Development of Melt-Castable Explosive: Targeted Synthesis of 3,5-Dinitro-4-Methylnitramino-1-Methylpyrazole and Functional Derivatization of Key Intermediates
Abstract
1. Introduction
2. Results and Discussion
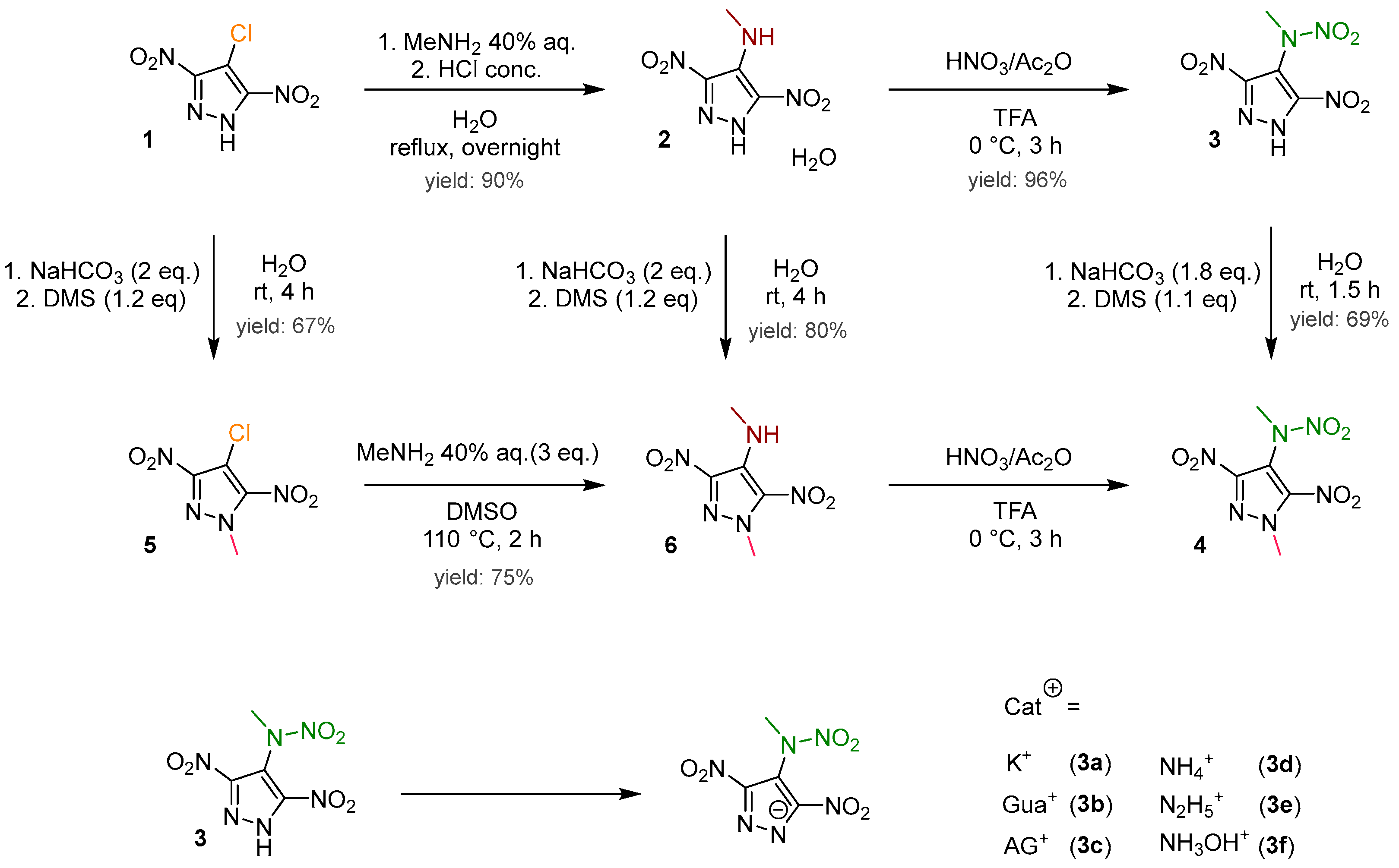
2.1. Synthesis
2.2. Crystal Structures
2.3. NMR Spectroscopy
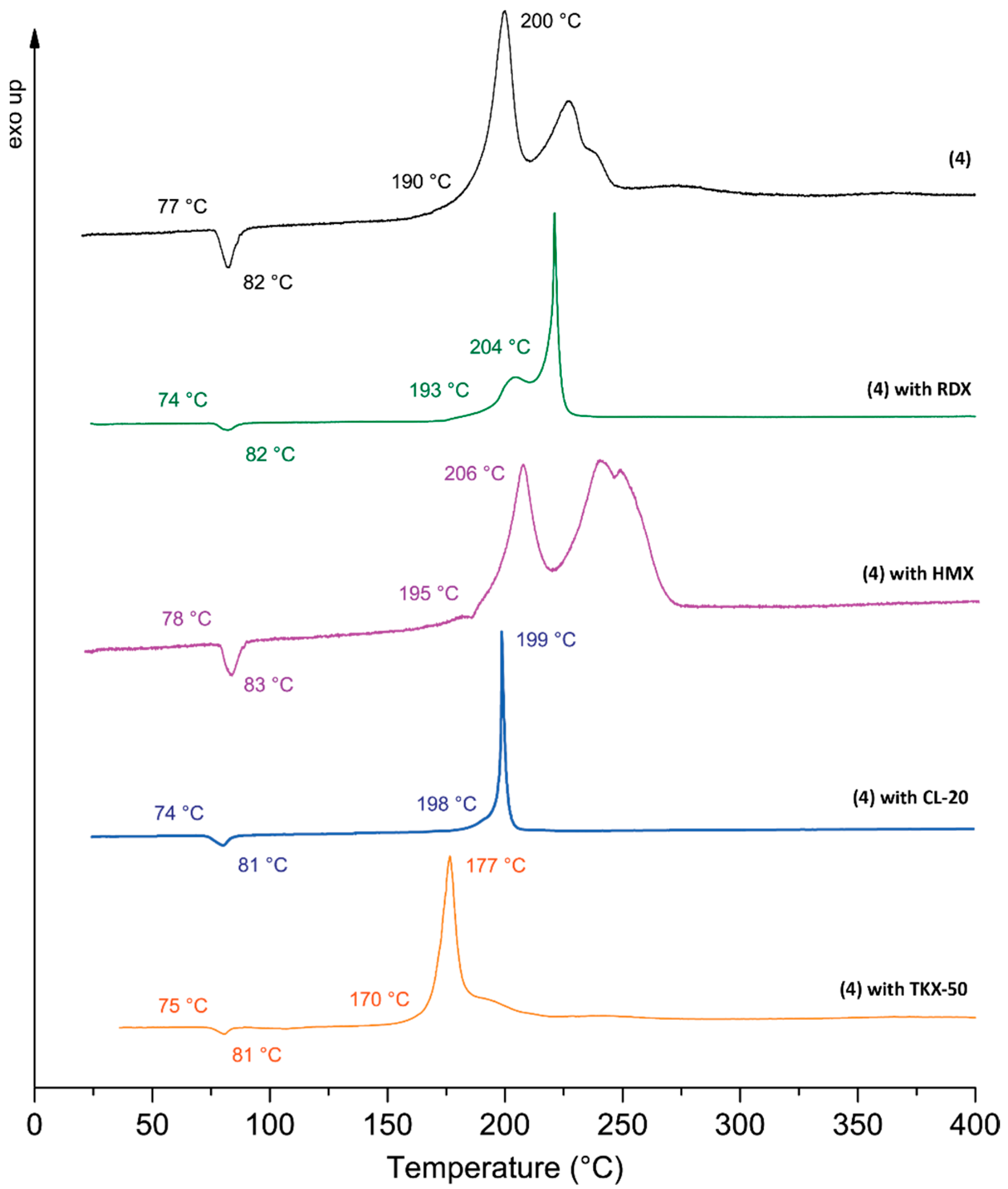
2.4. Physiochemical Properties
2.5. Small-Scale Shock Reactivity Test
2.6. Ames Test
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klapoetke, T.M. Chemistry of High-Energy Materials, 6th ed.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2022. [Google Scholar]
- Song, S.; Chen, F.; Wang, Y.; Wang, K.; Yan, M.; Zhang, Q. Accelerating the Discovery of Energetic Melt-Castable Materials by a High-Throughput Virtual Screening and Experimental Approach. J. Mater. Chem. A 2021, 9, 21723–21731. [Google Scholar] [CrossRef]
- Agrawal, J.P. High Energy Materials; Wiley: Weinheim, Germany, 2010. [Google Scholar]
- Urbański, T.; Laverton, S. Chemistry and Technology of Explosives; Macmillan: New York, NY, USA, 1964. [Google Scholar]
- Millar, R.W.; Anthony, A.W.; Hamid, J.; Endsor, R.M. Elimination of Redwater Formation from TNT Manufacture; QinetiQ: Farnborough, UK; Arlington, VA, USA, 2007. [Google Scholar]
- Freeman, D.; Colitti, O. Removal of Explosives from Load-Assemble-Pack Wastewater (Pink Water) Using Surfactant Technology. In Proceedings of the 36th Industrial Waste Conference, Purdue University, West Lafayette, IN, USA, 12–14 May 1981. [Google Scholar]
