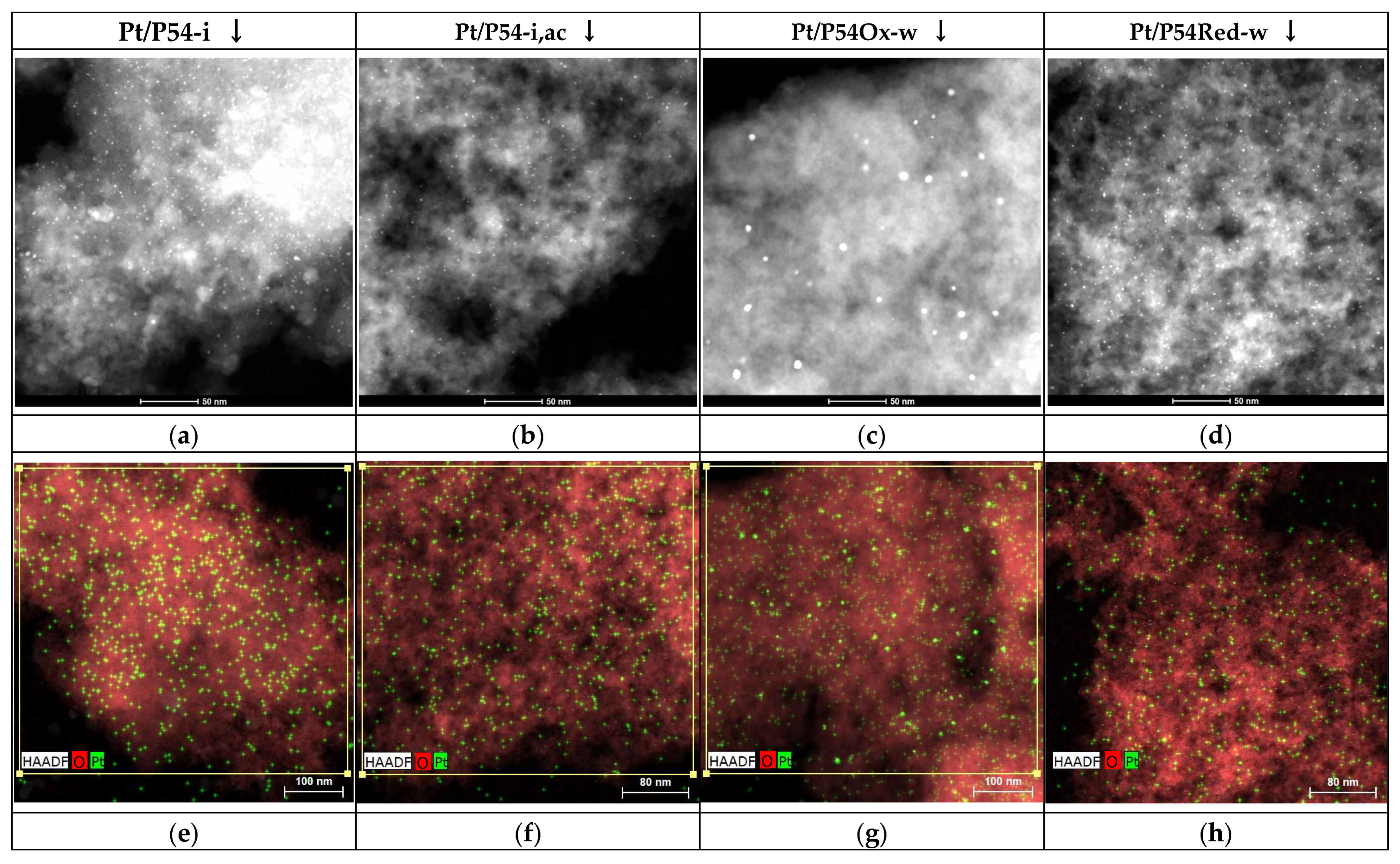
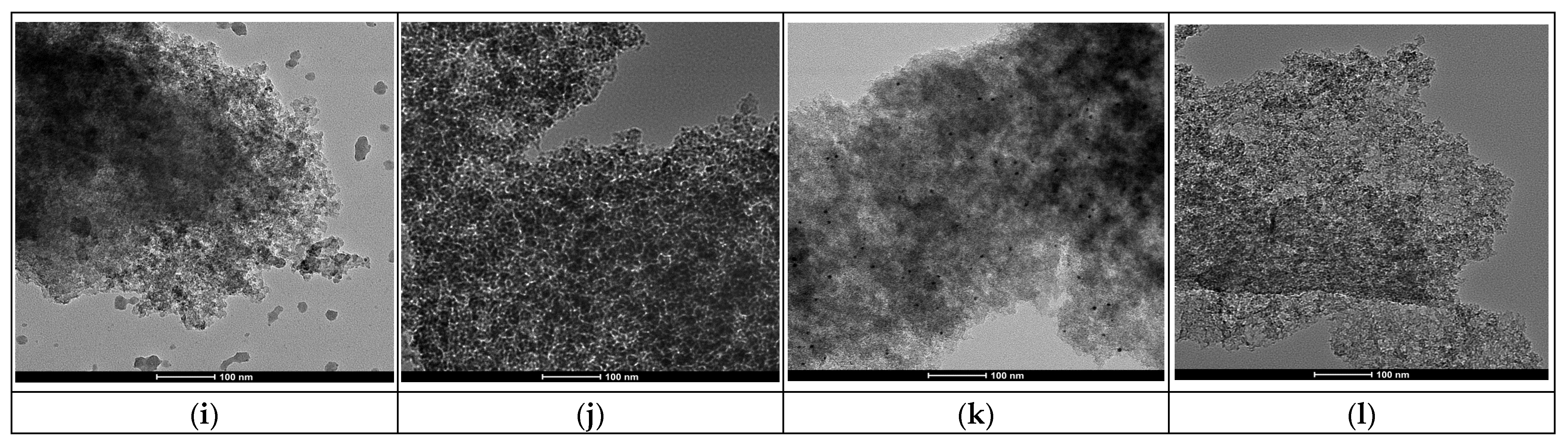
HR-TEM was carried out with a TALOS F200x instrument operating in STEM (scanning transmission electron microscopy) mode at 200 kV and 200 nA, equipped with a HAADF (high-angle annular dark-field) detector (Thermo Fisher Scientific, Waltham, MA, USA). The elemental mapping was carried out on an EDX Super-X system equipped with four X-ray detectors and an X-FEG (field emission gun) beam (Thermo Fisher Scientific, Waltham, MA, USA). Pt particle size distribution was calculated using the free software Image J 1.51k. At least 700 platinum particles from up to three different regions of each catalyst were analyzed to obtain a representative statistical analysis.
