The Role of Glycans in Human Immunity—A Sweet Code
Abstract
1. Introduction
2. Overview of Mammalian Glycosylation
2.1. N- and O-Glycosylation
2.2. The Structure of Glycosyltransferases
2.3. The Catalytic Mechanism of Glycosyltransferases
2.4. Glycosyltransferases Associated with the Immune System
3. Glycan Recognition in the Immune System
3.1. C-Type Lectins

3.2. Selectins
3.3. Siglecs
3.4. Galectins
4. Glycosylation in Immune Cell Trafficking
5. Glycosylation in Innate and Adaptive Immunity
5.1. Glycans in Bacterial Infections
5.2. Glycans in Fungal Infections
5.3. Glycans in Viral Infections
5.4. Glycans in Parasites
5.5. Glycans in Cancer
6. Antibody Glycosylation and Vaccines
6.1. Antibody Glycosylation
6.2. Antibody Glycosylation in Autoimmune Diseases and Infection
6.3. Antiglycan Antibodies
6.4. Glycan-Based Vaccines
7. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rabinovich, G.A.; van Kooyk, Y.; Cobb, B.A. Glycobiology of immune responses. Ann. N. Y. Acad. Sci. 2012, 1253, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.J.; He, M.; Lam, C.T. Congenital disorders of glycosylation. Ann. Transl. Med. 2018, 6, 477. [Google Scholar] [CrossRef] [PubMed]
- Lefeber, D.J.; Freeze, H.H.; Steet, R.; Kinoshita, T. Congenital Disorders of Glycosylation. In Essentials of Glycobiology; Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 2022; pp. 599–614. [Google Scholar] [CrossRef]
- Laine, R.A. A calculation of all possible oligosaccharide isomers both branched and linear yields 1.05 x 1012 structures for a reducing hexasaccharide: The Isomer Barrier to development of single-method saccharide sequencing or synthesis systems. Glycobiology 1994, 4, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.; Tvaroska, I. Carbohydrate-protein interactions: Molecular modeling insights. Adv. Carbohydr. Chem. Biochem. 2014, 71, 9–136. [Google Scholar] [CrossRef]
- Roseman, S. Reflections on glycobiology. J. Biol. Chem. 2001, 276, 41527–41542. [Google Scholar] [CrossRef]
- Cummings, R.D. The repertoire of glycan determinants in the human glycome. Mol. Biosyst. 2009, 5, 1087–1104. [Google Scholar] [CrossRef]
- Varki, A. Biological roles of glycans. Glycobiology 2017, 27, 3–49. [Google Scholar] [CrossRef]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef]
- Maverakis, E.; Kim, K.; Shimoda, M.; Gershwin, M.E.; Patel, F.; Wilken, R.; Raychaudhuri, S.; Ruhaak, L.R.; Lebrilla, C.B. Glycans in the immune system and The Altered Glycan Theory of Autoimmunity: A critical review. J. Autoimmun. 2015, 57, 1–13. [Google Scholar] [CrossRef]
- Marth, J.D.; Grewal, P.K. Mammalian glycosylation in immunity. Nat. Rev. Immunol. 2008, 8, 874–887. [Google Scholar] [CrossRef]
- Johnson, J.L.; Jones, M.B.; Ryan, S.O.; Cobb, B.A. The regulatory power of glycans and their binding partners in immunity. Trends Immunol. 2013, 34, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Harjunpaa, H.; Llort Asens, M.; Guenther, C.; Fagerholm, S.C. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef] [PubMed]
- van Kooyk, Y.; Rabinovich, G.A. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat. Immunol. 2008, 9, 593–601. [Google Scholar] [CrossRef]
- Pinho, S.S.; Alves, I.; Gaifem, J.; Rabinovich, G.A. Immune regulatory networks coordinated by glycans and glycan-binding proteins in autoimmunity and infection. Cell. Mol. Immunol. 2023, 20, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- Cummings, R.D. Stuck on sugars—How carbohydrates regulate cell adhesion, recognition, and signaling. Glycoconj. J. 2019, 36, 241–257. [Google Scholar] [CrossRef]
- Baum, L.G.; Cobb, B.A. The direct and indirect effects of glycans on immune function. Glycobiology 2017, 27, 619–624. [Google Scholar] [CrossRef]
- Alves, I.; Fernandes, A.; Santos-Pereira, B.; Azevedo, C.M.; Pinho, S.S. Glycans as a key factor in self and nonself discrimination: Impact on the breach of immune tolerance. FEBS Lett. 2022, 596, 1485–1502. [Google Scholar] [CrossRef]
- Wolfert, M.A.; Boons, G.J. Adaptive immune activation: Glycosylation does matter. Nat. Chem. Biol. 2013, 9, 776–784. [Google Scholar] [CrossRef]
- Sperandio, M.; Gleissner, C.A.; Ley, K. Glycosylation in immune cell trafficking. Immunol. Rev. 2009, 230, 97–113. [Google Scholar] [CrossRef]
- McEver, R.P. Selectins: Initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc. Res. 2015, 107, 331–339. [Google Scholar] [CrossRef]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef] [PubMed]
- von Itzstein, M. The war against influenza: Discovery and development of sialidase inhibitors. Nat. Rev. Drug Discov. 2007, 6, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Dings, R.P.M.; Miller, M.C.; Griffin, R.J.; Mayo, K.H. Galectins as Molecular Targets for Therapeutic Intervention. Int. J. Mol. Sci. 2018, 19, 905. [Google Scholar] [CrossRef] [PubMed]
- Macauley, M.S.; Crocker, P.R.; Paulson, J.C. Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 2014, 14, 653–666. [Google Scholar] [CrossRef]
- Smith, B.A.H.; Bertozzi, C.R. The clinical impact of glycobiology: Targeting selectins, Siglecs and mammalian glycans. Nat. Rev. Drug Discov. 2021, 20, 217–243. [Google Scholar] [CrossRef]
- Tvaroska, I.; Selvaraj, C.; Koca, J. Selectins-The Two Dr. Jekyll and Mr. Hyde Faces of Adhesion Molecules—A Review. Molecules 2020, 25, 2835. [Google Scholar] [CrossRef]
- Tvaroska, I. Glycosyltransferases as targets for therapeutic intervention in cancer and inflammation: Molecular modeling insights. Chem. Pap. 2022, 76, 1953–1988. [Google Scholar] [CrossRef]
- Sethi, A.; Sanam, S.; Alvala, R.; Alvala, M. An updated patent review of galectin-1 and galectin-3 inhibitors and their potential therapeutic applications (2016-present). Expert Opin. Ther. Pat. 2021, 31, 709–721. [Google Scholar] [CrossRef]
- Temme, J.S.; Butler, D.L.; Gildersleeve, J.C. Anti-glycan antibodies: Roles in human disease. Biochem. J. 2021, 478, 1485–1509. [Google Scholar] [CrossRef]
- Gillmann, K.M.; Temme, J.S.; Marglous, S.; Brown, C.E.; Gildersleeve, J.C. Anti-glycan monoclonal antibodies: Basic research and clinical applications. Curr. Opin. Chem. Biol. 2023, 74, 102281. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Toscano, M.A. Turning ‘sweet’ on immunity: Galectin-glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 2009, 9, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Y.; Cobb, B.A. Glycans in Immunologic Health and Disease. Annu. Rev. Immunol. 2021, 39, 511–536. [Google Scholar] [CrossRef] [PubMed]
- Schjoldager, K.T.; Narimatsu, Y.; Joshi, H.J.; Clausen, H. Global view of human protein glycosylation pathways and functions. Nat. Rev. Mol. Cell Biol. 2020, 21, 729–749. [Google Scholar] [CrossRef] [PubMed]
- Moremen, K.W.; Haltiwanger, R.S. Emerging structural insights into glycosyltransferase-mediated synthesis of glycans. Nat. Chem. Biol. 2019, 15, 853–864. [Google Scholar] [CrossRef]
- Stanley, P.; Taniguchi, N.; Aebi, M. N-Glycans. In Essentials of Glycobiology; Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 2015; pp. 99–111. [Google Scholar] [CrossRef]
- Nagae, M.; Yamaguchi, Y.; Taniguchi, N.; Kizuka, Y. 3D Structure and Function of Glycosyltransferases Involved in N-glycan Maturation. Int. J. Mol. Sci. 2020, 21, 437. [Google Scholar] [CrossRef]
- Vasconcelos-Dos-Santos, A.; Oliveira, I.A.; Lucena, M.C.; Mantuano, N.R.; Whelan, S.A.; Dias, W.B.; Todeschini, A.R. Biosynthetic Machinery Involved in Aberrant Glycosylation: Promising Targets for Developing of Drugs Against Cancer. Front. Oncol. 2015, 5, 138. [Google Scholar] [CrossRef]
- Brockhausen, I.; Stanley, P. O-GalNAc Glycans. In Essentials of Glycobiology; Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 2015; pp. 113–123. [Google Scholar] [CrossRef]
- Wandall, H.H.; Nielsen, M.A.I.; King-Smith, S.; de Haan, N.; Bagdonaite, I. Global functions of O-glycosylation: Promises and challenges in O-glycobiology. FEBS J. 2021, 288, 7183–7212. [Google Scholar] [CrossRef]
- Zhu, Y.; Hart, G.W. Targeting O-GlcNAcylation to develop novel therapeutics. Mol. Asp. Med. 2021, 79, 100885. [Google Scholar] [CrossRef]
- Campbell, J.A.; Davies, G.J.; Bulone, V.; Henrissat, B. A classification of nucleotide-diphospho-sugar glycosyltransferases based on amino acid sequence similarities. Biochem. J. 1997, 326, 929–942, Erratum in Biochem. J. 1998, 329 Pt 3, 719. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef]
- Ramakrishnan, B.; Qasba, P. Crystal Structure of Lactose Synthase Reveals a Large Conformational Change Change in its Catalytic Component, the b1,4-Galactosyltransferase-I. J. Mol. Biol. 2001, 310, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Acchione, M.; Lee, Y.C.; DeSantis, M.E.; Lipschultz, C.A.; Wlodawer, A.; Li, M.; Shanmuganathan, A.; Walter, R.L.; Smith-Gill, S.; Barchi, J.J., Jr. Specific fluorine labeling of the HyHEL10 antibody affects antigen binding and dynamics. Biochemistry 2012, 51, 6017–6027. [Google Scholar] [CrossRef] [PubMed]
- Lizak, C.; Gerber, S.; Numao, S.; Aebi, M.; Locher, K.P. X-ray structure of a bacterial oligosaccharyltransferase. Nature 2011, 474, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Yagi, H.; Takagi, K.; Kato, K. Exploring domain architectures of human glycosyltransferases: Highlighting the functional diversity of non-catalytic add-on domains. Biochim. Biophys. Acta Gen. Subj. 2024, 1868, 130687. [Google Scholar] [CrossRef]
- Unligil, U.M.; Rini, J.M. Glycosyltransferase structure and mechanism. Curr. Opin. Struct. Biol. 2000, 10, 510–517. [Google Scholar] [CrossRef]
- Breton, C.; Bettler, E.; Joziasse, D.H.; Geremia, R.A.; Imberty, A. Sequence-Function Relationships of Prokaryotic and Eukaryotic Galactosyltransferases. J. Biochem. 1998, 123, 1000–1009. [Google Scholar] [CrossRef]
- Breton, C.; Imberty, A. Structure/function studies of glycosyltransferases. Curr. Opin. Struct. Biol. 1999, 9, 563–571. [Google Scholar] [CrossRef]
- Breton, C.; Snajdrova, L.; Jeanneau, C.; Koca, J.; Imberty, A. Structures and mechanisms of glycosyltransferases. Glycobiology 2006, 16, 29r–37r. [Google Scholar] [CrossRef]
- Pak, J.E.; Arnoux, P.; Zhou, S.H.; Sivarajah, P.; Satkunarajah, M.; Xing, X.K.; Rini, J.M. X-ray crystal structure of leukocyte type core 2 beta 1,6-N-acetylglucosaminyltransferase—Evidence for a convergence of metal ion-independent glycosyltransferase mechanism. J. Biol. Chem. 2006, 281, 26693–26701. [Google Scholar] [CrossRef]
- Pak, J.E.; Satkunarajah, M.; Seetharaman, J.; Rini, J.M. Structural and Mechanistic Characterization of Leukocyte-Type Core 2 beta 1,6-N-Acetylglucosaminyltransferase: A Metal-Ion-Independent GT-A Glycosyltransferase. J. Mol. Biol. 2011, 414, 798–811. [Google Scholar] [CrossRef]
- Boix, E.; Zhang, Y.; Swaminathan, G.J.; Brew, K. Structural Basis of Orderer Binding of Donor and Acceptor Substrates to the Retaining Glycosyltransferase, a-1,3-Galactosyltransferase. J. Biol. Chem. 2002, 277, 28310–28318. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishan, B.; Balaji, P.V.; Qasba, P.K. Crystal Structure of b1,4-Galactosyltransferase Complex with UDP-Gal Reveals an Oligosaccharide Acceptor Binding Site. J. Mol. Biol. 2002, 318, 491–502. [Google Scholar] [CrossRef]
- Qasba, P.K.; Ramakrishnan, B.; Boeggeman, E. Substrate-induced conformational changes in glycosyltransferases. Trends Biochem. Sci. 2005, 30, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Wrabl, J.O.; Grishin, N.V. Homology Between O-linked GlcNAc Transferases and Proteins of the Glycogen Phosphorylase Superfamily. J. Mol. Biol. 2001, 314, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Lairson, L.L.; Henrissat, B.; Davies, G.J.; Withers, S.G. Glycosyltransferases: Structures, functions, and mechanisms. Annu. Rev. Biochem. 2008, 77, 521–555. [Google Scholar] [CrossRef]
- Igura, M.; Maita, N.; Kamishikiryo, J.; Yamada, M.; Obita, T.; Maenaka, K.; Kohda, D. Structure-guided identification of a new catalytic motif of oligosaccharyltransferase. EMBO J. 2008, 27, 234–243. [Google Scholar] [CrossRef]
- Hese, K.; Otto, C.; Routier, F.H.; Lehle, L. The yeast oligosaccharyltransferase complex can be replaced by STT3 from Leishmania major. Glycobiology 2009, 19, 160–171. [Google Scholar] [CrossRef]
- Wild, R.; Kowal, J.; Eyring, J.; Ngwa, E.M.; Aebi, M.; Locher, K.P. Structure of the yeast oligosaccharyltransferase complex gives insight into eukaryotic N-glycosylation. Science 2018, 359, 545–550. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Biwi, J.; Biot, C.; Guerardel, Y.; Vercoutter-Edouart, A.S.; Lefebvre, T. The Many Ways by Which O-GlcNAcylation May Orchestrate the Diversity of Complex Glycosylations. Molecules 2018, 23, 2858. [Google Scholar] [CrossRef]
- Tvaroska, I. Atomistic insight into the catalytic mechanism of glycosyltransferases by combined quantum mechanics/molecular mechanics (QM/MM) methods. Carbohydr. Res. 2015, 403, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, F.; Masgrau, L. Computational modeling of carbohydrate processingcenzymes reactions. Curr. Opin. Chem. Biol. 2021, 61, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Persson, K.; Ly, H.D.; Dieckelmann, M.; Wakarchuk, W.W.; Withers, S.G.; Strynadka, N.C.J. Crystal structure of the retaining galactosyltransferase LgtC from Neisseria meningitidis in complex with donor and acceptor sugar analogs: Towards a mechanism. Nat. Struct. Biol. 2001, 8, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, N.; Ohkawa, Y.; Maeda, K.; Harada, Y.; Nagae, M.; Kizuka, Y.; Ihara, H.; Ikeda, Y. True significance of N-acetylglucosaminyltransferases GnT-III, V and alpha1,6 fucosyltransferase in epithelial-mesenchymal transition and cancer. Mol. Asp. Med. 2021, 79, 100905. [Google Scholar] [CrossRef]
- Kizuka, Y.; Taniguchi, N. Enzymes for N-Glycan Branching and Their Genetic and Nongenetic Regulation in Cancer. Biomolecules 2016, 6, 25. [Google Scholar] [CrossRef]
- Taniguchi, N.; Korekane, H. Branched N-glycans and their implications for cell adhesion, signaling and clinical applications for cancer biomarkers and in therapeutics. BMB Rep. 2011, 44, 772–781. [Google Scholar] [CrossRef]
- Gordon, R.D.; Sivarajah, P.; Satkunarajah, M.; Ma, D.; Tarling, C.A.; Vizitiu, D.; Withers, S.G.; Rini, J.M. X-ray crystal structures of rabbit N-acetylglucosaminyltransferase I (GnT I) in complex with donor substrate analogues. J. Mol. Biol. 2006, 360, 67–79. [Google Scholar] [CrossRef]
