Antimicrobial Activity of Cinnamon, Tea Tree, and Thyme Essential Oils Against Pathogenic Bacteria Isolated from Tilapia (Oreochromis spp.) in Aquaculture Farms
Abstract
1. Introduction
2. Results
2.1. Antibiogram
2.2. Essential Oils (EOs) Disc Diffusion Test (DDt)
2.3. Minimal Inhibitory Concentrations (MICs)
3. Discussion
4. Materials and Methods
4.1. Essential Oils
4.2. Bacterial Strains
4.3. Inoculum Preparation
4.4. Antibiogram
4.5. Essential Oils (EOs) Disc Diffusion Test (DDt)
4.6. Determination of Minimum Inhibitory Concentration (MIC)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EOs | Essential Oils |
| CEO | Cinnamon Essential Oil |
| TEO | Thyme Essential Oil |
| TTEO | Tea Tree Essential Oil |
| MAR Index | Multiple Antibiotic Resistance Index |
| DDt | Disc Diffusion Test |
| MICs | Minimal Inhibitory Concentrations |
| DMSO | Dimethyl Sulfoxide |
References
- Islam, S.; Bhowmik, S.; Majumdar, P.R.; Srzednicki, G.; Rahman, M.; Hossain, M.A. Nutritional Profile of Wild, Pond-, Gher- and Cage-Cultured Tilapia in Bangladesh. Heliyon 2021, 7, e06968. [Google Scholar] [CrossRef] [PubMed]
- González Razo, F.D.J.; Sangerman-Jarquín, D.M.; Omaña Silvestre, J.M.; Rebollar Rebollar, S.; Hernández Martínez, J.; Ayllón Benítez, J.C. Marketing of tilapia (Oreochromis niloticus) in southern state of Mexico. Rev. Mex. De. Cienc. Agric. 2016, 7, 1985–1996. [Google Scholar]
- Prabu, E.; Rajagopalsamy, C.B.T.; Ahilan, B.; Jeevagan, I.J.M.A.; Renuhadevi, M. Tilapia—An Excellent Candidate Species for World Aquaculture: A Review. Annu. Res. Rev. Biol. 2019, 31, 1–14. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Vaseeharan, B.; Malaikozhundan, B.; Gobi, N.; Ravichandran, S.; Karthi, S.; Ashokkumar, B.; Sivakumar, N. A Novel Antimicrobial Therapy for the Control of Aeromonas hydrophila Infection in Aquaculture Using Marine Polysaccharide Coated Gold Nanoparticle. Microb. Pathog. 2017, 110, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Armwood, A.R.; Camus, A.C.; López-Porras, A.; Ware, C.; Griffin, M.J.; Soto, E. Pathologic Changes in Cultured Nile Tilapia (Oreochromis Niloticus) Associated with an Outbreak of Edwardsiella anguillarum. J. Fish. Dis. 2019, 42, 1463–1469. [Google Scholar] [CrossRef]
- Monir, S.; Yusoff, S.M.; Mohamad, A.; Ina-Salwany, M.Y. Vaccination of Tilapia against Motile Aeromonas Septicemia: A Review. J. Aquat. Anim. Health 2020, 32, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Ruzauskas, M.; Klimiene, I.; Armalyte, J.; Bartkiene, E.; Siugzdiniene, R.; Skerniskyte, J.; Krasauskas, R.; Suziedeliene, E. Composition and Antimicrobial Resistance Profile of Gram-Negative Microbiota Prevalent in Aquacultured Fish. J. Food Saf. 2018, 38, 1–10. [Google Scholar] [CrossRef]
- Karunasagar, I. Complexities Involved in Source Attribution of Antimicrobial Resistance Genes Found in Aquaculture Products. Asian Fish. Sci. 2020, 33 (Suppl. S1), 16–21. [Google Scholar] [CrossRef]
- Eissa, F.; Ghanem, K.; Al-Sisi, M. Occurrence and Human Health Risks of Pesticides and Antibiotics in Nile Tilapia along the Rosetta Nile Branch, Egypt. Toxicol. Rep. 2020, 7, 1640–1646. [Google Scholar] [CrossRef]
- Health for Animals. How Prevention Can Reduce the Need for Antibiotics Pathways to Reduce the Need for Antimicrobials on Farms for Sustainable Agrifood Systems Transformation (RENOFARM); Health for Animals: Brussels, Belgium, 2024. [Google Scholar]
- Zaidi, M.B.; Dreser, A.; Figueroa, I.M. A Collaborative Initiative for the Containment of Antimicrobial Resistance in Mexico. Zoonoses Public Health 2015, 62 (Suppl. S1), 52–57. [Google Scholar] [CrossRef]
- Matthews Mbokane, E.; Gukuta Moyo, N.A. Use of Medicinal Plants as Feed Additives in the Diets of Mozambique Tilapia (Oreochromis mossambicus) and the African Sharptooth Catfish (Clarias gariepinus) in Southern Africa. Front. Vet. Sci. 2022, 9, 1072369. [Google Scholar] [CrossRef]
- Gómez-Rincón, C.; Langa, E.; Murillo, P.; Sofía Valero, M.; Berzosa, C.; López, V. Activity of Tea Tree (Melaleuca alternifolia) Essential Oil against L3 Larvae of Anisakis simplex. BioMed Res. Int. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Bisht, D.; Pal, A.; Chanotiya, C.S.; Mishra, D.; Pandey, K.N. Terpenoid Composition and Antifungal Activity of Three Commercially Important Essential Oils against Aspergillus flavus and Aspergillus niger. Nat. Prod. Res. 2011, 25, 1993–1998. [Google Scholar] [CrossRef]
- Manion, C.R.; Widder, R.M. Essentials of Essential Oils. Am. J. Health-Syst. Pharm. 2017, 74, e153–e162. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; Alagawany, M.; Abdel-Moneim, A.-M.E.; Mohammed, N.G.; Khafaga, A.F.; Bin-Jumah, M.; Othman, S.I.; Allam, A.A.; Elnesr, S.S. Cinnamon (Cinnamomum zeylanicum) Oil as a Potential Alternative to Antibiotics in Poultry. Antibiotics 2020, 9, 210. [Google Scholar] [CrossRef]
- López, L.M.T. Tomillo. Ámbito Farm. 2006, 25, 74–77. [Google Scholar]
