Soil Pollution and Its Interrelation with Interfacial Chemistry
Abstract
1. Introduction
2. Significance of Soil Pollution
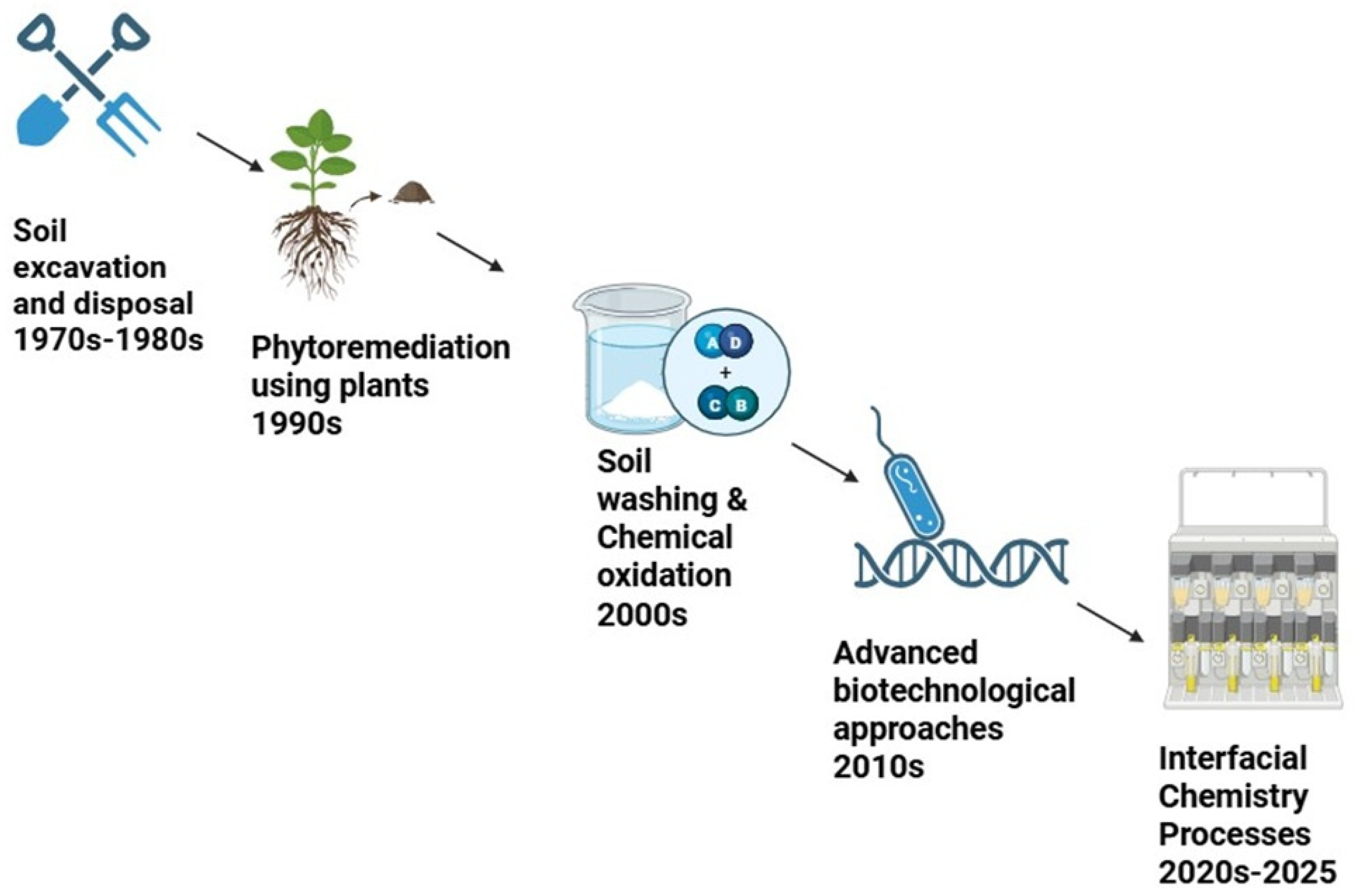
3. Historical Perspectives and Global Trends

4. Types and Characteristics of Selected Soil Pollutants
4.1. Inorganic Pollutants (Heavy Metals)
4.2. Organic Pollutants (Petroleum Hydrocarbons and Polycyclic Aromatic Hydrocarbons)
4.3. Emerging Pollutants (Pharmaceuticals, Microplastics, Perfluorinated Chemicals)
4.4. Instruments for Quantification of Selected Soil Pollutants
5. Relationship Between Soil Treatment and Interfacial Chemistry
5.1. Interfacial Chemistry Drivers
5.1.1. Soil–Contaminant Interactions
5.1.2. Adsorption Mechanisms
5.1.3. Mobility Modifiers
5.1.4. Contaminant Aging Effects
5.1.5. Molecular-Scale Dynamics Phenomena
- Slow Relaxation and Time-Dependent Transport Dynamics
- Dynamic Heterogeneity at Molecular Interfaces
- Confinement Effects
- Pollutant Retention at Soil Interfaces
6. Soil Treatment Technologies
6.1. Biological Treatment
6.2. Chemical Treatment
6.3. Physicochemical Treatment
7. Soil Remediation Investments
8. Future Directions
9. Conclusions
Funding
Conflicts of Interest
References
- Singh, S.P.; Singh, M.K. Soil pollution and human health. In Plant Responses to Soil Pollution; Springer: Berlin/Heidelberg, Germany, 2020; pp. 205–220. [Google Scholar]
- Ekka, P.; Patra, S.; Upreti, M.; Kumar, G.; Kumar, A.; Saikia, P. Land Degradation and its impacts on Biodiversity and Ecosystem services. In Land and Environmental Management Through Forestry; Wiley: Hoboken, NJ, USA, 2023; pp. 77–101. [Google Scholar]
- Jagaba, A.H.; Lawal, I.M.; Birniwa, A.H.; Affam, A.C.; Usman, A.K.; Soja, U.B.; Yaro, N.S.A. Sources of Water Contamination by Heavy Metals. In Membrane Technologies for Heavy Metal Removal from Water; CRC Press: Boca Raton, FL, USA, 2024; pp. 3–27. [Google Scholar]
- Nath, A.; Bhuyan, P.; Gogoi, N.; Deka, P. Pesticides and chemical fertilizers: Role in soil degradation, groundwater contamination, and human health. In Xenobiotics in Urban Ecosystems: Sources, Distribution and Health Impacts; Springer International Publishing: Cham, Switzerland, 2023; pp. 131–160. [Google Scholar]
- Sandil, S.; Kumar, R. Soil contamination from construction projects. In Ecological and Health Effects of Building Materials; Springer: Berlin/Heidelberg, Germany, 2022; pp. 205–244. [Google Scholar]
- Okorondu, J.; Umar, N.A.; Ulor, C.O.; Onwuagba, C.G.; Diagi, B.E.; Ajiere, S.I.; Nwaogu, C. Anthropogenic Activities as Primary Drivers of Environmental Pollution and Loss of Biodiversity A Review. Int. J. Trend Sci. Res. Dev. 2022, 6, 621–643. [Google Scholar]
- Ramakrishnan, B.; Megharaj, M.; Venkateswarlu, K.; Sethunathan, N.; Naidu, R. Mixtures of Environmental Pollutants: Effects on Microorganisms and Their Activities in Soils; Springer: New York, NY, USA, 2011; pp. 63–120. [Google Scholar]
- Ray, S.; Shaju, S.T. Bioaccumulation of pesticides in fish resulting toxicities in humans through food chain and forensic aspects. Environ. Anal. Health Toxicol. 2023, 38, e2023017. [Google Scholar] [CrossRef] [PubMed]
- Lawal, K.K.; Ekeleme, I.K.; Onuigbo, C.M.; Ikpeazu, V.O.; Obiekezie, S.O. A review on the public health implications of heavy metals. World J. Adv. Res. Rev. 2021, 10, 255–265. [Google Scholar] [CrossRef]
- Adigun, O.J.; Odeleye, D.A. The Devastating Consequences of Environmental Pollution on Human Health. Br. J. Multidiscip. Adv. Stud. 2025, 6, 37–46. [Google Scholar] [CrossRef]
- Vincent-Orugbo, E.; Bemgba, P.T. Assessing Chemical contamination of Nigeria’s agro-food systems and human security. Int. J. Confl. Secur. Manag. 2025, 4, 280–293. [Google Scholar] [CrossRef]
- Pignatello, J.J. Bioavailability of contaminants in soil. In Advances in Applied Bioremediation; Springer: Berlin/Heidelberg, Germany, 2009; pp. 35–71. [Google Scholar]
- Rodrigo-Comino, J.; López-Vicente, M.; Kumar, V.; Rodríguez-Seijo, A.; Valkó, O.; Rojas, C.; Pourghasemi, H.R.; Salvati, L.; Bakr, N.; Vaudour, E.; et al. Soil science challenges in a new era: A transdisciplinary overview of relevant topics. Air Soil Water Res. 2020, 13, 1178622120977491. [Google Scholar] [CrossRef]
