Portable and Rapid Smartphone-Based Colorimetric Assay of Peracetic Acid for Point-of-Use Medical/Pharmaceutical Disinfectant Preparation
Abstract
1. Introduction
2. Results and Discussion
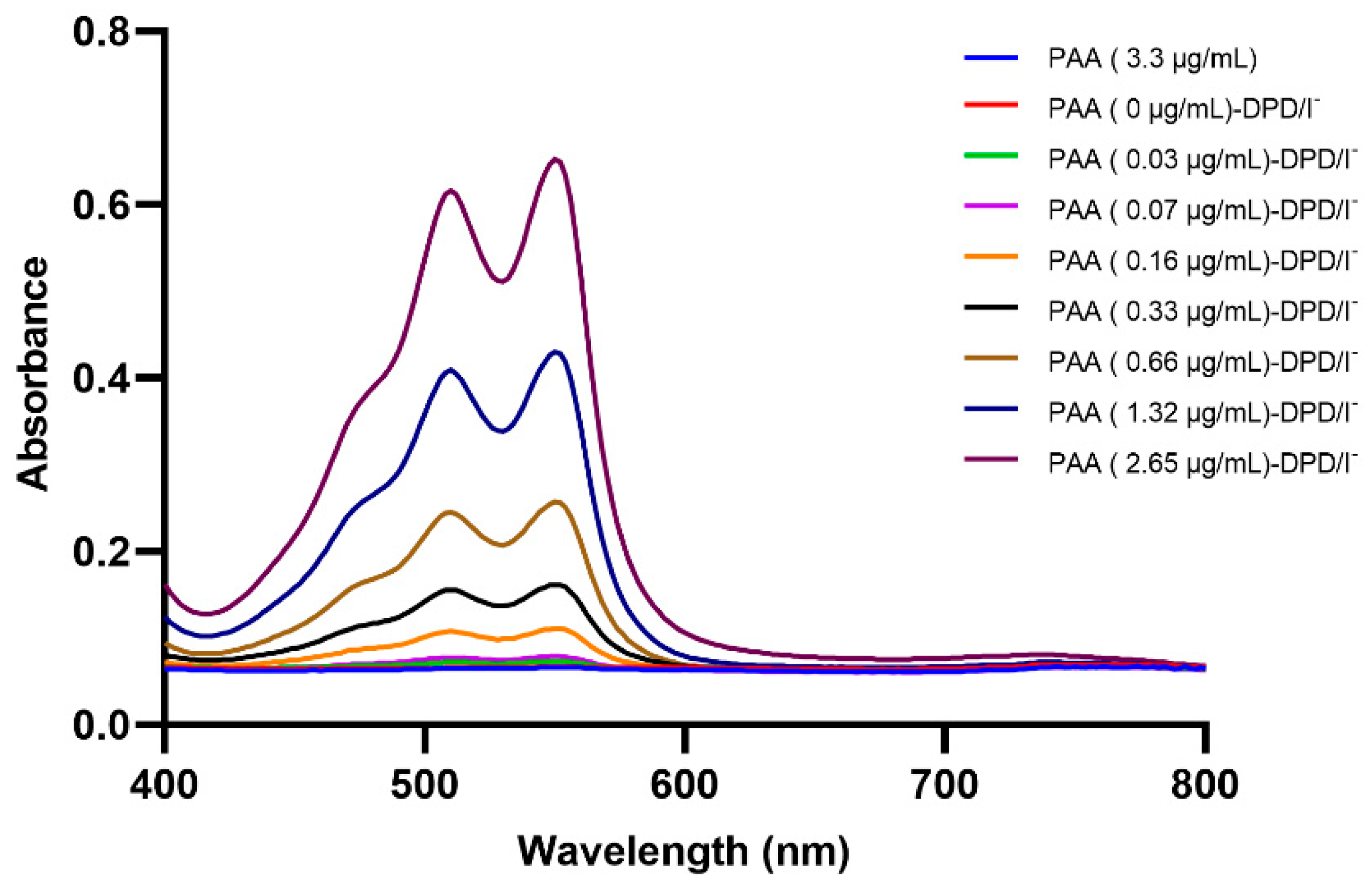
2.1. Spectrophotometric and Image Processing Investigation of the DPD-PAA Reaction
2.2. Investigation of the Optimum Conditions for PAA Determination
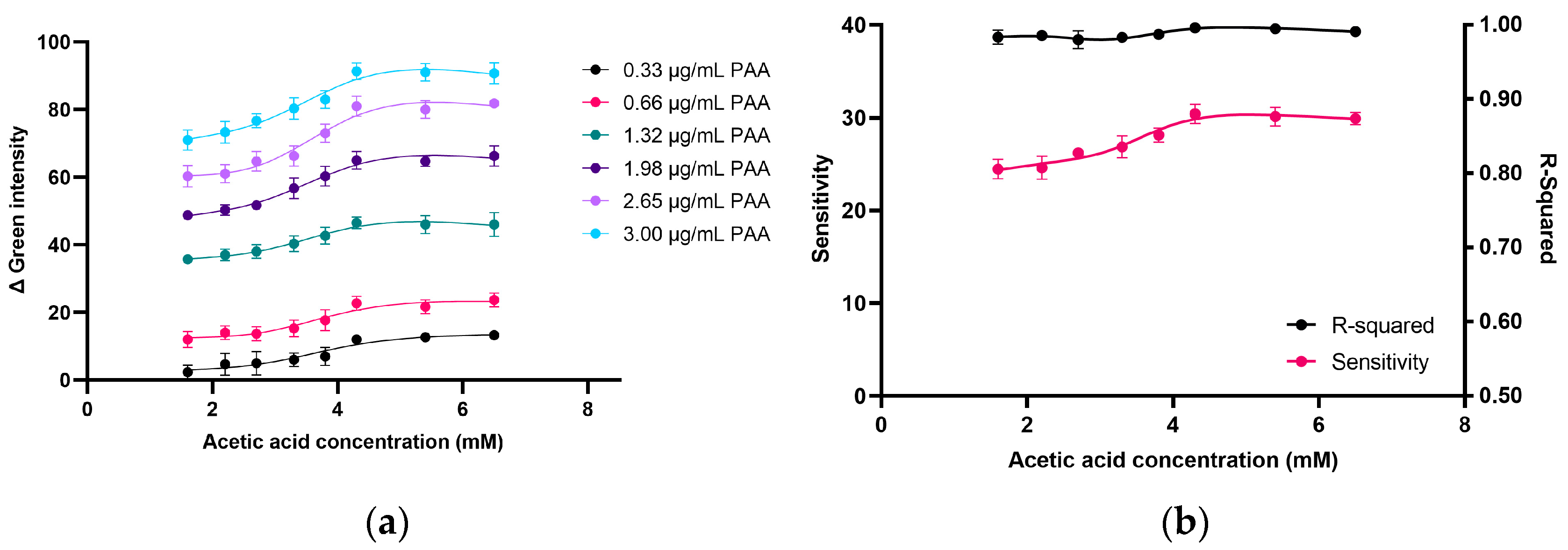
2.2.1. Effect of AcOH Concentration on Product Formation in PAA Determination
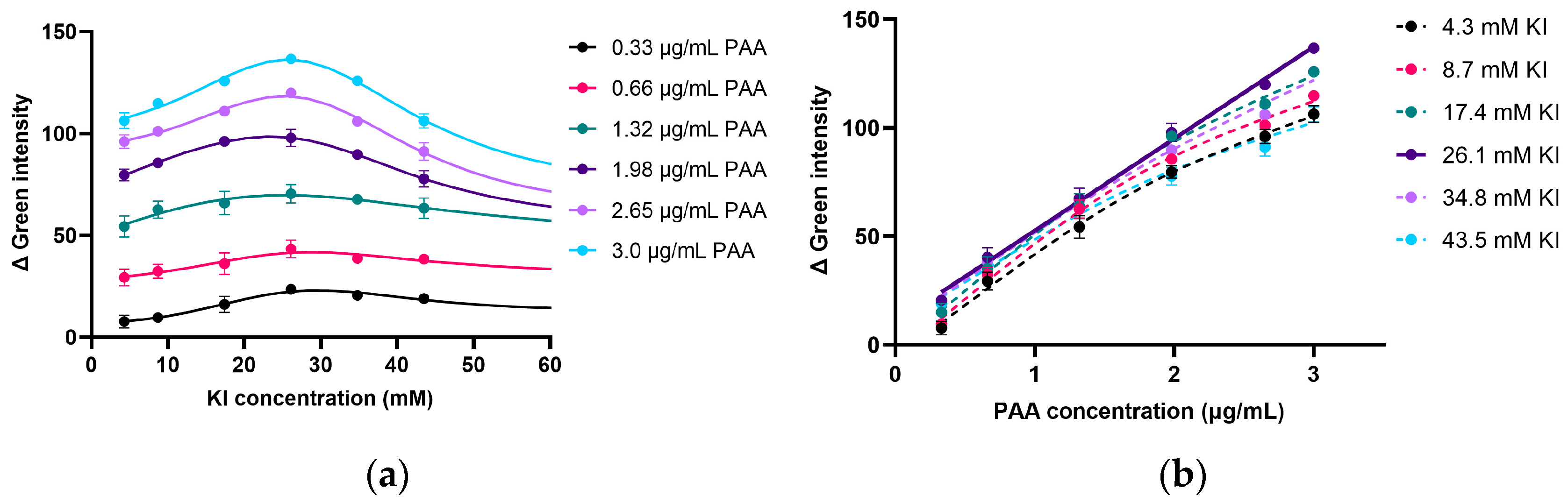
2.2.2. Effect of I− Concentration on Product Formation in PAA Determination
2.2.3. Effect of DPD Concentration on Product Formation
2.2.4. Investigation of the Period of DPD Oxidation by PAA
2.3. Validation of the Custom-Built Mobile Application
2.4. Analytical Characteristics of the Proposed Approach for PAA Determination