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef]
- Noui Mehidi, I.; Ait Ouazzou, A.; Tachoua, W.; Hosni, K. Investigating the Antimicrobial Properties of Essential Oil Constituents and Their Mode of Action. Molecules 2024, 29, 4119. [Google Scholar] [CrossRef]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef]
- Odeyemi, O.A.; Ahmad, A. Antibiotic Resistance Profiling and Phenotyping of Aeromonas Species Isolated from Aquatic Sources. Saudi J. Biol. Sci. 2017, 24, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Assane, I.M.; Valladão, G.M.R.; Pilarski, F. Chemical Composition, Cytotoxicity and Antimicrobial Activity of Selected Plant-Derived Essential Oils against Fish Pathogens. Aquac. Res. 2021, 52, 793–809. [Google Scholar] [CrossRef]
- Shaalan, M.; Mahboub, H.H.; Abdelwarith, A.A.; Younis, E.M.; Elnegiry, A.A.; Basher, A.W.; El Houseiny, W.; Shawky, S.M.; Orabi, S.H.; Davies, S.J.; et al. Dietary Tea Tree (Melaleucae aetheroleum) Oil Fortifies Growth, Biochemical, Immune antioxidant Trait, Gene Function, Tissue, and Aeromonas Sobria resistance in Nile Tilapia (Oreochromis niloticus). BMC Vet. Res. 2025, 21, 1–15. [Google Scholar] [CrossRef]
- Borotová, P.; Galovičová, L.; Vukovic, N.L.; Vukic, M.; Tvrdá, E.; Kačániová, M. Chemical and Biological Characterization of Melaleuca alternifolia Essential Oil. Plants 2022, 11, 558. [Google Scholar] [CrossRef]
- Borges, R.S.; Ortiz, B.L.S.; Pereira, A.C.M.; Keita, H.; Carvalho, J.C.T. Rosmarinus Officinalis Essential Oil: A Review of Its Phytochemistry, Anti-Inflammatory Activity, and Mechanisms of Action Involved. J. Ethnopharmacol. 2019, 229, 29–45. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; El Basuini, M.F.; Yilmaz, S.; Abdel-Latif, H.M.R.; Alagawany, M.; Kari, Z.A.; Razab, M.K.A.A.; Hamid, N.K.A.; Moonmanee, T.; Doan, H.V. Exploring the Roles of Dietary Herbal Essential Oils in Aquaculture: A Review. Animals 2022, 10, 823. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, J.G.; Sutili, F.J.; Gressler, L.T.; Ely, V.; Silveira, B.; Tasca, C.; Reghelin, M.; Matter, L.; Vargas, A.; Baldisserotto, B.; et al. Antibacterial Potential of Phytochemicals Alone or in Combination with Antimicrobials against Fish Pathogenic Bacteria. J. Appl. Microbiol. 2018, 125, 655–665. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef]
- Baginska, S.; Golonko, A.; Swislocka, R.; Lewandowski, W. Monoterpenes as Medicinal Agents: Exploring the Pharmaceutical Potential of p-Cymene, p-Cymenene, and Γ-Terpinene. Acta Pol. Pharm. Drug Res. 2023, 80, 879–892. [Google Scholar] [CrossRef]
- Zheng, X.; Feyaerts, A.F.; Van Dijck, P.; Bossier, P. Inhibitory Activity of Essential Oils against Vibrio campbellii and Vibrio parahaemolyticus. Microorganisms 2020, 8, 1946. [Google Scholar] [CrossRef]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial Mechanisms of Cinnamon and Its Constituents: A Review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Use of Asiatic Pennywort Centella Asiatica Aqueous Extract as a Bath Treatment to Control Columnaris in Nile Tilapia. J. Aquat. Anim. Health 2010, 22, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Wickramanayake, M.V.K.S.; Kumarage, P.M.; Majeed, S.; Heo, G.J. An Overview of the Antimicrobial Activity of Some Essential Oils against Fish Pathogenic Bacteria. Vet. Integr. Sci. 2022, 21, 99–119. [Google Scholar] [CrossRef]
- Sobhy, M.; Ali, S.S.; Cui, H.; Lin, L.; El-Sapagh, S. Exploring the Potential of 1,8-Cineole from Cardamom Oil against Food-Borne Pathogens: Antibacterial Mechanisms and Its Application in Meat Preservation. Microb. Pathog. 2023, 184, 106375. [Google Scholar] [CrossRef]
- Soković, M.; Glamočlija, J.; Marin, P.D.; Brkić, D.; Van Griensven, L.J.L.D. Antibacterial Effects of the Essential Oils of Commonly Consumed Medicinal Herbs Using an In Vitro Model. Molecules 2010, 15, 7532–7546. [Google Scholar] [CrossRef] [PubMed]
- Becerril, R.; Nerín, C.; Gómez-Lus, R. Evaluation of Bacterial Resistance to Essential Oils and Antibiotics after Exposure to Oregano and Cinnamon Essential Oils. Foodborne Pathog. Dis. 2012, 9, 699–705. [Google Scholar] [CrossRef]
- Zilberg, D.; Tal, A.; Froyman, N.; Abutbul, S.; Dudai, N.; Golan-Goldhirsh, A. Dried Leaves of Rosmarinus officinalis as a Treatment for Streptococcosis in Tilapia. J. Fish. Dis. 2010, 33, 361–369. [Google Scholar] [CrossRef]
- Nya, E.J.; Austin, B. Use of Garlic, Allium sativum, to Control Aeromonas Hydrophila Infection in Rainbow Trout, Oncorhynchus Mykiss Rainbow Trout, Oncorhynchus mykiss. J. Fish. Dis. 2009, 32, 963–970. [Google Scholar] [CrossRef]
- Öntaş, C.; Baba, E.; Kaplaner, E.; Küçükaydin, S.; Öztürk, M.; Ercan, M.D. Antibacterial Activity of Citrus Limon Peel Essential Oil and Argania Spinosa Oil Against Fish Pathogenic Bacteria. Kafkas Univ. Vet. Fak. Derg. 2016, 22, 741–749. [Google Scholar] [CrossRef]
