Abstract
We report the first syntheses—from commercially available 3-chloro-2-fluoroprop-1-ene (9)—of key garlic-derived compounds containing sp2-fluorine. We also report synthesis of fluoro-5,6-dihydrothiopyrans by trapping 2-fluorothioacrolein (15). Thus, difluoroallicin (12, S-(2-fluoro-2-propenyl) 2-fluoroprop-2-ene-1-sulfinothioate) is prepared by peracid oxidation of 1,2-bis(2-fluoro-2-propenyl)disulfane (11). S-2-Fluoro-2-propenyl-l-cysteine (2-fluorodeoxyalliin, 13), synthesized from cysteine and characterized by X-ray crystallography, is oxidized to its S-oxide, 2-fluoroalliin (22). The latter, with alliinase-containing powdered fresh garlic, gives a mixture of 12, allicin (1), and isomers of monofluoroallicin (23), indicating that 22 serves as a substrate for garlic alliinase. Upon heating, 12 generates transient 15, which dimerizes giving difluoro vinyl dithiins 6 and 7. Ethyl acrylate trapping of 15 affords 5- and 6-substituted 3-fluoro-5,6-dihydro-4H-thiopyrans (19 and 20). In 1,1,1,3,3,3-hexafluoro-2-propanol (HEFP) as solvent, 12 is converted into trifluoroajoene ((E,Z)-1-(2-fluoro-3-((2-fluoro-2-propenyl)sulfinyl)prop-1-en-1-yl)-2-(2-fluoro-2-propenyl)disulfane; 18). Liquid sulfur converts 11 to a (CH2=CFCH2)2Sn mixture (n = 4–15), characterized by UPLC-(Ag+)-coordination ion spray-mass spectrometry.
1. Introduction
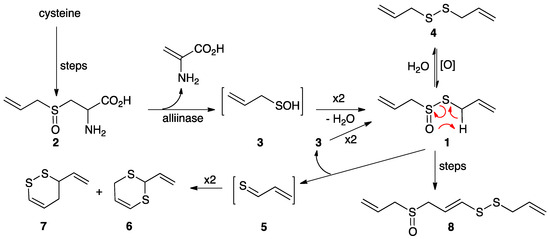
Since its discovery by Cavallito and Bailey in 1944 [1], allicin (1), the antibiotic active principle of garlic, has been the subject of extensive research [2,3,4,5,6,7,8,9,10]. Enzymatically released from its precursor alliin (2) when garlic is crushed, allicin is both unstable and reactive, readily decomposing by way of 2-propenesulfenic acid (3) and thioacrolein (5) to diallyl disulfide (4) and homologous polysulfanes, vinyl 1,2- and 1,3-dithiins (6 and 7, respectively) and ajoene (8) (Scheme 1). Other thiosulfinates, particularly those with methyl groups, such as MeS(O)SMe and mixed allyl methyl analogs, are also naturally produced, as are more complex compounds [11]. Many of these garlic-derived compounds are biologically active, e.g., as antimicrobial, antiplatelet and anticancer agents [1,2,3,4,5,6,7,8,10,12,13,14,15,16]. In particular, ajoene (8) [17,18] has been the subject of several reviews and structure–activity studies due to its notable biological activity as well as its stability, e.g., by Hunter, Kaschula and coworkers [19,20,21,22], Wirth and coworkers [23], and others [24,25,26]. Structure–activity studies and reviews of biological activity have appeared for several of the other garlic-derived compounds, including allicin and other thiosulfinates [27,28,29], S-allyl cysteine [30,31,32], disulfides and thiosulfonates [33].
Scheme 1.
Enzymatic formation of allicin (1) from alliin (2) by way of 2-propenesulfenic acid (3); synthesis of allicin by oxidation of diallyl disulfide (4); conversion of 1 to ajoene (8); decomposition of 1 to vinyl dithiins (6 and 7) via intramolecular elimination to thioacrolein (5). In addition to 2, garlic cloves contain methiin (MeS(O)CH2CH(NH2)CO2H) and isoalliin (MeCH=CHS(O)CH2CH(NH2)CO2H), which give rise to associated structures with methyl and 1-propenyl groups [2,3,4].
Given the great importance of fluorine in medicinal chemistry and chemical biology [34,35], we were interested in the effect of fluorine substitution for one or more sp2-hydrogen atoms in compounds 1–8 on the chemical reactivity and biological activity of these compounds. With the exception of CF3S(O)SCF3, CHF2S(O)SCF3, and CH2FS(O)SCF3, whose synthesis but not biological activity was reported [36,37,38], fluorinated analogs of garlic-derived organosulfur compounds are unknown. In line with the current interest in the biological activity of the allyl group [39], our initial goal was to replace one or more vinyl (sp2) hydrogens with fluorine, retaining the sp3 hydrogens to participate in key intramolecular elimination processes. Compounds with sp2 C–F bonds offer advantages associated with the small size, high electronegativity, and metabolic stability of fluorine as well as the altered molecular lipophilicity, membrane fluidity, binding affinity, enhanced volatility, and possibilities for halogen bonding [40] as well as conformational preferences of the fluorinated compounds compared to their hydrogen analogs. Reactivity toward thiol groups, a defining feature of the biological activity of allicin and other garlic compounds [41,42,43], should be enhanced by vinylic fluorine in the thioallylic groups in garlic compounds.
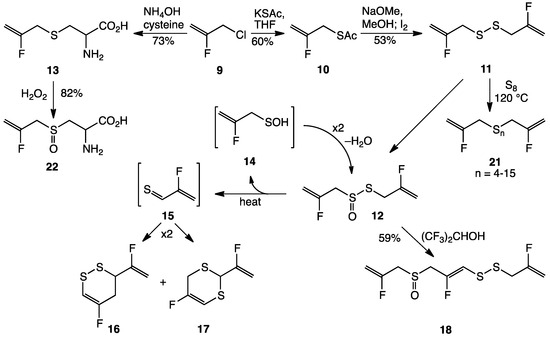
Two types of C–F substituted allyl groups were considered for this study, namely CH2=CFCH2–X (A) and CF2=CFCH2–X (B). We initially chose type A compounds based on commercial availability of CH2=CFCH2Cl (9; Scheme 2) [44,45]; other approaches to fluoroallylation are known [46,47,48,49]. Here, we describe our efforts to synthesize and study the reactivity and activity of difluoroallicin 12, prepared from 9, as well as related compounds, such as 2-fluorodeoxyalliin (13), 1,2-bis(2-fluoro-2-propenyl)disulfane (11), 5-fluoro-3-(1-fluorovinyl)-3,4-dihydro-1,2-dithiin (16), 5-fluoro-2-(1-fluorovinyl)-4H-1,3-dithiin (17), trifluoroajoene (18), and bis(2-fluoro-2-propenyl)polysulfanes (21).
Scheme 2.
Synthesis of difluoroallicin (12) from 3-chloro-2-fluoroprop-1-ene (9) by way of thioacetate 10 and bis(2-fluoro-2-propenyl) disulfide (11); synthesis of S-2-fluoro-2-propenyl-l-cysteine (13) from 9; conversion of 12 to vinyl dithiins 16 and 17 via 2-fluorothioacrolein (15) with loss of 2-fluoro-2-propenesulfenic acid (14); conversion of 12 to trifluoroajoene 18; conversion of 11 to polysulfane mixture 21; conversion of 13 to (+)-S-2-fluoro-2-propenyl-l-cysteine S-oxide (2-fluoroalliin; 22).