- Bendiak, B.; Schachter, H. Control of Glycoprotein Synthesis. Purification of UDP-N-Acetylglucosamine:a-D-Mannoside b 1-2 N- Acetylglucosaminyltransferase II from Rat Liver. J. Biol. Chem. 1987, 262, 5775–5783. [Google Scholar] [CrossRef]
- Kadirvelraj, R.; Yang, J.Y.; Sanders, J.H.; Liu, L.; Ramiah, A.; Prabhakar, P.K.; Boons, G.J.; Wood, Z.A.; Moremen, K.W. Human N-acetylglucosaminyltransferase II substrate recognition uses a modular architecture that includes a convergent exosite. Proc. Natl. Acad. Sci. USA 2018, 115, 4637–4642. [Google Scholar] [CrossRef]
- Nishikawa, A.; Ihara, Y.; Hatakeyama, M.; Kangawa, K.; Taniguchi, N. Purification, cDNA cloning, and expression of UDP-N-acetylglucosamine: Beta-D-mannoside beta-1,4N-acetylglucosaminyltransferase III from rat kidney. J. Biol. Chem. 1992, 267, 18199–18204. [Google Scholar] [CrossRef]
- Yoshida, A.; Minowa, M.T.; Takamatsu, S.; Hara, T.; Ikenaga, H.; Takeuchi, M. A novel second isoenzyme of the human UDP-N-acetylglucosamine:alpha1,3-D-mannoside beta1,4-N-acetylglucosaminyltransferase family: cDNA cloning, expression, and chromosomal assignment. Glycoconj. J. 1998, 15, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Nishikawa, A.; Tsuruoka, N.; Ohno, M.; Yamaguchi, N.; Kangawa, K.; Taniguchi, N. Purification and characterization of UDP-N-acetylglucosamine: Alpha-6-D-mannoside beta 1-6N-acetylglucosaminyltransferase (N-acetylglucosaminyltransferase V) from a human lung cancer cell line. J. Biochem. 1993, 113, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Brockhausen, I.; Carver, J.P.; Schachter, H. Control of glycoprotein synthesis. The use of oligosaccharide substrates and HPLC to study the sequential pathway for N-acetylglucosaminyltransferases I, II, III, IV, V, and VI in the biosynthesis of highly branched N-glycans by hen oviduct membranes. Biochem. Cell Biol. 1988, 66, 1134–1151. [Google Scholar] [CrossRef] [PubMed]
- Darby, J.F.; Gillio, A.K.; Piniello, B.; Roth, C.; Blagova, E.; Hubbard, R.E.; Rovira, C.; Davies, G.J.; Wu, L. Substrate Engagement and Catalytic Mechanism of N-Acetylglucosaminyltransferase V. ACS Catal. 2020, 10, 8590–8596. [Google Scholar] [CrossRef]
- Nagae, M.; Kizuka, Y.; Mihara, E.; Kitago, Y.; Hanashima, S.; Ito, Y.; Takagi, J.; Taniguchi, N.; Yamaguchi, Y. Structure and mechanism of cancer-associated N-acetylglucosaminyltransferase-V. Nat. Commun. 2018, 9, 3380. [Google Scholar] [CrossRef]
- Ju, T.; Brewer, K.; D’Souza, A.; Cummings, R.D.; Canfield, W.M. Cloning and expression of human core 1 beta1,3-galactosyltransferase. J. Biol. Chem. 2002, 277, 178–186. [Google Scholar] [CrossRef]
- Gonzalez-Ramirez, A.M.; Grosso, A.S.; Yang, Z.; Companon, I.; Coelho, H.; Narimatsu, Y.; Clausen, H.; Marcelo, F.; Corzana, F.; Hurtado-Guerrero, R. Structural basis for the synthesis of the core 1 structure by C1GalT1. Nat. Commun. 2022, 13, 2398. [Google Scholar] [CrossRef]
- Bierhuizen, M.F.; Fukuda, M. Expression cloning of a cDNA encoding UDP-GlcNAc:Gal beta 1-3-GalNAc-R (GlcNAc to GalNAc) beta 1-6GlcNAc transferase by gene transfer into CHO cells expressing polyoma large tumor antigen. Proc. Natl. Acad. Sci. USA 1992, 89, 9326–9330. [Google Scholar] [CrossRef]
- Schwientek, T.; Nomoto, M.; Levery, S.B.; Merkx, G.; van Kessel, A.G.; Bennett, E.P.; Hollingsworth, M.A.; Clausen, H. Control of O-glycan branch formation. Molecular cloning of human cDNA encoding a novel beta1,6-N-acetylglucosaminyltransferase forming core 2 and core 4. J. Biol. Chem. 1999, 274, 4504–4512. [Google Scholar] [CrossRef]
- Vanderplasschen, A.; Markine-Goriaynoff, N.; Lomonte, P.; Suzuki, M.; Hiraoka, N.; Yeh, J.C.; Bureau, F.; Willems, L.; Thiry, E.; Fukuda, M.; et al. A multipotential beta -1,6-N-acetylglucosaminyl-transferase is encoded by bovine herpesvirus type 4. Proc. Natl. Acad. Sci. USA 2000, 97, 5756–5761. [Google Scholar] [CrossRef]
- Tvaroska, I.; Kozmon, S.; Wimmerova, M.; Koca, J. A QM/MM investigation of the catalytic mechanism of metal-ion-independent core 2 beta1,6-N-acetylglucosaminyltransferase. Chem. Eur. J. 2013, 19, 8153–8162. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, B.; Shah, P.S.; Qasba, P.K. alpha-Lactalbumin (LA) stimulates milk beta-1,4-galactosyltransferase I (beta 4Gal-T1) to transfer glucose from UDP-glucose to N-acetylglucosamine. Crystal structure of beta 4Gal-T1 x LA complex with UDP-Glc. J. Biol. Chem. 2001, 276, 37665–37671. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Kurata-Miura, K.; Ujita, M.; Angata, K.; Nakagawa, S.; Sekine, S.; Nishi, T.; Fukuda, M. Expression cloning of cDNA encoding a human beta-1,3-N-acetylglucosaminyltransferase that is essential for poly-N-acetyllactosamine synthesis. Proc. Natl. Acad. Sci. USA 1997, 94, 14294–14299. [Google Scholar] [CrossRef] [PubMed]
- Togayachi, A.; Sato, T.; Narimatsu, H. Comprehensive enzymatic characterization of glycosyltransferases with a beta3GT or beta4GT motif. Methods Enzymol. 2006, 416, 91–102. [Google Scholar] [CrossRef]
- Hao, Y.; Crequer-Grandhomme, A.; Javier, N.; Singh, A.; Chen, H.; Manzanillo, P.; Lo, M.C.; Huang, X. Structures and mechanism of human glycosyltransferase beta1,3-N-acetylglucosaminyltransferase 2 (B3GNT2), an important player in immune homeostasis. J. Biol. Chem. 2021, 296, 100042. [Google Scholar] [CrossRef]
- de Vries, T.; Knegtel, R.M.; Holmes, E.H.; Macher, B.A. Fucosyltransferases: Structure/function studies. Glycobiology 2001, 11, 119R–128R. [Google Scholar] [CrossRef]
- Ma, B.; Simala-Grant, J.L.; Taylor, D.E. Fucosylation in prokaryotes and eukaryotes. Glycobiology 2006, 16, 158R–184R. [Google Scholar] [CrossRef]
- Grewal, R.K.; Shaikh, A.R.; Gorle, S.; Kaur, M.; Videira, P.A.; Cavallo, L.; Chawla, M. Structural Insights in Mammalian Sialyltransferases and Fucosyltransferases: We Have Come a Long Way, but It Is Still a Long Way Down. Molecules 2021, 26, 5203. [Google Scholar] [CrossRef]
- Rasko, D.A.; Wang, G.; Palcic, M.M.; Taylor, D.E. Cloning and characterization of the alpha(1,3/4) fucosyltransferase of Helicobacter pylori. J. Biol. Chem. 2000, 275, 4988–4994. [Google Scholar] [CrossRef]
- Sun, H.Y.; Lin, S.W.; Ko, T.P.; Pan, J.F.; Liu, C.L.; Lin, C.N.; Wang, A.H.; Lin, C.H. Structure and mechanism of Helicobacter pylori fucosyltransferase. A basis for lipopolysaccharide variation and inhibitor design. J. Biol. Chem. 2007, 282, 9973–9982. [Google Scholar] [CrossRef]
- Ihara, H.; Ikeda, Y.; Toma, S.; Wang, X.; Suzuki, T.; Gu, J.; Miyoshi, E.; Tsukihara, T.; Honke, K.; Matsumoto, A.; et al. Crystal structure of mammalian alpha1,6-fucosyltransferase, FUT8. Glycobiology 2007, 17, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Brzezinski, K.; Stepkowski, T.; Panjikar, S.; Bujacz, G.; Jaskolski, M. High-resolution structure of NodZ fucosyltransferase involved in the biosynthesis of the nodulation factor. Acta Biochim. Pol. 2007, 54, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Audry, M.; Jeanneau, C.; Imberty, A.; Harduin-Lepers, A.; Delannoy, P.; Breton, C. Current trends in the structure-activity relationships of sialyltransferases. Glycobiology 2011, 21, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhou, Y.; Guo, L.; Feng, S. Biological function of sialic acid and sialylation in human health and disease. Cell Death Discov. 2024, 10, 415. [Google Scholar] [CrossRef]
- Ortiz-Soto, M.E.; Reising, S.; Schlosser, A.; Seibel, J. Structural and functional role of disulphide bonds and substrate binding residues of the human beta-galactoside alpha-2,3-sialyltransferase 1 (hST3Gal1). Sci. Rep. 2019, 9, 17993. [Google Scholar] [CrossRef]
- Uslupehlivan, M.; Sener, E.; Izzetoglu, S. Computational analysis of the structure, glycosylation and CMP binding of human ST3GAL sialyltransferases. Carbohydr. Res. 2019, 486, 107823. [Google Scholar] [CrossRef]
- Rakic, B.; Rao, F.V.; Freimann, K.; Wakarchuk, W.; Strynadka, N.C.; Withers, S.G. Structure-based mutagenic analysis of mechanism and substrate specificity in mammalian glycosyltransferases: Porcine ST3Gal-I. Glycobiology 2013, 23, 536–545. [Google Scholar] [CrossRef]
- Kuhn, B.; Benz, J.; Greif, M.; Engel, A.M.; Sobek, H.; Rudolph, M.G. The structure of human alpha-2,6-sialyltransferase reveals the binding mode of complex glycans. Acta Crystallogr. D Biol. Crystallogr. 2013, 69, 1826–1838. [Google Scholar] [CrossRef]
- Meng, L.; Forouhar, F.; Thieker, D.; Gao, Z.; Ramiah, A.; Moniz, H.; Xiang, Y.; Seetharaman, J.; Milaninia, S.; Su, M.; et al. Enzymatic baslis for N-glycan sialylation: Structure of rat alpha2,6-sialyltransferase (ST6GAL1) reveals conserved and unique features for glycan sialylation. J. Biol. Chem. 2013, 288, 34680–34698. [Google Scholar] [CrossRef]
- Moremen, K.W.; Ramiah, A.; Stuart, M.; Steel, J.; Meng, L.; Forouhar, F.; Moniz, H.A.; Gahlay, G.; Gao, Z.; Chapla, D.; et al. Expression system for structural and functional studies of human glycosylation enzymes. Nat. Chem. Biol. 2018, 14, 156–162. [Google Scholar] [CrossRef]
- Harrus, D.; Harduin-Lepers, A.; Glumoff, T. Unliganded and CMP-Neu5Ac bound structures of human alpha-2,6-sialyltransferase ST6Gal I at high resolution. J. Struct. Biol. 2020, 212, 107628. [Google Scholar] [CrossRef] [PubMed]
- Volkers, G.; Worrall, L.J.; Kwan, D.H.; Yu, C.C.; Baumann, L.; Lameignere, E.; Wasney, G.A.; Scott, N.E.; Wakarchuk, W.; Foster, L.J.; et al. Structure of human ST8SiaIII sialyltransferase provides insight into cell-surface polysialylation. Nat. Struct. Mol. Biol. 2015, 22, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Hemmerich, S.; Rosen, S.D. Carbohydrate sulfotransferases in lymphocyte homing. Glycobiology 2000, 10, 849–856. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, K.; Li, C.; Zong, G.; Prabhu, S.K.; Chapla, D.G.; Moremen, K.W.; Wang, L.X. Site-selective sulfation of N-glycans by human GlcNAc-6-O-sulfotransferase 1 (CHST2) and chemoenzymatic synthesis of sulfated antibody glycoforms. Bioorganic Chem. 2022, 128, 106070. [Google Scholar] [CrossRef]
- Mehta, P.; Cummings, R.D.; McEver, R.P. Affinity and kinetic analysis of P-selectin binding to P-selectin glycoprotein ligand-1. J. Biol. Chem. 1998, 273, 32506–32513. [Google Scholar] [CrossRef]
- Stone, M.J.; Chuang, S.; Hou, X.; Shoham, M.; Zhu, J.Z. Tyrosine sulfation: An increasingly recognised post-translational modification of secreted proteins. New Biotechnol. 2009, 25, 299–317. [Google Scholar] [CrossRef]
- Teramoto, T.; Fujikawa, Y.; Kawaguchi, Y.; Kurogi, K.; Soejima, M.; Adachi, R.; Nakanishi, Y.; Mishiro-Sato, E.; Liu, M.C.; Sakakibara, Y.; et al. Crystal structure of human tyrosylprotein sulfotransferase-2 reveals the mechanism of protein tyrosine sulfation reaction. Nat. Commun. 2013, 4, 1572. [Google Scholar] [CrossRef]
- Moremen, K.W.; Tiemeyer, M.; Nairn, A.V. Vertebrate protein glycosylation: Diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 2012, 13, 448–462. [Google Scholar] [CrossRef]
- Nairn, A.V.; York, W.S.; Harris, K.; Hall, E.M.; Pierce, J.M.; Moremen, K.W. Regulation of glycan structures in animal tissues: Transcript profiling of glycan-related genes. J. Biol. Chem. 2008, 283, 17298–17313. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Oswald, D.M.; Oliva, K.D.; Kreisman, L.S.C.; Cobb, B.A. The Glycoscience of Immunity. Trends Immunol. 2018, 39, 523–535. [Google Scholar] [CrossRef]
- Brown, G.D.; Willment, J.A.; Whitehead, L. C-type lectins in immunity and homeostasis. Nat. Rev. Immunol. 2018, 18, 374–389. [Google Scholar] [CrossRef] [PubMed]
- Zelensky, A.N.; Gready, J.E. The C-type lectin-like domain superfamily. FEBS J. 2005, 272, 6179–6217. [Google Scholar] [CrossRef] [PubMed]
- Weis, W.I.; Taylor, M.E.; Drickamer, K. The C-type lectin superfamily in the immune system. Immunol. Rev. 1998, 163, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Barondes, S.H.; Castronovo, V.; Cooper, D.N.; Cummings, R.D.; Drickamer, K.; Feizi, T.; Gitt, M.A.; Hirabayashi, J.; Hughes, C.; Kasai, K.; et al. Galectins: A family of animal beta-galactoside-binding lectins. Cell 1994, 76, 597–598. [Google Scholar] [CrossRef]
- Barondes, S.H.; Cooper, D.N.; Gitt, M.A.; Leffler, H. Galectins. Structure and function of a large family of animal lectins. J. Biol. Chem. 1994, 269, 20807–20810. [Google Scholar] [CrossRef]
- Vasta, G.R. Galectins as pattern recognition receptors: Structure, function, and evolution. Adv. Exp. Med. Biol. 2012, 946, 21–36. [Google Scholar] [CrossRef]
- Varki, A.; Angata, T. Siglecs—The major subfamily of I-type lectins. Glycobiology 2006, 16, 1R–27R. [Google Scholar] [CrossRef]
- Crocker, P.R.; Paulson, J.C.; Varki, A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 2007, 7, 255–266. [Google Scholar] [CrossRef]
- Bornhofft, K.F.; Goldammer, T.; Rebl, A.; Galuska, S.P. Siglecs: A journey through the evolution of sialic acid-binding immunoglobulin-type lectins. Dev. Comp. Immunol. 2018, 86, 219–231. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Amarante-Mendes, G.P.; Adjemian, S.; Branco, L.M.; Zanetti, L.C.; Weinlich, R.; Bortoluci, K.R. Pattern Recognition Receptors and the Host Cell Death Molecular Machinery. Front. Immunol. 2018, 9, 2379. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A., Jr.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Drickamer, K.; Fadden, A.J. Genomic analysis of C-type lectins. Biochem. Soc. Symp. 2002, 69, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Zelensky, A.N.; Gready, J.E. C-type lectin-like domains in Fugu rubripes. BMC Genom. 2004, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Halberg, D.F.; Proulx, G.; Doege, K.; Yamada, Y.; Drickamer, K. A segment of the cartilage proteoglycan core protein has lectin-like activity. J. Biol. Chem. 1988, 263, 9486–9490. [Google Scholar] [CrossRef]
- Kilpatrick, D.C. Animal lectins: A historical introduction and overview. Biochim. Biophys. Acta 2002, 1572, 187–197. [Google Scholar] [CrossRef]
- Weis, W.I.; Drickamer, K.; Hendrickson, W.A. Structure of a C-type mannose-binding protein complexed with an oligosaccharide. Nature 1992, 360, 127–134. [Google Scholar] [CrossRef]
