Highly Engineered Cr-In/H-SSZ-39 Catalyst for Enhanced Performance in CH4-SCR of NOx
Abstract
:1. Introduction
2. Results and Discussion
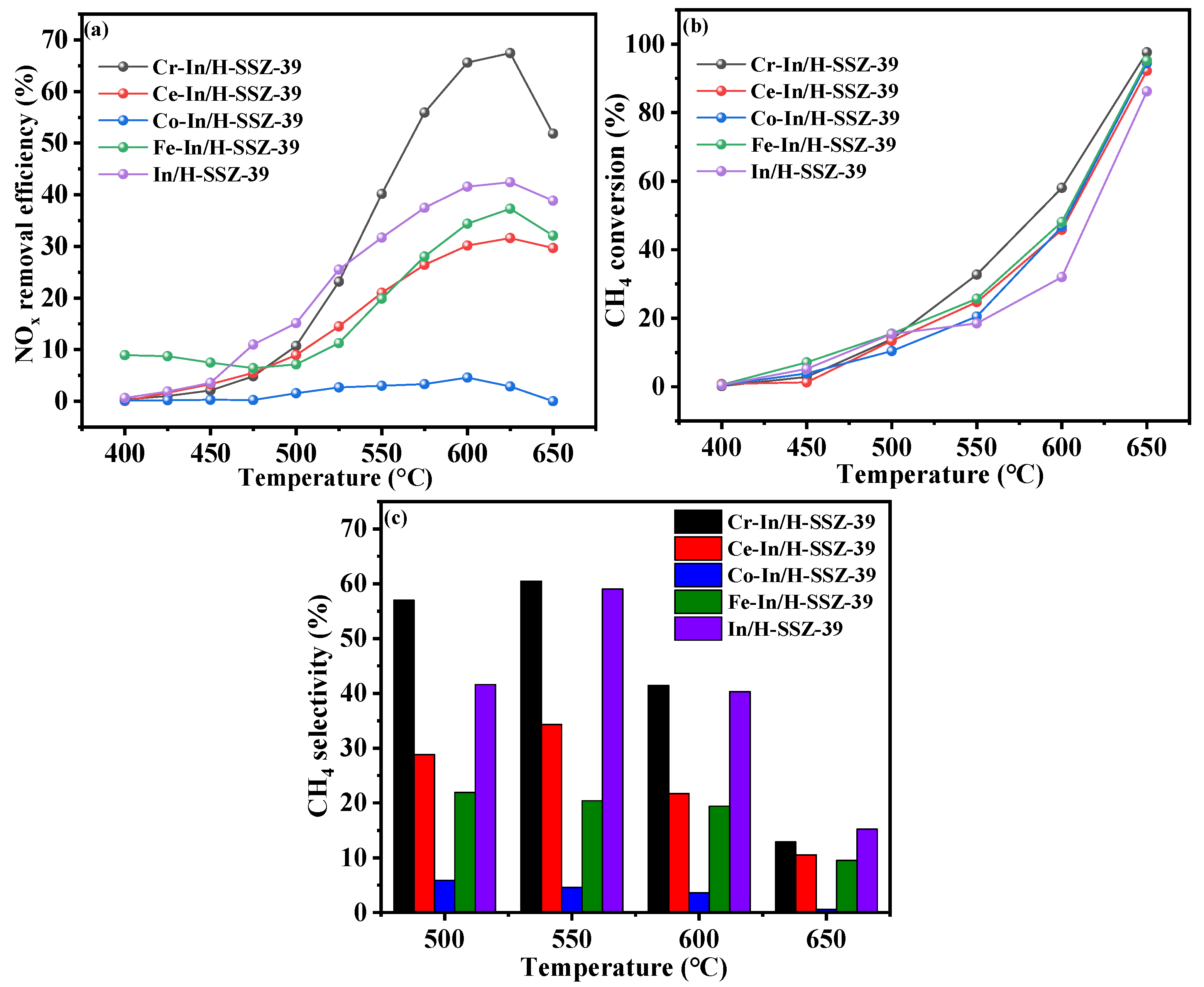
2.1. Screening of Bimetallic Catalysts
2.2. Effects of Preparation Conditions
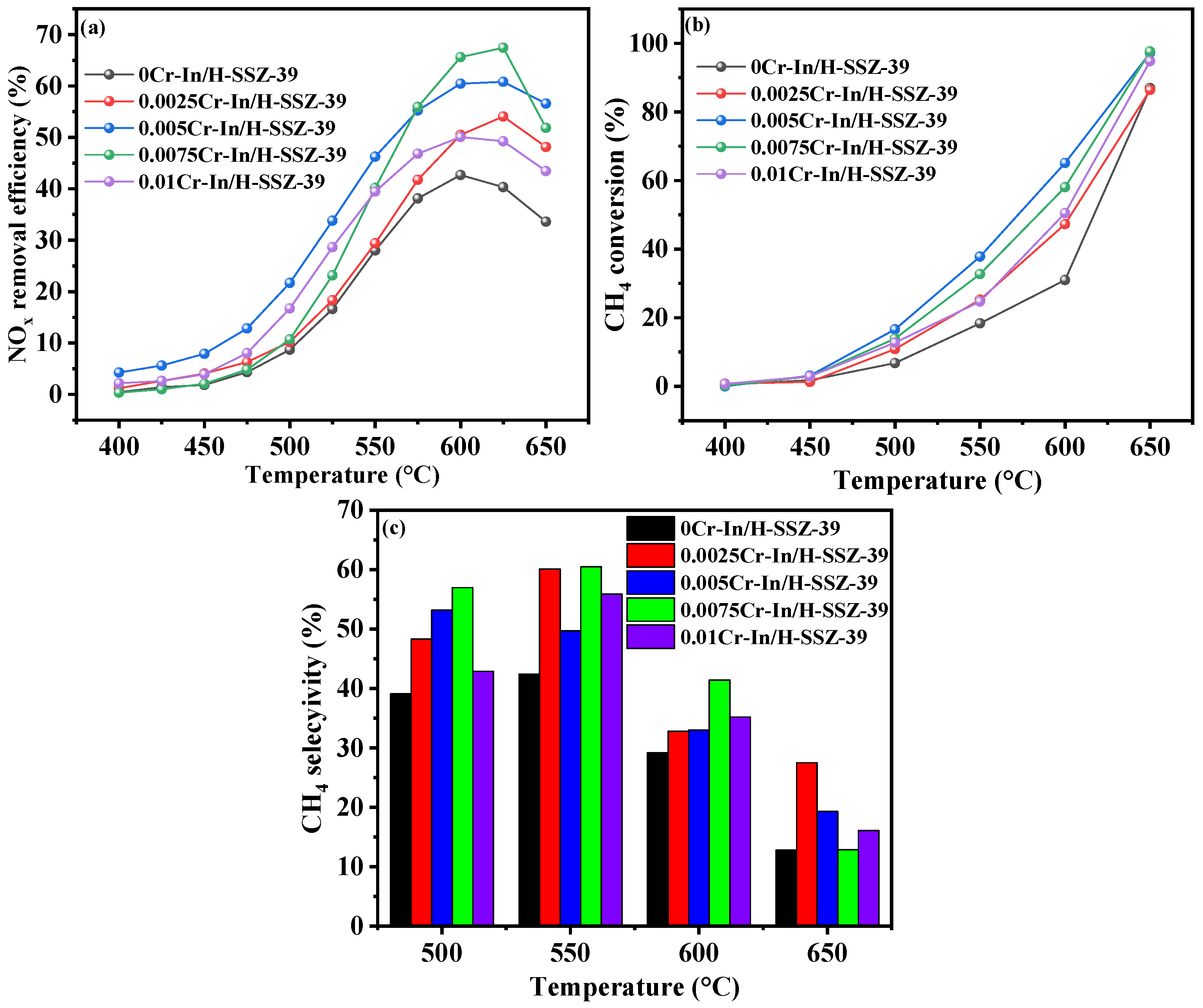
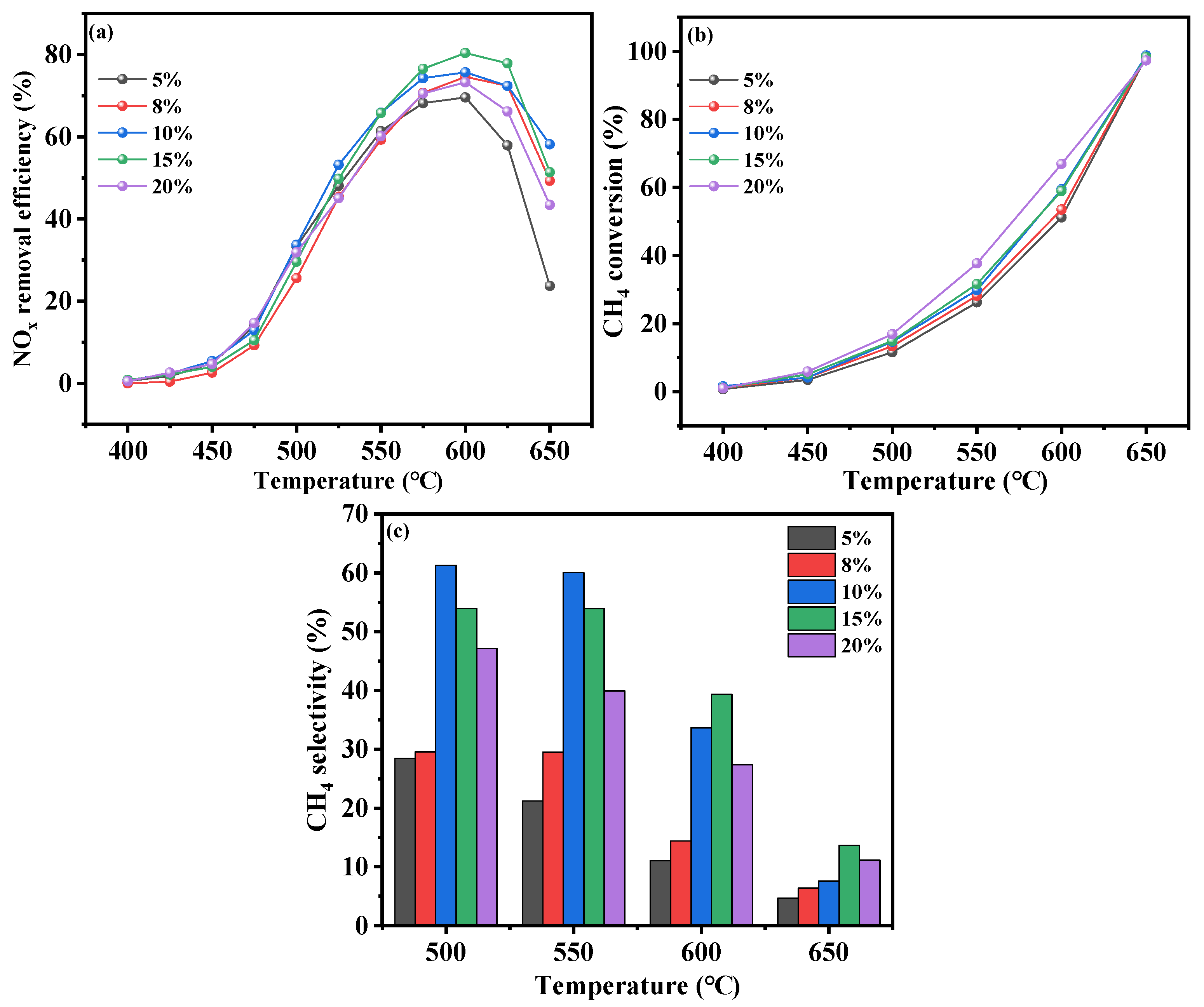
