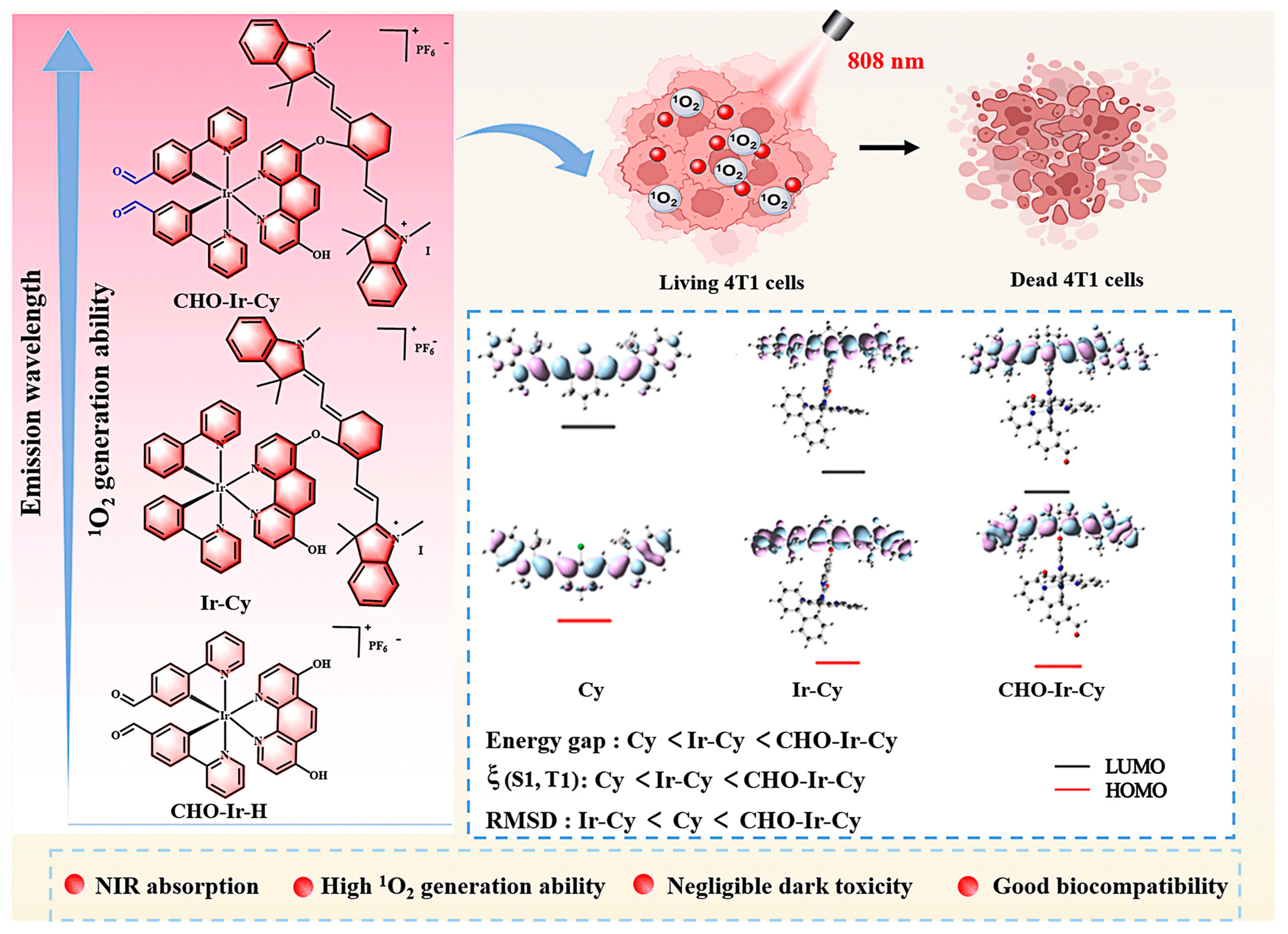
Constructing 1 + 1 > 2 Photosensitizers Based on NIR Cyanine–Iridium(III) Complexes for Enhanced Photodynamic Cancer Therapy
Abstract
1. Introduction
2. Results and Discussion
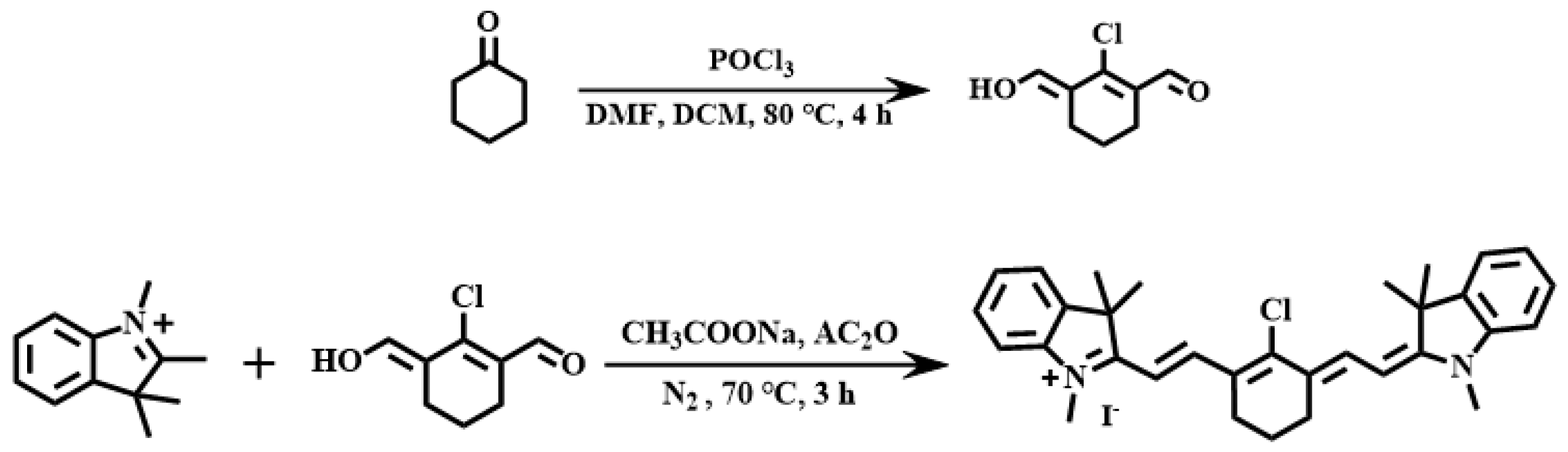
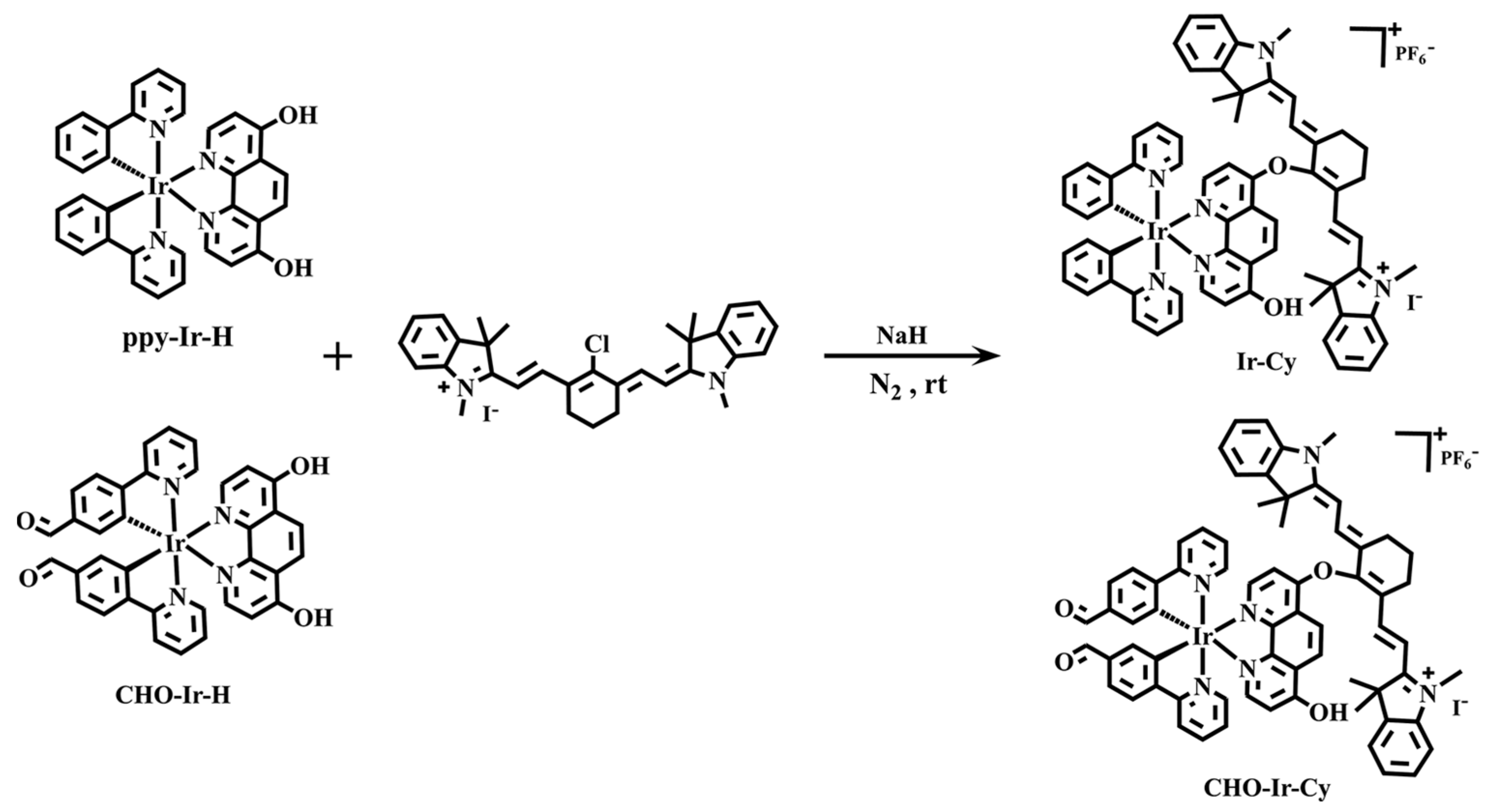
2.1. Synthesis of Ligands and Complexes
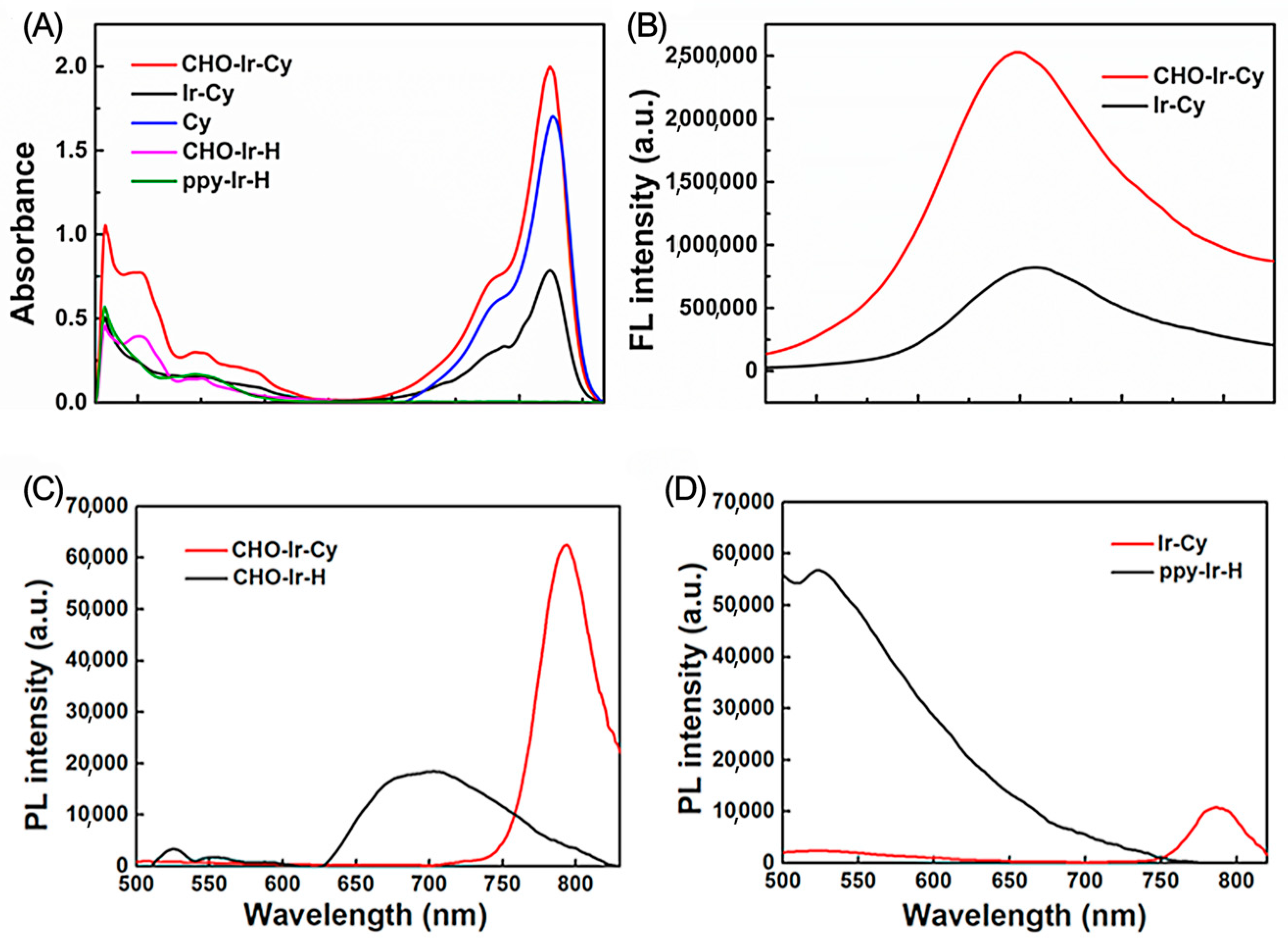
2.2. Analysis of Photophysical Properties of Complexes
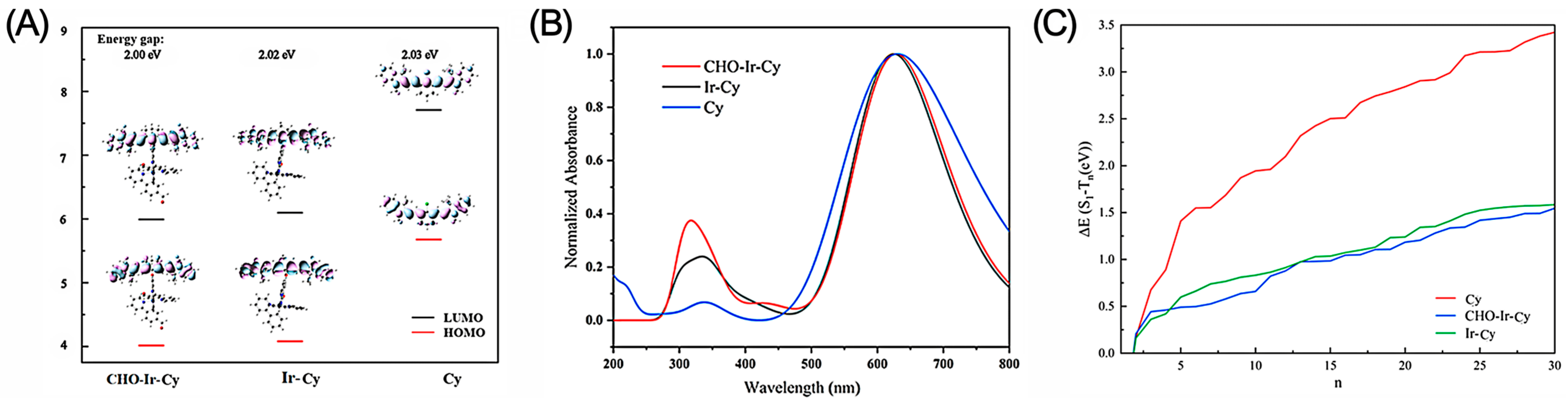
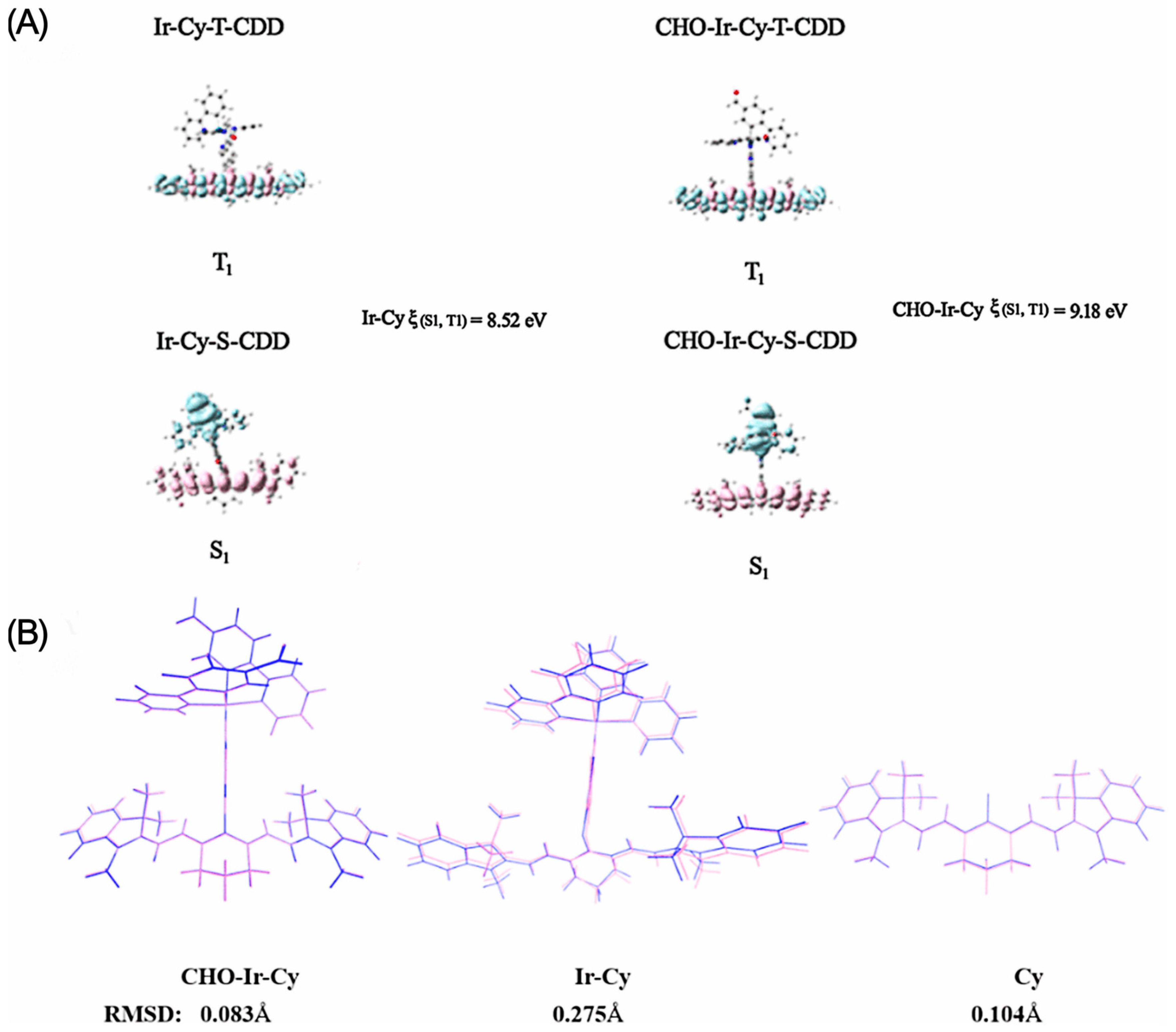
2.3. Density Functional Theory Calculations
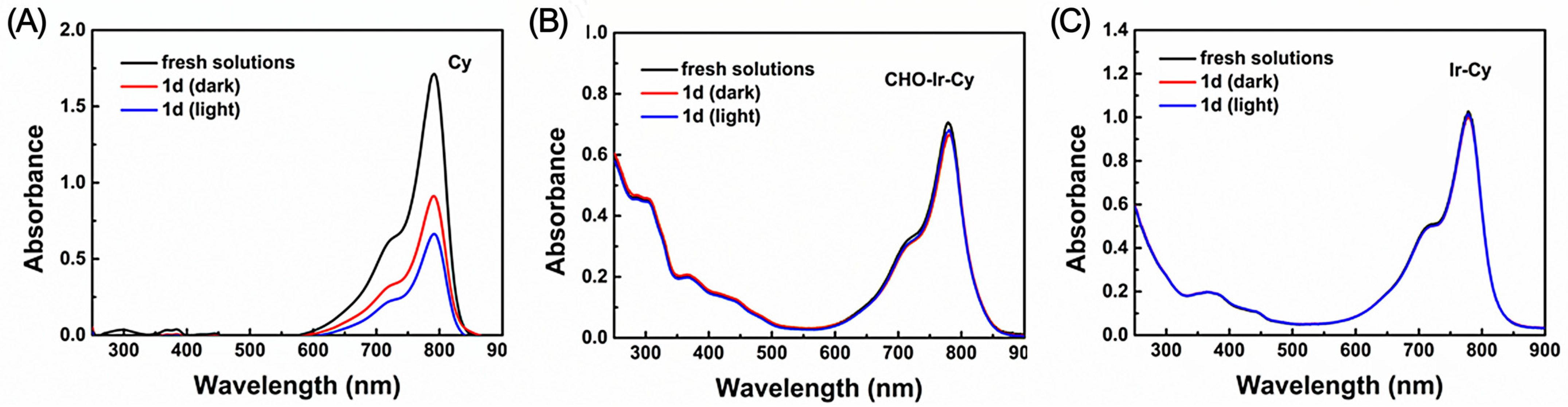
2.3.1. Analysis of Photostability Examination in Solution
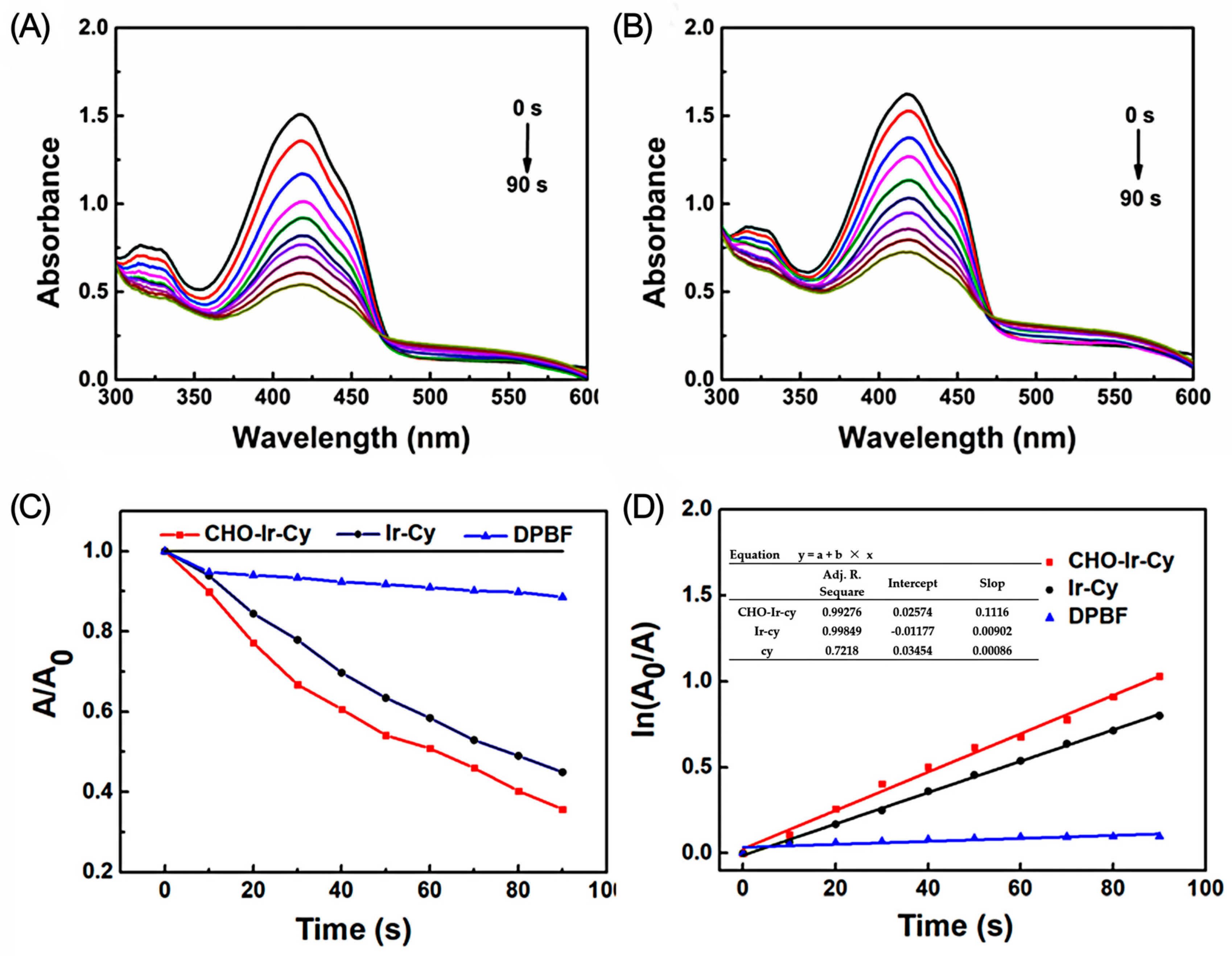
2.3.2. Analysis of Singlet Oxygen Production Capacity in Solution
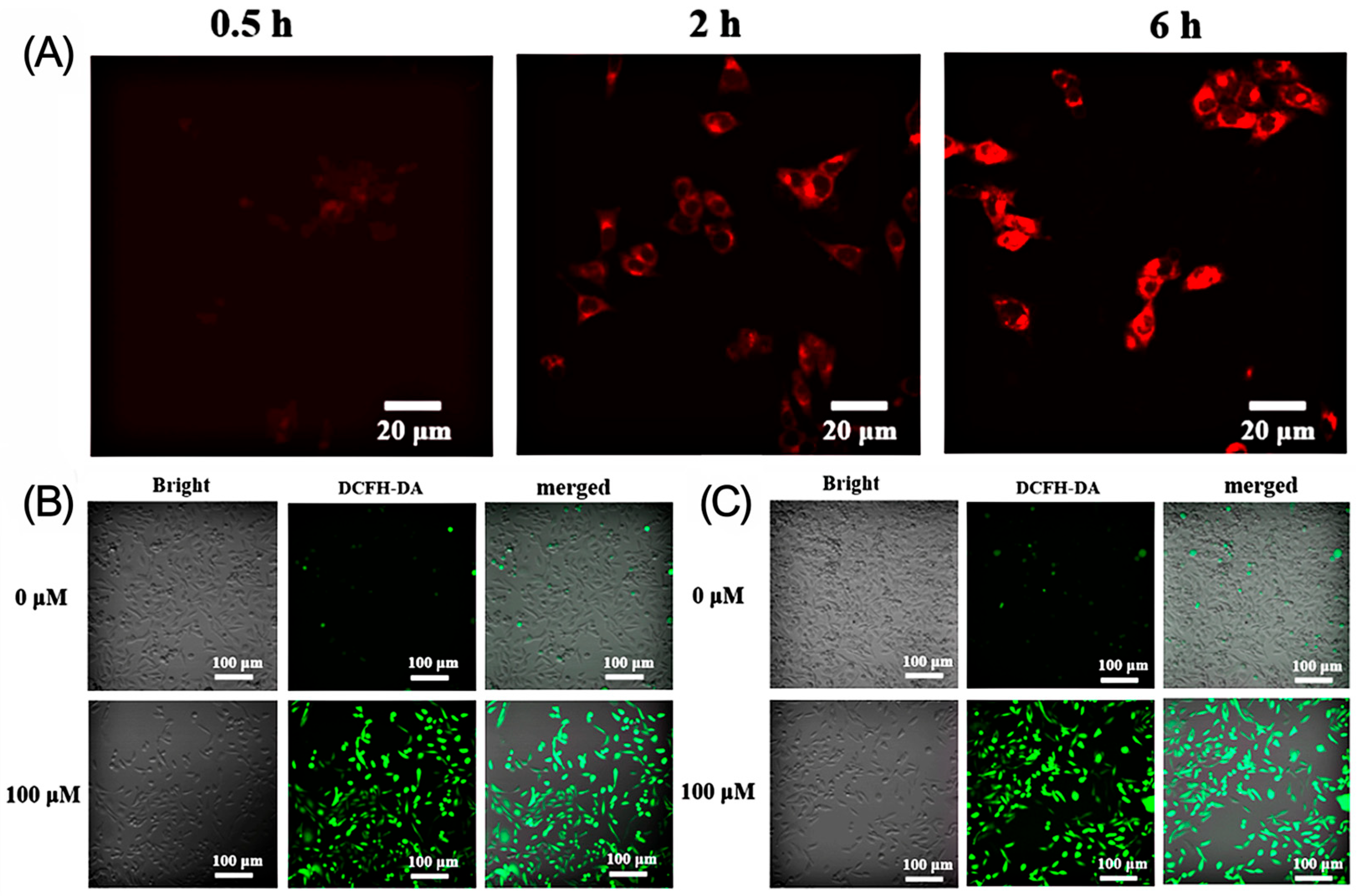
2.4. Study of Endocytosis Behavior
2.5. Analysis of Intracellular Singlet Oxygen Production Capacity
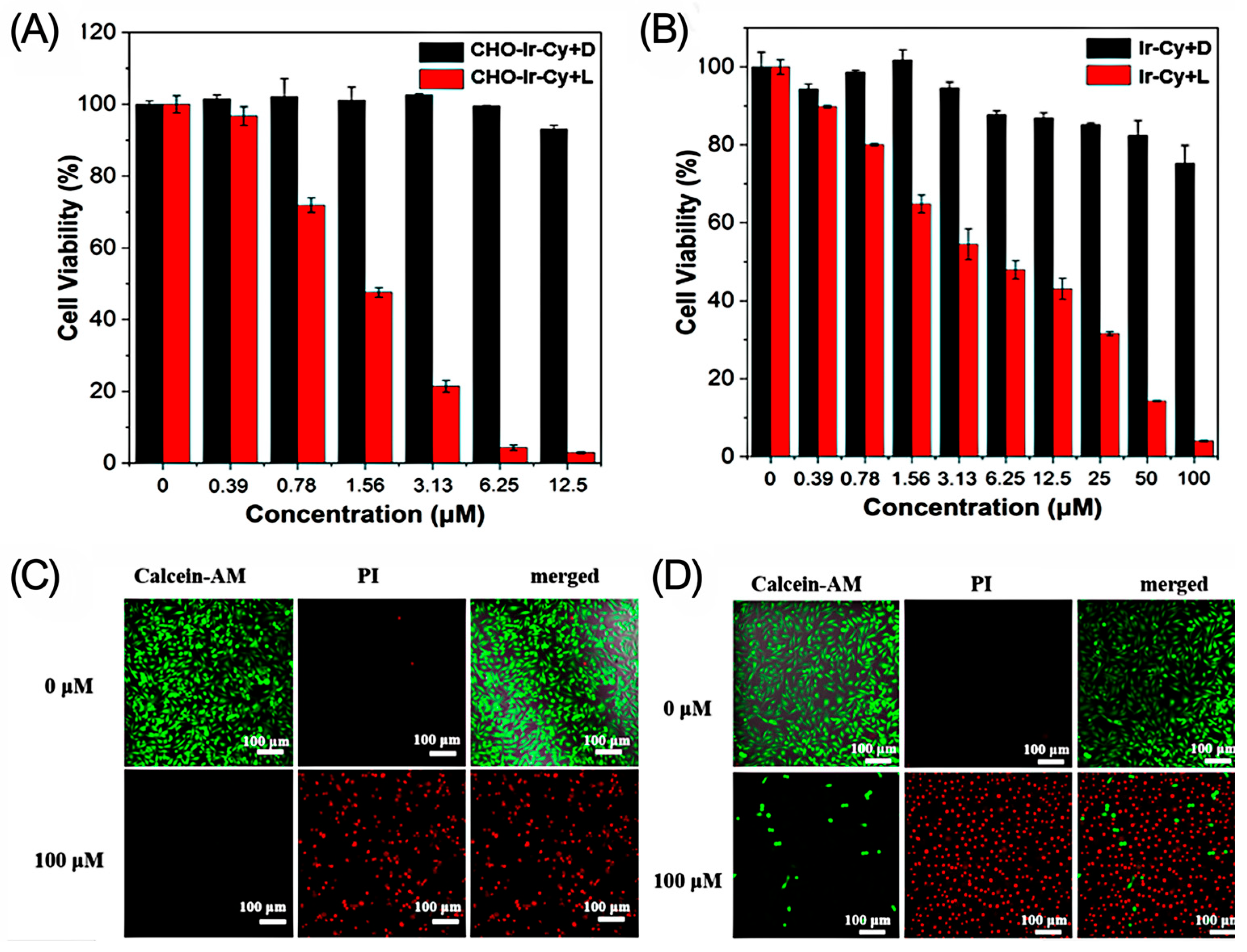
2.6. Cytotoxicity Research and Analysis
2.7. Living/Dead Cell Staining Experiment
3. Materials and Methods
3.1. General Information
3.2. Synthesis and Characterization of Complex
3.2.1. Synthesis of Cy
3.2.2. Preparation of Metal Iridium Complex Ir−Cy
3.2.3. Preparation of Metal Iridium Complex CHO−Ir−Cy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaiser, A.M.; Gatto, A.; Hanson, K.J.; Zhao, R.L.; Raj, N.; Ozawa, M.G.; Seoane, J.A.; Bieging-Rolett, K.T.; Wang, M.; Li, I.; et al. p53 governs an AT1 differentiation programme in lung cancer suppression. Nature 2023, 619, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Lewnard, J.A.; Kang, G.; Laxminarayan, R. Attributed causes ofexcess mortality during theCOVID-19pandemic ina south Indian city. Nat. Commun. 2023, 14, 3563. [Google Scholar] [CrossRef] [PubMed]
