Plasmonic Effect of Au Nanoparticles Deposited onto TiO2-Impact on the Photocatalytic Conversion of Acetaldehyde
Abstract
1. Introduction
2. Results
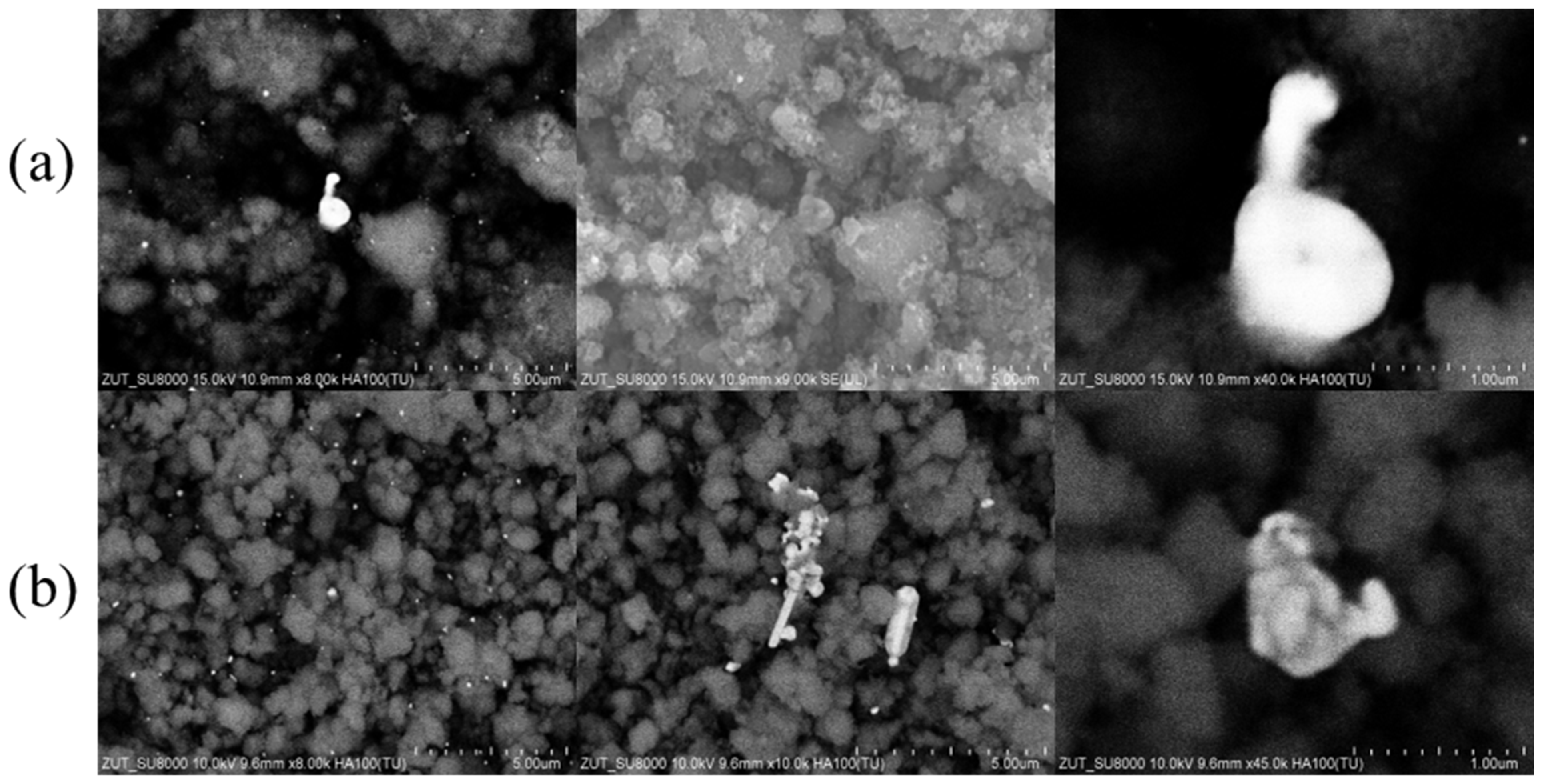
2.1. SEM Measurements
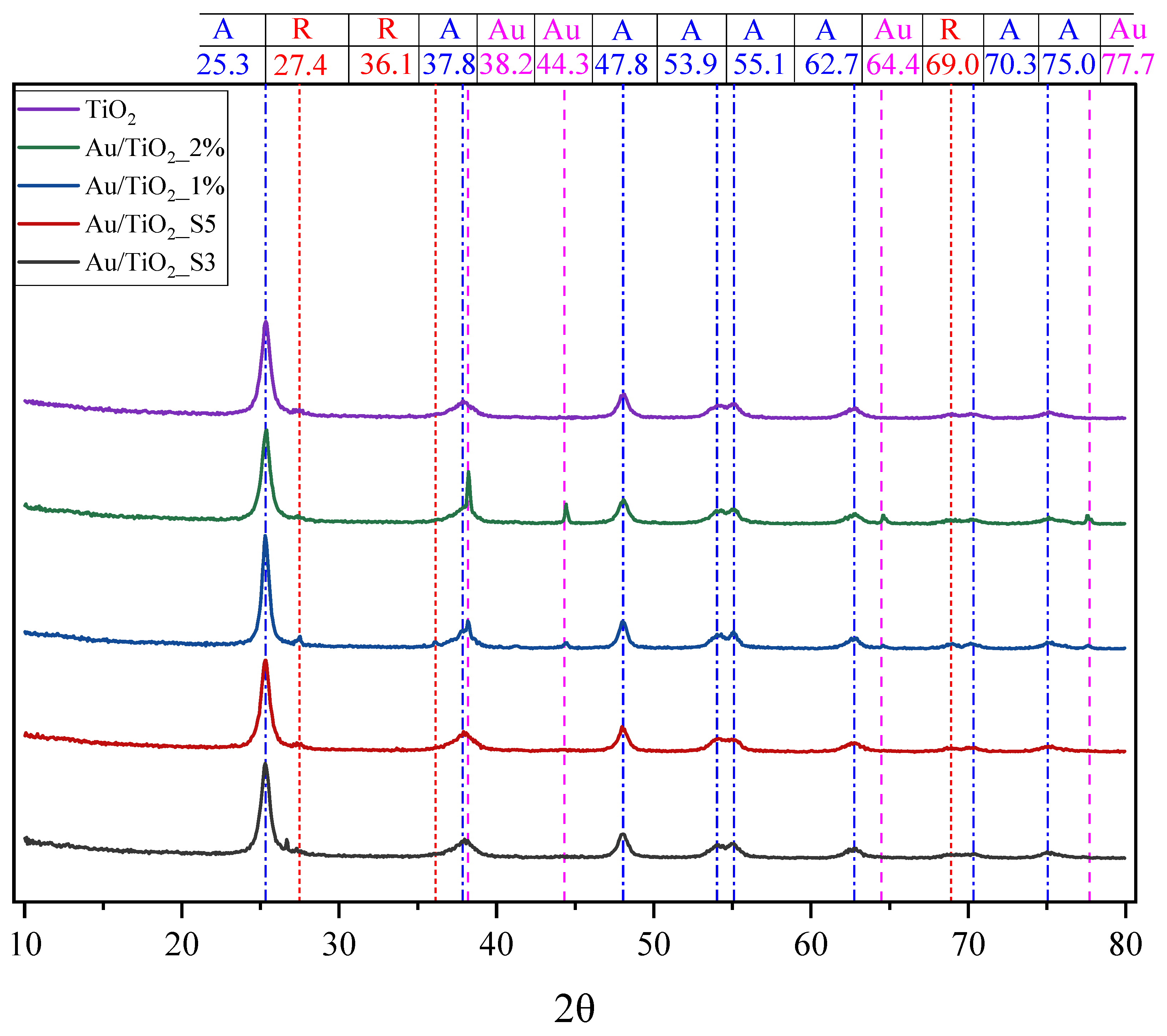
2.2. XRD Measurements
2.3. XRFS Measurements
2.4. UV-Vis/DR Measurements
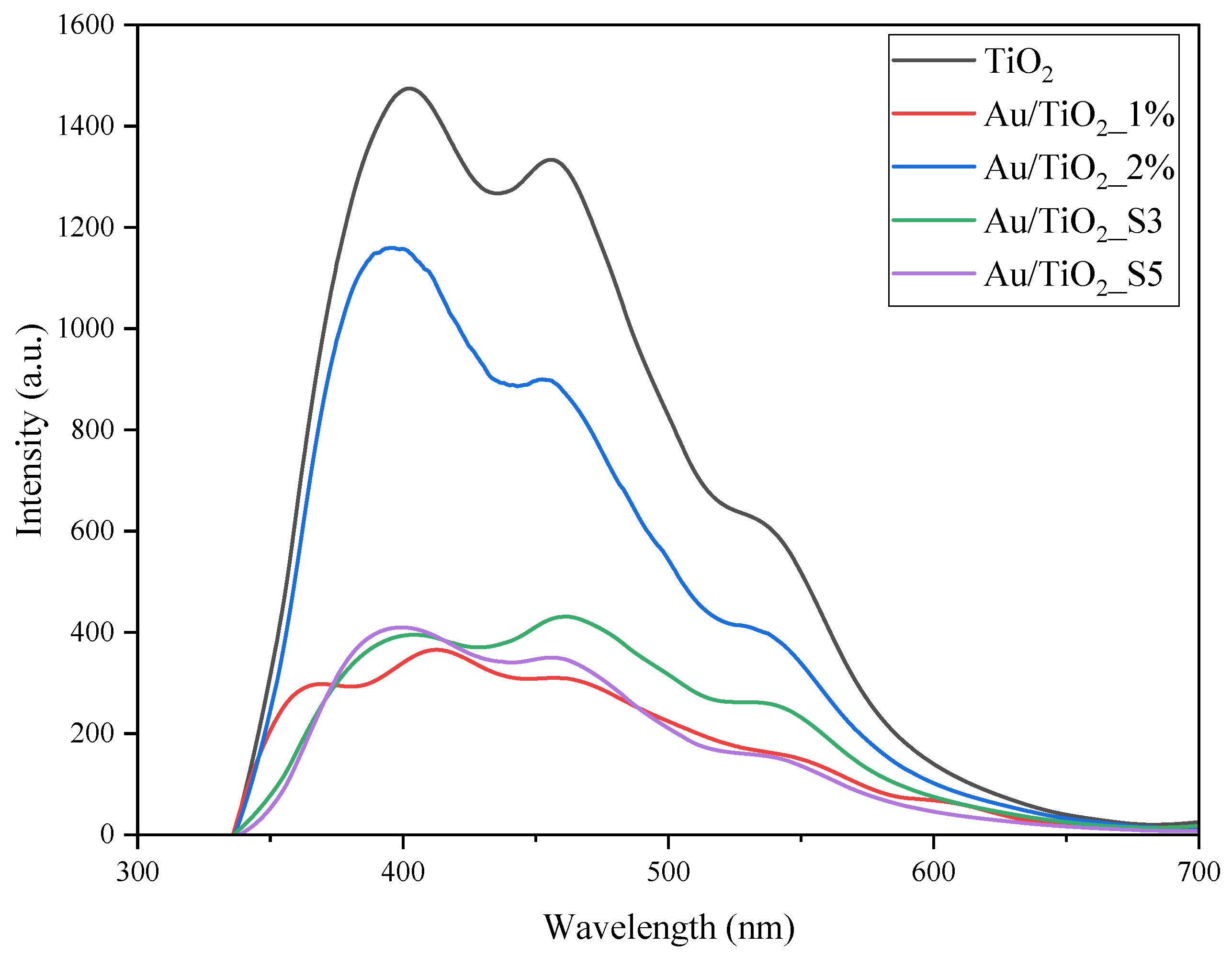
2.5. Photoluminescence Measurements
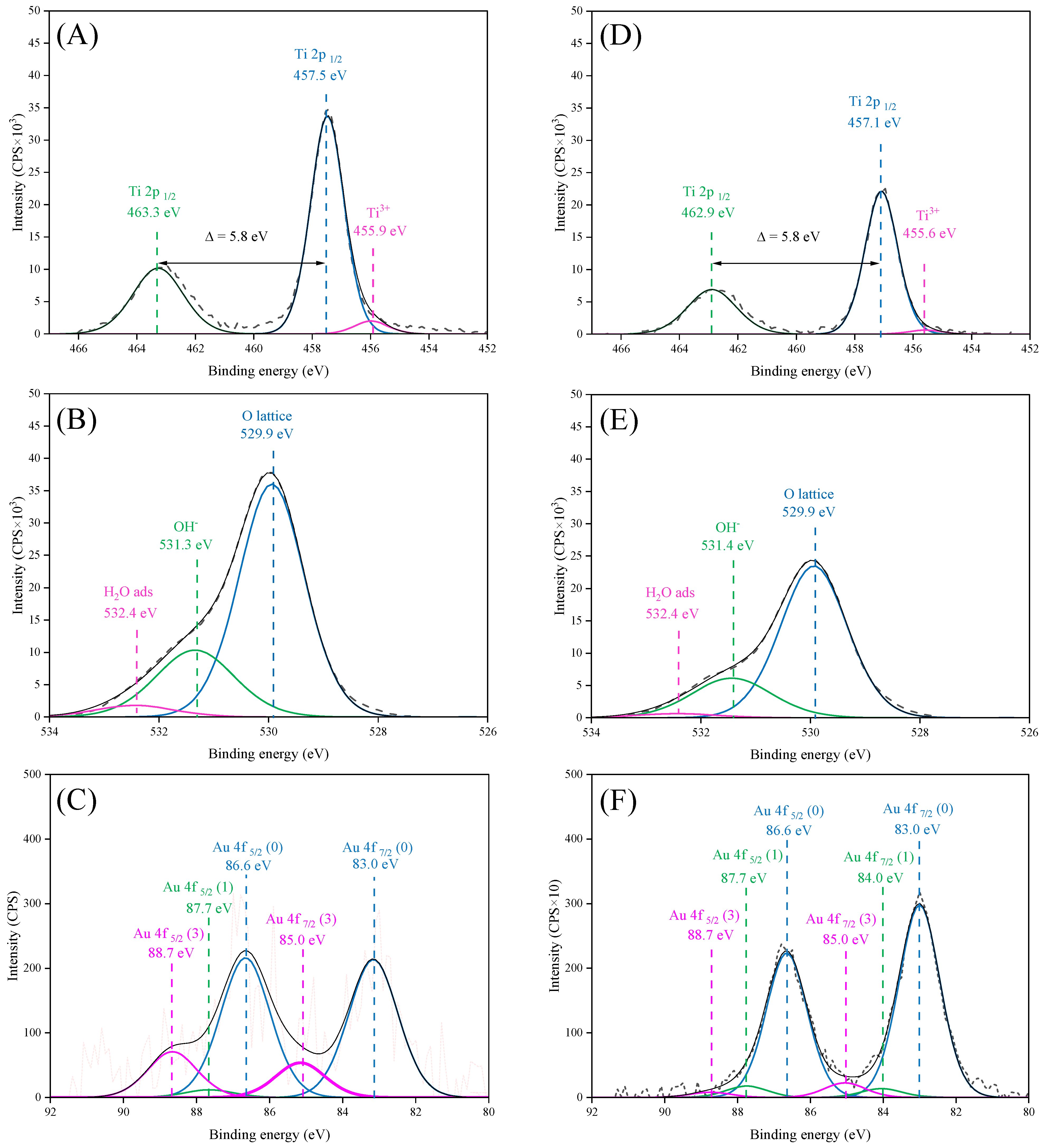
2.6. XPS Measurements
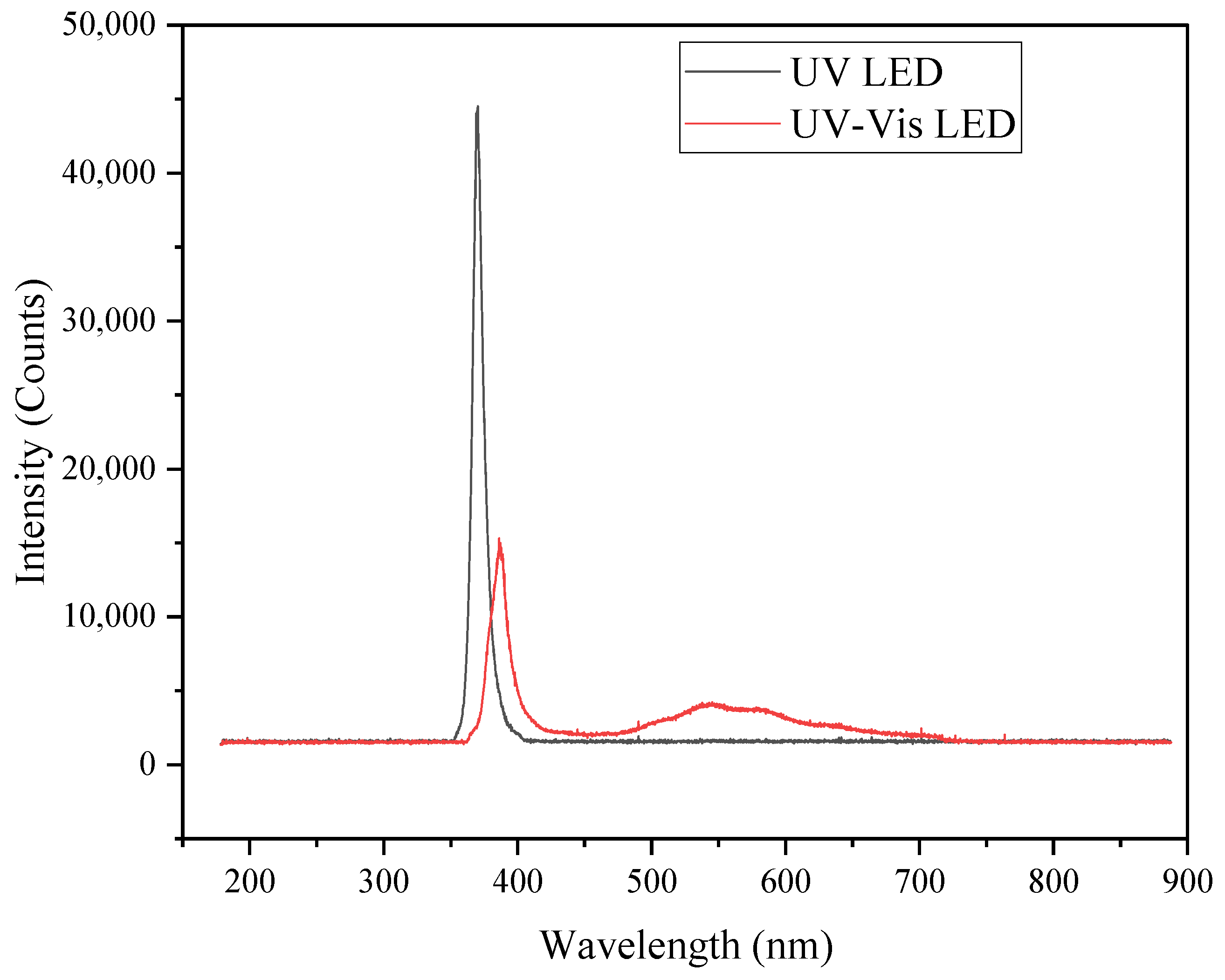
2.7. Spectral Characteristics of Irradiance Systems
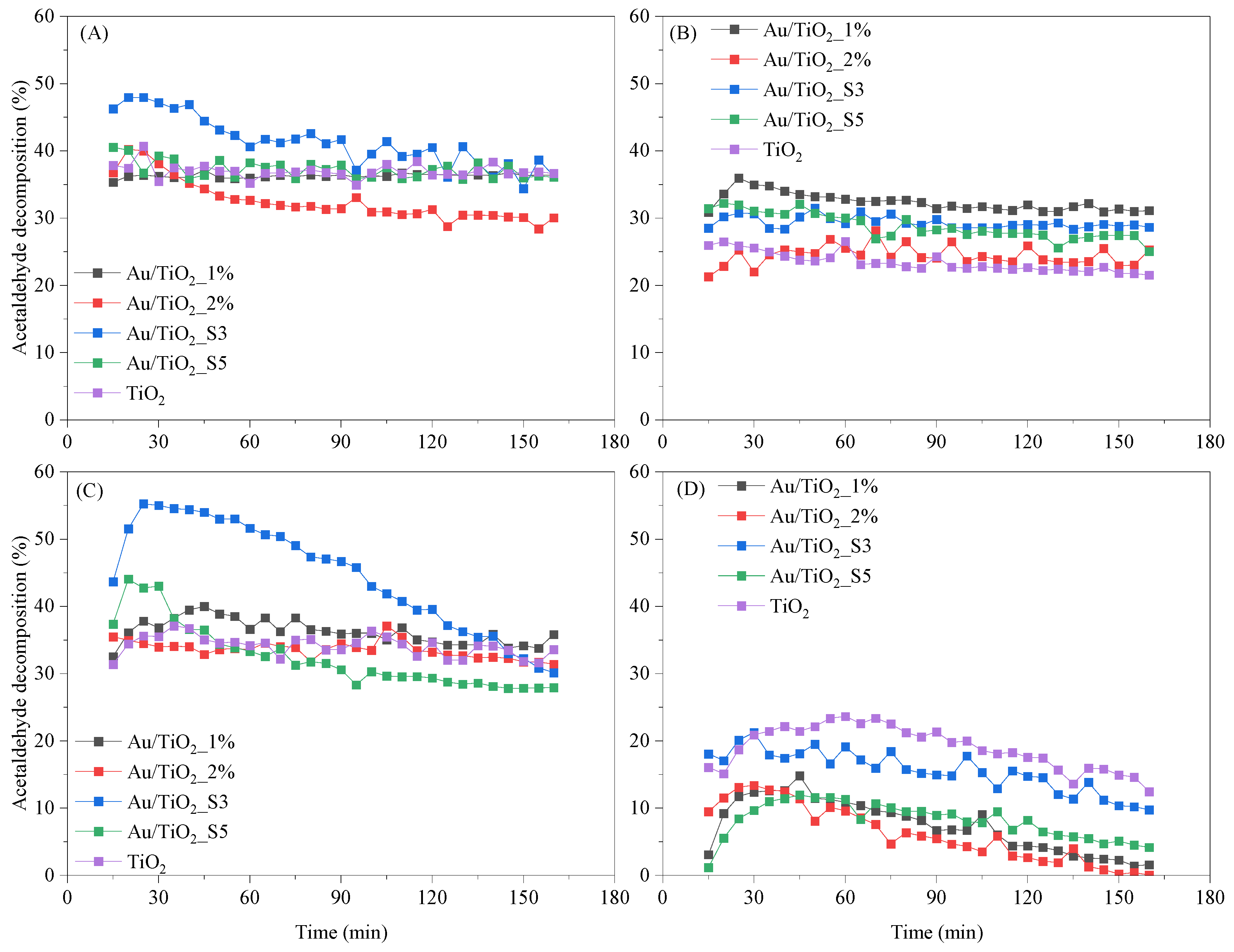
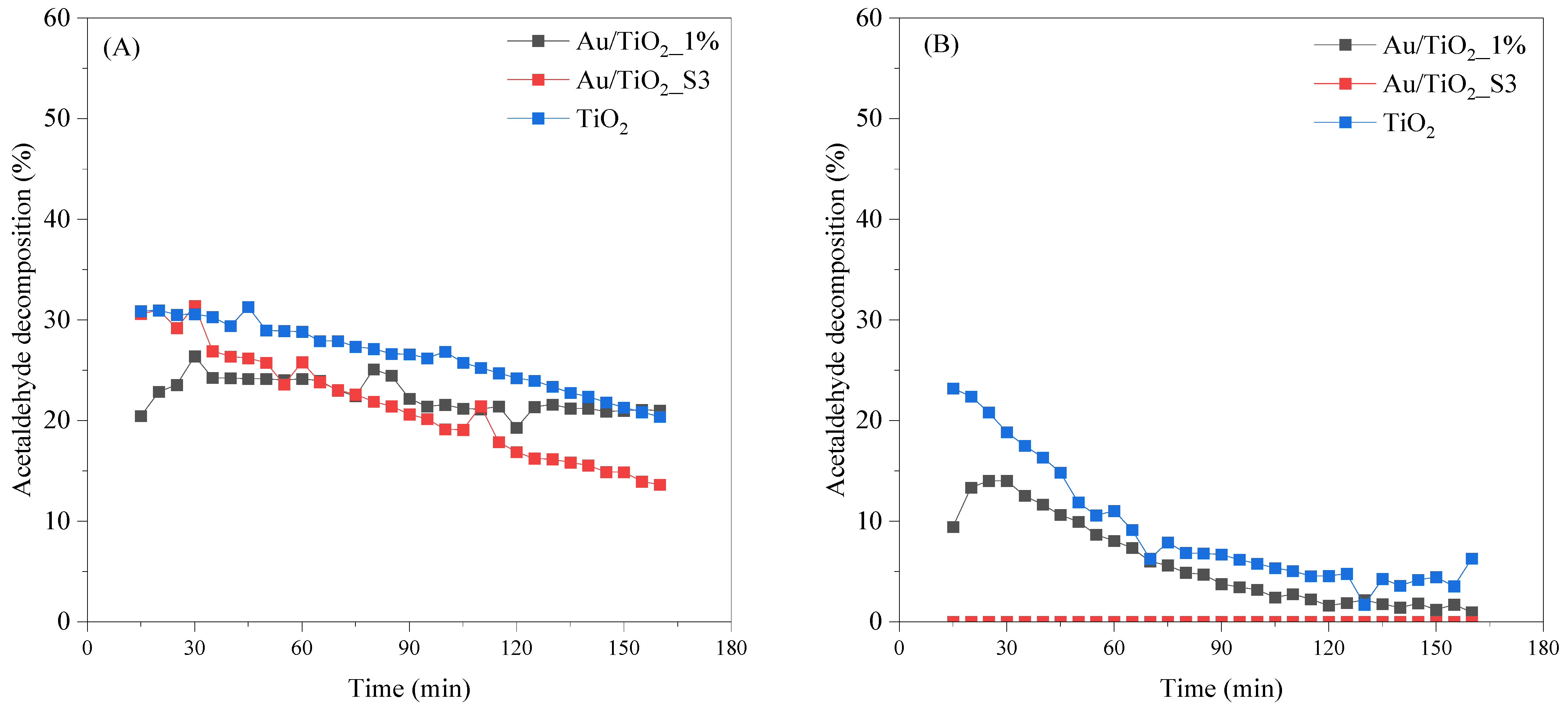
2.8. Photocatalytic Decomposition of Acetaldehyde
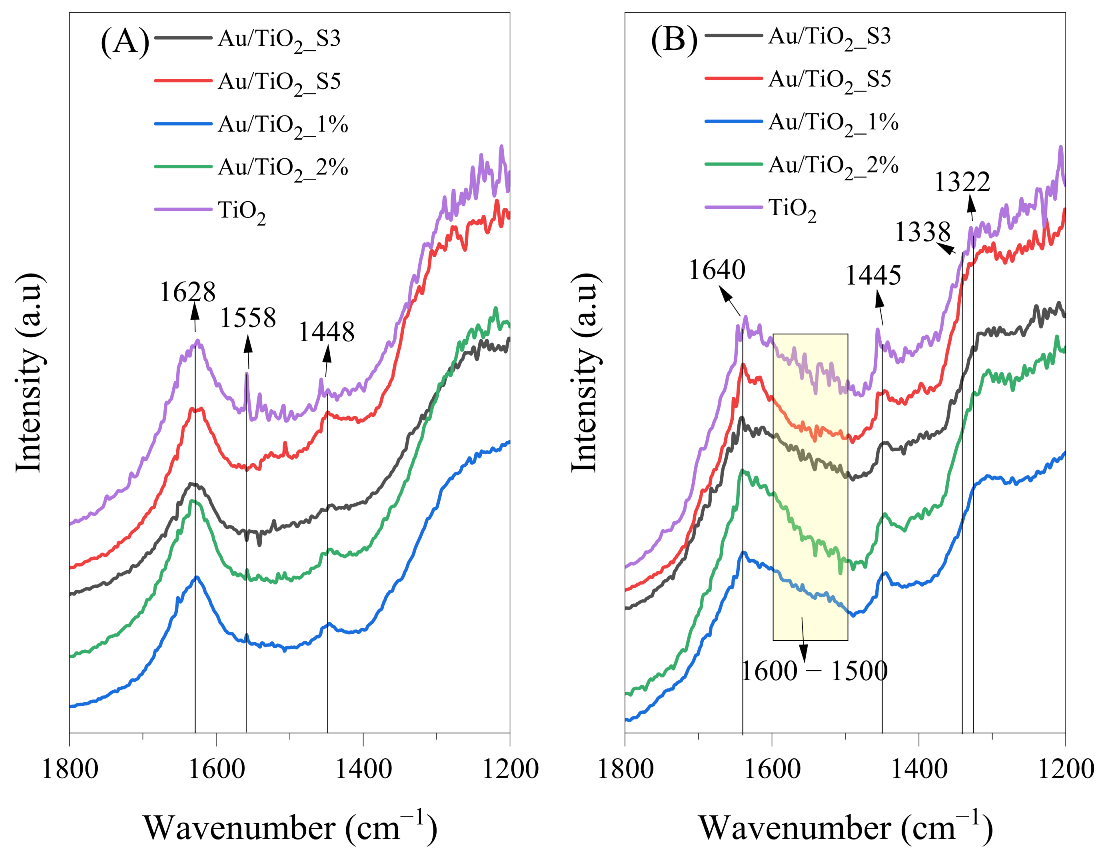
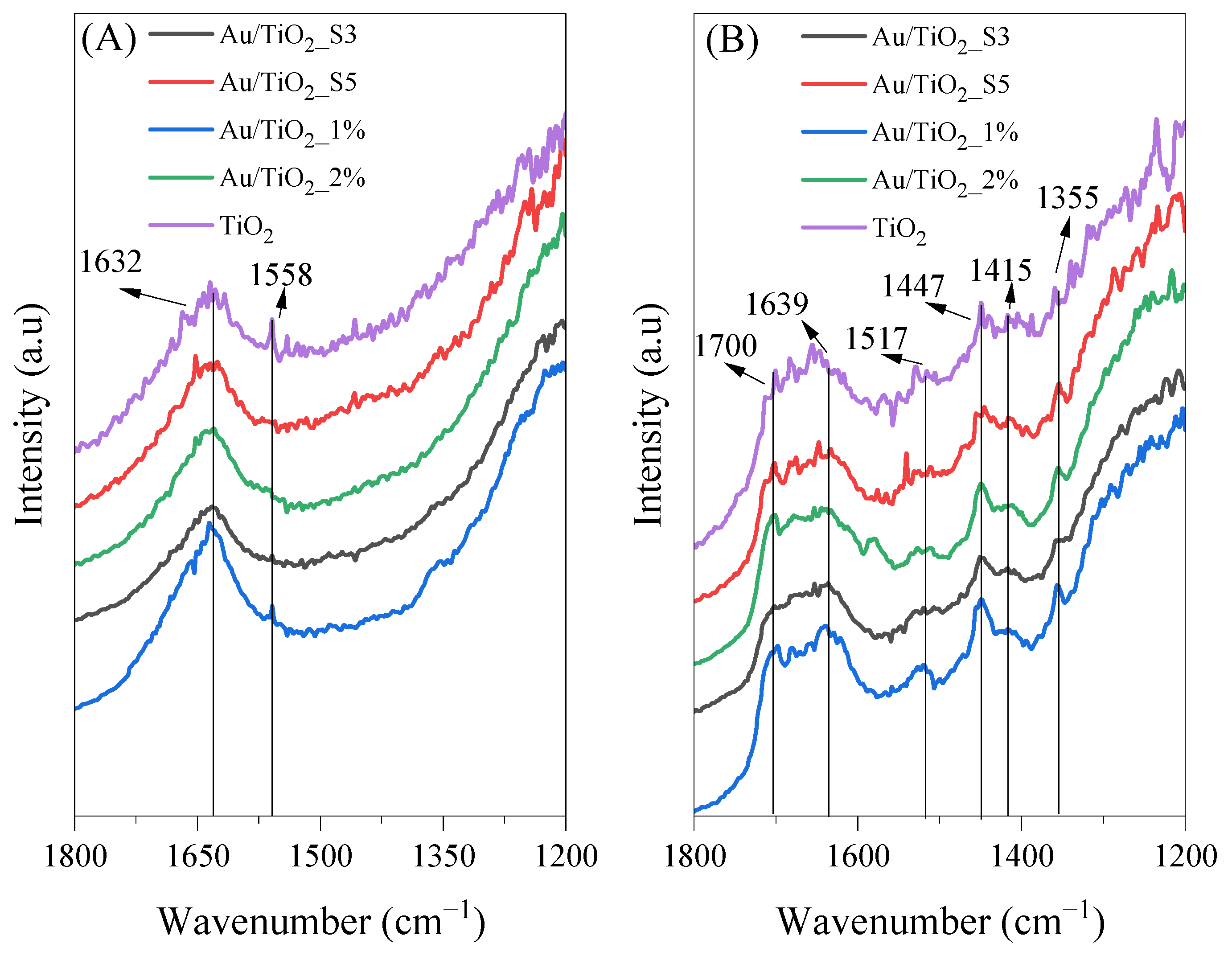
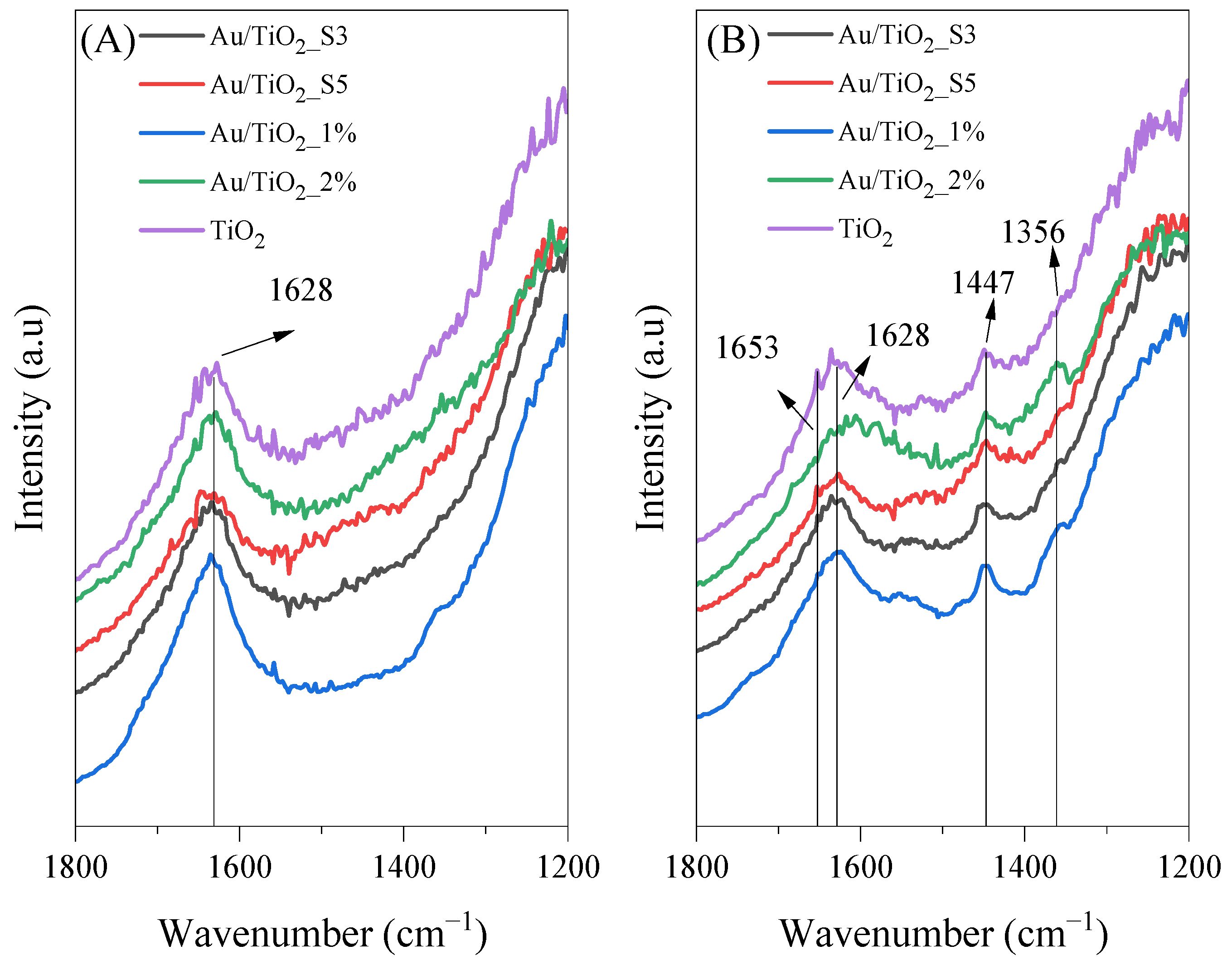
2.9. In Situ FTIR Measurements of the Photocatalysts Surface
3. Discussion
4. Materials and Methods
4.1. Preparation of TiO2
4.2. Preparation of Au-Deposited TiO2
4.3. Analytical Techniques
4.4. Photocatalytic Test
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tryba, B.; Rychtowski, P.; Markowska-Szczupak, A.; Przepiórski, J. Photocatalytic Decomposition of Acetaldehyde on Different TiO2-Based Materials: A Review. Catalysts 2020, 10, 1464. [Google Scholar] [CrossRef]
- Tryba, B.; Rychtowski, P.; Srenscek-Nazzal, J.; Przepiorski, J. The inflence of TiO2 structure on the complete decomposition of acetaldehyde gas. Mater. Res. Bull. 2020, 126, 110816. [Google Scholar] [CrossRef]
