Sustainable Production and Antioxidant Activity of Bacterial Xanthan Gum
Abstract
1. Introduction
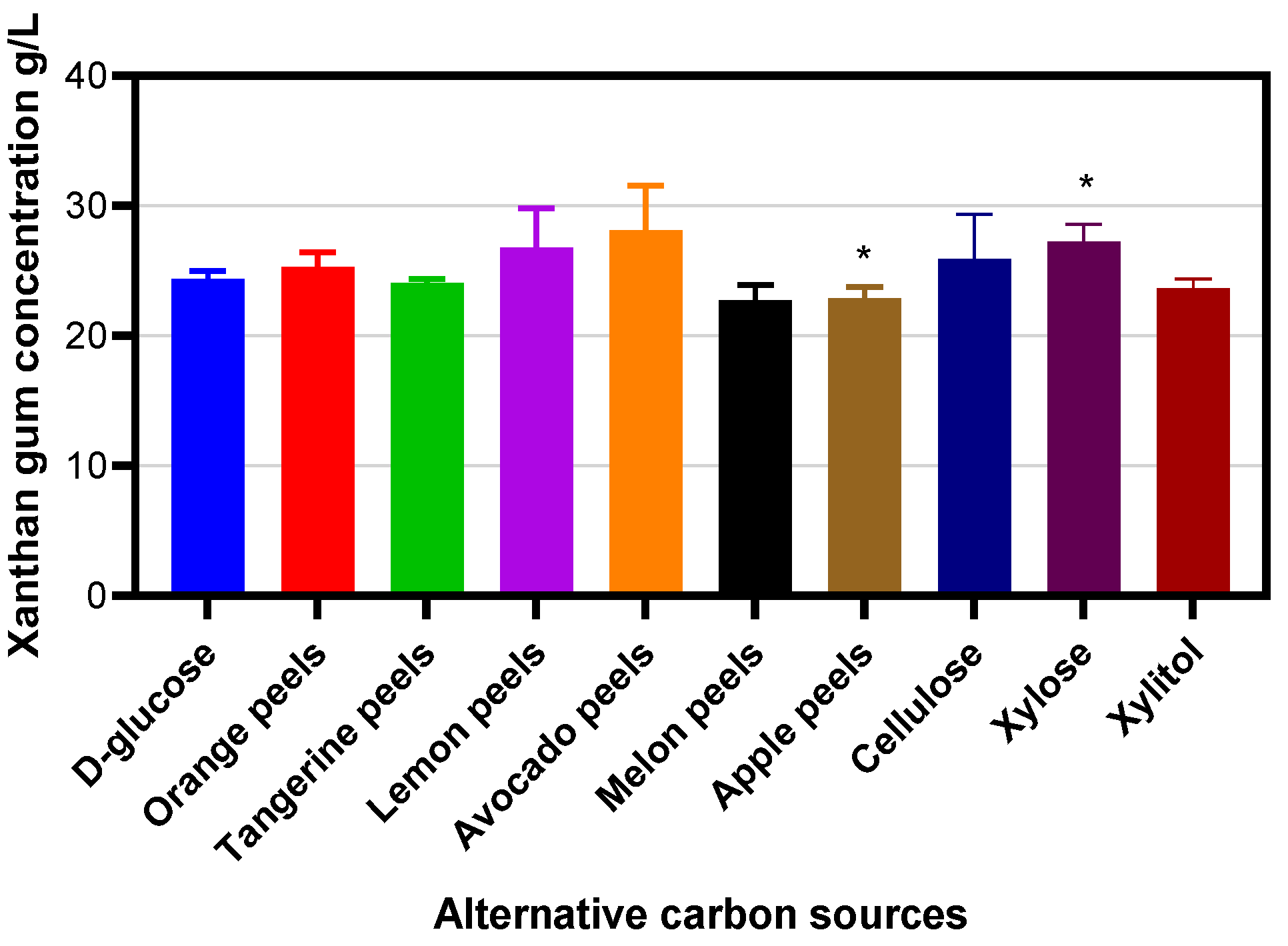
