2.1. Cross-Linking Kinetics Analysis
Assessing vulcanization kinetics provides important insights into the behavior of elastomers during cross-linking. Rheometers measure torque growth over time, indicating the progress of the cross-linking reaction. Parameters such as scorch time (t
02), curing time (t
90), and torque increment after selected time (ΔT) are analyzed to evaluate cure rate and cross-linking density. By testing these kinetics at different temperatures and with different curing systems, researchers can optimize processing conditions to achieve the desired mechanical and thermal properties of elastomeric compounds. The results of the vulcanization kinetics of the tested CR/BR blends are summarized in
Table 1. Analysis of these parameters allowed preliminary characterization of the resulting vulcanizates. The t
90 parameter represents the optimal curing time, indicating the duration required for the rubber compound to reach its maximum torque. A higher t
90 value implies a longer vulcanization process, consequently extending the overall processing duration. Moreover, scorch time (t
02) signifies the onset of vulcanization, marking the moment at which the curing process begins. This value is crucial for ensuring the safe processing of rubber before the vulcanization reaction becomes significant. Both parameters depend on temperature and curing system; therefore,
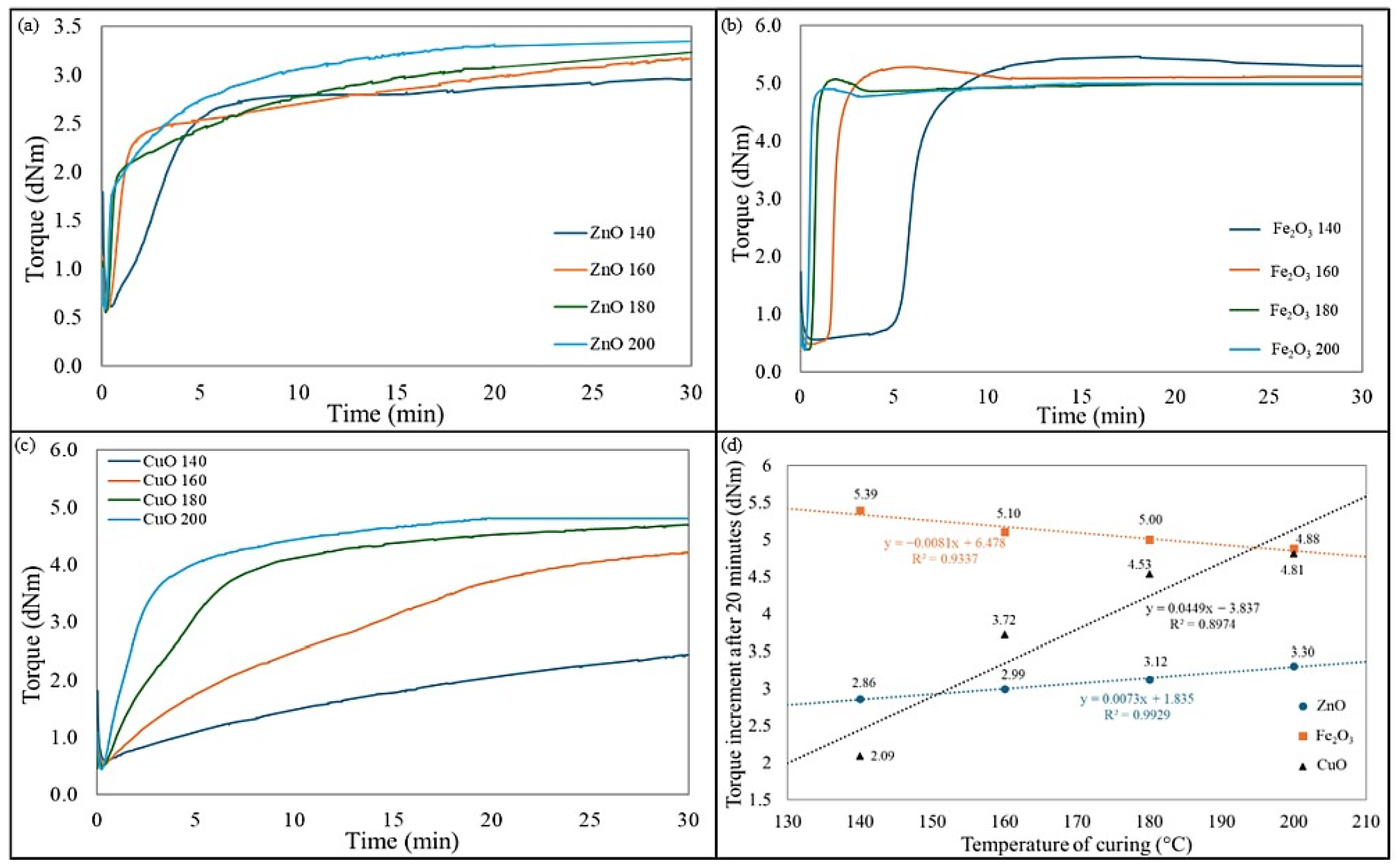
Figure 1 shows the effect of temperature and the cross-linking agent used on the scorch time and cure time values.
The use of zinc oxide in CR/BR blends allowed the achievement of great values of the torque increment in a short time. As the temperature increased, the scorch and vulcanization times decreased. It is worth noting that the CR/BR/ZnO blends were characterized by similar ΔT10 values. Moreover, it is worth pointing out that the higher the cross-linking temperature, the greater the torque increment. However, this growth is not as spectacular in the case of blends cross-linked with iron(III) oxide. For these blends, the higher temperature resulted in a shorter vulcanization time. It should be noted that blends cured at 140 °C achieved optimal vulcametric parameters and recorded the highest torque increments, which were responsible for a high degree of cross-linking. These compositions should be considered in the context of more sustainable vulcanization because the typical vulcanization temperature for rubber is 160 °C, but for this sample, the best properties were obtained when curing at 140 °C. This is an advantageous aspect in the context of less emissive elastomeric blends since a lower curing temperature means that less energy is required for the process. In the case of the CR/BR/CuO blends, the vulcanization time decreased with increasing temperature (from 36.9 min at 140 °C to 9 min at 200 °C). A similar trend was observed for the scorch time. Torque increments after 20 min also obtained greater values for cross-linking at higher temperatures, so using CuO as a curing agent was more effective at higher temperatures.
