Dense Hydrogen-Bonded Assembly of Hydrogen-Rich Cations and Pentazolate Anions: A Series of Highly Insensitive Ionic Salts
Abstract
1. Introduction
2. Results and Discussion
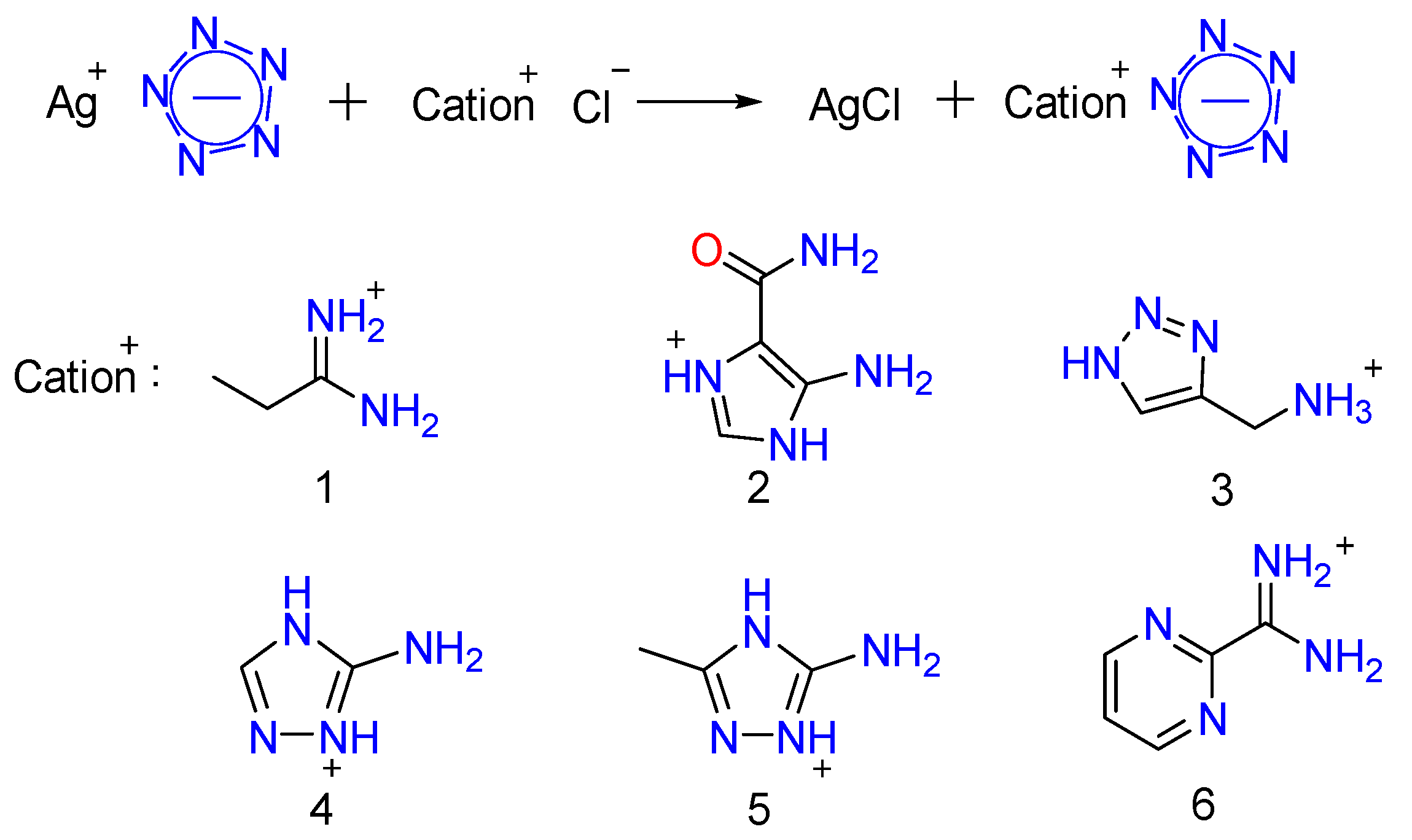
2.1. Design and Synthesis
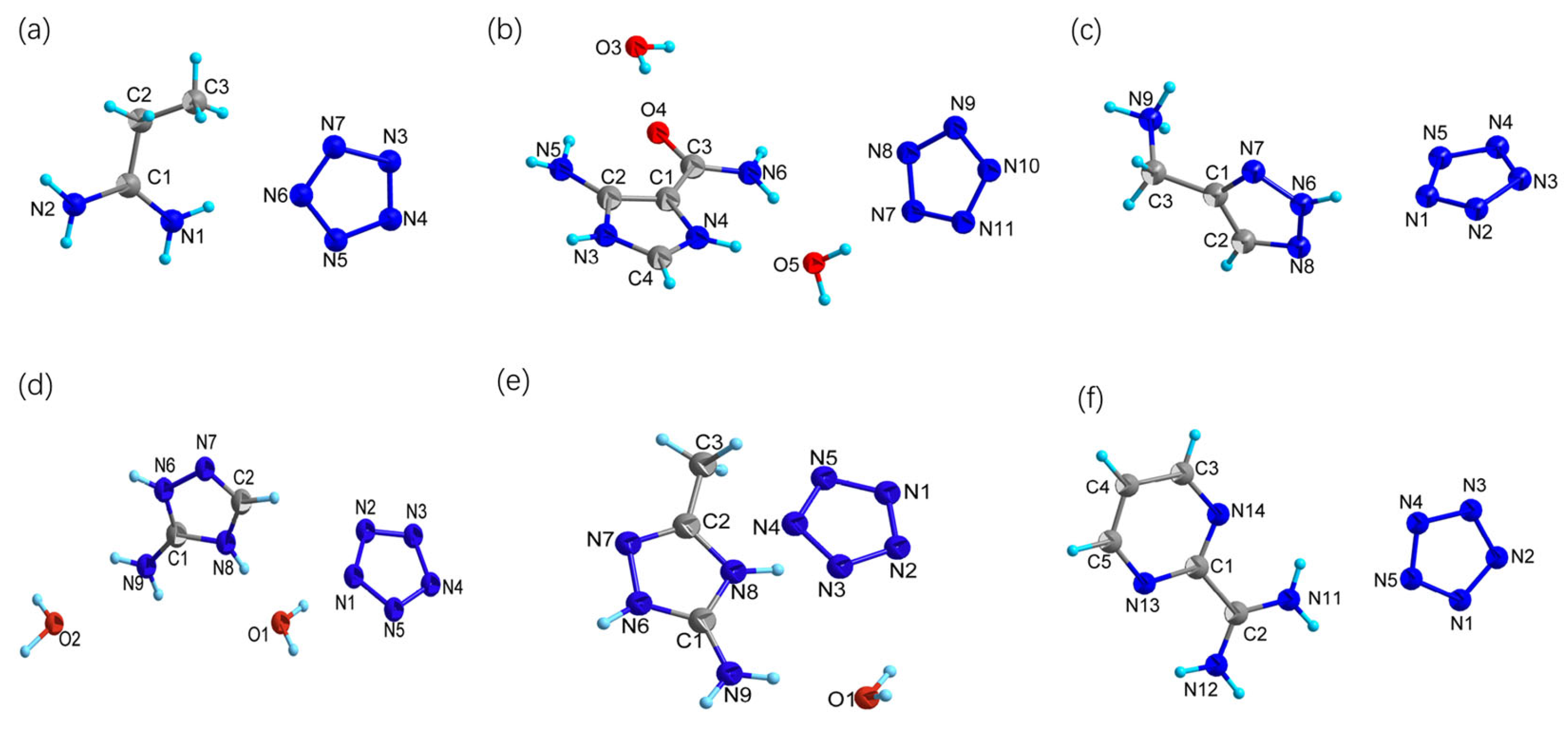
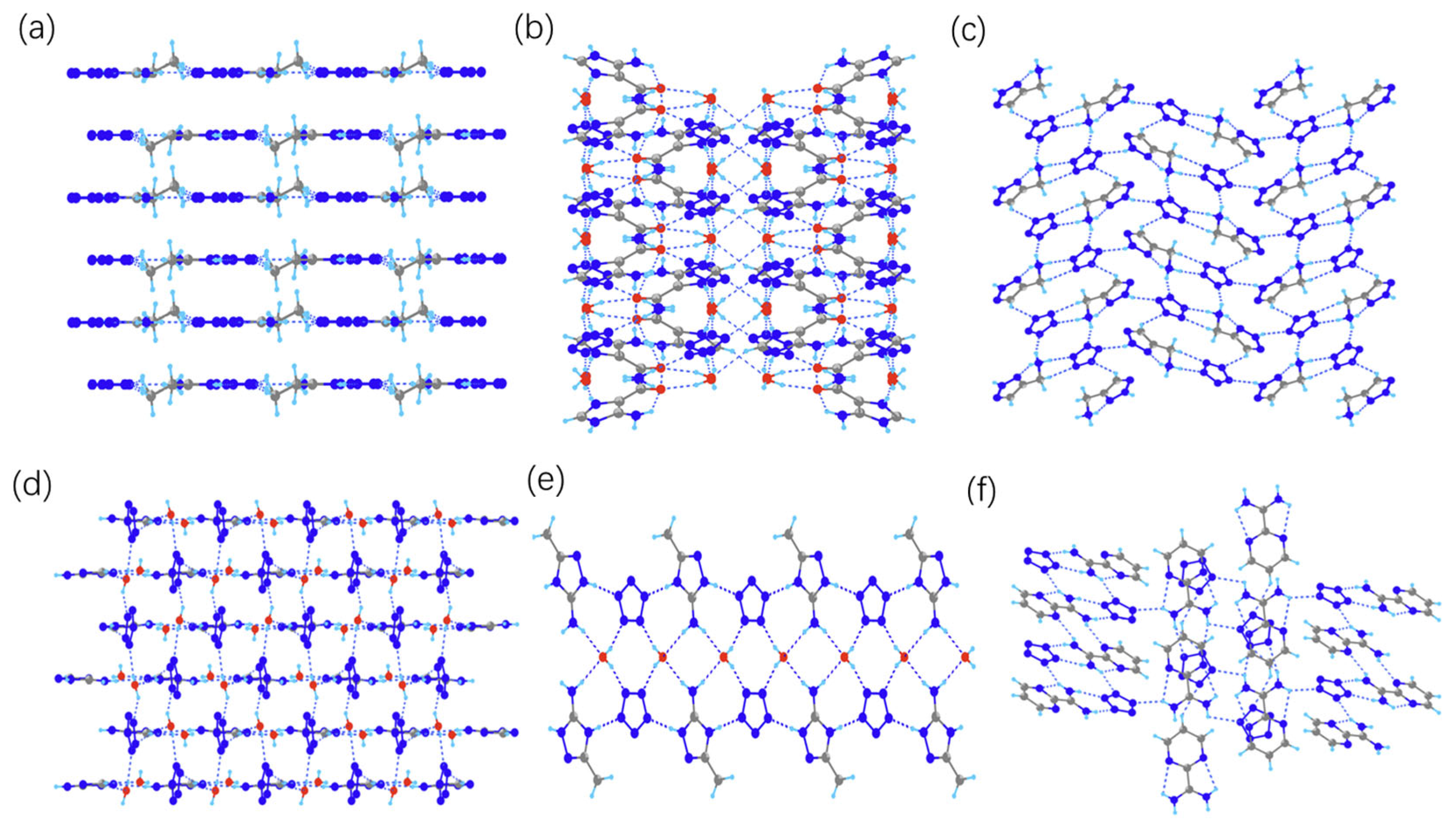
2.2. Crystal Structures
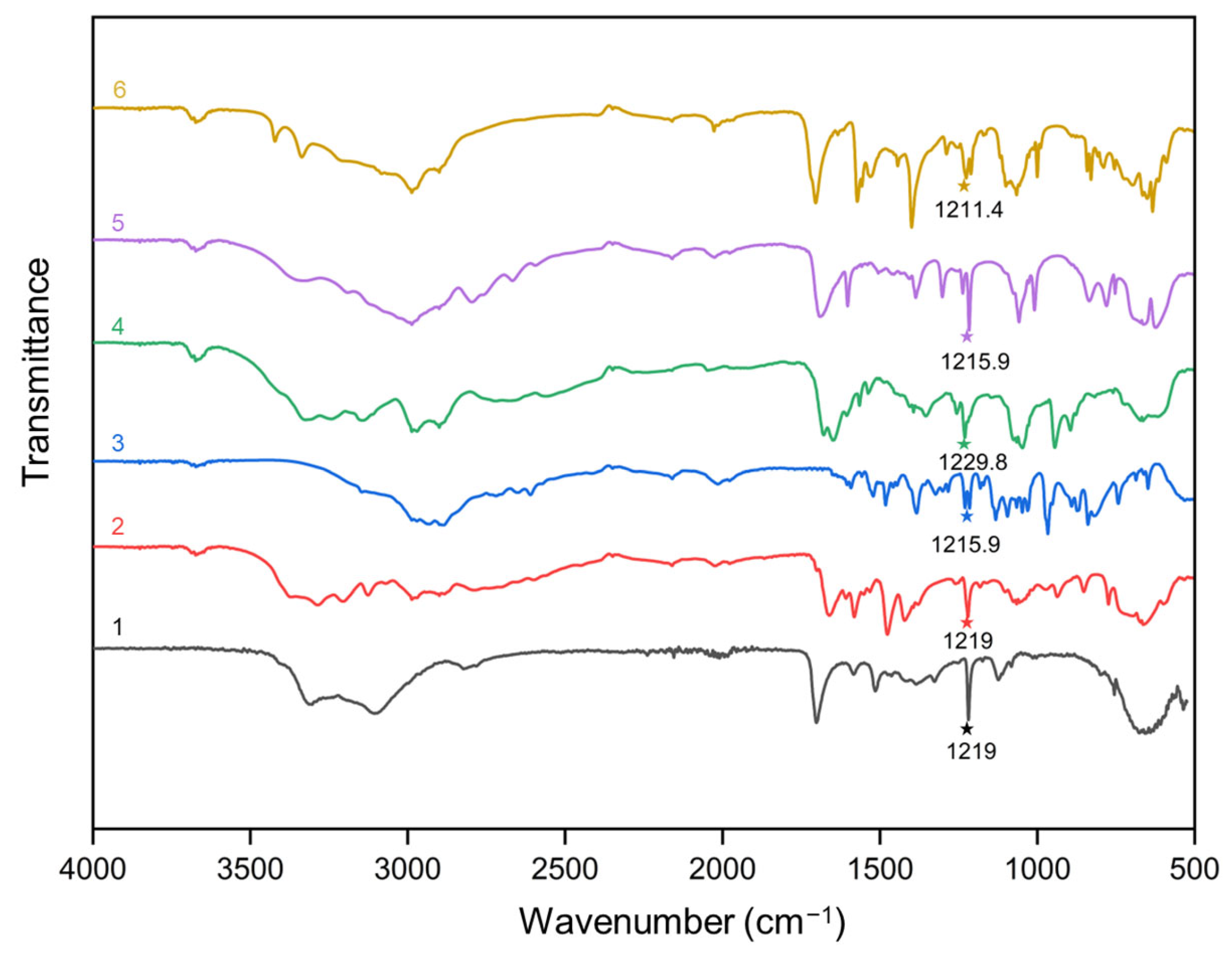
2.3. Vibrational Spectroscopy
2.4. Thermal Stability
2.5. Weak Interactions
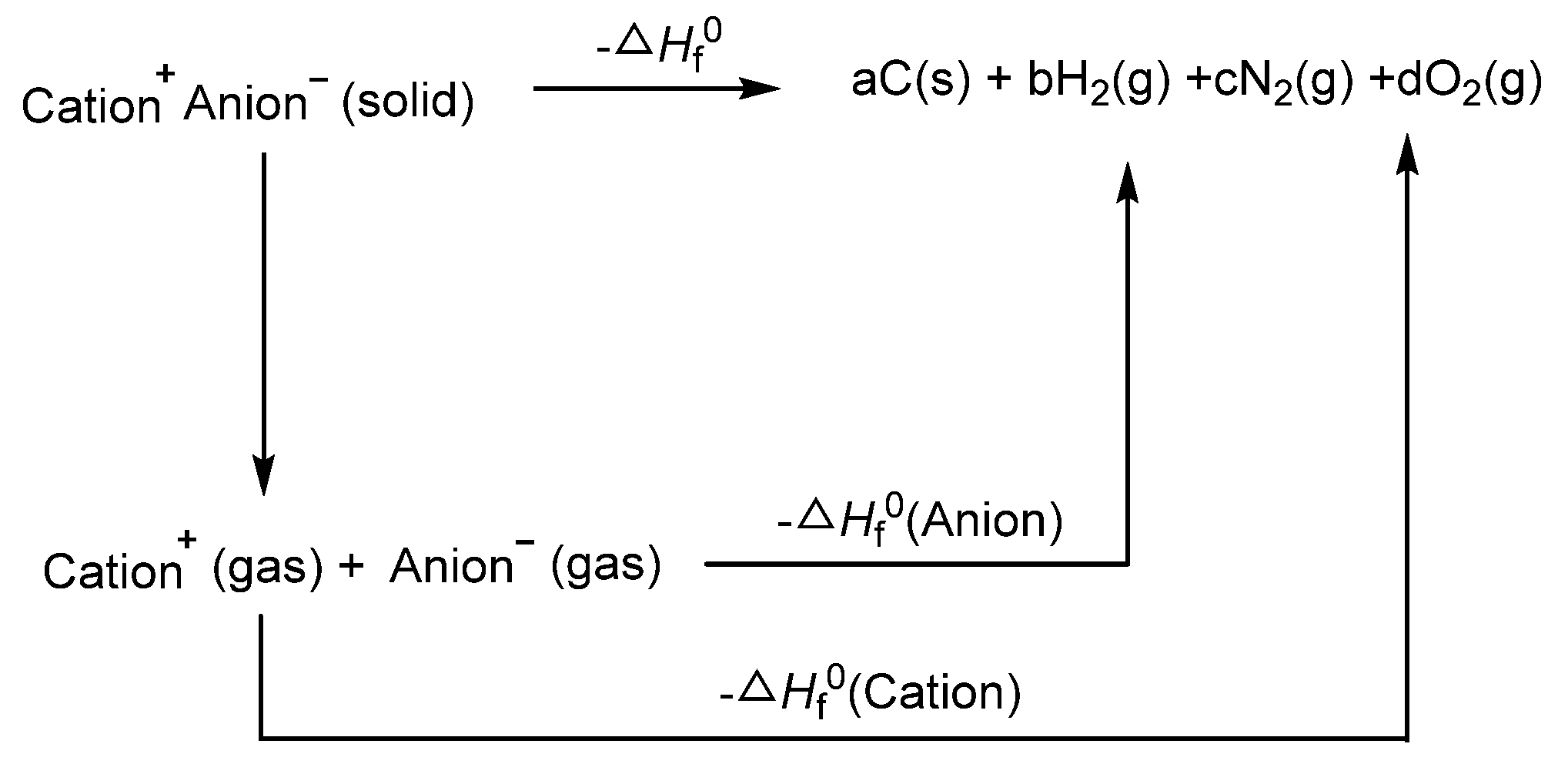
2.6. Physical Chemistry and Energetic Properties
2.7. Mechanical Sensitivity
3. Materials and Methods
3.1. Reagents and Instruments
3.2. Experimental Methods
3.3. Theoretical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klapötke, T.M.; Piercey, D.G. 1,1′-azobis(tetrazole): A highly energetic nitrogen-rich compound with a N10 chain. Inorg. Chem. 2011, 50, 2732–2734. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.H.; Twamley, B.; Garg, S.; Shreeve, J.M. Energetic nitrogen-rich derivatives of 1,5-diaminotetrazole. Angew. Chem. Int. Ed. 2008, 47, 6232–6239. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Pang, F.; Wang, G.; Huang, J.; Nie, F.; Chen, F.X. Pentazadiene: A high-nitrogen linkage in energetic materials. Chem. Commun. 2017, 53, 2327–2330. [Google Scholar] [CrossRef] [PubMed]