2. Results and Discussion
It was anticipated that difluoroallicin 12 could be prepared by oxidation of disulfane 11, which in turn could be synthesized from commercially available 9. Thus, 9 was stirred overnight with potassium thioacetate in THF giving S-(2-fluoro-2-propenyl)ethanethioate (10). Methanolysis of 10 (NaOMe/MeOH) followed by treatment with iodine gave 11. Treatment of a chloroform solution of 11 with peracetic acid gave difluoroallicin 12 in 42% yield as a yellow liquid with an onion-like odor. When a chloroform solution of 12 was heated in a sealed tube at 80 °C for 4 h, it decomposed, giving a mixture of dithiin (16) as the major product and dithiin (17) as the minor product.
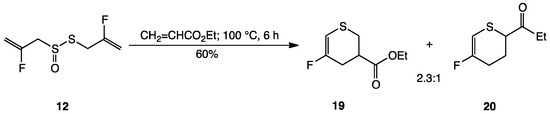
Formation of 16 and 17 follows from the proposed mechanism for intramolecular conversion of allicin to thioacrolein and 2-propenesulfenic acid followed by self-Diels-Alder reaction of thioacrolein 5 affording isomeric dithiins 6 and 7 (Scheme 1) [2,3]. To confirm this mechanism, when 12 was heated with a five-fold excess of ethyl acrylate in a sealed tube at 100 °C for 6 h, a 70:30 mixture of the regioisomeric Diels–Alder adducts (19 and 20) of ethyl acrylate and 15 was obtained in 60% yield and fully characterized by 1H, 13C and 19F NMR spectroscopy as well as HR-MS (Scheme 3). It is assumed that the second product in decomposition of 12, 2-fluoro-2-propenesulfenic acid (14), self-condenses back to 12 more rapidly than acrylate trapping. While only a single dienophile was used here, it is worth noting that trapping 15 with dienophiles represents a novel and potentially useful synthetic route to 5- and 6-substituted 3-fluoro-5,6-dihydro-4H-thiopyrans [50,51]. Thiopyrans display a broad range of biological activity [52] and new synthetic approaches are frequently reported [53,54]. 2-Fluorothioacrolein (15) has not been previously reported and represents a novel, reactive heterodiene.
Scheme 3.
Synthesis of 3-fluoro-5,6-dihydro-4H-thiopyran 19 and 20 from difluoroallicin 12.
Treatment of 9 with cysteine in NH4OH afforded 2-fluorodeoxyalliin (13) in 73% yield. After crystallization, 13 could be characterized by X-ray crystallography as the pure enantiomer shown in Figure 1. Structural data is given in Table 1. Two molecules co-crystallized in the structure; they are just slightly different in bond dimensions. Oxidation of an aqueous solution of 13 (30% H2O2) gave 2-fluoroalliin (22; 82% yield). When 22 was treated with powdered fresh garlic, the presence of 12 and isomers of mono-fluoroallicin 23a,b along with major amounts of allicin 4 was indicated by DART-MS analysis; the presence of 12 and 23a,b was also supported by 19F-NMR spectral data (Scheme 4).
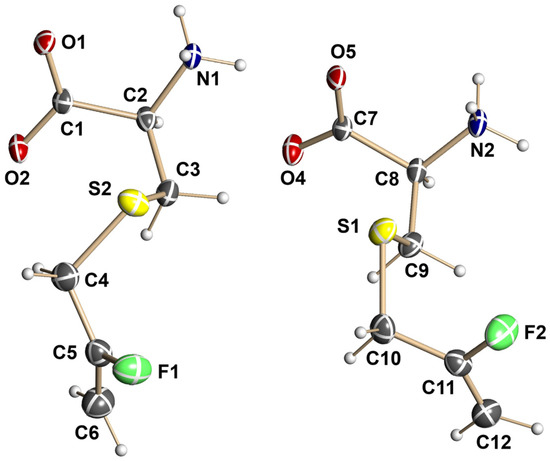
Figure 1.
Molecular structure of the l-enantiomer of fluorodeoxyalliin (13); non-hydrogen atoms are represented by thermal ellipsoids at the 50% probability level. Color coding: green = fluorine, yellow = sulfur, red = hydrogen, black = carbon.

Table 1.
Selected bond distances (Å) and angles (deg.) in 13. Atom labeling as in Figure 1.
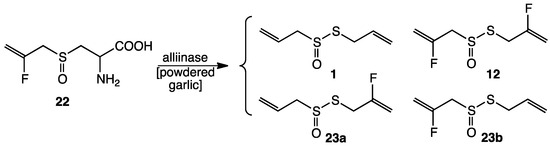
Scheme 4.
Enzymatic conversion of 2-fluoroalliin 22 to a mixture of 1, 12 and 23a,b. Alliin, present in the unpurified powdered garlic, provides the C3H5 group.
When a concentrated solution of 12 in 1,1,1,3,3,3-hexafluoro-2-propanol (HEFP) was kept at room temperature for 16 h, it was converted to a 1:1 mixture of 12 and trifluoroajoene 18 in 59% yield, based on converted 12. The choice of HEFP follows from the observation that partial conversion of 12 to a mixture of 16–18 occurred in isopropanol, and the belief that the higher acidity of HEFP would favor formation of 18. When the same reaction was conducted at 60 °C, a mixture of 12, 16 and 18 was formed, suggesting that heat favors formation of 16. While 18 could be characterized as a mixture with 12 by NMR and liquid chromatography–high resolution mass spectrometry, separation of 12 and 18 was not possible with the chromatographic methods employed, unfortunately precluding biological studies of 18.
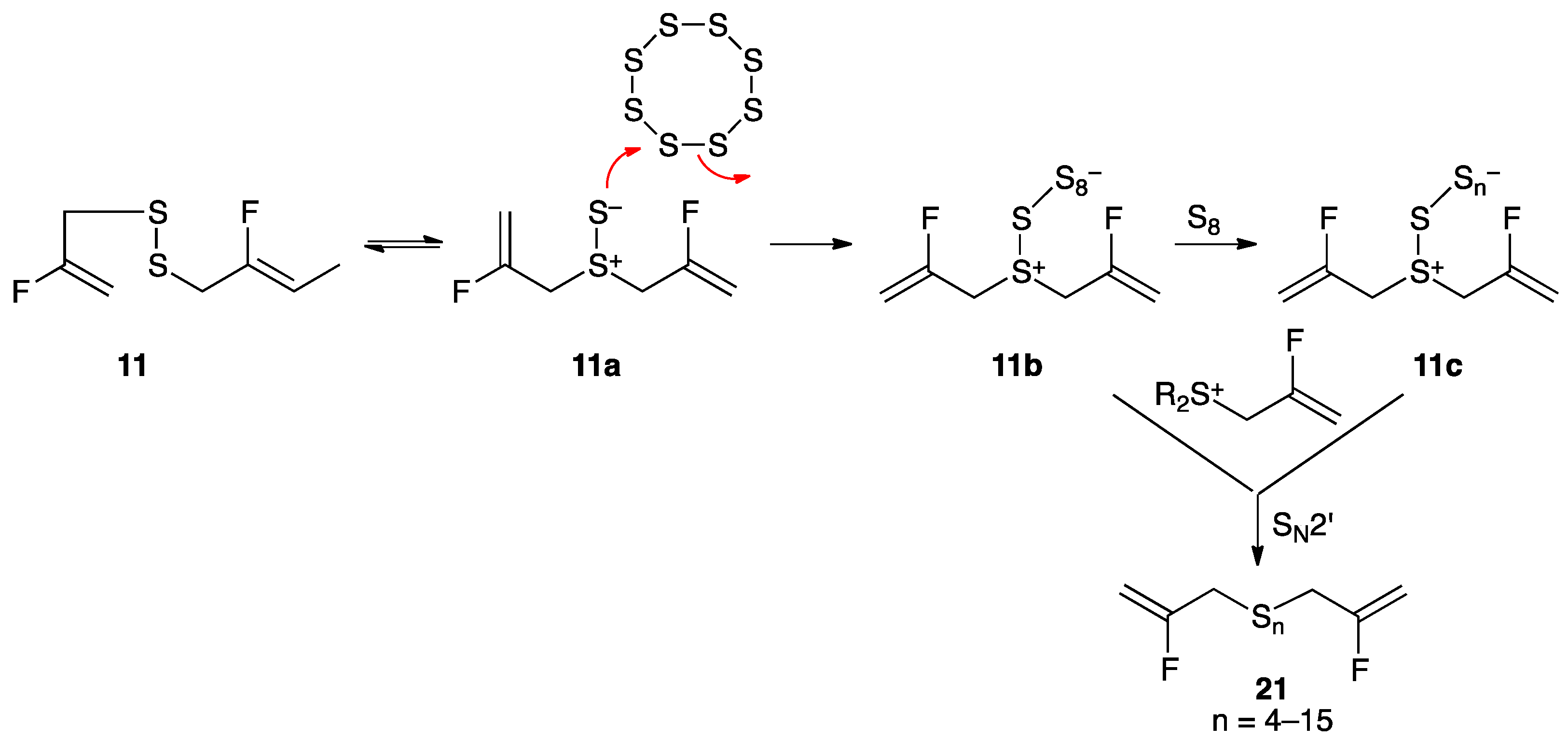
Treatment of disulfide 11 with liquid sulfur at its melting point of 120 °C for 1 h, as described previously for 4 [55], afforded polysulfane mixture 21 with 4–15 sulfur atoms, with compounds containing 4–6 sulfur atoms predominating. A proposed mechanism, initiated by [2,3]-sigmatropic rearrangement of disulfide 11 (Scheme 5) to the dipolar thiosulfoxide isomer, is consistent with computational studies showing that fluorine substitution on the 2-position of the allyl group has little effect on rearrangement to 11a [56].