- Brevik, E.C.; Slaughter, L.; Singh, B.R.; Steffan, J.J.; Collier, D.; Barnhart, P.; Pereira, P. Soil and human health: Current status and future needs. Air Soil Water Res. 2020, 13, 1178622120934441. [Google Scholar] [CrossRef]
- Innocent, M.O.; Mustapha, A.; Abdulsalam, M.; Livinus, M.U.; Samuel, J.O.; Elelu, S.A.; Muhammad, A.S. Soil Microbes and Soil Contamination. In Soil Microbiome in Green Technology Sustainability; Springer Nature: Cham, Switzerland, 2024; pp. 3–35. [Google Scholar]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Barathan, M.; Ng, S.L.; Lokanathan, Y.; Ng, M.H.; Law, J.X. Plant defense mechanisms against polycyclic aromatic hydrocarbon contamination: Insights into the role of extracellular vesicles. Toxics 2024, 12, 653. [Google Scholar] [CrossRef]
- Panagos, P.; Van Liedekerke, M.; Yigini, Y.; Montanarella, L. Contaminated sites in Europe: Review of the current situation based on data collected through a European network. J. Environ. Public Health 2013, 2013, 158764. [Google Scholar] [CrossRef]
- European Environmental Agency (EEA). Progress in Management of Contaminated Sites (CSI 015)—Assessment Published Aug 2007. 2007. Available online: http://www.eea.europa.eu/data-and-maps/indicators/progress-in-management-of-contaminated-sites/progress-in-management-of-contaminated-1 (accessed on 2 May 2025).
- Hoek, G.; Ranzi, A.; Alimehmeti, I.; Ardeleanu, E.R.; Arrebola, J.P.; Ávila, P.; Candeias, C.; Colles, A.; Crian, G.C.; Dack, S.; et al. A review of exposure assessment methods for epidemiological studies of health effects related to industrially contaminated sites. Epidemiol. Prev. 2018, 42, 21–36. [Google Scholar]
- Shukla, P.; Srivastava, P.; Mishra, A. Routes of Exposure (Inhalation, Ingestion, Dermal Contact) of Heavy Metals and Their Implications for Human Health. In Heavy Metal Contamination in the Environment; CRC Press: Boca Raton, FL, USA, 2024; pp. 114–123. [Google Scholar]
- Choi, J.; Bae, S.; Lim, H.; Lim, J.A.; Lee, Y.H.; Ha, M.; Kwon, H.J. Mercury exposure in association with decrease of liver function in adults: A longitudinal study. J. Prev. Med. Public Health 2017, 50, 377. [Google Scholar] [CrossRef]
- Perrelli, M.; Wu, R.; Liu, D.J.; Lucchini, R.G.; Del Bosque-Plata, L.; Vergare, M.; Gragnoli, C. Heavy Metals as Risk Factors for Human Diseases—A Bayesian Network Approach; Thomas Jefferson University: Philadelphia, PA, USA, 2022. [Google Scholar]
- Schug, T.T.; Janesick, A.; Blumberg, B.; Heindel, J.J. Endocrine disrupting chemicals and disease susceptibility. J. Steroid Biochem. Mol. Biol. 2011, 127, 204–215. [Google Scholar] [CrossRef]
- Ahn, C.; Jeung, E.B. Endocrine-disrupting chemicals and disease endpoints. Int. J. Mol. Sci. 2023, 24, 5342. [Google Scholar] [CrossRef]
- Lu, Y.; Song, S.; Wang, R.; Liu, Z.; Meng, J.; Sweetman, A.J.; Jenkins, A.; Ferrier, R.C.; Li, H.; Luo, W.; et al. Impacts of soil and water pollution on food safety and health risks in China. Environ. Int. 2015, 77, 5–15. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Ghosh, B.N.; Mishra, P.K.; Mandal, B.; Rao, C.S.; Sarkar, D.; Das, K.; Anil, K.S.; Lalitha, M.; Hati, K.M.; et al. Soil degradation in India: Challenges and potential solutions. Sustainability 2015, 7, 3528–3570. [Google Scholar] [CrossRef]
- Gomiero, T. Soil degradation, land scarcity and food security: Reviewing a complex challenge. Sustainability 2016, 8, 281. [Google Scholar] [CrossRef]
- Gao, Y.; Duan, Z.; Zhang, L.; Sun, D.; Li, X. The status and research progress of cadmium pollution in rice-(Oryza sativa L.) and wheat-(Triticum aestivum L.) cropping systems in China: A critical review. Toxics 2022, 10, 794. [Google Scholar] [CrossRef]
- Lebelo, K.; Malebo, N.; Mochane, M.J.; Masinde, M. Chemical contamination pathways and the food safety implications along the various stages of food production: A review. Int. J. Environ. Res. Public Health 2021, 18, 5795. [Google Scholar] [CrossRef]
- Ulko, Y.; Moskalenko, A.; Kucher, A.; Pavlenko, O.; Serbov, M. Economic evaluation of the consequences of soil pollution in the system of sustainable land management. Agric. Resour. Econ. Int. Sci. E 2022, 8, 266–300. [Google Scholar] [CrossRef]
- Government of Canada (GC). Reports 1 to 5 of the Commissioner of the Environment and Sustainable Development. 2024. Available online: https://www.oag-bvg.gc.ca/internet/English/parl_cesd_202404_01_e_44468.html (accessed on 31 May 2025).
