1. Introduction
Solid propellants served as the power source for various solid motors used in missiles, weaponry, and spacecraft for space launches. The energy performance of propellants determines their effective range and deterrence capability. Consequently, recent research in the field of high-energy solid propellants has focused primarily on exploring and developing new high-energy-density materials and enhancing the energy content of existing energetic materials. Hydrogen storage fuels [
1] release hydrogen upon thermal decomposition, which reduces the average molecular weight of combustion products, thereby increasing the specific impulse (
Isp) of the propellant. Therefore, incorporating hydrogen storage materials into propellant compositions could effectively enhance the energy level of solid propellants [
1]. Chemical hydride hydrogen storage materials mainly refer to hydrogen combined with other solid materials, such as metals, through ionic or coordination bonds. Chemical hydrogen storage is the most extensively researched and promising method of hydrogen storage. It is broadly categorized into metal-based and non-metal-based hydrogen storage materials. Metal-based hydrogen storage fuels primarily include metal hydrides such as aluminum hydride (AlH
3) and metal borohydrides [
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14]. However, AlH
3 is unstable and reacts with water and moist air to produce hydrogen, while borohydrides have high thermodynamic stability, which poses challenges in hydrogen release during use.
Ammonia borane (AB), as a non-metal hydrogen storage fuel [
7,
15], has garnered significant attention due to its high chemical hydrogen storage capacity (theoretical hydrogen content of 19.6 wt%). The first weight loss of AB occurs during the melting process, which is caused by the thermal dehydrogenation of AB, accompanied by the generation of foamy products [
16]. This exothermic process is very rapid and reaches the maximum decomposition rate between 107 and 125 °C, depending on factors such as heating rate, impurities, and particle size [
17,
18,
19,
20,
21,
22,
23]. The second decomposition step occurs at approximately 130 °C, accompanied by hydrogen release [
24]. In the temperature range of 95 °C to 177 °C, due to the slow decomposition reaction, two exothermic peaks are typically observed on the DSC curve. After the two-step decomposition, a polymeric residue (BNH)
x is obtained, which further dehydrogenates at temperatures well above 500 °C [
17,
18,
19,
20,
21,
22,
23]. When the temperature is raised to 1170–1500 °C, the product transforms into hexagonal boron nitride crystals.
Other by-products have been detected through TGA-MS or TGA-FTIR techniques, primarily borazine (B
3N
3H
6) [
25,
26]. During the first decomposition step, approximately 7 wt% of B
3N
3H
6 is formed within the temperature range of 100–200 °C, with the remainder being produced in the second step [
27]. Other minor gaseous products included diborane, ammonia, H
2N-BH
2, H
2BNH
2BH
3, and sublimated AB.
From these observations, three distinct reaction pathways for the thermal decomposition of AB have been summarized. The hydrogen release process of AB encompasses the three stages mentioned above, accompanied by the release of hydrogen gas and impurities, as well as the generation of (BNH)
x, which is mainly produced during the second stage. Due to the polarity differences between the B-H and N-H bonds in AB, the thermal decomposition of AB proceeds through anisotropic interactions and intermolecular dehydrogenation bonding [
28,
29,
30].
In the initial stages of decomposition, chain products such as BH
2, BH, and BH
3 are rapidly formed. These compounds subsequently transform into diammoniate of diborane (DADB) and H
2 [
30,
31,
32,
33,
34,
35,
36]. During this process, by-products such as ammonia diborane (ADB) are also produced, accompanied by the release of a significant amount of hydrogen. The chain substances undergo autocatalytic reactions to form polyaminoboranes (PABs). Under the catalysis of AB and NH
2BH
2, PABs participate in two competing reactions: propagation to form acyclic PABs or cyclization. Due to subsequent cyclization and cross-linking reactions, the formation of cyclic PABs requires higher temperatures, and these reactions also lead to the formation of borazine (BZ) and polyborazylene (PBZ). The presence of NH
3 can facilitate the reverse synthesis of AB from NH
3BH
2(μ-H)BH
3 [
37,
38]. DADB is the initial product of further decomposition of cyclic products of AB and can also be formed through dehydrogenation bonding between AB molecules. The ring-opening reaction of DADB leads to its gradual conversion into BZ, ultimately yielding polyborazylene (PBZ).
Notably, some progress has been made in the research on AB with oxidizers. Studies found that when AB is composited with AP, the presence of NH
4+ ions allows the thermal reaction of AB to follow an alternative pathway, generating [NH
3BH
3NH
3]
+[ClO
4]
− instead of DADB [
28,
29,
30,
31,
32,
33,
34,
35,
36,
39,
40].
AB is an attractive hydrogen storage material, not only due to its high theoretical hydrogen storage capacity but also because of its easier hydrogen release, which outperforms other hydrogen storage materials such as AlH
3 and metal borohydrides [
8,
9,
10,
11,
12]. However, its low density and poor thermal stability limit its applications. When AB is composited with AP, the presence of NH
4+ ions enables the thermal reaction of AB to follow an alternative pathway, generating [NH
3BH
2NH
3]
+[ClO
4]
− instead of DADB [
28,
29,
30,
31,
32,
33,
34,
35,
36,
40], which indicates the potential formation of ammonium perchlorate borane (APB).
Guillaume [
41] successfully synthesized high-energy-density ammonia dinitroamine borane through the reaction of dinitroamine with ammonia borane. This synthesis method provides a research idea to solve the problem of low density and low thermal stability of AB.
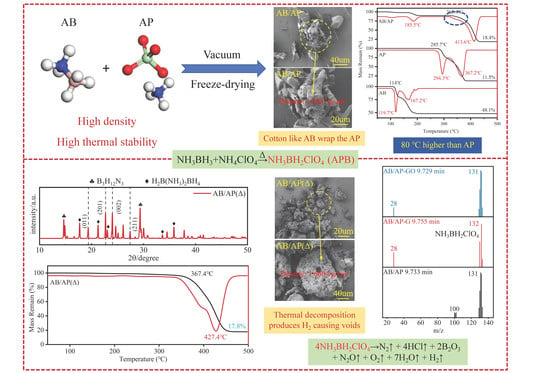
In view of the poor thermal stability and low density of AB, the researchers used AB, AP, graphene (GA), and graphene oxide (GO) as raw materials, weighed AB and AP according to the molar ratio of 1:1, and prepared AB/AP composite particles by the direct freeze-drying method. At the same time, 2 wt% GA or GO was added to prepare carbon material modified AB/AP-GA and AB/AP-GO. Then, AB/AP(Δ), AB/AP-GA(Δ), and AB/AP-GO(Δ) were synthesized by thermal reaction of the three under vacuum. The specific formula is shown in
Table 1 and
Table 2. APB was detected in three kinds of calcined AB/AP. The pyrolysis mechanism and physicochemical properties of APB were systematically studied [
28,
29,
30,
31,
32,
33,
34,
35,
36,
40]. The prepared oxidant/fuel integrated APB has high thermal stability and high density. APB has excellent energy performance and thermal stability, and is considered to be a promising high-energy additive for high-burning-rate solid propellant.
In response to AB’s poor thermal stability and low density, researchers prepared AB/AP composite particles via direct freeze-drying and thermally reacted them under vacuum to synthesize APB. The pyrolysis mechanism and physicochemical properties of APB were systematically studied. The resulting oxidant/fuel-integrated APB exhibited both high thermal stability and increased density. Due to its superior energy performance and excellent thermal stability, APB was considered a promising high-energy additive for high-burning-rate solid propellants.