2.4.1. Linear Regression Analysis, Detection Limit, and Quantitation Limit
2.4.2. Accuracy and Precision
2.4.3. Investigation of Interference of Active Compounds in Medical Formulations
2.5. Application in Medical Disinfectant Formulations as an Initial Model
2.6. Green and Sustainable Assessment
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Spectrophotometric Investigation of DPD-PAA Reaction
3.3. Proposed Analytical Procedure
3.4. Development and Validation of the Custom-Built Mobile App
3.5. Reference Method and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AcOH | Acetic acid |
| ABTS | bis(3-ethylbenzothiazoline-6-sulfonate) |
| DPD | N,N-diethyl-p-phenylenediamine |
| KHP | Potassium hydrogen phthalate |
| PAA | Peracetic acid |
| LODs | Limits of detection |
| LOQs | Limits of quantitation |
| AGREE | Analytical Greenness |
| ComplexGAPI | Complementary Green Analytical Procedure Index |
| BAGI | Blue Applicability Grade Index |
| NQS index | Need, Quality, and Sustainability Index |
| IPC | Infection Prevention and Control |
| RSDs | Relative Standard Deviations |
| SDGs | Sustainable Development Goals |
References
- U.S. Environmental Protection Agency. Peracetic Acid—Alternative Disinfection Methods Fact Sheet. Available online: https://www.epa.gov/sustainable-water-infrastructure/peracetic-acid-alternative-disinfection-methods-fact-sheet (accessed on 25 March 2025).
- Centers for Disease Control and Prevention. Guideline for Disinfection and Sterilization in Healthcare Facilities (2008). Available online: https://www.cdc.gov/infection-control/hcp/disinfection-and-sterilization/index.html (accessed on 25 March 2025).
- Regulation (EU) No 528/2012 Concerning the Making Available on the Market and Use of Biocidal Products; European Union: Brussels, Belgium, 2016.