- Chavez, D.E.; Hiskey, M.A.; Gilardi, R.D. 3,3′-Azobis(6-amino-1,2,4,5-tetrazine): A novel high-nitrogen energetic material. Angew. Chem. Int. Ed. 2000, 112, 1861–1863. [Google Scholar] [CrossRef]
- Chen, D.; Yang, H.; Yi, Z.; Xiong, H.; Zhang, L.; Zhu, S.; Cheng, G. C8N26H4: An Environmentally Friendly Primary Explosive with High Heat of Formation. Angew. Chem. Int. Ed. 2018, 130, 2103–2106. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Sabaté, C.M. Mater. Nitrogen-rich tetrazolium azotetrazolate salts: A new family of insensitive energetic materials. Chem. Mater. 2008, 20, 1750–1763. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Martin, F.A.; Stierstorfer, J. An energetic and highly sensitive binary azidotetrazole. Angew. Chem. Int. Ed. 2011, 50, 4227. [Google Scholar] [CrossRef]
- Shlomovich, A.; Pechersky, T.; Cohen, A.; Yan, Q.L.; Kosa, M.; Petrutik, N.; Tal, N.; Aizikovich, A.; Gozin, M. Energetic isomers of 1,2,4,5-tetrazine-bis-1,2,4-triazoles with low toxicity. Dalton Trans. 2017, 46, 5994–6002. [Google Scholar] [CrossRef]