- Guo, Y.; Feinberg, H.; Conroy, E.; Mitchell, D.A.; Alvarez, R.; Blixt, O.; Taylor, M.E.; Weis, W.I.; Drickamer, K. Structural basis for distinct ligand-binding and targeting properties of the receptors DC-SIGN and DC-SIGNR. Nat. Struct. Mol. Biol. 2004, 11, 591–598. [Google Scholar] [CrossRef]
- Drickamer, K. Engineering galactose-binding activity into a C-type mannose-binding protein. Nature 1992, 360, 183–186. [Google Scholar] [CrossRef]
- Gabba, A.; Bogucka, A.; Luz, J.G.; Diniz, A.; Coelho, H.; Corzana, F.; Canada, F.J.; Marcelo, F.; Murphy, P.V.; Birrane, G. Crystal Structure of the Carbohydrate Recognition Domain of the Human Macrophage Galactose C-Type Lectin Bound to GalNAc and the Tumor-Associated Tn Antigen. Biochemistry 2021, 60, 1327–1336. [Google Scholar] [CrossRef]
- Lee, R.T.; Hsu, T.L.; Huang, S.K.; Hsieh, S.L.; Wong, C.H.; Lee, Y.C. Survey of immune-related, mannose/fucose-binding C-type lectin receptors reveals widely divergent sugar-binding specificities. Glycobiology 2011, 21, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.K.; Drickamer, K.; Weis, W.I. Structural analysis of monosaccharide recognition by rat liver mannose-binding protein. J. Biol. Chem. 1996, 271, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Kolatkar, A.R.; Weis, W.I. Structural Basis of Galactose Recognition by C-type Animal Lectins. J. Biol. Chem. 1996, 271, 6679–6685. [Google Scholar] [CrossRef] [PubMed]
- Preston, R.C.; Jakob, R.P.; Binder, F.P.; Sager, C.P.; Ernst, B.; Maier, T. E-selectin ligand complexes adopt an extended high-affinity conformation. J. Mol. Cell Biol. 2016, 8, 62–72. [Google Scholar] [CrossRef]
- Figdor, C.G.; van Kooyk, Y.; Adema, G.J. C-type lectin receptors on dendritic cells and Langerhans cells. Nat. Rev. Immunol. 2002, 2, 77–84. [Google Scholar] [CrossRef]
- Osorio, F.; Reis e Sousa, C. Myeloid C-type lectin receptors in pathogen recognition and host defense. Immunity 2011, 34, 651–664. [Google Scholar] [CrossRef]
- Stoitzner, P.; Romani, N. Langerin, the “Catcher in the Rye”: An important receptor for pathogens on Langerhans cells. Eur. J. Immunol. 2011, 41, 2526–2529. [Google Scholar] [CrossRef]
- Deckers, J.; Hammad, H.; Hoste, E. Langerhans Cells: Sensing the Environment in Health and Disease. Front. Immunol. 2018, 9, 93. [Google Scholar] [CrossRef]
- Stambach, N.S.; Taylor, M.E. Characterization of carbohydrate recognition by langerin, a C-type lectin of Langerhans cells. Glycobiology 2003, 13, 401–410. [Google Scholar] [CrossRef]
- Feinberg, H.; Taylor, M.E.; Razi, N.; McBride, R.; Knirel, Y.A.; Graham, S.A.; Drickamer, K.; Weis, W.I. Structural basis for langerin recognition of diverse pathogen and mammalian glycans through a single binding site. J. Mol. Biol. 2011, 405, 1027–1039. [Google Scholar] [CrossRef]
- Feinberg, H.; Powlesland, A.S.; Taylor, M.E.; Weis, W.I. Trimeric structure of langerin. J. Biol. Chem. 2010, 285, 13285–13293. [Google Scholar] [CrossRef] [PubMed]
- Medve, L.; Achilli, S.; Guzman-Caldentey, J.; Thepaut, M.; Senaldi, L.; Le Roy, A.; Sattin, S.; Ebel, C.; Vives, C.; Martin-Santamaria, S.; et al. Enhancing Potency and Selectivity of a DC-SIGN Glycomimetic Ligand by Fragment-Based Design: Structural Basis. Chemistry 2019, 25, 14659–14668. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; O’Callaghan, C.A.; Marshall, A.S.; Gilbert, R.J.; Siebold, C.; Gordon, S.; Brown, G.D.; Jones, E.Y. Structure of the fungal beta-glucan-binding immune receptor dectin-1: Implications for function. Protein Sci. 2007, 16, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Nagae, M.; Ikeda, A.; Hanashima, S.; Kojima, T.; Matsumoto, N.; Yamamoto, K.; Yamaguchi, Y. Crystal structure of human dendritic cell inhibitory receptor C-type lectin domain reveals the binding mode with N-glycan. FEBS Lett. 2016, 590, 1280–1288. [Google Scholar] [CrossRef]
- Sheriff, S.; Chang, C.Y.; Ezekowitz, R.A. Human mannose-binding protein carbohydrate recognition domain trimerizes through a triple alpha-helical coiled-coil. Nat. Struct. Biol. 1994, 1, 789–794. [Google Scholar] [CrossRef]
- Geijtenbeek, T.B.; Torensma, R.; van Vliet, S.J.; van Duijnhoven, G.C.; Adema, G.J.; van Kooyk, Y.; Figdor, C.G. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 2000, 100, 575–585. [Google Scholar] [CrossRef]
- Khoo, U.S.; Chan, K.Y.; Chan, V.S.; Lin, C.L. DC-SIGN and L-SIGN: The SIGNs for infection. J. Mol. Med. 2008, 86, 861–874. [Google Scholar] [CrossRef]
- Cramer, J.; Lakkaichi, A.; Aliu, B.; Jakob, R.P.; Klein, S.; Cattaneo, I.; Jiang, X.; Rabbani, S.; Schwardt, O.; Zimmer, G.; et al. Sweet Drugs for Bad Bugs: A Glycomimetic Strategy against the DC-SIGN-Mediated Dissemination of SARS-CoV-2. J. Am. Chem. Soc. 2021, 143, 17465–17478. [Google Scholar] [CrossRef]
- Geijtenbeek, T.B.; den Dunnen, J.; Gringhuis, S.I. Pathogen recognition by DC-SIGN shapes adaptive immunity. Future Microbiol. 2009, 4, 879–890. [Google Scholar] [CrossRef]
- Svajger, U.; Anderluh, M.; Jeras, M.; Obermajer, N. C-type lectin DC-SIGN: An adhesion, signalling and antigen-uptake molecule that guides dendritic cells in immunity. Cell Signal. 2010, 22, 1397–1405. [Google Scholar] [CrossRef]
- van Kooyk, Y.; Geijtenbeek, T.B. DC-SIGN: Escape mechanism for pathogens. Nat. Rev. Immunol. 2003, 3, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Willment, J.A.; Brown, G.D. C-type lectin receptors in antifungal immunity. Trends Microbiol. 2008, 16, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D. Dectin-1: A signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 2006, 6, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Gordon, S. Immune recognition. A new receptor for beta-glucans. Nature 2001, 413, 36–37. [Google Scholar] [CrossRef]
- Palma, A.S.; Feizi, T.; Zhang, Y.; Stoll, M.S.; Lawson, A.M.; Diaz-Rodriguez, E.; Campanero-Rhodes, M.A.; Costa, J.; Gordon, S.; Brown, G.D.; et al. Ligands for the beta-glucan receptor, Dectin-1, assigned using “designer” microarrays of oligosaccharide probes (neoglycolipids) generated from glucan polysaccharides. J. Biol. Chem. 2006, 281, 5771–5779. [Google Scholar] [CrossRef]
- Anaya, E.U.; Amin, A.E.; Wester, M.J.; Danielson, M.E.; Michel, K.S.; Neumann, A.K. Dectin-1 multimerization and signaling depends on fungal beta-glucan structure and exposure. Biophys. J. 2023, 122, 3749–3767. [Google Scholar] [CrossRef]
- Kalia, N.; Singh, J.; Kaur, M. The role of dectin-1 in health and disease. Immunobiology 2021, 226, 152071. [Google Scholar] [CrossRef]
- Bates, E.E.; Fournier, N.; Garcia, E.; Valladeau, J.; Durand, I.; Pin, J.J.; Zurawski, S.M.; Patel, S.; Abrams, J.S.; Lebecque, S.; et al. APCs express DCIR, a novel C-type lectin surface receptor containing an immunoreceptor tyrosine-based inhibitory motif. J. Immunol. 1999, 163, 1973–1983. [Google Scholar] [CrossRef]
- Hsu, T.L.; Cheng, S.C.; Yang, W.B.; Chin, S.W.; Chen, B.H.; Huang, M.T.; Hsieh, S.L.; Wong, C.H. Profiling carbohydrate-receptor interaction with recombinant innate immunity receptor-Fc fusion proteins. J. Biol. Chem. 2009, 284, 34479–34489. [Google Scholar] [CrossRef]
- Bloem, K.; Vuist, I.M.; van den Berk, M.; Klaver, E.J.; van Die, I.; Knippels, L.M.; Garssen, J.; Garcia-Vallejo, J.J.; van Vliet, S.J.; van Kooyk, Y. DCIR interacts with ligands from both endogenous and pathogenic origin. Immunol. Lett. 2014, 158, 33–41. [Google Scholar] [CrossRef]
- Troegeler, A.; Mercier, I.; Cougoule, C.; Pietretti, D.; Colom, A.; Duval, C.; Vu Manh, T.P.; Capilla, F.; Poincloux, R.; Pingris, K.; et al. C-type lectin receptor DCIR modulates immunity to tuberculosis by sustaining type I interferon signaling in dendritic cells. Proc. Natl. Acad. Sci. USA 2017, 114, E540–E549. [Google Scholar] [CrossRef] [PubMed]
- Stoff, M.; Ebbecke, T.; Ciurkiewicz, M.; Pavasutthipaisit, S.; Mayer-Lambertz, S.; Stork, T.; Pavelko, K.D.; Baumgartner, W.; Jung, K.; Lepenies, B.; et al. C-type lectin receptor DCIR contributes to hippocampal injury in acute neurotropic virus infection. Sci. Rep. 2021, 11, 23819. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, T.; Fukawa, T.; Akaki, K.; Nagaoka, K.; Takeda, T.; Iwakura, Y.; Inaba, K.; Takahara, K. Absence of DCIR1 reduces the mortality rate of endotoxemic hepatitis in mice. Eur. J. Immunol. 2017, 47, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Tang, C.; Chung, S.H.; Ye, X.Q.; Makusheva, Y.; Han, W.; Kubo, M.; Shichino, S.; Ueha, S.; Matsushima, K.; et al. Blocking DCIR mitigates colitis and prevents colorectal tumors by enhancing the GM-CSF-STAT5 pathway. Cell Rep. 2022, 40, 111158. [Google Scholar] [CrossRef]
- Seno, A.; Maruhashi, T.; Kaifu, T.; Yabe, R.; Fujikado, N.; Ma, G.; Ikarashi, T.; Kakuta, S.; Iwakura, Y. Exacerbation of experimental autoimmune encephalomyelitis in mice deficient for DCIR, an inhibitory C-type lectin receptor. Exp. Anim. 2015, 64, 109–119. [Google Scholar] [CrossRef]
- Fujikado, N.; Saijo, S.; Yonezawa, T.; Shimamori, K.; Ishii, A.; Sugai, S.; Kotaki, H.; Sudo, K.; Nose, M.; Iwakura, Y. Dcir deficiency causes development of autoimmune diseases in mice due to excess expansion of dendritic cells. Nat. Med. 2008, 14, 176–180. [Google Scholar] [CrossRef]
- Fraser, I.P.; Koziel, H.; Ezekowitz, R.A. The serum mannose-binding protein and the macrophage mannose receptor are pattern recognition molecules that link innate and adaptive immunity. Semin. Immunol. 1998, 10, 363–372. [Google Scholar] [CrossRef]
- Dobo, J.; Kocsis, A.; Farkas, B.; Demeter, F.; Cervenak, L.; Gal, P. The Lectin Pathway of the Complement System-Activation, Regulation, Disease Connections and Interplay with Other (Proteolytic) Systems. Int. J. Mol. Sci. 2024, 25, 1566. [Google Scholar] [CrossRef]
- Kurata, H.; Sannoh, T.; Kozutsumi, Y.; Yokota, Y.; Kawasaki, T. Structure and function of mannan-binding proteins isolated from human liver and serum. J. Biochem. 1994, 115, 1148–1154. [Google Scholar] [CrossRef]
- Worthley, D.L.; Bardy, P.G.; Mullighan, C.G. Mannose-binding lectin: Biology and clinical implications. Intern. Med. J. 2005, 35, 548–555. [Google Scholar] [CrossRef]
- Kawasaki, N.; Kawasaki, T.; Yamashina, I. Isolation and characterization of a mannan-binding protein from human serum. J. Biochem. 1983, 94, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Yamamoto, K.; Toyoshima, S.; Osawa, T.; Irimura, T. Molecular cloning and expression of cDNA encoding human macrophage C-type lectin. Its unique carbohydrate binding specificity for Tn antigen. J. Immunol. 1996, 156, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Kolatkar, A.R.; Leung, A.K.; Isecke, R.; Brossmer, R.; Drickamer, K.; Weis, W.I. Mechanism of N-acetylgalactosamine binding to a C-type animal lectin carbohydrate-recognition domain. J. Biol. Chem. 1998, 273, 19502–19508. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, S.J.; Saeland, E.; van Kooyk, Y. Sweet preferences of MGL: Carbohydrate specificity and function. Trends Immunol. 2008, 29, 83–90. [Google Scholar] [CrossRef]
- Marcelo, F.; Garcia-Martin, F.; Matsushita, T.; Sardinha, J.; Coelho, H.; Oude-Vrielink, A.; Koller, C.; Andre, S.; Cabrita, E.J.; Gabius, H.J.; et al. Delineating binding modes of Gal/GalNAc and structural elements of the molecular recognition of tumor-associated mucin glycopeptides by the human macrophage galactose-type lectin. Chemistry 2014, 20, 16147–16155. [Google Scholar] [CrossRef]
- Diniz, A.; Coelho, H.; Dias, J.S.; van Vliet, S.J.; Jimenez-Barbero, J.; Corzana, F.; Cabrita, E.J.; Marcelo, F. The Plasticity of the Carbohydrate Recognition Domain Dictates the Exquisite Mechanism of Binding of Human Macrophage Galactose-Type Lectin. Chemistry 2019, 25, 13945–13955. [Google Scholar] [CrossRef]
- Gabba, A.; Murphy, P.V.; Kiessling, L.L.; Birrane, G. Beyond the Crystal Structure of Human Macrophage C-Type Lectin. Biochemistry 2024, 63, 191–193. [Google Scholar] [CrossRef]
- van Kooyk, Y.; Ilarregui, J.M.; van Vliet, S.J. Novel insights into the immunomodulatory role of the dendritic cell and macrophage-expressed C-type lectin MGL. Immunobiology 2015, 220, 185–192. [Google Scholar] [CrossRef]
- Zizzari, I.G.; Napoletano, C.; Battisti, F.; Rahimi, H.; Caponnetto, S.; Pierelli, L.; Nuti, M.; Rughetti, A. MGL Receptor and Immunity: When the Ligand Can Make the Difference. J. Immunol. Res. 2015, 2015, 450695. [Google Scholar] [CrossRef]
- Lasky, L.A. Selectin-carbohydrate interactions and the initiation of the inflammatory response. Annu. Rev. Biochem. 1995, 64, 113–139. [Google Scholar] [CrossRef]
- Kansas, G.S. Selectins and their ligands: Current concepts and controversies. Blood 1996, 88, 3259–3287. [Google Scholar] [CrossRef] [PubMed]
- Graves, B.J.; Crowther, R.L.; Chandran, C.; Rumberger, J.M.; Li, S.; Huang, K.S.; Presky, D.H.; Familletti, P.C.; Wolitzky, B.A.; Burns, D.K. Insight into E-selectin/ligand interaction from the crystal structure and mutagenesis of the lec/EGF domains. Nature 1994, 367, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Mehta-D’souza, P.; Klopocki, A.G.; Oganesyan, V.; Terzyan, S.; Mather, T.; Li, Z.; Panicker, S.R.; Zhu, C.; McEver, R.P. Glycan Bound to the Selectin Low Affinity State Engages Glu-88 to Stabilize the High Affinity State under Force. J. Biol. Chem. 2017, 292, 2510–2518. [Google Scholar] [CrossRef] [PubMed]
- Somers, W.S.; Tang, J.; Shaw, G.D.; Camphausen, R.T. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLe(X) and PSGL-1. Cell 2000, 103, 467–479. [Google Scholar] [CrossRef]
- Wedepohl, S.; Dernedde, J.; Vahedi-Faridi, A.; Tauber, R.; Saenger, W.; Bulut, H. Reducing Macro- and Microheterogeneity of N-Glycans Enables the Crystal Structure of the Lectin and EGF-Like Domains of Human L-Selectin To Be Solved at 1.9 A Resolution. Chembiochem 2017, 18, 1338–1345. [Google Scholar] [CrossRef]
- Ley, K.; Bullard, D.C.; Arbones, M.L.; Bosse, R.; Vestweber, D.; Tedder, T.F.; Beaudet, A.L. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J. Exp. Med. 1995, 181, 669–675. [Google Scholar] [CrossRef]
- Eppihimer, M.J.; Wolitzky, B.; Anderson, D.C.; Labow, M.A.; Granger, D.N. Heterogeneity of expression of E- and P-selectins in vivo. Circ. Res. 1996, 79, 560–569. [Google Scholar] [CrossRef]