2. Results
2.1. Microscopic Morphology and Elemental Compositions
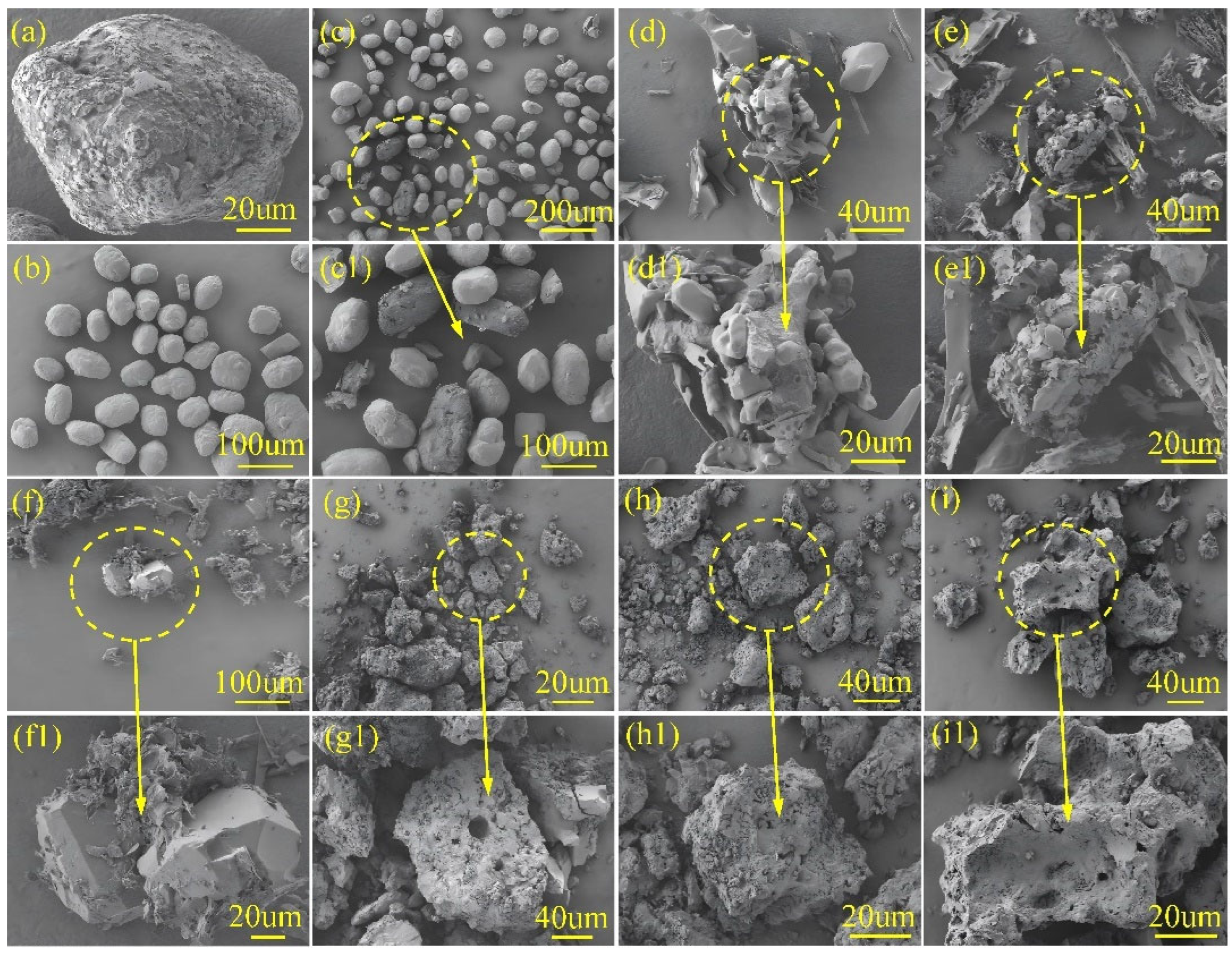
SEM morphological analysis revealed that AB particles exhibited irregular shapes, with most particles measuring approximately 100 μm and displaying numerous surface pits. In contrast, AP crystals predominantly adopted regular cubic or spherical morphologies, with an average particle size similarly around 100 μm.
Figure 1 shows the morphology of the AB/AP mechanical mixture, where well-defined AP particles and AB particles with surface cavities and cracks were observed, both with particle sizes close to 100 μm. The AB/AP crystal morphology, as depicted in
Figure 1, presented a fragmented structure alongside agglomerates of regular crystals. Notably, small, well-defined particles appeared enveloped by fibrous, cotton-like particles, with an average crystal size of only 30 μm. AB/AP-GA displayed agglomerative growth characteristics, featuring elongated graphene strands embedded within the composite particles. The overall particle morphology resembled that of AB/AP, with agglomerated particles measuring between 30 and 40 μm. Similarly, AB/AP-GO demonstrated crystal agglomeration but with larger dimensions, accompanied by numerous small, flake-like crystals filling the interparticle gaps. Comparative analysis indicated that the addition of GA or GO eliminated the cotton-like coating observed in AB/AP, instead promoting the growth of fine, fragmented structures interspersed among larger crystals. This observation suggested that the presence of GA and GO modified the AB/AP crystallization process, resulting in altered agglomeration morphologies. AB/AP(Δ) formed irregular bulk particles approximately 100 μm in size, containing abundant internal pores. AB/AP-GA(Δ) exhibited a morphology similar to APB, with comparable particle sizes around 100 μm. AB/AP-GO(Δ) similarly consisted of large, irregular particles with surface pores measuring about 100 μm. These surface pores originated from hydrogen evolution during the thermal treatment process, after which perchlorate ions combined with the foamed boron-nitrogen polymer to form the ammonia perchlorate borane derivative, APB.
Table 3 presents the elemental composition of the AB/AP composite. The elemental analysis results indicate that thermal treatment led to a reduction in the nitrogen (N) and hydrogen (H) content within the composite, which is attributed to both the release of hydrogen gas during the thermal treatment process and the generation of NH
3, N
2, and trace amounts of nitrogen oxides through AP’s redox reaction. The escape of these gaseous products consequently caused a significant decrease in the N and H content in the calcined product.
The oxygen (O) content of AB/AP-GO and AB/AP-GO(Δ) decreased significantly compared with the theoretical results. This is due to a large number of bubbles emerging from the sample with added GO during the preparation process. It is speculated that the poor compatibility between GO and AB in water may lead to the hydrolysis reaction of AB showing strong reducibility, and the oxidation–reduction reaction with a small amount of ClO4− may lead to the reduction of the measured O content of AB/AP-GO and AB/AP-GO(Δ).
2.2. Chemical Bond Structure and Energetic Properties
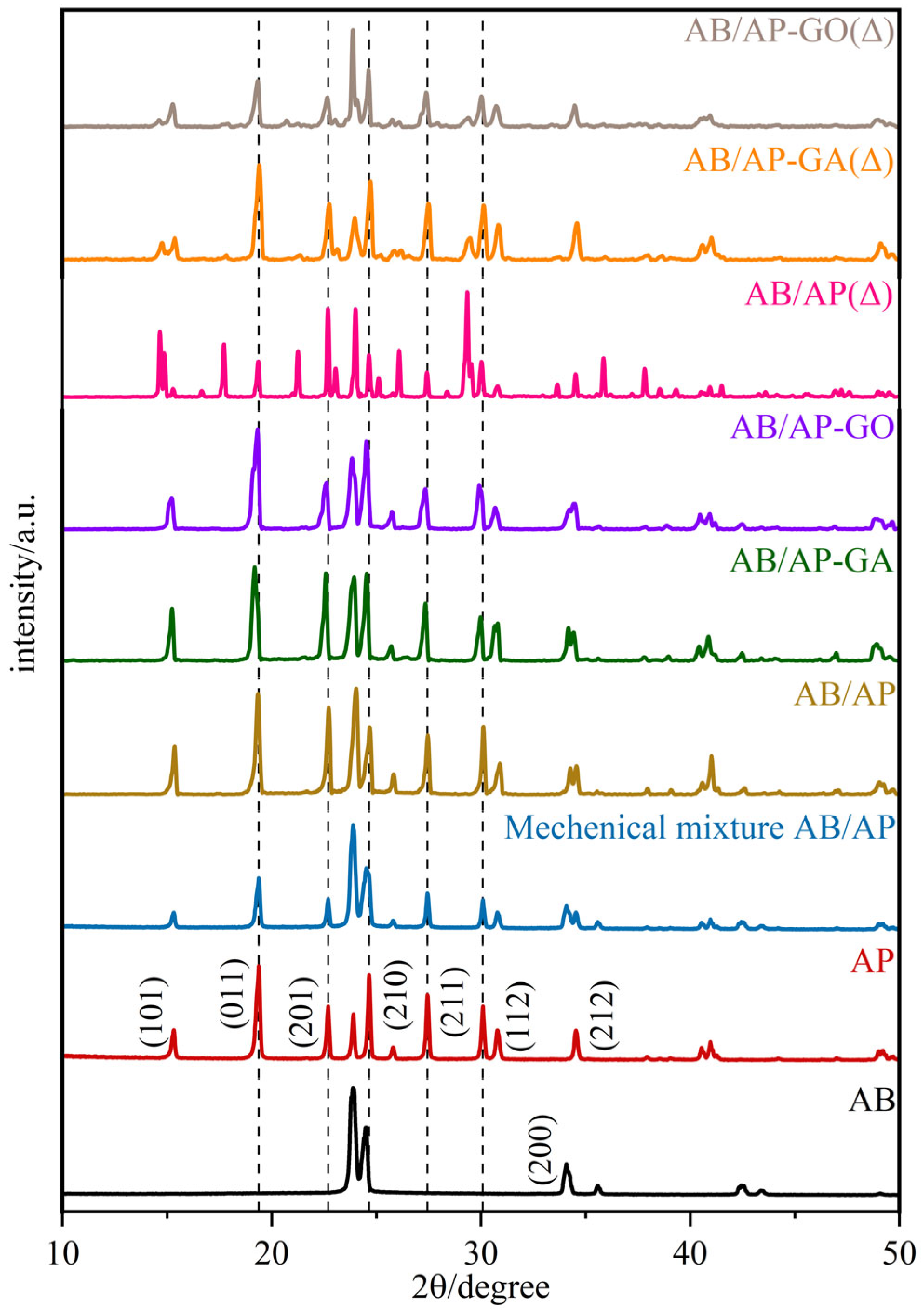
Simultaneously, the XRD diffraction patterns (
Figure 2) revealed that the diffraction peak positions of both AB/AP composite particles and modified AB/AP composites were essentially identical to those of the prepared mechanical mixture, differing only in peak intensity.