- Szopińska, M.; Prasuła, P.; Baran, P.; Kaczmarzyk, I.; Pierpaoli, M.; Nawała, J.; Szala, M.; Fudala-Książek, S.; Kamieńska-Duda, A.; Dettlaff, A. Efficient Removal of 2,4,6-Trinitrotoluene (TNT) from Industrial/Military Wastewater Using Anodic Oxidation on Boron-Doped Diamond Electrodes. Sci. Rep. 2024, 14, 4802. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, E.E.; Siele, V.I.; Chandler, C.D.; Mundy, R.A.; Spencer, J.R.; Gibson, G.R.; Bolleter, W.T. New Procedures for Purifying TNT. Propellants Explo. Pyrotec. 1982, 7, 150–154. [Google Scholar] [CrossRef]
- Larin, A.A.; Bystrov, D.M.; Fershtat, L.L.; Konnov, A.A.; Makhova, N.N.; Monogarov, K.A.; Meerov, D.B.; Melnikov, I.N.; Pivkina, A.N.; Kiselev, V.G.; et al. Nitro-, Cyano-, and Methylfuroxans, and Their Bis-Derivatives: From Green Primary to Melt-Cast Explosives. Molecules 2020, 25, 5836. [Google Scholar] [CrossRef] [PubMed]
- Ravi, P.; Badgujar, D.M.; Gore, G.M.; Tewari, S.P.; Sikder, A.K. Review on Melt Cast Explosives. Propellants Explo. Pyrotec. 2011, 36, 393–403. [Google Scholar] [CrossRef]
- Benz, M.; Delage, A.; Klapötke, T.M.; Kofen, M.; Stierstorfer, J. A Rocky Road toward a Suitable TNT Replacement—A Closer Look at Three Promising Azoles. Propellants Explo. Pyrotec. 2023, 48, e202300042. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Hwang, C.; Lu, K.; Yeh, T. Study on Thermal Characteristics of TNT Based Melt-Cast Explosives. Propellants Explo. Pyrotec. 2019, 44, 1270–1281. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, Z.; Lan, D.; Jia, Q.; Liu, N.; Zhang, J.; Kou, K. Recent Advances in Synthesis and Properties of Nitrated-Pyrazoles Based Energetic Compounds. Molecules 2020, 25, 3475. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, X.; Jiang, T.; Zhu, Y.; Zhou, L. Rheological Behavior of DNP/HMX Melt-Cast Explosives with Bimodal and Trimodal Particle-Size Distributions. Polymers 2023, 15, 1446. [Google Scholar] [CrossRef]