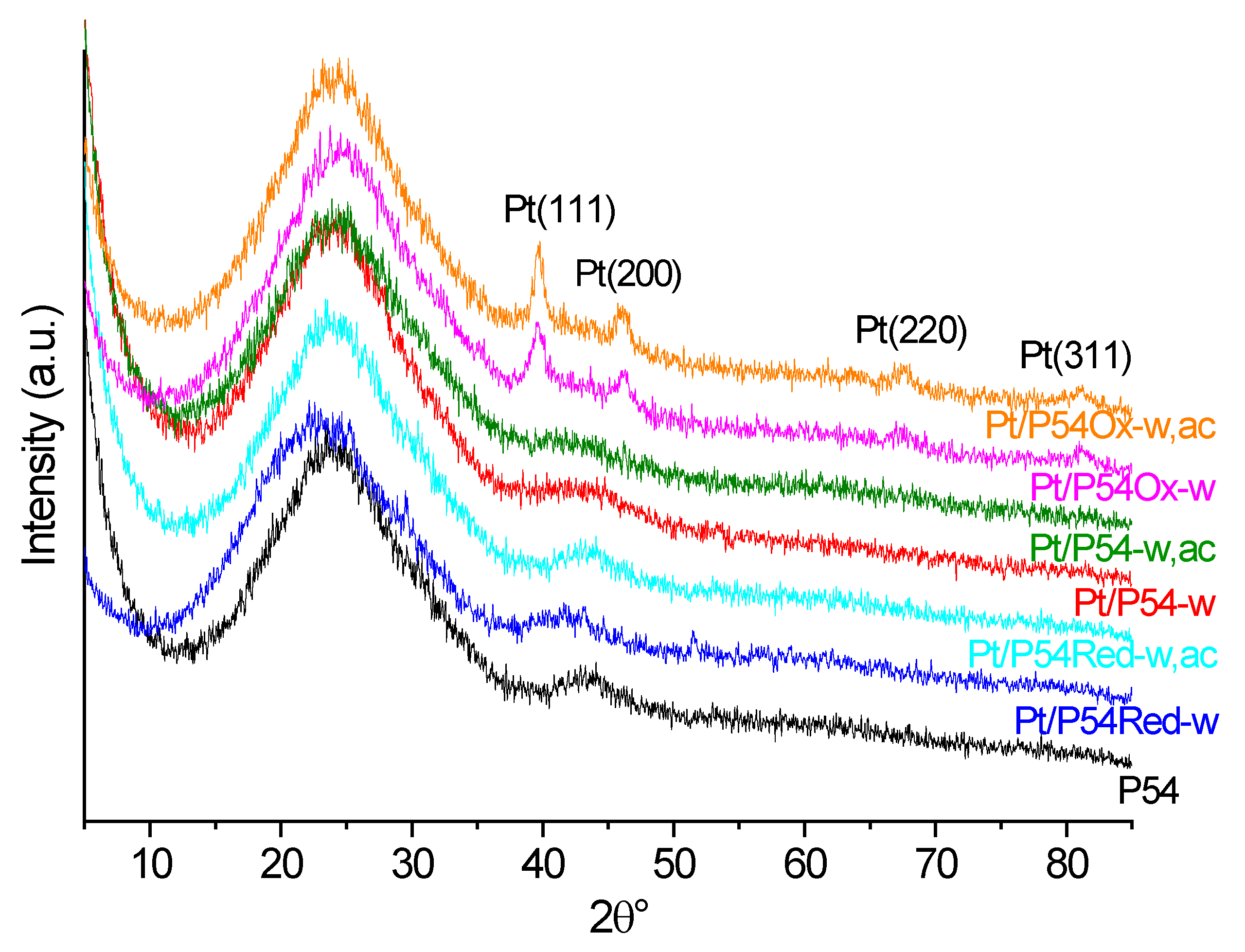
XRD diffractograms were obtained on a Rigaku instrument model Miniflex 300 (Akishima-shi, Tokyo, Japan) using Ni-filtered Cu-Kα radiation (λ = 0.15406 nm).
The contents of the acidic and basic groups in the ACs were determined through a procedure adapted from the Boehm titration methodology, as detailed elsewhere [
24].
3.5. HDO Tests
Lauric acid (99.0%, Sigma Aldrich) and extra virgin coconut oil (98%; Dr. Orgânico, Niterói, RJ, Brazil) were used without further purification as feedstocks in the HDO tests. The tests were carried out in a cylindrical stainless steel reactor with an internal volume of ~100 cm
3 (a scheme of the reactor is presented elsewhere [
50]). For in situ re-reduction, 0.500 g of catalyst was charged into the reactor, which was then purged and pressurized with H
2 at 20 bars and heated to 400 °C for one hour. After that, the system was cooled down to room temperature, and 10.0 g of feedstock was charged into the reactor under a N
2 flow. The reactor was purged and pressurized again with H
2 at 20 bars and heated to 375 °C. The heating took around 45 min, and the reaction time zero was considered to be the moment when the temperature reached 375 °C. After the desired HDO reaction time, the system was cooled down to room temperature, and the liquid product was dried over Na
2SO
4 and separated by centrifugation. For the experiments in which re-reduction was not performed, the first heating cycle was omitted.