Table 4 presents the unit cell parameters of the crystalline samples. The data indicate that the crystal structures of the three AB/AP composites underwent significant changes: while both AB and AP exhibit an orthorhombic system, the composite particles adopted a monoclinic system. Although the three composites share the same crystal system, AB/AP-GO belongs to a different space group compared to the other two. These results confirmed that the characteristic diffraction peaks of AB and AP remained present in the composites modified with GA or GO, but the incorporation of graphene-based materials did not facilitate the formation of new crystal planes between AB and AP. Instead, all composite particles retained a mechanically mixed co-particle morphology. The XRD powder diffraction patterns further demonstrate that AB and AP did not form co-crystals but rather produced a mechanically mixed co-particle system.
Table 4 lists the unit cell parameters of the thermally treated products, showing that while they share the same crystal system, their space groups differ. Furthermore, the diffraction peak positions of the three thermally treated products showed no significant overall differences, with only minor variations in intensity, indicating their fundamental structural similarity.
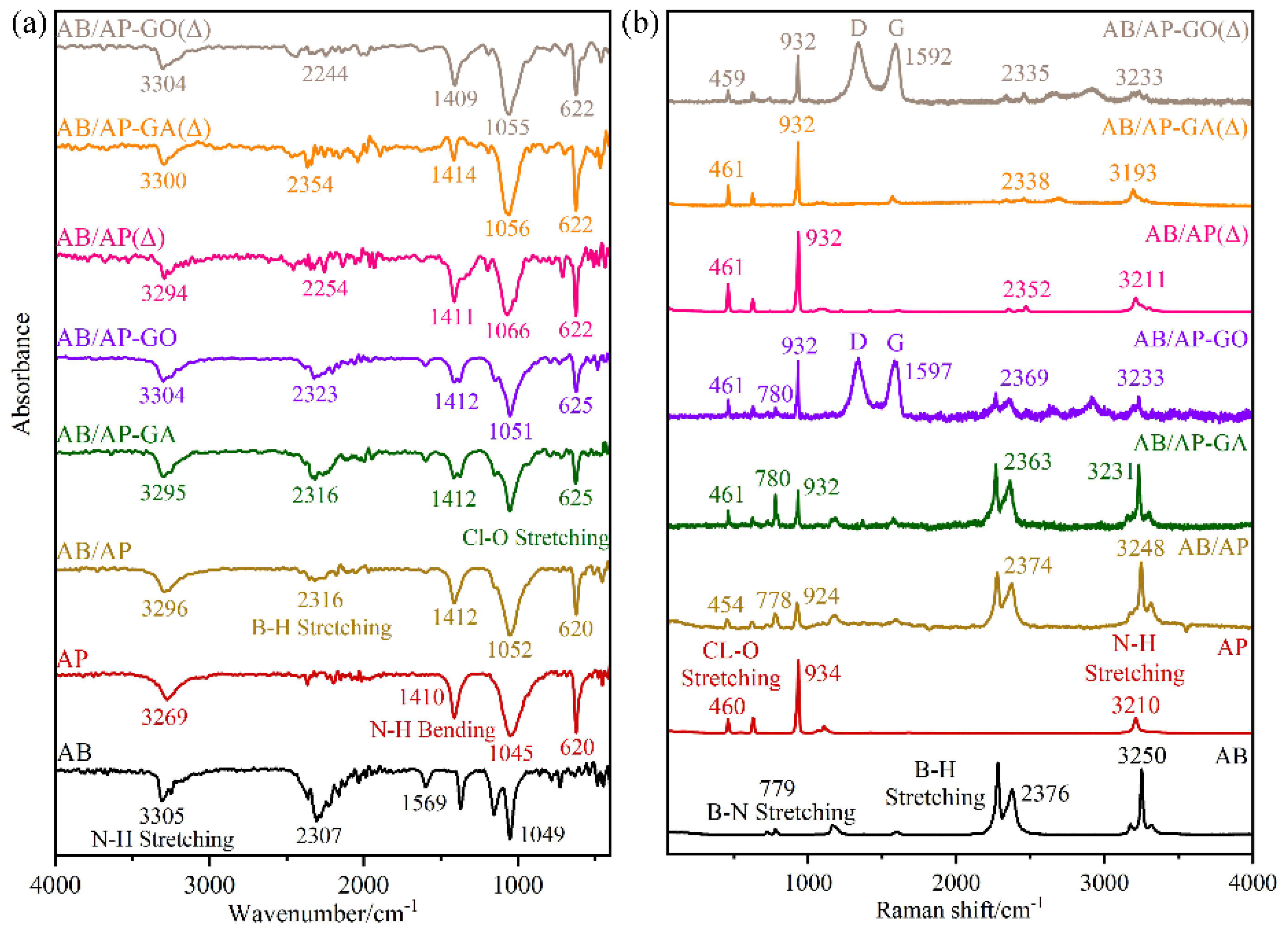
The FTIR and Raman spectra of the composite were tested, and the results are shown in
Figure 3. The characteristic peaks corresponding to each functional group are shown in
Table 5 and
Table 6.
The characteristic peaks of Cl-O and B-H were found in the three composite samples, which also proved that there were two kinds of substances, AB and AP, present. However, compared with the raw material, the positions of the three characteristic peaks have been shifted to a certain extent, mainly due to the obvious changes in the positions of the stretching vibration peaks of B-H and N-H. It is proven that the hydrogen bond between -NH3 and ClO4− has been enhanced due to the interaction of N-H···O, which leads to the change of the stretching vibration peak of N-H. The shift of the B-H stretching vibration may be due to the effect of the H-H bond in AB or the formation of a B-H···O hydrogen bond. The characteristic peak shifts of AB/AP-GO and AB/AP-GA were the same, and the D peak and G peak of graphene oxide were found at 1338 cm−1 and 1597 cm−1. The vibration mode of these three composite particles has been changed because of intermolecular interactions, such as hydrogen bonds, and the intensity and position of the absorption peak have also changed, and it is no longer caused by a single component intermolecular interaction. It was proved that the composite particles were not a simple mechanical mixture, and a new phase was formed, which was different from the mechanical mixture.
The main components of the three heat treatment products are related compounds of amino borane perchlorate. Among them, the stretching vibration peak intensity of ClO4− is particularly significant, and there are also relatively weak B-H stretching vibration peaks and bending vibration peaks. It is worth noting that the characteristic peak positions of these three products are shifted compared with AP, which is mainly due to the formation of APB. This process leads to significant changes in the original functional groups and their interactions, leading to the shift of the characteristic vibration peak positions of B-H and N-H. Compared with AB, the B-N stretching vibration peak of the three heat-treated products disappeared completely. Due to the low content of AB, only part of AP participated in the formation of APB during the heat-treatment process. During this process, some cyclo-borane and APB sublimate and precipitate, which reduces the proportion of amino borane in the system. At this stage, the concentration of cyclization products is very low, below the detection limits of both FTIR and Raman spectroscopy, and this leads to the disappearance of the B-N stretching vibration peak. The shift of the characteristic peak position is due to the change in the interaction between the original AB and AP molecules upon generation of APB. The Raman test results are consistent with those of the FTIR results, which proves that the three products contain APB after heat treatment. These derivatives change the interaction between molecules and form a new phase.