- Morris, J.N.; Esseili, M.A. Efficacy of peracetic acid and sodium hypochlorite against SARS-CoV-2 on contaminated surfaces. Appl. Environ. Microbiol. 2023, 89, e00622–e00623. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, Z.; Wang, H. Combination of ultrasound-peracetic acid washing and ultrasound-assisted aerosolized ascorbic acid: A novel rinsing-free disinfection method that improves the antibacterial and antioxidant activities in cherry tomato. Ultrason. Sonochem. 2022, 86, 106001. [Google Scholar] [CrossRef] [PubMed]
- Jaleta, M.; Junker, V.; Kolte, B.; Börger, M.; Werner, D.; Dolsdorf, C.; Schwenker, J.; Hölzel, C.; Zentek, J.; Amon, T.; et al. Improvements of weaned pigs barn hygiene to reduce the spread of antimicrobial resistance. Front. Microbiol. 2024, 15, 1393923. [Google Scholar] [CrossRef]
- Sode, F. Simultaneous determination of peracetic acid and acetic acid by titration with NaOH. Anal. Methods 2014, 6, 2406–2409. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, C.-H. Simultaneous quantification of peracetic acid and hydrogen peroxide in different water matrices using HPLC-UV. Chemosphere 2020, 257, 127229. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, T.; Sun, X.; Zhou, A.; Li, M. Determination of peracetic acid by an Ag nanoparticle decorated Cu-organic framework modified electrode. Microchem. J. 2021, 171, 106856. [Google Scholar] [CrossRef]
- Lim, D.S.; Lee, Y.J.; Yoo, J.H.; Choi, M.G.; Paek, K.; Koh, H.R.; Chang, S.-K. Fluorometric analysis of peracetic acid by the oxidative hydroxylation of a phenylboronic acid containing dye. Sens. Actuators B 2019, 298, 126824. [Google Scholar] [CrossRef]
- Binder, W.; Menger, F. Assay of peracid in the presence of excess hydrogen peroxide. Anal. Lett. 2000, 33, 479–488. [Google Scholar] [CrossRef]
- Domínguez-Henao, L.; Turolla, A.; Monticelli, D.; Antonelli, M. Assessment of a colorimetric method for the measurement of low concentrations of peracetic acid and hydrogen peroxide in water. Talanta 2018, 183, 209–215. [Google Scholar] [CrossRef]
- Stastny, A.L.; Doepke, A.; Streicher, R.P. A field-portable colorimetric method for the measurement of peracetic acid vapors: A comparison of glass and plastic impingers. J. Occup. Environ. Hyg. 2022, 19, 469–477. [Google Scholar] [CrossRef]
- CHEMetrics LLC. Peracetic Acid SAM Kit. Available online: https://www.chemetrics.com/product/peracetic-acid-sam-kit-i-2020/ (accessed on 23 March 2025).
- CHEMetrics LLC. Peracetic Acid Test Kits. Available online: https://www.chemetrics.com/product-category/test-kits/peracetic-acid/ (accessed on 23 March 2025).
- Katib, S.; Apichai, S.; Pattananandecha, T.; Jiaranaikulwanitch, J.; Sirithunyalug, B.; Grudpan, K.; Saenjum, C. Development of a sustainable procedure for smartphone-based colorimetric determination of benzalkonium chloride in pharmaceutical preparations. Heliyon 2024, 10, e28965. [Google Scholar] [CrossRef] [PubMed]
- Peracetic Acid (CAS No. 79-21-0) and Its Equilibrium Solutions; European Centre for Ecotoxicology and Toxicology of Chemicals: Brussels, Belgium, 2001.
- Cheng, C.; Li, H.; Wang, J.; Wang, H.; Yang, X. A review of measurement methods for peracetic acid (PAA). Front. Environ. Sci. Eng. 2020, 14, 87. [Google Scholar] [CrossRef]
- Quick, R. BioSide HS15%-1. Evaluation of the Envirotech Chemical Services, Inc., Submission for Peroxyacetic Acid Wastewater EUP; U.S. Environmental Protection Agency: Washington, DC, USA, 2006.