- Mani, K.; Deng, D.; Lin, C.; Wang, M.; Hsu, M.L.; Zaorsky, N.G. Causes ofdeath among people living with metastatic cancer. Nat. Commun. 2024, 15, 1519. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Zhang, X.; Lyu, Y.; Li, J.; Yang, X.; Lan, Z.; Chen, Z. Bimetallic Nanozymes-Integrated Parachute-Like Au2Pt @PMO@ICG Janus Nanomotor with Dual Propulsion for Enhanced Tumor Penetration and Synergistic PTT/PDT/CDT Cancer Therapy. Adv. Funct. Mater. 2024, 34, 2406059. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Y.; Chen, Y.; Cui, M.; Yang, F.; Wang, P.; Ji, M. Transmissible H-aggregated NIR-II fluorophore to the tumor cell membrane for enhanced PTT and synergistic therapy of cancer. Nano Converg. 2023, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Piipponen, M.; Liu, Z.; Luo, L.; Geara, J.; Chen, Y.; Sangsuwan, T.; Maselli, M.; Diaz, C.; Bain, C.A.; et al. Epigenetic memory of radiotherapy in dermal fibroblasts impairs wound repair capacity in cancer survivors. Nat. Commun. 2024, 15, 9286. [Google Scholar] [CrossRef]
- Jenniffer, L.; Anna, S.-A.; Jordi, B.-R.; Alba, R.-B.; Ana, M.; Noemí, M.-R.; Daniele, L.R.; Elisa, I.R.; Marc, G.; Melissa, Z.; et al. Long-term platinum-based drug accumulation in cancer-associated fibroblasts promotes colorectal cancer progression and resistance to therapy. Nat. Commun. 2023, 14, 746. [Google Scholar]
- Zhou, L.; Lyu, J.; Liu, F.; Su, Y.; Feng, L.; Zhang, X. Immunogenic PANoptosis-Initiated Cancer Sono-Immune Reediting Nanotherapy by Iteratively Boosting Cancer Immunity Cycle. Adv. Mater. 2023, 36, 2305361. [Google Scholar] [CrossRef]
- Ke, L.; Wei, F.; Xie, L.; Karges, J.; Chen, Y.; Ji, L.; Chao, H. A Biodegradable Iridium(III) Coordination Polymer for Enhanced Two-Photon Photodynamic Therapy Using an Apoptosis-Ferroptosis Hybrid Pathway. Angew. Chem. Int. Ed. Engl. 2022, 61, e202205429. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, J.; Fan, J.; Chao, H.; Peng, X. Recent progress in photosensitizers for overcoming the challenges of photodynamic therapy: From molecular design to application. Chem. Soc. Rev. 2021, 50, 4185–4219. [Google Scholar] [CrossRef]