2.2.1. Effects of Cr Concentration
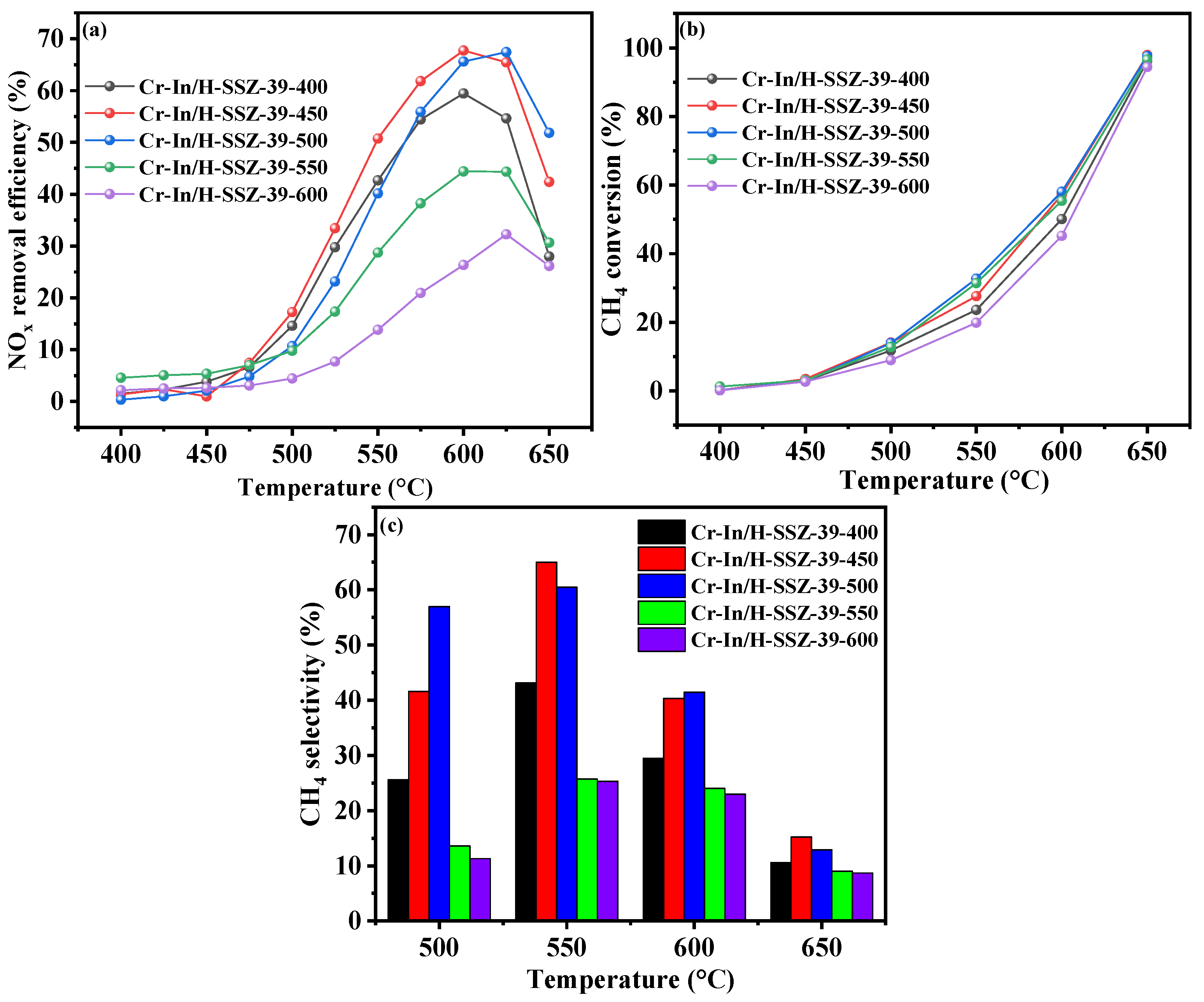
2.2.2. Effects of Calcination Temperature
2.3. Effects of Reaction Conditions
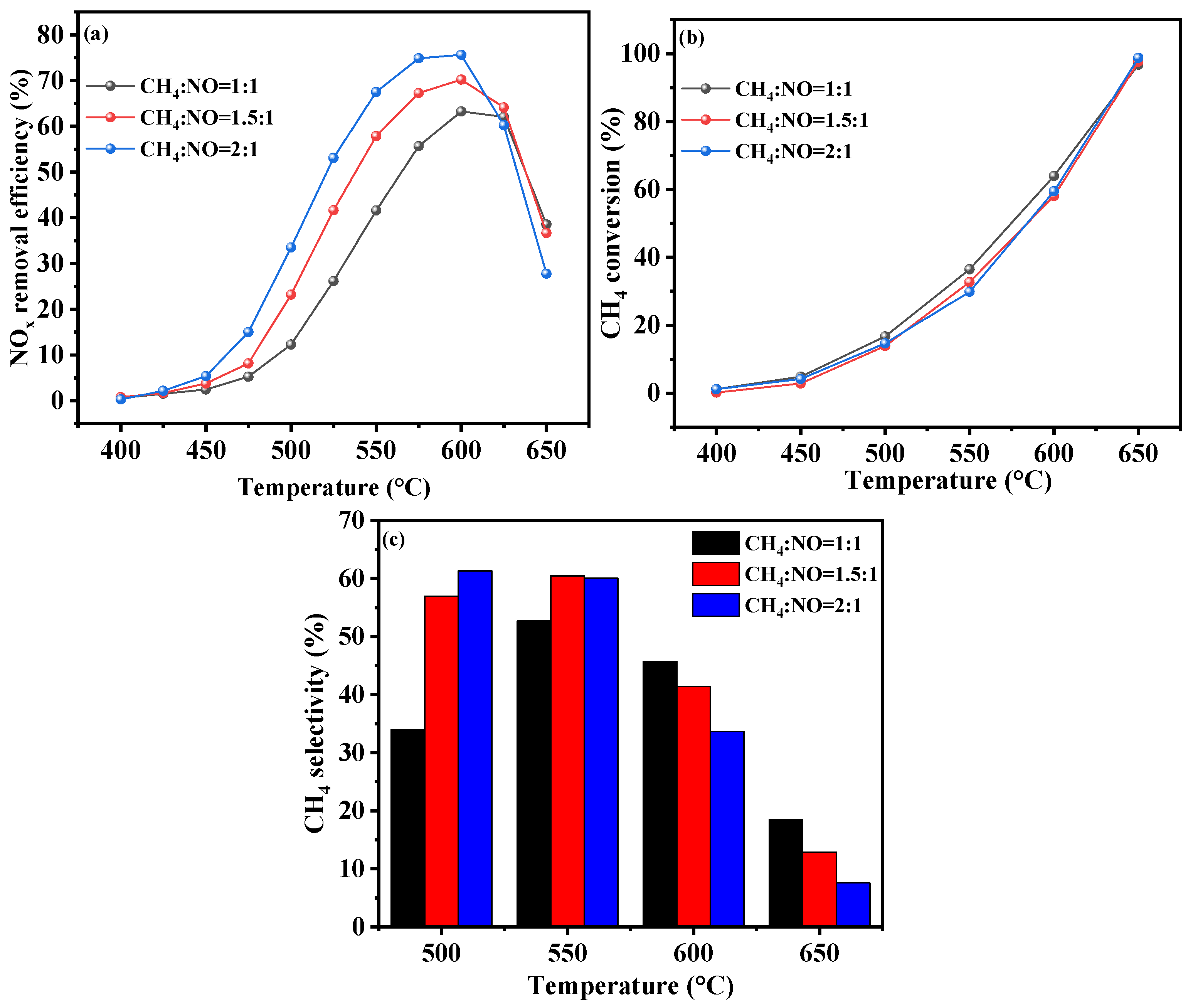
2.3.1. CH4/NO Ratio
2.3.2. O2 Concentration
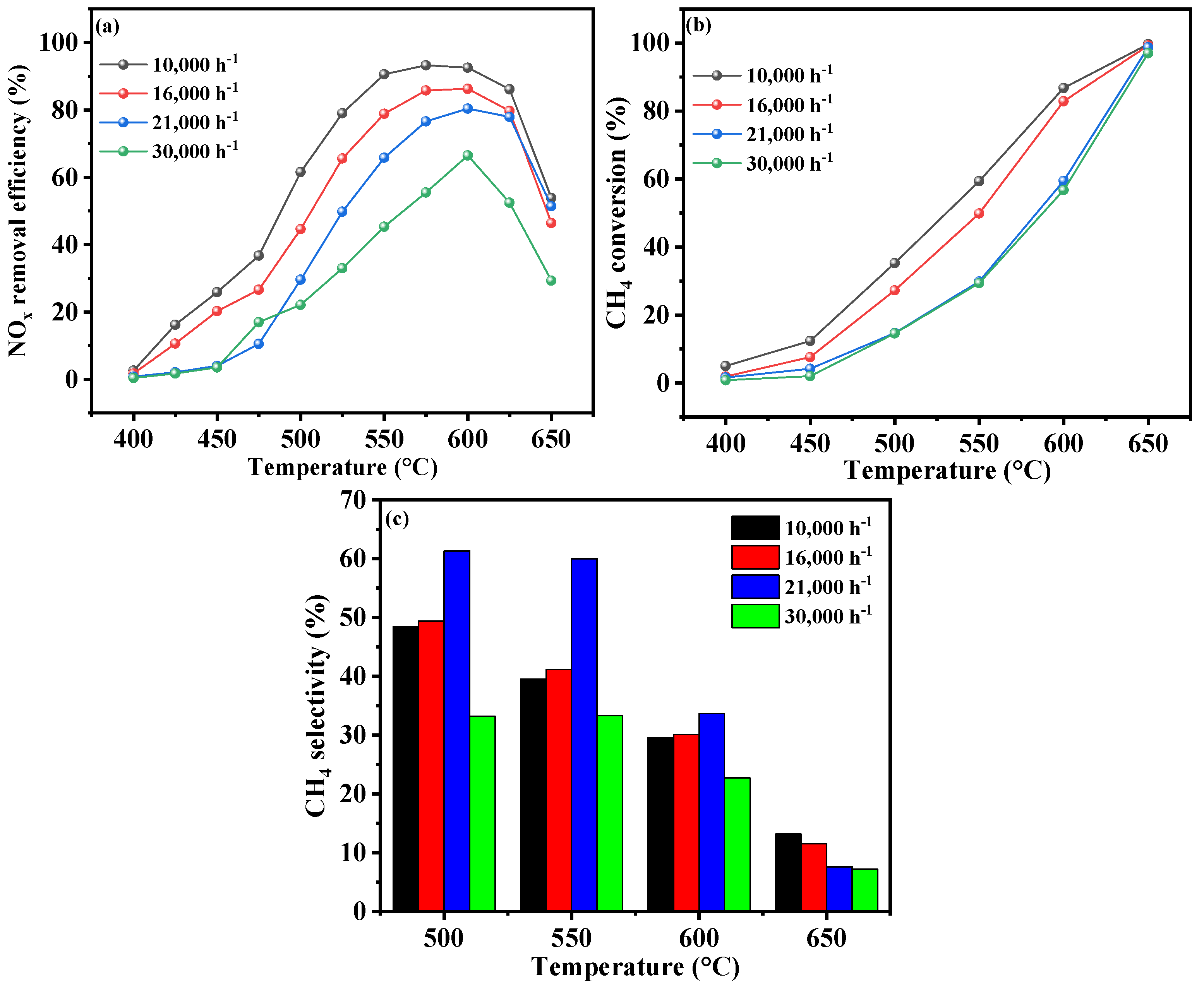
2.3.3. Gaseous Hourly Space Velocity