- Murdoch, M.; Waterhouse, G.I.N.; Nadeem, M.A.; Metson, J.B.; Keane, M.A.; Howe, R.F.; Llorca, J.; Idriss, H. The effect of gold loading and particle size on photocatalytic hydrogen production from ethanol over Au/TiO2 nanoparticles. Nat. Chem. 2011, 3, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, Z. Coupling plasmonic noble metal with TiO2 for efficient photocatalytic transfer hydrogenation: M/TiO2 (M = Au and Pt) for chemoselective transformation of cinnamaldehyde to cinnamyl alcohol under visible and 365 nm UV light. Appl. Surf. Sci. 2018, 452, 279–285. [Google Scholar] [CrossRef]
- Kong, T.; Liao, A.; Xu, Y.; Qiao, X.; Zhang, H.; Zhang, L.; Zhang, C. Recent advances and mechanism of plasmonic metal–semiconductor photocatalysis. RSC Adv. 2024, 14, 17041–17050. [Google Scholar] [CrossRef] [PubMed]
- Veziroglu, S.; Shondo, J.; Tjardts, T.; Sarwar, T.B.; Sünbül, A.; Mishra, Y.K.; Faupel, F.; Aktas, O.C. Photocatalytic deposition of noble metals on 0D, 1D, and 2D TiO2 structures: A review. Nanoscale Adv. 2024, 6, 6096–6108. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Xie, X.; Wang, X.; Wang, Y.; Lu, G.; Pui, D.Y.; Sun, J. Enhanced photocatalytic performance of Ag@TiO2 for the gaseous acetaldehyde photodegradation under fluorescent lamp. Chem. Eng. J. 2018, 341, 83–92. [Google Scholar] [CrossRef]
- Fang, H.; Liu, D.; Luo, Y.; Zhou, Y.; Liang, S.; Wang, X.; Lin, B.; Jiang, L. Challenges and Opportunities of Ru-Based Catalysts toward the Synthesis and Utilization of Ammonia. ACS Catal. 2022, 12, 3938–3954. [Google Scholar] [CrossRef]
- Janssen, M.; Weber, P.; Oezaslan, M. Recent advances of various Pt-based catalysts for oxygen reduction reaction (ORR) in polymer electrolyte membrane fuel cells (PEMFCs). Curr. Opin. Electrochem. 2023, 40, 101337. [Google Scholar] [CrossRef]
- Della Ca’, N. Palladium-Catalyzed Reactions. Catalysts 2021, 11, 588. [Google Scholar] [CrossRef]