- Fang, L.; Huang, R.; Gong, W.; Ji, Y.; Sun, Y.; Gou, S.; Zhao, J. A Self-Assembly-Induced Exciton Delocalization Strategy for Converting a Perylene Diimide Derivative from a Type-II to Type-I Photosensitizer. Small 2023, 20, 2307414. [Google Scholar] [CrossRef]
- Lu, B.; Xia, J.; Quan, H.; Huang, Y.; Zhang, Z.; Zhan, X. End Group Engineering for Constructing A−D−A Fused-Ring Photosensitizers with Balanced Phototheranostics Performance. Small 2023, 20, 2307664. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Chen, Y.; Lam, K.W.K.; Du, W.; Liu, Q.; Han, F.; Li, D.; Lam, J.W.Y.; Sun, J.; Kwok, R.T.K.; et al. Glutathione-responsive Aggregation-induced Emission Photosensitizers for Enhanced Photodynamic Therapy of Lung Cancer. Small 2024, 20, 2401334. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Q.; Zheng, Y.; Xie, Q.; Luo, X.; Zhou, S.; Pei, S.; Zhang, T.; Wu, X.; Xu, K.; Zhong, W. A Dual-Responsive Morphologically-Adaptable Nanoplatform for Targeted Delivery of Activatable Photosensitizers in Precision Photodynamic Therapy. Small 2023, 20, 2309054. [Google Scholar] [CrossRef]
- Cheng, H.B.; Qiao, B.; Li, H.; Cao, J.; Swamy, K.M.K.; Zhao, J.; Wang, Z.; Lee, J.; Liang, X.J.; Yoon, J. Protein-Activatable Diarylethene Monomer as a Smart Trigger of Noninvasive Control Over Reversible Generation of Singlet Oxygen: A Facile, Switchable, Theranostic Strategy for Photodynamic-Immunotherapy. J. Am. Chem. Soc. 2021, 143, 2413–2422. [Google Scholar] [CrossRef]
- Lu, H.; Jiang, X.; Chen, Y.; Peng, K.; Huang, Y.; Zhao, H.; Chen, Q.; Lv, F.; Liu, L.; Wang, S.; et al. Cyclometalated iridium(iii) complex nanoparticles for mitochondria-targeted photodynamic therapy. Nanoscale 2020, 12, 14061–14067. [Google Scholar] [CrossRef]
