Fluorinated Analogs of Organosulfur Compounds from Garlic (Allium sativum): Synthesis and Chemistry
Abstract
1. Introduction
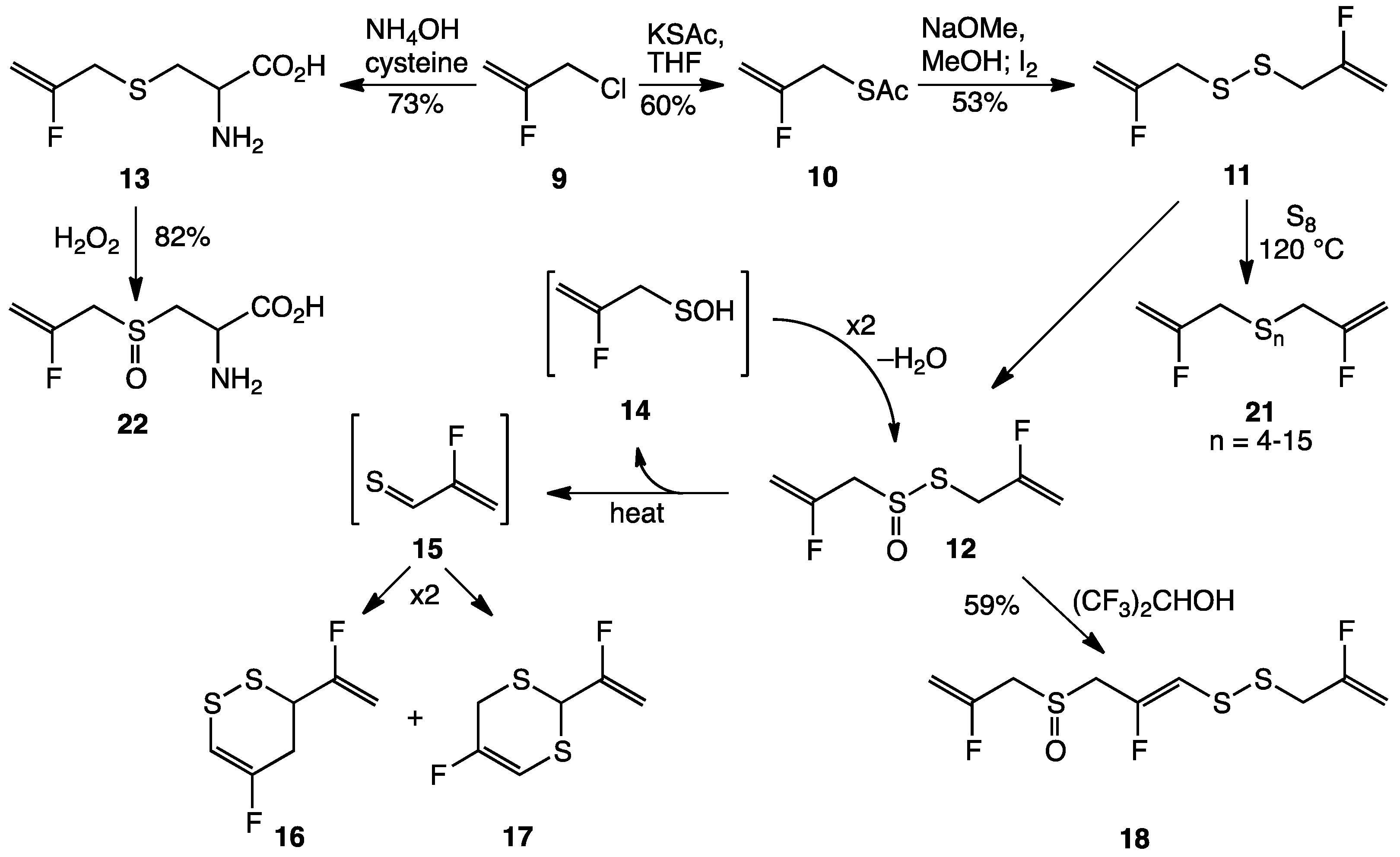
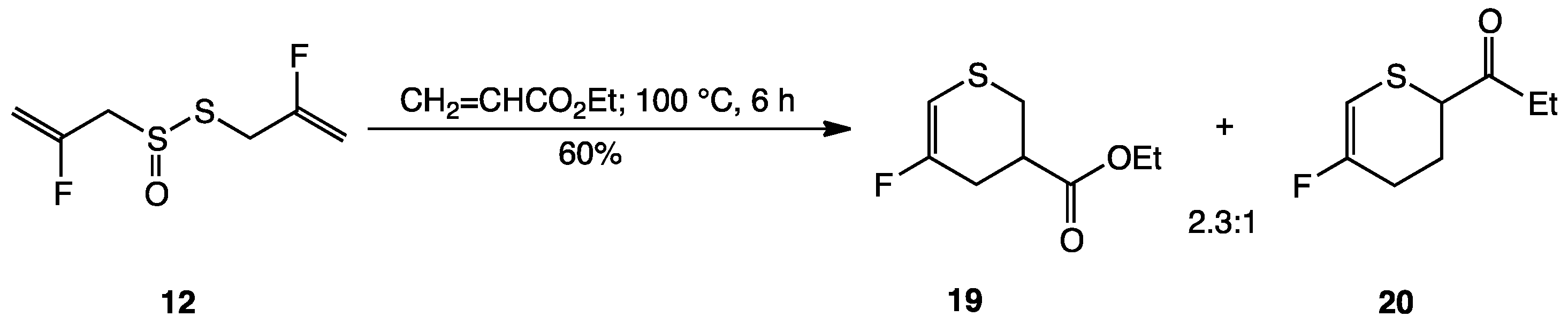
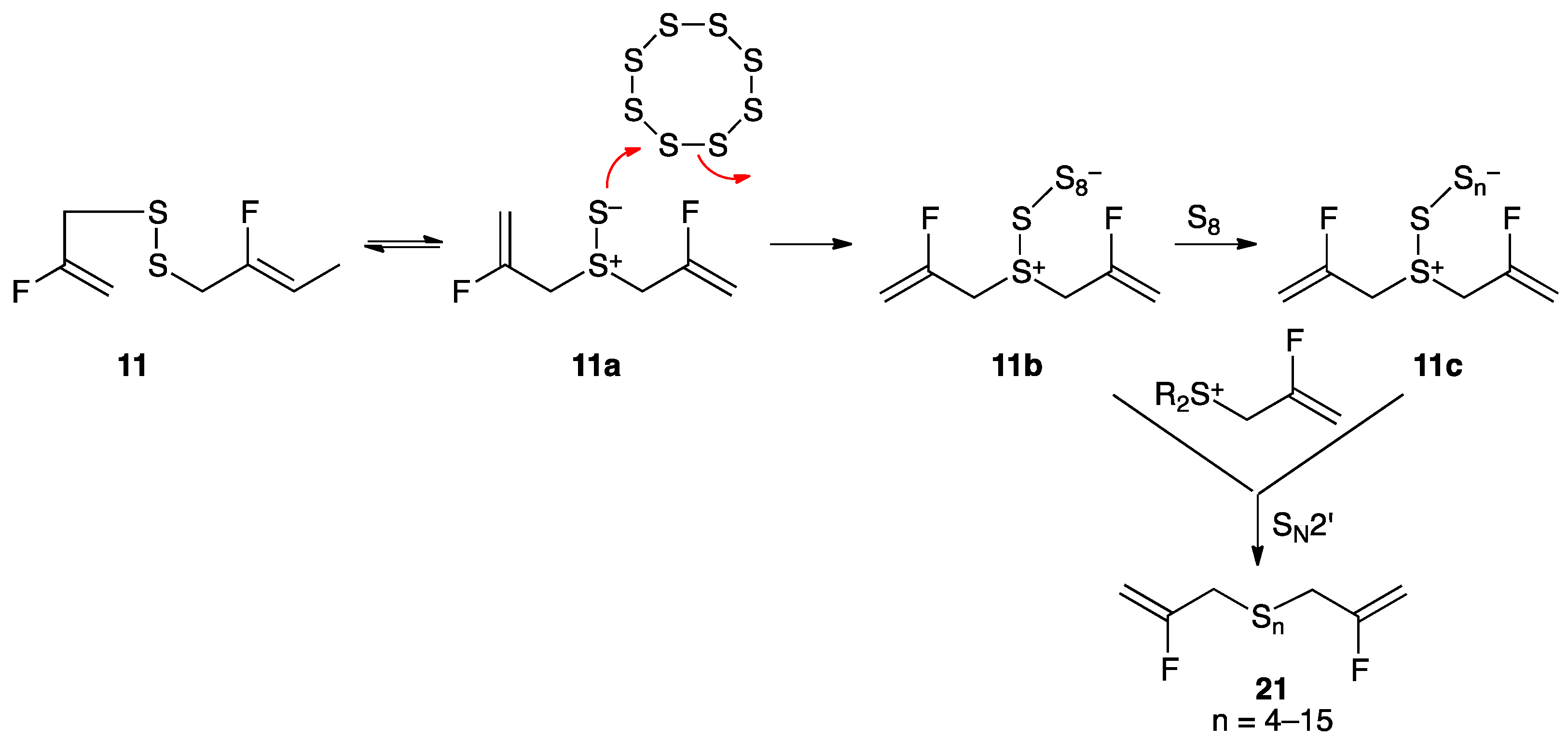
2. Results and Discussion
3. Materials and Methods
3.1. General Procedures
3.2. Synthesis of Fluorinated Garlic Organosulfur Compounds
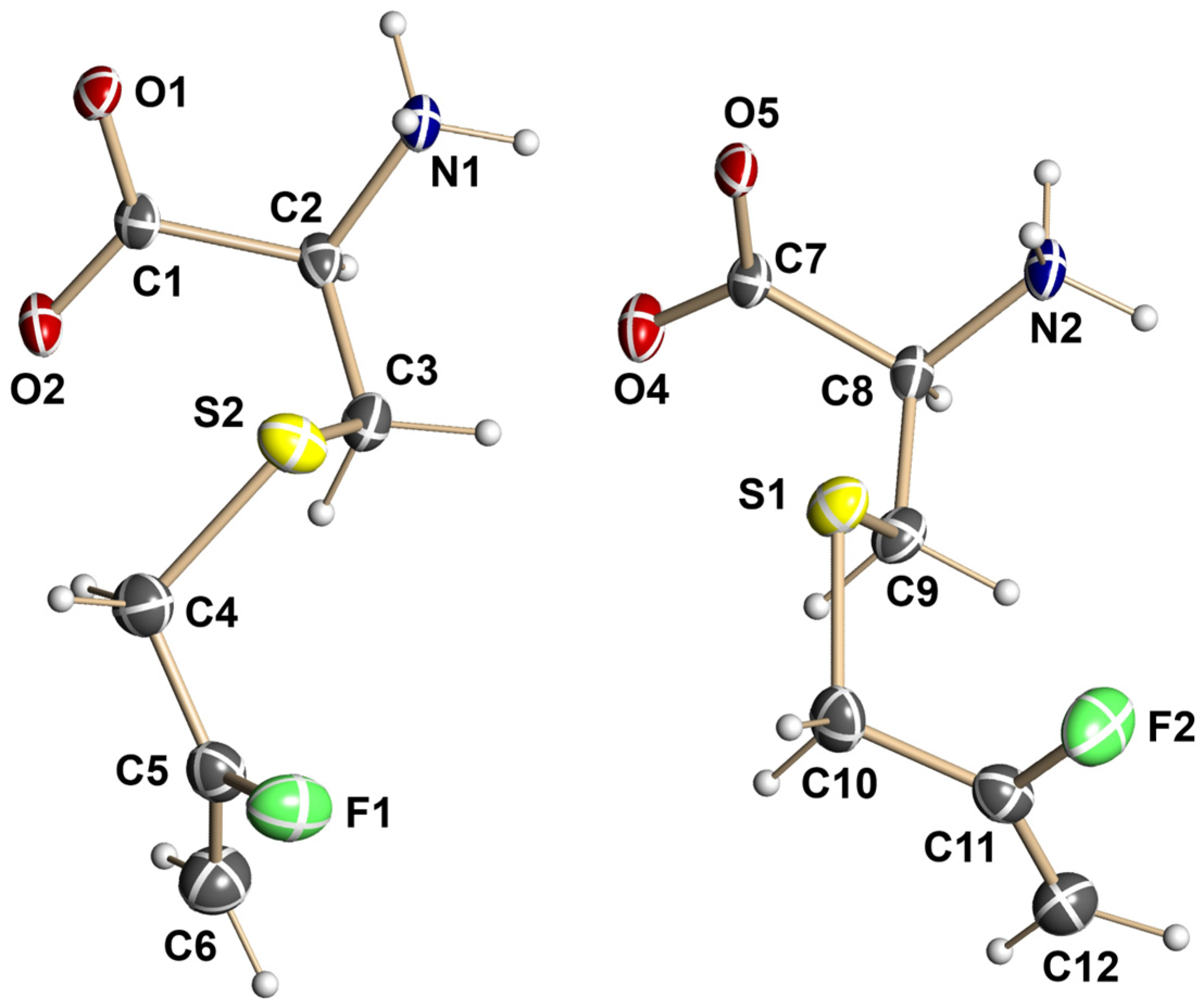
3.3. X-Ray Crystallographic Structural Determination for 13
4. Conclusions
X-Ray Crystallographic Data
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cavallito, C.J.; Bailey, J.H. Allicin, the Antibacterial Principle of Allium sativum. I. Isolation, Physical Properties and Antibacterial Action. J. Am. Chem. Soc. 1944, 66, 1950–1951. [Google Scholar] [CrossRef]
- Block, E. Garlic and Other Alliums: The Lore and the Science; Royal Society of Chemistry: Cambridge, UK, 2010. [Google Scholar] [CrossRef]
- Block, E. Magical Scallions and Garlics—The Lore and the Science; [神奇的葱蒜—传说与科学]; Chemistry Industry Press: Beijing, China, 2017; (In Chinese). ISBN 978-7-122-28930-8. [Google Scholar]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.; Nwachukwu, I.; Slusarenko, A. Allicin: Chemistry and Biological Properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [PubMed]
- Reiter, J.; Levina, N.; van der Linden, M.; Gruhlke, M.; Martin, C.; Slusarenko, A. Diallylthiosulfinate (Allicin), a Volatile Antimicrobial from Garlic (Allium sativum), Kills Human Lung Pathogenic Bacteria, Including MDR Strains, as a Vapor. Molecules 2017, 22, 1711. [Google Scholar] [CrossRef] [PubMed]
- Borlinghaus, J.; Foerster, J.; Kappler, U.; Antelmann, H.; Noll, U.; Gruhlke, M.C.H.; Slusarenko, A.J. Allicin, the Odor of Freshly Crushed Garlic: A Review of Recent Progress in Understanding Allicin’s Effects on Cells. Molecules 2021, 26, 1505. [Google Scholar] [CrossRef] [PubMed]