- Kowalska, E.; Mahaney, O.O.P.; Abe, R.; Ohtani, B. Visible-light-induced photocatalysis through surface plasmon excitation of gold on titania surfaces. Phys. Chem. Chem. Phys. 2010, 12, 2344–2355. [Google Scholar] [CrossRef] [PubMed]
- Atta, S.; Pennington, A.M.; Celik, F.E.; Fabris, L. TiO2 on Gold Nanostars Enhances Photocatalytic Water Reduction in the Near-Infrared Regime. Chem 2018, 4, 2140–2153. [Google Scholar] [CrossRef]
- Kao, K.-C.; Huang, S.-J.; Hsia, Y.-F.; Huang, J.-H.; Mou, C.-Y. Supported Au Nanoparticles on TiO2 for Visible Light Photocatalytic H2O2 Production: Effects of Au Particle Size and Density. ACS Appl. Nano Mater. 2024, 7, 218–229. [Google Scholar] [CrossRef]
- Okuno, T.; Kawamura, G.; Muto, H.; Matsuda, A. Fabrication of Shape-Controlled Au Nanoparticles in a TiO2-Containing Mesoporous Template Using UV Irradiation and Their Shape-Dependent Photocatalysis. J. Mater. Sci. Technol. 2014, 30, 8–12. [Google Scholar] [CrossRef]
- Kaur, R.; Pal, B. Size and shape dependent attachments of Au nanostructures to TiO2 for optimum reactivity of Au–TiO2 photocatalysis. J. Mol. Catal. A Chem. 2012, 355, 39–43. [Google Scholar] [CrossRef]
- Levchuk, I.; Sillanpää, M.; Guillard, C.; Gregori, D.; Chateau, D.; Chaput, F.; Lerouge, F.; Parola, S. Enhanced photocatalytic activity through insertion of plasmonic nanostructures into porous TiO2/SiO2 hybrid composite films. J. Catal. 2016, 342, 117–124. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, P.; Kumar, A.; Camargo, P.H.C.; Krishnan, V. Recent Advances in Plasmonic Photocatalysis Based on TiO2 and Noble Metal Nanoparticles for Energy Conversion, Environmental Remediation, and Organic Synthesis. Small 2022, 18, 2101638. [Google Scholar] [CrossRef] [PubMed]
