Closed-Loop Valorization of Annatto Seed Waste into Biochar: A Sustainable Platform for Phosphorus Adsorption and Safe Nutrient Recycling in Agro-Industries
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of Calcination and Temperature on the Adsorption Capacity of Annatto Biochar
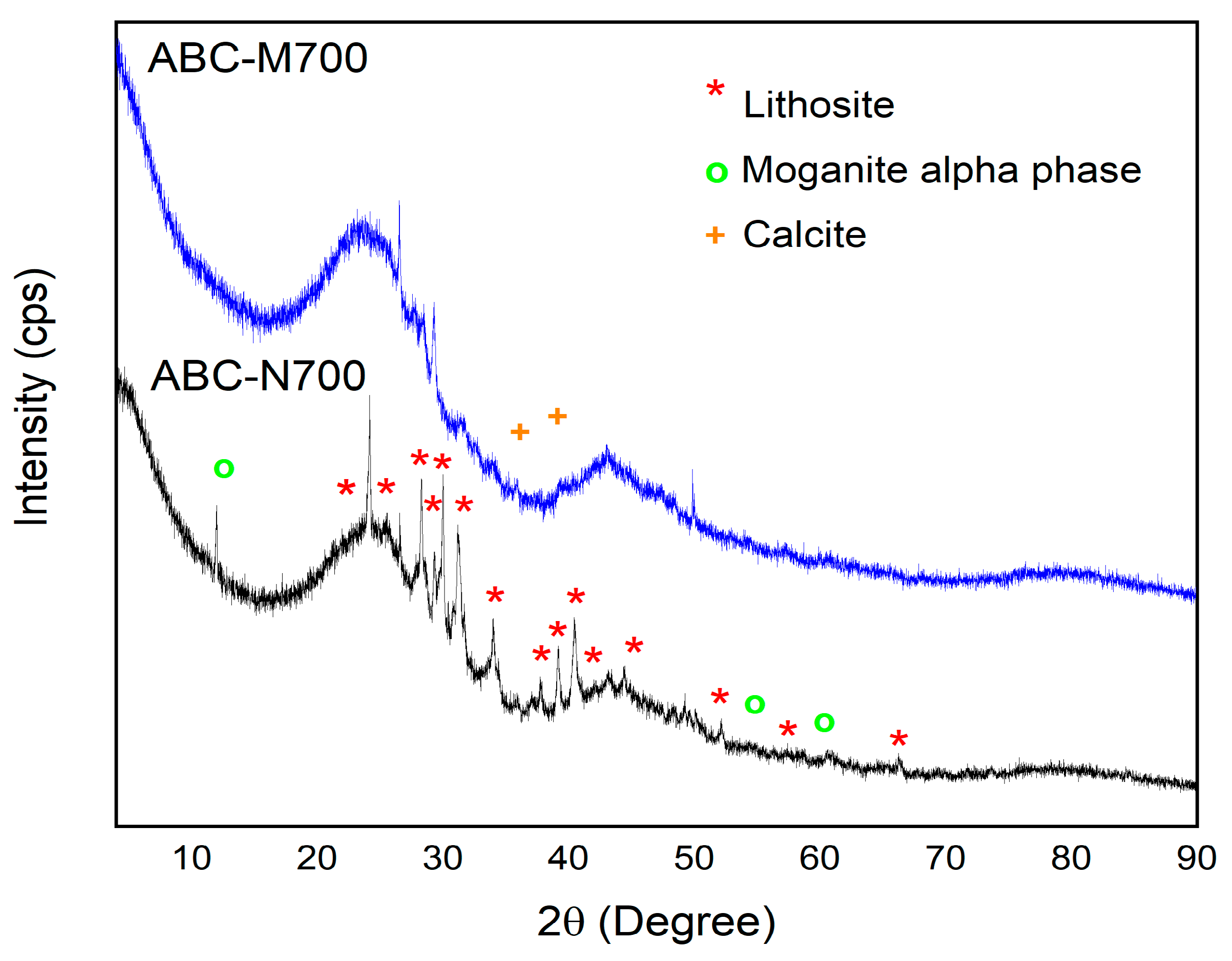
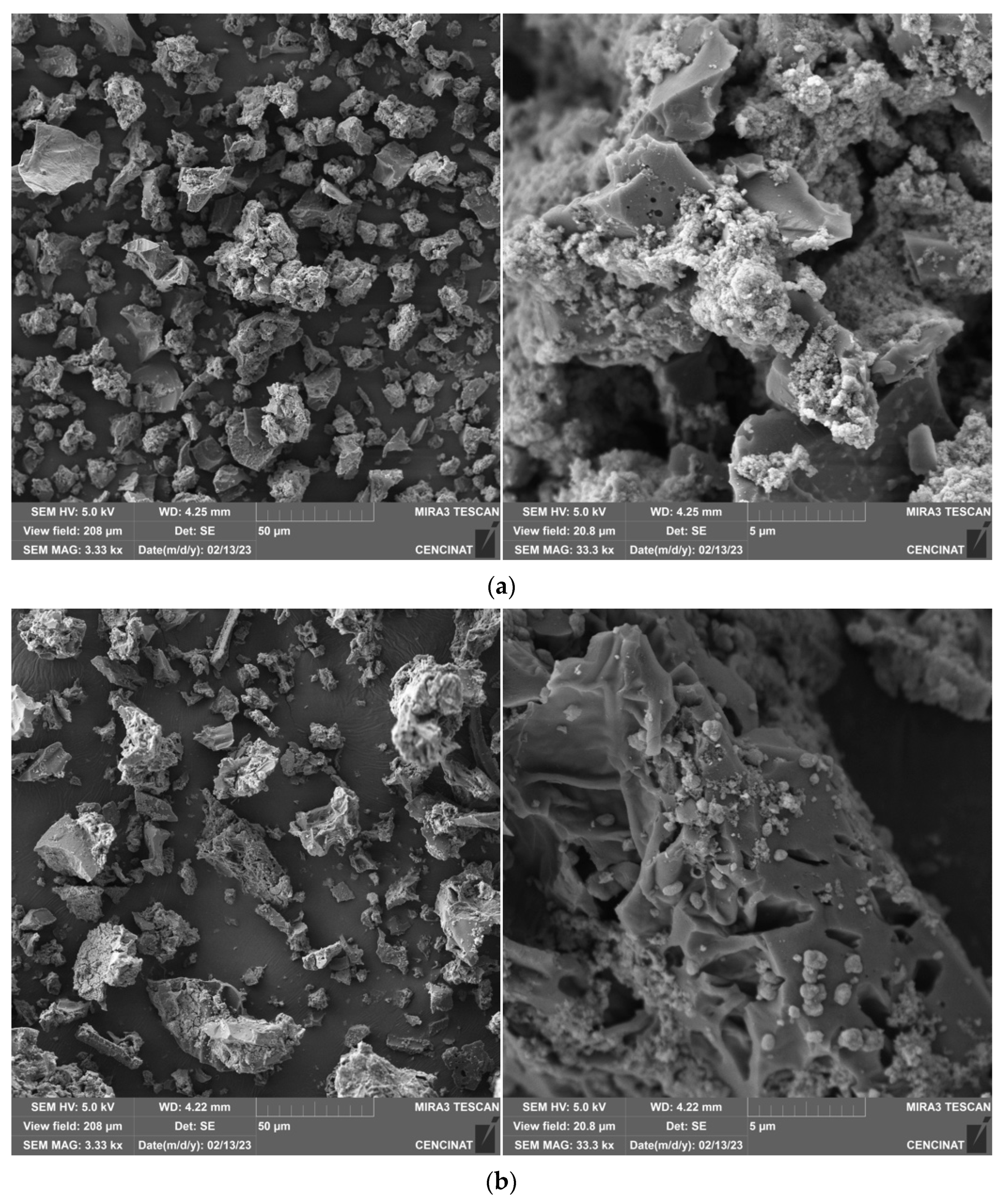
2.2. Biochar Physicochemical Characterization
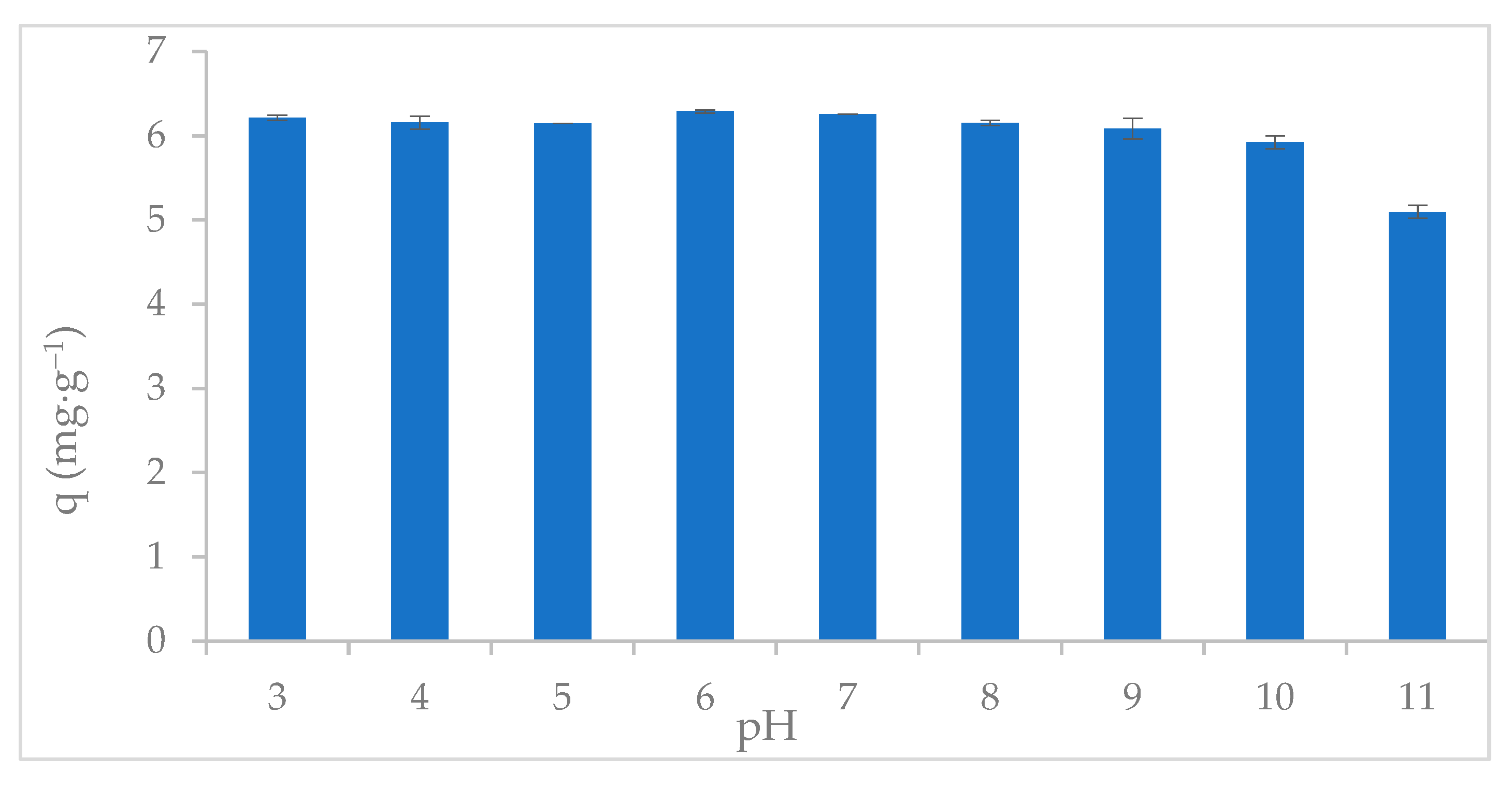
2.3. Uptake of Phosphate as a Function of pH
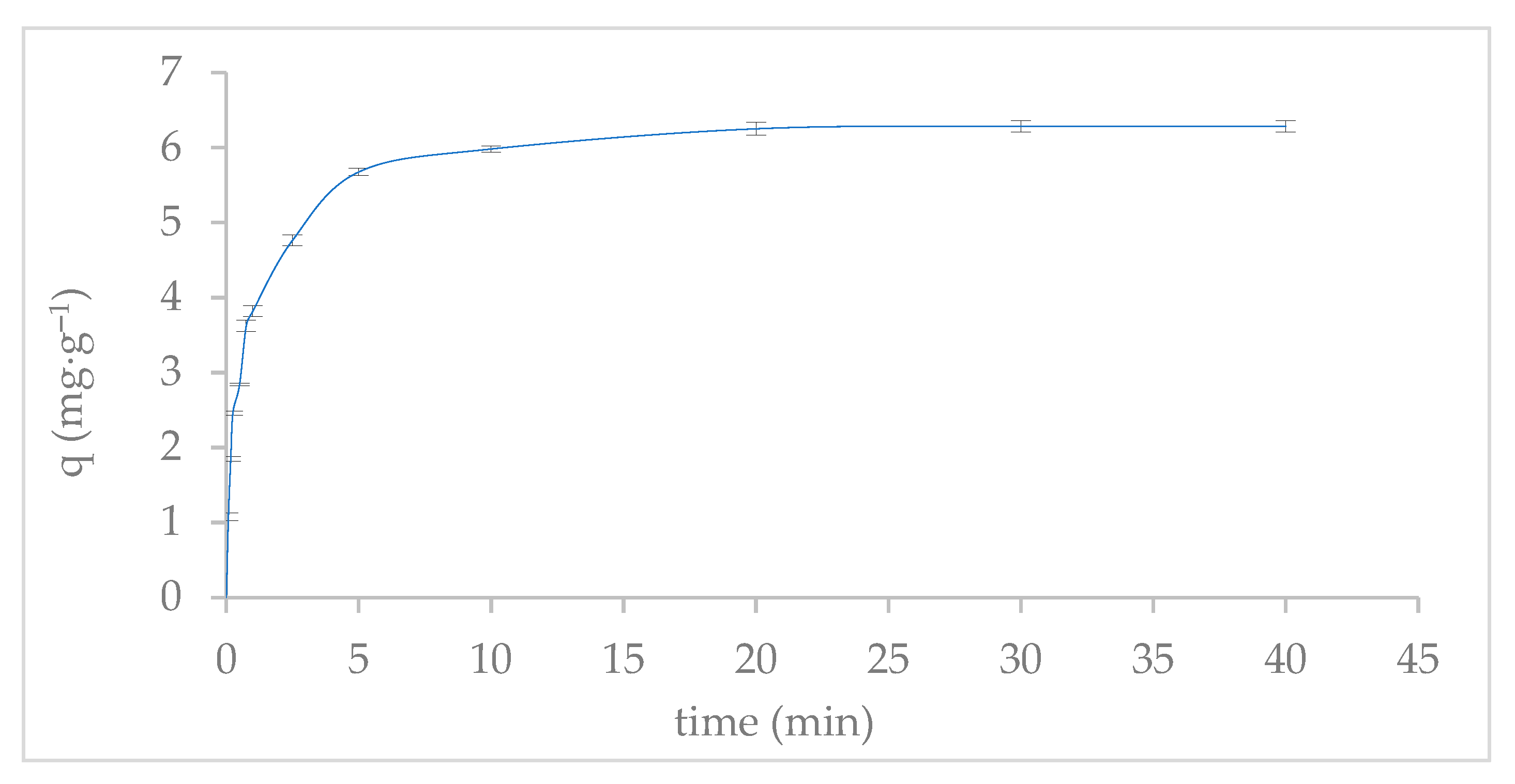
2.4. Adsorption Kinetics
2.5. Phosphate Adsorption Isotherms
2.6. Phosphate Fractionation
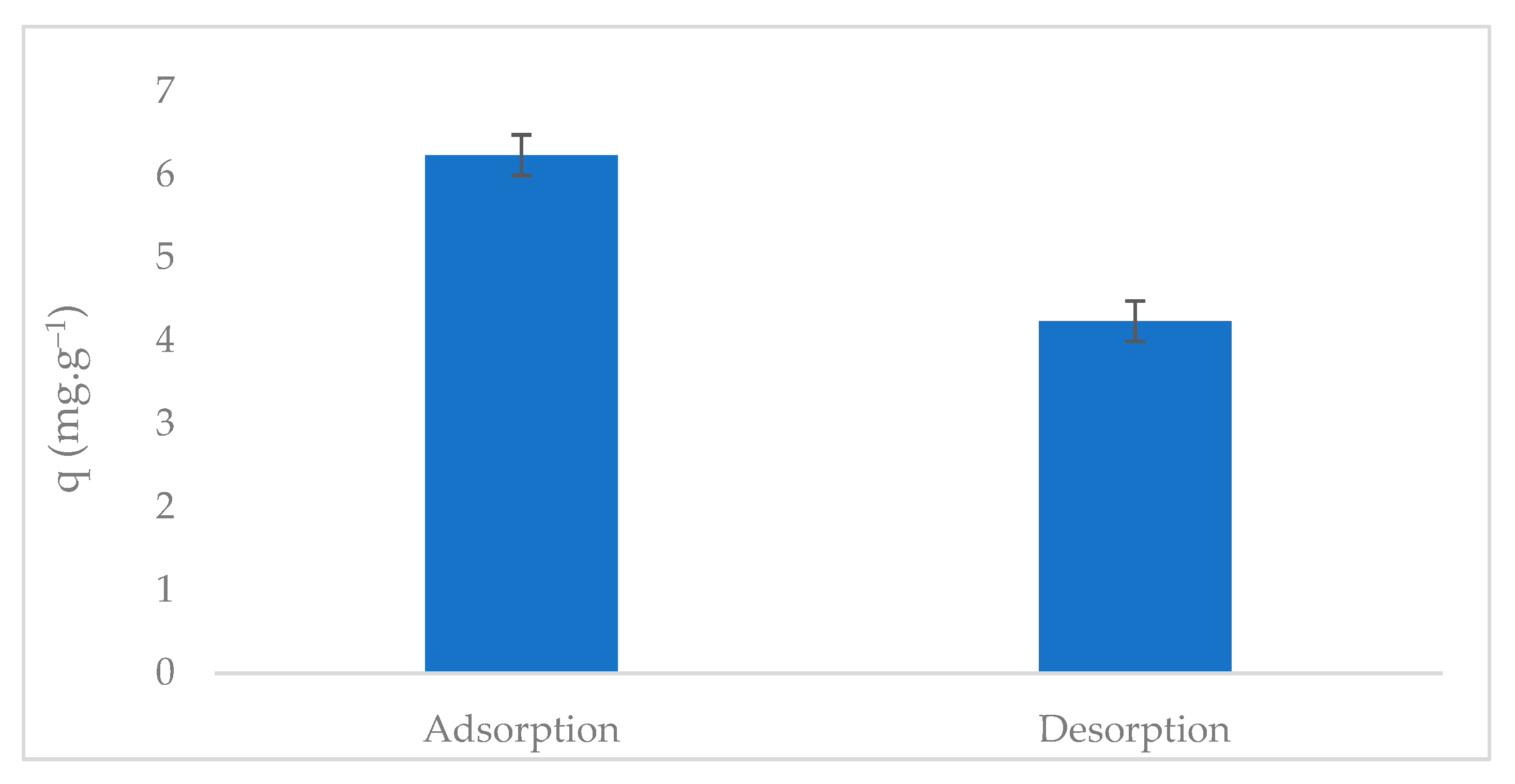
2.7. Phosphate Desorption
2.8. Phosphate Removal Efficiency in Real Wastewater
2.9. Practical Implications of Metal-Modified Annatto Biochar for Phosphate Adsorption
3. Materials and Methods
3.1. Annatto Residues Pretreatment and Chemicals
3.2. Biochar Production and Modification
3.3. Characterization of Materials
3.4. Phosphorus Adsorption Experiments
3.5. Application of Metal-Modified Biochar ABC-M700 for Phosphate Adsorption in Real Wastewater from Annatto Residue Processing Plant
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koul, B.; Yakoob, M.; Shah, M.P. Agricultural Waste Management Strategies for Environmental Sustainability. Environ. Res. 2022, 206, 112285. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.K.; Shetti, N.P.; Reddy, K.R.; Nadagouda, M.N.; Badawi, M.; Bonilla-Petriciolet, A.; Aminabhavi, T.M. Valorization of Biowastes for Clean Energy Production, Environmental Depollution and Soil Fertility. J. Environ. Manag. 2023, 332, 117410. [Google Scholar] [CrossRef] [PubMed]
- Masud, M.A.A.; Shin, W.S.; Sarker, A.; Septian, A.; Das, K.; Deepo, D.M.; Iqbal, M.A.; Islam, A.R.M.T.; Malafaia, G. A Critical Review of Sustainable Application of Biochar for Green Remediation: Research Uncertainty and Future Directions. Sci. Total Environ. 2023, 904, 166813. [Google Scholar] [CrossRef]
- Jayakumar, M.; Hamda, A.S.; Abo, L.D.; Daba, B.J.; Prabhu, S.V.; Rangaraju, M.; Jabesa, A.; Periyasamy, S.; Suresh, S.; Baskar, G. Comprehensive Review on Lignocellulosic Biomass Derived Biochar Production, Characterization, Utilization and Applications. Chemosphere 2023, 345, 140515. [Google Scholar] [CrossRef] [PubMed]
- Novair, S.B.; Cheraghi, M.; Faramarzi, F.; Lajayer, B.A.; Senapathi, V.; Astatkie, T.; Price, G.W. Reviewing the Role of Biochar in Paddy Soils: An Agricultural and Environmental Perspective. Ecotoxicol. Environ. Saf. 2023, 263, 115228. [Google Scholar] [CrossRef]
- Ginni, G.; Kavitha, S.; Kannah, R.Y.; Bhatia, S.K.; Kumar, S.A.; Rajkumar, M.; Kumar, G.; Pugazhendhi, A.; Chi, N.T.L.; Rajesh Banu, J. Valorization of Agricultural Residues: Different Biorefinery Routes. J. Environ. Chem. Eng. 2021, 9, 105435. [Google Scholar] [CrossRef]
- Biswal, B.K.; Balasubramanian, R. Use of Biochar as a Low-Cost Adsorbent for Removal of Heavy Metals from Water and Wastewater: A Review. J. Environ. Chem. Eng. 2023, 11, 110986. [Google Scholar] [CrossRef]
- Muddapur, U.M.; Turakani, B.; Jalal, N.A.; Ashgar, S.S.; Momenah, A.M.; Alshehri, O.M.; Mahnashi, M.H.; Shaikh, I.A.; Khan, A.A.; Dafalla, S.E.; et al. Phytochemical Screening of Bixa Orellana and Preliminary Antidiabetic, Antibacterial, Antifibrinolytic, Anthelmintic, Antioxidant, and Cytotoxic Activity against Lung Cancer (A549) Cell Lines. J. King Saud. Univ. Sci. 2023, 35, 102683. [Google Scholar] [CrossRef]