- Mösbauer, K.; Fritsch, V.N.; Adrian, L.; Bernhardt, J.; Gruhlke, M.C.H.; Slusarenko, A.J.; Niemeyer, D.; Antelmann, H. The Effect of Allicin on the Proteome of SARS-CoV-2 Infected Calu-3 Cells. Front. Microbiol. 2021, 12, 746795. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, E.; Canistro, D.; Pellicioni, V.; Vivarelli, F.; Fimognari, C. Anticancer Potential of Allicin: A Review. Pharmacol. Res. 2022, 177, 106118. [Google Scholar] [CrossRef] [PubMed]
- Mengers, H.G.; Schier, C.; Zimmermann, M.; Gruhlke, M.C.H.; Block, E.; Blank, L.M.; Slusarenko, A.J. Seeing the Smell of Garlic: Detection of Gas Phase Volatiles from Crushed Garlic (Allium sativum), Onion (Allium cepa), Ramsons (Allium ursinum) and Human Garlic Breath Using SESI-Orbitrap MS. Food Chem. 2022, 397, 133804. [Google Scholar] [CrossRef]
- Schultz, C.R.; Gruhlke, M.C.H.; Slusarenko, A.J.; Bachmann, A.S. In Vivo Antitumor Activity of Allicin in a Pediatric Neuroblastoma Patient-derived Xenograft (PDX) Mouse Model. In Vivo 2025, 39, 1283–1292. [Google Scholar] [CrossRef]
- Block, E.; Dethier, B.; Bechand, B.; Cotelesage, J.J.H.; George, G.N.; Goto, K.; Pickering, I.J.; Rengifo, E.M.; Sheridan, R.; Sneeden, E.Y.; et al. Ajothiolanes: 3,4-Dimethylthiolane Natural Products from Garlic (Allium sativum). J. Agric. Food Chem. 2018, 66, 10193–10204. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; Saad, A.M.; Korma, S.A.; Salem, H.M.; Abd El-Mageed, T.A.; Alkafaas, S.S.; Elsalahaty, M.I.; Elkafas, S.S.; Mosa, W.F.A.; Ahmed, A.E.; et al. Garlic Bioactive Substances and Their Therapeutic Applications for Improving Human Health: A Comprehensive Review. Front. Immunol. 2024, 15, 1277074. [Google Scholar] [CrossRef]
- Sela, U.; Brill, A.; Kalchenko, V.; Dashevsky, O.; Hershkoviz, R. Allicin Inhibits Blood Vessel Growth and Downregulates Akt Phosphorylation and Actin Polymerization. Nutr. Cancer 2008, 60, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Hosono, T. Functionality of Garlic Sulfur Compounds. Biomed. Rep. 2025, 23, 124. [Google Scholar] [CrossRef] [PubMed]