- Ivetic, A.; Hoskins Green, H.L.; Hart, S.J. L-selectin: A Major Regulator of Leukocyte Adhesion, Migration and Signaling. Front. Immunol. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Thiel, M.; Zourelidis, C.; Chambers, J.D.; von Andrian, U.H.; Arfors, K.E.; Messmer, K.; Peter, K. Expression of beta 2-integrins and L-selectin on polymorphonuclear leukocytes in septic patients. Eur. Surg. Res. 1997, 29, 160–175. [Google Scholar] [CrossRef]
- Lowe, J.B. Glycosylation in the control of selectin counter-receptor structure and function. Immunol. Rev. 2002, 186, 19–36. [Google Scholar] [CrossRef]
- Poppe, L.; Brown, G.S.; Philo, J.S.; Nokrad, P.V.; Shah, B.H. Conformation os sLex Tetrasaccharide, Free in Solution and Bound to E-, P-, and L-Selectiin. J. Am. Chem. Soc. 1997, 119, 1727–1736. [Google Scholar] [CrossRef]
- Scudder, P.R.; Shailubhai, K.; Duffin, K.L.; Streeter, P.R.; Jacob, G.S. Enzymatic synthesis of a 6′-sulphated sialyl-Lewisx which is an inhibitor of L-selectin binding to peripheral addressin. Glycobiology 1994, 4, 929–932. [Google Scholar] [CrossRef] [PubMed]
- Varki, A. Selectin ligands: Will the real ones please stand up? J. Clin. Investig. 1997, 100, S31–S35. [Google Scholar] [CrossRef] [PubMed]
- Ohtsubo, K.; Marth, J.D. Glycosylation in cellular mechanisms of health and disease. Cell 2006, 126, 855–867. [Google Scholar] [CrossRef]
- Cummings, R.D. Structure and function of the selectin ligand PSGL-1. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Medicas Biol. 1999, 32, 519–528. [Google Scholar] [CrossRef]
- Carlow, D.A.; Gold, M.R.; Ziltener, H.J. Lymphocytes in the peritoneum home to the omentum and are activated by resident dendritic cells. J. Immunol. 2009, 183, 1155–1165. [Google Scholar] [CrossRef]
- Tinoco, R.; Otero, D.C.; Takahashi, A.A.; Bradley, L.M. PSGL-1: A New Player in the Immune Checkpoint Landscape. Trends Immunol. 2017, 38, 323–335. [Google Scholar] [CrossRef]
- Leppanen, A.; Mehta, P.; Ouyang, Y.B.; Ju, T.; Helin, J.; Moore, K.L.; van Die, I.; Canfield, W.M.; McEver, R.P.; Cummings, R.D. A novel glycosulfopeptide binds to P-selectin and inhibits leukocyte adhesion to P-selectin. J. Biol. Chem. 1999, 274, 24838–24848. [Google Scholar] [CrossRef]
- Leppanen, A.; White, S.P.; Helin, J.; McEver, R.P.; Cummings, R.D. Binding of glycosulfopeptides to P-selectin requires stereospecific contributions of individual tyrosine sulfate and sugar residues. J. Biol. Chem. 2000, 275, 39569–39578. [Google Scholar] [CrossRef]
- Leppanen, A.; Yago, T.; Otto, V.I.; McEver, R.P.; Cummings, R.D. Model glycosulfopeptides from P-selectin glycoprotein ligand-1 require tyrosine sulfation and a core 2-branched O-glycan to bind to L-selectin. J. Biol. Chem. 2003, 278, 26391–26400. [Google Scholar] [CrossRef]
- Moore, K.L.; Eaton, S.F.; Lyons, D.E.; Lichenstein, H.S.; Cummings, R.D.; McEver, R.P. The P-selectin glycoprotein ligand from human neutrophils displays sialylated, fucosylated, O-linked poly-N-acetyllactosamine. J. Biol. Chem. 1994, 269, 23318–23327. [Google Scholar] [CrossRef] [PubMed]
- Sackstein, R. Glycoengineering of HCELL, the human bone marrow homing receptor: Sweetly programming cell migration. Ann. Biomed. Eng. 2012, 40, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Sako, D.; Comess, K.M.; Barone, K.M.; Camphausen, R.T.; Cumming, D.A.; Shaw, G.D. A sulfated peptide segment at the amino terminus of PSGL-1 is critical for P-selectin binding. Cell 1995, 83, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Asa, D.; Raycroft, L.; Ma, L.; Aeed, P.A.; Kaytes, P.S.; Elhammer, A.P.; Geng, J.G. The P-selectin glycoprotein ligand functions as a common human leukocyte ligand for P- and E-selectins. J. Biol. Chem. 1995, 270, 11662–11670. [Google Scholar] [CrossRef]
- Bruehl, R.E.; Springer, T.A.; Bainton, D.F. Quantitation of L-selectin distribution on human leukocyte microvilli by immunogold labeling and electron microscopy. J. Histochem. Cytochem. 1996, 44, 835–844. [Google Scholar] [CrossRef]
- Zarbock, A.; Ley, K.; McEver, R.P.; Hidalgo, A. Leukocyte ligands for endothelial selectins: Specialized glycoconjugates that mediate rolling and signaling under flow. Blood 2011, 118, 6743–6751. [Google Scholar] [CrossRef]
- Steegmaier, M.; Borges, E.; Berger, J.; Schwarz, H.; Vestweber, D. The E-selectin-ligand ESL-1 is located in the Golgi as well as on microvilli on the cell surface. J. Cell Sci. 1997, 110 Pt 6, 687–694. [Google Scholar] [CrossRef]
- Levinovitz, A.; Muhlhoff, J.; Isenmann, S.; Vestweber, D. Identification of a glycoprotein ligand for E-selectin on mouse myeloid cells. J. Cell Biol. 1993, 121, 449–459. [Google Scholar] [CrossRef]
- Huang, M.C.; Zollner, O.; Moll, T.; Maly, P.; Thall, A.D.; Lowe, J.B.; Vestweber, D. P-selectin glycoprotein ligand-1 and E-selectin ligand-1 are differentially modified by fucosyltransferases Fuc-TIV and Fuc-TVII in mouse neutrophils. J. Biol. Chem. 2000, 275, 31353–31360. [Google Scholar] [CrossRef]
- Hidalgo, A.; Peired, A.J.; Wild, M.; Vestweber, D.; Frenette, P.S. Complete identification of E-selectin ligands on neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44. Immunity 2007, 26, 477–489. [Google Scholar] [CrossRef]
- Sreeramkumar, V.; Leiva, M.; Stadtmann, A.; Pitaval, C.; Ortega-Rodriguez, I.; Wild, M.K.; Lee, B.; Zarbock, A.; Hidalgo, A. Coordinated and unique functions of the E-selectin ligand ESL-1 during inflammatory and hematopoietic recruitment in mice. Blood 2013, 122, 3993–4001. [Google Scholar] [CrossRef] [PubMed]
- Werneburg, S.; Buettner, F.F.; Erben, L.; Mathews, M.; Neumann, H.; Muhlenhoff, M.; Hildebrandt, H. Polysialylation and lipopolysaccharide-induced shedding of E-selectin ligand-1 and neuropilin-2 by microglia and THP-1 macrophages. Glia 2016, 64, 1314–1330. [Google Scholar] [CrossRef] [PubMed]
- Brandley, B.K.; Kiso, M.; Abbas, S.; Nikrad, P.; Srivasatava, O.; Foxall, C.; Oda, Y.; Hasegawa, A. Structure-function studies on selectin carbohydrate ligands. Modifications to fucose, sialic acid and sulphate as a sialic acid replacement. Glycobiology 1993, 3, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Jacob, G.S.; Kirmaier, C.; Abbas, S.Z.; Howard, S.C.; Steininger, C.N.; Welply, J.K.; Scudder, P. Binding of sialyl Lewis x to E-selectin as measured by fluorescence polarization. Biochemistry 1995, 34, 1210–1217. [Google Scholar] [CrossRef]
- Jacob, G.S.; Welply, J.K.; Scudder, P.R.; Kirmaier, C.; Abbas, S.Z.; Howard, S.C.; Keene, J.L.; Schmuke, J.J.; Broschat, K.; Steininger, C. Studies on selectin-carbohydrate interactions. Adv. Exp. Med. Biol. 1995, 376, 283–290. [Google Scholar] [CrossRef]
- Goodison, S.; Urquidi, V.; Tarin, D. CD44 cell adhesion molecules. Mol. Pathol. 1999, 52, 189–196. [Google Scholar] [CrossRef]
- Ponta, H.; Sherman, L.; Herrlich, P.A. CD44: From adhesion molecules to signalling regulators. Nat. Rev. Mol. Cell Biol. 2003, 4, 33–45. [Google Scholar] [CrossRef]
- Naor, D.; Sionov, R.V.; Ish-Shalom, D. CD44: Structure, function, and association with the malignant process. Adv. Cancer Res. 1997, 71, 241–319. [Google Scholar] [CrossRef]
- Cichy, J.; Pure, E. The liberation of CD44. J. Cell Biol. 2003, 161, 839–843. [Google Scholar] [CrossRef]
- Sackstein, R. The biology of CD44 and HCELL in hematopoiesis: The ‘step 2-bypass pathway’ and other emerging perspectives. Curr. Opin. Hematol. 2011, 18, 239–248. [Google Scholar] [CrossRef]
- Dimitroff, C.J.; Lee, J.Y.; Fuhlbrigge, R.C.; Sackstein, R. A distinct glycoform of CD44 is an L-selectin ligand on human hematopoietic cells. Proc. Natl. Acad. Sci. USA 2000, 97, 13841–13846. [Google Scholar] [CrossRef] [PubMed]
- Dimitroff, C.J.; Lee, J.Y.; Schor, K.S.; Sandmaier, B.M.; Sackstein, R. differential L-selectin binding activities of human hematopoietic cell L-selectin ligands, HCELL and PSGL-1. J. Biol. Chem. 2001, 276, 47623–47631. [Google Scholar] [CrossRef] [PubMed]
- Ouhtit, A.; Rizeq, B.; Saleh, H.A.; Rahman, M.; Zayed3, H. Novel CD44-downstream signaling pathways mediating breast tumor invasion. Int. J. Biol. Sci. 2018, 14, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Sackstein, R. The bone marrow is akin to skin: HCELL and the biology of hematopoietic stem cell homing. J. Investig. Dermatol. Symp. Proc. 2004, 9, 215–223. [Google Scholar] [CrossRef]
- Dimitroff, C.J.; Lee, J.Y.; Rafii, S.; Fuhlbrigge, R.C.; Sackstein, R. CD44 is a major E-selectin ligand on human hematopoietic progenitor cells. J. Cell Biol. 2001, 153, 1277–1286. [Google Scholar] [CrossRef]
- Silva, M.; Fung, R.K.F.; Donnelly, C.B.; Videira, P.A.; Sackstein, R. Cell-Specific Variation in E-Selectin Ligand Expression among Human Peripheral Blood Mononuclear Cells: Implications for Immunosurveillance and Pathobiology. J. Immunol. 2017, 198, 3576–3587. [Google Scholar] [CrossRef]
- Sackstein, R.; Dimitroff, C.J. A hematopoietic cell L-selectin ligand that is distinct from PSGL-1 and displays N-glycan-dependent binding activity. Blood 2000, 96, 2765–2774. [Google Scholar] [CrossRef]
- Hanley, W.D.; Burdick, M.M.; Konstantopoulos, K.; Sackstein, R. CD44 on LS174T colon carcinoma cells possesses E-selectin ligand activity. Cancer Res. 2005, 65, 5812–5817. [Google Scholar] [CrossRef]
- Silva, M.; Videira, P.A.; Sackstein, R. E-Selectin Ligands in the Human Mononuclear Phagocyte System: Implications for Infection, Inflammation, and Immunotherapy. Front. Immunol. 2018, 8, 1878. [Google Scholar] [CrossRef]
- Rosen, S.D. Ligands for L-selectin: Homing, inflammation, and beyond. Annu. Rev. Immunol. 2004, 22, 129–156. [Google Scholar] [CrossRef]
- Rosen, S.D. Endothelial ligands for L-selectin: From lymphocyte recirculation to allograft rejection. Am. J. Pathol. 1999, 155, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Jutila, M.A.; Kurk, S.; Jackiw, L.; Knibbs, R.N.; Stoolman, L.M. L-selectin serves as an E-selectin ligand on cultured human T lymphoblasts. J. Immunol. 2002, 169, 1768–1773. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H.; Li, Y.F.; Watanabe, N.; Hirose, J.; Hirose, M.; Miyasaka, M. Identification and characterization of ligands for L-selectin in the kidney. I. Versican, a large chondroitin sulfate proteoglycan, is a ligand for L-selectin. Int. Immunol. 1999, 11, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Celie, J.W.; Rutjes, N.W.; Keuning, E.D.; Soininen, R.; Heljasvaara, R.; Pihlajaniemi, T.; Drager, A.M.; Zweegman, S.; Kessler, F.L.; Beelen, R.H.; et al. Subendothelial heparan sulfate proteoglycans become major L-selectin and monocyte chemoattractant protein-1 ligands upon renal ischemia/reperfusion. Am. J. Pathol. 2007, 170, 1865–1878. [Google Scholar] [CrossRef]
- Kawashima, H.; Watanabe, N.; Hirose, M.; Sun, X.; Atarashi, K.; Kimura, T.; Shikata, K.; Matsuda, M.; Ogawa, D.; Heljasvaara, R.; et al. Collagen XVIII, a basement membrane heparan sulfate proteoglycan, interacts with L-selectin and monocyte chemoattractant protein-1. J. Biol. Chem. 2003, 278, 13069–13076. [Google Scholar] [CrossRef]
- Kitaya, K.; Yasuo, T. Dermatan sulfate proteoglycan biglycan as a potential selectin L/CD44 ligand involved in selective recruitment of peripheral blood CD16(-) natural killer cells into human endometrium. J. Leukoc. Biol. 2009, 85, 391–400. [Google Scholar] [CrossRef]
- Puri, K.D.; Finger, E.B.; Gaudernack, G.; Springer, T.A. Sialomucin CD34 is the major L-selectin ligand in human tonsil high endothelial venules. J. Cell Biol. 1995, 131, 261–270. [Google Scholar] [CrossRef]
- Sassetti, C.; Tangemann, K.; Singer, M.S.; Kershaw, D.B.; Rosen, S.D. Identification of podocalyxin-like protein as a high endothelial venule ligand for L-selectin: Parallels to CD34. J. Exp. Med. 1998, 187, 1965–1975. [Google Scholar] [CrossRef]
- Samulowitz, U.; Kuhn, A.; Brachtendorf, G.; Nawroth, R.; Braun, A.; Bankfalvi, A.; Bocker, W.; Vestweber, D. Human endomucin: Distribution pattern, expression on high endothelial venules, and decoration with the MECA-79 epitope. Am. J. Pathol. 2002, 160, 1669–1681. [Google Scholar] [CrossRef]
- Rosen, S.D.; Tsay, D.; Singer, M.S.; Hemmerich, S.; Abraham, W.M. Therapeutic targeting of endothelial ligands for L-selectin (PNAd) in a sheep model of asthma. Am. J. Pathol. 2005, 166, 935–944. [Google Scholar] [CrossRef]
- Chen, C.C.; Rosenbloom, C.L.; Anderson, D.C.; Manning, A.M. Selective inhibition of E-selectin, vascular cell adhesion molecule-1, and intercellular adhesion molecule-1 expression by inhibitors of I kappa B-alpha phosphorylation. J. Immunol. 1995, 155, 3538–3545. [Google Scholar] [CrossRef]
- Satomaa, T.; Renkonen, O.; Helin, J.; Kirveskari, J.; Makitie, A.; Renkonen, R. O-glycans on human high endothelial CD34 putatively participating in L-selectin recognition. Blood 2002, 99, 2609–2611. [Google Scholar] [CrossRef] [PubMed]
- Hernandez Mir, G.; Helin, J.; Skarp, K.P.; Cummings, R.D.; Makitie, A.; Renkonen, R.; Leppanen, A. Glycoforms of human endothelial CD34 that bind L-selectin carry sulfated sialyl Lewis x capped O- and N-glycans. Blood 2009, 114, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Berg, E.L.; McEvoy, L.M.; Berlin, C.; Bargatze, R.F.; Butcher, E.C. L-selectin-mediated lymphocyte rolling on MAdCAM-1. Nature 1993, 366, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Steeber, D.A.; Tedder, T.F. Adhesion molecule cascades direct lymphocyte recirculation and leukocyte migration during inflammation. Immunol. Res. 2000, 22, 299–317. [Google Scholar] [CrossRef]
- Angata, T.; Varki, A. Discovery, classification, evolution and diversity of Siglecs. Mol. Asp. Med. 2023, 90, 101117. [Google Scholar] [CrossRef]
- Pillai, S.; Netravali, I.A.; Cariappa, A.; Mattoo, H. Siglecs and immune regulation. Annu. Rev. Immunol. 2012, 30, 357–392. [Google Scholar] [CrossRef]
- Lim, J.; Sari-Ak, D.; Bagga, T. Siglecs as Therapeutic Targets in Cancer. Biology 2021, 10, 1178. [Google Scholar] [CrossRef]
- Crocker, P.R.; Clark, E.A.; Filbin, M.; Gordon, S.; Jones, Y.; Kehrl, J.H.; Kelm, S.; Le Douarin, N.; Powell, L.; Roder, J.; et al. Siglecs: A family of sialic-acid binding lectins. Glycobiology 1998, 8, v. [Google Scholar] [CrossRef]
- Siddiqui, S.S.; Vaill, M.; Do, R.; Khan, N.; Verhagen, A.L.; Zhang, W.; Lenz, H.J.; Johnson-Pais, T.L.; Leach, R.J.; Fraser, G.; et al. Human-specific polymorphic pseudogenization of SIGLEC12 protects against advanced cancer progression. FASEB Bioadvances 2021, 3, 69–82. [Google Scholar] [CrossRef]