- Pratibha, G.; Korwar, G.R.; Venkateswarlu, B.; Desai, S.; Chary, G.R.; Rao, M.S.; Srinivas, K.; Rao, K.S.; Rao, C.H.S.; Amalraj, D.K.L.D.; et al. Utilization of Composted Bixa Shell with Different Bioinoculants as Soil Amendment for Ashwagandha and Bixa Growth. Ecol. Eng. 2013, 61, 235–244. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, X.; Chen, J.; Zou, W.; He, F.; Hu, X.; Tsang, D.C.W.; Ok, Y.S.; Gao, B. Biochar Technology in Wastewater Treatment: A Critical Review. Chemosphere 2020, 252, 126539. [Google Scholar] [CrossRef]
- Otoni, J.P.; Matoso, S.C.G.; Pérez, X.L.O.; da Silva, V.B. Potential for Agronomic and Environmental Use of Biochars Derived from Different Organic Waste. J. Clean. Prod. 2024, 449, 141826. [Google Scholar] [CrossRef]
- Silveira, T.M.G.; Tapia-Blácido, D.R. Is Isolating Starch from the Residue of Annatto Pigment Extraction Feasible? Food Hydrocoll. 2018, 77, 117–125. [Google Scholar] [CrossRef]
- Guaya, D.; Hermassi, M.; Valderrama, C.; Farran, A.; Cortina, J.L. Recovery of Ammonium and Phosphate from Treated Urban Wastewater by Using Potassium Clinoptilolite Impregnated Hydrated Metal Oxides as N-P-K Fertilizer. J. Environ. Chem. Eng. 2016, 4, 3519–3526. [Google Scholar] [CrossRef]
- Guaya, D.; Valderrama, C.; Farran, A.; Sauras, T.; Cortina, J.L. Valorisation of N and P from Waste Water by Using Natural Reactive Hybrid Sorbents: Nutrients (N,P,K) Release Evaluation in Amended Soils by Dynamic Experiments. Sci. Total Environ. 2018, 612, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Guaya, D.; Mendoza, A.; Valderrama, C.; Farran, A.; Sauras-Yera, T.; Cortina, J.L. Use of Nutrient-Enriched Zeolite (NEZ) from Urban Wastewaters in Amended Soils: Evaluation of Plant Availability of Mineral Elements. Sci. Total Environ. 2020, 727, 138646. [Google Scholar] [CrossRef]
- Su, J.-Z.; Zhang, M.-Y.; Xu, W.-H.; Xu, W.-M.; Liu, C.; Rui, S.; Tuo, Y.-F.; He, X.-H.; Xiang, P. Preparation and Applications of Iron/Biochar Composites in Remediation of Heavy Metal Contaminated Soils: Current Status and Further Perspectives. Environ. Technol. Innov. 2024, 35, 103671. [Google Scholar] [CrossRef]
- Guaya, D.; Maza, L.; Angamarca, A.; Mendoza, E.; García, L.; Valderrama, C.; Cortina, J.L. Fe3+/Mn2+ (Oxy)Hydroxide Nanoparticles Loaded onto Muscovite/Zeolite Composites (Powder, Pellets and Monoliths): Phosphate Carriers from Urban Wastewater to Soil. Nanomaterials 2022, 12, 3848. [Google Scholar] [CrossRef] [PubMed]
- Guaya, D.; Cobos, H.; Valderrama, C.; Cortina, J.L. Effect of Mn2+/Zn2+/Fe3+ Oxy(Hydroxide) Nanoparticles Doping onto Mg-Al-LDH on the Phosphate Removal Capacity from Simulated Wastewater. Nanomaterials 2022, 12, 3680. [Google Scholar] [CrossRef] [PubMed]
- Rosa, D.; Petruccelli, V.; Iacobbi, M.C.; Brasili, E.; Badiali, C.; Pasqua, G.; Di Palma, L. Functionalized Biochar from Waste as a Slow-Release Nutrient Source: Application on Tomato Plants. Heliyon 2024, 10, e29455. [Google Scholar] [CrossRef]
- Muhammad, N.; Ge, L.; Chan, W.P.; Khan, A.; Nafees, M.; Lisak, G. Impacts of Pyrolysis Temperatures on Physicochemical and Structural Properties of Green Waste Derived Biochars for Adsorption of Potentially Toxic Elements. J. Environ. Manag. 2022, 317, 115385. [Google Scholar] [CrossRef]