- Beretta, H.V.; Bannoud, F.; Insani, M.; Berli, F.; Hirschegger, P.; Galmarini, C.R.; Cavagnaro, P.F. Relationships among Bioactive Compounds Content and the Antiplatelet and Antioxidant Activities of Six Allium Vegetable Species. Food Technol. Biotechnol. 2017, 55, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Atawneh, S.; Shakhatreh, A.N.; Hamed, R.A.; Al-Yasari, I.H. Anticancer Potential of Garlic Bioactive Constituents: Allicin, Z-Ajoene, and Organosulfur Compounds. Pharmacia 2024, 71, 1–23. [Google Scholar] [CrossRef]
- Block, E.; Ahmad, S.; Jain, M.K.; Crecely, R.W.; Apitz-Castro, R.; Cruz, M.R. (E,Z)-Ajoene: A Potent Antithrombotic Agent from Garlic. J. Am. Chem. Soc. 1984, 106, 8295–8296. [Google Scholar] [CrossRef]
- Block, E.; Ahmad, S.; Catalfamo, J.; Jain, M.K.; Apitz-Castro, R. Antithrombotic Organosulfur Compounds from Garlic: Structural, Mechanistic and Synthetic Studies. J. Am. Chem. Soc. 1986, 108, 7045–7055. [Google Scholar] [CrossRef]
- Kusza, D.A.; Venter, G.A.; Mabunda, M.; Biwi, J.; Samanta, S.K.; Klinck, J.D.; Singh, S.V.; Hunter, R.; Kaschula, C.H. Finding the Ajoene Sweet-Spot: Structure-Activity Relations that Govern its Blood Stability and Cancer Cytotoxicity. Chem. Med. Chem. 2024, 19, e202400087. [Google Scholar] [CrossRef]
- Kusza, D.A.; Hunter, R.; Schäfer, G.; Smith, M.; Katz, A.A.; Kaschula, C.H. Activity-Based Proteomic Identification of the S-Thiolation Targets of Ajoene in MDA-MB-231 Breast Cancer Cells. J. Agric. Food Chem. 2022, 70, 14679–14692. [Google Scholar] [CrossRef]
- Kaschula, C.H.; Hunter, R.; Stellenboom, N.; Caira, M.R.; Winks, S.; Ogunleye, T.; Richards, P.; Cotton, J.; Zilbeyaz, K.; Wang, Y.; et al. Structure–Activity Studies on the Anti-Proliferation Activity of Ajoene Analogues in WHCO1 Oesophageal Cancer Cells. Eur. J. Med. Chem. 2012, 50, 236–254. [Google Scholar] [CrossRef]
- Hunter, R.; Kaschula, C.H.; Parker, M.I.; Caira, M.R.; Richards, P.; Travis, S.; Taute, F.; Qwebani, T. Substituted Ajoenes as Novel Anti-Cancer Agents. Bioorg. Med. Chem. Lett. 2008, 18, 5277–5279. [Google Scholar] [CrossRef]
- Raynbird, M.Y.; Khokhar, S.S.; Neef, D.; Evans, G.J.S.; Wirth, T. Synthesis of Ajoene Analogues by Novel Synthetic Strategies. Chem. Eur. J. 2021, 27, 3008–3012. [Google Scholar] [CrossRef]