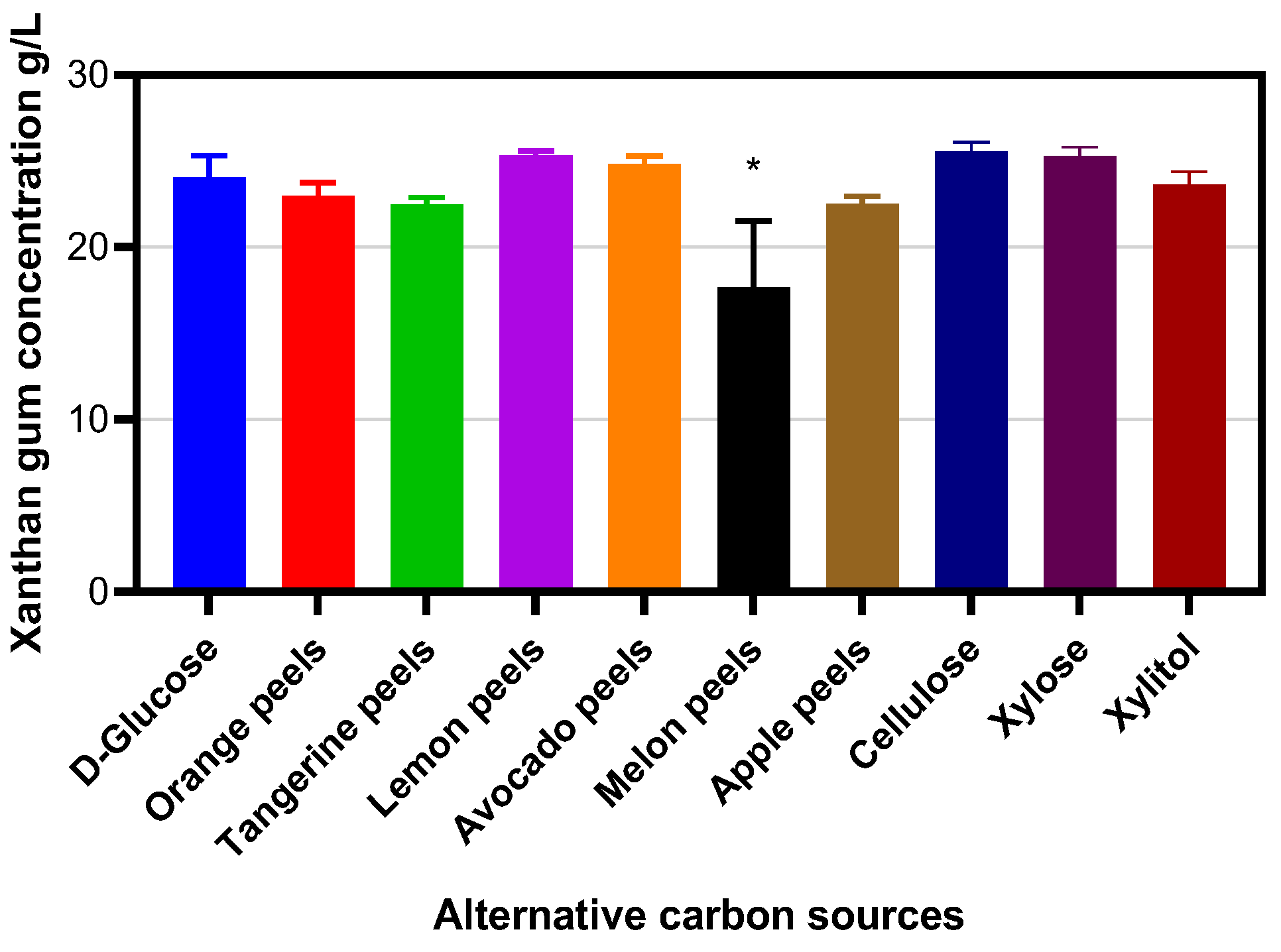
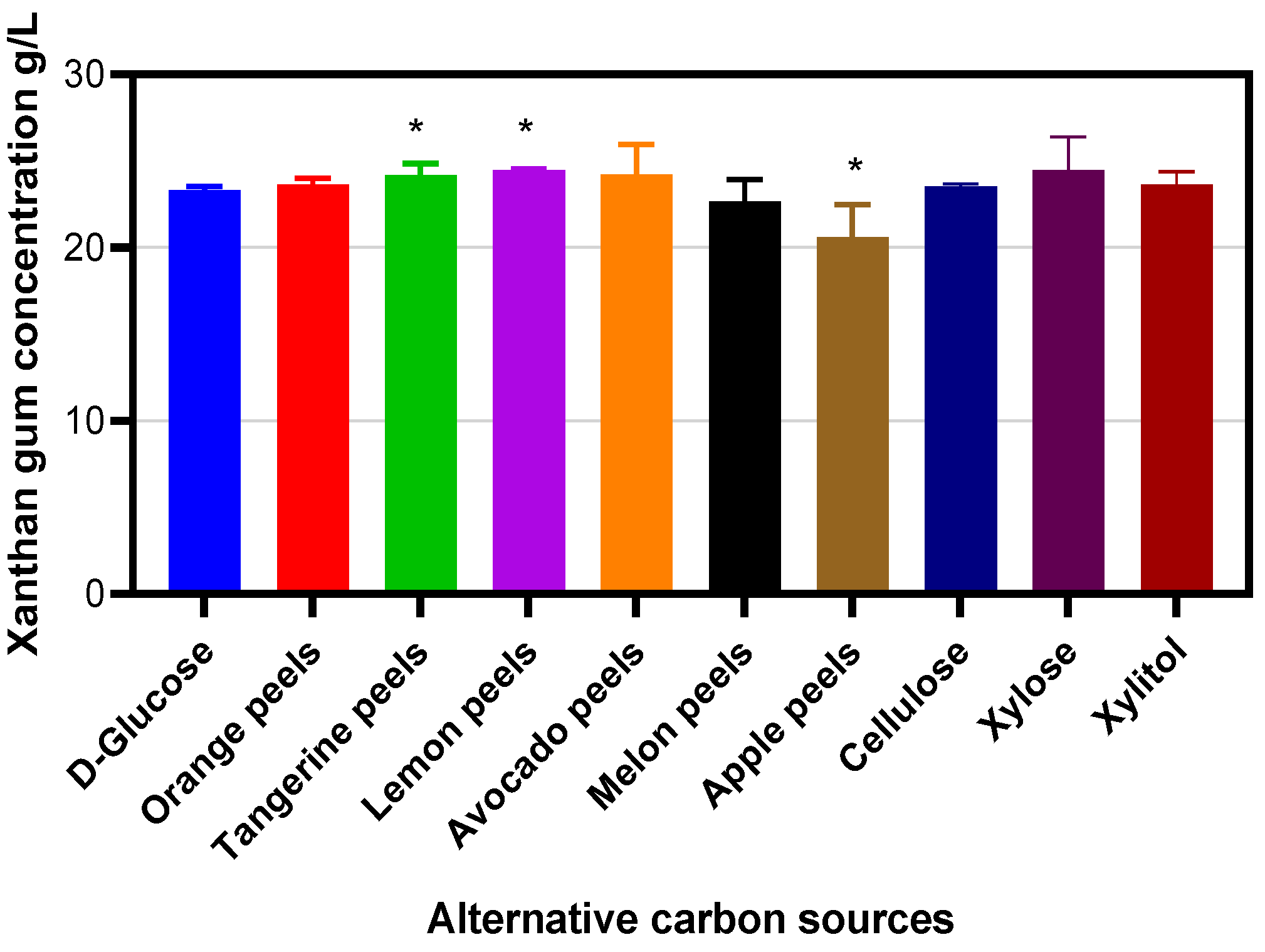
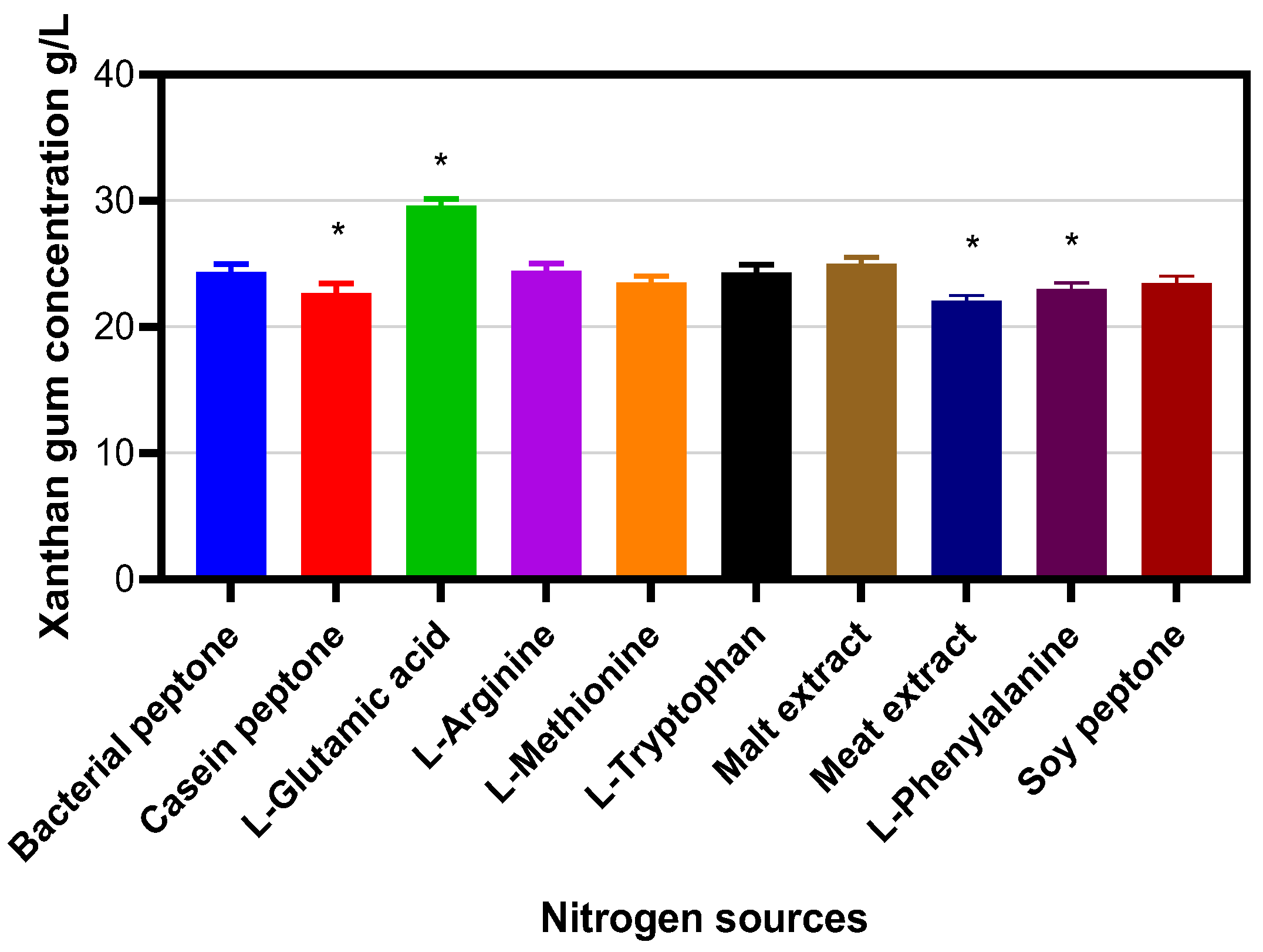
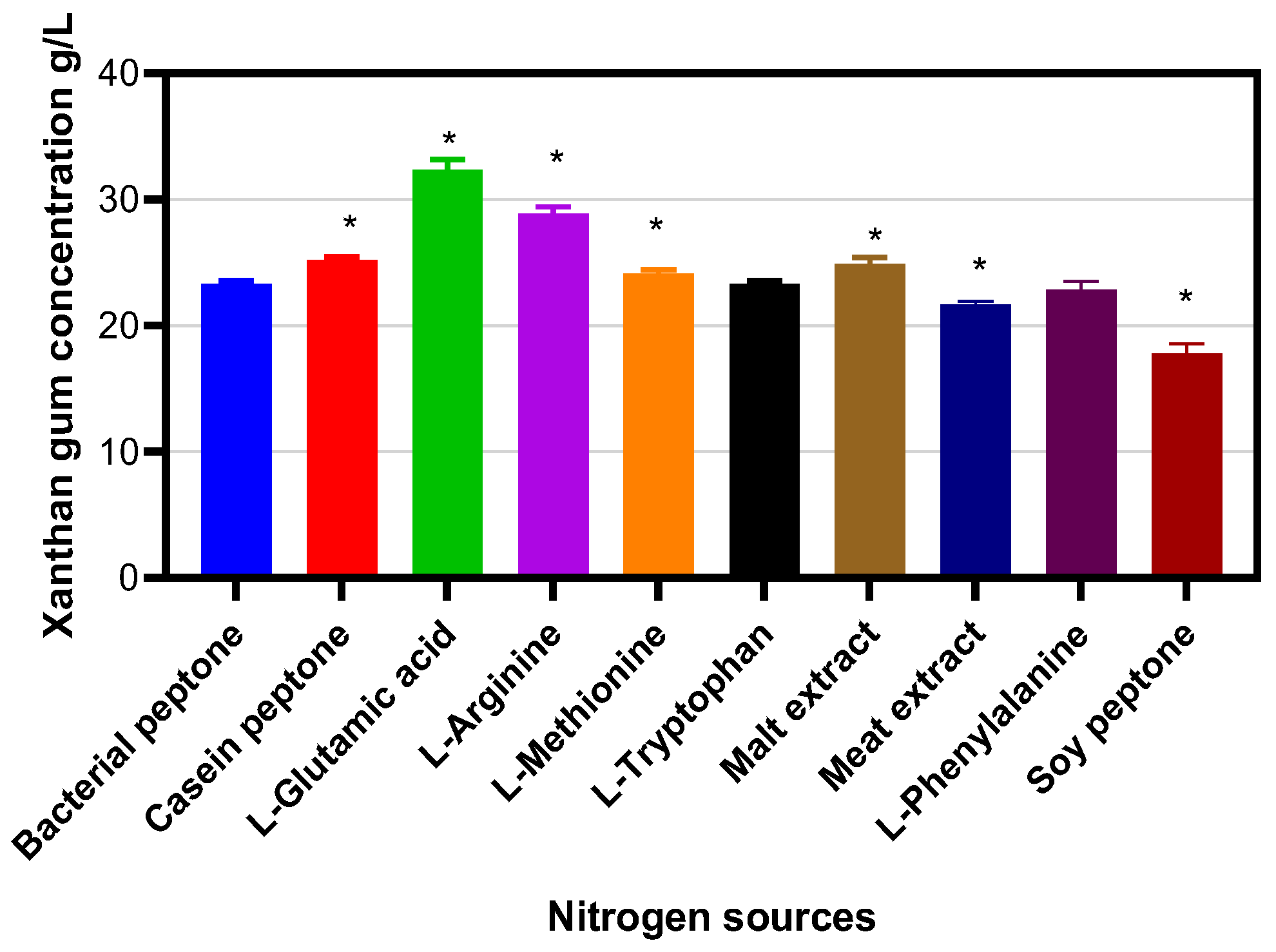