- Xue, L.; Liu, N.; Zhang, J.; Sun, Z.; Fu, S.; Zhan, X.; Yang, J.; Zhou, R.; Zhang, H.; Liu, H.; et al. Pyrolysis Temperature Had Effects on the Physicochemical Properties of Biochar. Plant Soil Environ. 2023, 69, 363–373. [Google Scholar] [CrossRef]
- Song, S.; Liu, S.; Liu, Y.; Shi, W.; Ma, H. Structural Characteristics and Adsorption of Phosphorus by Pineapple Leaf Biochar at Different Pyrolysis Temperatures. Agronomy 2024, 14, 2923. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, R.; Xia, B.; Ying, R.; Hu, Z.; Tao, X.; Yu, H.; Xiao, F.; Chu, Q.; Chen, H.; et al. Effect of Pyrolysis Temperature on Removal Efficiency and Mechanisms of Hg(II), Cd(II), and Pb (II) by Maize Straw Biochar. Sustainability 2022, 14, 9022. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; van Hullebusch, E.D.; Amrane, A.; Rtimi, S. Mechanisms and Adsorption Capacities of Biochar for the Removal of Organic and Inorganic Pollutants from Industrial Wastewater. Int. J. Environ. Sci. Technol. 2021, 18, 3273–3294. [Google Scholar] [CrossRef]
- Eduah, J.O.; Nartey, E.K.; Abekoe, M.K.; Henriksen, S.W.; Andersen, M.N. Mechanism of Orthophosphate (PO4-P) Adsorption onto Different Biochars. Environ. Technol. Innov. 2020, 17, 100572. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Xu, Y.; Qian, G. Phosphate Adsorption on Metal Oxides and Metal Hydroxides: A Comparative Review. Environ. Rev. 2016, 24, 319–332. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Gaston, L.A.; Zhou, B.; Li, M.; Xiao, R.; Wang, Q.; Zhang, Z.; Huang, H.; Liang, W.; et al. An Overview of Carbothermal Synthesis of Metal–Biochar Composites for the Removal of Oxyanion Contaminants from Aqueous Solution. Carbon 2018, 129, 674–687. [Google Scholar] [CrossRef]
- Nosratabad, N.A.; Yan, Q.; Cai, Z.; Wan, C. Exploring Nanomaterial-Modified Biochar for Environmental Remediation Applications. Heliyon 2024, 10, e37123. [Google Scholar] [CrossRef]
- Wang, S.; Shan, R.; Wang, Y.; Lu, L.; Yuan, H. Synthesis of Calcium Materials in Biochar Matrix as a Highly Stable Catalyst for Biodiesel Production. Renew Energy 2019, 130, 41–49. [Google Scholar] [CrossRef]
- Wang, S.; Kwak, J.-H.; Islam, M.S.; Naeth, M.A.; El-Din, M.G.; Chang, S.X. Biochar Surface Complexation and Ni(II), Cu(II), and Cd(II) Adsorption in Aqueous Solutions Depend on Feedstock Type. Sci. Total Environ. 2020, 712, 136538. [Google Scholar] [CrossRef]
- Yankovych, H.; Novoseltseva, V.; Kovalenko, O.; Marcin Behunova, D.; Kanuchova, M.; Vaclavikova, M.; Melnyk, I. New Perception of Zn(II) and Mn(II) Removal Mechanism on Sustainable Sunflower Biochar from Alkaline Batteries Contaminated Water. J. Environ. Manag. 2021, 292, 112757. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.-T.; Zhou, H.; Tang, S.-F.; Chen, Q.; Zhou, X.; Liu, X.-H.; Zeng, P.; Gu, J.-F.; Liao, B.-H. Simultaneous Alleviation of Cd Availability in Contaminated Soil and Accumulation in Rice (Oryza Sativa L.) by Fe-Mn Oxide-Modified Biochar. Sci. Total Environ. 2023, 858, 159730. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Zhang, Y.; Chen, Q.; Liu, R.; Wang, H. Unraveling Adsorption Characteristics and Removal Mechanism of Novel Zn/Fe-Bimetal-Loaded and Starch-Coated Corn Cobs Biochar for Pb(II) and Cd(II) in Wastewater. J. Mol. Liq. 2023, 391, 123375. [Google Scholar] [CrossRef]