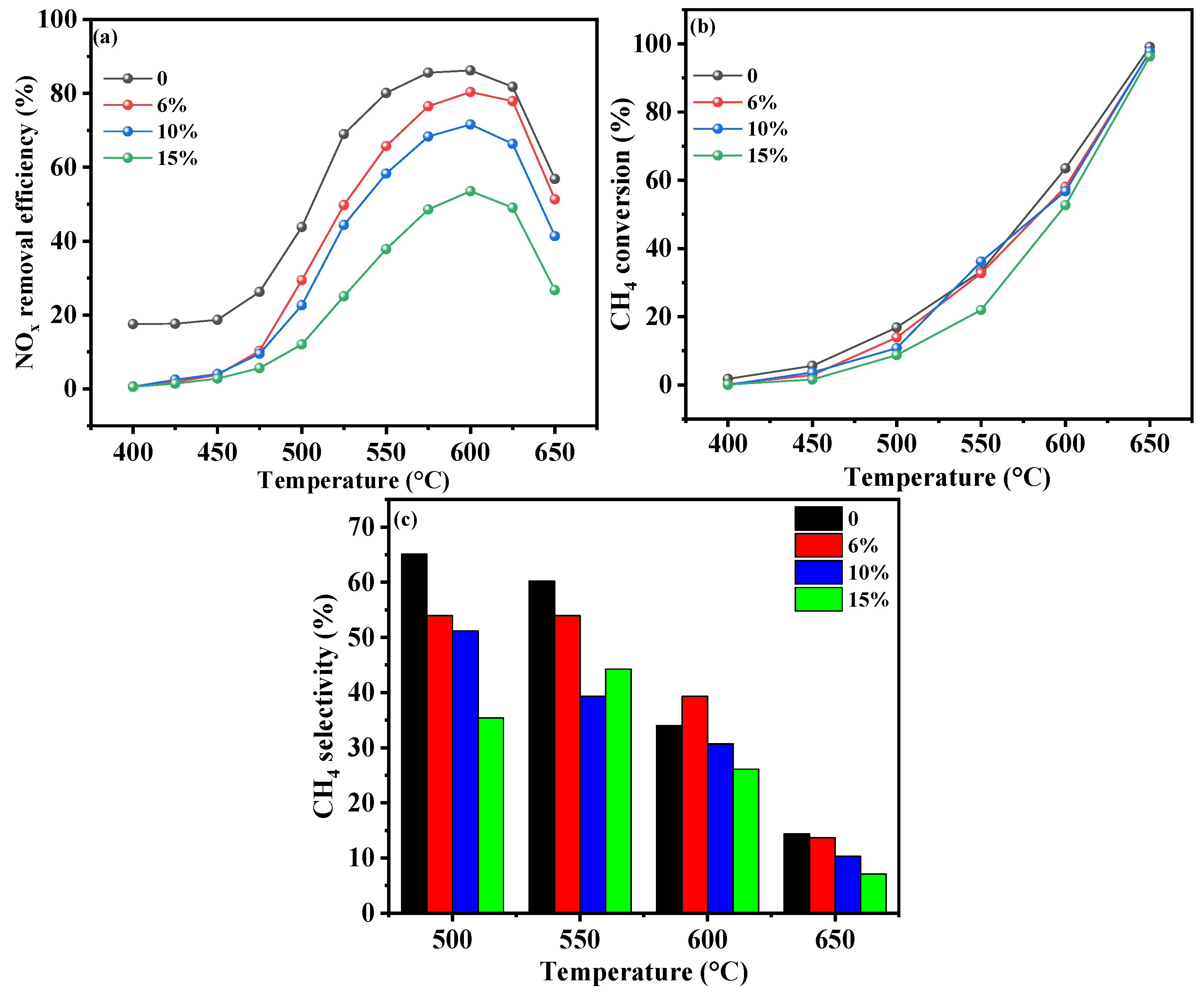
2.3.4. Water Vapor Content
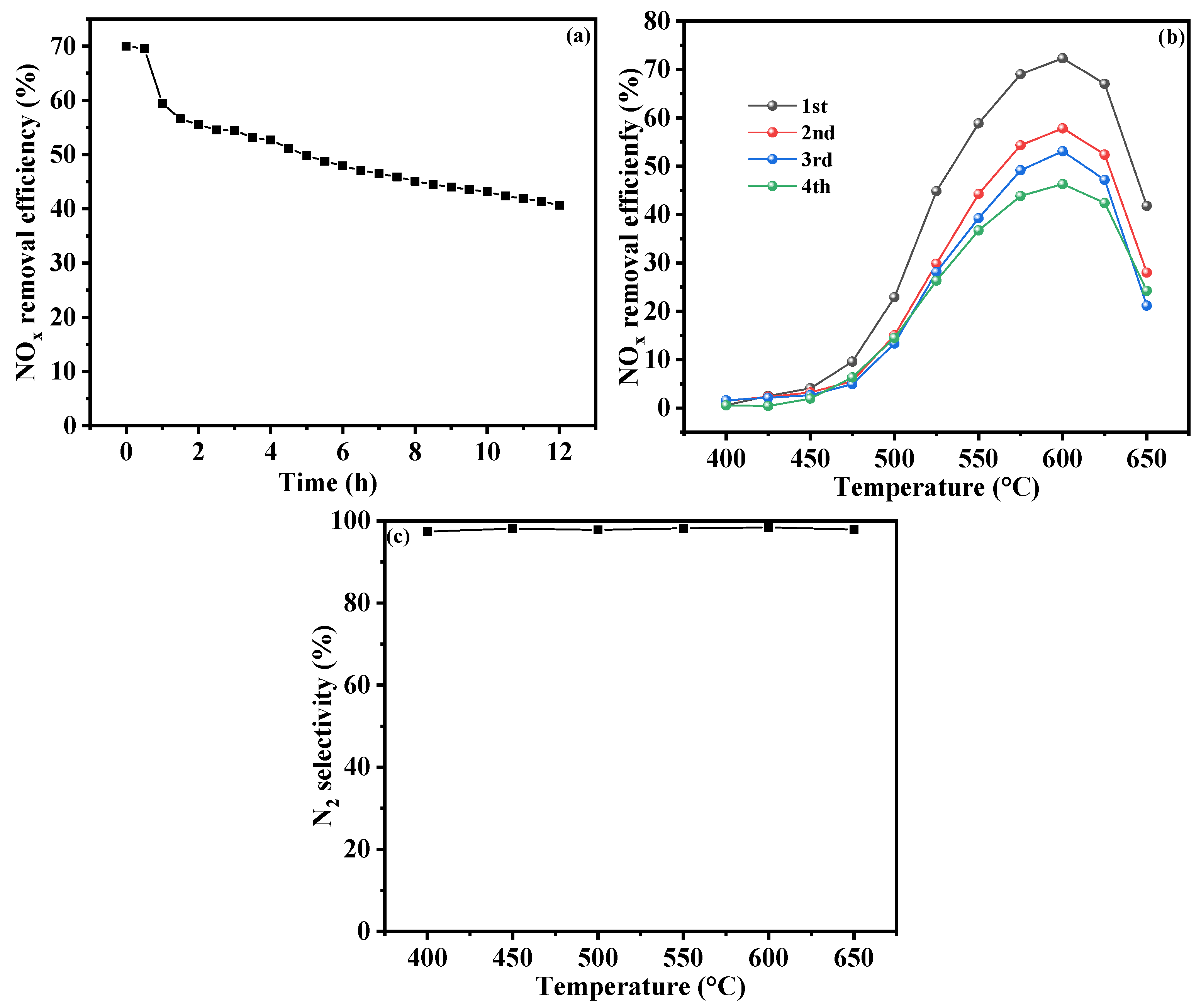
2.4. Cyclic and Stability Testing
2.5. Characterization and Analysis of the Cr-In/H-SSZ-39 Catalyst
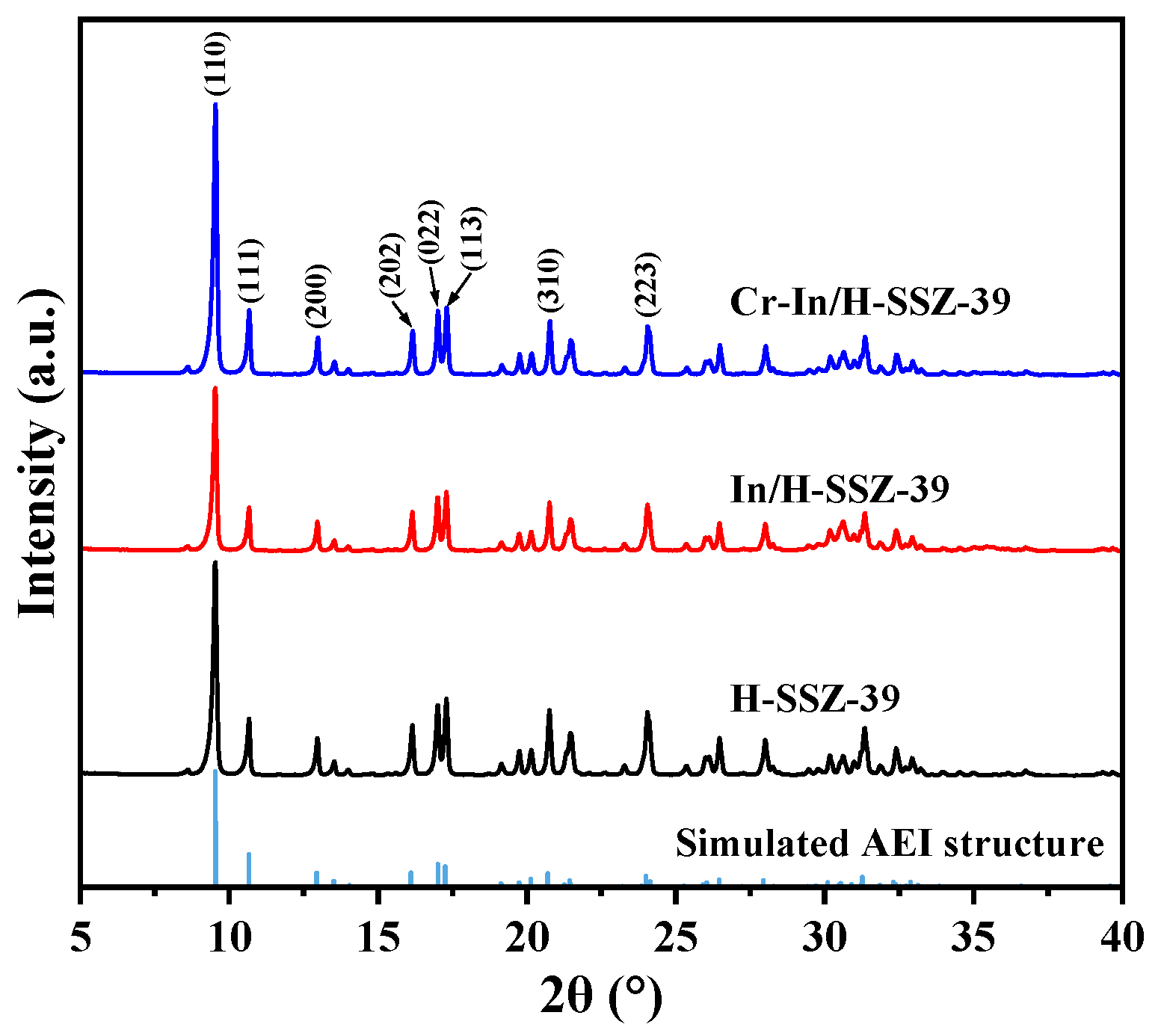
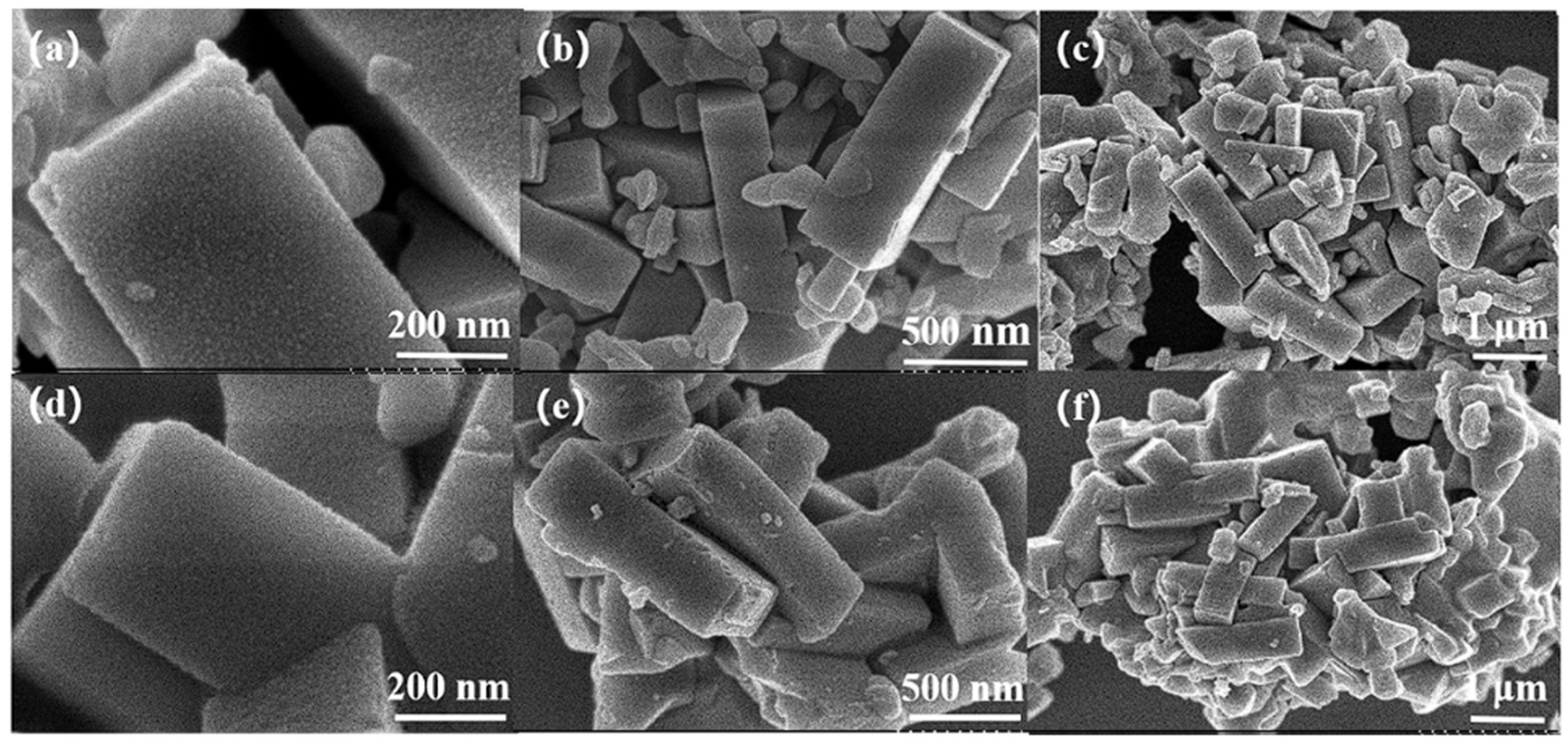
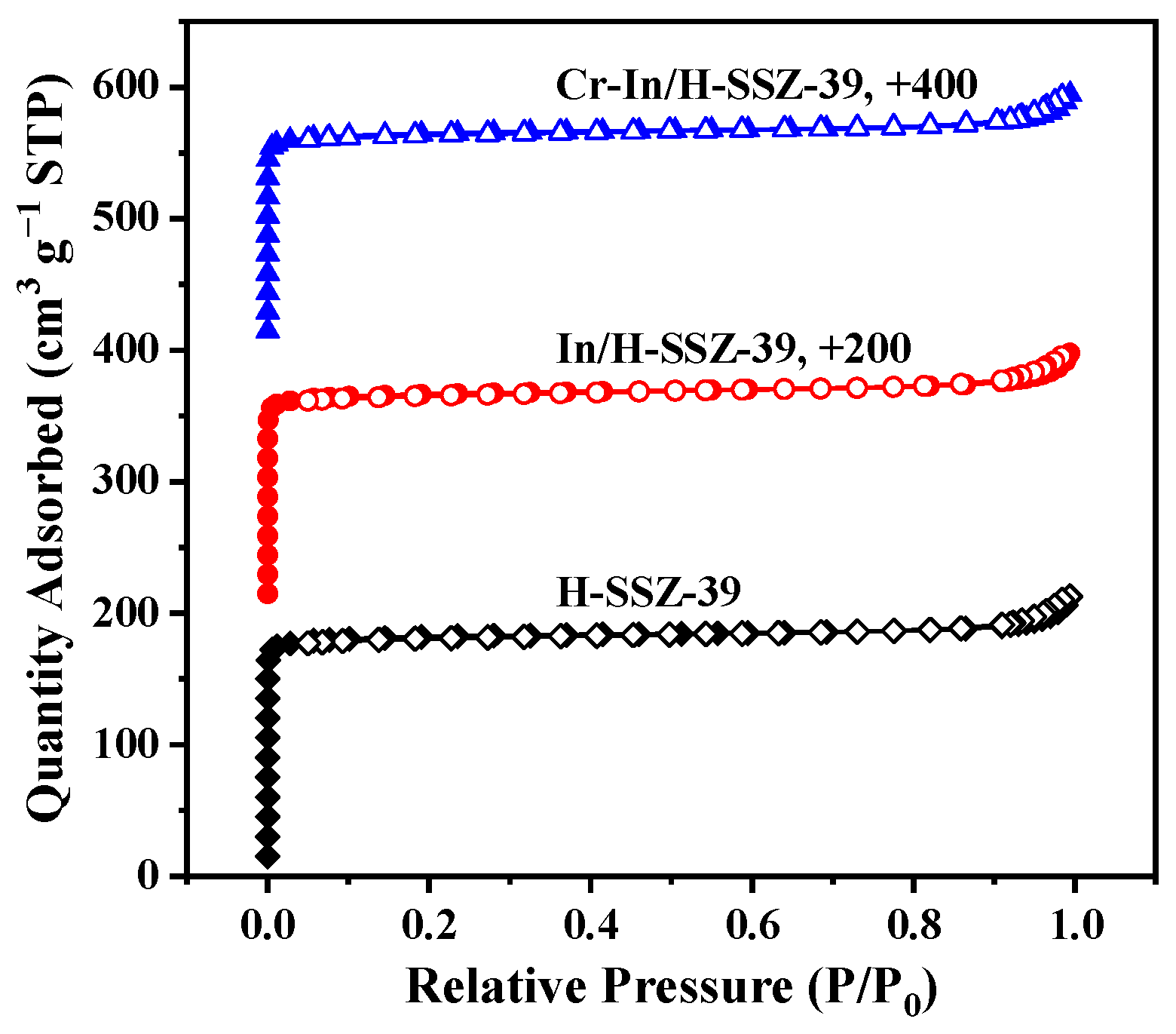