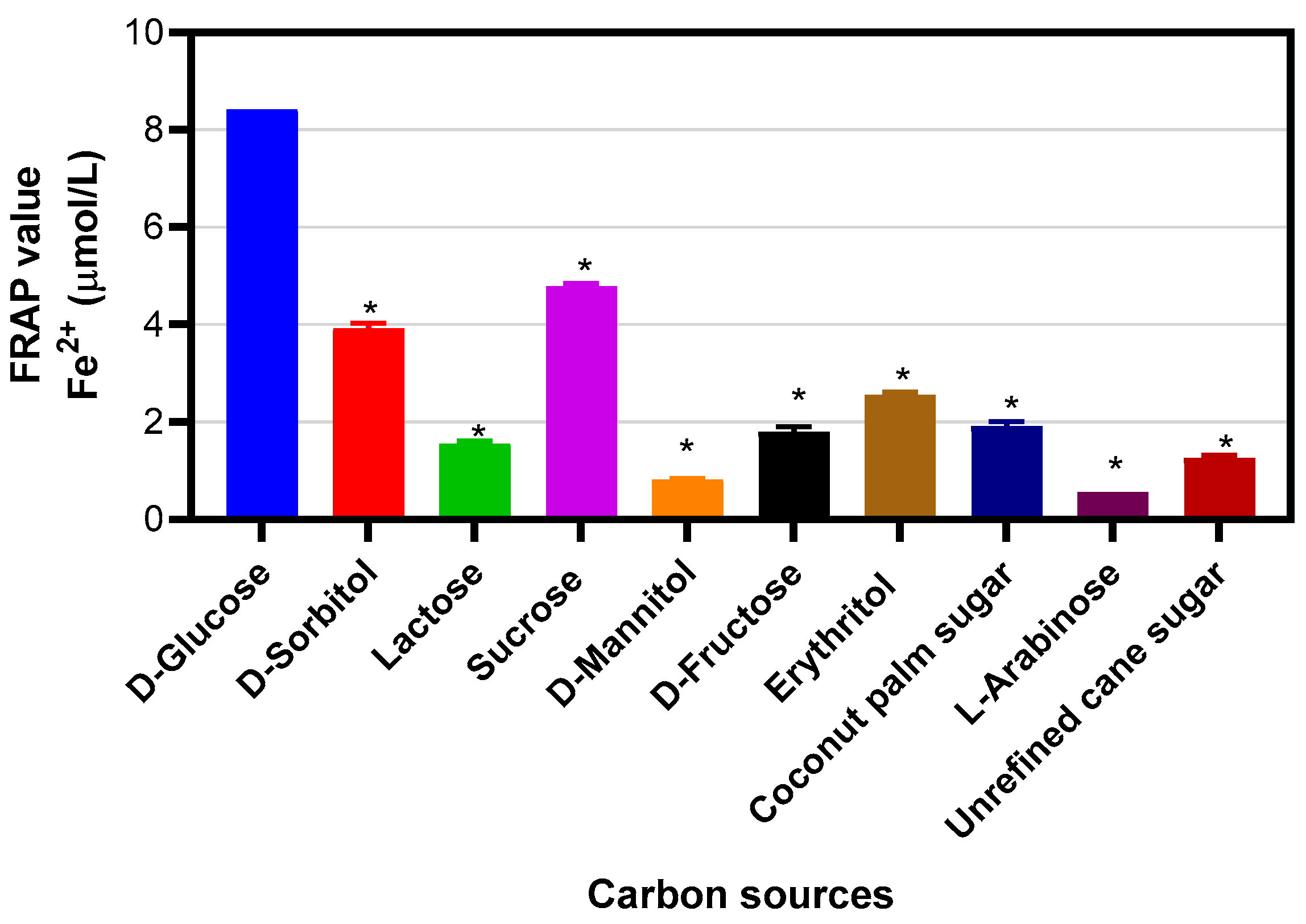
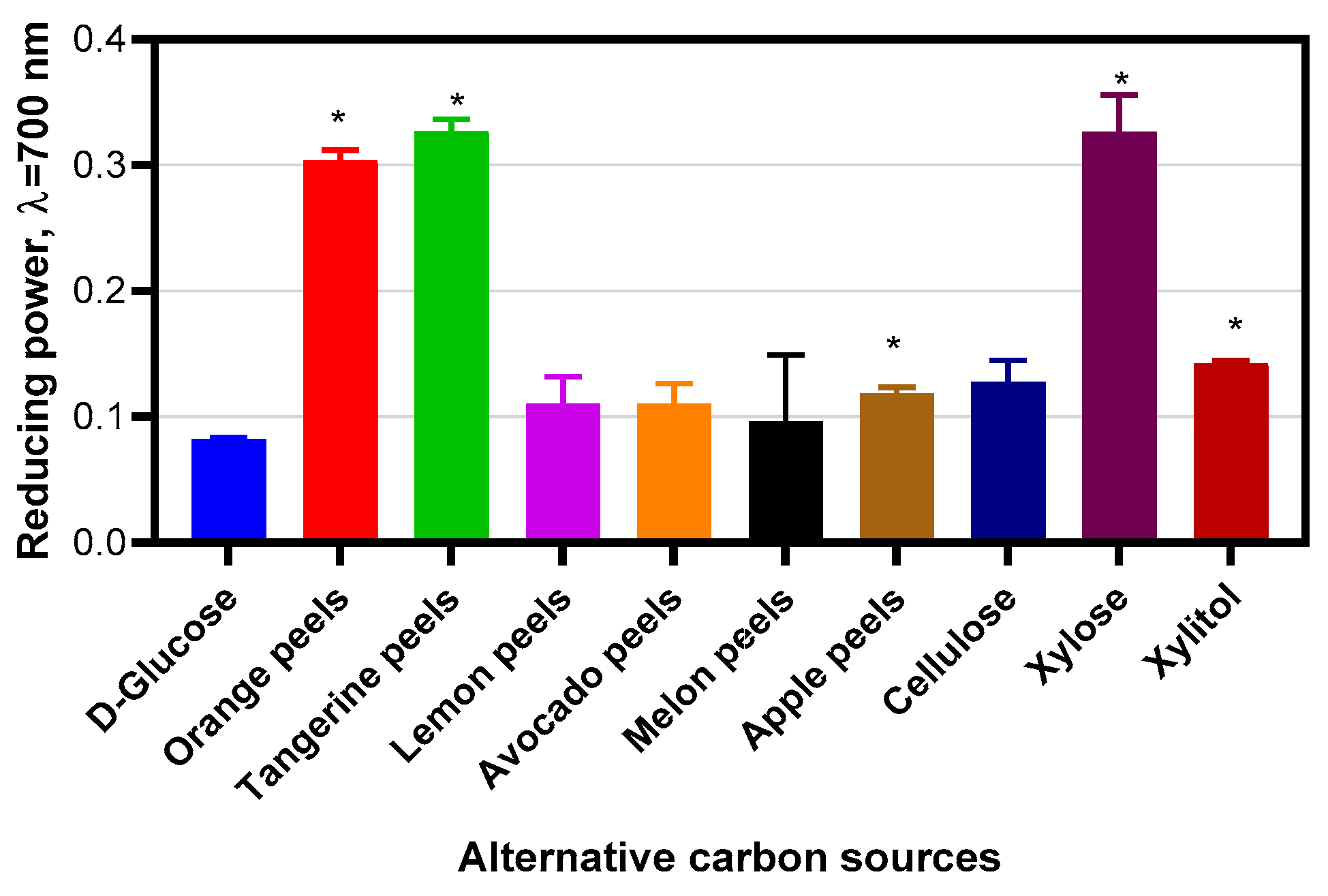
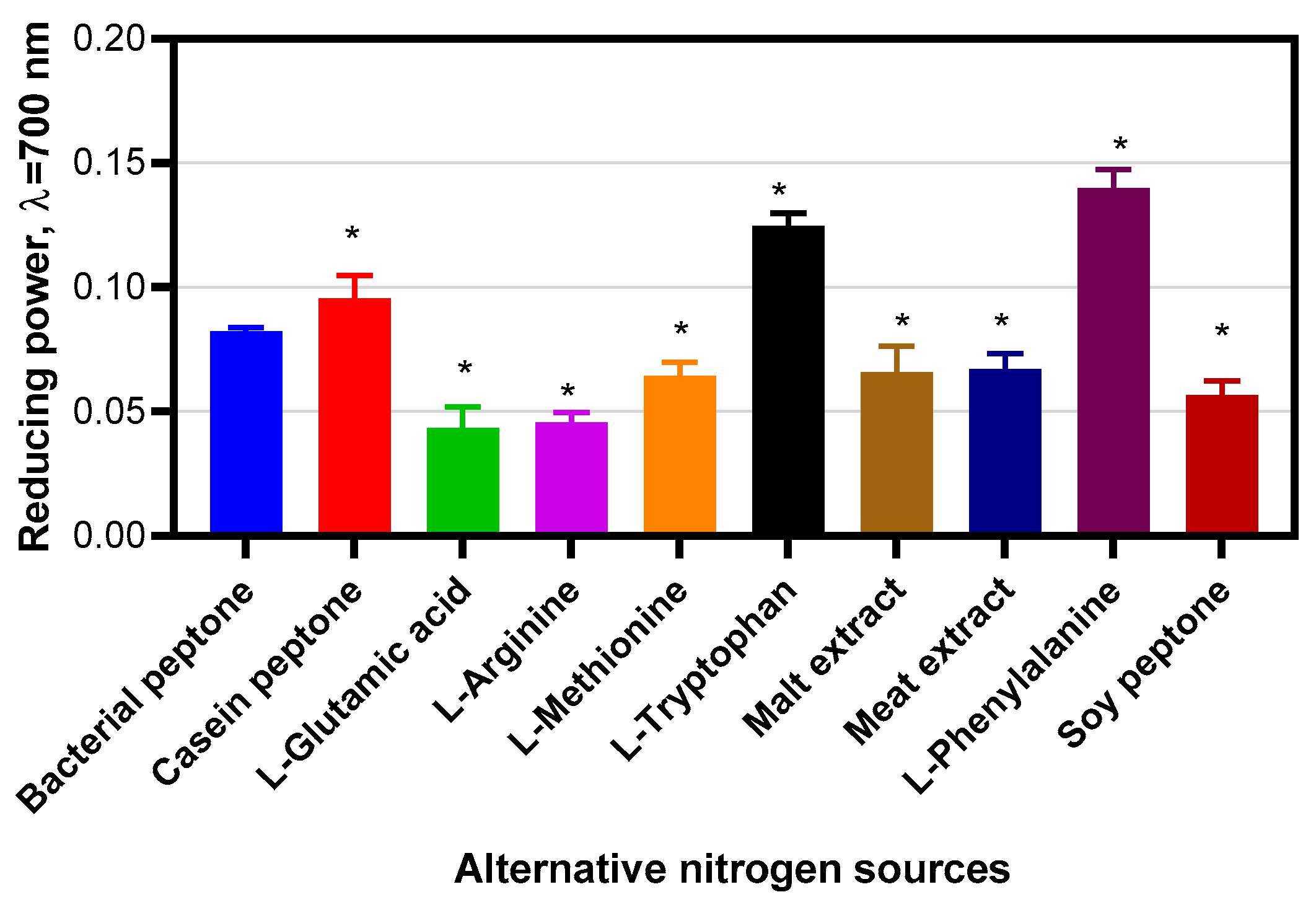
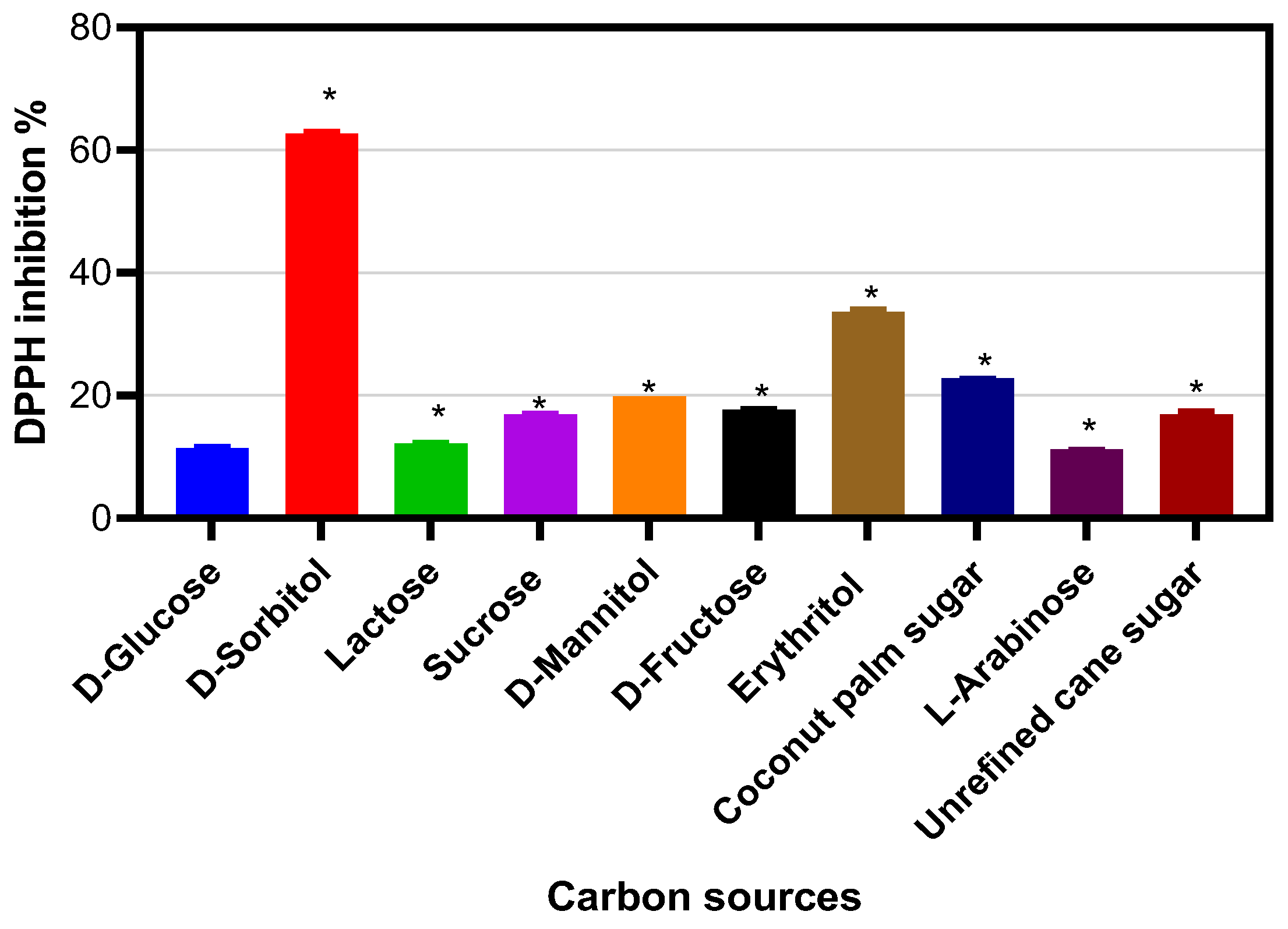
2. Results
3. Discussion
4. Materials and Methods
Measurement of Antioxidant Activities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xanthan Gum Market Size, Share | Forecasts Report 2022–2030. Available online: https://www.gminsights.com/ (accessed on 20 January 2025).