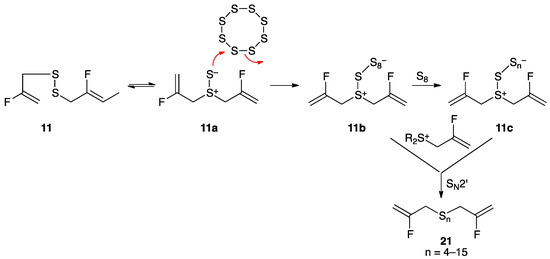
Scheme 5.
Conversion of disulfide 11 to polysulfane mixture 21 through condensation with molten sulfur. Several equivalents of S8 can react, losing different numbers of sulfur atom groupings, followed by SN2′ reaction with 2-fluoroallyl sulfonium species giving polysulfane mixture 21. By other techniques, compounds containing up to 26 sulfur atoms could be observed.
Because weak signals were obtained using standard LC-CIS-MS techniques, analysis of the complex polysulfane mixture was performed by ultra-performance liquid chromatography (UPLC) silver coordination ion spray mass spectrometry ((Ag+)CIS-MS) (Figure 2) as well as by 19F NMR and direct analysis in real time–mass spectrometry (DART-MS) techniques, as described more fully in the experimental section and elsewhere [55]. Reversed phase HPLC analysis of the mixture indicated the presence of higher polysulfides containing up to 26 sulfur atoms.
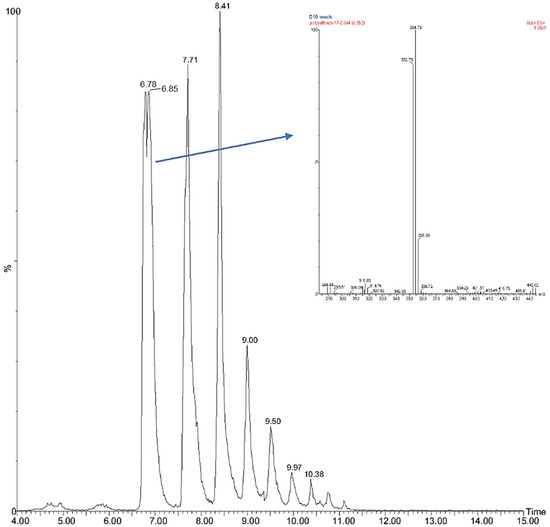
Figure 2.
UPLC (Ag+)-CIS-MS selective ion chromatogram of polysulfane mixture 21, with 4 to 15 sulfur atoms: C6H8F2S4-107Ag complex, m/z 352.7, 6.78/6.85 min (split peak due to column issues); C6H8F2S5-107Ag complex, m/z 384.7, 7.71 min; C6H8F2S6-107Ag complex, m/z 416.7, 8.41 min; C6H8F2S7-107Ag complex, m/z 448.7, 9.00 min; C6H8F2S8-107Ag complex, m/z 480.7, 9.50 min; C6H8F2S9-107Ag complex, m/z 512.6, 9.97 min; C6H8F2S10-107Ag complex, m/z 544.5, 10.38 min; C6H8F2S11-107Ag complex, m/z 576.4, 10.7 min; and C6H8F2S12-107Ag complex, m/z 608.4, 11.1 min. With additional signal amplification, other low abundance complexes could also be seen: C6H8F2S13-107Ag complex, m/z 640.3, 11.43 min; C6H8F2S14-107Ag complex, m/z 672.3, 11.73 min; C6H8F2S15-107Ag complex, m/z 704.2, 12.05 min. The insert shows molecular ions m/z 352.73 and 354.73 corresponding to C6H8F2S4-107Ag and C6H8F2S4-109Ag. The chromatogram selecting for C6H8F2Sn-109Ag complexes was very similar.
All new compounds were fully characterized by spectroscopic methods, either as pure compounds, or in the case of 18 mixed with 12, 19 mixed with 20, and 21, as mixtures. While synthesis of fluorine-substituted analogs of garlic-derived organosulfur compounds in Scheme 1 is straightforward, their accessibility is limited by the high cost of starting material 9. While 12 and 18 are chiral due to the sulfinyl group, thiosulfinates, such as 12, are stereochemically labile [2,3], so no effort was made to synthesize enantiomerically pure material.
3. Materials and Methods
3.1. General Procedures
NMR spectra were recorded in CDCl3, unless otherwise indicated, on a 400 Ultrashield, spectrometer (Bruker, Billerica, MA, USA) operating at 400 MHz for proton, 100 MHz for carbon, and a Bruker 600 Avance III HD with QCI Cryoprobe operating at 600 MHz for proton, 125 MHz for carbon and 376.5 MHz for 19F. The chemical shifts (δ) are indicated in ppm downfield from tetramethylsilane, with the internal standard being the residual CHCl3 (δ 7.27 ppm for proton and δ 77.23 for carbon spectra). 19F-NMR chemical shifts were relative to CFCl3 at δ 0.00 ppm. Infrared spectra of the neat compounds were recorded on a UATR 2 FTIR (Perkin Elmer, Boston, MA USA). GC-MS were obtained using the Hewlett Packard 6890 GC and a 5972A selective mass detector (Agilent Technologies, Inc., Santa Clara, CA, USA). A DART-AccuTOF (JEOL USA, Inc., Peabody, MA, USA) time-of-flight mass spectrometer operating in positive or negative ion mode was employed with a polyethylene glycol spectrum as reference standard for exact mass measurements (PEG average molecular weight: 600). The atmospheric pressure interface was typically operated at the following potentials: orifice 1 = 15 V; orifice 2 = 5 V; ring lens = 3 V. The RF ion guide voltage was generally set to 800 V to allow detection of ions greater than m/z 80. The DART ion source was operated with helium gas at 200 °C, and a grid voltage = 530. 3-Chloro-2-fluoroprop-1-ene (9) was obtained from Synquest Laboratories (Alachua, FL, USA). Selected 1H-, 13C- and 19F-NMR and DART-MS data for new compounds are available in the Supporting Materials.