- Díaz, B.; Sommer-Márquez, A.; Ordoñez, P.E.; Bastardo-González, E.; Ricaurte, M.; Navas-Cárdenas, C. Synthesis Methods, Properties, and Modifications of Biochar-Based Materials for Wastewater Treatment: A Review. Resources 2024, 13, 8. [Google Scholar] [CrossRef]
- Adilina, I.B.; Widjaya, R.R.; Hidayati, L.N.; Supriadi, E.; Safaat, M.; Oemry, F.; Restiawaty, E.; Bindar, Y.; Parker, S.F. Understanding the Surface Characteristics of Biochar and Its Catalytic Activity for the Hydrodeoxygenation of Guaiacol. Catalysts 2021, 11, 1434. [Google Scholar] [CrossRef]
- Gale, M.; Nguyen, T.; Moreno, M.; Gilliard-AbdulAziz, K.L. Physiochemical Properties of Biochar and Activated Carbon from Biomass Residue: Influence of Process Conditions to Adsorbent Properties. ACS Omega 2021, 6, 10224–10233. [Google Scholar] [CrossRef]
- Vaitkus, A.; Merkys, A.; Gražulis, S. Validation of the Crystallography Open Database Using the Crystallographic Information Framework. J. Appl. Crystallogr. 2021, 54, 661–672. [Google Scholar] [CrossRef]
- Profeta, D.O.; da Silva, M.A.; Faria, D.N.; Cipriano, D.F.; Freitas, J.C.C.; dos Santos, F.S.; Lima, T.M.; Vasconcelos, S.C.; Pietre, M.K. Zeolite/Calcium Carbonate Composite for a Synergistic Adsorption of Cadmium in Aqueous Solution. Next Mater. 2025, 6, 100493. [Google Scholar] [CrossRef]
- Faria, D.N.; dos Santos, F.S.; Teixeira, P.L.R.; Cipriano, D.F.; Schettino, M.A.; de Pietre, M.K.; Freitas, J.C.C. Synergistic Effect of CaCO3 Particles and Porous Carbon towards the Removal of Zn2+ Ions in Aqueous Solutions. Mater. Chem. Phys. 2024, 326, 129813. [Google Scholar] [CrossRef]
- Melo, V.M.e.; Ferreira, G.F.; Fregolente, L.V. Sustainable Catalysts for Biodiesel Production: The Potential of CaO Supported on Sugarcane Bagasse Biochar. Renew. Sustain. Energy Rev. 2024, 189, 114042. [Google Scholar] [CrossRef]
- Chiang, P.F.; Zhang, T.L.; Giwa, A.S.; Maurice, N.J.; Claire, M.J.; Ali, N.; Shafique, E.; Vakili, M. Effects of Calcium-Oxide-Modified Biochar on the Anaerobic Digestion of Vacuum Blackwater. Molecules 2025, 30, 215. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Yao, L.; Wang, Y.; Zuo, M.; Liu, Y.; Wu, K.; Zhang, W.; Xu, C. The Preparation, Layered Characterization and Potential Applications of Corncob Biochar. J. Anal. Appl. Pyrolysis 2024, 183, 106808. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, P.; Wang, C.; Jia, H.; Shang, X.; Tang, J.; Sun, H. Metal-Rich Hyperaccumulator-Derived Biochar as an Efficient Persulfate Activator: Role of Intrinsic Metals (Fe, Mn and Zn) in Regulating Characteristics, Performance and Reaction Mechanisms. J. Hazard. Mater. 2022, 424, 127225. [Google Scholar] [CrossRef] [PubMed]
- Alahakoon, Y.A.; Wilson, S.C.; Peiris, C.; Ranasinghe, Y.K.; Gunatilake, S.R.; Zhang, X.; Mlsna, T.E.; Kumarasinghe, U.; Mohideen, M.I.H.; Gandra, U.R.; et al. Carbothermally Synthesized, Lignin Biochar-Based, Embedded and Surface Deposited Nano Zero-Valent Iron Composites: Comparative Material Characterization, Selective Gas Adsorption and Nitroaromatics Remediation. Colloids Surf. C Environ. Asp. 2024, 2, 100048. [Google Scholar] [CrossRef]
- Wang, Y.; Lyu, H.; Du, Y.; Cheng, Q.; Liu, Y.; Ma, J.; Yang, S.; Lin, H. Unraveling How Fe-Mn Modified Biochar Mitigates Sulfamonomethoxine in Soil Water: The Activated Biodegradation and Hydroxyl Radicals Formation. J. Hazard. Mater. 2024, 465, 133490. [Google Scholar] [CrossRef]