- Cho, H.; Lee, E.; Kim, J.; Shin, S.; Kim, Y.-J.; Lee, H.; Yu, J.H.; Jeon, Y.H.; Lee, S.W.; Lee, S.Y.; et al. Discovery of Organosulfur-Based Selective HDAC8 Inhibitors with Anti-Neuroblastoma Activity. Eur. J. Pharm. Sci. 2024, 203, 106921. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-I.; Cho, H.; Jeon, R.; Sung, M.-K. Therapeutic Efficacy of Novel HDAC Inhibitors SPA3052 and SPA3074 against Intestinal Inflammation in a Murine Model of Colitis. Pharmaceuticals 2022, 15, 1515. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; Kamari, F.; El Omari, N.; Touhtouh, J.; Mssillou, I.; Aanniz, T.; Benali, T.; Khalid, A.; Abdalla, A.N.; Iesa, M.A.M.; et al. Unlocking the Natural Chemical Sources, Nutritional Properties, Biological Effects, and Molecular Actions of Ajoene. J. Funct. Foods 2025, 127, 106714. [Google Scholar] [CrossRef]
- Bhaumik, I.; Pal, K.; Debnath, U.; Karmakar, P.; Jana, K.; Misra, A.K. Natural Product Inspired Allicin Analogs as Novel Anti-Cancer Agents. Bioorg. Chem. 2019, 86, 259–272. [Google Scholar] [CrossRef]
- Puchkov, V.M.; Lyfenko, A.D.; Koval, V.S.; Revtovich, S.V.; Kulikova, V.V.; Anufrieva, N.V.; Zemskaya, A.S.; Morozova, E.A.; Solyev, P.N. Synthesis and Antimicrobial Activity of Thiosulfinates and Allicin Analogues. Mol. Biol. 2024, 58, 1074–1081. [Google Scholar] [CrossRef]
- Roseblade, A.; Ung, A.; Bebawy, M. Synthesis and in vitro Biological Evaluation of Thiosulfinate Derivatives for the Treatment of Human Multidrug-Resistant Breast Cancer. Acta Pharmacol. Sin. 2017, 38, 1353–1368. [Google Scholar] [CrossRef]
- Xu, Y.S.; Feng, J.G.; Zhang, D.; Zhang, B.; Luo, M.; Su, D.; Lin, N.M. S-Allylcysteine, a Garlic Derivative, Suppresses Proliferation and Induces Apoptosis in Human Ovarian Cancer Cells in vitro. Acta Pharmacol. Sin. 2014, 35, 267–274. [Google Scholar] [CrossRef]
- Xu, Y.; Su, D.; Zhu, L.; Zhang, S.; Ma, S.; Wu, K.; Yuan, Q.; Lin, N. S-Allylcysteine Suppresses Ovarian Cancer Cell Proliferation by DNA Methylation through DNMT1. J. Ovarian Res. 2018, 11, 39. [Google Scholar] [CrossRef]