3.2. Synthesis of Fluorinated Garlic Organosulfur Compounds
S-(2-Fluoro-2-propenyl)ethanethioate (10). Caution: 3-chloro-2-fluoroprop-1-ene is toxic (LD50 for skin penetration, 200 mg/kg) and, therefore, should be handled with great care [45,57,58]. Potassium thioacetate (2.50 g, 22 mmol) was added to a solution of 3-chloro-2-fluoroprop-1-ene (9, 1.89 g, 20 mmol) in THF (50 mL). The mixture was stirred overnight to give an orange-yellow solution with white precipitate. The solution was passed through a silica-gel pad and then concentrated in vacuo to give 1.60 g (60%, 11.9 mmol) of the title compound 10 as a red liquid, which is pure enough for further use; 1H NMR (400 MHz, CDCl3) δ 4.64 (dd, 3JHF = 15.6, 2JHH = 3.1 Hz, CF=CH(Z), 1H), 4.54 (dd, 3JHF = 43.9 Hz, 2JHH = 3.1 Hz, CF=CH(E), 1H), 3.66 (d, 3JHF = 17.2 Hz, CH2, 2H), 2.37 (s, CH3, 3H); 13C NMR (100 MHz, CDCl3) δ 194.2 (s), 161.6 (d, 1JCF = 256 Hz), 93.1 (d, 2JCF = 19.1 Hz), 30.7 (s), 30.0 (d, 2JCF = 31.3 Hz); 19F NMR (376.5 MHz, CDCl3) δ −98.23 (m). IR: νmax 2981, 1697, 1673, 1275, 1132, 1105, 944, 857, 846, 624 cm−1; HRMS (ESI) exact mass calculated for [M + H]+ (C5H8OFS) requires m/z 135.0279, found m/z 135.0290.
1,2-Bis(2-fluoro-2-propenyl)disulfane (11). Sodium chips (0.12 g, 5.0 mmol) were added to anhydrous methanol (25 mL) at 0 °C. The mixture was stirred until the sodium completely disappeared. Compound 10 (0.67 g, 5.0 mmol) was added to the above solution at 0 °C. Iodine (0.63 g, 2.5 mmol) was added to the solution after 2 h. The mixture was stirred for 30 min and then concentrated and purified by silica gel column chromatography (hexanes) to give the title compound as a light yellow liquid (0.24 g, 53%); 1H NMR (400 MHz, CDCl3) δ 4.72 (dd, J = 15.4, 3.0 Hz, 2H), 4.53 (dd, J = 47.5, 3.0 Hz, 2H), 3.41 (d, J = 18.9 Hz, 4H); 13C NMR (100 MHz, CDCl3) δ 161.1 (d, JCF = 256 Hz), 94.5 (d, JCF = 19.2 Hz), 40.4 (d, JCF = 30.1 Hz); 19F NMR (376.5 MHz, CDCl3) δ −100.37 (m); IR: νmax 2919, 1668, 1271, 1133, 946, 891, 855, 841, 732 cm−1; HRMS (ESI): calcd for C6H9F2S2 [M + H+] = 183.0114; found = 183.0121.
Difluoroallicin (12; S-(2-Fluoroallyl) 2-fluoroprop-2-ene-1-sulfinothioate). A 32% peracetic acid/acetic acid solution (0.58 g, 2.6 mmol, 1.04 equiv.) was added dropwise to a solution of 11 (0.46 g, 2.5 mmol) in chloroform (50 mL) at 0 °C. The reaction mixture was vigorously stirred at 0 °C for 45 min as anhydrous Na2CO3 (4.0 g, 37.7 mmol, 15.1 equiv.) was added in small portions. The mixture was stirred for an additional 1 h at 0 °C and then filtered through a pad of Celite and anhydrous MgSO4. The filtrate was concentrated in vacuo at 0 °C to yield the crude, water-soluble title compound 12, which was purified by preparative TLC (4:1 pentane:diethyl ether) to give pure 12 as yellow liquid with an onion-like odor (0.21 g, 1.06 mmol, 42%); 1H-NMR (400 MHz, CDCl3) δ 4.97 (dd, J = 15, 3 Hz, S(O)–CH2–CF=CH(Z), 1H), 4.78 (dd, J = 14, 3 Hz, S–CH2–CF=CH(Z), 1H), 4.70 (dd, J = 48, 3 Hz, S(O)–CH2–CF=CH(E), 1H), 4.63 (dd, J = 47, 3 Hz, S–CH2–CF=CH(E), 1H), 4.00–3.75 (m, 4H)); 13C-NMR (100 MHz, CDCl3) δ 32.5 (d, 2JCF = 31.0 Hz) 58.8 (d, 2JCF = 29.0 Hz), 94.1 (d, 2JCF = 16.9 Hz) 98.3 (d, 2JCF = 17.9 Hz), 155.4 (d, 1JCF = 259 Hz), 160.5 (d, 1JCF = 161 Hz); 19F-NMR (376.5 MHz, CDCl3) δ −99.65 to −99.44 (m, 1F), −94.90 to −94.62 (m, 1F); IR: νmax 2917, 1669, 1407, 1272, 1081 (S=O), 945 cm−1; HRMS (ESI) exact mass calculated for [M + H]+ (C6H9OF2S2) requires m/z 199.0063, found m/z 199.0080. Storage at −40 °C or lower is recommended to avoid decomposition. Vacuum distillation was not attempted due to the presumed thermal sensitivity of 12.
S-2-Fluoro-2-propenyl-l-cysteine (13; 2-Amino-3-((2-fluoroallyl)thio)propanoic acid). 3-Chloro-2-fluoroprop-1-ene (9) (0.94 g, 10 mmol) was added to an ice-cold solution of l-cysteine (1.21 g, 10 mmol) in ammonium hydroxide (40 mL) with vigorous stirring. The mixture was stirred at 0 °C for 60 min and filtered; the filtrate was concentrated in vacuo (<40 °C) to a small volume and filtered. The solid was washed repeatedly with ethanol, dried in vacuo, and recrystallized from 2:3 water/ ethanol to yield 13 as white needles (1.31 g, 7.31 mmol, 73% yield), mp 220–223 °C; 1H-NMR (400 MHz, D2O): δ 4.48 (dd, J = 3.2, 12 Hz, 1H), 4.58 (dd, J = 3.2, 50 Hz, 1H), 3.90 (dd, J = 4, 8 Hz, 1H), 3.32 (d, 2H, J = 16 Hz), 3.13–2.99 (m, 2H); 19F-NMR (D2O): δ −101.30 to −101.58 (m, 1F); 13C-NMR (100 MHz, D2O): δ 172.41, 161.73, 159.21, 53.27, 31.63–31.35 (d), 31.16. Single crystals were analyzed by X-ray crystallography as described in Section 3.3 below to provide the crystal structure of the l-enantiomer shown in Figure 1.