- Sonmez, A.Y.; Bilen, S.; Albayrak, M.; Yilmaz, S.; Biswas, G.; Hisar, O.; Yanik, T. Effects of Dietary Supplementation of Herbal Oils Containing 1,8-Cineole, Carvacrol or Pulegone on Growth Performance, Survival, Fatty Acid Composition, and Liver and Kidney Histology of Rainbow Trout (Oncorhynchus Mykiss) Fingerlings. Turk. J. Fish. Aquat. Sci. 2015, 15, 813–819. [Google Scholar] [CrossRef]
- Anandhi, P.; Tharani, M.; Rajeshkumar, S.; Lakshmi, T. Antibacterial Activity of Cinnamon and Clove Oil against Wound Pathogens. J. Popul. Ther. Clin. Pharmacol. 2022, 28, 41–46. [Google Scholar] [CrossRef]
- Sousa, L.G.V.; Castro, J.; Cavaleiro, C.; Salgueiro, L.; Tomás, M.; Palmeira-Oliveira, R.; Martinez-Oliveira, J.; Cerca, N. Synergistic Effects of Carvacrol, α-Terpinene, γ-Terpinene, ρ-Cymene and Linalool against Gardnerella Species. Sci. Rep. 2022, 12, 4417. [Google Scholar] [CrossRef]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Potential of Cinnamon (Cinnamomum verum) Oil to Control Streptococcus Iniae Infection in Tilapia (Oreochromis niloticus). Fish. Sci. 2010, 76, 287–293. [Google Scholar] [CrossRef]
- Hudecová, P.; Koščová, J.; Hajdučková, V.; Király, J.; Hoř Nak, P. Antibacterial and Antibiofilm Activity of Essential Oils Against Aeromonas Spp. Isolated from Rainbow Trout. Animals 2024, 14, 3202. [Google Scholar] [CrossRef]
- Kačániová, M.; Terentjeva, M.; Vukovic, N.; Puchalski, C.; Roychoudhury, S.; Kunová, S.; Klūga, A.; Tokár, M.; Kluz, M.; Ivanišová, E. The Antioxidant and Antimicrobial Activity of Essential Oils against Pseudomonas spp. Isolated from Fish. Saudi Pharm. J. 2017, 25, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Kabir Mumu, S.; Mahboob Hossain, M. Antimicrobial Activity of Tea Tree Oil against Pathogenic Bacteria and Comparison of Its Effectiveness with Eucalyptus Oil, Lemongrass Oil and Conventional Antibiotics. Am. J. Microbiol. Res. 2018, 6, 73–78. [Google Scholar] [CrossRef]
- Lyu, F.; Hong, Y.-L.; Cai, J.-H.; Wei, Q.-Q.; Zhou, X.; Ding, Y.-T.; Liu, Z.-F.; Liu, L. Antimicrobial Effect and Mechanism of Cinnamon Oil and Gamma Radiation on Shewanella putrefaciens. J. Food Sci. Technol. 2018, 55, 3353–3361. [Google Scholar] [CrossRef]
- Golus, J.; Sawicki, R.; Widelski, J.; Ginalska, G. The Agar Microdilution Method—A New Method for Antimicrobial Susceptibility Testing for Essential Oils and Plant Extracts. J. Appl. Microbiol. 2016, 121, 1291–1299. [Google Scholar] [CrossRef]
- Conapesca. Anuario Estadístico de Acuacultura y Pesca de la Comisión Nacional de Acuacultura y Pesca; Conapesca: Mazatlán, México, 2021. [Google Scholar]
- Chávez-Mejía, A.C.; Navarro-González, I.; Magaña-López, R.; Uscanga-Roldán, D.; Zaragoza-Sánchez, P.I.; Jiménez-Cisneros, B.E. Presence and Natural Treatment of Organic Micropollutants and Their Risks after 100 Years of Incidental Water Reuse in Agricultural Irrigation. Water 2019, 11, 2148. [Google Scholar] [CrossRef]
- Lesser, L.E.; Mora, A.; Moreau, C.; Mahlknecht, J.; Hernández-Antonio, A.; Ramírez, A.I.; Barrios-Piña, H. Survey of 218 Organic Contaminants in Groundwater Derived from the World’s Largest Untreated Wastewater Irrigation System: Mezquital Valley, Mexico. Chemosphere 2018, 198, 510–521. [Google Scholar] [CrossRef]
- Garduño-Jiménez, A.L.; Durán-Álvarez, J.C.; Ortori, C.A.; Abdelrazig, S.; Barrett, D.A.; Gomes, R.L. Delivering on Sustainable Development Goals in Wastewater Reuse for Agriculture: Initial Prioritization of Emerging Pollutants in the Tula Valley, Mexico. Water Res. 2023, 238, 119903. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Coyotl, I.; Galar-Martínez, M.; García-Medina, S.; Gómez-Oliván, L.M.; Gasca- Pérez, E.; Martínez-Galero, E.; Islas-Flores, H.; Pérez-Pastén, B.R.; Barceló, D.; López de Alda, M.; et al. Polluted Water from an Urban Reservoir (Madín Dam, México) Induces Toxicity and Oxidative Stress in Cyprinus carpio Embryos. Environ. Pollut. 2019, 251, 510–521. [Google Scholar] [CrossRef]
- Cortes-Sanchez, A.D.J.; Espinosa-Chaurand, L.D.; Garza-Torres, R.; Diaz-Ramirez, M.; Salgado-Cruz, M.D.L.P.; Sánchez-Minutii, L.; García-Barrientos, R. Foodborne Diseases, Fish and the Case of Aeromonas Spp. Afr. J. Agric. Res. 2019, 14, 617–628. [Google Scholar] [CrossRef]
- Hoel, S.; Vadstein, O.; Jakobsen, A.N. The Significance of Mesophilic Aeromonas spp. in Minimally Processed Ready-to-Eat Seafood. Microorganisms 2019, 7, 91. [Google Scholar] [CrossRef]
- Martin-Carnahan, A.; Joseph, S.W. Aeromonas. In Bergey’s Manual of Systematics of Archaea and Bacteria; Wiley: Hoboken, NJ, USA, 2015; pp. 1–44. [Google Scholar] [CrossRef]
- Zhai, W.; Wang, Q.; Zhu, X.; Jia, X.; Chen, L. Pathogenic Infection and Microbial Composition of Yellow Cat-fish (Pelteobagrus Fulvidraco) Challenged by Aeromonas veronii and Proteus mirabilis. Aquac. Fish. 2023, 8, 166–173. [Google Scholar] [CrossRef]
- Phumkhachorn, P.; Rattanachaikunsopon, P. Use of Cassia Alata Aqueous Extract as a Bath Treatment to Control Pseudomonas anguilliseptica Infection in Tilapia (Oreochromis niloticus). Arch. Biol. Sci. 2015, 67, 1165–1172. [Google Scholar] [CrossRef]