For the CR/BR/ZnO blends, a significant change in vulcanization time is noted between 160 °C and 180 °C. In the case of the CR/BR/Fe
2O
3 composites, a substantial difference occurred when cross-linking at temperatures of 160 °C and above, with an almost threefold reduction.
Figure 2 illustrates kinetics curves and changes in torque increment after 30 min under different curing temperatures and systems. Samples cross-linked with iron(III) oxide exhibited stable values, with the highest torque increment at 140 °C, while for other temperatures, a slight decrease was observed. In contrast, for samples cross-linked with ZnO or CuO, the opposite trend is noted (i.e., the higher the temperature, the greater the value of torque increment). For the CR/BR/ZnO blends, ΔT
20 increased from 2.86 dNm to 3.30 dNm. However, for samples cross-linked with CuO, a more than twofold increase in ΔT
20 values is observed, rising from 2.09 dNm to 4.81 dNm (
Figure 2d). Analyzing the behavior of the curves in
Figure 2a–c, it can be concluded that blends cross-linked with Fe
2O
3 do not need high temperatures to vulcanize optimally. The vulcametric curves for CR/BR/ZnO and CR/BR/CuO compositions vulcanized at 140, 160, 180, and 200 °C showed marching characteristics, while the CR/BR/Fe
2O
3 composition tended to reverse for all temperatures. It is also worth noting that the minimum torque (T
min), indicating the viscosity of tested CR/BR blends, did not depend significantly on the type of metal oxide used as a cross-linking agent. The T
min value for all compositions cured at 140 °C was 0.6 dNm. This parameter confirms that the viscosity of the CR/BR composites was comparable regardless of the curing substance used. The T
min values decreased slightly if the CR/BR blends were vulcanized at higher temperatures.
Table 2 summarizes the values of the activation energies of scorch or vulcanization calculated from the Arrhenius equation. The calculated values show that the cross-linking of blends was an exothermic reaction. The values of the activation energies of scorch and vulcanization were the lowest (E
scr.−54.28 kJ/mol, E
vulc.−21.23 kJ/mol) for the composition containing zinc oxide, which may be related to the smaller size of its particles and the lower energy requirement for the vulcanization process. For the composition cured with iron(III) oxide, the activation energies of scorch and vulcanization were close to each other, which means that once the energy barrier for scorch is exceeded, the blend begins to cross-link quickly (
Figure 2a–c), so this oxide was very active. In the case of the CR/BR/CuO blend, the value of the activation energy of the scorch is the highest, which means that a higher energy barrier needs to be crossed to start cross-linking. This is also confirmed by vulcametric curves.
2.2. Structural and Morphological Characterization of CR/BR Composites
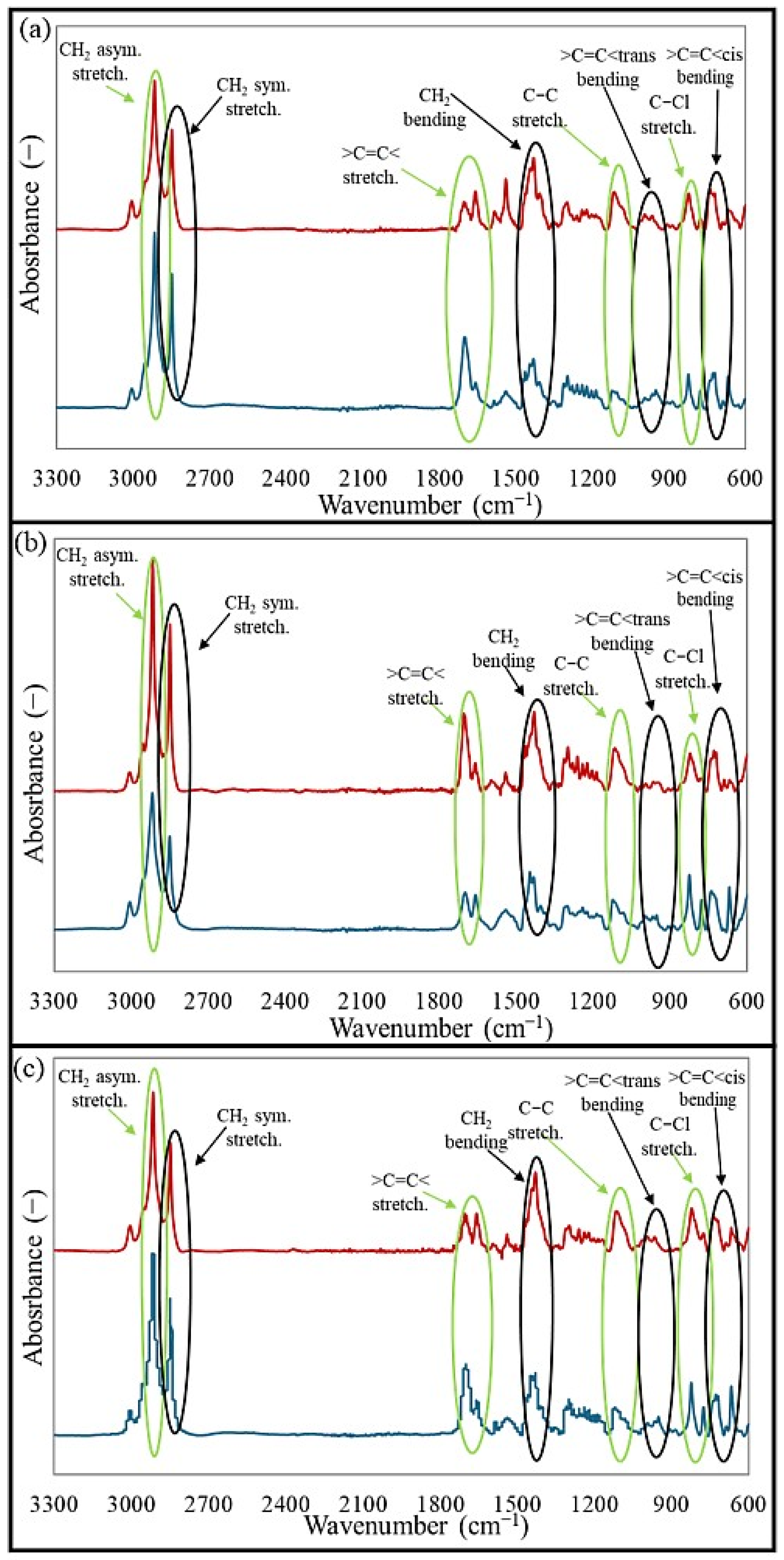
Fourier transform infrared spectroscopy (FT-IR) is widely used in polymer analysis because it provides valuable information on the chemical structure and composition of polymers. The method detects specific vibrations of molecular bonds in a polymer, enabling the identification of functional groups. In this study, the analysis of FT-IR spectra aims to determine whether any interelastomeric bonds were formed after vulcanization and to assess changes in the intensity of absorption signals. Fourier transform infrared spectroscopy was performed to analyze the structures of the studied blends and vulcanizates obtained during the cross-linking at 200 °C. In the obtained spectra, illustrated in