Trifluoroajoene (18) [(E,Z)-1-(2-Fluoro-3-((2-fluoroallyl)sulfinyl)prop-1-en-1-yl)-2-(2-fluoroallyl)disulfane]. A solution of 12 (200 mg; 1.01 mmol) was dissolved in 1,1,1,3,3,3-hexafluoro-2-propanol (0.50 mL) at room temperature. The mixture was immediately analyzed by DART-MS which showed the formation of trace amounts of trifluoroajoene 18. The mixture was stirred at room temperature for 16 h whereupon analysis by DART-MS showed a 1:1 mixture of F-allicin and F-ajoene, with no F-dithiin present. The mixture was concentrated in vacuo to yield a faintly yellow liquid (180 mg). 1H-, 13C- and 19F-NMR spectroscopy indicated a 1:1 mixture of difluoroallicin 12 and trifluoroajoene 18, with no difluorodithiins present, corresponding to a 59% yield of 18 based on 12 converted. A small sample was stirred for 32 h but no further conversion of 12 to 18 was observed. Attempts to purify 18 by silica gel chromatography or HPLC were unsuccessful; 1H-NMR (400 MHz, CDCl3) 18: δ 5.91 (d, 2H, 3JHF = 120.9), 5.90 (s, 1H), 4.4 (m, 2H), 3.8 (m, 6H); 12 as above; 13C-NMR (100 MHz, CDCl3) 18: δ 59.75 (d, 1C, J = 27.70 Hz), 60.02 (d, 1C, J = 27.59 Hz), 68.82 (d, 1C, J = 33.33 Hz), 69.32 (s, 1C), 69.81 (d, 1C, J = 33.23 Hz), 109.44 (d, 1C, J = 36.08 Hz), 108.57 (d, 1C, J = 283.20 Hz), 110.86 (d, 1C, J = 247.17 Hz), 121.75 (d, 1C, J = 278.34 Hz); 12 as above; 19F-NMR (376.5 MHz, CDCl3) 18: δ −78 (s, 1F), −128 (s,1F), −140 (s, 1F); 12 as above; HRMS (ESI) exact mass calculated for [M + H]+ (C9H12F3OS3) requires m/z 289.0002, found m/z 288.9992.
5-Fluoro-3-(1-fluorovinyl)-3,4-dihydro-1,2-dithiin (16). A solution of 12 (50.0 mg) in CHCl3 (1 mL) was placed in a glass tube which was sealed under vacuum and heated at 80 °C for 4.0 h. The reaction mixture was analyzed by GC-MS and the major dithiin isomer 16 was purified by flash chromatography (n-pentane); 1H-NMR (400 MHz, CDCl3) δ 3.61–2.84 (m, 2H), 3.93–3.99 (m, 1H), 4.44–4.57 (m, 1H), 4.39–4.94 (m, 1H), 6.09–6.13 (m, 1H). 13C-NMR (100 MHz, CDCl3) δ 30.3 (dd, J = 28.0, 5.0 Hz), 45.5 (dd, J = 30.0, 11.5 Hz), 93.3 (d, J = 19.5 Hz), 101.0 (d, J = 27.0 Hz) 157.0 (d, J = 395.0 Hz) 160.0 (d, J = 395.0 Hz); 19F-NMR (376.5 MHz CDCl3): δ −99.80 to −99.60 (m, 1F), −88.83 to −88.78 (m, 1F). IR: νmax 2853, 1668, 1420, 1257, 1192, 1100, 928 cm−1; DART-HR-TOF-MS [M + H]+ C6H7F2S2 found m/z 180.9958, requires m/z 180.9957.
2-Fluoroacrolein-ethyl acrylate Diels-Alder adducts: Ethyl 5-fluoro-3,4-dihydro-2H-thiopyran-2-carboxylate (19) and ethyl 5-fluoro-3,4-dihydro-2H-thiopyran-3-carboxylate (20). A mixture of difluoroallicin 12 (98.0 mg, 0.5 mmol) and ethyl acrylate (250.0 mg, 2.5 mmol) in CHCl3 (2.5 mL) were placed in a glass tube. The tube was sealed under vacuum and heated at 100 °C for 6.0 h. The reaction mixture was analyzed by NMR and the crude material was purified by using flash chromatography (n-pentane/Et2O), affording 70:30 (19:20) mixture (56.0 mg, 60% yield). It was not possible to separate completely 19 (major) from 20 (minor) by flash chromatography. DART-HR-TOF-MS (M + H) C8H12FO2S found m/z 191.0538, requires m/z 191.0542. 19 (Major 70%): 1H-NMR (400 MHz, CDCl3) δ 5.67 (d, J = 17.7 Hz, 1H), 4.15–4.22 (m, 2H, overlap with 20), 3.05–3.08 (m, 1H), 2.85–2.98 (m, 2H overlap with 20), 2.49–2.63 (m, 2H, overlap with 20), 1.26–1.30 (m, 3H, overlap with 20); 13C-NMR (100 MHz, CDCl3: δ 172.0, 153.5 (d, J = 259.7 Hz), 97.3 (d, J = 27.5 Hz), 61.2, 40.7 (m, overlap with 20), 27.2 (m, overlap with 20), 26.4, 14.0 (overlap with 20); 19F-NMR (376.5 MHz CDCl3): δ −96.26 to −96.21 (m). 20 (Minor 30%): 1H-NMR (400 MHz, CDCl3) δ 5.61 (d, J = 17.7 Hz, 1H), 4.15–4.22 (m, 2H, overlap with 19), 3.35–3.43 (m, 1H), 2.85–2.98 (m, 2H), 2.49–2.63 (m, 2H, overlap with 19), 1.26–1.30 (m, 3H, overlap with 19); 13C-NMR (100 MHz, CDCl3) δ 170.4, 151.4 (d, J = 259.7 Hz), 95.7 (d, J = 27.5 Hz), 61.6, 40.6 (m, overlap with 19), 27.2 (m, overlap with 19), 23.4, 14.0 (overlap with 19); 19F-NMR (376.5 MHz CDCl3): δ −96.09 to −96.04 (m).
Bis(2-fluoroallyl) polysulfane mixture (21). A 10 mL round-bottomed flask containing sublimed sulfur (S8, 0.256 g, 1.0 mmol) was placed in an oil bath pre-heated to 120 °C. When the sulfur had completely melted to a clear, straw-colored liquid, disulfide 11 (0.182 g, 1.0 mmol) was added all at once to the magnetically stirred liquid. After 30 min, the initial two-layer liquid mixture became a clear, homogeneous solution. Samples were withdrawn from the reaction mixture for analysis at various time points, e.g., 30 min, 1 h, 2 h, 4 h and 5 h. The withdrawn samples were dissolved in CDCl3 permitting both NMR and reversed phase (RP) HPLC analysis to be performed on the same sample. For the 2 h sample, disulfide 11 eluted at 2.65 min, S8 eluted between the heptasulfide and octasulfide at 22.2 min, and the highest polysulfane seen, eluting at 159 min, had a chain of 26 sulfur atoms. By UPLC-(Ag+)-CIS-MS, chains containing up to 15 sulfur atoms could be characterized as their 107Ag and 109Ag adducts (see Supporting Information and discussion elsewhere [53]). 1H-NMR spectroscopic analysis after 30 min shows the CH2 proton signals as doublets centered at δ 3.39 [area 0.35, S2], 3.52 [area 0.08, S3], 3.61 [area 0.08, S4], 3.63 [area 0.16, S5], 3.64 [area 0.50, ≥S6]; after 5 h 1H-NMR spectroscopic analysis shows 3.39 [area 0.06 S2], 3.52 [area 0.08 S3], and 3.64 [area 0.95, ≥S6]. After 1.25 h, 19F-NMR (376.5 MHz CDCl3) showed a series of multiplets centered at δ −100.3 (11; C6H8F2S2) and −100.0 (C6H8F2S3) down to −99.5 ppm (C6H8F2S>5). DART was used to obtain HRMS (ESI) exact mass data on C6H9F2Sn components in a mixture examined after 1.25 h of heating. The lighter components gave better exact mass agreement compared to the heavier components: calculated for [M + H]+ (11; C6H9F2S2) requires m/z 183.0114, found m/z 183.0117; calculated for [M + H]+ (C6H9F2S3) requires m/z 214.9835, found m/z 214.9835; calculated for [M + H]+ (C6H9F2S4) requires m/z 246.9555, found m/z 246.9563; calculated for [M + H]+ (C6H9F2S5) requires m/z 278.9276, found m/z 278.9242; calculated for [M + H]+ (C6H9F2S6) requires m/z 310.8997, found m/z 310.8985; calculated for [M + H]+ (C6H9F2S7) requires m/z 342.8717, found m/z 342.8808; calculated for [M + H]+ (C6H9F2S8) requires m/z 374.8438, found m/z 374.9639; calculated for [M + H]+ (C6H9F2S9) requires m/z 406.8159, found m/z 406.9403; calculated for [M + H]+ (C6H9F2S10) requires m/z 438.7880, found m/z 438.9084. UPLC-(Ag+)-CIS-MS analysis of (21) is listed in Table S2.
