From Logs to Bags: A Metabolic Blueprint of Sanghuang Cultivation Revealed by UPLC-Q-TOF-MS/MS and Amino Acid Profiling
Abstract
1. Introduction
2. Results
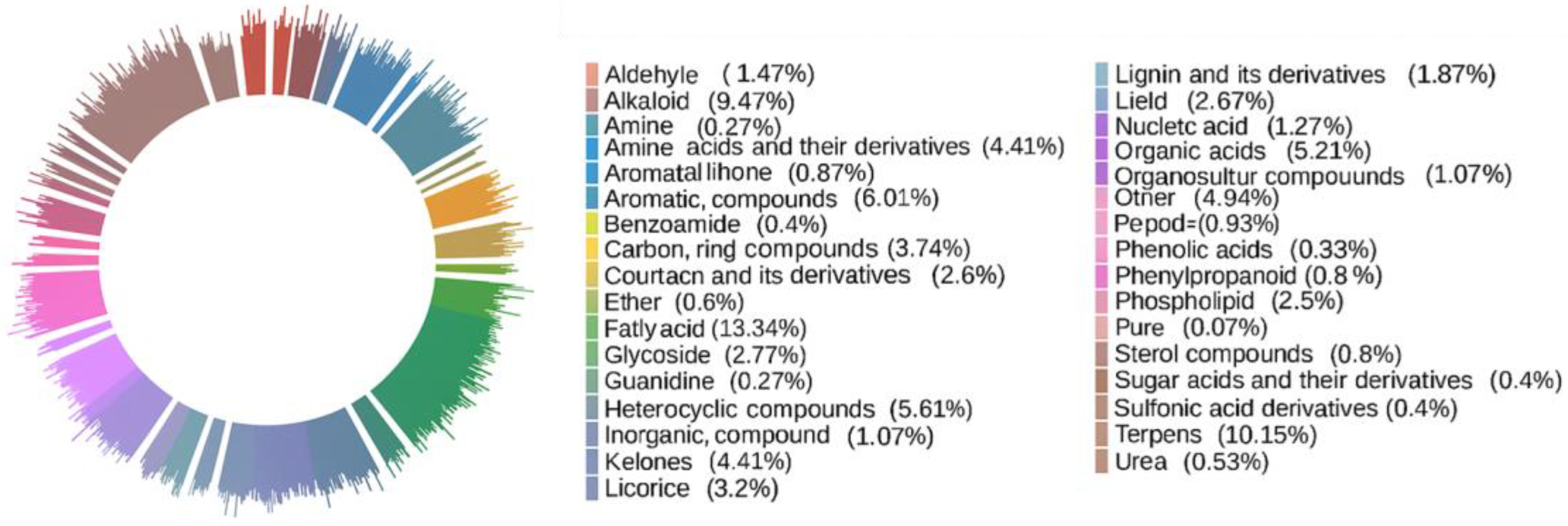
2.1. Characteristic Analysis of Secondary Metabolites in SH Samples
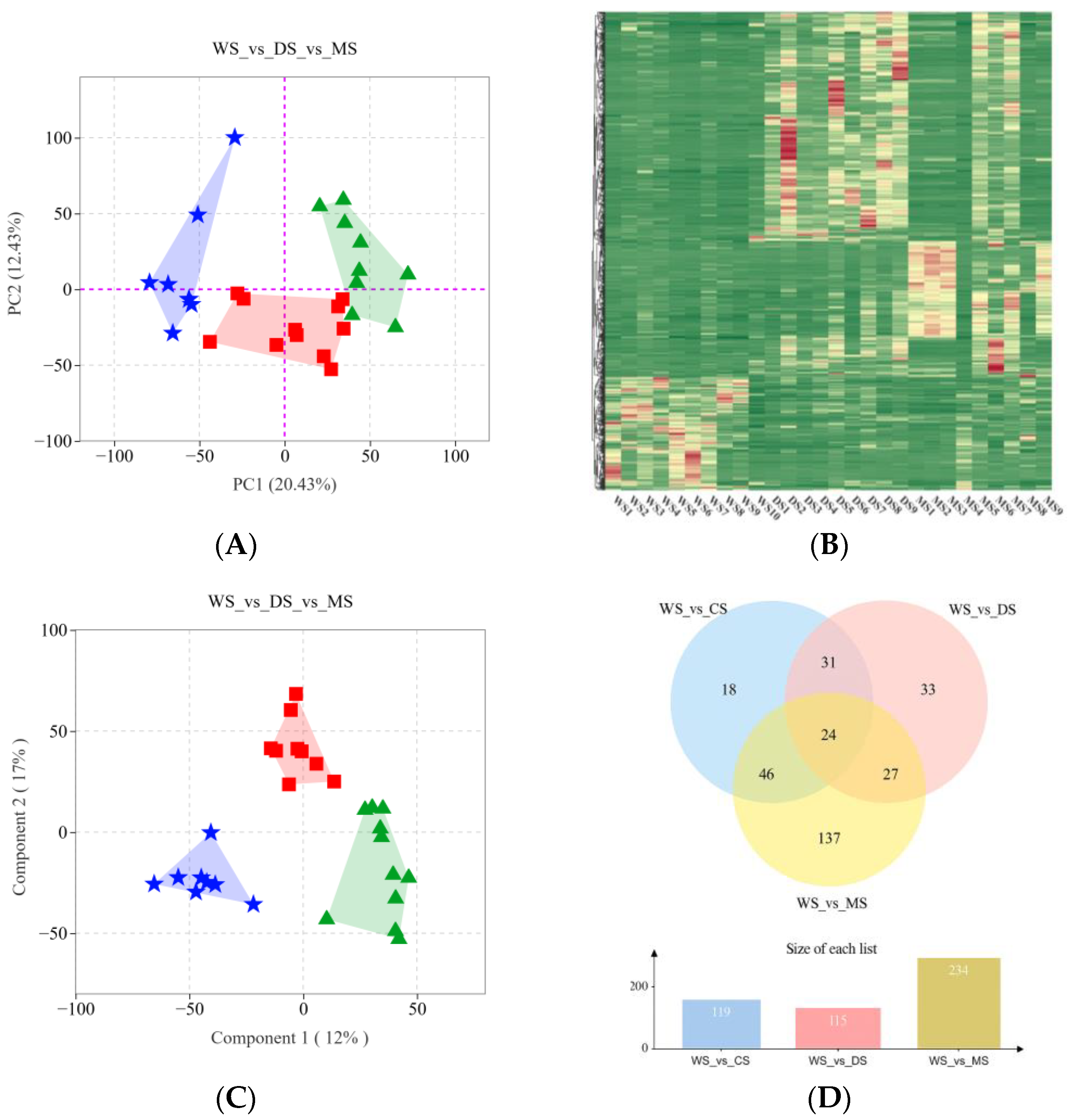
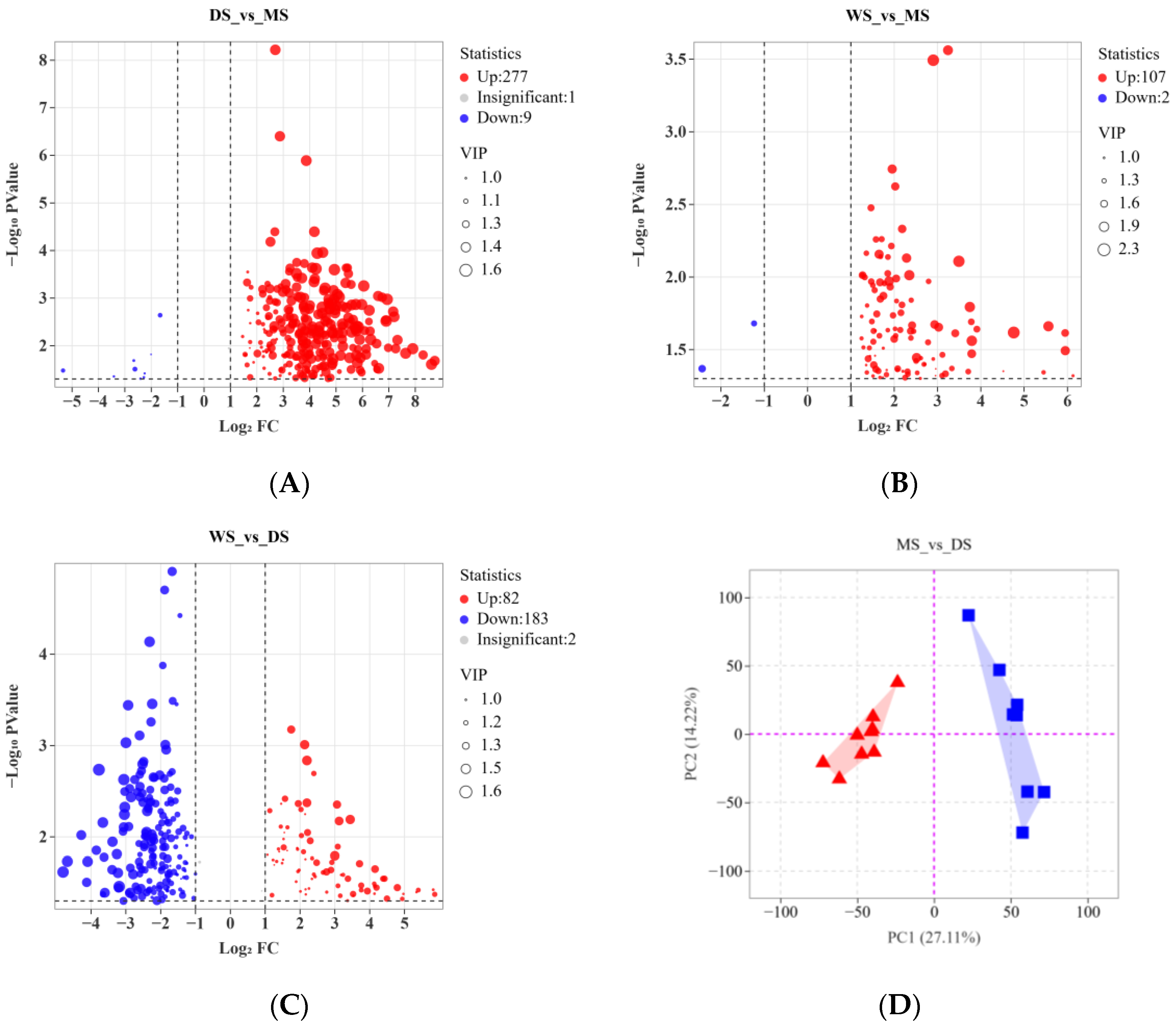
2.2. Analysis of Metabolite Variations Between Wild and Cultivated SH
2.3. Amino Acid Experiment Analysis
2.4. Evaluation of the Characteristics of Three Types of SH Amino Acids
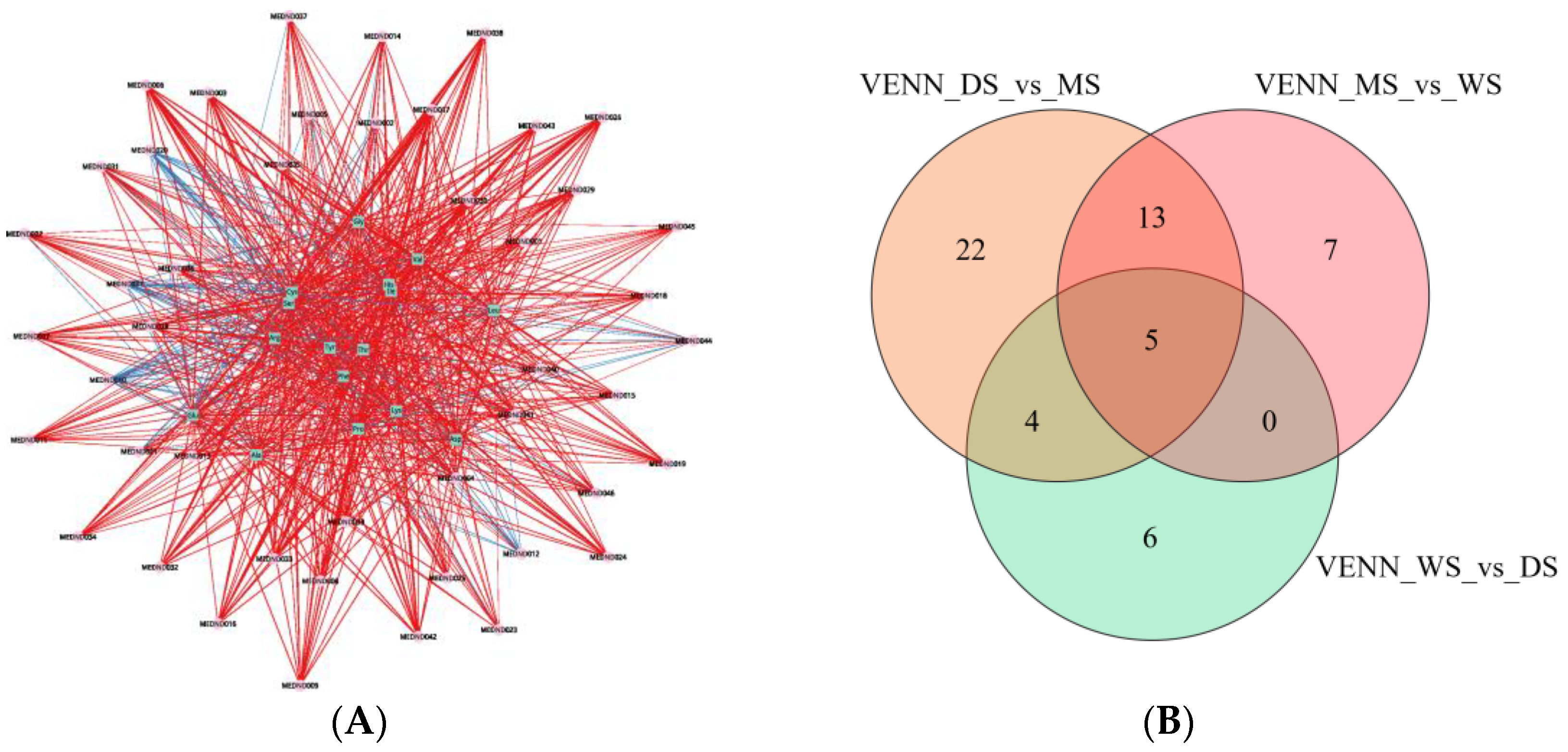
2.5. Correlation Analysis of Amino Acids and Their Derivatives in SH
2.6. Formatting of Mathematical Components
3. Discussion
4. Materials and Methods
4.1. Instruments
4.2. Sample Collection
4.3. Reagents
4.4. Sample Preparation
4.4.1. Preparation of Secondary Metabolites Solution
4.4.2. Preparation of Total Amino Acid Solution
4.5. Fully Automatic Amino Acid Analyzer Resolution and LC-Q-TOF-MS Analysis
4.5.1. Chromatographic Conditions
4.5.2. Mass Spectrometry Conditions
4.6. Statistical Analysis of SH Secondary Metabolites Data
4.7. Evaluation Indicators for the Amino Acid Content of SH
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SH | SH |
| WS | Wild SH |
| DS | Duanmu SH |
| MS | Mycelium SH |
| CS | Cultivated SH |
References
- Lv, Z.; Chen, L.; Ouyang, H.; Zhu, Y.; Ma, J.; Zhang, K.; Lin, W.; Li, W.; Zhang, Z.; He, J. Development, Basic Information, Classifications, Pharmacological Activities, and Underlying Mechanisms of Medicine Food Homology: A Review. J. Funct. Foods 2024, 122, 106552. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, X.; Liu, H.; Sun, B. Medicine and Food Homology Substances: A Review of Bioactive Ingredients, Pharmacological Effects and Applications. Food Chem. 2025, 463, 141111. [Google Scholar] [CrossRef]