- Yang, J.; Fang, H.J.; Cao, Q.; Mao, Z.W. The design of cyclometalated iridium(iii)-metformin complexes for hypoxic cancer treatment. Chem. Commun. 2021, 57, 1093–1096. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Wu, Z.G.; Wu, R.; Cao, X.; Zhou, L.; Zheng, Y.X.; Yang, C. Semitransparent Circularly Polarized Phosphorescent Organic Light-Emitting Diodes with External Quantum Efficiency over 30% and Dissymmetry Factor Close to 10−2. Adv. Funct. Mater. 2021, 31, 2102898. [Google Scholar] [CrossRef]
- Liu, S.; Han, J.; Wang, W.; Chang, Y.; Wang, R.; Wang, Z.; Li, G.; Zhu, D.; Martin, R.B. AIE-active Ir(III) complexes functionalised with a cationic Schiff base ligand: Synthesis, photophysical properties and applications in photodynamic therapy. Dalton Trans. 2022, 51, 16119–16125. [Google Scholar] [CrossRef]
- Liu, S.; Chen, H.; Wu, Q.; Sun, Y.; Pei, Y.; Wang, Z.; Zhu, D.; Li, G.; Bryce, M.R.; Chang, Y. Self-Chemiluminescence-Trig gered Ir(III) Complex Photosensitizer for Photodynamic Therapy against Hypoxic Tumor. Inorg. Chem. 2024, 63, 16404–16417. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, Y.; Xie, X.; Tong, J.; Shan, G.-G.; Qin, C.; Xiao, X.; Wang, Q.; Li, Y.; Wang, H. Enhanced ROS generation in AIE-active iridium(III) photosensitizers by cationization engineering for advanced photodynamic therapy. Inorg. Chem. Front. 2025, 12, 986–994. [Google Scholar] [CrossRef]
- Wen, L.L.; Zhang, J.M.; Han, Y.P.; Duan, Y.C.; Xie, W.F.; Shao, K.Z.; Shan, G.G.; Su, Z.M. Boosting the efficiency of deep-red Ir(iii) complexes by modulating nitrogen atoms for high-performance OLEDs. Inorg. Chem. Front. 2023, 11, 133–141. [Google Scholar] [CrossRef]
- Li, X.; Zang, C.-X.; Gao, Y.; Wen, L.-L.; Shao, K.-Z.; Ding, G.-Y.; Shan, G.-G.; Xie, W.-F.; Su, Z.-M. Novel Ir(III) Complexes with NHC-Based Ancillary Ligands for Efficient Nondoped OLEDs. Inorg. Chem. 2022, 61, 20299–20307. [Google Scholar] [CrossRef]