(+)-S-2-Fluoro-2-propenyl-l-cysteine S-oxide (22). With vigorous stirring, 13 (1.18 g, 6.59 mmol) was dissolved in 12 mL of water. Hydrogen peroxide (30%, 2.56 g) was added to the solution which was then stirred for 45 min at RT. The water was removed under vacuum, and the title compound 22 (1.04 g, 82%, 5.33 mmol) was obtained as a white powder, mp 168–171 °C; 1H-NMR (D2O) δ 5.03 (dd, 2H, J = 3 Hz), 4.86–4.82 (m, 2H) 4.08–3.75 (m, 2H), 3.50–3.26 (m, 2H); 19F-NMR (D2O) δ −94.84 to −95.12 (m, 1F), −95.18 to −95.47 (m, 1F).
3.3. X-Ray Crystallographic Structural Determination for 13
The single crystal diffraction data for 13 was measured on a SMART APEX CCD X-ray diffractometer (Bruker, Madison, WI, USA) equipped with a graphite monochromated Mo Kα radiation source (λ = 0.71073 Å) at T = 100(2) K. Data reduction and integration were performed with the Bruker software package SAINT (Version 8.38A). Data were corrected for absorption effects using the empirical methods as implemented in SADABS. Both SAINT and SADABS are part of Bruker APEX3 software package (Version 2016.9-0): Bruker AXS, 2016. The structure was solved by SHELXT (Version 2014/5) [59] and refined by full-matrix least-squares procedures using the Bruker SHELXTL (Version 2016/6) XL refinement program version 2016/6 software package [59]. All non-hydrogen atoms (including those in disordered parts) were refined anisotropically. The H-atoms were also included at calculated positions and refined as riders, with Uiso(H) = 1.2 Ueq(C) and Uiso(H) = 1.5 Ueq(N) for ammonia groups. Crystallographic data, details of the data collection and structure refinement for 13 are listed in Table S1.
4. Conclusions
We describe here the synthesis and characterization of a series of new fluorinated derivatives of key, biologically active garlic-derived organosulfur compounds, including difluoroallicin (12). Upon heating, 12 decomposes to 2-fluorothioacrolein (15), a previously unknown highly reactive fluorine-substituted heterodiene, trapped with ethyl acrylate to afford a mixture of isomeric 5- and 6-substituted 3-fluoro-5,6-dihydro-4H-thiopyrans. Our findings suggest a useful new synthetic route to fluorine-substituted thiopyrans, of potential pharmaceutical interest. Several of the other fluorinated organosulfur compounds prepared are anticipated to show interesting biological activity. Further studies on these new compounds and preparation of more heavily fluorinated homologues seem warranted.
X-Ray Crystallographic Data
CCDC1577315 contains the supplementary crystallographic data, which can be obtained free of charge from Cambridge Crystallographic Data Centre, www.ccdc.cam.ac.uk/data_request/cif (accessed on 20 June 2025).
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules30132841/s1: Table S1: Documentation for the X-ray structure; Table S2: UPLC-(Ag+)-CIS-MS analysis of bis(2-fluoro-2-propenyl) polysulfanes (21). Selected 1H-, 13C- and 19F-NMR and DART-MS data for new compounds are available online.
Author Contributions
E.B. conceived and designed the experiments; B.B., S.G., A.V. and K.W. performed the experiments and analyzed the data; E.B. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Foundation (CHE-0744578; CHE-1265679; CHE-1337594 [MRI]; CHE-1429329 [MRI]) and the University at Albany (E.B.). We thank Alexander Filatov and Zheng Wei for performing the X-ray structural analysis, and Robert Sheridan for the UPLC-MS studies.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
We dedicate this paper with our warmest wishes to E.J. Corey on his 97th birthday.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Cavallito, C.J.; Bailey, J.H. Allicin, the Antibacterial Principle of Allium sativum. I. Isolation, Physical Properties and Antibacterial Action. J. Am. Chem. Soc. 1944, 66, 1950–1951. [Google Scholar] [CrossRef]
- Block, E. Garlic and Other Alliums: The Lore and the Science; Royal Society of Chemistry: Cambridge, UK, 2010. [Google Scholar] [CrossRef]
- Block, E. Magical Scallions and Garlics—The Lore and the Science; [神奇的葱蒜—传说与科学]; Chemistry Industry Press: Beijing, China, 2017; (In Chinese). ISBN 978-7-122-28930-8. [Google Scholar]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.; Nwachukwu, I.; Slusarenko, A. Allicin: Chemistry and Biological Properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [PubMed]
- Reiter, J.; Levina, N.; van der Linden, M.; Gruhlke, M.; Martin, C.; Slusarenko, A. Diallylthiosulfinate (Allicin), a Volatile Antimicrobial from Garlic (Allium sativum), Kills Human Lung Pathogenic Bacteria, Including MDR Strains, as a Vapor. Molecules 2017, 22, 1711. [Google Scholar] [CrossRef] [PubMed]
- Borlinghaus, J.; Foerster, J.; Kappler, U.; Antelmann, H.; Noll, U.; Gruhlke, M.C.H.; Slusarenko, A.J. Allicin, the Odor of Freshly Crushed Garlic: A Review of Recent Progress in Understanding Allicin’s Effects on Cells. Molecules 2021, 26, 1505. [Google Scholar] [CrossRef] [PubMed]
- Mösbauer, K.; Fritsch, V.N.; Adrian, L.; Bernhardt, J.; Gruhlke, M.C.H.; Slusarenko, A.J.; Niemeyer, D.; Antelmann, H. The Effect of Allicin on the Proteome of SARS-CoV-2 Infected Calu-3 Cells. Front. Microbiol. 2021, 12, 746795. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, E.; Canistro, D.; Pellicioni, V.; Vivarelli, F.; Fimognari, C. Anticancer Potential of Allicin: A Review. Pharmacol. Res. 2022, 177, 106118. [Google Scholar] [CrossRef] [PubMed]
- Mengers, H.G.; Schier, C.; Zimmermann, M.; Gruhlke, M.C.H.; Block, E.; Blank, L.M.; Slusarenko, A.J. Seeing the Smell of Garlic: Detection of Gas Phase Volatiles from Crushed Garlic (Allium sativum), Onion (Allium cepa), Ramsons (Allium ursinum) and Human Garlic Breath Using SESI-Orbitrap MS. Food Chem. 2022, 397, 133804. [Google Scholar] [CrossRef]
- Schultz, C.R.; Gruhlke, M.C.H.; Slusarenko, A.J.; Bachmann, A.S. In Vivo Antitumor Activity of Allicin in a Pediatric Neuroblastoma Patient-derived Xenograft (PDX) Mouse Model. In Vivo 2025, 39, 1283–1292. [Google Scholar] [CrossRef]