- Du, J.; Jin, S.; Zhang, Y.; Qiu, W.; Dong, Y.; Liu, Y.; Yin, D.; Yang, Y.; Xu, W. In Vitro and in Vivo Inhibitory Effects of the Sanghuang Mushroom Extracts against Candida Albicans. Future Microbiol. 2024, 19, 983–996. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Su, M.; Zhou, X.; Li, D.; Niu, X.; Wang, Y. Research Progress of Bioactive Components in Sanghuangporus spp. Molecules 2024, 29, 1195. Molecules 2024, 29, 1195. [Google Scholar] [CrossRef]
- Li, T.; Mei, Y.; Li, J.; Yang, W.; He, F.; Ge, J.; Chen, F.; Yang, Y.; Xie, A.; Liu, Y.; et al. Comparative Compositions and Activities of Flavonoids from Nine Sanghuang Strains Based on Solid-State Fermentation and In Vitro Assays. Fermentation 2023, 9, 308. [Google Scholar] [CrossRef]
- Liu, K.; Xiao, X.; Wang, J.; Chen, C.-Y.O.; Hu, H. Polyphenolic Composition and Antioxidant, Antiproliferative, and Antimicrobial Activities of Mushroom Inonotus Sanghuang. LWT-Food Sci. Technol. 2017, 82, 154–161. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Q.-J.; Zhu, M.-J.; Wang, H.-X.; Zhang, G.-Q. An Extracellular Laccase with Antiproliferative Activity from the Sanghuang Mushroom Inonotus Baumii. J. Mol. Catal. B Enzym. 2014, 99, 20–25. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, L.; Zhu, Y.; Wu, J.; Hao, C.; Song, S.; Shi, W. Optimization of Culture Medium for Sanghuangporus Vaninii and a Study on Its Therapeutic Effects on Gout. Biomed. Pharmacother. 2021, 135, 111194. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Cao, N.; Huang, L.; Tang, L.; Liu, X.; Zhang, W.; Huang, S.; Xie, X.; Yan, Y. Structural Characterization, and Antioxidant, Hypoglycemic and Immunomodulatory Activity of Exopolysaccharide from Sanghuangporus Sanghuang JM-1. Molecules 2024, 29, 4564. [Google Scholar] [CrossRef]
- Yuan, W.; Yuan, W.; Zhou, R.; Lv, G.; Sun, M.; Zhao, Y.; Zheng, W. Production of Hispidin Polyphenols from Medicinal Mushroom Sanghuangporus Vaninii in Submerged Cultures. Chin. Herb. Med. 2023, 15, 594–602. [Google Scholar] [CrossRef]