Figure 3, the intensity of the bands for both the blends and vulcanizates in the wavenumber range of 2945–2840 cm
−1 corresponded to the asymmetric and symmetric stretching of -CH
2- groups [
28,
29]. The intensities of vibrations in the region of 1660–1656 cm
−1 corresponded to >C=C< stretching [
30], which decreased for the CR/BR/ZnO and CR/BR/CuO samples cross-linked at 200 °C. This decrease may be related to the breaking of double bonds in macromolecular chains, followed by the formation of interelastomeric bonds. In the area of 1430–1420 cm
−1, the increase in band intensity, caused by changes in the structure of the vulcanizates, is associated with -CH
2- deformations [
31,
32]. An increase in absorption for the vulcanizates was observed in the range of 1123–1121 cm
−1, attributed to the stretching of C-C bonds in the main macromolecular chains [
33], which resulted from structural reorganization during the cross-linking with oxides at elevated temperatures. In the region of 996–950 cm
−1, bands corresponding to C-H deformation vibrations in the >CH=CH<
trans configuration were observed [
34,
35], while in the absorption range of 689–668 cm
−1, peaks characteristic of C-H deformation vibrations in the >CH=CH<
cis configuration were detected [
36,
37]. The increase in trans units in the studied vulcanizates is probably related to the formation of intermolecular bonds between butadiene rubber and chloroprene rubber. The reduction in absorption band intensity at 825–820 cm
−1 for vulcanizates (C-Cl stretching vibrations) is associated with elastomer cross-linking. FT-IR spectral analysis suggests that the type of oxide used for cross-linking does not significantly affect band intensity, indicating the formation of a comparable network structure [
32].
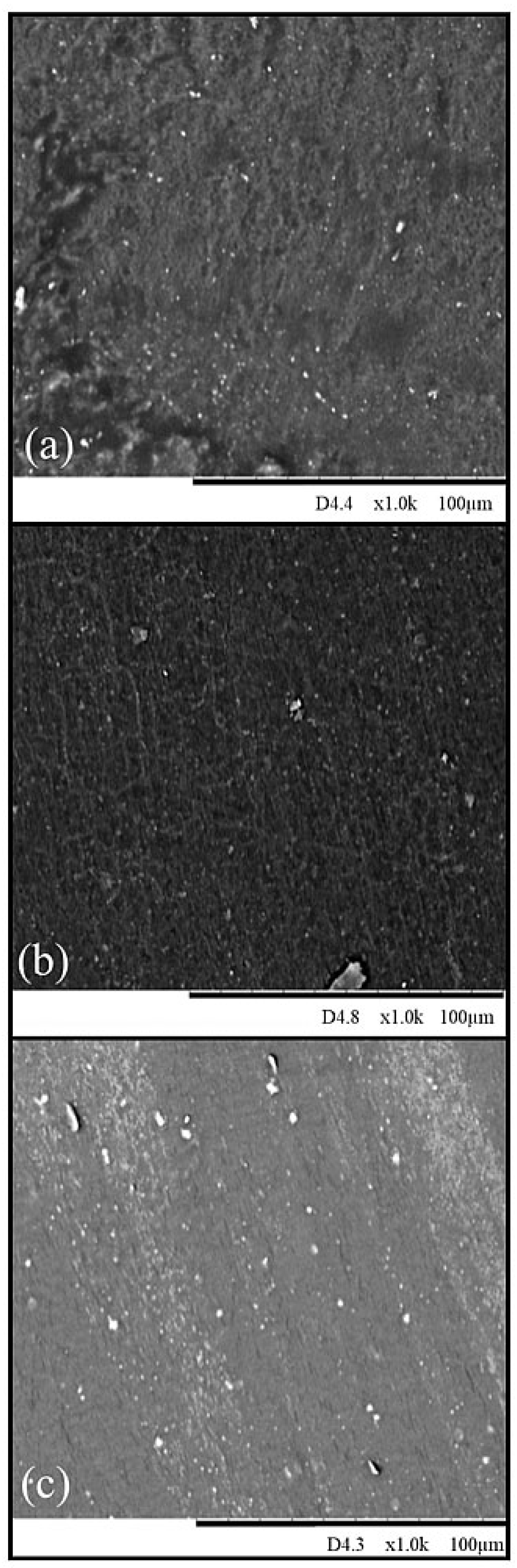
The study of the structure and morphology of materials is an important aspect of materials science. It involves understanding the composition and chemistry related with processing, micro- or nano-structure, and properties. Analysis of SEM images enabled checking the compatibility of non-polar butadiene rubber with polar chloroprene rubber and evaluating the effect of the cross-linking agent (ZnO, Fe
2O
3, and CuO) on the surface morphology of the vulcanizates. The SEM images of composites cross-linked with zinc oxide, iron(III) oxide, and copper(II) oxide at 200 °C are shown in
Figure 4 (at 1000× magnification). It is noted that the surfaces of the samples were homogeneous and there was no visible separation of the rubber phases, which indicates the activity of the cross-linking substances interacting between the two elastomers in the tested CR/BR blends. The surface of the sample cured with zinc oxide was rough and contained oxide clusters (dark fragments) (
Figure 4a). The composition containing iron(III) oxide had cracks on its surface (
Figure 4b). In contrast, the composition cross-linked with copper(II) oxide (
Figure 4c) had small and regularly spaced particles in the elastomeric matrix on its surface.
2.3. Analysis of the Degree of Cross-Linking Determined by the Equilibrium Swelling
Swelling properties are crucial for elastomeric products as they determine the material’s resistance to solvents, oils, and chemicals, directly affecting performance and durability. Excessive swelling can lead to softening, loss of mechanical strength, and dimensional instability, necessitating the optimization of cross-link density and curing conditions. Swelling behavior analysis helps predict long-term stability, chemical resistance, and compatibility with different environments, which is particularly important for seals, gaskets, fuel hoses, and industrial applications.