- Tong, J.; Liu, A.; Huang, S.; Zhou, D.; Gao, Y.; Wang, Y.; Shan, G.G. Precise ligand engineering of Ir(III)-based photo sensitizer with aggregation-induced emission for image-guided photodynamic therapy. Luminescence 2023, 39, e4656. [Google Scholar] [CrossRef] [PubMed]
- Song, W.L.; Mao, H.T.; Gao, Y.; Yao, Y.X.; Shan, G.G.; Su, Z.M. Understanding AIE and ACQ phenomenon of organ ometallic iridium(III) complexes by simple cationization engineering. Chin. Chem. Lett. 2024, 35, 108309. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, G.; Che, W.; Liu, Y.; Xu, B.; Shan, G.; Zhu, D.; Su, Z.; Bryce, M.R. A neutral dinuclear Ir(III) complex for anti-counterfeiting and data encryption. Chem. Commun. 2017, 53, 3022–3025. [Google Scholar] [CrossRef]
- Song, W.; Gao, J.; Gao, Y.; Shan, G.-G.; Geng, Y.; Shao, K.; Su, Z.-M. Constructing anion–π interactions in cationic iridium(III) complexes to achieve aggregationinduced emission properties. Inorg. Chem. Front. 2024, 11, 1198–1206. [Google Scholar] [CrossRef]
- Li, G.; Ren, X.; Shan, G.; Che, W.; Zhu, D.; Yan, L.; Su, Z.; Bryce, M.R. New AIE-active dinuclear Ir(III) complexes with reversible piezochromic phosphorescence behaviour. Chem. Commun. 2015, 51, 13036–13039. [Google Scholar] [CrossRef]
- Liu, B.; Monro, S.; Li, Z.; Jabed, M.A.; Ramirez, D.; Cameron, C.G.; Colón, K.; Roque, J., III; Kilina, S.; Tian, J.; et al. A New Class of Homoleptic and Heteroleptic Bis(terpyridine) Iridium(III) Complexes with Strong Photodynamic Therapy Effects. ACS Appl. Bio Mater. 2019, 2, 2964–2977. [Google Scholar] [CrossRef]