3.6. Characterization of Reaction Products
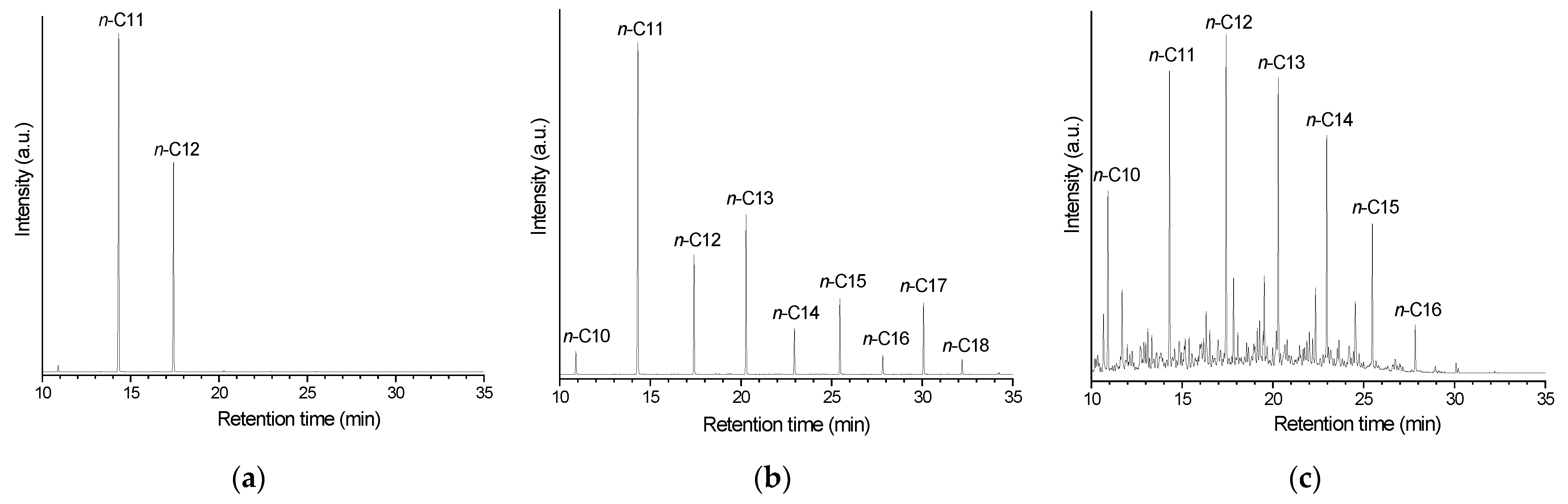
The liquid reaction products were qualitatively analyzed by GC/MS (gas chromatography/mass spectrometry) using a GC-17a chromatograph interfaced with a QP5050A spectrometer. For quantitative analyses, the GC/FID chromatograms were recorded on a GC-2010 device (Kyoto, Japan). A Rtx-5MS polydimethylsiloxane column (30 m, 25 mm) was used in both GC/MS and GC/FID analyses. All chromatographs, spectrometers, and columns were manufactured by Shimadzu (Kyoto, Japan).
The AI of the reaction products was determined according to the AOCS methodology Cd-3d-63-O.
4. Conclusions
A mesopore-rich AC was produced from chemical activation of a dried endocarp of coconut shell with H3PO4. This AC was modified by two treatments: (i) heating to 800 °C in a reductive H2 atmosphere, which removed the acidic oxygenated functional groups, and (ii) treatment in a refluxing HNO3 solution, which increased the content of the acidic oxygenated functional groups.
The unmodified and modified ACs were employed as supports in the preparation of Pt catalysts aimed at the synthesis of hydrocarbon biofuels via the hydroprocessing of lipid feedstocks. A systematic study on the effects of the preparation conditions on the properties and performance of the obtained catalysts has been carried out for the first time.
Compared to incipient wetness impregnation, wet impregnation enabled better diffusion of the Pt-containing species through the pore network of the supports, thus leading to higher Pt dispersion and consequently, more active catalysts.
The oxygenated functional groups and π-electrons on the carbon basal planes acted as active sites for the coordination of Pt-containing species. However, the presence of carboxylic acids is unfavorable, as these groups decompose during the Pt reduction step, allowing the Pt to become mobile and to sinter. For this reason, catalysts prepared using the HNO3-pretreated AC as the support exhibited low Pt dispersion and consequently, poor HDO activity.
Acidification of the impregnating solution with HCl led to an alternative adsorption mechanism when a more basic AC was used as the support. The low pH of the impregnating solution favored the protonation of the basic sites, resulting in a positively charged surface that promoted strong attractive electrostatic interaction with the Pt-containing anions, thus inhibiting Pt sintering and increasing Pt dispersion.