- Bölter, M.F.; Harter, A.; Klapötke, T.M.; Stierstorfer, J. Isomers of Dinitropyrazoles: Synthesis, Comparison and Tuning of Their Physicochemical Properties. ChemPlusChem 2018, 83, 804–811. [Google Scholar] [CrossRef]
- Kuehl, V.A.; Cleveland, A.H.; Snyder, C.J.; Chavez, D.E. Synthesis and Energetic Properties of N-Substituted 3,4- and 3,5-Dinitropyrazoles. ACS Omega 2023, 8, 18408–18413. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yang, Y.; Zhu, S.; Zhang, S.; Liu, Y.; Chen, Y. Anisotropic Shock Response of 3,4-Dinitropyrazole Revealed by First-Principles Calculations. J. Phys. Chem. A 2025, 129, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; He, C.; Gao, H.; Shreeve, J.M. Energized Nitro-Substituted Azoles through Ether Bridges. J. Mater. Chem. A 2015, 3, 15576–15582. [Google Scholar] [CrossRef]
- Reinhardt, E.; Lenz, T.; Bauer, L.; Stierstorfer, J.; Klapötke, T.M. Synthesis and Characterization of Azido- and Nitratoalkyl Nitropyrazoles as Potential Melt-Cast Explosives. Molecules 2023, 28, 6489. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Stierstorfer, J.; Reinhardt, E. Nitramine Pyrazole Energetic Compounds. WO 2023/072812 A1 Patent, 14 December 2023. [Google Scholar]
- Baus, U.; Reuther, W. Verfahren Zur Herstellung von 4-Chloropyrazolen 1990. DE 3840342 A1 Patent, 23 June 2021. [Google Scholar]
- Fischer, D.; Gottfried, J.L.; Klapötke, T.M.; Karaghiosoff, K.; Stierstorfer, J.; Witkowski, T.G. Synthesis and Investigation of Advanced Energetic Materials Based on Bispyrazolylmethanes. Angew. Chem. Int. Ed. 2016, 55, 16132–16135. [Google Scholar] [CrossRef]