To measure the mass calorific value of the materials, the combustion heat of the composite particles was determined through ignition under a 3 MPa oxygen atmosphere. The density of each material was measured using a densitometer, and the corresponding volumetric calorific values were then calculated. The specific experimental data are presented in
Table 7.
The energetic properties of the materials were evaluated by measuring the combustion heat of composite particles under a 3 MPa oxygen atmosphere using bomb calorimetry, while the material densities were determined with a densitometer to obtain volumetric heat values (specific data presented in
Table 7). The results demonstrate that pure AB achieved the mass-specific heat value of 42.54 kJ·g
−1 in oxygen, whereas pure AP exhibited a mass-specific heat value of 3.64 kJ·g
−1.
As shown in
Table 7, the theoretical mass combustion heat of the AB/AP mechanical mixture reached 11.42 kJ·g
−1, while the combustion heats of the three composite particles were relatively close to this value, indicating that the actual molar ratios of AB to AP in the composites were consistent with the theoretical design. However, the unmodified AB/AP exhibited higher combustion heat, demonstrating that the incorporation of graphene-based materials reduced the energetic performance of the composites. The combustion heats of all three thermally treated products showed significant decreases compared to the raw mechanical mixture: AB/AP(Δ) decreased by 15.3%, AB/AP-GA(Δ) by 21.8%, and AB/AP-GO(Δ) by 30.5%. This reduction was primarily attributed to the sublimation of some ammonia borane derivatives [
32,
33,
34,
35,
36], which led to decreased energetic performance of the system. Nevertheless, the remaining energy performance still maintained a relatively high level, suggesting that the formation of APB [
32,
33,
34,
35,
36,
40] partially compensated for the energy loss. Furthermore, the mass combustion heat data revealed that APB itself possessed relatively high combustion heat. This finding further confirmed that the addition of graphene-based materials negatively impacted the energetic properties of the composite system. The experimental results demonstrated that while graphene modification affected combustion performance, the as-prepared APB derivatives helped maintain considerable energy output in the composites.
The density data in
Table 7 reveal that the measured densities of the composite materials were generally higher than their theoretical values, demonstrating that the molecular interactions between AB and AP components extended beyond simple mechanical mixing to include significant intermolecular forces that promoted tight binding. Notably, the unmodified composites exhibited higher densities compared to their modified counterparts, as the incorporation of low-density graphene-based materials increased molecular dispersion within the modified composites, thereby reducing their overall density. After thermal treatment, all materials showed substantial density increases relative to theoretical values: AB/AP(Δ) by 30.8%, AB/AP-GA(Δ) by 26.2%, and AB/AP-GO(Δ) by 24.4%. These enhancements confirmed that the formation of APB during thermal processing altered the intermolecular interactions, leading to significant modifications in product density. Furthermore, the inherently high density of APB itself may have contributed to the observed increases in system density. The thermal treatment significantly enhanced the density of AB/AP composites, effectively addressing the issue of low density inherent to pure AB materials.
2.3. Thermal Behavior and Decomposition Kinetics
2.3.1. Non-Isothermal Mass Loss and Heat Flow Properties
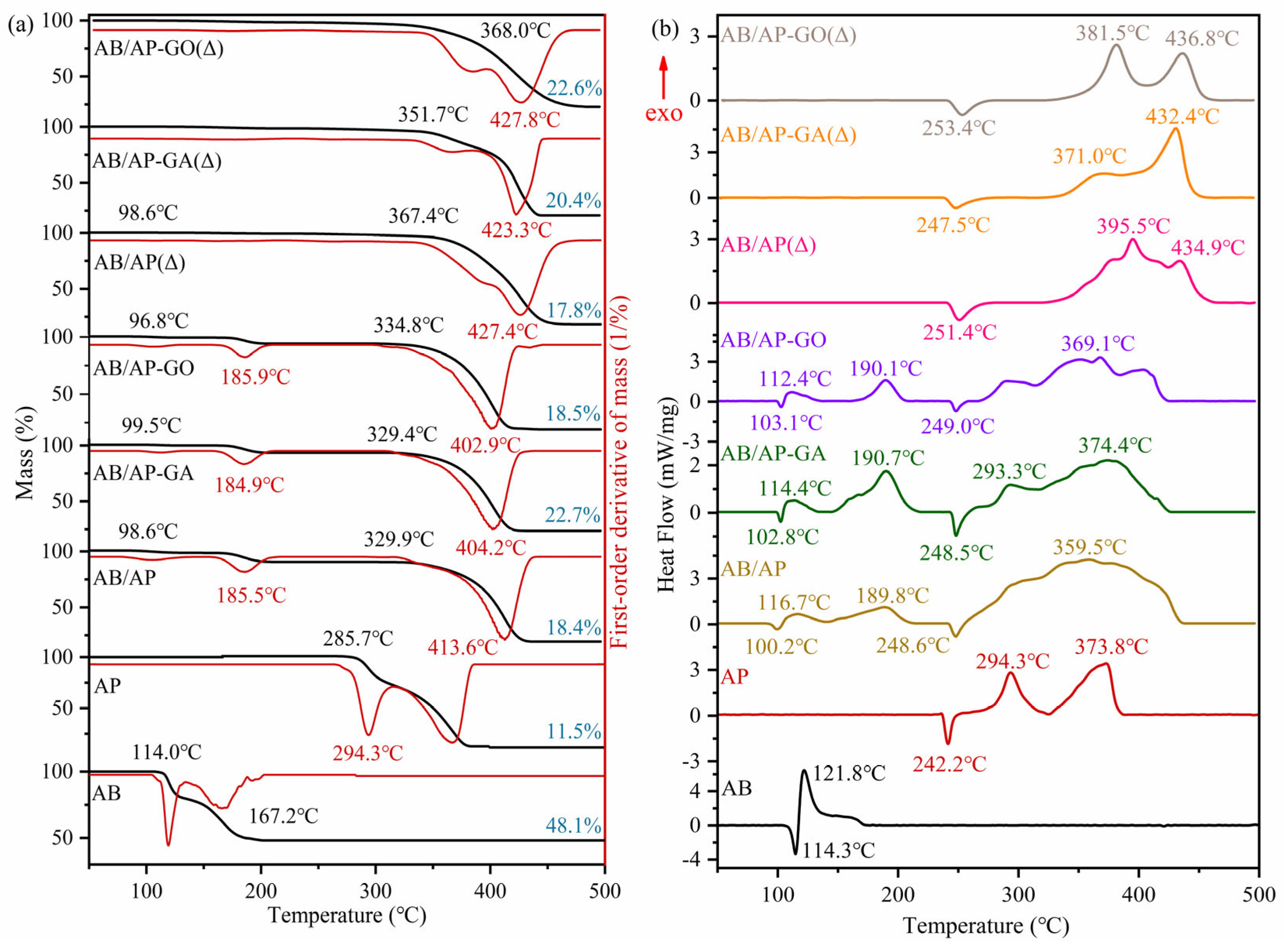
The thermal reactivity of the raw materials AB, AP, and the composite particles was investigated using a differential scanning calorimeter (DSC). The experimental conditions were maintained as follows: argon gas flow rate at 40 mL/min, heating rate of 10 K·min
−1, and a temperature range from 50 to 500 °C. The resulting TG-DTG and DSC curves are presented in
Figure 4, and the thermal decomposition parameters are summarized in
Table 8 and
Table 9.