- Gupta, G.S. Land degradation and challenges of food security. Rev. Eur. Stud. 2019, 11, 63. [Google Scholar] [CrossRef]
- Osman, K.T.; Osman, K.T. Polluted soils. In Management of Soil Problems; Springer: Berlin/Heidelberg, Germany, 2018; pp. 333–408. [Google Scholar]
- Xu, Y.; Yu, X.; Xu, B.; Peng, D.; Guo, X. Sorption of pharmaceuticals and personal care products on soil and soil components: Influencing factors and mechanisms. Sci. Total Environ. 2021, 753, 141891. [Google Scholar] [CrossRef] [PubMed]
- Delang, C.O. Causes and distribution of soil pollution in China. Environ. Socio-Econ. Stud. 2017, 5, 1–17. [Google Scholar] [CrossRef]
- Tindwa, H.J.; Singh, B.R. Soil pollution and agriculture in sub-Saharan Africa: State of the knowledge and remediation technologies. Front. Soil Sci. 2023, 2, 1101944. [Google Scholar] [CrossRef]
- Zahoor, I.; Mushtaq, A. Water pollution from agricultural activities: A critical global review. Int. J. Chem. Biochem. Sci 2023, 23, 164–176. [Google Scholar]
- Annar, S. The characteristics, toxicity and effects of heavy metals arsenic, mercury and cadmium: A review. Int. J. Multidiscip. Educ. Res. 2022, 11, 35–43. [Google Scholar]
- Basu, M. Impact of mercury and its toxicity on health and environment: A general perspective. In Mercury Toxicity: Challenges and Solutions; Springer Nature: Singapore, 2023; pp. 95–139. [Google Scholar]
- Maxim, L. The Birth of Green Chemistry: A Political History. Sci. Technol. Hum. Values 2025, 50, 144–168. [Google Scholar] [CrossRef]
- Svatos, R.L. Contaminated Sites. In Women in Infrastructure; Springer International Publishing: Cham, Switzerland, 2022; pp. 361–389. [Google Scholar]
- Najam, L.; Alam, T. Occurrence, distribution, and fate of emerging persistent organic pollutants (POPs) in the environment. In Emerging Contaminants and Plants: Interactions, Adaptations and Remediation Technologies; Springer International Publishing: Cham, Switzerland, 2023; pp. 135–161. [Google Scholar]
- Virto, I.; Imaz, M.J.; Fernández-Ugalde, O.; Gartzia-Bengoetxea, N.; Enrique, A.; Bescansa, P. Soil degradation and soil quality in Western Europe: Current situation and future perspectives. Sustainability 2014, 7, 313–365. [Google Scholar] [CrossRef]
- Hannam, I. Soil governance and land degradation neutrality. In Soil Security; Elsevier: Amsterdam, The Netherlands, 2022; Volume 6, p. 100030. [Google Scholar]
- Rillig, M.C. Microplastic in terrestrial ecosystems and the soil? Environ. Sci. Technol. 2012, 46, 6453–6454. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic use in agriculture and its consequential resistance in environmental sources: Potential public health implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef]
- Samuel, P.O.; Edo, G.I.; Oloni, G.O.; Ugbune, U.; Ezekiel, G.O.; Essaghah, A.E.A.; Agbo, J.J. Effects of chemical contaminants on the ecology and evolution of organisms a review. Chem. Ecol. 2023, 39, 1071–1107. [Google Scholar] [CrossRef]
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Zlati, M.L.; Georgescu, L.P.; Iticescu, C.; Ionescu, R.V.; Antohi, V.M. New approach to modelling the impact of heavy metals on the European Union’s water resources. Int. J. Environ. Res. Public Health 2022, 20, 45. [Google Scholar] [CrossRef]
- European Environmental Agency (EEA). Heavy Metal Emissions in Europe. 2024. Available online: https://www.eea.europa.eu/en/analysis/indicators/heavy-metal-emissions-in-europe?activeAccordion=ecdb3bcf-bbe9-4978-b5cf-0b136399d9f8 (accessed on 28 May 2025).