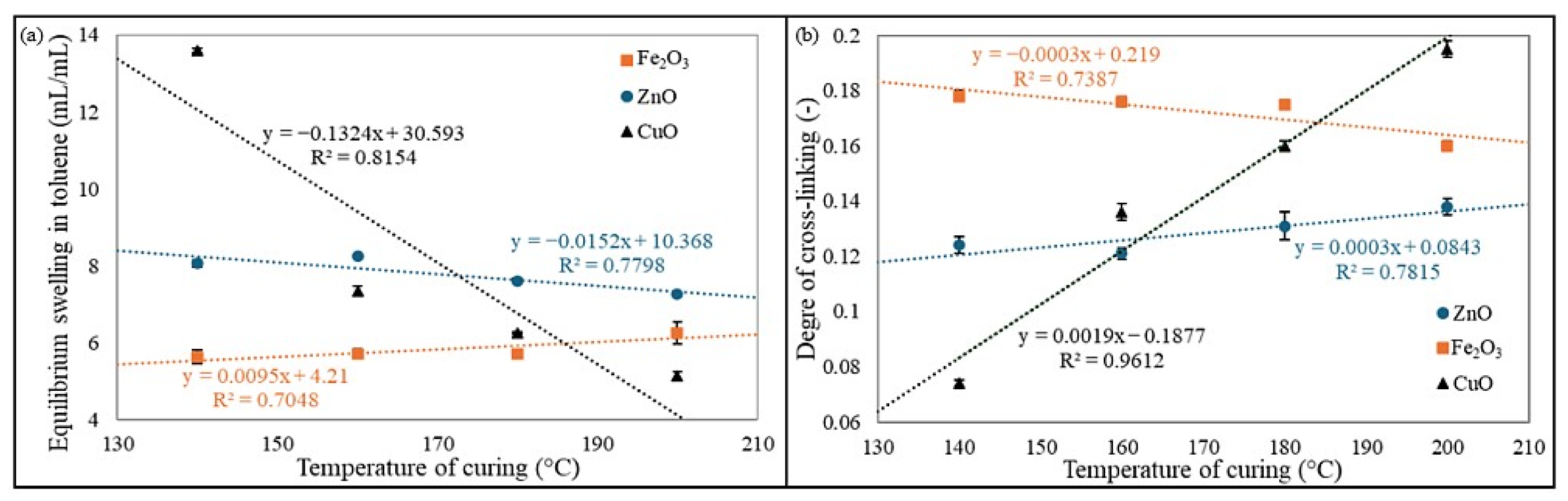
The obtained results of equilibrium swelling are presented in
Table 3. For the CR/BR/ZnO vulcanizate, the highest equilibrium volume swelling in toluene (Q
VT = 8.24 mL/mL) was observed for the CR/BR/ZnO-160 sample, followed by a slightly lower value (Q
VT = 8.06 mL/mL) for the CR/BR/ZnO-140 vulcanizates. As the curing temperature increased (180 and 200 °C), the Q
VT values decreased, indicating a higher cross-link density and a lower amount of unbound rubber, which swells upon contact with the solvent. For the CR/BR/Fe
2O
3 composites, the lowest equilibrium swelling value in toluene (Q
VT = 5.63 mL/mL) was obtained at the lowest curing temperature, while increasing temperature led to higher Q
VT values. The greatest equilibrium volume swelling in toluene (Q
VT = 13.61 mL/mL) was found for the CR/BR/CuO-140 vulcanizate. For the composite cured with CuO at 160 °C, the equilibrium swelling in toluene was significantly lower (Q
VT = 7.35 mL/mL). As the curing temperature of CR/BR/CuO blends increased, the equilibrium volume swelling in toluene decreased, with the lowest value (Q
VT = 5.14 mL/mL) observed at 200 °C. For swelling in n-hexane, a similar trend was observed for CR/BR/ZnO vulcanizates, where the highest volume swelling (Q
VH = 1.28 mL/mL) was obtained for the sample cured at 160 °C, and with higher temperatures (180 and 200 °C), the Q
VH values decreased. The equilibrium swelling values in n-hexane for all CR/BR/Fe
2O
3 vulcanizates were comparable (~1 mL/mL). However, for the CR/BR/CuO products, Q
VH decreased with increasing temperature, with the lowest value (Q
VH = 0.77 mL/mL) recorded for the composite cured at 200 °C.
The leached fraction eluted in toluene was higher than in n-hexane, indicating that the tested elastomeric composites were more sensitive to toluene. The highest values (−Q
WT = 0.31 mg/mg) were recorded for the CR/BR/CuO-140 vulcanizate, while the lowest (−Q
WT = 0.13 mg/mg) was for the CR/BR/CuO-200 sample. For blends cross-linked with ZnO or CuO, the values decreased with increasing temperature, while for the CR/BR/Fe
2O
3 products, no important changes were observed. Moreover, the leached fraction eluted in n-hexane, ranging from 0.05 to 0.08 mg/mg for all tested samples. The rubber volume fraction in the swollen sample immersed in toluene increased with curing temperature for CR/BR/ZnO and CR/BR/CuO samples but decreased for CR/BR/Fe
2O
3 vulcanizates. A higher rubber volume fraction indicates a greater degree of cross-linking. The lowest V
RT value in toluene (0.0685) was observed for the sample cured with CuO at 140 °C, while the highest V
RT value (0.163) was recorded for the CR/BR/CuO-200. The highest degree of cross-linking for samples tested in toluene (α
cT = 0.195) was obtained for the CR/BR/CuO-200 vulcanizate, while the lowest α
cT value (0.0735) was calculated for the CR/BR/CuO-140 composite. For blends cross-linked with CuO and ZnO, higher curing temperatures resulted in higher α
c values, while an opposite trend was observed for CR/BR/Fe
2O
3 samples.
Figure 5 shows the relationship between cross-linking temperature and curing agent and such values as Q
vT and α
c.
The equilibrium swelling results indicate that the CR/BR blend cross-linked with copper(II) oxide at 200 °C exhibited the highest resistance to solvents, confirming a well-developed cross-linking network in this vulcanizate. Additionally, as the curing temperature of the CR/BR/CuO blend increased, the Q
VT and Q
VH values decreased, indicating a higher degree of cross-linking and the formation of a more complex network structure. For samples cross-linked with iron(III) oxide, the opposite trend was observed. However, these differences were less pronounced than those observed for CR/BR/CuO products.