As shown in
Figure 4, the thermal decomposition of AB occurred in two distinct stages: (i) Between approximately 112 and 130 °C, the first decomposition stage took place (NH
3-BH
3 → -[NH
2-BH
2]
n- + H
2), releasing one hydrogen molecule. AB melted between 97 and 120 °C, indicating that the first decomposition step occurred simultaneously with melting. (ii) In the temperature range of 130–200 °C, the second decomposition stage proceeded (-[NH
2-BH
2]
n- → -[NH=BH]
n- + H
2), releasing an additional hydrogen molecule and leaving a residue of 41%. The total heat released during AB decomposition was measured at 616.3 J·g
−1, consistent with previously reported thermal stability data for AB [
32,
33,
34,
35,
36,
37]. Additionally, an endothermic peak at 242.2 °C was identified, corresponding to the phase transition of AP from orthorhombic to cubic crystal structure. AP decomposition also exhibited two stages: (i) The low-temperature decomposition stage occurred between 274 and 300 °C, with an associated heat release of 361 J·g
−1. (ii) The high-temperature decomposition stage took place from 300 to 382.2 °C, releasing 631 J·g
−1 of heat and resulting in a final residue of 11.5%.
The AB/AP composite particles prepared by direct freeze-drying exhibited a two-stage decomposition profile, with each stage corresponding to the decomposition of AB and AP components, respectively. Notably, the low-temperature decomposition of AP disappeared, retaining only its high-temperature decomposition. The initial decomposition temperature of AP in the composite showed a significant increase of 70–80 °C compared to pristine AP. Furthermore, the heat release during AP decomposition in the composite (2997 J·g−1) substantially exceeded that of pristine AP (962 J·g−1). The residual mass ratio after decomposition of the AB/AP composite particles was measured at 18.4%. Similarly, the modified AB/AP composites only displayed high-temperature decomposition of AP with equivalent temperature elevation (70–80 °C). The decomposition heats for AB/AP-GA (1184 J·g−1) and AB/AP-GO (1777 J·g−1) composites both surpassed that of pristine AP (962 J·g−1). The residual mass ratios after decomposition were determined to be 22.7% and 18.5% for AB/AP-GA and AB/AP-GO composites, respectively.
Comparative analysis revealed that all three types of prepared composite particles induced the appearance of two distinct exothermic peaks during AB decomposition, while eliminating the low-temperature decomposition of AP and retaining only its high-temperature decomposition with significantly elevated decomposition temperatures. This alteration in thermodynamic properties provided clear evidence that the prepared composite particles were not simple mechanical mixtures but rather demonstrated enhanced thermal stability due to the formation of intermolecular interactions. Furthermore, the experimental residue quantities of all three composites (18.4%, 22.7%, and 18.5%) showed close agreement with the theoretically calculated value of 18.8%. This consistency confirmed that the actual AB/AP ratios in the synthesized composites corresponded well with the designed experimental proportions.
Notably, the unmodified AB/AP composite particles exhibited significantly higher heat release compared to their modified counterparts, which aligned consistently with the combustion heat data. Pure AB typically left behind boron–nitrogen polymeric chains and borazine derivatives after decomposition at 200 °C, which retained certain hydrogen storage capabilities but required extremely high temperatures (2000 °C) for complete conversion to boron nitride. According to Refs. [
32,
33,
34,
35,
36,
37,
38] and experimental phenomena, we found that the presence of AP fundamentally altered this decomposition pathway. The residual boron–nitrogen compounds participated in redox reactions with oxidative species generated during AP’s high-temperature decomposition, thereby significantly enhancing the exothermic output. In contrast, while the modified composites (AB/AP-GA and AB/AP-GO) still demonstrated increased heat release, their energetic performance was compromised because: (1) the incorporated graphene materials were non-energetic themselves, and (2) they competed for reaction with the oxidative intermediates during AP decomposition. This competition resulted in incomplete oxidation of the boron–nitrogen species, ultimately leading to reduced heat release compared to the unmodified system.
In
Figure 4, it can be observed that a portion of the substance had already decomposed before 200 °C, although the overall weight loss ratio remained extremely low (less than 1%). This phenomenon might have been caused by incomplete hyperchlorination of B-N polymers during the dehydrogenation process. Comparative analysis of the three thermal decomposition curves revealed that while the endothermic peak of AP persisted, no low-temperature decomposition of AP was detected in the AB/AP(Δ) sample. However, the characteristic two-stage decomposition of AP was clearly evident in both the AB/AP-GA(Δ) and AB/AP-GO(Δ) samples. Notably, all three heat-treated samples exhibited decomposition temperatures as high as 350 °C, approximately 250 °C higher than the decomposition temperature of the raw AB material, demonstrating the significantly enhanced thermal stability of the composite materials after heat treatment.
The decomposition heat release values of AB/AP(Δ) and AB/AP-GA(Δ) after heat treatment (1098 J·g−1 and 1213 J·g−1, respectively) were higher than that of pristine AP (962 J·g−1). In contrast, AB/AP-GO(Δ) showed a lower decomposition heat release (801 J·g−1) compared to AP. Notably, all three samples exhibited residual rates close to 18.8%, suggesting minimal loss of AB through sublimation during the heat treatment process.
Comparative analysis revealed that the thermal stability of all three heat-treated products was significantly enhanced relative to AP. Based on Refs. [
32,
33,
34,
35,
36,
37,
38] and experimental phenomena, during heat treatment, only trace amounts of ammonia borane derivatives underwent sublimation. While AB/AP(Δ) showed no low-temperature AP decomposition, both AB/AP-GA(Δ) and AB/AP-GO(Δ) displayed this characteristic. This difference was attributed to the establishment of a new intermolecular interaction system between the APB and AP formed during heat treatment, which fundamentally altered the thermal stability of the products. However, the introduction of graphene-like materials weakened these newly formed intermolecular interactions, thereby allowing the low-temperature decomposition of AP to persist in both AB/AP-GA(Δ) and AB/AP-GO(Δ).
2.3.2. Decomposition Kinetic Parameters and Physical Models
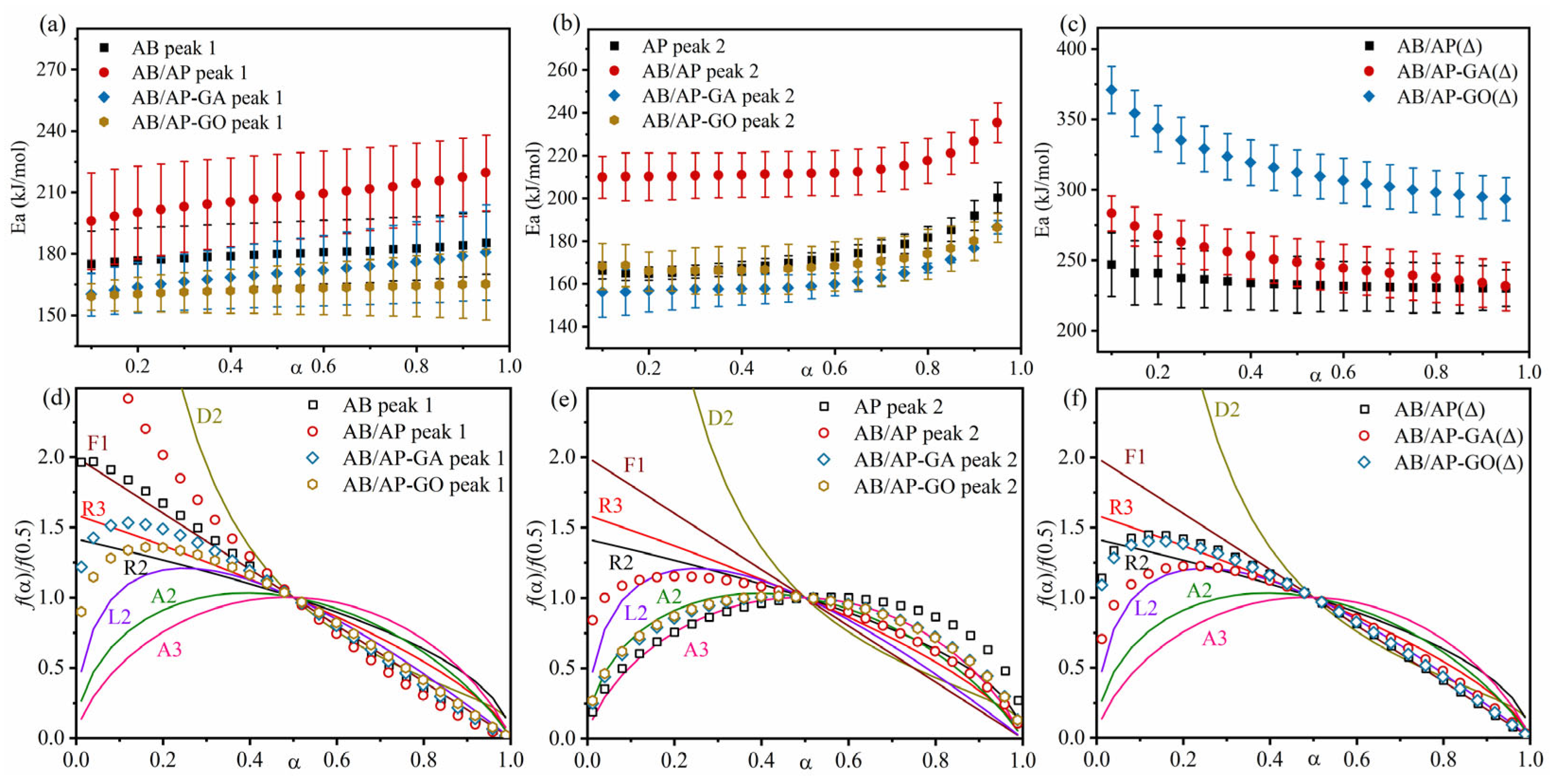
Thermal analysis kinetics is widely employed in the field of energetic materials, enabling the investigation of reaction mechanisms and thermal stability through kinetic parameters [
41,
42,
43,
44]. To determine the thermal decomposition activation energy (
Ea) of AB/AP composite particles, TG-DSC tests were performed at heating rates of 2.5, 5, 7.5, and 10 K·min
−1. However, the experimental data obtained at 2.5 K·min
−1 were unsuitable for calculating kinetic parameters. The DTG curves in
Figure 4 reveal two distinct decomposition peaks at approximately 180 °C (Peak I) and 410 °C (Peak II) during AB/AP thermal decomposition. Additionally, calcinated AB/AP exhibited a prominent peak around 420 °C. Molecular dynamics calculations were subsequently conducted for these characteristic peaks using DTG data from different heating rates.