- Block, E.; Dethier, B.; Bechand, B.; Cotelesage, J.J.H.; George, G.N.; Goto, K.; Pickering, I.J.; Rengifo, E.M.; Sheridan, R.; Sneeden, E.Y.; et al. Ajothiolanes: 3,4-Dimethylthiolane Natural Products from Garlic (Allium sativum). J. Agric. Food Chem. 2018, 66, 10193–10204. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; Saad, A.M.; Korma, S.A.; Salem, H.M.; Abd El-Mageed, T.A.; Alkafaas, S.S.; Elsalahaty, M.I.; Elkafas, S.S.; Mosa, W.F.A.; Ahmed, A.E.; et al. Garlic Bioactive Substances and Their Therapeutic Applications for Improving Human Health: A Comprehensive Review. Front. Immunol. 2024, 15, 1277074. [Google Scholar] [CrossRef]
- Sela, U.; Brill, A.; Kalchenko, V.; Dashevsky, O.; Hershkoviz, R. Allicin Inhibits Blood Vessel Growth and Downregulates Akt Phosphorylation and Actin Polymerization. Nutr. Cancer 2008, 60, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Hosono, T. Functionality of Garlic Sulfur Compounds. Biomed. Rep. 2025, 23, 124. [Google Scholar] [CrossRef] [PubMed]
- Beretta, H.V.; Bannoud, F.; Insani, M.; Berli, F.; Hirschegger, P.; Galmarini, C.R.; Cavagnaro, P.F. Relationships among Bioactive Compounds Content and the Antiplatelet and Antioxidant Activities of Six Allium Vegetable Species. Food Technol. Biotechnol. 2017, 55, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Atawneh, S.; Shakhatreh, A.N.; Hamed, R.A.; Al-Yasari, I.H. Anticancer Potential of Garlic Bioactive Constituents: Allicin, Z-Ajoene, and Organosulfur Compounds. Pharmacia 2024, 71, 1–23. [Google Scholar] [CrossRef]
- Block, E.; Ahmad, S.; Jain, M.K.; Crecely, R.W.; Apitz-Castro, R.; Cruz, M.R. (E,Z)-Ajoene: A Potent Antithrombotic Agent from Garlic. J. Am. Chem. Soc. 1984, 106, 8295–8296. [Google Scholar] [CrossRef]
- Block, E.; Ahmad, S.; Catalfamo, J.; Jain, M.K.; Apitz-Castro, R. Antithrombotic Organosulfur Compounds from Garlic: Structural, Mechanistic and Synthetic Studies. J. Am. Chem. Soc. 1986, 108, 7045–7055. [Google Scholar] [CrossRef]
- Kusza, D.A.; Venter, G.A.; Mabunda, M.; Biwi, J.; Samanta, S.K.; Klinck, J.D.; Singh, S.V.; Hunter, R.; Kaschula, C.H. Finding the Ajoene Sweet-Spot: Structure-Activity Relations that Govern its Blood Stability and Cancer Cytotoxicity. Chem. Med. Chem. 2024, 19, e202400087. [Google Scholar] [CrossRef]
- Kusza, D.A.; Hunter, R.; Schäfer, G.; Smith, M.; Katz, A.A.; Kaschula, C.H. Activity-Based Proteomic Identification of the S-Thiolation Targets of Ajoene in MDA-MB-231 Breast Cancer Cells. J. Agric. Food Chem. 2022, 70, 14679–14692. [Google Scholar] [CrossRef]
- Kaschula, C.H.; Hunter, R.; Stellenboom, N.; Caira, M.R.; Winks, S.; Ogunleye, T.; Richards, P.; Cotton, J.; Zilbeyaz, K.; Wang, Y.; et al. Structure–Activity Studies on the Anti-Proliferation Activity of Ajoene Analogues in WHCO1 Oesophageal Cancer Cells. Eur. J. Med. Chem. 2012, 50, 236–254. [Google Scholar] [CrossRef]
- Hunter, R.; Kaschula, C.H.; Parker, M.I.; Caira, M.R.; Richards, P.; Travis, S.; Taute, F.; Qwebani, T. Substituted Ajoenes as Novel Anti-Cancer Agents. Bioorg. Med. Chem. Lett. 2008, 18, 5277–5279. [Google Scholar] [CrossRef]
- Raynbird, M.Y.; Khokhar, S.S.; Neef, D.; Evans, G.J.S.; Wirth, T. Synthesis of Ajoene Analogues by Novel Synthetic Strategies. Chem. Eur. J. 2021, 27, 3008–3012. [Google Scholar] [CrossRef]
- Cho, H.; Lee, E.; Kim, J.; Shin, S.; Kim, Y.-J.; Lee, H.; Yu, J.H.; Jeon, Y.H.; Lee, S.W.; Lee, S.Y.; et al. Discovery of Organosulfur-Based Selective HDAC8 Inhibitors with Anti-Neuroblastoma Activity. Eur. J. Pharm. Sci. 2024, 203, 106921. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-I.; Cho, H.; Jeon, R.; Sung, M.-K. Therapeutic Efficacy of Novel HDAC Inhibitors SPA3052 and SPA3074 against Intestinal Inflammation in a Murine Model of Colitis. Pharmaceuticals 2022, 15, 1515. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; Kamari, F.; El Omari, N.; Touhtouh, J.; Mssillou, I.; Aanniz, T.; Benali, T.; Khalid, A.; Abdalla, A.N.; Iesa, M.A.M.; et al. Unlocking the Natural Chemical Sources, Nutritional Properties, Biological Effects, and Molecular Actions of Ajoene. J. Funct. Foods 2025, 127, 106714. [Google Scholar] [CrossRef]
- Bhaumik, I.; Pal, K.; Debnath, U.; Karmakar, P.; Jana, K.; Misra, A.K. Natural Product Inspired Allicin Analogs as Novel Anti-Cancer Agents. Bioorg. Chem. 2019, 86, 259–272. [Google Scholar] [CrossRef]
- Puchkov, V.M.; Lyfenko, A.D.; Koval, V.S.; Revtovich, S.V.; Kulikova, V.V.; Anufrieva, N.V.; Zemskaya, A.S.; Morozova, E.A.; Solyev, P.N. Synthesis and Antimicrobial Activity of Thiosulfinates and Allicin Analogues. Mol. Biol. 2024, 58, 1074–1081. [Google Scholar] [CrossRef]
- Roseblade, A.; Ung, A.; Bebawy, M. Synthesis and in vitro Biological Evaluation of Thiosulfinate Derivatives for the Treatment of Human Multidrug-Resistant Breast Cancer. Acta Pharmacol. Sin. 2017, 38, 1353–1368. [Google Scholar] [CrossRef]
- Xu, Y.S.; Feng, J.G.; Zhang, D.; Zhang, B.; Luo, M.; Su, D.; Lin, N.M. S-Allylcysteine, a Garlic Derivative, Suppresses Proliferation and Induces Apoptosis in Human Ovarian Cancer Cells in vitro. Acta Pharmacol. Sin. 2014, 35, 267–274. [Google Scholar] [CrossRef]
- Xu, Y.; Su, D.; Zhu, L.; Zhang, S.; Ma, S.; Wu, K.; Yuan, Q.; Lin, N. S-Allylcysteine Suppresses Ovarian Cancer Cell Proliferation by DNA Methylation through DNMT1. J. Ovarian Res. 2018, 11, 39. [Google Scholar] [CrossRef]
- Rais, N.; Ved, A.; Ahmad, R.; Kumar, M.; Barbhai, M.D.; Radha; Chandran, D.; Dey, A.; Dhumal, S.; Senapathy, M.; et al. S-Allyl-L-cysteine—A Garlic Bioactive: Physicochemical Nature, Mechanism, Pharmacokinetics, and Health Promoting Activities. J. Funct. Foods 2023, 107, 105657. [Google Scholar] [CrossRef]