- Žerjav, G.; Roškarič, M.; Zavašnik, J.; Kovač, J.; Pintar, A. Effect of Au loading on Schottky barrier height in TiO2 + Au plasmonic photocatalysts. Appl. Surf. Sci. 2022, 579, 152196. [Google Scholar] [CrossRef]
- Panayotov, D.A.; Frenkel, A.I.; Morris, J.R. Catalysis and Photocatalysis by Nanoscale Au/TiO2: Perspectives for Renewable Energy. ACS Energy Lett. 2017, 2, 1223–1231. [Google Scholar] [CrossRef]
- Mateo, D.; Cerrillo, J.L.; Durini, S.; Gascon, J. Fundamentals and applications of photo-thermal catalysis. Chem. Soc. Rev. 2021, 50, 2173–2210. [Google Scholar] [CrossRef] [PubMed]
- Nikawa, T.; Naya, S.; Tada, H. Rapid removal and decomposition of gaseous acetaldehyde by the thermo- and photo-catalysis of gold nanoparticle-loaded anatase titanium(IV) oxide. J. Colloid Interface Sci. 2015, 456, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Falconer, J.L.; Magrini-Bair, K.A. Photocatalytic and Thermal Catalytic Oxidation of Acetaldehyde on Pt/TiO2. J. Catal. 1998, 179, 171–178. [Google Scholar] [CrossRef]
- Tryba, B.; Miądlicki, P.; Rychtowski, P.; Trzeciak, M.; Wróbel, R.J. The Superiority of TiO2 Supported on Nickel Foam over Ni-Doped TiO2 in the Photothermal Decomposition of Acetaldehyde. Materials 2023, 16, 5241. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.B.; Buttiglieri, G.; Babić, S. State-of-the-art and current challenges for TiO2/UV-LED photocatalytic degradation of emerging organic micropollutants. Environ. Sci. Pollut. Res. 2021, 28, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Naik, G.K.; Ahn, Y. LED light-induced enhanced photocatalytic hydrogen evolution on Au@TiO2 core–shell modified by nitrogen doping and reduced graphene oxide. Int. J. Hydrogen Energy 2022, 47, 32121–32132. [Google Scholar] [CrossRef]