- Singh, R.P.; Verma, R.D.; Meshri, D.T.; Shreeve, J.M. Energetic nitrogen-rich salts and ionic liquids. Angew. Chem. Int. Ed. 2006, 45, 3584–3601. [Google Scholar] [CrossRef]
- Gao, H.; Shreeve, J.M. Azole-Based Energetic Salts. Chem. Rev. 2011, 111, 7377–7436. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Witkowski, T.G. Nitrogen-Rich Energetic 1,2,5-Oxadiazole-Tetrazole–Based Energetic Materials. Propell. Explos. Pyrotech. 2015, 40, 366–373. [Google Scholar] [CrossRef]
- Wozniak, D.R.; Piercey, D.G. Review of the current synthesis and properties of energetic pentazolate and derivatives thereof. Engineering 2020, 6, 981–991. [Google Scholar] [CrossRef]
- Wang, P.; Xu, Y.; Lin, Q.; Lu, M. Recent advances in the syntheses and properties of polynitrogen pentazolate anion cyclo-N5- and its derivatives. Chem. Soc. Rev. 2018, 47, 7522–7538. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, C.; Zheng, Z.; Jiang, C.; Luo, J.; Du, Y.; Hu, B.; Sun, C.; Christe, K.O. Synthesis and characterization of cyclo-pentazolate salts of NH4+, NH3OH+, N2H5+, C(NH2)3+, and N(CH3)4+. J. Am. Chem. Soc. 2018, 140, 16488–16494. [Google Scholar] [CrossRef]