- Zhou, Q.; Yang, N.; Li, Y.; Ren, B.; Ding, X.; Bian, H.; Yao, X. Total concentrations and sources of heavy metal pollution in global river and lake water bodies from 1972 to 2017. Glob. Ecol. Conserv. 2020, 22, e00925. [Google Scholar] [CrossRef]
- Mititelu, M.; Neacșu, S.M.; Busnatu, Ș.S.; Scafa-Udriște, A.; Andronic, O.; Lăcraru, A.E.; Ioniță-Mîndrican, C.-B.; Lupuliasa, D.; Negrei, C.; Olteanu, G. Assessing Heavy Metal Contamination in Food: Implications for Human Health and Environmental Safety. Toxics 2025, 13, 333. [Google Scholar] [CrossRef]
- Saint-Laurent, D.; Hähni, M.; St-Laurent, J.; Baril, F. Comparative assessment of soil contamination by lead and heavy metals in riparian and agricultural areas (Southern Québec, Canada). Int. J. Environ. Res. Public Health 2010, 7, 3100–3114. [Google Scholar] [CrossRef]
- Nahlik, A.M.; Blocksom, K.A.; Herlihy, A.T.; Kentula, M.E.; Magee, T.K.; Paulsen, S.G. Use of national-scale data to examine human-mediated additions of heavy metals to wetland soils of the US. Environ. Monit. Assess. 2019, 191, 336. [Google Scholar] [CrossRef]
- Gonçalves Jr, A.C.; Nacke, H.; Schwantes, D.; Coelho, G.F. Heavy metal contamination in Brazilian agricultural soils due to application of fertilizers. In Environmental Risk Assessment of Soil Contamination; IntechOpen: London, UK, 2014. [Google Scholar]
- Huang, P.M.; Iskandar, I.K. Soils and Groundwater Pollution and Remediation: Asia, Africa, and Oceania; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Clark, J.H.A.; Tredoux, M.; Van Huyssteen, C.W. Heavy metals in the soils of Bloemfontein, South Africa: Concentration levels and possible sources. Environ. Monit. Assess. 2015, 187, 439. [Google Scholar] [CrossRef]
- Ogundele, D.T.; Adio, A.A.; Oludele, O.E. Heavy metal concentrations in plants and soil along heavy traffic roads in North Central Nigeria. J. Environ. Anal. Toxicol. 2015, 5, 1. [Google Scholar]
- Rabbani, A.; Bag, R.; Samui, P.; Kumari, S. Remediation of Heavy Metals from Contaminated Soil: State of the Art Review of Sources, Risk, Policies and Available Remediation Techniques. Trans. Indian Natl. Acad. Eng. 2025, 10, 19–31. [Google Scholar] [CrossRef]
- Dhara, A.; Dutta, R. A review on sources and distribution of polycyclic aromatic hydrocarbons (PAHs) in wetland ecosystem: Focusing on plant-biomonitoring and phytoremediation. Environ. Sci. Pollut. Res. 2025, 32, 8743–8765. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, S.; Huang, F.; Luo, Q.; Ren, B.; Abo El-Maati, M.F.; El-Sappah, A.H. Fate of polycyclic aromatic hydrocarbons in the phytoremediation of different hydrocarbon contaminated soils with cotton, ryegrass, tall fescue, and wheat. Front. Plant Sci. 2025, 16, 1550234. [Google Scholar] [CrossRef]
- Boxall, A.B.; Rudd, M.A.; Brooks, B.W.; Caldwell, D.J.; Choi, K.; Hickmann, S.; Van Der Kraak, G. Pharmaceuticals and personal care products in the environment: What are the big questions? Environ. Health Perspect. 2012, 120, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Pullagurala, V.L.R.; Rawat, S.; Adisa, I.O.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Plant uptake and translocation of contaminants of emerging concern in soil. Sci. Total Environ. 2018, 636, 1585–1596. [Google Scholar] [CrossRef] [PubMed]
- Rajandran, P.; Masngut, N.; Manas, N.H.A.; Azelee, N.I.W.; Fuzi, S.F.Z.M.; Bunyamin, M.A.H. Fixed-bed adsorption for industrial wastewater purification: An in-depth review. Int. J. Environ. Sci. Technol. 2025, 22, 3943–3964. [Google Scholar] [CrossRef]
- Gunasekara, M.I.; Mahawaththa, I.; Madhubhashini, D.; Amarasena, K. Pollution from Land-Based Sources: Industrial and Urban Runoff. In Coastal and Marine Pollution: Source to Sink, Mitigation and Management; Wiley: Hoboken, NJ, USA, 2025; pp. 27–44. [Google Scholar]
- Lead, J.R.; Batley, G.E.; Alvarez, P.J.; Croteau, M.N.; Handy, R.D.; McLaughlin, M.J.; Schirmer, K. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects—An updated review. Environ. Toxicol. Chem. 2018, 37, 2029–2063. [Google Scholar] [CrossRef]
- Shyamalagowri, S.; Shanthi, N.; Manjunathan, J.; Kamaraj, M.; Manikandan, A.; Aravind, J. Techniques for the detection and quantification of emerging contaminants. Phys. Sci. Rev. 2023, 8, 2191–2218. [Google Scholar] [CrossRef]
- Liu, N.; Zhao, J.; Du, J.; Hou, C.; Zhou, X.; Chen, J.; Zhang, Y. Non-phytoremediation and phytoremediation technologies of integrated remediation for water and soil heavy metal pollution: A comprehensive review. Sci. Total Environ. 2024, 948, 174237. [Google Scholar] [CrossRef]
- Li, F.; Zhao, X.; Qi, R.; He, L.; Wan, D.; Zhang, J.; Zhang, X.; Wang, Y.; Wu, G.; Huang, H.; et al. Remediation of Heavy Metal Contaminated Soil by Functional Pellets of Charcoal Organic Fertilizer: Rhizosphere and Non-Rhizosphere Soil Microorganisms. Water Air Soil Pollut. 2025, 236, 408. [Google Scholar] [CrossRef]
- Mukherjee, R.; Pattanaik, J.K.; Purushothaman, P. Understanding Zinc: Its Genesis, Distribution and Uses. In Zinc; CRC Press: Boca Raton, FL, USA, 2024; pp. 1–13. [Google Scholar]
- Thakur, A.; Anadebe, V.C.; Kaur, H.; Kumar, A. Influences of Biochar on Phytoremediation Potential of Heavy Metals Contaminated Soils. In Biochar Revolution: Transforming Agriculture and Environment Management; Springer Nature: Cham, Switzerland, 2025; pp. 37–61. [Google Scholar]
- Adnan, M.; Xiao, B.; Ali, M.U.; Xiao, P.; Zhao, P.; Wang, H.; Bibi, S. Heavy metals pollution from smelting activities: A threat to soil and groundwater. Ecotoxicol. Environ. Saf. 2024, 274, 116189. [Google Scholar] [CrossRef]
- Angon, P.B.; Islam, M.S.; Das, A.; Anjum, N.; Poudel, A.; Suchi, S.A. Sources, effects and present perspectives of heavy metals contamination: Soil, plants and human food chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.M.; Li, H.K.; Xu, Z.L.; Fu, R.B. How do the occurrence patterns of potentially toxic elements (PTEs) control their release behaviours from Pb/Zn smelter contaminated soils? J. Clean. Prod. 2024, 434, 140334. [Google Scholar] [CrossRef]
- Xu, W.; Jin, Y.; Zeng, G. Introduction of heavy metals contamination in the water and soil: A review on source, toxicity and remediation methods. Green Chem. Lett. Rev. 2024, 17, 2404235. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Nepovimova, E.; Kuca, K.; Valko, M. Heavy metals: Toxicity and human health effects. Arch. Toxicol. 2025, 99, 153–209. [Google Scholar] [CrossRef]
- Nyika, J.; Dinka, M.O. (Eds.) Global Industrial Impacts of Heavy Metal Pollution in Sub-Saharan Africa; IGI Global: Hershey, PA, USA, 2023. [Google Scholar]
- Caporale, A.G.; Violante, A. Chemical processes affecting the mobility of heavy metals and metalloids in soil environments. Curr. Pollut. Rep. 2016, 2, 15–27. [Google Scholar] [CrossRef]