- He, S.; Zhao, K.; Ma, L.; Yang, J.; Chang, Y. Effects of Different Cultivation Material Formulas on the Growth and Quality of Morchella Spp. Saudi J. Biol. Sci. 2018, 25, 719–723. [Google Scholar] [CrossRef]
- Lee, C.; Lee, S.; Cho, K.-J.; Hwang, S. Mycelial Cultivation of Phellinus Linteus Using Cheese-Processing Waste and Optimization of Bioconversion Conditions. Biodegradation 2011, 22, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xue, F.; Xu, H.; Yuan, Y.; Wu, X.; Zhang, J.; Fu, J. Optimization of Solid-State Fermentation Extraction of Inonotus Hispidus Fruiting Body Melanin. Foods 2021, 10, 2893. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.C.; Bae, J.T.; Pyo, H.B.; Choe, T.B.; Kim, S.W.; Hwang, H.J.; Yun, J.W. Submerged Culture Conditions for the Production of Mycelial Biomass and Exopolysaccharides by the Edible Basidiomycete Grifola Frondosa. Enzym. Microb. Technol. 2004, 35, 369–376. [Google Scholar] [CrossRef]
- Mohan, A.; Gurumurthy, D.M.; Anand Iyer, V.; Mukherjee, T.K.; Patel, V.H.; Kumar, R.; Siddiqui, N.; Nayak, M.; Maurya, P.K.; Kumar, P. Dietary Flavonoids in Health and Diseases: A Concise Review of Their Role in Homeostasis and Therapeutics. Food Chemistry 2025, 487, 144674. [Google Scholar] [CrossRef]
- Naik, K.; Mishra, S.; Srichandan, H.; Singh, P.K.; Sarangi, P.K. Plant Growth Promoting Microbes: Potential Link to Sustainable Agriculture and Environment. Biocatal. Agric. Biotechnol. 2019, 21, 101326. [Google Scholar] [CrossRef]
- Brestenský, M.; Nitrayová, S.; Patráš, P.; Nitray, J. Dietary Requirements for Proteins and Amino Acids in Human Nutrition. CNF 2019, 15, 638–645. [Google Scholar] [CrossRef]