- Laniel, D.; Weck, G.; Gaiffe, G.; Garbarino, G.; Loubeyre, P. High-pressure synthesized lithium pentazolate compound metastable under ambient conditions. J. Phys. Chem. Lett. 2018, 9, 1600–1604. [Google Scholar] [CrossRef]
- Yang, C.; Chen, L.; Wu, W.; Zhang, C.; Sun, C.; Du, Y.; Hu, B. Investigating the Stabilizing Forces of Pentazolate Salts. ACS Appl. Energy Mater. 2021, 4, 146–153. [Google Scholar] [CrossRef]
- Li, X.; Long, Y.; Zhang, C.; Sun, C.; Hu, B.; Lu, P.; Chen, J. Symmetrical cyclo-N5- hydrogen bonds: Stabilization mechanism of four non-metallic cyclo-pentazolate energetic salts. Phys. Chem. Chem. Phys. 2022, 24, 3970–3983. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Q.; Wang, P.; Lu, M. Syntheses, crystal structures and properties of a series of 3D metal-inorganic frameworks containing pentazolate anion. Chem. Asian J. 2018, 13, 1669–1673. [Google Scholar] [CrossRef]
- Xu, Y.; Tian, L.; Li, D.; Wang, P.; Lu, M. A series of energetic cyclo-pentazolate salts: Rapid synthesis, characterization, and promising performance. J. Mater. Chem. A 2019, 7, 12468–12479. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Q.; Shen, C.; Lin, Q.; Wang, P.; Lu, M. A series of energetic metal pentazolate hydrates. Nature 2017, 549, 78–81. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, C.; Hu, B.; Yu, C.; Zheng, Z.; Sun, C. Asymmetric Co(N5)2(H2O)4·4H2O high-nitrogen compound formed by Cobalt (II) cation trapping of a cyclo-N5ˉ anion. Angew. Chem. Int. Ed. 2017, 56, 4512–4514. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Xu, Y.; Lin, Q.; Wang, P.; Lu, M. Syntheses of energetic cyclo-pentazolate salts. Chem. Asian J. 2019, 14, 2877–2882. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Qi, X.; Zhang, W.; Huang, S.; Song, S.; Liu, Y.; Luo, J.; Zhang, Q. New Insight into the Aromaticity of cyclo-N5− by Constructing 3D Arrays in Crystal Structures. Cryst. Growth Des. 2021, 21, 33–39. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17; University of Western Australia: Perth, Australia, 2017. [Google Scholar]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Gao, H.; Ye, C.; Piekarski, C.M.; Shreeve, J.M. Computational Characterization of Energetic Salts. J. Phys. Chem. C 2007, 111, 10718–10731. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Parrish, D.A.; Shreeve, J.M. 4-Amino-3,5-dinitropyrazolate salts-highly insensitive energetic materials. J. Mater. Chem. A 2011, 21, 6891–6897. [Google Scholar] [CrossRef]