- Karges, J.; Heinemann, F.; Jakubaszek, M.; Maschietto, F.; Subecz, C.; Dotou, M.; Vinck, R.; Blacque, O.; Tharaud, M.; Goud, B.; et al. Rationally designed long-wavelength absorbing Ru(II) polypyridyl complexes as photosensitizers for photodynamic therapy. J. Am. Chem. Soc. 2020, 142, 6578–6587. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, L.; Wang, W.; Wang, R.; Li, G.; Bian, H.; Zhu, D.; Bryce, M.R. Self-assembled nanoparticles based on cationic mono-/AIE tetra-nuclear Ir(III) complexes: Long wavelength absorption/near-infrared emission photosensitizers for photodynamic therapy†. Dalton Trans. 2023, 52, 1595–1601. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, S.; Wang, Z.; Shi, C.; Zhang, Q.; Wu, Z.; Li, G.; Zhu, D. An AIE Metal Iridium Complex: Photophysical Properties and Singlet Oxygen Generation Capacity. Molecules 2023, 28, 7914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Che, W.; Yang, Z.; Liu, X.; Liu, S.; Xie, Z.; Zhu, D.; Su, Z.; Tang, B.Z.; Bryce, M.R. Bright red aggregation-induced emission nanoparticles for multifunctional applications in cancer therapy. Chem. Sci. 2020, 11, 2369–2374. [Google Scholar] [CrossRef]
- Pei, Y.; Sun, Y.; Zhu, D. Phosphorescent Sensor Based on Iridium(III) Complex with Aggregation-Induced Emission Activity for Facile Detection of Volatile Acids. Molecules 2024, 29, 6041. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; He, S.; Cheng, Z.; Hu, J. Enhancing the fluorescence emission of the NIR-II fluorophores: Strategies, mechanisms, challenges, and opportunities. Coord. Chem. Rev. 2025, 532, 216511. [Google Scholar] [CrossRef]
- Wilson, J.S.; Chawdhury, N.; Al-Mandhary, M.R.; Younus, M.; Khan, M.S.; Raithby, P.R.; Kohler, A.; Friend, R.H. The energy gap law for triplet states in Pt-containing conjugated polymers and monomers. J. Am. Chem. Soc. 2001, 123, 9412–9417. [Google Scholar] [CrossRef]
- Wang, S.F.; Su, B.K.; Wang, X.Q.; Wei, Y.C.; Kuo, K.H.; Wang, C.H.; Liu, S.H.; Liao, L.S.; Hung, W.Y.; Fu, L.W.; et al. Polyatomic molecules with emission quantum yields > 20% enable efficient organic light-emitting diodes in the NIR (II) window. Nat. Photonics 2022, 16, 843–850. [Google Scholar] [CrossRef]