- Magesh, S.; Ando, H.; Tsubata, T.; Ishida, H.; Kiso, M. High-affinity ligands of Siglec receptors and their therapeutic potentials. Curr. Med. Chem. 2011, 18, 3537–3550. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, N.; Ilarregui, J.M.; Toscano, M.A.; Rabinovich, G.A. The role of galectins in the initiation, amplification and resolution of the inflammatory response. Tissue Antigens 2004, 64, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mitra, N.; Banda, K.; Altheide, T.K.; Schaffer, L.; Johnson-Pais, T.L.; Beuten, J.; Leach, R.J.; Angata, T.; Varki, N.; Varki, A. SIGLEC12, a human-specific segregating (pseudo)gene, encodes a signaling molecule expressed in prostate carcinomas. J. Biol. Chem. 2011, 286, 23003–23011. [Google Scholar] [CrossRef] [PubMed]
- Bochner, B.S.; Zimmermann, N. Role of siglecs and related glycan-binding proteins in immune responses and immunoregulation. J. Allergy Clin. Immunol. 2015, 135, 598–608. [Google Scholar] [CrossRef]
- Muller, J.; Obermeier, I.; Wohner, M.; Brandl, C.; Mrotzek, S.; Angermuller, S.; Maity, P.C.; Reth, M.; Nitschke, L. CD22 ligand-binding and signaling domains reciprocally regulate B-cell Ca2+ signaling. Proc. Natl. Acad. Sci. USA 2013, 110, 12402–12407. [Google Scholar] [CrossRef]
- Duan, S.; Paulson, J.C. Siglecs as Immune Cell Checkpoints in Disease. Annu. Rev. Immunol. 2020, 38, 365–395. [Google Scholar] [CrossRef]
- Blixt, O.; Collins, B.E.; van den Nieuwenhof, I.M.; Crocker, P.R.; Paulson, J.C. Sialoside specificity of the siglec family assessed using novel multivalent probes: Identification of potent inhibitors of myelin-associated glycoprotein. J. Biol. Chem. 2003, 278, 31007–31019. [Google Scholar] [CrossRef]
- O’Reilly, M.K.; Paulson, J.C. Multivalent ligands for siglecs. Methods Enzymol. 2010, 478, 343–363. [Google Scholar] [CrossRef]
- Laubli, H.; Kawanishi, K.; George Vazhappilly, C.; Matar, R.; Merheb, M.; Sarwar Siddiqui, S. Tools to study and target the Siglec-sialic acid axis in cancer. FEBS J. 2021, 288, 6206–6225. [Google Scholar] [CrossRef]
- Gonzalez-Gil, A.; Schnaar, R.L. Siglec Ligands. Cells 2021, 10, 1260. [Google Scholar] [CrossRef]
- Gonzalez-Gil, A.; Li, T.A.; Kim, J.; Schnaar, R.L. Human sialoglycan ligands for immune inhibitory Siglecs. Mol. Asp. Med. 2023, 90, 101110. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Nizet, V. Siglecs at the Host-Pathogen Interface. Adv. Exp. Med. Biol. 2020, 1204, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Collins, B.E.; Bengtson, P.; Paulson, J.C. Homomultimeric complexes of CD22 in B cells revealed by protein-glycan cross-linking. Nat. Chem. Biol. 2005, 1, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Bull, C.; den Brok, M.H.; Adema, G.J. Sweet escape: Sialic acids in tumor immune evasion. Biochim. Biophys. Acta 2014, 1846, 238–246. [Google Scholar] [CrossRef]
- Cao, H.; Crocker, P.R. Evolution of CD33-related siglecs: Regulating host immune functions and escaping pathogen exploitation? Immunology 2011, 132, 18–26. [Google Scholar] [CrossRef]
- Varki, A. Since there are PAMPs and DAMPs, there must be SAMPs? Glycan “self-associated molecular patterns” dampen innate immunity, but pathogens can mimic them. Glycobiology 2011, 21, 1121–1124. [Google Scholar] [CrossRef]
- Thiemann, S.; Baum, L.G. Galectins and Immune Responses-Just How Do They Do Those Things They Do? Annu. Rev. Immunol. 2016, 34, 243–264. [Google Scholar] [CrossRef]
- Yang, R.Y.; Rabinovich, G.A.; Liu, F.T. Galectins: Structure, function and therapeutic potential. Expert Rev. Mol. Med. 2008, 10, e17. [Google Scholar] [CrossRef]
- Liu, F.T.; Stowell, S.R. The role of galectins in immunity and infection. Nat. Rev. Immunol. 2023, 23, 479–494. [Google Scholar] [CrossRef]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a glance. J. Cell Sci. 2018, 131, jcs208884. [Google Scholar] [CrossRef]
- He, J.; Baum, L.G. Galectin interactions with extracellular matrix and effects on cellular function. Methods Enzymol. 2006, 417, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Elola, M.T.; Wolfenstein-Todel, C.; Troncoso, M.F.; Vasta, G.R.; Rabinovich, G.A. Galectins: Matricellular glycan-binding proteins linking cell adhesion, migration, and survival. Cell. Mol. Life Sci. 2007, 64, 1679–1700. [Google Scholar] [CrossRef] [PubMed]
- Garner, O.B.; Baum, L.G. Galectin-glycan lattices regulate cell-surface glycoprotein organization and signalling. Biochem. Soc. Trans. 2008, 36, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Fred Brewer, C. Binding and cross-linking properties of galectins. Biochim. Biophys. Acta 2002, 1572, 255–262. [Google Scholar] [CrossRef]
- Mkhikian, H.; Sy, M.; Dennis, J.W.; Demetriou, M. The galectin-lattice: A decoder of bio-equivalent glycans. Glycoforum 2021, 24, A10. [Google Scholar] [CrossRef]
- Zetterberg, F.R.; Diehl, C.; Hakansson, M.; Kahl-Knutson, B.; Leffler, H.; Nilsson, U.J.; Peterson, K.; Roper, J.A.; Slack, R.J. Discovery of Selective and Orally Available Galectin-1 Inhibitors. J. Med. Chem. 2023, 66, 16980–16990. [Google Scholar] [CrossRef]
- Saraboji, K.; Hakansson, M.; Genheden, S.; Diehl, C.; Qvist, J.; Weininger, U.; Nilsson, U.J.; Leffler, H.; Ryde, U.; Akke, M.; et al. The carbohydrate-binding site in galectin-3 is preorganized to recognize a sugarlike framework of oxygens: Ultra-high-resolution structures and water dynamics. Biochemistry 2012, 51, 296–306. [Google Scholar] [CrossRef]
- Manzoni, F.; Wallerstein, J.; Schrader, T.E.; Ostermann, A.; Coates, L.; Akke, M.; Blakeley, M.P.; Oksanen, E.; Logan, D.T. Elucidation of Hydrogen Bonding Patterns in Ligand-Free, Lactose- and Glycerol-Bound Galectin-3C by Neutron Crystallography to Guide Drug Design. J. Med. Chem. 2018, 61, 4412–4420. [Google Scholar] [CrossRef]
- Zetterberg, F.R.; MacKinnon, A.; Brimert, T.; Gravelle, L.; Johnsson, R.E.; Kahl-Knutson, B.; Leffler, H.; Nilsson, U.J.; Pedersen, A.; Peterson, K.; et al. Discovery and Optimization of the First Highly Effective and Orally Available Galectin-3 Inhibitors for Treatment of Fibrotic Disease. J. Med. Chem. 2022, 65, 12626–12638. [Google Scholar] [CrossRef]
- Carlsson, S.; Oberg, C.T.; Carlsson, M.C.; Sundin, A.; Nilsson, U.J.; Smith, D.; Cummings, R.D.; Almkvist, J.; Karlsson, A.; Leffler, H. Affinity of galectin-8 and its carbohydrate recognition domains for ligands in solution and at the cell surface. Glycobiology 2007, 17, 663–676. [Google Scholar] [CrossRef]
- Rapoport, E.M.; Andre, S.; Kurmyshkina, O.V.; Pochechueva, T.V.; Severov, V.V.; Pazynina, G.V.; Gabius, H.J.; Bovin, N.V. Galectin-loaded cells as a platform for the profiling of lectin specificity by fluorescent neoglycoconjugates: A case study on galectins-1 and -3 and the impact of assay setting. Glycobiology 2008, 18, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.C.; Lin, H.Y.; Tu, Z.; Kuo, Y.H.; Hsu, S.D.; Lin, C.H. Dissecting the Structure-Activity Relationship of Galectin-Ligand Interactions. Int. J. Mol. Sci. 2018, 19, 392. [Google Scholar] [CrossRef]
- Hsieh, T.J.; Lin, H.Y.; Tu, Z.; Huang, B.S.; Wu, S.C.; Lin, C.H. Structural Basis Underlying the Binding Preference of Human Galectins-1, -3 and -7 for Galbeta1-3/4GlcNAc. PLoS ONE 2015, 10, e0125946. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, J.; Hashidate, T.; Arata, Y.; Nishi, N.; Nakamura, T.; Hirashima, M.; Urashima, T.; Oka, T.; Futai, M.; Muller, W.E.; et al. Oligosaccharide specificity of galectins: A search by frontal affinity chromatography. Biochim. Biophys. Acta 2002, 1572, 232–254. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, S.K.; Potvin, B.; Carlsson, S.; Sturm, D.; Leffler, H.; Stanley, P. Complex N-glycans are the major ligands for galectin-1, -3, and -8 on Chinese hamster ovary cells. Glycobiology 2006, 16, 305–317. [Google Scholar] [CrossRef]
- Stowell, S.R.; Arthur, C.M.; Mehta, P.; Slanina, K.A.; Blixt, O.; Leffler, H.; Smith, D.F.; Cummings, R.D. Galectin-1, -2, and -3 exhibit differential recognition of sialylated glycans and blood group antigens. J. Biol. Chem. 2008, 283, 10109–10123. [Google Scholar] [CrossRef]
- Arthur, C.M.; Baruffi, M.D.; Cummings, R.D.; Stowell, S.R. Evolving mechanistic insights into galectin functions. Methods Mol. Biol. 2015, 1207, 1–35. [Google Scholar] [CrossRef]
- Bouffette, S.; Botez, I.; De Ceuninck, F. Targeting galectin-3 in inflammatory and fibrotic diseases. Trends Pharmacol. Sci. 2023, 44, 519–531. [Google Scholar] [CrossRef]
- Novak, J.; Takacs, T.; Tilajka, A.; Laszlo, L.; Oravecz, O.; Farkas, E.; Than, N.G.; Buday, L.; Balogh, A.; Vas, V. The sweet and the bitter sides of galectin-1 in immunity: Its role in immune cell functions, apoptosis, and immunotherapies for cancer with a focus on T cells. Semin. Immunopathol. 2025, 47, 24. [Google Scholar] [CrossRef]
- Rodriguez, E.; Lindijer, D.V.; van Vliet, S.J.; Garcia Vallejo, J.J.; van Kooyk, Y. The transcriptional landscape of glycosylation-related genes in cancer. iScience 2024, 27, 109037. [Google Scholar] [CrossRef]
- Wu, R.; Wu, T.; Wang, K.; Luo, S.; Chen, Z.; Fan, M.; Xue, D.; Lu, H.; Zhuang, Q.; Xu, X. Prognostic significance of galectin-1 expression in patients with cancer: A meta-analysis. Cancer Cell Int. 2018, 18, 108. [Google Scholar] [CrossRef] [PubMed]
- Lo, T.-H.; Weng, I.-C.; Chen, H.-L.; Liu, F.-T. The role of galectins in the regulation of autophagy and inflammasome in host immunity. Semin. Immunopathol. 2024, 46, 6. [Google Scholar] [CrossRef] [PubMed]
- Vasta, G.R. Roles of galectins in infection. Nat. Rev. Microbiol. 2009, 7, 424–438. [Google Scholar] [CrossRef] [PubMed]
- Baum, L.G.; Garner, O.B.; Schaefer, K.; Lee, B. Microbe-Host Interactions are Positively and Negatively Regulated by Galectin-Glycan Interactions. Front. Immunol. 2014, 5, 284. [Google Scholar] [CrossRef][Green Version]
- Diaz-Alvarez, L.; Ortega, E. The Many Roles of Galectin-3, a Multifaceted Molecule, in Innate Immune Responses against Pathogens. Mediat. Inflamm. 2017, 2017, 9247574. [Google Scholar] [CrossRef]
- Hara, A.; Niwa, M.; Noguchi, K.; Kanayama, T.; Niwa, A.; Matsuo, M.; Hatano, Y.; Tomita, H. Galectin-3 as a Next-Generation Biomarker for Detecting Early Stage of Various Diseases. Biomolecules 2020, 10, 389. [Google Scholar] [CrossRef]
- Guo, Y.; Shen, R.; Yu, L.; Zheng, X.; Cui, R.; Song, Y.; Wang, D. Roles of galectin-3 in the tumor microenvironment and tumor metabolism (Review). Oncol. Rep. 2020, 44, 1799–1809. [Google Scholar] [CrossRef]
- Ley, K.; Kansas, G.S. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat. Rev. Immunol. 2004, 4, 325–335. [Google Scholar] [CrossRef]
- Vestweber, D. How leukocytes cross the vascular endothelium. Nat. Rev. Immunol. 2015, 15, 692–704. [Google Scholar] [CrossRef]
- McEver, R.P.; Zhu, C. Rolling cell adhesion. Annu. Rev. Cell Dev. Biol. 2010, 26, 363–396. [Google Scholar] [CrossRef]
- Butcher, E.C.; Picker, L.J. Lymphocyte homing and homeostasis. Science 1996, 272, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, O.; Sanchez-Madrid, F. Molecular basis of leukocyte-endothelium interactions during the inflammatory response. Rev. Esp. Cardiol. 2009, 62, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Cheung, L.S.; Raman, P.S.; Balzer, E.M.; Wirtz, D.; Konstantopoulos, K. Biophysics of selectin-ligand interactions in inflammation and cancer. PhBio 2011, 8, 015013. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yago, T.; Lou, J.; Zarnitsyna, V.I.; McEver, R.P. Mechanisms for flow-enhanced cell adhesion. Ann. Biomed. Eng. 2008, 36, 604–621. [Google Scholar] [CrossRef]
- Ludwig, R.J.; Schon, M.P.; Boehncke, W.H. P-selectin: A common therapeutic target for cardiovascular disorders, inflammation and tumour metastasis. Expert Opin. Ther. Targets 2007, 11, 1103–1117. [Google Scholar] [CrossRef]
- Ley, K. The role of selectins in inflammation and disease. Trends Mol. Med. 2003, 9, 263–268. [Google Scholar] [CrossRef]
- Frommhold, D.; Ludwig, A.; Bixel, M.G.; Zarbock, A.; Babushkina, I.; Weissinger, M.; Cauwenberghs, S.; Ellies, L.G.; Marth, J.D.; Beck-Sickinger, A.G.; et al. Sialyltransferase ST3Gal-IV controls CXCR2-mediated firm leukocyte arrest during inflammation. J. Exp. Med. 2008, 205, 1435–1446. [Google Scholar] [CrossRef]
- Woodard-Grice, A.V.; McBrayer, A.C.; Wakefield, J.K.; Zhuo, Y.; Bellis, S.L. Proteolytic shedding of ST6Gal-I by BACE1 regulates the glycosylation and function of alpha4beta1 integrins. J. Biol. Chem. 2008, 283, 26364–26373. [Google Scholar] [CrossRef]
- Laubli, H.; Spanaus, K.S.; Borsig, L. Selectin-mediated activation of endothelial cells induces expression of CCL5 and promotes metastasis through recruitment of monocytes. Blood 2009, 114, 4583–4591. [Google Scholar] [CrossRef]
- Laubli, H.; Borsig, L. Selectins promote tumor metastasis. Semin. Cancer Biol. 2010, 20, 169–177. [Google Scholar] [CrossRef]
- St Hill, C.A. Interactions between endothelial selectins and cancer cells regulate metastasis. Front. Biosci. 2011, 16, 3233–3251. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Shin, J.S.; Nahm, M.H. NOD-Like Receptors in Infection, Immunity, and Diseases. Yonsei Med. J. 2016, 57, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Platnich, J.M.; Muruve, D.A. NOD-like receptors and inflammasomes: A review of their canonical and non-canonical signaling pathways. Arch. Biochem. Biophys. 2019, 670, 4–14. [Google Scholar] [CrossRef]
- Wieczorek, M.; Abualrous, E.T.; Sticht, J.; Alvaro-Benito, M.; Stolzenberg, S.; Noe, F.; Freund, C. Major Histocompatibility Complex (MHC) Class I and MHC Class II Proteins: Conformational Plasticity in Antigen Presentation. Front. Immunol. 2017, 8, 292. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Bao, Z.; Tang, P.; Gong, W.; Yoshimura, T.; Wang, J.M. Chemokines in homeostasis and diseases. Cell. Mol. Immunol. 2018, 15, 324–334. [Google Scholar] [CrossRef]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From Clinical Significance to Quantification. Adv. Sci. 2021, 8, e2004433. [Google Scholar] [CrossRef]
- Sun, L.; Su, Y.; Jiao, A.; Wang, X.; Zhang, B. T cells in health and disease. Signal Transduct. Target. Ther. 2023, 8, 235. [Google Scholar] [CrossRef]
- Toscano, M.A.; Bianco, G.A.; Ilarregui, J.M.; Croci, D.O.; Correale, J.; Hernandez, J.D.; Zwirner, N.W.; Poirier, F.; Riley, E.M.; Baum, L.G.; et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat. Immunol. 2007, 8, 825–834. [Google Scholar] [CrossRef]