- He, W.; Huang, X.; Ma, X.; Zhang, J. Significant temperature effect on the LSPR properties of noble metal nanoparticles. J. Opt. 2022, 51, 142–153. [Google Scholar] [CrossRef]
- Rahulan, K.M.; Ganesan, S.; Aruna, P. Synthesis and optical limiting studies of Au-doped TiO2 nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 2, 025012. [Google Scholar] [CrossRef]
- Cojocaru, B.; Neaţu, Ş.; Sacaliuc-Pârvulescu, E.; Lévy, F.; Pârvulescu, V.I.; Garcia, H. Influence of gold particle size on the photocatalytic activity for acetone oxidation of Au/TiO2 catalysts prepared by dc-magnetron sputtering. Appl. Catal. B 2011, 107, 140–149. [Google Scholar] [CrossRef]
- Kunthakudee, N.; Ramakul, P.; Serivalsatit, K.; Hunsom, M. Photosynthesis of Au/TiO2 nanoparticles for photocatalytic gold recovery from industrial gold-cyanide plating wastewater. Sci. Rep. 2022, 12, 21956. [Google Scholar] [CrossRef] [PubMed]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wang, X.; Liu, J.; Tian, Z.; Dai, L.; He, B.; Han, C.; Wu, Y.; Zeng, Z.; Hu, Z. On the role of localized surface plasmon resonance in UV-Vis light irradiated Au/TiO2 photocatalysis systems: Pros and cons. Nanoscale 2015, 7, 4114–4123. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Cervantes, O.R.; Pérez-Larios, A.; Arellano, V.H.R.; Sulbaran-Rangel, B.; González, C.A.G. Effects in Band Gap for Photocatalysis in TiO2 Support by Adding Gold and Ruthenium. Processes 2020, 8, 1032. [Google Scholar] [CrossRef]
- Fan, L.; Ichikuni, N.; Shimazu, S.; Uematsu, T. Preparation of Au/TiO2 catalysts by suspension spray reaction method and their catalytic property for CO oxidation. Appl. Catal. A Gen. 2003, 246, 87–95. [Google Scholar] [CrossRef]