- Economopoulou, A.; Chochlakis, D.; Almpan, M.A.; Sandalakis, V.; Maraki, S.; Tselentis, Y.; Psaroulaki, A. Environmental Investigation for the Presence of Vibrio Species Following a Case of Severe Gastroenteritis in a Touristic Island. Environ. Sci. Pollut. Res. 2017, 24, 4835–4840. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Liu, Y.; Dong, J.; Xu, N.; Yang, Q.; Zhou, S.; Ai, X. Identification of Vibrio cholerae as a Bacterial Pathogen of Bluegill Sunfish. Aquac. Rep. 2022, 23, 101092. [Google Scholar] [CrossRef]
- Sood, N.; Pradhan, P.K.; Ravindra; Verma, D.K.; Yadav, M.K.; Mishra, R.K.; Kumar, U.; Swaminathan, T.R.; Sood, N.K. Large-Scale Mortality in Cultured Tilapia Oreochromis niloticus Due to Infection with Shewanella putrefaciens in India. J. World Aquac. Soc. 2020, 51, 563–570. [Google Scholar] [CrossRef]
- Pȩkala-Safińska, A. Contemporary Threats of Bacterial Infections in Freshwater Fish. J. Vet. Res. 2018, 62, 261–267. [Google Scholar] [CrossRef]
- Cao, H.; Ye, W.; He, S.; Li, Y.; Yang, Y. Acinetobacter Lwoffii: An Emerging Pathogen for Red Disease in Farmed Channel Catfish Ictalurus. Isr. J. Aquac. 2016, 68, 20799. [Google Scholar]
- Willems, A.; Gillis, M. Comamonas. In Bergey’s Manual of Systematics of Archaea and Bacteria; Wiley: Hoboken, NJ, USA, 2015; pp. 1–17. [Google Scholar] [CrossRef]
- Traglia, G.M.; Dixon, C.; Chiem, K.; Almuzara, M.; Barberis, C.; Montaña, S.; Merino, C.; Mussi, M.A.; Tolmasky, M.E.; Iriarte, A.; et al. Draft Genome Sequence of Empedobacter (Formerly Wautersiella) falsenii Comb. Nov. Wf282, a Strain Isolated from a Cervical Neck Abscess. Genome Announc. 2016, 3, e00235-15. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Chowdhury, B. Pseudomonas anguilliseptica As a Pathogen of Tilapia (Oreochromis niloticus) Culture in Bangladesh. Bangladesh Res. Pub. J. 2009, 2, 712–721. [Google Scholar]
- Hassan, S.; Abdel-Rahman, M.; Mansour, E.S.; Monir, W. Isolation, Phenotypic Characterization and Antibiotic Susceptibility of Prevalent Bacterial Pathogens Implicating the Mortality of Cultured Nile Tilapia, Oreochromis niloticus. Egypt. J. Aquac. 2020, 10, 23–43. [Google Scholar] [CrossRef]
- Preena, P.G.; Dharmaratnam, A.; Swaminathan, T.R. Antimicrobial Resistance Analysis of Pathogenic Bacteria Isolated from Freshwater Nile Tilapia (Oreochromis niloticus) Cultured in Kerala, India. Curr. Microbiol. 2020, 77, 3278–3287. [Google Scholar] [CrossRef] [PubMed]
- Elgendy, M.Y.; Abdelsalam, M.; Kenawy, M.A.; Shimaa, E.A. Vibriosis Outbreaks in Farmed Nile Tilapia (Oreochromis niloticus) Caused by Vibrio mimicus and V. cholerae. Aquac. Int. 2022, 30, 2661–2677. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, X.; Li, L.; Niu, C.; Pei, C.; Zhu, L.; Kong, X.; Yang, Y.; Qi, Z.; Kong xhkong, X. Identification of Shewanella putrefaciens as a Novel Pathogen of the Largemouth Bass (Micropterus salmoides) and Histopathological Analysis of Diseased Fish. Front. Cell Infect. Microbiol. 2022, 12. [Google Scholar] [CrossRef]
- Kozińska, A.; Paździor, E.; Pȩkala, A.; Niemczuk, W. Acinetobacter Johnsonii and Acinetobacter Lwoffii—The Emerging Fish Pathogens. Bull. Vet. Inst. Pulawy 2014, 58, 193–199. [Google Scholar] [CrossRef]
- Zhang, M.; Dou, Y.; Xiao, Z.; Xue, M.; Jiang, N.; Liu, W.; Xu, C.; Fan, Y.; Zhang, Q.; Zhou, Y. Identification of an Acinetobacter Lwoffii Strain Isolated from Diseased Hybrid Sturgeon (Acipenser Baerii♀ × Acipenser Schrenckii♂). Aquaculture 2023, 574, 739649. [Google Scholar] [CrossRef]
- Cao, S.; Geng, Y.; Yu, Z.; Deng, L.; Gan, W.; Wang, K.; Ou, Y.; Chen, D.; Huang, X.; Zuo, Z.; et al. Acinetobacter Lwoffii, an Emerging Pathogen for Fish in Schizothorax Genus in China. Transbound. Emerg. Dis. 2018, 65, 1816–1822. [Google Scholar] [CrossRef]
- Ryan, M.P.; Sevjahova, L.; Gorman, R.; White, S. The Emergence of the Genus Comamonas as Important Opportunistic Pathogens. Pathogens 2022, 11, 1032. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, Q.; Xu, H.; He, X.; Guo, L.; Liu, S.; Wen, P.; Gou, J. Emergence of IMP-8-Producing Comamonas thiooxydans Causing Urinary Tract Infection in China. Front. Microbiol. 2021, 12, 585716. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.; Matabang, M.A.; Miller, D.; Aggarwal, R.; LaFortune, A. First Case Report on Empedobacter Falsenii Bacteremia. IDCases 2023, 33, e01814. [Google Scholar] [CrossRef] [PubMed]
- Zepeda-Velazquez, A.P.; Gómez-De-Anda, F.-R.; Aguilar-Mendoza, L.F.; Castrejón-Jiménez, N.S.; Hernán-dez-González, J.C.; Varela-Guerrero, J.A.; de-la-Rosa-Arana, J.-L.; Vega-Sánchez, V.; Reyes-Rodríguez, N.E. Bullfrogs (Lithobates Catesbeianus) as a Potential Source of Foodborne Disease. J. Food Prot. 2023, 86, 100067. [Google Scholar] [CrossRef]
- M02-A11; Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard—Eleventh Edition. CLSI: Wayne, PA, USA, 2012.