- Moody, A.M.; Chui, D.; Reche, P.A.; Priatel, J.J.; Marth, J.D.; Reinherz, E.L. Developmentally regulated glycosylation of the CD8alphabeta coreceptor stalk modulates ligand binding. Cell 2001, 107, 501–512. [Google Scholar] [CrossRef]
- Vicente, M.M.; Alves, I.; Fernandes, A.; Dias, A.M.; Santos-Pereira, B.; Perez-Anton, E.; Santos, S.; Yang, T.; Correia, A.; Munster-Kuhnel, A.; et al. Mannosylated glycans impair normal T-cell development by reprogramming commitment and repertoire diversity. Cell. Mol. Immunol. 2023, 20, 955–968. [Google Scholar] [CrossRef]
- Gomez-Henao, W.; Tenorio, E.P.; Sanchez, F.R.C.; Mendoza, M.C.; Ledezma, R.L.; Zenteno, E. Relevance of glycans in the interaction between T lymphocyte and the antigen presenting cell. Int. Rev. Immunol. 2021, 40, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Demetriou, M.; Granovsky, M.; Quaggin, S.; Dennis, J.W. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature 2001, 409, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Mortales, C.L.; Lee, S.U.; Demetriou, M. N-Glycan Branching Is Required for Development of Mature B Cells. J. Immunol. 2020, 205, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Shinzaki, S.; Iijima, H.; Wakamatsu, K.; Iwamoto, C.; Sobajima, T.; Kuwahara, R.; Hiyama, S.; Hayashi, Y.; Takamatsu, S.; et al. Core Fucosylation on T Cells, Required for Activation of T-Cell Receptor Signaling and Induction of Colitis in Mice, Is Increased in Patients with Inflammatory Bowel Disease. Gastroenterology 2016, 150, 1620–1632. [Google Scholar] [CrossRef]
- Vicente, M.M.; Leite-Gomes, E.; Pinho, S.S. Glycome dynamics in T and B cell development: Basic immunological mechanisms and clinical applications. Trends Immunol. 2023, 44, 585–597. [Google Scholar] [CrossRef]
- Li, H.; Debowski, A.W.; Liao, T.; Tang, H.; Nilsson, H.O.; Marshall, B.J.; Stubbs, K.A.; Benghezal, M. Understanding protein glycosylation pathways in bacteria. Future Microbiol. 2017, 12, 59–72. [Google Scholar] [CrossRef]
- Nothaft, H.; Szymanski, C.M. Protein glycosylation in bacteria: Sweeter than ever. Nat. Rev. Microbiol. 2010, 8, 765–778. [Google Scholar] [CrossRef]
- Morrison, M.J.; Imperiali, B. The renaissance of bacillosamine and its derivatives: Pathway characterization and implications in pathogenicity. Biochemistry 2014, 53, 624–638. [Google Scholar] [CrossRef]
- Tan, F.Y.; Tang, C.M.; Exley, R.M. Sugar coating: Bacterial protein glycosylation and host-microbe interactions. Trends Biochem. Sci. 2015, 40, 342–350. [Google Scholar] [CrossRef]
- Turner, J.; Torrelles, J.B. Mannose-capped lipoarabinomannan in Mycobacterium tuberculosis pathogenesis. Pathog. Dis. 2018, 76, fty026. [Google Scholar] [CrossRef]
- Chang, Y.C.; Nizet, V. The interplay between Siglecs and sialylated pathogens. Glycobiology 2014, 24, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Vimr, E.R.; Kalivoda, K.A.; Deszo, E.L.; Steenbergen, S.M. Diversity of microbial sialic acid metabolism. Microbiol. Mol. Biol. Rev. 2004, 68, 132–153. [Google Scholar] [CrossRef] [PubMed]
- Carlin, A.F.; Uchiyama, S.; Chang, Y.C.; Lewis, A.L.; Nizet, V.; Varki, A. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood 2009, 113, 3333–3336. [Google Scholar] [CrossRef] [PubMed]
- Park, A.M.; Hagiwara, S.; Hsu, D.K.; Liu, F.T.; Yoshie, O. Galectin-3 Plays an Important Role in Innate Immunity to Gastric Infection by Helicobacter pylori. Infect. Immun. 2016, 84, 1184–1193. [Google Scholar] [CrossRef]
- Tana, F.L.; Guimaraes, E.S.; Cerqueira, D.M.; Campos, P.C.; Gomes, M.T.R.; Marinho, F.V.; Oliveira, S.C. Galectin-3 regulates proinflammatory cytokine function and favours Brucella abortus chronic replication in macrophages and mice. Cell. Microbiol. 2021, 23, e13375. [Google Scholar] [CrossRef]
- Deshpande, N.; Wilkins, M.R.; Packer, N.; Nevalainen, H. Protein glycosylation pathways in filamentous fungi. Glycobiology 2008, 18, 626–637. [Google Scholar] [CrossRef]
- De Pourcq, K.; De Schutter, K.; Callewaert, N. Engineering of glycosylation in yeast and other fungi: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 87, 1617–1631. [Google Scholar] [CrossRef]
- Loibl, M.; Strahl, S. Protein O-mannosylation: What we have learned from baker’s yeast. Biochim. Biophys. Acta 2013, 1833, 2438–2446. [Google Scholar] [CrossRef]
- Bai, L.; Li, H. Protein N-glycosylation and O-mannosylation are catalyzed by two evolutionarily related GT-C glycosyltransferases. Curr. Opin. Struct. Biol. 2021, 68, 66–73. [Google Scholar] [CrossRef]
- Wu, S.Y.; Huang, J.H.; Chen, W.Y.; Chan, Y.C.; Lin, C.H.; Chen, Y.C.; Liu, F.T.; Wu-Hsieh, B.A. Cell Intrinsic Galectin-3 Attenuates Neutrophil ROS-Dependent Killing of Candida by Modulating CR3 Downstream Syk Activation. Front. Immunol. 2017, 8, 48. [Google Scholar] [CrossRef]
- Almeida, F.; Wolf, J.M.; da Silva, T.A.; DeLeon-Rodriguez, C.M.; Rezende, C.P.; Pessoni, A.M.; Fernandes, F.F.; Silva-Rocha, R.; Martinez, R.; Rodrigues, M.L.; et al. Galectin-3 impacts Cryptococcus neoformans infection through direct antifungal effects. Nat. Commun. 2017, 8, 1968. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Bowden, T.A.; Wilson, I.A.; Crispin, M. Exploitation of glycosylation in enveloped virus pathobiology. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 1480–1497. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.K.; Sharma, R.; Prajapati, G.K.; Mohanta, T.K.; Mishra, A.K. N-glycosylation, a leading role in viral infection and immunity development. Mol. Biol. Rep. 2022, 49, 8109–8120. [Google Scholar] [CrossRef] [PubMed]
- Ming, A.; Zhao, J.; Liu, Y.; Wang, Y.; Wang, X.; Li, J.; Zhang, L. O-glycosylation in viruses: A sweet tango. mLife 2024, 3, 57–73. [Google Scholar] [CrossRef]
- Feng, T.; Zhang, J.; Chen, Z.; Pan, W.; Chen, Z.; Yan, Y.; Dai, J. Glycosylation of viral proteins: Implication in virus-host interaction and virulence. Virulence 2022, 13, 670–683. [Google Scholar] [CrossRef]
- Vigerust, D.J.; Shepherd, V.L. Virus glycosylation: Role in virulence and immune interactions. Trends Microbiol. 2007, 15, 211–218. [Google Scholar] [CrossRef]
- Bayer, K.; Banning, C.; Bruss, V.; Wiltzer-Bach, L.; Schindler, M. Hepatitis C Virus Is Released via a Noncanonical Secretory Route. J. Virol. 2016, 90, 10558–10573. [Google Scholar] [CrossRef]
- Luther, K.B.; Hulsmeier, A.J.; Schegg, B.; Deuber, S.A.; Raoult, D.; Hennet, T. Mimivirus collagen is modified by bifunctional lysyl hydroxylase and glycosyltransferase enzyme. J. Biol. Chem. 2011, 286, 43701–43709. [Google Scholar] [CrossRef]
- Markine-Goriaynoff, N.; Gillet, L.; Van Etten, J.L.; Korres, H.; Verma, N.; Vanderplasschen, A. Glycosyltransferases encoded by viruses. J. Gen. Virol. 2004, 85, 2741–2754. [Google Scholar] [CrossRef]
- Woolhouse, M.; Scott, F.; Hudson, Z.; Howey, R.; Chase-Topping, M. Human viruses: Discovery and emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 2864–2871. [Google Scholar] [CrossRef]
- Deshpande, K.L.; Fried, V.A.; Ando, M.; Webster, R.G. Glycosylation affects cleavage of an H5N2 influenza virus hemagglutinin and regulates virulence. Proc. Natl. Acad. Sci. USA 1987, 84, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Ohuchi, M.; Feldmann, A.; Ohuchi, R.; Klenk, H.D. Neuraminidase is essential for fowl plague virus hemagglutinin to show hemagglutinating activity. Virology 1995, 212, 77–83. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakamura, S.; Horie, M.; Daidoji, T.; Honda, T.; Yasugi, M.; Kuno, A.; Komori, T.; Okuzaki, D.; Narimatsu, H.; Nakaya, T.; et al. Influenza A Virus-Induced Expression of a GalNAc Transferase, GALNT3, via MicroRNAs Is Required for Enhanced Viral Replication. J. Virol. 2016, 90, 1788–1801. [Google Scholar] [CrossRef] [PubMed]
- McKimm-Breschkin, J.L. Influenza neuraminidase inhibitors: Antiviral action and mechanisms of resistance. Influenza Other Respir. Viruses 2013, 7 (Suppl. 1), 25–36. [Google Scholar] [CrossRef]
- Sagar, M.; Wu, X.; Lee, S.; Overbaugh, J. Human immunodeficiency virus type 1 V1-V2 envelope loop sequences expand and add glycosylation sites over the course of infection, and these modifications affect antibody neutralization sensitivity. J. Virol. 2006, 80, 9586–9598. [Google Scholar] [CrossRef]
- Mathys, L.; Francois, K.O.; Quandte, M.; Braakman, I.; Balzarini, J. Deletion of the highly conserved N-glycan at Asn260 of HIV-1 gp120 affects folding and lysosomal degradation of gp120, and results in loss of viral infectivity. PLoS ONE 2014, 9, e101181. [Google Scholar] [CrossRef]
- de Witte, L.; Nabatov, A.; Pion, M.; Fluitsma, D.; de Jong, M.A.; de Gruijl, T.; Piguet, V.; van Kooyk, Y.; Geijtenbeek, T.B. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat. Med. 2007, 13, 367–371. [Google Scholar] [CrossRef]
- van der Vlist, M.; Geijtenbeek, T.B. Langerin functions as an antiviral receptor on Langerhans cells. Immunol. Cell Biol. 2010, 88, 410–415. [Google Scholar] [CrossRef]
- Walls, A.C.; Tortorici, M.A.; Frenz, B.; Snijder, J.; Li, W.; Rey, F.A.; DiMaio, F.; Bosch, B.J.; Veesler, D. Glycan shield and epitope masking of a coronavirus spike protein observed by cryo-electron microscopy. Nat. Struct. Mol. Biol. 2016, 23, 899–905. [Google Scholar] [CrossRef]
- Bagdonaite, I.; Thompson, A.J.; Wang, X.; Sogaard, M.; Fougeroux, C.; Frank, M.; Diedrich, J.K.; Yates, J.R., 3rd; Salanti, A.; Vakhrushev, S.Y.; et al. Site-Specific O-Glycosylation Analysis of SARS-CoV-2 Spike Protein Produced in Insect and Human Cells. Viruses 2021, 13, 551. [Google Scholar] [CrossRef]
- Yang, Q.; Hughes, T.A.; Kelkar, A.; Yu, X.; Cheng, K.; Park, S.; Huang, W.C.; Lovell, J.F.; Neelamegham, S. Inhibition of SARS-CoV-2 viral entry upon blocking N- and O-glycan elaboration. eLife 2020, 9, e61552. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, D.; Mereiter, S.; Jin Oh, Y.; Monteil, V.; Elder, E.; Zhu, R.; Canena, D.; Hain, L.; Laurent, E.; Grunwald-Gruber, C.; et al. Identification of lectin receptors for conserved SARS-CoV-2 glycosylation sites. EMBO J. 2021, 40, e108375. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Du, M.; Bosse, Y.; Albrecht, H.; Qin, F.; Luo, X.; Androulakis, X.M.; Cheng, C.; Nagarkatti, M.; Nagarkatti, P.; et al. SARS-CoV-2 Impairs Dendritic Cells and Regulates DC-SIGN Gene Expression in Tissues. Int. J. Mol. Sci. 2021, 22, 9228. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, I.; Amarasinghe, G.K.; Basler, C.F. Filovirus pathogenesis and immune evasion: Insights from Ebola virus and Marburg virus. Nat. Rev. Microbiol. 2015, 13, 663–676. [Google Scholar] [CrossRef]
- Lee, J.E.; Saphire, E.O. Ebolavirus glycoprotein structure and mechanism of entry. Future Virol. 2009, 4, 621–635. [Google Scholar] [CrossRef]
- Zhao, Y.; Ren, J.; Harlos, K.; Jones, D.M.; Zeltina, A.; Bowden, T.A.; Padilla-Parra, S.; Fry, E.E.; Stuart, D.I. Toremifene interacts with and destabilizes the Ebola virus glycoprotein. Nature 2016, 535, 169–172. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Frabutt, D.A.; Zhang, X.; Yao, X.; Hu, D.; Zhang, Z.; Liu, C.; Zheng, S.; Xiang, S.H.; et al. Mechanistic understanding of N-glycosylation in Ebola virus glycoprotein maturation and function. J. Biol. Chem. 2017, 292, 5860–5870. [Google Scholar] [CrossRef]
- Peng, W.; Rayaprolu, V.; Parvate, A.D.; Pronker, M.F.; Hui, S.; Parekh, D.; Shaffer, K.; Yu, X.; Saphire, E.O.; Snijder, J. Glycan shield of the ebolavirus envelope glycoprotein GP. Commun. Biol. 2022, 5, 785. [Google Scholar] [CrossRef]
- Collar, A.L.; Clarke, E.C.; Anaya, E.; Merrill, D.; Yarborough, S.; Anthony, S.M.; Kuhn, J.H.; Merle, C.; Theisen, M.; Bradfute, S.B. Comparison of N- and O-linked glycosylation patterns of ebolavirus glycoproteins. Virology 2017, 502, 39–47. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, L.; Sun, Y.; Nabel, G.J. The assembly of Ebola virus nucleocapsid requires virion-associated proteins 35 and 24 and posttranslational modification of nucleoprotein. Mol. Cell 2002, 10, 307–316. [Google Scholar] [CrossRef]
- Raich-Regue, D.; Resa-Infante, P.; Gallemi, M.; Laguia, F.; Muniz-Trabudua, X.; Munoz-Basagoiti, J.; Perez-Zsolt, D.; Chojnacki, J.; Benet, S.; Clotet, B.; et al. Role of Siglecs in viral infections: A double-edged sword interaction. Mol. Asp. Med. 2023, 90, 101113. [Google Scholar] [CrossRef] [PubMed]
- Perez-Zsolt, D.; Munoz-Basagoiti, J.; Rodon, J.; Elosua-Bayes, M.; Raich-Regue, D.; Risco, C.; Sachse, M.; Pino, M.; Gumber, S.; Paiardini, M.; et al. SARS-CoV-2 interaction with Siglec-1 mediates trans-infection by dendritic cells. Cell. Mol. Immunol. 2021, 18, 2676–2678. [Google Scholar] [CrossRef] [PubMed]
- Markovic, S.S.; Gajovic, N.; Jurisevic, M.; Jovanovic, M.; Jovicic, B.P.; Arsenijevic, N.; Mijailovic, Z.; Jovanovic, M.; Dolicanin, Z.; Jovanovic, I. Galectin-1 as the new player in staging and prognosis of COVID-19. Sci. Rep. 2022, 12, 1272. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Alvarez, E.; la Rosa, N.L.; la Mora, M.S.; Valdez-Sandoval, P.; Palacios-Jimenez, M.; Rodriguez-Alvarez, F.; Vera-Maldonado, B.I.; Aguirre-Aguilar, E.; Escobar-Valderrama, J.M.; Alanis-Mendizabal, J.; et al. Galectin-3 as a potential prognostic biomarker of severe COVID-19 in SARS-CoV-2 infected patients. Sci. Rep. 2022, 12, 1856. [Google Scholar] [CrossRef]
- Bozorgmehr, N.; Mashhouri, S.; Perez Rosero, E.; Xu, L.; Shahbaz, S.; Sligl, W.; Osman, M.; Kutsogiannis, D.J.; MacIntyre, E.; O’Neil, C.R.; et al. Galectin-9, a Player in Cytokine Release Syndrome and a Surrogate Diagnostic Biomarker in SARS-CoV-2 Infection. mBio 2021, 12, 10–1128. [Google Scholar] [CrossRef]
- Nguyen, L.; McCord, K.A.; Bui, D.T.; Bouwman, K.M.; Kitova, E.N.; Elaish, M.; Kumawat, D.; Daskhan, G.C.; Tomris, I.; Han, L.; et al. Sialic acid-containing glycolipids mediate binding and viral entry of SARS-CoV-2. Nat. Chem. Biol. 2022, 18, 81–90. [Google Scholar] [CrossRef]
- Chen, Y.J.; Wang, S.F.; Weng, I.C.; Hong, M.H.; Lo, T.H.; Jan, J.T.; Hsu, L.C.; Chen, H.Y.; Liu, F.T. Galectin-3 Enhances Avian H5N1 Influenza A Virus-Induced Pulmonary Inflammation by Promoting NLRP3 Inflammasome Activation. Am. J. Pathol. 2018, 188, 1031–1042. [Google Scholar] [CrossRef]