- Boyde, S.; Strouse, G.F.; Jones, W.E., Jr.; Meyer, T.J. The effect on MLCT excited states of electronic delocalization in the acceptor ligand. J. Am. Chem. Soc. 1990, 112, 7395–7396. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, Y.; Huang, R.; Chi, C.; Sun, Y.; Xu, G.; Xia, X.H.; Gou, S. Design of Near-Infrared-Triggered metallo-photosensitizers via a self-assembly-induced vibronic decoupling strategy. J. Am. Chem. Soc. 2023, 145, 11633–11642. [Google Scholar] [CrossRef]
- Treadway, J.A.; Loeb, B.; Lopez, R.; Anderson, P.A.; Keene, F.R.; Meyer, T.J. Effect of delocalization and rigidity in the acceptor ligand on MLCT excited-state decay. Inorg. Chem. 1996, 35, 2242–2246. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Z.; Wu, Z.; Chen, H.; Zhu, D.; Li, G.; Yan, M.; Martin, R.B.; Chang, Y. Long-wavelength triggered iridium(III) complex nanoparticles for photodynamic therapy against hypoxic cancer. Chem. Commun. 2024, 60, 9938. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yan, K.; Xu, G.; Liu, X.; Zhao, Q.; Xu, C.; Gou, S. An Iridium (III) Complex Bearing a Donor–Acceptor–Donor Type Ligand for NIR-Triggered Dual Phototherapy. Adv. Funct. Mater. 2021, 31, 2008325. [Google Scholar] [CrossRef]
- Liu, B.; Jiao, J.; Xu, W.; Zhang, M.; Cui, P.; Guo, Z.; Deng, Y.; Chen, H.; Sun, W. Highly Efficient Far-Red/NIR-Absorbing Neutral Ir(III) Complex Micelles for Potent Photodynamic/Photothermal Therapy. Adv. Mater. 2021, 33, 2100795. [Google Scholar] [CrossRef]
- Lv, F.; Feng, E.; Lv, S.; Liu, D.; Song, F. Metal-Coordination-Mediated H-Aggregates of Cyanine Dyes for Effective Photothermal Therapy. Chem.—A Eur. J. 2023, 29, e202301483. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L.; Liu, S.; Xie, Z. Metal−Organic Frameworks@Polymer Composites Containing Cyanines for Near-Infrared Fluorescence Imaging and Photothermal Tumor Therapy. Bioconjug. Chem. 2017, 28, 2784–2793. [Google Scholar] [CrossRef]