- Rais, N.; Ved, A.; Ahmad, R.; Kumar, M.; Barbhai, M.D.; Radha; Chandran, D.; Dey, A.; Dhumal, S.; Senapathy, M.; et al. S-Allyl-L-cysteine—A Garlic Bioactive: Physicochemical Nature, Mechanism, Pharmacokinetics, and Health Promoting Activities. J. Funct. Foods 2023, 107, 105657. [Google Scholar] [CrossRef]
- Smith, M.; Hunter, R.; Stellenboom, N.; Kusza, D.A.; Parker, M.I.; Hammouda, A.M.I.; Jackson, G.; Kaschula, C.H. The Cytotoxicity of Garlic-Related Disulphides and Thiosulfonates in WHCO1 Oesophageal Cancer Cells is Dependent on S-Thiolation and not Production of ROS. Biochim. Biophys. Acta 2016, 1860, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sánchez-Roselló, M.; Aceña, J.L.; del Pozo, C.; Sorochinsky, A.E.; Fustero, S.; Soloshonok, V.A.; Liu, H. Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, T.; Yamamoto, H. Biologically active organofluorine compounds. In Organofluorine Compounds: Chemistry and Applications; Yamamoto, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 137–182. [Google Scholar] [CrossRef]
- Gombler, W. Bis(trifluoromethyl)disulfane Oxide CF3S(O)SCF3. Angew. Chem. Int. Ed. 1977, 16, 723–724. [Google Scholar] [CrossRef]
- Zhu, B.; Wu, Z.; Wang, L.; Lu, B.; Trabelsi, T.; Francisco, J.S.; Zeng, X. Matrix-Isolated Trifluoromethylthiyl Radical: Sulfur Atom Transfer, Isomerization and Oxidation Reactions. Chem. Commun. 2021, 57, 12143–12146. [Google Scholar] [CrossRef]
- Zhu, B.; Jiang, J.; Lu, B.; Li, X.; Zeng, X. Fluoromethylsulfinyl Radicals: Spectroscopic Characterization and Photoisomerization via Intramolecular Hydrogen Shift. Phys. Chem. Chem. Phys. 2022, 24, 8881–8889. [Google Scholar] [CrossRef]
- Astrain-Redin, N.; Sanmartin, C.; Sharma, A.K.; Plano, D. From Natural Sources to Synthetic Derivatives: The Allyl Motif as a Powerful Tool for Fragment-Based Design in Cancer Treatment. J. Med. Chem. 2023, 66, 3703–3731. [Google Scholar] [CrossRef]
- Han, Z.; Czap, G.; Chiang, C.; Xu, C.; Wagner, P.J.; Wei, X.; Zhang, Y.; Wu, R.; Ho, W. Imaging the Halogen Bond in Self-Assembled Halogenbenzenes on Silver. Science 2017, 358, 206–210. [Google Scholar] [CrossRef]