- Wang, L.; Putnis, C.V. Dissolution and precipitation dynamics at environmental mineral interfaces imaged by in situ atomic force microscopy. Acc. Chem. Res. 2020, 53, 1196–1205. [Google Scholar] [CrossRef]
- Khatun, J.; Intekhab, A.; Dhak, D. Effect of uncontrolled fertilization and heavy metal toxicity associated with arsenic (As), lead (Pb) and cadmium (Cd), and possible remediation. Toxicology 2022, 477, 153274. [Google Scholar] [CrossRef]
- Hafsteinsdóttir, E.G.; Camenzuli, D.; Rocavert, A.L.; Walworth, J.; Gore, D.B. Chemical immobilization of metals and metalloids by phosphates. Appl. Geochem. 2015, 59, 47–62. [Google Scholar] [CrossRef]
- Sakshi Singh, S.K.; Haritash, A.K. Polycyclic aromatic hydrocarbons: Soil pollution and remediation. Int. J. Environ. Sci. Technol. 2019, 16, 6489–6512. [Google Scholar] [CrossRef]
- Hong, H.; Liu, C.; Li, Z. Chemistry of soil-type dependent soil matrices and its influence on behaviors of pharmaceutical compounds (PCs) in soils. Heliyon 2023, 9, e22931. [Google Scholar] [CrossRef]
- Chen, C.H.; Liu, P.W.G.; Whang, L.M. Effects of natural organic matters on bioavailability of petroleum hydrocarbons in soil-water environments. Chemosphere 2019, 233, 843–851. [Google Scholar] [CrossRef]
- Shah, A.; Shahzad, S.; Munir, A.; Nadagouda, M.N.; Khan, G.S.; Shams, D.F.; Rana, U.A. Micelles as soil and water decontamination agents. Chem. Rev. 2016, 116, 6042–6074. [Google Scholar] [CrossRef] [PubMed]
- Keiluweit, M.; Kleber, M. Molecular-level interactions in soils and sediments: The role of aromatic π-systems. Environ. Sci. Technol. 2009, 43, 3421–3429. [Google Scholar] [CrossRef]
- Saeedi, M.; Li, L.Y.; Grace, J.R. Effect of co-existing heavy metals and natural organic matter on sorption/desorption of polycyclic aromatic hydrocarbons in soil: A review. Pollution 2020, 6, 1–24. [Google Scholar]
- Ren, X.; Zeng, G.; Tang, L.; Wang, J.; Wan, J.; Liu, Y.; Deng, R. Sorption, transport and biodegradation–an insight into bioavailability of persistent organic pollutants in soil. Sci. Total Environ. 2018, 610, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Li, M.; Qian, T.; Chen, J.; Pan, T. Influence of Surfactants on Interfacial Microbial Degradation of Hydrophobic Organic Compounds. Catalysts 2025, 15, 187. [Google Scholar] [CrossRef]
- Prasad, M.N.V.; Elchuri, S.V. Environmental contaminants of emerging concern: Occurrence and remediation. Chem.-Didact.-Ecol.-Metrol. 2023, 28, 57–77. [Google Scholar] [CrossRef]
- Tang, K.H.D. Environmental co-existence of microplastics and perfluorochemicals: A review of their interactions. Biointerface Res. Appl. Chem. 2023, 13, 587. [Google Scholar]
- Maity, S.; Guchhait, R.; Chatterjee, A.; Pramanick, K. Co-occurrence of co-contaminants: Cyanotoxins and microplastics, in soil system and their health impacts on plant–A comprehensive review. Sci. Total Environ. 2021, 794, 148752. [Google Scholar] [CrossRef]
- Leung, S.C.E.; Wanninayake, D.; Chen, D.; Nguyen, N.T.; Li, Q. Physicochemical properties and interactions of perfluoroalkyl substances (PFAS)-Challenges and opportunities in sensing and remediation. Sci. Total Environ. 2023, 905, 166764. [Google Scholar] [CrossRef]
- Alves, A.V.; Tsianou, M.; Alexandridis, P. Fluorinated surfactant adsorption on mineral surfaces: Implications for PFAS fate and transport in the environment. Surfaces 2020, 3, 516–566. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L.M. Fate of antibiotics in soil and their uptake by edible crops. Sci. Total Environ. 2017, 599, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Conde-Cid, M.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Fernández-Calviño, D.; Arias-Estévez, M. Tetracycline and sulfonamide antibiotics in soils: Presence, fate and environmental risks. Processes 2020, 8, 1479. [Google Scholar] [CrossRef]
- Khalid, N.; Aqeel, M.; Noman, A. Microplastics could be a threat to plants in terrestrial systems directly or indirectly. Environ. Pollut. 2020, 267, 115653. [Google Scholar] [CrossRef]
- Qi, Y.; Cao, H.; Pan, W.; Wang, C.; Liang, Y. The role of dissolved organic matter during Per-and Polyfluorinated Substance (PFAS) adsorption, degradation, and plant uptake: A review. J. Hazard. Mater. 2022, 436, 129139. [Google Scholar] [CrossRef]
- Adu, O.; Ma, X.; Sharma, V.K. Bioavailability, phytotoxicity and plant uptake of per-and polyfluoroalkyl substances (PFAS): A review. J. Hazard. Mater. 2023, 447, 130805. [Google Scholar] [CrossRef]
- Rasmusson, K.; Fagerlund, F. Per-and polyfluoroalkyl substances (PFAS) as contaminants in groundwater resources–A comprehensive review of subsurface transport processes. Chemosphere 2024, 362, 142663. [Google Scholar] [CrossRef] [PubMed]
- Aretaki, M.A.; Desmet, J.; Viana, M.; van Drooge, B.L. Comprehensive methodology for semi-volatile organic compound determination in ambient air with emphasis on polycyclic aromatic hydrocarbons analysis by GC–MS/MS. J. Chromatogr. A 2024, 1730, 465086. [Google Scholar] [CrossRef] [PubMed]
- Poole, C. An interphase model for retention in liquid chromatography. JPC-J. Planar Chromatogr.-Mod. TLC 2015, 28, 98–105. [Google Scholar] [CrossRef]
- Dhull, P.; Dunuweera, S.; Bietsch, J.; Bandu, R.; Wannere, C.; Achanta, S.; Krishnamurthy, D.; Qu, B.; Senanayake, C. Recent advances and application of liquid chromatography in pharmaceutical industry. J. Liq. Chromatogr. Relat. Technol. 2025, 48, 168–187. [Google Scholar] [CrossRef]
- Xu, J.L.; Thomas, K.V.; Luo, Z.; Gowen, A.A. FTIR and Raman imaging for microplastics analysis: State of the art, challenges and prospects. TrAC Trends Anal. Chem. 2019, 119, 115629. [Google Scholar] [CrossRef]
- Khosrowshahi, E.M.; Khataee, A.; Arefi-Oskoui, S.; Orooji, Y. Dispersive solid phase extraction of perfluorooctanoic acid from wastewater using chromium-doped CoFe layered double hydroxide for determination by LC-MS/MS. Microchem. J. 2024, 199, 109918. [Google Scholar] [CrossRef]
- Tang, H.; Zhao, Y.; Shan, S.; Yang, X.; Liu, D.; Cui, F.; Xing, B. Wrinkle-and edge-adsorption of aromatic compounds on graphene oxide as revealed by atomic force microscopy, molecular dynamics simulation, and density functional theory. Environ. Sci. Technol. 2018, 52, 7689–7697. [Google Scholar] [CrossRef]
- Yang, Z.; Ren, J.; Du, M.; Zhao, Y.; Yu, K. Enhanced laser-induced breakdown spectroscopy for heavy metal detection in agriculture: A review. Sensors 2022, 22, 5679. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, S.; Jiang, Y.; Chen, A.; Jin, M. Direct analysis of heavy metal elements in liquid water using femtosecond laser-induced breakdown spectroscopy for high-sensitivity detection. Talanta 2025, 286, 127512. [Google Scholar] [CrossRef]
- Khan, S.R.; Sharma, B.; Chawla, P.A.; Bhatia, R. Inductively coupled plasma optical emission spectrometry (ICP-OES): A powerful analytical technique for elemental analysis. Food Anal. Methods 2022, 15, 666–688. [Google Scholar] [CrossRef]