- Li, M.; Tang, Y.; Ren, N.; Zhang, Z.; Cao, Y. Effect of Mineral Constituents on Temperature-Dependent Structural Characterization of Carbon Fractions in Sewage Sludge-Derived Biochar. J. Clean. Prod. 2018, 172, 3342–3350. [Google Scholar] [CrossRef]
- Hasan, M.; Chakma, S.; Liang, X.; Sutradhar, S.; Kozinski, J.; Kang, K. Engineered Biochar for Metal Recycling and Repurposed Applications. Energies 2024, 17, 4674. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Wang, X.; Zhao, Q.; Jiang, J.; Jiang, M. Effect of Different Production Methods on Physicochemical Properties and Adsorption Capacities of Biochar from Sewage Sludge and Kitchen Waste: Mechanism and Correlation Analysis. J. Hazard. Mater. 2024, 461, 132690. [Google Scholar] [CrossRef]
- Liu, X.; Li, G.; Chen, C.; Zhang, X.; Zhou, K.; Long, X. Banana Stem and Leaf Biochar as an Effective Adsorbent for Cadmium and Lead in Aqueous Solution. Sci. Rep. 2022, 12, 1584. [Google Scholar] [CrossRef]
- Zhou, Q.; Liao, B.; Lin, L.; Qiu, W.; Song, Z. Adsorption of Cu(II) and Cd(II) from Aqueous Solutions by Ferromanganese Binary Oxide–Biochar Composites. Sci. Total Environ. 2018, 615, 115–122. [Google Scholar] [CrossRef]
- Maneechakr, P.; Mongkollertlop, S. Investigation on Adsorption Behaviors of Heavy Metal Ions (Cd2+, Cr3+, Hg2+ and Pb2+) through Low-Cost/Active Manganese Dioxide-Modified Magnetic Biochar Derived from Palm Kernel Cake Residue. J. Environ. Chem. Eng. 2020, 8, 104467. [Google Scholar] [CrossRef]
- Guaya, D.; Valderrama, C.; Farran, A.; Armijos, C.; Cortina, J.L. Simultaneous Phosphate and Ammonium Removal from Aqueous Solution by a Hydrated Aluminum Oxide Modified Natural Zeolite. Chem. Eng. J. 2015, 271, 204–213. [Google Scholar] [CrossRef]
- Guaya, D.; Valderrama, C.; Farran, A.; Cortina, J.L. Modification of a Natural Zeolite with Fe(III) for Simultaneous Phosphate and Ammonium Removal from Aqueous Solutions. J. Chem. Technol. Biotechnol. 2016, 91, 1737–1746. [Google Scholar] [CrossRef]
- Moraes, C.S.; Carneiro, P.A.; Faria, D.N.; Cipriano, D.F.; Freitas, J.C.C.; Amorim, R.G.; da Silva, R.S.; Pietre, M.K. High Efficiency of Myclobutanil Adsorption by CTAB-Zeolite Structures: Experimental Evidence Meets Theoretical Investigation. Silicon 2024, 16, 3737–3753. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption Kinetic Models: Physical Meanings, Applications, and Solving Methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Yang, S.; Katuwal, S.; Zheng, W.; Sharma, B.; Cooke, R. Capture and Recover Dissolved Phosphorous from Aqueous Solutions by a Designer Biochar: Mechanism and Performance Insights. Chemosphere 2021, 274, 129717. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Chen, J.; Yang, L. Engineered Biochar Reclaiming Phosphate from Aqueous Solutions: Mechanisms and Potential Application as a Slow-Release Fertilizer. Environ. Sci. Technol. 2013, 47, 8700–8708. [Google Scholar] [CrossRef]
- Almanassra, I.W.; Mckay, G.; Kochkodan, V.; Ali Atieh, M.; Al-Ansari, T. A State of the Art Review on Phosphate Removal from Water by Biochars. Chem. Eng. J. 2021, 409, 128211. [Google Scholar] [CrossRef]