- Wallock-Richards, D.; Doherty, C.J.; Doherty, L.; Clarke, D.J.; Place, M.; Govan, J.R.W.; Campopiano, D.J. Garlic Revisited: Antimicrobial Activity of Allicin-Containing Garlic Extracts against Burkholderia cepacia Complex. PLoS ONE 2014, 9, e112726. [Google Scholar] [CrossRef]
- Müller, A.; Eller, J.; Albrecht, F.; Prochnow, P.; Kuhlmann, K.; Bandow, J.E.; Slusarenko, A.J.; Leichert, L.I.O. Allicin Induces Thiol Stress in Bacteria through S-Allylmercapto Modification of Protein Cysteines. J. Biol. Chem. 2016, 291, 11477–11490. [Google Scholar] [CrossRef]
- Gruhlke, M.C.H.; Schlembach, I.; Leontiev, R.; Uebachs, A.; Gollwitzer, P.U.G.; Weiss, A.; Delaunay, A.; Toledano, M.; Slusarenko, A.J. Yap1p, the Central Regulator of the S. cerevisiae Oxidative Stress Response, Is Activated by Allicin, a Natural Oxidant and Defence Substance of Garlic. Free Radic. Biol. Med. 2017, 108, 793–802. [Google Scholar] [CrossRef]
- Jubault, P. 3-Chloro-2-fluoropropene. Encycl. Reagents Org. Synth. 2019, 1, 1–3. [Google Scholar]
- Moore, L.O.; Henry, J.P.; Clark, J.W. Chlorination of 2-Fluoropropene. 3-Chloro-2-fluoropropene and some of its Derivatives. J. Org. Chem. 1970, 35, 4201–4204. [Google Scholar] [CrossRef]
- Laue, K.W.; Haufe, G. 3-Bromo-2-Fluoropropene—A Fluorinated Building Block. 2-Fluoroallylation of Glycine and Alanine Ester Imines. Synthesis 1998, 1998, 1453–1456. [Google Scholar] [CrossRef]
- Bobrovo, A.Y.; Novikov, M.A.; Tomilov, Y.V. (2-Fluoroallyl)pyridinium Tetrafluoroborates: Novel Fluorinated Electrophiles for Pd-Catalyzed Allylic Substitution. Org. Biomol. Chem. 2021, 19, 4678–4684. [Google Scholar] [CrossRef]
- Ai, Y.; Yang, H.; Duan, C.; Li, X.; Yu, S. Cobalt-Catalyzed Fluoroallyllation of Carbonyls via C–C Activation of gem-Difluorocyclopropanes. Org. Lett. 2022, 24, 5051–5055. [Google Scholar] [CrossRef]
- Yan, Y.; Qian, H.; Lv, L.; Li, Z. Pd-IHept-Catalyzed Ring-Opening of gem-Difluorocyclopropanes with Malonates Via Selective C–CBond Cleavage: Synthesis of Monofluoroalkenes. J. Org. Chem. 2024, 89, 16253–16261. [Google Scholar] [CrossRef]