- Yu, R.J.; Liu, Y.J.; Huang, W.; Tang, Y.X. A hybrid of tetrazolium and pentazolate: An energetic salt with ultrahigh nitrogen content and energy. Energ. Mater. Front. 2023, 4, 63–67. [Google Scholar] [CrossRef]
- Chand, D.; Parrish, D.A.; Shreeve, J.M. Di(1H-tetrazol-5-yl)methanone oxime and 5,5′-(hydrazonomethylene)bis(1H-tetrazole) and their salts: A family of highly useful new tetrazoles and energetic materials. J. Mater. Chem. A 2013, 1, 15383–15389. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Sućeska, M. EXPLO5 V6.05.04; Brodarski Institute: Zagreb, Croatia, 2020. [Google Scholar]
- United Nations; Committee of Experts on the Transport of Dangerous Goods. Recommendations on the Transport of Dangerous Goods: Manual of Tests and Criteria; United Nations Publications: New York, NY, USA, 2009. [Google Scholar]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Ochterski, J.W.; Petersson, G.A.; Montgomery, J.A. A complete basis set model chemistry. V. Extensions to six or more heavy atoms. J. Chem. Phys. 1996, 104, 2598–2619. [Google Scholar] [CrossRef]
- Jenkins, H.D.B.; Tudela, D.; Glasser, L. Lattice potential energy estimation for complex ionic salts from density measurements. Inorg. Chem. 2002, 41, 2364–2367. [Google Scholar] [CrossRef]
- Jenkins, H.D.B.; Roobottom, H.K.; Passmore, J.; Glasser, L. Relationships among ionic lattice energies, molecular (Formula Unit) volumes, and thermochemical radii. Inorg. Chem. 1999, 38, 3609–3620. [Google Scholar] [CrossRef] [PubMed]




| Comp. | ρ a (g cm−3) | Td b (°C) | IS c (J) | FS d (N) | ΔHf e (kJ mol−1) | D f (m s−1) | P g (GPa) |
|---|---|---|---|---|---|---|---|
| 1 | 1.307 | 121 | >40 | >360 | 213.97 | 7122 | 14.6 |
| 2 | 1.575 | 107 | >40 | >360 | 210.66 | 7791 | 23.3 |
| 3 | 1.541 | 118 | >35 | >360 | 533.86 | 8288 | 22.6 |
| 4 | 1.580 | 120 | >35 | >360 | 440.90 | 8162 | 25.1 |
| 5 | 1.430 | 108 | >40 | >360 | 402.99 | 7561 | 20.0 |
| 6 | 1.485 | 117 | >40 | >360 | 376.29 | 8094 | 21.0 |
| C2H6N10 [19] | 1.583 | 107 | >40 | >360 | 639.7 | 7824 | 24.5 |
| NH4N5 [19] | 1.486 | 106.5 | 8 | 130 | 269.1 | 7757 | 23.2 |
| N(CH3)4N5 [14] | 1.245 | 81.6 | 35 | >360 | 296.1 | 6300 | 14.0 |
| [DAG]N5 [19] | 1.438 | 100.5 | 25 | 240 | 508.0 | 7505 | 21.2 |