- Akindolie, M.S.; Choi, H.J. Fe12LaO19 Fabricated Biochar for Removal of Phosphorus in Water and Exploration of Its Adsorption Mechanism. J. Environ. Manag. 2023, 329, 117053. [Google Scholar] [CrossRef]
- Chanda, R.; Jahid, T.; Karmokar, A.; Hossain, B.; Moktadir, M.D.; Islam, M.D.S.; Aich, N.; Biswas, B.K. Functionalized Biochar from Vegetable Waste for Phosphorus Removal from Aqueous Solution and Its Potential Use as a Slow-Release Fertilizer. Clean. Mater. 2025, 15, 100287. [Google Scholar] [CrossRef]
- Beiyuan, J.; Wu, X.; Ruan, B.; Chen, Z.; Liu, J.; Wang, J.; Li, J.; Xu, W.; Yuan, W.; Wang, H. Highly Efficient Removal of Aqueous Phosphate via Iron-Manganese Fabricated Biochar: Performance and Mechanism. Chemosphere 2024, 364, 143207. [Google Scholar] [CrossRef]
- Nakarmi, A.; Bourdo, S.E.; Ruhl, L.; Kanel, S.; Nadagouda, M.; Alla, P.K.; Pavel, I.; Viswanathan, T. Benign Zinc Oxide Betaine-Modified Biochar Nanocomposites for Phosphate Removal from Aqueous Solutions. J. Environ. Manag. 2020, 272, 111048. [Google Scholar] [CrossRef]
- Teixeira, R.S.; Schmidt, D.V.C.; dos Santos, F.S.; Cipriano, D.F.; Faria, D.N.; Freitas, J.C.C.; Pietre, M.K. Nanostructured Faujasites with Different Structural and Textural Properties for Adsorption of Cobalt and Nickel. Braz. J. Chem. Eng. 2024, 41, 1271–1283. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Zhou, B.; Awasthi, M.K.; Ali, A.; Zhang, Z.; Gaston, L.A.; Lahori, A.H.; Mahar, A. Enhancing Phosphate Adsorption by Mg/Al Layered Double Hydroxide Functionalized Biochar with Different Mg/Al Ratios. Sci. Total Environ. 2016, 559, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Hermassi, M.; Valderrama, C.; Font, O.; Moreno, N.; Querol, X.; Batis, N.H.; Cortina, J.L. Phosphate Recovery from Aqueous Solution by K-Zeolite Synthesized from Fly Ash for Subsequent Valorisation as Slow Release Fertilizer. Sci. Total Environ. 2020, 731, 139002. [Google Scholar] [CrossRef] [PubMed]
- Guaya, D.; Jiménez, R.; Sarango, J.; Valderrama, C.; Cortina, J.L. Iron-Doped Natural Clays: Low-Cost Inorganic Adsorbents for Phosphate Recovering from Simulated Urban Treated Wastewater. J. Water Process Eng. 2021, 43, 102274. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, M.; Wang, H.; Xue, J.; Lv, Q.; Pang, G. Efficient Recovery of Phosphate from Aqueous Solution Using Biochar Derived from Co-Pyrolysis of Sewage Sludge with Eggshell. J. Environ. Chem. Eng. 2021, 9, 105354. [Google Scholar] [CrossRef]
- Shin, H.; Tiwari, D.; Kim, D.-J. Phosphate Adsorption/Desorption Kinetics and P Bioavailability of Mg-Biochar from Ground Coffee Waste. J. Water Process Eng. 2020, 37, 101484. [Google Scholar] [CrossRef]
- Hermassi, M.; Guaya, D.; Gibert, O.; Valderrama, C.; Cortina, J.L. Valorisation of Nutrients in Wastewaters Using Reactive Inorganic Sorbents BT—Phosphorus Recovery and Recycling. In Phosphorus Recovery and Recycling; Ohtake, H., Tsuneda, S., Eds.; Springer: Singapore, 2019; pp. 457–482. ISBN 978-981-10-8031-9. [Google Scholar]
- Yang, Q.; Wang, X.; Luo, W.; Sun, J.; Xu, Q.; Chen, F.; Zhao, J.; Wang, S.; Yao, F.; Wang, D.; et al. Effectiveness and Mechanisms of Phosphate Adsorption on Iron-Modified Biochars Derived from Waste Activated Sludge. Bioresour. Technol. 2018, 247, 537–544. [Google Scholar] [CrossRef]