- M100; Performance Standards for Antimicrobial Susceptibility Testing. 34th ed. CLSI Supplement M100. CLSI: Wayne, PA, USA, 2024.
- EUCAST—The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters; EUCAST—The European Committee on Antimicrobial Susceptibility Testing: Växjö, Sweden, 2024. [Google Scholar]
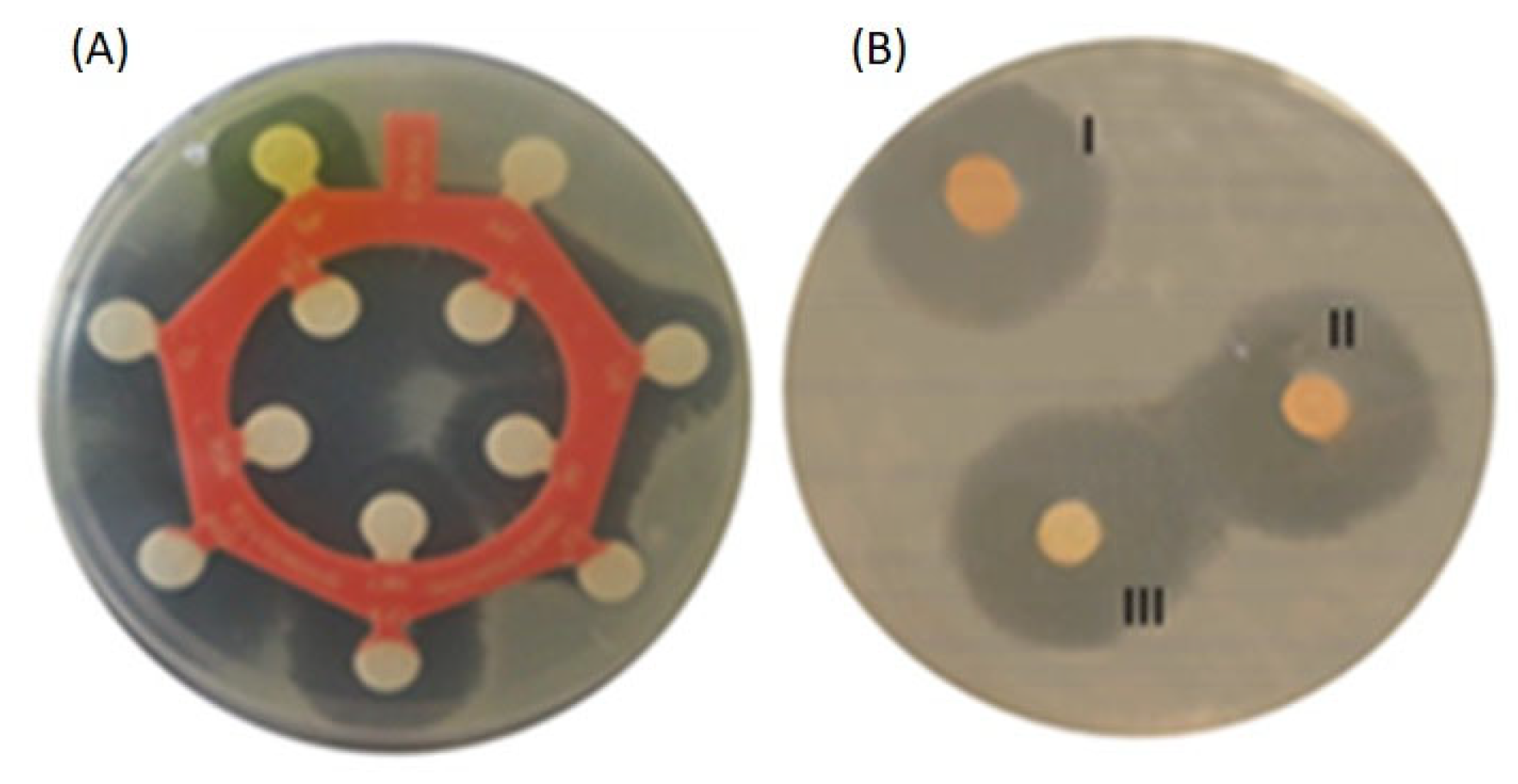
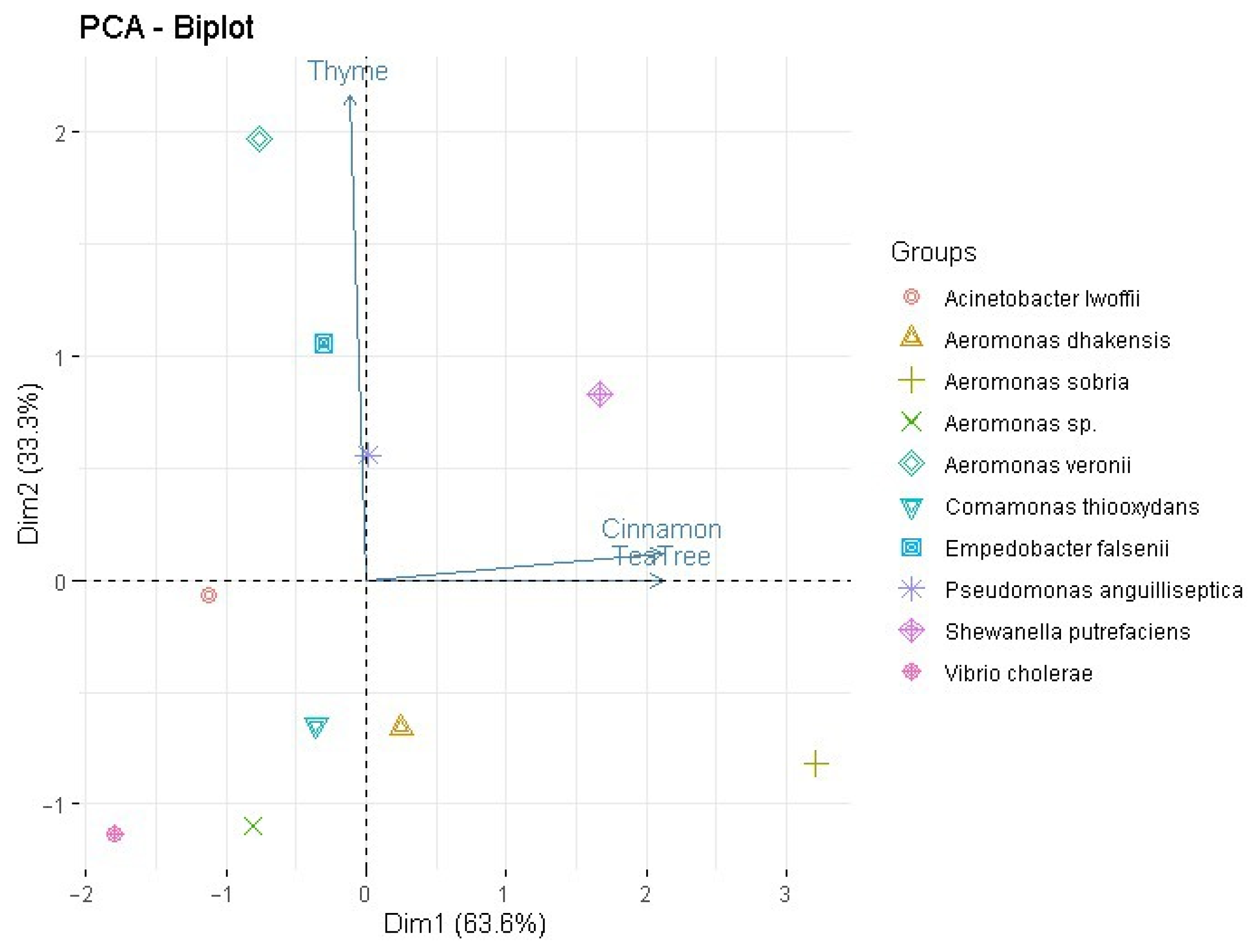
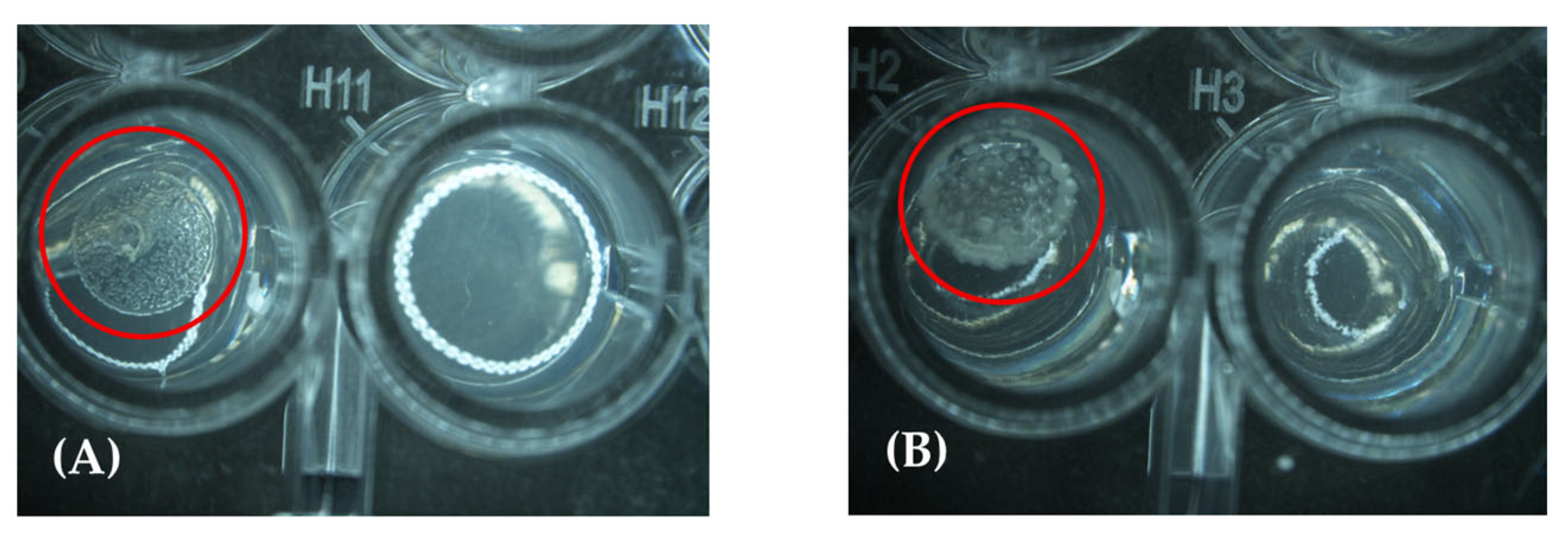

| Microorganism | Resistant to | MAR Index |
|---|---|---|
| Aeromonas sp. | AM, AK, CB, GE, CF, CFX, CPF, NOF, CL, STX, NF, NET | 1.00 |
| Aeromonasdhakensis | AM, AK, CB, GE, CF, CFX, CPF, NOF, CL, STX, NF, NET | 1.00 |
| Aeromonas veronii | AM, AK, CB, GE, CF, CFX, CPF, NOF, CL, STX, NF, NET | 1.00 |
| Aeromonas veronii | AM, AK, CB, GE, CF, CFX, CPF, NOF, CL, STX, NF, NET | 1.00 |
| Aeromonas veronii | AM, AK, CB, GE, CF, CFX, CPF, NOF, CL, STX, NF, NET | 1.00 |
| Aeromonas sobria | AM, AK, CB, GE, CF, CFX, CPF, NOF, CL, STX, NF, NET | 1.00 |
| Pseudomonas anguilliseptica | AM, AK, CB, GE, CF, CFX, CPF, NOF, CL, STX, NF, NET | 1.00 |
| Shewanella putrefaciens | AM, AK, CB, GE, CF, CFX, CPF, NOF, CL, STX, NF, NET | 1.00 |
| Comamonas thiooxydans | AM, AK, CB, GE, CF, CFX, CPF, NOF, CL, STX, NF, NET | 1.00 |
| Vibrio cholerae | AM, AK, CB, GE, CF, CFX, CPF, NOF, CL, STX, NF, NET | 1.00 |
| Acinetobacter Iwoffii | AM, AK, CB, GE, CF, CFX, CPF, NOF, CL, NF, NET | 0.83 |
| Empedobacter falsenii | AK, GE, CPF, STX, NET | 0.50 |
| Microorganisms | Vol EOs (µL) | Cinnamon | Thyme | Tea tree |
|---|---|---|---|---|
| Aeromonas sp. | 5 10 15 | 7.33 ± 0.57 Aa 8.33 ± 1.15 ABc 9.67 ± 0.57 Be | 5.33 ± 1.55 Cab 6.67 ± 1.55 Ccd 8.67 ± 2.31 Ce | 3.33 ± 0.57 Db 4.67 ± 0.57 Dd 8.00 ± 1.00 Ee |
| Aeromonasdhakensis | 5 10 15 | 9.33 ± 0.57 Ff 9.67 ± 0.57 Fg 10.67 ± 0.57 Fh | 6.67 ± 1.15 Gf 8.33 ± 2.08 Gg 11.00 ± 2.65 Gh | 5.67 ± 2.89 Hf 6.67 ± 2.08 Hg 10.33 ± 2.08 Hh |