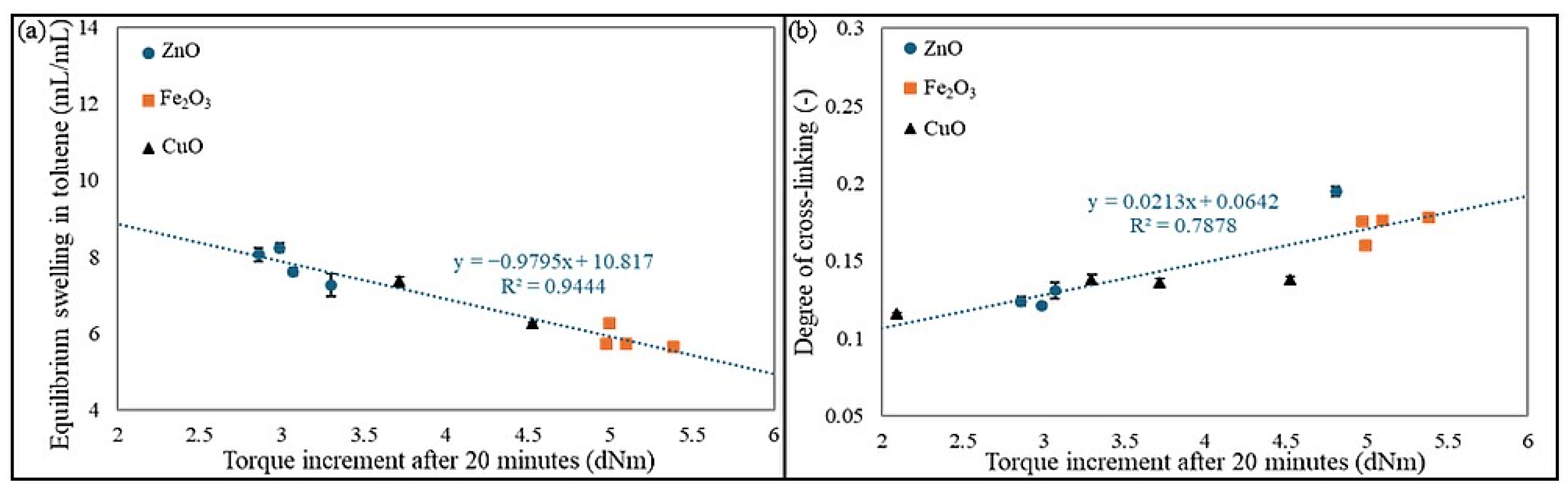
Figure 6 shows the correlation of equilibrium swelling and degree of cross-linking with the torque increment after 20 min for all tested samples.
Figure 6a indicates that the higher the ΔT
20 value, the lower the Q
vT parameter, which is related to the higher number of cross-links in the materials and affects the lower possibility of solvent penetration into the material [
38,
39]. Meanwhile,
Figure 6b shows that the larger the ΔT
20 value, the greater the degree of cross-linking, resulting from the increasing number of cross-links [
40].
2.4. Analysis of the Degree of Cross-Linking Determined by the Elasticity Constants
The next method used in the study to determine the networks formed during the cross-linking was the determination of the Mooney–Rivlin constants. The value of the first elasticity constant (2C
1) is directly proportional to the degree of cross-linking (the higher its value, the greater the degree of cross-linking of the vulcanizate), while the second elasticity constant (2C
2) is a measure of the deviation of the formed network from an ideal network [
41]. The obtained test results are presented in
Table 4.
After analyzing the study, it should be noted that for compositions cross-linked with ZnO or CuO, the 2C
1 values increase with increasing vulcanization temperature, indicating a higher degree of cross-linking in these samples. For the CR/BR/Fe
2O
3 vulcanizate, no significant trend was observed. Moreover, for all temperatures, the CR/BR/Fe
2O
3 vulcanizates exhibited similar 2C
1 values (3.21–4.03 kG/cm
2). Comparable to previous studies, the highest degree of cross-linking was obtained for the CR/BR/CuO sample cross-linked at 200 °C, where the first elasticity constant was 4.25 kG/cm
2. For the CR/BR/CuO sample cross-linked at 160 °C, a similar value of the first elasticity constant (3.61 kG/cm
2) was observed as for the CR/BR/ZnO-180 sample (3.67 kG/cm
2). However, for the sample containing copper(II) oxide, the value of the second elasticity constant was significantly higher (4.54 kG/cm
2), which may suggest that structures are formed in this vulcanizate that hinder the creation of an ideal network despite the high degree of cross-linking. Additionally, these structures contribute to the strain-hardening effect, making the material more resistant to stretching under large deformations [
42].
Furthermore, the 2C
2 parameter in the Mooney–Rivlin model suggests that the material behaves similarly to a neo-Hookean rubber, exhibiting a simpler elastic response with minimal strain stiffening [
43]. The results for the CR/BR/Fe
2O
3-200 demonstrated greater resistance to high deformations (2C
2 = 2.63 kG/cm
2), while the remaining samples exhibited lower network stiffening and behaved more like neo-Hookean rubbers [
42,
43]. This means that at low deformations, the stress-strain relationship remains nearly linear. This behavior aligns with the formation of a more uniform cross-linked network, resulting in a predictable, linear stress-strain response [
44].