- Stojanovic, B.; Milovanovic, J.; Arsenijevic, A.; Stojanovic, B.; Strazic Geljic, I.; Arsenijevic, N.; Jonjic, S.; Lukic, M.L.; Milovanovic, M. Galectin-3 Deficiency Facilitates TNF-alpha-Dependent Hepatocyte Death and Liver Inflammation in MCMV Infection. Front. Microbiol. 2019, 10, 185. [Google Scholar] [CrossRef]
- Cummings, R.D.; Hokke, C.H.; Haslam, S.M. Parasitic Infections. In Essentials of Glycobiology, 4th ed.; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Mohnen, D., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022; pp. 569–582. [Google Scholar]
- Verissimo, C.M.; Graeff-Teixeira, C.; Jones, M.K.; Morassutti, A.L. Glycans in the roles of parasitological diagnosis and host-parasite interplay. Paras 2019, 146, 1217–1232. [Google Scholar] [CrossRef]
- Hokke, C.H.; van Diepen, A. Helminth glycomics—Glycan repertoires and host-parasite interactions. Mol. Biochem. Parasitol. 2017, 215, 47–57. [Google Scholar] [CrossRef]
- Cummings, R.D. Glycosphingolipids in human parasites. FEBS Open Bio 2023, 13, 1625–1635. [Google Scholar] [CrossRef] [PubMed]
- Damian, R.T. Molecular mimicry revisited. Parasitol. Today 1987, 3, 263–266. [Google Scholar] [CrossRef] [PubMed]
- van Die, I.; Cummings, R.D. Glycan gimmickry by parasitic helminths: A strategy for modulating the host immune response? Glycobiology 2010, 20, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Prasanphanich, N.S.; Mickum, M.L.; Heimburg-Molinaro, J.; Cummings, R.D. Glycoconjugates in host-helminth interactions. Front. Immunol. 2013, 4, 240. [Google Scholar] [CrossRef]
- Stahl, P.D.; Rodman, J.S.; Miller, M.J.; Schlesinger, P.H. Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc. Natl. Acad. Sci. USA 1978, 75, 1399–1403. [Google Scholar] [CrossRef]
- Oliveira, R.M.; Teixeira, T.L.; Rodrigues, C.C.; da Silva, A.A.; Borges, B.C.; Brigido, R.T.S.; Teixeira, S.C.; Dos Santos, M.A.; Servato, J.P.S.; de O Santos, D.; et al. Galectin-3 plays a protective role in Leishmania (Leishmania) amazonensis infection. Glycobiology 2021, 31, 1378–1389. [Google Scholar] [CrossRef]
- Poncini, C.V.; Ilarregui, J.M.; Batalla, E.I.; Engels, S.; Cerliani, J.P.; Cucher, M.A.; van Kooyk, Y.; Gonzalez-Cappa, S.M.; Rabinovich, G.A. Trypanosoma cruzi Infection Imparts a Regulatory Program in Dendritic Cells and T Cells via Galectin-1-Dependent Mechanisms. J. Immunol. 2015, 195, 3311–3324. [Google Scholar] [CrossRef]
- Bernardes, E.S.; Silva, N.M.; Ruas, L.P.; Mineo, J.R.; Loyola, A.M.; Hsu, D.K.; Liu, F.T.; Chammas, R.; Roque-Barreira, M.C. Toxoplasma gondii infection reveals a novel regulatory role for galectin-3 in the interface of innate and adaptive immunity. Am. J. Pathol. 2006, 168, 1910–1920. [Google Scholar] [CrossRef]
- Debierre-Grockiego, F.; Niehus, S.; Coddeville, B.; Elass, E.; Poirier, F.; Weingart, R.; Schmidt, R.R.; Mazurier, J.; Guerardel, Y.; Schwarz, R.T. Binding of Toxoplasma gondii glycosylphosphatidylinositols to galectin-3 is required for their recognition by macrophages. J. Biol. Chem. 2010, 285, 32744–32750. [Google Scholar] [CrossRef]
- Pereira-Chioccola, V.L.; Acosta-Serrano, A.; Correia de Almeida, I.; Ferguson, M.A.; Souto-Padron, T.; Rodrigues, M.M.; Travassos, L.R.; Schenkman, S. Mucin-like molecules form a negatively charged coat that protects Trypanosoma cruzi trypomastigotes from killing by human anti-alpha-galactosyl antibodies. J. Cell Sci. 2000, 113 Pt 7, 1299–1307. [Google Scholar] [CrossRef]
- Hakomori, S. Tumor-associated carbohydrate antigens defining tumor malignancy: Basis for development of anti-cancer vaccines. Adv. Exp. Med. Biol. 2001, 491, 369–402. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, N.; Kizuka, Y. Glycans and cancer: Role of N-glycans in cancer biomarker, progression and metastasis, and therapeutics. Adv. Cancer Res. 2015, 126, 11–51. [Google Scholar] [CrossRef] [PubMed]
- Hauselmann, I.; Borsig, L. Altered tumor-cell glycosylation promotes metastasis. Front. Oncol. 2014, 4, 28. [Google Scholar] [CrossRef]
- Läubli, H.; Borsig, L. Altered Cell Adhesion and Glycosylation Promote Cancer Immune Suppression and Metastasis. Front. Immunol. 2019, 10, 2120. [Google Scholar] [CrossRef]
- Pearce, O.M.T. Cancer glycan epitopes: Biosynthesis, structure and function. Glycobiology 2018, 28, 670–696. [Google Scholar] [CrossRef]
- Dimitroff, C.J. I-branched carbohydrates as emerging effectors of malignant progression. Proc. Natl. Acad. Sci. USA 2019, 116, 13729–13737. [Google Scholar] [CrossRef]
- Christiansen, M.N.; Chik, J.; Lee, L.; Anugraham, M.; Abrahams, J.L.; Packer, N.H. Cell surface protein glycosylation in cancer. Proteomics 2014, 14, 525–546. [Google Scholar] [CrossRef]
- Kannagi, R. Molecular mechanism for cancer-associated induction of sialyl Lewis X and sialyl Lewis A expression-The Warburg effect revisited. Glycoconj. J. 2004, 20, 353–364. [Google Scholar] [CrossRef]
- Hakomori, S. Tumor-associated glycolipid antigens, their metabolism and organization. Chem. Phys. Lipids 1986, 42, 209–233. [Google Scholar] [CrossRef]
- Dall’Olio, F.; Malagolini, N.; Trinchera, M.; Chiricolo, M. Mechanisms of cancer-associated glycosylation changes. Front. Biosci. (Landmark) 2012, 17, 670–699. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, M.; den Hollander, P.; Kuburich, N.A.; Rosen, J.M.; Mani, S.A. Mechanisms of cancer metastasis. Semin. Cancer Biol. 2022, 87, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Hapach, L.A.; Mosier, J.A.; Wang, W.; Reinhart-King, C.A. Engineered models to parse apart the metastatic cascade. NPJ Precis. Oncol. 2019, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Bindeman, W.E.; Fingleton, B. Glycosylation as a regulator of site-specific metastasis. Cancer Metastasis Rev. 2022, 41, 107–129. [Google Scholar] [CrossRef]
- Borsig, L. Selectins in cancer immunity. Glycobiology 2018, 28, 648–655. [Google Scholar] [CrossRef]
- Geng, Y.; Marshall, J.R.; King, M.R. Glycomechanics of the metastatic cascade: Tumor cell-endothelial cell interactions in the circulation. Ann. Biomed. Eng. 2012, 40, 790–805. [Google Scholar] [CrossRef]
- Witz, I.P. The selectin-selectin ligand axis in tumor progression. Cancer Metastasis Rev. 2008, 27, 19–30. [Google Scholar] [CrossRef]
- Kang, S.A.; Blache, C.A.; Bajana, S.; Hasan, N.; Kamal, M.; Morita, Y.; Gupta, V.; Tsolmon, B.; Suh, K.S.; Gorenstein, D.G.; et al. The effect of soluble E-selectin on tumor progression and metastasis. BMC Cancer 2016, 16, 331. [Google Scholar] [CrossRef]
- Laubli, H.; Stevenson, J.L.; Varki, A.; Varki, N.M.; Borsig, L. L-selectin facilitation of metastasis involves temporal induction of Fut7-dependent ligands at sites of tumor cell arrest. Cancer Res. 2006, 66, 1536–1542. [Google Scholar] [CrossRef]
- Borsig, L.; Wong, R.; Hynes, R.O.; Varki, N.M.; Varki, A. Synergistic effects of L- and P-selectin in facilitating tumor metastasis can involve non-mucin ligands and implicate leukocytes as enhancers of metastasis. Proc. Natl. Acad. Sci. USA 2002, 99, 2193–2198. [Google Scholar] [CrossRef]
- Rodriguez, E.; Schetters, S.T.T.; van Kooyk, Y. The tumour glyco-code as a novel immune checkpoint for immunotherapy. Nat. Rev. Immunol. 2018, 18, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.G.; Balmana, M.; Macedo, J.A.; Pocas, J.; Fernandes, A.; de-Freitas-Junior, J.C.M.; Pinho, S.S.; Gomes, J.; Magalhaes, A.; Gomes, C.; et al. Glycosylation in cancer: Selected roles in tumour progression, immune modulation and metastasis. Cell. Immunol. 2018, 333, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Chang, Y.C.; Chan, M.H.; Yang, Y.F.; Liang, S.M.; Hsiao, M. Galectins in Cancer and the Microenvironment: Functional Roles, Therapeutic Developments, and Perspectives. Biomedicines 2021, 9, 1159. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.T.; Rabinovich, G.A. Galectins as modulators of tumour progression. Nat. Rev. Cancer 2005, 5, 29–41. [Google Scholar] [CrossRef]
- Compagno, D.; Gentilini, L.D.; Jaworski, F.M.; Perez, I.G.; Contrufo, G.; Laderach, D.J. Glycans and galectins in prostate cancer biology, angiogenesis and metastasis. Glycobiology 2014, 24, 899–906. [Google Scholar] [CrossRef]
- Song, S.; Ji, B.; Ramachandran, V.; Wang, H.; Hafley, M.; Logsdon, C.; Bresalier, R.S. Overexpressed galectin-3 in pancreatic cancer induces cell proliferation and invasion by binding Ras and activating Ras signaling. PLoS ONE 2012, 7, e42699. [Google Scholar] [CrossRef]
- Nagy, N.; Bronckart, Y.; Camby, I.; Legendre, H.; Lahm, H.; Kaltner, H.; Hadari, Y.; Van Ham, P.; Yeaton, P.; Pector, J.C.; et al. Galectin-8 expression decreases in cancer compared with normal and dysplastic human colon tissue and acts significantly on human colon cancer cell migration as a suppressor. Gut 2002, 50, 392–401. [Google Scholar] [CrossRef]
- Adams, O.J.; Stanczak, M.A.; von Gunten, S.; Laubli, H. Targeting sialic acid-Siglec interactions to reverse immune suppression in cancer. Glycobiology 2018, 28, 640–647. [Google Scholar] [CrossRef]
- Bull, C.; Stoel, M.A.; den Brok, M.H.; Adema, G.J. Sialic acids sweeten a tumor’s life. Cancer Res. 2014, 74, 3199–3204. [Google Scholar] [CrossRef]
- Wang, J.; Sun, J.; Liu, L.N.; Flies, D.B.; Nie, X.; Toki, M.; Zhang, J.; Song, C.; Zarr, M.; Zhou, X.; et al. Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat. Med. 2019, 25, 656–666. [Google Scholar] [CrossRef]
- He, X.; Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Lin, S.; Guan, F.; Kang, H. Glycosylation Targeting: A Paradigm Shift in Cancer Immunotherapy. Int. J. Biol. Sci. 2024, 20, 2607–2621. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Peng, Q.; Jiang, X.; Tan, S.; Yang, W.; Han, Y.; Oyang, L.; Lin, J.; Shen, M.; Wang, J.; et al. Altered glycosylation in cancer: Molecular functions and therapeutic potential. Cancer Commun. 2024, 44, 1316–1336. [Google Scholar] [CrossRef]
- Berois, N.; Pittini, A.; Osinaga, E. Targeting Tumor Glycans for Cancer Therapy: Successes, Limitations, and Perspectives. Cancers 2022, 14, 645. [Google Scholar] [CrossRef]
- Supruniuk, K.; Radziejewska, I. MUC1 is an oncoprotein with a significant role in apoptosis (Review). Int. J. Oncol. 2021, 59, 68. [Google Scholar] [CrossRef]
- Nath, S.; Mukherjee, P. MUC1: A multifaceted oncoprotein with a key role in cancer progression. Trends Mol. Med. 2014, 20, 332–342. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Z.; Zhang, S.; Zhu, P.; Ko, J.K.; Yung, K.K. MUC1: Structure, Function, and Clinic Application in Epithelial Cancers. Int. J. Mol. Sci. 2021, 22, 6567. [Google Scholar] [CrossRef]
- Lau, S.K.; Weiss, L.M.; Chu, P.G. Differential expression of MUC1, MUC2, and MUC5AC in carcinomas of various sites: An immunohistochemical study. Am. J. Clin. Pathol. 2004, 122, 61–69. [Google Scholar] [CrossRef]
- Awaya, H.; Takeshima, Y.; Yamasaki, M.; Inai, K. Expression of MUC1, MUC2, MUC5AC, and MUC6 in atypical adenomatous hyperplasia, bronchioloalveolar carcinoma, adenocarcinoma with mixed subtypes, and mucinous bronchioloalveolar carcinoma of the lung. Am. J. Clin. Pathol. 2004, 121, 644–653. [Google Scholar] [CrossRef]
- Singh, A.P.; Chauhan, S.C.; Bafna, S.; Johansson, S.L.; Smith, L.M.; Moniaux, N.; Lin, M.F.; Batra, S.K. Aberrant expression of transmembrane mucins, MUC1 and MUC4, in human prostate carcinomas. Prostate 2006, 66, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Hattrup, C.L.; Gendler, S.J. Structure and function of the cell surface (tethered) mucins. Annu. Rev. Physiol. 2008, 70, 431–457. [Google Scholar] [CrossRef] [PubMed]
- Haddon, L.; Hugh, J. MUC1-mediated motility in breast cancer: A review highlighting the role of the MUC1/ICAM-1/Src signaling triad. Clin. Exp. Metastasis 2015, 32, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, B.; Gupta, N.; Konowalchuk, J.D. MUC1 Mucin: A Putative Regulatory (Checkpoint) Molecule of T Cells. Front. Immunol. 2018, 9, 2391. [Google Scholar] [CrossRef]
- Xu, F.; Liu, F.; Zhao, H.; An, G.; Feng, G. Prognostic Significance of Mucin Antigen MUC1 in Various Human Epithelial Cancers: A Meta-Analysis. Medicine 2015, 94, e2286. [Google Scholar] [CrossRef]
- Taylor-Papadimitriou, J.; Burchell, J.M.; Graham, R.; Beatson, R. Latest developments in MUC1 immunotherapy. Biochem. Soc. Trans. 2018, 46, 659–668. [Google Scholar] [CrossRef]
- Sterner, E.; Flanagan, N.; Gildersleeve, J.C. Perspectives on Anti-Glycan Antibodies Gleaned from Development of a Community Resource Database. ACS Chem. Biol. 2016, 11, 1773–1783. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Giddens, J.; Pincetic, A.; Lomino, J.V.; Ravetch, J.V.; Wang, L.X.; Bjorkman, P.J. Structural characterization of anti-inflammatory immunoglobulin G Fc proteins. J. Mol. Biol. 2014, 426, 3166–3179. [Google Scholar] [CrossRef]
- Schroeder, H.W., Jr.; Cavacini, L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 2010, 125, S41–S52. [Google Scholar] [CrossRef]
- Arnold, J.N.; Wormald, M.R.; Sim, R.B.; Rudd, P.M.; Dwek, R.A. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu. Rev. Immunol. 2007, 25, 21–50. [Google Scholar] [CrossRef]
- Remesh, S.G.; Armstrong, A.A.; Mahan, A.D.; Luo, J.; Hammel, M. Conformational Plasticity of the Immunoglobulin Fc Domain in Solution. Structure 2018, 26, 1007–1014.e12. [Google Scholar] [CrossRef] [PubMed]
- Jay, J.W.; Bray, B.; Qi, Y.; Igbinigie, E.; Wu, H.; Li, J.; Ren, G. IgG Antibody 3D Structures and Dynamics. Antibodies 2018, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, L.; Tong, H.; Peng, B.; Rames, M.J.; Zhang, S.; Ren, G. 3D Structural Fluctuation of IgG1 Antibody Revealed by Individual Particle Electron Tomography. Sci. Rep. 2015, 5, 9803. [Google Scholar] [CrossRef] [PubMed]
- Jaszczyszyn, I.; Bielska, W.; Gawlowski, T.; Dudzic, P.; Satlawa, T.; Konczak, J.; Wilman, W.; Janusz, B.; Wrobel, S.; Chomicz, D.; et al. Structural modeling of antibody variable regions using deep learning-progress and perspectives on drug discovery. Front. Mol. Biosci. 2023, 10, 1214424. [Google Scholar] [CrossRef]
- Irvine, E.B.; Alter, G. Understanding the role of antibody glycosylation through the lens of severe viral and bacterial diseases. Glycobiology 2020, 30, 241–253. [Google Scholar] [CrossRef]
- Jennewein, M.F.; Alter, G. The Immunoregulatory Roles of Antibody Glycosylation. Trends Immunol. 2017, 38, 358–372. [Google Scholar] [CrossRef]