- Chen, S.; He, L.; Zou, J.; Yang, S.; Chen, L.; Hou, Y.; Xu, D.; Yan, Z.; Li, S.; Wu, J. Rapid and selective spectrophotometric determination of peracetic acid in water via oxidative coloration of N,N-diethyl-p-phenylenediamine with the assistance of Co(II). J. Environ. Chem. Eng. 2025, 13, 115214. [Google Scholar] [CrossRef]
- Chhetri, R.K.; Kaarsholm, K.M.S.; Andersen, H.R. Colorimetric quantification methods for peracetic acid together with hydrogen peroxide for water disinfection process control. Int. J. Environ. Res. Public Health 2020, 17, 4656. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cai, H.; Zou, J.; Pang, Z.; Yuan, B.; Zhou, Z.; Cheng, Q. Spectrophotometric determination of trace permanganate in water with N,N-diethyl-p-phenylenediamine (DPD). Chemosphere 2018, 212, 604–610. [Google Scholar] [CrossRef]
- Li, T.; Dai, L.; Huang, Y.; Pan, S.; Pang, Z.; Zou, J. Spectrophotometric determination of Cr(VI) in water using N,N-diethyl-p-phenylenediamine (DPD) as the indicator. J. Environ. Chem. Eng. 2021, 9, 105517. [Google Scholar] [CrossRef]
- Awad, M.I.; Oritani, T.; Ohsaka, T. Kinetic studies on the oxidation of iodide by peroxyacetic acid. Inorg. Chim. Acta 2003, 344, 253–256. [Google Scholar] [CrossRef]
- Kim, G.-H.; Jung, J.-Y.; Lim, H.-J.; Jung, S.M.; Kwon, J.-H. Photometric determination of peracetic acid by reaction with potassium iodide solution. Anal. Sci. 2023, 39, 383–388. [Google Scholar] [CrossRef]
- Cavallini, G.S.; de Campos, S.X.; de Souza, J.B.; Vidal, C.M.d.S. Comparison of methodologies for determination of residual peracetic acid in wastewater disinfection. Int. J. Environ. Anal. Chem. 2013, 93, 906–918. [Google Scholar] [CrossRef]
- Pinkernell, U.; Lüke, H.-J.; Karst, U. Selective photometric determination of peroxycarboxylic acids in the presence of hydrogen peroxide. Analyst 1997, 122, 567–571. [Google Scholar] [CrossRef]
- Yuan, N.; Li, H.; Qian, J. Determination of peracetic acid in the presence of hydrogen peroxide based on the catalytic oxidation of ABTS. Chem. Eng. J. Adv. 2022, 10, 100247. [Google Scholar] [CrossRef]
- Lin, J.; Xiao, J.; Cai, H.; Huang, Y.; Li, J.; Yang, H.; Li, T.; Zou, J. Multi-wavelength spectrophotometric determination of peracetic acid and the coexistent hydrogen peroxide via oxidative coloration of ABTS with the assistance of Fe2+ and KI. Chemosphere 2022, 287, 132242. [Google Scholar] [CrossRef] [PubMed]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness metric approach and software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Wojnowski, W. Complementary green analytical procedure index (ComplexGAPI) and software. Green Chem. 2021, 23, 8657–8665. [Google Scholar] [CrossRef]
- Manousi, N.; Wojnowski, W.; Płotka-Wasylka, J.; Samanidou, V. Blue applicability grade index (BAGI) and software: A new tool for the evaluation of method practicality. Green Chem. 2023, 25, 7598–7604. [Google Scholar] [CrossRef]
- Kiwfo, K.; Suteerapataranon, S.; McKelvie, I.D.; Meng Woi, P.; Kolev, S.D.; Saenjum, C.; Christian, G.D.; Grudpan, K. A new need, quality, and sustainability (NQS) index for evaluating chemical analysis procedures using natural reagents. Microchem. J. 2023, 193, 109026. [Google Scholar] [CrossRef]