- Dalinger, I.L.; Vatsadze, I.A.; Shkineva, T.K.; Popova, G.P.; Shevelev, S.A. Efficient Procedure for High-Yield Synthesis of 4-Substituted 3,5-Dinitropyra- Zoles Using 4-Chloro-3,5-Dinitropyrazole. Synthesis 2012, 44, 2058–2064. [Google Scholar] [CrossRef]
- Reinhardt, E.; Thamm, S.; Stierstorfer, J.; Klapötke, T.M. Alkyl-Bridged Nitropyrazoles—Adjustment of Performance and Sensitivity Parameters. Eur. J. Org. Chem. 2023, 26, e202300304. [Google Scholar] [CrossRef]
- Dalinger, I.L.; Vatsadze, I.A.; Shkineva, T.K.; Popova, G.P.; Shevelev, S.A. The Specific Reactivity of 3,4,5-Trinitro-1H-Pyrazole. Mendeleev Commun. 2010, 20, 253–254. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Penger, A.; Pflüger, C.; Stierstorfer, J. Melt-Cast Materials: Combining the Advantages of Highly Nitrated Azoles and Open-Chain Nitramines. New J. Chem. 2016, 40, 6059–6069. [Google Scholar] [CrossRef]
- Bauer, L.; Benz, M.; Klapötke, T.M. Linear Correlation Between Confined Explosive Quantity and Dent Volume of an Underlying Aluminium Block Using the SSRT Setup. Propellants Explo. Pyrotec. 2022, 47, e202100332. [Google Scholar] [CrossRef]
- CrysAlisPro Software, Rigaku Corporation: Wroclaw, Poland, 2009. Available online: http://www.rigaku.com (accessed on 25 May 2025).
- Sheldrick, G.M. SHELXT Integrated space-group and structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H.J. OLEX2: A Complete Structure Solution, Refinement and Analysis Programm. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- SCALE3 ABSPACK—An Oxford Diffraction Program, Version 1.0.4; Rigaku Corporation: Wroclaw, Poland, 2009. Available online: http://www.rigaku.com (accessed on 25 May 2025).