- Sarwar, G. The Protein Digestibility–Corrected Amino Acid Score Method Overestimates Quality of Proteins Containing Antinutritional Factors and of Poorly Digestible Proteins Supplemented with Limiting Amino Acids in Rats. J. Nutr. 1997, 127, 758–764. [Google Scholar] [CrossRef]
- Tavano, O.L.; Neves, V.A.; Da Silva Júnior, S.I. In Vitro versus in Vivo Protein Digestibility Techniques for Calculating PDCAAS (Protein Digestibility-Corrected Amino Acid Score) Applied to Chickpea Fractions. Food Res. Int. 2016, 89, 756–763. [Google Scholar] [CrossRef]
- Ouyang, J.; Huang, N.; Jiang, Y. A Single-Model Quality Assessment Method for Poor Quality Protein Structure. BMC Bioinform. 2020, 21, 157. [Google Scholar] [CrossRef]
- Dunn, M.F. Key Roles of Microsymbiont Amino Acid Metabolism in Rhizobia-Legume Interactions. Crit. Rev. Microbiol. 2015, 41, 411–451. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.J.L.; Krasnikov, B.F.; Niatsetskaya, Z.V.; Pinto, J.T.; Callery, P.S.; Villar, M.T.; Artigues, A.; Bruschi, S.A. Cysteine S-Conjugate β-Lyases: Important Roles in the Metabolism of Naturally Occurring Sulfur and Selenium-Containing Compounds, Xenobiotics and Anticancer Agents. Amino Acids 2011, 41, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.; Macià, A.; Romero, M.-P.; Reguant, J.; Ortega, N.; Motilva, M.-J. Metabolic Pathways of the Colonic Metabolism of Flavonoids (Flavonols, Flavones and Flavanones) and Phenolic Acids. Food Chem. 2012, 130, 383–393. [Google Scholar] [CrossRef]
- Arul Prakash, S.; Kamlekar, R.K. Function and Therapeutic Potential of N-Acyl Amino Acids. Chem. Phys. Lipids 2021, 239, 105114. [Google Scholar] [CrossRef]
- Tang, C.; Wang, T.; Fan, Y.; Wang, J.; Xiao, M.; He, M.; Chang, X.; Li, Y.; Li, X. Integrated Proteomics and Metabolomics Reveal Regulatory Pathways Underlying Quality Differences Between Wild and Cultivated Ophiocordyceps Sinensis. JoF 2025, 11, 469. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Sun, J.; Luo, Z.-Y.; Rao, S.-Q.; Su, Y.-J.; Xu, R.-R.; Yang, Y.-J. Chemical Composition of Five Wild Edible Mushrooms Collected from Southwest China and Their Antihyperglycemic and Antioxidant Activity. Food Chem. Toxicol. 2012, 50, 1238–1244. [Google Scholar] [CrossRef]