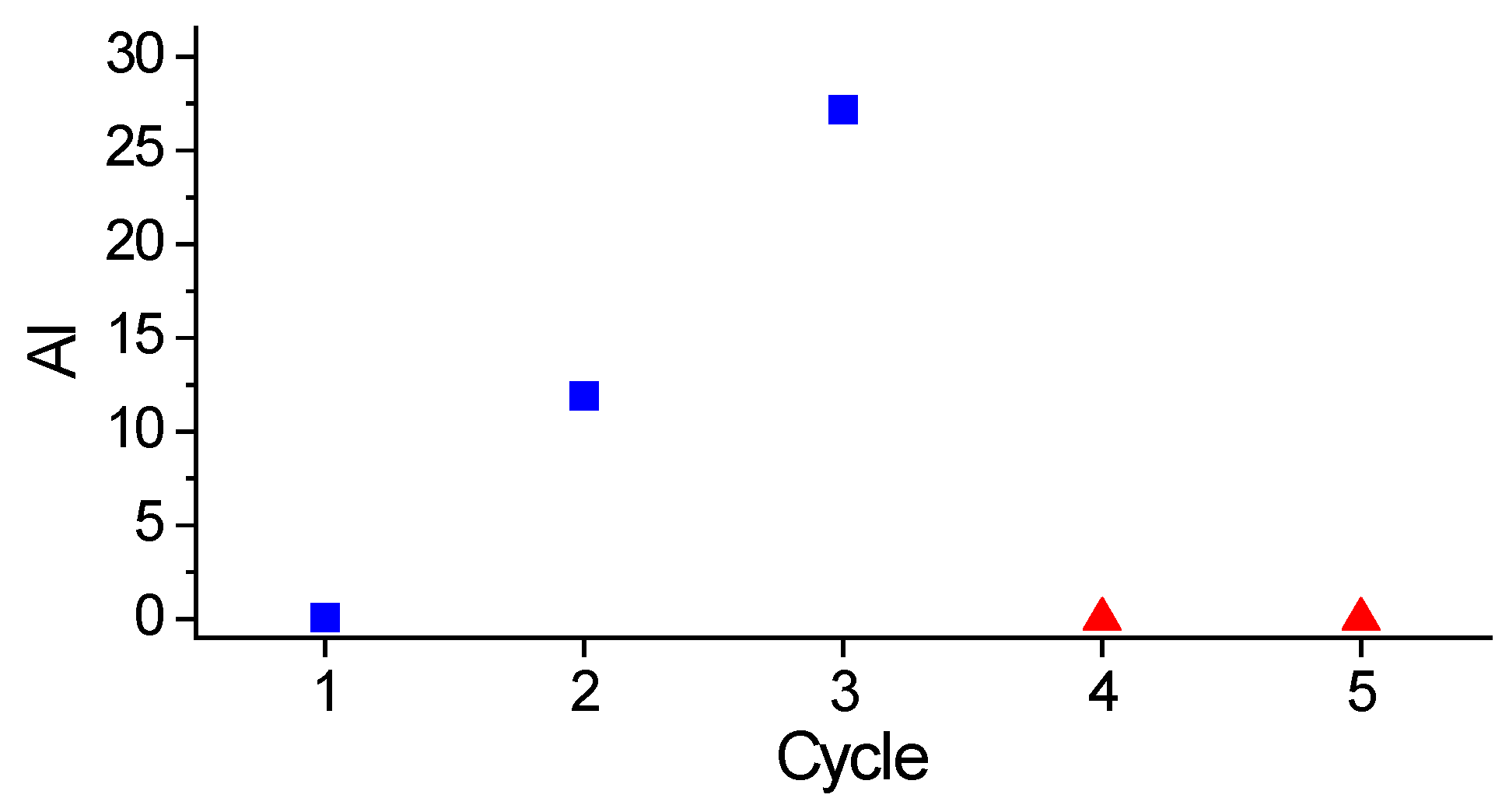
In this context, the catalyst with the highest HDO activity was the one prepared using the AC subjected to thermal pre-treatment in a H2 atmosphere as the support, with Pt deposited by wet impregnation of a H2PtCl6 solution acidified with HCl. The resulting catalyst promoted a complete deoxygenation of lauric acid and coconut oil, yielding products composed mainly of n-alkanes. Catalyst stability was achieved by maintaining Pt in its reduced form throughout successive HDO cycles.
The obtained results showed that AC-supported Pt catalysts show great potential to be used in the production of hydrocarbon biofuels through the hydroprocessing of lipid feedstocks.