- APEX3, Version 2018.7-2; Bruker AXS Inc.: Madison, WI, USA, 2018. Available online: http://www.bruker.com (accessed on 25 May 2025).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.04; Gaussian, Inc.: Wallingford, CT, USA, 2016.
- Montgomery, J.A.; Frisch, M.J.; Ochterski, J.W.; Petersson, G.A. A complete basis set model chemistry. VII. Use of the minimum population localization method. J. Chem. Phys. 2000, 12, 6532–6542. [Google Scholar]
- Curtiss, L.A.; Raghavachari, K.; Redfern, P.C.; Pople, J.A. Assessment of Gaussian-2 and density functional theories for the computation of enthalpies of formation. J. Chem. Phys. 1997, 106, 1063–1079. [Google Scholar] [CrossRef]
- Rice, B.M.; Pai, S.V.; Hare, J. Predicting heats of formation of energetic materials using quantum mechanical calculations. Combust. Flame 1999, 118, 445–458. [Google Scholar] [CrossRef]
- Byrd, E.F.C.; Rice, B.M. Improved Prediction of Heats of Formation of Energetic Materials Using Quantum Mechanical Calculations. J. Phys. Chem. A 2006, 110, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Linstrom, J.; Mallard, W.G. National Institute of Standards and Technology Standard Reference Database Number 69. Available online: http://www.webbook.nist.gov/chemistry (accessed on 25 May 2025).
- Trouton, F. IV. On molecular latent heat. Philosophical Magazine Series 5 1884, 18, 54–57. [Google Scholar] [CrossRef]
- Westwell, M.S.; Searle, M.S.; Wales, D.J.; Williams, D.H. Empirical Correlations between Thermodynamic Properties and Intermolecular Forces. J. Am. Chem. Soc. 1995, 117, 5013–5015. [Google Scholar] [CrossRef]
- Rossini, F.D. Experimental Thermochemistry: Measurement of Heats of Reaction; Interscience Publishers Inc.: New York, NY, USA, 1956. [Google Scholar]
- Paulechka, E.; Riccardi, D. Combustion Calorimetry Tool—NIST Standard Reference Database 206; National Institute of Standard and Technology: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Koehler, J.; Meyer, R.; Homburg, A. Explosivstoffe, 6th ed.; Wiley: Weinheim, Germany, 2007. [Google Scholar]
- STANAG 4147; Chemical Compatibility of Ammunition Components with Explosives (Non-Nuclear Application). NATO: Brussels, Belgium, 2001.
- Bienta. Available online: https://bienta.net (accessed on 25 May 2025).
- Bundesanstalt für Materialforschung und -Prüfung. Available online: http://www.bam.de (accessed on 25 May 2025).
- STANAG 4489; Explosives, Impact Sensitivity Tests. NATO: Brussels, Belgium, 1999.
- Standardarbeitsanweisung 4-5.1.02; Ermittlung der Explosionsgefährlichkeit, hier der Schlagempfindlichkeit mit dem Fallhammer. WIWEB: Erding, Germany, 2002.
- STANAG 4487; Explosives, Friction Sensitivity Tests. NATO: Brussels, Belgium, 2002.
- Standardarbeitsanweisung 4-5.1.03; Ermittlung der Explosionsgefährlichkeit oder der Reibeempfindlichkeit mit dem Reibeapparat. WIWEB: Erding, Germany, 2002.
- OZM Research. Available online: http://www.ozm.cz (accessed on 25 May 2025).
- Impact: Insensitive > 40 J, Less Sensitive ≤35 J, Sensitive ≤4 J, Very Sensitive ≤3 J; Friction: Insensitive > 360 N, Less Sensitive = 360 N, Sensitive 80 N, Very Sensitive ≤80 N, Extreme Sensitive ≤10 N; According to the UN Recommendations on the Transport of Dangerous Goods (+) Indicates: Not Safe for Transport. Available online: https://www.un-ilibrary.org/content/books/9789210019057 (accessed on 25 May 2025).