- Salam, U.; Ullah, S.; Tang, Z.-H.; Elateeq, A.A.; Khan, Y.; Khan, J.; Khan, A.; Ali, S. Plant Metabolomics: An Overview of the Role of Primary and Secondary Metabolites against Different Environmental Stress Factors. Life 2023, 13, 706. [Google Scholar] [CrossRef]
- Ouyang, N.; Hu, W.; Meng, J.; Wang, B. Amino Acid Derivatives as Regulatory Molecules: Mechanisms in Plant Growth and Stress Tolerance. Crop J. 2025, 13, 668–680. [Google Scholar] [CrossRef]
- Preiner, J.; Steccari, I.; Oburger, E.; Wienkoop, S. Rhizobium Symbiosis Improves Amino Acid and Secondary Metabolite Biosynthesis of Tungsten-Stressed Soybean (Glycine Max). Front. Plant Sci. 2024, 15, 1355136. [Google Scholar] [CrossRef]
- Zhang, D.-X.; Liu, B.-Y.; Xue, F.-F.; Tang, Y.-L.; Yan, M.-J.; Wang, S.-X.; Guo, L.; Tong, T.; Wan, L.-N.; Liu, Y.-N.; et al. Paclobutrazol Induces Triterpenoid Biosynthesis via Downregulation of the Negative Transcriptional Regulator SlMYB in Sanghuangporus Lonicericola. Commun. Biol. 2025, 8, 551. [Google Scholar] [CrossRef]
| Amino Acid | Strains | |||
|---|---|---|---|---|
| MS | DS | WS | ||
| EAA | Thr | 2.08 ± 0.07 | 2.62 ± 0.05 | 2.84 ± 0.07 |
| Val | 2.34 ± 0.07 | 2.84 ± 0.05 | 2.95 ± 0.07 | |
| Met | ND | ND | ND | |
| Try | ND | ND | ND | |
| Ile | 2.21 ± 0.06 | 2.7 ± 0.04 | 2.82 ± 0.07 | |
| Leu | 3.5 ± 0.11 | 4.06 ± 0.08 | 4.41 ± 0.11 | |
| Phe | 1.98 ± 0.07 | 2.24 ± 0.05 | 2.49 ± 0.07 | |
| Lys | 1.8 ± 0.07 | 2.01 ± 0.04 | 2.2 ± 0.09 | |
| NEAA | Tyr | 1.73 ± 0.07 | 1.61 ± 0.03 | 1.85 ± 0.05 |
| Glu | 2.79 ± 0.1 | 3.96 ± 0.07 | 4.05 ± 0.1 | |
| Ser | 3.04 ± 0.12 | 3.54 ± 0.07 | 3.75 ± 0.13 | |