- Yoneda, T.; Kojima, N.; Matsumoto, T.; Imahori, D.; Ohta, T.; Yoshida, T.; Nakamura, S. Construction of Sulfur-Containing Compounds with Anti-Cancer Stem Cell Activity Using Thioacrolein Derived from Garlic Based on Nature-Inspired Scaffolds. Org. Biomol. Chem. 2022, 20, 196–207. [Google Scholar] [CrossRef]
- Mousavi-Ebadia, M.; Safaei-Ghomi, J.; Nejad, M.J. Synthesis of thiopyran derivatives via [4 + 2] cycloaddition reactions. RSC Adv. 2025, 15, 11160–11188. [Google Scholar] [CrossRef]
- Pathania, S.; Narang, R.K.; Rawal, R.K. Role of Sulphur-Heterocycles in Medicinal Chemistry: An Update. Eur. J. Med. Chem. 2019, 180, 486–508. [Google Scholar] [CrossRef]
- Mandal, P.K.; Chakrawarti, R.; Katuk, S. [3+3] Annulation of Diazoenals and α-Mercapto Ketones via Protic Sulfonium Ylides: Direct Synthesis of 2H-Thiopyrans, Innovative Progenitors for Unstudied 2H-Thiopyran-2-ones and 4H-Thiopyran-4-ones. Org. Lett. 2024, 26, 3463–3468. [Google Scholar] [CrossRef]
- Soni, S.; Shukla, G.; Singh, M.S. Magnesium catalyzed [3 + 3] heteroannulation of α-enolic dithioesters with MBH acetate: Access to functionalized 3,4-dihydro-2H-thiopyrans. Org. Biomol. Chem. 2022, 20, 6784–6798. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Groom, M.; Sheridan, R.; Zhang, S.; Block, E. Liquid Sulfur as a Reagent: Synthesis of Polysulfanes with 20 or More Sulfur Atoms with Characterization by UPLC-(Ag+)-Coordination Ion Spray-MS. J. Sulfur Chem. 2013, 34, 55–66. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C.; Fu, Y.; Guo, Q.-X.; Liu, L. Substituent Effect on the Efficiency of Desulfurizative Rearrangement of Allylic Disulfides. J. Org. Chem. 2008, 73, 6127–6136. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, A.N.; Radchenko, D.S.; Mykhailiuk, P.K.; Grygorenko, O.O.; Komarov, I.V. 4-Fluoro-2,4-methanoproline. Org. Lett. 2009, 11, 5674–5676. [Google Scholar] [CrossRef]