- GarcõÂa-Ochoa, F.; Santosa, V.E.; Casasb, J.A.; GoÂmez, E. Xanthan gum: Production, recovery, and properties. Biotechnol. Adv. 2000, 18, 549. [Google Scholar] [CrossRef]
- Rana, S.; Upadhyay, L.S.B. Microbial exopolysaccharides: Synthesis pathways, types and their commercial applications. Int. J. Biol. Macromol. 2020, 157, 577–583. [Google Scholar] [CrossRef]
- Petri, D.F.S. Xanthan gum: A versatile biopolymer for biomedical and technological applications. J. Appl. Polym. Sci. 2015, 132, 2–13. [Google Scholar] [CrossRef]
- Bhat, I.M.; Wani, S.M.; Mir, S.A.; Masoodi, F.A. Advances in xanthan gum production, modifications and its applications. Biocatal. Agric. Biotechnol. 2022, 42, 102328. [Google Scholar] [CrossRef]
- Rosalan, S.; England, R. Review of xanthan gum production from unmodified starches by Xanthomonas campestris sp. Enzym. Microb. Technol. 2006, 39, 197–207. [Google Scholar] [CrossRef]
- Dey, R.; Chatterji, B.P. Sources and methods of manufacturing xanthan by fermentation of various carbon sources. Biotechnol. Prog. 2023, 39, e3379. [Google Scholar] [CrossRef] [PubMed]
- Steffens, T.; Vorhölter, F.J.; Teckentrup, J.; Hublik, G.; Walhorn, V.; Anselmetti, D.; Pühler, A.; Niehaus, K.; Ortseifen, V. Two Flagellar mutants of Xanthomonas campestris are characterized by enhanced xanthan production and higher xanthan viscosity. J. Biotechnol. 2022, 347, 9–17. [Google Scholar] [CrossRef]
- Moreira, A.S.; Vendruscolo, J.L.S.; Gil-Turnes, C.; Vendruscolo, C.T. Screening among 18 novel strains of Xanthomonas campestris pv pruni. Food Hydrocoll. 2001, 15, 469–474. [Google Scholar] [CrossRef]
- Kalogiannis, S.; Iakovidou, G.; Liakopoulou-Kyriakides, M.; Kyriakidis, D.A.; Skaracis, G.N. Optimization of xanthan gum production by Xanthomonas campestris grown in molasses. Process Biochem. 2003, 39, 249–256. [Google Scholar] [CrossRef]
- Chaitali, M.; Kapadi, M.; Suraishkumar, G.K.; Gudi, R.D. Productivity improvement in xanthan gum fermentation using multiple substrate optimization. Biotechnol. Progr. 2003, 19, 1190–1198. [Google Scholar] [CrossRef]
- Sun, H.; Ni, J.; Yang, G.; Liu, Z.; Wang, Z.; Zhu, S.; Li, Z.; Jiang, Y.; Zhan, X.; Wang, Y.; et al. A novel coupled fermentation system for low-molecular-weight xanthan gum with diverse biological activities. Int. J. Biol. Macromol. 2024, 279, 135283. [Google Scholar] [CrossRef] [PubMed]
- Letisse, F.; Chevallereau, P.; Simon, J.L.; Lindley, N.D. Kinetic analysis of growth and xanthan gum production with Xanthomonas campestris on sucrose, using sequentially consumed nitrogen sources. Appl. Microbiol. Biotechnol. 2001, 55, 417–422. [Google Scholar] [CrossRef]
- Silva, M.F.; Fornari, R.C.G.; Mazutti, M.A.; de Oliveira, D.; Padilha, F.F.; Cichoski, A.J.; Cansian, R.L.; Di Luccio, M.; Treichel, H. Production and characterization of xantham gum by Xanthomonas campestris using cheese whey as sole carbon source. J. Food Eng. 2009, 90, 119–123. [Google Scholar] [CrossRef]
- Demirci, A.S.; Palabiyik, I.; Apaydın, D.; Mirik, M.; Gumus, T. Xanthan gum biosynthesis using Xanthomonas isolates from waste bread: Process optimization and fermentation kinetics. LWT 2019, 101, 40–47. [Google Scholar] [CrossRef]
- Psomas, S.K.; Liakopoulou-Kyriakides, M.; Kyriakidis, M. Optimization study of xanthan gum production using response surface methodology. Biochem. Eng. J. 2007, 35, 273–280. [Google Scholar] [CrossRef]
- Oliveira, D.B.; Kundlastsch, G.E.; Cruz, R.D.; Batista, B.; de Arruda Ribeiro, M.P.; Novo-Mansur, M.T.M.; da Silva, A.J. Xanthan gum production in Xanthomonas campestris is increased by favoring the biosynthesis of its monomers. Bioresour. Technol. 2025, 416, 131808. [Google Scholar] [CrossRef]
- Lopez, M.J.; Moreno, J.; Ramos, A.C. Xanthomonas campestris strain selection for xanthan production from olive mill wastewaters. Water Res. 2001, 35, 1828–1830. [Google Scholar] [CrossRef]
- Lopez, M.J.; Vargas-Garcia, M.C.; Suarez-Estrella, F.; Moreno, J. Properties of xanthan obtained from agricultural wastes acid hydrolysates. J. Food Eng. 2004, 63, 111–115. [Google Scholar] [CrossRef]
- Lopes, B.M.; Lessa, V.L.; Silva, B.M.; Filho, M.A.S.C.; Schnitzler, E.; Lacerda, L.G. Xanthan gum: Properties, production conditions, quality and economic perspective. J. Food Nutr. Res. 2015, 54, 185–194. [Google Scholar]
- Mudoi, P.; Bharali, P.; Konwar, B.K. Study on the Effect of pH, temperature and aeration on the cellular growth and xanthan production by Xanthomonas campestris using waste residual molasses. J. Bioprocess. Biotech. 2013, 3, 135. [Google Scholar] [CrossRef]
- Palaniraj, A.; Jayaraman, V. Production, recovery and applications of xanthan gum by Xanthomonas campestris. J. Food Eng. 2011, 106, 1–12. [Google Scholar] [CrossRef]
- Brunchi, C.E.; Bercea, M.; Morariu, S.; Dascalu, M. Some properties of xanthan gum in aqueous solutions: Effect of temperature and pH. J. Polym. Res. 2016, 23, 123. [Google Scholar] [CrossRef]
- Cacik, F.; Dondo, R.G.; Marques, D. Optimal control of a batch bioreactor for the production of xanthan gum. Comput. Chem. Eng. 2001, 25, 409–418. [Google Scholar] [CrossRef]
- Rehm, B.H.A. Bacterial polymers: Biosynthesis, modifications, and applications. Nat. Rev. Microbiol. 2010, 8, 578–592. [Google Scholar] [CrossRef]
- de Jesus, A.D.; Brandão, L.V.; de Sousa, C.L.A.; Figueiredo, T.V.; Sousa, L.S.; Padilha, F.F.; Druzian, J.I. A study of the effects of aeration and agitation on the properties and production of xanthan gum from crude glycerin derived from biodiesel using the response surface methodology. Appl. Biochem. Biotechnol. 2014, 172, 2769–2785. [Google Scholar] [CrossRef]
- Garcia-Ochoa, F.; Gomez, E. Bioreactor scale-up and oxygen transfer rate in microbial processes: An overview. Biotechnol. Adv. 2009, 27, 153–176. [Google Scholar] [CrossRef] [PubMed]
- Sadino-Riquelme, M.C.; Rivas, J.; Jeison, D.; Donoso-Bravo, A.; Hayes, R.E. Investigating a stirred bioreactor: Impact of evolving fermentation broth pseudoplastic rheology on mixing mechanisms. Fermentation 2022, 8, 102. [Google Scholar] [CrossRef]