2.5.1. Analysis of Composition and Texture Properties
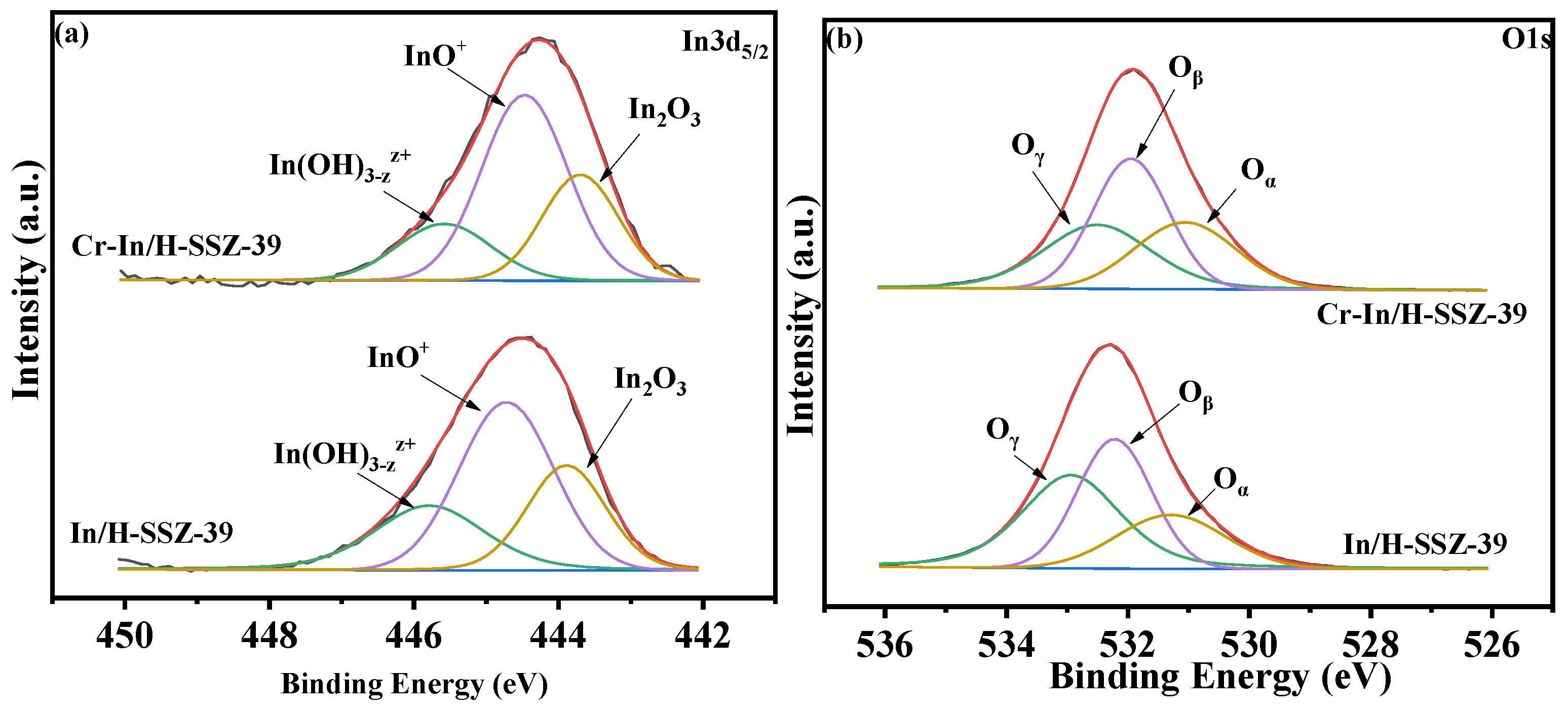
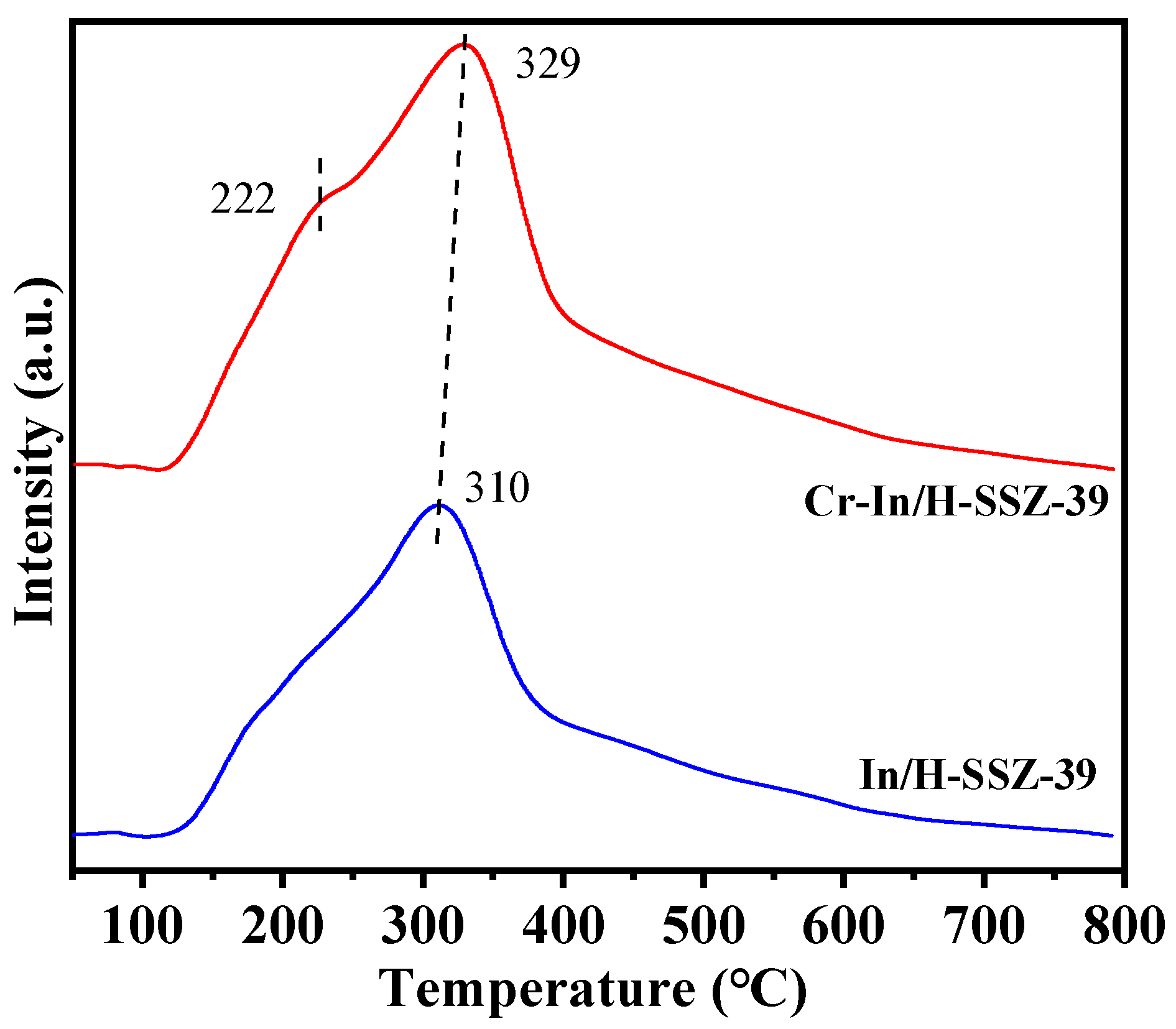
2.5.2. Analysis of Chemical States and Redox Properties
2.6. Characterization of the Cr-In/H-SSZ-39 Catalyst After Reaction
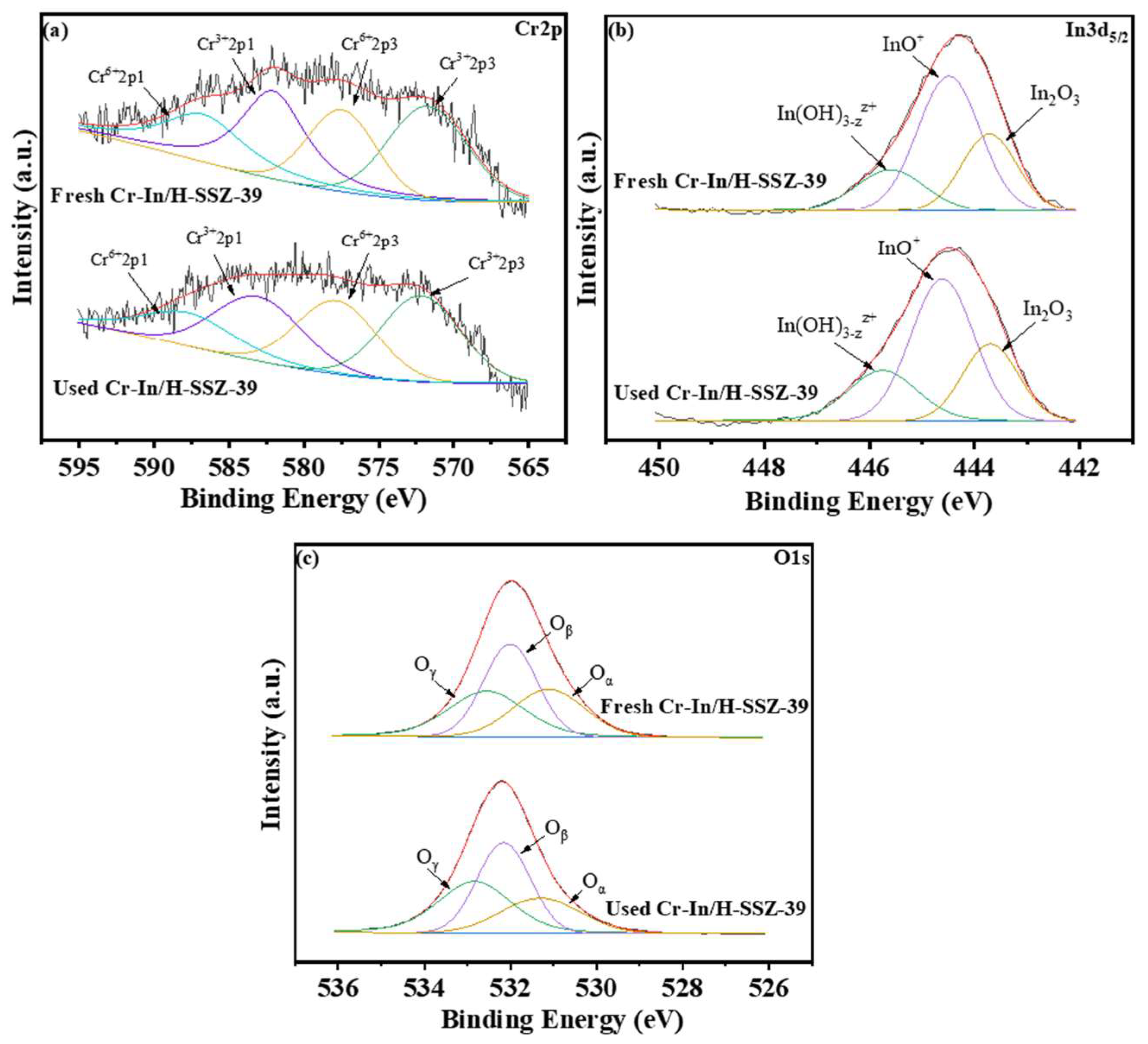
2.6.1. Analysis of Chemical States