AB/AP systems were further investigated using the joint kinetic method [
45] to calculate kinetic parameters and determine the most probable mechanism functions (
Figure 5,
Table 10). The analysis revealed that at Peak I, AB decomposition followed the F1 model (Unimolecular decay law), while AB/AP exhibited between F1 and D2 model (bidimensional particle shape). The high-temperature decomposition of AP consistently conformed from the A2 to the A3 model. At Peak II, AB/AP followed the L2 model (Random scission), AB/AP-GA and AB/AP-GO exhibited between the A2 and A3 model, confirming the disappearance of AP’s low-temperature decomposition while retaining its high-temperature pathway. Notably, the calcinated products displayed distinct decomposition mechanisms: AB/AP(Δ), AB/AP-GA(Δ), and AB/AP-GO(Δ) all preferentially followed from the L2 to the R3 model (Phase boundary-controlled reaction). These findings indicated that: (1) AB/AP(Δ)’s decomposition mechanism differed significantly from raw AP, and that (2) the simultaneous decomposition of APB and AP under high-temperature conditions substantially modified the reaction mechanism.
The reactive molecular dynamic simulations on thermal decomposition confirmed that AP in the composite particles exclusively underwent high-temperature decomposition, validating the previously proposed decomposition mechanism for AB/AP composites. Based on the theoretical results, the thermal decomposition process revealed distinct stages: (1) AB initially decomposed, releasing hydrogen gas while generating boron–nitrogen polymeric chains and cyclic borazine derivatives. (2) These boron–nitrogen polymers subsequently encapsulated AP particles, altering the intermolecular interactions between AB and AP components. (3) With continued temperature increase, the modified molecular environment caused AP to bypass its characteristic low-temperature decomposition and proceed directly to high-temperature decomposition. Comparative analysis demonstrated significant mechanistic differences between AB/AP(Δ) and pure AP systems. The simultaneous decomposition of thermally generated APB and AP fundamentally modified the reaction pathway, resulting in a distinct decomposition mechanism for AB/AP(Δ).
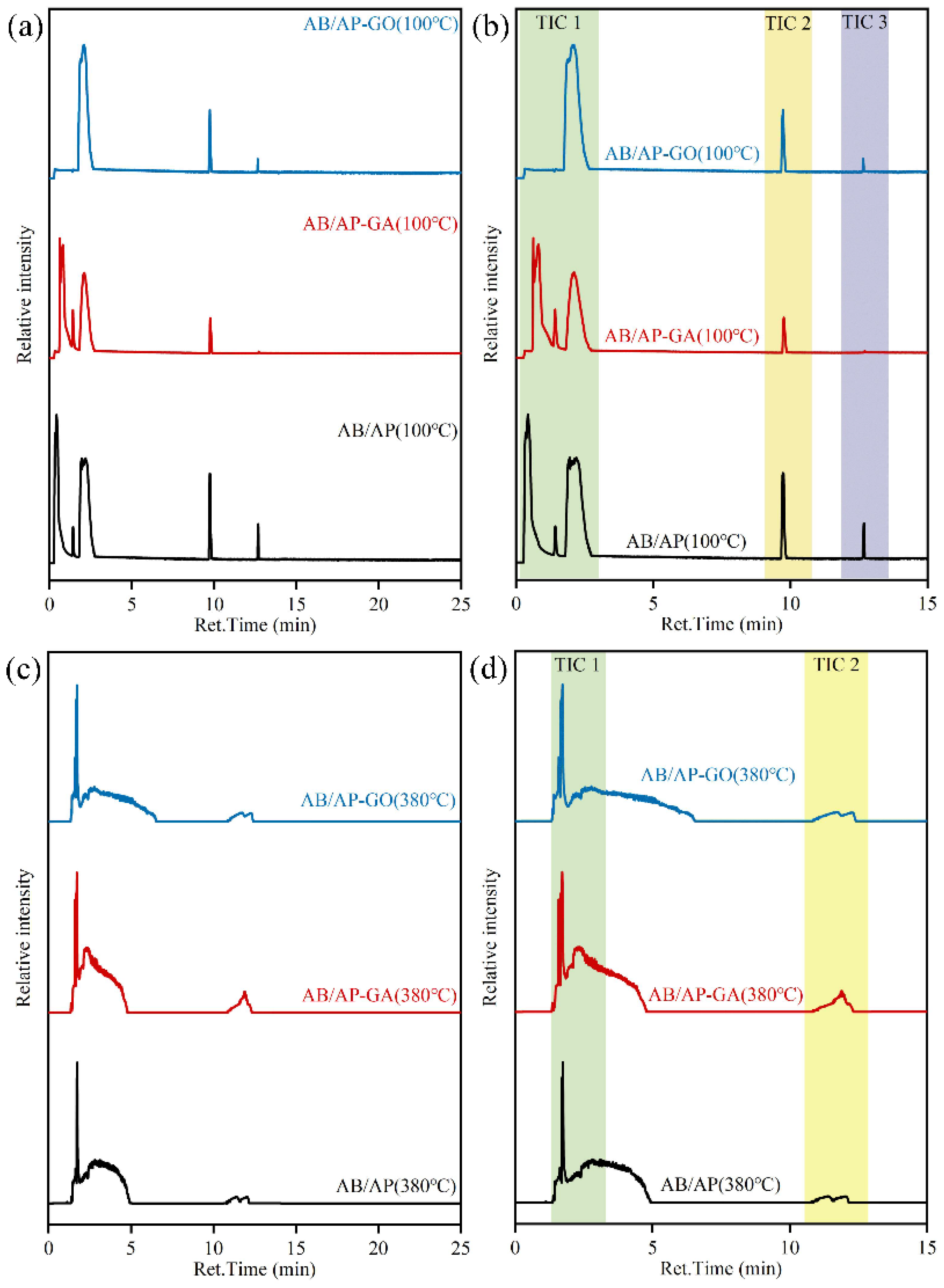
2.4. Isothermal Pyrolysis Products and Decomposition Mechanisms
Pyrolysis GC-MS analysis was conducted to characterize the gaseous pyrolysis products of the three composite particle types. To simulate the heat treatment process, the pyrolysis chamber temperature was initially maintained at 100 °C with a heating rate of 20 °C·min
−1, yielding the TIC curves presented in
Figure 6. Subsequently, the chamber temperature was elevated to 380 °C at the same heating rate for additional gas-phase product analysis, with corresponding TIC results shown in
Figure 6. Since the predominant peaks in the TIC profiles appeared within the first 15 min, mass spectral analysis focused on the strong peak regions (TIC 1 and TIC 2) during this initial period. The time-resolved pyrolysis mass spectra are displayed in
Figure S1, while the identified gaseous products are summarized in
Table 11.