- Borrill, A.J.; Reily, N.E.; Macpherson, J.V. Addressing the practicalities of anodic stripping voltammetry for heavy metal detection: A tutorial review. Analyst 2019, 144, 6834–6849. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, J.; Wang, L.; Shang, L.; Cui, L.; Gao, Y.; Li, B.; Li, Y.F. Synchrotron-based techniques for studying the environmental health effects of heavy metals: Current status and future perspectives. TrAC Trends Anal. Chem. 2020, 122, 115721. [Google Scholar] [CrossRef]
- Majumdar, S.; Peralta-Videa, J.R.; Castillo-Michel, H.; Hong, J.; Rico, C.M.; Gardea-Torresdey, J.L. Applications of synchrotron μ-XRF to study the distribution of biologically important elements in different environmental matrices: A review. Anal. Chim. Acta 2012, 755, 1–16. [Google Scholar] [CrossRef]
- Hussain, C.M.; Kecili, R. Modern Environmental Analysis Techniques for Pollutants; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Biswas, B.; Qi, F.; Biswas, J.K.; Wijayawardena, A.; Khan, M.A.I.; Naidu, R. The fate of chemical pollutants with soil properties and processes in the climate change paradigm—A review. Soil Syst. 2018, 2, 51. [Google Scholar] [CrossRef]
- Hao, Y.; Ma, H.; Wang, Q.; Zhu, C.; He, A. Complexation behaviour and removal of organic-Cr (III) complexes from the environment: A review. Ecotoxicol. Environ. Saf. 2022, 240, 113676. [Google Scholar] [CrossRef] [PubMed]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhao, M.; Chen, L.; Gong, Z.; Hu, J.; Ma, D. Electrokinetic remediation for the removal of heavy metals in soil: Limitations, solutions and prospection. Sci. Total Environ. 2023, 903, 165970. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Jellali, S.; Anastopoulos, I.; Charabi, Y.; Hameed, B.H.; Hanna, K. Fenton oxidation for soil remediation: A critical review of observations in historically contaminated soils. J. Hazard. Mater. 2022, 424, 127670. [Google Scholar] [CrossRef]
- Arif, M.; Liu, G.; Yousaf, B.; Ahmed, R.; Irshad, S.; Ashraf, A.; Rashid, M.S. Synthesis, characteristics and mechanistic insight into the clays and clay minerals-biochar 11. surface interactions for contaminants removal-A review. J. Clean. Prod. 2021, 310, 127548. [Google Scholar] [CrossRef]
- Remucal, C.K.; Ginder-Vogel, M. A critical review of the reactivity of manganese oxides with organic contaminants. Environ. Sci. Process. Impacts 2014, 16, 1247–1266. [Google Scholar] [CrossRef]
- Boguta, P.; Sokołowska, Z. Zinc binding to fulvic acids: Assessing the impact of pH, metal concentrations and chemical properties of fulvic acids on the mechanism and stability of formed soluble complexes. Molecules 2020, 25, 1297. [Google Scholar] [CrossRef]
- Omo-Okoro, P.N.; Curtis, C.J.; Karásková, P.; Melymuk, L.; Oyewo, O.A.; Okonkwo, J.O. Kinetics, isotherm, and thermodynamic studies of the adsorption mechanism of PFOS and PFOA using inactivated and chemically activated maize tassel. Water Air Soil Pollut. 2020, 231, 485. [Google Scholar] [CrossRef]
- Omo-Okoro, P.N.; Adeiga, O.I.; Velempini, T.; Prabakaran, E.; Curtis, C.J.; Pillay, K. Nickel ion removal from aqueous media using polyaniline–macadamia nutshells and its reuse for photodegradation of orange dye. Int. J. Environ. Sci. Technol. 2023, 20, 8655–8672. [Google Scholar] [CrossRef]
- Babalola, B.M.; Wilson, L.D. Valorization of Eggshell as Renewable Materials for Sustainable Biocomposite Adsorbents—An Overview. J. Compos. Sci. 2024, 8, 414. [Google Scholar] [CrossRef]
- Alsharif, M.A. Understanding Adsorption: Theories, Techniques, and Applications; IntechOpen: London, UK, 2025; p. 14. [Google Scholar]
- Putra, N.R.; Zaini, M.A.A.; Kusuma, H.S.; Darmokoesoemo, H.; Faizal, A.N.M. Advances in chromium removal using biomass-derived activated carbon: A comprehensive review and bibliometric analysis. Environ. Prog. Sustain. Energy 2025, 44, e14598. [Google Scholar] [CrossRef]
- Strawn, D.G. Sorption mechanisms of chemicals in soils. Soil Syst. 2021, 5, 13. [Google Scholar] [CrossRef]
- Borchert, K.B.; Steinbach, C.; Reis, B.; Lappan, U.; Gerlach, N.; Mayer, M.; Schwarz, S.; Schwarz, D. Adsorption vs. surface precipitation of Cu2+ onto porous Poly (melamine-co-formaldehyde) particles. Microporous Mesoporous Mater. 2023, 348, 112383. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Xu, Y.; Qian, G. Phosphate adsorption on metal oxides and metal hydroxides: A comparative review. Environ. Rev. 2016, 24, 319–332. [Google Scholar] [CrossRef]
- Emenike, C.U.; Agamuthu, P.; Fauziah, S.H.; Omo-Okoro, P.N.; Jayanthi, B. Enhanced bioremediation of metal-contaminated soil by consortia of Proteobacteria. Water Air Soil Pollut. 2023, 234, 731. [Google Scholar] [CrossRef]
- Chen, W.; Li, W.; Wang, T.; Wen, Y.; Shi, W.; Zhang, W.; Yang, Y. Isolation of functional bacterial strains from chromium-contaminated site and bioremediation potentials. J. Environ. Manag. 2022, 307, 114557. [Google Scholar] [CrossRef]
- Rinklebe, J.; Shaheen, S.M.; Frohne, T. Amendment of biochar reduces the release of toxic elements under dynamic redox conditions in a contaminated floodplain soil. Chemosphere 2016, 142, 41–47. [Google Scholar] [CrossRef]
- Jiang, X.; Long, W.; Peng, L.; Xu, T.; He, F.; Tang, Y.; Zhang, W. Reductive immobilization of Cr (VI) in contaminated water by tannic acid. Chemosphere 2022, 297, 134081. [Google Scholar] [CrossRef]
- Fu, H.; Wei, C.; Qu, X.; Li, H.; Zhu, D. Strong binding of apolar hydrophobic organic contaminants by dissolved black carbon released from biochar: A mechanism of pseudomicelle partition and environmental implications. Environ. Pollut. 2018, 232, 402–410. [Google Scholar] [CrossRef]
- Lee, S.; Kim, D.H.; Kim, K.W. The enhancement and inhibition of mercury reduction by natural organic matter in the presence of Shewanella oneidensis MR-1. Chemosphere 2018, 194, 515–522. [Google Scholar] [CrossRef]
- Zhao, L.; Bian, J.; Zhang, Y.; Zhu, L.; Liu, Z. Comparison of the sorption behaviors and mechanisms of perfluorosulfonates and perfluorocarboxylic acids on three kinds of clay minerals. Chemosphere 2014, 114, 51–58. [Google Scholar] [CrossRef]
- Lyu, X.; Liu, X.; Sun, Y.; Ji, R.; Gao, B.; Wu, J. Transport and retention of perfluorooctanoic acid (PFOA) in natural soils: Importance of soil organic matter and mineral contents, and solution ionic strength. J. Contam. Hydrol. 2019, 225, 103477. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Schlenk, D.; Gan, J. A direct method for quantifying the effects of aging on the bioavailability of legacy contaminants in soil and sediment. Environ. Sci. Technol. Lett. 2019, 6, 148–152. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Exploring Linkages Between Soil Health and Human Health. 2024. Available online: https://nap.nationalacademies.org/read/27459/chapter/8#248 (accessed on 11 May 2025).