| Gly | 1.86 ± 0.07 | 2.24 ± 0.05 | 2.52 ± 0.08 | |
| Ala | 2.2 ± 0.08 | 2.71 ± 0.05 | 2.85 ± 0.08 | |
| Cys | 0.03 ± 0.01 | 0.11 ± 0.01 | 0.11 ± 0.01 | |
| Asp | 3.73 ± 0.14 | 4.53 ± 0.08 | 4.93 ± 0.14 | |
| His | 0.64 ± 0.03 | 0.78 ± 0.02 | 0.83 ± 0.03 | |
| Arg | 1.9 ± 0.07 | 2.24 ± 0.05 | 2.25 ± 0.09 | |
| Pro | 1.73 ± 0.06 | 2.39 ± 0.04 | 2.34 ± 0.07 | |
| TAA TEAA TNEAA | 33.5 ± 0.06 | 40.6 ± 0.04 | 43.2 ± 0.07 | |
| 13.91 ± 0.07 | 16.47 ± 0.06 | 17.71 ± 0.08 | ||
| 16.02 ± 0.06 | 19.48 ± 0.05 | 20.89 ± 0.07 | ||
| Amino Acid | Strains | WHO/FAO (mg/g) | IOM (mg/g) | ||
|---|---|---|---|---|---|
| MS | DS | WS | |||
| His | 0.64 ± 0.03 | 0.78 ± 0.02 | 0.83 ± 0.03 | 15 | 17 |
| Thr | 2.08 ± 0.07 | 2.62 ± 0.05 | 2.84 ± 0.07 | 40 | 27 |
| Lys | 1.8 ± 0.07 | 2.01 ± 0.04 | 2.2 ± 0.09 | 55 | 51 |
| Leu | 3.5 ± 0.11 | 4.06 ± 0.08 | 4.41 ± 0.11 | 70 | 55 |
| Ile | 2.21 ± 0.06 | 2.7 ± 0.04 | 2.82 ± 0.07 | 40 | 25 |
| Met + Cys | 0.03 ± 0.01 | 0.11 ± 0.01 | 0.11 ± 0.01 | 35 | 25 |
| Phe + Tyr | 4.59 ± 0.17 | 5.97 ± 0.11 | 6.25 ± 0.19 | 60 | 47 |
| Val | 2.34 ± 0.07 | 2.84 ± 0.05 | 2.95 ± 0.07 | 50 | 32 |
| Amino Acid | Amino Acid Score (AAS, %) | Ratio Coefficient (RC, %) | Ratio Coefficient (RC, %) | Score of RC (SRC, %) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MS | DS | WS | MS | DS | WS | MS | DS | WS | MS | DS | WS | |
| His | 4.27 | 5.22 | 5.53 | 0.85 | 1.04 | 1.11 | 37.65 | 45.88 | 48.82 | 31.35 | 79.62 | 94.41 |
| Thr | 5.24 | 6.55 | 7.11 | 0.83 | 1.04 | 1.13 | 77.04 | 97.04 | 105.19 | |||
| Lys | 3.27 | 3.65 | 4.08 | 0.93 | 1.01 | 1.10 | 35.29 | 39.41 | 43.14 | |||
| Leu | 5.05 | 5.81 | 6.30 | 0.88 | 1.02 | 1.11 | 63.64 | 73.82 | 80.18 | |||
| Ile | 5.53 | 6.75 | 7.05 | 0.86 | 1.05 | 1.09 | 88.43 | 108.02 | 112.82 | |||
| Met + Cys | 0.09 | 0.31 | 0.31 | 0.36 | 1.32 | 1.32 | 1.23 | 4.41 | 4.42 | |||