- Yang, Y.-F.; Sangeetha, P.; Chen, Y.-W. Au/TiO2 catalysts prepared by photo-deposition method for selective CO oxidation in H2 stream. Int. J. Hydrogen Energy 2009, 34, 8912–8920. [Google Scholar] [CrossRef]
- Radnik, J.; Mohr, C.; Claus, P. On the origin of binding energy shifts of core levels of supported gold nanoparticles and dependence of pretreatment and material synthesis. Phys. Chem. Chem. Phys. 2003, 5, 172–177. [Google Scholar] [CrossRef]
- Melchers, S.; Schneider, J.; Emeline, A.V.; Bahnemann, D.W. Effect of H2O and O2 on the Adsorption and Degradation of Acetaldehyde on Anatase Surfaces—An In Situ ATR-FTIR Study. Catalysts 2018, 8, 417. [Google Scholar] [CrossRef]
- Hauchecorne, B.; Terrens, D.; Verbruggen, S.; Martens, J.A.; Van Langenhove, H.; Demeestere, K.; Lenaerts, S. Elucidating the photocatalytic degradation pathway of acetaldehyde: An FTIR in situ study under atmospheric conditions. Appl. Catal. B Environ. 2011, 106, 630–638. [Google Scholar] [CrossRef]
- Rychtowski, P.; Tryba, B.; Fuks, H.; Lillo-Ródenas, M.Á.; Román-Martínez, M.C. Impact of TiO2 Surface Defects on the Mechanism of Acetaldehyde Decomposition under Irradiation of a Fluorescent Lamp. Catalysts 2021, 11, 1281. [Google Scholar] [CrossRef]
- Topalian, Z.; Stefanov, B.; Granqvist, C.; Österlund, L. Adsorption and photo-oxidation of acetaldehyde on TiO2 and sulfate-modified TiO2: Studies by in situ FTIR spectroscopy and micro-kinetic modeling. J. Catal. 2013, 307, 265–274. [Google Scholar] [CrossRef]
- Tryba, B.; Tygielska, M.; Colbeau-Justin, C.; Kusiak-Nejman, E.; Kapica-Kozar, J.; Wróbel, R.; Żołnierkiewicz, G.; Guskos, N. Influence of pH of sol-gel solution on phase composition and photocatalytic activity of TiO2 under UV and visible light. Mater. Res. Bull. 2016, 84, 152–161. [Google Scholar] [CrossRef]