| Aeromonas veronii | 5 10 15 | 9.00 ± 3.46 Iij 9.33 ± 3.21 Ikl 10.67 ± 2.89 Imn | 15.33 ± 4.16 Ji 16.00 ± 4.00 Jk 19.67 ± 7.51 Jm | 2.67 ± 0.57 Kj 4.67 ± 0.57 Ll 6.33 ± 0.57 Mn |
| Aeromonas veronii | 5 10 15 | 10.00 ± 0.57 Nñ 10.67 ± 0.57 Ño 14.67 ± 0.57 Ñp | 4.67 ± 1.5 Oñ 5.00 ± 1.73 Oo 6.33 ± 2.31 Oq | 6.67 ± 2.36 Pñ 7.33 ± 4.04 Po 5.17 ± 2.36 Pq |
| Aeromonas veronii | 5 10 15 | 9.67 ± 1.52 Qr 10.67 ± 0.57 Qt 13.33 ± 2.31 Qu | 5.00 ± 1.00 Rrs 7.00 ± 2.65 Rt 7.67 ± 2.52 Ruv | 3.00 ± 3.61 Ss 5.33 ± 3.51 St 5.67 ± 2.31 Sv |
| Aeromonas sobria | 5 10 15 | 18.00 ± 1.73 Tx 16.00 ± 1.00 Tz 17.00 ± 0.00 Tb’ | 5.67 ± 0.57 Uy 7.00 ± 1.73 UVa’ 10.00 ± 1.73 Vc’ | 9.67 ± 3.51 Wy 9.00 ± 1.00 Wa’ 9.67 ± 1.52 Wc’ |
| Pseudomonas anguilliseptica | 5 10 15 | 9.33 ± 2.89 Xd’ 10.00 ± 2.00 Xe’ 11.33 ± 3.51 Xf’ | 10.67 ± 6.03 Yd’ 11.00 ± 4.58 Ye’ 12.33 ± 4.04 Yf’ | 5.00 ± 2.00 Zd’ 10.00 ± 1.00 A’e’ 13.67 ± 1.528 A’f’ |
| Shewanella putrefaciens | 5 10 15 | 13.67 ± 1.15 B’g’ 13.67 ± 0.57 B’i’ 16.67 ± 1.528 C’k’ | 11.33 ± 2.08 D’g’h’ 12.33 ± 3.06 D’i’ 14.00 ± 3.61 D’k’l’ | 7.67 ± 2.52 E’h’ 6.33 ± 1.52 E’j’ 7.67 ± 3.06 E’l’ |
| Vibrio cholerae | 5 10 15 | 5.33 ± 0.57 F’m’ 6.67 ± 0.57 F’G’n’ 8.33 ± 1.15 G’ñ’ | 5.33 ± 4.04 H’m’ 7.33 ± 2.08 H’n’ 8.33 ± 2.08 H’ñ’ | 1.333 ± 0.57 I’m’ 3.33 ± 2.08 I’n’ 4.00 ± 2.65 I’ñ’ |
| Acinetobacter Iwoffii | 5 10 15 | 8.33 ± 1.528 J’o’ 10.00 ± 2.65 J’q’ 11.67 ± 2.89 J’s’ | 8.67 ± 1.15 K’o’ 9.67 ± 0.57 K’q’ 11.67 ± 2.89 K’s’ | 1.60 ± 0.693 L’p’ 1.30 ± 0.608 L’r’ 0.93 ± 0.115 L’t’ |
| Comamonas thiooxydans | 5 10 15 | 10.00 ± 0.00 M’u’ 16.67 ± 0.577 N’w’ 16.67 ± 2.08 N’y’ | 6.67 ± 2.89 Ñ’u’v’ 7.67 ± 1.528 Ñ’x’ 9.67 ± 2.08 Ñ’z’ | 3.00 ± 1.00 O’v’ 8.00 ± 1.73 P’x’ 9.67 ± 2.31 P’z’ |