| Phe + Tyr | 7.65 | 9.95 | 10.42 | 0.82 | 1.07 | 1.12 | 97.66 | 127.02 | 132.98 | |||
| Val | 4.68 | 5.68 | 5.92 | 0.86 | 1.05 | 1.09 | 73.13 | 88.75 | 92.19 | |||
| Number | Batch Number | Latitude (°N) | Longitude (°E) | Place of Origin | Collection Time |
|---|---|---|---|---|---|
| WS01 | SH20181201 | 29.565583 | 94.304296 | Zayul, Tibet | 01122018 |
| WS02 | SH20191201 | 29.565583 | 94.304296 | Zayul, Tibet | 01122019 |
| WS03 | SH20191202 | 29.565583 | 94.304296 | Zayul, Tibet | 02122019 |
| WS04 | SH20201201 | 29.565583 | 94.304296 | Zayul, Tibet | 01122020 |
| WS05 | SH20201202 | 29.565583 | 94.304296 | Zayul, Tibet | 02122020 |
| WS06 | SH20181101 | 29.565583 | 94.304296 | Zayul, Tibet | 01112018 |
| WS07 | SH20181202 | 29.565583 | 94.304296 | Zayul, Tibet | 02122018 |
| WS08 | SH20180602 | 29.565583 | 94.304296 | Zayul, Tibet | 02062018 |
| WS09 | SH20191203 | 29.565583 | 94.304296 | Zayul, Tibet | 03122019 |
| WS10 | SH20191204 | 29.565583 | 94.304296 | Zayul, Tibet | 04122019 |
| DS01 | SH20201203 | 115.704881 | 36.838277 | Linqing, Shandong | 03122020 |
| DS02 | SH20191205 | 115.704881 | 36.838277 | Linqing, Shandong | 05122019 |
| DS03 | SH20201204 | 115.704881 | 36.838277 | Linqing, Shandong | 04122020 |
| DS04 | SH20201206 | 115.704881 | 36.838277 | Linqing, Shandong | 06122020 |
| DS05 | SH20201207 | 115.704881 | 36.838277 | Linqing, Shandong | 07122020 |
| DS06 | SH20191201 | 115.704881 | 36.838277 | Linqing, Shandong | 01122019 |
| DS07 | SH20191202 | 115.704881 | 36.838277 | Linqing, Shandong | 02122019 |
| DS08 | SH20201201 | 115.704881 | 36.838277 | Linqing, Shandong | 01122020 |
| DS09 | SH20201202 | 115.704881 | 36.838277 | Linqing, Shandong | 02122020 |
| MS01 | SH20181101 | 31.454120 | 115.567047 | Jinzhai, Anhui | 01112018 |
| MS02 | SH20181202 | 31.454120 | 115.567047 | Jinzhai, Anhui | 02122018 |