Comparative analysis of the TIC profiles revealed distinct differences in the strong peak positions among the three composite particles during 100 °C pyrolysis. Mass spectral characterization demonstrated that while peak positions varied, the gaseous products at these TIC strong peaks were essentially identical across all three materials. Specifically: N2 and O2 were detected in AB/AP and AB/AP-GA composites between 0.30 and 1.50 min. Cyclotriborazane derivatives appeared at 1.90–2.20 min. Modified composites exhibited N2 and APB signatures at 9.70–9.80 min, while AB/AP showed perchloric acid and APB. During 12.60–12.80 min, N2 and APB were consistently observed in modified composite products.
Comparing all gas products’ mass spectra, it can be seen that at 100 °C, AP did not reach the decomposition temperature, and pure AP would not produce any gas products under heating conditions at 100 °C. However, H
2O was found in the gas-phase products N
2, O
2, H
35Cl, N
2O, and nitric acid [
46] substances were also found, and the formation of cyclic compounds such as cyclotriborazane and APB was also discovered during the heat treatment process.
Comparative analysis of the TIC curve strong peak positions revealed that AB/AP-GO exhibited no detectable peaks between 0.30 and 1.50 min, indicating that graphene oxide incorporation significantly altered the decomposition pathway of AB/AP composites during heat treatment. Unlike AB/AP and AB/AP-GA, which sequentially released O
2 and N
2, AB/AP-GO directly co-generated multiple products at 1.90 min, including N
2, nitric acid [
46], and cyclotriborazane derivatives [
35,
36,
37,
38,
39,
40]. These findings demonstrated that graphene oxide addition modified the reaction mechanism by suppressing AP’s characteristic decomposition into O
2 and N
2 in the composite system.
During the heat treatment of AB/AP composites, AB decomposition produced H
2, cyclotriborazane derivatives, and boron–nitrogen polymeric species. The presence of AP induced significant chemical interactions, where the highly electronegative ClO
4− ions promoted the formation of NH
3BH
2+ species from AB, which subsequently combined with ClO
4− to form APB [
40]. Concurrently, boron–nitrogen polymers generated from AB decomposition also reacted with ClO
4− to produce additional APB. This process caused partial AP to undergo redox reactions through ClO
4− loss, while the remaining unreacted AP and newly formed APB collectively constituted the final heat-treated product.
Comparative analysis of the TIC profiles at 380 °C pyrolysis revealed nearly identical peak positions among the three composite particles. Mass spectral characterization confirmed similar gaseous product distributions at corresponding TIC peaks. Specifically: NH3, N2, N2O, and cyclotriborazane derivatives were detected in all three composites between 1.58 and 1.59 min. Additional hypochlorous acid and nitric acid appeared at 1.70–1.75 min. During 2.30–2.85 min, NH3, H2O, and HCl were consistently identified in the decomposition products of all materials.
Comparative analysis of the mass spectra for all gaseous products revealed that the three composite materials generated essentially identical gas species during thermal cracking. Notably, the two modified AB/AP composites (AB/AP-GA and AB/AP-GO) produced additional CO and CO2, demonstrating that oxidative species (particularly O2) derived from AP decomposition oxidized the graphene-based additives during the process.
Gas-phase products N
2, O
2, H
35Cl, N
2O, and nitric acid [
46] substances both originated from the gas products of the AP thermal cracking process [
46]. Due to the stable presence of
35Cl and
37Cl in the natural chlorine element, the industrial grade ammonium perchlorate used may contain these two chlorine isotopes. The macromolecular compounds were B
3N
3H
6, which were gaseous products produced during the thermal decomposition of AB [
32,
33,
34,
35,
36]. These substances still had a certain hydrogen storage capacity, but they would sublime into gas above 80 °C [
34,
40].
Comparative analysis of the TIC curves and TG-DSC data reveals that during heating, AB and AP in the AB/AP composite underwent separate decomposition processes. The thermal decomposition occurred in two distinct stages: Between 100 and 200 °C, AB decomposed to release hydrogen gas while generating boron–nitrogen polymeric chains and borazine derivatives, both of which maintained hydrogen storage capacity. These boron–nitrogen polymers subsequently encapsulated AP particles, significantly altering the AB–AP intermolecular interactions. Upon further heating to 350 °C, the modified molecular environment caused AP to bypass its characteristic low-temperature decomposition and proceed directly to high-temperature decomposition. This process generated oxidizing gases (H2O, N2, O2, HCl) that reacted exothermically with the boron–nitrogen polymers, thereby enhancing the overall heat release during AP decomposition in the composite system.
3. Discussion
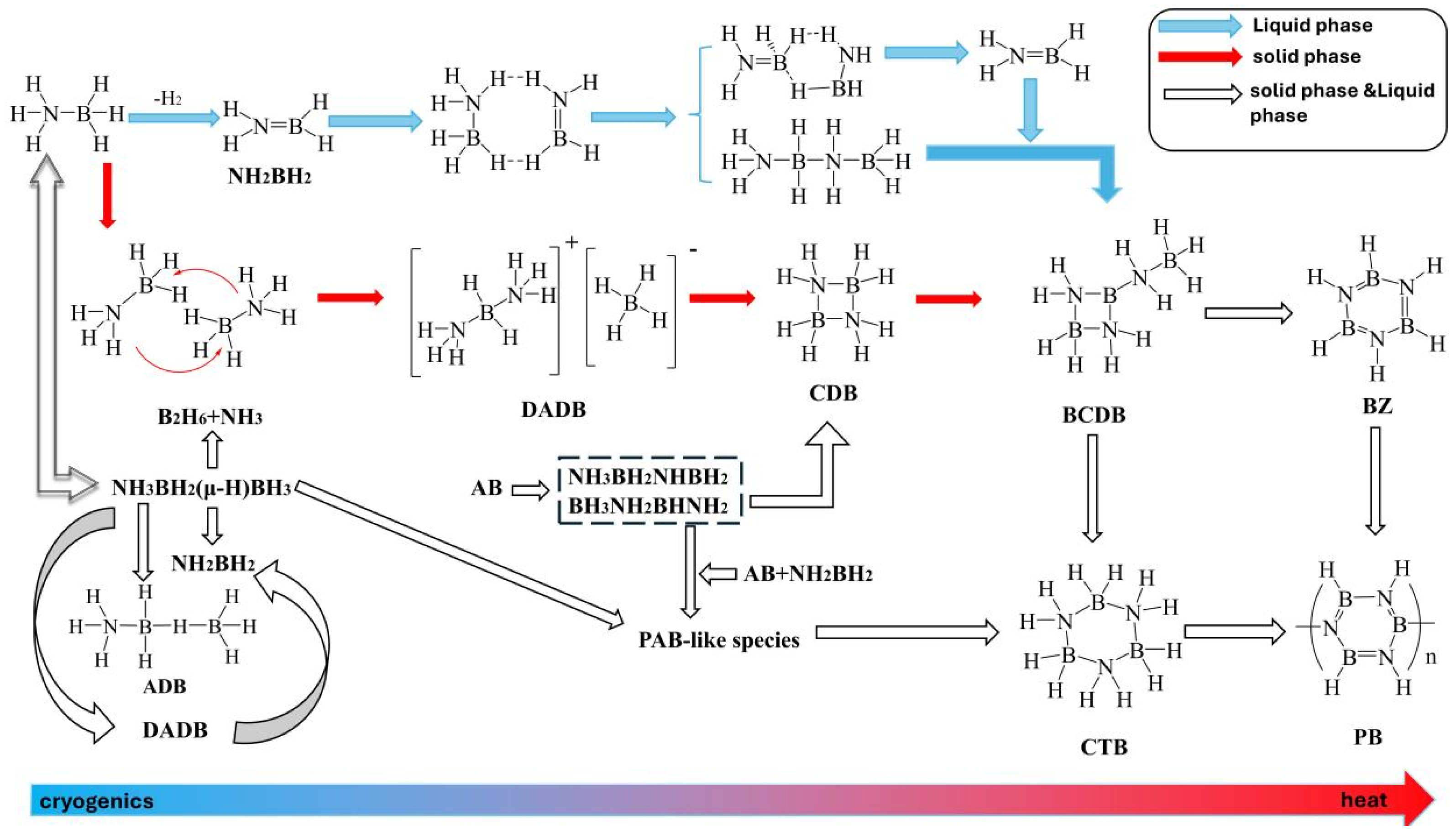
The thermal decomposition process of pure AB can be summarized from previous studies (see
Figure 7): the initial stage of AB decomposition forms BH
2, BH, BH
3, etc. These compounds are subsequently converted into DADB, chain-like products, and H
2 [
32,
33,
34,
35,
36]. Chainlike substances undergo self-catalytic reactions to form PABs. Under the catalysis of AB and NH
2BH
2, PAB would proliferate to form acyclic PABs or undergo cyclization to form BZ and PBZ. At the same time, the open-loop reaction of DADB gradually converts it into BZ, ultimately producing PBZ.