| [EDA](N5)2 [22] | 1.395 | 98.5 | 25 | 240 | 556.1 | 6896 | 17.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Zhu, H.; Jiang, S.; Yuan, X.; Lu, G.; Lu, M.; Xu, Y. Dense Hydrogen-Bonded Assembly of Hydrogen-Rich Cations and Pentazolate Anions: A Series of Highly Insensitive Ionic Salts. Molecules 2025, 30, 2613. https://doi.org/10.3390/molecules30122613
Sun L, Zhu H, Jiang S, Yuan X, Lu G, Lu M, Xu Y. Dense Hydrogen-Bonded Assembly of Hydrogen-Rich Cations and Pentazolate Anions: A Series of Highly Insensitive Ionic Salts. Molecules. 2025; 30(12):2613. https://doi.org/10.3390/molecules30122613
Chicago/Turabian StyleSun, Lianghe, Hongwei Zhu, Shuaijie Jiang, Xiaofeng Yuan, Guoping Lu, Ming Lu, and Yuangang Xu. 2025. "Dense Hydrogen-Bonded Assembly of Hydrogen-Rich Cations and Pentazolate Anions: A Series of Highly Insensitive Ionic Salts" Molecules 30, no. 12: 2613. https://doi.org/10.3390/molecules30122613
APA StyleSun, L., Zhu, H., Jiang, S., Yuan, X., Lu, G., Lu, M., & Xu, Y. (2025). Dense Hydrogen-Bonded Assembly of Hydrogen-Rich Cations and Pentazolate Anions: A Series of Highly Insensitive Ionic Salts. Molecules, 30(12), 2613. https://doi.org/10.3390/molecules30122613