| Detection Technique | Chromogenic Agent | Range (µg/mL) | R2 | LOD (µg/mL) | LOQ (µg/mL) | References |
|---|---|---|---|---|---|---|
| Image processing | DPD | 0.15–3.0 | 0.9974 | 0.11 | 0.34 | Proposed approach |
| Spectrophotometric method | DPD | 0.10–1.65 | 0.9967 | - | - | [12] |
| 0.125–2.5 | 0.9944 | 0.0015 | 0.0025 | [21] | ||
| ABTS | 0.04–0.76 | 0.9999 | 0.0008 | - | [28] | |
| 0.16–1.2 | 0.999 | 0.0023 | - | [29] |
| PAA Concentration Spiked (µg/mL) | Repeatability | Intermediate Precision | ||||||
|---|---|---|---|---|---|---|---|---|
| Day1 | Day2 | Day3 | ||||||
| %Recovery | %RSD | %Recovery | %RSD | %Recovery | %RSD | %Recovery | %RSD | |
| 0.5 | 98 ± 5 | 5 | 105 ± 4 | 3 | 101 ± 4 | 4 | 102 ± 4 | 3 |
| 1.0 | 99 ± 2 | 2 | 94 ± 3 | 3 | 102 ± 2 | 2 | 98 ± 4 | 4 |
| 1.5 | 100 ± 5 | 5 | 102 ± 5 | 5 | 101 ± 2 | 2 | 101 ± 1 | 1 |
| 2.0 | 104 ± 5 | 5 | 102 ± 5 | 5 | 100 ± 1 | 1 | 102 ± 3 | 2 |
| 2.5 | 104 ± 1 | 1 | 99 ± 4 | 4 | 97 ± 1 | 1 | 100 ± 4 | 4 |
| Major Interfering Substances in Disinfectant | %Recovery |
|---|---|
| Iodine | 95 ± 1 |
| Chlorhexidine | 92 ± 1 |
| Hypochlorite | 94 ± 3 |
| Hydrogen peroxide | 97 ± 1 |
| Acetic acid | 94 ± 1 |
| Benzalkonium chloride | 102 ± 3 |
| DDAC 1 | 93 ± 2 |
| No. | Titration | Proposed Approach | ||
|---|---|---|---|---|
| PAA Concentration (%) | %RSD | PAA Concentration (%) | %RSD | |
| S1 | 0.14 ± 0.000 | 0.0 | 0.16 ± 0.002 | 1.5 |
| S2 | 0.15 ± 0.001 | 0.7 | 0.16 ± 0.004 | 2.4 |
| S3 | 0.18 ± 0.003 | 1.7 | 0.18 ± 0.006 | 3.2 |
| S4 | 5.84 ± 0.003 | 0.1 | 5.81 ± 0.120 | 2.1 |
| S5 | 4.56 ± 0.000 | 0.0 | 4.44 ± 0.093 | 2.1 |
| S6 | 0.15 ± 0.000 | 0.0 | 0.15 ± 0.002 | 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katib, S.; Apichai, S.; Jiaranaikulwanitch, J.; Sirithunyalug, B.; Ogata, F.; Kawasaki, N.; Grudpan, K.; Saenjum, C. Portable and Rapid Smartphone-Based Colorimetric Assay of Peracetic Acid for Point-of-Use Medical/Pharmaceutical Disinfectant Preparation. Molecules 2025, 30, 2798. https://doi.org/10.3390/molecules30132798
Katib S, Apichai S, Jiaranaikulwanitch J, Sirithunyalug B, Ogata F, Kawasaki N, Grudpan K, Saenjum C. Portable and Rapid Smartphone-Based Colorimetric Assay of Peracetic Acid for Point-of-Use Medical/Pharmaceutical Disinfectant Preparation. Molecules. 2025; 30(13):2798. https://doi.org/10.3390/molecules30132798
Chicago/Turabian StyleKatib, Suphakorn, Sutasinee Apichai, Jutamas Jiaranaikulwanitch, Busaban Sirithunyalug, Fumihiko Ogata, Naohito Kawasaki, Kate Grudpan, and Chalermpong Saenjum. 2025. "Portable and Rapid Smartphone-Based Colorimetric Assay of Peracetic Acid for Point-of-Use Medical/Pharmaceutical Disinfectant Preparation" Molecules 30, no. 13: 2798. https://doi.org/10.3390/molecules30132798
APA StyleKatib, S., Apichai, S., Jiaranaikulwanitch, J., Sirithunyalug, B., Ogata, F., Kawasaki, N., Grudpan, K., & Saenjum, C. (2025). Portable and Rapid Smartphone-Based Colorimetric Assay of Peracetic Acid for Point-of-Use Medical/Pharmaceutical Disinfectant Preparation. Molecules, 30(13), 2798. https://doi.org/10.3390/molecules30132798