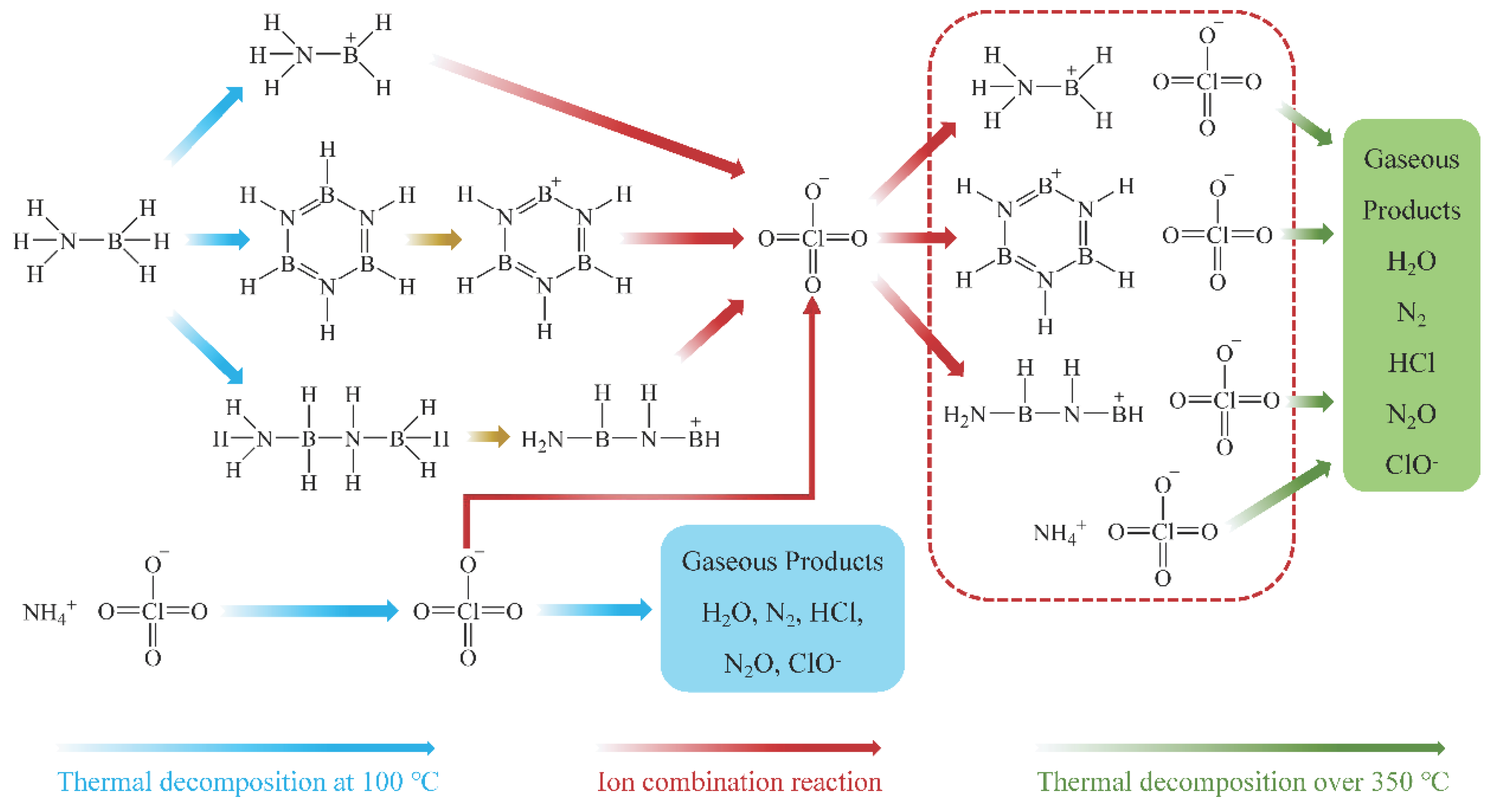
Based on gas-phase product analysis from thermal cracking at 100 °C and 380 °C, the thermal decomposition process of AB/AP composite particles can be summarized (see
Figure 8). During calcination at 100 °C, the binding between AP and AB initiates the following reactions: AB releases H
2 while forming borazine (BZ) and boron–nitrogen polymeric chains. The highly electronegative ClO
4− from AP promotes NH
3BH
2+ formation, which combines with ClO
4− to yield APB [
40]. Boron–nitrogen polymers and BZ from AB decomposition further react with ClO
4− through dehydrogenation to produce additional APB. Concurrently, partial AP undergoes redox reactions via ClO
4− loss, with the remaining AP and newly formed APB constituting the heat-treated product. Upon further heating above 350 °C, modified intermolecular interactions induce two parallel processes: direct high-temperature decomposition of AP, and oxidative decomposition of APB (major products: 4NH
3BH
2ClO
4 → N
2↑ + 4HCl↑ + 2B
2O
3 + N
2O↑ + O
2↑ + 7H
2O↑ + H
2↑).
High-density AB/AP composite particles were successfully synthesized through direct freeze-drying at a 1:1 molar ratio (AB: AP). The formation of novel intermolecular interactions increased the composite density by 26.4% compared to mechanical mixtures, while simultaneously eliminating AP’s low-temperature decomposition and raising its high-temperature decomposition onset by 80 °C. This method effectively resolved AB’s inherent low-density limitation.
Subsequent vacuum calcination of the composites produced AB/AP(Δ), which demonstrated superior thermal stability with a decomposition temperature 250 °C higher than pure AB and achieved a density of 1.6604 g/cm3. APB in AB/AP(Δ) overcomes the limitations of low density and thermal instability of AB, and has the potential to be used as a high-energy-density additive for high-burning-rate solid propellant.
5. Conclusions
The AB/AP composite particles were prepared using a direct freeze-drying method, followed by thermal treatment to obtain calcinated AB/AP with enhanced thermal stability and density. The reaction mechanism of the thermal treatment process was investigated through pyrolysis gas-phase product analysis. The main findings were as follows:
High-density AB/AP composite particles with a 1:1 molar ratio were successfully prepared via direct freeze-drying. The establishment of novel intermolecular interactions between AB and AP increased the composite density by 26.4% compared to mechanical mixtures. The composite particles retained only AP’s high-temperature decomposition, with an 80 °C higher initial decomposition temperature than pure AP, effectively addressing AB’s low-density limitation.
Three types of composite particles were heated in a vacuum oven at 100 °C for 2 h, yielding oxygen/fuel-integrated APB with superior thermal stability. The thermal decomposition mechanism study revealed that: AB/AP initially decomposed during heat treatment, producing borazine derivatives and boron–nitrogen polymeric chains; AP presence facilitated NH3BH2+ formation, which combined with ClO4− to generate APB; and partial AP underwent redox reactions through ClO4− loss, with the remaining AP and newly formed APB constituting AB/AP(Δ). Upon further heating to 350 °C, modified intermolecular interactions induced simultaneous AP high-temperature decomposition and APB redox reactions, generating substantial gaseous products. However, carbon additives remained non-reactive during heat treatment, forming heterogeneous interfaces with APB and AP that weakened molecular interactions and reduced thermal stability in modified composites. APB demonstrated remarkable thermal stability (250 °C higher decomposition temperature than AB) and achieved a density of 1.6604 g/cm3, overcoming AB’s limitations of low density and poor thermal stability. These properties establish APB as a promising high-energy-density additive for high-burning-rate solid propellants.