- Dalinger, I.L.; Vatsadze, I.A.; Shkineva, T.K.; Papova, G.P.; Shevelev, S.A.; Nelyubina, Y.V. Synthesis and Comparison of the Reactivity of 3,4,5-1H-Trinitropyrazole and Its N-Methyl Derivative. J. Heterocycl. Chem. 2013, 50, 911–924. [Google Scholar] [CrossRef]




| IS [a] [J] | FS [b] [N] | Tendo [c] [°C] | Texo [d] [°C] | Ρ [e] [g cm−3] | ΔfH° [f] [kJ∙mol−1] | DC-J [g] [m∙s−1] | pC-J [h] [GPa] | |
|---|---|---|---|---|---|---|---|---|
| 3a | 6 | 80 | / | 217 | 1.906 | −446.5 | 7296 | 21.7 |
| 3b | >40 | >360 | 140 | 158 | 1.638 | 60.0 | 7719 | 22.7 |
| 3c | 8 | 168 | 116 | 148 | 1.628 | 165.7 | 7848 | 23.3 |
| 3d | 3 | 192 | / | 169 | 1.681 | 101.9 | 8129 | 26.8 |
| 3e | 5 | 120 | / | 165 | 1.660 | 248.5 | 8261 | 27.3 |
| 3f | 5 | 192 | / | 147 | 1.724 | 157.7 | 8470 | 30.5 |
| 3 | 8 | 144 | / | 157 | 1.725 | 145 | 8228 | 28.8 |
| 6 | >40 | >360 | 133 | 203 | 1.629 | 76.1 | 7273 | 19.6 |
| 4 | 15 | >360 | 77 | 190 | 1.648 | 135.2/106.4 [j] | 7721/7682 [j] | 24.1/23.8 [j] |
| TNT [i] | 15 | >360 | 81 | 289 | 1.65 | −185 | 6950 | 20.5 |
| DNAN [26] | >40 | >360 | 94 | 315 | 1.59 | −177 | 6705 | 16.1 |
| 4 | PETN | TNT | |
|---|---|---|---|
| Dent−Volume [mm3] | 1037.34 | 1107.81 | 845.89 |
| m [g] [a] | 443 | 478 | 443 |
| ρ [g cm−3] [b] | 1.648 | 1.778 | 1.648 |
| pC-J [GPa] [c] | 24.1 [e] | 30.8 [e] | 19.4 [e] |
| Vdet [m s−1] [d] | 7721 [e] | 8429 [e] | 6839 [e] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reinhardt, E.; Bauer, L.; Stadler, A.H.; Wilke, H.R.; Delage, A.; Stierstorfer, J.; Klapötke, T.M. Development of Melt-Castable Explosive: Targeted Synthesis of 3,5-Dinitro-4-Methylnitramino-1-Methylpyrazole and Functional Derivatization of Key Intermediates. Molecules 2025, 30, 2796. https://doi.org/10.3390/molecules30132796
Reinhardt E, Bauer L, Stadler AH, Wilke HR, Delage A, Stierstorfer J, Klapötke TM. Development of Melt-Castable Explosive: Targeted Synthesis of 3,5-Dinitro-4-Methylnitramino-1-Methylpyrazole and Functional Derivatization of Key Intermediates. Molecules. 2025; 30(13):2796. https://doi.org/10.3390/molecules30132796
Chicago/Turabian StyleReinhardt, Elena, Lukas Bauer, Antonia H. Stadler, Henrik R. Wilke, Arthur Delage, Jörg Stierstorfer, and Thomas M. Klapötke. 2025. "Development of Melt-Castable Explosive: Targeted Synthesis of 3,5-Dinitro-4-Methylnitramino-1-Methylpyrazole and Functional Derivatization of Key Intermediates" Molecules 30, no. 13: 2796. https://doi.org/10.3390/molecules30132796
APA StyleReinhardt, E., Bauer, L., Stadler, A. H., Wilke, H. R., Delage, A., Stierstorfer, J., & Klapötke, T. M. (2025). Development of Melt-Castable Explosive: Targeted Synthesis of 3,5-Dinitro-4-Methylnitramino-1-Methylpyrazole and Functional Derivatization of Key Intermediates. Molecules, 30(13), 2796. https://doi.org/10.3390/molecules30132796