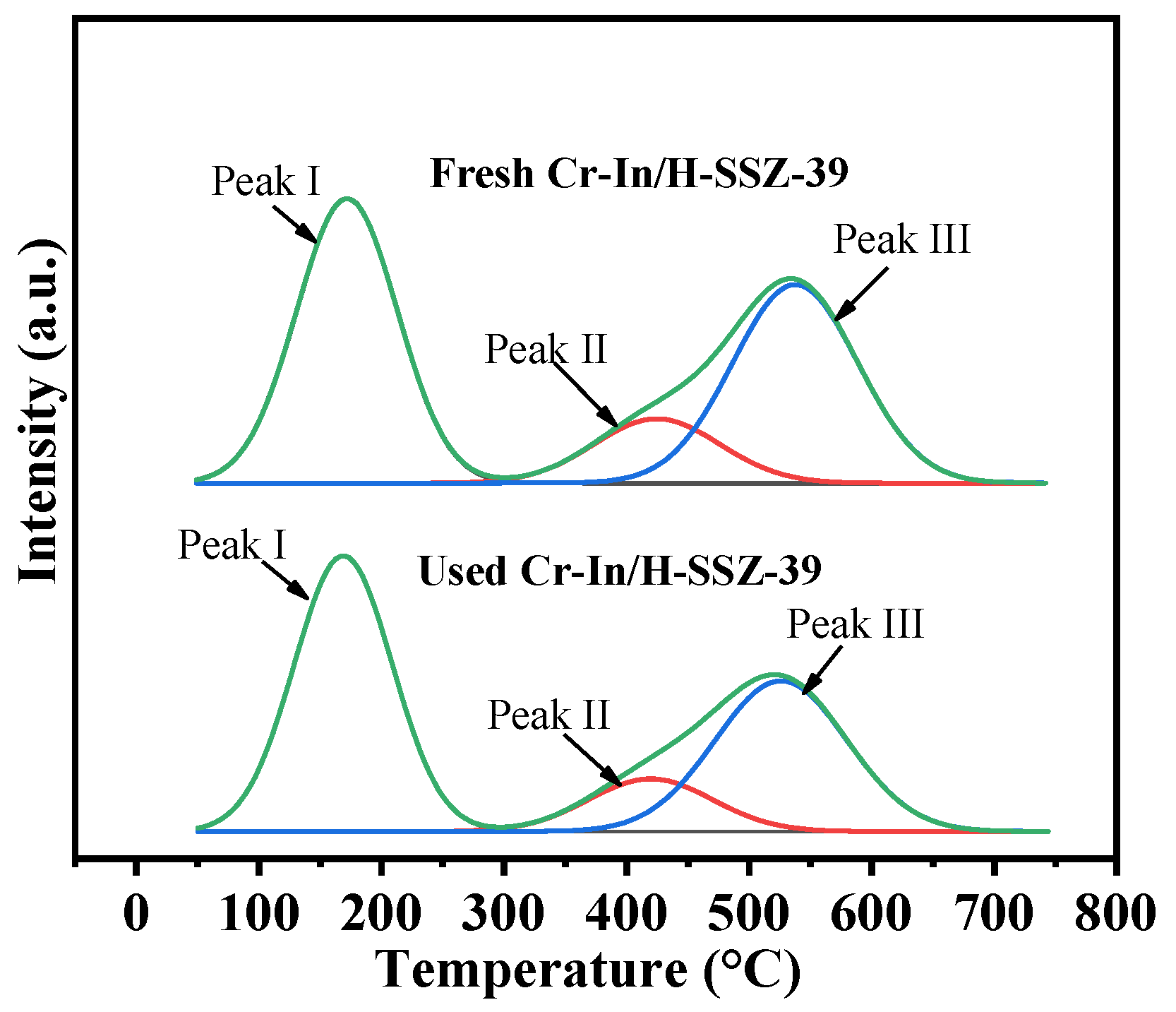
2.6.2. Analysis of Acidity
2.7. Analysis of Water Resistance Mechanism
- It could be seen from PXRD that the catalyst maintained its crystalline properties to a great extent after introducing Cr and In species;
- The NH3-TPD and XPS results showed that the incorporation of Cr into In/H-SSZ-39 increased the number of BAS and generated more InO+ species, thus promoting NO oxidation and CH4 activation;
- A strong interaction between Cr and In species was found from the XPS and H2-TPR results; introducing Cr species increased the redox properties of the catalyst, thus promoting NO oxidation.