- Zhao, X.B.; Ha, W.; Gao, K.; Shi, Y.P. Precisely Traceable Drug Delivery of Azoreductase-Responsive Prodrug for Colon Targeting via Multimodal Imaging. Anal. Chem. 2020, 92, 9039–9047. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.Y.; Li, C.X.; Gao, M.; Han, X.; Zhang, Y.-J.; Zhang, W.-J.; Li, W.-W. Inside Cover: Mn−O Covalency Governs the Intrinsic Activity of Co-Mn Spinel Oxides for Boosted Peroxymonosulfate Activation. Angew. Chem. Int. Ed. 2021, 60, 2–11. [Google Scholar] [CrossRef]
- Zheng, Y.; Lu, H.; Jiang, Z.; Guan, Y.; Zou, J.; Wang, X.; Cheng, R.; Gao, H. Low-power white light triggered AIE polymer nanoparticles with high ROS quantum yield for mitochondria-targeted and image-guided photodynamic therapy. J. Mater. Chem. B 2017, 5, 6277–6281. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wang, W.; Wu, Q.; Zhu, D. Constructing 1 + 1 > 2 Photosensitizers Based on NIR Cyanine–Iridium(III) Complexes for Enhanced Photodynamic Cancer Therapy. Molecules 2025, 30, 2662. https://doi.org/10.3390/molecules30122662
Wang Z, Wang W, Wu Q, Zhu D. Constructing 1 + 1 > 2 Photosensitizers Based on NIR Cyanine–Iridium(III) Complexes for Enhanced Photodynamic Cancer Therapy. Molecules. 2025; 30(12):2662. https://doi.org/10.3390/molecules30122662
Chicago/Turabian StyleWang, Ziwei, Weijin Wang, Qi Wu, and Dongxia Zhu. 2025. "Constructing 1 + 1 > 2 Photosensitizers Based on NIR Cyanine–Iridium(III) Complexes for Enhanced Photodynamic Cancer Therapy" Molecules 30, no. 12: 2662. https://doi.org/10.3390/molecules30122662
APA StyleWang, Z., Wang, W., Wu, Q., & Zhu, D. (2025). Constructing 1 + 1 > 2 Photosensitizers Based on NIR Cyanine–Iridium(III) Complexes for Enhanced Photodynamic Cancer Therapy. Molecules, 30(12), 2662. https://doi.org/10.3390/molecules30122662