- Kaiser, K.; Mikutta, R.; Guggenberger, G. Increased stability of organic matter sorbed to ferrihydrite and goethite on aging. Soil Sci. Soc. Am. J. 2007, 71, 711–719. [Google Scholar] [CrossRef]
- Leonardi, V.; Šašek, V.; Petruccioli, M.; D’Annibale, A.; Erbanová, P.; Cajthaml, T. Bioavailability modification and fungal biodegradation of PAHs in aged industrial soils. Int. Biodeterior. Biodegrad. 2007, 60, 165–170. [Google Scholar] [CrossRef]
- Bourg, I.C.; Sposito, G. Connecting the molecular scale to the continuum scale for diffusion processes in smectite-rich porous media. Environ. Sci. Technol. 2010, 44, 2085–2091. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Yang, X.; Lin, Y.; Shi, J.; Prominski, A.; Clayton, C.; Ostroff, E.; Tian, B. Dissecting biological and synthetic soft–hard interfaces for tissue-like systems. Chem. Rev. 2021, 122, 5233–5276. [Google Scholar] [CrossRef]
- Grathwohl, P. Diffusion in Natural Porous Media: Contaminant Transport, Sorption/Desorption and Dissolution Kinetics; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 1. [Google Scholar]
- Wang, C.; Wang, R.; Huo, Z.; Xie, E.; Dahlke, H.E. Colloid transport through soil and other porous media under transient flow conditions—A review. Wiley Interdiscip. Rev. Water 2020, 7, e1439. [Google Scholar] [CrossRef]
- Li, Y.; Huo, Z.; Ying, Y.; Duan, L.; Jiang, C.; Chen, W. Effects of transient flow conditions on colloid-facilitated release of decabromodiphenyl ether: Implications for contaminant mobility at e-waste recycling sites. Eco-Environ. Health 2024, 3, 317–324. [Google Scholar] [CrossRef]
- Essaid, H.I.; Bekins, B.A.; Cozzarelli, I.M. Organic contaminant transport and fate in the subsurface: Evolution of knowledge and understanding. Water Resour. Res. 2015, 51, 4861–4902. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Li, Y.; Li, L.; Tang, M.; Hu, W.; Chen, L.; Ai, S. Speciation of heavy metals in soils and their immobilization at micro-scale interfaces among diverse soil components. Sci. Total Environ. Health 2022, 825, 153862. [Google Scholar] [CrossRef] [PubMed]
- Rimola, A.; Costa, D.; Sodupe, M.; Lambert, J.F.; Ugliengo, P. Silica surface features and their role in the adsorption of biomolecules: Computational modeling and experiments. Chem. Rev. 2013, 113, 4216–4313. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, J.; An, P.; Yang, B.; Hou, D.; Pu, S. Understanding the dilemmas and breakdown of the reactive migration of in situ groundwater injection reagents from an environmental geology perspective. Crit. Rev. Environ. Sci. Technol. 2024, 54, 747–770. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, J.; Huang, Z.; Jiang, Y.; Zeng, Z.; Han, L.; Yu, J. Migration of total petroleum hydrocarbon and heavy metal contaminants in the soil–groundwater interface of a petrochemical site using machine learning: Impacts of convection and diffusion. RSC Adv. 2024, 14, 32304–32313. [Google Scholar] [CrossRef]
- Knight, A.W.; Ilani-Kashkouli, P.; Harvey, J.A.; Greathouse, J.A.; Ho, T.A.; Kabengi, N.; Ilgen, A.G. Interfacial reactions of Cu (II) adsorption and hydrolysis driven by nano-scale confinement. Environ. Sci. Nano 2020, 7, 68–80. [Google Scholar] [CrossRef]
- Xiao, C.; Shi, P.; Yan, W.; Chen, L.; Qian, L.; Kim, S.H. Thickness and structure of adsorbed water layer and effects on adhesion and friction at nanoasperity contact. Colloids Interfaces 2019, 3, 55. [Google Scholar] [CrossRef]
- Bie, C.; Yang, J.; Zeng, X.; Wang, Z.; Sun, X.; Yang, Z.; Zhang, X. Nanoconfinement Effects in Electrocatalysis and Photocatalysis. Small 2025, 21, 2411184. [Google Scholar] [CrossRef]
- Dai, J.; Zhang, H. Recent advances in catalytic confinement effect within micro/meso-porous crystalline materials. Small 2021, 17, 2005334. [Google Scholar] [CrossRef]
- Grommet, A.B.; Feller, M.; Klajn, R. Chemical reactivity under nanoconfinement. Nat. Nanotechnol. 2020, 15, 256–271. [Google Scholar] [CrossRef]
- Voigtländer, A.; Houssais, M.; Bacik, K.A.; Bourg, I.C.; Burton, J.C.; Daniels, K.E.; Datta, S.S.; Del Gado, E.; Deshpande, N.S.; Devauchelle, O.; et al. Soft matter physics of the ground beneath our feet. Soft Matter 2024, 20, 5859–5888. [Google Scholar] [CrossRef]
- Teixeira, W.G.; Ceddia, M.B.; Ottoni, M.V.; Donnagema, G.K. Application of Soil Physics in Environmental Analyses; Springer: Cham, Switzerland, 2014; 499p. [Google Scholar]
- Nabipour, I.; Raoof, A.; Cnudde, V.; Aghaei, H.; Qajar, J. A computationally efficient modeling of flow in complex porous media by coupling multiscale digital rock physics and deep learning: Improving the tradeoff between resolution and field-of-view. Adv. Water Resour. 2024, 188, 104695. [Google Scholar] [CrossRef]
- Faraone, A.; Magazù, S.; Maisano, G.; Ponterio, R.; Villari, V. Experimental evidence of slow dynamics in semidilute polymer solutions. Macromolecules 1999, 32, 1128–1133. [Google Scholar] [CrossRef]