| Empedobacter falsenii | 5 10 15 | 9.00 ± 0.00 Q’a” 10.00 ± 1.00 Q’c”d” 10.67 ± 1.528 Q’e”f | 12.33 ± 2.52 R’a” 12.33 ± 2.08 R’c” 16.00 ± 4.36 R’e” | 4.17 ± 2.02 S’b” 5.33 ± 3.21 S’d” 6.33 ± 3.06 S’f” |
| Strain | Cinnamon MIC | Thyme MIC | Tea Tree MIC |
|---|---|---|---|
| Aeromonas sp. | 1 | <0.12 | 2 |
| Aeromonas dhakensis | <0.12 | <0.12 | 2 |
| Aeromonas veronii | 1 | <0.12 | 1 |
| Aeromonas veronii | <0.12 | <0.12 | 2 |
| Aeromonas veronii | <0.12 | <0.12 | 2 |
| Aeromonas sobria | <0.12 | <0.12 | 4 |
| Pseudomonas anguilliseptica | 0.5 | <0.12 | 0.5 |
| Shewanella putrefaciens | 2 | <0.12 | 2 |
| Comamonas thiooxydans | 1 | <0.12 | 0.5 |
| Vibrio cholerae | 1 | <0.12 | 2 |
| Acinetobacter Iwoffii | 1 | <0.12 | 1 |
| Empedobacter falsenii | 0.25 | <0.12 | >0.12 |
| Essential Oil | Chemical Composition |
|---|---|
| Cinnamon oil (Cinnamomum zeylanicum) | trans-cinnamaldehyde (53.79%) trans-cinnamyl acetate (9.83%) β-phellandrene (5.29%) β-caryophyllene (4.17%) linalool (3.01%) α-pinene (2.51%) para-cymene (2.33%) eugenol (2.12%) α-phellandrene (1.72%) limonene (1.51%) α-terpinene (1.37%) camphene (1.10%) |
| Tea tree (Melaleuca alternifolia) | terpinen-4-ol (38.26%) γ-terpinene (17.01%) α-terpinene (8.59%) α-terpineol (4.69%) terpinolene (3.15%) α-pinene (2.23%) delta-cadinene (2.22%) p-cymene (2.10%) 1,8-Cineole (1.97%) viridiflorene (1.90%) bicyclogermacrene (1.88%) |
| Thyme (Thymus vulgaris) | Thymol (54.88%) p-cymene (17.30%) carvacrol (3.33%) γ-terpinene (9.80%) Linalool (3.87%) myrcene (1.29%) β-caryophyllene (1.17%) borneol (1.13%) α-terpinene (1.12%) |
| Microorganism | Accession Number |
|---|---|
| Aeromonas sp. | EF491849.1 |
| Aeromonas aquariorum | FN796727.1 |
| Aeromonas veronii | CP014774.1 |
| Aeromonas veronii | JF490068.1 |
| Aeromonas veronii | FR682763.1 |
| Aeromonas sobria | AB526508.1 |
| Pseudomonas anguilliseptica | MH185879.1 |
| Shewanella putrefaciens | CP046329.1 |
| Vibrio cholerae | CP026531.1 |