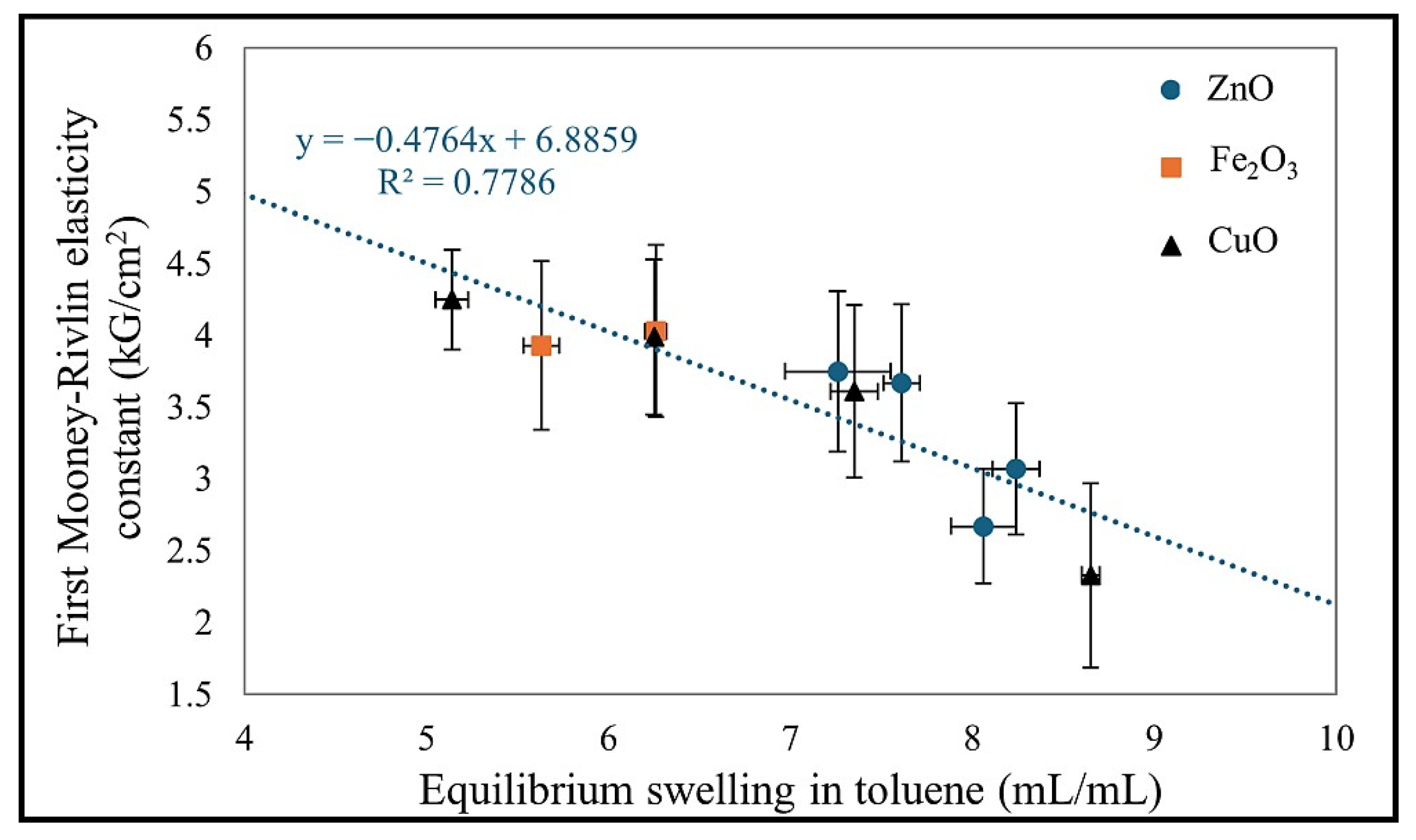
Figure 7 represents the correlation of the first elasticity constant with the value of the equilibrium volume swelling in toluene for all the samples tested. The value of Q
vT is inversely proportional to the value of 2C
1.
2.5. Mechanical Properties of Vulcanizates Before and After Thermo-Oxidative Aging
The tensile strength properties before and after thermo-oxidative aging of the vulcanizates are summarized in
Table 5. The mechanical properties of rubber materials are directly related to cross-linking parameters. The conducted studies showed that the type of cross-linking agent used and the vulcanization temperature affect the tensile strength properties. The mechanical properties of elastomeric products before and after aging are crucial for assessing their durability and long-term performance. Before aging, properties such as tensile strength, elongation at break, and hardness indicate the initial quality and suitability of the material for specific applications. After aging, changes in these properties reflect the material’s resistance to thermal, oxidative, and environmental degradation. This evaluation helps predict the lifespan, mechanical stability, and risk of failure in applications such as automotive seals, tires, and industrial components.
The results of mechanical tests showed that the best mechanical properties (TS
b = 11.4 MPa) were achieved by the CR/BR/Fe
2O
3-140. Additionally, it is worth noting that samples cross-linked with Fe
2O
3 generally exhibited the best mechanical parameters, regardless of the curing temperature. Samples cured with ZnO achieved the highest tensile strength (7.29 MPa) at 200 °C. Vulcanizates containing CuO had the highest TS
b value (9.17 MPa) at 180 °C. Surprisingly low strength (TS
b = 2.59 MPa) was observed for the composition cross-linked with CuO at 200 °C. It is important to mention that cross-linking temperatures affect the mechanical properties of the studied systems differently, making it difficult to identify a clear trend. However, optimal curing temperatures can be indicated for specific elastomeric blends: 200 °C for CR/BR/ZnO, 140 °C for CR/BR/Fe
2O
3, and 180 °C for CR/BR/CuO. When analyzing the elongation at break (E
b), all samples (except the CR/BR/CuO-200 compound) exhibited elongation above 800%, whereas the CR/BR/CuO-200 vulcanizates stretched only to 393%. Such a low value indicates over-cross-linking of the composition and a lack of balance between elasticity and flexibility. This is further supported by the S
E100 value of 0.94 MPa, the highest among the tested samples, despite the lowest TS
b value. The CR/BR/CuO-200 vulcanizate had the highest degree of cross-linking (α
c = 0.195) among all samples and was characterized by increased stiffness while simultaneously reducing mechanical strength.
Figure 8 shows the relationship between the degree of cross-linking and the tensile strength values of the CR/BR vulcanizates tested. It should be noted that, in general, tensile strength increased in direct proportion to the degree of cross-linking. This is because a higher number of cross-links creates a tighter polymer network, improving stress transfer between polymer chains. It also affects less chain slippage, making the material stronger under tension [
45,
46,
47]. However, not all samples fulfilled this relationship. An example is the CR/BR/CuO-200 compound mentioned early on, which is over-cross-linked [
48].