- Smith, M.; Hunter, R.; Stellenboom, N.; Kusza, D.A.; Parker, M.I.; Hammouda, A.M.I.; Jackson, G.; Kaschula, C.H. The Cytotoxicity of Garlic-Related Disulphides and Thiosulfonates in WHCO1 Oesophageal Cancer Cells is Dependent on S-Thiolation and not Production of ROS. Biochim. Biophys. Acta 2016, 1860, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sánchez-Roselló, M.; Aceña, J.L.; del Pozo, C.; Sorochinsky, A.E.; Fustero, S.; Soloshonok, V.A.; Liu, H. Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, T.; Yamamoto, H. Biologically active organofluorine compounds. In Organofluorine Compounds: Chemistry and Applications; Yamamoto, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 137–182. [Google Scholar] [CrossRef]
- Gombler, W. Bis(trifluoromethyl)disulfane Oxide CF3S(O)SCF3. Angew. Chem. Int. Ed. 1977, 16, 723–724. [Google Scholar] [CrossRef]
- Zhu, B.; Wu, Z.; Wang, L.; Lu, B.; Trabelsi, T.; Francisco, J.S.; Zeng, X. Matrix-Isolated Trifluoromethylthiyl Radical: Sulfur Atom Transfer, Isomerization and Oxidation Reactions. Chem. Commun. 2021, 57, 12143–12146. [Google Scholar] [CrossRef]
- Zhu, B.; Jiang, J.; Lu, B.; Li, X.; Zeng, X. Fluoromethylsulfinyl Radicals: Spectroscopic Characterization and Photoisomerization via Intramolecular Hydrogen Shift. Phys. Chem. Chem. Phys. 2022, 24, 8881–8889. [Google Scholar] [CrossRef]
- Astrain-Redin, N.; Sanmartin, C.; Sharma, A.K.; Plano, D. From Natural Sources to Synthetic Derivatives: The Allyl Motif as a Powerful Tool for Fragment-Based Design in Cancer Treatment. J. Med. Chem. 2023, 66, 3703–3731. [Google Scholar] [CrossRef]
- Han, Z.; Czap, G.; Chiang, C.; Xu, C.; Wagner, P.J.; Wei, X.; Zhang, Y.; Wu, R.; Ho, W. Imaging the Halogen Bond in Self-Assembled Halogenbenzenes on Silver. Science 2017, 358, 206–210. [Google Scholar] [CrossRef]
- Wallock-Richards, D.; Doherty, C.J.; Doherty, L.; Clarke, D.J.; Place, M.; Govan, J.R.W.; Campopiano, D.J. Garlic Revisited: Antimicrobial Activity of Allicin-Containing Garlic Extracts against Burkholderia cepacia Complex. PLoS ONE 2014, 9, e112726. [Google Scholar] [CrossRef]
- Müller, A.; Eller, J.; Albrecht, F.; Prochnow, P.; Kuhlmann, K.; Bandow, J.E.; Slusarenko, A.J.; Leichert, L.I.O. Allicin Induces Thiol Stress in Bacteria through S-Allylmercapto Modification of Protein Cysteines. J. Biol. Chem. 2016, 291, 11477–11490. [Google Scholar] [CrossRef]
- Gruhlke, M.C.H.; Schlembach, I.; Leontiev, R.; Uebachs, A.; Gollwitzer, P.U.G.; Weiss, A.; Delaunay, A.; Toledano, M.; Slusarenko, A.J. Yap1p, the Central Regulator of the S. cerevisiae Oxidative Stress Response, Is Activated by Allicin, a Natural Oxidant and Defence Substance of Garlic. Free Radic. Biol. Med. 2017, 108, 793–802. [Google Scholar] [CrossRef]
- Jubault, P. 3-Chloro-2-fluoropropene. Encycl. Reagents Org. Synth. 2019, 1, 1–3. [Google Scholar]
- Moore, L.O.; Henry, J.P.; Clark, J.W. Chlorination of 2-Fluoropropene. 3-Chloro-2-fluoropropene and some of its Derivatives. J. Org. Chem. 1970, 35, 4201–4204. [Google Scholar] [CrossRef]
- Laue, K.W.; Haufe, G. 3-Bromo-2-Fluoropropene—A Fluorinated Building Block. 2-Fluoroallylation of Glycine and Alanine Ester Imines. Synthesis 1998, 1998, 1453–1456. [Google Scholar] [CrossRef]
- Bobrovo, A.Y.; Novikov, M.A.; Tomilov, Y.V. (2-Fluoroallyl)pyridinium Tetrafluoroborates: Novel Fluorinated Electrophiles for Pd-Catalyzed Allylic Substitution. Org. Biomol. Chem. 2021, 19, 4678–4684. [Google Scholar] [CrossRef]
- Ai, Y.; Yang, H.; Duan, C.; Li, X.; Yu, S. Cobalt-Catalyzed Fluoroallyllation of Carbonyls via C–C Activation of gem-Difluorocyclopropanes. Org. Lett. 2022, 24, 5051–5055. [Google Scholar] [CrossRef]
- Yan, Y.; Qian, H.; Lv, L.; Li, Z. Pd-IHept-Catalyzed Ring-Opening of gem-Difluorocyclopropanes with Malonates Via Selective C–CBond Cleavage: Synthesis of Monofluoroalkenes. J. Org. Chem. 2024, 89, 16253–16261. [Google Scholar] [CrossRef]
- Yoneda, T.; Kojima, N.; Matsumoto, T.; Imahori, D.; Ohta, T.; Yoshida, T.; Nakamura, S. Construction of Sulfur-Containing Compounds with Anti-Cancer Stem Cell Activity Using Thioacrolein Derived from Garlic Based on Nature-Inspired Scaffolds. Org. Biomol. Chem. 2022, 20, 196–207. [Google Scholar] [CrossRef]
- Mousavi-Ebadia, M.; Safaei-Ghomi, J.; Nejad, M.J. Synthesis of thiopyran derivatives via [4 + 2] cycloaddition reactions. RSC Adv. 2025, 15, 11160–11188. [Google Scholar] [CrossRef]
- Pathania, S.; Narang, R.K.; Rawal, R.K. Role of Sulphur-Heterocycles in Medicinal Chemistry: An Update. Eur. J. Med. Chem. 2019, 180, 486–508. [Google Scholar] [CrossRef]
- Mandal, P.K.; Chakrawarti, R.; Katuk, S. [3+3] Annulation of Diazoenals and α-Mercapto Ketones via Protic Sulfonium Ylides: Direct Synthesis of 2H-Thiopyrans, Innovative Progenitors for Unstudied 2H-Thiopyran-2-ones and 4H-Thiopyran-4-ones. Org. Lett. 2024, 26, 3463–3468. [Google Scholar] [CrossRef]
- Soni, S.; Shukla, G.; Singh, M.S. Magnesium catalyzed [3 + 3] heteroannulation of α-enolic dithioesters with MBH acetate: Access to functionalized 3,4-dihydro-2H-thiopyrans. Org. Biomol. Chem. 2022, 20, 6784–6798. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Groom, M.; Sheridan, R.; Zhang, S.; Block, E. Liquid Sulfur as a Reagent: Synthesis of Polysulfanes with 20 or More Sulfur Atoms with Characterization by UPLC-(Ag+)-Coordination Ion Spray-MS. J. Sulfur Chem. 2013, 34, 55–66. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C.; Fu, Y.; Guo, Q.-X.; Liu, L. Substituent Effect on the Efficiency of Desulfurizative Rearrangement of Allylic Disulfides. J. Org. Chem. 2008, 73, 6127–6136. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, A.N.; Radchenko, D.S.; Mykhailiuk, P.K.; Grygorenko, O.O.; Komarov, I.V. 4-Fluoro-2,4-methanoproline. Org. Lett. 2009, 11, 5674–5676. [Google Scholar] [CrossRef]
- Smyth, H.F.; Carpenter, C.P.; Well, C.S.; Pozzani, U.C.; Striegel, J.A. Range-Finding Toxicity Data: List VI. Am. Ind. Hyg. Assoc. J. 1962, 23, 95–107. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).