- Colbeau-Justin, C.; Kunst, M.; Huguenin, D. Structural influence on charge-carrier lifetimes in TiO2 powders studied by microwave absorption. J. Mater. Sci. 2003, 38, 2429–2437. [Google Scholar] [CrossRef]
- Tryba, B.; Tygielska, M.; Orlikowski, J.; Przepiórski, J. Influence of TiO2 hydrophilicity on the photocatalytic decomposition of gaseous acetaldehyde in a circulated flow reactor. React. Kinet. Catal. Lett. 2016, 119, 349–365. [Google Scholar] [CrossRef]
- Otto, K.; Acik, I.O.; Krunks, M.; Tõnsuaadu, K.; Mere, A. Thermal decomposition study of HAuCl4·3H2O and AgNO3 as precursors for plasmonic metal nanoparticles. J. Therm. Anal. Calorim. 2014, 118, 1065–1072. [Google Scholar] [CrossRef]
- Montero, M.A.; de Chialvo, M.R.G.; Chialvo, A.C. Preparation of gold nanoparticles supported on glassy carbon by direct spray pyrolysis. J. Mater. Chem. 2009, 19, 3276–3280. [Google Scholar] [CrossRef]





| Sample | Content/wt% Au |
|---|---|
| Au/TiO2_1% | 1.4 |
| Au/TiO2_2% | 3.0 |
| Au/TiO2_S3 | 0.8 |
| Au/TiO2_S5 | 1.4 |
| Sample | Elemental Surface Content [% at.] | ||
|---|---|---|---|
| O1s | Ti2p | Au4f | |
| Au/TiO2_1% | 72.19 | 27.56 | 0.25 |
| Au/TiO2_S5 | 72.36 | 25.64 | 2.01 |
| Sample | Ti2p | O1s | Au4f | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ti2p 3/2 [%] | Ti2p 1/2 [%] | Ti3+ [%] | O lattice [%] | −OH [%] | H2O ads [%] | Au(0) (metallic Au) [%] | Au(1) [%] | Au(3) [%] | |
| Au/TiO2_1% | 66.16 | 29.94 | 3.9 | 71.22 | 24.54 | 4.24 | 75.89 | 2.10 | 22.01 |
| Au/TiO2_S5 | 66.84 | 31.27 | 1.9 | 74.3 | 23.28 | 2.42 | 89.43 | 5.35 | 5.22 |
| Illumination System | 315–400 nm (Irradiance, W/m−2) | 400–1050 nm (Irradiance, W/m−2) |
|---|---|---|
| UV-LED | 40.00 | 0.05 |
| UV-VIS LED | 0.65 | 3.70 |