- Lukichev, A. Physical meaning of the stretched exponential Kohlrausch function. Phys. Lett. A 2019, 383, 2983–2987. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.; Jiang, Y.; Wang, H.; Mosa, A.; Ling, W. Potential interaction mechanisms between PAHs and glomalin related-soil protein (GRSP). Chemosphere 2023, 337, 139287. [Google Scholar] [CrossRef] [PubMed]
- Cipelletti, L.; Ramos, L. Slow dynamics in glassy soft matter. J. Phys. Condens. Matter 2005, 17, R253. [Google Scholar] [CrossRef]
- Guo, B.; Zeng, J.; Brusseau, M.L. A mathematical model for the release, transport, and retention of per-and polyfluoroalkyl substances (PFAS) in the vadose zone. Water Resour. Res. 2020, 56, e2019WR026667. [Google Scholar] [CrossRef]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Rinklebe, J. Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation–A review. Earth-Sci. Rev. 2017, 171, 621–645. [Google Scholar] [CrossRef]
- Wang, L.; Hou, D.; Shen, Z.; Zhu, J.; Jia, X.; Ok, Y.S.; Tack, F.M.G.; Rinklebe, J. Field trials of phytomining and phytoremediation: A critical review of influencing factors and effects of additives. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2724–2774. [Google Scholar] [CrossRef]
- Guemiza, K.; Coudert, L.; Metahni, S.; Mercier, G.; Besner, S.; Blais, J.F. Treatment technologies used for the removal of As, Cr, Cu, PCP and/or PCDD/F from contaminated soil: A review. J. Hazard. Mater. 2017, 333, 194–214. [Google Scholar] [CrossRef]
- Karna, R.R.; Noerpel, M.R.; Luxton, T.P.; Scheckel, K.G. Point of zero charge: Role in pyromorphite formation and bioaccessibility of lead and arsenic in phosphate-amended soils. Soil Syst. 2018, 2, 22. [Google Scholar] [CrossRef]
- Dzhardimalieva, G.I.; Uflyand, I.E. Design strategies of metal complexes based on chelating polymer ligands and their application in nanomaterials science. J. Inorg. Organomet. Polym. Mater. 2018, 28, 1305–1393. [Google Scholar] [CrossRef]
- Petrillo, A.; Fraternali, F.; Acampora, A.; Di Chiara, G.; Colangelo, F.; Farina, I. Innovative Solidification and Stabilization Techniques Using Industrial By-Products for Soil Remediation. Appl. Sci. 2025, 15, 4002. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Tyrer, M.; Hills, C.D.; Yang, X.M.; Carey, P. Immobilisation of heavy metal in cement-based solidification/stabilisation: A review. Waste Manag. 2009, 29, 390–403. [Google Scholar] [CrossRef]
- Ma, J.; Lei, L.; Gong, Z.; Wang, Z.; Liu, H.; Chen, G.; Guo, G. Strategies and mechanisms for improving groundwater remediation efficiency of chlorinated ethenes by controlling the particle size of polyhydroxyalkanoate. Chem. Eng. J. 2024, 496, 154038. [Google Scholar] [CrossRef]
- African Forest Landscape Restoration Initiative (AFR100). “AFR100 Infographic”. 2021. Available online: https://www.ideassonline.org/public/pdf/AFR100-Initiative-ENG.pdf (accessed on 31 May 2025).
- Tye, S.; Pool, J.R.; Lomeli, L.G. The Potential for Nature-Based Solutions Initiatives to Incorporate and Scale Climate Adaptation; World Resources Institute: Washington, DC, USA, 2022. [Google Scholar]
- Zhang, J.; Lin, X. Cleaning up Toxic Soils in China: A trillion-Dollar Question. International Institute for Sustainable Management (IISM). 2018. Available online: https://www.iisd.org/articles/toxic-soil-china (accessed on 2 June 2025).
- Tierney, A. Bipartisan Infrastructure Law Spending on Environmental Remediation as of 2022. 2024. Available online: https://www.statista.com/statistics/1395287/bipartisan-infrastructure-law-spending-environmental-remediation/ (accessed on 2 June 2025).
- European Environmental Agency (EEA). Soil Contamination Widespread in Europe. 2020. Available online: https://www.eea.europa.eu/highlights/soil-contamination-widespread-in-europe (accessed on 2 June 2025).
- Pittock, J. Yes, Australia’s Environment Is on a Depressing Path—But $7 Billion a Year Would Transform It. In Environment, Energy Climate. The Australian National University Report, Canberra, Australia. 2024. Available online: https://reporter.anu.edu.au/all-stories/yes-australias-environment-is-on-a-depressing-path-but-7-billion-a-year-would-transform-it (accessed on 2 June 2025).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omo-Okoro, P.; Ofori, P.; Amalapridman, V.; Dadrasnia, A.; Abbey, L.; Emenike, C. Soil Pollution and Its Interrelation with Interfacial Chemistry. Molecules 2025, 30, 2636. https://doi.org/10.3390/molecules30122636
Omo-Okoro P, Ofori P, Amalapridman V, Dadrasnia A, Abbey L, Emenike C. Soil Pollution and Its Interrelation with Interfacial Chemistry. Molecules. 2025; 30(12):2636. https://doi.org/10.3390/molecules30122636
Chicago/Turabian StyleOmo-Okoro, Patricia, Peter Ofori, Vijitha Amalapridman, Arezoo Dadrasnia, Lord Abbey, and Chijioke Emenike. 2025. "Soil Pollution and Its Interrelation with Interfacial Chemistry" Molecules 30, no. 12: 2636. https://doi.org/10.3390/molecules30122636
APA StyleOmo-Okoro, P., Ofori, P., Amalapridman, V., Dadrasnia, A., Abbey, L., & Emenike, C. (2025). Soil Pollution and Its Interrelation with Interfacial Chemistry. Molecules, 30(12), 2636. https://doi.org/10.3390/molecules30122636