3. Experimental Section
3.1. Catalyst Preparation
3.1.1. Chemicals
3.1.2. Preparation of Monometallic In/H-SSZ-39 Catalyst
3.1.3. Preparation of Bimetallic M-In/H-SSZ-39 Catalyst
3.2. Catalytic Activity Measurement
3.3. Catalyst Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Q.-J.; Feng, G.-F.; Wang, H.-J.; Chang, C.-P. The Influence of Political Ideology on Greenhouse Gas Emissions. Glob. Environ. Change 2022, 74, 102496. [Google Scholar] [CrossRef]
- Thomson, H.; Corbett, J.J.; Winebrake, J.J. Natural Gas as a Marine Fuel. Energy Policy 2015, 87, 153–167. [Google Scholar] [CrossRef]
- Zhu, N.; Hong, Y.; Cai, Y.; Dong, F.; Song, J. The Removal of CH4 and NOx from Marine LNG Engine Exhaust by NTP Combined with Catalyst: A Review. Materials 2023, 16, 4969. [Google Scholar] [CrossRef]
- Li, Q.; Cui, K.; Lv, J.; Zhang, J.; Peng, C.; Li, Y.; Gu, Z.; Song, X. Biochar Amendments Increase Soil Organic Carbon Storage and Decrease Global Warming Potentials of Soil CH4 and N2O under N Addition in a Subtropical Moso Bamboo Plantation. For. Ecosyst. 2022, 9, 100054. [Google Scholar] [CrossRef]
- Boningari, T.; Smirniotis, P.G. Impact of Nitrogen Oxides on the Environment and Human Health: Mn-Based Materials for the NOx Abatement. Curr. Opin. Chem. Eng. 2016, 13, 133–141. [Google Scholar] [CrossRef]
- Ricciardolo, F.L.M.; Sterk, P.J.; Gaston, B.; Folkerts, G. Nitric Oxide in Health and Disease of the Respiratory System. Physiol. Rev. 2004, 84, 731–765. [Google Scholar] [CrossRef] [PubMed]
- De Vries, W. Impacts of Nitrogen Emissions on Ecosystems and Human Health: A Mini Review. Curr. Opin. Environ. Sci. 2021, 21, 100249. [Google Scholar] [CrossRef]
- Bellmann, A.; Atia, H.; Bentrup, U.; Brückner, A. Mechanism of the Selective Reduction of NOx by Methane over Co-ZSM-5. Appl. Catal. B 2018, 230, 184–193. [Google Scholar] [CrossRef]
- Li, Y.; Armor, J.N. Selective Catalytic Reduction of NOx with Methane over Metal Exchange Zeolites. Appl. Catal. B 1993, 2, 239–256. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; Qin, M.; Li, Q. Selective Catalytic Reduction of NOx with CH4 over Zeolite Catalysts: Research Progress, Challenges and Perspectives. J. Environ. Chem. Eng. 2022, 10, 107270. [Google Scholar] [CrossRef]
- Kaucký, D.; Vondrová, A.; Dědeček, J.; Wichterlová, B. Activity of Co Ion Sites in ZSM-5, Ferrierite, and Mordenite in Selective Catalytic Reduction of NO with Methane. J. Catal. 2000, 194, 318–329. [Google Scholar] [CrossRef]
- Huang, F.; Hu, W.; Chen, J.; Wu, Y.; Qu, P.; Yuan, S.; Zhong, L.; Chen, Y. Insight into Enhancement of NO Reduction with Methane by Multifunctional Catalysis over a Mixture of Ce/HZSM-5 and CoOx in Excess of Oxygen. Ind. Eng. Chem. Res. 2018, 57, 13312–13317. [Google Scholar] [CrossRef]
- Bustamante, F.; Córdoba, F.; Yates, M.; Montes de Correa, C. The Promotion of Cobalt Mordenite by Palladium for the Lean CH4-SCR of NOx in Moist Streams. Appl. Catal. A 2002, 234, 127–136. [Google Scholar] [CrossRef]
- Gutierrez, L.; Lombardo, E.A. Steam Resistant CoLa-Mordenite Catalysts for the SCR of NOx with CH4. Appl. Catal. A 2009, 360, 107–119. [Google Scholar] [CrossRef]
- Costilla, I.O.; Sanchez, M.D.; Volpe, M.A.; Gigola, C.E. Ce Effect on the Selective Catalytic Reduction of NO with CH4 on Pd-Mordenite in the Presence of O2 and H2O. Catal. Today 2011, 172, 84–89. [Google Scholar] [CrossRef]
- Anunziata, O.A.; Beltramone, A.R.; Requejo, F.G. In-Containing BEA Zeolite for Selective Catalytic Reduction of NOx: Part I: Synthesis, Characterization and Catalytic Activity. J. Mol. Catal. A Chem. 2007, 267, 194–201. [Google Scholar] [CrossRef]
- Pan, H.; Jian, Y.; Yu, Y.; Chen, N.; He, C.; He, C. Promotional Mechanism of Propane on Selective Catalytic Reduction of NOx by Methane over In/H-BEA at Low Temperature. Appl. Surf. Sci. 2016, 390, 608–616. [Google Scholar] [CrossRef]
- Feng, B.; Zhao, T.; Du, J.; Hu, J.; Shi, Y.; Zhao, J.; Chen, J. Reaction and Deactivation Mechanisms of a CeIn/HBEA Catalyst with Dual Active Sites for Selective Catalytic Reduction of NOx by CH4. Appl. Catal. B 2024, 358, 124343. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Y.; Wang, Y.; Li, Z.; Nkinahamira, F.; Zhu, R.; Zhang, J.; Sun, S.; Zhu, Y.; Li, H.; et al. The Poisoning Mechanism of H2O/SO2 to In/H-Beta for Selective Catalytic Reduction of NOx with Methane. Appl. Catal. A 2023, 649, 118973. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, L.; Wang, Y.; Zhang, J.; Zhu, R.; Li, C.; Hong, M. Amino-Acid Modulated Hierarchical In/H-Beta Zeolites for Selective Catalytic Reduction of NO with CH4 in the Presence of H2O and SO2. Nanoscale 2022, 14, 5915–5928. [Google Scholar] [CrossRef]
- Shan, Y.; Shan, W.; Shi, X.; Du, J.; Yu, Y.; He, H. A Comparative Study of the Activity and Hydrothermal Stability of Al-Rich Cu-SSZ-39 and Cu-SSZ-13. Appl. Catal. B 2020, 264, 118511. [Google Scholar] [CrossRef]
- Du, J.; Tang, X.; Huang, C.; Liu, J.; Shan, Y.; Zhang, Y.; Ren, M.; Wang, Y.; Shan, W.; Yu, Y.; et al. Facile One-Pot Synthesis of Cu-SSZ-39 Catalysts with Excellent Catalytic Performance in NH3-SCR Reaction. Appl. Catal. B 2024, 356, 124258. [Google Scholar] [CrossRef]
- Fickel, D.W.; D’Addio, E.; Lauterbach, J.A.; Lobo, R.F. The Ammonia Selective Catalytic Reduction Activity of Copper-Exchanged Small-Pore Zeolites. Appl. Catal. B 2011, 102, 441–448. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, Y.; Shan, Y.; Chen, J.; Du, J.; He, G.; He, H. Unexpected Promotion Effect of H2O on the Selective Catalytic Reduction of NOx with NH3 over Cu-SSZ-39 Catalysts. Environ. Sci. Technol. 2024, 58, 3520–3529. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Han, S.; Huang, C.; Shan, Y.; Zhang, Y.; Shan, W.; He, H. Comparison of Precursors for the Synthesis of Cu-SSZ-39 Zeolite Catalysts for NH3-SCR Reaction. Appl. Catal. B 2023, 338, 123072. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Ma, Y.; Fan, K.; Zhang, J.; Wu, J.; Yan, W.; Meng, X.; Wu, Q.; Yang, F.; et al. Selective Catalytic Reduction of NOx with Methane over Cobalt-Exchanged SSZ-39 Zeolite. Chem. Eng. J. 2024, 496, 153191. [Google Scholar] [CrossRef]