| Molecule | Vibration Mode | Wavenumber (cm−1) | References |
|---|---|---|---|
| CH3CH=CHCHO | ν(CO) | 1653 | [37] |
| H2O | d(OH) | 1620–1630 | [40] |
| HCOO− | νs(CO) | 1355 | [1] |
| CH3CH=CHCHO | δas(CH3) | 1445–1448 | [37] |
| CH3COO− | ns(COO) | 1444 | [36] |
| HCOO−ad | νas(COO) | 1558 | [37] |
| CH3COOHad | ν(C=O) | 1700 | [37] |
| CH3COOHad | ν(C=O) | 1676 | [37] |
| CH3CH=CHCHO | n(C=C) | 1632 | [37,39] |
| CH3COOHad | δas(CH3) | 1412–1415 | [1,36,37] |
| CH3COOH | δas(CH3) | 1306 | [37] |
| CH3COO−ad | δas(CH3) | 1338 | [37] |
| CH3COO− | ν(COO) | 1517 | [38] |
| HCOO− | δ(CH) | 1322 | [37] |
| HCOO− | νas(COO) | 1581 | [37] |
| CH3COO−ad | νas(COO) | 1545 | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trzeciak, M.; Przepiórski, J.; Kałamaga, A.; Tryba, B. Plasmonic Effect of Au Nanoparticles Deposited onto TiO2-Impact on the Photocatalytic Conversion of Acetaldehyde. Molecules 2025, 30, 3118. https://doi.org/10.3390/molecules30153118
Trzeciak M, Przepiórski J, Kałamaga A, Tryba B. Plasmonic Effect of Au Nanoparticles Deposited onto TiO2-Impact on the Photocatalytic Conversion of Acetaldehyde. Molecules. 2025; 30(15):3118. https://doi.org/10.3390/molecules30153118
Chicago/Turabian StyleTrzeciak, Maciej, Jacek Przepiórski, Agnieszka Kałamaga, and Beata Tryba. 2025. "Plasmonic Effect of Au Nanoparticles Deposited onto TiO2-Impact on the Photocatalytic Conversion of Acetaldehyde" Molecules 30, no. 15: 3118. https://doi.org/10.3390/molecules30153118
APA StyleTrzeciak, M., Przepiórski, J., Kałamaga, A., & Tryba, B. (2025). Plasmonic Effect of Au Nanoparticles Deposited onto TiO2-Impact on the Photocatalytic Conversion of Acetaldehyde. Molecules, 30(15), 3118. https://doi.org/10.3390/molecules30153118