| Acinetobacter Iwoffii | PP762073.1 |
| Comamonas thiooxydans | AP026738.1 |
| Empedobacter falsenii | MH712956.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terrazas-Pineda, K.A.; Alamilla-Beltrán, L.; Acero-Ortega, C.A.; Damas-Espinoza, J.A.; Calderón-Domínguez, G.; Mora-Escobedo, R.; Vega-Sánchez, V.; Gómez-de Anda, F.R. Antimicrobial Activity of Cinnamon, Tea Tree, and Thyme Essential Oils Against Pathogenic Bacteria Isolated from Tilapia (Oreochromis spp.) in Aquaculture Farms. Molecules 2025, 30, 2799. https://doi.org/10.3390/molecules30132799
Terrazas-Pineda KA, Alamilla-Beltrán L, Acero-Ortega CA, Damas-Espinoza JA, Calderón-Domínguez G, Mora-Escobedo R, Vega-Sánchez V, Gómez-de Anda FR. Antimicrobial Activity of Cinnamon, Tea Tree, and Thyme Essential Oils Against Pathogenic Bacteria Isolated from Tilapia (Oreochromis spp.) in Aquaculture Farms. Molecules. 2025; 30(13):2799. https://doi.org/10.3390/molecules30132799
Chicago/Turabian StyleTerrazas-Pineda, Karen A., Liliana Alamilla-Beltrán, Claudia Ariadna Acero-Ortega, Juan Antonio Damas-Espinoza, Georgina Calderón-Domínguez, Rosalva Mora-Escobedo, Vicente Vega-Sánchez, and Fabián Ricardo Gómez-de Anda. 2025. "Antimicrobial Activity of Cinnamon, Tea Tree, and Thyme Essential Oils Against Pathogenic Bacteria Isolated from Tilapia (Oreochromis spp.) in Aquaculture Farms" Molecules 30, no. 13: 2799. https://doi.org/10.3390/molecules30132799
APA StyleTerrazas-Pineda, K. A., Alamilla-Beltrán, L., Acero-Ortega, C. A., Damas-Espinoza, J. A., Calderón-Domínguez, G., Mora-Escobedo, R., Vega-Sánchez, V., & Gómez-de Anda, F. R. (2025). Antimicrobial Activity of Cinnamon, Tea Tree, and Thyme Essential Oils Against Pathogenic Bacteria Isolated from Tilapia (Oreochromis spp.) in Aquaculture Farms. Molecules, 30(13), 2799. https://doi.org/10.3390/molecules30132799