- Hu, Z.-T.; Wang, X.-F.; Xiang, S.; Ding, Y.; Zhao, D.-Y.; Hu, M.; Pan, Z.; Varjani, S.; Wong, J.W.-C.; Zhao, J. Self-Cleaning MnZn Ferrite/Biochar Adsorbents for Effective Removal of Tetracycline. Sci. Total Environ. 2022, 844, 157202. [Google Scholar] [CrossRef]
- Sarkhot, D.V.; Ghezzehei, T.A.; Berhe, A.A. Effectiveness of Biochar for Sorption of Ammonium and Phosphate from Dairy Effluent. J. Environ. Qual. 2013, 42, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Novais, S.V.; Zenero, M.D.O.; Barreto, M.S.C.; Montes, C.R.; Cerri, C.E.P. Phosphorus Removal from Eutrophic Water Using Modified Biochar. Sci. Total Environ. 2018, 633, 825–835. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Zhou, B.; Awasthi, M.K.; Ali, A.; Zhang, Z.; Lahori, A.H.; Mahar, A. Recovery of Phosphate from Aqueous Solution by Magnesium Oxide Decorated Magnetic Biochar and Its Potential as Phosphate-Based Fertilizer Substitute. Bioresour. Technol. 2016, 215, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a Sorbent for Contaminant Management in Soil and Water: A Review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, C.; Gray, E.M.; Boyd, S.E.; Yang, H.; Zhang, D. Roles of Biochar in Improving Phosphorus Availability in Soils: A Phosphate Adsorbent and a Source of Available Phosphorus. Geoderma 2016, 276, 1–6. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, B.; Gao, Q.; Gao, Y.; Liu, S. Adsorption of Phosphorus by Different Biochars. Spectrosc. Lett. 2017, 50, 73–80. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Inyang, M.; Zimmerman, A.R.; Cao, X.; Pullammanappallil, P.; Yang, L. Biochar Derived from Anaerobically Digested Sugar Beet Tailings: Characterization and Phosphate Removal Potential. Bioresour. Technol. 2011, 102, 6273–6278. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Liu, M.; Ren, H. Biochar Produced from the Co-Pyrolysis of Sewage Sludge and Walnut Shell for Ammonium and Phosphate Adsorption from Water. J. Environ. Manag. 2019, 249, 109410. [Google Scholar] [CrossRef]
- Tran, T.C.P.; Nguyen, T.P.; Nguyen, X.C.; Nguyen, X.H.; Nguyen, T.A.H.; Nguyen, T.T.N.; Vo, T.Y.B.; Nguyen, T.H.G.; Nguyen, T.T.H.; Vo, T.D.H.; et al. Adsorptive Removal of Phosphate from Aqueous Solutions Using Low-Cost Modified Biochar-Packed Column: Effect of Operational Parameters and Kinetic Study. Chemosphere 2022, 309, 136628. [Google Scholar] [CrossRef]
- APHA (2017). Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1999. [Google Scholar]
- Hieltjes, A.H.M.; Lijklema, L. Fractionation of Inorganic Phosphates in Calcareous Sediments. J. Environ. Qual. 1980, 9, 405–407. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists: Official Methods of Analysis of AOAC International, 21st ed.; AOAC: Washington, DC, USA, 2019. [Google Scholar]


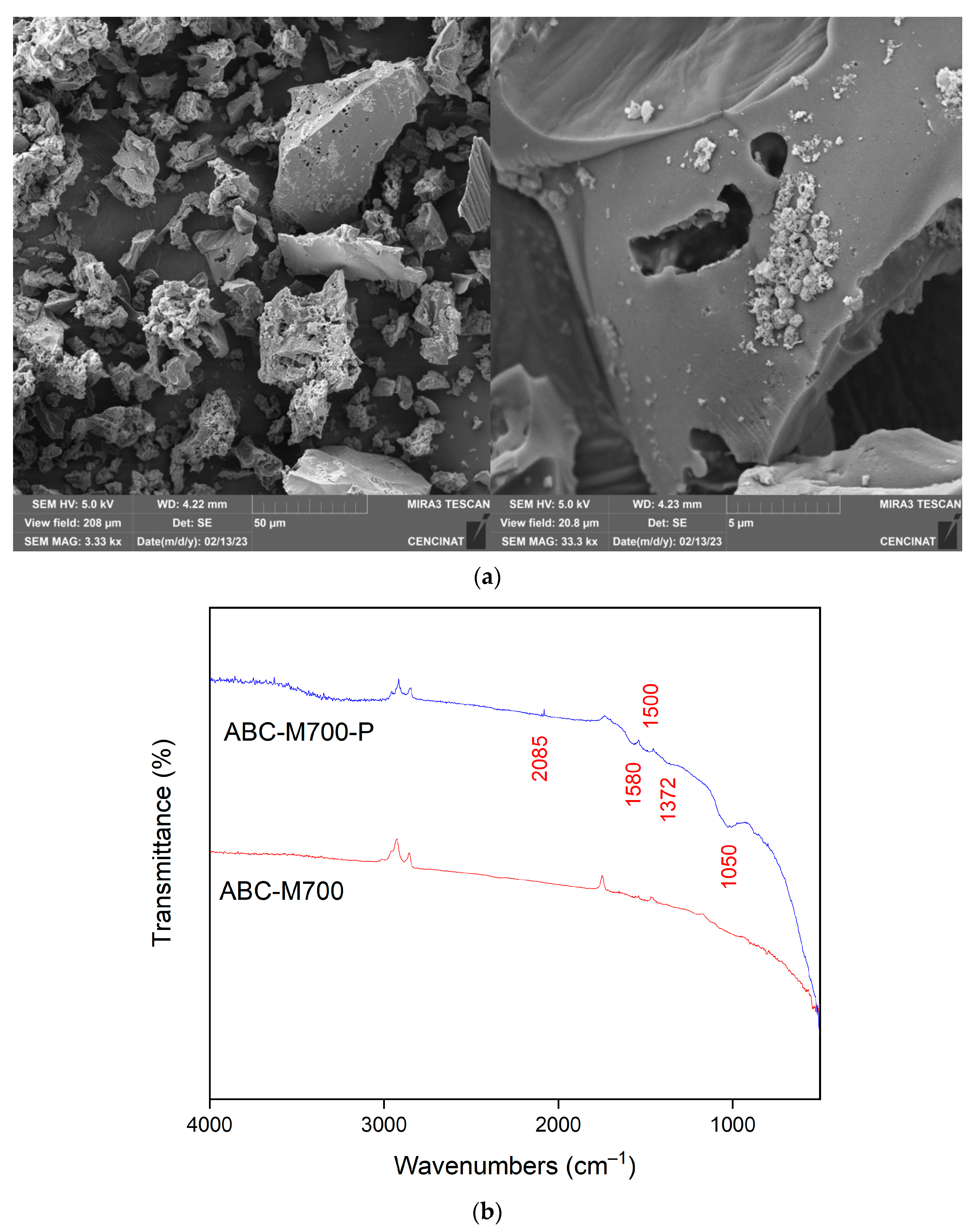

| Elements | ABC-N700 (%) | ABC-M700 (%) | ||||
|---|---|---|---|---|---|---|
| MgO | 3.26 | ± | 0.48 | 0.91 | ± | 0.05 |
| Al2O3 | 5.32 | ± | 0.67 | 4.72 | ± | 0.58 |
| SiO2 | 4.24 | ± | 0.27 | 3.07 | ± | 0.26 |
| P2O5 | 2.57 | ± | 0.08 | 2.39 | ± | 0.08 |
| S | 1.38 | ± | 0.02 | 1.08 | ± | 0.02 |
| K2O | 11.10 | ± | 0.05 | 2.81 | ± | 0.03 |
| CaO | 2.63 | ± | 0.02 | 3.60 | ± | 0.02 |
| Cr2O3 | ND | 0.04 | ± | 0.02 | ||
| Fe2O3 | 0.14 | ± | 0.00 | 0.52 | ± | 0.00 |
| Mn | 0.04 | ± | 0.00 | 0.24 | ± | 0.01 |
| Zn | 0.03 | ± | 0.00 | 0.71 | ± | 0.01 |
| Ba | 0.01 | ± | 0.04 | 0.02 | ± | 0.08 |
| Model | Kinetic Parameters | |