- Smyth, H.F.; Carpenter, C.P.; Well, C.S.; Pozzani, U.C.; Striegel, J.A. Range-Finding Toxicity Data: List VI. Am. Ind. Hyg. Assoc. J. 1962, 23, 95–107. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]



| Bond Lengths | |||||
|---|---|---|---|---|---|
| S(1)-C(10) | 1.806(3) | C(10)-C(11) | 1.472(5) | F(1)-C(5) | 1.360(4) |
| S(1)-C(9) | 1.812(3) | C(9)-C(8) | 1.512(5) | C(1)-C(2) | 1.524(4) |
| O(5)-C(7) | 1.242(4) | C(11)-C(12) | 1.287(5) | C(5)-C(6) | 1.286(5) |
| O(4)-C(7) | 1.238(4) | S(2)-C(4) | 1.803(4) | C(5)-C(4) | 1.477(5) |
| N(2)-C(8) | 1.479(4) | S(2)-C(3) | 1.815(3) | C(2)-N(1) | 1.488(4) |
| F(2)-C(11) | 1.362(4) | O(1)-C(1) | 1.242(4) | C(2)-C(3) | 1.510(4) |
| C(7)-C(8) | 1.525(4) | O(2)-C(1) | 1.247(4) | ||
| Angles | |||||
| C(4)-S(2)-C(3) | 100.4(2) | C(6)-C(5)-F(1) | 119.4(3) | N(1)-C(2)-C(1) | 109.6(3) |
| O(1)-C(1)-O(2) | 125.8(3) | C(6)-C(5)-C(4) | 129.0(4) | C(3)-C(2)-C(1) | 112.4(3) |
| O(1)-C(1)-C(2) | 118.5(3) | F(1)-C(5)-C(4) | 111.5(3) | C(2)-C(3)-S(2) | 113.4(2) |
| O(2)-C(1)-C(2) | 115.7(3) | N(1)-C(2)-C(3) | 110.8(3) | C(5)-C(4)-S(2) | 113.4(3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Block, E.; Bechand, B.; Gundala, S.; Vattekkatte, A.; Wang, K. Fluorinated Analogs of Organosulfur Compounds from Garlic (Allium sativum): Synthesis and Chemistry. Molecules 2025, 30, 2841. https://doi.org/10.3390/molecules30132841
Block E, Bechand B, Gundala S, Vattekkatte A, Wang K. Fluorinated Analogs of Organosulfur Compounds from Garlic (Allium sativum): Synthesis and Chemistry. Molecules. 2025; 30(13):2841. https://doi.org/10.3390/molecules30132841
Chicago/Turabian StyleBlock, Eric, Benjamin Bechand, Sivaji Gundala, Abith Vattekkatte, and Kai Wang. 2025. "Fluorinated Analogs of Organosulfur Compounds from Garlic (Allium sativum): Synthesis and Chemistry" Molecules 30, no. 13: 2841. https://doi.org/10.3390/molecules30132841
APA StyleBlock, E., Bechand, B., Gundala, S., Vattekkatte, A., & Wang, K. (2025). Fluorinated Analogs of Organosulfur Compounds from Garlic (Allium sativum): Synthesis and Chemistry. Molecules, 30(13), 2841. https://doi.org/10.3390/molecules30132841