- Vattepu, R.; Sneed, S.L.; Anthony, R.M. Sialylation as an Important Regulator of Antibody Function. Front. Immunol. 2022, 13, 818736. [Google Scholar] [CrossRef]
- Huang, J.; Guerrero, A.; Parker, E.; Strum, J.S.; Smilowitz, J.T.; German, J.B.; Lebrilla, C.B. Site-specific glycosylation of secretory immunoglobulin A from human colostrum. J. Proteome Res. 2015, 14, 1335–1349. [Google Scholar] [CrossRef]
- Alter, G.; Ottenhoff, T.H.M.; Joosten, S.A. Antibody glycosylation in inflammation, disease and vaccination. Semin. Immunol. 2018, 39, 102–110. [Google Scholar] [CrossRef]
- Ding, L.; Chen, X.; Cheng, H.; Zhang, T.; Li, Z. Advances in IgA glycosylation and its correlation with diseases. Front. Chem. 2022, 10, 974854. [Google Scholar] [CrossRef]
- Cymer, F.; Beck, H.; Rohde, A.; Reusch, D. Therapeutic monoclonal antibody N-glycosylation—Structure, function and therapeutic potential. Biologicals 2018, 52, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, Y.; Yamaguchi, H.; Nakajima, K.; Mizuno, T.; Fukamachi, Y.; Yokoi, Y.; Tsuboi, N.; Inaguma, D.; Hasegawa, M.; Renfrow, M.B.; et al. Analysis of O-glycoforms of the IgA1 hinge region by sequential deglycosylation. Sci. Rep. 2020, 10, 671. [Google Scholar] [CrossRef] [PubMed]
- Gudelj, I.; Lauc, G.; Pezer, M. Immunoglobulin G glycosylation in aging and diseases. Cell. Immunol. 2018, 333, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Motta, F.; Selmi, C.; Ridgway, W.M.; Gershwin, M.E.; Zhang, W. Antibody glycosylation in autoimmune diseases. Autoimmun. Rev. 2021, 20, 102804. [Google Scholar] [CrossRef]
- Pucic, M.; Knezevic, A.; Vidic, J.; Adamczyk, B.; Novokmet, M.; Polasek, O.; Gornik, O.; Supraha-Goreta, S.; Wormald, M.R.; Redzic, I.; et al. High throughput isolation and glycosylation analysis of IgG-variability and heritability of the IgG glycome in three isolated human populations. Mol. Cell. Proteom. 2011, 10, M111.010090. [Google Scholar] [CrossRef]
- Shkunnikova, S.; Mijakovac, A.; Sironic, L.; Hanic, M.; Lauc, G.; Kavur, M.M. IgG glycans in health and disease: Prediction, intervention, prognosis, and therapy. Biotechnol. Adv. 2023, 67, 108169. [Google Scholar] [CrossRef]
- Vadrevu, S.K.; Trbojevic-Akmacic, I.; Kossenkov, A.V.; Colomb, F.; Giron, L.B.; Anzurez, A.; Lynn, K.; Mounzer, K.; Landay, A.L.; Kaplan, R.C.; et al. Frontline Science: Plasma and immunoglobulin G galactosylation associate with HIV persistence during antiretroviral therapy. J. Leukoc. Biol. 2018, 104, 461–471. [Google Scholar] [CrossRef]
- Goulabchand, R.; Vincent, T.; Batteux, F.; Eliaou, J.F.; Guilpain, P. Impact of autoantibody glycosylation in autoimmune diseases. Autoimmun. Rev. 2014, 13, 742–750. [Google Scholar] [CrossRef]
- Tanaka, T.; Yoneyama, T.; Noro, D.; Imanishi, K.; Kojima, Y.; Hatakeyama, S.; Tobisawa, Y.; Mori, K.; Yamamoto, H.; Imai, A.; et al. Aberrant N-Glycosylation Profile of Serum Immunoglobulins is a Diagnostic Biomarker of Urothelial Carcinomas. Int. J. Mol. Sci. 2017, 18, 2632. [Google Scholar] [CrossRef]
- Theodoratou, E.; Thaci, K.; Agakov, F.; Timofeeva, M.N.; Stambuk, J.; Pucic-Bakovic, M.; Vuckovic, F.; Orchard, P.; Agakova, A.; Din, F.V.; et al. Glycosylation of plasma IgG in colorectal cancer prognosis. Sci. Rep. 2016, 6, 28098. [Google Scholar] [CrossRef]
- Parekh, R.; Isenberg, D.; Rook, G.; Roitt, I.; Dwek, R.; Rademacher, T. A comparative analysis of disease-associated changes in the galactosylation of serum IgG. J. Autoimmun. 1989, 2, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Decker, Y.; Schomburg, R.; Nemeth, E.; Vitkin, A.; Fousse, M.; Liu, Y.; Fassbender, K. Abnormal galactosylation of immunoglobulin G in cerebrospinal fluid of multiple sclerosis patients. Mult. Scler. 2016, 22, 1794–1803. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Ito, K.; Furukawa, J.; Nakata, J.; Alvarez, M.; Verbeek, J.S.; Shinohara, Y.; Izui, S. Galactosylation of IgG1 modulates FcgammaRIIB-mediated inhibition of murine autoimmune hemolytic anemia. J. Autoimmun. 2013, 47, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Lou, Y.; Zhang, Y.; Liu, S.; Li, J.; Tao, J. Sialylated immunoglobulin G: A promising diagnostic and therapeutic strategy for autoimmune diseases. Theranostics 2021, 11, 5430–5446. [Google Scholar] [CrossRef]
- Tomana, M.; Novak, J.; Julian, B.A.; Matousovic, K.; Konecny, K.; Mestecky, J. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J. Clin. Investig. 1999, 104, 73–81. [Google Scholar] [CrossRef]
- Novak, J.; Tomana, M.; Kilian, M.; Coward, L.; Kulhavy, R.; Barnes, S.; Mestecky, J. Heterogeneity of O-glycosylation in the hinge region of human IgA1. Mol. Immunol. 2000, 37, 1047–1056. [Google Scholar] [CrossRef]
- Novak, J.; Julian, B.A.; Mestecky, J.; Renfrow, M.B. Glycosylation of IgA1 and pathogenesis of IgA nephropathy. Semin. Immunopathol. 2012, 34, 365–382. [Google Scholar] [CrossRef]
- Novak, J.; Barratt, J.; Julian, B.A.; Renfrow, M.B. Aberrant Glycosylation of the IgA1 Molecule in IgA Nephropathy. Semin. Nephrol. 2018, 38, 461–476. [Google Scholar] [CrossRef]
- Novak, J.; King, R.G.; Yother, J.; Renfrow, M.B.; Green, T.J. O-glycosylation of IgA1 and the pathogenesis of an autoimmune disease IgA nephropathy. Glycobiology 2024, 34, cwae060. [Google Scholar] [CrossRef]
- Suzuki, H.; Kiryluk, K.; Novak, J.; Moldoveanu, Z.; Herr, A.B.; Renfrow, M.B.; Wyatt, R.J.; Scolari, F.; Mestecky, J.; Gharavi, A.G.; et al. The pathophysiology of IgA nephropathy. J. Am. Soc. Nephrol. 2011, 22, 1795–1803. [Google Scholar] [CrossRef]
- Suzuki, H.; Fan, R.; Zhang, Z.; Brown, R.; Hall, S.; Julian, B.A.; Chatham, W.W.; Suzuki, Y.; Wyatt, R.J.; Moldoveanu, Z.; et al. Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J. Clin. Investig. 2009, 119, 1668–1677. [Google Scholar] [CrossRef] [PubMed]
- Stuchlova Horynova, M.; Raska, M.; Clausen, H.; Novak, J. Aberrant O-glycosylation and anti-glycan antibodies in an autoimmune disease IgA nephropathy and breast adenocarcinoma. Cell. Mol. Life Sci. 2013, 70, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Herget, S.; Toukach, P.V.; Ranzinger, R.; Hull, W.E.; Knirel, Y.A.; von der Lieth, C.W. Statistical analysis of the Bacterial Carbohydrate Structure Data Base (BCSDB): Characteristics and diversity of bacterial carbohydrates in comparison with mammalian glycans. BMC Struct. Biol. 2008, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Bovin, N.; Obukhova, P.; Shilova, N.; Rapoport, E.; Popova, I.; Navakouski, M.; Unverzagt, C.; Vuskovic, M.; Huflejt, M. Repertoire of human natural anti-glycan immunoglobulins. Do we have auto-antibodies? Biochim. Biophys. Acta 2012, 1820, 1373–1382. [Google Scholar] [CrossRef]
- Watkins, W.M. The ABO blood group system: Historical background. Transfus. Med. 2001, 11, 243–265. [Google Scholar] [CrossRef]
- Wuttke, N.J.; Macardle, P.J.; Zola, H. Blood group antibodies are made by CD5+ and by CD5- B cells. Immunol. Cell Biol. 1997, 75, 478–483. [Google Scholar] [CrossRef]
- Yamamoto, F. Review: ABO blood group system--ABH oligosaccharide antigens, anti-A and anti-B, A and B glycosyltransferases, and ABO genes. Immunohematology 2004, 20, 3–22. [Google Scholar] [CrossRef]
- Patenaude, S.I.; Seto, N.O.; Borisova, S.N.; Szpacenko, A.; Marcus, S.L.; Palcic, M.M.; Evans, S.V. The structural basis for specificity in human ABO(H) blood group biosynthesis. Nat. Struct. Biol. 2002, 9, 685–690. [Google Scholar] [CrossRef]
- Pereira, E.; Felipe, S.; de Freitas, R.; Araujo, V.; Soares, P.; Ribeiro, J.; Henrique Dos Santos, L.; Alves, J.O.; Canabrava, N.; van Tilburg, M.; et al. ABO blood group and link to COVID-19: A comprehensive review of the reported associations and their possible underlying mechanisms. Microb. Pathog. 2022, 169, 105658. [Google Scholar] [CrossRef]
- Soejima, M.; Koda, Y. Molecular mechanisms of Lewis antigen expression. Leg. Med. 2005, 7, 266–269. [Google Scholar] [CrossRef]
- Galili, U. Biosynthesis of alpha-Gal Epitopes (Galalpha1-3Galbeta1-4GlcNAc-R) and Their Unique Potential in Future alpha-Gal Therapies. Front. Mol. Biosci. 2021, 8, 746883. [Google Scholar] [CrossRef] [PubMed]
- Joziasse, D.H.; Oriol, R. Xenotransplantation: The importance of the Galalpha1,3Gal epitope in hyperacute vascular rejection. Biochim. Biophys. Acta 1999, 1455, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Hamanova, M.; Chmelikova, M.; Nentwich, I.; Thon, V.; Lokaj, J. Anti-Gal IgM, IgA and IgG natural antibodies in childhood. Immunol. Lett. 2015, 164, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Van Nunen, S.A.; O’Connor, K.S.; Clarke, L.R.; Boyle, R.X.; Fernando, S.L. An association between tick bite reactions and red meat allergy in humans. Med. J. Aust. 2009, 190, 510–511. [Google Scholar] [CrossRef]
- Ghaderi, D.; Taylor, R.E.; Padler-Karavani, V.; Diaz, S.; Varki, A. Implications of the presence of N-glycolylneuraminic acid in recombinant therapeutic glycoproteins. Nat. Biotechnol. 2010, 28, 863–867. [Google Scholar] [CrossRef]
- Padler-Karavani, V.; Tremoulet, A.H.; Yu, H.; Chen, X.; Burns, J.C.; Varki, A. A simple method for assessment of human anti-Neu5Gc antibodies applied to Kawasaki disease. PLoS ONE 2013, 8, e58443. [Google Scholar] [CrossRef]
- Julien, S.; Videira, P.A.; Delannoy, P. Sialyl-tn in cancer: (how) did we miss the target? Biomolecules 2012, 2, 435–466. [Google Scholar] [CrossRef]
- Pinho, S.; Marcos, N.T.; Ferreira, B.; Carvalho, A.S.; Oliveira, M.J.; Santos-Silva, F.; Harduin-Lepers, A.; Reis, C.A. Biological significance of cancer-associated sialyl-Tn antigen: Modulation of malignant phenotype in gastric carcinoma cells. Cancer Lett. 2007, 249, 157–170. [Google Scholar] [CrossRef]
- Munkley, J. The Role of Sialyl-Tn in Cancer. Int. J. Mol. Sci. 2016, 17, 275. [Google Scholar] [CrossRef]
- Prendergast, J.M.; Galvao da Silva, A.P.; Eavarone, D.A.; Ghaderi, D.; Zhang, M.; Brady, D.; Wicks, J.; DeSander, J.; Behrens, J.; Rueda, B.R. Novel anti-Sialyl-Tn monoclonal antibodies and antibody-drug conjugates demonstrate tumor specificity and anti-tumor activity. MAbs 2017, 9, 615–627. [Google Scholar] [CrossRef]
- Soares, C.O.; Lugieri, M.E. Decoding the Molecular Basis of the Specificity of an Anti-sTn Antibody. JACS Au 2025, 5, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Dochez, A.R.; Avery, O.T. Soluble substance of pneumococcus origin in the blood and urine during lobar pneumonia. Exp. Biol. Med. 1917, 14, 126–127. [Google Scholar] [CrossRef]
- Heidelberger, M.; Mac, L.C.; Di Lapi, M.M. The human antibody response to simultaneous injection of six specific polysaccharides of pneumococcus. J. Exp. Med. 1948, 88, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, S.; Lepenies, B. Glycans as Vaccine Antigens and Adjuvants: Immunological Considerations. Methods Mol. Biol. 2015, 1331, 11–26. [Google Scholar] [CrossRef]
- Hutter, J.; Lepenies, B. Carbohydrate-Based Vaccines: An Overview. Methods Mol. Biol. 2015, 1331, 1–10. [Google Scholar] [CrossRef]
- Costantino, P.; Rappuoli, R.; Berti, F. The design of semi-synthetic and synthetic glycoconjugate vaccines. Expert Opin. Drug Discov. 2011, 6, 1045–1066. [Google Scholar] [CrossRef]
- Landers, C.D.; Chelvarajan, R.L.; Bondada, S. The role of B cells and accessory cells in the neonatal response to TI-2 antigens. Immunol. Res. 2005, 31, 25–36. [Google Scholar] [CrossRef]
- Astronomo, R.D.; Burton, D.R. Carbohydrate vaccines: Developing sweet solutions to sticky situations? Nat. Rev. Drug Discov. 2010, 9, 308–324. [Google Scholar] [CrossRef]
- Krumm, S.A.; Doores, K.J. Targeting Glycans on Human Pathogens for Vaccine Design. Curr. Top. Microbiol. Immunol. 2020, 428, 129–163. [Google Scholar] [CrossRef]
- Cecioni, S.; Imberty, A.; Vidal, S. Glycomimetics versus multivalent glycoconjugates for the design of high affinity lectin ligands. Chem. Rev. 2015, 115, 525–561. [Google Scholar] [CrossRef]
- Schlingmann, B.; Castiglia, K.R.; Stobart, C.C.; Moore, M.L. Polyvalent vaccines: High-maintenance heroes. PLoS Pathog. 2018, 14, e1006904. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Ho, M.; Hu, Y.; Shi, Y. Vaccine adjuvants: Current status, research and development, licensing, and future opportunities. J. Mater. Chem. B 2024, 12, 4118–4137. [Google Scholar] [CrossRef]
- Micoli, F.; Del Bino, L.; Alfini, R.; Carboni, F.; Romano, M.R.; Adamo, R. Glycoconjugate vaccines: Current approaches towards faster vaccine design. Expert Rev. Vaccines 2019, 18, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Del Bino, L.; Osterlid, K.E.; Wu, D.Y.; Nonne, F.; Romano, M.R.; Codee, J.; Adamo, R. Synthetic Glycans to Improve Current Glycoconjugate Vaccines and Fight Antimicrobial Resistance. Chem. Rev. 2022, 122, 15672–15716. [Google Scholar] [CrossRef] [PubMed]
- Adamo, R.; Nilo, A.; Castagner, B.; Boutureira, O.; Berti, F.; Bernardes, G.J. Synthetically defined glycoprotein vaccines: Current status and future directions. Chem. Sci. 2013, 4, 2995–3008. [Google Scholar] [CrossRef]
- Mettu, R.; Chen, C.Y.; Wu, C.Y. Synthetic carbohydrate-based vaccines: Challenges and opportunities. J. Biomed. Sci. 2020, 27, 9. [Google Scholar] [CrossRef]
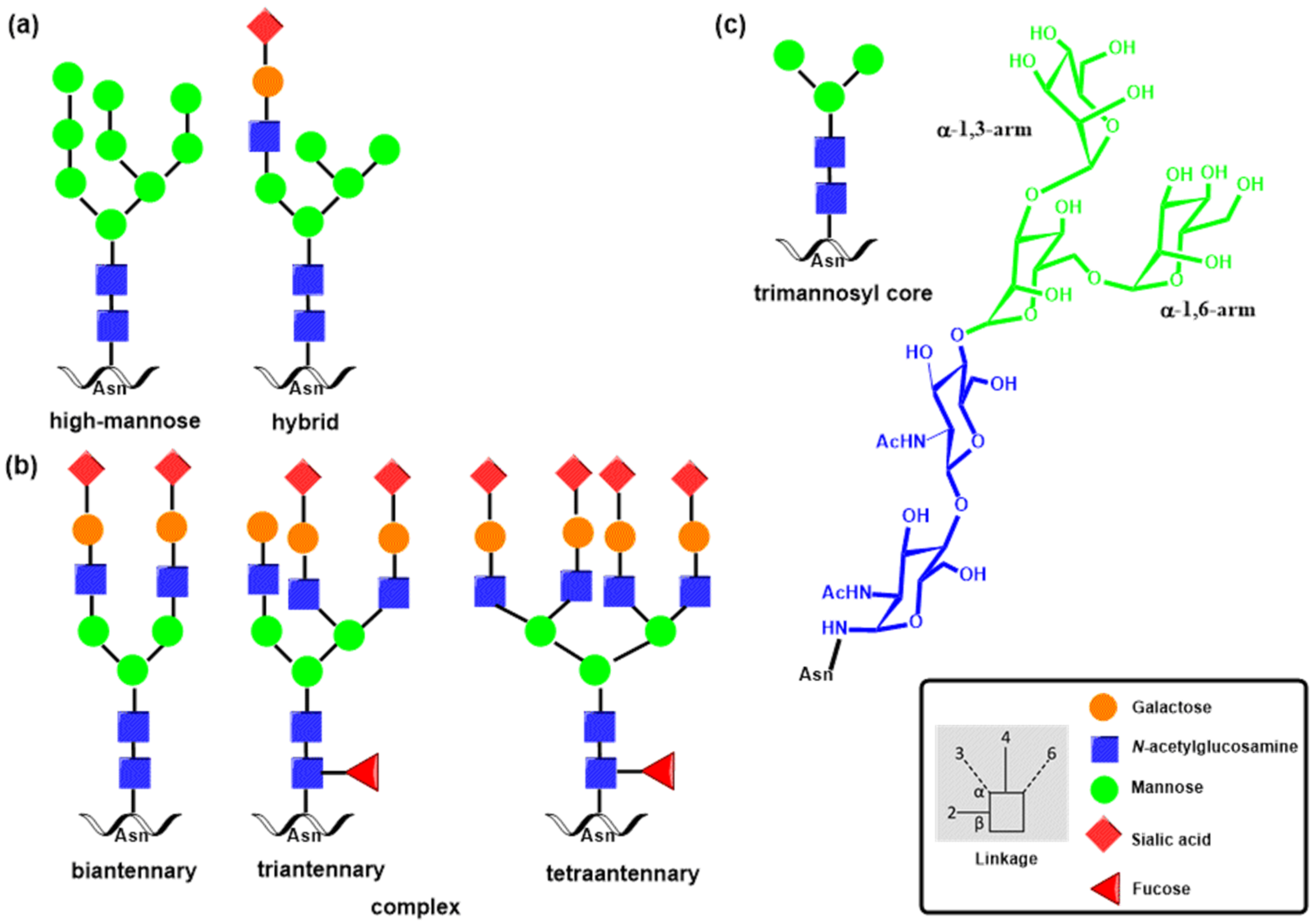


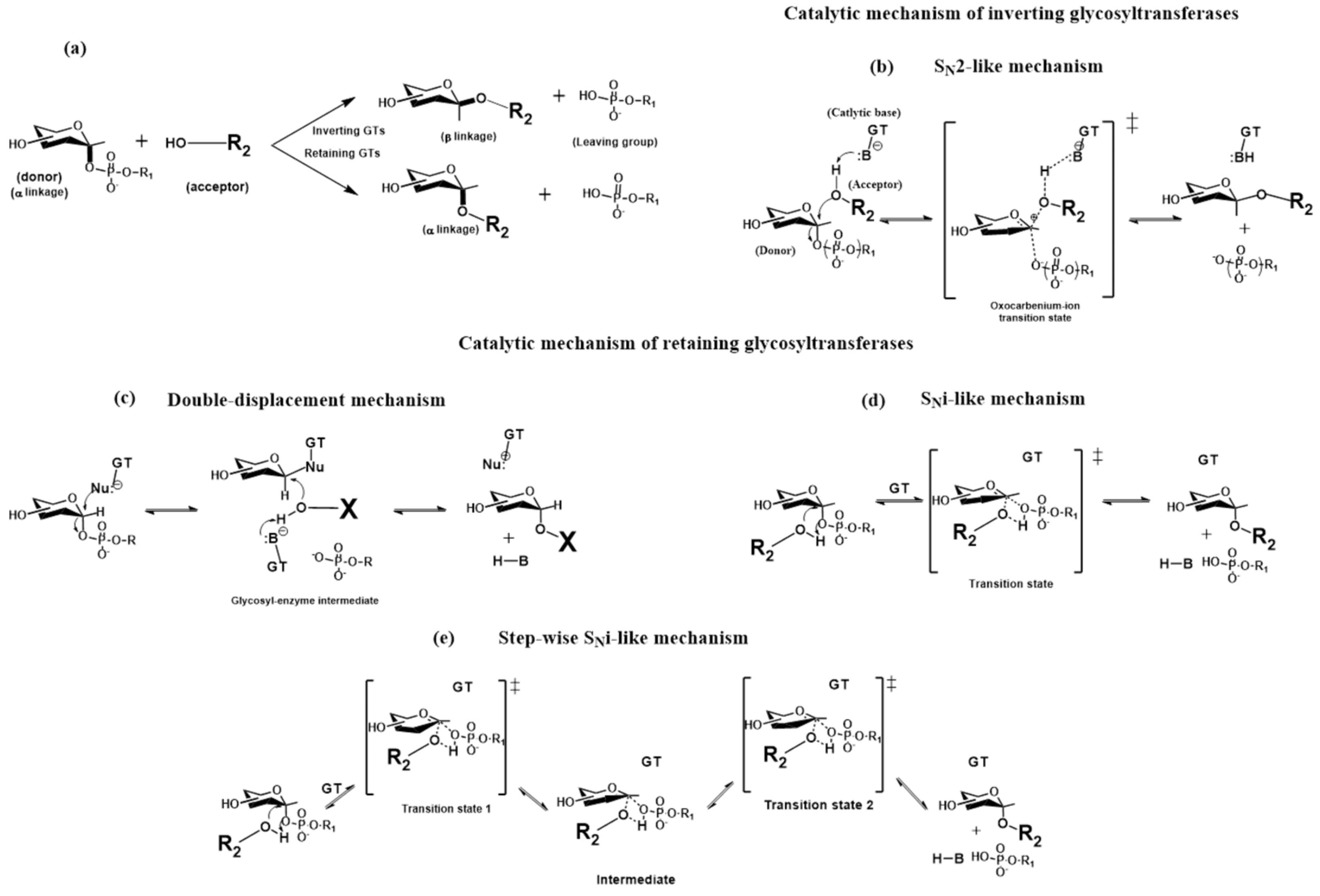

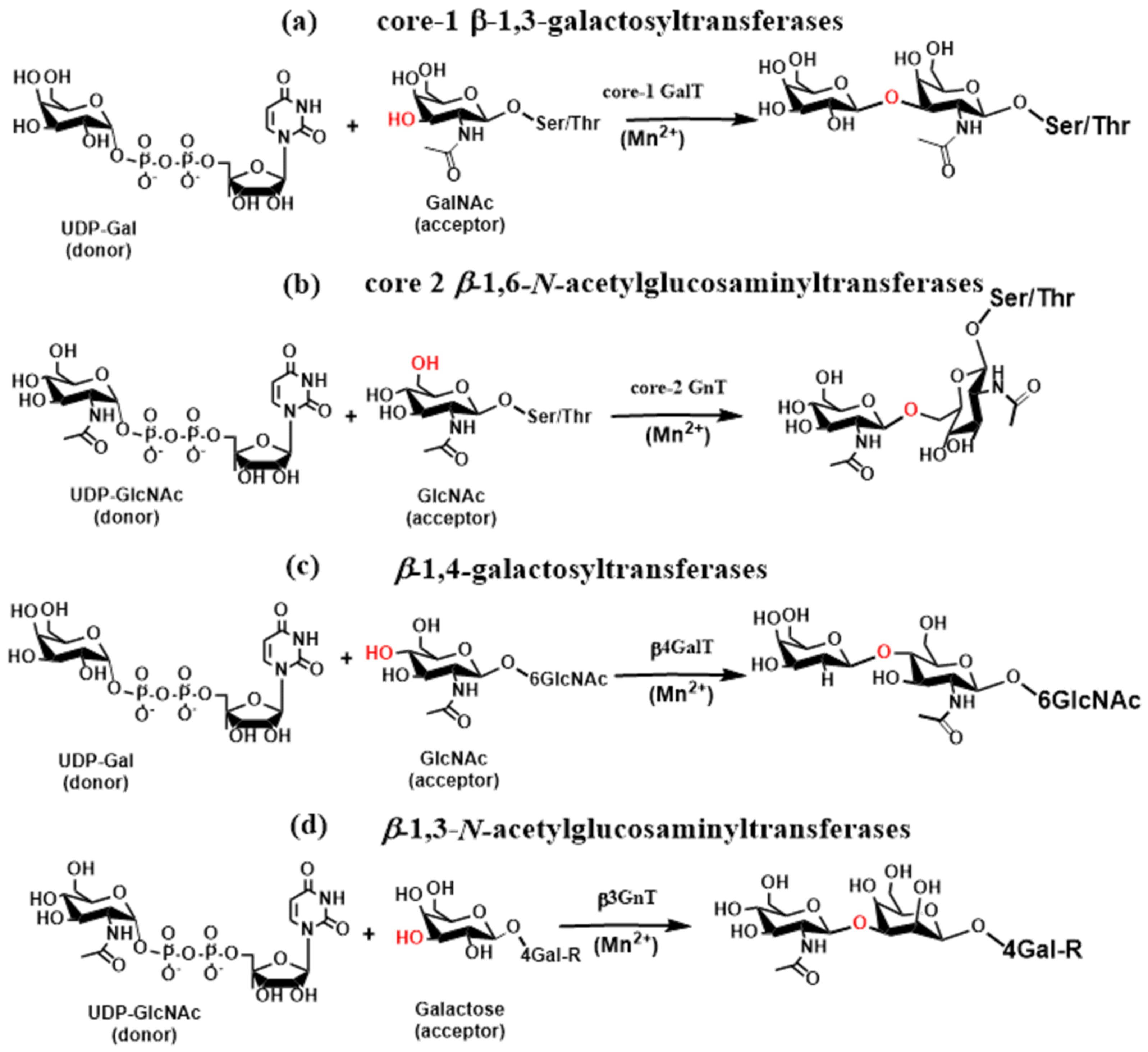
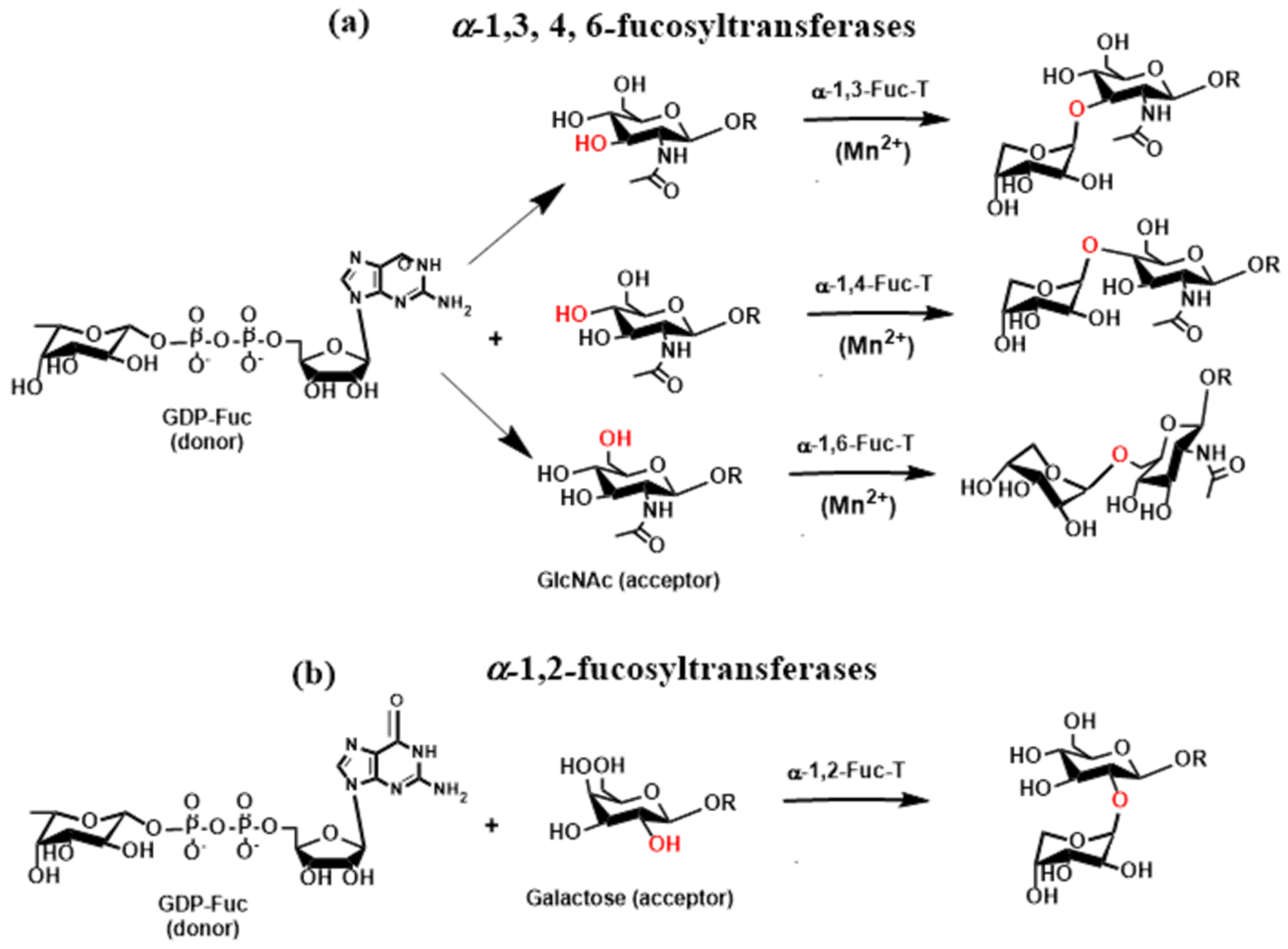


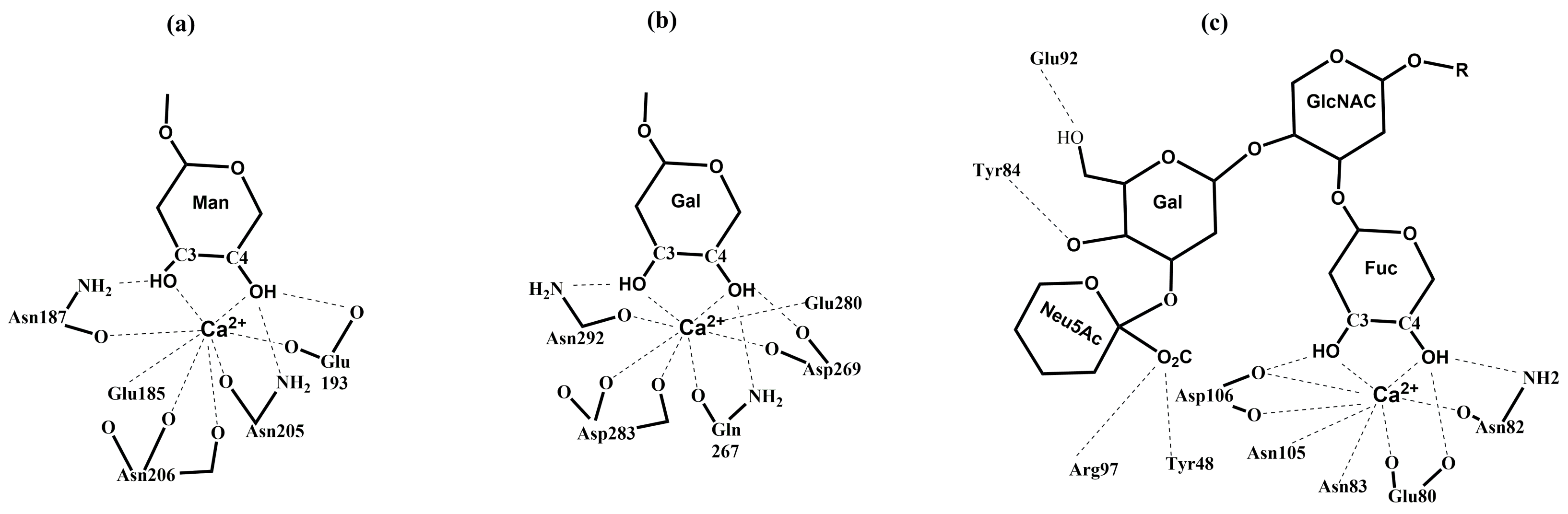

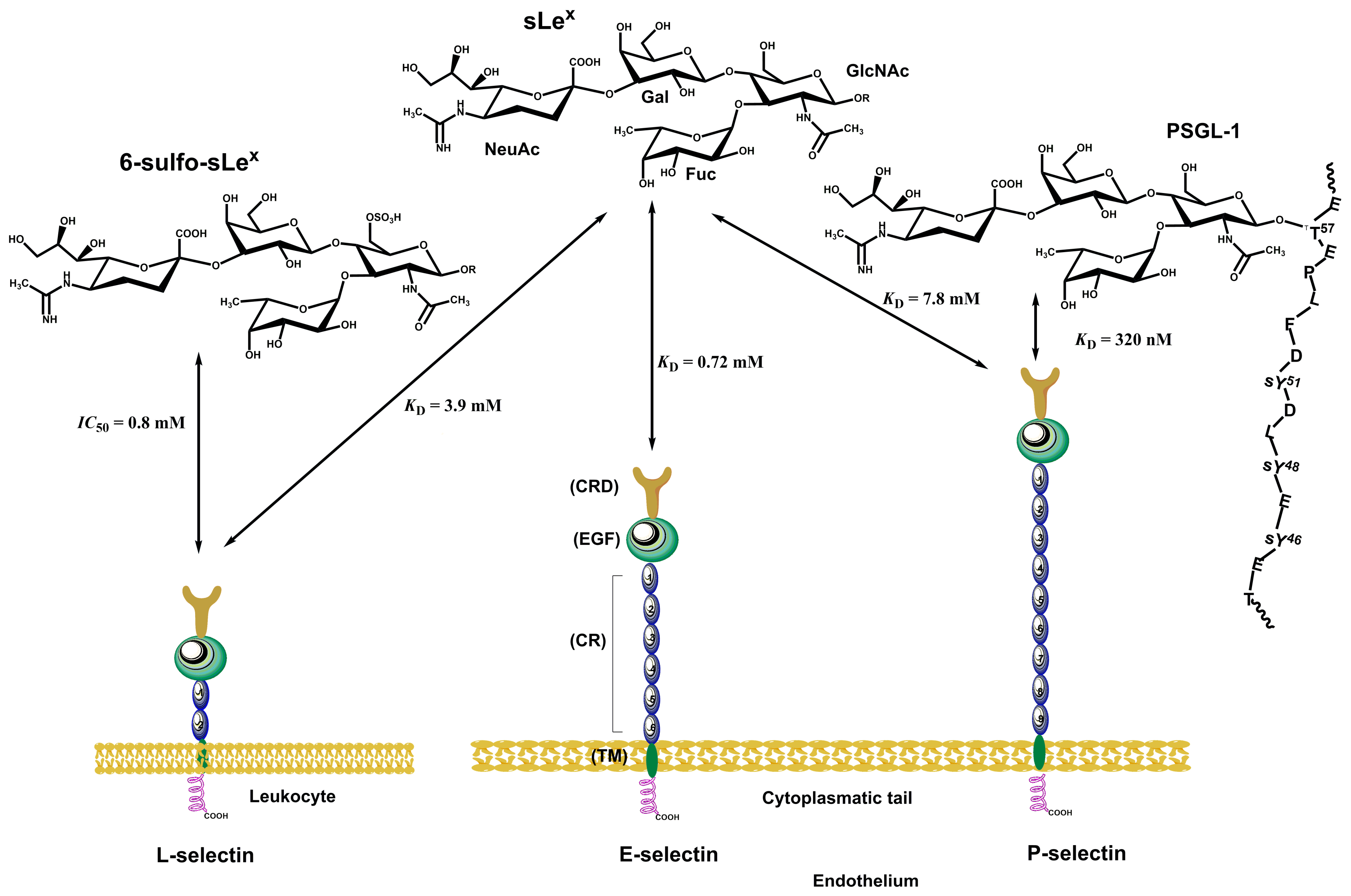
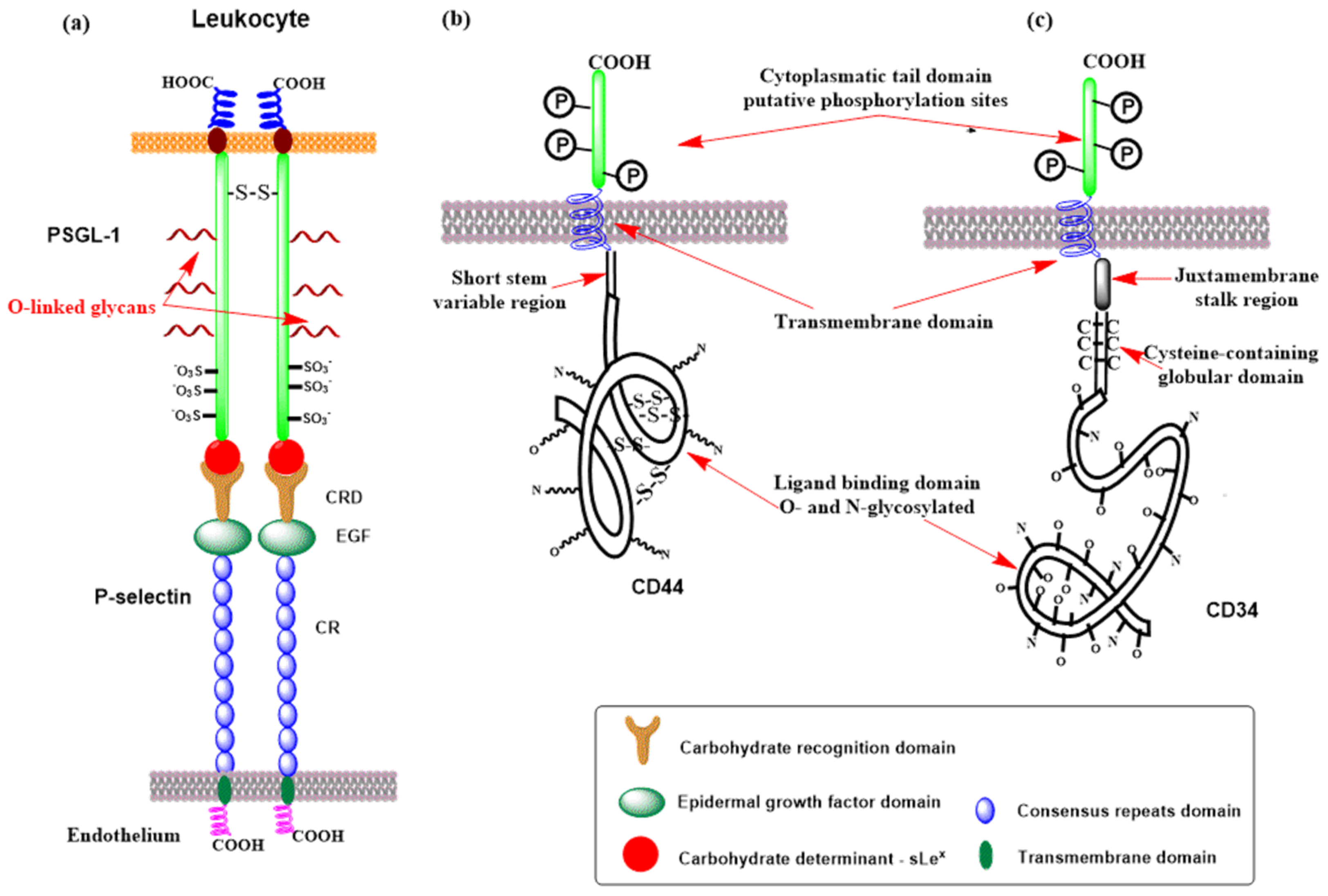
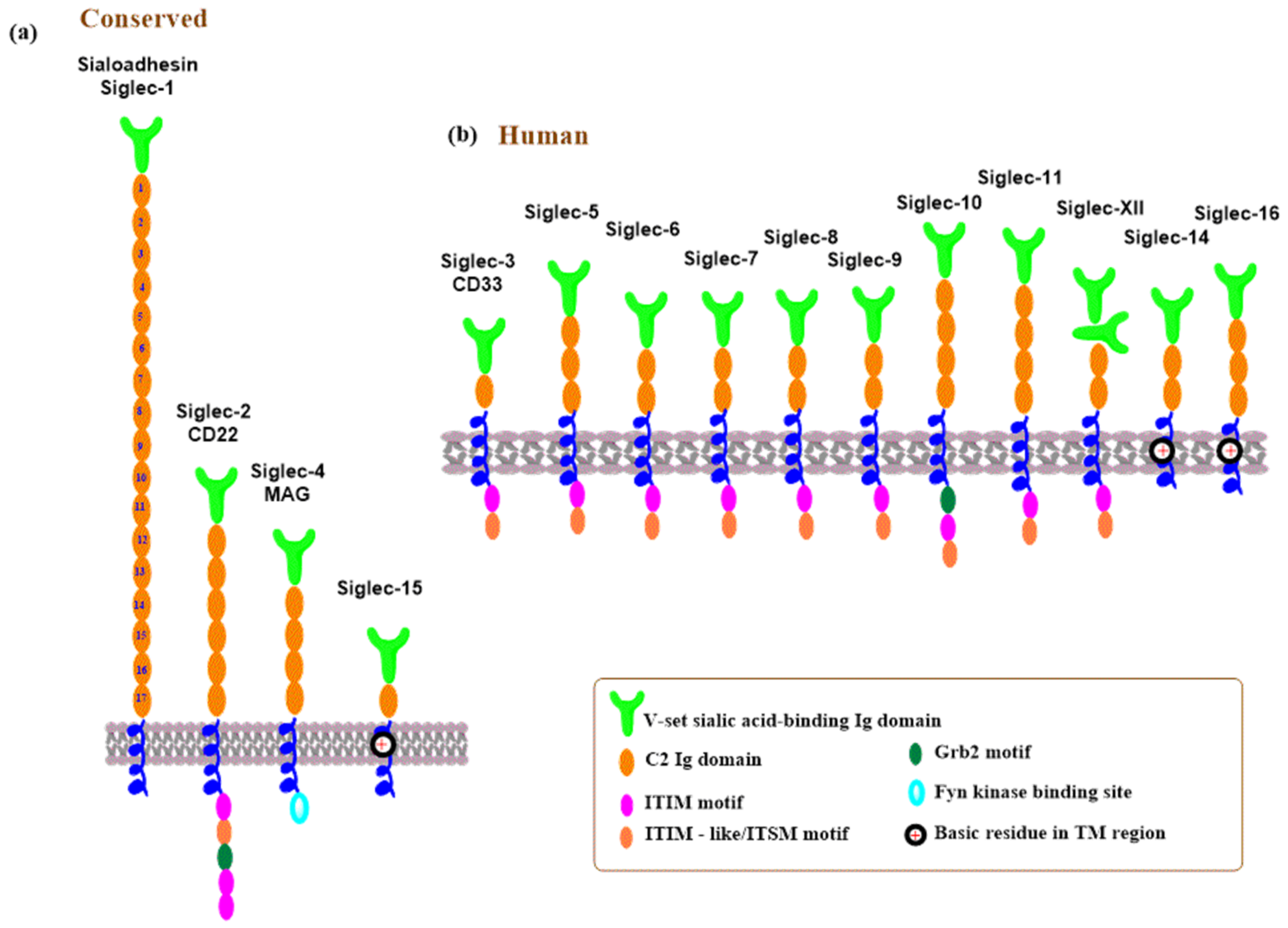

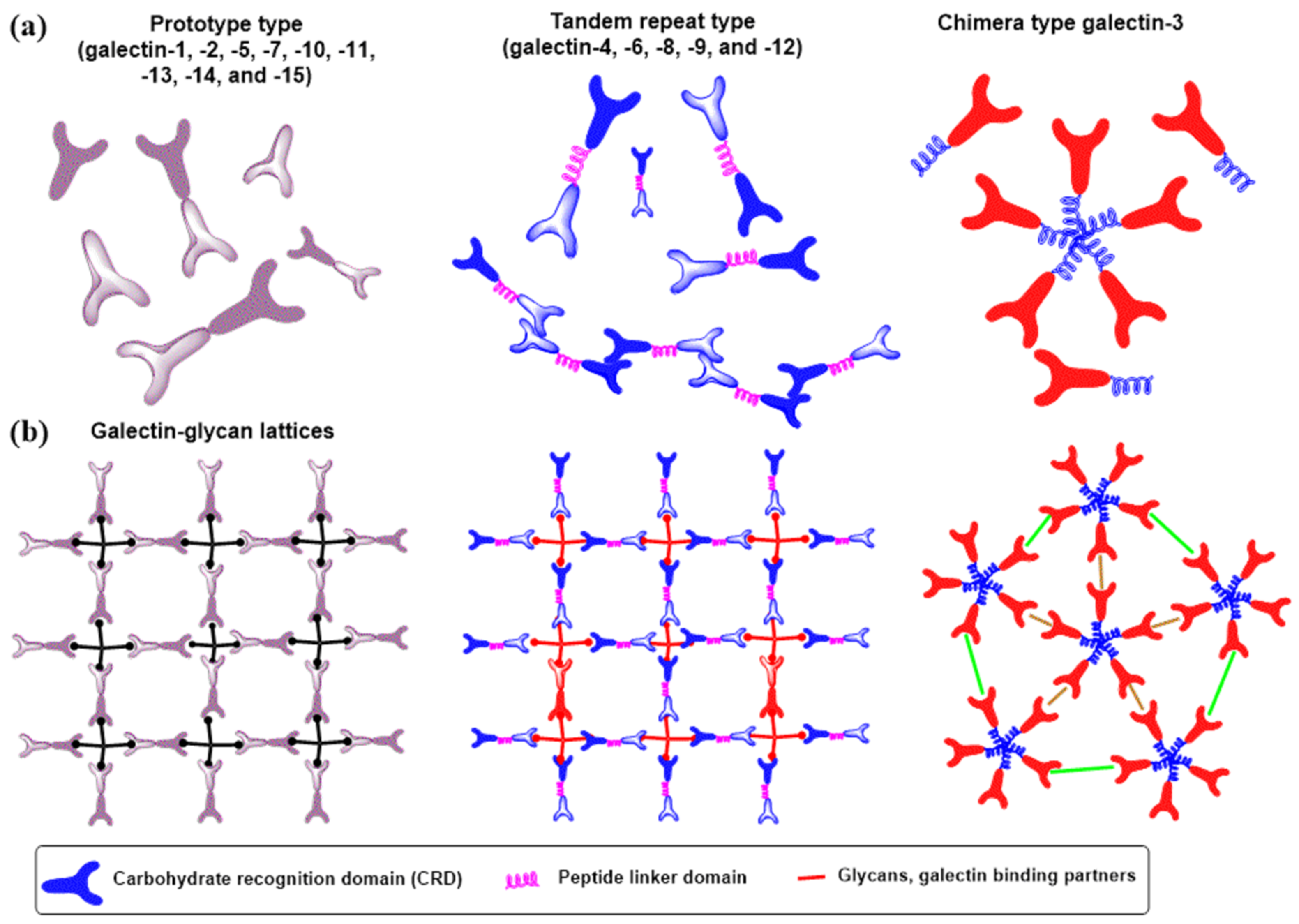
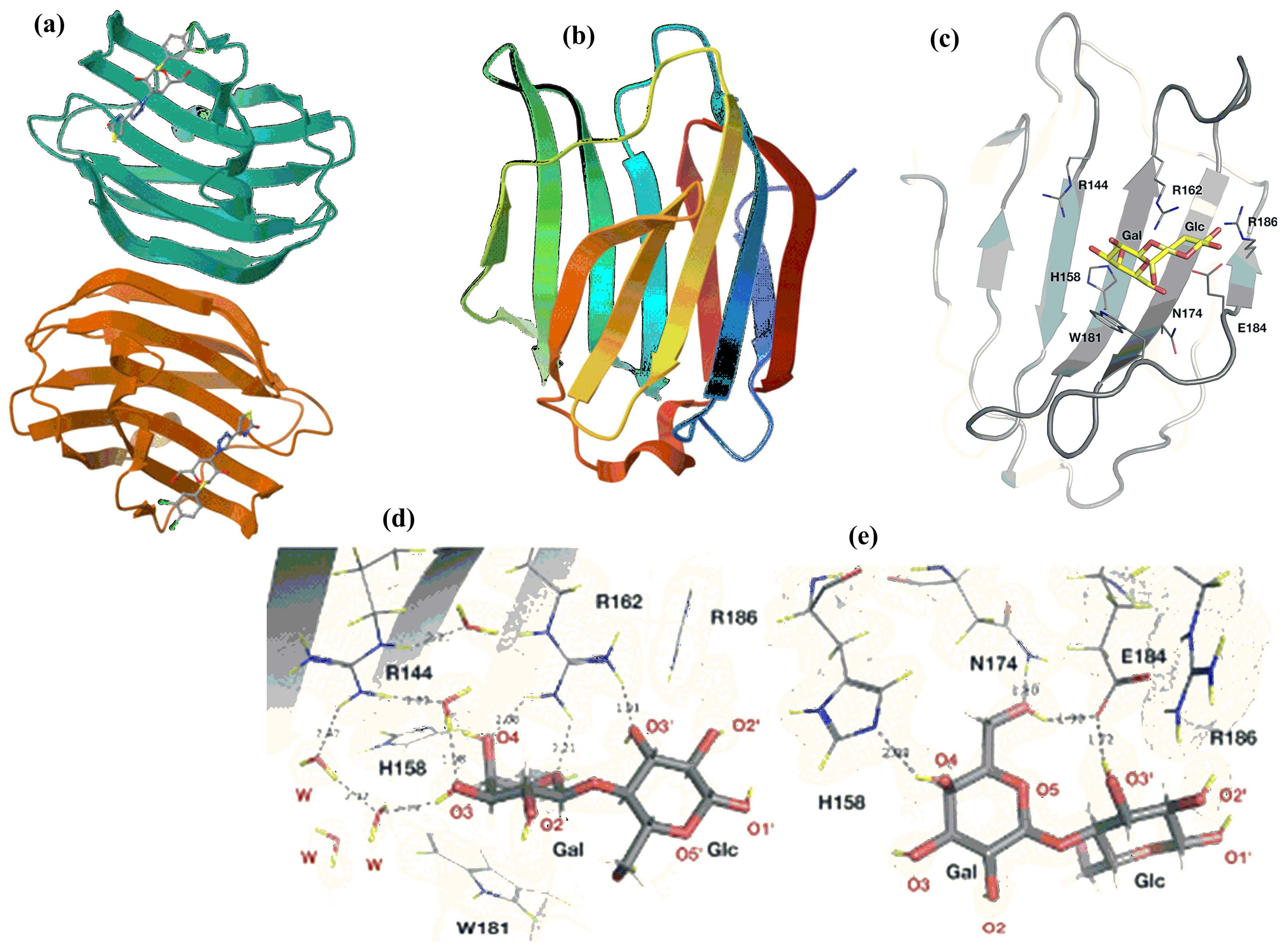

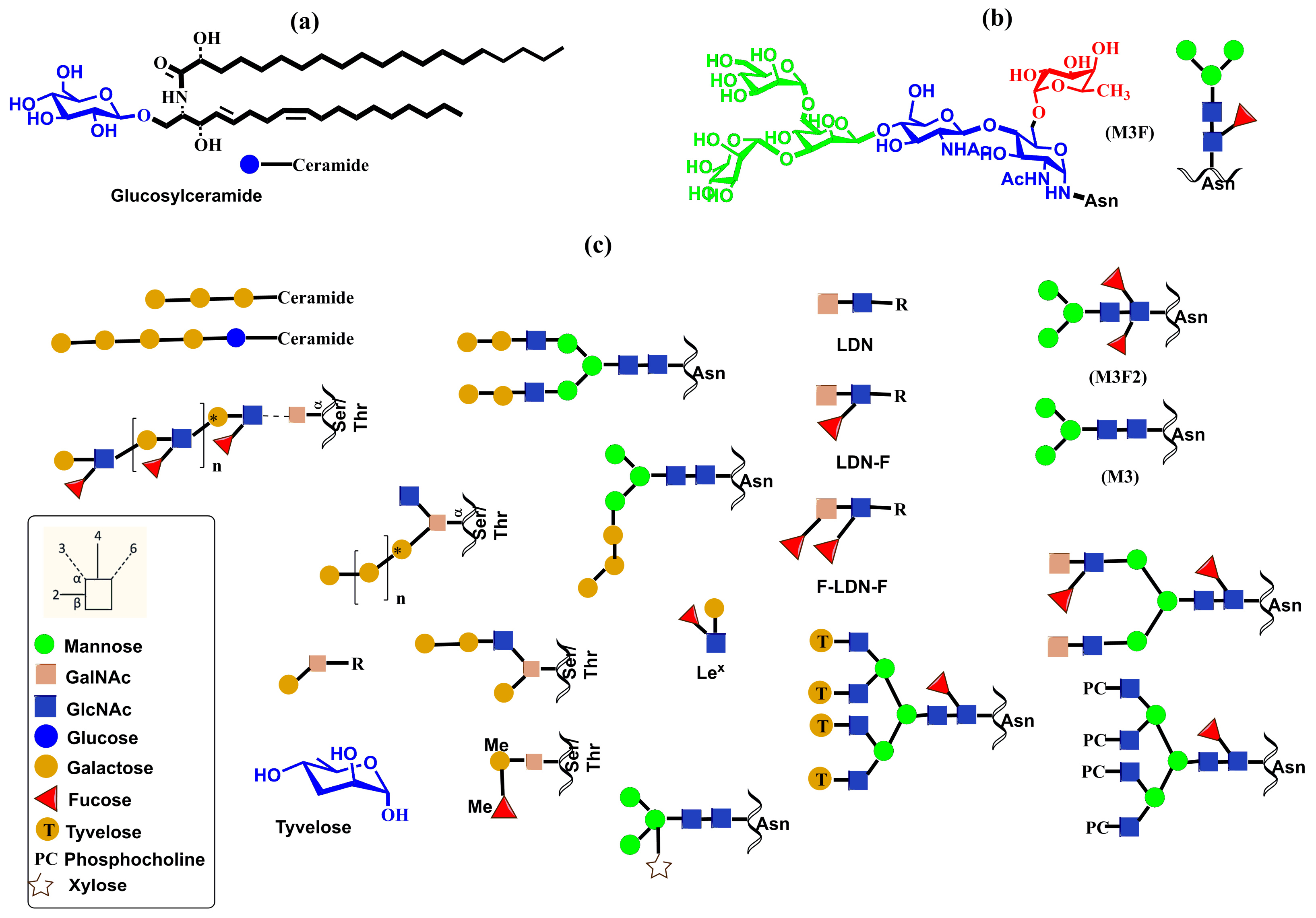

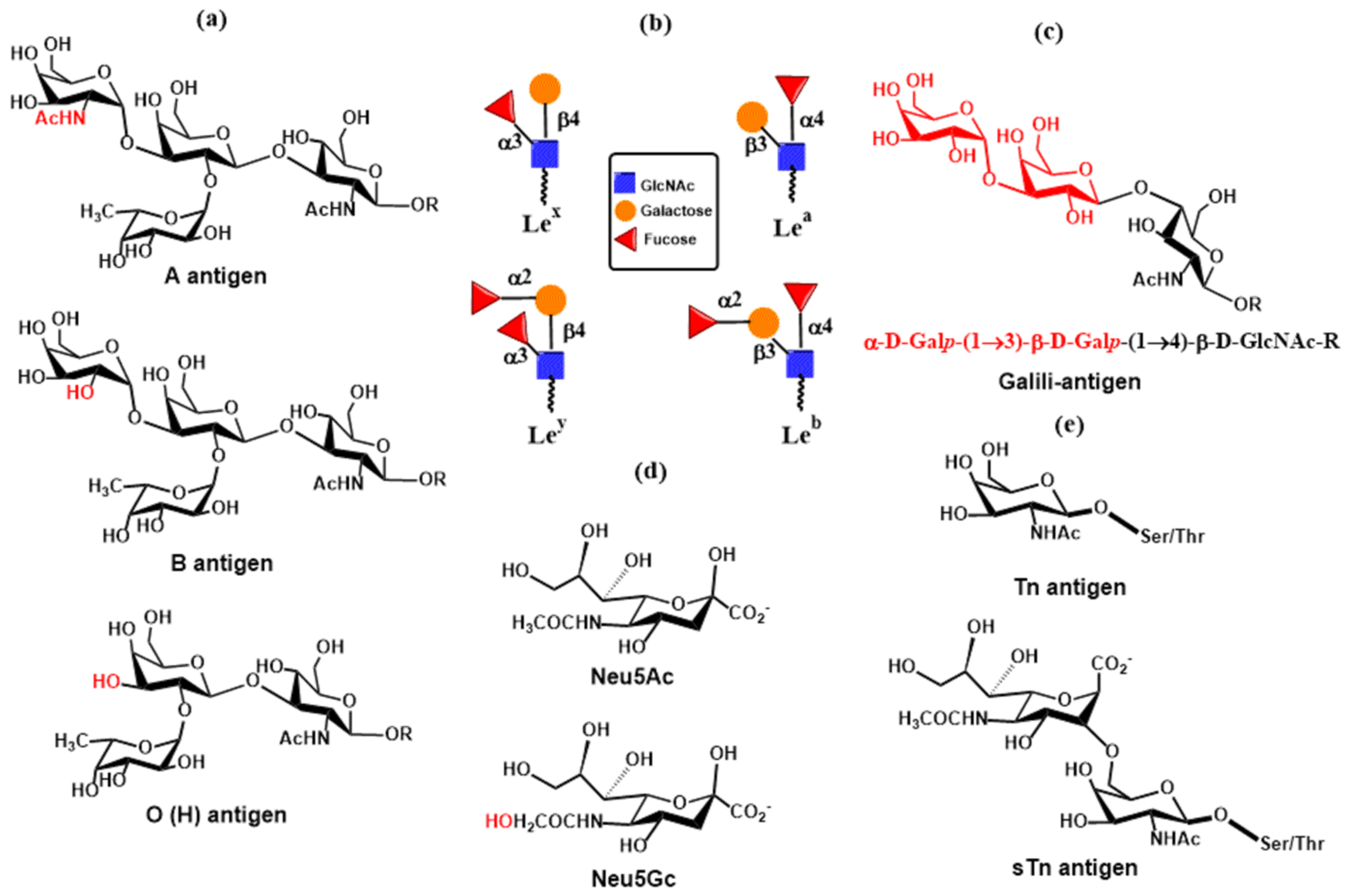
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tvaroška, I. The Role of Glycans in Human Immunity—A Sweet Code. Molecules 2025, 30, 2678. https://doi.org/10.3390/molecules30132678
Tvaroška I. The Role of Glycans in Human Immunity—A Sweet Code. Molecules. 2025; 30(13):2678. https://doi.org/10.3390/molecules30132678
Chicago/Turabian StyleTvaroška, Igor. 2025. "The Role of Glycans in Human Immunity—A Sweet Code" Molecules 30, no. 13: 2678. https://doi.org/10.3390/molecules30132678
APA StyleTvaroška, I. (2025). The Role of Glycans in Human Immunity—A Sweet Code. Molecules, 30(13), 2678. https://doi.org/10.3390/molecules30132678