|---|---|---|
| Pseudo-first-order | qe (mg∙g−1) | 3.56 |
| k1 (h−1) | 15.26 | |
| R2 | 0.87 | |
| Pseudo-second-order | qe (mg∙g−1) | 6.29 |
| k2 (g·mg−1·h−1) | 31.73 | |
| R2 | 1.00 | |
| Intraparticular diffusion | kt1 (mg·g−1·h−1/2) | 25.00 |
| R2 | 0.95 | |
| kt2 (mg·g−1·h−1/2) | 2.10 | |
| R2 | 0.92 | |
| Film diffusion | Df (m2∙h−1) | 3.84 × 10−6 |
| R2 | 0.87 | |
| Particle diffusion | Dp (m2∙h−1) | 6.45 × 10−9 |
| R2 | 0.93 | |
| Langmuir | Freundlich | ||
|---|---|---|---|
| qm (mg∙g−1) | 73.22 | kF (mg∙g−1) | 6.41 |
| kL (L∙mg−1) | 4.88 × 10−3 | 1/n | 0.28 |
| R2 | 0.85 | R2 | 0.82 |
| Biochar Source | Phosphate Adsorption Capacity (mg·g−1)/ Removal (%) | Water Type | Reference |
|---|---|---|---|
| Annatto biochar ABC-M700 | 73.2 | Synthetic solution | This study |
| 34/80% | Real wastewater | ||
| Mallee (Eucalyptus polybractea) biochar | 3.7 | Synthetic solution | [76] |
| Pine biochar Maiza-straw biochar | 13.9 8.8 | Synthetic solution | [77] |
| Anaerobically digested sugar beet tailings biochar | 73% | Synthetic solution | [78] |
| Sewage sludge biochar | 303.5 | Synthetic solution | [79] |
| Sugarcane leaves Mg/Al-LDHs biochar | 81.8 at pH 3 | Synthetic solution | [64] |
| Mimosa pigra trees (trunks) modified with AlCl3 biochar | 70.6 | Synthetic solution | [80] |
| Fe12LaO19 spent coffee ground biochar | 81.6 at 40 °C | Synthetic solution | [59] |
| Non-edible vegetable waste biochar modified with ZnCl2 | 47.8 | Synthetic solution | [60] |
| Sawdust biomass treated with lime sludge biochar | 16.7 | Synthetic solution | [56] |
| Rice straw powder BC, Fe/MnBC (comprising Fe3O4 and MnO2), and Fe–MnBC (comprising MnFe2O4) biochar | Fe–MnBC: 135.9 Fe/MnBC: 17.9 | Synthetic solution | [61] |
| ZnO/betaine-modified biochar adsorbent | 256.5 | Synthetic solution | [62] |
| Parameter | Value (mg·L−1) |
|---|---|
| Phosphate (PO43−) | 82 |
| Total phosphorus | 41.2 |
| QOD | 63 |
| BOD5 | 30 |
| Nitrates | 83.5 |
| Nitrites | 0.1 |
| Total nitrogen | 5.9 |
| Sulfates (SO42−) | 28.2 |
| Chloride (Cl−) | 491.3 |
| Oil and grease | 157.7 |
| pH | 7.05 |
| Temperature | 21.4 |
| * Surfactants | 0.57 |
| * Total suspended solids TSS | 116 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guaya, D.; Piedra, C.; Carmona, I. Closed-Loop Valorization of Annatto Seed Waste into Biochar: A Sustainable Platform for Phosphorus Adsorption and Safe Nutrient Recycling in Agro-Industries. Molecules 2025, 30, 2842. https://doi.org/10.3390/molecules30132842
Guaya D, Piedra C, Carmona I. Closed-Loop Valorization of Annatto Seed Waste into Biochar: A Sustainable Platform for Phosphorus Adsorption and Safe Nutrient Recycling in Agro-Industries. Molecules. 2025; 30(13):2842. https://doi.org/10.3390/molecules30132842
Chicago/Turabian StyleGuaya, Diana, Camilo Piedra, and Inmaculada Carmona. 2025. "Closed-Loop Valorization of Annatto Seed Waste into Biochar: A Sustainable Platform for Phosphorus Adsorption and Safe Nutrient Recycling in Agro-Industries" Molecules 30, no. 13: 2842. https://doi.org/10.3390/molecules30132842
APA StyleGuaya, D., Piedra, C., & Carmona, I. (2025). Closed-Loop Valorization of Annatto Seed Waste into Biochar: A Sustainable Platform for Phosphorus Adsorption and Safe Nutrient Recycling in Agro-Industries. Molecules, 30(13), 2842. https://doi.org/10.3390/molecules30132842