| MS03 | SH20180602 | 31.454120 | 115.567047 | Jinzhai, Anhui | 02062018 |
| MS04 | SH20191203 | 31.454120 | 115.567047 | Jinzhai, Anhui | 03122019 |
| MS05 | SH20191204 | 31.454120 | 115.567047 | Jinzhai, Anhui | 04122019 |
| MS06 | SH20201203 | 31.454120 | 115.567047 | Jinzhai, Anhui | 03122020 |
| MS07 | SH20191204 | 31.454120 | 115.567047 | Jinzhai, Anhui | 04122019 |
| MS08 | SH20201203 | 31.454120 | 115.567047 | Jinzhai, Anhui | 03122020 |
| MS09 | SH20191205 | 31.454120 | 115.567047 | Jinzhai, Anhui | 05122019 |
| Time (min) | Mobile Phase A (%) | Mobile Phase B (%) |
|---|---|---|
| 0~6 | 95→88 | 5→12 |
| 6~16 | 88→85 | 12→15 |
| 16~20 | 85→75 | 15→25 |
| 20~25 | 75→50 | 25→50 |
| 25~31 | 50→33 | 50→67 |
| 31~37 | 33→25 | 67→75 |
| 37~39 | 25→20 | 75→80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, K.; Chen, L.; Wu, C.; Wang, H.; Wu, F.; Pu, J.; Zhang, Y. From Logs to Bags: A Metabolic Blueprint of Sanghuang Cultivation Revealed by UPLC-Q-TOF-MS/MS and Amino Acid Profiling. Molecules 2025, 30, 2829. https://doi.org/10.3390/molecules30132829
Xu K, Chen L, Wu C, Wang H, Wu F, Pu J, Zhang Y. From Logs to Bags: A Metabolic Blueprint of Sanghuang Cultivation Revealed by UPLC-Q-TOF-MS/MS and Amino Acid Profiling. Molecules. 2025; 30(13):2829. https://doi.org/10.3390/molecules30132829
Chicago/Turabian StyleXu, Kefan, Lingli Chen, Chenchen Wu, Haiyang Wang, Fei Wu, Jingzhe Pu, and Yazhong Zhang. 2025. "From Logs to Bags: A Metabolic Blueprint of Sanghuang Cultivation Revealed by UPLC-Q-TOF-MS/MS and Amino Acid Profiling" Molecules 30, no. 13: 2829. https://doi.org/10.3390/molecules30132829
APA StyleXu, K., Chen, L., Wu, C., Wang, H., Wu, F., Pu, J., & Zhang, Y. (2025). From Logs to Bags: A Metabolic Blueprint of Sanghuang Cultivation Revealed by UPLC-Q-TOF-MS/MS and Amino Acid Profiling. Molecules, 30(13), 2829. https://doi.org/10.3390/molecules30132829