The mechanical strength analysis after thermo-oxidative aging revealed low resistance to aging factors in the samples cross-linked with CuO or Fe
2O
3. The mechanical properties of these vulcanizates significantly deteriorated after thermo-oxidative aging. For samples cross-linked with iron(III) oxide, the TS
′b value was approximately 1.2 MPa, with an aging coefficient of ~0.01. In the case of CR/BR/CuO samples, it was observed that the vulcanizate at 200 °C had an aging coefficient (~0.46) that deviated significantly from those obtained at other temperatures (~0.06). This discrepancy arises from the fact that, compared to the other samples, the tensile strength and elongation at break before thermo-oxidative aging were very low, possibly due to exceeding the cross-linking threshold and initiating sample degradation. As a result, a significant decline in these values after aging was not observed. Additionally, S
′E100 values increased, indicating polymer chain stiffening and reduced deformation ability. However, this had a significantly negative effect on the tensile strength values after aging. It can therefore be concluded that these metal oxides, due to their variable valency, have a significant impact on the aging resistance of CR/BR vulcanizates. Transition metal oxides (Fe
2O
3, CuO) with variable oxidation states can act as redox catalysts in the presence of heat and oxygen, accelerating the decomposition of hydroperoxides into free radicals (RO•, HO•) [
49]. These radicals cause chain scission, leading to a loss of mechanical properties, accelerated oxidative degradation, and rubber embrittlement [
17,
18,
50,
51]. Tensile strength after thermo-oxidative aging increased for CR/BR/ZnO blends cross-linked at 140 and 160 °C, possibly due to additional cross-linking of the samples. However, for samples cured with ZnO at 180 or 200 °C, tensile strength decreased, which corresponded to a decrease in the aging coefficient (0.60 and 0.42, respectively). The CR/BR/ZnO-160 vulcanizates were the most aging resistant, and their aging coefficient was approximately 1.0. This high aging resistance is attributed to the fact that zinc, unlike copper and iron, has a filled “d” subshell, making it more stable and preventing it from catalyzing aging processes. Conversely, since iron and copper atoms exist in multiple oxidation states, CR/BR blends cross-linked with these oxides exhibit poorer aging resistance compared to samples containing ZnO [
19,
24].
Figure 9 summarizes comparisons of the mechanical parameters before and after thermo-oxidative aging of the tested elastomer compositions (
Figure 9a–c), as well as the aging coefficient values for all the samples tested.
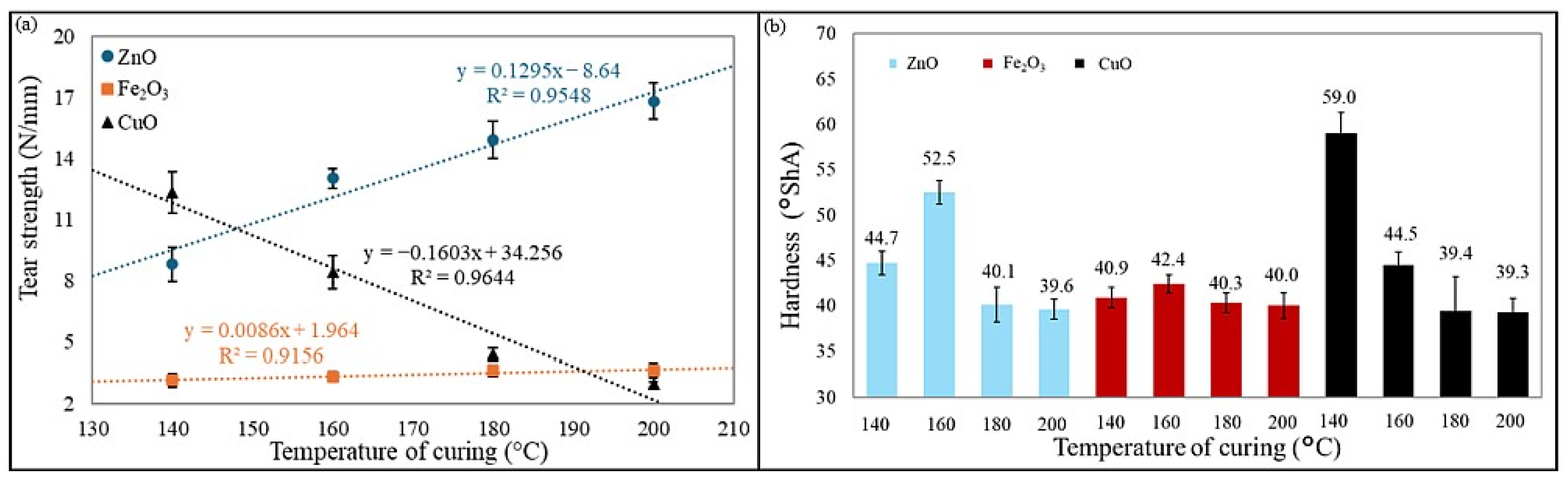
Hardness is another important mechanical parameter, as it directly affects wear resistance, load-bearing capacity, and elasticity. It determines how well an elastomer can withstand deformation under applied forces. Optimal hardness provides a balance between elasticity and durability, preventing excessive deformation or brittleness. Hardness is widely used for an indicative assessment of rubber and the degree of vulcanization, and it depends on the elastic properties of the material under test (Young’s modulus) [
52,
53]. The CR/BR/CuO-140 vulcanizate was characterized by the highest hardness (59 °Sh A), and the lowest hardness (39.3 °Sh A) was observed for the CR/BR/CuO-200 product. The highest hardness values for CR/BR/ZnO and CR/BR/Fe
2O
3 vulcanizates were obtained for samples cured at 160 °C (52.5 °Sh A and 42.4 °Sh A, respectively).
Figure 10 presents the effect of the curing system and curing temperature on the tear strength of the samples, as well as a comparison of the hardness of the CR/BR vulcanizates tested.
Tear strength is a critical property of elastomeric compounds, reflecting their resistance to crack initiation and propagation under mechanical stress. This is particularly important for applications exposed to repeated bending, stretching, and impact. High tear strength enhances durability, reliability, and service life, preventing premature failure under demanding conditions. The tear strength of rubber materials is measured as the force required to tear a pre-cut sample and is expressed as the ratio of the maximum force causing sample failure to its thickness. Among the tested materials, the CR/BR/ZnO-200 vulcanizate exhibited the highest tear resistance (16.8 N/mm), whereas the CR/BR/CuO composite cured at 200 °C showed the lowest tear strength (2.95 N/mm). For the CR/BR/ZnO composition, tear strength increased with rising vulcanization temperature, while an inverse trend was observed for the CR/BR/CuO samples. The CR/BR/Fe2O3 vulcanizates demonstrated the lowest tear strength (2.6–3.6 N/mm).
2.6. Analysis of Thermal Properties
Thermal properties, as analyzed by differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA), are crucial for understanding the stability and performance of elastomeric blends under various temperature conditions. DSC helps determine glass transition temperature (Tg), melting behavior, and phase transitions that affect flexibility and processing. TGA provides insight into thermal degradation, weight loss, and thermal stability, essential for predicting material life and high temperature resistance. These analyses help optimize elastomer formulations for heat resistance, processing conditions, and long-term durability in demanding applications.