- Xie, M.-h.; Xia, J.-y.; Zhou, Z.; Zhou, G.-z.; Chu, J.; Zhuang, Y.-p.; Zhang, S.-l.; Noorman, H. Power consumption, local and average volumetric mass transfer coefficient in multiple-impeller stirred bioreactors for xanthan gum solutions. Chem. Eng. Sci. 2014, 106, 144–156. [Google Scholar] [CrossRef]
- Chachi, M.; Kamla, Y.; Alhaffar, M.T.; Bouzit, M.; Meliani, M.H.; Al-Badour, F.A.; Rahman, M.M.; Suleiman, R.K. Quantitative assessment of agitator performance in an anchor-stirred tank: Investigating the impact of geometry, eccentricity, and rheological characteristics. Arab. J. Sci. Eng. 2024, 49, 13885–13895. [Google Scholar] [CrossRef]
- Woiciechowski, A.L.; Soccol, C.R.; Rocha, S.N.; Pandey, A. Xanthan gum production from cassava bagasse hydrolysate with Xanthomonas campestris using alternative sources of nitrogen. Appl. Biochem. Biotechnol. 2004, 118, 305–312. [Google Scholar] [CrossRef]
- Habibi, H.; Khosravi-Daranib, K. Effective variables on production and structure of xanthan gum and its food applications: A review. Biocatal. Agric. Biotechnol. 2017, 10, 130–140. [Google Scholar] [CrossRef]
- Wu, S.J.; Wu, J.H.; Xia, L.Z.; Chu, C.; Liu, D.; Gong, M. Preparation of xanthan-derived oligosaccharides and their hydroxyl radical scavenging activity. Carbohydr. Polym. 2013, 92, 1612–1614. [Google Scholar] [CrossRef] [PubMed]
- Klaic, P.M.A.; Vendruscolo, C.T.; Furlan, L.; Moreira, A.S. Ion exchange as post-fermentative process enhancer of viscosity of xanthan produced by Xanthomonas arboricola pv pruni. Food Hydrocoll. 2016, 56, 118–126. [Google Scholar] [CrossRef]
- da Silva, L.C.C.; Brenda, N.T.; Furtado, M.M.; de Oliveira Pinto, M.A.; Rodarte, M.P.; Hungaro, H.M. Xanthan: Biotechnological production and applications. In Microbial Production of Food Ingredients and Additives; Elsevier: Oxford, UK, 2017; pp. 385–422. [Google Scholar] [CrossRef]
- Chilakamarry, C.T.; Sakinah, A.M.M.; Zularisam, A.W.; Sirohi, R.; Khilji, I.A.; Ahmad, N.; Pandey, A. Advances in solid-state fermentation for bioconversion of agricultural wastes to value-added products: Opportunities and challenges. Bioresour. Technol. 2022, 343, 126065. [Google Scholar] [CrossRef]
- Barcelos, M.C.S.; Vespermann, K.A.C.; Pelissari, F.M.; Molina, G. Current status of biotechnological production and applications of microbial exopolysaccharides. Crit. Rev. Food Sci. Nutr. 2020, 60, 1475–1495. [Google Scholar] [CrossRef]
- Murad, H.A.; Mohamed, S.H.; Abu-El-Khair, A.G. Impact of amino acids, nitrogen source and buffering system on xanthan yield produced on hydrolysed whey lactose. Biotechnology 2017, 16, 69–76. [Google Scholar] [CrossRef]
- Rončević, Z.Z.; Bajić, B.Ž.; Grahovac, J.A.; Dodić, S.N.; Dodić, J.M. Effect of the initial glycerol concentration in the medium on the xanthan biosynthesis. APTEFF 2014, 45, 239–246. [Google Scholar] [CrossRef]
- Leela, K.J.; Sharma, G. Studies on xanthan production from Xanthomonas campestris. Bioprocess Eng. 2000, 23, 687–689. [Google Scholar] [CrossRef]
- Mohsin, A.; Akyliyaevna, K.A.; Zaman, W.Q.; Hussain, M.H.; Mohsin, M.Z.; Al-Rashed, S.; Tan, X.; Tian, X.; Aida, K.; Tariq, M.; et al. Kinetically modelled approach of xanthan production using different carbon sources: A study on molecular weight and rheological properties of xanthan. Int. J. Biol. Macromol. 2021, 193, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Rico, M.; Rodríguez-Lamazares, S.; Barral, L.; Bouza, R.; Montero, B. Processing and characterization of polyols plasticized-starch reinforced with microcrystalline cellulose. Carbohydr. Polym. 2016, 149, 83–93. [Google Scholar] [CrossRef]
- Rzechonek, D.A.; Dobrowolski, A.; Rymowicz, W.; Mirończuk, A.M. Recent advances in biological production of erythritol. Crit. Rev. Biotechnol. 2018, 38, 620–633. [Google Scholar] [CrossRef] [PubMed]
- Mensi, M.; Scotti, E.; Sordillo, A.; Dalè, M.; Calza, S. Clinical evaluation of air polishing with erythritol powder followed by ultrasonic calculus removal versus conventional ultrasonic debridement and rubber cup polishing for the treatment of gingivitis: A split-mouth randomized controlled clinical trial. Int. J. Dent. Hyg. 2022, 20, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, A.; Zhang, K.; Hu, J.; Salim-ur-Rehman; Tariq, M.; Zaman, W.Q.; Khan, I.M.; Zhuang, Y.; Guo, M. Optimized biosynthesis of xanthan via effective valorization of orange peels using response surface methodology: A kinetic model approach. Carbohydr. Polym. 2018, 181, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Meydanju, N.; Pirsa, S.; Farzi, J. Biodegradable film based on lemon peel powder containing xanthan gum and TiO2–Ag nanoparticles: Investigation of physicochemical and antibacterial properties. Polym. Test. 2022, 106, 107445. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U. Changes in the antioxidant activities of vegetables as a consequence of interactions between active compounds. J. Funct. Foods 2012, 4, 872–882. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Wang, Y.; Nie, S.; Li, C.; Xie, M. Acetylation and carboxymethylation of the polysaccharide from Ganoderma atrum and their antioxidant and immunomodulating activities. Food Chem. 2014, 156, 279–288. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, J.; Zhu, L.; Zhan, X. Characterization of xanthan gum produced from glycerol by a mutant strain Xanthomonas campestris CCTCC M2015714. Carbohydr. Polym. 2017, 157, 521–526. [Google Scholar] [CrossRef]
- Xiong, X.; Li, M.; Xie, J.; Jin, Q.; Xue, B.; Sun, T. Antioxidant activity of xanthan oligosaccharides prepared by different degradation methods. Carbohydr. Polym. 2013, 92, 1166–1171. [Google Scholar] [CrossRef]
- Delattre, C.; Pierre, G.; Gardarin, C.; Traikia, M.; Elboutachfaiti, R.; Isogai, A.; Michaud, P. Antioxidant activities of a polyglucuronic acid sodium salt obtained from TEMPO-mediated oxidation of xanthan. Carbohydr. Polym. 2015, 116, 34–41. [Google Scholar] [CrossRef]