- An, S.; Wang, P.; Wang, K.; Wang, X.; Li, B.; Guo, X. Efficient in/SSZ-39 Catalysts for the Selective Catalytic Reduction of NO with CH4. Front. Chem. 2024, 12, 1439581. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, W.; Zhu, R.; Chen, Y.; Zhao, M.; Hong, M. Engineering In-Co3O4/H-SSZ-39(OA) Catalyst for CH4-SCR of NOx: Mild Oxalic Acid (OA) Leaching and Co3O4 Modification. Molecules 2024, 29, 3747. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, J.; Wang, M.; Chen, J.; Li, J.; Wang, X.; Zhu, R. Innovative in/H-SSZ-39 Catalysts: An Exploration in NOx Reduction via CH4-SCR. Catalysts 2024, 14, 582. [Google Scholar] [CrossRef]
- Yang, J.; Chang, Y.; Dai, W.; Wu, G.; Guan, N.; Li, L. Bimetallic Cr-In/H-SSZ-13 for Selective Catalytic Reduction of Nitric Oxide by Methane. Chin. J. Catal. 2018, 39, 1004–1011. [Google Scholar] [CrossRef]
- Yang, J.; Chang, Y.; Dai, W.; Wu, G.; Guan, N.; Li, L. Ru-In/H-SSZ-13 for the Selective Reduction of Nitric Oxide by Methane: Insights from Temperature-Programmed Desorption Studies. Appl. Catal. B 2018, 236, 404–412. [Google Scholar] [CrossRef]
- Zhao, J.; Wen, Z.; Zhu, R.; Li, Z.; Ding, R.; Zhu, Y.; Gu, T.; Yang, R.; Zhu, Z. In/H-Beta Modified by Co3O4 and Its Superior Performance in the Presence of H2O and SO2 for Selective Catalytic Reduction of NOx with CH4. Chem. Eng. J. Adv. 2020, 3, 100029. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, G.; Zhang, Y.; Chang, C.; Jiang, M.; Ruan, L.; Xiao, M.; Yan, Z.; Yu, Y.; He, H. Designing a Ce/In-CHA OXZEO Catalyst for High-Efficient Selective Catalytic Reduction of Nitrogen Oxide with Methane. Appl. Catal. B 2024, 348, 123820. [Google Scholar] [CrossRef]
- Ge, W.; Wang, X.; Liu, Y.; Miao, B.; Li, Q. Enhanced Activity and Water Resistance of SiO2-Coated Co/In-SSZ-13 Catalysts in the Selective Catalytic Reduction of NOx with CH4. J. Environ. Chem. Eng. 2024, 12, 113746. [Google Scholar] [CrossRef]
- Wang, C.; Lv, G.; Li, Y.; Liu, Y.; Song, C. Selective Catalytic Reduction of NOX with CH4 over in/SSZ-13 Zeolites: The Enhancement of High-Temperature Catalytic Activity by Ce Modification. J. Environ. Chem. Eng. 2024, 12, 111830. [Google Scholar] [CrossRef]
- Shi, Y.; Pu, J.; Gao, L.; Shan, S. Selective Catalytic Reduction of NOx with NH3 and CH4 over Zeolite Supported Indium-Cerium Bimetallic Catalysts for Lean-Burn Natural Gas Engines. Chem. Eng. J. 2021, 403, 126394. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, J.; Ding, R.; Zhu, R.; Li, H.; Li, Z.; Zhang, J.; Zhu, Y.; Li, H. Insights into the Superior Resistance of In-Co3O4-Ga2O3/H-Beta to SO2 and H2O in the Selective Catalytic Reduction of NOx by CH4. J. Colloid Interface Sci. 2022, 626, 89–100. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, G.; He, J.; Wen, Z.; Li, Z.; Gu, T.; Ding, R.; Zhu, Y.; Zhu, R. Effect of Preparation and Reaction Conditions on the Performance of In/H-Beta for Selective Catalytic Reduction of NOx with CH4. Chemosphere 2020, 252, 126458. [Google Scholar] [CrossRef]
- Deng, W.; Tang, Q.; Huang, S.; Zhang, L.; Jia, Z.; Guo, L. Low Temperature Catalytic Combustion of Chlorobenzene over Cobalt Based Mixed Oxides Derived from Layered Double Hydroxides. Appl. Catal. B 2020, 278, 119336. [Google Scholar] [CrossRef]
- Yoo, J.S.; Khan, T.S.; Abild-Pedersen, F.; Nørskov, J.K.; Studt, F. On the Role of the Surface Oxygen Species during A–H (A = C, N, O) Bond Activation: A Density Functional Theory Study. Chem. Commun. 2015, 51, 2621–2624. [Google Scholar] [CrossRef]
- Xing, M.; Liu, N.; Dai, C.; Chen, B. CO2 Oxidative Dehydrogenation of Propane to Olefin over Cr-M (M = Zr, La, Fe) Based Zeolite Catalyst. Catalysts 2024, 14, 370. [Google Scholar] [CrossRef]
| Sample | SBET a | Vtotal b | Vmicro c |
|---|---|---|---|
| (m2 g−1) | (cm3 g−1) | (cm3 g−1) | |
| H-SSZ-39 | 754.5 | 0.293 | 0.269 |
| In/H-SSZ-39 | 685.5 | 0.271 | 0.243 |
| Cr-In/H-SSZ-39 | 676.7 | 0.267 | 0.240 |
| Sample | In Content | Cr Content | Al Content | Si Content | Si/Al |
|---|---|---|---|---|---|
| (wt%) | (wt%) | (wt%) | (wt%) | ||
| H-SSZ-39 | / | / | 2.44 | 39.41 | 16 |
| In/H-SSZ-39 | 5.5 | / | 2.45 | 40.33 | 16 |
| Cr-In/H-SSZ-39 | 4.7 | 0.075 | 2.90 | 43.06 | 15 |
| Sample | Oβ/(Oα + Oβ + Oγ) | Cr3+/(Cr3+ + Cr6+) | |
|---|---|---|---|
| In/H-SSZ-39 | 0.49 | 0.36 | / |
| Cr-In/H-SSZ-39 | 0.54 | 0.40 | 0.58 |
| Used Cr-In/H-SSZ-39 | 0.54 | 0.40 | 0.57 |
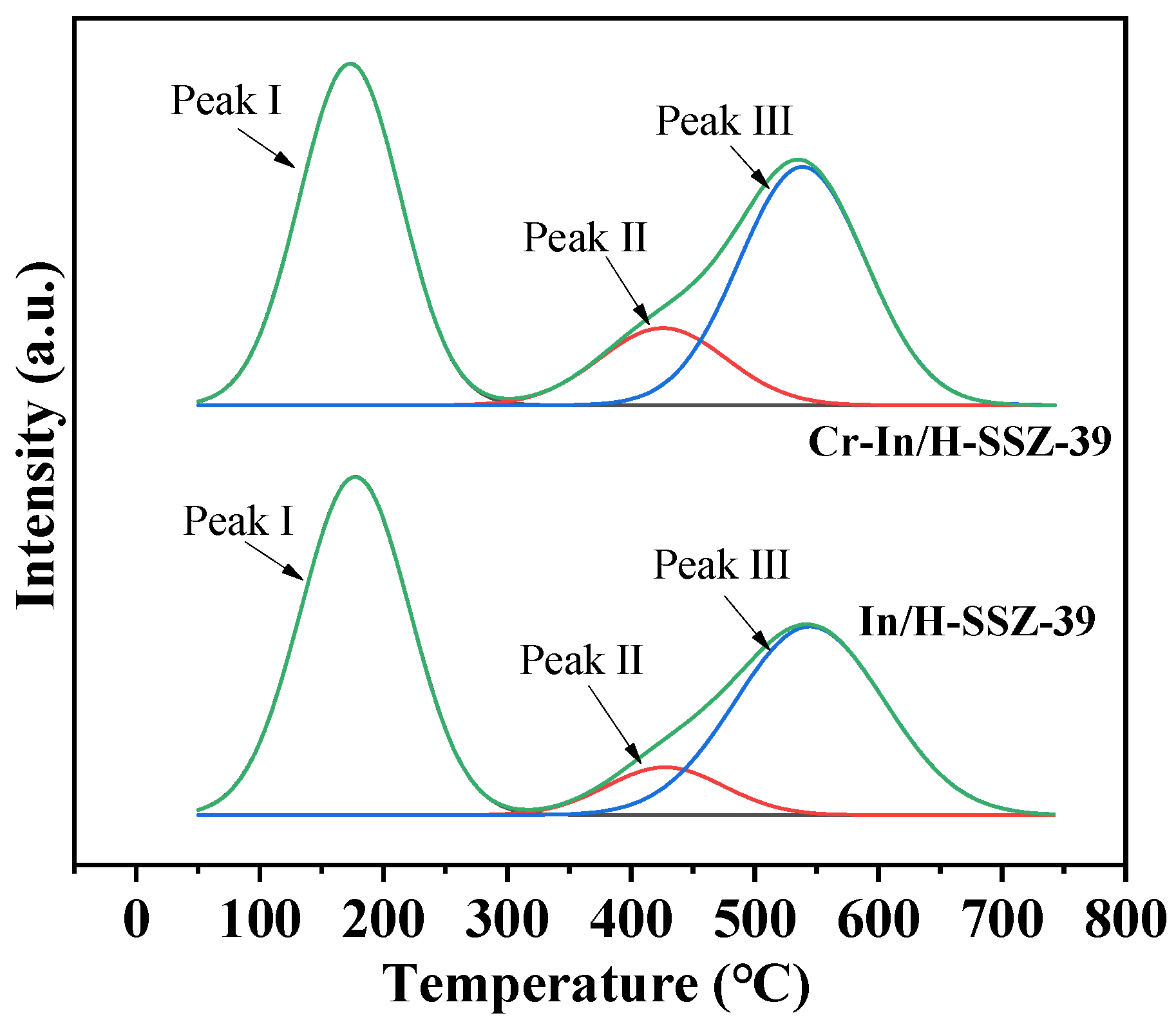
| Sample | Peak I | Peak II | Peak III | Qtotal | |||
|---|---|---|---|---|---|---|---|
| T (°C) | Q (mmol g−1) | T (°C) | Q (mmol g−1) | T (°C) | Q (mmol g−1) | (mmol g−1) | |
| In/H-SSZ-39 | 178 | 1.098 | 477 | 0.186 | 546 | 0.529 | 1.813 |
| Cr-In/H-SSZ-39 | 180 | 1.236 | 471 | 0.195 | 540 | 0.587 | 2.018 |
| Used Cr-In/H-SSZ-39 | 182 | 1.145 | 470 | 0.184 | 538 | 0.552 | 1.881 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Jiang, J.; Chen, G.; Wang, M.; Zuo, X.; Bi, Y.; Zhu, R. Highly Engineered Cr-In/H-SSZ-39 Catalyst for Enhanced Performance in CH4-SCR of NOx. Molecules 2025, 30, 2691. https://doi.org/10.3390/molecules30132691
Zhao J, Jiang J, Chen G, Wang M, Zuo X, Bi Y, Zhu R. Highly Engineered Cr-In/H-SSZ-39 Catalyst for Enhanced Performance in CH4-SCR of NOx. Molecules. 2025; 30(13):2691. https://doi.org/10.3390/molecules30132691
Chicago/Turabian StyleZhao, Jiuhu, Jingjing Jiang, Guanyu Chen, Meng Wang, Xiaoyuan Zuo, Yanjiao Bi, and Rongshu Zhu. 2025. "Highly Engineered Cr-In/H-SSZ-39 Catalyst for Enhanced Performance in CH4-SCR of NOx" Molecules 30, no. 13: 2691. https://doi.org/10.3390/molecules30132691
APA StyleZhao, J., Jiang, J., Chen, G., Wang, M., Zuo, X., Bi, Y., & Zhu, R. (2025). Highly Engineered Cr-In/H-SSZ-39 Catalyst for Enhanced Performance in CH4-SCR of NOx. Molecules, 30(13), 2691. https://doi.org/10.3390/molecules30132691