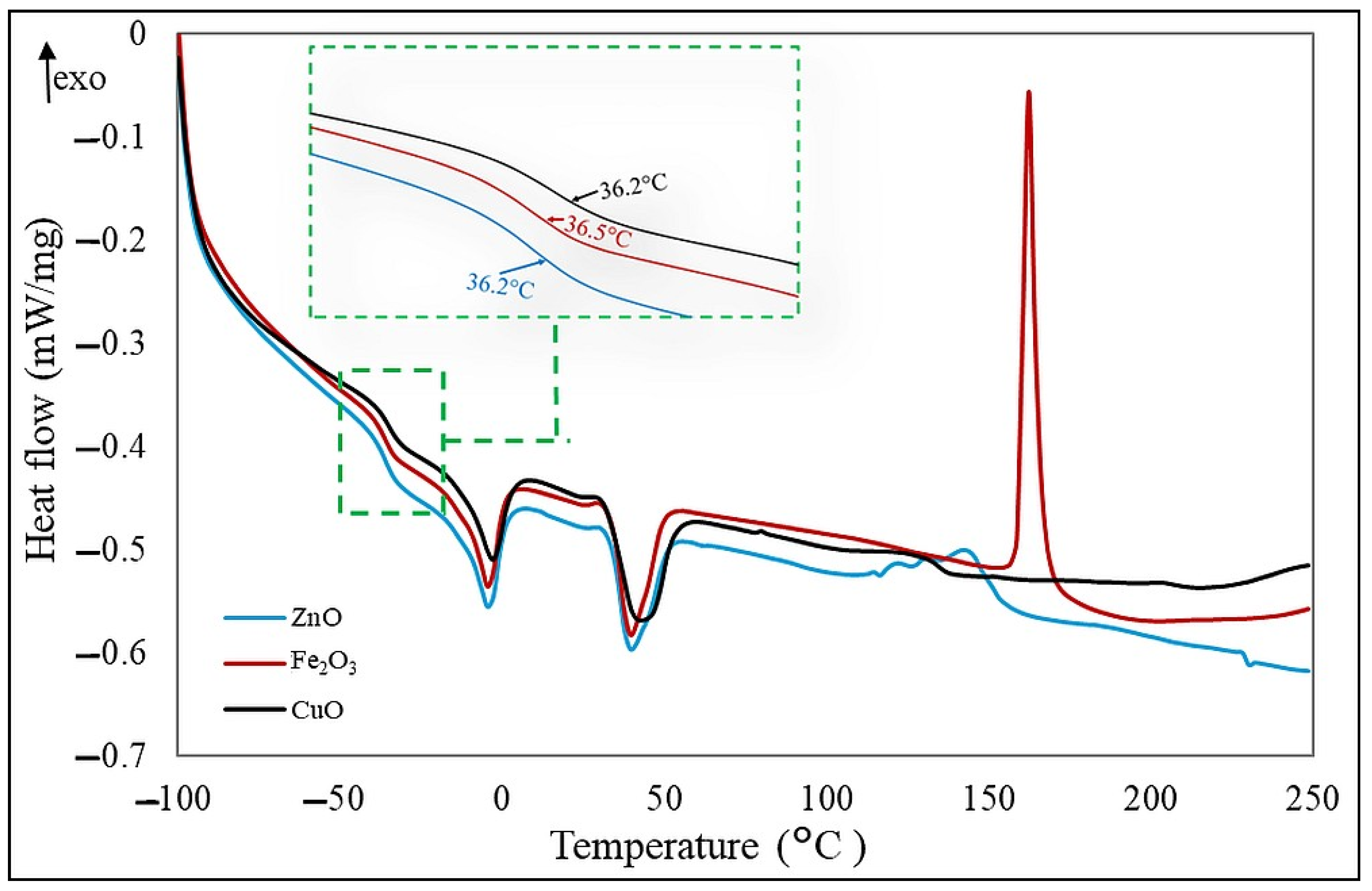
The glass transition temperature (T
g) values obtained for the compositions tested are shown in
Table 6. For all samples, the T
g values do not differ significantly. The type of cross-linking agent added to the CR/BR compositions caused changes in the cross-linking temperatures. The presence of zinc oxide or copper(II) oxide in the blends caused a shift in the exothermic peak, corresponding to cross-linking, toward a lower temperature than when iron(III) oxide was used. However, the type of oxide used had no noticeable effect on the change in glass transition temperature. Appearing exothermic peaks in the temperature ranges in
Table 6 indicated cross-linking of the samples. Cross-linking temperatures show the largest values of the peaks, which were where the cross-linking process occurs most rapidly. The use of iron(III) oxide results in more efficient cross-linking of the elastomeric mix, as evidenced by the increasing amount of energy released during the process. The lowest enthalpy of cross-linking was determined for the sample cured with copper(II) oxide, which means that cross-linking in this case is the least effective.
Figure 11 shows DSC curves for the three tested elastomeric blends differing in curing system.
Figure 12a shows the TGA curve, which identifies the lost weight of the sample as a function of temperature and illustrates the thermal stability of the material. For the composition cross-linked with zinc oxide, the first stage of thermal decomposition began above 285 °C and was 49.8%. The use of iron(III) oxide as a cross-linking agent increased the decomposition temperature of the samples to 325 °C, and the weight loss was 37.9%. The elastomeric composition with the addition of copper(II) oxide is pyrolyzed at 345 °C. The first stage of decomposition for all samples ended at about 420 °C, and then the course of the thermogravimetric curves is almost identical. In
Figure 12b, the mass loss during the first stage could be seen as the first peak and was mainly due to the elimination of HCl. In the second stage of decomposition taking place in the temperature range of 420–500 °C, a less rapid mass loss of the CR/BR/ZnO and CR/BR/Fe
2O
3 samples is noticeable than in the first stage, while the initial mass loss is greater for the CR/BR/CuO sample. At ~620 °C, there was a final initial weight loss of 18–20% for the samples. In summary, the CR/BR/ZnO product had the highest weight loss in the first stage (49.8%), while the CR/BR/CuO composite had the lowest weight loss (37.9%). The differences in the weight loss may be due to the thermal stability of their cross-linking agents. Therefore, the use of iron(III) oxide or copper(II) oxide improved thermal stability, but these compositions aged faster than zinc-oxide-cured blends after extended thermal exposition, confirming the mechanical properties after thermo-oxidative aging.