- Spasojević, I.; Mojović, M.; Blagojević, D.; Spasić, S.D.; Jones, D.R.; Nikolić-Kokić, A.; Spasić, M.B. Relevance of the capacity of phosphorylated fructose to scavenge the hydroxyl radical. Carbohydr. Res. 2009, 344, 80–84. [Google Scholar] [CrossRef]
- Hu, X.; Wang, K.; Yu, M.; He, P.; Qiao, H.; Zhang, H.; Wang, Z. Characterization and antioxidant activity of a low-molecular-weight xanthan gum. Biomolecules 2019, 9, 730. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Li, P.; Zeng, X.; Chen, X.; Xie, Y.; Zeng, Y.; Zhang, Y.; Xie, T. Biosynthesis, structure and antioxidant activities of xanthan gum from Xanthomonas campestris with additional furfural. Carbohydr. Polym. 2019, 216, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Niknezhad, S.V.; Asadollahi, M.A.; Zamani, A.; Biria, D.; Doostmohammadi, M. Optimization of xanthan gum production using cheese whey and response surface methodology. Food Sci. Biotechnol. 2015, 24, 453–460. [Google Scholar] [CrossRef]
- Swami, S.B.; Thakor, N.J.; Haldankar, P.M.; Kalse, S.B. Jackfruit and its many functional components as related to human health: A review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 565–576. [Google Scholar] [CrossRef]
- Dos Santos, F.P.; Jr, A.M.O.; Nunes, T.P.; De Farias Silva, C.E.; De Souza Abud, A.K. Bioconversion of agro-industrial wastes into xanthan gum. Chem. Eng. Trans. 2016, 49, 145–150. [Google Scholar] [CrossRef]
- Katherine, R.F.; Muthukumaran, C.; Sharmila, G.; Kumar, N.M.; Tamilarasan, K.; Jaiganesh, R. Xanthan gum production using jackfruit-seed-powder-based medium: Optimization and characterization. 3 Biotech 2017, 7, 248. [Google Scholar] [CrossRef]
- Nejadmansouri, M.; Razmjooei, M.; Safdarianghomsheh, R.; Shad, E.; Delvigne, F.; Khalesi, M. Semi-continuous production of xanthan in biofilm reactor using Xanthomonas campestris. J. Biotechnol. 2021, 328, 1–11. [Google Scholar] [CrossRef]
- Pereira, M.A.F.; Monteiro, C.R.M.; Pereira, G.N.; Júnior, S.E.B.; Zanella, E.; Ávila, P.F.; Stambuk, B.U.; Goldbeck, R.; de Oliveira, D.; Poletto, P. Deconstruction of banana peel for carbohydrate fractionation. Bioprocess Biosyst. Eng. 2021, 44, 297–306. [Google Scholar] [CrossRef]
- Jonuškienė, I.; Stankevičienė, R.; Kantminienė, K.; Tumosienė, I. The influence of phytohormones on antioxidative and antibacterial activities in callus cultures of Hypericum perforatum L. Agriculture 2023, 13, 1543. [Google Scholar] [CrossRef]
- Minickaitė, R.; Grybaitė, B.; Vaickelionienė, R.; Kavaliauskas, P.; Petraitis, V.; Petraitienė, R.; Tumosienė, I.; Jonuškienė, I.; Mickevičius, V. Synthesis of novel aminothiazole derivatives as promising antiviral, antioxidant and antibacterial candidates. Int. J. Mol. Sci. 2022, 23, 7688. [Google Scholar] [CrossRef]
- Virzì, N.F.; Diaz-Rodriguez, P.; Concheiro, A.; Pittalà, V.; Alvarez-Lorenzo, C. Xanthan gum/Guar gum-based 3D-printed scaffolds for wound healing: Production, characterization, and biocompatibility screening. Carbohydr. Polym. Technol. Appl. 2024, 7, 100523. [Google Scholar] [CrossRef]
- Revin, V.V.; Liyaskina, E.V.; Parchaykina, M.V.; Kurgaeva, I.V.; Efremova, K.V.; Novokuptsev, N.V. Production of bacterial exopolysaccharides: Xanthan and bacterial cellulose. Int. J. Mol. Sci. 2023, 24, 14608. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zheng, M.; Tan, K.B.; Lin, J.; Chen, M.; Zhu, Y. Development of xanthan gum/hydroxypropyl methyl cellulose composite films incorporating tea polyphenol and its application on fresh-cut green bell peppers preservation. Int. J. Biol. Macromol. 2022, 201, 198–206. [Google Scholar] [CrossRef]
- Kumar, N.; Pratibha; Upadhyay, A.; Petkoska, A.T.; Gniewosz, M.; Kieliszek, M. Extending the shelf life of mango (Mangifera indica L.) fruits by using edible coating based on xanthan gum and pomegranate peel extract. J. Food Meas. Charact. 2023, 17, 1300–1308. [Google Scholar] [CrossRef]
- Guo, J.; Ge, L.; Li, X.; Mu, C.; Li, D. Periodate oxidation of xanthan gum and its crosslinking effects on gelatin-based edible film. Food Hydrocoll. 2014, 39, 243–250. [Google Scholar] [CrossRef]
- Furtado, I.F.S.P.C.; Sydney, E.B.; Rodrigues, S.A.; Sydney, A.C.N. Xanthan gum: Applications, challenges, and advantages of this asset of biotechnological origin. Biotechnol. Res. Innov. 2022, 6, e202204. [Google Scholar] [CrossRef]
- de Mello, A.F.M.; de Souza Vandenberghe, L.P.; Herrmann, L.W.; Letti, L.A.J.; Burgos, W.J.M.; Scapini, T.; Manzoki, M.C.; de Oliveira, P.Z.; Soccol, C.R. Strategies and engineering aspects on the scale-up of bioreactors for different bioprocesses. Syst. Microbiol. Biomanuf. 2023, 4, 365–385. [Google Scholar] [CrossRef]
- Piccinno, F.; Hischier, R.; Seeger, S.; Som, C. From laboratory to industrial scale: A scale-up framework for chemical processes in life cycle assessment studies. J. Clean. Prod. 2016, 135, 1085–1097. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonuškienė, I.; Davicijonaitė, E.; Vaškevičiūtė, M.; Kala, I.; Stankevičienė, R.; Kantminienė, K.; Tumosienė, I. Sustainable Production and Antioxidant Activity of Bacterial Xanthan Gum. Molecules 2025, 30, 2734. https://doi.org/10.3390/molecules30132734
Jonuškienė I, Davicijonaitė E, Vaškevičiūtė M, Kala I, Stankevičienė R, Kantminienė K, Tumosienė I. Sustainable Production and Antioxidant Activity of Bacterial Xanthan Gum. Molecules. 2025; 30(13):2734. https://doi.org/10.3390/molecules30132734
Chicago/Turabian StyleJonuškienė, Ilona, Erika Davicijonaitė, Monika Vaškevičiūtė, Ihsan Kala, Rima Stankevičienė, Kristina Kantminienė, and Ingrida Tumosienė. 2025. "Sustainable Production and Antioxidant Activity of Bacterial Xanthan Gum" Molecules 30, no. 13: 2734. https://doi.org/10.3390/molecules30132734
APA StyleJonuškienė, I., Davicijonaitė, E., Vaškevičiūtė, M., Kala, I., Stankevičienė, R., Kantminienė, K., & Tumosienė, I. (2025). Sustainable Production and Antioxidant Activity of Bacterial Xanthan Gum. Molecules, 30(13), 2734. https://doi.org/10.3390/molecules30132734





